Abstract
Traumatic brain injury survivors often experience cognitive deficits and neuropsychiatric symptoms. However, the neurobiological mechanisms underlying specific impairments are not fully understood. Advances in neuroimaging techniques (such as diffusion tensor imaging and functional MRI) have given us new insights on structural and functional connectivity patterns of the human brain in both health and disease. The connectome derived from connectivity maps reflects the entire constellation of distributed brain networks. Using these powerful neuroimaging approaches, changes at the microstructural level can be detected through regional and global properties of neuronal networks. Here we will review recent developments in the study of brain network abnormalities in traumatic brain injury, mainly focusing on structural and functional connectivity. Some connectomic studies have provided interesting insights into the neurological dysfunction that occurs following traumatic brain injury. These techniques could eventually be helpful in developing imaging biomarkers of cognitive and neurobehavioral sequelae, as well as predicting outcome and prognosis.
Keywords: nerve regeneration, traumatic brain injury, brain trauma, connectivity, diffusion tensor imaging, resting-state fMRI, connectome, default mode network, cognition, neural regeneration
Introduction
Traumatic brain injury (TBI) is a leading cause of death and disability in the US and is associated with substantial socioeconomic burden (Langlois et al., 2006). The Center for Disease Control estimates that 1.1 million emergency department visits, 275,000 hospitalizations, and 52,000 deaths occur every year in the US as a result of TBI (Faul et al., 2010). Nonetheless, given the limited range and efficacy of available therapeutic strategies, even state-of-the-art intensive care that adheres to best practices is unable to entirely alleviate the long-term morbidity associated with TBI. As such, TBI survivors are often left with cognitive, emotional, and behavioral changes that result in persistent disability and psychosocial difficulties (Millis et al., 2001; Whitnall et al., 2006; Stuss, 2011). For example, deficits in speed of information processing, complex attention, memory, and executive function are common after moderate to severe TBI, even several years post-injury (Millis et al., 2001; Stuss, 2011). In the emotional domain, many individuals develop depression and anxiety after sustaining a brain injury (Hibbard et al., 1998). The cognitive and emotional sequelae of TBI are heterogeneous across individuals. Our ability to predict specific deficits and their trajectory, from the early stages post-injury, remains less than definitive.
Imaging modalities have played a pivotal role in advancing TBI research over the past three decades. More recently, advanced MRI approaches such as diffusion tensor imaging (DTI) and resting state functional MRI (RS-fMRI) are further extending our understanding of normal brain organization and the neurobiological basis of neurocognitive and neuropsychiatric disorders (Greicius, 2008; Hagmann et al., 2008). In the past decade, DTI has been widely applied to detect subtle white matter abnormalities in patients with TBI (Hulkower et al., 2013). The value of DTI lies in its sensitivity to microstructural axonal injury, a pathological condition that presumably contributes to persistent cognitive and behavioral impairments in at least a subset of TBI patients. Using RS-fMRI, the blood oxygenation level dependent signal can be used to measure spontaneous (but correlated) fluctuations that occur between functionally related regions during the resting state (Biswal et al., 2010).
These advances in MRI techniques now permit the structural and functional connectivity of the brain to be studied in vivo (Beckmann et al., 2005; Jones, 2008). Optimized DTI methodology can be used to shed new light on structural connectivity, while RS-fMRI can be used to explore the functional connectivity of neuronal networks. These methods can be used individually or in parallel. Recent work has shown that functional connectivity reflects underlying structural connectivity (Hagmann et al., 2008; Greicius et al., 2009). The connectome represents the whole brain network in its entirety. It includes the entire constellation of distributed functionally-related neural networks, including all of their elements and interconnections (Sporns, 2013). Further investigation of the connectome after brain injury may lead to powerful insights about TBI. For example, these techniques could be used to study the disruption of distributed brain networks underlying specific cognitive or neurobehavioral symptoms after TBI, as well as changes to these networks during recovery and with neurorehabilitative interventions. In theory, adopting a connectomic approach to the study of TBI may eventually allow us to more precisely predict the nature of an individual patient's residual long-term deficits by estimating the impact of damage to nodes within distributed functional networks on specific neurobehavioral systems. This ability would facilitate accurate prognostication and effective treatment planning.
In this review, our intent is to focus on recent developments in the study of structural and functional connectivity in TBI. First, we will briefly introduce basic concepts relevant to human brain connectomics. Then, we will discuss imaging modalities and applications relevant to structural and functional connectivity, as well as recent empirical findings involving connectivity in TBI. Finally, we will propose considerations for future studies of TBI connectomics.
Basic Concepts of Human Brain Connectomics
The field of connectomics is a new area of scientific inquiry, which seeks comprehensive description of the structural and functional connectivity patterns of the human brain (Sporns et al., 2005). The term “connectome” emphasizes that the brain is a single large and unique structural entity with a network composed of neural connections (edges) and neural units (nodes). The word “connectomics” emphasizes a comprehensive research field, which maps the nodes and edges in the brain at an individual and population level, while developing the tools to analyze its organization and structure. Functional (RS-fMRI) and structural (DTI) imaging provide noninvasive methods for studying human brain networks. Sporns et al. (2005) outlined a five-step strategy for describing the human connectome. Step 1 is to perform DTI tractography to obtain a voxel-wise probabilistic all-to-all structural connectivity matrix for the human brain. Step 2 is to perform correlation analysis through fMRI data to find strong functional relationships that are consistent across tasks; the aim is to capture a voxel-wise all-to-all functional connectivity matrix for the human brain. Step 3 is to identify regions of consistent structure–function relationships in the human brain by performing a cluster analysis of correspondences between the structural and functional connectivity matrix obtained under steps 1 and 2. Step 4 is to identify correspondences and deviations by comparing the results obtained by cluster analysis through step 3 with macaque data. Lastly, step 5 is to certify the strongest predictions generated by assembling the final combined structural-functional connectivity matrix using custom-designed stimuli and perturbational techniques such as transcranial magnetic stimulation.
Connectomics might help us to better understand brain damage and recovery. The connectome can be obtained and studied in various conditions, such as TBI, neurodegenerative disease, and neurodevelopmental disorders. Various abnormalities can be seized as specific structural and functional variants of the human connectome.
Structural Connectomics in TBI
DTI tractography is a commonly used technique for reconstructing white matter structures and assessing structural connectivity in vivo (Mori and van Zijl, 2002). The reconstruction is based on algorithms that use information from each imaging voxel (such as direction of diffusion), and assume continuity from voxel to voxel, to eventually track the whole pathway. This method allows one to map the macroscopic connections of the human brain and provides a large amount of information about fiber tracts, such as length and density (Mori and van Zijl, 2002; Irimia, 2012).
