Abstract
Dysfunction in corticolimbic circuits that mediate the extinction of learned fear responses is thought to underlie the perseveration of fear in stress-related psychopathologies, including post-traumatic stress disorder. Chronic stress produces dendritic hypertrophy in basolateral amygdala (BLA) and dendritic hypotrophy in medial prefrontal cortex, whereas acute stress leads to hypotrophy in both BLA and prelimbic cortex. Additionally, both chronic and acute stress impair extinction retrieval. Here, we examined the effects of a single elevated platform stress on extinction learning and dendritic morphology in infralimbic cortex, a region considered to be critical for extinction. Acute stress produced resistance to extinction, as well as dendritic retraction in infralimbic cortex. Spine density on apical and basilar terminal branches was unaffected by stress. However, animals that underwent conditioning and extinction had decreased spine density on apical terminal branches. Thus, whereas dendritic morphology in infralimbic cortex appears to be particularly sensitive to stress, changes in spines may more sensitively reflect learning. Further, in stressed rats that underwent conditioning and extinction, the level of extinction learning was correlated with spine densities, in that rats with poorer extinction retrieval had more immature spines and fewer thin spines than rats with better extinction retrieval, suggesting that stress may have impaired learning-related spine plasticity. These results may have implications for understanding the role of medial prefrontal cortex in learning deficits associated with stress-related pathologies.
Keywords: Prefrontal cortex, Stress, Dendritic spines, Dendrites, Extinction, Extinction retrieval
Abbreviations: BLA, basolateral amygdala; IL, infralimbic; PL, prelimbic; mPFC, medial prefrontal cortex
1. Introduction
Deficits in fear extinction are characterized by an inability to suppress a conditioned fear response following the removal of the unconditioned stimulus. This impairment may contribute to the perseveration of fear in stress-sensitive psychopathologies, including post-traumatic stress disorder (PTSD), and may impede any therapeutic techniques targeting the extinction process (Akirav and Maroun, 2007, Myers and Davis, 2007, Herry et al., 2010, Holmes and Quirk, 2010).
Connections between the amygdala and medial prefrontal cortex (mPFC) are critical for extinction learning, and dysfunctional communication between these two structures may lead to extinction deficits (Maroun, 2013). Both acute (Akirav et al., 2009, Maroun et al., 2013) and chronic stress (Izquierdo et al., 2006, Miracle et al., 2006) produce deficits in fear extinction and alter the morphology and function of neurons in basolateral amygdala (BLA; Vyas et al., 2002, Maroun et al., 2013) and mPFC (Cook and Wellman, 2004, Izquierdo et al., 2006). Indeed, BLA is a critical site of plasticity for fear extinction (Orsini and Maren, 2012) and is vulnerable to the effects of stress. For example, acute stress enhances LTP in BLA (Maroun and Richter-Levin, 2003, Maroun, 2006) and leads to dendritic retraction and a rapid increase in spine density here (Maroun et al., 2013). In contrast, chronic stress produces dendritic proliferation in BLA, as well as an increase in spine density (Vyas et al., 2002, Vyas et al., 2004, Vyas et al., 2006, Mitra et al., 2005, Roozendaal et al., 2009). In spite of the opposite effects stress has on dendritic morphology in BLA, both acute and chronic stress produce deficits in fear extinction (Maroun et al., 2013) and extinction recall (Miracle et al., 2006, Maroun et al., 2013). Therefore, cortico–amygdalar interactions are likely responsible for the observed behavioral deficits following stress exposure.
Projections from infralimbic (IL) cortex to BLA result in inhibition of BLA projection neurons, and therefore a decrease in fear responding, via the activation of either inhibitory intercalated cells of the amygdala or intra-BLA circuits (Quirk and Mueller, 2008). Indeed, CS-induced firing in IL is correlated with extinction retrieval, and stress-induced decreases in CS-related firing in IL are associated with deficits in extinction retrieval (Wilber et al., 2011). Lesions (Quirk et al., 2000), temporary inactivation (Sierra-Mercado et al., 2011), or inhibition of protein synthesis in IL (Santini et al., 2004) impairs extinction retrieval, which is rescued in chronically stressed animals with IL lesions (Farrell et al., 2010). Potentiation in the mPFC, which is critical for the success of extinction (Herry and Garcia, 2002, Vouimba and Maroun, 2011), is altered following acute stress (Maroun and Richter-Levin, 2003, Rocher et al., 2004, Richter-Levin and Maroun, 2010, Schayek and Maroun, 2015). Additionally, chronic (Cook and Wellman, 2004), mild (Brown et al., 2005), and acute stress (Izquierdo et al., 2006) have all been shown to produce apical dendritic retraction in mPFC. Chronic stress has also been shown to decrease spine density in mPFC (Radley et al., 2006, Liu and Aghajanian, 2008, Radley et al., 2008, Radley et al., 2013), while the effect of acute stress on spine density in mPFC has yet to be examined. Thus, we examined the effect of an acute elevated platform stressor on fear extinction and the morphology of IL neurons.
2. Materials and methods
2.1. Subjects and stressor
All procedures were conducted in accordance with NIH Guidelines and International Guiding Principles for Biomedical Research Involving Animals, and were approved by the Bloomington Animal Care and Use Committee and the University of Haifa Ethics and Animal Care Committee. Young adult male Sprague Dawley rats (age ∼60 days; 250–300 g; Harlan Laboratories, Jerusalem, Israel) weighing 200–280 g were group housed (two rats per cage) in Plexiglas cages and maintained on a 12:12 light/dark cycle with free access to food and water. Stress was evoked as previously described (Xu et al., 1997, Maroun et al., 2013). Briefly, each rat was placed on an elevated platform (12 × 12 cm) in a brightly lit room for 30 min. This was previously demonstrated to effectively impair fear extinction (Akirav and Maroun, 2007, Maroun et al., 2013), as well as LTP in mPFC (Maroun and Richter-Levin, 2003, Rocher et al., 2004, Richter-Levin and Maroun, 2010).
2.2. Fear conditioning and extinction
Fear conditioning was conducted in ‘context A,’ a chamber with a grid floor and transparent Plexiglas walls. The conditioning procedure was performed as previously described (Hikind and Maroun, 2008, Maroun et al., 2013), and was comprised of three pairings of a conditioned stimulus and unconditioned stimulus (120-s inter-pairing interval) after a 120-s no-stimulus baseline. The CS was a 4-kHz, 80-dB, 30-s tone that co-terminated with delivery of the 0.8-mA, 1-s footshock unconditioned stimulus. On the day after conditioning, rats were placed in ‘context B,’ a chamber with transparent Plexiglas walls and a black Plexiglas floor, and were subjected to fear retention testing via three CS presentations (note that rats had been habituated to context B for 20 min on each of 3 days prior to conditioning).
Immediately after retention testing, stressed rats (n = 11) were exposed to the elevated platform, whereas unstressed rats (n = 8) were returned to their home cages. One day later, rats were given an extinction session composed of 10 CSs in context B. Extinction retrieval was carried out in context B on the following day, by presentation of three CSs. Additional groups of rats (n = 8, unstressed; n = 7, stressed) underwent stress/no-stress, but were not subjected to fear conditioning or extinction testing.
Freezing, i.e. the absence of all movement except for respiration (Blanchard and Blanchard, 1972, Kim et al., 1992), was quantified from video with image-based software (P. Schmid, Behavioral Neurobiology Laboratory, Swiss Federal Institute of Technology, Zurich), and expressed as the percentage of time spent freezing during tone presentation. The behavioral data reported here were used in previous analyses (Maroun et al., 2013).
2.3. Golgi histology and dendritic analysis
Following extinction retrieval testing, tissue was processed using Glaser and van der Loos' modified Golgi stain, as described previously (Glaser and Van der Loos, 1981, Martin and Wellman, 2011). Rats were deeply anesthetized with equithesin and perfused with 0.9% saline. Brains were then removed and immersed in Golgi-Cox solution for 12 day and then moved to 30% sucrose in saline. Brains were then dehydrated, embedded in 8% celloidin, and sectioned at 180 μm on a sliding microtome (AO860; American Optical Company, Buffalo, NY, USA). Free-floating sections were alkalinized, developed in Dektol (Kodak), fixed in Ilford rapid fixer, dehydrated in a graded series of ethanols, cleared in xylenes, mounted, and coverslipped.
Pyramidal neurons in IL of mPFC were drawn. IL is readily identifiable by its position on the medial wall of rostral cortex, and its location just ventral to prelimbic cortex, which has more well-defined layers (Zilles and Wree, 1995). Pyramidal neurons were identified by a distinct, single apical branch extending from the apex of the soma towards the pial surface of the cortex, two or more basilar dendrites, and dendritic spines. Neurons selected for reconstruction did not have truncated branches and were not obscured by neighboring neurons, with dendrites that were easily discernable by focusing through the depth of the tissue. In 3 evenly spaced sections through the rostral-caudal extent of IL, all pyramidal neurons meeting these criteria were identified. A random number generator was used to select four of these neurons per section, two from each hemisphere (one superficial, one deep, for a total of 12 neurons per animal). All neurons were drawn at 600× and morphology of apical and basilar arbors was quantified in three dimensions using a computer-based neuron tracing system (Neurolucida; MBF, Bioscience, Williston, VT) with the experimenter blind to the condition.
Spines were also counted on 12 neurons per animal. For each neuron, a segment averaging 40 μm in length was drawn from one terminal apical and one terminal basilar dendrite. We examined distal branches because corticosterone has been shown to induce dendritic remodeling in these branches (Wellman, 2001, Cook and Wellman, 2004, Liu and Aghajanian, 2008). Spines were classified as stubby, thin, or mushroom, based on standard morphological criteria (Peters and Kaiserman-Abramof, 1970).
