The peripheral nervous system (PNS) has an intrinsic ability for repair and regeneration even after severe injury. Peripheral nerve injury is followed by the normal rapid progression of Wallerian degeneration (anterograde or orthograde degeneration). During this process, the distal detached nerve segment degenerates, and the surrounding myelin breaks down. Simultaneously, the proximal segment of the axon undergoes degeneration that extends proximally only as far as the first node of Ranvier. The proximal segment can undergo a chromatolytic reaction; the Nissl bodies dissolve; and the nucleus migrates towards the periphery of the cell and the size of the nucleolus, nucleus and neuronal cell body increases, initiating a process of protein synthesis in an attempt to regenerate. Schwann cells play a major role in PNS axon regeneration. They are able to dedifferentiate, reenter the cell cycle and promote axonal regrowth by phagocytosis of the axonal and myelin debris, recruiting macrophages to the injury site, secreting several neurotrophic and key transcription factors and forming a unique column of cells called bands of Büngner within their endoneurial tubes for guiding the axonal sprouts from the proximal stumps (Burnett and Zager, 2004) (Figure 1).
Figure 1.
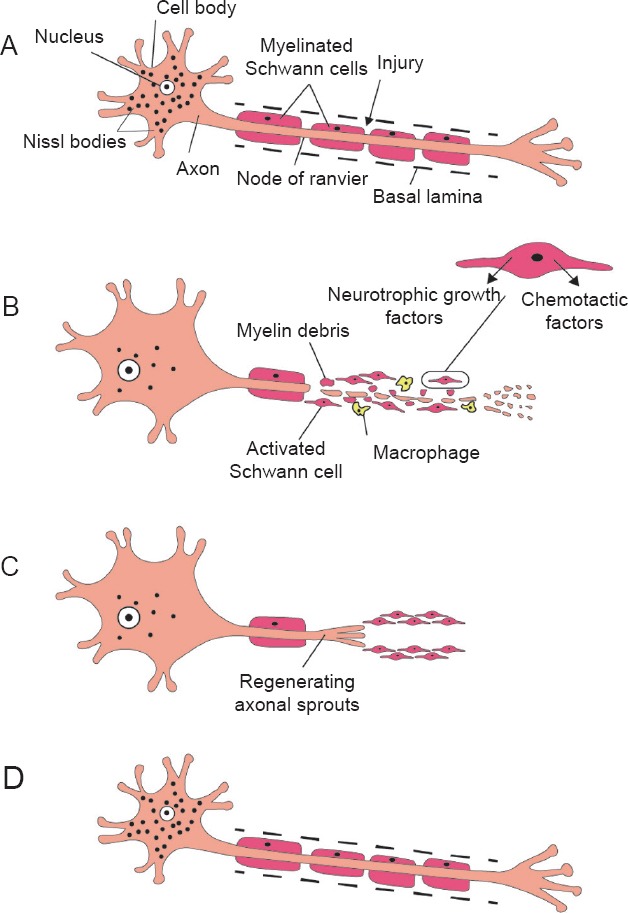
Wallerian degeneration in peripheral nervous system and axonal regeneration.
A typical motor neuron with its axon surrounded by myelin sheath formed by Schwann cells (A). After injury, the distal detached nerve segment degenerates, and the surrounding myelin breaks down. The proximal segment of the axon undergoes degeneration that extends proximally only as far as the first node of Ranvier. The proximal segment undergoes chromatolysis; the Nissl bodies dissolve, the nucleus migrates towards the periphery of the cell and the size of the neuronal cell body and nucleus increase. Schwann cells become activated; they rapidly demyelinate, proliferate and dedifferentiate to a more progenitor-like cell type. Schwann cells also induce chemotactic migration of macrophages and secrete several neurotrophic factors needed for regeneration. Axonal and myelin debris are phagosytosed by both Schwann cells and recruited macrophages (B). The proliferating Schwann cells form a line of cells called bands of Büngner within their basal lamina. These bands guide axonal sprouts during regeneration (C). In the later stages of regeneration, Schwann cells redifferentiate into myelinating cells and evidence of chromatolysis is no longer present (D).
