Populations of large frugivores are declining in tropical rainforests with potential consequences for carbon storage and climate.
Keywords: Ecosystems, tropical ecosystems, defaunation, carbon storage, seed dispersal, conservation, biodiversity, rainforests, bushmeat, Atlantic Forest
Abstract
Carbon storage is widely acknowledged as one of the most valuable forest ecosystem services. Deforestation, logging, fragmentation, fire, and climate change have significant effects on tropical carbon stocks; however, an elusive and yet undetected decrease in carbon storage may be due to defaunation of large seed dispersers. Many large tropical trees with sizeable contributions to carbon stock rely on large vertebrates for seed dispersal and regeneration, however many of these frugivores are threatened by hunting, illegal trade, and habitat loss. We used a large data set on tree species composition and abundance, seed, fruit, and carbon-related traits, and plant-animal interactions to estimate the loss of carbon storage capacity of tropical forests in defaunated scenarios. By simulating the local extinction of trees that depend on large frugivores in 31 Atlantic Forest communities, we found that defaunation has the potential to significantly erode carbon storage even when only a small proportion of large-seeded trees are extirpated. Although intergovernmental policies to reduce carbon emissions and reforestation programs have been mostly focused on deforestation, our results demonstrate that defaunation, and the loss of key ecological interactions, also poses a serious risk for the maintenance of tropical forest carbon storage.
INTRODUCTION
Tropical forests store ~40% of the world’s terrestrial carbon (1), and their deforestation contributes to ~7 to 17% of the global carbon emissions (2, 3). However, tropical carbon has another silent threat. The disappearance of large frugivores may represent a loss in seed dispersal and natural regeneration of large-seeded hardwood plant species, which are key contributors to carbon storage. Therefore, defaunation is a largely unrecognized threat that can affect the sustainability of tropical forest carbon.
Forest degradation is related to selective logging, harvesting of natural products, fragmentation, fire events, and overhunting (4). The intensity of unsustainable hunting is a worldwide problem that has increased in the last few decades over tropical forests (5, 6). All studies on the effects of bushmeat hunting indicate unsustainable levels (7). Hunting threatens approximately 19% of all tropical forest vertebrates (8). However, it does not equally affect all animal community species, with large vertebrates being affected at disproportionately higher rates (9).
The local or functional extinction of large-bodied frugivores has profound implications to forest composition and dynamics because they perform unique ecological roles such as efficient fruit removal, long-distance dispersal, and dispersal of large-seeded plants (5, 10–13). The efficient consumption and dispersal of large seeds are primarily restricted to wide-gaped large frugivores (14); therefore, seed size is an obvious limiting trait for successful dispersal by frugivores that ingest whole fruits or seeds (10). In contrast, small-seeded species can be dispersed by nonthreatened generalist frugivores, which typically inhabit small forest fragments (10, 15). Some frugivorous bats (for example, Artibeus spp.) and terrestrial caviomorph rodents (Dasyprocta spp.) may occasionally eat large-seeded fruits (16), but bats disperse seeds mostly in forest edges and gaps (17), a habitat not suitable for recruitment of these species (18), whereas large rodents are mainly seed eaters (19) and can be also locally extinct in overhunted areas (20).
In addition, there is a well-supported tendency for large hardwood species to have larger fruits and seeds (21–23), mainly in relatively intact forests where carbon stocks are greatest owing to the distinct contribution of large trees (24, 25). Wood density, diameter at breast height, and tree height are keys traits positively related to potential carbon storage capacity across tree species (26). Variation across communities in these traits, which are associated with changes in species composition, has been demonstrated to directly influence variation in biomass estimates by a staggering 70% (27); thus, we hypothesize that defaunation of large frugivores, which limits the recruitment of large-seeded species and induces compositional changes, can alter the community-aggregated values of wood density and height and eventually result in a markedly limited carbon storage capacity.
RESULTS
Here, we quantified the potential effect of defaunation of large-bodied seed dispersers on carbon storage on the basis of the relationship between dispersal and carbon storage traits of 2014 tree species from a tropical biodiversity hot spot, the Atlantic Forest (table S1). We then simulated how this relationship affects the carbon storage potential of 31 sites that represent the largest forest remnants (table S2) (28).
In each forest site, we simulated extinctions of large-seeded trees induced by the lack of large frugivores and compared the carbon loss between replicated scenarios of defaunation-driven extinctions and a null model with random extinctions (Fig. 1). We defined large-seeded species on the basis of the analysis of more than 5000 fruit-frugivore interactions and their seed traits for the Atlantic Forest biome (see the Supplementary Materials). We found that resilient frugivores such as small birds, bats, and marsupials, which are not targeted by hunters (9), can disperse seeds up to 12.0 ± 1.1 mm in width (fig. S1). This threshold also corresponds to a seed size limit where successful dispersal would be seriously impaired under post-defaunation scenarios in the Atlantic Forest (10). The simulated defaunation scenarios consisted of the extinction of large-seeded species (10 to 100% of the individuals) and its replacement by any other tree species remnant in the community. The simulated scenarios are governed by a zero-sum game where communities retain the same number of individuals and the same basal area (29). We assume that the probability of extinction is proportional to seed size and the probability of recruitment is proportional to the species abundance. We also allow any remaining large-seeded species to enter the replacement game because dispersal by bats or rodents and near-parent recruitment can occur (fig. S1).
Fig. 1. Simulation pathway of frugivore defaunation on carbon storage.
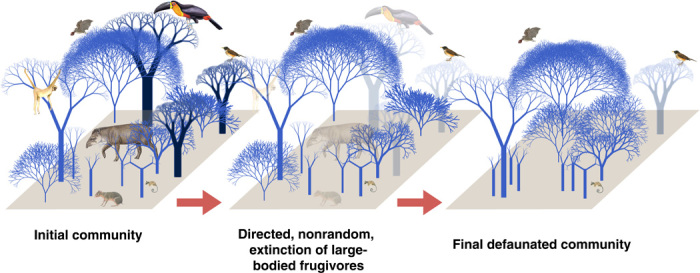
We generated downgraded communities with altered species composition. Each simulation had two main steps. First, we simulated directed extinctions induced by defaunation (loss of tree species with seed size ≥12.0 mm) or random extinction (that is, tree species removal independent of seed size). Second, we simulated a compensatory replacement of the individuals by the remaining species pool after defaunation by adding the same number of individuals and basal area removed. Dark blue indicates tree individuals of hardwood species with large seeds (≥12.0 mm) and different trunk diameters, light blue represents other tree species.
A total of 813 species and 101,211 individuals were represented in these 31 communities, which are large forest fragments (that is, minimum area ≥1000 ha) spread through the whole range of Atlantic Forest types. This patch size is not prone to dispersal limitation and edge effects (30, 31). Finally, we explore how abiotic forest site (elevation, forest type, temperature, precipitation, latitude) and forest compositional characteristics (richness and abundance of abiotic and resilient species) may explain changes in carbon storage.
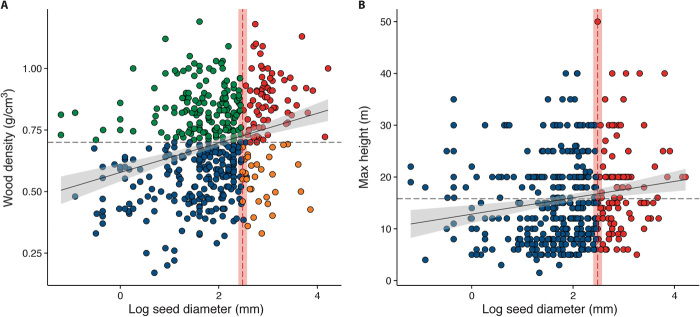
We observed an important contribution of large-seeded trees to carbon storage potential. Species with large animal-dispersed seeds (≥12.0 ± 1.1 mm) represented 21% of our sample, 70% of which had high wood density (>0.7 g/cm3) and tended to be higher-stature trees (fig. S2). Fifty-four percent of these species have recalcitrant seeds that cannot tolerate drought prior to germination (table S1). In addition, we found a functional relationship between seed diameter and traits related to carbon storage. We found a positive correlation between seed diameter and wood density (rs = 0.22, P < 0.001, N = 732) and between seed diameter and maximum tree height (rs = 0.21, P < 0.001, N = 1087), especially for animal-dispersed species (Fig. 2 and table S3). Conversely, wind- or gravity-dispersed species did not show a significant association between seed size and wood density (fig. S3). Therefore, trees bearing seeds larger than 12 mm have high carbon stock capacity, and large-bodied dispersers are functionally connected to forest carbon storage, given their distinct link with large-seeded trees.
Fig. 2. Relationships between seed diameter and carbon storage–related traits in animal-dispersed trees.
The black solid line shows the linear regression fit for the trend and the confidence interval (gray envelopes). The red vertical line indicates the seed diameter threshold of 12 mm. Points represent tree species. (A) Wood density and seed diameter (rs = 0.28, P < 0.001, N = 486). The gray dashed horizontal line indicates a wood density = 0.7 g/cm3. Red points are endangered species with dense wood; orange points are endangered species with light wood; green points are nonendangered species with dense wood (resilient hardwood species); and blue points are nonendangered species with light wood. (B) Maximum tree height (m) and seed diameter (mm) (rs = 0.25, P < 0.001, N = 783). Red points are endangered species, and blue points are nonendangered species.
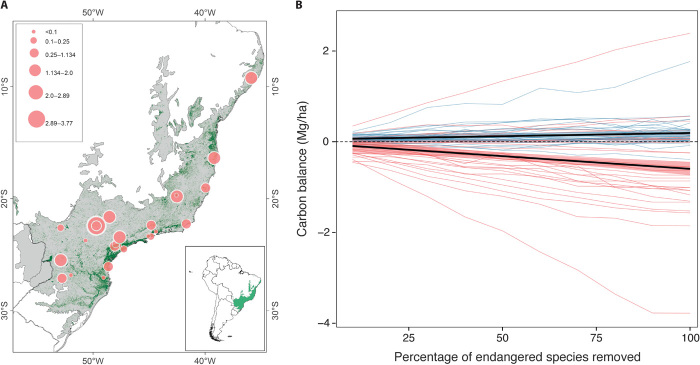
We found strong support for the hypothesis that removal of large-seeded trees will erode carbon stocks in defaunated tropical forests. We observed a greater loss of carbon as the percentage of removed large-seeded tree species increases, as a consequence of defaunation of large frugivores. This response significantly deviates from a random extinction scenario, even when few species are removed (for example, 10%) (Fig. 3B and table S4). Those changes were consistent at the landscape scale throughout the heterogeneous conditions of the different communities, being more pronounced in warmer sites (fig. S4). However, in plant communities where the dominance of hardwood resilient species (that is, small-seeded species with high wood density) exceeds ~50% of individuals (fig. S5 and tables S5 and S6), carbon loss is slowed down. Moreover, we found that the compensatory role of large frugivore substitutes that are not affected by hunting in defaunated rainforests, such as rodents and bats, remains questionable (fig. S1).