Diffusion tensor tractography is a valuable tool for detecting structural connectivity changes occurring in different stages of TBI and for studying the utility of acute tractography in predicting patients’ long-term cognitive outcomes (Wang et al., 2011). However, the tractography algorithms may sometimes fail because of the disruption of voxel to voxel continuity based on white matter damage. To circumvent this problem, Squarcina et al. (2012) recently proposed a method for studying thalamo-cortical connectivity using a template based on probabilistic tractography of healthy individuals, which eliminates the need for individual tractography. This method appears robust to varying degrees of traumatic axonal injury and, therefore, appears appropriate for investigating specific thalamo-cortical white matter connections after TBI.
Another methodological challenge that hinders DTI research in TBI and other disorders is that DTI has limited capacity to resolve the direction of individual fiber tracts at junctions between overlapping bundles. A technique called High-Angular-Resolution Diffusion Imaging (HARDI) can overcome this “fiber-crossing problem” without substantially increasing scan lengths or hardware requirements (Tuch et al., 2002; Jansons and Alexander, 2003; Tournier et al., 2004). As such, this technique has become quite popular and may be useful for many TBI researchers.
Newer techniques, with greater technical requirements, have been developed to measure structural connectivity in even greater detail than early DTI. Diffusion spectrum imaging (DSI) is a new DTI technique characterized by the acquisition of a very large number of images applying strong motion sensitizing magnetic gradients in multiple directions (Hagmann et al., 2010). It allows one to map complex tissue fiber architectures by imaging the 3D spectra of tissue water diffusion (Wedeen et al., 2005). DSI tractography has the capacity to image crossing fibers in neural tissue, which is essential to image the connectional neuroanatomy non-invasively (Wedeen et al., 2008) (Figure 1). Strong DSI connections can predict stronger functional connectivity quantitatively.
Figure 1.
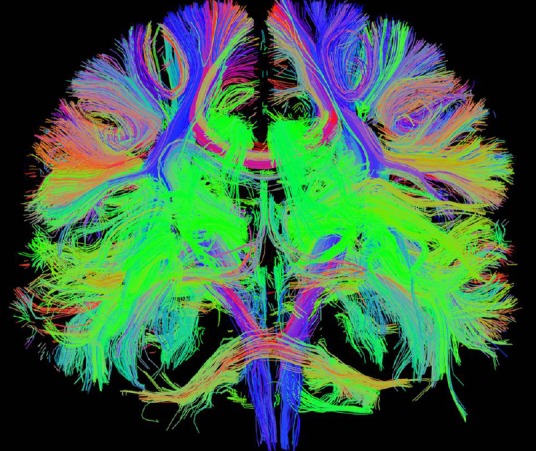
Structural connectivity from diffusion spectrum imaging tractography in a normal human participant in 3T MRI, which gives a delicate connectional neuroanatomy.
In brief, DTI constitutes an excellent imaging modality for the noninvasive study of brain architecture. It is well suited for the study of brain network properties in TBI patients. We are optimistic that ongoing TBI research, along with refinements in DTI techniques, will provide important insights into brain connectivity in TBI in the future.
DTI-derived structural connectivity techniques have been used to detect microstructural abnormalities in multiple neurologic disorders, such as schizophrenia (White et al., 2007; Camchong et al., 2011), Alzheimer's disease (Zhou et al., 2008; Zarei et al., 2010), epilepsy (Holt et al., 2011; Vulliemoz et al., 2011), and autism (Ameis et al., 2011; Billeci et al., 2012). These DTI-derived techniques are also being used to study structural connectivity in TBI. Accurate measurement of white matter structure and connectivity is presumably critical to the study of TBI. This would be particularly true in cases involving traumatic axonal injury (TAI), which contributes to cognitive and neuropsychiatric impairment (Lux, 2007). Numerous studies have shown that disrupted structural connectivity (as measured by DTI metrics) corresponds to deficits in cognitive domains, including sustained attention (Bonnelle et al., 2011), working memory (Palacios et al., 2012), executive function (Wang et al., 2011; Caeyenberghs et al., 2014), information processing speed (Wang et al., 2011), and learning and memory (Wang et al., 2011; Rohling et al., 2012; Tallus et al., 2012). A complete synthesis of DTI studies in TBI is beyond the scope of this review, but the interested reader should refer to prior reviews (e.g., Sharp and Ham, 2011; Wortzel et al., 2011; Gardner et al., 2012; Irimia, 2012; Hulkower et al., 2013).
One recent study used discriminant correspondence analysis (DCA), a type of discriminant analysis, to study tractography measurements from 28 fiber regions in TAI of varying severities from the acute to chronic stages of TBI. DCA factor scores discriminated acute TBI patients from healthy controls. The longitudinal trajectory of tractography measurements was heterogeneous across patients, reflecting improved, deteriorated, or stable connectivity over time. However, the change in connectivity over time predicted cognitive outcome (Wang et al., 2011).
Recently, Caeyenberghs et al. (2014) studied brain behavioral switching networks in 23 patients with chronic moderate to severe TBI using diffusion tensor tractography. They calculated topological properties of the networks using a graph theoretical method, and found that both TBI patients and controls exhibited a small-world topology in their white matter networks. The TBI patients demonstrated increased shortest path length and decreased global efficiency of the structural network. The network property of degree, which reflects how interconnected a node is with other brain nodes, correlated with behavioral performance. In particular, increased connectivity degree predicted poorer switching. Another study about working memory networks using whole-brain DTI maps revealed that structural damage in specific tracts such as the inferior and superior longitudinal fasciculi, inferior fronto-occipital fasciculi, cingulum, anterior thalamic radiations, and corpus callosum were associated with working memory functional activations (Palacios et al., 2012). These findings suggest that TBI patients have a weaker globally integrated structural brain network, resulting in a limited capacity of executive control function.
Other recent work has also revealed that structural and functional connectivity in TBI are closely related (Bonnelle et al., 2011, 2012; Sharp et al., 2011, 2014; Palacios et al., 2012; Goh et al., 2014). For example, one DTI study showed that white matter damage within the tracts of the salience network (SN, which includes the anterior cingulate cortex, presupplementary motor area, and anterior insula) predicted abnormal deactivation of the default mode network (DMN), which in turn was associated with deficient inhibition (Bonnelle et al., 2012). Using DTI, structural disconnection within the DMN also correlated with sustained attention performance (Bonnelle et al., 2011). Sharp et al. (2011) found that as white matter disruption within the splenium of the corpus callosum increased (as indicated by mean diffusivity), posterior cingulate cortex functional connectivity decreased. It is very likely that a variable degree of structural disconnection modulates a compensatory increase in functional connectivity within DMN.
