1954 Nanodrug-coated three-dimensional microelectronic stent used in axon regeneration after spinal cord injury
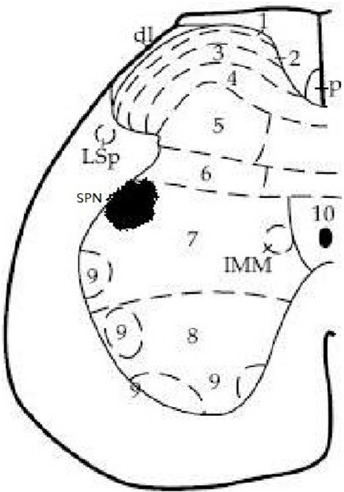
1955 Macrophage polarization and spinal cord injury repair
1957 Inhibiting the RhoA signaling pathway promotes regeneration of axons and myelin sheath
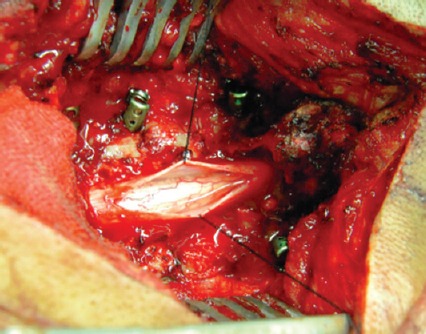
1958 Microsurgical lysis of spinal cord nerve roots for the treatment of spinal cord injury
1959 Local manipulation of the growth cone cytoskeleton: new strategies to promote axon regeneration
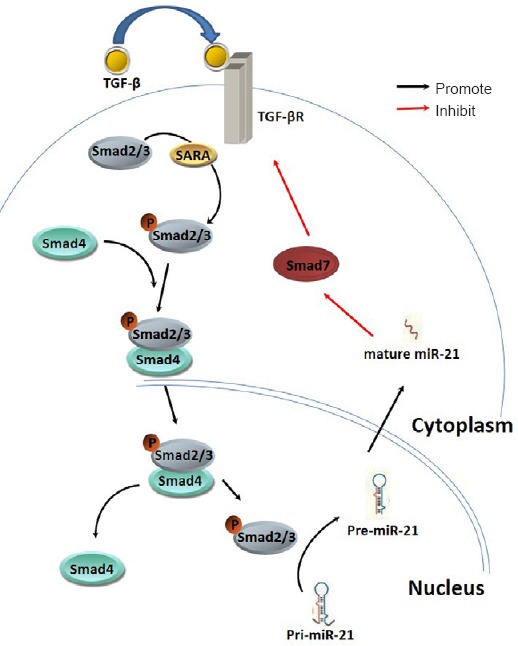
1961 Interaction of miR-21 and TGF-β/SMADs signaling pathway affects the formation of fibrotic scars after spinal cord injury
1962 Elucidating the molecular mechanisms of CSPG-mediated axon growth inhibition through phosphoproteomics analysis
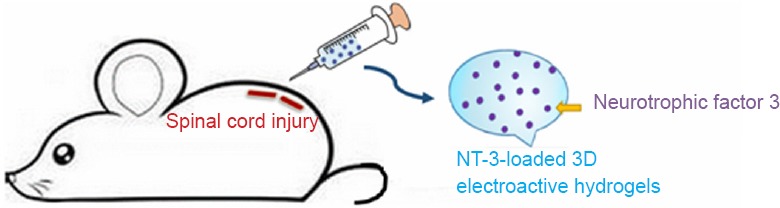
1963 The repair of spinal cord injury with multifunctional, three-dimensional, electroactive scaffolds
1964 A strategy for treating spinal cord injury: targeting the variation of immune cells in the local microenvironment
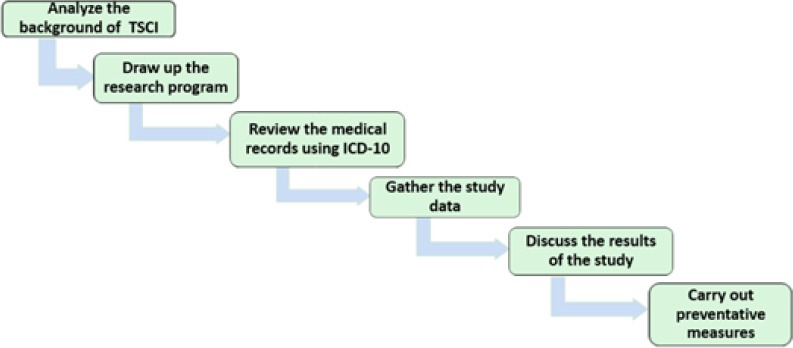
1965 Epidemiological data is critical for decreasing the incidence of spinal cord injury
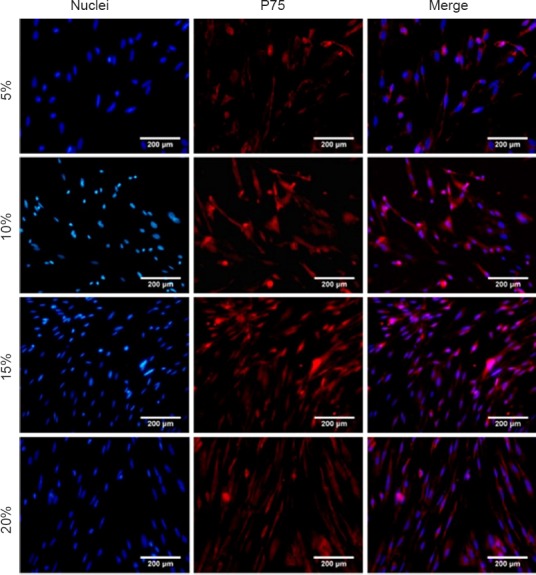
1966 The importance of olfactory ensheathing cells for spinal cord injury
1967 Optogenetics: a new method to repair urination dysfunction after spinal cord injury?
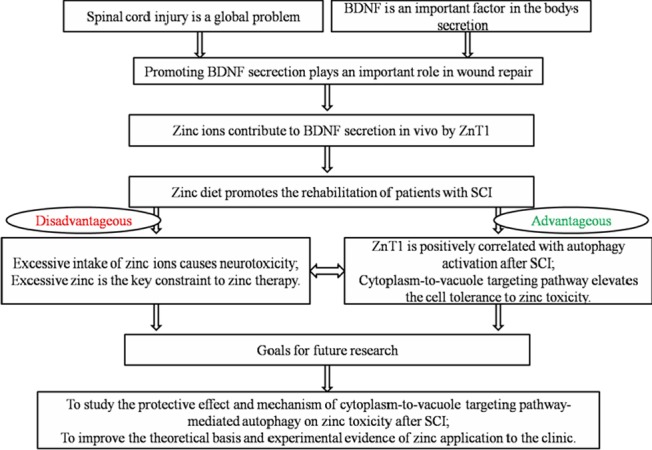
1968 Effects of zinc on the recovery of neurological function after spinal cord injury
1969 Durotomy and dural grafting to treat lower cervical spine injuries with extensive spinal cord edema
1971 Reconstruction of artificial micturition reflex arc for neurogenic bladder after spinal nerve injury