2.4. Statistical analyses
For statistical analyses, extinction trials were collapsed into blocks of two trials. Percentage freezing during each testing phase was compared between unstressed and stressed groups using two-way repeated measures ANOVAS (for conditioning, retention, and extinction retrieval, stress × trial; for initial extinction, stress × block). When appropriate, subsequent planned comparisons were conducted, consisting of two-group F-tests performed within the overall ANOVA (Hayes, 1994, Maxwell and Delaney, 2003). Freezing during the pre-tone baseline periods was compared between groups by the use of a two-way repeated measures ANOVA (stress × testing phase).
The amount and distribution of apical and basilar dendritic material was quantified via a three-dimensional Sholl analysis and compared between groups using four-way repeated measures ANOVAs (stress x conditioning-extinction x layer x distance from soma). In the unstressed group, at least 98% of the apical dendritic material of the pyramidal neurons reconstructed from the deep layers of IL was localized within 340 μm of the soma. Therefore, for simplicity and presentation purposes, for the apical Sholl analysis, dendritic lengths were assessed between 20-μm concentric spheres from 0 to 340 μm from the soma, with the remainder of dendritic material collapsed into one bin (>340 μm). This strategy is consistent with previous analyses (e.g., Cook and Wellman, 2004, Brown et al., 2005, Garrett and Wellman, 2009, Godar et al., 2015). Likewise, ∼98% of the basilar arbor is contained within 140 μm of the soma, and therefore for the basilar Sholl analysis, lengths were assessed between 20-μm concentric spheres from 0 to 140 μm from the soma, with the remainder collapsed into one bin (>140 μm). Total spine density and densities of each spine type were compared using three-way ANOVAs (stress x conditioning-extinction x layer). When appropriate, subsequent planned comparisons were conducted, consisting of two-group F-tests performed within the context of the overall ANOVA (Hayes, 1994, Maxwell and Delaney, 2003).
Finally, to examine potential relationships between dendritic or spine morphology and extinction, Pearson correlations were calculated between mean freezing (averaged across trials) during each phase of extinction and overall branch length and number, spine density, and total number of spines on terminal branches (estimated by calculating the mean terminal branch length per neuron and multiplying this value by the total spine density, as well as the density of each spine type). Fisher's r to z transformations were used to compare r-coefficients between unstressed and stressed rats.
3. Results
3.1. Acute stress produces resistance to extinction
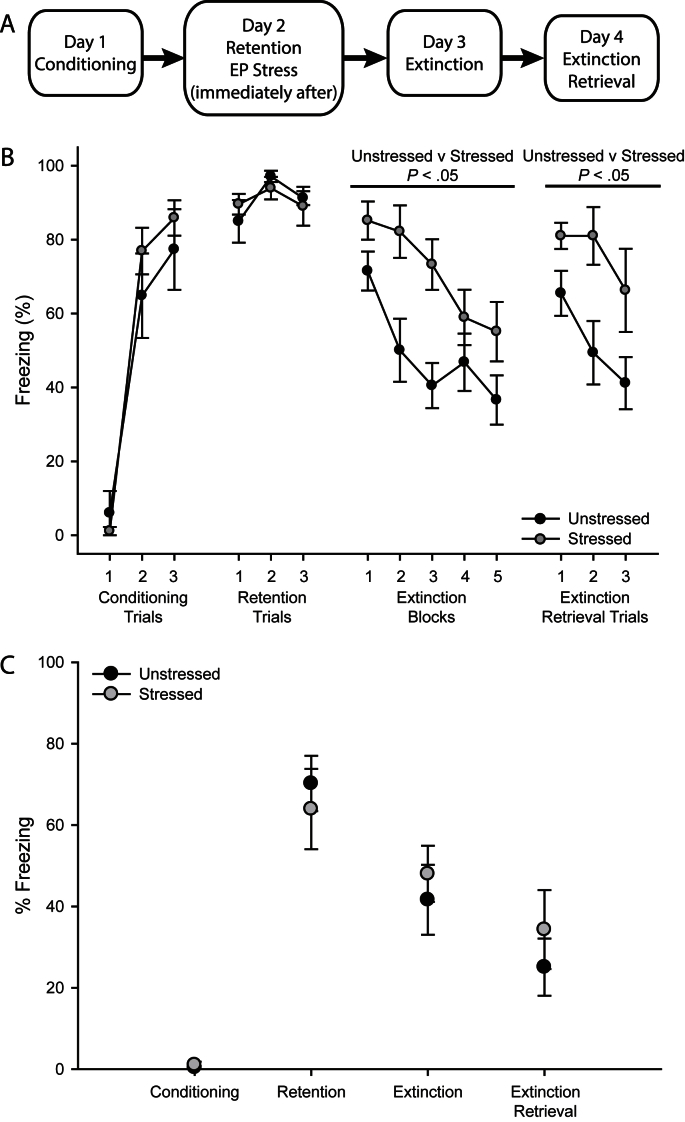
As previously reported (Maroun et al., 2013), acquisition of conditioned fear did not differ across groups (Fig. 1B; effect of trial, F2,34 = 122.67, P < 0.05; effect of stress, F1,17 = 0.03, not significant (NS); stress × trial interaction, F2,34 = 0.67, NS), and retention of the conditioned fear response was also comparable across groups (effect of stress, F1,17 = 0.25, NS; effect of trial, F2,34 = 1.71, NS; stress × trial interaction, F2,34 = 0.16, NS). Subsequent exposure to acute stress produced resistance to extinction, with stressed rats showing significantly more freezing across all extinction blocks than unstressed controls (effect of stress, F1,17 = 9.53, P < 0.05; effect of extinction block, F4,68 = 14.71, P < 0.05; stress × block interaction, F2,68 = 2.36, NS). Likewise, stressed rats showed significantly increased freezing relative to unstressed rats during extinction retrieval trials on the following day (effect of stress, F1,17 = 7.58, P < 0.05; effect of trial, F2,34 = 5.24, P < 0.05; stress × trial interaction, F2,34 = 0.88, NS). Freezing did not differ significantly between groups during the baseline period during any phase of conditioning and extinction (Fig. 1C; main effect of stress, F1,17 = 0.29, NS; stress × phase interaction, F3,51 = 0.42, NS).
Fig. 1.
Acute stress impairs extinction. (A) Schematic diagram of experimental design. (B) Percent freezing (mean ± SEM) to conditioned stimulus during conditioning, fear retention, extinction and extinction retrieval in rats that were either unstressed (gray; n = 8) or stressed (black; n = 11). (C) Percent freezing (mean ± SEM) during the 120-s baseline period during conditioning, fear retention, extinction and extinction retrieval in rats that were either unstressed (gray; n = 8) or stressed (black; n = 11).
3.2. Acute stress produces apical dendritic retraction in IL
As expected, apical dendritic length varied across the arbor (main effect of distance from soma, F17,510 = 190.37, P < 0.05). Although overall apical dendritic length did not vary across superficial and deep layers, (main effect of layer, F1,30 = 1.07, NS), the distribution of dendritic length varied across layers (layer x distance from soma, F17,510 = 13.53; P < 0.05).
Overall, exposure to acute stress significantly decreased apical dendritic length compared to unstressed rats (Fig. 2A, B, and C; main effect of stress, F1,30 = 15.39, P < 0.05). Overall, behavioral testing did not significantly alter apical dendritic length (main effect of conditioning-extinction, F1,30 = 0.28, NS), and the effect of stress did not vary across testing conditions (stress × conditioning interaction, F1,30 = 1.44, NS). Moreover, the effects of stress and conditioning-extinction conditions did not vary significantly across layers (layer × stress interaction, F1,30 = 0.60; layer × conditioning-extinction interaction, F1,30 = 0.32; layer × stress × conditioning-extinction interaction, F1,30 = 2.05; all NS). However, the effects of both stress and conditioning-extinction varied across the apical dendritic arbor (distance from soma × stress interaction, F17,510 = 3.13; distance from soma x conditioning-extinction interaction, F17,510 = 1.98; both P < 0.05). No other interactions were significant (for distance from soma x stress x conditioning-extinction, layer × distance from soma x stress, layer × distance from soma x conditioning-extinction, and layer × distance from soma x stress x conditioning-extinction, all Fs17,510 ≤ 0.99, NS).
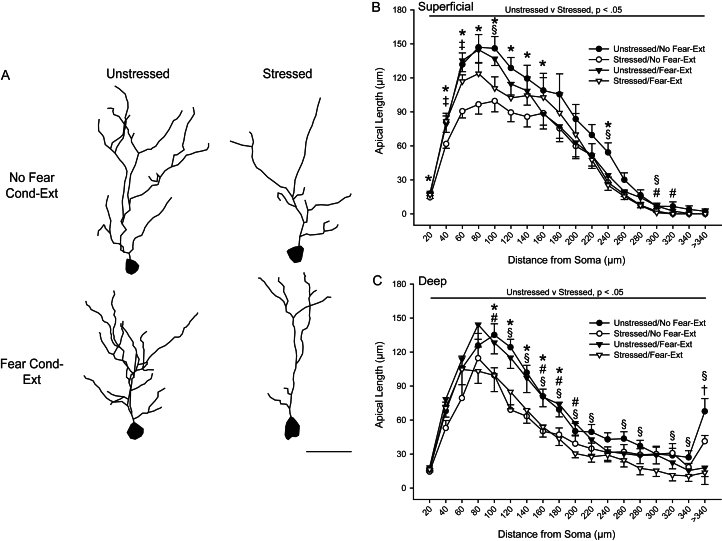
Fig. 2.
