The striatum is the main input structure of the basal ganglia and is involved in voluntary motor control, habit learning and reward processing. Medium spiny neurons (MSNs) comprise 80% and 95% of striatal neurons in primates and rodents, respectively, while the remaining population is made up of gamma-aminobutyric acid-ergic (GABAergic) and cholinergic interneurons. Up to 90% of MSNs are specifically lost in Huntington's disease (HD), which is an inherited neurodegenerative disorder caused by an extended CAG-repeat mutation in the Huntingtin (HTT) gene. Although the exact mechanism by which mutant Htt protein disrupts striatal cell homeostasis and leads to MSN loss remains largely unknown, several studies using induced pluripotent stem cells (iPSCs) derived from patients have proven that this could be a powerful platform for understanding HD (Camnasio et al., 2012; Consortium, 2012). Furthermore, with no disease-modifying treatment currently available, cell replacement has long been recognized as a potential therapy for HD. Human fetal tissue from the ganglionic eminences, the developmental birthplace of striatal neurons, has been used as a proof-of-principle in both pre-clinical animal studies and clinical trials (Dunnett and Rosser, 2014). However, fetal tissue is in limited supply, involves ethical concerns and therapeutic product derived from it is impossible to quality-control; all of which could be avoided by using human PSCs (hPSCs) as a source of tissue for transplantation. In order to exploit the full potential of hPSCs, a robust differentiation paradigm is required to obtain an enriched population of MSNs in vitro and in vivo following transplantation. This article will be focused on a recent discovery of a novel approach to generate MSNs from hPSCs using Activin A (Arber et al., 2015).
A novel, reliable and efficient paradigm for directing striatal fate in hPSCs: MSNs are born in the lateral ganglionic eminence (LGE), which is the dorsal region of the embryonic ventral telencephalon located dorsolateral to the medial ganglionic eminence (MGE) and ventral to the developing cortex. The cellular identity of MSN progenitors can be identified by the combinatorial expression of several transcription factor genes shown in Figure 1 (Onorati et al., 2014). All mature MSNs in the striatum express a zinc finger protein BCL11B (also named CTIP2) and the dopamine- and cyclic adenosine monophosphate (cAMP)-regulated phosphoprotein (DARPP32). Previous studies have demonstrated that components of the transforming growth factor-β (TGF-β) superfamily, Activin receptors and their transcriptional effectors, the Smad proteins, are expressed in the developing striatum (Feijen et al., 1994; Maira et al., 2010), suggesting potential regulation of LGE development by TGF-β family signalling. Indeed, we found that Activin A, a multifunctional TGF-β family protein referred to hereafter as Activin, is capable of inducing LGE fate in hPSC-derived forebrain progenitors. This was demonstrated by a substantial increase in LGE specific gene transcripts as well as a higher yield of cells immunoreactive for MSN progenitor markers (Arber et al., 2015). We further showed that this increase in striatal precursor cell population was driven by a direct induction of LGE fate in forebrain progenitors by Activin, rather than a higher rate of proliferation of pre-patterned striatal precursors. The observed induction of LGE characteristics translated to MSN production – around 40% yield of DARPP32-expressing neurons with Activin compared to < 1% in untreated controls. The DARPP32+ cells co-expressed other MSN neuronal markers and with 2–3 months maturation time in culture exhibited multipolar morphology with numerous spines on dendritic processes. Patch clamping of these cells revealed MSN-like firing properties and synaptic connection to other GABAergic neurons in vitro, despite their functional immaturity as evidenced by a relatively depolarised membrane potential. Upon transplantation into a quinolinic acid (QA)-lesioned rat model of HD, Activin-induced LGE progenitors survived and differentiated into DARPP32+ cells, which constituted 50% of the grafts. Our approach differs from previously published methods that apply the ventralising morphogen Sonic hedgehog (SHH) (Aubry et al., 2008; Ma et al., 2012; Carri et al., 2013; Nicoleau et al., 2013). We did not observe a further enhancement of Activin-induced LGE fate by exogenous SHH, nor a compromise of MSN production by blocking endogenous SHH signalling with cyclopamine. Moreover, in our hands, whilst SHH could induce the MGE (NKX2.1) and pan-GE transcription factors (DLX2, MEIS2, ASCL1 and GSX2), it failed to upregulate genes preferentially expressed by the LGE (BCL11B, NOLZ1 and FOXP1).
