Abstract
The GABAergic synapse undergoes structural and functional maturation during early brain development. Maternal stress alters GABAergic synapses in the pup's brain that are associated with the pathophysiology of neuropsychiatric disorders in adults; however, the mechanism for this is still unclear. In this study, we examined the effects of maternal restraint stress on the development of Cation-Chloride Cotransporters (CCCs) and the GABAA receptor α1 and α5 subunits in the hippocampus of rat pups at different postnatal ages. Our results demonstrate that maternal restraint stress induces a transient but significant increase in the level of NKCC1 (Sodium–Potassium Chloride Cotransporter 1) only at P14, followed by a brief, yet significant increase in the level of KCC2 (Potassium-Chloride Cotransporter 2) at P21, which then decreases from P28 until P40. Thus, maternal stress alters NKCC1 and KCC2 ratio in the hippocampus of rat pups, especially during P14 to P28. Maternal restraint stress also caused biphasic changes in the level of GABAA receptor subunits in the pup's hippocampus. GABAA receptor α1 subunit gradually increased at P14 then decreased thereafter. On the contrary, GABAA receptor α5 subunit showed a transient decrease followed by a long-term increase from P21 until P40. Altogether, our study suggested that the maternal restraint stress might delay maturation of the GABAergic system by altering the expression of NKCC1, KCC2 and GABAA receptor α1 and α5 subunits in the hippocampus of rat pups. These changes demonstrate the dysregulation of inhibitory neurotransmission during early life, which may underlie the pathogenesis of psychiatric diseases at adolescence.
Keywords: Maternal restraint stress, Cation-chloride cotransporters, NKCC1, KCC2, GABAA receptor, Hippocampus
1. Introduction
Early life stressors that shape the stress response in offspring have profound effects on mood and cognition in adulthood (Davidson & McEwen, 2012). Chronic exposure to glucocorticoids contribute to the dysfunction of the inhibitory network and impairment of rhythmic oscillations, which are critical for the regulation of brain activity and complex cognitive processes (Hu & et al, 2010). A dysfunctional GABAergic system is associated with the pathogenesis of neuropsychiatric diseases such as schizophrenia, anxiety and depression (Hines & et al, 2012).
During brain development, GABAergic synapses are formed prior to the formation of glutamatergic synapses and the activation of the GABAA receptor depolarizes immature neurons (Ben-Ari and et al, 2012, Ben-Ari, 2002, Ben-Ari and et al, 2007). Excitatory GABA transmission plays important roles in various neurodevelopmental processes including; neuronal migration, cell proliferation, neurite outgrowth and generating synchronized network activity (Cherubini & et al, 2011). Cation Chloride Cotransporter (CCC) is the key controlling factor in controlling the switch of the GABAA receptor function. CCCs control the reversal potential of the GABAA receptor-mediated current (EGABA), which is important for the modulation of the GABAA receptor function. There are two main types of CCCs; the outwardly directed Potassium-Chloride Cotransporter 2 (KCC2), and the inwardly directed Sodium–Potassium-Chloride Cotransporter 1 (NKCC1). In immature neurons, NKCC1 increases the chloride reversal potential thus it accumulates Cl− inside the cell. KCC2, on the other hand, reduces the chloride reversal potential thus it extrudes Cl− out of the cell and shifts the actions of the GABA from excitation to inhibition. Although the other chloride regulators channels and transporters also take part in this sequence (Blaesse and et al, 2009, Medina and Chudotvorova, 2006), the expression of KCC2 is thought to initiate the developmental switch of the GABAA receptor function from excitatory to inhibitory transmission (Ben-Ari, 2002).
In addition to the expression of Cation-Chloride Cotransporters, the GABAA receptor undergoes postnatal changes in its structure and function by the differential expression of different subunits' composition (Jacob et al., 2008). The presence of GABAA receptor α1 subunits mediates phasic inhibition by inducing a more rapid decay rate in GABAA-mediated synaptic currents (Dunning & et al, 1999). In contrast, the GABAA receptor α5 subunits mediate tonic inhibition, which can be characterized by a slow decay rate of the synaptic current (Jacob et al., 2008). The α1 subunits are located at the synaptic sites and mostly found in mature neurons, while the α5 subunits are located at the extrasynaptic sites and found mostly in immature neurons prior to the formation of the inhibitory synapse (Jacob et al., 2008, Farrant and Nusser, 2005, Owens and Kriegstein, 2002). Thus, the maturation of the GABAergic function requires the precise expression of specific subunits of the GABAA receptor during postnatal brain development. The early expression of the GABAA receptor α5 subunit is required for the tonic inhibitory function of GABA, while the late expression of the α1 subunit is required for the phasic inhibition that indicates the maturation of the GABAA receptor function.
It is well documented that stress increases glucocorticoid hormones and thereby potentiates excitotoxic damage in hippocampal GABAergic neurons (Elliott and Sapolsky, 1992, Stein-Behrens and Sapolsky, 1992). Early life stress exerts an effect on the hippocampal neurons and predisposes individuals to psychosis (Stumpf and et al, 1989, Tornello and et al, 1982, Zhang and et al, 1990, Scheller-Gilkey and et al, 2003). The hippocampus exhibits subtle alterations subsequent to neuropsychiatric diseases such as schizophrenia and mania depressive disorder (Benes, 1999). Previous studies in postmortem brains from schizophrenia patients have shown a decrease in hippocampal GABAergic activity that could potentiate excitotoxic damage to hippocampal interneurons, consistent with abnormal oscillatory rhythms and increased basal metabolic activity (Benes, 1999). It is still unclear how prenatal stress affects the development of GABAergic synapses in the hippocampus of the offspring. In this study we hypothesized that prenatal stress may affect the structural and functional maturation of the GABAA receptor in the hippocampus of rat pups. Therefore, the purpose of this study was to examine the effect of maternal restraint stress on the levels of NKCC1 and KCC2, as well as GABAA receptors α1 and α5 subunits in the hippocampus of the offspring to provide insights about the involved mechanisms of maternal stress as a cause of dysregulation of GABAergic synapses that are known to be associated with the pathogenesis of psychiatric diseases at adulthood.