Acute stress produces apical dendritic retraction in IL pyramidal neurons, and fear conditioning and/or extinction ameliorates this effect on superficial but not deep neurons. (A) Reconstructions of representative apical dendrites of IL pyramidal neurons from superficial layers of unstressed vs. stressed rats that were either non-conditioned or underwent fear conditioning and extinction. Neurons are at or near the mean for each group. Scale bar: 50 μm. (B) Mean length (±SEM) of apical dendrites between 20-μm concentric spheres for pyramidal neurons in superficial IL in unstressed and stressed rats that underwent either no behavioral testing (No Fear-Ext) or fear conditioning and extinction (Fear-Ext). (C) Mean length (±SEM) of apical dendrites between 20-μm concentric spheres for pyramidal neurons in deep IL in unstressed and stressed rats that underwent either no behavioral testing (No Fear-Ext) or fear conditioning and extinction (Fear-Ext). For both graphs, *P < 0.05 for Unstressed v Stressed, No Fear-Ext; #P < 0.05 for Unstressed v Stressed, Fear-Ext; †P < 0.05 for No Fear-Ext v Fear-Ext, Unstressed; ‡P < 0.05 for No Fear-Ext v Fear-Ext, Stressed; §P < 0.05 for Unstressed/No Fear-Ext v Stressed/Fear-Ext.
Follow-up planned comparisons revealed that for pyramidal neurons in the superficial layers of IL in rats that did not undergo fear conditioning and extinction, stress significantly reduced dendritic length at 20–140 μm and 240 μm from the soma relative to unstressed rats (Fig. 2B; all Fs1,14 ≥ 5.01, P < 0.05; all other Fs1,14 ≤ 2.76, NS). This effect was attenuated in rats that had undergone conditioning and extinction, with significant stress-induced reductions at only 300–320 μm from the soma relative to unstressed rats (F1,18 = 9.22 and 5.55, respectively, both P < 0.05; all other Fs1,18 ≤ 4.06, NS). In unstressed rats, no significant differences between unconditioned and conditioned groups were present at any point in the arbor (all Fs1,15 ≤ 3.66, NS). Likewise, within stressed rats, unconditioned animals showed larger decreases in dendritic material at 40–60 μm from the soma relative to animals that had undergone conditioning-extinction (F1,17 = 8.24 and 9.06, respectively, both P < 0.05; all other Fs1,17 ≤ 3.63, NS). Finally, stressed, rats that had undergone conditioning-extinction showed significant reductions in arbor relative to unstressed, unconditioned rats at 100, 240, and 300 μm from the soma (Fs1,18 ≥ 5.65, P < 0.05; all other Fs1,18 ≤ 3.76, NS). Overall, this pattern of results suggests an attenuation of stress-induced dendritic remodeling in rats that underwent conditioning and extinction.
On the other hand, planned comparisons demonstrated that stress produced robust apical dendritic retraction in pyramidal neurons in the deep layers of IL, regardless of conditioning-extinction. In rats that did not undergo conditioning and extinction, stress significantly reduced dendritic length at 100–180 μm from the soma relative to unstressed rats (Fig. 2C; all Fs1,14 ≥ 6.27, P < 0.05; all other Fs1,14 ≤ 4.15, NS). In rats that had undergone conditioning and extinction, significant stress-induced reductions were present at 80 and 160–200 μm from the soma relative to unstressed unconditioned rats (all Fs1,18 ≥ 4.34, all P < 0.05; all other Fs1,18 ≤ 4.65, NS), and at 120–220, 260–280, 320–340, and >340 μm from the soma relative to unstressed, unconditioned rats (all Fs1,18 ≥ 4.43, P < 0.05; all other Fs1,18 ≤ 3.64, NS). Within unstressed rats, no significant differences between unconditioned and conditioned groups were present at any point in the arbor except for >340 μm (F1,15 = 13.25, P < 0.05; all other Fs1,15 ≤ 2.27, NS). Likewise, within stressed rats, dendritic length was comparable in unconditioned versus conditioned rats at all points in the arbor except 320 μm (F1,17 = 4.44, P < 0.05; all other Fs1,17 ≤ 3.99, NS).
Thus, overall, acute stress produces apical dendritic retraction in pyramidal neurons in IL, and conditioning-extinction appears to ameliorate this effect in the superficial, but not deep layers of IL.
3.3. Conditioning-extinction produces dendritic proliferation of basilar dendrites in IL
As expected, basilar dendritic length varied across layers and across the dendritic arbor (main effect of layer, F1,30 = 9.55; main effect of distance from soma, F7,210 = 565.69, both P < 0.05). Acute stress did not alter the amount and distribution of basilar dendritic material (Fig. 3A, B; main effect of stress, F1,30 = 0.67; layer × stress interaction, F1,30 = 0.08; distance from soma × stress interaction, F7,210 = 0.45, NS; layer × distance from soma × stress interaction, F7,210 = 0.31; all NS), nor did the effect of stress vary with testing condition (stress × conditioning-extinction interaction, F1,30 = 0.91; layer x stress x conditioning-extinction interaction, F1,30 = 0.10; layer x distance from soma x stress x conditioning-extinction, F7,210 = 1.19; all NS).
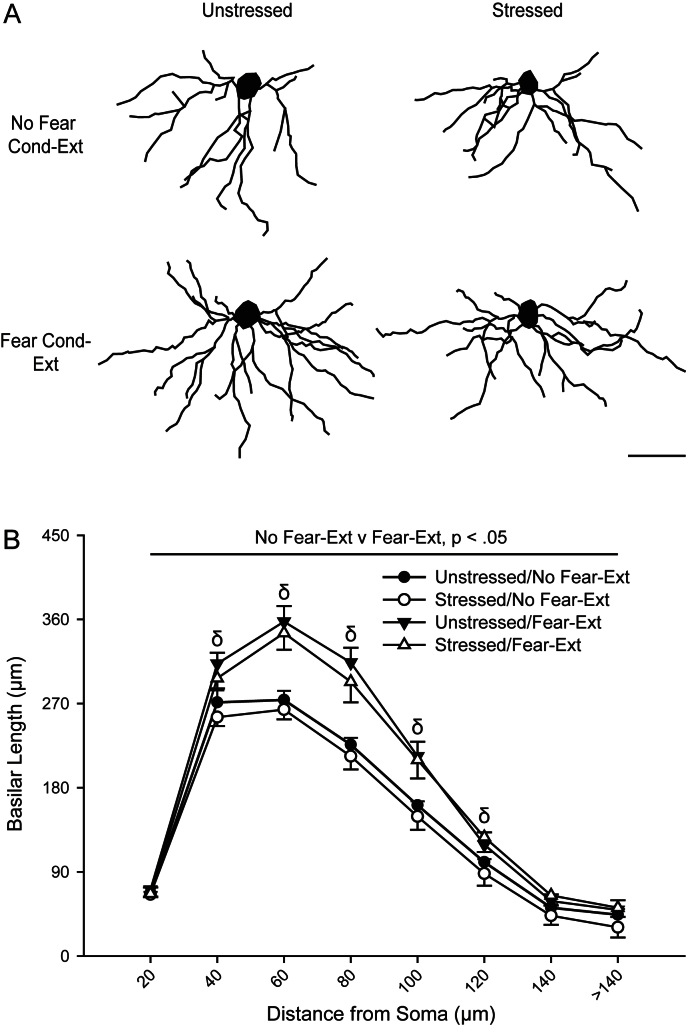
Fig. 3.
Fear conditioning and extinction produces basilar dendritic proliferation in IL pyramidal neurons. (A) Reconstructions of representative basilar dendrites of IL pyramidal neurons from unstressed vs. stressed rats that were either non-conditioned or underwent fear conditioning and extinction. Neurons are at or near the mean for each group. Scale bar: 50 μm. (B) Mean length (±SEM) of basilar dendrites between 20-μm concentric spheres for pyramidal neurons in IL collapsed across superficial and deep layers in unstressed and stressed rats that underwent either no behavioral testing (No Fear-Ext) or fear conditioning and extinction (Fear-Ext). δP < 0.05 for No Fear-Ext v Fear-Ext collapsed across stress conditions.
However, conditioning-extinction significantly altered the amount of basilar dendritic material (Fig. 3A, B; main effect of conditioning-extinction, F1,30 = 17.49, P < 0.05). This effect did not vary across superficial and deep layers (layer × conditioning-extinction interaction, F1,30 = 0.70; layer × distance from soma × conditioning-extinction interaction, F7,210 = 0.15; both NS). However, the effect varied with distance from the soma (distance from soma × conditioning-extinction interaction, F7,210 = 11.60, P < 0.05) Follow-up planned comparisons comparing untested versus tested rats collapsed across stress conditions demonstrated that rats that underwent fear conditioning and extinction had a significant increase in basilar dendritic length at 40–120 μm from the soma (Fs1,33 ≥ 4.85, P < 0.05; all other Fs1,33 ≤ 2.77, NS). This indicates that, while not particularly sensitive to stress, basilar dendrites of IL pyramidal neurons may undergo dendritic remodeling in response to fear conditioning and/or extinction.
