Abstract
Transfection of the human telomerase reverse transcriptase (hTERT) gene has been shown to increase cell proliferation and enhance tissue repair. In the present study, hTERT was transfected into rat Schwann cells. A rat model of acute spinal cord injury was established by the modified free-falling method. Retrovirus PLXSN was injected at the site of spinal cord injury as a vector to mediate hTERT gene-transfected Schwann cells (1 × 1010/L; 10 μL) or Schwann cells (1 × 1010/L; 10 μL) without hTERT gene transfection. Between 1 and 4 weeks after model establishment, motor function of the lower limb improved in the hTERT-transfected group compared with the group with non-transfected Schwann cells. Terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling and reverse transcription-polymerase chain reaction results revealed that the number of apoptotic cells, and gene expression of aquaporin 4/9 and matrix metalloproteinase 9/2 decreased at the site of injury in both groups; however, the effect improved in the hTERT-transfected group compared with the Schwann cells without hTERT transfection group. Hematoxylin and eosin staining, PKH26 fluorescent labeling, and electrophysiological testing demonstrated that compared with the non-transfected group, spinal cord cavity and motor and sensory evoked potential latencies were reduced, while the number of PKH26-positive cells and the motor and sensory evoked potential amplitude increased at the site of injury in the hTERT-transfected group. These findings suggest that transplantation of hTERT gene-transfected Schwann cells repairs the structure and function of the injured spinal cord.
Keywords: nerve regeneration, spinal cord injury, Schwann cells, transplantation, motor function, telomerase, reverse transcriptase, proliferation, modification, cells, neural regeneration
Introduction
The regeneration of injured axons after spinal cord injury (SCI) can be promoted by changing the microenvironment of regenerating nerves (Ban et al., 2009; Allodi et al., 2014). Schwann cells are the myelin-forming cells of peripheral nerve fibers and thus, are the main structural and functional cells of these nerves (Chen et al., 2014; Deng et al., 2014; Wu et al., 2015). Schwann cells play a major role after SCI. Numerous studies have shown that Schwann cell transplantation contributes to the functional recovery of the spinal cord (Kang et al., 2004; Dong et al., 2013; Hakim et al., 2015).
Human telomerase reverse transcriptase (hTERT) is a key nutritional factor, which is not expressed in most normal tissues; however, in primary tumor and carcinoma cell lines, hTERT is highly expressed (Marcol et al., 2015; Peterson et al., 2015; Sparling et al., 2015). hTERT has multiple biological effects and an important clinical value. After a local application, hTERT is diluted and metabolized rapidly; therefore, it should be administered repeatedly in large dosages (Siddiqui et al., 2015; Thumm et al., 2015; Vasudeva et al., 2015).
pLXSN is a commonly used retrovirus vector (Liu and Wang, 2015), which was used in the current study to transfect rat Schwann cells with the hTERT gene. The aim of the study was to investigate the effect of transplanting hTERT gene-modified Schwann cells on the electrophysiology of these cells in SCI rats.
Materials and Methods
Experimental animals
Eighty-five healthy, inbred-line female, 1-month-old Sprague-Dawley rats, weighing 200–250 g were purchased from the Animal Laboratory of Chinese Academy of Medical Sciences, China (animal certificate No. SCXK(Jin)20050076). Experiments were approved by the Animal Ethics Committee of the Department of Orthopedics of Tianjin Nankai Hospital of China.
Culture and identification of Schwann cells
The sciatic nerve was aseptically stripped under a microscope from two rats. The tissue was digested with 0.25% trypsin/0.2% collagenase for 40 minutes, and then centrifuged at 300 × g for 5 minutes. The cells were incubated in Dulbecco's Modified Eagle's medium (DMEM)/F12 medium containing 10% fetal bovine serum (Gibco, Carlsbad, CA, USA) at 37°C in a 5% CO2 incubator for 30 minutes. Fibroblasts were then removed by differential adherence. Any remaining fibroblasts were killed 24 hours later by adding 100 μL cytarabine (10–5 mM; Gibco). The fourth passage of Schwann cells was incubated on coverslips for 48 hours, then washed three times with phosphate-buffered saline (PBS), fixed with 4% paraformaldehyde (pH 7.4) at room temperature (20 minutes), and then washed three times with PBS. These cells were incubated with rat anti-myelin basic protein monoclonal antibody (1:1,000; Amyjet Scientific Inc., Wuhan, China) in a wet box at 4°C overnight, and then washed three times with PBS followed by incubation with goat anti-rat IgG (1:1,000) at 37°C for 2 hours. The cells were then treated with 4′,6-diamidino-2-phenylindole (DAPI) for 10 minutes and then washed three times with PBS. The samples were mounted with mounting medium. Schwann cells were digested with 0.25% trypsin (Gibco) and the single-cell suspension was obtained. Schwann cells (2 × 107 cells/mL) were washed once with serum-free DMEM/F12 medium and resuspended with 0.5 mL of diluent C. All protocols were conducted in accordance with PKH26 dye instructions (Sigma-Aldrich, St. Louis, MO, USA). Immediately after labeling, 1 × 105 cells were collected and washed once with PBS, then fixed with 1% paraformaldehyde. Cell proliferation rate was detected using flow cytometry (BIOS biological, Wuhan, China). Cell characteristics were observed with a fluorescence microscope (Olympus IX71; Olympus, Tokyo, Japan) 24 hours after the culture. Green fluorescence revealed the bodies and processes of normal Schwann cells. For nestin immunofluorescence staining, the most immunoreactive materials were fine particles and partial immunoreactive materials were coarse particles. Furthermore, some particles were scattered or clustered, while some were filamentous of different sizes. Nestin was mainly present in the cytoplasm and seldom present in cell processes.
