Abstract
Studies have shown that sensory nerve damage can activate the p38 mitogen-activated protein kinase (MAPK) pathway, but whether the same type of nerve injury after exercise activates the p38MAPK pathway remains unclear. Several studies have demonstrated that nerve growth factor may play a role in the repair process after peripheral nerve injury, but there has been little research focusing on the hypoglossal nerve injury and repair. In this study, we designed and established rat models of hypoglossal nerve crush injury and gave intraperitoneal injections of exogenous nerve growth factor to rats for 14 days. p38MAPK activity in the damaged neurons was increased following hypoglossal nerve crush injury; exogenous nerve growth factor inhibited this increase in acitivity and increased the survival rate of motor neurons within the hypoglossal nucleus. Under transmission electron microscopy, we found that the injection of nerve growth factor contributed to the restoration of the morphology of hypoglossal nerve after crush injury. Our experimental findings indicate that exogenous nerve growth factor can protect damaged neurons and promote hypoglossal nerve regeneration following hypoglossal nerve crush injury.
Keywords: nerve regeneration, p38MAPK, mitogen-activated protein kinase, nerve growth factor, hypoglossal nerve, crush injury, nerve injury, neural regeneration
Introduction
The p38MAPK pathway is one of the mitogen-activated protein kinase (MAPK) cascade signal transduction pathways. The p38MAPK pathway can be activated by a variety of stressors, and phosphorylated p38MAPK (p-p38MAPK) is the actived form of p38MAPK (Bu et al., 2007; Chaparro-Huerta et al., 2008; de Rivero Vaccari et al., 2009; Gwak et al., 2009). Growing evidence has demonstrated that central or peripheral sensory nerve injury can activate the p38MAPK pathway (Lee et al., 2010; Jeon et al., 2011; Mizukoshi et al., 2013; Katome, 2014; Zhou et al., 2014b). However, whether the same type of nerve injury after exercise can activate the p38MAPK pathway remains unclear. In addition, the activation of p38MAPK in the repair process after nerve injury is poorly understood. Nerve growth factor (NGF) is a member of neurotrophic factor family, and can protect sympathetic nerves, sensory nerves and cholinergic nerves, as well as promote nerve cell differentiation and development (Liu et al., 2014). In the peripheral nervous system, NGF has been shown to increase the numbers of sympathetic and sensory ganglia, and to promote nerve fiber growth (Kemp et al., 2008; Fortun et al., 2009; Hood et al., 2009; Delaviz et al., 2011; Liu et al., 2013; Ma et al., 2013; Wang et al., 2015). However, the NGF-mediated repair process after nerve injury is very complex and multifactorial, and whether the p38MAPK signal transduction pathway is involved in this process remains unclear.
The hypoglossal nerve is a cranial nerve that innervates the muscles of the tongue and is an important motor nerve in the maxillofacial region. When the internal jugular vein is ligated or the submandibular triangle is involved during lymphadenectomy, surgery in the hypoglossal area causes damage to the hypoglossal nerve, causing tongue movement disorders. In addition, the goal when repairing tongue defects is to ensure that the regenerated hypoglossal nerve can control the remaining tongue muscle and the transplanted muscle (Zhang and Tu, 2005). Therefore, nerve restoration after hypoglossal nerve injury is important. In this study, we established rat models of hypoglossal nerve crush injury and observed the activation of p38MAPK and the survival of neurons within the hypoglossal nucleus, before and after injury, and before and after intervention with exogenous NGF. We also explored the effects of motor nerve injury and NGF intervention on the p38MAPK pathway, as well as the protective effect and regeneration effect of NGF after hypoglossal nerve injury.
Materials and Methods
Establishing the hypoglossal nerve crush injury model
Sixty healthy adult Sprague-Dawley rats, aged 8 weeks, half male and half female, weighing 200–250 g, were provided by the Experimental Animal Laboratory, Xiangya School of Medicine, Central South University (China, license No. SCXK (Xiang) 2009-0004). Animals were housed in a room at 19–26°C with 40–70% humidity. Animal experiments were approved by the Animal Ethics Committee of Central South University, China. The 60 rats were randomly divided into a control group (n = 20), a model group (n = 20) and an NGF group (n = 20).
Rats in the model and NGF groups were anesthetized with 10% chloral hydrate through intraperitoneal injection. A vertical incision was made in the submaxillary region, the left hypoglossal nerve stem was exposed below the left digastric tendon, and the nerve was clamped with a serrated microsurgical forceps 2 mm lateral to the hypoglossal nerve bifurcation below the left digastric tendon. After the nerve was clamped for 30 seconds, the forceps were rotated through 90° and the nerve was clamped for an additional 30 seconds. During the operations, the left hypoglossal nerve was clamped but not severed in all rats, the clamping force on the left hypoglossal nerve was the same in all rats, and the wounds were sutured (Armstrong et al., 1991; Bussmann and Sofroniew, 1999; Zhang et al., 2009). Control animals were operated on to expose the hypoglossal nerve only.
NGF intervention
After injury, rats in the NGF group were intraperitoneally injected with 200 U NGF (R & D, Minneapolis, MN, USA), once per day, until death. Rats in the control and model groups were given 1 mL of saline, once per day, until death.
