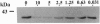Abstract
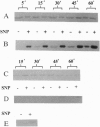
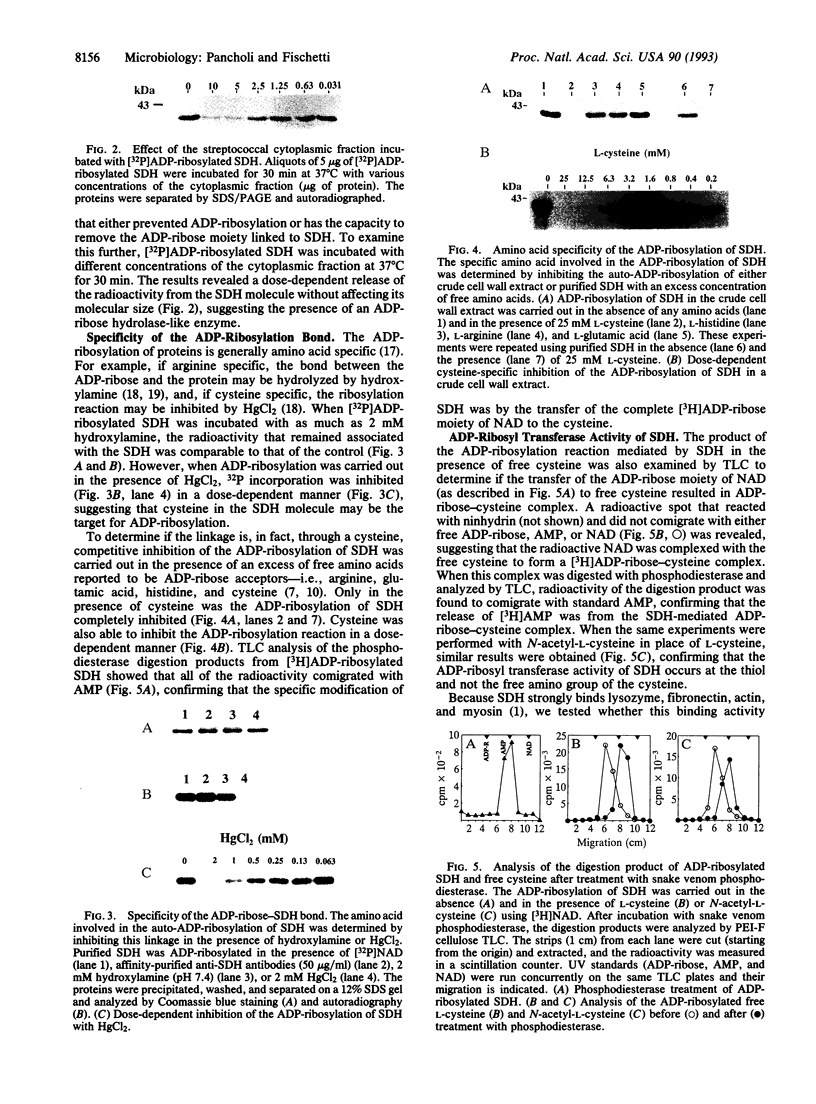
We recently identified an enzymatically active glyceraldehyde-3-phosphate dehydrogenase (EC 1.2.1.12; GAPDH) as a major protein on the surface of group A streptococci (SDH), which exhibits multiple binding activity to various mammalian proteins. We now report that the SDH molecule also functions as an ADP-ribosylating enzyme, which, in the presence of NAD, is auto-ADP-ribosylated. In a crude cell wall extract of group A streptococci, SDH is the only protein that is ADP-ribosylated. SDH found in the streptococcal cytoplasmic fraction could not be ADP-ribosylated in the presence of NAD. Treatment of ADP-ribosylated SDH with the cytoplasmic fraction removed the ADP-ribose from SDH, suggesting the presence of an ADP-ribosyl hydrolase in the cytoplasmic compartment. The covalent linkage of ADP-ribose to SDH was stable to neutral hydroxylamine, sensitive to HgCl2, and inhibitable by free cysteine, indicating that the modification was at a cysteine residue of SDH. In addition to its auto-ADP-ribosylation activity, purified SDH or streptococcal cell wall extracts were able to transfer the ADP-ribose moiety of NAD specifically to free cysteine, resulting in a true thioglycosidic linkage. Treatment of purified SDH or the crude cell wall extract with sodium nitroprusside, which spontaneously generates nitric oxide, was found to stimulate the ADP-ribosylation of SDH in a time-dependent manner. ADP-ribosylation and nitric oxide treatment inhibited the GAPDH activity of SDH. Since ADP-ribosylation and nitric oxide are involved in signal transduction events, the ADP-ribosylating activity of SDH may enable communication between host and parasite during infection by group A streptococci.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alefounder P. R., Perham R. N. Identification, molecular cloning and sequence analysis of a gene cluster encoding the class II fructose 1,6-bisphosphate aldolase, 3-phosphoglycerate kinase and a putative second glyceraldehyde 3-phosphate dehydrogenase of Escherichia coli. Mol Microbiol. 1989 Jun;3(6):723–732. doi: 10.1111/j.1365-2958.1989.tb00221.x. [DOI] [PubMed] [Google Scholar]
- Allen R. W., Trach K. A., Hoch J. A. Identification of the 37-kDa protein displaying a variable interaction with the erythroid cell membrane as glyceraldehyde-3-phosphate dehydrogenase. J Biol Chem. 1987 Jan 15;262(2):649–653. [PubMed] [Google Scholar]
- Bode J., Blumenstein M., Raftery M. A. Nonidentical alkylation sites in rabbit muscle glyceraldehyde-3-phosphate dehydrogenase. Biochemistry. 1975 Mar 25;14(6):1146–1152. doi: 10.1021/bi00677a008. [DOI] [PubMed] [Google Scholar]
- Brüne B., Lapetina E. G. Activation of a cytosolic ADP-ribosyltransferase by nitric oxide-generating agents. J Biol Chem. 1989 May 25;264(15):8455–8458. [PubMed] [Google Scholar]
- Brüne B., Lapetina E. G. Properties of a novel nitric oxide-stimulated ADP-ribosyltransferase. Arch Biochem Biophys. 1990 Jun;279(2):286–290. doi: 10.1016/0003-9861(90)90493-i. [DOI] [PubMed] [Google Scholar]
