Abstract
In this study, microstructural brain damage in Parkinson's disease patients was examined using diffusion tensor imaging and tract-based spatial statistics. The analyses revealed the presence of neuronal damage in the substantia nigra and putamen in the Parkinson's disease patients. Moreover, disease symptoms worsened with increasing damage to the substantia nigra, confirming that the substantia nigra and basal ganglia are the main structures affected in Parkinson's disease. We also found that microstructural damage to the putamen, caudate nucleus and frontal lobe positively correlated with depression. Based on the tract-based spatial statistics, various white matter tracts appeared to have microstructural damage, and this correlated with cognitive disorder and depression. Taken together, our results suggest that diffusion tensor imaging and tract-based spatial statistics can be used to effectively study brain function and microstructural changes in patients with Parkinson's disease. Our novel findings should contribute to our understanding of the histopathological basis of cognitive dysfunction and depression in Parkinson's disease.
Keywords: nerve regeneration; Parkinson's disease, cognitive dysfunction, depression, functional magnetic resonance imaging, diffusion tensor imaging, tract-based spatial statistical analysis, basal ganglia, substantia nigra, neural regeneration
Introduction
Parkinson's disease (PD) has an insidious onset and a slow progression (Katunina and Bezdolny, 2013; Hφlscher, 2014), and it is difficult to identify specific structural changes that have diagnostic value using routine brain imaging analysis. The most widely used assessment methods for PD are the Unified Parkinson's Disease Rating Scale (UPDRS) (Martinez-Martin et al., 1994) and the Hoehn and Yahr (H-Y) Scale; however, there is a lack of laboratory tests that can clearly identify the disease. Early diagnosis and early detection of non-motor symptoms in PD are the key to allowing clinicians to provide appropriate treatment as early as possible and improve the quality of life and prognosis for PD patients.
In recent years, functional magnetic resonance imaging (fMRI) techniques such as diffusion tensor imaging (DTI) have greatly advanced the study of PD (Karceski, 2014). These techniques are able to detect neuronal and white matter damage at early, even subclinical, stages that cannot be identified by conventional MRI scans. Fractional anisotropy (FA) and apparent diffusion coefficient (ADC), commonly used parameters in DTI, can be used to assess neuronal damage and morphological changes in the white matter in the brain (Cnyrim et al., 2014). In the present study, tract-based spatial statistics (TBSS) analysis was used to visualize white matter tracts because it is a very sensitive method for identifying damage to these structures. TBSS is often used to evaluate the microstructure of white matter fiber tracts and has been widely used in the treatment of nervous system diseases (Ibarretxe-Bilbao et al., 2010). Here, we evaluated microstructural changes in the white matter in the brain of PD patients, and we also investigated their association with PD and depression, with the aim of providing insight into the pathogenesis of cognitive dysfunction and depression in PD patients.
Subjects and Methods
Participants
The study protocol was in accordance with the Declaration of Helsinki and the International Ethical Guidelines for Biomedical Research Involving Human Subjects, and was approved by the Ethics Committee of the Affiliated Hospital of Inner Mongolia Medical University, China. All the participants and their relatives provided written informed consent.
PD group: Thirty-one PD patients who had been hospitalized in the Department of Neurology, the Affiliated Hospital of Inner Mongolia Medical University, China, from May 2013 to December 2014 were enrolled in the study.
Patients were eligible for inclusion in the study if they met the following criteria: (1) meeting the UK Parkinson's Disease Society Brain Bank Clinical Diagnostic Criteria (Zhang et al, 2015); (2) being able to tolerate and undergo brain MRI and DTI examinations; (3) being able to tolerate and undergo various scale assessments and examinations; (4) no prior history of surgery for PD.
Patients were excluded if they appeared to: (1) have pyramidal signs, symptoms of cerebellar damage, eye movement disorders, or a clear diagnosis of Parkinson's-plus syndrome; (2) have clear trauma, cerebrovascular disease, a history of infection, history of specific medication, intracranial lesion, or Parkinson's syndrome associated with these factors; (3) have clear and severe cognitive dysfunction, unapparent or atypical extrapyramidal symptoms; (4) have a history of mental illness, or long-term use or misuse of antipsychotics; (5) have severe medical disorders, including liver and kidney failure, acute myocardial infarction, coronary heart disease, or heart failure; (6) have brain lesions, tissue softening foci, remarkable ischemia or demyelinating lesions revealed on cranial MRI.