In summary, DTI remains a promising tool to study white matter damage and to assist in understanding pathological changes in the structural connectivity of the brain after TBI. Although DTI-derived structural connectivity has provided novel insights into structural network disruptions after TBI and their relationship to cognitive deficits, DTI only reflects the essence of disease from a structural point of view. Functional connectivity based on RS-fMRI can help us to more fully understand aberrations in brain network properties following TBI.
Functional Connectomics in TBI
Task-dependent fMRI experiments deliberately investigate the activity of the brain in specific regions, which is usually distinct, while resting state activations are produced by spontaneous, intrinsic fluctuations. RS-fMRI is well-matched to the view that the brain largely activates intrinsically, with sensory information modulating system processes (Burton et al., 2004). RS-fMRI data are usually used to identify intrinsic neural networks or areas of connectivity (fcMRI) distributed throughout the brain. Recent evidence suggests that it may be possible to extract information about the connectivity and functionality of specific networks from RS-fMRI fluctuations (Hampson et al., 2002; Greicius et al., 2003; Seeley et al., 2007). The ability to use fcMRI to assess the functionality of specific networks while the patient is at rest would be of particular interest for clinical applications. For example, the ability to image network integrity or disruptions might provide valuable prognostic or treatment planning information in severely injured patients with limited ability to comply with active fMRI paradigms, as in disorders of consciousness.
According to Mantini et al. (2007), there are six main resting state networks (RSNs) that have been identified. RSN 1 is the DMN, which typically comprises bilateral inferior parietal lobe, bilateral superior frontal gyrus, the posterior cingulate cortex (PCC) and precuneus, medial prefrontal cortex (MPFC). RSN 2 is a network of dorsal attention, which includes bilateral intraparietal sulcus/cortex at the intersection of the precentral and superior frontal sulcus, ventral precentral, and middle frontal gyrus. RSN 3 is a posterior visual processing network, which involves the retinotopic occipital cortex and temporal-occipital regions. RSN 4 primarily involves the bilateral superior temporal cortex, and is the auditory-phonological network. RSN 5 is the sensory-motor network including the precentral, postcentral, and medial frontal gyri, the primary sensory-motor cortices, and the supplementary motor area. RSN 6 is a network related to self-referential mental activity, which includes the medial-ventral prefrontal cortex, the pregenual anterior cingulate, the hypothalamus, and the cerebellum. Among all the resting state patterns, the default mode is the most prominent, and was originally discovered in resting metabolism positron emission tomography (PET) data (Raichle et al., 2001); it was subsequently assumed to reflect an organized, baseline default mode of brain function. Thus far, default mode RS-fMRI has been the most commonly used technique to research functional connectivity in TBI.
Currently, two methodological approaches can be used to analyze RS-fMRI data to study functional connectivity. The first one is an ROI-based method, which relies on prior anatomical hypotheses to restrict the analysis to a predefined anatomical region of interest or to a specific seed (Greicius et al., 2003). With this approach, seed ROIs are selected by the user. Signals within the seed ROI are then compared and correlated to the signals within other parts of the brain. Statistical measures can be derived to understand the correlation structure of the seed ROI in relation to other brain regions. The other method used to analyze RS-fMRI to evaluate the connectivity within different areas of the brain is independent components analysis (ICA) (Calhoun et al., 2002). ICA allows the analysis of fMRI data using only minimal assumptions about the spatiotemporal structure of the component signals (Lewine et al., 1999). ICA separates spatial components by making the assumption that the spatial network patterns are statistically independent in their time courses. Essentially, ICA is a data-driven statistical method that attempts to decompose the total RS-fMRI signal into several independent components arising from various spatial locations, the sum of which most closely reconstructs the total measured RS-fMRI signal. The technique segregates components, each of which contains a network of interrelated structures whose activity correlates closely, while the various components are independent of one another (Figure 2). It does not require user selection of an ROI, as this analysis is performed on the signal arising from the whole brain. This approach has a number of advantages over the use of seed voxel-based approaches and has been used to probe functional connectivity in multiple populations (Damoiseaux et al., 2008; Filippini et al., 2009).
Figure 2.
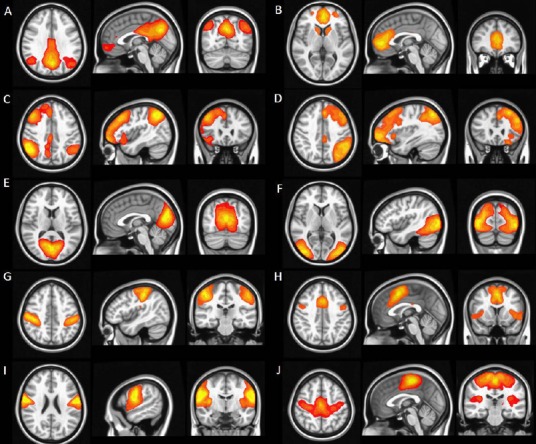
Functional connectivity from resting state functional MRI.
Six of the most common and consistent resting state networks identified by independent components analysis: (A, B) default mode network; (C, D) left- and right-lateral executive networks; (E, F) visual network; (G, H) motor network; (I) auditory network; and (J) salience network.
Resting state fMRI network analysis has been applied to a variety of diseases and conditions, such as Alzheimer's disease (AD) (Rombouts et al., 2007; Sorg et al., 2007), aging (Andrews-Hanna et al., 2007), schizophrenia (Jafri et al., 2008), and epilepsy (Laufs et al., 2007). These studies have identified various abnormalities in resting state network patterns across disorders. A substantial body of literature now confirms that RS-fMRI can be used to extract information about network functionality and connectivity (Hampson et al., 2002; Seeley et al., 2007). Nowadays RS-fMRI is widely used to evaluate functional connectivity in TBI, and both increases and decreases in connectivity have been observed in a number of networks (Hillary et al., 2011; Mayer et al., 2011; Sharp et al., 2011; Shumskaya et al., 2012; Stevens et al., 2012). Several studies have reported that these abnormalities correlate with cognitive impairment or post-concussive symptoms (Tang et al., 2011; Messé et al., 2013; Caeyenberghs et al., 2014).
Table 1.
