Abstract
Neural electrodes, the core component of neural prostheses, are usually encapsulated in polydimethylsiloxane (PDMS). However, PDMS can generate a tissue response after implantation. Based on the physicochemical properties and excellent biocompatibility of polyurethane (PU) and poly(vinyl alcohol) (PVA) when used as coating materials, we synthesized PU/PVA hydrogel coatings and coated the surface of PDMS using plasma treatment, and the cytocompatibility to rat pheochromocytoma (PC12) cells was assessed. Protein adsorption tests indicated that the amount of protein adsorption onto the PDMS substrate was reduced by 92% after coating with the hydrogel. Moreover, the PC12 cells on the PU/PVA-coated PDMS showed higher cell density and longer and more numerous neurites than those on the uncoated PDMS. These results indicate that the PU/PVA hydrogel is cytocompatible and a promising coating material for neural electrodes to improve their biocompatibility.
Keywords: nerve regeneration, cerebral injury, neural electrodes, hydrogel coatings, polyurethane, polydimethylsiloxane, poly(vinyl alcohol), cytocompatibility, protein adsorption, nerve growth factor, rat pheochromocytoma cells, synaptic differentiation, neural regeneration
Introduction
Neural electrical stimulation has been used over the last few decades as an alternative treatment to help patients suffering from neurological disorders such as stroke, paralysis, blindness, deafness, Parkinson's disease, and epilepsy (Sloviter et al., 1987; Makeig et al., 2002; Rauschecker et al., 2002; Hochberg et al., 2006; Chan CKL et al., 2008; Denheyer et al., 2009). Neural prostheses and modulators are used to recover or replace damaged neurological functions by stimulating target neurons (Talwar et al., 2002; Jackson et al., 2006). The implantable neural electrodes are a crucial component and play a key role in recording and stimulating the nerve tissue (Cogan et al., 2008). However, one major obstacle for the long-term use of neural electrodes is their unstable performance caused by tissue responses after clinical implantation (Zhong et al., 2001; Polikov et al., 2005). These tissue reactions not only lead to a large increase in the impedance between the electrode materials and neural tissue, but also decrease the number of neurons surrounding the electrodes. Over time, the effect of the electrical stimulation is weakened and can even disappear (Biran et al., 2005; He et al., 2007). Because certain biomaterials with excellent biocompatibility show decreased tissue responses, there is an urgent need to improve the biocompatibility of implantable electrode materials.
Polydimethylsiloxane (PDMS) has been used extensively as an insulation material for implantable neural electrodes because of its good biocompatibility (Erlich et al., 2003; Stieglitz et al., 2010). However, PDMS induces strong protein adsorption because it is hydrophobic (Anderson et al., 1995), which results in both acute and chronic tissue responses after implantation, leading to a noticeable increase in the electrode-tissue interface impedance caused by tissue encapsulation (Anderson et al., 2008). While several bioactive coating materials have previously been investigated to determine whether they improve neural cell ingrowth and reduce the interactions between the electrode and neural tissue (Zhong et al., 2001; He et al., 2007), most of the research was focused on improving the silicon substrate-tissue interface and few studies reported improvements in the PDMS substrate-tissue interface.
Hydrogels, on the other hand, have been extensively used in the biomedical and pharmaceutical fields because of their low interfacial tension and large capacity for water uptake in the biological environment (Hamidi et al., 2008). For these reasons, considerable effort has been devoted to improving the hydrophobicity of PDMS by coating with hydrophilic hydrogels. Han et al. (2011) demonstrated that composite coatings mated with hydrogel-electrospun fibers promote the integration of neuron-prostheses and the formation of a stable long-term interface. Winter et al (2007) reported that coating neural electrodes with a neurotrophin-eluting hydrogel improved neurite extension on the electrode surface and decreased the threshold for electrical stimulation.
In our previous work, we synthesized poly(vinyl alcohol)/poly(acrylic acid) (PVA/PAA) and polyethylene glycol-containing polyurethane hydrogels as coatings for PDMS-based neural electrodes. The results demonstrated the ability of those hydrogel coatings to improve the electrode-tissue interface in vitro and in vivo (Lu et al., 2009; Rao et al., 2012). Another of our previous studies demonstrated that hydrophilicity is an important material property when modifying neural electrodes (Zhou et al., 2012). PVA is a type of poly hydroxyl polymer that is broadly used as a bioactive material in tissue engineering. It has appropriate physicochemical properties for use as a coating such as hydrophilicity and ease of film-formation, as well as good biocompatibility. To develop a better coating material for neural electrodes based on the biocompatible polyurethane (PU) hydrogel coating of PDMS substrates, we introduced PVA and synthesized PU/PVA hydrogel as a coating for PDMS.
