Abstract
Drugs for the treatment and prevention of nervous system diseases must permeate the blood-brain barrier to take effect. In vitro models of the blood-brain barrier are therefore important in the investigation of drug permeation mechanisms. However, to date, no unified method has been described for establishing a blood-brain barrier model. Here, we modified an in vitro model of the blood-brain barrier by seeding brain microvascular endothelial cells and astrocytes from newborn rats on a polyester Transwell cell culture membrane with 0.4-µm pores, and conducted transepithelial electrical resistance measurements, leakage tests and assays for specific blood-brain barrier enzymes. We show that the permeability of our model is as low as that of the blood-brain barrier in vivo. Our model will be a valuable tool in the study of the mechanisms of action of neuroprotective drugs.
Keywords: nerve regeneration, blood-brain barrier, astrocytes, brain microvascular endothelial cells, permeability, co-culture, Transwell chamber, neural regeneration
Introduction
The blood-brain barrier (BBB) is a low-permeability cell system between the central nervous system and circulating blood (Hou et al., 2014). It mainly comprises brain microvascular endothelial cells (BMECs), basement membrane, and astrocytic foot, processes. The specific structure and function of the BBB mean it is difficult to replicate in an in vitro model. It is therefore important to establish a model that is as close to the in vivo environment as possible, and can be easily replicated; however, primary culture of brain cells can be difficult. Closely-spaced low-permeability cell lines, such as the umbilical vein endothelial (ECV-304) and C6 glioma cell lines, have previously been used to simulate BBB function (Kuhlmann et al., 2008; Wang et al., 2011b). The BBB skeleton is made up of BMECs (Wang et al, 2011a), so a compact monolayer of BMECs has also been used as a model (Winter et al., 2008; Bernas et al., 2010; Patabendige et al., 2013; Stephan et al., 2013). However, although these models simulate some characteristics of the BBB, in vivo the BBB contains a wide variety of cell types from different sources. For example, although ECV-304 cells simulate the low permeability of the BBB, this cell type is not found in the BBB in vivo; similarly, the BMEC model contains only a single cell type, which inadequately represents the structure of the BBB. Furthermore, BMECs and astrocytes in the BBB are not in direct contact, but have a space between them (Abbott, 2005; Daneman and Barres, 2005). For this reason, Transwell chambers have been developed for the culture of BMECs and astrocytes and the construction of low-permeability BBB models (Kang et al., 2013; Sun et al., 2013). In the present study, we sought to establish a simple and effective method by which to model the BBB in vitro using modified cell culture techniques.
Materials and Methods
Experimental animals
Newborn male and female Sprague-Dawley rats, aged 1–3 days, were obtained from the Animal Center of Anhui Medical University, China (Certification No. SXCK (Wan) 2011-002). All rats were housed at 18–26°C and 40–70% humidity under a 12 hour light-dark cycle. All animal procedures were overseen and approved by the Animal Center of Anhui University of Chinese Medicine in China. Every effort was made to minimize animal suffering.
Primary culture and identification of astrocytes
Five rat pups were decapitated and disinfected in 75% ethanol for 3–5 minutes. The whole brain was dissected out and put into precooled D-Hanks’ solution, and the meninges and blood vessels were carefully removed on ice. The cerebral cortex was separated and washed three times in D-Hanks’ solution before being cut into blocks (1 mm3) in Dulbecco's Modified Eagle's Medium (DMEM; Gibco, Grand Island, NY, USA). The tissue was digested in 0.25% trypsin (Sigma, St. Louis, MO, USA) and filtered through a 200-mesh screen. The filtrate was centrifuged at 140 × g for 10 minutes and the supernatant discarded. The cells were resuspended in DMEM, and placed in a 75-cm2 disposable culture bottle. To aid in the removal of fibroblasts by the differential adhesion method (Ki et al., 2010; Aremu et al., 2011; Tanahashi et al., 2012), the cell suspension was removed and placed in another bottle, and cultured at 37°C and 5% CO2. The DMEM was then replaced every 3 days, and the cells were observed under an inverted microscope (Olympus, Tokyo, Japan) (Anderson and Swanson, 2000; Schmuck et al., 2002; Schousboe and Waagepetersen, 2005; Jayakumar et al., 2006; Sun et al., 2007; Pan et al., 2012; Yin et al., 2012; Zeng et al., 2013).
Astrocytes were selected at different growth stages, rinsed with PBS three times for 5 minutes each, fixed in 4% paraformaldehyde for 20 minutes, rinsed in PBS as before, and blocked in normal goat serum for 20 minutes at 37°C. The serum was discarded, and residual moisture was absorbed using filter paper. Mouse anti-rat glial fibrillary acidic protein (GFAP) monoclonal antibody (1:100; Beyotime Institute of Biotechnology, Shanghai, China) was added to the cells and incubated for 30 minutes at room temperature. After three further PBS washes, fluorescein isothiocyanate (FITC)-labeled sheep anti-mouse IgG (1:100; Beyotime Institute of Biotechnology) was added and the cells were incubated for 30 minutes at room temperature. Samples were observed and photographed under a fluorescence microscope (Olympus) (Anderson and Swanson, 2000; Schmuck et al., 2002; Schousboe and Waagepetersen, 2005; Jayakumar et al., 2006; Sun et al., 2007; Pan et al., 2012; Yin et al., 2012; Zeng et al., 2013).