2. Materials and methods
2.1. Animals
Pregnant Sprague Dawley rats and their offspring were used in this experiment. Pregnant rats were obtained from the National Laboratory Animal Centre, Mahidol University, Salaya, Thailand. They were housed in a single housing condition with a temperature and humidity controlled environment and maintained on a 12 h light/dark cycle with free access to food and water. Each pregnant female rat was weighed on gestation day (GD) 7–21. In the morning of GD 21, each pregnant rat received nesting material, and thereafter, the cage was checked twice daily for the appearance of a litter. The day a litter gets discovered becomes designated as postnatal day 0 (P0), and the length of gestation was noted. All experiments were conducted according to the Guidelines for Care and Use of the Laboratory Animals and approved by the Experimental Animal Ethics Committee of the Institute of Molecular Biosciences, Mahidol University, Thailand (COA.MB-ACUC 2015/003). Every effort was taken to minimize the number of animals used and their suffering.
2.2. Maternal restraint stress
Pregnant rats were divided into two groups; 1) control group, 2) maternal restraint stress group (N = 4/group). For the restraint stress, each pregnant rat was put into a small Plexiglas cylindrical cage in which the length can be adjusted to accommodate the size of each animal. The restraint stress was performed during GD14-20, at four hours daily intervals during the light phase of the cycle as previously described (Surakul et al., 2011, Chutabhakdikul and Surakul, 2013). The control group was left undisturbed in their home cages. Gestation days 14–20 were selected because they represent the most sensitive period for the behavioral teratogenic effect of prenatal stress (Fride & Weinstock, 1984).
2.3. Tissue preparation
Whole hippocampal tissues were collected from rat pups at different postnatal days (P) from P7, P14, P21, P28 and P40, with n = 4/group. Brain tissues were then suspended in a lysis buffer composed of 50 mM Tris pH 7.4, 150 mM NaCl, 1 mM EDTA, 0.5% Sodium Deoxycholate, 1% SDS, 1 mM PMSF, 1% Triton-X-100 and supplemented with a complete protease and phosphatase inhibitor cocktail set (Calbiochem, Germany), then homogenized twice with a sonicator for 10 s each. The homogenized samples were centrifuged at 14,000 rpm, 4 °C for 15 min. The supernatant was collected for protein determination. The protein concentration from each sample was determined by a Bradford protein assay.
2.4. Western blot analysis
Cell lysates were mixed with a sodium dodecyl sulfate (SDS) sample buffer and boiled. Equal amounts (20 μg) of extracted protein samples were resolved in 10% SDS-PAGE and electrophoresis at 100 V for 150 min. The protein bands were then transferred to the PVDF membrane (Amersham, USA) at 100V for 135 min. The membranes were then incubated in a blocking solution containing 3% skimmed milk for NKCC1, KCC2 and GABAA receptor α1 and α5 subunits, and 5% skimmed milk for actin at room temperature for 60 min. Then the membranes were incubated with the following specific primary antibodies purchased from the available commercial sources. Polyclonal goat anti-NKCC1 (SC-21545; 1:500), polyclonal goat anti-KCC2 (SC-19420; 1:500), polyclonal goat anti-GABAA receptor α1 subunit (SC-7348; 1:500), polyclonal goat anti-GABAA receptor α5 subunit (SC-7357; 1:500), and monoclonal mouse anti-actin (SC-69879; 1:5000), all antibodies were purchased from Santa Cruz Biotechnology, USA. The membranes were then thoroughly washed 3 times using 0.1% Tween-TBS (TTBS) for 5 min each, and then incubated with an appropriate HRP-conjugated secondary antibody. After that, the membranes were washed 3 times using 0.1% TTBS for 5 min each, and then the signals were detected by an Enhanced Chemiluminescence System (ECL Prime, Amersham Biosciences, USA) and film exposure. The films were then scanned and digitally processed using Adobe Photoshop software. The intensities of the band were quantified using densitometry software (Image J, National Institutes of Health, USA). The immunoblot data were corrected for corresponding product of the β-actin extracted from the same tissue which serve as an internal control.
2.5. Data and statistical analysis
The data was statistically analyzed using GraphPad Prism software. Quantitative results were expressed as mean ± SEM, calculated from the duplicate experiments. The statistical significance of difference between means was evaluated using Student's t-test (unpaired, unless otherwise stated). Changes produced by prenatal stress were analyzed at different postnatal ages using a two way ANOVA with the prenatal stress and postnatal ages as independent variables and the protein levels as dependent variables; followed by a Tukey's post hoc multiple comparison test. The probability level of p ≤ 0.05 was considered to have a statistically significant difference between the two sets of data.