3.4. Fear conditioning-extinction leads to decreased spine density on apical terminal dendrites in IL
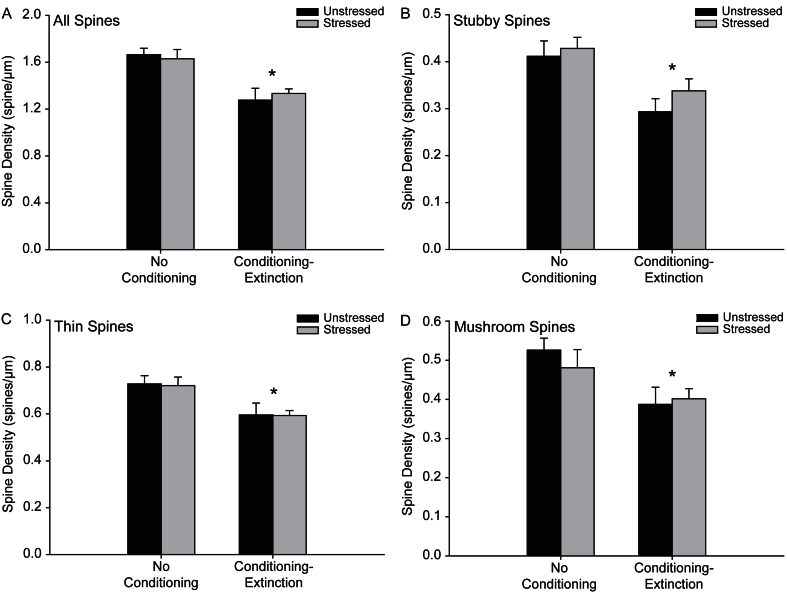
Acute stress did not alter total spine density (F1,30 = 0.03, NS) on apical terminal branches, nor the density of any spine type on apical terminal branches (all Fs1,30 ≤ 1.19, NS). Likewise, no changes following stress were seen in basilar spines on terminal branches (all Fs1,30 ≤ 2.77, NS). In contrast, total spine density on apical terminals was significantly reduced in rats that had undergone fear conditioning and extinction (Fig. 4A; F1,30 = 24.96, P < 0.05). Further, this decrease was observed in all spine types (Fig. 4B, C, and D; stubby, F1,30 = 13.78, P < 0.05; thin, F1,30 = 13.13, P < 0.05; mushroom, F1,30 = 9.32, P < 0.05). Fear conditioning and extinction also decreased the density of stubby spines on basilar terminal dendrites (F1,30 = 14.53, P < 0.05), but did not influence total, thin or mushroom spine density (all Fs1,30 ≤ 2.43, NS). Apical and basilar terminal spine density varied by layer for each spine type (main effect of layer, all Fs1,30 ≥ 12.48, P < 0.05). The layer x testing condition interaction was significant for apical stubby spines only (F1,30 = 8.32, P < 0.05). A follow-up planned comparison revealed that in both superficial and deep layers, conditioning-extinction decreased stubby spine density (superficial, F1,33 = 4.15, P = 0.05; deep, F1,33 = 17.28, P < 0.01). However, this effect was more pronounced in the deep layers (mean ± SEM; no conditioning-extinction, 0.48 ± 0.02; conditioning-extinction, 0.34 ± 0.02; 29.17% decrease) than in the superficial layers (no conditioning-extinction, 0.31 ± 0.02; conditioning-extinction, 0.36 ± 0.03; 13.89% decrease). No other interactions were present for any spine type on apical (layer x conditioning-extinction, all Fs(1,30) ≤ 0.81, NS; layer x stress, all Fs1,30 ≤ 2.45, NS; layer x conditioning-extinction x stress, all Fs1,30 ≤ 2.27, NS) or basilar dendrites (layer x conditioning-extinction, all Fs1,30 ≤ 0.20, NS; layer x stress, all Fs1,30 ≤ 0.13, NS; layer x conditioning-extinction x stress, all Fs1,30 ≤ 0.40, NS).
Fig. 4.
Fear conditioning and extinction leads to a decrease in spine density. (A) Total spine density (mean ± SEM) in unstressed (black bars) and stressed (gray bars) rats that were either non-conditioned or underwent fear conditioning and extinction. (B) Density of stubby spines (mean ± SEM) in unstressed (black bars) and stressed (gray bars) rats that were either non-conditioned or underwent fear conditioning and extinction. (C) Density of thin spines (mean ± SEM) in unstressed (black bars) and stressed (gray bars) rats that were either non-conditioned or underwent fear conditioning and extinction. (D) Density of mushroom spines (mean ± SEM) in unstressed (black bars) and stressed (gray bars) rats that were either non-conditioned or underwent fear conditioning and extinction. Unstressed, no conditioning, n = 8. Stressed, no conditioning, n = 7. Unstressed, conditioning and extinction, n = 8. Stressed, conditioning and extinction, n = 11. For all graphs, *P < 0.05 relative to non-conditioned groups.
3.5. Neuronal morphology correlates with freezing during extinction and extinction retrieval in stressed rats
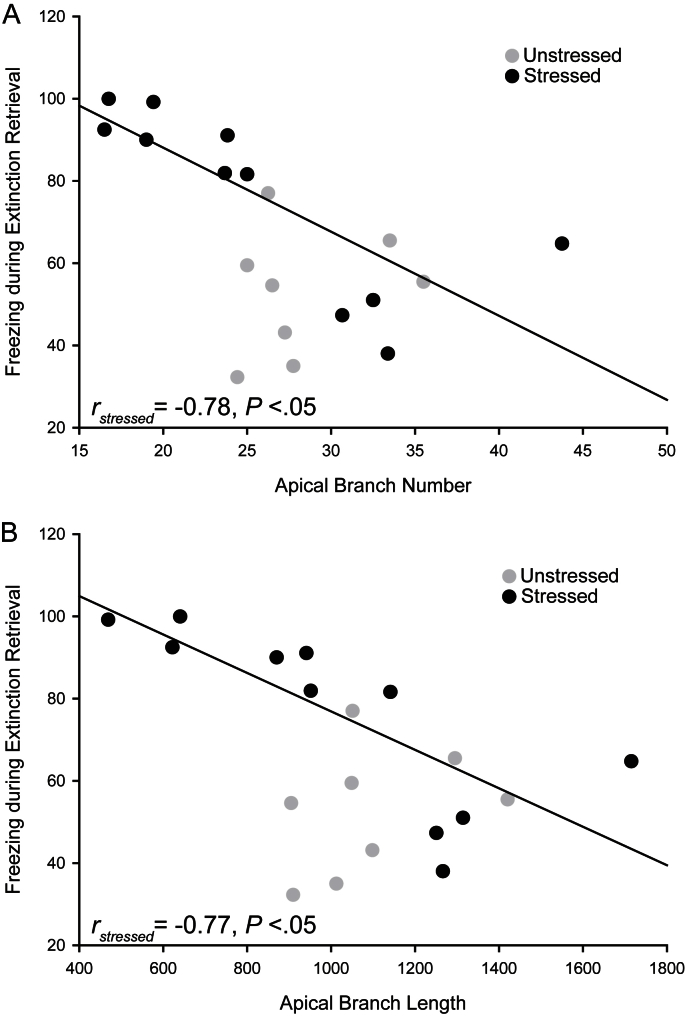
Across both unstressed and stressed rats, the number and length of apical dendrites of neurons in the superficial IL were negatively correlated with freezing during retrieval of extinction (Fig. 5A and B; r = −0.58 and −0.53, respectively, both P < 0.05). This relationship was driven by the association between dendritic morphology and extinction retrieval in stressed rats: whereas no significant extinction–morphology correlations were present in unstressed rats, apical branch number and length were strongly and negatively correlated with freezing during extinction retrieval in stressed rats (Fig. 5A and B; r = −0.78 and −0.77, respectively, both P < 0.05). Further, correlations between apical branch number and length and freezing during extinction retrieval differed significantly between unstressed and stressed rats (number, z = 2.30, P < 0.05; length, z = 2.47, P < 0.05).
Fig. 5.
In stressed rats, apical branch number and length correlate with percent freezing during extinction retrieval. (A) Linear regression for average IL branch number in stressed (black; n = 11) and unstressed rats (gray; n = 8) vs. percent freezing during extinction retrieval (averaged across trials). (B) Linear regression for average IL branch length in stressed (black; n = 11) and unstressed rats (gray; n = 8) vs. percent freezing during extinction retrieval (averaged across trials). For both A and B, regression line is for stressed rats only.
