Abstract
Purpose
Inflammation is associated with the development of atrial fibrillation (AF). Activity in hematopoietic tissues, which produce inflammatory leukocytes, is closely related to systemic inflammation, arterial inflammation and cardiovascular events, but its relationship to AF is unknown. Using 18F-fluorodeoxyglucose (FDG) positron emission tomography (PET) imaging, we examined whether AF associates with splenic metabolic activity and vascular inflammation.
Methods
We conducted a cross sectional study of 70 participants: 35 with AF, who were matched (by age, sex and history of active cancer) to 35 controls without AF. Splenic metabolic activity and vascular aortic inflammation were measured by the mean FDG maximum standard uptake values (SUV Max) by PET. We examined 1) the relationship between AF and splenic activity, and 2) AF and aortic inflammation.
Results
The mean age of the population was 68.13 (SD 8.98) years and 46 (65%) participants were male. Splenic activity was higher in AF participants (2.31 (SD 0.45) vs. 2.07 (0.37), p = 0.024), and remained significant after adjusting for demographic and clinical covariates. Aortic inflammation was also higher in AF participants (2.22 (SD 0.44) vs. 1.91 (SD 0.44), p = 0.004), and remained significant on multivariable analysis. Aortic inflammation and splenic activity were highly correlated (Pearson R = 0.61, p<0.001)
Conclusions
Atrial fibrillation is associated with higher hematopoietic tissue activation and arterial inflammation. Further studies are needed to clarify the mechanisms by which this cardio-splenic axis is implicated in AF.
Keywords: Atrial Fibrillation, Hematopoietic Activation, Positron Emission Tomography
1 Introduction
Atrial fibrillation (AF) is the most common sustained arrhythmia, affecting approximately 1–2% of adults [1–3]. Epidemiological evidence suggests that systemic inflammation is an important pathological feature in AF development and persistence [4]. Still, the processes by which chronic inflammation beget AF remain poorly understood; and a greater understanding of these mechanisms may inform strategies aimed at reducing AF burden by targeting its associated inflammatory pathways.
Hematopoiesis and the differentiation of hematopoietic cells to monocytes and macrophages is a crucial mechanism in the inflammatory process, and chronic hematopoietic activation has been shown to be a predictor of adverse cardiovascular outcomes [5–7]. The spleen is the primary extramedullary reservoir for inflammatory immune cells, including monocytes, and is an important capacitor and regulator of monocyte mediated inflammation [8]. 18F-fluorodeoxyglucose (FDG) is a glucose analogue, and its uptake measured by positron emission tomography (PET) is an effective method of quantifying increased glucose mediated metabolic activity in multiple tissues. Consistent with studies that have observed an association between hematopoietic activation and cardiovascular outcomes, we have previously observed that increased splenic activity measured by FDG-PET is associated with increased arterial inflammation and cardiovascular events, providing evidence for splenic activation as a mechanism for promoting vascular inflammation and atherosclerotic disease progression [6, 9–12].
Given the strong relationship between AF and inflammation, it is possible that hematopoietic tissue activation may also contribute to AF development, although this potential mechanism has not been extensively evaluated [4]. The primary objective of this study was to examine whether AF is associated with evidence of increased splenic activation measured by FDG-PET. We also examined the relationship between AF and evidence of arterial inflammation by FDG-PET.
2 Methods
2.1 Study Design and Participants
We performed a single-center, cross-sectional imaging study of 70 participants from a clinical database of patients who underwent whole body FDG-PET/Computed Tomography (CT) imaging at the Massachusetts General Hospital for clinical indications (e.g. atherosclerosis inflammation or cancer screening) between 2005–2008. Thirty-five participants with a prior clinical diagnosis of AF were matched (based on age, sex, date of FDG-PET/CT imaging and a history of active cancer) to a comparator group of 35 participants without a history of AF.
2.2 FDG-PET and Computed Tomography Imaging
All participants underwent FDG-PET/CT imaging at the Massachusetts General Hospital. Imaging was performed as per clinical protocols. All patients were required to fast overnight prior to the study. Patients underwent injection of approximately 370 MBq (10 mCi) of FDG intravenously, and data was acquired using a Siemens, Biograph 64 (Siemens, Forchheim, Germany) PET/CT scanner (or a similar system) in 3-dimensional mode approximately 60 minutes after injection of the radiotracer. Patients were imaged in the supine position over 15–20 minutes. Attenuation correction was performed with low-dose, non-gated, non-contrast CT (120 keV, ~50 mAs). Attenuation-corrected images were reconstructed using a conventional, filtered back-projection algorithm. PET and CT images were then coregistered using a workstation that enables multi-modal standard image fusion (Leonardo-TrueD, Siemens Solutions, Malvern, Pennsylvania).
