Abstract
Lycium barbarum is a widely used Chinese herbal medicine prescription for protection of optic nerve. However, it remains unclear regarding the effects of Lycium barbarum polysaccharides, the main component of Lycium barbarum, on in vivo proliferation of adult ciliary body cells. In this study, adult rats were intragastrically administered low- and high-dose Lycium barbarum polysaccharides (1 and 10 mg/kg) for 35 days and those intragastrically administered phosphate buffered saline served as controls. The number of Ki-67-positive cells in rat ciliary body in the Lycium barbarum polysaccharides groups, in particular low-dose Lycium barbarum polysaccharides group, was significantly greater than that in the phosphate buffered saline group. Ki-67-positive rat ciliary body cells expressed nestin but they did not express glial fibrillary acidic protein. These findings suggest that Lycium barbarum polysaccharides can promote the proliferation of adult rat retinal progenitor cells and the proliferated cells present with neuronal phenotype.
Keywords: nerve regeneration, traditional Chinese medicine, Lycium barbarum polysaccharides, Lycium barbarum (wolfberry), retinal disease, neurogenesis, adult, neural regeneration
Introduction
Lycium barbarum, also known as wolfberry, is a traditional Chinese medicine that can increase visual acuity, regulate immunity, prevent liver damage, and reduce the side effects of chemotherapy and radiotherapy. Although Lycium barbarum has been used over centuries as a traditional Chinese medicine for nourishing the eyes and liver, its exact biochemical function remains elusive. The mechanism underlying the effect of Lycium barbarum has been clarified (Chang and So, 2008). There is evidence that the key player of the therapeutic effect of Lycium barbarum is its antioxidative function, which alleviates conditions related to free radicals (Amagase et al., 2009; Zhang et al., 2010; Bucheli et al., 2011).
Lycium barbarum polysaccharide (LBP), which accounts for more than 40% of compositions in Lycium barbarum, is a major component expected to have the beneficial effect of Lycium barbarum. Increasing lines of evidence discloses the therapeutic effects of LBP from different aspects, including neuroprotection, which verifies the use of Lycium barbarum in the traditional Chinese medicine practice. Studies involving primary cultured cortical neurons showed that LBP treatment reduced cellular death induced by amyloid-beta peptides (Ho et al., 2007) and homocysteine insults (Ho et al., 2009). Because increased levels of both amyloid-beta peptides and homosysteine are risk factors for Alzheimer's disease, so these results provide evidence for the anti-aging property of Lycium barbarum.
Chan et al. (2007) reported that intragastric administration of LBP can reduce the death of retinal ganglion cells induced by experimental ocular hypertension. The protective effect of LBP has also been shown in ischemic models of retina (Li et al., 2011). Chronic consumption of a drink prepared from Lycium barbarum can protect elderly human subjects from hypopigmentation and soft drusen accumulation in the macula (Bucheli et al., 2011). The abovementioned studies provide scientific evidence for the protective functions of Lycium barbarum in the eyes and nervous system. However, further investigation is needed to clarify the molecular mechanisms underlying the therapeutic effects, such as immunomodulation and anti-oxidative stress.
Cell renewal is a common mechanism underlying repair of damaged tissues. Generation of new neurons under physiological conditions is limited in adult mammalian retina (Kubota et al., 2002), so the existence of a quiescent population of retinal progenitor cells in the ciliary body of mammalian retina suggests a possible source to generate new neurons in adulthood (Ahmad et al., 2000; Tropepe et al., 2000). This population of cells is responsive to retinal injury (Nickerson et al., 2007; Wohl et al., 2009), provides trophic support or immunomodulation to injured neurons (Gamm et al., 2007; Stanke and Fischer, 2010), and differentiates to replace the injured neurons (Tropepe et al., 2000). An investigation on how to enhance the proliferation of the retinal progenitor cells that exhibit great potential for treating retina-related diseases will be helpful for development of neuroprotective or cell-renewal treatment for retina-related diseases.
Few studies have been reported on the effect of Lycium barbarum on ciliary body cell proliferation. According to abovementioned studies, LBP is neuroprotective for retina-related diseases, so we hypothesized that LBP treatment can promote the proliferation of retinal progenitor cells in the ciliary body, which is beneficial for retina-related diseases.