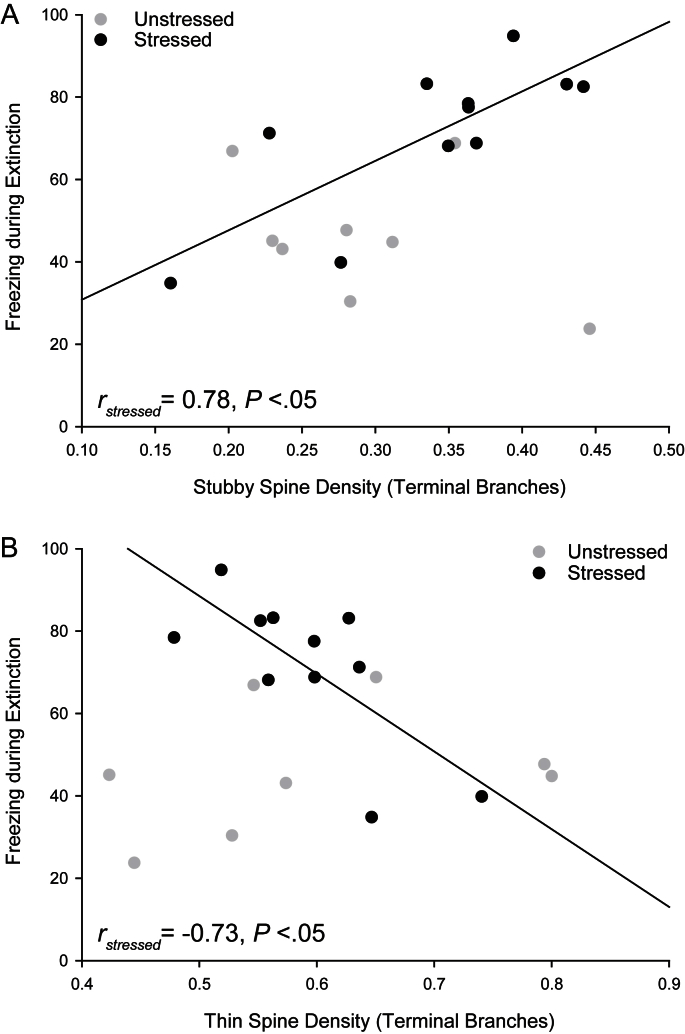
Although acute stress did not significantly alter spine density or morphology on terminal branches, spine densities were also strongly correlated with freezing behavior in stressed, but not unstressed, animals. In stressed rats, the density of stubby spines on apical terminal branches was positively correlated with mean percent freezing during extinction trials (Fig. 6A; for stressed, r = 0.78, P < 0.05; for unstressed, r = −0.45, NS; z = −2.45, P < 0.05), whereas density of thin spines on apical dendrites was negatively correlated with percent freezing during extinction trials (Fig. 6B; for stressed, r = −0.73, P < 0.05; for unstressed, r = 0.45, NS; z = 2.19, P < 0.05).
Fig. 6.
Spine densities correlate with percent freezing during extinction acquisition. (A) Linear regression for mean density of stubby spines in IL in stressed (black; n = 11) and unstressed rats (gray; n = 8) vs. percent freezing during extinction (averaged across trials). (B) Linear regression for mean density of thin spines in IL in stressed (black; n = 11) and unstressed rats (gray; n = 8) vs. percent freezing during extinction (averaged across trials). For both A and B, regression line is for stressed rats only.
For extinction retrieval trials, total (r = 0.72, P < 0.05), stubby (r = 0.74, P < 0.05), and mushroom (r = 0.68, P < 0.05) densities on apical terminals were all positively correlated with time spent freezing in stressed rats (for unstressed, all r values ≤ −0.42, NS). Each of these correlations was significantly different between unstressed and stressed rats (all z values ≤ −2.19, all P < 0.05). Finally, basilar stubby spine density was also positively correlated with freezing during extinction retrieval in stressed (r = 0.60, P < 0.05), but not unstressed rats (r = −0.25, NS), although these correlations did not differ significantly (z = −0.167, NS).
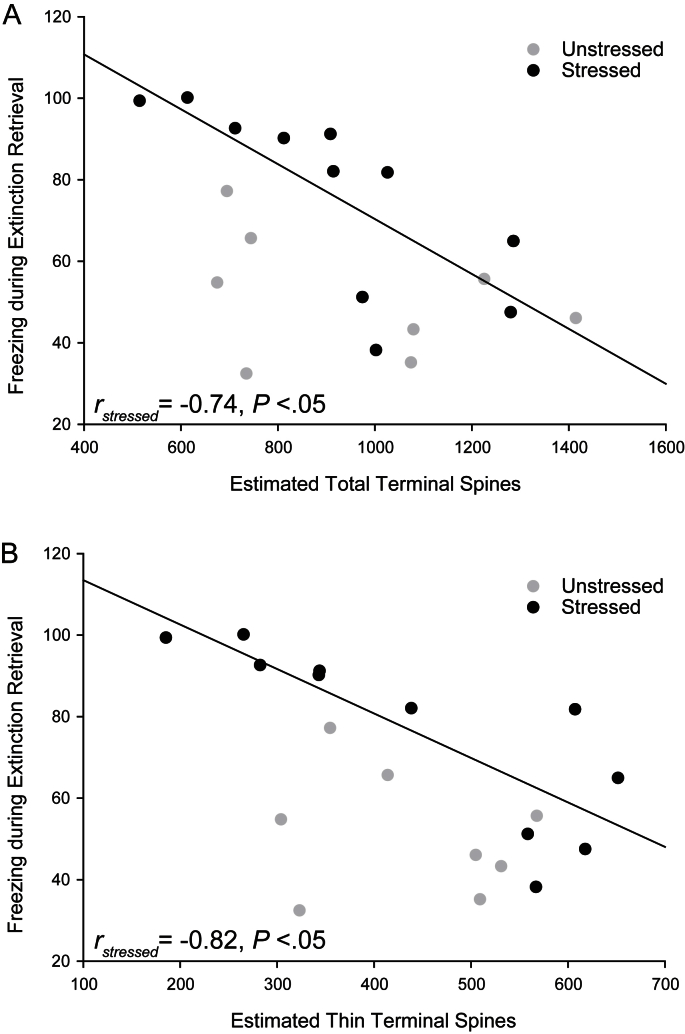
However, estimates of total spine numbers on apical terminals revealed a different relationship with freezing during extinction retrieval trials. In stressed animals, the overall estimate of the total number of spines, as well as the total number of thin spines, on the terminal portion of apical dendrites were negatively correlated with freezing during extinction retrieval (Fig. 7A and B; stressed, overall, r = −0.74, P < 0.05; for thin, r = −0.82, P < 0.05; unstressed, both r values ≥ −0.36, both NS; both z values ≤ 1.6, both NS). These correlations suggest that individual variation in spine number and morphology following stress may be associated with the level of extinction learning, as well as extinction retrieval.
Fig. 7.
Estimated number of spines on IL terminal branches correlates with percent freezing during extinction retrieval. (A) Linear regression for estimated total number of spines on IL terminal branches in stressed (black; n = 11) and unstressed rats (gray; n = 8) vs. percent freezing during extinction retrieval (averaged across trials). (B) Linear regression for estimated number of thin spines on IL terminal branches in stressed (black; n = 11) and unstressed rats (gray; n = 8) vs. percent freezing during extinction retrieval (averaged across trials). For both A and B, regression line is for stressed rats only.
4. Discussion
As reported previously (Maroun et al., 2013), acute stress following fear conditioning produced resistance to extinction, which was observed both in the initial extinction blocks and during extinction retrieval trials 24 h later. As we discussed previously (Maroun et al., 2013), given that the acute stress occurred shortly after fear retention testing, the increased freezing during extinction may be due to enhanced fear reconsolidation, as opposed to impairments in extinction learning per se. This would agree with well-documented literature regarding the effects of stress-related glucocorticoids and monoamines on memory consolidation (reviewed in Roozendaal et al., 2009). On the other hand, the relatively long retrieval procedure employed here, comprised of three CS presentations, may favor extinction over reactivation, suggesting that post-retrieval stress may have instead impaired the consolidation of a partial extinction memory. However, we cannot rule out an effect of acute stress on reconsolidation. Thus, it remains for future studies to determine which process acute stress is impacting to alter fear expression during extinction.
Nonetheless, the main findings of the current study were that acute stress, concurrent with producing resistance to extinction, produced changes in morphology of pyramidal neurons in IL. Acute stress produced apical dendritic retraction in both deep and superficial layers of IL; however, in animals that underwent fear conditioning and extinction, the effect of acute stress was attenuated in the superficial layers of IL. Unexpectedly, fear conditioning and extinction resulted in basilar dendritic proliferation, as well as decreases in apical spine density, which were not specific to any spine type. Finally, apical dendritic branch number and length, spine density, and estimates of total spine numbers on terminal dendrites following acute stress were strongly correlated with freezing during both extinction and extinction retrieval trials. Together, these findings provide evidence that alterations in IL pyramidal neuron morphology occur quickly and differentially in response to acute stress and fear conditioning/extinction.
4.1. Morphology of IL apical dendrites is not affected by fear conditioning-extinction
Previously, we demonstrated that fear conditioning and extinction produces hemisphere-specific morphological changes in BLA (Maroun et al., 2013). In that study, both unstressed and stressed rats showed dendritic retraction in the left BLA in response to fear conditioning/extinction, whereas retraction in the right BLA was seen only following stress. In contrast, here we found that behavioral testing does not alter apical dendritic morphology in IL. These differences between behavioral testing effects in BLA and IL may be due to their respective roles in fear conditioning and extinction (Quirk and Mueller, 2008). Alternatively, the remodeling seen in BLA following fear conditioning and extinction could reflect the stressfulness of the testing. If this is the case, it would suggest differential sensitivity of BLA and IL to stress, as well as differential sensitivity of IL apical dendrites to different types of acute stressors (repeated footshock during fear conditioning versus elevated platform exposure).
In contrast to the lack of effect of fear conditioning/extinction on apical dendritic morphology, we found that, regardless of stress condition, rats that underwent fear conditioning and extinction had basilar dendritic proliferation. As pyramidal neurons segregate their inputs, with apical and basilar dendritic arbors receiving information from distinct areas of cortical and subcortical regions (Price, 1973, Spratling, 2002), it is not surprising that we found differential effects of stress and behavioral testing in IL pyramidal neurons. Although to our knowledge this is the first demonstration of prefrontal basilar dendritic changes in response to fear conditioning and extinction, there is evidence that a single day of water maze training can produce morphological changes in basilar dendrites of hippocampal CA1 neurons (Diamond et al., 2006). Thus, it is possible that, although relatively insensitive to stress, basilar dendrites may show rapid and robust changes as a result of learning.