The aim of this study was to synthesize PU/PVA hydrogel and determine its effect on the cytocompatibility of PDMS. The adsorption of nonspecific proteins and cytocompatibility to pheochromocytoma (PC12) cells, including cell attachment and differentiation, on PDMS and PU/PVA-coated PDMS were compared. The PU hydrogel coatings were also assessed as a comparison.
Materials and Methods
Preparation of PU/PVA
Before use, polyethylene glycol (CP, molecular weight = 1,000) (Sinopharm, Shanghai, China) was dried at 80°C for 1 day, and the N,N-dimethylformamide (Sinopharm) was dehydrated. As shown in Figure 1, the synthesis of PU/PVA included two steps: first, the prepolymers of PU were synthesized. Next, the PU prepolymers were mixed into a PVA (CP, molecular weight = 80,000, Bodi Chemical, Tianjin, China) solution, and then a cross-linking reaction was initiated. The PU prepolymers were synthesized as previously described (Rao et al., 2012). In brief, the N,N-dimethylformamide, polyethylene glycol, and isophorone diisocyanate (Sigma, St. Louis, MO, USA) were mixed in a three-necked flask, and dibutyltin dilaurate (Sinopharm) was added as a catalyst. Prior to initiating the reaction, the oxygen in the mixture was replaced with argon. Then, the solution was slowly heated to 70°C and placed in an argon atmosphere to react for 12 hours. For the cross-linking reaction, the PVA was dehydrated in a vacuum-drying oven for 8 hours, and then dissolved in dimethyl sulfoxide (AR, Sinopharm) at 100°C for 30 minutes. After the PVA solution in the three-necked flask was cooled to room temperature, a certain amount of N,N-dimethylformamide was added, and then the fixed solution was cooled to 0°C. Next, the PU prepolymers were slowly added into the mixture with intense stirring and purging with argon. Finally, the reaction conditions were maintained for 2 days. In the above mixture, the volume ratio of dimethyl sulfoxide and N,N-dimethylformamide was 1:1, and the molar ratio of PVA and PU prepolymers was kept at n(–NCO): m(–OH) of 1:12.5. In this study, the PU as the comparison was synthesized using a chain-extending reaction based on the methods from a previous study (Rao et al., 2012).
Figure 1.

Schematic diagram of the polyurethane/poly(vinyl alcohol) (PU/PVA) synthesis.
(a) Polyurethane (PU) prepolymers were formed using the polymerization reaction between polyethylene glycol and isophorone diisocyanate. (b) PU/PVA formation by cross-linking reactions between PU prepolymers and poly(vinyl alcohol) (PVA).
Fabrication of samples
Platinum silicone elastomer (medical-grade, MDX4-4210, Factor II, Dow Corning Corporation, Midland, MI, USA) and its cross-linking catalyst were degassed under a vacuum after being completely mixed. The mixture was placed in a stainless steel mold and heated to 80°C for 2 hours to create PDMS films. After cooling them to room temperature, the PDMS films were removed from the mold and cut into small round pieces approximately 0.2 mm thick and 14 mm in diameter.
The PDMS films were ultrasonically cleaned with deionized water and acetone and dried under a vacuum at 40°C for 12 hours. The films were water plasma treated with a CTP-2000k plasma generator (Corona Lab, China) for 2 minutes at 60 W. Next, a 50-µL drop of 1 wt. % polymer solution (PU/PVA or PU) was placed onto the plasma-treated films and spread equally over the entire surface to create the PU/PVA- and PU-coated PDMS films. The coated PDMS films were dried at 80°C for 12 hours under a vacuum. Finally, all of the samples, including the PU/PVA- and PU-coated PDMS films and the control PDMS films, were washed three times with sterile water and placed in 24-well tissue culture plates (TCPs) after drying at 40°C for 12 hours. All of the prepared samples were sterilized with ethylene oxide before use.