Studies of functional and structural connectivity in TBI
The DMN is the most readily identified and most commonly studied of the resting state networks in TBI. DMN represents an organized, baseline default mode of brain function that is active at rest and suppressed during specific goal-directed behaviors (Raichle et al., 2001). Several recent studies have described DMN abnormalities after TBI, both with respect to resting state DMN functional connectivity and DMN deactivation during task performance (Bonnelle et al., 2011, 2012; Hillary et al., 2011; Mayer et al., 2011; Sharp et al., 2011; Zhou et al., 2012; Sours et al., 2013).
Consistent with earlier PET studies, several fMRI studies have reported reduced connectivity in severely injured TBI patients with disorders of consciousness. Connectivity within the DMN correlates with level of consciousness, with minimally conscious patients showing an intermediate degree of connectivity relative to locked-in syndrome and vegetative state (Zhang et al., 2012). In general, disruptions in thalamo-cortical and cortico-cortical frontoparietal connectivity are thought to contribute to the impaired levels of consciousness seen in vegetative or minimally conscious states (Cauda et al., 2009; Noirhomme et al., 2010).
In moderate to severe TBI patients who recover consciousness, DMN abnormalities have been associated with the nature and magnitude of cognitive impairment. For example, higher relative activation of the DMN (particularly within the precuneus and PCC) during an fMRI choice reaction time task is associated with deficits in sustained attention (Bonnelle et al., 2011). This finding is compatible with the view that the DMN must be deactivated to switch from the internally-directed resting state towards externally-directed or task-positive goal-directed behavior. Failure to suppress DMN structures such as the precuneus and PCC is associated with attentional lapses in healthy controls (Weissman et al., 2006). Furthermore, DMN deactivation is enhanced during performance of cognitively demanding tasks with increasing cognitive load (Singh and Fawcett, 2008, Pyka et al., 2009). As such, it appears that TBI patients with inconsistent attention and vigilance sometimes fail to deactivate the DMN, as would be required for consistent and focused engagement in goal-directed behavior in the external world. Moreover, functional connectivity within the DMN and structural connectivity (as demonstrated by DTI) also correlated with the degree of impairment in sustained attention (Bonnelle et al., 2011). Specifically, reduced functional connectivity of the precuneus within the DMN and lower FA of the right cingulum predicted poor sustained attention.
Another recent study provided corroborating evidence that lesser deactivation of DMN during cognitively demanding tasks is associated with cognitive deficits. Chronic-stage severe TBI patients with better working memory demonstrated greater deactivation of DMN structures during performance of a visual n-back working memory task, along with greater activations in task-related working memory structures (Palacios et al., 2012). Furthermore, white matter integrity (as measured by global FA) correlated with DMN deactivations (Palacios et al., 2012).
Sharp and colleagues (2011) identified increased functional connectivity within resting-state DMN in chronic TBI (> 6 months post-injury). Greater functional connectivity within the DMN (particularly the PCC) during rest was associated with lesser impairments in information processing speed. Patients with evidence of DTI abnormalities within the splenium of the corpus callosum demonstrated lower functional connectivity within adjacent DMN structures (i.e., the PCC). The authors suggested that increased connectivity within the DMN at rest may reflect a dynamic and adaptive process in chronic TBI, which ultimately promotes more effective deactivation of the DMN during focused goal-directed behavior, while these network changes may be modulated by the degree of structural disconnection secondary to axonal injury (Sharp et al., 2011). Consistent with this hypothesis, one longitudinal study that evaluated changes in connectivity at 3 and 6 months following resolution of posttraumatic amnesia after moderate to severe TBI did indeed identify increased DMN connectivity over this recovery period (Hillary et al., 2011).
Other studies have documented DMN alterations in mild TBI. Zhou et al. (2012) reported increased resting state functional connectivity in the anterior medial prefrontal cortical region and decreased connectivity in the PCC/parietal regions in mild TBI compared with healthy controls. Lower functional connectivity of the posterior DMN regions predicted reduced speed/mental flexibility on the Trail Making Test, while increased functional connectivity of the anterior DMN regions was associated with symptomatic report of depression, anxiety, and post-concussive symptoms. Mayer et al. (2011) examined mTBI patients within 3 weeks of injury, with repeat evaluation of a subsample of participants at 3–5 months. They reported decreased DMN connectivity, but increased connectivity between DMN and lateral prefrontal cortex in semiacute mTBI patients. While no changes in functional connectivity were found in recovery period. They also reported some evidence of a relationship between abnormal functional connectivity and white matter microstructure, as well as a relationship between functional connectivity measures and cognitive complaints. The results indicated that functional connectivity may serve as a biomarker of both mTBI and underlying cognitive impairment.
Abnormal functional connectivity after TBI has also been observed in several other networks (Kasahara et al., 2010, 2011; Marquez de la Plata et al., 2011; Tang et al., 2011; Hillary et al., 2012; Shumskaya et al., 2012; Stevens et al., 2012). For example, functional connectivity among motor system regions was altered during self-paced finger-thumb opposition, potentially reflecting post-injury reorganization (Kasahara et al., 2011). Abnormal frontoparietal network connectivity during working memory task performance was recently described, raising the possibility that abnormal interhemispheric connectivity between the left and right inferior frontal gyri may contribute to working memory deficits after TBI (Kasahara et al., 2011). In fact, functional connectivity abnormalities were recently identified in all twelve networks generated by ICA analysis of resting-state fMRI data from mTBI patients. These included networks subserving visual processing, motor abilities, and cognitive-executive function. Furthermore, abnormal connectivity within various networks, particularly the anterior cingulate, was associated with postconcussive symptom severity (Stevens et al., 2012).
In summary, RS-fMRI may have the potential to become an important tool to assess large-scale connectivity and functional integrity of neuronal networks in TBI patients. It may give us a better understanding of the structural origins of cognitive dysfunction, and, not only useful as a research tool, it may permit the design of strategies for recovery based on network analysis in the future, as well as serving as a biomarker to monitor disease progression and recovery in TBI.
Future Considerations
Advanced MRI methods, such as DTI and RS-fMRI, are useful tools to reveal the multifaceted nature of brain injury and study connectome abnormalities in TBI. Studies to date raise some optimism that these methods could eventually yield biomarkers of the assorted pathologies in TBI to improve diagnosis, prognosis, and treatment planning. Despite the encouraging findings emerging in the recent literature, TBI connectomics is only in its infancy. Findings require further investigation and replication.