Materials and Methods
Animals
Eighteen adult male Sprague-Dawley rats (Laboratory Animal Unit, The University of Hong Kong, China), weighting 250 ± 20 g, were included in this study. The experimental procedures were carried out according to the guidelines provided by the Committee on the Use of Live Animals in Teaching and Research (CULATR, The University of Hong Kong, China). All rats were allowed free access to food and water and were maintained under a 12-hour light/dark cycle. These rats were randomly intragrastrically administered LBP solution at 1mg/kg per day (low-dose LBP group, n = 6), 10 mg/kg per day (high-dose LBP group, n = 6) or vehicle phosphate-buffered saline (PBS group, n = 6) for 35 days.
Preparation of LBP
LBP was prepared as described in previous reports (Yu et al., 2006; Chiu et al., 2009). In brief, fruits of Lycium barbarum were purchased from Ningxia, China and the aqueous extract of the fruit was prepared by decoloration and delipidation in alcohol and boiling in distilled water. Then the extracts were then freeze-dried and stored in the form of powder. To prepare different concentrations of LBP solution, the dried extract was dissolved in 0.01M PBS.
Preparation of sections
After 35 days of treatment, animals were killed by overdose of anesthetic injection. To preserve delicate neural cells, transcardial perfusion with 4% paraformaldehyde was performed immediately after the injection. Eyes were then dissected, enucleated and post-fixed in paraformaldehyde at 4˚C overnight. The eyes were then cryoprotected in 30% sucrose until sectioning. Frozen sections of 10 µm thickness were prepared by a cryostat (Leica Microsystems, Wetzlar, Germany).
Immunohistochemistry
Frozen sections affixed on gelatin-coated slides were used for immunohistochemistry. After two washes with 0.01M PBS containing 0.5% triton (PBST) for 5 minutes each, sections were subjected to antigen retrieval by heating in 0.01M sodium citrate buffer (pH6.0; Sigma-Aldrich, St. Louis, MO, USA) at 90˚C for 20 minutes. After three washes in PBS-Tween, 10% bovine serum albumin was used to block non-specific staining for 1 hour. Then the sections were incubated with primary antibodies. The primary antibodies used were rabbit anti-Ki-67 (1:200; Abcam, Cambridge, UK), mouse anti-nestin (1:400; Abcam, Cambridge, MA, USA) and mouse anti-glial fibrillary acidic protein (GFAP; 1:400, Abcam, USA). These antibodies are able to label different cell types via labelling different target molecules. Ki-67, nestin and GFAP are expressed in newly generated cells, neurons and glial cells only, so these cells can be labeled by corresponding antibodies mentioned above. Sections were incubated with primary antibodies at 4˚C overnight and then with secondary antibodies (sheep anti-mouse, 1:600, Alexor-fluor 568; goat anti-rabbit, 1:250, Alexor-fluor 488, Molecular Probe, Eugene, OR, USA) at room temperature for 1 hour. Then the sections were washed again and 4′,6-diamidino-2-phenylindol (DAPI, Molecular Probes) was used to label cell nucleus. The sections were then stored at 4˚C before quantification.
Quantification of proliferative cells
Eye sections from 18 animals (n = 6 per group) were used for cell quantification. For each animal, eye sections were prepared in 1-in-8 manner and three sections of each rat were used for quantification. The ciliary body regions were observed under 40× objectives with a fluorescent microscope (Eclipse 80i; Nikon, Tokyo, Japan). Ki-67-positive cells and DAPI-stained cell nuclei were quantified, and the results were expressed as the number of Ki-67-positive cells/the number of DAPI-stained nuclei × 100%.
Statistical analysis
Statistical analysis was performed by one-way analysis of variance with the least significant difference post-hoc test (version 20, IBM Corp., Armonk, NY, USA). Statistically significant difference was defined as P < 0.05.