Characterization
Fourier transform-infrared spectroscopy (FT-IR) measurements
PVA, PU/PVA, and PU solutions were coated on potassium bromide plates and dried with an infrared lamp. The FT-IR spectra of these materials were measured with an FT-IR spectrometer (Thermo Nicolet Avatar 360) over the range of 4,000–400 cm−1.
Protein adsorption
As previously described (Chen et al., 2004, 2005a, b), fibrinogen (Calbiochem, San Diego, CA, USA) was labeled with 125I (ICN Pharmaceuticals, Irvine, CA, USA) using the iodogen method to determine the amount of nonspecific protein adsorption. For the assessment of fibrinogen adsorption, labeled and unlabeled fibrinogens were mixed at a ratio of 1:19 and the total fibrinogen concentration of the solution was 1 mg/mL. Before the adsorption experiments, the surfaces of the samples were equilibrated in Tris-buffered saline overnight. Samples (PU/PVA-coated PDMS, PU-coated PDMS, and PDMS) were incubated in the 125I-labeled protein solution at room temperature for 3 hours, and then rinsed three times with Tris-buffered saline for 10 minutes each, blotted with filter paper, and moved to stock tubes. The tubes were placed in a gamma counter (1480 Perkin Elmer) for radioactivity testing. Each sample group was assayed in triplicate. The amount of radioactivity was converted to calculate the amount of adsorbed protein.
Cell culture
Cell attachment
PC12 cells (China Center for Type Culture Collection, Wuhan, China) are widely used as a neural cell model for determining neuronal viability and neurite extension. Here, cell culture was performed for the PU/PVA-coated PDMS, PU-coated PDMS, and PDMS groups, with a TCP group as a control. The different samples were affixed to the bottom of 24-well tissue culture plates and coated with 0.1 mg/mL poly L-lysine (Sigma) at 37°C for 6 hours to promote PC12 cell attachment. The PC12 cells were cultured in RPMI 1640 culture medium containing 5% fetal bovine serum, 2 mM L-glutamine, 10% horse serum, 100 µg/mL streptomycin, and 100 units/mL of penicillin. The cultures were maintained in a 95% humidified incubator with 5% CO2 at 37°C. The PC12 cells were plated at a density of 2 × 104 cells per well in the 24-well plates. DAPI staining was performed as previously described (Webb et al., 2001; Lopez et al., 2006). Digital images of the stained cells were taken on a Ti/S inverted fluorescence microscope equipped with a DS-5MC-U2 digital camera (Nikon, Japan). At least five randomly selected fields of each well were imaged at 100× magnification. The cell attachment was quantified as the cell number per unit area using Image-Pro Plus® 6.0 software (Media Cybernetics, Silver Spring, MD, USA). The experiments were performed in triplicate.
Cell differentiation
After 24 hours of culture, the cells were serum starved for 6 hours. The culture medium was replaced with low-serum medium containing 50 ng/mL nerve growth factor (Sigma). Half of the culture medium was replaced every 2 days. After culture in the presence of nerve growth factor for 7 days, digital images of the PC12 cells were taken with an inverted phase-contrast microscope (Nikon). At least five randomly selected fields were imaged from each well at 200× magnification. To assess the cell differentiation, 50 cells from each well were randomly selected and the average number and length of their neurites were calculated. The cellular differentiation was quantified as the number and length of neurites per cell using IPP 6.0 software (Media Cybernetics).
The cells were stained with calcein to visualize the neurite outgrowths from the PC12 cells on the different samples, as was done in a previous study (Hashimoto et al., 2001). Briefly, the culture medium was removed and the samples were washed with 0.1 M PBS. The cells were then cultured in RPMI 1640 medium with 5 mM calcein AM (Sigma) at 37°C for 30 minutes in a CO2 atmosphere. The samples were washed with 0.1 M PBS after calcein loading. Fluorescence images were taken with a Ti/S inverted fluorescence microscope (Nikon) using a 520-nm emission filter and 490-nm excitation filter. The cell culture experiments were conducted in triplicate. Each sample was assayed for cell attachment and differentiation in 16 independent wells of the 24-well plates (n = 16).