4.2. Stress-induced changes in IL dendritic morphology
Given the role IL is thought to play in the retrieval of conditioned fear extinction memories (Quirk et al., 2000), it is not surprising that we found dendritic retraction that occurred in tandem with stress-induced resistance to extinction. Our data complement previous work demonstrating dendritic retraction in IL after acute swim stress in mice (Izquierdo et al., 2006), and suggests that dendritic retraction in IL may occur after a variety of acute stressors, across multiple species.
Our results further add to the complicated nature of stress effects on corticolimbic morphology. As mentioned above, acute elevated platform stress leads to dendritic retraction in BLA (Maroun et al., 2013), whereas chronic immobilization or restraint stress has been shown to induce dendritic proliferation (Vyas et al., 2002, Vyas et al., 2004, Govindarajan et al., 2006, Vyas et al., 2006, Padival et al., 2013). In contrast to these findings, however, others have found either no change in morphology following an acute stressor (Mitra et al., 2005), or dendritic retraction following chronic stress (Grillo et al., 2015). Further, dendritic morphology in both CA1 and CA3 regions of hippocampus appears to be unaffected by acute or short-term stress (McLaughlin et al., 2007), but shows dendritic retraction following chronic stress (Woolley et al., 1990, Watanabe et al., 1992, Christian et al., 2011). As for IL, we have demonstrated here and previously (Izquierdo et al., 2006), that acute stress produces rapid dendritic retraction. This is consistent with the notion that morphology in this region is especially sensitive to the effect of stress, regardless of chronicity or type of stressor. Finally, dendritic retraction is also seen following chronic stress in both infralimbic (Goldwater et al., 2009) and prelimbic (Cook and Wellman, 2004) cortices. Taken together, these contrasting results suggest that the morphology of different corticolimbic regions responds differentially to varying durations and intensities of stressors, and suggests that the weighting of inputs among the corticolimbic structures involved in emotion regulation is profoundly reorganized as a result of stress—and differentially reorganized in response to various stressors. An important issue is how such differential remodeling among critical structures in the emotion-regulation circuit influences the function of the circuit as a whole. In addition, it is important to note that the present study, and all of the above studies, focused exclusively on males. We have previously shown that in contrast to the dendritic retraction seen in males, chronic stress produces dendritic proliferation in anterior cingulate/prelimbic cortices of females (Garrett and Wellman, 2009). Subsequent reports suggest similar sexually dimorphic effects of stress in infralimbic cortex (Shansky et al., 2010). Thus, sexually dimorphic patterns of stress-induced dendritic remodeling in the circuit will likely add yet another layer of complexity to these stress effects.
Interestingly, although there was no direct effect of fear conditioning and extinction on apical dendritic morphology, we found that, in the superficial layers of IL, behavioral testing appeared to ameliorate the effects of stress on dendritic morphology. The superficial and deep layers of mPFC are functionally and anatomically distinct. For instance, the superficial layers of IL receive inputs from dorsolateral entorhinal and perirhinal cortices (Delatour and Witter, 2002), whereas the deep layers of IL receive inputs from different regions, including hippocampus (Mulder et al., 1997). Such differences in inputs may be responsible for the localized effect of behavioral testing seen here. One explanation for the partial occlusion of the stress-induced dendritic remodeling is that the prior fear conditioning and extinction may have acted as a kind of stress inoculation, with the extinction following the uncontrollable stressor conferring resilience to the subsequent elevated platform stress (Maier, 2015). Alternatively, extinction learning induces physiological plasticity in both hippocampus-to-mPFC and BLA-to-mPFC pathways (Herry and Garcia, 2002, Garcia et al., 2008, Vouimba and Maroun, 2011); perhaps the induction of this plasticity following the elevated platform stressor counterbalanced the plasticity that results in stress-induced dendritic retraction in this population of IL neurons.
4.3. Fear conditioning-extinction but not acute stress decreases spine density on terminal branches of IL neurons
Perhaps the most surprising finding from the current study was the lack of stress-induced alterations in dendritic spine density and morphology—measures not previously examined in IL following acute stress (Moench and Wellman, 2015). As spines are the primary sites of excitatory inputs on pyramidal neurons, changes in spine density could significantly alter the excitability of neurons in IL. As spine density in prelimbic cortex seems especially sensitive to stress, as evidenced by daily injections of vehicle driving an increase in spine density (Seib and Wellman, 2003), it is surprising that we did not find an effect of acute stress in the present study. This likely demonstrates that, while the gross morphology of pyramidal neurons in IL is sensitive to acute stress, dendritic spines do not show the same sensitivity. Given the reciprocal connections of the infralimbic and prelimbic cortices (Hoover and Vertes, 2007) as well as their opposing modulatory effects on fear conditioning and extinction (Maren and Holmes, 2016) and HPA axis activity (Herman, 2012), such subregional specificity of structural remodeling after stress likely has important implications for stress-induced changes in emotionality and stress responsivity.
Although acute stress did not significantly alter spine density, fear conditioning and extinction decreased total spine density. This finding contrasts with previous research showing that fear extinction increased spine formation in layer V of secondary motor cortex (Lai et al., 2012). Although seemingly contradictory, the finding in the current study may demonstrate the differential effects of fear conditioning and extinction on critical structures in the fear conditioning/extinction circuitry, rather than incidental effects on structures that are not critical to fear conditioning and extinction.
We also found that all spine types were affected by behavioral testing. There are at least two plausible explanations for the observed decrease in spine density. First, the decrease in spine density may be a compensatory response by neurons in IL to limit excitability due to the acute stress of repeated footshock during fear conditioning, as opposed to a decrease in morphological flexibility, which has been suggested to occur following chronic stress (Radley et al., 2008). We have shown here that acute stress produces dendritic retraction, which has been hypothesized to reduce neuronal responsivity (Wilber et al., 2011). There is also evidence, however, that dendritic retraction leads to increased excitability (Kole et al., 2004), likely due to an increase in membrane resistance following retraction. Thus, if we posit that fear conditioning and extinction are acting as an acute stressor to increase the excitability of neurons, a resultant decrease in spine density may reduce neuronal excitability, thus limiting excitotoxicity.
Alternatively, there is evidence that plasticity thresholds vary along the length of dendrites, especially when considering dendrites proximal to the soma compared to those in the most distal branches (Sajikumar and Korte, 2011). We examined spines on distal, terminal portions of dendrites because of the known corticosterone- and stress-induced changes to dendritic morphology observed in this dendritic compartment (Wellman, 2001, Cook and Wellman, 2004, Liu and Aghajanian, 2008). A stress-induced increase in proximal dendritic spines has been observed in prelimbic cortex (Seib and Wellman, 2003); therefore, the neurons examined here may have also had an increase in spine density on proximal branches, which may have induced compensation via a reduction of spines at the distal branches. Future studies are required to discriminate between these possibilities.
4.4. Neuronal morphology predicts resistance to extinction in stressed rats
Although we found no direct effect of acute stress on spine density, we demonstrated that certain spine type densities were correlated with freezing behavior in stressed rats. Stubby spines are considered immature and are most abundant during early postnatal development, whereas thin spines, are considered to be the most plastic spine type (Harris et al., 1992). We found that an increase in stubby spine density was strongly and positively correlated with freezing during extinction and extinction retrieval in stressed, but not unstressed rats, whereas thin spine density was strongly and negatively correlated with freezing during extinction. These correlations were even stronger when dendritic morphology was taken into account to estimate total numbers of spines on terminal apical dendrites. This is consistent with the notion that as extinction learning occurs, stubby spines may mature into thin spines as a reflection of learning-related plasticity. It further suggests that stress impaired this plasticity. To complicate this however, we also found a positive correlation between mushroom spine density and freezing during extinction retrieval trials, which appears to contradict the notion of moving towards formation of more stable spines as learning proceeds. However, given that IL is involved in other stress-sensitive functions (e.g. modulation of HPA axis activity) that could influence the expression of learned fear, and the correlational nature of our data, a more direct examination of the role of dendritic spine plasticity in IL during fear conditioning and extinction, and the effects of acute stress on plasticity of IL spines is needed.
4.5. Conclusion
In conclusion, the present data demonstrate that the dendritic morphology of pyramidal neurons in infralimbic cortex is remarkably sensitive to acute stress, whereas dendritic spines may more sensitively reflect learning-related changes. These findings have important implications for stress-induced dysfunction of medial prefrontal cortex and stress-sensitive disorders.
Acknowledgments
Supported by US-Israel Binational Science Foundation grant number 2013292 to M. Maroun and C.L. Wellman. BSF was not involved in the study design; data collection, analysis, or interpretation; or writing or decision to submit the article for publication.