Harvesting specimens
At 1, 3, 5, 7 and 14 days after injury, four rats selected from each group were anesthetized and the whole animals were fixed with paraformaldehyde; then, a 1-cm brain stem segment containing the hypoglossal motor nucleus was cut (Paxinos and Watson, 2005). Specimens were labeled on the right ventral side and cut into frozen transverse slices at a thickness of 30 µm.
Immunohistochemical staining
Rat brain tissue slices were prepared, blocked with serum, and incubated with rabbit anti-rat p-p38MAPK monoclonal antibody (1:100; Cell Signaling Technology, Inc., Danvers, MA, USA) at 4°C for 24 hours, then with biotinylated goat anti-rabbit IgG (ready-to-use; Beijing Zhongshan Golden Bridge Biotechnology Co., Ltd., Beijing, China) at 37°C for 10-15 minutes, and finally with horseradish peroxidase-conjugated streptavidin. Subsequently, slices were developed with DAB, cleared with xylene, and mounted and observed under a light microscope (Olympus, Tokyo, Japan). Negative control slices were treated with phosphate-buffered saline rather than primary and secondary antibodies. The number of p-p38MAPK-positive cells was counted in each of two fields of vision for each slice, in five slices from each animal, under 200× magnification. The average value was obtained and grayscale analysis was performed using the Advance3.2 system (Motic Medical Laboratory, Xiamen, Fujian Province, China).
Nissl staining
At 7 and 14 days after injury, hypoglossal nucleus tissue from rats in the model and NGF groups was prepared for Nissl staining. Tissue was stained with 1% toluidine blue at room temperature for 3 minutes, rinsed with distilled water, treated with ethanol, cleared with xylene, and mounted. Five slices from each animal were randomly selected for counting the number of neurons within the hypoglossal nucleus on the normal and injury sides. The survival rate of neurons = the number of neurons within the hypoglossal nucleus on the injury side/the number of neurons within the hypoglossal nucleus on the normal side × 100% (Schmalbruch, 1984).
Transmission electron microscopy
At 7 and 14 days after injury, five rats randomly selected from the model and NGF groups were anesthetized, and underwent thoracotomy, intubation and perfusion. The skin in the submandibular area was cut, the muscle and the left hypoglossal nerve stem were bluntly dissected, and the hypoglossal nerve 0.5 cm lateral to the left hypoglossal nerve crush site was harvested and prepared into ultrathin slices. Then, slices were observed under a transmission electron microscope (Hitachi, Tokyo, Japan).
Statistical analysis
Data are represented as the mean ± SD, and the mean values were compared between groups by one-way analysis of variance using SPSS 13.0 software (SPSS, Chicago, IL, USA). P < 0.05 was considered to represent a statistically significant difference.
Results
Effects of exogenous NGF on the expression of p-p38MAPK in the brains of rats with hypoglossal nerve crush injury
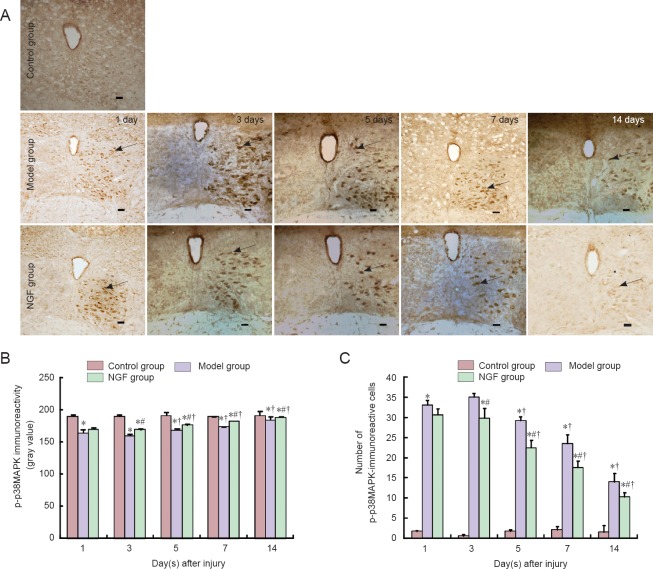
Immunohistochemical staining showed that no p-p38MAPK was expressed within the hypoglossal nucleus in the control group of rats. Compared with the control group, p-p38MAPK expression within the hypoglossal nucleus was obviously increased in the model and NGF groups. The expression levels in the two groups increased with time post-injury, reaching a peak at 3 days, and then decreased (P < 0.05). The level of p-p38MAPK expression within the hypoglossal nucleus in the NGF group was lower than that in the model group (P < 0.05; Figure 1).
Figure 1.
Effect of exogenous nerve growth factor (NGF) on the immunoreactivity of p-p38MAPK in rat brain tissue after hypoglossal nerve crush injury.
(A) Immunoreactivity for p-p38MAPK in rat brain tissue (immunohistochemical staining). Arrows indicate immunoreactive cells. Scale bars: 100 μm. (B) The immunoreactivity of p-p38MAPK in rat brain tissue. (C) The number of p-p38MAPK immunoreactive cells in rat brain tissue. Data are the mean ± SD of four rats in each group. Differences between groups were compared using one-way analysis of variance. *P < 0.05 vs. control group; #P < 0.05 vs. model group; †P < 0.05 vs. previous time point.