Statistical analysis
The statistical analysis was performed using SPSS 20.0 software (IBM Corp, Armonk, NY, USA). The measurement data were normally distributed and are expressed as the mean ± SEM. Differences between the PU-coated PDMS, PU/PVA-coated PDMS, PDMS, and TCP films were analyzed by one-way analysis of variance followed by Student-Newman-Keuls post hoc analysis. P-values less than 0.05 were considered statistically significant.
Results
FT-IR spectra
The FT-IR spectra of PVA, PU/PVA, and PU are shown in Figure 2. The main characteristic spectrum bands for the pure PVA films were: the O-H stretching vibration at 3,300 cm−1, the C-H stretching vibration at 2,941 and 2,910 cm−1, the C-H deformation vibration at 1,421 and 1,328 cm−1, the C-O stretching vibration at 1,241 cm−1, the C-O stretching vibration at 1,090 cm−1, and the O-H bend at 917 cm−1. The main characteristic FT-IR spectrum bands for the pure PU films were: the C-O-C stretching vibration at 1,106 cm−1, C-O and C-N stretching vibrations at 1,252 cm−1, C-H bending vibration of O-CH2 at 1,452 cm−1, N-H stretching vibration of carbamate at 1,541 cm−1, C=O stretching vibration of carbamate at 1,712 cm−1, C-H stretching vibration of O-CH2 at 2,872 cm−1, and N-H stretching vibration at 3,330 cm−1.
Figure 2.

Fourier transform-infrared spectra of the poly(vinyl alcohol) (PVA), polyurethane/poly(vinyl alcohol) (PU/PVA), and polyurethane (PU) films.
In the PU/PVA spectrum, the -OH absorption peak at 3,330 cm−1 was clearly decreased compared with that in the PVA spectrum, while the absorption peaks of C-O-C at 1,106 cm−1 and N-H from carbamates at 1,541 cm−1 were increased.
The -OH absorption peak at 3,330 cm−1 was clearly decreased in the PU/PVA spectrum compared with that in the PVA spectrum, while the absorption peaks of C-O-C at 1,106 cm−1 and N-H from carbamates at 1,541 cm−1 were stronger, indicating that the -OH of the PVA reacted with the -N=C=O of the PU. The FT-IR characterization of the sample spectra indicated that the PU/PVA hydrogel was successfully created.
Protein adsorption
Fibrinogen has been widely used as a model for the nonspecific adsorption of proteins on implants (Chen et al., 2004; Chen et al., 2005; Rao et al., 2012). The amounts of fibrinogen adsorbed onto the surface of the PDMS, PU-coated PDMS, and PU/PVA-coated PDMS films are shown in Figure 3.
Figure 3.

Fibrinogen adsorption on polydimethylsiloxane (PDMS), polyurethane/poly (vinyl alcohol) (PU/PVA)-coated PDMS, and polyurethane (PU)-coated PDMS films.
Data are expressed as the mean ± SEM of n = 3 wells, and comparisons were made with one-way analysis of variance followed by Student-Newman-Keuls post hoc analysis. The amount of nonspecific protein adsorption was 442.67 ng/cm2 on the surface of the PDMS, while fibrinogen adsorption was decreased to 36.67 ng/cm2 on the PU/ PVA-coated PDMS surfaces (a reduction of ~92 %) and to 32 ng/cm2 on the PU-coated PDMS surfaces. PU/PVA: PU/PVA-coated PDMS group; PU: PU-coated PDMS group.
The amount of nonspecific protein adsorption on the uncoated PDMS surface was 442.67 ng/cm2, indicating that a significant amount of fibrinogen had assembled and formed a protein layer. In contrast, the fibrinogen adsorption was only 36.67 ng/cm2 on the PU/PVA-coated surfaces (a reduction of ~92 %), and 32 ng/cm2 on the PU-coated surfaces.
PC12 cell attachment
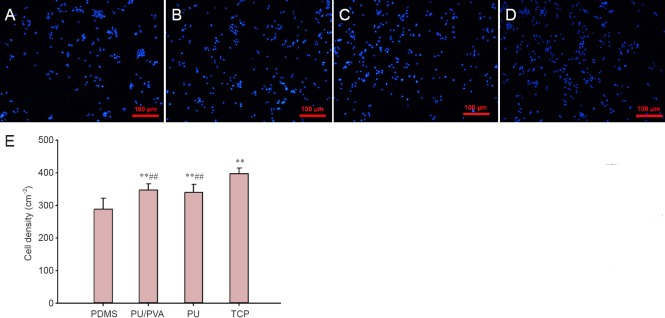
As shown in Figure 4, the cells on the PDMS films were more aggregated and showed a lower density per area than those on the PU-PU/PVA-coated PDMS films. The PC12 cells on the PU- and PU/PVA-coated PDMS displayed a similar degree of dispersion and cell density. As expected, the smallest number of PC12 cells attached to the TCP. The quantitative comparisons of cell density on the different samples are shown in Figure 4E, which mirror the qualitative results described above.