2.3 Measurements of FDG-Uptake in the Spleen, Aorta and Bone Marrow
Imaging analysis was performed blinding to clinical data. Following co-registration, the spleen was first identified on CT. Then, using the co-registered PET images, splenic FDG uptake was measured by placing 3 regions of interest in orthogonal planes (axial, sagittal and coronal), which was consistent with previous studies. The maximum standardized uptake value (SUVmax) was identified in each region, which represents the decay-corrected tissue concentration of FDG (in kilobecquerels per milliliter) divided by the injected dose per body weight (kilobecquerels per gram)[9]. For each participant, the mean splenic SUVmax was measured as the average of the three SUVmax values and used as the primary measure of splenic metabolic activity for this analysis.
Aortic FDG uptake was measured using previously published methods[9]. Measurements were taken beginning from just distal to the origin of the left main coronary artery to the aortic arch. SUVmax values were measured on axial images at 5mm intervals within regions of interest that were drawn around the aortic vessel wall. For each participant, the mean aortic SUVmax was calculated across all measured segments and used as the primary measure of aortic inflammation for this analysis.
For an exploratory analysis, FDG-uptake in the bone marrow was also measured using previously described methods. For each thoracic and lumbar vertebra, a region of interest was drawn mid-vertebra and the SUVmax value was obtained. The mean SUVmax value was then calculated as the average of all individual vertebral SUVmax values.
2.4 Statistical Analysis
The primary outcome for the study was splenic metabolic activity (mean SUVmax), and the primary independent variable of interest was a history of AF. Baseline continuous parameters were reported as means ± standard deviations (SD) and compared using the Students T-test if normally distributed or Spearman correlation if skewed. Baseline categorical values were reported as proportions and formally compared using the chi-square test. For the primary analysis, splenic metabolic activity was compared in participants with and without a history of AF using a Student’s T test. Multi-variable analyses were also performed using linear regression, adjusting for age, sex and additional clinical covariates associated with splenic metabolic activity on univariate analysis. To determine whether markers of aortic and splenic inflammation were independent of traditional cardiovascular risk factors, additional multivariable modeling was performed adjusting for the Framingham Risk Score (FRS). Finally, the analysis was performed adjusting for the mean aortic SUVmax.
Aortic inflammation (mean aortic SUVmax) was also compared in participants with and without a history of AF using a Student’s T test. As above, separate multi-variable analyses were also performed using linear regression, adjusting for age and sex, additional clinical covariates significant on univariate analysis, FRS and the mean splenic SUVmax. Data were analyzed using R Version 3.1.2, with a p-value < 0.05 considered statistically significant.
3 Results
3.1 Baseline Clinical Characteristics
Baseline clinical characteristics of the study population are summarized in Table 1. The mean age of the population was 68.13 (SD 8.98) years and 46 (0.65%) of participants were male. Twenty-one (30%) participants had a history of cardiovascular disease (CVD), 54 (78%) had a history of hypertension, 22 (32%) were diabetic, and 21 (30%) had a history of smoking. Statins were used by 29 (42%) participants. Mean Framingham risk score was 8.50 (SD 5.89). No participants were actively receiving treatment for cancer at the time of PET imaging. Among the measured baseline characteristics, no significant differences were observed between those with and without AF.
Table 1.