Results
LBP treatment increased the number of Ki-67-positive cells in the ciliary body
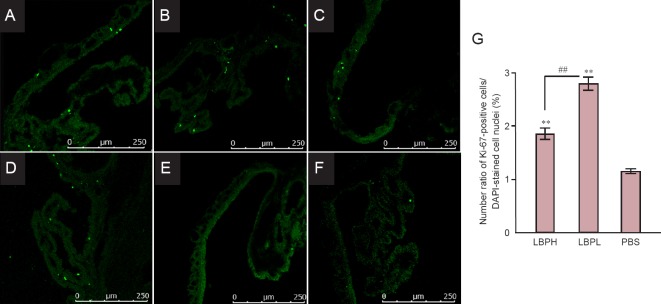
Ki-67 was used as a cell proliferation marker. Ki-67-positive cells were observed in the ciliary bodies, but not in the retina, in all three groups (Figure 1). The number of Ki-67-positive cells in the high-dose LBP (Figure 1A, B) and low-dose LBP groups (Figure 1E, D) was significantly greater than that in the PBS group (Figure 1E, F). The ratio of number of Ki-67-positive cells/number of DAPI-stained nuclei in the high-dose LBP (1.85 ± 0.64%) and low-dose LBP groups (2.79 ± 0.74%) was significantly higher than in the PBS group (1.15 ± 0.27%; both P < 0.01 for both comparisons), and the ratio in the low-dose LBP group was significantly higher than in the high-dose LBP group (P < 0.01).
Figure 1.
Ki-67-positive cells in the adult ciliary body following LBP administration.
(A–F) Representative fluorescence images of Ki-67-positive cells in the ciliary body in LBPH (A, B), LBPL (C, D) and PBS (E, F) groups. Scale bars: 250 μm. (G) Quantification of Ki-67-positive cells in the ciliary body. Both LBPH and LBPL groups showed significantly greater number of Ki-67-positive cells than PBS group (**P < 0.01, vs. PBS group), and the number of Ki-67-positive cells in the LBPL group was significantly greater than in LBPH group (##P < 0.01). One-way analysis of variance with the least significance difference post-hoc test was used. LBPH or LBPL group: Adult rats were intragastrically administrated 10 or 1 mg/kg LBP for 35 days. PBS group: Adult rats intragastrically administrated PBS for 35 days. LBP: Lycium barbarum polysaccharide; LBPH: high-dose Lycium barbarum polysaccharide; LBPL: low-dose Lycium barbarum polysaccharide; PBS: phosphate buffered saline; DAPI: 4′,6-diamidino-2-phenylindol.
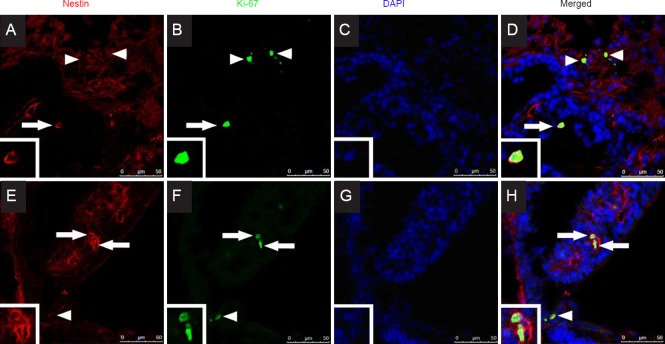
Co-expression of Ki-67 and nestin in the ciliary body
Co-expression of Ki-67 and nestin was observed in the ciliary bodies in both high-dose LBP and low-dose LBP groups (Figure 2), while no Ki-67 expression was observed in the neural retina. Ki-67 expression was observed, but no co-expression of Ki-67 and nestin was observed, in the ciliary bodies in both high-dose LBP and low-dose LBP groups. No co-expression of Ki-67 and nestin was observed in the ciliary bodies in the PBS group.
Figure 2.
Fluorescence microscopy images of nestin and Ki-67 co-expression and DAPI labeling in the ciliary body of LBPH (A–D) and LBPL (E–F)-treated adult rats.
Nestin or Ki-67 expression is indicated by arrows and arrowheads. The nestin and Ki-67 co-expression indicated with arrow is magnified for better morphological observation in the box at the left-bottom corner. Scale bars: 250 μm. LBPH or LBPL group: Adult rats administrated intragastrically with 10 or 1 mg/kg LBP for 35 days. DAPI: 4′,6-Diamidino-2-phenylindol; LBP: Lycium barbarum polysaccharide.