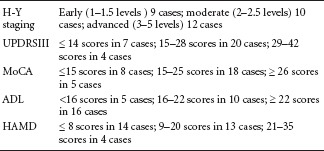
In the PD group, there were 15 females and 16 males, aged 53–84 years, with a mean age of 69.4 ± 8.0 years. The duration of the disease was < 3 years in 15 cases, 3–5 years in 9 cases, and 5–10 years in 7 cases. According to the education level, there were 5 cases of illiteracy, 9 cases of primary school, 13 cases of middle school and secondary school, and 4 cases of college and above. Among the 31 patients, before admission, 10 patients had no therapy for PD, 8 patients had irregular/no use of levodopa, benserazide hydrochloride and dopamine receptor agonists (piribedil sustained-release tablets or pramipexole hydrochloride tablets), and 13 patients had regular use of levodopa, benserazide hydrochloride and dopamine receptor agonists (2 of these 13 patients, who developed side effects of dyskinesia and orthostatic hypotension, used other PD drugs). Among the patients, 6 were from the Mongol ethnic group, 2 from the Hui ethnic group, and 23 from the Han ethnic group. All the patients were subjected to assessments using the Montreal Cognitive Assessment Scale (MoCA) (Krishnan et al., 2015), the Hamilton Depression Rating Scale (HAMD) (Luckenbaugh et al., 2015), the H-Y Scale (Kataoka et al., 2011), and the UPDRSIII and Activity of Daily Living Scale (ADL) (Delva et al., 2014). The patients were grouped according to the grades obtained from these scales. MoCA was used to assess cognitive function, HAMD to assess depression, H-Y Scale to assess PD staging, UPDRSIII to evaluate motor function, and ADL to evaluate self-care ability. Patients in the PD group were enrolled without considering educational level, onset side or severity of impairment of a particular side.
Control group: 34 healthy control subjects, matched with the PD patients for age and gender, were recruited from healthy persons undergoing physical examination at the Medical Examination Center, the Affiliated Hospital of Inner Mongolia Medical University, China. This group was composed of 16 females and 18 males, aged 52–85 years, with a mean age of 69.3 ± 8.0 years. These healthy controls had no serious medical disorder, focal symptoms or signs of nervous system disorder, no history of cerebrovascular disease or other nervous system diseases, no mental disease, and no long-term medication use. Furthermore, brain MRI revealed no abnormalities.
PD patients were grouped according to the various scale results as follows:
Imaging tests
Imaging equipment: A GE Signal HDx 3.0T MRI scanner (GE Medical Systems, Milwaukee, WI, USA) was employed, and an eight-channel phased-array head coil served as a transmitter/receiver coil. Canthomeatal lines were used as base lines for conventional MRI and DTI examination. All scans were performed by the same physician.
Conventional MRI examination: All subjects underwent brain MRI, and specific scanning parameters were as follows: (1) T1-weighted imaging: repetition time (TR)/echo time (TE)/number of excitations (NEX) = 2,014 ms/24.5 ms/1.0, field of view (FOV) = 24 cm × 24 cm, matrix = 320 × 224, slice thickness/gap = 5.0/1.5 mm, scan time = 114 seconds. (2) T2-weighted imaging: TR/TE/NEX = 6,280 ms/104.2 ms/1.0, FOV = 24 cm × 24cm, matrix = 320 × 320, slice thickness/gap = 5.0/1.5 mm, scan time = 84 seconds. (3) Diffusion weighted imaging: TR/TE/NEX = 5,000 ms/75.8 ms/2.0, FOV = 24 cm × 24 cm, matrix = 160 × 160, slice thickness/gap = 5.0/1.5 mm, scan time = 45 seconds. Conventional MRI images of all subjects were reviewed blindly by two experienced physicians, and their conclusions were identical. Slight differences in procedures and descriptions were not considered in this study.
DTI scans: Echo-planar technique was used for DTI examination of the transverse view, and the specific parameters were as follows: b-value = 1,000 s/mm, TR/TE/NEX = 8,500 ms/87.6 ms/1.0, the number of diffusion gradient sensitive directions = 30, FOV = 24 cm × 24 cm, matrix = 130 × 128, slice thickness/gap = 5.0/0 mm, scan time = 272 seconds. Functool software from GE Advantage Workstation 4.4 was used for postprocessing DTI images, followed by original data correction and noise reduction. Then, the parameters were calculated automatically to generate FA and ADC maps. Nigrostriatal degeneration is a major pathological hallmark of PD, and cognitive dysfunction, depression and pain are common non-motor symptoms in PD (Christopher et al., 2014). Therefore, the substantia nigra, red nucleus, basal ganglia, thalamus and frontal white matter were selected as regions of interest (ROI) in this study. Circular ROIs of similar sizes (30–50 mm2) were manually placed by two physicians independently within the target anatomical area (Figure 1). FA and ADC values of bilateral ROIs were measured and averaged in all the subjects.
Figure 1.
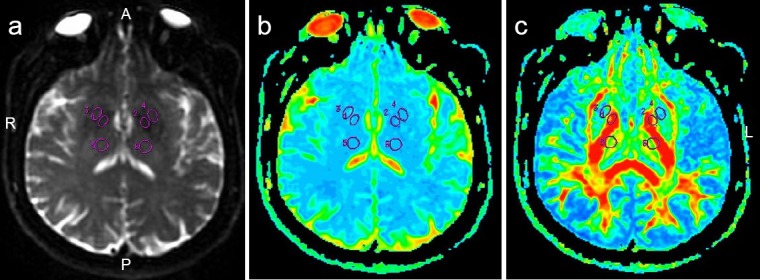
Regions of interest in a female Parkinson's disease patient (63 years old; Hoehn and Yahr Scale stage 1.5).