3. Results
3.1. Maternal restraint stress alters NKCC1 and KCC2 in the hippocampus of rat pups at puberty
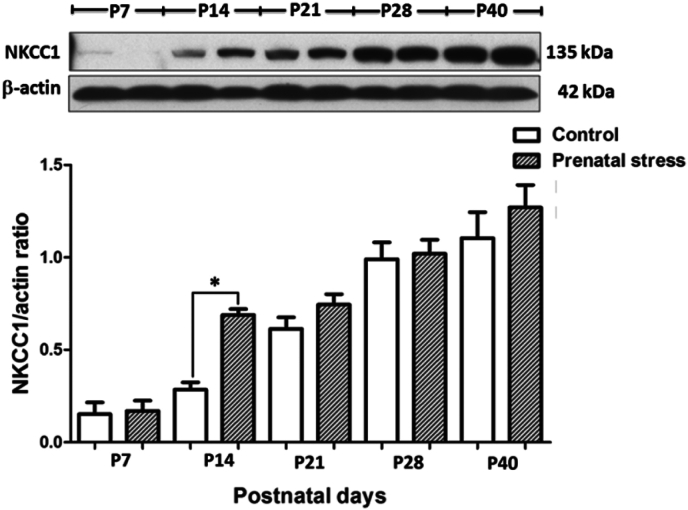
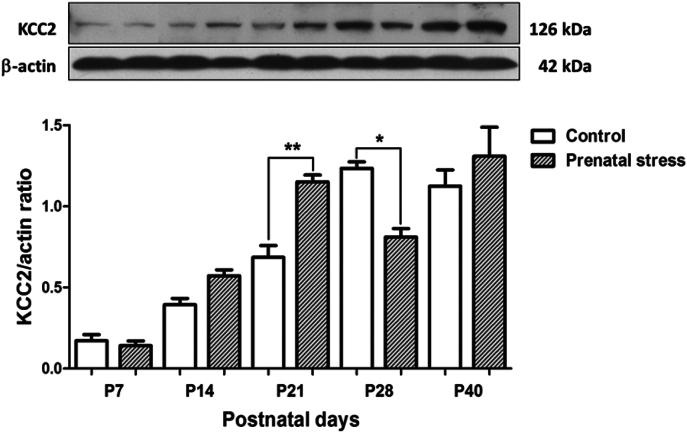
We examined the effects of maternal restraint stress, during the gestation day (GD) 14–20, on the levels of NKCC1 and KCC2 in the hippocampus of rat pups and compared between the groups at different postnatal ages. The results showed that maternal restraint stress caused a transient but significant increase in the level of NKCC1 in the hippocampus at P14 (p < 0.05) but no significant difference when observed at the other periods (Fig. 1). For KCC2, the results show that maternal restraint stress caused a transient but significant increase in the KCC2 level in the hippocampus of rat pups during the weaning period (P21) (p < 0.01) and this was followed by a transient but significant decrease during the preadolescence period (P28) (p < 0.05) (Fig. 2). However, there was no difference in the level of KCC2 when compare between groups during the adolescence period (P40).
Fig. 1.
Effects of maternal restraint stress on the level of NKCC1 in the hippocampus of rat pups. The (Upper) western blot analysis of NKCC1 in the hippocampal tissue comparing the prenatal stress groups and the control groups during P7–P40. The (Lower) bar graph displays the results from western blot analysis. Data were expressed as band densities/β-actin ratio; values represent Mean ± SEM, N = 4. There was a significant difference when compared with control group at *p < 0.05.
Fig. 2.
Effects of maternal restraint stress on the levels of KCC2 in the hippocampus of rat pups. The (Upper) western blot analysis of KCC2 in the hippocampal tissue comparing the prenatal stress groups and control groups during P7–P40. The (Lower) bar graph displays the results from the western blot analysis. Data were expressed as band densities/β-actin ratio; values represent Mean ± SEM, N = 4. There was a significant difference when compared with the control group at **p < 0.01 and *p < 0.05.
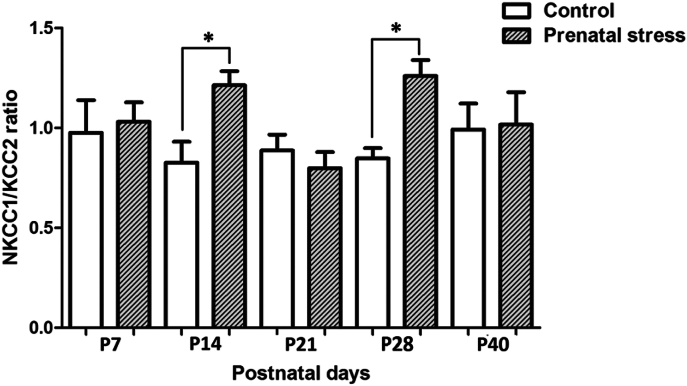
The NKCC1/KCC2 ratios were calculated and compared between groups across the different postnatal ages. We found that maternal restraint stress significantly increases the NKCC1/KCC2 ratio in the pup's hippocampus at P14 and P28 (p < 0.01). During this period, the NKCC1/KCC2 ratios in the hippocampus of prenatally stress pups exhibited more fluctuations than those observed in the control group (Fig. 3).
Fig. 3.
Bar graph comparing the NKCC1/KCC2 ratios between prenatal stress pups and the control pups at different postnatal ages. Data were expressed as Mean ± SEM, N = 4. There was a significant difference when compared with the control group at *p < 0.05.
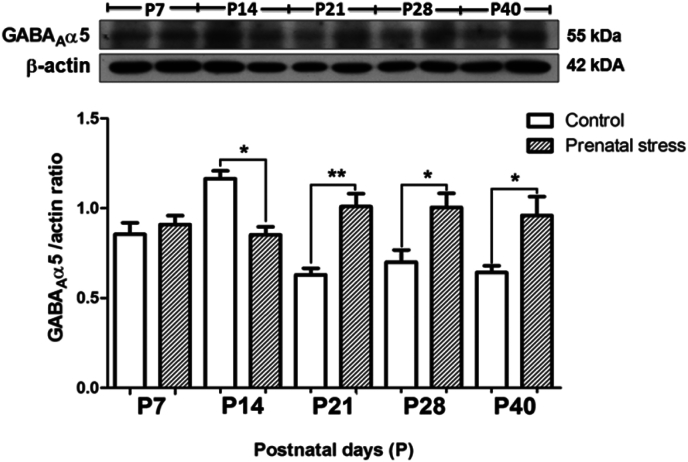
3.2. Maternal restraint stress delays development of the GABAA receptor α1 and α5 subunits in the hippocampus of rat pups
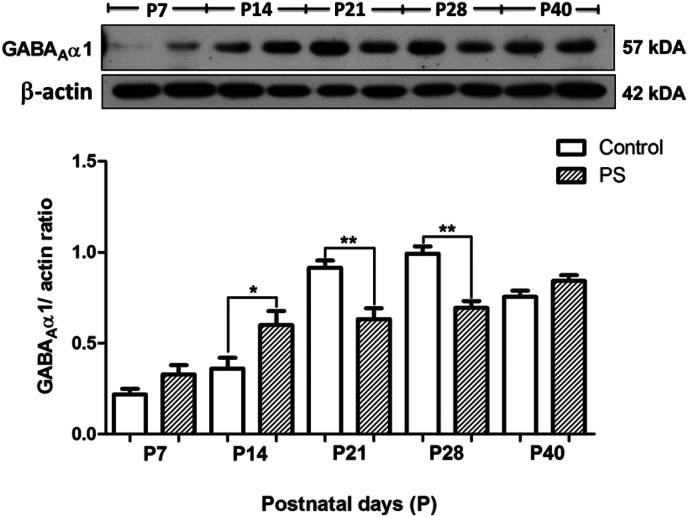
In the control pups, the developmental expressions of GABAA receptor α5 and α1 subunits in the hippocampus appear in the opposite way. The α5 subunit was highly expressed during P7–P14, and then declined during P21–P40 (Fig. 4, white bar) while the α1 subunit was expressed at a very low level during P7–P14, then continually increased during P21–P28 (Fig. 5, white bar). Maternal restraint stress caused a transient but significant decrease in the level of the GABAA receptor α5 subunit at P14 (p < 0.05) and followed by a long term increase at P21 (p < 0.01), P28 (p < 0.05) and P40 (p < 0.05) as compared to the control (Fig. 4). In contrast, maternal restraint stress caused a transient but significant increase in the level of the GABAA α1 subunit at P14 (p < 0.05) followed by a significant decrease at P21 (p < 0.01) and P28 (p < 0.01) (Fig. 5). We found no significant difference in the level of the α1 subunit when comparing between the groups at P40.