Fig. 3. Carbon deficit after defaunation simulation in Atlantic forest sites.
(A) Locations of the 31 communities studied. The size of the points represents the magnitude of carbon loss (Mg/ha). (B) Carbon balance after simulated changes in carbon storage capacity in the random (blue) and defaunated (red) scenarios over the 31 selected communities. Initial carbon was used as the 0 or neutral point. A negative balance represents a net carbon loss, and positive values indicate gains in carbon storage. Lines represent the simulated trajectories for each community. The black lines show the mean combined values for all communities in each scenario and their confidence interval. The width of the confidence interval for the random scenario trend was increased 2× to improve visualization.
DISCUSSION
Defaunation is a human-induced process that significantly erodes key ecosystem services and functions through direct and indirect cascading effects (5, 32, 33). Defaunation has been shown to affect pollination, seed dispersal, pest control, nutrient cycling, decomposition, water quality, and soil erosion (34). Now, we have evidence that defaunation will, over time, result in significantly decreased carbon storage ecosystem service in tropical forests where animal-dispersed plants are abundant (35) and crucially dependent on large frugivores. Our findings may also translate into the Amazonian forests where most of the tree species that retain 50% of the carbon are also dispersed by large frugivores (36, 37), but they will be slowed down in forests that are dominated by abiotic hardwood species, such as the Dipterocarpaceae forests in Southeast Asia (32).
Our result highlights the fragility of carbon storage service in tropical forests under the current global change conditions. Processes such as fragmentation (30, 38–40), climate change, liana overabundance (41–43), and human-ignited fires (44–46) will enhance the effects of carbon loss in defaunated ecosystems.
Halting the ongoing, fast-paced defaunation of tropical forests will not only save large charismatic animals and the plants they disperse but also have effects on climate change, carbon markets, and reforestation processes. For instance, restoration and REDD+ programs should achieve a complete vision of biotic interactions and processes to guarantee carbon storage capacity and its co-benefits. Their effectiveness over climate change will be improved by ensuring the array of biotic processes that support the target ecological services addressed by these initiatives.
MATERIALS AND METHODS
Study site
The Atlantic Forest spans from 3° to 31° latitude south, from 35° to 60° longitude west, and from sea level to approximately 2800 m above sea level, which ensures a wide latitudinal and altitudinal gradient from tropical to subtropical regions (47). In this biome, about 89% of all woody species are animal-dispersed (48). We selected 31 independent large forest communities across the latitudinal and altitudinal gradients of the Atlantic Forest to simulate the effects of defaunation on carbon storage. These tree communities were obtained from a recent assessment of the existing knowledge on the Atlantic Forest that included more than 1000 tree community surveys (28). To obtain the 31 forest communities, we filtered this database by selecting only the studies (i) with a sampling area larger than or equal to 1 ha, (ii) with a cutoff criterion of stem diameter at breast height ≥5 cm, (iii) conducted in forest fragments ≥1000 ha of the whole range of Atlantic Forest types [because this is the minimum patch size at which the effect of carbon loss due to edge effect is minimized (30)], (iv) with a robust taxonomic resolution at species level recognized by REFLORA (49), and (v) with information on dispersal mode and carbon traits in more than 50% of each community species (table S2). All the communities’ surveys were carried out after 1990. These large fragments represent just 0.05% of all remaining fragments of the Atlantic Forest, but concentrate 41% of the remaining area (6.6 million ha of the remnant 16 million ha of the Atlantic Forest) (50). For each community, we obtained species name, number of individuals (N), basal area [BA (m2)], absolute density (DA = N/ha), and absolute dominance (DO = BA/ha).
Plant traits
We compiled information on tree species of the Atlantic Forest from the TreeAtlan 2.0 database (51) and TreeCo (28). We explored quantitative traits related to seed dispersal (seed and fruit diameter and length) and to potential carbon storage (wood density and maximum height). Seed and fruit traits were obtained from previous studies (48, 52–101), our own measurements in herbaria, and private collections. Carbon traits were obtained from different literature sources (60, 102–104). For the simulation process, we used information at species level for wood density; however, when such information was not available, we used the means of the genera.
We tested the relationship between carbon and dispersal traits for 2014 species (table S1), which represent 28% of the trees and shrub species described for the Atlantic Forest (49). We used simple correlations for the whole set of species differentiated by dispersal mode. We used log transformation and Spearman correlations because not all traits satisfied a normal distribution.
Delimitation of endangered species
To determine which plant species will be threatened by the local extinction of large frugivore defaunation, we examined a plant-frugivore interaction data set combined with information on seed traits. This data set contains information on ca. 5000 fruit-frugivore interactions from the entire Atlantic Forest (105–181). This data set includes animal- and plant-oriented studies that reported the occurrence of interactions, that is, a given animal species feeding on fruits of a particular plant species. From these interactions, we recorded plant and animal taxonomy and related each plant with its carbon traits (wood density, maximum height) and dispersal traits (fruit and seed diameter and length).
We selected the maximum seed diameter dispersed by frugivores that are not threatened by hunting, such as small birds, bats, and marsupials (9), as the threshold limit for defining species endangered by defaunation of large frugivores. We also used the confidence interval of the mean seed size distribution (±1.09 mm) around the threshold limit to allow variability in this threshold value (fig. S1). Therefore, we classified those tree species having animal-dispersed seeds and seed diameter ≥12 ±1.09 mm as endangered because large frugivores are the only effective dispersers with gapes wide enough to effectively consume and disperse such large seeds (10, 14). We also classified as hardwood species those with a wood density >0.7 g/cm3, according to UNE 56-540-78 (182).
Simulated scenarios
For each of the 31 large forest communities (table S2), we generated two hypothetical scenarios of downgraded communities with altered species composition: the random extinctions scenario with tree species removal independent of seed size, and the directed extinctions scenario induced by defaunation of large-bodied frugivores with removal of tree species with seed size ≥12.0 ± 1.09 mm (Fig. 1). Each simulation had two main steps. First, we simulated extinctions; and second, we simulated a compensatory replacement of the individuals by adding the numbers of individuals removed, but of species drawn from the remaining community pool, to construct a new final community.
In each scenario, we removed a crescent percentage (from 10 to 100%) of large-seeded species and did 1000 repetitions for each percentage class. These numbers ranged from 1 species (10%) to the maximum number of endangered species (100%) in each community. For the random extinctions scenarios, we randomly removed the same number of species.
In the simulations, we assumed saturated communities with zero-sum game dynamic and immigration is equal to zero (29). We made sure that the basal area and the total number of individuals remained constant. Further, we assumed that the extinction probability of a large-seeded species is proportional to its seed size. The recruitment probability of each species is proportional to its abundance, and we allowed the remaining large-seeded species to enter in the replacement game because dispersal by bats and rodents and near-parent recruitment could occur (for detailed information, see Code file S1).
We explored the carbon balance and the magnitude of carbon loss for each percentage of endangered species removed. We assessed the carbon balance by comparing the estimated carbon of the final (downgraded) scenario community and the carbon in the initial (pristine) community for each percentage of removed species. The carbon of the initial community was used as the 0 or neutral point; therefore, carbon balance was calculated as
| (1) |
where Cf is the carbon in the final community and Ci is the carbon in the initial community, both expressed in megagrams per hectare (Mg/ha).
The magnitude of carbon loss was estimated as the difference between the final carbon in the defaunated scenario and the final carbon in the random scenario at each percentage of endangered species removed. The simulations were applied independently for each community and then aggregated in the mean response for all communities. We also explored the relationship between the magnitude of carbon loss against abiotic variables (altitude, forest type, temperature, precipitation, and latitude) and species compositional variables (richness and abundance of abiotic and resilient species) using generalized linear models. We used the Gaussian family for the error distribution. We obtained the abiotic variables using the community location and climatic information from Hijmans et al. (183) and the forest size information from Ribeiro et al. (50). Compositional data were calculated from the reported abundance data of each community (table S2). The abiotic variables of the community sites were altitude, latitude, annual precipitation, mean annual temperature, and forest size. For compositional variables, we explored the percentage, quantity, and dominance of three types of species: (i) endangered species (large-seeded trees; seed diameter >12 mm), (ii) animal-dispersed resilient species (seed diameter <12 mm and dense wood), and (iii) abiotically dispersed hardwood species.
Carbon estimation
We estimated the carbon stock in each community twice: first at the initial community [initial carbon (Ci)] and then at the final community [final carbon (Cf)], in each scenario. To estimate the amount of above-ground biomass (AGB), we used a proxy for biomass that related the three main traits related to carbon storage potential: basal area (related to diameter at breast height), wood density, and maximum height (26). In particular, we used total basal area (BA) in hectares (DO) of the species. BA is widely used as a proxy for biomass and carbon stock (184, 185), and we weighted it by the effects of the wood density and tree height.
Here, we show that these estimates are linearly and closely related to AGB of Atlantic Forest communities (fig. S6), so we can have a fair estimate of the population AGB for each site based on the population BA, which is the only information available for all sites at the species level.
To inspect the relationship between this estimate, we used the data from four 10.24-ha forest plots placed at four contrasting types of forest from southeastern Brazil: rainforest, seasonal forest, white-sand (Restinga) forest, and savanna forest (locally known as “Cerradão”) (186). The plots vary greatly in their tree density, basal area, and species richness. Thus, they represent a good sample among the wide spectrum of possible types of Atlantic Forests. Although we have not included any savanna forest site in the main analysis (see the text), we decided to include it here to have a wider variation in total basal area estimates. Population values of BA for all four plots varied between 0.002 and 56.3 m2 per 10.24 ha, whereas AGB varied between 0.003 and 444.5 Mg per 10.24 ha. These ranges cover the entire variation of BA found in the 31 sites studied here because these 10.24-ha forest plots were the sites with the largest sample sizes included in the simulations presented in the text.
For each species at each plot, we calculated the BA (m2) and AGB (Mg). Estimates of AGB were obtained using the allometric equations for moist forests provided by Chave et al. (26) based on individual field measurements of tree diameter at breast height and tree height. The mean values of wood specific gravity (WSG) for each species were obtained from the literature as stated above, and when this mean value was not available at the species level, we again used the generic means from the study of Chave et al. (26). We then used linear regression to relate the AGB for each species as a function of basal area × wood density × tree height. The variables were log-transformed prior to analysis, which was performed separately for each permanent forest plot. Thus, the analysis corresponds to a total of 601 populations of 483 tree species.