In clinical practice, nerve autografting is considered to be the “clinical golden standard” for promoting repair of segmental peripheral nerve injury and bridging a critical gap defect, but the results are still unsatisfactory. The limited availability of donor nerves, the size incompatibilities and other associated donor site morbidities are the major drawbacks (Alluin et al., 2009). In addition, the reported outcomes of synthetic nerve conduits and allogenic nerve grafts have been voluminous and often conflicting. For example, synthetic nerve conduits have been developed for sensory gaps of 30 mm or less, but it remains unclear whether these conduits are beneficial for longer gaps (Schlosshauer et al., 2006). On the other hand, nerve allografts were shown to be very effective in regeneration of large nerves. However, the nerve allograft immunology and the risk of rejection present a major concern (Mackinnon et al., 2001). Therefore, cell-based therapies by transplantation of Schwann cells within appropriate scaffolds have been introduced as a promising treatment modality for peripheral nerve regeneration.
Schwann cells: Schwann cells (SCs) originate from the neural crest. They are the principal glia cell supporting neurons of the PNS, and they are required for normal nerve development and function. Culturing SCs in vitro is a challenge. Nerve donors undergo a surgical procedure, in which the nerve is separated from a vast amount of connective tissue. Afterwards, SCs are cultured in the laboratory utilizing various methods to retrieve the appropriate number of cells and to induce them to proliferate and survive for an acceptable period of time in the culture enough to consider them reliable for use in in vitro experiments (Turnbull, 2005). It should be noted that it is possible for patients to experience an infection, neuroma or loss of function and/or sensation of the donor site in response to the surgical procedure. Accordingly, the search for an alternative suitable SC source for cell therapy of PNS injury is necessary.
Dental pulp stem cells: Dental pulp stem cells (DPSCs) are considered to be a strong candidate for PNS regeneration because both the dental pulp and SCs originate from the neural crest. Accordingly, DPSCs have been suggested as a new source for in vitro differentiated SCs. DPSCs cultured under special serum-free conditions show neural crest cell properties (neural crest-derived DPSCs, neural crest (NC)-derived DPSCs) and can be induced to differentiate into SCs in vitro (Al-Zer et al., 2015) (Figure 2A–C). Furthermore, DPSCs are reported to secrete several neurotrophic factors (Nosrat et al., 2001) and to guide neurons in vitro (Martens et al., 2014) and in an in vivo animal model (Arthur et al., 2009). DPSC-derived SCs were capable of secreting higher levels of neurotrophic factors compared with undifferentiated cells. In addition, when they were co-cultured with dorsal root ganglion neurons, a significant increase in survival, neuritogenesis and myelination of the growing neuritis was observed, compared with undifferentiated DPSCs (Martens et al., 2014). DPSCs can be cultured for a relatively long time in vitro before they can be induced to differentiate into SCs, providing an appropriate amount of time for in vitro investigations of cells without the fear of their death compared with the culture of fully differentiated SCs from a donor nerve, which have a short lifespan in culture (Turnbull, 2005). DPSC-derived SCs should be thoroughly investigated before they are utilized in any future therapies for peripheral nerve injury regeneration. In our laboratory, we will characterize DPSC-derived SCs, optimize the freezing and thawing conditions of NC-derived DPSCs and the induced SCs, as well as experiment with the ability of the induced SCs to guide neurons in vitro and in vivo. Furthermore, we will investigate the molecular signaling pathways responsible for the differentiation of NC-derived DPSCs into SCs as they are not well understood.
Figure 2.