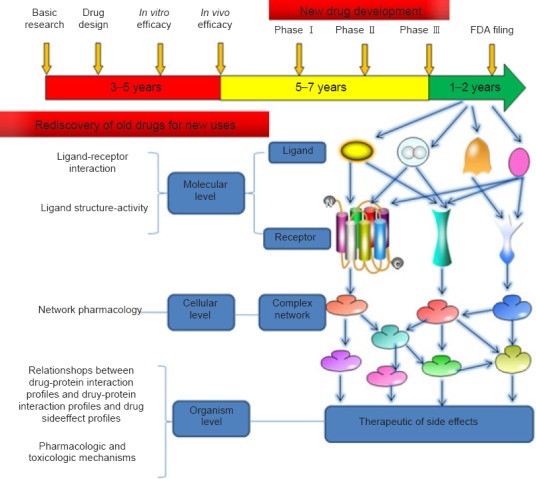
1972 Old drugs, new tricks: the strategy for new drug development in spinal cord injury
First authors and corresponding authors:
Xiao-jian Cao* Department of Spine Surgery, First Affiliated Hospital of Nanjing Medical University
Shi-qing Feng* Department of Orthopedics, Tianjin Medical University General Hospital
Chang-feng Fu* Department of Spine Surgery, First Hospital of Jilin University
Kai Gao# Department of Orthopedics, First Affiliated Hospital of Liaoning Medical University
Jia-song Guo* Department of Histology and Embryology, Southern Medical University; Key Laboratory of Tissue Construction and Detection of Guangdong Province; Institute of Bone Biology, Academy of Orthopedics of Guangdong Province
Xiao-dong Guo* Department of Orthopedics, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology
Xi-jing He* Department of Spine Surgery, The Second Hospital of Xi’an Jiaotong University
Zi-wei Huang# Department of Orthopedics, the First Affiliated Hospital, Orthopedic Institute, Soochow University
Zhong-hai Li#, * Department of Orthopedics, the First Affiliated Hospital of the PLA General Hospital, Orthopedics Institute of PLA
Ling Liu# Institute of Neuroscience, the Fourth Military Medical University of Chinese PLA
Rong-han Liu# Department of Spine Surgery, Jinan Central Hospital Affiliated to Shandong University
He-Zuo Lü#, * Anhui Key Laboratory of Tissue Transplantation, Bengbu Medical College, Bengbu, Anhui Province, China
Xi-fan Mei* Department of Orthopedics, First Affiliated Hospital of Liaoning Medical University
Bin Ning* Department of Spine Surgery, Jinan Central Hospital Affiliated to Shandong University
Guang-zhi Ning# Department of Orthopedics, Tianjin Medical University General Hospital