Primary culture and identification of BMECs
Ten rat pups were selected for cell isolation. The separation procedure was the same as that described for astrocytes. The meninx and meningeal vessels were absorbed by the filter paper. The isolated brain tissue was cut into 1-mm3 blocks in DMEM, and digested in 10 mL 0.1% type II collagenase containing 30 U/mL DNase I (Invitrogen, Carlsbad, CA, USA) for 15 minutes, and centrifuged at 140 × g for 10 minutes. The supernatant was discarded. The tissue was blocked with 20% bovine serum albumin (Sigma) and centrifuged at 1,000 × g at 4°C for 20 minutes. After removal of nerve tissue and blood vessels in the upper layer, the isolated tissue was digested with 2-mL 0.1% collagenase containing 20 U/mL DNase I (Roche, Basel, Switzerland) for 10 minutes, and centrifuged at 140 × g for 10 minutes before the supernatant was discarded. Cells were incubated with 800 mL DMEM/nutrient mixture F12 (DMEM/F12) (Hyclone, Logan, UT, USA), 4 mM L-glutamine, 0.05 g heparin sodium, 1 g NaHCO3, 75 mg endothelial cell growth supplement, and 200 mL fetal bovine serum in a 75 cm2 disposable culture bottle prepared by coating with 1% gelatin at 37°C and 5% CO2. The medium was replaced every 3 days (Fischer et al., 1999; Gaillard et al., 2000; Gomez and Reich, 2003; Shen et al., 2004; Calabria and Shusta, 2008; Demarest et al., 2012; Hsieh et al., 2012; Chen et al., 2013).
The identification method was the same as that described for astrocytes, except the primary and secondary antibodies were rabbit anti-factor VIII heavy chain polyclonal antibody (H-140) (1:500; Santa Cruz Biotechnology, Shanghai, China) and FITC-labeled goat anti-rabbit IgG (1:200; BeiJing ZhongShan JinQiao Company, Beijing, China), respectively (Fischer et al., 1999; Gaillard et al., 2000; Gomez and Reich, 2003; Shen et al., 2004; Calabria and Shusta, 2008; Demarest et al., 2012; Hsieh et al., 2012; Chen et al., 2013).
Construction of in vitro BBB model
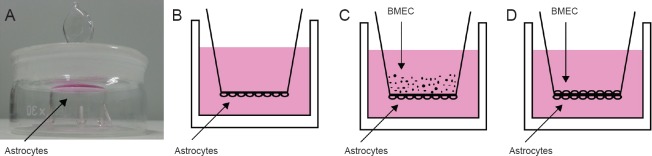
Transwell chamber inserts with a 0.4-µm aperture (Corning, New York, USA) were placed into volumetric flasks. Astrocytes (1 mL at 1 × 106 /mL) were added to the underside of the Transwell (Figure 1A) and incubated at 37°C and 5% CO2 for 24 hours. The chambers were then placed carefully into a 6-well plate (Corning) (Figure 1B). When the astrocytes reached 60% confluence under an inverted microscope, BMECs (1 × 107 /mL) were seeded on top of the Transwell (Figure 1C) at 37°C and 5% CO2. The cells were observed under an inverted microscope until the astrocytes and BMECs reached a high-density co-culture (Figure 1D) (Abbott et al., 2006; Ribatti et al., 2006; Kuo and Lu, 2011; Takata et al., 2013; Zhou et al., 2013).
Figure 1.
Establishing the in vitro blood-brain barrier model.
(A) Astrocytes were seeded (1 mL at 1 × 106/mL) on the underside of a Transwell chamber, which was placed upside-down in a weighing bottle. (B) Astrocytes were allowed to adhere to the wall for 24 hours, and the Transwell chamber was carefully turned up-right and placed in a 6-well plate. (C) When astrocytes reached 60% confluence, BMECs (1 × 107/mL) were seeded on the top of the Transwell chamber. (D) Astrocytes and BMECs were co-cultured to a high density. BMECs: Brain microvascular endothelial cells.