Future studies should take into account a number of considerations. First, the pathology underlying TBI is so heterogeneous that outcomes can vary from death, to survival with severe cognitive disability, to complete recovery. Every brain injury is unique and has a complex effect on multiple interacting networks. Given the significant pathophysiological differences between mild and moderate-to-severe traumatic brain injuries (Wang et al., 2011), we should not generalize functional connectivity findings across injury severities, which definitely limits our ability to predict specific neurobehavioral trajectories of recovery in TBI. It is possible that the nature or magnitude of structural and functional connectivity abnormalities should be differentiated across the TBI populations and across the timing post-injury in the future.
Second, there have been a limited number of TBI studies fully exploring structural and resting state networks or patterns of connectivity in TBI. Thus far, most of the TBI network studies have focused on identifying differences between TBI and control groups. While these studies appear to confirm the presence of network abnormalities secondary to TBI, little is known about the relationship between networks and disease progression. Longitudinal studies are currently limited, but are needed to characterize the natural history of network changes over the course of spontaneous recovery and in response to rehabilitation. Furthermore, the relationship of network abnormalities to the nature or magnitude of specific neurobehavioral deficits warrants further investigation and replication, as does the relationship of network abnormalities to long-term outcome.
Finally, existing studies on TBI brain networks are at the macroscale. More detailed and comprehensive information could become available in the future, should methods ultimately progress to the microscale or mesoscale level.
Conclusions
Modern fMRI and DTI techniques have provided us with tools to view the human brain connectome by assessing tract structure, tract connectivity, and functional connectivity. Studies performed thus far in TBI confirm the presence of both structural and functional connectivity abnormalities across the spectrum of injury severity. They also suggest that these abnormalities are in some way related to specific cognitive impairments demonstrated after TBI. It can plausibly be hypothesized that cortical and white matter damage arising from TBI can disrupt structural connectivity in TBI patients, thereby affecting patterns of functional brain activity and connectivity. Therefore, further research investigating the structural and functional connectivity of large-scale cognitive networks in TBI certainly appears warranted. These studies should eventually be combined with investigation of rapid changes in brain activity associated with abnormal behavior and cognition, because persistent alterations in the connectivity of these larger-scale networks are likely to influence a network's short-term response to transient and changing environmental demands. In the future, longitudinal studies using such MRI techniques from the acute setting to the chronic stage, in conjunction with neurocognitive assessment, are warranted to reveal the dynamic picture of brain injury and determine whether these techniques might someday be helpful in the clinical context for the benefit of our patients.
Footnotes
Funding: This work was supported by a grant from the Medical Scientific Research Programs of Nanjing Military Command, No. 14MS122.
Conflicts of interest: None declared.
Plagiarism check: This paper was screened twice using Cross-Check to verify originality before publication.
Peer review: This paper was double-blinded and stringently reviewed by international expert reviewers.
Copyedited by Jackson C, Li CH, Song LP, Liu WJ, Zhao M
References
- 1.Ameis SH, Fan J, Rockel C, Voineskos AN, Lobaugh NJ, Soorya L, Wang AT, Hollander E, Anagnostou E. Impaired structural connectivity of socio-emotional circuits in autism spectrum disorders: a diffusion tensor imaging study. PLoS One. 2011;6:e28044. doi: 10.1371/journal.pone.0028044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andrews-Hanna JR, Snyder AZ, Vincent JL, Lustig C, Head D, Raichle ME, Buckner RL. Disruption of large-scale brain systems in advanced aging. Neuron. 2007;56:924–935. doi: 10.1016/j.neuron.2007.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beckmann CF, DeLuca M, Devlin JT, Smith SM. Investigations into resting-state connectivity using independent component analysis. Philos Trans R Soc Lond B Biol Sci. 2005;360:1001–1013. doi: 10.1098/rstb.2005.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Billeci L, Calderoni S, Tosetti M, Catani M, Muratori F. White matter connectivity in children with autism spectrum disorders: a tract-based spatial statistics study. BMC Neurol. 2012;12:148. doi: 10.1186/1471-2377-12-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Biswal BB, Mennes M, Zuo XN, Gohel S, Kelly C, Smith SM, Beckmann CF, Adelstein JS, Buckner RL, Colcombe S, Dogonowski AM, Ernst M, Fair D, Hampson M, Hoptman MJ, Hyde JS, Kiviniemi VJ, Kotter R, Li SJ, Lin CP, et al. Toward discovery science of human brain function. Proc Natl Acad Sci U S A. 2010;107:4734–4739. doi: 10.1073/pnas.0911855107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bonnelle V, Ham TE, Leech R, Kinnunen KM, Mehta MA, Greenwood RJ, Sharp DJ. Salience network integrity predicts default mode network function after traumatic brain injury. Proc Natl Acad Sci U S A. 2012;109:4690–4695. doi: 10.1073/pnas.1113455109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bonnelle V, Leech R, Kinnunen KM, Ham TE, Beckmann CF, De Boissezon X, Greenwood RJ, Sharp DJ. Default mode network connectivity predicts sustained attention deficits after traumatic brain injury. J Neurosci. 2011;31:13442–13451. doi: 10.1523/JNEUROSCI.1163-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burton H, Snyder AZ, Raichle ME. Default brain functionality in blind people. Proc Natl Acad Sci U S A. 2004;101:15500–15505. doi: 10.1073/pnas.0406676101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Caeyenberghs K, Leemans A, Leunissen I, Gooijers J, Michiels K, Sunaert S, Swinnen SP. Altered structural networks and executive deficits in traumatic brain injury patients. Brain Struct Funct. 2014;219:193–209. doi: 10.1007/s00429-012-0494-2. [DOI] [PubMed] [Google Scholar]