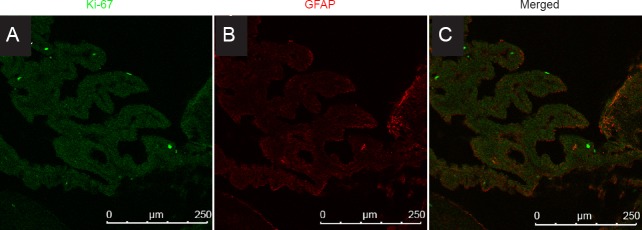
No expression of GFAP in the ciliary body
GFAP, a marker of astroglia, was expressed in the neural retina, but not in the ciliary body (Figure 3). No co-expression of Ki-67 and GFAP was observed in the ciliary body.
Figure 3.
Co-expression of Ki-67 and GFAP in the adult rat ciliary body cells following LBP administration.
No co-expression of Ki-67 and GFAP was observed in the ciliary body cells in the LBP groups and PBS group. Scale bars: 250 μm. LBPH or LBPL group: Adult rats intragastrically administrated 10 or 1mg/kg LBP for 35 days. PBS group: Adult rats intragastrically administrated PBS for 35 days. GFAP: Glial fibrillary acidic protein.
Discussion
Beside LBP, goji berry also contains taurine, which has been proven to be an effective component for eye protection (Lim, 2012). Both taurine and taurine containing methanol Lycium barbarum extract can protect against blood-retinal barrier disruption caused by retinal pigment epithelial cell exposure to high levels of glucose via various mechanisms (Song et al., 2011; Pavan et al., 2014). However, this study is unlikely affected by the taurine content of Lycium barbarum, as the preparation route of LBP used in this study is completely different. First, column chromatography with diethylaminoethylcellulose (DEAE)-sepharose fast flow column was applied in this study for purification of LBP. Second, a dialysis procedure with the eluted fraction was performed, in which the molecular weight cut-off is 3,000-5,000 Da (Yu et al., 2007). These two preparation steps are very effective to remove small molecules like taurine (125.15 g/mol).
Previous reports showed that LBP have neuroprotective effects in different experimental models, including acute hepatotoxicity (Xiao et al., 2012), cytotoxic insults (Yu et al., 2005; Ho et al., 2010), secondary degeneration (Li et al., 2013), experimental glaucoma (Chan et al., 2007) and retinal ischemia (Li et al., 2011). The present study showed another physiological effect of LBP, i.e., promotion of retinal progenitor cell proliferation in the ciliary body. With chronic intragastric administration of either 10 or 1 mg/kg LBP, ciliary body cells were greatly proliferated, showing a neuronal phenotype. Although the underlying mechanism is unclear yet, our results provide a new insight into the biological effect of LBP and may be useful for future studies on the proliferation of retinal progenitor cells.
With advances in stem cell biology, there is a hope that degenerative retinopathies, which are incurable by medication at the present stage, can be treated with stem cell therapy to replace the damaged retinal neurons (Volarevic et al., 2011). Although promising results were gained in generation of photoreceptors from isolated retinal progenitor cells (Klassen et al., 2004), the generation of retinal ganglion cells was shown to be more problematic due to complex connection of retinal ganglion cells within the retina and the long distance for axons to extend and reinnvervate the brain (Johnson et al., 2009). However, possible development of oncological diseases from exogenous source of stem cells suggests caution to develop a cell replacement therapy (Anisimov et al., 2010).
Rather than replacing the injured neurons, several studies suggested that the proliferative progenitor cells may serve another function for recovery: providing trophic support for the existing neurons or regulating the immunological microenvironment (Martino and Pluchino, 2006; Lau et al., 2011). As endogenous retinal progenitor cells can be found in the ciliary body, stimulation of this quiescent stem cell population may be an alternative method to increase the progenitor cell pool in the retina. This approach has the advantage over exogenous cell transplantation since teratoma is unlikely to occur due to treatment manipulation.
Different groups are exploring the ways to increase ciliary body cell proliferation for potential treatments. For instance, Abdouh and Bernier (2006) reported that injection of growth factors induced the proliferative cells in the ciliary body and iris to re-enter the cell cycle in adult rats. In this study, we investigated whether LBP, a traditional Chinese medicine for improving vision, has effect on the proliferation of ciliary body retinal progenitor cells. To the best of our knowledge, this is the first report to show that LBP treatment increases cell proliferation in the ciliary body. This finding provides another support to the effect of LBP on the eyes. The present study did not investigate the effect of LBP in animal disease models, but the discovered pro-proliferative effect will be tested in future studies to determine its potential application in stem cell therapy.