Chang-hui Qian# Department of Histology and Embryology, Southern Medical University
Jie Qin# Department of Spine Surgery, The Second Hospital of Xi’an Jiaotong University
Yan-zhen Qu# Department of Orthopedics, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology
Saijilafu* Department of Orthopedics, the First Affiliated Hospital, Orthopedic Institute, Soochow University
Bo Shi# Department of Spine Surgery, First Hospital of Jilin University
Tao Sui# Department of Spine Surgery, First Affiliated Hospital, Nanjing Medical University
Tian-sheng Sun* Institute of Trauma Orthopedic Surgery of Chinese PLA, General Hospital of Beijing Military Region
Jian Wang* Institute of Neuroscience, the Fourth Military Medical University
Jin-kun Wen# Department of Histology and Embryology, Southern Medical University
Jian Xiao* Molecular Pharmacology Research Center, School of Pharmacy, Wenzhou Medical University
Bin Xu* Department of Orthopedics, Jinling Hospital, Nanjing University (Nanjing General Hospital of Nanjing Military Command)
Hai-dong Xu# Department of Orthopedics, Jinling Hospital, Nanjing University (Nanjing General Hospital of Nanjing Military Command)
Pan-pan Yu#, * Guangdong-Hongkong-Macau Institute of CNS Regeneration (GHMICR), Jinan University
Zhi-cheng Zhang# Institute of Trauma Orthopedic Surgery of Chinese PLA, General Hospital of Beijing Military Region
Yong Zhou# Department of Orthopedics, Tianjin Medical University General Hospital
Yu-long Zhou# Department of Orthopedics, the Second Affiliated Hospital, Wenzhou Medical University; Molecular Pharmacology Research Center, School of Pharmacy, Wenzhou Medical University
Note: Arranged vertically in alphabetical order by their last name; #First author; *Corresponding author.