Fig. 4.
Effects of maternal restraint stress on the levels of the GABAA receptor α5 subunit in the hippocampus of rat pups. The (Upper) western blot analysis of GABAA receptor α5 subunit in the hippocampal tissue comparing the prenatal stress and control groups during P7–P40. The (Lower) bar graph displays the results from the western blot analysis. Data were expressed as a band densities/β-actin ratio; values represent Mean ± SEM, N = 4. There was a significant difference when compared with the control group at **p < 0.01 and *p < 0.05.
Fig. 5.
Effects of maternal restraint stress on the levels of the GABAA receptor α1 subunit in the hippocampus of rat pups. The (Upper) western blot analysis of the GABAA receptor α1 subunit in the hippocampal tissue comparing the prenatal stress and control groups during P7–P40. The (Lower) bar graph displays the results from the western blot analysis. Data were expressed as band densities/β-actin ratio; values represent Mean ± SEM, N = 3. There was a significant difference when compared with the control group at **p < 0.01 and *p < 0.05.
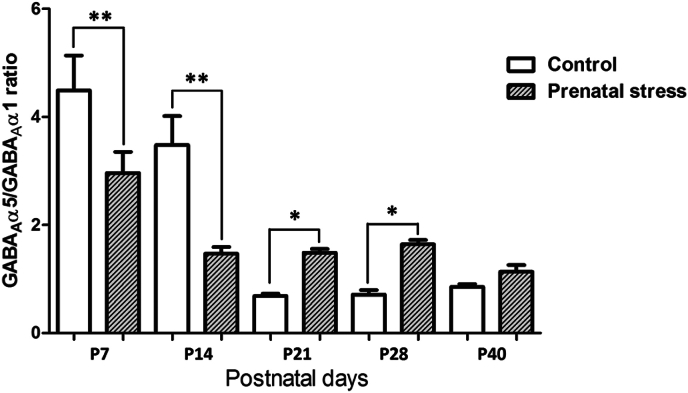
When the ratios of the α5/α1 subunit of the GABAA receptor were calculated and compared across the different postnatal ages, we found that maternal restraint stress causes a significant decrease in the ratio of the α5/α1 subunits during P7–P14 (p < 0.01), but a significant increase in the ratios of the α5/α1 subunits during P21–P28 (p < 0.05) (Fig. 6). In fact, we found small increase in the α5/α1 ratios at P40; however, there was no statistically significant difference when compared with the control group.
Fig. 6.
Bar graph comparing the ratios of the α5/α1 subunit of the GABAA receptor between the prenatal stress and the control groups at different postnatal ages. Data were expressed as Mean ± SEM, N = 4. There was a significant difference when compared with the control group at **p < 0.01 and *p < 0.05.
4. Discussion
NKCC1 induces depolarizing GABA transmission and is mostly active in immature neurons. GABAA receptor depolarization maintained by NKCC1 is important for proper brain development since it is a key factor in the control of several Ca2+-dependent developmental phenomena, including neuronal proliferation, migration and targeting (Rivera & et al, 1999). KCC2, in contrast, shifts the GABAA receptor activity from depolarization to hyperpolarization in mature neurons. In a developing hippocampus, the level of NKCC1 continuously increases starting from P21 to adulthood (Yan et al., 2001) while the hyperpolarizing GABA is completed by the second postnatal week due to the progressive reduction of NKCC1 activity in parallel with the enhanced activity of KCC2 (Rivera and et al, 1999, Emri and et al, 2001, Gulyas and et al, 2001, Stein and et al, 2004). Previous studies reported that KCC2 expression significantly increases during the second postnatal week, which is the co-incidence time point when the developmental switch of GABAA receptor activity is observed (Rivera and et al, 1999, Clayton and et al, 1998) and continually increased until P28 (Lu et al., 1999). In the hippocampus, NKCC1 and KCC2 expressions show relatively similar developmental patterns indicating that both are required for the reciprocal regulation of Cl− homeostasis, which is important for the functional maturation of the GABAA receptor.
In this study, maternal restraint stress induced a transient increase in the level of NKCC1 in the hippocampus of rat pups only at P14, while there was a KCC2 increase at P21 and then a decrease at P28. Previous studies reported that the alteration in NKCC1 under a stress response has no effect on the GABAA receptor function. In contrast, the stress-altered KCC2 level has a profound effect on the modulation of intracellular Cl− concentrations (Hewitt & et al, 2009). Our finding, that maternal restraint stress increases the KCC2 level in the pup's hippocampus at P21, indicates the protective mechanism that counteracts the higher level of NKCC1 at P14. Maternal restraint stress, which increases KCC2 level at P21 and then decreases it at P28, indicates the dysregulation of intracellular Cl− concentrations and the GABAA receptor mediated current during the preadolescence period. In the mature pyramidal neurons, KCC2 inhibition positively shifts the GABAA receptor reversal potential, thus, GABAA receptor activation causes depolarization (Rivera & et al, 1999). Our results indicate that maternal restraint stress may induce prolonged depolarizing of GABA in the hippocampus of rat pups until the pre-adolescence period, while in the control pups, hyperpolarizing GABA was completed by the early postnatal week.