Figure 1.
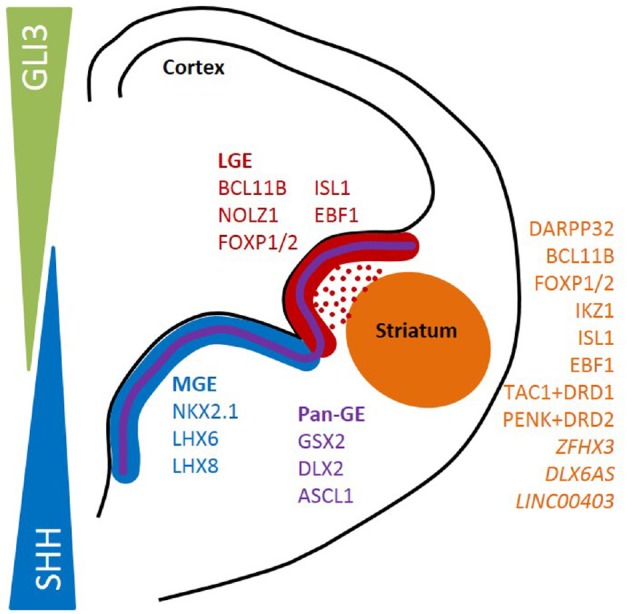
Schematic representation of a coronal hemisection of the developing telencephalon, showing the gene expression profile of morphologically defined progenitor domains of the cortex and medial (MGE) and lateral ganglionic eminences (LGE).
Dorso-ventral patterning of the telencephalon is achieved through gradients of morphogens Sonic hedgehog (SHH) and GLI family zinc finger 3 (GLI3). Genes in italics represent novel striatum-enriched genes discovered by Onorati et al. (2014).
A recent study reported the generation of DARPP32+ neurons from human fibroblasts by direct transfection of microRNAs and LGE-specific transcription factors (Victor et al., 2014). These induced MSNs (iMSNs) appear to exhibit a similar molecular profile and electrophysiological properties to those derived from hPSCs and maintained the key MSN marker expression following transplantation in mouse brain. However, the distinct conceptual and experimental approach between PSC in vitro differentiation and induced neuron (iN) may impact on the potential applications of the respective cellular products. iNs undergo direct conversion from fibroblasts to neurons, bypassing the developmental stages in-between. In contrast, hPSC differentiation plays out the sequential steps of neural development, allowing both the study of this important process in a human setting and cellular pathology and molecular events leading to neurological diseases. The developmental stage of transplanted tissue has a big impact on its survival and functional integration into the host brain, so the direct nature of iN may limit the scope for optimisation of the grafting procedure.
Does TGF-β signalling play a role in striatal development and homeostasis? The TGF-β family of proteins, including TGF-β, Activins and BMPs, are involved in many aspects of cell proliferation, lineage determination, differentiation, motility, adhesion, and death. Their intracellular effector proteins, the Smads, are phosphorylated upon receptor-ligand binding and translocate to the nucleus where they can activate gene transcription. Phosphorylated Smad2 (pSmad2), which is downstream of Activin and TGF-β signalling via the ALK4/5 receptors, was detected in the ganglionic eminences, and co-localised with DLX2 throughout. The two proteins were also confirmed to interact in vivo by immunoprecipitation, and pSmad2 was found to bind to the DLX2 gene enhancer by chromatin immunoprecipitation (Maira et al., 2010). This is in line with our findings that Activin upregulated LGE marker expression within just 24 hours, supporting a direct regulatory mechanism. In order to investigate whether continuous Activin signalling was necessary for the differentiation of LGE progenitors towards mature MSNs, we treated hPSC-derived forebrain progenitors at day 9 with Activin for 5, 11, 19 and 34 days in culture. We found that the maximum yield of DARPP32+ neurons (40%) was achieved after 11 days of exposure to Activin, while the number of BCL11B+ neurons continued to increase with longer Activin treatment (Arber et al., 2015). In contrast, cultures supplemented with Activin for 5 days produced only half the number of DARPP32+ neurons and the lowest yield of BCL11B+ cells. This suggests that there might be a well-defined short developmental window when Activin signalling is essential for normal MSN development. To confirm that this LGE marker upregulation was a result of specific Activin signalling we added a potent ALK4/5 inhibitor, SB431542, and observed a complete elimination of Activin's effects. Activin is known to have pro-survival neurotrophic effects, and was tested as such to improve striatal neuron survival in a QA-induced rat model of HD. Intrastriatal administration of Activin was able to recover a large proportion of the degenerated neurons in the lesioned striatum including MSNs, parvalbuminergic and cholinergic interneurons (Hughes et al., 1999). Similarly, we previously demonstrated that Activin had pro-differentiation effects that were dependent on retinoic acid (RA) signalling (Cambray et al., 2012). While RA has been shown to double the number of DARPP32+ MSNs generated from cultured primary mouse LGE cells (Toresson et al., 1999), a hPSC differentiation protocol using RA as the sole patterning factor yielded no MSN-like cells, rather inducing a GABAergic interneuron fate (Chatzi et al., 2011). Together, these data suggest that Activin may be functioning via multiple mechanisms to induce MSN fate in hPSCs, highlighting a possible physiological role of TGF-β signalling in striatal development and homeostasis that warrants further investigation.