- 10.Calhoun VD, Adali T, Pearlson GD, van Zijl PC, Pekar JJ. Independent component analysis of fMRI data in the complex domain. Magn Reson Med. 2002;48:180–192. doi: 10.1002/mrm.10202. [DOI] [PubMed] [Google Scholar]
- 11.Camchong J, MacDonald AW, 3rd, Bell C, Mueller BA, Lim KO. Altered functional and anatomical connectivity in schizophrenia. Schizophr Bull. 2011;37:640–650. doi: 10.1093/schbul/sbp131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cauda F, Micon BM, Sacco K, Duca S, D’Agata F, Geminiani G, Canavero S. Disrupted intrinsic functional connectivity in the vegetative state. J Neurol Neurosurg Psychiatry. 2009;80:429–431. doi: 10.1136/jnnp.2007.142349. [DOI] [PubMed] [Google Scholar]
- 13.Damoiseaux JS, Beckmann CF, Arigita EJ, Barkhof F, Scheltens P, Stam CJ, Smith SM, Rombouts SA. Reduced resting-state brain activity in the “default network” in normal aging. Cereb Cortex. 2008;18:1856–1864. doi: 10.1093/cercor/bhm207. [DOI] [PubMed] [Google Scholar]
- 14.Faul M, Xu L, Wald MM, Coronado VG. Atlanta (GA): Centers for Disease Control and Prevention, National Center for Injury Prevention and Control; 2010. Traumatic Brain Injury in the United States: Emergency Department Visits, Hospitalizations and Deaths 2002–2006. [Google Scholar]
- 15.Filippini N, MacIntosh BJ, Hough MG, Goodwin GM, Frisoni GB, Smith SM, Matthews PM, Beckmann CF, Mackay CE. Distinct patterns of brain activity in young carriers of the APOE-epsilon4 allele. Proc Natl Acad Sci U S A. 2009;106:7209–7214. doi: 10.1073/pnas.0811879106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gardner A, Kay-Lambkin F, Stanwell P, Donnelly J, Williams WH, Hiles A, Schofield P, Levi C, Jones DK. A systematic review of diffusion tensor imaging findings in sports-related concussion. J Neurotrauma. 2012;29:2521–2538. doi: 10.1089/neu.2012.2628. [DOI] [PubMed] [Google Scholar]
- 17.Goh SY, Irimia A, Torgerson CM, Horn JD. Neuroinformatics challenges to the structural, connectomic, functional and electrophysiological multimodal imaging of human traumatic brain injury. Front Neuroinform. 2014;8:19. doi: 10.3389/fninf.2014.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Greicius M. Resting-state functional connectivity in neuropsychiatric disorders. Curr Opin Neurol. 2008;21:424–430. doi: 10.1097/WCO.0b013e328306f2c5. [DOI] [PubMed] [Google Scholar]
- 19.Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci U S A. 2003;100:253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Greicius MD, Supekar K, Menon V, Dougherty RF. Resting-state functional connectivity reflects structural connectivity in the default mode network. Cereb Cortex. 2009;19:72–78. doi: 10.1093/cercor/bhn059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hagmann P, Cammoun L, Gigandet X, Meuli R, Honey CJ, Wedeen VJ, Sporns O. Mapping the structural core of human cerebral cortex. PLoS Biol. 2008;6:e159. doi: 10.1371/journal.pbio.0060159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hagmann P, Cammoun L, Gigandet X, Gerhard S, Grant PE, Wedeen V, Meuli R, Thiran JP, Honey CJ, Sporns O. MR connectomics: Principles and challenges. J Neurosci Methods. 2010;194:34–45. doi: 10.1016/j.jneumeth.2010.01.014. [DOI] [PubMed] [Google Scholar]
- 23.Hampson M, Peterson BS, Skudlarski P, Gatenby JC, Gore JC. Detection of functional connectivity using temporal correlations in MR images. Hum Brain Mapp. 2002;15:247–262. doi: 10.1002/hbm.10022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hibbard MR, Uysal S, Kepler K, Bogdany J, Silver J. Axis I psychopathology in individuals with traumatic brain injury. J Head Trauma Rehabil. 1998;13:24–39. doi: 10.1097/00001199-199808000-00003. [DOI] [PubMed] [Google Scholar]
- 25.Hillary FG, Medaglia JD, Gates KM, Molenaar PC, Good DC. Examining network dynamics after traumatic brain injury using the extended unified SEM approach. Brain Imaging Behav. 2014;8:435–445. doi: 10.1007/s11682-012-9205-0. [DOI] [PubMed] [Google Scholar]
- 26.Hillary FG, Slocomb J, Hills EC, Fitzpatrick NM, Medaglia JD, Wang J, Good DC, Wylie GR. Changes in resting connectivity during recovery from severe traumatic brain injury. Int J Psychophysiol. 2011;82:115–123. doi: 10.1016/j.ijpsycho.2011.03.011. [DOI] [PubMed] [Google Scholar]
- 27.Holt RL, Provenzale JM, Veerapandiyan A, Moon WJ, De Bellis MD, Leonard S, Gallentine WB, Grant GA, Egger H, Song AW, Mikati MA. Structural connectivity of the frontal lobe in children with drug-resistant partial epilepsy. Epilepsy Behav. 2011;21:65–70. doi: 10.1016/j.yebeh.2011.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hulkower MB, Poliak DB, Rosenbaum SB, Zimmerman ME, Lipton ML. A Decade of DTI in Traumatic Brain Injury: 10 Years and 100 Articles Later. AJNR Am J Neuroradiol. 2013;34:2064–2074. doi: 10.3174/ajnr.A3395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Irimia A. Neuroimaging of structural pathology and connectomics in traumatic brain injury: Toward personalized outcome prediction. Neuroimage Clin. 2012;1:1–17. doi: 10.1016/j.nicl.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jafri MJ, Pearlson GD, Stevens M, Calhoun VD. A method for functional network connectivity among spatially independent resting-state components in schizophrenia. Neuroimage. 2008;39:1666–1681. doi: 10.1016/j.neuroimage.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jansons KM, Alexander DC. Persistent Angular Structure: new insights from diffusion MRI data. Dummy version. Inf Process Med Imaging. 2003;18:672–683. doi: 10.1007/978-3-540-45087-0_56. [DOI] [PubMed] [Google Scholar]