- Dimmeler S., Brüne B. Characterization of a nitric-oxide-catalysed ADP-ribosylation of glyceraldehyde-3-phosphate dehydrogenase. Eur J Biochem. 1992 Nov 15;210(1):305–310. doi: 10.1111/j.1432-1033.1992.tb17422.x. [DOI] [PubMed] [Google Scholar]
- Dimmeler S., Lottspeich F., Brüne B. Nitric oxide causes ADP-ribosylation and inhibition of glyceraldehyde-3-phosphate dehydrogenase. J Biol Chem. 1992 Aug 25;267(24):16771–16774. [PubMed] [Google Scholar]
- Goudot-Crozel V., Caillol D., Djabali M., Dessein A. J. The major parasite surface antigen associated with human resistance to schistosomiasis is a 37-kD glyceraldehyde-3P-dehydrogenase. J Exp Med. 1989 Dec 1;170(6):2065–2080. doi: 10.1084/jem.170.6.2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson M. K., Loflin P. T., Aboul-Ela N., Mingmuang M., Moss J., Jobson E. L. Modification of plasma membrane protein cysteine residues by ADP-ribose in vivo. J Biol Chem. 1990 Jul 5;265(19):10825–10828. [PubMed] [Google Scholar]
- Kots AYa, Skurat A. V., Sergienko E. A., Bulargina T. V., Severin E. S. Nitroprusside stimulates the cysteine-specific mono(ADP-ribosylation) of glyceraldehyde-3-phosphate dehydrogenase from human erythrocytes. FEBS Lett. 1992 Mar 23;300(1):9–12. doi: 10.1016/0014-5793(92)80153-8. [DOI] [PubMed] [Google Scholar]
- Lehmann A. R., Kirk-Bell S., Shall S., Whish W. J. The relationship between cell growth, macromolecular synthesis and poly ADP-ribose polymerase in lymphoid cells. Exp Cell Res. 1974 Jan;83(1):63–72. doi: 10.1016/0014-4827(74)90688-0. [DOI] [PubMed] [Google Scholar]
- Lottenberg R., Broder C. C., Boyle M. D., Kain S. J., Schroeder B. L., Curtiss R., 3rd Cloning, sequence analysis, and expression in Escherichia coli of a streptococcal plasmin receptor. J Bacteriol. 1992 Aug;174(16):5204–5210. doi: 10.1128/jb.174.16.5204-5210.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald L. J., Wainschel L. A., Oppenheimer N. J., Moss J. Amino acid-specific ADP-ribosylation: structural characterization and chemical differentiation of ADP-ribose-cysteine adducts formed nonenzymatically and in a pertussis toxin-catalyzed reaction. Biochemistry. 1992 Dec 1;31(47):11881–11887. doi: 10.1021/bi00162a029. [DOI] [PubMed] [Google Scholar]
- Michels P. A., Marchand M., Kohl L., Allert S., Wierenga R. K., Opperdoes F. R. The cytosolic and glycosomal isoenzymes of glyceraldehyde-3-phosphate dehydrogenase in Trypanosoma brucei have a distant evolutionary relationship. Eur J Biochem. 1991 Jun 1;198(2):421–428. doi: 10.1111/j.1432-1033.1991.tb16031.x. [DOI] [PubMed] [Google Scholar]
- Molina y Vedia L., McDonald B., Reep B., Brüne B., Di Silvio M., Billiar T. R., Lapetina E. G. Nitric oxide-induced S-nitrosylation of glyceraldehyde-3-phosphate dehydrogenase inhibits enzymatic activity and increases endogenous ADP-ribosylation. J Biol Chem. 1992 Dec 15;267(35):24929–24932. [PubMed] [Google Scholar]
- Moss J., Stanley S. J. Amino acid-specific ADP-ribosylation. Identification of an arginine-dependent ADP-ribosyltransferase in rat liver. J Biol Chem. 1981 Aug 10;256(15):7830–7833. [PubMed] [Google Scholar]