Our results showed that intragastric adminstration of LBP either at a low (1 mg/kg) or a high (10 mg/kg) dose for 35 days significantly increased cell proliferation in the ciliary body and iris, which is indicated by quantification of Ki-67 expression. The low-dose LBP group had a greater number of proliferative cells in the ciliary body than the high-dose LBP group. Co-expression of nestin and Ki-67 in the ciliary body indicates that newly born cells differentiate into neuronal lineage. Our results showed that cell proliferation in the iris was not observed in the PBS group, and the number of new cells in the ciliary body was significantly lower in the high- and low-dose LBP groups than in the PBS group. In addition, no co-expression of nestin and Ki-67 was observed in the PBS group. This suggests that LBP administration can induce the proliferative cells in the ciliary body to re-enter the cell cycle and express nestin. As no co-expression of Ki-67 and GFAP was observed, it is unlikely that the newborn cells belong to the glial cell lineage. The number of proliferated cells in the low-dose LBP group was greater than in the high-dose LBP group. The dose-dependent effect needs to be elucidated in future studies, such as quantification of Ki-67 expression by western blot analysis.
The cell fate, potential migration, differentiation and functional significances of the newly born cells also need to be further clarified. For instance, investigation of the expression of markers specific to immature and mature neurons like doublecortin, beta-III tubulin, rhodopsin and recoverin in those newborn cells will provide basis for characterization of the new cells.
Lycium barbarum contains a wide array of components speculated to exert its biological function. For instance, lutein and zeaxanthin, which are two common components studied in Lycium barbarum (Chiu et al., 2010), have been shown to prevent light-induced retinal injury (Sasaki et al., 2012) and be potentially beneficial for the prevention of age-related macular degeneration (Huang et al., 2015). Lycium barbarum exhibits antioxidant property by lowering abnormal oxidation index back to normal (Amagase et al., 2009).
LBP, a major component of Lycium barbarum, has also been shown to exhibit a variety of biological effects, including anti-aging (Yu et al., 2005), lowering blood glucose level (Luo et al., 2004), reducing the side effects of chemotherapy or radiotherapy (Gong et al., 2005), anti-tumor progression (Zhang et al., 2005) and protecting the streptozotocin-induced diabetic rats from liver and kidney injury (Zhao et al., 2009). But the underlying mechanisms are still unclear. Alternation of immune functions may be one mechanism by which LBP exerts biological function (Yu et al., 2006; Chiu et al., 2009; London et al., 2011). LBP has been shown to prevent apoptosis of retinal ganglion cells in an experimental model of ocular hypertension/glaucoma (Chan et al., 2007), which is induced by laser photocoagulation of episcleral and limbic veins in the retina. Feeding of LBP at a dose range from 0.01 to 1,000 mg/kg per day can significantly prevent the loss of retinal ganglion cells with the intraocular pressure unaltered, while the optimal effect was found at the doses of 1 and 10 mg/kg. Later studies revealed that LBP may exert the protective effect on the retina through the activation of microglia, a type of macrophages in the central nervous system. A previous study showed that with inhibition of microglial activation by macrophage/microglia inhibitory factors, the protective effect of LBP on retinal ganglion cells was diminished (Chiu et al., 2009). A similar conclusion was also drawn in a study which utilized another animal model to induce retinal damage (Li et al., 2011). Li et al. (2011) used carotid artery occlusion to induce retinal cell death and showed that LBP treatment significantly attenuated the apoptosis of retinal interneurons, oxidative stress and blood-retinal barrier disruption. In addition to the neuroprotective effect of LBP, the dose range at which LBP affects ciliary cell proliferation requires further investigation.
Previous studies showed that paroxetine and corticosterone, which are known for their effect on neural stem cell proliferation in neurogenic zones of the brain (Lau et al., 2007), also affected the proliferation of dividing cells residing in the ciliary body (Wang et al., 2010).
Our results demonstrated that LBP treatment increased ciliary body cell proliferation, suggesting that LBP exerts protective effect on the eye, and providing hints for subsequent treatment to promote retinal progenitor cell proliferation. A better understanding of the effect of LBP on different retinal disease models will be beneficial to reveal the potential use of LBP.
Footnotes
Conflicts of interest: None declared.
Plagiarism check: This paper was screened twice using Cross-Check to verify originality before publication.
Peer review: This paper was double-blinded and stringently reviewed by international expert reviewers.