Figure 4.
Representative 4′6-diamidino-2-phenylindole (DAPI)-stained fluorescent images of the PC12 cells cultured on different materials.
PC12 cells were cultured on polydimethylsiloxane (PDMS) films (A), polyurethane (PU)-coated PDMS films (B), polyurethane/poly(vinyl alcohol) (PU/PVA)-coated PDMS films (C), and tissue culture plates (TCP) (D), as a control. Scale bars: 100 μm. The PC12 cells on the PDMS films were more aggregated and at a lower density per area unit compared with those on the PU- and PU/PVA-coated PDMS films. (E) Quantitative comparison of the cell density on different materials. **P < 0.01, vs. PDMS, ##P < 0.01, vs. TCP (mean ± SEM, n = 16 wells, one-way analysis of variance followed by Student-Newman-Keuls post hoc analysis). The experiment was performed in triplicate. PU/PVA: PU/ PVA-coated PDMS films; PU: PU-coated PDMS films.
The cell densities on the PU- and PU/PVA-coated films were significantly higher than that on the PDMS film (P < 0.01), but were not different to each other. These results demonstrated that the PU/PVA films had a better affinity for the PC12 cells than the PDMS alone, and that the PU/PVA and PU had similar affinities for the PC12 cells. Thus, the hydrophilic surfaces improved PC12 cell viability and neurite outgrowth. The introduction of PVA, a type of hydrophilic poly hydroxyl polymer, into the PU polymers resulted in a more hydrophilic surface that had more affinity to the PC12 cells than the hydrophobic PDMS surface.
PC12 cell differentiation
Neurite outgrowth from PC12 cells can be induced in the presence of nerve growth factor, and cytocompatible biomaterials promote neurite outgrowth in PC12 cells (Rao et al., 2012). The differentiation of PC12 cells on the different materials is shown in Figure 5A–D. The cells on the PDMS films aggregated together and extended fewer and shorter neurites than those on the other films. In contrast, the PC12 cells exhibited better neurite extension and formed an interconnected neurite network when cultured on the surface of the PU, PU/PVA, and TCP materials on day 7. The quantitative results indicated that the neurite number per cell (Figure 5E) and neurite length per cell (Figure 5F) for cells on the PU/PVA were significantly higher and longer than those on the PDMS (P < 0.01). The PC12 cells on the PU/PVA and PU showed more and longer neurites than those on the TCP.
Figure 5.
Representative calcein AM-stained fluorescent images of the PC12 cells cultured on different materials.
The PC12 cells were cultured on polydimethylsiloxane (PDMS) films (A), polyurethane (PU)-coated PDMS films (B), polyurethane/poly(vinyl alcohol) (PU/PVA)-coated PDMS films (C), and tissue culture plates (TCP) (D) in the presence of 50 ng/mL nerve growth factor for 7 days. Scale bars: 50 μm The PC12 cells exhibited better neurite extension and formed an interconnected neurite network on the surface of the PU/PVA-coated PDMS films, but not on the uncoated PDMS films. Quantitative comparisons of the neurite number per cell (E) and neurite length per cell (F) on the different samples. *P < 0.05 and **P < 0.01, vs. PDMS; #P < 0.05 and ##P < 0.01, vs. TCP (mean ± SEM, n = 16 wells, one-way analysis of variance followed by Student-Newman-Keuls post hoc analysis). The experiment was performed in triplicate. PU/PVA: PU/PVA-coated PDMS films; PU: PU-coated PDMS films.