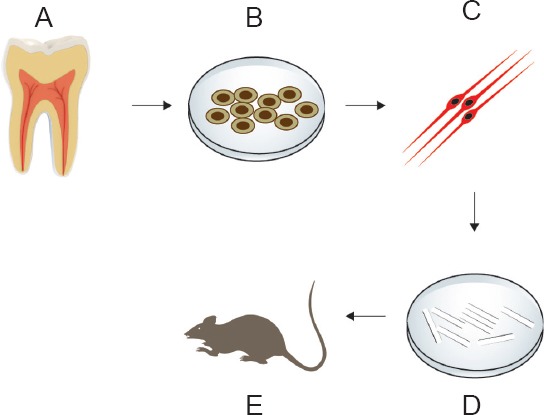
Dental pulp stem cell (DPSC)-derived SCs in peripheral nerve injury regeneration.
Dental pulp is extracted from healthy adult human teeth (A). DPSCs are cultured in a special serum-free medium to ensure that the resulting stem cells are of neural crest ontogeny (B). Nerual crest-derived DPSCs are induced to differentiate into Schwann cells, which is characterized by Schwann cell marker expression and neurotrophic factor secretion (C). Schwann cells are seeded on scaffolds in vitro to study their ability to survive and multiply in a 3D structure (D). Schwann cells seeded on a suitable scaffold are transplanted into an in vivo PNS injury model (e.g., rat sciatic nerve injury) and healing is monitored according to specific criteria (E).
Induction of NC-derived DPSCs into Schwann cells: To culture DPSCs, we experimented with former DPSC culturing protocols, and these protocols have always used serum in the composition of the culture medium. The resulting stem cell population was not able to differentiate into SCs, leading to the conclusion that conventional cell culture methods of DPSCs did not succeed in preserving the stemness of NC-derived DPSCs. On the other hand, dental pulp is a small-sized tissue, and expanding the low cell-numbered primary cell culture without serum is a great challenge. To overcome these challenges, we established a serum-free culture method, which supplies factors that support NC-derived DPSC survival and proliferation while eliminating factors that cause spontaneous differentiation or death of NC-derived DPSCs such as serum and enzymatic digestion (Al-Zer et al., 2015). In our original work, dental pulp was extracted from human wisdom teeth and cultured as explants on fibronectin-coated plates in serum-free neurobasal medium supplemented with B27, basic fibroblast growth factor (bFGF), epidermal growth factor (EGF), insulin, L-glutamine and neuregulin-β1. The resulting stem cell population expressed nestin, CD271 and SOX10, which are well-known markers for NC cells. Consequently, this population of cells was successfully induced to differentiate into SCs utilizing a protocol of three different steps that depended mainly on the addition of retinoic acid, mercaptoethanol and neuregulin β1 to the differentiation medium. After four weeks of glial differentiation induction, the differentiated SCs resembled normal SC morphology; the cells were thin, bipolar spindle shaped, with two to three main processes and an oval, blunt-ended nucleus oriented longitudinally in relation to the main axis of the cell. Furthermore, strong expression of the well-known SC marker, S100β, was detectable. The induced SCs survived and multiplied in the culture for three passages while showing increased expression of S100β, and their cryopreservation was possible (unpublished observation).
Induction of differentiation starts from the step of an appropriate DPSC culturing method through the development of an accurate differentiation protocol. Several critical points determine whether the differentiation occurs or not. First, DPSCs should be cultured in serum-free special medium to ensure that the resulting stem cells are of neural crest ontogeny instead of mesenchymal ontogeny as mesenchymal DPSCs are not able to differentiate into SCs (Al-Zer et al., 2015). Second, the resulting NC-derived DPSCs should be maintained in EGF- and bFGF-supplemented stem cell medium for a relatively long time before the induction can take place. Finally, the induction should be performed in a step-wise manner. NC-derived DPSCs are cultured on laminated eight-well chamber slides at a medium density, and the culture medium is supplemented with mercaptoethanol for one day. Then, the medium is supplemented with retinoic acid for three days. For the following three weeks, the medium is supplemented with a mixture of platelet derived growth factor (PDGF), forskolin, bFGF, EFG and neuregulin β1; these components are reported to induce neural crest cell differentiation into SCs (Hall, 2009).