Copyedited by Lupo G, Pavan B, Li CH, Song LP, Zhao M
References
- 1.Abdouh M, Bernier G. In vivo reactivation of a quiescent cell population located in the ocular ciliary body of adult mammals. Exp Eye Res. 2006;83:153–164. doi: 10.1016/j.exer.2005.11.016. [DOI] [PubMed] [Google Scholar]
- 2.Ahmad I, Tang L, Pham H. Identification of neural progenitors in the adult mammalian eye. Biochem Biophys Res Commun. 2000;270:517–521. doi: 10.1006/bbrc.2000.2473. [DOI] [PubMed] [Google Scholar]
- 3.Amagase H, Sun B, Borek C. Lycium barbarum (goji) juice improves in vivo antioxidant biomarkers in serum of healthy adults. Nutr Res. 2009;29:19–25. doi: 10.1016/j.nutres.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 4.Anisimov SV, Morizane A, Correia AS. Risks and mechanisms of oncological disease following stem cell transplantation. Stem Cell Rev. 2010;6:411–424. doi: 10.1007/s12015-010-9134-5. [DOI] [PubMed] [Google Scholar]
- 5.Bucheli P, Vidal K, Shen L, Gu Z, Zhang C, Miller LE, Wang J. Goji berry effects on macular characteristics and plasma antioxidant levels. Optom Vis Sci. 2011;88:257–262. doi: 10.1097/OPX.0b013e318205a18f. [DOI] [PubMed] [Google Scholar]
- 6.Chan HC, Chang RC, Koon-Ching Ip A, Chiu K, Yuen WH, Zee SY, So KF. Neuroprotective effects of Lycium barbarum Lynn on protecting retinal ganglion cells in an ocular hypertension model of glaucoma. Exp Neurol. 2007;203:269–273. doi: 10.1016/j.expneurol.2006.05.031. [DOI] [PubMed] [Google Scholar]
- 7.Chang RC, So KF. Use of anti-aging herbal medicine, Lycium barbarum, against aging-associated diseases. What do we know so far? Cell Mol Neurobiol. 2008;28:643–652. doi: 10.1007/s10571-007-9181-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chiu K, Chan HC, Yeung SC, Yuen WH, Zee SY, Chang RC, So KF. Modulation of microglia by Wolfberry on the survival of retinal ganglion cells in a rat ocular hypertension model. J Ocul Biol Dis Infor. 2009;2:47–56. doi: 10.1007/s12177-009-9023-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chiu K, Zhou Y, Yeung SC, Lok CK, Chan OO, Chang RC, So KF, Chiu JF. Up-regulation of crystallins is involved in the neuroprotective effect of wolfberry on survival of retinal ganglion cells in rat ocular hypertension model. J Cell Biochem. 2010;110:311–320. doi: 10.1002/jcb.22539. [DOI] [PubMed] [Google Scholar]
- 10.Gamm DM, Wang S, Lu B, Girman S, Holmes T, Bischoff N, Shearer RL, Sauve Y, Capowski E, Svendsen CN, Lund RD. Protection of visual functions by human neural progenitors in a rat model of retinal disease. PLoS One. 2007;2:e338. doi: 10.1371/journal.pone.0000338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gong H, Shen P, Jin L, Xing C, Tang F. Therapeutic effects of Lycium barbarum polysaccharide (LBP) on irradiation or chemotherapy-induced myelosuppressive mice. Cancer Biother Radiopharm. 2005;20:155–162. doi: 10.1089/cbr.2005.20.155. [DOI] [PubMed] [Google Scholar]
- 12.Ho YS, Yu MS, Lai CS, So KF, Yuen WH, Chang RC. Characterizing the neuroprotective effects of alkaline extract of Lycium barbarum on beta-amyloid peptide neurotoxicity. Brain Res. 2007;1158:123–134. doi: 10.1016/j.brainres.2007.04.075. [DOI] [PubMed] [Google Scholar]
- 13.Ho YS, Yu MS, Yang XF, So KF, Yuen WH, Chang RC. Neuroprotective effects of polysaccharides from wolfberry the fruits of Lycium barbarum, against homocysteine-induced toxicity in rat cortical neurons. J Alzheimers Dis. 2010;19:813–827. doi: 10.3233/JAD-2010-1280. [DOI] [PubMed] [Google Scholar]