Different brain regions may exhibit differential vulnerability to the effects of stress on the level of KCC2 and its activity. For examples, in the rat hypothalamic paraventricular nuclei, acute restraint stress has no effect on the level of KCC2, but attenuates the KCC2 activity (Hewitt & et al, 2009). Maternal restraint stress has no effect on the level of KCC2 in the amygdala of male pups at P14 and P22 (Laloux & et al, 2012). In contrast, prenatal stress causes a significant decrease in the KCC2 level and its activity in the hippocampus, as measured by phosphorylation of KCC2 on Ser 940 residue (Sarkar & et al, 2011). These findings, together with our results, indicate that maternal restraint stress might alter GABAA transmission in the hippocampus of prenatal stress pups during the post-weaning (P21) to pre-adolescence period (P28) and this mechanism might be due to the alteration in the levels of KCC2.
The underlying mechanism by which prenatal stress alters KCC2 levels remains unclear. Studies have shown that brain a derived neurotrophic factor (BDNF) regulates the expression of KCC2 in both the young and adult brains (Aguado and et al, 2003, Rivera and et al, 2002). BDNF is seen to down-regulate KCC2 expression in the adult hippocampal slices via the activation of tyrosine receptor kinase B (TrkB) (Rivera & et al, 2002). In addition, BDNF promotes KCC2 expression in the developing mice forebrain (Aguado & et al, 2003). Therefore, BDNF regulation of KCC2 expression varies depending on the developmental stages and brain regions. Prenatal stress has been reported to decrease BDNF levels in the rat hippocampus at P21 (Van den Hove & et al, 2006). Taken together, the results suggest that an alteration in BDNF levels caused by prenatal stress might affect the KCC2 level in the pup's hippocampus. Although it has been noted that BDNF-induced alteration in KCC2 expression was not caused by neuronal excitability and network activity (Aguado & et al, 2003), the endogenous action that is regulating the changes is still elusive.
It is assumed that maternal restraint stress altering KCC2 level in the rat pups hippocampus at preadolescence period could affect the excitatory glutamatergic synapses as well. It has been reported that KCC2 has an important roles in the modulation of the dendritic spines and AMPA receptor diffusion by interacting with sub-membranous actin cytoskeleton (Gauvain & et al, 2011), therefore indicating KCC2 is also require for the production of long-term potentiation (LTP) in the hippocampus of young animals.
Prenatal stress has been linked to an increased risk of psychiatric disorders such as schizophrenia and depression (Charil & et al, 2010). Recent studies show that the KCC2 level is significantly decreased in the hippocampus of schizophrenia patients, while there was no change in the NKCC1 level (Hyde & et al, 2011). Thus, an increase in the NKCC1/KCC2 ratio indicates the delayed maturation of Cation-Chloride Cotransporters in the patient's brain, which may underlie the pathology of neuropsychiatric diseases. Recently, it was demonstrated the significant increase in the level of OXSR1 (Oxidative stress response kinase1) and WNK3 (With no K [lysine] protein kinase3) in the post-mortem brain of schizophrenia patients (Arion & Lewis, 2011). These proteins are kinases that regulate the activities of NKCC1 and KCC2, respectively. Consequently, changes in the level of OXSR1 and WNK3 can shift the balance of chloride transport and leading to an abnormal GABAergic transmission in the prefrontal cortex, thereby contributing to the impaired neural network synchrony and cognitive dysfunction in affected individuals (Arion & Lewis, 2011).
For the development of GABAA receptor subunits, our results are consistent with those that have been previously reported (Ramos and et al, 2004, Laurie et al., 1992). We found the α5 subunit is highly expressed during the early postnatal period and declined to the adult level around the 3rd postnatal week, while the α1 subunit was initially expressed at a small level during the 1st and the 2nd postnatal week and gradually increased until reaching its peak around the 3rd postnatal week. Prenatal stress delays the developmental shift of the GABAA receptor α1 and α5 subunits that normally occur around P21 in the control pups. This was clearly observed in the control pups that were seen to have manifested developmental increments in the α1 subunit at P21, but not in the prenatal stress pups, at least until P40. On the contrary, the control pups show a developmental decrease in the α5 subunit at P21, but not in the prenatal stress pups that were seen to maintain the expression of the α5 subunit at least until P40. As a result, the ratio of the α5/α1 subunits in the prenatal stress pups exhibits a significant increase during P7 and P14, but shows a significant decrease when observed at P21 and P28, as compared to the control group.
The underlying mechanism of prenatal stress that induces a prolonged increase of α5 subunits in the rat pup's hippocampus at preadolescence is still elusive. A prolonged increase in the α5 subunit in the pup's brain may create an unpredictable effect on GABA inhibitory transmission; especially during puberty (Shen & et al, 2007). In the hippocampal CA1 and neocortical pyramidal neurons, the extrasynaptic α5 subunit of the GABAA receptor mediates tonic inhibition and plays an important role in memory and learning (Rudolph & Mohler, 2014). An increased expression of the GABAA receptors' α5 subunit is associated with memory loss (Wang & et al, 2012) while the antagonist of the receptor can enhance learning and memory processes (Rudolph and Mohler, 2014, Ling and et al, 2015). Thus, our results suggest that prenatal stress induces an increase in the GABAA receptor's α5 subunit expression and the α5/α1 ratios in the hippocampus at the preadolescence period may underlie the long term effects of prenatal stress on learning and memory impairment in the offspring at adulthood. For the GABAA receptors α1 subunit, our findings are consistent with previous report that reveal exposure to stress in juvenile rats can induce biphasic changes in their behavior, including hyperactivity at juveniles which in adulthood becomes hypoactivity accompanied by behavioral anxiety that are associated with the decrease of α1 subunits in the hippocampus and amygdala (Jacobson-Pick & Richter-Levin, 2012). Taken together the results are in agreement, the juvenile period is a sensitive time and is more vulnerable to stress than other periods and this supports the hypothesis that prenatal stress is a predisposing factor for various neuropsychiatric diseases and memory impairment at later life.