hTERT transfection and the western immunoblot assay
The fourth passage of Schwann cells was incubated in DMEM containing 10% fetal bovine serum at saturated humidity with 5% CO2 and at 37°C. The cells were subcultured once every 3 days. Schwann cells (6 × 104 /well) in the logarithmic phase were seeded in a 24-well plate for 3 days. For the PLXSN-hTERT transfection, the medium was discarded and the cells were washed twice with PBS. In accordance with multiple of infection = 105, PLXSN-hTERT diluted with serum-free L-DMEM including 10 mM nicotinamide + 1 mM b-mercaptoethanol + 200 mL/L fetal calf serum was added to the cell culture flask and incubated at 37°C for 2 hours. Cells were then incubated with fetal bovine serum and L-DMEM medium for 1 week. The cell culture medium was not replenished 3 days before detection. Under the same conditions, the EV group was exposed to the empty virus (EV) transfection.
Three groups were designed in the in vitro experiment: Schwann cells without hTERT transfection (SCs group), EV, and hTERT-transfected group (n = 6 per group). The cell suspension from each group was centrifuged at 800 r/min for 5 minutes. After removal of the culture medium, 400 μL of lysate was added and the total protein was extracted. Protein concentration was determined using the Bradford method (Wang and Zhang, 2015). The proteins were separated on a 5% stacking gel (40 V for 1 hour) and 10% running gel (60 V for 3.5 hours). Proteins were then transferred (via a wet transfer) onto a membrane (14 V for 14 hours). The membrane was blocked in a swing bed at 37°C for 2 hours, then treated with rabbit anti-human fibroblast polyclonal antibody (1:800; Hyclone, Logan, UT, USA) at 37°C for 2 hours in a swing bed. After four 5-minute washes with Tris-buffered saline with Tween-20 (TBST), the membrane was treated with goat anti-rabbit IgG (1:700; Hyclone) at 37°C for 1.5 hours then washed four times (5 minutes) with TBST. Bands were visualized with 3,3′-dimethylbenzidine. The experiment was performed in triplicate. Results were analyzed with Quantity One image software (Bio-Rad, Hercules, CA, USA). Protein expression was determined from the ratio of the target band to the β-actin band.
Cell counting kit-8 (CCK-8) assay
Cells from the exponential growth phase were seeded in 96-well plates at 2 × 103 and incubated in DMEM containing 10% fetal bovine serum (200 μL) for 24 hours. Six wells were used for each group. CCK-8 (10 μL; Gibco, Carlsbad, CA, USA) was added to each well followed by incubation for 4 hours. The blank well was considered as the control. Optical density values were measured at 450 nm using a microplate reader. Schwann cells before and after transfection were made into single-cell suspensions. After quantification, cells were incubated in a 24-well plate (1.5 × 104 /well) in a 5% CO2 incubator at 37°C. Three wells were used for each group. From day 2 of the culture, the cells from the three wells of each group were digested daily and at the same time for 5 days to obtain an accurate count. Cell growth curves in each group were then drawn.
Model establishment and maintenance
The remaining 83 Sprague-Dawley rats were acclimatized in the laboratory for 2 weeks. They were intraperitoneally anesthetized with 2.5% ketamine (20 mg/kg) and fixed on the bench in the prone position. After the lower back was shaved, a median incision was made on the back using the T8–9 spinous processes as a center to expose the T7–10 spinous processes and the lamina. The T8–9 spinous processes and part of the lamina tissue were removed. This exposed spinal cord tissue was the lesion area. Based on the method by Allen (Xie et al., 2010; Zhang et al., 2010; Chen et al., 2011; Wang and Zhang, 2015), but with some modifications, a 10-g object was vertically dropped from a 2.5-cm height, which impacted directly on the rat spinal cord. Paralysis of the lower limbs was observed after the rat's tail suffered from swinging and spasms. These responses confirmed a successful establishment of the model. The wound was washed with penicillin-saline, and the tissue was sutured layer by layer. Abdominal massage and extrusion were performed in the morning and afternoon on a daily basis to assist in urination of the rats. The 75 successful models were equally and randomly assigned to the following groups: SCI, Schwann cells without hTERT transfection, and hTERT transfection. Six hours after model establishment, 10 μL of culture medium was injected in rats of the SCI group. At the same time point, 10 μL of Schwann cell without hTERT transfection suspension (1 × 1010 /L) was slowly injected over 3 minutes at the site of injury (dura mater was retained) to the Schwann cells without hTERT transfection group. The microinjector was maintained in place for 5 minutes. The pinhole was blocked with a biological adhesive to prevent suspension overflow. The wound was sutured layer by layer. In the hTERT transfection group, 10 μL of hTERT-transfected Schwann cell suspension (1 × 1010 /L) was slowly injected, with the subsequent procedures the same as those of the Schwann cells without hTERT transfection group. Rats in each group were used for the terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) assay (n = 5), reverse transcription polymerase chain reaction (RT-PCR) (n = 5), hematoxylin and eosin staining (n = 5), chlormethyl-benzamidodialkylcarbocyanine (CM-DiI) count (n = 5), and detection of motor and electrophysiological function. The rats were not sacrificed. After scoring, they were returned to their respective groups. The remaining five rats were kept to prevent data loss.