(a) T2-weighted image; (b) ADC map; (c) FA map. 1–6 refer to regions of interest on FA and ADC maps. A: Anterior; P: posterior; R: right side; L: left side; ADC: apparent diffusion coefficient; FA: fractional anisotropy.
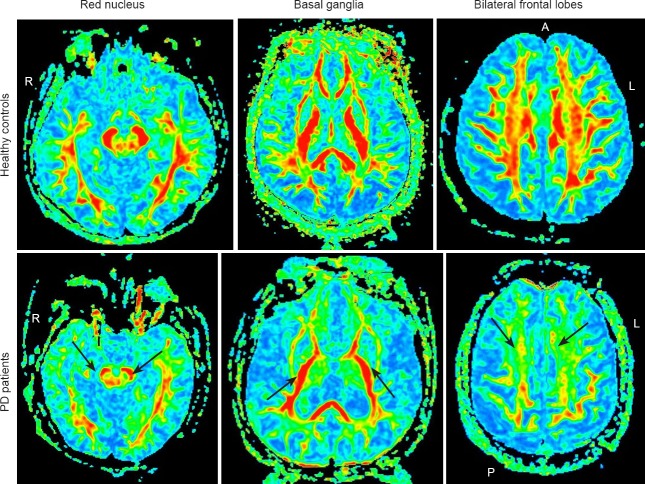
Figure 2.
FA characteristics of PD patients and healthy controls.
Compared with healthy controls, the FA values (arrows) in the substantia nigra, red nucleus, basal ganglia and frontal white matter in PD patients were significantly reduced. PD: Parkinson's disease; FA: fractional anisotropy; A: anterior; P: posterior; R: right side; L: left side.
TBSS analysis
Extraction of FA maps: FSL software (FMRIB Analysis Group, Oxford, UK) was used to process DTI images. First, the image format was converted, the eddy current was corrected, and a brain mask was created. B0 field maps were registered with T1 images in a linear manner followed by a nonlinear registration of T1 and MNI, and finally, relevant parameters were input into B0 and other indicator diagrams to generate an initial FA map.
TBSS analysis: First, all the FA maps were registered into FMRIB58_FA_1 mm templates for nonparametric estimation to generate average FA maps and mean FA skeleton images. Then, skeletonization was realized using an FA threshold of 0.2, and then, the FA data were projected onto the mean FA skeleton representing the centers of each tract in the brain for case-by-voxel analysis. Permutation value was set to 5,000, and P < 0.05 was considered to indicate a statistically disparate point. Furthermore, we analyzed the correlation of FA values at all the disparate points with MoCA, HAMD, ADL and UPDRSIII scores. Positive voxels were projected onto the white matter skeleton using different colors.
Statistical analysis
Data are expressed as the mean ± SD, and were analyzed using SPSS 20.0 software (IBM, Armonk, NY, USA). Two-sample t-tests were used to assess differences in DTI measurements between the PD and control groups. Analysis of variance was used for testing the difference in H-Y staging, UPDRSIII scores, MoCA scores, HAMD scores and ADL scores among PD patients. A value of P < 0.05 was considered statistically significant. Linear correlation analysis was used for testing the association of measured values at each part with H-Y staging, UPDRSIII scores, MoCA scores, HAMD scores and ADL scores.