Detection of transepithelial electrical resistance (TEER)
Permeability changes in the BBB can be detected by changes in TEER, which directly reflects the structural integrity of the BBB (Czupalla et al., 2014). We measured TEER in the co-culture model and single-layer BMEC groups at 2, 4, 8, 12, 16, 20, 24, 48, 72, and 96 hours after BMEC seeding. Measurements were adjusted for insertion of the Millicell ERS-2 Volt-Ohm Meter (Millipore, Boston, MA, USA) into 70% ethanol for 15 minutes. The samples were air-dried for 15 seconds, and washed in sterilized electrolyte solution. The short and long electrode probes were inserted vertically into the medium inside and outside the Transwell chamber, respectively, and the values were recorded (Rubin et al., 1991; Nakagawa et al., 2009; Ribeiro et al., 2010).
Liquid surface leakage test
Once the TEER value was constant, DMEM was added to the interior of the Transwell chamber so that the difference between the depth of the medium inside and outside the Transwell was 0.5 cm. The depths of the media above and below the Transwell were measured again after culturing for 4 hours at 37°C and 5% CO2. If the difference between the liquids remained at 0.4–0.5 cm, this showed that the cells had formed a dense barrier (Steiner et al., 2011; Burek et al., 2012; Wuest and Lee, 2012; Zhou et al., 2013; Hanada et al., 2014).
Quantification of specific BBB enzymes
Gamma-glutamyl transpeptidase (γ-GT)
BMECs in the co-culture model and single layer BMEC groups were washed three times in cold PBS, collected gently using a cell scraper (Corning), centrifuged at 700 × g or 5 minutes, and the supernatant was discarded. Cells in each well were lysed with 150 µL cell lysate (YuanYe Biological Technology Co., Ltd., Shanghai, China) on ice for 30 minutes (shaken every 10 minutes), and centrifuged at 1,104 × g or 30 minutes at 4°C. The filtrate was aliquoted and stored. γ-GT was measured using an enzyme linked immunosorbent assay kit (YuanYe Biological Technology Co., Ltd.) according to the manufacturer's instructions. The optical density of each well was measured with a microplate reader (Awareness Technology, Palm City, FL, USA) at a wavelength of 450 nm.
Alkaline phosphatase (ALP)
The Bradford protein concentration determination kit was used to measure the protein concentrations of each sample in the co-culture model and single layer BMEC groups. ALP was measured using an ALP determination kit (Jiancheng Biological Engineering Research Institute, Nanjing, Jingsu Province, China), according to the manufacturer's instructions. The optical density of each well was measured in a spectrophotometer (Shimadzu Corporation, Kyoto, Japan) at a wavelength of 520 nm.
Statistical analysis
Data are expressed as the mean ± SD. Statistical analysis was performed using independent sample t-tests of variance in SPSS 17.0 software (SPSS, Chicago, IL, USA). P = 0.05 was considered significant.
Results
Morphology of primary cultured astrocytes
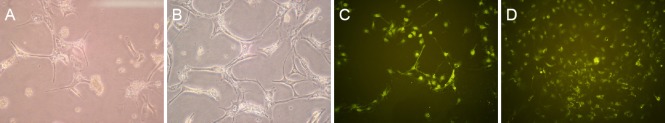
Most primary cultured astrocytes had adhered to the wall by the third day of culture, and a small number of short branching processes were observed between cell bodies (Figure 2A). At 7 days, all cells had adhered to the wall, and the number of cell bodies had markedly increased, with many slender branching processes stretching between neighboring cells (Figure 2B).
Figure 2.
Morphology of primary cultured astrocytes.
(A, B) Astrocytes under an inverted microscope (200× magnification). At 3 days of primary culture (A), astrocyte bodies were observed with a small number of short processes. At 7 days (B), large astrocyte bodies were observed, with several slender branching processes. (C, D) Immunofluorescence staining for glial fibrillary acidic protein, an astrocyte marker (100× magnification). Between 3 (C) and 7 days of primary culture (D), the cell bodies swelled markedly and formed a distinct cell configuration.
After immunofluorescence staining for GFAP, the cytoplasm and branches of cultured astrocytes at 3 and 7 days fluoresced green and showed distinct cellular contours (Figure 2C, D).
Morphology of primary cultured BMECs
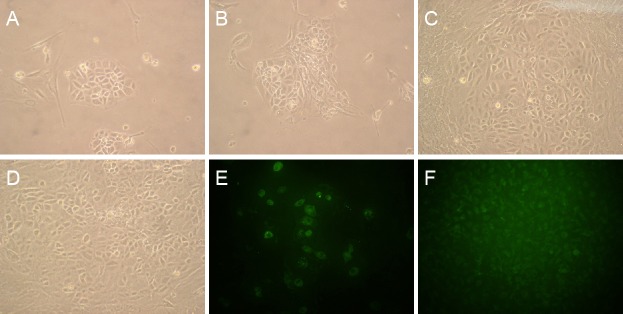
Primary culture BMECs were short, polygonal, and spin-dle-like, showing regional monolayer growth at 3 days (Figure 3A). At 7 days, the medium was replaced. The number of cells had increased, and the area of growth had expanded (Figure 3B). As incubation time and number of medium changes increased, other cells disappeared while endothelial cells proliferated, appearing pale with a polygonal or fusiform shape, a nuclear membrane, and spiral distribution. By 14 days, the endothelial cells had integrated, forming a dense, cobblestone-like monolayer (Figure 3C, D).