TUNEL assay
Rats were anesthetized with chloral hydrate 72 hours after transplantation. The chest was then opened and aortic cannulation was conducted through the left ventricle, followed by fixation with 4% paraformaldehyde. Spinal cord tissue (2.0 cm) was collected by using the injury site as the center. The tissue was then fixed with paraformaldehyde. Paraffin sections were dewaxed. TUNEL-positive cells were quantified in the samples using the TUNEL assay kit (Roche, Shanghai, China). Cells with brown nuclei were deemed TUNEL positive. Under high power microscopy (× 200; Olympus), ten fields were selected from each section. TUNEL-positive cells in each field were quantified and the mean value was calculated.
RT-PCR
Seventy-two hours after transplantation, 50 mg of spinal cord tissue was obtained. In accordance with the manufacturer's instructions, total RNA was extracted from the spinal cord tissue with Trizol reagent (Gibco). The RNA content was determined by an ultraviolet spectrophotometer. The two-step RT-PCR (Yi et al., 2005) using the TaKaRa kit was performed according to the kit's instructions. mRNA was reverse transcribed into cDNA, which was then amplified by PCR. Primers are listed in Table 1. The amplified products underwent electrophoresis and the optical density of the bands was analyzed by Image-ProPlus 6.0 software (Media Cybernetics, Washington, DC, USA). The ratio of integrated optical density of aquaporin 4/9 (AQP4/9) or matrix metalloproteinase 9/2 (MMP9/2) to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was calculated as an index of AQP4/9 and MMP9/2 mRNA expression.
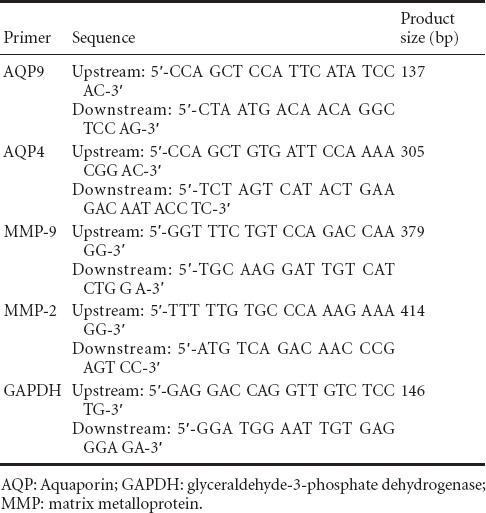
Table 1.
Primer sequences and product size
Evaluation of motor function
Motor function was assessed in rats from the three groups before surgery, and then 1 and 3 days and 1, 2, 3, and 4 weeks after transplantation. After scoring, the rats were not sacrificed but returned to their respective groups. The Basso, Beattie, and Bresnahan (BBB) scale (Barros Filho and Molina, 2008) and the inclined plane test (Wang et al., 2010; Liu et al., 2011) were carried out. The BBB scores ranged from 0 to 21, where 21 = normal and 0 = complete paralysis. The scores were based on the number and range of joint activities, the degree of loading, front and rear limb coordination, and the activities of the front and back claws and tail. In the inclined plane test, rats were placed horizontally on a smooth tiltboard with their heads to the front. The angle was increased every 5°, and the maximum angle at which the rats stayed on the board for 5 seconds was recorded. The evaluation began at 8:00 a.m. The score was obtained by two investigators, and the average value was calculated.
Electrophysiology
Four weeks after transplantation, a Keypoint 4 induced potential instrument (Beijing Weidi Kangtai Medical Instrument Co., Ltd., Beijing, China) was used to measure somatosensory-evoked potentials and motor-evoked potentials, based on a previous method (Chi et al., 2010). The rats were intraperitoneally anesthetized with 10% chloral hydrate and then laid on the horizontal plane. Hind limbs were stimulated with the stimulating electrode. A recording electrode was placed at the intersection of the coronal suture and sagittal suture healing line under the scalp (i.e., the hindlimb cortical sensory area). A reference electrode was placed 0.5 cm posterior to the hindlimb cortical sensory area. A direct current, square wave, and electrical pulses were given until the hind limb suffered from a slight tic. The conditions were as follows: current intensity: 5–15 mA; pulse width: 0.2 ms; frequency: 3 Hz; superimposed: 50–60 times. Neurophysiological recovery was monitored by recording the somatosensory-evoked potential latency and amplitude. Motor-evoked potentials were detected by the latencies and amplitudes. These were obtained after anesthesia by placing the stimulating electrodes 2 mm anterior to the coronal suture and 2 mm lateral to the sagittal suture under the scalp (i.e. the motor cortex). The electrodes were stimulated as follows: stimulus intensity: 40 mA; pulse width: 0.1 m; frequency: 1 Hz; superimposed: 300–500 times; scanning speed: 5 ms/D; sensitivity: 5 μV/D.
Hematoxylin and eosin staining and fluorescence microscopy
Four weeks after transplantation, five rats from each of the three groups were subjected to hematoxylin and eosin staining. The degree of injury was verified by a histological examination. Spinal cord tissue was fixed with 4% paraformaldehyde and sliced into frozen sections. Sections were stained with hematoxylin and eosin, and observed under fluorescence microscopy. Under high power microscopy (× 200), ten fields were selected from each section. The number of PKH26-positive cells in each field was quantified and the average value was calculated. The apoptotic rate was then calculated.