Results
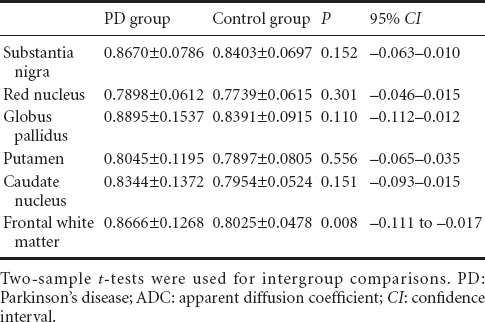
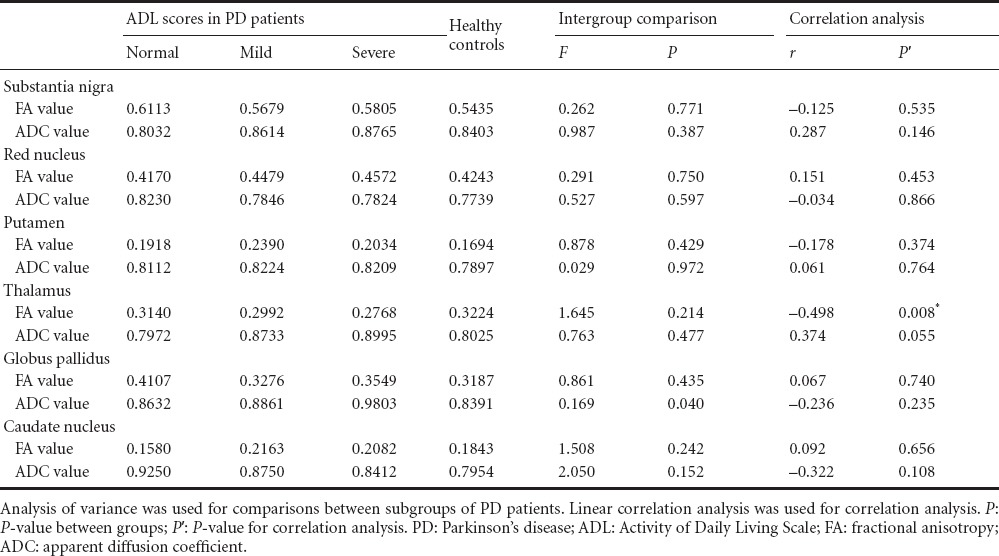
The FA values for the substantia nigra, red nucleus, basal ganglia and frontal white matter were significantly lower in the PD group than in the control group (P < 0.05), but the participants in the two groups had similar ADC values at the detection sites (P > 0.05; Figure 1, Tables 1 and 2).
Table 1.
Comparison of FA values between PD and control groups
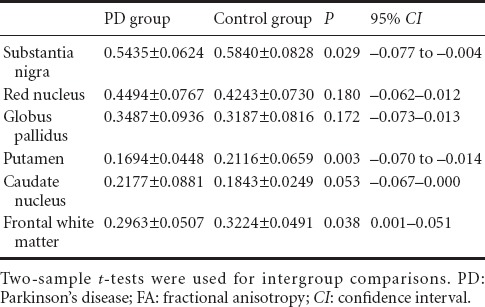
Table 2.
Comparison of ADC values between PD and control groups
FA and ADC values were compared between healthy controls and PD patients with different H-Y staging and ADL scores, as shown in Tables 3 and 4. PD patients who were grouped according to MoCA scores showed similar FA and ADC values. Moreover, the MoCA scores had no correlation with FA or ADC values (P > 0.05), which is in contrast to previous findings (Zheng et al., 2014), and which may possibly result from averaging the values for bilateral ROIs in the present study. PD patients who were grouped according to HAMD scores showed statistically significant differences in ADC values in the putamen, caudate nucleus and frontal white matter (P < 0.05). The ADC values were highest in PD patients with moderate depression and lowest in the PD patients with no depression, but there was no correlation between the ADC values and HAMD scores.
Table 3.
Comparison of FA and ADC values between healthy controls and PD patients with different H-Y stages
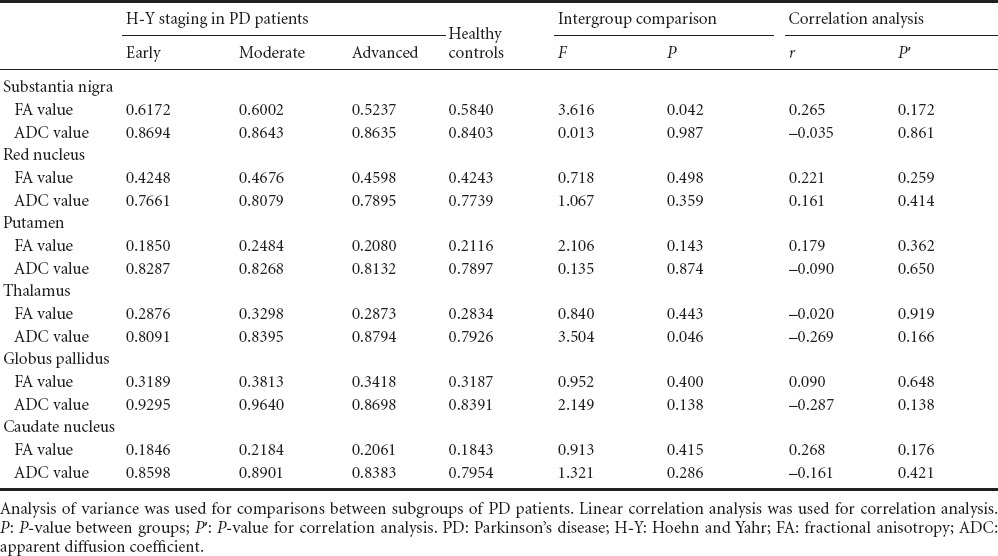
Table 4.