Discussion
Neural prostheses such as cochlear implants, deep brain stimulation devices, and eye prostheses have played an important role in the treatment of brain injury and neurological disability. However, tissue encapsulation of the electrode-tissue interface caused by tissue responses after implantation increases the distance between the electrode and neurons, leading to large increases in the impedance between the electrode materials and the neural tissue, which weakens the effect of the electrical stimulation over time. Therefore, there is an urgent need to decrease the tissue responses by improving the biocompatibility of the implantable electrode materials. Coating the electrode surface with a biocompatible hydrogel is one feasible and simple technique to achieve this goal. The aim of the present study was to synthesize a PU/PVA hydrogel and investigate its effect on the cytocompatibility of PDMS.
First, the PU/PVA hydrogel was synthesized and the FT-IR spectrum was measured to verify the formation of the PU/PVA hydrogel. Previous studies indicated that the common, nonspecific adsorption of a protein layer was related to the tissue response and harmful to the performance of implants (Chen et al., 2004). The amount of nonspecific protein adsorption was 442 ng/cm2 on the PDMS surface, but the fibrinogen adsorption was decreased to 36.7 ng/cm2 on the PU/PVA-coated surfaces and 32 ng/cm2 on the PU-coated surfaces. PU had previously exhibited good biocompatibility and protein resistance (Rao et al., 2012). The PU/PVA hydrogel coatings showed similar levels of nonspecific protein adsorption to the PU hydrogel coatings. These results indicate that the PU/PVA hydrogel possesses good nonspecific protein resistance, which should improve the stability of the electrode/tissue interface.
PC12 cells are a commonly used model for assessing cell viability and neurite outgrowth, which were done here on the PU/PVA hydrogel coatings. Based on the protein adsorption and PC12 cell attachment and differentiation results, the PU/PVA coating improved the cytocompatibility of PDMS to PC12 cells, which can be attributed to the hydrophilicity of the coating yielding higher cell viability and more neurite extension. Furthermore, the cytocompatibility of the PU/PVA is similar to that of PU, which is a biocompatible material appropriate for long-term use in implantable neural prostheses (Rao et al., 2012). These results suggest that the PU/PVA hydrogel coating improves the cell attachment and neurite outgrowth of PC12 cells and is cytocompatible. However, hydrogel coatings have certain disadvantages, including being insulators and swelling as a result of water absorption. Improving the ionic conductivity and decreasing the swelling of PU/PVA hydrogels should be investigated further in future studies.
In summary, the PU/PVA hydrogel coatings prepared here could improve the cytocompatibility of the PDMS used in neural electrodes. The PU/PVA-coated PDMS films decreased the amount of protein adsorption by 92%, while the PC12 cell assays revealed that the PU/PVA coatings enhanced the attachment and neurite extension of PC12 cells. All of the measured characteristics of the PU/PVA hydrogels satisfy the requirements necessary for hydrogel coatings on implantable neural electrodes. The tissue responses after implantation could be assessed to further verify the improvement in PDMS biocompatibility using the PU/PVA hydrogel coatings.
Acknowledgments:
The authors would like to thank Yi Lu from Shenzhen Institute of Advanced Technology, China and Ding-fang Wang (College of Chemistry and Molecular Science, Wuhan University, China) for their assistance in the synthesis of polyurethane and its characterization.
Footnotes
Funding: This study was supported by the National Natural Science Foundation of China, No. 81170768; a grant from the Fundamental Research Project of Shanxi Province of China, No. 2015021079.
Conflicts of interest: None declared.
Plagiarism check: This paper was screened twice using Cross-Check to verify originality before publication.
Peer review: This paper was double-blinded and stringently reviewed by international expert reviewers.