Figure 3.
Morphology of primary cultured BMECs.
(A–D) BMECs under an inverted microscope (100× magnification). At 3 days of primary culture (A), BMECs showed single-layer growth. At 7 days (B), the area of growth had expanded. At 14 days (C), cells were polygonal or fusiform, pale, with nuclear membranes; spiral distribution was observed. At 16 days (D), cells formed a tight single layer with a typical cobblestone-like appearance. (E, F) Immunofluorescence staining for the BMEC marker factor VIII (200× magnification). At 7 days of primary culture (E), green fluorescence was observed in the cytoplasm of several cells. At 14 days (F), cells were densely arranged and showed marked green fluorescence. BMECs: Brain microvascular endothelial cells.
Green immunofluorescence was observed in the cytoplasm of only a few BMECs at 7 days (Figure 3E). At 14 days, however, the cells were tightly connected to each other, and strong green fluorescence was observed (Figure 3F).
Characterization of the in vitro BBB model
Under an inverted phase contrast microscope, we ob-served good astrocyte growth, with interconnecting branches, underneath the Transwell chamber (Figure 4A). The BMECs seeded in the Transwell chamber were also well-formed, and were connected to the astrocytes after 24 hours (Figure 4B).
Figure 4.
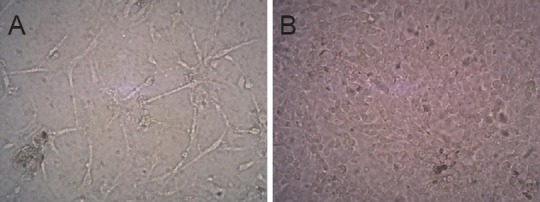
Cellular morphology of the simulated in vitro blood-brain barrier (inverted microscope, × 200).
(A) Astrocyte lower layer showed good growth and adherence to the wall; branching processes connected the cells. (B) BMEC upper layer showed dense growth and defined morphology. BMEC: Brain microvascular endothelial cell.
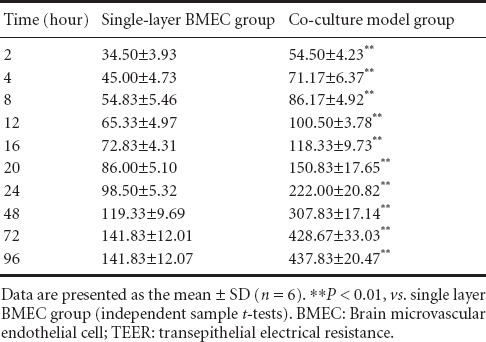
The TEER value in the co-culture model and single layer BMEC groups increased with time. No significant difference in TEER value was detected between the co-culture model group and the single-layer BMEC group (P < 0.01). TEER values plateaued by 72 hours in both groups (Table 1).
Table 1.
TEER values (Ω/pore) in co-culture model and single-layer BMEC groups
When the TEER value was constant, the difference between the levels of the internal and external media was ≥ 0.5 cm (Figure 5A). After 4 hours of culture, this difference remained ≥ 0.5 cm (Figure 5B), indicating that the cultured cells had formed a dense barrier.
Figure 5.
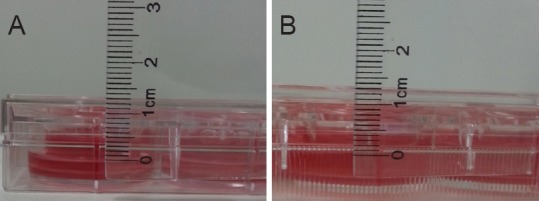
Leakage test of liquid surface in co-culture model group.
(A) Measurement of the difference in levels between the medium on either side of the Transwell chamber (0.5 cm). (B) After 4 hours of culture, the difference in levels remained significant (0.4–0.5 cm, indicating that the co-cultured cells had formed a dense barrier).
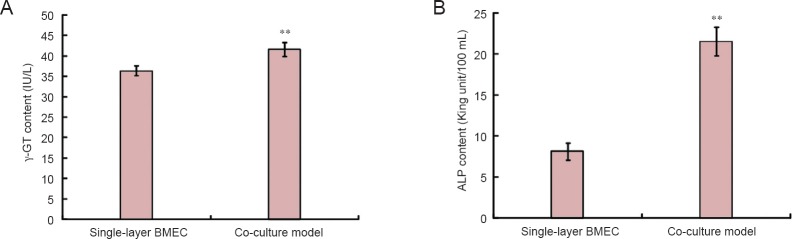
γ-GT and ALP contents in the co-culture model group were greater than those in the single-layer BMEC group (P < 0.01; Figure 6).
Figure 6.