Exogenous NGF increased the number of motor neurons within the hypoglossal nucleus in rats with hypoglossal nerve crush injury
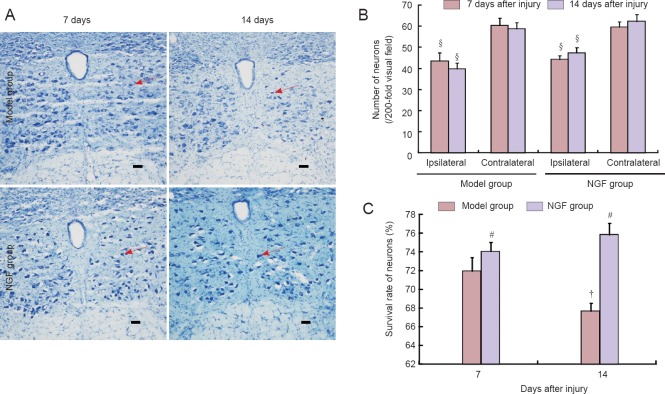
Nissl staining showed that at 7 and 14 days after injury, the number of motor neurons within the hypoglossal nucleus on the injury side was lower than that on the normal side in the model and NGF groups (P < 0.05). The survival rate of motor neurons within the hypoglossal nucleus at 14 days was lower than that at 7 days in the model group (P < 0.05), while the survival rate was similar in the NGF group at 7 and 14 days (P > 0.05). The survival rate of motor neurons within hypoglossal nucleus in the NGF group was decreased compared with the model group (P < 0.05; Figure 2).
Figure 2.
Effect of exogenous nerve growth factor (NGF) on the motor neurons within the hypoglossal nucleus in rats with hypoglossal nerve crush injury.
(A) Motor neurons within the hypoglossal nucleus (Nissl staining, × 200). The survival rate of motor neurons within the hypoglossal nucleus was increased in the NGF group compared with the model group. Arrows indicate Nissl-stained neurons. Scale bars: 100 μm. (B) The number of neurons within the hypoglossal nucleus. (C) The survival rate of neurons within the ipsilateral hypoglossal nucleus. Survival rate of neurons in the ipsilateral hypoglossal nucleus = the number of neurons within the hypoglossal nucleus on the ipsilateral side/the number of neurons within the hypoglossal nucleus on the contralateral side × 100%. Data are the mean ± SD of four rats in each group. Differences between groups were compared using one-way analysis of variance. #P < 0.05, vs. model group; †P < 0.05, vs. previous time point; §P < 0.05, vs. contralateral side.
Exogenous NGF improved the ultrastructure of motor neurons within hypoglossal nucleus in rats with hypoglossal nerve crush injury
Under transmission electron microscopy, tissue edema and myelinated fiber layer loosening and degeneration were found in the model and NGF groups at 7 and 14 days post-injury. In the NGF group, myelinated nerve fiber morphology and lamellar structure were dense and clearly visible at the distal end of the hypoglossal nerve, while the organelle structure within the axon and Schwann cell morphology were better than in the model group.
At 7 days post-injury, myelinated nerve fibers showed swelling and the lamellar structure showed loosening, even becoming isolated, in the model group. Swelling of mitochondria was found within axons and Schwann cells, and the structure of microfilaments and microtubules was not clear. In the NGF group, myelinated nerve fibers were porous and twisted, but no lamellar structure was isolated, and although partial mitochondrial swelling was found within axons, it was within acceptable limits within Schwann cells. At 14 days post-injury, all of the myelinated nerve fibers degenerated, the axonal cavity disappeared, normal structure was lost, the myelin lamellar structure showed loosening, axons disappeared, and pyknotic Schwann cells were found in the model group. While the morphology of myelinated nerve fibers was normal, the lamellar structure showed thickening, and a small amount of mitochondrial swelling was found within the axons of rats in the NGF group. Microtubules and microfilaments showed a clear structure, and organelles within Schwann cells were clearly visible (Figure 3).
Figure 3.
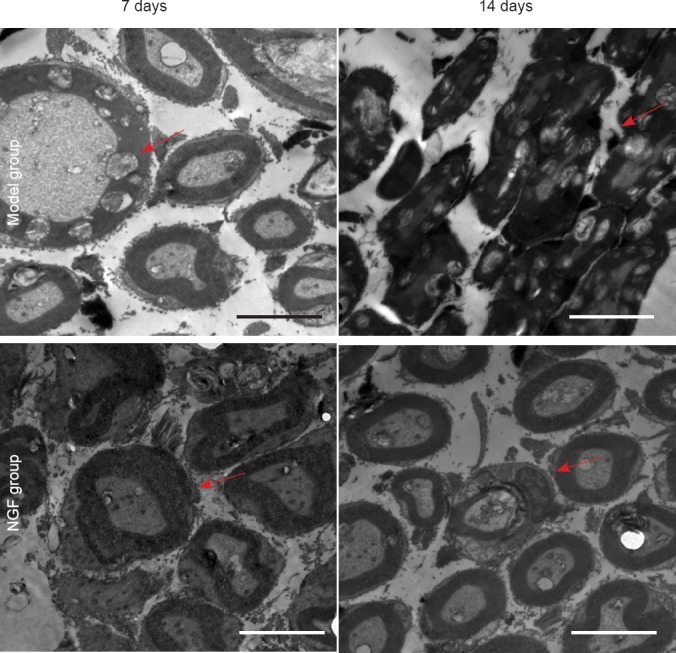
Effect of exogenous nerve growth factor (NGF) on the ultrastructure of the hypoglossal nucleus in rats after hypoglossal nerve crush injury (transmission electron microscope, × 7,000).