Might BCL11B be an effector of Activin A-induced striatal fate? We consider BCL11B the likely key mediator down-stream of Activin-Smad signalling. BCL11B is expressed by all nascent and mature MSNs but is excluded from striatal interneurons. Loss of BCL11B leads to reduced expression levels of DARPP32 and other MSN markers and disrupted patch-matrix organization of the striatum with heterotopic cellular aggregates (Arlotta et al., 2008), demonstrating a requirement for BCL11B in MSN development and the establishment of the striatal architecture. In the induced neuron study, BCL11B was the only LGE transcription factor tested that was able to produce DARPP32-expressing MSNs in conjunction with the neural inducing microRNAs (Victor et al., 2014). Indeed, we found that BCL11B is the first LGE transcription factor gene to be robustly induced upon Activin treatment. The increased levels of BCL11B transcript can be detected as early as 12 hours post-treatment (8-fold) and continue to further increase with prolonged exposure to Activin (Arber et al., 2015). Such rapid upregulation of BCL11B strongly suggests a direct mechanism by which Activin targets BCL11B transcription, potentially via phosphorylation of Smad2. Interestingly, loss of BCL11B did not have an effect on the birth of MSN progenitors in mice but rather severely impeded their postmitotic maturation and connectivity (Arlotta et al., 2008). This suggests that Activin may have other direct targets in the developing LGE, either together with or upstream of BCL11B, to specify MSN progenitor fate. One such target could be a closely related transcription factor BCL11A, which shares a high degree of homology with BCL11B and is also strongly expressed in the developing striatum. Indeed, BCL11A expression was found to be upregulated in the BCL11B-mutant striatum, which may have had a compensatory effect, resulting in a milder phenotype. Furthermore, both transcription factors are sequence-specific DNA binding proteins that act primarily as transcriptional repressors, either independently or by interacting with chicken ovalbumin upstream promoter transcription factor (COUP-TF) family members (Avram et al., 2000, 2002). BCL11B consensus binding sites have been identified in the 5’ UTR of DARPP32 and FOXP1 genes (Arlotta et al., 2008; Tang et al., 2011), while a COUP-TF1 consensus binding site has been identified within the first intron of DARPP32 (Arlotta et al., 2008). It would be interesting to demonstrate direct binding of these transcription factors to DARPP32 and potentially other MSN genes.