- 14.Huang YM, Dou HL, Huang FF, Xu XR, Zou ZY, Lin XM. Effect of supplemental lutein and zeaxanthin on serum, macular pigmentation, and visual performance in patients with early age-related macular degeneration. Biomed Res Int 2015. 2015:564738. doi: 10.1155/2015/564738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnson TV, Bull ND, Martin KR. Transplantation prospects for the inner retina. Eye (Lond) 2009;23:1980–1984. doi: 10.1038/eye.2008.376. [DOI] [PubMed] [Google Scholar]
- 16.Klassen HJ, Ng TF, Kurimoto Y, Kirov I, Shatos M, Coffey P, Young MJ. Multipotent retinal progenitors express developmental markers differentiate into retinal neurons and preserve light-mediated behavior. Invest Ophthalmol Vis Sci. 2004;45:4167–4173. doi: 10.1167/iovs.04-0511. [DOI] [PubMed] [Google Scholar]
- 17.Kubota R, Hokoc JN, Moshiri A, McGuire C, Reh TA. A comparative study of neurogenesis in the retinal ciliary marginal zone of homeothermic vertebrates. Brain Res Dev Brain Res. 2002;134:31–41. doi: 10.1016/s0165-3806(01)00287-5. [DOI] [PubMed] [Google Scholar]
- 18.Lau BW, Yau SY, Lee TM, Ching YP, Tang SW, So KF. Effect of corticosterone and paroxetine on masculine mating behavior: possible involvement of neurogenesis. J Sex Med. 2011;8:1390–1403. doi: 10.1111/j.1743-6109.2010.02081.x. [DOI] [PubMed] [Google Scholar]
- 19.Lau WM, Qiu G, Helmeste DM, Lee TM, Tang SW, So KF. Corticosteroid decreases subventricular zone cell proliferation which could be reversed by paroxetine. Restor Neurol Neurosci. 2007;25:17–23. [PubMed] [Google Scholar]
- 20.Li H, Liang Y, Chiu K, Yuan Q, Lin B, Chang RC, So KF. Lycium Barbarum (Wolfberry) reduces secondary degeneration and oxidative stress and inhibits jnk pathway in retina after partial optic nerve transection. PLoS One. 2013;8:e68881. doi: 10.1371/journal.pone.0068881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li SY, Yang D, Yeung CM, Yu WY, Chang RC, So KF, Wong D, Lo AC. Lycium barbarum polysaccharides reduce neuronal damage blood-retinal barrier disruption and oxidative stress in retinal ischemia/reperfusion injury. PLoS One. 2011;6:e16380. doi: 10.1371/journal.pone.0016380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lim TK. Springer; 2012. Edible Medicinal And Non-Medicinal Plants. [Google Scholar]
- 23.London A, Itskovich E, Benhar I, Kalchenko V, Mack M, Jung S, Schwartz M. Neuroprotection and progenitor cell renewal in the injured adult murine retina requires healing monocyte-derived macrophages. J Exp Med. 2011;208:23–39. doi: 10.1084/jem.20101202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luo Q, Cai Y, Yan J, Sun M, Corke H. Hypoglycemic and hypolipidemic effects and antioxidant activity of fruit extracts from Lycium barbarum. Life Sci. 2004;76:137–149. doi: 10.1016/j.lfs.2004.04.056. [DOI] [PubMed] [Google Scholar]
- 25.Martino G, Pluchino S. The therapeutic potential of neural stem cells. Nat Rev Neurosci. 2006;7:395–406. doi: 10.1038/nrn1908. [DOI] [PubMed] [Google Scholar]
- 26.Nickerson PE, Emsley JG, Myers T, Clarke DB. Proliferation and expression of progenitor and mature retinal phenotypes in the adult mammalian ciliary body after retinal ganglion cell injury. Invest Ophthalmol Vis Sci. 2007;48:5266–5275. doi: 10.1167/iovs.07-0167. [DOI] [PubMed] [Google Scholar]
- 27.Pavan B, Capuzzo A, Forlani G. High glucose-induced barrier impairment of human retinal pigment epithelium is ameliorated by treatment with Goji berry extracts through modulation of cAMP levels. Exp Eye Res. 2014;120:50–54. doi: 10.1016/j.exer.2013.12.006. [DOI] [PubMed] [Google Scholar]