Myelin sheath impairment in myelinated nerve fibers at the distal end of the hypoglossal nerve was obviously less in the NGF group than in the model group. Arrows indicate myelin. Scale bars: 5 μm.
Discussion
p38MAPK activity in damaged neurons is enhanced after hypoglossal nerve crush injury
In a variety of nerve injury models, p38MAPK is activated in the early stages after central and sensory nerve injuries; its expression levels are increased at the central injury area in a time-dependent fashion (Suter et al., 2009; Dapper et al., 2013; Semba et al., 2014; Zhou et al., 2014a; Lee et al., 2015a). Chiang et al. (2013) found that 1 day after chronic constriction injury in the median nerve of rats, p-p38MAPK expression was significantly increased and reached a peak at 7 days, indicating that median nerve injury can activate the p38MAPK pathway. Agthong et al. (2012) found that at 2 weeks after sciatic nerve transection injury, the expression of p-p38MAPK at the L4/5 dorsal root ganglia was increased, highlighting the contribution of p38MAPK to the loss of neurons after nerve transection. Terayama et al. (2008) demonstrated that 3–21 days after peripheral nerve injury, p-p38MAPK expression was increased in glial cells in the ipsilateral dorsal horn and gracile nucleus. Kwon et al. (2014) demonstrated increased p-p38MAPK expression in rats with neuropathic pain caused by spinal nerve ligation. Terayama et al. (2011) found that p-MAPK expression was gradually increased within the trigeminal sensory nuclear complex following lingual nerve injury.
In this study, after rat models of hypoglossal nerve crush injury were produced, p-p38MAPK was not expressed in the cell body within the hypoglossal nucleus in the control group. Subsequently, p-p38MAPK expression within the hypoglossal nucleus was obviously increased at 1 day after crush injury on the ipsilateral (left) side, reaching a peak at 3 days and then gradually decreased, but the expression level at 14 days was still higher than that in the control group. Nissl staining showed that the number of neurons within the hypoglossal nucleus on the ipsilateral side was lower than that on the contralateral side, indicating the death of neurons. These results suggest that stimuli after hypoglossal nerve injury can activate the p38MAPK pathway, and that while the activated p38MAPK pathway is involved in the death of nerve cells after hypoglossal nerve injury, the activation is more obvious in the early state after injury.
NGF inhibits p38MAPK activation induced by hypoglossal nerve injury
NGF is a typical cell growth factor that can regulate neuronal differentiation, growth, death, and connection remodeling through binding to the surface receptor of neurons (Kernie and Parada, 2000; Namiki et al., 2000; Chen et al., 2014b). Recent studies have shown that NGF exerts biological effects through binding its receptors, and that the process is mediated by the intracellular MAPK signal transduction pathway (Diolaiti et al., 2007; Santos et al., 2007; Nguyen et al., 2009; Hong et al., 2012; Wuhanqimuge et al., 2013; Yuan et al., 2013). Muroi et al. (2004) found that shortening the duration of p38MAPK phosphorylation may increase axon growth effects induced by NGF. Marampon et al. (2008) proposed that NGF regulates the expression of cyclin D1 in PC12 cells through influencing the p38MAPK and other extracellular signaling pathways. Morill et al. (2012) found that NGF triggered the p38MAPK-mediated phosphorylation of the transcription factor E2F4, ultimately leading to a reboot of the cell cycle in newborn neurons. Holub et al. (2009) demonstrated that NGF plays a crucial role in the transfection of SK-N-SH neuroblastoma cells through regulating the activation of the p38MAPK apoptotic pathway. Together, these findings indicate that NGF may affect the p38MAPK pathway in some way. The results of the present study suggest that exogenous NGF reduced p-p38MAPK expression within the hypoglossal nucleus after hypoglossal nerve crush injury in rats, and that its expression levels decreased with time post-injury.
NGF protects hypoglossal neurons and promotes neural regeneration
Growing evidence has shown that NGF not only maintains the development and function of sensory, motor and sympathetic neurons, but is also involved in peripheral nerve regeneration (Madduri et al., 2009; Scholz et al., 2010; Liu et al., 2011; Pan et al., 2011; de Boer et al., 2012; Ma et al., 2013, 2014; Tang et al., 2013; Asanome et al., 2014; Chen et al., 2014a; Kuihua et al., 2014; Leng et al., 2014, Wang et al., 2014a, b; Yu et al., 2014; da Silva et al., 2015; Lee et al., 2015b). The results of the present study showed that exogenous NGF increases the survival rate of neurons after hypoglossal nerve crush injury, and reduces the loss of normal neurons. This evidence supports a protective effect of exogenous NGF after hypoglossal nerve crush injury. Meanwhile, after hypoglossal nerve crush injury, exogenous NGF can slow ultrastructural changes at the distal end of the damaged nerve, suggesting that exogenous NGF can promote regeneration of the hypoglossal nerve after injury.