Quantification of specific blood-brain barrier enzymes in the co-culture model and single-layer BMEC groups.
(A) γ-GT; (B) ALP. Data are presented as the mean ± SD (n = 6). **P < 0.01, vs. single-layer BMEC group (independent sample t-test). ALP: Alkaline phosphatase; γ-GT: gamma-glutamyl transpeptidase; BMEC: brain microvascular endothelial cell.
Discussion
Primary culture of astrocytes
In the BBB, a small number of microglia and other cells are found on the astrocyte side and have no special growth requirements. The original method for BBB modeling is therefore simpler than BMEC culture. Here, we used five newborn rat brains to grow BMECs in a 75-cm2 culture bottle after trypsin digestion and filtration purification. Removal of contaminating fibroblasts is important during BMEC culture. Because fibroblasts attach faster than astrocytes, we used a fresh cell culture bottle after 1 hour of culture, and used the differential adhesion method to remove the fibroblasts. DMEM containing 20% fetal bovine serum is nutritionally sufficient for astrocyte growth. Astrocyte density is particularly important, because when the density is low, astrocyte growth is slow and may even cease. It is therefore important to maintain high astrocyte density during co-culture generation.
Primary culture of BMECs
BMECs are difficult to culture and demand a strict growth environment. They are sensitive to hybrid cells, divide slowly, and require the addition of growth factors. A unified approach for the separation, purification and culture of BMECs has not yet been described. Here, we built a culture system by selecting experimental animals, specifying anatomic location, and separating and purifying brain capillaries. We selected newborn rats (1–3 days), instead of those 3–7-weeks-old as previously described (Molino et al., 2014), because despite gray matter being easier to separate at 3–7 weeks, rats at that age should be killed by cervical dislocation, a time-consuming method requiring removal of meningeal blood vessels and multiple rounds of mechanical homogenization and centrifugation, which may result in tissue damage. In 1–3-day-old rats, however, the tissues are easy to separate and cell proliferation ability is strong. We were able to remove the pia mater and meningeal blood vessels carefully and accurately, effectively reducing contamination by hybrid cells.
We also used enzyme digestion combined with gradient centrifugation to separate and purify the capillaries in the brain. Cerebral microvascular tissue was completely separated from the brain by digestion with collagenase II. Nerve tissue and blood vessels were removed by gradient centrifugation after adding 20% bovine serum albumin, and hybrid cells were removed by a second digestion with collagenase and other enzymes. To ensure endothelial cell vitality, a digestion time of 10–15 minutes must be used. A previous study (Molino et al., 2014) reported a large cell loss with a 2–3-hour experiment. Our method avoided cell damage by mechanical homogenization and centrifugation, and only needed 1-hour experimental time; we thus obtained a high percentage of isolated cells.
Because BMECs are more fragile than cells of other tissues, it is important that their environment is suitable for their growth, and not that of other cells. We modified the DMEM/F12 culture medium by adding endothelial cell growth supplement to promote the growth of BMECs, and heparin sodium was used to inhibit that of smooth muscle cells. Because of the weak adherence of BMECs, we chose 1% gelatin to pre-coat the disposable culture bottles to facilitate adherence of capillaries and the growth of BMECs.
Evaluation index of the in vitro BBB model
Cells were observed through transparent polyester Transwell material (aperture 0.4 µm). The cells grew normally on both sides of the Transwell and a dense structure was observed under the inverted phase contrast microscope. TEER dynamic measurements showed that resistance on either side of the Transwell increased with cell growth. At 72 hours, TEER was constant, indicative of a dense structure. The difference in resistance was greater in the single-layer BMECs than in the co-culture model, indicating that the structure is more compact in the monolayer cells after astrocyte and BMEC co-culture. This may lead to cellular interaction, promote the expression of tight junctions, and make the structure more compact. When TEER values were constant, we performed a 4-hour leakage test to confirm the density of the structure and ensure experimental rigor; the results showed that the levels of medium inside and outside the Transwell chamber remained significantly different after 4 hours, indicating that the compact structure could withstand strong osmotic pressure.
We have also shown here that the γ-GT and ALP content of in vitro cultured endothelial cells is low; but when co-cultured, endothelial cells showed high levels of γ-GT and ALP. Our results support those from previous studies comparing the activities of γ-GT and ALP after BMEC monoculture (Reichel et al., 2003; Tóth et al., 2011).
Conclusion
Our approach comprises the following: (1) selection of newborn rats, instead of 3–7-week-old rats as commonly used, for the primary culture of BMECs to ensure homology of astrocytes; (2) enzymatic digestion to avoid mechanical damage by centrifugation; (3) minimization of centrifugation during primary culture to avoid cell loss; (4) shortening of the time required for primary culture, resulting in improved cell yield; (5) use of transparent polyester Transwell inserts for real-time direct observation of cell growth on the membrane under the inverted microscope.