Statistical analysis
All data are expressed as the mean ± SD. The difference of intergroup data was compared using one-way analysis of variance followed by the least significant difference test. All data were analyzed using SPSS 15.0 software (SPSS, Chicago, IL, USA). A value of P < 0.05 was considered statistically significant.
Results
Identification of Schwann cells
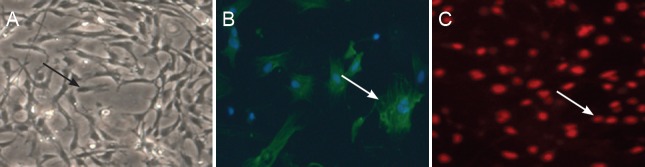
Inverted phase-contrast microscopy demonstrated a confluent layer of cells 1 week after culturing. Most of these cells were determined to be Schwann cells, while a few were found to be fibroblasts. After purification, more than 96% of the total number of cells were Schwann cells. These cells occupied a long, spindle, narrow shape, with small nuclei (Figure 1A). This shape was also confirmed under fluorescence microscopy (Figure 1B). Immunofluorescence for myelin basic protein revealed green fluorescence of cell bodies and processes of Schwann cells. Furthermore, DAPI immunofluorescence revealed blue-stained nuclei of Schwann cells and fibroblasts. The cytoplasm of the fibroblasts was not stained and was thus transparent. Therefore, the proportion of Schwann cells could be roughly estimated.
Figure 1.
Identification of Schwann cells 4 days after the primary culture.
(A) Inverted microscope image (40× magnification) of the morphology of primary cultured Schwann cells; cells are spindle in shape (arrow). Immunofluorescence staining showing (B) nestin-positive Schwann cells (arrow) where bodies and processes of Schwann cells are labelled green (40× magnification) and (C) PKH-26-labeled Schwann cells (stained red and indicated by arrow; 100× magnification).
hTERT gene transfection
Western immunoblots confirmed the expression of the hTERT protein in rats 48 hours after hTERT transfection. The expression of this protein was low in the EV group and in the group without hTERT transfection of Schwann cells. These findings confirmed that rats transfected with the hTERT gene into Schwann cells were indeed the hTERT-transfected group (Figure 2).
Figure 2.
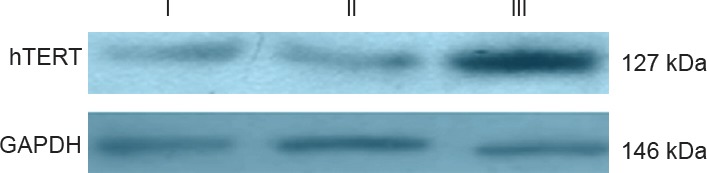
Protein expression of hTERT in Schwann cells 48 hours after transfection (western blot assay).
Protein expression for hTERT is noticeably higher in the hTERT-transfected group compared with the EV group and in the group without hTERT transfection of Schwann cells. hTERT: Human telomerase reverse transcriptase; GAPDH: glyceraldehyde phosphate dehydrogenase; EV: empty virus transfection; I: EV group; II: Schwann cells without hTERT transfection group; III: hTERT transfection group.
Effect of the transplantation of hTERT gene-transfected Schwann cells at the site of injury on cell proliferation
The proliferation of Schwann cells was significantly (P < 0.05) higher in the hTERT-transfected group compared with the Schwann cells without hTERT transfection group, which was markedly (P < 0.05) higher than the EV group (Figure 3).
Figure 3.
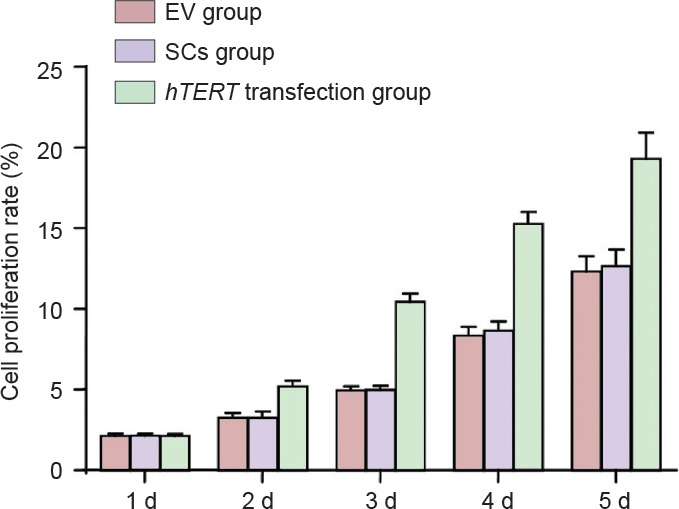
Effect of the transplantation of hTERT gene-transfected Schwann cells at the site of injury on cell proliferation.
Between 2 and 5 days from the time of culturing, the proliferation of Schwann cells was significantly (P < 0.05) higher in the hTERT-transfected group compared with the Schwann cells without hTERT transfection group, which was markedly (P < 0.05) higher than the SCI group (n = 6; paired comparison; one-way analysis of variance with the least significant difference test). hTERT: Human telomerase reverse transcriptase; EV: empty virus transfection; d: day(s); SCs group: Schwann cells without hTERT transfection.