Comparison of FA and ADC values between healthy controls and PD patients with different ADL scores
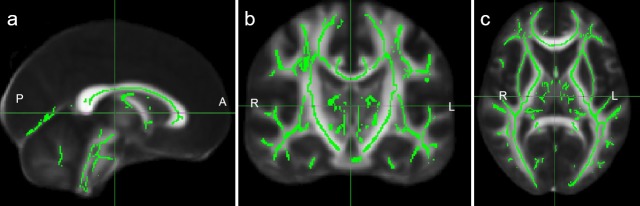
FA maps were extracted using FSL software, which were further registered into the standard templates to generate the average FA maps and mean FA skeleton maps. An FA threshold of 0.2 was employed to exclude the gray matter and cerebrospinal fluid, and the white matter skeleton for all the subjects was obtained (Figure 3).
Figure 3.
White matter skeleton of the brain.
(a) Sagittal; (b) coronal; (CT) axial. Green represents the white matter skeleton. A: Anterior; P: posterior; R: right side; L: left side.
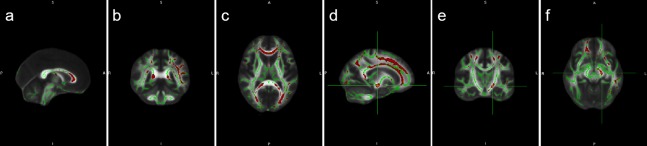
Results from TBSS analysis showed that, compared with the control group, the FA values in the corpus callosum, superior longitudinal fasciculus, inferior longitudinal fasciculus, cingulum bundle, optic radiation, left internal capsule and subcortical arcuate fibers were significantly lower in the PD group (P < 0.05; Figure 4).
Figure 4.
Mean FA skeletons in the PD patients and healthy controls using TBSS analysis.
a–f represent the number of different levels. Green represents the white matter skeleton, and red represents the reduced FA value in the white matter tracts of PD patients. PD: Parkinson's disease; FA: fractional anisotropy; TBSS: tract-based spatial statistics; A: anterior; P: posterior; R: right side; L: left side; S: superior: I: inferior.
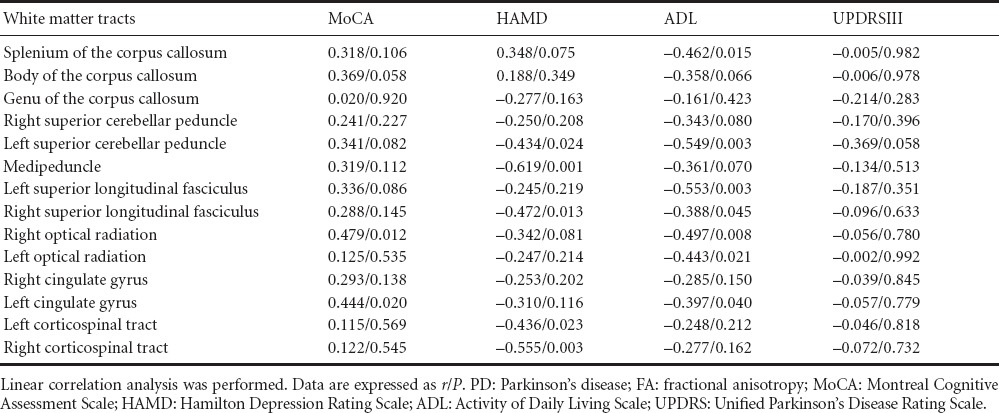
The FA values in the right optical radiation and left cingulate gyrus were positively correlated with MoCA scores (P < 0.05). The FA values in the right superior longitudinal fasciculus, left corticospinal tract, left superior cerebellar peduncle, right corticospinal tract and middle peduncle were negatively correlated with HAMD scores (P < 0.05). The FA values in the white matter tracts showed no correlation with UPDRSIII scores (P > 0.05). The FA values in the splenium of the corpus callosum, left and right superior longitudinal fasciculus, right and left optic radiations, left superior cerebellar peduncle and left cingulate gyrus were negatively correlated with ADL scores (P < 0.05; Table 5).
Table 5.
Correlation of reduced FA values in the white matter tracts with various scale scores in PD patients
Discussion
Application of DTI technology in PD
The motor symptoms of PD result from nigrostriatal degeneration, but the causes of cognitive dysfunction, depression and other non-motor symptoms are poorly understood (Prodoehl et al., 2013; Teng et al., 2014). When brain lesions result in neuronal/axonal loss and damage, there are associated changes in ADC and FA values. In this study, in the PD group compared with the control group, the FA values in the substantia nigra, putamen and frontal white matter were lower, but the ADC value in the frontal white matter was higher, consistent with previous studies (Prakash et al., 2012). PD patients were grouped into three subgroups according to their H-Y stage: early-stage, middle-stage and advanced-stage. The FA values were lowest in the advanced-stage patients and highest in the early-stage patients. The ADC values were also different between the advanced-stage and early-stage groups, as well as between the middle-stage and advanced-stage groups. These findings show that the degeneration of neurons in the substantia nigra of PD patients shows a worsening trend with time, and the FA value in the substantia nigra of PD patients is negatively correlated with the progression of disease. Therefore, DTI appears to be an effective means for early diagnosis and reasonable staging of PD (Hess et al., 2014; Zheng et al., 2014).