Copyedited by McCarty W, PacK M, Wang J, Qiu Y, Li CH, Song LP, Zhao M
References
- 1.Anderson JM, Rodriguez A, Chang DT. Foreign body reaction to biomaterials. Semin Immunol. 2008;20:86–100. doi: 10.1016/j.smim.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson JM, Ziats NP, Azeez A, Brunstedt MR, Stack S, Bonfield TL. Protein adsorption and macrophage activation on polydimethylsiloxane and silicone rubber. J Biomater Sci Polym Ed. 1995;7:159–169. doi: 10.1163/156856295x00670. [DOI] [PubMed] [Google Scholar]
- 3.Biran R, Martin DC, Tresco PA. Neuronal cell loss accompanies the brain tissue response to chronically implanted silicon microelectrode arrays. Exp Neurol. 2005;195:115–126. doi: 10.1016/j.expneurol.2005.04.020. [DOI] [PubMed] [Google Scholar]
- 4.Chan CKL. A preliminary study of functional electrical stimulation in upper limb rehabilitation after stroke: an evidence-based review. Hong Kong J Occup Th. 2008;18:52–58. [Google Scholar]
- 5.Chen H, Brook MA, Sheardown H. Silicone elastomers for reduced protein adsorption. Biomaterials. 2004;25:2273–2282. doi: 10.1016/j.biomaterials.2003.09.023. [DOI] [PubMed] [Google Scholar]
- 6.Chen H, Chen Y, Sheardown H, Brook MA. Immobilization of heparin on a silicone surface through a heterobifunctional PEG spacer. Biomaterials. 2005a;26:7418–424. doi: 10.1016/j.biomaterials.2005.05.053. [DOI] [PubMed] [Google Scholar]
- 7.Chen H, Zhang Z, Chen Y, Brook MA, Sheardown H. Protein repellant silicone surfaces by covalent immobilization of poly (ethylene oxide) Biomaterials. 2005b;26:2391–2399. doi: 10.1016/j.biomaterials.2004.07.068. [DOI] [PubMed] [Google Scholar]
- 8.Choi J, Bodugoz-Senturk H, Kung HJ, Malhi AS, Muratoglu OK. Effects of solvent dehydration on creep resistance of poly (vinyl alcohol) hydrogel. Biomaterials. 2007;28:772–780. doi: 10.1016/j.biomaterials.2006.09.049. [DOI] [PubMed] [Google Scholar]
- 9.Cogan SF. Neural stimulation and recording electrodes. Annu Rev Biomed Eng. 2008;10:275–309. doi: 10.1146/annurev.bioeng.10.061807.160518. [DOI] [PubMed] [Google Scholar]
- 10.Denheyer M, Kiss ZH, Haffenden AM. Behavioral effects of subthalamic deep brain stimulation in Parkinson's disease. Neuropsychologia. 2009;47:3203–3209. doi: 10.1016/j.neuropsychologia.2009.07.022. [DOI] [PubMed] [Google Scholar]
- 11.Erlich MA, Parhiscar A. Nasal dorsal augmentation with silicone implants. Facial Plast Surg. 2003;19:325–330. doi: 10.1055/s-2004-815652. [DOI] [PubMed] [Google Scholar]
- 12.Hamidi M, Azadi A, Rafiei P. Hydrogel nanoparticles in drug delivery. Adv Drug Deliv Rev. 2008;60:1638–1649. doi: 10.1016/j.addr.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 13.Han N, Rao SS, Johnson J, Parikh KS, Bradley PA, Lannutti JJ, Winter JO. Hydrogel-electrospun fiber mat composite coatings for neural prostheses. Front Neuroeng. 2011;4:2. doi: 10.3389/fneng.2011.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hashimoto Y, Niikura T, Tajima H, Yasukawa T, Sudo H, Ito Y. A rescue factor abolishing neuronal cell death by a wide spectrum of familial Alzheimer's disease genes and A beta. Proc Natl Acad Sci U S A. 2001;98:6336–6341. doi: 10.1073/pnas.101133498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.He W, McConnell GC, Schneider TM, Bellamkonda RV. A novel anti-inflammatory surface for neural electrodes. Adv Mater. 2007;19:3529–3533. [Google Scholar]
- 16.Hochberg LR, Serruya MD, Friehs GM, Mukand JA, Saleh M, Caplan AH, Branner A, Chen D, Penn RD, Donoghue JP. Neuronal ensemble control of prosthetic devices by a human with tetraplegia. Nature. 2006;442:164–171. doi: 10.1038/nature04970. [DOI] [PubMed] [Google Scholar]