Our carbon proxy (BA × WSG × height) explained a large amount of the variation in species AGB (adjusted R2 ≥ 93.7%). For all sites, our proxy explained from 93.7 to 96% of the variation in species AGB. It was more efficient in predicting AGB in seasonal forests and less efficient in rainforests (fig. S6). Although we did find a site effect on the relationship between AGB and BA × WSG, the regression performed by combining populations from the four sites had a good development (fig. S7) and still explained a large amount of AGB variation (adjusted R2 = 94.6%), resulting in the following general relationship
where AGB is the above-ground biomass (Mg/ha), wooden is the wood density (g/cm3), BA is the basal area (m2/ha), and height is the reported maximum height. Finally, to determine the carbon concentration in the AGB, we used the estimation of 40% of water in the AGB and 48.5% of carbon in the dry biomass (187).
Supplementary Material
Acknowledgments
We thank E. Cazetta, V. Staggemeier, and P. Brancalion for sending additional data on seed size; Museu de Zoologia de São Paulo (MZUSP) for providing access to the collection; R. R. Rodrigues for providing the raw field data; and P. Brancalion, T. Siqueira, R. Chazdon, M. Ribeiro, T. Hoffman, M. C. Côrtes, and B. Anderson for ideas and comments on the manuscript. Funding: C.B., M.G., and R.A.F.L. thank the São Paulo Research Foundation (FAPESP) (grant nos. 2014/01986-0, 2013/22492-2, and 2013/08722-5). P.J. received financial support from Conselho Nacional de Desenvolvimento Científico (CNPq), Excellence Grant-Junta Andalucía. M.G. is a research fellow at Conselho Nacional de Desenvolvimento Científico e Tecnológico. P.J. is a visiting research fellow at CAPES (Programa Ciências Sem Fronteiras). L.F.S.M. was supported by Projeto Floresta Escola and by a postdoctoral grant from CAPES/PNPD. M.F.R. received a postdoctoral grant from Projeto Floresta Escola; O.O. was supported by the Academy of Finland (grant nos. 273523 and 284601). Author contributions: C.B., P.J., R.A.F.L., L.F.S.M., M.G., and O.O. designed the analyses. C.B., P.J., and L.F.S.M. analyzed the data. C.B., P.J., and M.G. created the figures. M.G., M.P., M.F.R., C.B., L.F.S.M., and R.A.F.L. performed the data collection. All authors contributed to the writing and extensive revisions of the manuscript. Competing interests: The authors declare that they have no competing interests. Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. Additional data related to this paper may be requested from the authors. Simulation R code and example data are available in the repository. Plant trait information is available as an Excel file.
SUPPLEMENTARY MATERIALS
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/1/11/e1501105/DC1
Fig. S1. Distribution function of seed size diameter (mm) dispersed by the major frugivores in the Atlantic forest, Brazil.
Fig. S2. Maximum tree height by class of species according to its seed diameter and wood density.
Fig. S3. Relationship between wood density and seed diameter by dispersal mode.
Fig. S4. Relationships between abiotic variables and magnitude of carbon loss.
Fig. S5. Relationships between the compositional variables of each community and its magnitude of carbon loss.
Fig. S6. Linear regression of the above-ground biomass (AGB) and the proxy for basal area (BA) times the wood specific gravity (WSG) times maximum height for the different types of forest.
Fig. S7. Diagnostic plots of the regression model using basal area (BA) times the wood specific gravity (WSG) times tree maximum height (MaxHeight) as a proxy for AGB.
Table S1. Trait information of the 2014 species analyzed (available in the data repository).
Table S2. Atlantic Forest communities analyzed, their spatial localization in Brazil, and abiotic characteristics.
Table S3. Spearman correlations among dispersal traits and carbon traits.
Table S4. T test between carbon loss in random scenarios and defaunated scenarios at different intervals of species removed.
Table S5. Generalized linear model results showing the influence of abiotical and compositional variables on the magnitude of carbon loss of each community.
Table S6. Compositional characteristics of Atlantic Forest communities.
Supplementary code and data file available at
https://github.com/pedroj/MS_Carbon (DOI:10.5281/zenodo.31880).
Code file S1. Simulation code in R (Simulation_Code.RMD).
Code file S2. Read me (Simulation_Code.html).
Data file S1. Trait information of the 2014 species analyzed (Table S1_Trait Data. xls).
Data file S2. Community data example for the simulation code (prove_community.csv).
REFERENCES AND NOTES
- 1.Dixon R. K., Solomon A. M., Brown S., Houghton R. A., Trexier M. C., Wisniewski J., Carbon pools and flux of global forest ecosystems. Science 263, 185–190 (1994). [DOI] [PubMed] [Google Scholar]
- 2.Harris N. L., Brown S., Hagen S. C., Saatchi S. S., Petrova S., Salas W., Hansen M. C., Potapov P. V., Lotsch A., Baseline map of carbon emissions from deforestation in tropical regions. Science 336, 1573–1576 (2012). [DOI] [PubMed] [Google Scholar]
- 3.van der Werf G. R., Morton D. C., DeFries R. S., Olivier J. G. J., Kasibhatla P. S., Jackson R. B., Collatz G. J., Randerson J. T., CO2 emissions from forest loss. Nat. Geosci. 2, 737–738 (2009). [Google Scholar]
- 4.Putz F. E., Redford K. H., The importance of defining “forest”: Tropical forest degradation, deforestation, long-term phase shifts, and further transitions. Biotropica 42, 10–20 (2010). [Google Scholar]
- 5.Poulsen J. R., Clark C. J., Palmer T. M., Ecological erosion of an Afrotropical forest and potential consequences for tree recruitment and forest biomass. Biol. Conserv. 163, 122–130 (2013). [Google Scholar]
- 6.Peres C. A., Palacios E., Basin-wide effects of game harvest on vertebrate population densities in Amazonian forests: Implications for animal-mediated seed dispersal. Biotropica 39, 304–315 (2007). [Google Scholar]
- 7.Milner-Gulland E. J., Bennett E. L., Wild meat: The bigger picture. Trends Ecol. Evol. 18, 351–357 (2003). [Google Scholar]
- 8.IUCN, Red List of Threatened Species (International Union for Conservation of Nature and Natural Resources, Cambridge, UK, 2007). [Google Scholar]
- 9.Peres C. A., Effects of subsistence hunting on vertebrate community structure in Amazonian forests. Conserv. Biol. 14, 240–253 (2000). [Google Scholar]
- 10.Galetti M., Guevara R., Côrtes M. C., Fadini R., Von Matter S., Leite A. B., Labecca F., Ribeiro T., Carvalho C. S., Collevatti R. G., Pires M. M., Guimarães P. R. Jr, Brancalion P. H., Ribeiro M. C., Jordano P., Functional extinction of birds drives rapid evolutionary changes in seed size. Science 340, 1086–1090 (2013). [DOI] [PubMed] [Google Scholar]
- 11.Nuñez-lturri G., Howe H. F., Bushmeat and the fate of trees with seeds dispersed by large primates in a lowland rain forest in Western Amazonia. Biotropica 39, 348–354 (2007). [Google Scholar]
- 12.Terborgh J., Nuñez-Iturri G., Pitman N. C. A., Valverde F. H. C., Alvarez P., Swamy V., Pringle E. G., Paine C. E. T., Tree recruitment in an empty forest. Ecology 89, 1757–1768 (2008). [DOI] [PubMed] [Google Scholar]
- 13.Kurten E. L., Wright S. J., Carson W. P., Hunting alters seedling functional trait composition in a Neotropical forest. Ecology 96, 1923–1932 (2015). [DOI] [PubMed] [Google Scholar]
- 14.Levey D. J., Seed size and fruit-handling techniques of avian frugivores. Am. Nat. 129, 471–485 (1987). [Google Scholar]
- 15.Cardoso da Silva J. M., Tabarelli M., Tree species impoverishment and the future flora of the Atlantic forest of northeast Brazil. Nature 404, 72–74 (2000). [DOI] [PubMed] [Google Scholar]
- 16.Melo F. P. L., Rodriguez-Herrera B., Chazdon R. L., Medellin R. A., Ceballos G. G., Small tent-roosting bats promote dispersal of large-seeded plants in a Neotropical forest. Biotropica 41, 737–743 (2009). [Google Scholar]
- 17.Peters S. L., Malcolm J. R., Zimmerman B. L., Effects of selective logging on bat communities in the southeastern Amazon. Conserv. Biol. 20, 1410–1421 (2006). [DOI] [PubMed] [Google Scholar]
- 18.de Melo F. P. L., Dirzo R., Tabarelli M., Biased seed rain in forest edges: Evidence from the Brazilian Atlantic forest. Biol. Conserv. 132, 50–60 (2006). [Google Scholar]
- 19.Jansen P. A., Bongers F., Hemerik L., Seed mass and mast seeding enhance dispersal by a Neotropical scatter-hoarding rodent. Ecol. Monogr. 74, 569–589 (2004). [Google Scholar]
- 20.Galetti M., Guevara R., Neves C. L., Rodarte R. R., Bovendorp R. S., Moreira M., Hopkins J. B. III, Yeakel J. D., Defaunation affects the populations and diets of rodents in Neotropical rainforests. Biol. Conserv. 190, 2–7 (2015). [Google Scholar]