PD patients were divided into normal, mild and severe dysfunction groups according to their ADL score. The FA value in the frontal white matter was lower in the severe group than in the mild group. According to the HAMD scores, the ADC values in the caudate nucleus and frontal white matter were highest in the PD patients with moderate depression, and lowest in those with no depression. These findings provide support for an association between frontal lobe lesions and depression, as well as between non-motor symptoms in PD and neurodegeneration. Neurodegeneration of the nigrostriatal system directly results in motor dysfunction in PD patients and indirectly affects the patient's emotional and mental state, thereby impacting HAMD scores (Meijer et al, 2013). This likely accounts for the association between the nigrostriatal system and depression. Additionally, dopamine is involved in memory, emotion and cognition, and a reduction or depletion of dopamine in the brain is an important factor contributing to depression in PD patients.
In this study, the FA and ADC values in the frontal white matter were strongly correlated with depression in PD patients. Therefore, DTI appears to be an effective assessment tool for depression in PD patients. In this study, we did not take into consideration differences in the side of symptom onset or lateral differences in severity, and only the average FA and ADC values for the bilateral ROIs were used for comparison between PD and control groups. This may have impacted the findings in this study, and may have prevented the identification of some correlations. Furthermore, the patients had no follow-up, preventing an assessment of disease progression. Nonetheless, our findings provide a basis for future studies on PD using DTI and other fMRI techniques.
Application of TBSS techniques in microstructural brain damage
In this study, TBSS analysis showed that the FA values in the corpus callosum, superior longitudinal fasciculus, cingulum bundle, optic radiation, frontal and temporal subcortical arcuate fibers, brain stem and internal capsule were significantly lower in the PD group compared with the control group, suggesting that the PD group had more extensive white matter lesions (Melzer et al., 2013). The corpus callosum is the largest association fiber linking the neocortices, from the frontal pole to the occipital pole (except the primary visual cortex (area 17) and the somatosensory hand and foot areas). Currently, little is known about the function of the corpus callosum. The significant reduction in FA value suggests microstructural damage to the corpus callosum in PD patients, but the association with clinical outcome is unclear (Schwarz et al., 2013). A previous clinical study on cognitive dysfunction found that the FA value in the splenium of the corpus callosum was a useful indicator, distinguishing patients with memory disorders from normal controls (Zhuang et al., 2010). The superior longitudinal fasciculus is an important association fiber bundle connecting the frontal, occipital, parietal and temporal lobes, and is closely related to frontal lobe functions (Urger et al., 2015).
In the present study, the superior longitudinal fasciculus exhibited microstructural damage to the white matter, and the FA values in the bilateral superior longitudinal fasciculi were negatively correlated with ADL scores. In addition, the FA value in the right superior longitudinal fasciculus showed a negative correlation with the HAMD scores, suggesting that depressive symptoms in PD patients worsen with decreasing FA values (Perea et al., 2013). Because the frontal lobe contains the motor cortex, prefrontal lobe, the orbital gyri and the eloquent cortex, which are closely related to mind, emotion and thinking, the emergence of depression, cognitive dysfunction and abnormal self-care ability can result from damage to the superior longitudinal fasciculus. We can further speculate that the presence of language deficits in advanced PD patients may be related to damage to the superior longitudinal fasciculus (Tang et al., 2014).
The corticospinal tract is the longest projection fiber bundle, and is an important component of the pyramidal tract. This tract is responsible for the voluntary movement of the limbs and body. In PD patients, corticospinal tract damage is associated with dyskinesia (Cnyrim et al., 2014). In this study, the FA values in the right corticospinal tract were reduced significantly, and it was significantly negatively correlated with HAMD scores. The cingulate gyrus is an integral part of the limbic system, which links the corpus callosum and the parahippocampal gyrus. The cingulate gyrus, especially the posterior cingulate gyrus, constitutes a memory loop that is an important part of the cholinergic system (Ding et al., 2013). Our results showed that the FA values in the posterior cingulate gyrus were dramatically reduced in PD patients, suggesting that damage to this structure is directly related to cognitive dysfunction in PD (Skidmore et al., 2015).
There are a number of limitations in this study, including the small number of PD patients and the lack of follow-up comparison and analysis. Furthermore, TBSS analysis may not have been sufficiently sensitive for small fibers, making it difficult to distinguish whether the changes in FA values were caused by lesions or variations in the diameter of white matter fibers. Additionally, the TBSS method may not have accurately revealed the white matter skeleton at white matter tract crossings (Worker et al., 2014). These limitations may have impacted our present findings and need to be addressed in future studies.