Prospects of hPSC-derived MSNs in disease modelling and regenerative medicine: Our robust approach for generating MSNs from hPSCs provides a useful platform to study human MSN development in vitro, revealing a novel pathway by which Activin may play a role in striatal development. Further probing into in vivo striatal development will be necessary to draw more parallels between the two processes. The high proportion of MSNs we can produce is also of interest to the field of cell replacement therapy for HD. Our initial data demonstrates that it is possible to achieve high proportions of cells expressing the correct markers for MSNs, but that much more investigation into the functional integration of these cells within the host brain is needed to assess their true potential. Recording graft activity from brain slices, for example, would reveal if the grafted neurons are able to form functional synapses with host neurons as a first step to addressing whether they will be able to produce detectable changes in motor function. However, it must be contemplated whether transplantation of MSNs alone will be sufficient to yield functional recovery in animal models or patients. Striatal parvalbumin interneurons are also severely affected in HD, and reports comparing transplantation of LGE alone with whole GE primary tissue have been conflicting. Co-transplantation of hPSC-derived LGE with MGE progenitors, the source of striatal interneurons, is one avenue that should be considered going forward. Finally, we have been able to replicate Activin induction of MSN fate in every embryonic and induced PSC line tested, providing a highly reliable tool for studying MSNs derived from HD patient iPSCs, rather than a heterogeneous pool of neurons not specific to the disease. This will allow the field to build upon previous studies that have begun to find differences between patient and control iPSC-derived neurons in electrophysiology, metabolism, cell adhesion and cell death (Consortium, 2012). Our platform may provide new insight into mutant HTT protein function within a disease-relevant population of MSNs and a robust in vitro model for drug screening, which has been missing from our field so far.
This work was supported by funding from the UK Medical Research Council, EU Framework Programme 7 Neurostemcell and Repair-HD.
Presentations: BNA2015 Festival of Neuroscience; INTR12 2013.
References
- 1.Arber C, Precious SV, Cambray S, Risner-Janiczek JR, Kelly C, Noakes Z, Fjodorova M, Heuer A, Ungless MA, Rodriguez TA, Rosser AE, Dunnett SB, Li M. Activin A directs striatal projection neuron differentiation of human pluripotent stem cells. Development. 2015;142:1375–1386. doi: 10.1242/dev.117093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arlotta P, Molyneaux BJ, Jabaudon D, Yoshida Y, Macklis JD. Ctip2 controls the differentiation of medium spiny neurons and the establishment of the cellular architecture of the striatum. J Neurosci. 2008;28:622–632. doi: 10.1523/JNEUROSCI.2986-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aubry L, Bugi A, Lefort N, Rousseau F, Peschanski M, Perrier AL. Striatal progenitors derived from human ES cells mature into DARPP32 neurons in vitro and in quinolinic acid-lesioned rats. Proc Natl Acad Sci U S A. 2008;105:16707–16712. doi: 10.1073/pnas.0808488105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Avram D, Fields A, Senawong T, Topark-Ngarm A, Leid M. COUP-TF (chicken ovalbumin upstream promoter transcription factor)-interacting protein 1 (CTIP1) is a sequence-specific DNA binding protein. Biochem J. 2002;368:555–563. doi: 10.1042/BJ20020496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Avram D, Fields A, Top KPO, Nevrivy DJ, Ishmael JE, Leid M. Isolation of a novel family of C2H2 zinc finger proteins implicated in transcriptional repression mediated by chicken ovalbumin upstream promoter transcription factor (COUP-TF) orphan nuclear receptors. J Biol Chem. 2000;275:10315–10322. doi: 10.1074/jbc.275.14.10315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cambray S, Arber C, Little G, Dougalis AG, de Paola V, Ungless MA, Li M, Rodriguez TA. Activin induces cortical interneuron identity and differentiation in embryonic stem cell-derived telencephalic neural precursors. Nat Commun. 2012;3:841. doi: 10.1038/ncomms1817. [DOI] [PubMed] [Google Scholar]