- 17.Jackson A, Mavoori J, Fetz EE. Long-term motor cortex plasticity induced by an electronic neural implant. Nature. 2006;444:56–60. doi: 10.1038/nature05226. [DOI] [PubMed] [Google Scholar]
- 18.Lopez CA, Fleischman AJ, Roy S, Desai TA. Evaluation of silicon nanoporous membranes and ECM-based microenvironments on neurosecretory cells. Biomaterials. 2006;27:3075–3083. doi: 10.1016/j.biomaterials.2005.12.017. [DOI] [PubMed] [Google Scholar]
- 19.Lu Y, Wang D, Li T, Zhao X, Cao Y, Yang H, Duan YY. Poly (vinyl alcohol)/poly (acrylic acid) hydrogel coatings for improving electrode-neural tissue interface. Biomaterials. 2009;30:4143–4151. doi: 10.1016/j.biomaterials.2009.04.030. [DOI] [PubMed] [Google Scholar]
- 20.Makeig S, Westerfield M, Jung TP, Enghoff S, Townsend J, Courchesne E, Sejnowski TJ. Dynamic brain sources of visual evoked responses. Science. 2002;295:690–694. doi: 10.1126/science.1066168. [DOI] [PubMed] [Google Scholar]
- 21.Mawad D, Martens PJ, Odell RA, Poole-Warren LA. The effect of redox polymerisation on degradation and cell responses to poly (vinyl alcohol) hydrogels. Biomaterials. 2007;28:947–955. doi: 10.1016/j.biomaterials.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 22.Polikov VS, Tresco PA, Reichert WM. Response of brain tissue to chronically implanted neural electrodes. J Neurosci Methods. 2005;148:1–18. doi: 10.1016/j.jneumeth.2005.08.015. [DOI] [PubMed] [Google Scholar]
- 23.Rao L, Zhou H, Li T, Li C, Duan YY. Polyethylene glycol-containing polyurethane hydrogel coatings for improving the biocompatibility of neural electrodes. Acta Biomater. 2012;8:2233–2242. doi: 10.1016/j.actbio.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 24.Rauschecker JP, Shannon RV. Sending sound to the brain. Science. 2002;295:1025–1029. doi: 10.1126/science.1067796. [DOI] [PubMed] [Google Scholar]
- 25.Sloviter RS. Decreased hippocampal inhibition and a selective loss of interneurons in experimental epilepsy. Science. 1987;235:73–76. doi: 10.1126/science.2879352. [DOI] [PubMed] [Google Scholar]
- 26.Stieglitz T, Huang W, Chen SC, Morley JW, Lovell NH, Suaning G. A transparent electrode array for simultaneous cortical potential recording and intrinsic signal optical imaging. Conf Proc IEEE Eng Med Biol Soc. 2010;2010:1796–1799. doi: 10.1109/IEMBS.2010.5626394. [DOI] [PubMed] [Google Scholar]
- 27.Talwar SK, Xu SH, Hawley ES, Weiss SA, Moxon KA, Chapin JK. Behavioural neuroscience: Rat navigation guided by remote control-Free animals can be ‘virtually’ trained by microstimulating key areas of their brains. Nature. 2002;417:37–38. doi: 10.1038/417037a. [DOI] [PubMed] [Google Scholar]
- 28.Teramura Y, Kaneda Y, Iwata H. Islet-encapsulation in ultra-thin layer-by-layer membranes of poly(vinyl alcohol) anchored to poly(ethylene glycol)-lipids in the cell membrane. Biomaterials. 2007;28:4818–4825. doi: 10.1016/j.biomaterials.2007.07.050. [DOI] [PubMed] [Google Scholar]
- 29.Webb K, Budko E, Neuberger TJ, Chen S, Schachner M, Tresco PA. Substrate-bound human recombinant L1 selectively promotes neuronal attachment and outgrowth in the presence of astrocytes and fibroblasts. Biomaterials. 2001;22:1017–1028. doi: 10.1016/s0142-9612(00)00353-7. [DOI] [PubMed] [Google Scholar]
- 30.Winter J O, Cogan S F, Rizzo J F. Neurotrophin-eluting hydrogel coatings for neural stimulating electrodes. J Biomed Mater Res B Appl Biomater. 2007;81:551–563. doi: 10.1002/jbm.b.30696. [DOI] [PubMed] [Google Scholar]
- 31.Zhong YH, Yu XJ, Gilbert R, Bellamkonda RV. Stabilizing electrode-host interfaces: a tissue engineering approach. J Rehabil Res Dev. 2001;38:627–632. [PubMed] [Google Scholar]
- 32.Zhou HH, Li T, Duan YY. Reduce impedance of intracortical iridium oxide microelectrodes by hydrogel coatings. Sensor Actuat B-Chem. 2012;161:198–202. [Google Scholar]