- 28.Sasaki M, Yuki K, Kurihara T, Miyake S, Noda K, Kobayashi S, Ishida S, Tsubota K, Ozawa Y. Biological role of lutein in the light-induced retinal degeneration. J Nutr Biochem. 2012;23:423–439. doi: 10.1016/j.jnutbio.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 29.Song M, Roufogalis BD, Huang TH. Modulation of RAGE and the downstream targets of RAGE signaling cascades by taurine in Lycium barbarum (Goji Berry): protection of human retinal pigment epithelial barrier function and its potential benefit in diabetic retinopathy. J Diabetes Metab. 2011;2:1000162. [Google Scholar]
- 30.Stanke JJ, Fischer AJ. Embryonic retinal cells and support to mature retinal neurons. Invest Ophthalmol Vis Sci. 2010;51:2208–2218. doi: 10.1167/iovs.09-4447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tropepe V, Coles BL, Chiasson BJ, Horsford DJ, Elia AJ, McInnes RR, van der Kooy D. Retinal stem cells in the adult mammalian eye. Science. 2000;287:2032–2036. doi: 10.1126/science.287.5460.2032. [DOI] [PubMed] [Google Scholar]
- 32.Volarevic V, Arsenijevic N, Lukic ML, Stojkovic M. Concise review: Mesenchymal stem cell treatment of the complications of diabetes mellitus. Stem Cells. 2011;29:5–10. doi: 10.1002/stem.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang H, Lau BW, Yau SY, Li SY, Leung N, Wang NL, Tang SW, Lee TM, So KF. Roles of paroxetine and corticosterone on adult mammalian ciliary body cell proliferation. Chin Med J (Engl) 2010;123:1305–1310. [PubMed] [Google Scholar]
- 34.Wohl SG, Schmeer CW, Kretz A, Witte OW, Isenmann S. Optic nerve lesion increases cell proliferation and nestin expression in the adult mouse eye in vivo. Exp Neurol. 2009;219:175–186. doi: 10.1016/j.expneurol.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 35.Xiao J, Liong EC, Ching YP, Chang RC, So KF, Fung ML, Tipoe GL. Lycium barbarum polysaccharides protect mice liver from carbon tetrachloride-induced oxidative stress and necroinflammation. J Ethnopharmacol. 2012;139:462–470. doi: 10.1016/j.jep.2011.11.033. [DOI] [PubMed] [Google Scholar]
- 36.Yu MS, Ho YS, So KF, Yuen WH, Chang RC. Cytoprotective effects of Lycium barbarum against reducing stress on endoplasmic reticulum. Int J Mol Med. 2006;17:1157–1161. [PubMed] [Google Scholar]
- 37.Yu MS, Leung SK, Lai SW, Che CM, Zee SY, So KF, Yuen WH, Chang RC. Neuroprotective effects of anti-aging oriental medicine Lycium barbarum against beta-amyloid peptide neurotoxicity. Exp Gerontol. 2005;40:716–727. doi: 10.1016/j.exger.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 38.Yu MS, Lai CS, Ho YS, Zee SY, So KF, Yuen WH, Chang RC. Characterization of the effects of anti-aging medicine Fructus lycii on beta-amyloid peptide neurotoxicity. Int J Mol Med. 2007;20:261–268. [PubMed] [Google Scholar]
- 39.Zhang M, Chen H, Huang J, Li Z, Zhu C, Zhang S. Effect of lycium barbarum polysaccharide on human hepatoma QGY7703 cells: inhibition of proliferation and induction of apoptosis. Life Sci. 2005;76:2115–2124. doi: 10.1016/j.lfs.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 40.Zhang R, Kang KA, Piao MJ, Kim KC, Kim AD, Chae S, Park JS, Youn UJ, Hyun JW. Cytoprotective effect of the fruits of Lycium chinense Miller against oxidative stress-induced hepatotoxicity. J Ethnopharmacol. 2010;130:299–306. doi: 10.1016/j.jep.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 41.Zhao R, Li QW, Li J, Zhang T. Protective effect of Lycium barbarum polysaccharide 4 on kidneys in streptozotocin-induced diabetic rats. Can J Physiol Pharmacol. 2009;87:711–719. doi: 10.1139/y09-068. [DOI] [PubMed] [Google Scholar]