- 21.Wright I. J.,Ackerly D. D., Bongers F., Harms K. E., Ibarra-Manriquez G., Martinez-Ramos M., Mazer S. J., Muller-Landau H. C., Paz H., Pitman N. C. A., Poorter L., Silman M. R., Vriesendorp C. F., Webb C. O., Westoby M., Wright S. J., Relationships among ecologically important dimensions of plant trait variation in seven Neotropical forests. Ann. Bot. 99, 1003–1015 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Diaz S., Hodgson J. G., Thompson K., Cabido M., Cornelissen J. H. C., Jalili A., Montserrat-Martí G., Grime J. P., Zarrinkamar F., Asri Y., Band S. R., Basconcelo S., Castro-Díez P., Funes G., Hamzehee B., Khoshnevi M., Pérez-Harguindeguy N., Pérez-Rontomé M. C., Shirvany F. A., Vendramini F., Yazdani S., Abbas-Azimi R., Bogaard A., Boustani S., Charles M., Dehghan M., de Torres-Espuny L., Falczuk V., Guerrero-Campo J., Hynd A., Jones G., Kowsary E., Kazemi-Saeed F., Maestro-Martínez M., Romo-Díez A., Shaw S., Siavash B., Villar-Salvador P., Zak M. R., The plant traits that drive ecosystems: Evidence from three continents. J. Veg. Sci. 15, 295–304 (2004). [Google Scholar]
- 23.Thompson K., Rabinowitz D., Do big plants have big seeds? Am. Nat. 133, 722–728 (1989). [Google Scholar]
- 24.Lindner A., Biomass storage and stand structure in a conservation unit in the Atlantic Rainforest—The role of big trees. Ecol. Eng. 36, 1769–1773 (2010). [Google Scholar]
- 25.Stephenson N. L., Das A. J., Condit R., Russo S. E., Baker P. J., Beckman N. G., Coomes D. A., Lines E. R., Morris W. K., Rüger N., Álvarez E., Blundo C., Bunyavejchewin S., Chuyong G., Davies S. J., Duque Á., Ewango C. N., Flores O., Franklin J. F., Grau H. R., Hao Z., Harmon M. E., Hubbell S. P., Kenfack D., Lin Y., Makana J.-R., Malizia A., Malizia L. R., Pabst R. J., Pongpattananurak N., Su S.-H., Sun I.-F., Tan S., Thomas D., van Mantgem P. J., Wang X., Wiser S. K., Zavala M. A., Rate of tree carbon accumulation increases continuously with tree size. Nature 507, 90–93 (2014). [DOI] [PubMed] [Google Scholar]
- 26.Chave J., Andalo C., Brown S., Cairns M. A., Chambers J. Q., Eamus D., Fölster H., Fromard F., Higuchi N., Kira T., Lescure J.-P., Nelson B. W., Ogawa H., Puig H., Riéra B., Yamakura T., Tree allometry and improved estimation of carbon stocks and balance in tropical forests. Oecologia 145, 87–99 (2005). [DOI] [PubMed] [Google Scholar]
- 27.Baker T. R., Phillips O. L., Laurance W. F., Pitman N. C. A., Almeida S., Arroyo L., DiFiore A., Erwin T., Higuchi N., Killeen T. J., Laurance S. G., Nascimento H., Monteagudo A., Neill D. A., Silva J. N. M., Malhi Y., López Gonzalez G., Peacock J., Quesada C. A., Lewis S. L., Lloyd J., Do species traits determine patterns of wood production in Amazonian forests? Biogeosciences 6, 297–307 (2009). [Google Scholar]
- 28.de Lima R. A. F., Mori D. P., Pitta G., Melito M. O., Bello C., Magnago L. F., Zwiener V. P., Saraiva D. D., Marques M. C. M., de Oliveira A. A., Prado P. I., How much do we know about the endangered Atlantic Forest? Reviewing nearly 70 years of information on tree community surveys. Biodivers. Conserv. 24, 2135–2148 (2015). [Google Scholar]
- 29.S. P. Hubbell, The Unified Neutral Theory of Biodiversity and Biogeography (Princeton Univ. Press, Princeton, NJ, 2001). [Google Scholar]
- 30.Pütz S. Groeneveld J., Henle K., Knogge C., Martensen A. C., Metz M., Metzger J. P., Ribeiro M. C., de Paula M. D., Huth A., Long-term carbon loss in fragmented Neotropical forests. Nat. Commun. 5, 5037 (2014). [DOI] [PubMed] [Google Scholar]
- 31.Lôbo D., Leão T., Melo F. P. L., Santos A. M. M., Tabarelli M., Forest fragmentation drives Atlantic forest of northeastern Brazil to biotic homogenization. Divers. Distrib. 17, 287–296 (2011). [Google Scholar]
- 32.Kurten E. L., Cascading effects of contemporaneous defaunation on tropical forest communities. Biol. Conserv. 163, 22–32 (2013). [Google Scholar]
- 33.Markl J. S., Schleuning M., Forget P. M., Jordano P., Lambert J. E., Traveset A., Wright S. J., Böhning-Gaese K., Meta-analysis of the effects of human disturbance on seed dispersal by animals. Conserv. Biol. 26, 1072–1081 (2012). [DOI] [PubMed] [Google Scholar]
- 34.Dirzo R., Young H. S., Galetti M., Ceballos G., Isaac N. J. B., Collen B., Defaunation in the Anthropocene. Science 345, 401–406 (2014). [DOI] [PubMed] [Google Scholar]
- 35.Howe H. F., Smallwood J., Ecology of seed dispersal. Annu. Rev. Ecol. Syst. 13, 201–228 (1982). [Google Scholar]
- 36.Fauset S., Johnson M. O., Gloor M., Baker T. R., Monteagudo M. A., Brienen R. J. W., Feldpausch T. R., Lopez-Gonzalez G., Malhi Y., ter Steege H., Pitman N. C. A., Baraloto C., Engel J., Pétronelli P., Andrade A., Camargo J. L. C., Laurance S. G. W., Laurance W. F., Chave J., Allie E., Núñez Vargas P., Terborgh J. W., Ruokolainen K., Silveira M., Aymard C. G. A., Arroyo L., Bonal D., Ramirez-Angulo H., Araujo-Murakami A., Neill D., Hérault B., Dourdain A., Torres-Lezama A., Marimon B. S., Salomão R. P., Comiskey J. A., Réjou-Méchain M., Toledo M., Licona J. C., Alarcón A., Prieto A., Rudas A., van der Meer P. J., Killeen T. J., Marimon Junior B.-H., Poorter L., Boot R. G. A., Stergios B., Torre E. V., Costa F. R. C., Levis C., Schietti J., Souza P., Groot N., Arets E., Moscoso V. C., Castro W., Coronado E. N. H., Peña-Claros M., Stahl C., Barroso J., Talbot J., Vieira I. C. G., van der Heijden G., Thomas R., Vos V. A., Almeida E. C., Davila E. Á., Aragão L. E. O. C., Erwin T. L., Morandi P. S., de Oliveira E. A., Valadão M. B. X., Zagt R. J., van der Hout P., Loayza P. A., Pipoly J. J., Wang O., Alexiades M., Cerón C. E., Huamantupa-Chuquimaco I., Di Fiore A., Peacock J., Camacho N. C. P., Umetsu R. K., de Camargo P. B., Burnham R. J., Herrera R., Quesada C. A., Stropp J., Vieira S. A., Steininger M., Rodríguez C. R., Restrepo Z., Muelbert A. E., Lewis S. L., Pickavance G. C., Phillips O. L., Hyperdominance in Amazonian forest carbon cycling. Nat. Commun. 6, 6857 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.C. Peres, M. G. M. Roosmalen, in Seed Dispersal and Frugivory: Ecology, Evolution and Conservation, D. Levey, W. R. Silva, M. Galetti, Eds. (CAB International, Oxon, UK, 2002), pp. 407–421. [Google Scholar]
- 38.Laurance W. F., Laurance S. G., Ferreira L. V., Merona J. M. R.-de, Gascon C., Lovejoy T. E., Biomass collapse in Amazonian forest fragments. Science 278, 1117–1118 (1997). [Google Scholar]
- 39.Michalski F., Peres C. A., Disturbance-mediated mammal persistence and abundance-area relationships in Amazonian forest fragments. Conserv. Biol. 21, 1626–1640 (2007). [DOI] [PubMed] [Google Scholar]
- 40.de Paula M. D., Costa C. P. A., Tabarelli M., Carbon storage in a fragmented landscape of Atlantic forest: The role played by edge-affected habitats and emergent trees. Trop. Conserv. Sci. 4, 349–358 (2011). [Google Scholar]
- 41.Wright S. J., Hernandéz A., Condit R., The bushmeat harvest alters seedling banks by favoring lianas, large seeds, and seeds dispersed by bats, birds, and wind. Biotropica 39, 363–371 (2007). [Google Scholar]
- 42.Durán S. M., Gianoli E., Carbon stocks in tropical forests decrease with liana density. Biol. Lett. 9, 20130301 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van der Heijden G. M., Schnitzer S. A., Powers J. S., Phillips O. L., Liana impacts on carbon cycling, storage and sequestration in tropical forests. Biotropica 45, 682–692 (2013). [Google Scholar]
- 44.Nepstad D. C.,Verssimo A., Alencar A., Nobre C., Lima E., Lefebvre P., Schlesinger P., Potter C., Moutinho P., Mendoza E., Cochrane M., Brooks V., Large-scale impoverishment of Amazonian forests by logging and fire. Nature 398, 505–508 (1999). [Google Scholar]
- 45.Cochrane M. A., Alencar A., Schulze M. D., Souza C. M. Jr, Nepstad D. C., Lefebvre P., Davidson E. A., Positive feedbacks in the fire dynamic of closed canopy tropical forests. Science 284, 1832–1835 (1999). [DOI] [PubMed] [Google Scholar]
- 46.Cochrane M. A., Schulze M. D., Fire as a recurrent event in tropical forests of the eastern Amazon: Effects on forest structure, biomass, and species composition. Biotropica 31, 2–16 (1999). [Google Scholar]
- 47.Morellato L. P. C., Haddad C. F. B., Introduction: The Brazilian Atlantic Forest. Biotropica 32, 786–792 (2000). [Google Scholar]
- 48.Almeida-Neto M., Campassi F., Galetti M., Jordano P., Oliveira-Filho A., Vertebrate dispersal syndromes along the Atlantic forest: Broad-scale patterns and macroecological correlates. Glob. Ecol. Biogeogr. 17, 503–513 (2008). [Google Scholar]
- 49.REFLORA (Jardim Botânico do Rio de Janeiro, 2014); http://reflora.jbrj.gov.br/jabot/listaBrasil/ConsultaPublicaUC/ConsultaPublicaUC.do.
- 50.Ribeiro M. C., Metzger J. P., Martensen A. C., Ponzoni F. J., Hirota M. M., The Brazilian Atlantic Forest: How much is left, and how is the remaining forest distributed? Implications for conservation. Biol. Conserv. 142, 1141–1153 (2009). [Google Scholar]
- 51.A. T. Oliveira-Filho, in Um banco de dados envolvendo biogeografia, diversidade e conservação, www.icb.ufmg.br/treeatlan/ (2010).