In this study, we added further information that the developmental expression of the GABAA receptor α1 subunit is similar to the developmental pattern of the KCC2. Both the GABAA α1 subunit and KCC2 reach their peak around P21–P28 and this indicates that the GABAA α1 subunit and KCC2 might coordinate in enhancing the GABAA receptor mediated synaptic inhibition that occurs around this period. Interestingly, we found that prenatal stress induces changes in the levels of the GABAA α1 subunit and the KCC2 in a similar way. Our results correspond to what has been previously documented in that KCC2 could modulate the expression level of the GABAA receptor α1 subunit via an alteration in intracellular [Cl−] and the decay rate of GABA-mediated inhibitory transmission (Houston & et al, 2009). Indeed, lower intracellular [Cl−] resulted in a faster decay rate of the GABA transmission (Moroni & et al, 2011). It has been shown that KCC2 can manipulate the expression of GABAA receptor subunits, i.e., overexpression of KCC2 results in the reduction of intracellular [Cl−] and leads to an increase in the level of α1 and δ subunits (Succol & et al, 2012). Taken together, these results are in accordance with the hypothesis that prenatal stress reduces the KCC2 levels, which might lower the intracellular [Cl−], that acts as the intracellular signal and induces a faster decay rate of the GABAA receptor gating and, thus, decreases the expression of the α1 subunit of the GABAA receptor (Succol & et al, 2012). These changes indicate a delayed maturation of the GABAergic function in the hippocampus of prenatal stress pups, especially during the preadolescence period.
Additionally, it was reported that stress disrupts the GABAergic function in the brain in many ways. Stress induces dysfunction of the inhibitory network and impairs rhythmic oscillations leading to cognitive deficits commonly found in psychiatric disorders (Hu & et al, 2010). Prenatal stress disturbs the distribution of GABAergic interneurons in the cortical plate, reflecting the changes occurring in tangential migration and radial integration in the developing cortex (Stevens & et al, 2013). Prenatal stress causes a significant decrease in the frequency of spontaneous IPSCs in the immature hippocampal neurons (Grigoryan & Segal, 2013) and increasing the vulnerability to stressful situations in the offspring during adulthood accompanied by a reduction of benzodiazepine binding in the hippocampus (Fride & et al, 1985).
In summary, this study has shown that maternal restraint stress has the ability to differentially alter the levels of NKCC1, KCC2, and GABAA receptor α1 and α5 subunits in the hippocampus of rat pups. Consequently, these changes can lead to an imbalance of inhibitory transmission that may delineate the linkage between prenatal stress and neuropsychiatric disorders in later life. Our findings reveal that there is a strong connection between early life stress exposures with an increased risk of developing psychiatric disorders at adulthood. Furthermore, a reduction of KCC2 levels has been linked to the cause of epilepsy, which is considered as a risk factor for schizophrenia and autism. Moreover, a prolonged increase in the α5 subunits in the hippocampus of rat pups during adolescence indicates a prolongation of the slow decay rate of inhibitory transmission in the pup's hippocampus and predisposes it for neuropsychiatric diseases and memory impairment in adulthood.
5. Concluding summary
Experiencing adverse events during pregnancy has a negative impact on brain development and may increase vulnerability to developing neurological and psychiatric disorders later in life. The delayed maturation of the GABAergic function has been reported in the schizophrenic brain. As we demonstrated, fetal exposure to maternal stress hormones delays structural and functional development of GABA transmission in the rat pup's hippocampus during the preadolescence period. These changes may lead to the dis-regulation of GABA inhibitory transmission in the developing hippocampus. Similar patterns of changes in the KCC2 and GABAA receptor α1 subunits were observed in response to early life stress, accompanied by supporting evidence that indicates changes in KCC2 levels may underlie the effect of maternal stress on the alterations in the α1 subunit of the GABAA receptors. Moreover, prenatal stress also increases GABAA receptor α5 subunit expression throughout the preadolescence period, which may underlie the learning and memory impairments in the offspring at adulthood. In summary, we have provided an explanation of certain prenatal factors mediating structural and functional development of the GABAergic synapse that may be the link between prenatal stress and the emergence of neuro-psychiatric disorders at adulthood.
Acknowledgments
This work was supported by Thailand Research Fund to N. Chutabhakdikul (RSA-5780016). The authors have no conflicts of interest to declare.