Baseline Clinical Characteristics of the Study Population
| Characteristic | Total Population | Atrial Fibrillation (N=35) | No Atrial Fibrillation (N=35) | P-value for difference |
|---|---|---|---|---|
| Age, yrs, mean (SD) | 68.13 (8.98) | 68.03 (8.64) | 68.23 (9.43) | 0.92 |
| Male, n (%) | 46/70 (0.65) | 23/35 (0.65) | 23/35 (0.65) | 1.00 |
| History of cardiovascular disease, n (%) | 21/69 (0.30) | 12/35 (0.34) | 9/34 (0.26) | 0.65 |
| Active Cancer, n (%) | 46/70 (0.66) | 23/35 (0.66) | 23/35 (0.66) | 1.00 |
| History of hypertension, n (%) | 54/69 (0.78) | 29/35 (0.83) | 25/34 (0.74) | 0.51 |
| History of Diabetes, n (%) | 22/69 (0.32) | 13/35 (0.37) | 9/34 (0.26) | 0.49 |
| Current or prior smoker, n (%) | 21/69 (0.30) | 12/35 (0.34) | 9/34 (0.26) | 0.65 |
| Statin Use, n (%) | 29/69 (0.42) | 14/35 (0.40) | 15/34 (0.44) | 0.91 |
| Systolic Blood pressure, mmHg, mean (SD) | 129.00 (17.92) | 127.84 (18.03) | 129.96 (18.17) | 0.70 |
| Low density lipoprotein, mg/dL, mean (SD) | 90.74 (34.13) | 94.55 (37.27) | 86.37 (30.24) | 0.36 |
| High density lipoprotein, mg/dL, mean (SD) | 47.69 (22.71) | 44.39 (13.51) | 51.10 (29.23) | 0.25 |
| Body mass index, kg/m2 mean (SD) | 27.87 (6.33) | 28.57 (6.77) | 27.18 (5.86) | 0.36 |
| Framingham Risk Score, mean (SD) | 8.50 (5.89) | 8.75 (5.87) | 8.28 (6.07) | 0.81 |
SD = Standard deviation
3.2 Splenic and bone marrow activity in participants with and without AF
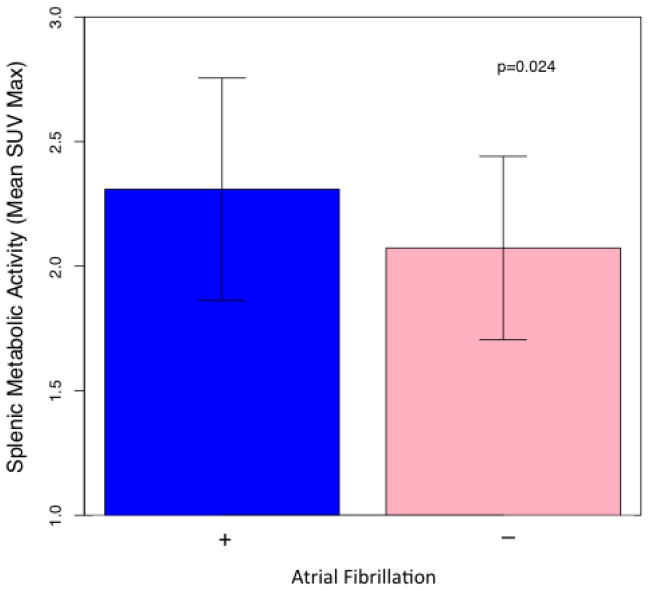
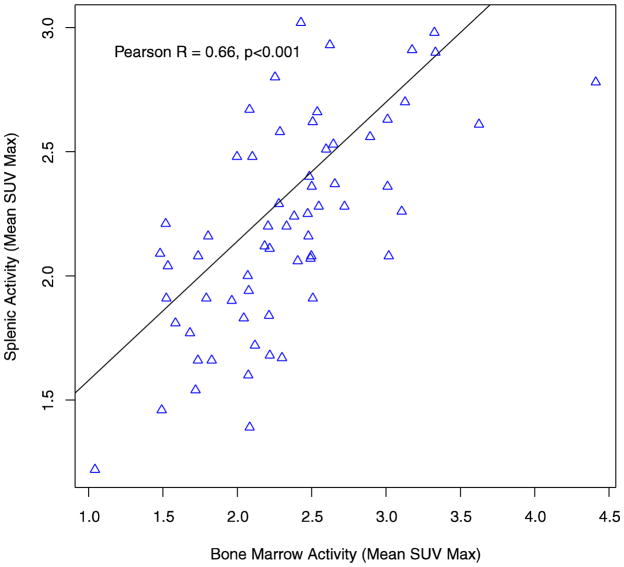
The mean splenic SUVmax for the overall study population was 2.19 (SD 0.42). In patients with AF, splenic metabolic activity was significantly higher when compared to patients without a history of AF (mean SUVmax = 2.31 (SD 0.45) vs. 2.07 (SD 0.37), p = 0.024) (Figure 1). In separate multivariable analyses, atrial fibrillation remained significantly associated with splenic metabolic activity after adjusting for demographic variables, significant clinical variables (see supplementary table 1 for covariates), and the FRS. Bone marrow activity was not significantly associated with AF (p=0.20). However, in the overall study population, splenic and bone marrow activity were highly correlated (Pearson R = 0.66 (0.50, 0.79), p<0.001) (Figure 3).