- 52.Secco R. D. S., Alchorneae (Euphorbiaceae) (Alchornea, Aparisthmium e Conceveiba). Flora Neotrop. 93, 1–194 (2004). [Google Scholar]
- 53.Gentry A. H., Bignoniaceae. Part II (tribe Tecomeae). Flora Neotrop. 25, 1–370 (1992). [Google Scholar]
- 54.Marquete R., da Fonseca Vaz A. M. S., O gênero Casearia no estado do Rio de Janeiro, Brasil. Rodriguésia 58, 705–738 (2007). [Google Scholar]
- 55.Prance G. T., Chrysobalanaceae. Fl. Neotrop. Monogr. 9, 1–409 (1972). [Google Scholar]
- 56.Forero E., Connaraceae. Fl. Neotrop. Monogr. 36, 1–208 (1983). [Google Scholar]
- 57.de Moraes P., Taxonomy of Cryptocarya species of Brazil. Abc Taxa 3, 191 (2007). [Google Scholar]
- 58.Maas P. J. M., Westra L. Y. T., Chatrou L., Duguetia (Annonaceae). Floral Neotrop. 88, 1–274 (2003). [Google Scholar]
- 59.Dutra S. M., Salimena F. R. G., Menini Neto L., Annonaceae na Serra Negra, Minas Gerais, Brasil. Rodriguésia 63, 785–793 (2012). [Google Scholar]
- 60.H. Lorenzi, Árvores brasileiras. Manual de identificacao e cultivo de plantas arboreas nativas do Brasil (Instituto Plantarum de Estudos da Flora, Nova Odessa, Brazil, 2009), 384 pp. [Google Scholar]
- 61.Lobão A. Q., Mello-Silva R. d., Forzza R. C., Guatteria (Annonaceae) da Floresta Atlântica brasileira. Rodriguésia 63, 1039–1064 (2012). [Google Scholar]
- 62.Kubitzki K., Renner S., Lauraceae—I: Aniba and Aiouea. Fl. Neotrop. Monogr. 31, 1–125 (1982). [Google Scholar]
- 63.Mori S. A., Prance G. T., De Zeeuw C. H., Lecythidaceae.- Part II: The zygomorphic-flowered new world genera (Couroupita, Corythophora, Bertholletia, Couratari, Eschweilera, & Lecythis). With a study of secondary xylem of neotropical Lecythidaceae. Flora Neotrop. 21, 1–376 (1990). [Google Scholar]
- 64.Rogers D. J., Appan S. G., Manihot, Manihotoides (Euphorbiaceae). Fl. Neotrop. Monogr. 13, 1–272 (1973). [Google Scholar]
- 65.Pennington T. D., Styles B. T., Taylor D. A. H., Meliaceae with accounts of Swietenioideae and Chemotaxonomy. Fl. Neotrop. Monogr. 28, 1–470 (1981). [Google Scholar]
- 66.de Mello-Silva R., Lopes J. d. C., Pirani J. R., Flora da Serra do Cipó, Minas Gerais: Annonaceae. Boletim de Botânica 30, 37–56 (2012). [Google Scholar]
- 67.Acevedo-Rodríguez P., Melicocceae (Sapindaceae): Melicoccus and Talisia. Flora Neotrop. 87, 1–178 (2003). [Google Scholar]
- 68.Berg C. C., Olmedieae Brosimeae (Moraceae). Fl. Neotrop. Monogr. 7, 1–228 (1972). [Google Scholar]
- 69.Cowan R. S., Swartzia (Leguminosae, Caesalpinioideae Swartzieae). Flora Neotrop. 1, 1–228 (1967). [Google Scholar]
- 70.Delprete P. G., Rondeletieae (Rubiaceae): Part I (Rustia, Tresanthera, Condaminea, Picardaea, Pogonopus, Chimarrhis, Dioicodendron, Molopanthera, Dolichodelphys, and Parachimarrhis). Fl. Neotrop. Monogr. 77, 1–225 (1999). [Google Scholar]
- 71.Hekking W. H. A., Violaceae-Part I. Rinorea and Rinoreocarpus. Fl. Neotrop. Monogr. 46, 1–207 (1988). [Google Scholar]
- 72.Henderson A., Bactris (Palmae). Fl. Neotrop. Monogr. 79, 1–181 (2000). [Google Scholar]
- 73.Hopkins H. C. F., Parkia (Leguminosae: Mimosoideae). Flora Neotrop. 43, 1–123 (1986). [Google Scholar]
- 74.Kaastra R. C., Pilocarpinae (Rutaceae). Fl. Neotrop. Monogr. 33, 1–197 (1982). [Google Scholar]
- 75.Knapp S., Solanum section Geminata (Solanaceae). Fl. Neotrop. Monogr. 84, 1–404 (2002). [Google Scholar]
- 76.Landrum L. R., A Monograph of the genus Myrceugenia (Myrtaceae). Flora Neotrop. 29, 1–135 (1981). [Google Scholar]
- 77.Landrum L. R., Campomanesia, Pimenta, Blepharocalyx, Legrandia, Acca, Myrrhinium, and Luma (Myrtaceae). Flora Neotrop. 45, 1–178 (1986). [Google Scholar]
- 78.Madriñán S., Rhodostemonodaphne (Lauraceae). Flora Neotrop. 92, 1–102 (2004). [Google Scholar]
- 79.Pennington T. D., Sapotaceae. Fl. Neotrop. Monogr. 52, 1–770 (1990). [Google Scholar]
- 80.Prance G. T., Plana V., Edwards K. S., Pennington R. T., Proteaceae. Fl. Neotrop. Monogr. 100, 1–218 (2007). [Google Scholar]
- 81.Rohwer J. G., Lauraceae: Nectandra. Flora Neotrop. 60, 1332 (1993). [Google Scholar]
- 82.Sleumer H. O., Olacaceae. Flora Neotrop. 38, 1–158 (1984). [Google Scholar]
- 83.Smith G. L., Coile N. C., Piptocarpha (Compositae: Vernonieae). Flora Neotrop. 99, 1–94 (2007). [Google Scholar]
- 84.Fabris L. C., Peixoto A. L., Sapotaceae das Restingas do Espírito Santo, Brasil. Rodriguésia 64, 265–283 (2013). [Google Scholar]
- 85.Maas P. J. M., Westra L. Y. T., Revision of the Neotropical genus Pseudoxandra (Annonaceae). Blumea Biodivers. Evol. Biogeogr. Plants 48, 201–259 (2003). [Google Scholar]
- 86.S. D. O. Santos, Lauraceae Juss. Ao Norte da Floresta Atlantica, thesis, Universidade Federal de Pernambuco (2012). [Google Scholar]
- 87.C. Mez, Lauraceae Americanae monographice descriptae (L. S.-H. S. A. Wheldon & Wesley Inc., New York, 1963), p. 556. [Google Scholar]
- 88.A. C. C. Moreira, A. A. de Souza, D. C. Reis, M. d. M. S. Conde, R. C. Alves, Seeds and Other Diaspores of Marambia Island (UFRRJ, Rio de Janeiro, 2010), vol. 1.0, p. 4; http://fieldguides.fieldmuseum.org/sites/default/files/rapid-color-guides-pdfs/433_1.pdf.
- 89.Centro de Referência em Informação Ambiental Ed., Brasil (2014), http://www.splink.org.br/
- 90.A. Alves-Araujo, Taxonomia e Filogenia de Pouteria Aubl. (Sapotaceae) na Mata Atlantica setentrional Centro de Ciencias Biologicas, thesis, Universidade Federal de Pernambuco Recife (2012). [Google Scholar]
- 91.Grokoviski L., Cervi A. C., Tardivo R. C., O gênero Piptocarpha R.Br. (Asteraceae: Vernonieae) no estado do Paraná, Brasil. Acta. Bot. Brasilica 23, 486–498 (2009). [Google Scholar]
- 92.Staggemeier V. G., Diniz-Filho J. A. F., Morellato L. P. C., The shared influence of phylogeny and ecology on the reproductive patterns of Myrteae (Myrtaceae). J. Ecol. 98, 1409–1421 (2010). [Google Scholar]
- 93.A. S. P. Boeira, O gênero Sloanea L. (Elaeocarpaceae) na Reserva Florestal Adolpho Ducke, thesis, Instituto Nacional De Pesquisas Da Amazônia INPA, Manaus, Amazonas (2010). [Google Scholar]
- 94.Silva G. G. d., Souza P. A. d., Morais P. L. D. d., Santos E. C. d., Moura R. D., Menezes J. B., Caracterização do fruto de ameixa silvestre (Ximenia americana L.). Rev. Bras. Frutic. 30, 311–314 (2008). [Google Scholar]
- 95.Lima M. P. M. d., Lima H. C. d., Parapiptadenia Brenam (Leguminosae-Mimosoideae)—Estudo taxonômico das espécies brasileiras. Rodriguésia 36, 23–30 (1984). [Google Scholar]
- 96.Silva M. d. S., Borges E. E. d. L. e., Leite H. G., Corte V. B., Biometria de frutos e sementes Melanoxylon brauna Schott (Fabaceae-Caesalpinioideae). Cerne 19, 517–524 (2013). [Google Scholar]
- 97.Camargo E. A. d., Souza C. M. F. d., Caddah M. K., Goldenberg R., O gênero Leandra, seções Carassanae, Chaetodon, Niangae, Oxymeris e Secundiflorae (Melastomataceae) no estado do Paraná. Rodriguésia 60, 595–631 (2009). [Google Scholar]
- 98.M. C. Rodrigues, Bignoniáceas de dezoito fragmentos florestais remanescentes no noroeste paulista, Brasil, thesis, Universidade Estadual Paulista UNESP, Botucatu-SP (2012). [Google Scholar]
- 99.L. R. d. Mendonça-Souza, Ficus (Moraceae) no Estado de São Paulo (Instituto de Botânica da Secretaria do Meio Ambiente, São Paulo, 2006); www.ambiente.sp.gov.br/pgibt/files/2013/09/Livia_Ribeiro_de_Mendonca_Souza_MS.pdf. [Google Scholar]
- 100.Melo M. d. F. F., Zickel C. S., Os gêneros Zanthoxylum L. e Esenbeckia Kunth (Rutaceae) no Estado de Pernambuco, Brasil. Acta Bot. Bras. 18, 73–90 (2004). [Google Scholar]
- 101.C. F. P. von Martius, A. W. Eichler, I. Urban, Flora Brasiliensis, Eds. (Jardim Botânico de Missouri, Departamento de Botânica do Instituto de Biologia da Unicamp, Centro de Referência em Informação Ambiental (CRIA), Alemania); http://florabrasiliensis.cria.org.br/.
- 102.Chave J., Réjou-Méchain M., Búrquez A., Chidumayo E., Colgan M. S., Delitti W. B. C., Duque A., Eid T., Fearnside P. M., Goodman R. C., Henry M., Martínez-Yrízar A., Mugasha W. A., Muller-Landau H. C., Mencuccini M., Nelson B. W., Ngomanda A., Nogueira E. M., Ortiz-Malavassi E., Pélissier R., Ploton P., Ryan C. M., Saldarriaga J. G., Vieilledent G., Improved allometric models to estimate the aboveground biomass of tropical trees. Glob. Chang. Biol. 20, 3177–3190 (2014). [DOI] [PubMed] [Google Scholar]
- 103.Chave J., Coomes D., Jansen S., Lewis S. L., Swenson N. G., Zanne A. E., Towards a worldwide wood economics spectrum. Ecol. Lett. 12, 351–366 (2009). [DOI] [PubMed] [Google Scholar]
- 104.G. Reyes, S. Brown, J. Chapman, A. E. Lugo, Wood densities of tropical tree species (Forest Service, United States Deparment of Agriculture, New Orleans, LA, 1992); www.treesearch.fs.fed.us/pubs/30311.