Conclusion
DTI is used to evaluate microstructural brain damage in PD patients through measurement of FA and ADC in selected ROIs. This technique is useful for early diagnosis and staging, and for understanding the neuropathological changes underlying non-motor symptoms in PD. In this study, we used the TBSS method to analyze the white matter microstructure in PD patients. Our findings provide valuable insight that lays the foundation for future studies on the pathogenesis of PD, especially of the non-motor symptoms.
Footnotes
Conflicts of interest: None declared.
Plagiarism check: This paper was screened twice using Cross-Check to verify originality before publication.
Peer review: This paper was double-blinded and stringently reviewed by international expert reviewers.
Copyedited by Patel B, Ge HL, Yin JZ, Qiao J, Jiang GH, Yu J, Wang L, Li CH, Song LP, Zhao M
References
- 1.Christopher L, Koshimori Y, Lang AE, Criaud M, Strafella AP. Uncovering the role of the insula in non-motor symptoms of Parkinson's disease. Brain. 2014;137:2143–2154. doi: 10.1093/brain/awu084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cnyrim CD, Kupsch A, Ebersbach G, Hoffmann KT. Diffusion tensor imaging in idiopathic Parkinson's disease and multisystem atrophy (Parkinsonian type) Neurodegener Dis. 2014;13:1–8. doi: 10.1159/000348512. [DOI] [PubMed] [Google Scholar]
- 3.Delva F, Edjolo A, Pérès K, Berr C, Barberger-Gateau P, Dartigues JF. Hierarchical structure of the activities of daily living scale in dementia. J Nutr Health Aging. 2014;18:698–704. doi: 10.1007/s12603-014-0503-7. [DOI] [PubMed] [Google Scholar]
- 4.Ding B, Ling HW, Wang T, Huang J, Zhang H, Chen KM, Yan FH. Investigation of white matter by tract-based spatial statistics in patients with Alzheimer's disease. Zhenduan Xue Lilun yu Shijian. 2013;12:269–273. [Google Scholar]
- 5.Hölscher C. New drug treatments show neuroprotective effects in Alzheimer's and Parkinson's diseases. Neural Regen Res. 2014;9:1870–1873. doi: 10.4103/1673-5374.145342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hess CP, Christine CW, Apple AC, Dillon WP, Aminoff MJ. Changes in the thalamus in atypical parkinsonism detected using shape analysis and diffusion tensor imaging. Am J Neuroradiol. 2014;35:897–903. doi: 10.3174/ajnr.A3832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ibarretxe-Bilbao N, Junque C, Marti MJ, Valldeoriola F, Vendrell P, Bargallo N, Zarei M, Tolosa E. Olfactory impairment in Parkinson's disease and white matter abnormalities in central olfactory areas: A voxel-based diffusion tensor imaging study. Mov Disord. 2010;25:1888–1894. doi: 10.1002/mds.23208. [DOI] [PubMed] [Google Scholar]
- 8.Kamagata K, Tomiyama H, Hatano T, Motoi Y, Abe O, Shimoji K, Kamiya K, Suzuki M, Hori M, Yoshida M, Hattori N, Aoki S. A preliminary diffusional kurtosis imaging study of Parkinson disease: comparison with conventional diffusion tensor imaging. Neuroradiology. 2014;56:251–258. doi: 10.1007/s00234-014-1327-1. [DOI] [PubMed] [Google Scholar]
- 9.Karceski S. New ways to assess brain function in Parkinson disease. Neurology. 2014;83:e199–201. doi: 10.1212/WNL.0000000000001091. [DOI] [PubMed] [Google Scholar]
- 10.Kataoka H, Tanaka N, Eng M, Saeki K, Kiriyama T, Eura N, Ikeda M, Izumi T, Kitauti T, Furiya Y, Sugie K, Ikada Y, Ueno S. Risk of falling in Parkinson's disease at the Hoehn-Yahr stage III. Eur Neurol. 2011;66:298–304. doi: 10.1159/000331635. [DOI] [PubMed] [Google Scholar]
- 11.Katunina EA, Bezdolny YN. Epidemiology of Parkinson's disease. Zh Nevrol Psikhiatr Im S S Korsakova. 2013;113:81–88. [PubMed] [Google Scholar]
- 12.Krishnan S, Justus S, Meluveettil R, Menon RN, Sarma SP, Kishore A. Validity of Montreal Cognitive Assessment in non-english speaking patients with Parkinson's disease. Neurol India. 2015;63:63–67. doi: 10.4103/0028-3886.152637. [DOI] [PubMed] [Google Scholar]
- 13.Luckenbaugh DA, Ameli R, Brutsche NE, Zarate CA. Rating depression over brief time intervals with the Hamilton Depression Rating Scale: Standard vs. abbreviated scales. J Psychiatr Res. 2015;61:40–45. doi: 10.1016/j.jpsychires.2014.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martínez-Martín P, Gil-Nagel A, Gracia LM, Gómez JB, Martínez-Sarriés J, Bermejo F. Unified Parkinson's Disease Rating Scale characteristics and structure. The Cooperative Multicentric Group. Mov Disord. 1994;9:76–83. doi: 10.1002/mds.870090112. [DOI] [PubMed] [Google Scholar]