Figure 1.
Splenic Metabolic Activity in Participants with and without Atrial Fibrillation
Figure 3.
Correlation between Splenic and Bone Marrow Metabolic Activity
3.3 Aortic inflammation in participants with and without AF
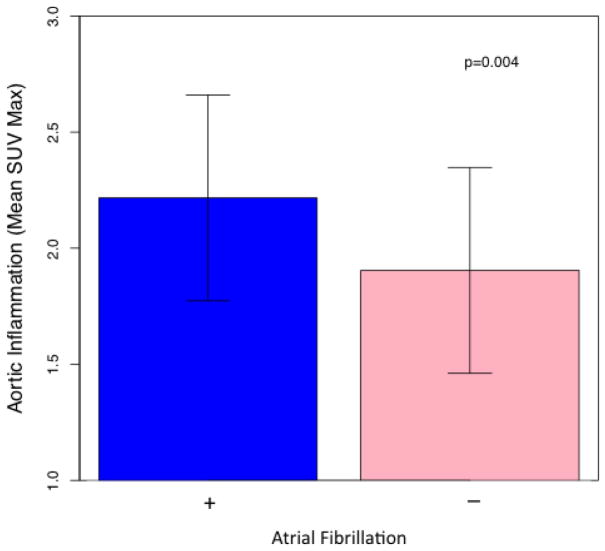
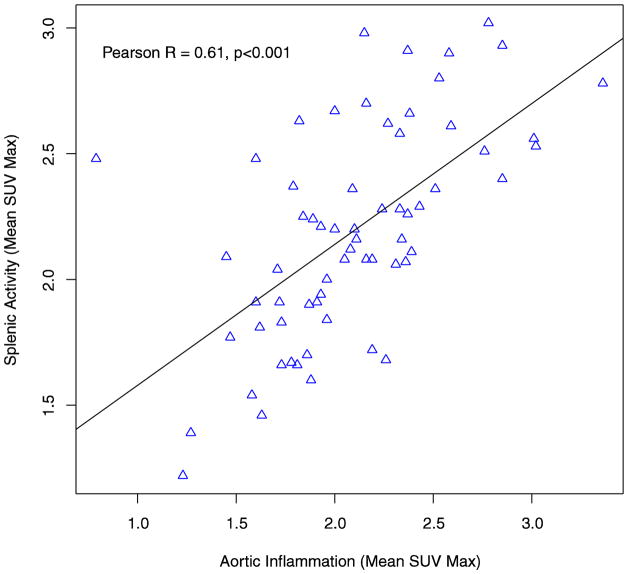
The mean Aortic SUVmax in the overall study population was 2.06 (SD 0.47). In patients with AF, average TBR was significantly higher compared to patients without a history of AF (2.22 (SD 0.44) vs. 1.91 (SD 0.44), p = 0.004) (Figure 2). In separate multivariable analyses, atrial fibrillation remained significantly associated with arterial inflammation after adjusting for demographic variables, significant clinical variables (see supplementary table 2 for covariates), and the FRS (table 2). Aortic inflammation was highly correlated with splenic metabolic activity (Pearson R = 0.61 (0.43, 0.74), p<0.001) (Figure 4). Aortic inflammation was also highly correlated with bone marrow activity in the overall study population (Pearson R = 0.64 (0.47, 0.76), p<0.001) (Supplementary Figure 1).
Figure 2.
Aortic Inflammation in Participants with and without Atrial Fibrillation
Table 2.