- 32.Jones DK. Studying connections in the living human brain with diffusion MRI. Cortex. 2008;44:936–952. doi: 10.1016/j.cortex.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 33.Kasahara M, Menon DK, Salmond CH, Outtrim JG, Tavares JV, Carpenter TA, Pickard JD, Sahakian BJ, Stamatakis EA. Traumatic brain injury alters the functional brain network mediating working memory. Brain Inj. 2011;25:1170–1187. doi: 10.3109/02699052.2011.608210. [DOI] [PubMed] [Google Scholar]
- 34.Kasahara M, Menon DK, Salmond CH, Outtrim JG, Taylor Tavares JV, Carpenter TA, Pickard JD, Sahakian BJ, Stamatakis EA. Altered functional connectivity in the motor network after traumatic brain injury. Neurology. 2010;75:168–176. doi: 10.1212/WNL.0b013e3181e7ca58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Langlois JA, Rutland-Brown W, Wald MM. The epidemiology and impact of traumatic brain injury: a brief overview. J Head Trauma Rehabil. 2006;21:375–378. doi: 10.1097/00001199-200609000-00001. [DOI] [PubMed] [Google Scholar]
- 36.Laufs H, Hamandi K, Salek-Haddadi A, Kleinschmidt AK, Duncan JS, Lemieux L. Temporal lobe interictal epileptic discharges affect cerebral activity in “default mode” brain regions. Hum Brain Mapp. 2007;28:1023–1032. doi: 10.1002/hbm.20323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lewine JD, Davis JT, Sloan JH, Kodituwakku PW, Orrison WW., Jr Neuromagnetic assessment of pathophysiologic brain activity induced by minor head trauma. AJNR Am J Neuroradiol. 1999;20:857–866. [PMC free article] [PubMed] [Google Scholar]
- 38.Lux WE. A neuropsychiatric perspective on traumatic brain injury. J Rehabil Res Dev. 2007;44:951–962. doi: 10.1682/jrrd.2007.01.0009. [DOI] [PubMed] [Google Scholar]
- 39.Mantini D, Perrucci MG, Del Gratta C, Romani GL, Corbetta M. Electrophysiological signatures of resting state networks in the human brain. Proc Natl Acad Sci U S A. 2007;104:13170–13175. doi: 10.1073/pnas.0700668104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marquez de la Plata CD, Garces J, Shokri Kojori E, Grinnan J, Krishnan K, Pidikiti R, Spence J, Devous MD, Sr, Moore C, McColl R, Madden C, Diaz-Arrastia R. Deficits in functional connectivity of hippocampal and frontal lobe circuits after traumatic axonal injury. Arch Neurol. 2011;68:74–84. doi: 10.1001/archneurol.2010.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mayer AR, Mannell MV, Ling J, Gasparovic C, Yeo RA. Functional connectivity in mild traumatic brain injury. Hum Brain Mapp. 2011;32:1825–1835. doi: 10.1002/hbm.21151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Messé A, Caplain S, Pélégrini-Issac M, Blancho S, Lévy R, Aghakhani N, Montreuil M, Benali H, Lehéricy S. Specific and Evolving Resting-State Network Alterations in Post-Concussion Syndrome Following Mild Traumatic Brain Injury. PLoS One. 2013;8:e65470. doi: 10.1371/journal.pone.0065470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Millis SR, Rosenthal M, Novack TA, Sherer M, Nick TG, Kreutzer JS, High WM, Jr, Ricker JH. Long-term neuropsychological outcome after traumatic brain injury. J Head Trauma Rehabil. 2001;16:343–355. doi: 10.1097/00001199-200108000-00005. [DOI] [PubMed] [Google Scholar]
- 44.Mori S, van Zijl PC. Fiber tracking: principles and strategies - a technical review. NMR Biomed. 2002;15:468–480. doi: 10.1002/nbm.781. [DOI] [PubMed] [Google Scholar]
- 45.Noirhomme Q, Soddu A, Lehembre R, Vanhaudenhuyse A, Boveroux P, Boly M, Laureys S. Brain connectivity in pathological and pharmacological coma. Front Syst Neurosci. 2010;4:160. doi: 10.3389/fnsys.2010.00160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Palacios EM, Sala-Llonch R, Junque C, Roig T, Tormos JM, Bargallo N, Vendrell P. White matter integrity related to functional working memory networks in traumatic brain injury. Neurology. 2012;78:852–860. doi: 10.1212/WNL.0b013e31824c465a. [DOI] [PubMed] [Google Scholar]
- 47.Pyka M, Beckmann CF, Schoning S, Hauke S, Heider D, Kugel H, Arolt V, Konrad C. Impact of working memory load on FMRI resting state pattern in subsequent resting phases. PLoS One. 2009;4:e7198. doi: 10.1371/journal.pone.0007198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci U S A. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rohling ML, Larrabee GJ, Millis SR. The “Miserable Minority” following mild traumatic brain injury: who are they and do meta-analyses hide them? Clin Neuropsychol. 2012;26:197–213. doi: 10.1080/13854046.2011.647085. [DOI] [PubMed] [Google Scholar]
- 50.Rombouts SA, Scheltens P, Kuijer JP, Barkhof F. Whole brain analysis of T2* weighted baseline FMRI signal in dementia. Hum Brain Mapp. 2007;28:1313–1317. doi: 10.1002/hbm.20349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sharp DJ, Beckmann CF, Greenwood R, Kinnunen KM, Bonnelle V, De Boissezon X, Powell JH, Counsell SJ, Patel MC, Leech R. Default mode network functional and structural connectivity after traumatic brain injury. Brain. 2011;134:2233–2247. doi: 10.1093/brain/awr175. [DOI] [PubMed] [Google Scholar]
- 53.Sharp DJ, Ham TE. Investigating white matter injury after mild traumatic brain injury. Curr Opin Neurol. 2011;24:558–563. doi: 10.1097/WCO.0b013e32834cd523. [DOI] [PubMed] [Google Scholar]
- 54.Sharp DJ, Scott G, Leech R. Network dysfunction after traumatic brain injury. Nat Rev Neurol. 2014;10:156–166. doi: 10.1038/nrneurol.2014.15. [DOI] [PubMed] [Google Scholar]
- 55.Shumskaya E, Andriessen TM, Norris DG, Vos PE. Abnormal whole-brain functional networks in homogeneous acute mild traumatic brain injury. Neurology. 2012;79:175–182. doi: 10.1212/WNL.0b013e31825f04fb. [DOI] [PubMed] [Google Scholar]
- 56.Singh KD, Fawcett IP. Transient and linearly graded deactivation of the human default-mode network by a visual detection task. Neuroimage. 2008;41:100–112. doi: 10.1016/j.neuroimage.2008.01.051. [DOI] [PubMed] [Google Scholar]