In summary, after hypoglossal nerve injury, p38MAPK activity is increased in damaged neurons, and the activated p38MAPK pathway may mediate the death of neurons within the hypoglossal nucleus. Administration of exogenous NGF can inhibit the activation of p38MAPK caused by hypoglossal nerve injury, while NGF functions to protect damaged neurons and promote nerve regeneration after hypoglossal nerve injury.
Footnotes
Conflicts of interest: None declared.
Plagiarism check: This paper was screened twice using Cross-Check to verify originality before publication.
Peer review: This paper was double-blinded and stringently reviewed by international expert reviewers.
Copyedited by McGowan D, Norman C, Yang Y, Li CH, Song LP, Zhao M
References
- 1.Agthong S, Kaewsema A, Chentanez V. Inhibition of p38 MAPK reduces loss of primary sensory neurons after nerve transection. Neurol Res. 2012;34:714–720. doi: 10.1179/1743132812Y.0000000070. [DOI] [PubMed] [Google Scholar]
- 2.Armstrong DM, Brady R, Hersh LB, Hayes RC, Wiley RG. Expression of choline acetyltransferase and nerve growth factor receptor within hypoglossal motoneurons following nerve injury. J Comp Neurol. 1991;304:596–607. doi: 10.1002/cne.903040407. [DOI] [PubMed] [Google Scholar]
- 3.Asanome A, Kawabe J, Matsuki M, Kabara M, Hira Y, Bochimoto H, Yamauchi A, Aonuma T, Takehara N, Watanabe T, Hasebe N. Nerve growth factor stimulates regeneration of perivascular nerve and induces the maturation of microvessels around the injured artery. Biochem Biophys Res Commun. 2014;443:150–155. doi: 10.1016/j.bbrc.2013.11.070. [DOI] [PubMed] [Google Scholar]
- 4.Bu X, Huang P, Qi Z, Zhang N, Han S, Fang L, Li J. Cell type-specific activation of p38 MAPK in the brain regions of hypoxic preconditioned mice. Neurochem Int. 2007;51:459–466. doi: 10.1016/j.neuint.2007.04.028. [DOI] [PubMed] [Google Scholar]
- 5.Bussmann KAV, Sofroniew MV. Re-expression of p75NTR by adult motor neurons after axotomy is triggered by retrograde transport of a positive signal from axons regrowing through damaged or denervated peripheral nerve tissue. Neuroscience. 1999;91:273–281. doi: 10.1016/s0306-4522(98)00562-4. [DOI] [PubMed] [Google Scholar]
- 6.Chaparro-Huerta V, Flores-Soto ME, Gudiño-Cabrera G, Rivera-Cervantes MC, Bitzer-Quintero OK, Beas-Zárate C. Role of p38 MAPK and pro-inflammatory cytokines expression in glutamate-induced neuronal death of neonatal rats. Int J Dev Neurosci. 2008;26:487–495. doi: 10.1016/j.ijdevneu.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 7.Chen L, Wei RH, Tan DT, Beuerman RW, Li W, Zhao S. Nerve growth factor expression and nerve regeneration in monkey corneas after LASIK. J Refract Surg. 2014a;30:134–139. doi: 10.3928/1081597X-20140120-10. [DOI] [PubMed] [Google Scholar]
- 8.Chen W, Xiong Q, Ren Q, Guo Y, Li G. Can amino-functionalized carbon nanotubes carry functional nerve growth factor? Neural Regen Res. 2014b;9:285–292. doi: 10.4103/1673-5374.128225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chiang RP, Huang CT, Tsai YJ. Melatonin reduces median nerve injury-induced mechanical hypersensitivity via inhibition of microglial p38 mitogen-activated protein kinase activation in rat cuneate nucleus. J Pineal Res. 2013;54:232–244. doi: 10.1111/jpi.12029. [DOI] [PubMed] [Google Scholar]
- 10.da Silva JT, Santos FM, Giardini AC, Martins Dde O, de Oliveira ME, Ciena AP, Gutierrez VP, Watanabe IS, Britto LR, Chacur M. Neural mobilization promotes nerve regeneration by nerve growth factor and myelin protein zero increased after sciatic nerve injury. Growth Factors. 2015;33:8–13. doi: 10.3109/08977194.2014.953630. [DOI] [PubMed] [Google Scholar]
- 11.Dapper JD, Crish SD, Pang IH, Calkins DJ. Proximal inhibition of p38 MAPK stress signaling prevents distal axonopathy. Neurobiol Dis. 2013;59:26–37. doi: 10.1016/j.nbd.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Boer R, Borntraeger A, Knight AM, Hébert-Blouin MN, Spinner RJ, Malessy MJ, Yaszemski MJ, Windebank AJ. Short- and long-term peripheral nerve regeneration using a poly-lactic-co-glycolic-acid scaffold containing nerve growth factor and glial cell line-derived neurotrophic factor releasing microspheres. J Biomed Mater Res A. 2012;100:2139–2146. doi: 10.1002/jbm.a.34088. [DOI] [PubMed] [Google Scholar]