References
- Aguado F. BDNF regulates spontaneous correlated activity at early developmental stages by increasing synaptogenesis and expression of the K+/Cl- co-transporter KCC2. Development. 2003;130(7):1267–1280. doi: 10.1242/dev.00351. [DOI] [PubMed] [Google Scholar]
- Arion D., Lewis D.A. Altered expression of regulators of the cortical chloride transporters nkcc1 and kcc2 in schizophrenia. Arch. Gen. Psychiatry. 2011;68(1):21–31. doi: 10.1001/archgenpsychiatry.2010.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Ari Y. GABA: a pioneer transmitter that excites immature neurons and generates primitive oscillations. Physiol. Rev. 2007;87(4):1215–1284. doi: 10.1152/physrev.00017.2006. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y. The GABA excitatory/inhibitory shift in brain maturation and neurological disorders. Neurosci. 2012;18(5):467–486. doi: 10.1177/1073858412438697. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y. Excitatory actions of gaba during development: the nature of the nurture. Nat. Rev. Neurosci. 2002;3(9):728–739. doi: 10.1038/nrn920. [DOI] [PubMed] [Google Scholar]
- Benes F.M. Evidence for altered trisynaptic circuitry in schizophrenic hippocampus. Biol. Psychiatry. 1999;46(5):589–599. doi: 10.1016/s0006-3223(99)00136-5. [DOI] [PubMed] [Google Scholar]
- Blaesse P. Cation-chloride cotransporters and neuronal function. Neuron. 2009;61(6):820–838. doi: 10.1016/j.neuron.2009.03.003. [DOI] [PubMed] [Google Scholar]
- Charil A. Prenatal stress and brain development. Brain Res. Rev. 2010;65(1):56–79. doi: 10.1016/j.brainresrev.2010.06.002. [DOI] [PubMed] [Google Scholar]
- Cherubini E. The depolarizing action of GABA controls early network activity in the developing hippocampus. Mol. Neurobiol. 2011;43(2):97–106. doi: 10.1007/s12035-010-8147-z. Epub 2010 Nov 3. [DOI] [PubMed] [Google Scholar]
- Chutabhakdikul N., Surakul P. Prenatal stress increased Snk Polo-like kinase 2, SCF beta-TrCP ubiquitin ligase and ubiquitination of SPAR in the hippocampus of the offspring at adulthood. Int. J. Dev. Neurosci. 2013;31(7):560–567. doi: 10.1016/j.ijdevneu.2013.06.011. Epub 2013 Jul 10. [DOI] [PubMed] [Google Scholar]
- Clayton G.H. Ontogeny of cation–Cl− cotransporter expression in rat neocortex. Dev. Brain Res. 1998;109(2):281–292. doi: 10.1016/s0165-3806(98)00078-9. [DOI] [PubMed] [Google Scholar]
- Davidson R.J., McEwen B.S. Social influences on neuroplasticity: stress and interventions to promote well-being. Nat. Neurosci. 2012;15(5):689–695. doi: 10.1038/nn.3093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunning D.D. GABAA receptor–mediated miniature postsynaptic currents and α-subunit expression in developing cortical neurons. J. Neurophysiology. 1999;82(6):3286–3297. doi: 10.1152/jn.1999.82.6.3286. [DOI] [PubMed] [Google Scholar]
- Elliott E.M., Sapolsky R.M. Corticosterone enhances kainic acid-induced calcium elevation in cultured hippocampal neurons. J. Neurochem. 1992;59(3):1033–1040. doi: 10.1111/j.1471-4159.1992.tb08345.x. [DOI] [PubMed] [Google Scholar]
- Emri Z. Electrotonic profile and passive propagation of synaptic potentials in three subpopulations of hippocampal CA1 interneurons. Neuroscience. 2001;104(4):1013–1026. doi: 10.1016/s0306-4522(01)00136-1. [DOI] [PubMed] [Google Scholar]
- Farrant M., Nusser Z. Variations on an inhibitory theme: phasic and tonic activation of GABAA receptors. Nat. Rev. Neurosci. 2005;6(3):215–229. doi: 10.1038/nrn1625. [DOI] [PubMed] [Google Scholar]
- Fride E. Prenatal stress impairs maternal behavior in a conflict situation and reduces hippocampal benzodiazepine receptors. Life Sci. 1985;36(22):2103–2109. doi: 10.1016/0024-3205(85)90306-6. [DOI] [PubMed] [Google Scholar]
- Fride E., Weinstock M. The effects of prenatal exposure to predictable or unpredictable stress on early development in the rat. Dev. Psychobiol. 1984;17(6):651–660. doi: 10.1002/dev.420170607. [DOI] [PubMed] [Google Scholar]
- Gauvain G. The neuronal K-Cl cotransporter KCC2 influences postsynaptic AMPA receptor content and lateral diffusion in dendritic spines. Proc. Natl. Acad. Sci. 2011;108(37):15474–15479. doi: 10.1073/pnas.1107893108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigoryan G., Segal M. Prenatal stress affects network properties of rat hippocampal neurons. Biol. Psychiatry. 2013;73(11):1095–1102. doi: 10.1016/j.biopsych.2013.02.003. [DOI] [PubMed] [Google Scholar]
- Gulyas A.I. The KCl cotransporter, KCC2, is highly expressed in the vicinity of excitatory synapses in the rat hippocampus. Eur. J. Neurosci. 2001;13(12):2205–2217. doi: 10.1046/j.0953-816x.2001.01600.x. [DOI] [PubMed] [Google Scholar]
- Hewitt S.A. Altered chloride homeostasis removes synaptic inhibitory constraint of the stress axis. Nat. Neurosci. 2009;12(4):438–443. doi: 10.1038/nn.2274. [DOI] [PubMed] [Google Scholar]
- Hines R.M. Functional regulation of GABAA receptors in nervous system pathologies. Curr. Opin. Neurobiol. 2012;22(3):552–558. doi: 10.1016/j.conb.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houston C.M. Intracellular chloride ions regulate the time course of GABA-mediated inhibitory synaptic transmission. J. Neurosci. 2009;29(33):10416–10423. doi: 10.1523/JNEUROSCI.1670-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu W. Stress impairs GABAergic network function in the hippocampus by activating nongenomic glucocorticoid receptors and affecting the integrity of the parvalbumin-expressing neuronal network. Neuropsychopharmacology. 2010;35(8):1693–1707. doi: 10.1038/npp.2010.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde T.M. Expression of GABA signaling molecules KCC2, NKCC1, and GAD1 in cortical development and schizophrenia. J. Neurosci. 2011;31(30):11088–11095. doi: 10.1523/JNEUROSCI.1234-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob T.C., Moss S.J., Jurd R. GABAA receptor trafficking and its role in the dynamic modulation of neuronal inhibition. Nat. Rev. Neurosci. 2008;9(5):331–343. doi: 10.1038/nrn2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson-Pick S., Richter-Levin G. Short- and long-term effects of juvenile stressor exposure on the expression of GABAA receptor subunits in rats. Stress. 2012;15(4):416–424. doi: 10.3109/10253890.2011.634036. [DOI] [PubMed] [Google Scholar]