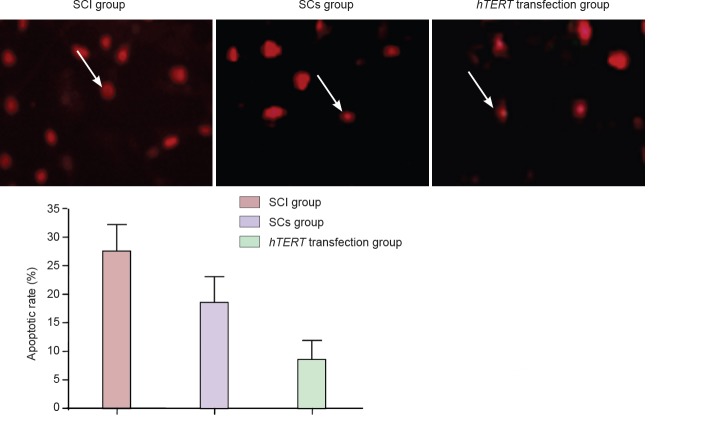
Effect of the transplantation of hTERT gene-transfected Schwann cells at the site of injury on cell apoptosis
Apoptotic cells were distributed all over the site of injury, including the edge of the injury site. The TUNEL assay revealed that the apoptotic rate was significantly (P < 0.05) less in both the Schwann cells without hTERT transfection group and the hTERT-transfected group, with the latter group exhibiting the lowest rate (P < 0.05; Figure 4).
Figure 4.
Effect of the transplantation of hTERT-transfected Schwann cells at the site of injury on cell apoptosis (TUNEL assay, fluorescence microscope).
Specific brown particles were seen in the nuclei of apoptotic nerve cells (arrow; 200× magnification). Compared with the SCI group, the apoptotic rate was significantly (P < 0.05) less in both the Schwann cells without hTERT transfection group and the hTER-transfected group, with the latter group exhibiting the lowest rate (P < 0.05) (n = 5; paired comparison; one-way analysis of variance with the least significant difference test). hTERT: Human telomerase reverse transcriptase; SCI: spinal cord injury; TUNEL: terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling; SCs group: Schwann cells without hTERT transfection.
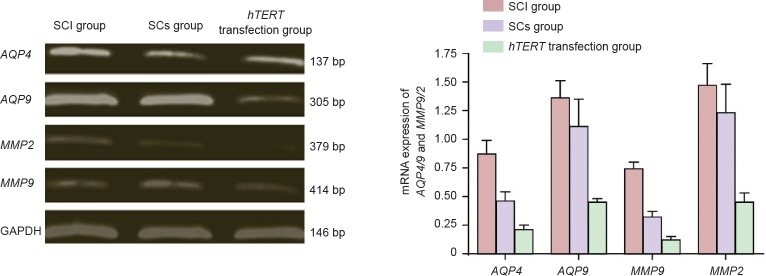
Effect of the transplantation of hTERT gene-transfected Schwann cells at the site of injury on mRNA expression of AQP4/9 and MMP9/2
AQP4/9 and MMP9/2 mRNA expression was markedly (P < 0.05) higher in the Schwann cells without hTERT transfection group compared with the hTERT-transfected group 72 hours after injury. The expression of these two genes was significantly (P < 0.05) lower in the non-transfected group compared with the SCI group (Figure 5).
Figure 5.
Effect of the transplantation of hTERT-transfected Schwann cells at the site of injury on mRNA expression of AQP4/9 and MMP9/2.
AQP4/9 and MMP9/2 mRNA expression was lower in the SCs group compared with the SCI group. AQP4/9 and MMP9/2 mRNA expression (/GAPDH optical density) is the lowest in the hTERT-transfected group followed by the SCs group, and the highest in the SCI group (n = 6; one-way analysis of variance followed by the least significant difference test; P < 0.05). AQP: Aquaporin; GAPDH: glyceraldehyde phosphate dehydrogenase; hTERT: human telomerase reverse transcriptase; MMP: matrix metalloproteinases; SCI: spinal cord injury; SCs group: Schwann cells without hTERT transfection group.
Motor function
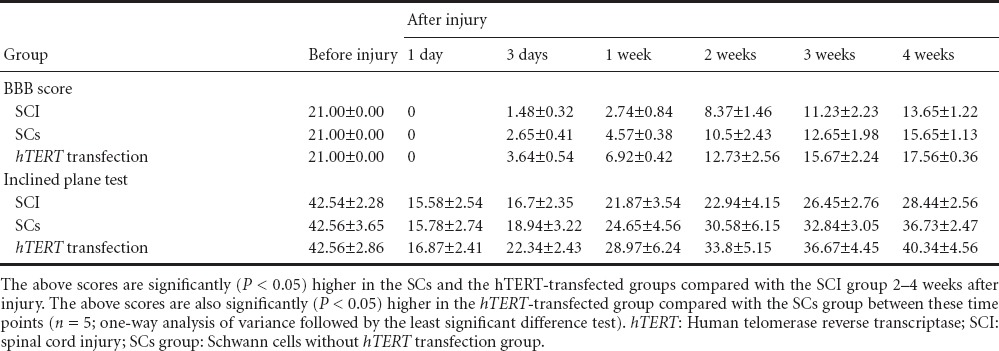
No significant difference in motor function was detected in all rats before injury. Two to four weeks after injury, motor function significantly (P < 0.05) improved in the Schwann cells without hTERT transfection group and in the hTERT-transfected group compared with the SCI group (Table 2).