- 105.K. J. F. Alves, Composição da avifauna e frugivoria por aves em um mosaico sucessional na Mata Atlântica, thesis, UNESP, Rio Claro (2008). [Google Scholar]
- 106.Allenspach N., Dias M. M., Frugivory by birds on Miconia albicans (Melastomataceae), in a fragment of cerrado in São Carlos, southeastern Brazil. Braz. J. Biol. 72, 407–413 (2012). [DOI] [PubMed] [Google Scholar]
- 107.Amâncio S., Melo C., Frugivoria por aves em bordas de fragmentos florestais, Uberlândia-MG. Horizonte Científico 1, 1–19 (2008). [Google Scholar]
- 108.S. Athiê, Composição da avifauna e frugivoria por aves em um mosaico de vegetação secundária em Rio Claro, região centro-leste do estado de São Paulo, thesis, UFSCar, São Carlos, SP (2009). [Google Scholar]
- 109.M. M. Argel de Oliveira, Frugivoria por aves em um fragmento de floresta de restinga no Estado do Espírito Santo, Brasil, thesis, UNICAMP, Campinas, SP (1999). [Google Scholar]
- 110.Andreani D. V. d. M., Macedo M. M., Evangelista M. M., Almeida S. M., Aves como potenciais dispersoras de Trema micrantha (L.) Blume (Cannabaceae) em um fragmento florestal no estado de Mato Grosso. Atual. Ornito. 180, 33–37 (2014). [Google Scholar]
- 111.Andrade P. C., Mota J. V. L., Carvalho A. A. F., Interações mutualísticas entre aves frugívoras e plantas em um fragmento urbano de Mata Atlântica, Salvador, BA. Rev. Bras. Ornitol. 19, 63–73 (2011). [Google Scholar]
- 112.Côrtes M. C., Cazetta E., Staggemeier V. G., Galetti M., Linking frugivore activity to early recruitment of a bird dispersed tree, Eugenia umbelliflora (Myrtaceae) in the Atlantic rainforest. Austral Ecol. 34, 249–258 (2009). [Google Scholar]
- 113.Oniki Y., Júnior T. A. d. M., Scopel E. T., Willis E. O., Bird use of Cecropia (Cecropiaceae) and nearby trees in Espirito Santo State, Brazil. Ornitol. Neotrop. 5, 109–114 (1994). [Google Scholar]
- 114.Zimmermann C. E., Santos D. S., Santos C. A. K., Assunção L. G., O uso de poleiros naturais para recuperação de florestas ciliares. Simpósio Regional de Mata Ciliar 1, 70–75 (2002). [Google Scholar]
- 115.Zimmerman C. E., Observações preliminares sobre a frugivoria por aves em Alchornea glandulosa (Endl. and Poepp.) (Euphorbiaceae) em vegetação secundária. Rev. Bras. Zool. 13, 533–538 (1996). [Google Scholar]
- 116.Vasconcellos-Neto J., Albuquerque L. B. d., Silva W. R., Seed dispersal of Solanum thomasiifolium Sendtner (Solanaceae) in the Linhares Forest, Espírito Santo state, Brazil. Acta Bot. Brasil. 23, 1171–1179 (2009). [Google Scholar]
- 117.Valente R. d. M., Comportamento alimentar de aves em Alchornea glandulosa (Euphorbiaceae) em Rio Claro, São Paulo. Iheringia Sér. Zool. 91, 61–66 (2001). [Google Scholar]
- 118.Silva L. B. d., Leite A. V. d. L., Castro C. C., Frugivoria por aves em Miconia prasina D. C. (Melastomataceae) em um fragmento de Mata Atlântica no nordeste do Brasil. Atual. Ornito. 174, 4–7 (2013). [Google Scholar]
- 119.da Silva J. C. B., Junior J. F. C., Vogel H. F., Campos J. B., Dispersão por aves de Psidium guajava L.(Myrtaceae) em ambiente ripário na bacia do rio Paraná, Brasil. Semina: Ciências Biológicas e da Saúde 34, 195 (2013). [Google Scholar]
- 120.Silva F., Frugivoria e dispersão de sementes de Ficus organensis (Moraceae) por aves em um fragmento de Mata de Restinga, Pelotas, RS. Rev. Bras. Ornitol. 18, 19–25 (2010). [Google Scholar]
- 121.Silva P. A.d., Predação de sementes pelo maracanã-nobre (Diopsittaca nobilis, Psittacidae) em uma planta exótica (Melia azedarach, Meliaceae) no oeste do Estado de São Paulo, Brasil. Rev. Bras. Ornitol. 13, 183–185 (2005). [Google Scholar]
- 122.Silva W. R., Ornitocoria em Cereus peruvianus (Cactaceae) na Serra do Japi, estado de Sao Paulo (Ornithochory of Cereus peruvianus (Cactaceae) in the Serra do Japi, State of Sao Paulo). Rev. Bras. Biol. 48, 381–389 (1988). [Google Scholar]
- 123.Rabello A. M., Ramos F. N., Hasui E., Effect of fragment size on Copaifera langsdorffii seeds dispersal. Biota Neotrop. 10, 47–54 (2010). [Google Scholar]
- 124.Scheibler D. R., De Melo T. A. Jr, Frugivory by birds on two exotic Ligustrum species (Oleaceae) in Brazil. Ararajuba 11, 89–91 (2003). [Google Scholar]
- 125.D. C. Rother, Dispersão de sementes e processos de limitação demográfica em ambientes com e sem bambus na Floresta Pluvial Atlântica, thesis, UNESP, Rio Claro, Brasil (2010). [Google Scholar]
- 126.Purificação K. N., Pascotto M. C., Mohr A., Lenza E., Frugivory by birds on Schefflera morototoni (Araliaceae) in a Cerrado-Amazon Forest transition area, eastern Mato Grosso, Brazil. Acta Amaz. 45, 57–64 (2015). [Google Scholar]
- 127.Pascotto M. C., Caten H. T., Oliveira J. P. F. d., Birds as potential seed dispersers of Curatella americana L. (Dilleniaceae) in the Brazilian Cerrado. Ornitol. Neotrop. 23, 585–595 (2012). [Google Scholar]
- 128.Pascotto M. C., Rapanea ferruginea (Ruiz & Pav.) Mez. (Myrsinaceae) como importante fonte alimentar para as aves em uma mata de galeria no interior do Estado de São Paulo. Rev. Bras. Zool. 24, 735–741 (2007). [Google Scholar]
- 129.Pascotto M. C., Avifauna dispersora de sementes de Alchornea glandulosa (Euphorbiaceae) em uma área de mata ciliar no estado de São Paulo. Rev. Bras. Ornitol. 14, 291–296 (2006). [Google Scholar]
- 130.Parrini R., Raposo M. A., da Silva J d H. e A. R., Schefflera morototoni (Araliaceae) como importante recurso alimentar para as aves durante a estação seca na Amazônia. Cotinga 35, 3–6 (2013). [Google Scholar]
- 131.Parrini R., Pacheco J. F., Haefeli L., Observações de aves se alimentando dos frutos de Miconia sellowiana (Melastomataceae) na Floresta Atlântica Alto-Montana do Parque Nacional da Serra dos Órgãos e do Parque Nacional do Itatiaia, região Sudeste do Brasil. Atual. Ornito. 146, 4–7 (2008). [Google Scholar]
- 132.Parrini R., Pacheco J. F., Mallet-Rodrigues F., Frugivoria em Tangara desmaresti (Passeriformes: Thraupidae) na Floresta Atlântica do Parque Nacional da Serra dos Órgãos e adjacências, Estado do Rio de Janeiro, sudeste do Brasil. Atual. Ornito. 142, 10–13 (2008). [Google Scholar]
- 133.Parrini R., Pacheco J. F., Aspectos da frugivoria por aves em Cupania oblongifolia (Sapindaceae) na Mata Atlântica do Parque Nacional da Serra dos Órgãos, estado do Rio de Janeiro, Brasil. A. O. On-Line 178, 55–62 (2014). [Google Scholar]
- 134.Parrini R., Pacheco J. F., Frugivoria por aves em seis espécies arbóreas do gênero Miconia (Melastomataceae) na Mata Atlântica do Parque Nacional da Serra dos Órgãos, Região Sudeste do Brasil. Atual. Ornitol. 159, 51–58 (2011). [Google Scholar]
- 135.Parrini R., Pacheco J. F., Frugivoria por aves em Alchornea triplinervia (Euphorbiaceae) na Mata Atlântica do Parque Estadual dos Três Picos, estado do Rio de Janeiro, Brasil. Atual. Ornitol. 162, 33–41 (2011). [Google Scholar]
- 136.Parrini R., Pacheco J. F., Frugivoria por aves em Coussapoa microcarpa (Cecropiaceae) na Mata Atlântica do estado do Rio de Janeiro, sudeste do Brasil. Atual. Ornitol. 157, 18–21 (2010). [Google Scholar]
- 137.Parrini R., Raposo M. A., Aves se alimentando de Alchornea glandulosa (Euphorbiaceae) na Mata Atlântica do sudeste do Brasil. Bol. Mus. Biol. Mello Leitão (N. Sér.) 27, 75–83 (2010). [Google Scholar]
- 138.D. S. F. Oliveira, Disponibilidade e consumo de frutos de Michelia champaca L. (Magnoliaceae) na área urbana de Uberlândia, MG: Uma interação ave-planta exótica, thesis, Universidade Federal de Uberlândia (2010). [Google Scholar]
- 139.Oliveira A. P. D., Machado C. G., Sigrist M. R., Matayba guianensis (Sapindaceae): Frugivoria por aves em remanescente de cerrado do Centro-Oeste brasileiro. Sitientibus (série Ciências Biológicas) 13, 13–20 (2013). [Google Scholar]
- 140.E. S. Muller, Frugivoria por aves em quatro espécies arbóreas no Parque Nacional dos Aparados da Serra/RS, sul do Brasil, thesis, UNISINOS, São Leopoldo—RS (2006). [Google Scholar]
- 141.Motta-Junior J. C., Lombardi J. A., Aves como agentes dispersores da Copaíba (Copaifera langsdorffii, Caesalpiniaceae) em São Carlos, estado de São Paulo. Ararajuba 1, 105–106 (1990). [Google Scholar]
- 142.J. C. Motta Jr., A exploração de frutos como alimento por aves de mata ciliar numa região do Distrito Federal, thesis, UNESP, Rio Claro, SP (1991). [Google Scholar]
- 143.Melo C., Bento E. C., Oliveira P. E., Frugivory and dispersal of Faramea cyanea (Rubiaceae) in Cerrado woody plant formations. Braz. J. Biol. 63, 75–82 (2003). [DOI] [PubMed] [Google Scholar]
- 144.Melo C., Oliveira P. E., Frugivory in Lacistema hasslerianum Chodat (Lacistemaceae), a gallery forest understory treelet in Central Brazil. Braz. J. Biol. 69, 201–207 (2009). [DOI] [PubMed] [Google Scholar]
- 145.Maruyama P. K., Borges M. R., Silva P. A., Burns K. C., Melo C., Avian frugivory in Miconia (Melastomataceae): Contrasting fruiting times promote habitat complementarity between savanna and palm swamp. J. Trop. Ecol. 29, 99–109 (2013). [Google Scholar]