- Laloux C. Anxiety-like behaviour and associated neurochemical and endocrinological alterations in male pups exposed to prenatal stress. Psychoneuroendocrinology. 2012;37(10):1646–1658. doi: 10.1016/j.psyneuen.2012.02.010. [DOI] [PubMed] [Google Scholar]
- Laurie D.J., Wisden W., Seeburg P.H. The distribution of thirteen GABAA receptor subunit mRNAs in the rat brain. III. Embryonic and postnatal development. J. Neurosci. 1992;12(11):4151–4172. doi: 10.1523/JNEUROSCI.12-11-04151.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling I. A novel GABA alpha 5 receptor inhibitor with therapeutic potential. Eur. J. Pharmacol. 2015;764:497–507. doi: 10.1016/j.ejphar.2015.07.005. [DOI] [PubMed] [Google Scholar]
- Lu J., Karadsheh M., Delpire E. Developmental regulation of the neuronal-specific isoform of K-CL cotransporter KCC2 in postnatal rat brains. J. Neurobiol. 1999;39(4):558–568. [PubMed] [Google Scholar]
- Medina I., Chudotvorova I. GABA neurotransmission and neural cation-chloride co-transporters: actions beyond ion transport. Crit. Rev. Neurobiol. 2006;18(1–2):105–112. doi: 10.1615/critrevneurobiol.v18.i1-2.110. [DOI] [PubMed] [Google Scholar]
- Moroni M. Chloride ions in the pore of glycine and GABA channels shape the time course and voltage dependence of agonist currents. J. Neurosci. 2011;31(40):14095–14106. doi: 10.1523/JNEUROSCI.1985-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens D.F., Kriegstein A.R. Is there more to gaba than synaptic inhibition? Nat. Rev. Neurosci. 2002;3(9):715–727. doi: 10.1038/nrn919. [DOI] [PubMed] [Google Scholar]
- Ramos B. Expression of α5 GABAA receptor subunit in developing rat hippocampus. Dev. Brain Res. 2004;151(1–2):87–98. doi: 10.1016/j.devbrainres.2004.04.003. [DOI] [PubMed] [Google Scholar]
- Rivera C. The K+/Cl- co-transporter KCC2 renders GABA hyperpolarizing during neuronal maturation. Nature. 1999;397(6716):251–255. doi: 10.1038/16697. [DOI] [PubMed] [Google Scholar]
- Rivera C. BDNF-induced TrkB activation down-regulates the K+–Cl− cotransporter KCC2 and impairs neuronal Cl− extrusion. J. Cell Biol. 2002;159(5):747–752. doi: 10.1083/jcb.200209011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph U., Mohler H. GABAA receptor subtypes: therapeutic potential in down syndrome, affective disorders, schizophrenia, and autism. Annu. Rev. Pharmacol. Toxicol. 2014;54:483–507. doi: 10.1146/annurev-pharmtox-011613-135947. (doi): p. 10.1146/annurev-pharmtox-011613-135947. Epub 2013 Oct 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar J. Neurosteroidogenesis is required for the physiological response to stress: role of neurosteroid-sensitive GABAA receptors. J. Neurosci. 2011;21(50):18198–18210. doi: 10.1523/JNEUROSCI.2560-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheller-Gilkey G. Relationship of clinical symptoms and substance use in schizophrenia patients on conventional versus atypical antipsychotics. Am. J. Drug Alcohol Abuse. 2003;29(3):553–566. doi: 10.1081/ada-120023458. [DOI] [PubMed] [Google Scholar]
- Shen H. Reversal of neurosteroid effects at alpha4beta2delta GABAA receptors triggers anxiety at puberty. Nat. Neurosci. 2007;10(4):469–477. doi: 10.1038/nn1868. Epub 2007 Mar 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein V. Expression of the KCl cotransporter KCC2 parallels neuronal maturation and the emergence of low intracellular chloride. J. Comp. Neurol. 2004;468(1):57–64. doi: 10.1002/cne.10983. [DOI] [PubMed] [Google Scholar]
- Stein-Behrens B.A., Sapolsky R.M. Stress, glucocorticoids, and aging. Aging Milano. 1992;4(3):197–210. doi: 10.1007/BF03324092. [DOI] [PubMed] [Google Scholar]
- Stevens H.E. Prenatal stress delays inhibitory neuron progenitor migration in the developing neocortex. Psychoneuroendocrinology. 2013;38(4):509–521. doi: 10.1016/j.psyneuen.2012.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stumpf W.E. Dexamethasone and corticosterone receptor sites. Differential topographic distribution in rat hippocampus revealed by high resolution autoradiography. Histochemistry. 1989;92(3):201–210. doi: 10.1007/BF00500919. [DOI] [PubMed] [Google Scholar]
- Succol F. Intracellular chloride concentration influences the GABAA receptor subunit composition. Nat. Commun. 2012;3:738. doi: 10.1038/ncomms1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surakul P., Weerachatyanukul W., Chutabhakdikul N. Repeated carbenoxolone injections during late pregnancy alter Snk-SPAR and PSD-95 expression in the hippocampus of rat pups. Neurosci. Lett. 2011;494(1):75–79. doi: 10.1016/j.neulet.2011.02.060. Epub 2011 Mar 6. [DOI] [PubMed] [Google Scholar]
- Tornello S. Regulation of glucocorticoid receptors in brain by corticosterone treatment of adrenalectomized rats. Neuroendocrinology. 1982;35(6):411–417. doi: 10.1159/000123429. [DOI] [PubMed] [Google Scholar]
- Van den Hove D.L.A. Prenatal stress and neonatal rat brain development. Neuroscience. 2006;137(1):145–155. doi: 10.1016/j.neuroscience.2005.08.060. [DOI] [PubMed] [Google Scholar]
- Wang D.S. Memory deficits induced by inflammation are regulated by alpha5-subunit-containing GABAA receptors. Cell Rep. 2012;2(3):488–496. doi: 10.1016/j.celrep.2012.08.022. Epub 2012 Sep. 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Y., Dempsey R.J., Sun D. Expression of Na+-K+-Cl− cotransporter in rat brain during development and its localization in mature astrocytes. Brain Res. 2001;911(1):43–55. doi: 10.1016/s0006-8993(01)02649-x. [DOI] [PubMed] [Google Scholar]
- Zhang W.Q. Systemic administration of kainic acid increases GABA levels in perfusate from the hippocampus of rats in vivo. Neurotoxicology. 1990;11(4):593–600. [PubMed] [Google Scholar]