Table 2.
Effect of the transplantation of hTERT-transfected Schwann cells in SCI rats on motor function
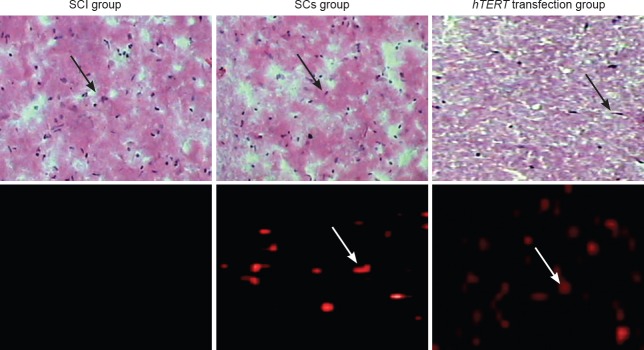
Effect of the transplantation of hTERT gene-transfected Schwann cells on the number of nerve cells
Four weeks after injury, hematoxylin and eosin staining results revealed that the spinal cord tissue was disrupted; i.e. scarring, a clear cavity, and structural disorder were visible at the injury site (Figure 6). These typical morphological changes to nerve cells were seen in the transplanted region of Schwann cells without hTERT transfection group. The cavity was smaller in this group compared with the SCI group, but larger compared with the hTERT-transfected group. Typical nerve cell-like morphological changes were visible, and the cavity disappeared in the hTERT-transfected group. Scattered distribution of PKH26 (red fluorescence) was visible both in the group without Schwann cell hTERT transfection and in the hTERT-transfected group. Significant (P < 0.01) differences were found between the groups under high power: SCI group: 0; Schwann cells without hTERT transfection group: 18.64 ± 4.57; hTERT-transfected group: 29.86 ± 7.26.
Figure 6.
Morphology of nerve cells (arrows) in the rat spinal cord after transplantation with hTERT-transfected Schwann cells.
Hematoxylin and eosin staining reveals spinal cord loss and cavitation 4 weeks after injury. The cavities are small in the SCs group, and no cavities are found in the hTERT-transfected group (upper panels; 40× magnification). Immunofluorescence staining shows that the number of PKH-26-positive cells is the highest in the hTERT-transfected group followed by the SCs, and the lowest in the SCI group (lower panels; 200× magnification). hTERT: Human telomerase reverse transcriptase; SCI: spinal cord injury. SCs group: Schwann cells without hTERT transfection group.
Changes in electrophysiological indices after transplantation
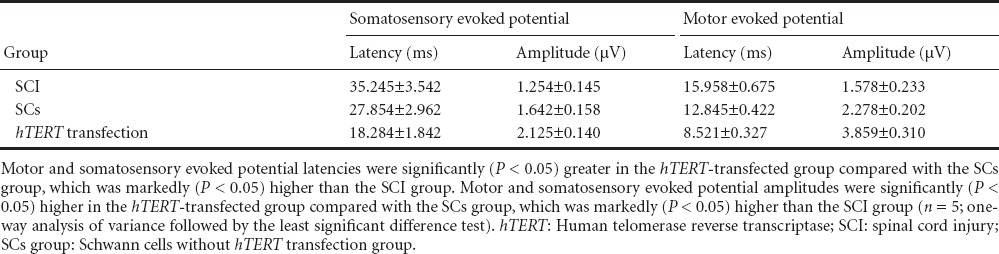
After injury, electrophysiological recordings revealed that evoked potential waveforms completely disappeared in all rats. Four weeks after transplantation, motor and sensory evoked potential latencies were significantly (P < 0.05) greater in the hTERT-transfected group compared with the Schwann cells without hTERT transfection group, which was markedly (P < 0.05) higher than the SCI group. Motor and sensory evoked potential amplitudes were significantly (P < 0.05) higher in the hTERT-transfected group compared with the Schwann cells without hTERT transfection group, which was markedly (P < 0.05) higher than the SCI group (Table 3).
Table 3.
Effects of hTERT-transfected Schwann cell transplantation on electrophysiological function in SCI rats
Discussion
This study explored the effects of the transplantation of hTERT gene-transfected Schwann cells on electrophysiological changes in SCI rats, the results of which were consistent with previous studies (Jin et al., 2013; Wu et al., 2014). Our results showed that gene products were detectable in Schwann cells—mainly in the nuclei—48 hours after transfection. Western immunoblots revealed that rat Schwann cells transfected with the hTERTT gene expressed hTERT. Seventy-two hours after model establishment, results from RT-PCR showed that gene expression of AQP4/9 and MMP9/2 reduced in the hTERT-transfected group compared the SCI group. Lower limb motor function improved in the hTERT-transfected group compared with the group without the transplantation of hTERT-transfected Schwann cells, which was more improved than the SCI group. Hematoxylin and eosin staining in the SCI group revealed spinal cord disruption; the formation of cavities and loss of axons. A few axon-like structures and a small cavity were observed at the injury site in the Schwann cells without hTERT transfection group. In the hTERT-transfected group, numerous axon-like structures were seen, but no cavity was found. The TUNEL assay revealed fewer apoptotic cells in the hTERT-transfected group, suggesting that the transplantation of hTERT gene-transfected Schwann cells may diminish apoptosis of nerve cells at the injury site. The number of PKH-26-positive cells was highest in the hTERT-transfected group followed by the Schwann cells without hTERT transfection group, and was the lowest in the SCI group. Sensory and motor evoked potential latencies were as follows (from lowest to highest): hTERT transfected group < the Schwann cells without hTERT transfection group < the SCI group. Motor and sensory evoked potential amplitudes were as follows (from highest to lowest): hTERT transfection group > the Schwann cells without hTERT transfection group > the SCI group.