- 13.de Rivero Vaccari JP, Marcillo A, Nonner D, Dietrich WD, Keane RW. Neuroprotective effects of bone morphogenetic protein 7 (BMP7) treatment after spinal cord injury. Neurosci Lett. 2009;465:226–229. doi: 10.1016/j.neulet.2009.09.013. [DOI] [PubMed] [Google Scholar]
- 14.Delaviz H, Faghihi A, Azizzadeh Delshad A, Bahadori Mh, Mohamadi J, Roozbehi A. Repair of peripheral nerve defects using a polyvinylidene fluoride channel containing nerve growth factor and collagen gel in adult rats. Cell J. 2011;13:137–142. [PMC free article] [PubMed] [Google Scholar]
- 15.Diolaiti D, Bernardoni R, Trazzi S, Papa A, Porro A, Bono F, Herbert JM, Perini G, Della Valle G. Functional cooperation between TrkA and p75NTR accelerates neuronal differentiation by increased transcription of GAP-43 and p21(CIP/WAF) genes via ERK1/2 and AP-1 activities. Exp Cell Res. 2007;313:2980–2992. doi: 10.1016/j.yexcr.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 16.Fortun J, Hill CE, Bunge MB. Combinatorial strategies with Schwann cell transplantation to improve repair of the injured spinal cord. Neurosci Lett. 2009;456:124–132. doi: 10.1016/j.neulet.2008.08.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gwak YS, Unabia GC, Hulsebosch CE. Activation of p-38á MAPK contributes to neuronal hyperexcitability in caudal regions remote from spinal cord injury. Exp Neurol. 2009;220:154–161. doi: 10.1016/j.expneurol.2009.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holub JL, Qiu YY, Madonna MB. 2009 Nerve growth factor activation of the p-38 apoptotic pathway in p-75 transfected SK-N-SH neuroblastoma cells. J Am Coll Surg. 209:S65–66. [Google Scholar]
- 19.Hong J, Qian T, Le Q, Sun X, Wu J, Chen J, Yu X, Xu J. NGF promotes cell cycle progression by regulating D-type cyclins via PI3K/Akt and MAPK/Erk activation in human corneal epithelial cells. Mol Vis. 2012;18:758–764. [PMC free article] [PubMed] [Google Scholar]
- 20.Hood B, Levene HB, Levi AD. Transplantation of autologous Schwann cells for the repair of segmental peripheral nerve defects. Neurosurg Focus. 2009;26:E4. doi: 10.3171/FOC.2009.26.2.E4. [DOI] [PubMed] [Google Scholar]
- 21.Jeon HJ, Han SR, Lim KH, Won KA, Bae YC, Ahn DK. Intracisternal administration of NR2 subunit antagonists attenuates the nociceptive behavior and p-p38 MAPK expression produced by compression of the trigeminal nerve root. Mol Pain. 2011;7:46. doi: 10.1186/1744-8069-7-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Katome T. Inhibition of stress-responsive signaling pathway prevents neural cell death following optic nerve injury. Nihon Ganka Gakkai Zasshi. 2014;118:907–915. [PubMed] [Google Scholar]
- 23.Kemp SWP, Walsh SK, Midha R. Growth factor and stem cell enhanced conduits in peripheral nerve regeneration and repair. Neurol Res. 2008;30:1030–1038. doi: 10.1179/174313208X362505. [DOI] [PubMed] [Google Scholar]
- 24.Kernie SG, Parada LF. The molecular basis for understanding neurotrophins and their relevance to neurologic disease. Arch Neurol. 2000;57:654–657. doi: 10.1001/archneur.57.5.654. [DOI] [PubMed] [Google Scholar]
- 25.Kuihua Z, Chunyang W, Cunyi F, Xiumei M. Aligned SF/P(LLA-CL)-blended nanofibers encapsulating nerve growth factor for peripheral nerve regeneration. J Biomed Mater Res A. 2014;102:2680–2691. doi: 10.1002/jbm.a.34922. [DOI] [PubMed] [Google Scholar]
- 26.Kwon SY, Yeom JH, Joo JD. Ketamine reduces the induced spinal p38 MAPK and pro-inflammatory cytokines in a neuropathic rats. Korean J Anesthesiol. 2014;66:52–58. doi: 10.4097/kjae.2014.66.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee JY, Kang SR, Yune TY. Fluoxetine prevents oligodendrocyte cell death by inhibiting microglia activation after spinal cord injury. J Neurotrauma. 2015a doi: 10.1089/neu.2014.3527. doi:10.1089/neu.2014.3527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee JY, Jahng JW, Kim SM, Kim MJ, Lee JH. Simultaneous inferior alveolar nerve regeneration and osseointegration with a nerve growth factor-supplying implant: a preliminary study. J Oral Maxillofac Surg. 2015b;73:410–423. doi: 10.1016/j.joms.2014.07.009. [DOI] [PubMed] [Google Scholar]
- 29.Lee KM, Jeon SM, Cho HJ. Interleukin-6 induces microglial CX3CR1 expression in the spinal cord after peripheral nerve injury through the activation of p38 MAPK. Eur J Pain. 2010;14:e681–612. doi: 10.1016/j.ejpain.2009.10.017. [DOI] [PubMed] [Google Scholar]