- 146.Maruyama P. K., Mendes-Rodrigues C., Alves-Silva E., Cunha A. F., Parasites in the neighbourhood: Interactions of the mistletoe Phoradendron affine (Viscaceae) with its dispersers and hosts in urban areas of Brazil. Flora Morphol. Distrib. Funct. Ecol. Plants 207, 768–773 (2012). [Google Scholar]
- 147.Masteguin M. A., Figueiredo R. A., Consumo de frutos de Prunus sellowii Koehne (Rosaceae) por aves em um fragmento florestal em Jundiaí, SP. Ciên. e Nat. 17, 51–56 (1995). [Google Scholar]
- 148.Marcondes-Machado L. O., Comportamento alimentar de aves em Miconia rubiginosa (Melastomataceae) em fragmento de Cerrado, São Paulo. Iheringia, Sér. Zool. 92, 97–100 (2002). [Google Scholar]
- 149.Manhães M. A., Assis L. C. S., Castro R. M., Frugivoria e dispersão de sementes de Miconia urophylla (Melastomataceae) por aves em um fragmento de Mata Atlântica secundária em Juiz de Fora, Minas Gerais, Brasil. Ararajuba 11, 173–180 (2003). [Google Scholar]
- 150.R. F. Lopes, Frugivoria e dispersão de sementes através da avifauna, em quatro espécies de vegetais na região de Botucatu—SP, thesis, ESALQ/USP, Piracicaba (2000). [Google Scholar]
- 151.Lombardi J. A., Motta-Júnior J. C., Possibilidade de dispersão endozoocórica das sementes de Rhipsalis (Cactaceae). Ararajuba 3, 61–62 (1995). [Google Scholar]
- 152.Lima D. M., Neves E. L., Machado C. G., Frugivoria por aves em Elaeis guineensis Jacq. (Arecaceae) na Costa do Dendê, Valença, Bahia, Brasil. Sitientibus série Ciências Biol. 7, 354–359 (2007). [Google Scholar]
- 153.R. R. Laps, Frugivoria e dispersão de sementes de palmiteiro (Euterpe edulis, Martius, Arecaceae) na Mata Atlântica, sul do estado de São Paulo, thesis, UNICAMP, Campinas, SP (1996). [Google Scholar]
- 154.Krügel M. M., Burger M. I., Alves M. A., Frugivoria por aves em Nectandra megapotamica (Lauraceae) em uma área de Floresta Estacional Decidual no Rio Grande do Sul, Brasil. Iheringia, Sér. Zool. 96, 17–24 (2006). [Google Scholar]
- 155.Guerta R. S., Lucon L. G., Motta-Junior J. C., Vasconcellos L. A. d. S., Figueiredo R. A. d., Bird frugivory and seed germination of Myrsine umbellata and Myrsine lancifolia (Myrsinaceae) seeds in a cerrado fragment in southeastern Brazil. Biota Neotrop. 11, 59–65 (2011). [Google Scholar]
- 156.Guimarães M. A., Frugivoria por aves em Tapirira guianensis (Anacardiaceae) na zona urbana do município de Araruama, estado do Rio de Janeiro, sudeste brasileiro. Atual. Ornito. 116, 12–22 (2003). [Google Scholar]
- 157.T. J. A. Guerra, Frugivoria e dispersão de sementes de Struthanthus flexicaulis (Loranthaceae): Aspectos quantitativos e qualitativos da dispersão direcionada, thesis, UNESP, Rio Claro, SP (2005). [Google Scholar]
- 158.Guaraldo A. d. C., Boeni B. d. O., Pizo M. A., Specialized seed dispersal in epiphytic cacti and convergence with mistletoes. Biotropica 45, 465–473 (2013). [Google Scholar]
- 159.Guerra T. J., Marini M. Â., Bird frugivory on Struthantus concinnus (Loranthaceae) in southeastern Brazil. Ararajuba 10, 187–192 (2002). [Google Scholar]
- 160.Figueiredo R. D., Vertebrates at neotropical fig species in a forest fragment. Trop. Ecol. 37, 139–141 (1996). [Google Scholar]
- 161.Gridi-Papp C. O., Gridi-Papp M., Silva W. R., Differential fruit consumption of two Melastomataceae by birds in Serra da Mantiqueira, southeastern Brazil. Ararajuba 12, 5–10 (2004). [Google Scholar]
- 162.Francisco M. R., Lunardi V. O., Galetti M., Características dos propágulos, atributos das aves e a dispersão das sementes de Pera glabrata (Schott, 1858) (Euphorbiaceae) numa área degradada de cerrado. Braz. J. Biol. 67, 627–634 (2007). [DOI] [PubMed] [Google Scholar]
- 163.Francisco M. R., Galetti M., Consumo dos frutos de Davilla rugosa (Dilleniaceae) por aves numa área de cerrado em São Carlos, Estado de São Paulo. Ararajuba 10, 193–198 (2002). [Google Scholar]
- 164.Francisco M. R., Galetti M., Aves como potenciais dispersoras de sementes de Ocotea pulchella Mart. (Lauraceae) numa área de vegetação de cerrado do sudeste brasileiro. Rev. Bras. Bot. 25, 11–17 (2002). [Google Scholar]
- 165.Francisco M. R., Galetti M., Frugivoria e dispersão de sementes de Rapanea lancifolia (Myrsinaceae) por aves numa área de cerrado do Estado de São Paulo, sudeste do Brasil. Ararajuba 9, 13–19 (2001). [Google Scholar]
- 166.Figueiredo R. A. d., Aparecida de Oliveira A., Zacharias M. A., Barbosa S. M., Fontes Pereira F., Cazela G. N., Pedroso Viana J., Andreza de Camargo R., Reproductive ecology of the exotic tree Muntingia calabura (Muntingiaceae) in southeastern Brazil. Rev. Árvore 32, 993–999 (2008). [Google Scholar]
- 167.Figueiredo R. A., Motta J. C. Jr, Vasconcellos L. A. S., Pollination, seed dispersal, seed germination and establishment of seedlings of Ficus microcarpa, Moraceae, in southeastern Brazil. Rev. Bras. Biol. 55, 233–239 (1995). [Google Scholar]
- 168.Jesus S., Monteiro-Filho E. L. A., Frugivoria por aves em Schinus terebinthifolius (Anacardiaceae) e Myrsine coriacea (Myrsinaceae). Rev. Bras. Ornitol. 15, 585–591 (2007). [Google Scholar]
- 169.da Rosa G. A. B., Marcondes-Machado L. O., Frugivoria por aves em Cytharexylum myrianthum Cham (Verbenaceae) em áreas de pastagens de Campinas, SP. Ararajuba 13, 113–115 (2005). [Google Scholar]
- 170.M. C. Côrtes, Frugivoria e dispersão de sementes de Euterpe edulis (Arecaceae) em três tipos florestais no Parque Estadual da Ilha do Cardoso—SP, thesis, UNESP, Rio Claro, SP (2003). [Google Scholar]
- 171.Colussi J., Prestes N. P., Frugivoria realizada por aves em Myrciaria trunciflora (Mart) O. Berg. (Myrtaceae), Eugenia uniflora L. (Myrtaceae) e Ilex paraguariensis St. Hil. no norte do estado do Rio Grande do Sul. Rev. Bras. Ornitol. 19, 48–55 (2011). [Google Scholar]
- 172.Cazetta E., Rubim P., Lunardi V. d. O., Francisco M. R., Galetti M., Frugivoria e dispersão de sementes de Talauma ovata (Magnoliaceae) no sudeste brasileiro. Ararajuba 10, 199–206 (2002). [Google Scholar]
- 173.R. Castro, Variação espaço-temporal na fenologia e frugivoria do palmito juçara Euterpe edulis Martius (Arecaceae) em três tipos de Floresta Atlântica, thesis, UNESP, Rio Claro-SP. (2003). [Google Scholar]
- 174.M. G. G. Camargo, Padrões de frutificação e diversidade na produção, cor e composição química de frutos no cerrado: Uma visão integrada, thesis, UNESP, Rio Claro—SP (2014). [Google Scholar]
- 175.Gondim M. J. C., Dispersão de sementes de Trichilia spp. (Meliaceae) por aves em um fragmento de mata mesófila semidecídua, Rio Claro, SP, Brasil. Ararajuba 9, 101–112 (2001). [Google Scholar]
- 176.Galetti M., Pizo M. A., Fruit eating by birds in a forest fragment in southeastern Brazil. Ararajuba 4, 71–79 (1996). [Google Scholar]
- 177.M. J. C. Gondim, A exploração de frutos por aves frugívoras de uma área de cerrado no Estado de São Paul, thesis, UNESP, Rio Claro (2002). [Google Scholar]
- 178.Ikuta K. G., Martins F. C., Interação entre aves frugívoras e plantas no Parque Estadual da Cantareira, estado de São Paulo. Atualidades Ornitológicas 172, 33–36 (2013). [Google Scholar]
- 179.A. Kindel, Interações entre plantas ornitocóricas e aves frugívoras na estação ecológica de Aracuri, Muitos Capões, RS, thesis, UFRGS, Porto Alegre (1996). [Google Scholar]
- 180.Pizo M. A., Seed dispersal and predation in two populations of Cabralea canjerana (Meliaceae) in the Atlantic forest of southeastern Brazil. J. Trop. Ecol. 13, 559–577 (1997). [Google Scholar]
- 181.Zimmermann C. E., O uso da grandiúva, Trema micrantha Blume (Ulmaceae), na recuperação de áreas degradadas: O papel das aves que se alimentam de seus frutos. Tangara 1, 177–182 (2001). [Google Scholar]
- 182.UNE 56-540-78, Abril 1978: Características físico-mecánicas de la madera: Interpretación de los resultados de los ensayos (IRANOR, 1978). [Google Scholar]
- 183.Hijmans R. J., Cameron S. E., Parra J. L., Jones P. G., Jarvis A., Very high resolution interpolated climate surfaces for global land areas. Int. J. Climatol. 25, 1965–1978 (2005). [Google Scholar]
- 184.Chiba Y., Architectural analysis of relationship between biomass and basal area based on pipe model theory. Ecol. Model. 108, 219–225 (1998). [Google Scholar]
- 185.Baker T. R., Phillips O. L., Malhi Y., Almeida S., Arroyo L., Di Fiore A., Erwin T., Higuchi N., Killeen T. J., Laurance S. G., Laurance W. F., Lewis S. L., Monteagudo A., Neill D. A., Vargas P. N., Pitman N. C. A., Silva J. N. M., Martínez R. V., Increasing biomass in Amazonian forest plots. Philos. Trans. R. Soc. Lond. B 11, 353–365 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.R. R. Rodrigues, Parcelas Permanentes em 40ha de florestas do Estado de São Paulo: Uma experiência interdisciplinar. ESALQ/USP (ESALQ/USP, Piracicaba, 2005); www.lerf.esalq.usp.br/old/parcelas/relatorio2005/introducao.pdf.