In conclusion, compared with the transplantation of Schwann cells without hTERT transfection in SCI rats, the gene and protein expression of AQP4/9 and MMP9/2 was lower, fewer apoptotic cells and improved tissue repair were observed, and the number of PKH-26-positive cells at the injury site was increased in the hTERT-transfected group. There was also an improvement in the recovery of lower limb motor function. Our results suggest that transplantation of hTERT gene-transfected Schwann cells promotes the protein expression of hTERT in these cells. Compared with the transplantation of Schwann cells without hTERT transfection in SCI rats, the transplantation of hTERT gene-transfected Schwann cells provided superior results whether it was histological or functional. Therefore, this treatment may be a novel strategy for optimizing stem cell transplantation in the treatment of nervous system injury.
Acknowledgments:
We are very grateful to Dr. Bao-bin Liu from General Hospital of Tianjin Medical University of China for presenting hTERT PLXSN expression vector.
Footnotes
Funding: This study was supported by a grant from the Science and Technology Development Plan Program of Jilin Province of China, No. 2011084.
Conflicts of interest: None declared.
Plagiarism check: This paper was screened twice using Cross-Check to verify originality before publication.
Peer review: This paper was double-blinded and stringently reviewed by international expert reviewers.
Copyedited by Mark F, Raye W, Wang J, Qiu Y, Li CH, Song LP, Zhao M
References
- 1.Allodi I, Mecollari V, Eggers R. Schwann cells transduced with a lentiviral vector encoding Fgf-2 promote motor neuron regeneration following sciatic nerve injury. Glia. 2014;62:1736–1746. doi: 10.1002/glia.22712. [DOI] [PubMed] [Google Scholar]
- 2.Ban DX, Kong XH, Feng SQ. Intraspinal cord graft of autologous activated Schwann cells efficiently promotes axonal regeneration and functional recovery after rat's spinal cord injury. Brain Res. 2009;1256:149–161. doi: 10.1016/j.brainres.2008.11.098. [DOI] [PubMed] [Google Scholar]
- 3.Barros Filho TE, Molina AE. Analysis of the sensitivity and reproducibility of the Basso, Beattie, Bresnahan (BBB) scale in Wistar rats. Clinics (Sao Paulo) 2008;63:103–108. doi: 10.1590/s1807-59322008000100018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen G, Wan H, Yang F. Observation of spinal cord injury after intramedullary PLGA scaffold cells transplanted tissue. Zhonghua Shenjing Waike Zazhi. 2011;25:83–86. [Google Scholar]
- 5.Chen HH, Yang PS, Zhang JX. Pleural effusion cell lung cancer shedding flow cytometry and preliminary clinical application. Guoji Jianyan Yixue Zazhi. 2015;38:106–110. [Google Scholar]
- 6.Chen L, Huang H, Xi H. A prospective randomized double-blind clinical trial using a combination of olfactory ensheathing cells and Schwann cells for the treatment of chronic complete spinal cord injuries. Cell Transplant. 2014;23:35–44. doi: 10.3727/096368914X685014. [DOI] [PubMed] [Google Scholar]
- 7.Chi GF, Kim MR, Kim DW. Schwann cells differentiated from spheroid-forming cells of rat subcutaneous fat tissue myelinate axons in the spinal cord injury. Exp Neurol. 2010;222:304–317. doi: 10.1016/j.expneurol.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 8.Deng LX, Walker C, Xu XM. Schwann cell transplantation and descending propriospinal regeneration after spinal cord injury. Brain Res. 2014;26:1260–1268. doi: 10.1016/j.brainres.2014.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hakim JS, Esmaeili Rad M, Grahn PJ. Positively charged oligo [poly (ethylene glycol) fumarate] scaffold implantation results in a permissive lesion environment after spinal cord injury in rat. Tissue Eng Part A 2015. 2015:17. doi: 10.1089/ten.tea.2015.0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jin B, Wang W, Liu ZY. exogenous hTERT gene transfection on the aged rats liver ischemia-reperfusion injury protective effect. Shandong Daxue Xuebao. 2013;51:13–16. [Google Scholar]
- 11.Kang SK, Putnam L, Dufour J. Expression of telomerase extends the lifespan and enhances osteogenic differentiation of adipose tissue derived stromal cells. Stem Cells. 2004;22:l356–1372. doi: 10.1634/stemcells.2004-0023. [DOI] [PubMed] [Google Scholar]
- 12.Liu LL, Deng WM. Biological characteristics of Schwann cells of human telomerase reverse transcriptase transfected rat. Zhongguo Zuzhi Gongcheng Yanjiu. 2015;14:2250–2254. [Google Scholar]
- 13.Liu YY, Chun QX, Zhang DZ. hTERT antisense oligodeoxynucleotide on chordoma cell cycle and proliferation. Modern Oncol. 2011;19:1922–1924. [Google Scholar]
- 14.Marcol W, lusarczyk W, Larysz-Brysz M. Grafted activated schwann cells support survival of injured rat spinal cord white matter. World Neurosurg. 2015;21:1878–1887. doi: 10.1016/j.wneu.2015.04.027. [DOI] [PubMed] [Google Scholar]