Future perspective: The proposition of a feasible SC source for peripheral nerve regeneration after injury is the first step in a long step-wise protocol for treating such an injury. The in vitro differentiated SCs should be able to secrete neurotrophic factors, guide neurons and survive for a long term within a 3D scaffold in vitro. Afterwards, these in vitro differentiated SCs could be used in an in vivo animal model to investigate the appropriate scaffold and the appropriate surgical procedures needed for such a treatment modality (Figure 2D and E). Finally, a human clinical trial could be designed to combine these steps together.
Conclusion: The dental pulp of adult humans contains different stem cells populations, which show broad diversity and potentials. Here, the NC-derived DPSC population is discussed in term of their culturing method and induction of differentiation into SCs. The NC-derived DPSC population is to be recruited in the future for peripheral nerve injury regeneration after their induction into SCs in vitro. We recommend that these DPSC-derived SCs be considered as a superior alternative source of SCs compared with a nerve donor source. DPSCs are feasible, are cost and time efficient and do not require complicated surgical procedures.
We thank Mrs Hala Shahrori for the graphics and artwork.
References
- 1.Al-Zer H, Apel C, Heiland M, Friedrich RE, Jung O, Kroeger N, Eichhorn W, Smeets R. Enrichment and schwann cell differentiation of neural crest-derived dental pulp stem cells. In Vivo. 2015;29:319–326. [PubMed] [Google Scholar]
- 2.Alluin O, Wittmann C, Marqueste T, Chabas JF, Garcia S, Lavaut MN, Guinard D, Feron F, Decherchi P. Functional recovery after peripheral nerve injury and implantation of a collagen guide. Biomaterials. 2009;30:363–373. doi: 10.1016/j.biomaterials.2008.09.043. [DOI] [PubMed] [Google Scholar]
- 3.Arthur A, Shi S, Zannettino AC, Fujii N, Gronthos S, Koblar SA. Implanted adult human dental pulp stem cells induce endogenous axon guidance. Stem Cells. 2009;27:2229–2237. doi: 10.1002/stem.138. [DOI] [PubMed] [Google Scholar]
- 4.Burnett MG, Zager EL. Pathophysiology of peripheral nerve injury: a brief review. Neurosurg Focus. 2004;16:E1. doi: 10.3171/foc.2004.16.5.2. [DOI] [PubMed] [Google Scholar]
- 5.Hall BK. Delamination, Migration and Potential. The Neural Crest and Neural Crest Cells in Vertebrate Development and Evolution. In: Hall BK, editor. New York: Springer; 2009. [Google Scholar]
- 6.Mackinnon SE, Doolabh VB, Novak CB, Trulock EP. Clinical outcome following nerve allograft transplantation. Plast Reconstr Surg. 2001;107:1419–1429. doi: 10.1097/00006534-200105000-00016. [DOI] [PubMed] [Google Scholar]
- 7.Martens W, Sanen K, Georgiou M, Struys T, Bronckaers A, Ameloot M, Phillips J, Lambrichts I. Human dental pulp stem cells can differentiate into Schwann cells and promote and guide neurite outgrowth in an aligned tissue-engineered collagen construct in vitro. FASEB J. 2014;28:1634–1643. doi: 10.1096/fj.13-243980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nosrat IV, Widenfalk J, Olson L, Nosrat CA. Dental pulp cells produce neurotrophic factors, interact with trigeminal neurons in vitro, and rescue motoneurons after spinal cord injury. Dev Biol. 2001;238:120–132. doi: 10.1006/dbio.2001.0400. [DOI] [PubMed] [Google Scholar]
- 9.Schlosshauer B, Dreesmann L, Schaller HE, Sinis N. Synthetic nerve guide implants in humans: a comprehensive survey. Neurosurgery. 2006;59:740–747. doi: 10.1227/01.NEU.0000235197.36789.42. [DOI] [PubMed] [Google Scholar]
- 10.Turnbull VJ. Culturing human Schwann cells. Methods Mol Med. 2005;107:173–182. doi: 10.1385/1-59259-861-7:173. [DOI] [PubMed] [Google Scholar]