- Moss J., Stanley S. J., Nightingale M. S., Murtagh J. J., Jr, Monaco L., Mishima K., Chen H. C., Williamson K. C., Tsai S. C. Molecular and immunological characterization of ADP-ribosylarginine hydrolases. J Biol Chem. 1992 May 25;267(15):10481–10488. [PubMed] [Google Scholar]
- Moss J., Vaughan M. ADP-ribosylation of guanyl nucleotide-binding regulatory proteins by bacterial toxins. Adv Enzymol Relat Areas Mol Biol. 1988;61:303–379. doi: 10.1002/9780470123072.ch6. [DOI] [PubMed] [Google Scholar]
- Nathan C. Nitric oxide as a secretory product of mammalian cells. FASEB J. 1992 Sep;6(12):3051–3064. [PubMed] [Google Scholar]
- Pancholi V., Fischetti V. A. A major surface protein on group A streptococci is a glyceraldehyde-3-phosphate-dehydrogenase with multiple binding activity. J Exp Med. 1992 Aug 1;176(2):415–426. doi: 10.1084/jem.176.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pancholi V., Fischetti V. A. Identification of an endogenous membrane anchor-cleaving enzyme for group A streptococcal M protein. Its implication for the attachment of surface proteins in gram-positive bacteria. J Exp Med. 1989 Dec 1;170(6):2119–2133. doi: 10.1084/jem.170.6.2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pancholi V., Fischetti V. A. Isolation and characterization of the cell-associated region of group A streptococcal M6 protein. J Bacteriol. 1988 Jun;170(6):2618–2624. doi: 10.1128/jb.170.6.2618-2624.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope M. R., Murrell S. A., Ludden P. W. Covalent modification of the iron protein of nitrogenase from Rhodospirillum rubrum by adenosine diphosphoribosylation of a specific arginine residue. Proc Natl Acad Sci U S A. 1985 May;82(10):3173–3177. doi: 10.1073/pnas.82.10.3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh R., Green M. R. Sequence-specific binding of transfer RNA by glyceraldehyde-3-phosphate dehydrogenase. Science. 1993 Jan 15;259(5093):365–368. doi: 10.1126/science.8420004. [DOI] [PubMed] [Google Scholar]
- Tanuma S., Endo H. Identification in human erythrocytes of mono(ADP-ribosyl) protein hydrolase that cleaves a mono(ADP-ribosyl) Gi linkage. FEBS Lett. 1990 Feb 26;261(2):381–384. doi: 10.1016/0014-5793(90)80597-c. [DOI] [PubMed] [Google Scholar]
- Tanuma S., Kawashima K., Endo H. Eukaryotic mono(ADP-ribosyl)transferase that ADP-ribosylates GTP-binding regulatory Gi protein. J Biol Chem. 1988 Apr 15;263(11):5485–5489. [PubMed] [Google Scholar]
- Ueda K., Hayaishi O. ADP-ribosylation. Annu Rev Biochem. 1985;54:73–100. doi: 10.1146/annurev.bi.54.070185.000445. [DOI] [PubMed] [Google Scholar]
- West R. E., Jr, Moss J., Vaughan M., Liu T., Liu T. Y. Pertussis toxin-catalyzed ADP-ribosylation of transducin. Cysteine 347 is the ADP-ribose acceptor site. J Biol Chem. 1985 Nov 25;260(27):14428–14430. [PubMed] [Google Scholar]
- Zhang J., Snyder S. H. Nitric oxide stimulates auto-ADP-ribosylation of glyceraldehyde-3-phosphate dehydrogenase. Proc Natl Acad Sci U S A. 1992 Oct 15;89(20):9382–9385. doi: 10.1073/pnas.89.20.9382. [DOI] [PMC free article] [PubMed] [Google Scholar]