- 57.Sorg C, Riedl V, Muhlau M, Calhoun VD, Eichele T, Laer L, Drzezga A, Forstl H, Kurz A, Zimmer C, Wohlschlager AM. Selective changes of resting-state networks in individuals at risk for Alzheimer's disease. Proc Natl Acad Sci U S A. 2007;104:18760–18765. doi: 10.1073/pnas.0708803104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sours C, Zhuo J, Janowich J, Aarabi B, Shanmuganathan K, Gullapalli RP. Default mode network interference in mild traumatic brain injury - a pilot resting state study. Brain Res. 2013;1537:201–215. doi: 10.1016/j.brainres.2013.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sporns O. The human connectome: origins and challenges. Neuroimage. 2013;80:53–61. doi: 10.1016/j.neuroimage.2013.03.023. [DOI] [PubMed] [Google Scholar]
- 60.Sporns O, Tononi G, Kotter R. The human connectome: A structural description of the human brain. PLoS Comput Biol. 2005;1:e42. doi: 10.1371/journal.pcbi.0010042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Squarcina L, Bertoldo A, Ham TE, Heckemann R, Sharp DJ. A robust method for investigating thalamic white matter tracts after traumatic brain injury. Neuroimage. 2012;63:779–788. doi: 10.1016/j.neuroimage.2012.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Stevens MC, Lovejoy D, Kim J, Oakes H, Kureshi I, Witt ST. Multiple resting state network functional connectivity abnormalities in mild traumatic brain injury. Brain Imaging Behav. 2012;6:293–318. doi: 10.1007/s11682-012-9157-4. [DOI] [PubMed] [Google Scholar]
- 63.Stuss DT. Traumatic brain injury: relation to executive dysfunction and the frontal lobes. Curr Opin Neurol. 2011;24:584–589. doi: 10.1097/WCO.0b013e32834c7eb9. [DOI] [PubMed] [Google Scholar]
- 64.Tallus J, Lioumis P, Hamalainen H, Kahkonen S, Tenovuo O. Long-lasting TMS motor threshold elevation in mild traumatic brain injury. Acta Neurol Scand. 2012;126:178–182. doi: 10.1111/j.1600-0404.2011.01623.x. [DOI] [PubMed] [Google Scholar]
- 65.Tang L, Ge Y, Sodickson DK, Miles L, Zhou Y, Reaume J, Grossman RI. Thalamic resting-state functional networks: disruption in patients with mild traumatic brain injury. Radiology. 2011;260:831–840. doi: 10.1148/radiol.11110014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tournier JD, Calamante F, Gadian DG, Connelly A. Direct estimation of the fiber orientation density function from diffusion-weighted MRI data using spherical deconvolution. Neuroimage. 2004;23:1176–1185. doi: 10.1016/j.neuroimage.2004.07.037. [DOI] [PubMed] [Google Scholar]
- 67.Tuch DS, Reese TG, Wiegell MR, Makris N, Belliveau JW, Wedeen VJ. High angular resolution diffusion imaging reveals intravoxel white matter fiber heterogeneity. Magn Reson Med. 2002;48:577–582. doi: 10.1002/mrm.10268. [DOI] [PubMed] [Google Scholar]
- 68.Vulliemoz S, Vollmar C, Koepp MJ, Yogarajah M, O’Muircheartaigh J, Carmichael DW, Stretton J, Richardson MP, Symms MR, Duncan JS. Connectivity of the supplementary motor area in juvenile myoclonic epilepsy and frontal lobe epilepsy. Epilepsia. 2011;52:507–514. doi: 10.1111/j.1528-1167.2010.02770.x. [DOI] [PubMed] [Google Scholar]
- 69.Wang JY, Bakhadirov K, Abdi H, Devous MD, Sr, Marquez de la Plata CD, Moore C, Madden CJ, Diaz-Arrastia R. Longitudinal changes of structural connectivity in traumatic axonal injury. Neurology. 2011;77:818–826. doi: 10.1212/WNL.0b013e31822c61d7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wedeen VJ, Hagmann P, Tseng WY, Reese TG, Weisskoff RM. Mapping complex tissue architecture with diffusion spectrum magnetic resonance imaging. Magn Reson Med. 2005;54:1377–1386. doi: 10.1002/mrm.20642. [DOI] [PubMed] [Google Scholar]
- 71.Wedeen VJ, Wang RP, Schmahmann JD, Benner T, Tseng WY, Dai G, Pandya DN, Hagmann P, D’Arceuil H, de Crespigny AJ. Diffusion spectrum magnetic resonance imaging (DSI) tractography of crossing fibers. Neuroimage. 2008;41:1267–1277. doi: 10.1016/j.neuroimage.2008.03.036. [DOI] [PubMed] [Google Scholar]
- 72.Weissman DH, Roberts KC, Visscher KM, Woldorff MG. The neural bases of momentary lapses in attention. Nat Neurosci. 2006;9:971–978. doi: 10.1038/nn1727. [DOI] [PubMed] [Google Scholar]
- 73.White T, Kendi AT, Lehericy S, Kendi M, Karatekin C, Guimaraes A, Davenport N, Schulz SC, Lim KO. Disruption of hippocampal connectivity in children and adolescents with schizophrenia--a voxel-based diffusion tensor imaging study. Schizophr Res. 2007;90:302–307. doi: 10.1016/j.schres.2006.09.032. [DOI] [PubMed] [Google Scholar]
- 74.Whitnall L, McMillan TM, Murray GD, Teasdale GM. Disability in young people and adults after head injury: 5-7 year follow up of a prospective cohort study. J Neurol Neurosurg Psychiatry. 2006;77:640–645. doi: 10.1136/jnnp.2005.078246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wortzel HS, Kraus MF, Filley CM, Anderson CA, Arciniegas DB. Diffusion tensor imaging in mild traumatic brain injury litigation. J Am Acad Psychiatry Law. 2011;39:511–523. [PubMed] [Google Scholar]
- 76.Xie T, He Y. Mapping the Alzheimer's brain with connectomics. Front Psychiatry. 2011;2:77. doi: 10.3389/fpsyt.2011.00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zarei M, Patenaude B, Damoiseaux J, Morgese C, Smith S, Matthews PM, Barkhof F, Rombouts SA, Sanz-Arigita E, Jenkinson M. Combining shape and connectivity analysis: an MRI study of thalamic degeneration in Alzheimer's disease. Neuroimage. 2010;49:1–8. doi: 10.1016/j.neuroimage.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 78.Zhang K, Johnson B, Gay M, Horovitz SG, Hallett M, Sebastianelli W, Slobounov S. Default mode network in concussed individuals in response to the YMCA physical stress test. J Neurotrauma. 2012;29:756–765. doi: 10.1089/neu.2011.2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhou Y, Dougherty JH, Jr, Hubner KF, Bai B, Cannon RL, Hutson RK. Abnormal connectivity in the posterior cingulate and hippocampus in early Alzheimer's disease and mild cognitive impairment. Alzheimers Dement. 2008;4:265–270. doi: 10.1016/j.jalz.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 80.Zhou Y, Milham MP, Lui YW, Miles L, Reaume J, Sodickson DK, Grossman RI, Ge Y. Default-mode network disruption in mild traumatic brain injury. Radiology. 2012;265:882–892. doi: 10.1148/radiol.12120748. [DOI] [PMC free article] [PubMed] [Google Scholar]