Relationship between Atrial Fibrillation and FDG-Uptake in the Aorta and Spleen
| Estimate (SE) | p-value | Adjusted R2 of Model | |
|---|---|---|---|
| Atrial fibrillation as a predictor of splenic metabolic activity (mean SUVmax) | |||
| Univariate Model | 0.236 (0.102) | 0.024 | 0.064 |
| Multivariable Model 1* | 0.238 (0.102) | 0.024 | 0.061 |
| Multivariable Model 2** | 0.222 (0.110) | 0.048 | 0.152 |
| Multivariable Model 3*** | 0.299 (0.117) | 0.017 | 0.306 |
| Atrial fibrillation as a predictor of aortic inflammation (mean SUVmax) | |||
| Univariate Model | 0.312 (0.106) | 0.004 | 0.100 |
| Multivariable Model 1^ | 0.310 (0.104) | 0.004 | 0.133 |
| Multivariable Model 2^^ | 0.293 (0.099) | 0.005 | 0.251 |
| Multivariable Model 3^^^ | 0.496 (0.137) | 0.001 | 0.319 |
Covariates: Age + Sex,
Covariates: Age + Sex + BMI + HDL,
Covariates: Age + Sex + FRS
Covariates: Age + Sex,
Covariates: Age + Sex + CVD + HTN + Statin Use,
Covariates: Age + Sex + FRS
Please refer to supplementary Tables 1 and 2 for the summary of variables associated with aortic and splenic mean SUVmax (using a threshold p-value <0.05) that were carried forwards as covariates in multivariable analysis (Model 2 in each analysis). BMI = body mass index, CVD = cardiovascular disease, FRS = Framingham risk score, HDL = high density lipoprotein, HTN = hypertension, SE = standard error.
Figure 4.
Correlation between Splenic Metabolic Activity and Aortic Inflammation
Discussion
Our study is the first to observe evidence of increased hematopoietic tissue activity in individuals with AF. Evidence of increased vascular inflammation was also observed in participants with AF. These associations may be linked via a common pathway.
Current epidemiologic evidence suggests that inflammation is an important mediator in AF development and persistence [4, 13]. In 24,734 participants enrolled in the Women’s Health Study, Conen et al. observed a significant and graded increase in the risk of incident AF associated with markers of systemic inflammation[14]. Similarly, Marott et al. observed that C-reactive protein (CRP) was associated with incident AF, although at the same time CRP polymorphisms were not related, suggesting that CRP was a marker of AF risk rather than a causal factor [15]. In fact, the processes by which inflammation beget AF remain poorly understood [4]. The spleen plays a key role in moderating the inflammatory response as the major extra-medullary site of leukocyte storage. Prior studies, in animal models, have shown that a large fraction of bone marrow derived monocyte precursors traffic to the spleen, from where they can later contribute to inflammatory disorders such as atherosclerosis.[16, 17] Prior studies have provided evidence for a cardio-splenic axis (in the case of atherosclerosis) [9]. However, the current study is the first to provide direct evidence of higher splenic activity in AF.
Although we did not observe a significant association between bone marrow activity and AF, we did observe that splenic and bone marrow activity were highly correlated in the overall cohort, suggesting that the increase in splenic metabolic activity observed was related to a generalized process of hematopoietic activation. Our findings are consistent with observations from prior studies exploring the role of hematopoietic activation in AF. In the Framingham study, Reinstra et al. observed a direct association between total white blood cell count and incident AF at 5 years of follow-up, although individual cell lineages were not assessed, making it difficult to directly examine the role of hematopoietic mechanisms [18]. Preliminary data from Suzuki et al. report a higher occurrence of CD14++CD16+ intermediate monocytes in blood of patients with AF compared to those without AF, and in patients with persistent compared to paroxysmal AF, suggesting that a direct relationship exists between the burden of AF and activation of the hematopoietic system [19]. Peripheral monocyte recruitment in AF is further supported by evidence of increased serum concentrations of monocyte chemotactic protein 1 (MCP1), although whether this is due to AF itself or secondary to inflammatory processes that also act to promote AF is unknown [20].
The second novel finding of our study is that AF is also associated with increased arterial inflammation. Although atherosclerosis and AF share multiple risk factors (e.g. age, hypertension), this relationship remained independent of global cardiovascular risk (measured by the FRS), suggesting that additional factors account for this observed relationship. It is possible that systemic processes regulating inflammation act to promote both arterial inflammation and AF, which again may be related to activation of the hematopoietic system. For example, pre-clinical studies have observed that increased granulocyte/macrophage colony-stimulating factor (GM-CSF) production by several classes of immune cells can co-stimulate hematopoietic and pro-inflammatory processes in both the bone marrow and spleen [21, 22]. Once this persistent state of hematopoietic activation occurs, it is associated with an increased risk of adverse CV events [5, 6]. Consistent with these findings, we have previously observed that increased splenic metabolic activity measured by FDG-PET was associated with increasing markers of systemic inflammation, increased arterial inflammation, and a higher risk of future adverse cardiovascular events [9]. In this current study, we also observed that arterial inflammation was highly correlated to both splenic and bone marrow activity providing evidence for a common pathway linking hematopoietic activation, arterial inflammation and AF. Alternatively, some studies have also observed that AF can promote inflammation, and its potential role as an inciting factor for hematopoietic activation and arterial inflammation is an intriguing hypothesis [4, 23]. Overall, our findings add to a growing body of evidence supporting the role of hematopoietic activation in a broad range of cardiovascular disorders, and further studies are needed in order to better understand pathophysiological mechanisms that underlie ‘cardio-splenic’ activation and its consequences.