- 30.Leng CH, Hao TC, Ding D, Jiao XW, Li JP, Wang XJ, Ma JB, He ZY. The effects of co-application of NGF and GM1 on obsolete injured sciatic nerve repaired with xenogeneic acellular nerve scaffold. Shenjing Jiepou Xue Zazhi. 2014;30:86–92. [Google Scholar]
- 31.Liu F, Zhang H, Zhang K, Wang X, Li S, Yin Y. Rapamycin promotes Schwann cell migration and nerve growth factor secretion. Neural Regen Res. 2014;9:602–609. doi: 10.4103/1673-5374.130101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu HW, Wen WS, Hu M, Bi WT, Chen LJ, Liu SX, Chen P, Tan XY. Chitosan conduits combined with nerve growth factor microspheres repair facial nerve defects. Neural Regen Res. 2013;8:3139–3147. doi: 10.3969/j.issn.1673-5374.2013.33.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu JJ, Wang CY, Wang JG, Ruan HJ, Fan CY. Peripheral nerve regeneration using composite poly(lactic acid-caprolactone)/nerve growth factor conduits prepared by coaxial electrospinning. J Biomed Mater Res A. 2011;96A:13–20. doi: 10.1002/jbm.a.32946. [DOI] [PubMed] [Google Scholar]
- 34.Ma K, Yan N, Huang Y, Cao G, Deng J, Deng Y. Effects of nerve growth factor on nerve regeneration after corneal nerve damage. Int J Clin Exp Med. 2014;7:4584–4589. [PMC free article] [PubMed] [Google Scholar]
- 35.Madduri S, Papaloïzos M, Gander B. Synergistic effect of GDNF and NGF on axonal branching and elongation in vitro. Neurosci Res. 2009;65:88–97. doi: 10.1016/j.neures.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 36.Marampon F, Casimiro MC, Fu M, Powell MJ, Popov VM, Lindsay J, Zani BM, Ciccarelli C, Watanabe G, Lee RJ, Pestell RG. Nerve Growth factor regulation of cyclin D1 in PC12 cells through a p21RAS extracellular signal-regulated kinase pathway requires cooperative interactions between Sp1 and nuclear factor-kappaB. Mol Biol Cell. 2008;19:2566–2578. doi: 10.1091/mbc.E06-12-1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mizukoshi K, Sasaki M, Izumi Y, Miura M, Watanabe M, Amaya F. Activation of p38 mitogen-activated protein kinase in the dorsal root ganglion contributes to pain hypersensitivity after plantar incision. Neuroscience. 2013;234:77–87. doi: 10.1016/j.neuroscience.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 38.Morillo SM, Abanto EP, Román MJ, Frade JM. Nerve growth factor-induced cell cycle reentry in newborn neurons is triggered by p38MAPK-dependent E2F4 phosphorylation. Mol Cell Biol. 2012;32:2722–2737. doi: 10.1128/MCB.00239-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Muroi Y, Ishii T, Teramoto K, Hori M, Nishimura M. Calcineurin contributes to the enhancing effect of adenosine on nerve growth factor-induced neurite outgrowth via the decreased duration of p38 mitogen-activated protein kinase phosphorylation. J Pharmacol Sci. 2004;95:124–131. doi: 10.1254/jphs.95.124. [DOI] [PubMed] [Google Scholar]
- 40.Namiki J, Kojima A, Tator CH. Effect of brain-derived neurotrophic factor nerve growth factor and neurotrophin-3 on functional recovery and regeneration after spinal cord injury in adult rats. J Neurotrauma. 2000;17:1219–1231. doi: 10.1089/neu.2000.17.1219. [DOI] [PubMed] [Google Scholar]
- 41.Nguyen N, Lee SB, Lee YS, Lee KH, Ahn JY. Neuroprotection by NGF and BDNF against neurotoxin-exerted apoptotic death in neural stem cells are mediated through Trk receptors, activating PI3-kinase and MAPK pathways. Neurochem Res. 2009;34:942–951. doi: 10.1007/s11064-008-9848-9. [DOI] [PubMed] [Google Scholar]
- 42.Pan WD, Yin ZS, Zhang H, Hu Y, Li GB, Shen Z. Effect of intrathecal injection of nerve growth factor on functional recovery and nerve cells after spinal cord injury in rats. Anhui Yike Daxue Xuebao. 2011;46:875–878. [Google Scholar]
- 43.Paxinos G, Watson C. London: Academic Press; 2005. The Rat Brain in Stereotaxic Coordinates. [Google Scholar]
- 44.Santos SD, Verveer PJ, Bastiaens PI. Growth factor-induced MAPK network topology shapes Erk response determining PC-12 cell fate. Nat Cell Biol. 2007;9:324–330. doi: 10.1038/ncb1543. [DOI] [PubMed] [Google Scholar]
- 45.Schmalbruch H. Motoneuron death after sciatic nerve section in newborn rats. J Comp Neurol. 1984;224:252–258. doi: 10.1002/cne.902240206. [DOI] [PubMed] [Google Scholar]
- 46.Scholz T, Rogers JM, Krichevsky A, Dhar S, Evans GRD. Inducible nerve growth factor delivery for peripheral nerve regeneration in vivo. Plast Reconstr Surg. 2010;126:1874–1889. doi: 10.1097/PRS.0b013e3181f5274e. [DOI] [PubMed] [Google Scholar]