In conclusion, the modeling method we have presented here is simple and feasible, and consumables and instruments required are easy to obtain. However, it is a basic model, and we encourage researchers to make appropriate adjustments according to different research purposes.
Footnotes
Funding: This study was supported by the National Natural Science Foundation of China, No. 81374005, 30973979; a grant from the National Science and Technology Support Program during the Twelfth “Five-Year” Plan Period of China, No. 2012BAI26B03.
Conflicts of interest: None declared.
Plagiarism check: This paper was screened twice using Cross-Check to verify originality before publication.
Peer review: This paper was double-blinded and stringently reviewed by international expert reviewers.
Copyedited by Slone-Murphy J, Robens J, Yu J, Qiu Y, Li CH, Song LP, Zhao M
References
- 1.Abbott N. Dynamics of CNS barriers: evolution, differentiation, and modulation. Cell Mol Neurobiol. 2005;25:5–23. doi: 10.1007/s10571-004-1374-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abbott NJ, Ronnback L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci. 2006;7:41–53. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- 3.Anderson CM, Swanson RA. Astrocyte glutamate transport: review of properties, regulation, and physiological functions. Glia. 2000;32:1–14. [PubMed] [Google Scholar]
- 4.Aremu DA, Ezomo OF, Meshitsuka S. Gene expression in primary cultured astrocytes affected by aluminum: alteration of chaperons involved in protein folding. Environ Health Prev Med. 2011;16:16–24. doi: 10.1007/s12199-010-0161-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bernas MJ, Cardoso FL, Daley SK, Weinand ME, Campos AR, Ferreira AJG, Hoying JB, Witte MH, Brites D, Persidsky Y, Ramirez SH, Brito MA. Establishment of primary cultures of human brain microvascular endothelial cells: a new and simplified method to obtain cells for an in vitro model of the blood-brain barrier. Nat Protoc. 2010;5:1265–1272. doi: 10.1038/nprot.2010.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burek M, Salvador E, Förster CY. Generation of an immortalized murine brain microvascular endothelial cell line as an in vitro blood brain barrier model. J Vis Exp. 2012:e4022. doi: 10.3791/4022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Calabria AR, Shusta EV. A genomic comparison of in vivo and in vitro brain microvascular endothelial cells. J Cereb Blood Flow Metab. 2008;28:135–148. doi: 10.1038/sj.jcbfm.9600518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen C, Mei H, Shi W, Deng J, Zhang B, Guo T, Wang H, Hu Y. EGFP-EGF1-conjugated PLGA nanoparticles for targeted delivery of siRNA into injured brain microvascular endothelial cells for efficient RNA interference. PLoS One. 2013;8:e60860. doi: 10.1371/journal.pone.0060860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Czupalla C, Liebner S, Devraj K. In vitro models of the blood-brain barrier. Cerebral Angiogenesis. 2014;1135:415–437. doi: 10.1007/978-1-4939-0320-7_34. [DOI] [PubMed] [Google Scholar]
- 10.Daneman R, Barres BA. The blood-brain barrier--lessons from moody flies. Cell. 2005;123:9–12. doi: 10.1016/j.cell.2005.09.017. [DOI] [PubMed] [Google Scholar]
- 11.Demarest TG, Murugesan N, Shrestha B, Pachter JS. Rapid expression profiling of brain microvascular endothelial cells by immuno-laser capture microdissection coupled to TaqMan(®) low density array. J Neurosci Methods. 2012;206:200–204. doi: 10.1016/j.jneumeth.2012.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fischer S, Clauss M, Wiesnet M, Renz D, Schaper W, Karliczek GF. Hypoxia induces permeability in brain microvessel endothelial cells via VEGF and NO. Am J Physiol. 1999;276:C812–820. doi: 10.1152/ajpcell.1999.276.4.C812. [DOI] [PubMed] [Google Scholar]
- 13.Gaillard PJ, van der Sandt IC, Voorwinden LH, Vu D, Nielsen JL, de Boer AG, Breimer DD. Astrocytes increase the functional expression of P-glycoprotein in an in vitro model of the blood-brain barrier. Pharm Res. 2000;17:1198–1205. doi: 10.1023/a:1026406528530. [DOI] [PubMed] [Google Scholar]
- 14.Gomez D, Reich NC. Stimulation of primary human endothelial cell proliferation by IFN. J Immunol. 2003;170:5373–5381. doi: 10.4049/jimmunol.170.11.5373. [DOI] [PubMed] [Google Scholar]