- 15.Meijer FJ, Bloem BR, Mahlknecht P, Seppi K, Goraj B. Update on diffusion MRI in Parkinson's disease and atypical parkinsonism. J Neurol Sci. 2013;332:21–29. doi: 10.1016/j.jns.2013.06.032. [DOI] [PubMed] [Google Scholar]
- 16.Melzer TR, Watts R, MacAskill MR, Pitcher TL, Livingston L, Keenan RJ, Dalrymple-Alford JC, Anderson TJ. White matter microstructure deteriorates across cognitive stages in Parkinson disease. Neurology. 2013;80:1841–1849. doi: 10.1212/WNL.0b013e3182929f62. [DOI] [PubMed] [Google Scholar]
- 17.Perea RD, Rada RC, Wilson J, Vidoni ED, Morris JK, Lyons KE, Pahwa R, Burns JM, Honea RA. A comparative white matter study with Parkinson's disease Parkinson's disease with dementia and Alzheimer's disease. J Alzheimers Dis Parkinsonism. 2013;3:123. doi: 10.4172/2161-0460.1000123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prakash BD, Sitoh YY, Tan LC, Au WL. Asymmetrical diffusion tensor imaging indices of the rostral substantia nigra in Parkinson's disease. Parkinsonism Relat D. 2012;18:1029–1033. doi: 10.1016/j.parkreldis.2012.05.021. [DOI] [PubMed] [Google Scholar]
- 19.Prodoehl J, Li H, Planetta PJ, Goetz CG, Shannon KM, Tangonan R, Comella CL, Simuni T, Zhou XJ, Leurgans S, Corcos DM, Vaillancourt DE. Diffusion tensor imaging of Parkinson's disease, atypical parkinsonism, and essential tremor. Mov Disord. 2013;28:1816–1822. doi: 10.1002/mds.25491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schwarz ST, Abaei M, Gontu V, Morgan PS, Bajaj N, Auer DP. Diffusion tensor imaging of nigral degeneration in Parkinson's disease: A region-of-interest and voxel-based study at 3 T and systematic review with meta-analysis. Neuroimage Clin. 2013;3:481–488. doi: 10.1016/j.nicl.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Skidmore FM, Spetsieris PG, Anthony T, Cutter GR, von Deneen KM, Liu Y, White KD, Heilman KM, Myers J, Standaert DG, Lahti AC, Eidelberg D, Ulug AM. A full-brain bootstrapped analysis of diffusion tensor imaging robustly differentiates Parkinson disease from healthy controls. Neuroinformatics. 2015;13:7–18. doi: 10.1007/s12021-014-9222-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tang JT, Hong XN, Cheng H, Wang Z, Chen YJ, Yu YF. Diffusion tensor imaging study of cerebral white matter in Alzheimer's disease: a tract-based spatial statistics analysis. Linchuang Fangshe Xue Zazhi. 2014;33:968–971. [Google Scholar]
- 23.Teng L, Hong F, Zhang C, He J, Wang H. Compound Formula Rehmannia alleviates levodopa-induced dyskinesia in Parkinson's disease. Neural Regen Res. 2014;9:407–412. doi: 10.4103/1673-5374.128246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Urger SE, De Bellis MD, Hooper SR, Woolley DP, Chen SD, Provenzale J. The superior longitudinal fasciculus in typically developing children and adolescents: diffusion tensor imaging and neuropsychological correlates. J Child Neurol. 2015;30:9–20. doi: 10.1177/0883073813520503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Worker A, Blain C, Jarosz J, Chaudhuri KR, Barker GJ, Williams SCR, Brown RG, Leigh PN, Dell’Acqua F, Simmons A. Diffusion tensor imaging of Parkinson's disease, multiple system atrophy and progressive supranuclear palsy: a tract-based spatial statistics study. PLoS One. 2014;9:e112638. doi: 10.1371/journal.pone.0112638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang N, Liu W, Ye M, Cohen AD, Zhang Y. The heterogeneity of non-motor symptoms of Parkinson's disease. Neurol Sci. 2015;36:577–584. doi: 10.1007/s10072-014-1993-0. [DOI] [PubMed] [Google Scholar]
- 27.Zheng Z, Shemmassian S, Wijekoon C, Kim W, Bookheimer SY, Pouratian N. DTI correlates of distinct cognitive impairments in Parkinson's disease. Hum Brain Mapp. 2014;35:1325–1333. doi: 10.1002/hbm.22256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhuang L, Wen W, Zhu W, Trollor J, Kochan N, Crawford J, Reppermund S, Brodaty H, Sachdev P. White matter integrity in mild cognitive impairment: A tract-based spatial statistics study. Neuroimage. 2010;53:16–25. doi: 10.1016/j.neuroimage.2010.05.068. [DOI] [PubMed] [Google Scholar]