- 47.Semba K, Namekata K, Kimura A, Harada C, Katome T, Yoshida H, Mitamura Y, Harada T. Dock3 overexpression and p38 MAPK inhibition synergistically stimulate neuroprotection and axon regeneration after optic nerve injury. Neurosci Lett. 2014;581:89–93. doi: 10.1016/j.neulet.2014.08.034. [DOI] [PubMed] [Google Scholar]
- 48.Suter MR, Berta T, Gao YJ, Decosterd I, Ji RR. Large A-fiber activity is required for microglial proliferation and p38 MAPK activation in the spinal cord: different effects of resiniferatoxin and bupivacaine on spinal microglial changes after spared nerve injury. Mol Pain. 2009;5:53. doi: 10.1186/1744-8069-5-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tang S, Zhu J, Xu Y, Xiang AP, Jiang MH, Quan D. The effects of gradients of nerve growth factor immobilized PCLA scaffolds on neurite outgrowth in vitro and peripheral nerve regeneration in rats. Biomaterials. 2013;34:7086–7096. doi: 10.1016/j.biomaterials.2013.05.080. [DOI] [PubMed] [Google Scholar]
- 50.Terayama R, Omura S, Fujisawa N, Yamaai T, Ichikawa H, Sugimoto T. Activation of microglia and p38 mitogen-activated protein kinase in the dorsal column nucleus contributes to tactile allodynia following peripheral nerve injury. Neuroscience. 2008;153:1245–1255. doi: 10.1016/j.neuroscience.2008.03.041. [DOI] [PubMed] [Google Scholar]
- 51.Terayama R, Fujisawa N, Yamaguchi D, Omura S, Ichikawa H, Sugimoto T. Differential activation of mitogen-activated protein kinases and glial cells in the trigeminal sensory nuclear complex following lingual nerve injury. Neurosci Res. 2011;69:100–110. doi: 10.1016/j.neures.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 52.Wang L, Zhao Y, Cao J, Yang X, Lei D. Mesenchymal stem cells modified with nerve growth factor improve recovery of the inferior alveolar nerve after mandibular distraction osteogenesis in rabbits. Br J Oral Maxillofac Surg. 2015;53:279–284. doi: 10.1016/j.bjoms.2014.12.014. [DOI] [PubMed] [Google Scholar]
- 53.Wang P, Zang XY, Zhang YC. Electrophysiological study of the effect of NGF on facail nerve crush injuries. Kouqiang Hemian Waike Zazhi. 2014a;24:345–347. [Google Scholar]
- 54.Wang Z, Han N, Wang J, Zheng H, Peng J, Kou Y, Xu C, An S, Yin X, Zhang P, Jiang B. Improved peripheral nerve regeneration with sustained release nerve growth factor microspheres in small gap tubulization. Am J Transl Res. 2014b;6:413–421. [PMC free article] [PubMed] [Google Scholar]
- 55.Wuhanqimuge, Itakura A, Matsuki Y, Tanaka M, Arioka M. Lysophosphatidylcholine enhances NGF-induced MAPK and Akt signals through the extracellular domain of TrkA in PC12 cells. FEBS Open Bio. 2013;3:243–251. doi: 10.1016/j.fob.2013.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yu H, Liu J, Ma J, Xiang L. Local delivery of controlled released nerve growth factor promotes sciatic nerve regeneration after crush injury. Neurosci Lett. 2014;566:177–181. doi: 10.1016/j.neulet.2014.02.065. [DOI] [PubMed] [Google Scholar]
- 57.Yuan J, Huang G, Xiao Z, Lin L, Han T. Overexpression of β-NGF promotes differentiation of bone marrow mesenchymal stem cells into neurons through regulation of AKT and MAPK pathway. Mol Cell Biochem. 2013;383:201–211. doi: 10.1007/s11010-013-1768-6. [DOI] [PubMed] [Google Scholar]
- 58.Zhang CX, Tu L. Nerve growth factor and its receptor and hypoglossal nerve injury. Kouqiang Yixue Yanjiu. 2005;21:470–471. [Google Scholar]
- 59.Zhang CX, Tu L, Tang YP, Liu LK. Effect of Nao yi-an on the expression of P75 in rats with hypoglossal nerve injury. Shiyong Kouqiang Yixue Zazhi. 2009;25:658–662. [Google Scholar]
- 60.Zhou C, Shi X, Huang H, Zhu Y, Wu Y. Montelukast attenuates neuropathic pain through inhibiting p38 mitogen-activated protein kinase and nuclear factor-kappa B in a rat model of chronic constriction injury. Anesth Analg. 2014a;118:1090–1096. doi: 10.1213/ANE.0000000000000174. [DOI] [PubMed] [Google Scholar]
- 61.Zhou CH, Li X, Zhu YZ, Huang H, Li J, Liu L, Hu Q, Ma TF, Shao Y, Wu YQ. Ghrelin alleviates neuropathic pain through GHSR-1a-mediated suppression of the p38 MAPK/NF-êB pathway in a rat chronic constriction injury model. Reg Anesth Pain Med. 2014b;39:137–148. doi: 10.1097/AAP.0000000000000050. [DOI] [PubMed] [Google Scholar]