- 15.Hanada S, Fujioka K, Inoue Y, Kanaya F, Manome Y, Yamamoto K. Cell-based in vitro blood-brain barrier model can rapidly evaluate nanoparticles’ brain permeability in association with particle size and surface modification. Int J Mol Sci. 2014;15:1812–1825. doi: 10.3390/ijms15021812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hou H, Zhang G, Wang H, Gong H, Wang C, Zhang X. High matrix metalloproteinase-9 expression induces angiogenesis and basement membrane degradation in stroke-prone spontaneously hypertensive rats after cerebral infarction. Neural Regen Res. 2014;9:1154–1162. doi: 10.4103/1673-5374.135318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hsieh HL, Lin CC, Chan HJ, Yang CM, Yang CM. c-Src-dependent EGF receptor transactivation contributes to ET-1-induced COX-2 expression in brain microvascular endothelial cells. J Neuroinflammation. 2012;9:152. doi: 10.1186/1742-2094-9-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jayakumar AR, Panickar KS, Murthy ChR, Norenberg MD. Oxidative stress and mitogen-activated protein kinase phosphorylation mediate ammonia-induced cell swelling and glutamate uptake inhibition in cultured astrocytes. J Neurosci. 2006;26:4774–4784. doi: 10.1523/JNEUROSCI.0120-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kang YB, Rawat S, Cirillo J, Bouchard M, Noh H. Layered long-term co-culture of hepatocytes and endothelial cells on a transwell membrane: toward engineering the liver sinusoid. Biofabrication. 2013;5:045008. doi: 10.1088/1758-5082/5/4/045008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ki KH, Park DY, Lee SH, Kim NY, Choi BM, Noh GJ. The optimal concentration of siRNA for gene silencing in primary cultured astrocytes and microglial cells of rats. Korean J Anesthesiol. 2010;59:403–410. doi: 10.4097/kjae.2010.59.6.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuhlmann CRW, Gerigk M, Bender B, Closhen D, Lessmann V, Luhmann HJ. Fluvastatin prevents glutamate-induced blood-brain-barrier disruption in vitro. Life Sci. 2008;82:1281–1287. doi: 10.1016/j.lfs.2008.04.017. [DOI] [PubMed] [Google Scholar]
- 22.Kuo YC, Lu CH. Effect of human astrocytes on the characteristics of human brain-microvascular endothelial cells in the blood-brain barrier. Colloids Surf B Biointerfaces. 2011;86:225–231. doi: 10.1016/j.colsurfb.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 23.Molino Y, Jabès F, Lacassagne E, Gaudin N, Khrestchatisky M. Setting-up an in vitro model of rat blood-brain barrier (BBB): a focus on BBB impermeability and receptor-mediated transport. J Vis Exp. 2014:e51278. doi: 10.3791/51278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nakagawa S, Deli MA, Kawaguchi H, Shimizudani T, Shimono T, Kittel Á, Tanaka K, Niwa M. A new blood–brain barrier model using primary rat brain endothelial cells, pericytes and astrocytes. Neurochem Int. 2009;54:253–263. doi: 10.1016/j.neuint.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 25.Pan H, Wang H, Wang X, Zhu L, Mao L. The absence of Nrf2 enhances NF-êB-dependent inflammation following scratch injury in mouse primary cultured astrocytes. Mediators Inflamm 2012. 2012:217580. doi: 10.1155/2012/217580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Patabendige A, Skinner RA, Morgan L, Joan Abbott N. A detailed method for preparation of a functional and flexible blood-brain barrier model using porcine brain endothelial cells. Brain Res. 2013;1521:16–30. doi: 10.1016/j.brainres.2013.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reichel A, Begley DJ, Abbott NJ. An overview of in vitro techniques for blood-brain barrier studies. Methods Mol Med. 2003;89:307–324. doi: 10.1385/1-59259-419-0:307. [DOI] [PubMed] [Google Scholar]
- 28.Ribatti D, Nico B, Crivellato E, Artico M. Development of the blood-brain barrier: a historical point of view. Anat Rec B New Anat. 2006;289B:3–8. doi: 10.1002/ar.b.20087. [DOI] [PubMed] [Google Scholar]
- 29.Ribeiro MM, Castanho MA, Serrano I. In vitro blood-brain barrier models--latest advances and therapeutic applications in a chronological perspective. Mini Rev Med Chem. 2010;10:262–270. doi: 10.2174/138955710791185082. [DOI] [PubMed] [Google Scholar]
- 30.Rubin LL, Hall DE, Porter S, Barbu K, Cannon C, Horner HC, Janatpour M, Liaw CW, Manning K, Morales J, Tanner LI, Tomaselli KJ, Bard F. A cell culture model of the blood-brain barrier. J Cell Biol. 1991;115:1725–1735. doi: 10.1083/jcb.115.6.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schmuck G, Röhrdanz E, Tran-Thi QH, Kahl R, Schlüter G. Oxidative stress in rat cortical neurons and astrocytes induced by paraquatin vitro. Neurotox Res. 2002;4:1–13. doi: 10.1080/10298420290007574. [DOI] [PubMed] [Google Scholar]