- 7.Camnasio S, Delli Carri A, Lombardo A, Grad I, Mariotti C, Castucci A, Rozell B, Lo Riso P, Castiglioni V, Zuccato C, Rochon C, Takashima Y, Diaferia G, Biunno I, Gellera C, Jaconi M, Smith A, Hovatta O, Naldini L, Di Donato S, et al. The first reported generation of several induced pluripotent stem cell lines from homozygous and heterozygous Huntington's disease patients demonstrates mutation related enhanced lysosomal activity. Neurobiol Dis. 2012;46:41–51. doi: 10.1016/j.nbd.2011.12.042. [DOI] [PubMed] [Google Scholar]
- 8.Carri AD, Onorati M, Lelos MJ, Castiglioni V, Faedo A, Menon R, Camnasio S, Vuono R, Spaiardi P, Talpo F, Toselli M, Martino G, Barker RA, Dunnett SB, Biella G, Cattaneo E. Developmentally coordinated extrinsic signals drive human pluripotent stem cell differentiation toward authentic DARPP-32(+) medium-sized spiny neurons. Development. 2013;140:301–312. doi: 10.1242/dev.084608. [DOI] [PubMed] [Google Scholar]
- 9.Chatzi C, Brade T, Duester G. Retinoic acid functions as a key GABAergic differentiation signal in the basal ganglia. PLoS Biol. 2011;9:e1000609. doi: 10.1371/journal.pbio.1000609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Consortium HDi. Induced pluripotent stem cells from patients with Huntington's disease show CAG-repeat-expansion-associated phenotypes. Cell Stem Cell. 2012;11:264–278. doi: 10.1016/j.stem.2012.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dunnett SB, Rosser AE. Challenges for taking primary and stem cells into clinical neurotransplantation trials for neurodegenerative disease. Neurobiol Dis. 2014;61:79–89. doi: 10.1016/j.nbd.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 12.Feijen A, Goumans MJ, van den Eijnden-van Raaij AJ. Expression of activin subunitsactivin receptors and follistatin in postimplantation mouse embryos suggests specific developmental functions for different activins. Development. 1994;120:3621–3637. doi: 10.1242/dev.120.12.3621. [DOI] [PubMed] [Google Scholar]
- 13.Hughes PE, Alexi T, Williams CE, Clark RG, Gluckman PD. Administration of recombinant human Activin-A has powerful neurotrophic effects on select striatal phenotypes in the quinolinic acid lesion model of Huntington's disease. Neuroscience. 1999;92:197–209. doi: 10.1016/s0306-4522(98)00724-6. [DOI] [PubMed] [Google Scholar]
- 14.Ma L, Hu B, Liu Y, Vermilyea SC, Liu H, Gao L, Sun Y, Zhang X, Zhang SC. Human embryonic stem cell-derived GABA neurons correct locomotion deficits in quinolinic acid-lesioned mice. Cell Stem Cell. 2012;10:455–464. doi: 10.1016/j.stem.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maira M, Long JE, Lee AY, Rubenstein JLR, Stifani S. Role for TGF-beta superfamily signaling in telencephalic GABAergic neuron development. J Neurodev Disord. 2010;2:48–60. doi: 10.1007/s11689-009-9035-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nicoleau C, Varela C, Bonnefond C, Maury Y, Bugi A, Aubry L, Viegas P, Bourgois-Rocha F, Peschanski M, Perrier AL. Embryonic stem cells neural differentiation qualifies the role of Wnt/β-Catenin signals in human telencephalic specification and regionalization. Stem Cells. 2013;31:1763–1774. doi: 10.1002/stem.1462. [DOI] [PubMed] [Google Scholar]
- 17.Onorati M, Castiglioni V, Biasci D, Cesana E, Menon R, Vuono R, Talpo F, Goya RL, Lyons PA, Bulfamante GP, Muzio L, Martino G, Toselli M, Farina C, Barker RA, Biella G, Cattaneo E. Molecular and functional definition of the developing human striatum. Nat Neurosci. 2014;17:1804–1815. doi: 10.1038/nn.3860. [DOI] [PubMed] [Google Scholar]
- 18.Tang B, Di Lena P, Schaffer L, Head SR, Baldi P, Thomas EA. Genome-wide identification of Bcl11b gene targets reveals role in brain-derived neurotrophic factor signaling. PLos One. 2011;6:e23691. doi: 10.1371/journal.pone.0023691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Toresson H, Mata de Urquiza A, Fagerström C, Perlmann T, Campbell K. Retinoids are produced by glia in the lateral ganglionic eminence and regulate striatal neuron differentiation. Development. 1999;126:1317–1326. doi: 10.1242/dev.126.6.1317. [DOI] [PubMed] [Google Scholar]
- 20.Victor MB, Richner M, Hermanstyne TO, Ransdell JL, Sobieski C, Deng PY, Klyachko VA, Nerbonne JM, Yoo AS. Generation of human striatal neurons by microRNA-dependent direct conversion of fibroblasts. Neuron. 2014;84:311–323. doi: 10.1016/j.neuron.2014.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]