- 15.Peterson SL, Nguyen HX, Mendez OA. Complement protein C1q modulates neurite outgrowth in vitro and spinal cord axon regeneration in vivo. J Neurosci. 2015;35:4332–4349. doi: 10.1523/JNEUROSCI.4473-12.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Siddiqui AM, Khazaei M, Fehlings MG. Translating mechanisms of neuroprotection, regeneration, and repair to treatment of spinal cord injury. Prog Brain Res. 2015;218:45–54. doi: 10.1016/bs.pbr.2014.12.007. [DOI] [PubMed] [Google Scholar]
- 17.Sparling JS, Bretzner F, Biernaskie J. Schwann cells generated from neonatal skin-derived precursors or neonatal peripheral nerve improve functional recovery after acute transplantation into the partially injured cervical spinal cord of the rat. J Neurosci. 2015;35:6714–6730. doi: 10.1523/JNEUROSCI.1070-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thumm M, Simons M. Myelinophagy: schwann cells dinein. J Cell Biol. 2015;210:9–10. doi: 10.1083/jcb.201506039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vasudeva VS, Abd-El-Barr MM, Chi JH. implantation of neonatal skin-derived precursor schwann cells improves outcomes after incomplete cervical spinal cord injury in rats. Neurosurgery. 2015;77:15–17. doi: 10.1227/01.neu.0000467294.10763.69. [DOI] [PubMed] [Google Scholar]
- 20.Wakao S, Matsuse D, Dezawa M. Mesenchymal stem cells as a source of Schwann cells: their anticipated use in peripheral nerve regeneration. Cells Tissues Organs. 2015;200:31–41. doi: 10.1159/000368188. [DOI] [PubMed] [Google Scholar]
- 21.Wang D, Zhang JJ. Effects of hypothermia combined with neural stem cell transplantation on recovery of neurological function in rats with spinal cord injury. Mol Med Rep. 2015;11:1759–1766. doi: 10.3892/mmr.2014.2905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang D, Fan YD, Zhang JJ. Transplantation of Nogo-66 receptor gene-silenced cells of in a poly (D L-lactic-co-glycolic acid) scaffold for the treatment of spinal cord injury. Neural Regen Res. 2013;8:677–685. doi: 10.3969/j.issn.1673-5374.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang QY, Liu WG, Wang ZY. FTY720 On RhoA expression in rats after acute spinal cord injury in rats. Zhongguo Guke Linchuang yu Jichu Yanjiu Zazhi. 2010;4:292–296. [Google Scholar]
- 24.Weaver FM, Burns SP, Evans CT. Provider perspectives on soldiers with new spinal cord injuries returning from iraq and afghanistan. Arch Phys Med Rehabil. 2009;90:517–521. doi: 10.1016/j.apmr.2008.09.560. [DOI] [PubMed] [Google Scholar]
- 25.Wu SH, Huang SH, Cheng KI. Third-degree hindpaw burn injury induced apoptosis of lumbar spinal cord ventral horn motor neurons and sciatic nerve and muscle atrophy in rats. Biomed Res Int 2015. 2015:372819. doi: 10.1155/2015/372819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu SH, Huang SH, Lo YC. Autologous adipose-derived stem cells attenuate muscular atrophy and protect spinal cord ventral horn motor neurons in an animal model of burn injury. Cytotherapy. 2015;17:1066–1075. doi: 10.1016/j.jcyt.2015.03.687. [DOI] [PubMed] [Google Scholar]
- 27.Xie QS, Xu XL, Wei XJ. spinal cord tissue engineering scaffolds: a polylactic glycolic acid optimum pore size filter water. Zhongguo Zuzhi Gongcheng Yanjiu. 2010;14:393–396. [Google Scholar]
- 28.Xue F, Wu EJ, Zhang PX. Biodegradable chitin conduit tubulation combined with bone marrow mesenchymal stem cell transplantation for treatment of spinal cord injury by reducing glial scar and cavity formation. Neural Regen Res. 2015;10:104–111. doi: 10.4103/1673-5374.150715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yi CL, Chen AM, Bai XJ, Song XZ, Xu WG. The effect of bcl-xL gene transfection in vivo on the expression of Caspase-3 and neuroprotective function following spinal cord injury. Zhonghua Shiyan Waike Zazhi. 2005;22:984–986. [Google Scholar]
- 30.Zeng X, Qiu XC, Ma YH. Integration of donor mesenchymal stem cell-derived neuron-like cells into host neural network after rat spinal cord transection. Biomaterials. 2015;53:184–201. doi: 10.1016/j.biomaterials.2015.02.073. [DOI] [PubMed] [Google Scholar]
- 31.Zhang SD, Yu HS, Li JH. polylactic acid stent graft evoked potentials of spinal cord hemisection hind legs and exercise in rats. Zhongguo Zuzhong Zazhi. 2010;5:286–290. [Google Scholar]
- 32.Zhang SQ, Wu MF, Piao Z. Edaravone combined with Schwann cell transplantation may repair spinal cord injury in rats. Neural Regen Res. 2015;10:230–236. doi: 10.4103/1673-5374.152376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhi WL, Li SB, Cheng GW. hTERT gene transfection on human neuronal viability. Gannan Yixueyuan Xuebao. 2014;34:170–173. [Google Scholar]