- 32.Schousboe A, Waagepetersen HS. Role of astrocytes in glutamate homeostasis: implications for excitotoxicity. Neurotox Res. 2005;8:221–225. doi: 10.1007/BF03033975. [DOI] [PubMed] [Google Scholar]
- 33.Shen Q, Goderie SK, Jin L, Karanth N, Sun Y, Abramova N, Vincent P, Pumiglia K, Temple S. Endothelial cells stimulate self-renewal and expand neurogenesis of neural stem cells. Science. 2004;304:1338–1340. doi: 10.1126/science.1095505. [DOI] [PubMed] [Google Scholar]
- 34.Steiner O, Coisne C, Engelhardt B, Lyck R. Comparison of immortalized bEnd5 and primary mouse brain microvascular endothelial cells as in vitro blood-brain barrier models for the study of T cell extravasation. J Cereb Blood Flow Metab. 2011;31:315–327. doi: 10.1038/jcbfm.2010.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stephan D, Sbai O, Wen J, Couraud P-O, Putterman C, Khrestchatisky M, Desplat-Jégo S. TWEAK/Fn14 pathway modulates properties of a human microvascular endothelial cell model of blood brain barrier. J Neuroinflammation. 2013;10:9. doi: 10.1186/1742-2094-10-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sun X, Liu X, Zhang Y, Kuang X, Lv B, Ge J. A simple and effective pressure culture system modified from a transwell cell culture system. Biol Res. 2013;46:47–52. doi: 10.4067/S0716-97602013000100007. [DOI] [PubMed] [Google Scholar]
- 37.Sun XL, Zeng XN, Zhou F, Dai CP, Ding JH, Hu G. KATP channel openers facilitate glutamate uptake by GluTs in rat primary cultured astrocytes. Neuropsychopharmacology. 2007;33:1336–1342. doi: 10.1038/sj.npp.1301501. [DOI] [PubMed] [Google Scholar]
- 38.Tóth A, Veszelka S, Nakagawa S, Niwa M, Deli MA. Patented in vitro blood-brain barrier models in CNS drug discovery. Recent Pat CNS Drug Discov. 2011;6:107–118. doi: 10.2174/157488911795933910. [DOI] [PubMed] [Google Scholar]
- 39.Takata F, Dohgu S, Yamauchi A, Matsumoto J, Machida T, Fujishita K, Shibata K, Shinozaki Y, Sato K, Kataoka Y, Koizumi S. In vitro blood-brain barrier models using brain capillary endothelial cells isolated from neonatal and adult rats retain age-related barrier properties. PLoS One. 2013;8:e55166. doi: 10.1371/journal.pone.0055166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tanahashi S, Yamamura S, Nakagawa M, Motomura E, Okada M. Clozapine, but not haloperidol, enhances glial d-serine and L-glutamate release in rat frontal cortex and primary cultured astrocytes. Br J Pharmacol. 2012;165:1543–1555. doi: 10.1111/j.1476-5381.2011.01638.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang J, Li PT, Du H, Hou JC, Li WH, Pan YS, Hua Q, Chen HC. Impact of paracrine signals from brain microvascular endothelial cells on microglial proliferation and migration. Brain Res Bull. 2011a;86:53–59. doi: 10.1016/j.brainresbull.2011.06.016. [DOI] [PubMed] [Google Scholar]
- 42.Wang Q, Luo W, Zhang W, Liu M, Song H, Chen J. Involvement of DMT1+IRE in the transport of lead in an in vitro BBB model. Toxicol In Vitro. 2011b;25:991–998. doi: 10.1016/j.tiv.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 43.Winter S, Konter J, Scheler S, Lehmann J, Fahr A. Permeability changes in response to NONOate and NONOate prodrug derived nitric oxide in a blood-brain barrier model formed by primary porcine endothelial cells. Nitric Oxide. 2008;18:229–239. doi: 10.1016/j.niox.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 44.Wuest DM, Lee KH. Optimization of endothelial cell growth in a murine in vitro blood-brain barrier model. Biotechnol J. 2012;7:409–417. doi: 10.1002/biot.201100189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yin P, Knolhoff AM, Rosenberg HJ, Millet LJ, Gillette MU, Sweedler JV. Peptidomic analyses of mouse astrocytic cell lines and rat primary cultured astrocytes. J Proteome Res. 2012;11:3965–3973. doi: 10.1021/pr201066t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zeng X, Zhang S, Xu L, Yang H, He S. Activation of protease-activated receptor 2-mediated signaling by mast cell tryptase modulates cytokine production in primary cultured astrocytes. Mediat Inflamm 2013. 2013:140812. doi: 10.1155/2013/140812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhou JX, Ding GR, Zhang J, Zhou YC, Zhang YJ, Guo GZ. Detrimental effect of electromagnetic pulse exposure on permeability of in vitro blood-brain-barrier model. Biomed Environ Sci. 2013;26:128–137. doi: 10.3967/0895-3988.2013.02.007. [DOI] [PubMed] [Google Scholar]