References
- Akirav I., Maroun M. The role of the medial prefrontal cortex-amygdala circuit in stress effects on the extinction of fear. Neural Plast. 2007:1–11. doi: 10.1155/2007/30873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akirav I., Segev A., Motanis H., Maroun M. D-cycloserine into the bla reverses the impairing effects of exposure to stress on the extinction of contextual fear, but not conditioned taste aversion. Learn Mem. 2009;16:682–686. doi: 10.1101/lm.1565109. [DOI] [PubMed] [Google Scholar]
- Blanchard D.C., Blanchard R.J. Innate and conditioned reactions to threat in rats with amygdaloid lesions. J. Comp. Physiol. Psychol. 1972;81:281. doi: 10.1037/h0033521. [DOI] [PubMed] [Google Scholar]
- Brown S.M., Henning S., Wellman C.L. Mild, short-term stress alters dendritic morphology in rat medial prefrontal cortex. Cereb. Cortex. 2005;15:1714–1722. doi: 10.1093/cercor/bhi048. [DOI] [PubMed] [Google Scholar]
- Christian K.M., Miracle A.D., Wellman C.L., Nakazawa K. Chronic stress-induced hippocampal dendritic retraction requires ca3 nmda recpetora. Neuroscience. 2011;174:26–36. doi: 10.1016/j.neuroscience.2010.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook S.C., Wellman C.L. Chronic stress alters dendritic morphology in rat medial prefrontal cortex. J. Neurobiol. 2004;60:236–248. doi: 10.1002/neu.20025. [DOI] [PubMed] [Google Scholar]
- Delatour B., Witter M.P. Projections from the parahippocampal region to the prefrontal cortex in the rat: evidence of multiple pathways. Eur. J. Neurosci. 2002;15:1400–1407. doi: 10.1046/j.1460-9568.2002.01973.x. [DOI] [PubMed] [Google Scholar]
- Diamond D.M., Campbell A.M., Park C.R., Woodson J.C., Conrad C.D., Bachstetter A.D., Mervis R.F. Influence of predator stress on the consolidation versus retrieval of long-term spatial memory and hippocampal spinogenesis. Hippocampus. 2006;16:571–576. doi: 10.1002/hipo.20188. [DOI] [PubMed] [Google Scholar]
- Farrell M.R., Sayed J.A., Underwood A.R., Wellman C.L. Lesions of infralimbic cortex occludes stress effects on retrieval of extinction but not fear conditioning. Neurobiol. Learn Mem. 2010;94:240–246. doi: 10.1016/j.nlm.2010.06.001. [DOI] [PubMed] [Google Scholar]
- Garcia R., Spennato G., Nilsson-Todd L., Moreau J.-L., Deschaux O. Hippocampal low-frequency stimulation and chronic mild stress similarly disrupt fear extinction memory in rats. Neurobiol. Learn Mem. 2008;89:560–566. doi: 10.1016/j.nlm.2007.10.005. [DOI] [PubMed] [Google Scholar]
- Garrett J.E., Wellman C.L. Chronic stress effects on dendritic morphology in medial prefrontal cortex: sex differences and estrogen dependence. Neuroscience. 2009;162:195–207. doi: 10.1016/j.neuroscience.2009.04.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser E.M., Van der Loos H. Analysis of thick brain sections by obverse-reverse computer microscopy – application of a new, high clarity golgi-nissl stain. J. Neurosci. Methods. 1981;4:117–125. doi: 10.1016/0165-0270(81)90045-5. [DOI] [PubMed] [Google Scholar]
- Godar S.C., Bortolato M., Richards S.E., Li F.G., Chen K., Wellman C.L., Shih J.C. Monoamine oxidase a enables rapid dendritic remodeling in response to stress. Int. J. Neuropsychopharmacol./Off. Sci. J. Coll. Int. Neuropsychopharmacol. (CINP) 2015;18 doi: 10.1093/ijnp/pyv035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldwater D.S., Pavlides C., Hunter R.G., Bloss E.B., Hof P.R., McEwen B.S., Morrison J.H. Structural and functional alterations to rat medial prefrontal cortex following chronic restraint stress and recovery. Neuroscience. 2009;164:798–808. doi: 10.1016/j.neuroscience.2009.08.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govindarajan A., Rao B.S.S., Nair D., Trinh M., Mawjee N., Tonegawa S., Chattarji S. Transgenic brain-derived neurotrophic factor expression causes both anxiogenic and antidepressant effects. Proc. Natl. Acad. Sci. U. S. A. 2006;103:13208–13213. doi: 10.1073/pnas.0605180103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillo C.A., Risher M., Macht V.A., Bumgardner A.L., Hang A., Gabriel C., Mocaer E., Piroli G.G., Fadel J.R., Reagan L.P. Repeated restraint stress-induced atrophy of glutamatergic pyramidal neurons and decreases in glutamatergic efflux in the rat amygdala are prevented by the antidepressant agomelatine. Neuroscience. 2015;284:430–443. doi: 10.1016/j.neuroscience.2014.09.047. [DOI] [PubMed] [Google Scholar]
- Harris K.M., Jensen F.E., Tsao B. 3-dimensional structure of dendritic spines and synapses in rat hippocampus (ca1) and postnatal day-15 and adult ages – implications for the maturation of synaptic physiology and long-term potentiation. J. Neurosci. 1992;12:2685–2705. doi: 10.1523/JNEUROSCI.12-07-02685.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes W.L. Harcourt Brace; Fort Worth, TX: 1994. Statistics. [Google Scholar]
- Herman J.P. Neural pathways of stress integration: relevance to alcohol abuse. Alcohol Res. Curr. Rev. 2012;34:441–447. doi: 10.35946/arcr.v34.4.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herry C., Garcia R. Prefrontal cortex long-term potentiation, but not long-term depression, is associated with the maintenance of extinction of learned fear in mice. J. Neurosci. Off. J. Soc. Neurosci. 2002;22:577–583. doi: 10.1523/JNEUROSCI.22-02-00577.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herry C., Ferraguti F., Singewald N., Letzkus J.J., Ehrlich I., Luthi A. Neuronal circuits of fear extinction. Eur. J. Neurosci. 2010;31:599–612. doi: 10.1111/j.1460-9568.2010.07101.x. [DOI] [PubMed] [Google Scholar]
- Hikind N., Maroun M. Microinfusion of the d1 receptor antagonist, sch23390 into the il but not the bla impairs consolidation of extinction of auditory fear conditioning. Neurobiol. Learn Mem. 2008;90:217–222. doi: 10.1016/j.nlm.2008.03.003. [DOI] [PubMed] [Google Scholar]
- Holmes A., Quirk G.J. Pharmacological facilitation of fear extinction and the search for adjunct treatments for anxiety disorders – the case of yohimbine. Trends Pharmacol. Sci. 2010;31:2–7. doi: 10.1016/j.tips.2009.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover W.B., Vertes R.P. Anatomical analysis of afferent projections to the medial prefrontal cortex in the rat. Brain Struct. Funct. 2007;212:149–179. doi: 10.1007/s00429-007-0150-4. [DOI] [PubMed] [Google Scholar]
- Izquierdo A., Wellman C.L., Holmes A. Brief uncontrollable stress causes dendritic retraction in infralimbic cortex and resistance to fear extinction in mice. J. Neurosci. 2006;26:5733–5738. doi: 10.1523/JNEUROSCI.0474-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.J., Fanselow M.S., DeCola J.P., Landeira-Fernandez J. Selective impairment of long-term but not short-term conditional fear by the n-methyl-d-aspartate antagonist apv. Behav. Neurosci. 1992;106:591–596. doi: 10.1037//0735-7044.106.4.591. [DOI] [PubMed] [Google Scholar]
- Kole M.H.P., Costoli T., Koolhaas J.M., Fuchs E. Bidirectional shift in the cornu ammonis 3 pyramidal dendritic organization following brief stress. Neuroscience. 2004;125:337–347. doi: 10.1016/j.neuroscience.2004.02.014. [DOI] [PubMed] [Google Scholar]
- Lai C.S.W., Franke T.F., Gan W.B. Opposite effects of fear conditioning and extinction on dendritic spine remodelling. Nature. 2012;483:87–U130. doi: 10.1038/nature10792. [DOI] [PubMed] [Google Scholar]
- Liu R.J., Aghajanian G.K. Stress blunts serotonin- and hypocretin-evoked epscs in prefrontal cortex: role of corticosterone-mediated apical dendritic atrophy. Proc. Natl. Acad. Sci. U. S. A. 2008;105:359–364. doi: 10.1073/pnas.0706679105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier S.F. Behavioral control blunts reactions to contemporaneous and future adverse events: medial prefrontal cortex plasticity and a corticostriatal network. Neurobiol. Stress. 2015;1:12–22. doi: 10.1016/j.ynstr.2014.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S., Holmes A. Stress and fear extinction. Neuropsychopharmacology. 2016;41:58–79. doi: 10.1038/npp.2015.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maroun M. Stress reverses plasticity in the pathway projecting from the ventromedial prefrontal cortex to the basolateral amygdala. Eur. J. Neurosci. 2006;24:2917–2922. doi: 10.1111/j.1460-9568.2006.05169.x. [DOI] [PubMed] [Google Scholar]
- Maroun M. Medial prefrontal cortex: multiple roles in fear and extinction. Neurosci. 2013;19:370–383. doi: 10.1177/1073858412464527. [DOI] [PubMed] [Google Scholar]
- Maroun M., Richter-Levin G. Exposure to acute stress blocks the induction of long-term potentiation of the amygdala-prefrontal cortex pathway in vivo. J. Neurosci. 2003;23:4406–4409. doi: 10.1523/JNEUROSCI.23-11-04406.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maroun M., Ioannides P.J., Bergman K.L., Kavushansky A., Holmes A., Wellman C.L. Fear extinction deficits following acute stress associate with increased spine density and dendritic retraction in basolateral amygdala neurons. Eur. J. Neurosci. 2013;38:2611–2620. doi: 10.1111/ejn.12259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin K.P., Wellman C.L. Nmda receptor blockade alters stress-induced dendritic remodeling in medial prefrontal cortex. Cereb. Cortex. 2011;21:2366–2373. doi: 10.1093/cercor/bhr021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell S., Delaney H. Lawrence Erlbaum Associates; Mahway, NJ: 2003. Designing Experiments and Analyzing Data: a Model Comparison Perspective. [Google Scholar]