- 187.Higuchi N., Carvalho J. A. Jr, Fitomassa e Conteúdo de Carbono de Espécies Arbóreas da Amazônia, in Anais do Seminário “Emissão x Seqüestro de CO2—Uma Nova Oportunidade de Negócios para o Brasil” (Companhia Vale do Rio Doce, Rio de Janeiro, 1994), pp. 127–153. [Google Scholar]
- 188.N. T. Mendonça, Florística e fitossociologia em fragmento de Mata Atlântica—Serra da Bananeira, Estação Ecológica de Murici, Alagoas, thesis, Universidade Federal Rural de Pernambuco, Recife (2005). [Google Scholar]
- 189.G. M. Carvalho, Influência de processos estocásticos sobre a estruturação de comunidades em Floresta de Tabuleiros, Bahia, Brasil, thesis, Universidade Estadual de Santa Cruz, Ilhé (2011). [Google Scholar]
- 190.Paula A., Soares J. J., Estrutura horizontal de um trecho de floresta ombrófila densa das terras baixas na Reserva Biológica de Sooretama, Linhares, ES. Floresta 41, 321–334 (2011). [Google Scholar]
- 191.P. V. Pompeu, Composição e estrutura de uma floresta ombrófila densa ao longo de um gradiente altitudinal na serra da Mantiqueira, Minas Gerais, thesis, Universidade Federal de Lavras, Lavras, MG, Brasil (2011). [Google Scholar]
- 192.P. B. Souza, Diversidade florística e atributos pedológicos ao longo de uma encosta com floresta estacional semidecidual submontana, zona de amortecimento do Parque Estadual do Rio Doce, MG, thesis, Universidade Federal de Vicousa, MG (2008). [Google Scholar]
- 193.J. R. Scolforo, J. M. Mello, C. P. d. C. Silva, Inventário florestal de Minas Gerais: Floresta estacional semidecidual e ombrófila: Florística, estrutura, diversidade, similaridade, distribuição diamétrica e de altura, volumetria, tendências de crescimento e áreas aptas para menejo florestal (UFLA, Lavras, MG, 2008); www.inventarioflorestal.mg.gov.br/publicacoes/semidecidua/indice.pdf.
- 194.J. Dálgima, Fitossociologia em um fragmento de floresta estacional semidecidual na Estação Ecológica do Caiuá, Paraná, Brasi, thesis, UNESP, Botucatu (2009). [Google Scholar]
- 195.Viani R. A. G., Costa J. C., Rozza A. d. F., Bufo L. V. B., Ferreira M. A. P., Oliveira A. C. P. d., Caracterização florística e estrutural de remanescentes florestais de Quedas do Iguaçu, Sudoeste do Paraná. Biota Neotrop. 11, 115–128 (2011). [Google Scholar]
- 196.K. L. V. R. D. S. Furlanete, Padrões e relações florísticas do componente arbóreo na floresta Atlântica lato sensu do Brasil Meridional, thesis, Universidade Estadual de Campinas, Campinas (2011). [Google Scholar]
- 197.L. P. G. Rosa, Florística e fitossociologia da Floresta Atlântica montana no Parque Estadual da Ilha Grande, thesis, UERJ, RJ (2013). [Google Scholar]
- 198.B. C. Kurtz, Fitogeografia e fitossociologia do componente arbóreo de florestas pantanosas de restinga no norte-fluminense. Rio de Janeiro, thesis, Universidade Federal do Rio de Janeiro (2009). [Google Scholar]
- 199.L. A. Schorn, Estrutura e dinâmica de estágios sucessionais de uma floresta ombrófila densa em Blumenau, Santa Catarina, thesis, Universidade Federal do Paraná, Curitiba (2005). [Google Scholar]
- 200.P. A. Floss, Aspectos ecológicos e fitossociológicos no entorno de nascentes em formações florestais do Oeste de Santa Catarina, thesis, Universidade Federal de Santa Maria, Santa Maria (2011). [Google Scholar]
- 201.Negrelle R. R. B., Composição florística e estrutura vertical de um trecho de Floresta Ombrófila Densa de Planície Quaternária. Hoehnea 33, 261–289 (2006). [Google Scholar]
- 202.R. R. Rodrigues, S. Gandolfi, V. C. Souza, Diversidade, dinâmica e conservação em florestas do estado de São Paulo: 40,96ha de parcelas permanentes. 4° Relatório Científico à FAPESP (Universidade de São Paulo, 2006); www.bv.fapesp.br/pt/auxilios/1006/diversidade-dinamica-e-conservacao-de-arvores-em-florestas-do-estado-de-sao-paulo-estudos-em-parce/.
- 203.F. T. Rocha, Levantamento florestal na Estação Ecológica dos Caetetus como subsídio para laudos de desapropriação ambiental, thesis, ESALQ/USP, Piracicaba (2003). [Google Scholar]
- 204.H. T. Z. Couto, Métodos de Inventário da Biodiversidade de Espécies Arbóreas. Relatório Final de Projeto Temático FAPESP (ESALQ, Piracicaba, 2005); www.bv.fapesp.br/pt/auxilios/1004/metodos-de-inventario-da-biodiversidade-de-especies-arboreas/.
- 205.G. A. D. C. Franco, Florística e fitossociologia de duas unidades do mosaico florestal da Estação Ecológica dos Caetetus - Floresta Estacional Semidecidual, Gália—SP, thesis, ESALQ/USP, Piracicaba (2002). [Google Scholar]
- 206.Melo M. M. R. F., Oliveira R. J., Rossi L., Mamede M. C. H., Cordeiro I., Estrutura de um trecho de Floresta Atlântica de planície na Estação Ecológica de Juréia-Itatins, Iguape, SP, Brasil. Hoehnea 27, 299–322 (2000). [Google Scholar]
- 207.Albuquerque G. B. d., Rodrigues R. R., A vegetação do Morro de Araçoiaba, Floresta Nacional de Ipanema, Iperó (SP). Scientia Forestalis 58, 145–159 (2000). [Google Scholar]
- 208.A. C. Marcondelli, Estrutura de uma comunidade arbórea de floresta estacional semidecídua não perturbada no noroeste paulista em relação à outra comunidade com indicadores de perturbação, thesis, Universidade Estadual Paulista “Júlio de Mesquita Filho”, Botucatu (2010). [Google Scholar]
- 209.A. C. Dias, Composição florística, fitossociológica, diversidade de espécies arbóreas e comparação de métodos de amostragem na floresta ombrófila densa do Parque Estadual de Carlos Botelho/SP, Brasil, thesis, ESALQ/USP, Piracicaba (2005). [Google Scholar]
- 210.O. T. Aguiar, Comparação entre os métodos de quadrantes e parcelas na caracterização da composição florística e fitossociológica de um trecho de floresta ombrófila densa no Parque Estadual “Carlos Botelho”—São Miguel Arcanjo, São Paulo, thesis, ESALQ/USP, Piracicaba (2003). [Google Scholar]
- 211.Guilherme F. A. G., Morellato L. P., Assis M. A., Horizontal and vertical tree community structure in a lowland Atlantic Rain Forest, Southeastern Brazil. Rev. Bras. Bot. 27, 725–737 (2004). [Google Scholar]
- 212.M. S. Lacerda, Composição florística e estrutura da comunidade arbórea num gradiente altitudinal da Mata Atlântica, thesis, Universidade Estadual de Campinas (2001). [Google Scholar]
- 213.Gomes J. A. M. A., Bernacci L. C., Joly C. A., Diferenças florísticas e estruturais entre duas cotas altiduninais da Floresta Ombrófila Densa Submontana Atlântica, do Parque Estadual da Serra do Mar, município de Ubatuba/SP, Brasil. Biota Neotrop. 11, 123–137 (2011). [Google Scholar]
- 214.Padgurschi M. C. G., Pereira L. P., Tamashiro J. Y., Joly C. A., Composição e similaridade florística entre duas áreas de Floresta Atlântica Montana, São Paulo, Brasil. Biota Neotrop. 11, 139–152 (2011). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/1/11/e1501105/DC1
Fig. S1. Distribution function of seed size diameter (mm) dispersed by the major frugivores in the Atlantic forest, Brazil.
Fig. S2. Maximum tree height by class of species according to its seed diameter and wood density.
Fig. S3. Relationship between wood density and seed diameter by dispersal mode.
Fig. S4. Relationships between abiotic variables and magnitude of carbon loss.
Fig. S5. Relationships between the compositional variables of each community and its magnitude of carbon loss.
Fig. S6. Linear regression of the above-ground biomass (AGB) and the proxy for basal area (BA) times the wood specific gravity (WSG) times maximum height for the different types of forest.
Fig. S7. Diagnostic plots of the regression model using basal area (BA) times the wood specific gravity (WSG) times tree maximum height (MaxHeight) as a proxy for AGB.
Table S1. Trait information of the 2014 species analyzed (available in the data repository).
Table S2. Atlantic Forest communities analyzed, their spatial localization in Brazil, and abiotic characteristics.
Table S3. Spearman correlations among dispersal traits and carbon traits.
Table S4. T test between carbon loss in random scenarios and defaunated scenarios at different intervals of species removed.
Table S5. Generalized linear model results showing the influence of abiotical and compositional variables on the magnitude of carbon loss of each community.
Table S6. Compositional characteristics of Atlantic Forest communities.
Supplementary code and data file available at
https://github.com/pedroj/MS_Carbon (DOI:10.5281/zenodo.31880).
Code file S1. Simulation code in R (Simulation_Code.RMD).
Code file S2. Read me (Simulation_Code.html).
Data file S1. Trait information of the 2014 species analyzed (Table S1_Trait Data. xls).
Data file S2. Community data example for the simulation code (prove_community.csv).