Some important limitations of our study warrant consideration. Firstly, our analysis was performed cross-sectionally, therefore causation cannot be determined from our observations. Nevertheless, our findings provide important hypothesis generating data supporting common mechanisms in AF, atherosclerosis and hematopoietic activity; and additional prospective studies are needed to further characterize the causal pathways underlying this cardio-splenic axis. Splenic FDG uptake may arise from various immune cell lines that utilize glucose production and are present in the spleen (e.g. monocytes, lymphocytes) and therefore does not necessarily correspond to monocyte activation. However, prior studies have shown that splenic activity measured by FDG is associated with serum evidence of leukocytes gene expression, and further studies are needed in order to clarify the specific cell lines that are activated in the presence of higher splenic FDG uptake. Also, examining how splenic activity measured by FDG corresponds to serological evidence of increased monocyte expression, and their sub-types, would be of considerable interest in future studies, given their associations with both AF and CVD. Our study population was derived from a clinical database of patient’s who underwent FDG-PET for clinical reasons, primarily cancer surveillance. Although we did not have any participants receiving treatment for active cancer, further studies are needed to determine the generalizability of our findings to broader patient populations. Our clinical database also did not allow for the differentiation of paroxysmal, persistent and permanent AF, and it would be of considerable interest in future studies to determine whether the degree of hematopoietic activation differs across different AF types. Finally, because PET studies were performed as per routine clinical protocols, methods were not employed that would optimize arterial uptake of FDG. However, we would expect that this lack of optimization would lead to underestimation of observed associations.
In conclusion, our findings demonstrate that patients with AF have evidence of both increased hematopoietic tissue activation and arterial inflammation. Further studies are needed to clarify the mechanisms by which this cardio-splenic axis is implicated in AF.
Supplementary Material
Footnotes
Disclosures and Conflicts of Interest:
None of the authors report any relevant disclosures or conflicts of interest related to this study.
References
- 1.Lip GY, Brechin CM, Lane DA. The global burden of atrial fibrillation and stroke: a systematic review of the epidemiology of atrial fibrillation in regions outside North America and Europe. Chest. 2012;142:1489–98. doi: 10.1378/chest.11-2888. [DOI] [PubMed] [Google Scholar]
- 2.Chugh SS, Havmoeller R, Narayanan K, Singh D, Rienstra M, Benjamin EJ, et al. Worldwide epidemiology of atrial fibrillation: a Global Burden of Disease 2010 Study. Circulation. 2014;129:837–47. doi: 10.1161/CIRCULATIONAHA.113.005119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.January CT, Wann LS, Alpert JS, Calkins H, Cleveland JC, Jr, Cigarroa JE, et al. 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. Journal of the American College of Cardiology. 2014 doi: 10.1016/j.jacc.2014.03.022. [DOI] [PubMed] [Google Scholar]
- 4.Guo Y, Lip GY, Apostolakis S. Inflammation in atrial fibrillation. Journal of the American College of Cardiology. 2012;60:2263–70. doi: 10.1016/j.jacc.2012.04.063. [DOI] [PubMed] [Google Scholar]
- 5.Waterhouse DF, Cahill RA, Sheehan F, McCreery C. Prediction of calculated future cardiovascular disease by monocyte count in an asymptomatic population. Vascular health and risk management. 2008;4:177–87. doi: 10.2147/vhrm.2008.04.01.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rogacev KS, Cremers B, Zawada AM, Seiler S, Binder N, Ege P, et al. CD14++CD16+ monocytes independently predict cardiovascular events: a cohort study of 951 patients referred for elective coronary angiography. Journal of the American College of Cardiology. 2012;60:1512–20. doi: 10.1016/j.jacc.2012.07.019. [DOI] [PubMed] [Google Scholar]