- McLaughlin K.J., Gomez J.L., Baran S.E., Conrad C.D. The effects of chronic stress on hippocampal morphology and function: an evaluation of chronic restraint paradigms. Brain Res. 2007;1161:56–64. doi: 10.1016/j.brainres.2007.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miracle A.D., Brace M.F., Huyck K.D., Singler S.A., Wellman C.L. Chronic stress impairs recall of extinction of conditioned fear. Neurobiol. Learn Mem. 2006;85:213–218. doi: 10.1016/j.nlm.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Mitra R., Jadhav S., McEwen B.S., Vyas A., Chattarji S. Stress duration modulates the spatiotemporal patterns of spine formation in the basolateral amygdala. Proc. Natl. Acad. Sci. U. S. A. 2005;102:9371–9376. doi: 10.1073/pnas.0504011102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moench K.M., Wellman C.L. Stress-induced alterations in prefrontal dendritic spines: Implications for post-traumatic stress disorder. Neurosci. Lett. 2015;601:41–45. doi: 10.1016/j.neulet.2014.12.035. [DOI] [PubMed] [Google Scholar]
- Mulder A.B., Arts M.P.M., daSilva F.H.L. Short- and long-term plasticity of the hippocampus to nucleus accumbens and prefrontal cortex pathways in the rat, in vivo. Eur. J. Neurosci. 1997;9:1603–1611. doi: 10.1111/j.1460-9568.1997.tb01518.x. [DOI] [PubMed] [Google Scholar]
- Myers K.M., Davis M. Mechanisms of fear extinction. Mol. Psychiatr. 2007;12:120–150. doi: 10.1038/sj.mp.4001939. [DOI] [PubMed] [Google Scholar]
- Orsini C.A., Maren S. Neural and cellular mechanisms of fear and extinction memory formation. Neurosci. Biobehav. Rev. 2012;36:1773–1802. doi: 10.1016/j.neubiorev.2011.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padival M.A., Blume S.R., Rosenkranz J.A. Repeated restraint stress exerts different impact on structure of neurons in the lateral and basal nuclei of the amygdala. Neuroscience. 2013;246:230–242. doi: 10.1016/j.neuroscience.2013.04.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters A., Kaiserman-Abramof I.R. Small pryamidal neuron of rat cerebral cortex – perikayron, dendrites and spines. Am. J. Anat. 1970;127:321. doi: 10.1002/aja.1001270402. [DOI] [PubMed] [Google Scholar]
- Price J.L. Autoradiographic study of complementary laminar patterns of termination of afferent terminations to olfactory cortex. J. Comp. Neurol. 1973;150:87–108. doi: 10.1002/cne.901500105. [DOI] [PubMed] [Google Scholar]
- Quirk G.J., Mueller D. Neural mechanisms of extinction learning and retrieval. Neuropsychopharmacology. 2008;33:56–72. doi: 10.1038/sj.npp.1301555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk G.J., Russo G.K., Barron J.L., Lebron K. The role of ventromedial prefrontal cortex in the recovery of extinguished fear. J. Neurosci. 2000;20:6225–6231. doi: 10.1523/JNEUROSCI.20-16-06225.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radley J.J., Anderson R.M., Hamilton B.A., Alcock J.A., Romig-Martin S.A. Chronic stress-induced alterations of dendritic spine subtypes predict functional decrements in an hypothalamo-pituitary-adrenal-inhibitory prefrontal circuit. J. Neurosci. 2013;33:14379. doi: 10.1523/JNEUROSCI.0287-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radley J.J., Rocher A.B., Miller M., Janssen W.G.M., Liston C., Hof P.R., McEwen B.S., Morrison J.H. Repeated stress induces dendritic spine loss in the rat medial prefrontal cortex. Cereb. Cortex. 2006;16:313–320. doi: 10.1093/cercor/bhi104. [DOI] [PubMed] [Google Scholar]
- Radley J.J., Rocher A.B., Rodriguez A., Ehlenberger D.B., Dammann M., McEwen B.S., Morrison J.H., Wearne S.L., Hof P.R. Repeated stress alters dendritic spine morphology in the rat medial prefrontal cortex. J. Comp. Neurol. 2008;507:1141–1150. doi: 10.1002/cne.21588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter-Levin G., Maroun M. Stress and amygdala suppression of metaplasticity in the medial prefrontal cortex. Cereb. Cortex. 2010;20:2433–2441. doi: 10.1093/cercor/bhp311. [DOI] [PubMed] [Google Scholar]
- Rocher C., Spedding M., Munoz C., Jay T.M. Acute stress-induced changes in hippocampal/prefrontal circuits in rats: effects of antidepressants. Cereb. Cortex. 2004;14:224–229. doi: 10.1093/cercor/bhg122. [DOI] [PubMed] [Google Scholar]
- Roozendaal B., McEwen B.S., Chattarji S. Stress, memory and the amygdala. Nat. Rev. Neurosci. 2009;10:423–433. doi: 10.1038/nrn2651. [DOI] [PubMed] [Google Scholar]
- Sajikumar S., Korte M. Different compartments of apical ca1 dendrites have different plasticity thresholds for expressing synaptic tagging and capture. Learn Mem. 2011;18:327–331. doi: 10.1101/lm.2095811. [DOI] [PubMed] [Google Scholar]
- Santini E., Ge H., Ren K.Q., de Ortiz S.P., Quirk G.J. Consolidation of fear extinction requires protein synthesis in the medial prefrontal cortex. J. Neurosci. 2004;24:5704–5710. doi: 10.1523/JNEUROSCI.0786-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schayek R., Maroun M. Differences in stress-induced changes in extinction and prefrontal plasticity in postweanling and adult animals. Biol. Psychiatry. 2015;78:159–166. doi: 10.1016/j.biopsych.2014.10.004. [DOI] [PubMed] [Google Scholar]
- Seib L.M., Wellman C.L. Daily injections alter spine density in rat medial prefrontal cortex. Neurosci. Lett. 2003;337:29–32. doi: 10.1016/s0304-3940(02)01287-9. [DOI] [PubMed] [Google Scholar]
- Shansky R.M., Hamo C., Hof P.R., Lou W., McEwen B.S., Morrison J.H. Estrogen promotes stress sensitivity in a prefrontal cortex-amygdala pathway. Cereb. Cortex (New York, N. Y. 1991) 2010;20:2560–2567. doi: 10.1093/cercor/bhq003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra-Mercado D., Padilla-Coreano N., Quirk G.J. Dissociable roles of prelimbic and infralimbic cortices, ventral hippocampus, and basolateral amygdala in the expression and extinction of conditioned fear. Neuropsychopharmacology. 2011;36:529–538. doi: 10.1038/npp.2010.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spratling M.W. Cortical region interactions and the functional role of apical dendrites. Behav. Cogn. Neurosci. Rev. 2002;1:219–228. doi: 10.1177/1534582302001003003. [DOI] [PubMed] [Google Scholar]
- Vouimba R.M., Maroun M. Learning-induced changes in mpfc-bla connections after fear conditioning, extinction, and reinstatement of fear. Neuropsychopharmacology. 2011;36:2276–2285. doi: 10.1038/npp.2011.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyas A., Pillai A.G., Chattarji S. Recovery after chronic stress fails to reverse amygdaloid neuronal hypertrophy and enhanced anxiety-like behavior. Neuroscience. 2004;128:667–673. doi: 10.1016/j.neuroscience.2004.07.013. [DOI] [PubMed] [Google Scholar]
- Vyas A., Jadhav S., Chattarji S. Prolonged behavioral stress enhances synaptic connectivity in the basolateral amygdala. Neuroscience. 2006;143:387–393. doi: 10.1016/j.neuroscience.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Vyas A., Mitra R., Rao B.S.S., Chattarji S. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. J. Neurosci. 2002;22:6810–6818. doi: 10.1523/JNEUROSCI.22-15-06810.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe Y., Gould E., Cameron H.A., Daniels D.C., McEwen B.S. Phenytoin prevents stress-induced and corticosterone-induced atrophy of ca3 pyramidal neurons. Hippocampus. 1992;2:431–435. doi: 10.1002/hipo.450020410. [DOI] [PubMed] [Google Scholar]
- Wellman C.L. Dendritic reorganization in pyramidal neurons in medial prefrontal cortex after chronic corticosterone administration. J. Neurobiol. 2001;49:245–253. doi: 10.1002/neu.1079. [DOI] [PubMed] [Google Scholar]
- Wilber A.A., Walker A.G., Southwood C.J., Farrell M.R., Lin G.L., Rebec G.V., Wellman C.L. Chronic stress alters neural activity in medial prefrontal cortex during retrieval of extinction. Neuroscience. 2011;174:115–131. doi: 10.1016/j.neuroscience.2010.10.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley C.S., Gould E., McEwen B.S. Exposure to excess glucocorticoids alters dendritic morphology of adult hippocampal pyramidal neurons. Brain Res. 1990;531:225–231. doi: 10.1016/0006-8993(90)90778-a. [DOI] [PubMed] [Google Scholar]
- Xu L., Anwyl R., Rowan M.J. Behavioural stress facilitates the induction of long-term depression in the hippocampus. Nature. 1997;387:497–500. doi: 10.1038/387497a0. [DOI] [PubMed] [Google Scholar]
- Zilles K., Wree A. Cortex: areal and laminal structure. In: Paxinos G., editor. The Rat Nervous System. Academic Press; San Diego, CA: 1995. pp. 649–685. [Google Scholar]