- 7.Seita J, Weissman IL. Hematopoietic stem cell: self-renewal versus differentiation. Wiley interdisciplinary reviews Systems biology and medicine. 2010;2:640–53. doi: 10.1002/wsbm.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim CH. Homeostatic and pathogenic extramedullary hematopoiesis. Journal of blood medicine. 2010;1:13–9. doi: 10.2147/JBM.S7224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Emami H, Singh P, MacNabb M, Vucic E, Lavender Z, Rudd JH, et al. Splenic metabolic activity predicts risk of future cardiovascular events: demonstration of a cardiosplenic axis in humans. JACC Cardiovascular imaging. 2015;8:121–30. doi: 10.1016/j.jcmg.2014.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Swirski FK, Nahrendorf M. Leukocyte behavior in atherosclerosis, myocardial infarction, and heart failure. Science. 2013;339:161–6. doi: 10.1126/science.1230719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tapp LD, Shantsila E, Wrigley BJ, Pamukcu B, Lip GY. The CD14++CD16+ monocyte subset and monocyte-platelet interactions in patients with ST-elevation myocardial infarction. Journal of thrombosis and haemostasis : JTH. 2012;10:1231–41. doi: 10.1111/j.1538-7836.2011.04603.x. [DOI] [PubMed] [Google Scholar]
- 12.Rogacev KS, Seiler S, Zawada AM, Reichart B, Herath E, Roth D, et al. CD14++CD16+ monocytes and cardiovascular outcome in patients with chronic kidney disease. European heart journal. 2011;32:84–92. doi: 10.1093/eurheartj/ehq371. [DOI] [PubMed] [Google Scholar]
- 13.Siu CW, Watson T, Lai WH, Lee YK, Chan YH, Ng KM, et al. Relationship of circulating endothelial progenitor cells to the recurrence of atrial fibrillation after successful conversion and maintenance of sinus rhythm. Europace : European pacing, arrhythmias, and cardiac electrophysiology : journal of the working groups on cardiac pacing, arrhythmias, and cardiac cellular electrophysiology of the European Society of Cardiology. 2010;12:517–21. doi: 10.1093/europace/eup382. [DOI] [PubMed] [Google Scholar]
- 14.Conen D, Ridker PM, Everett BM, Tedrow UB, Rose L, Cook NR, et al. A multimarker approach to assess the influence of inflammation on the incidence of atrial fibrillation in women. European heart journal. 2010;31:1730–6. doi: 10.1093/eurheartj/ehq146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marott SC, Nordestgaard BG, Zacho J, Friberg J, Jensen GB, Tybjaerg-Hansen A, et al. Does elevated C-reactive protein increase atrial fibrillation risk? A Mendelian randomization of 47,000 individuals from the general population. Journal of the American College of Cardiology. 2010;56:789–95. doi: 10.1016/j.jacc.2010.02.066. [DOI] [PubMed] [Google Scholar]
- 16.Heidt T, Sager HB, Courties G, Dutta P, Iwamoto Y, Zaltsman A, et al. Chronic variable stress activates hematopoietic stem cells. Nature medicine. 2014;20:754–8. doi: 10.1038/nm.3589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dutta P, Courties G, Wei Y, Leuschner F, Gorbatov R, Robbins CS, et al. Myocardial infarction accelerates atherosclerosis. Nature. 2012;487:325–9. doi: 10.1038/nature11260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rienstra M, Sun JX, Magnani JW, Sinner MF, Lubitz SA, Sullivan LM, et al. White blood cell count and risk of incident atrial fibrillation (from the Framingham Heart Study) The American journal of cardiology. 2012;109:533–7. doi: 10.1016/j.amjcard.2011.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Suzuki A, Fukuzawa K, Yamashita T, Yoshida A, Sasaki N, Emoto T, et al. Circulating interMediate Cd14++Cd16+ Monocytes are increased in patients with atrial fibrillation and reflect functional remodeling of left atrium. Journal of the American College of Cardiology. 65:A466. doi: 10.1093/europace/euv422. (conference abstract) [DOI] [PubMed] [Google Scholar]
- 20.Li J, Solus J, Chen Q, Rho YH, Milne G, Stein CM, et al. Role of inflammation and oxidative stress in atrial fibrillation. Heart rhythm : the official journal of the Heart Rhythm Society. 2010;7:438–44. doi: 10.1016/j.hrthm.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takahashi T, Kalka C, Masuda H, Chen D, Silver M, Kearney M, et al. Ischemia- and cytokine-induced mobilization of bone marrow-derived endothelial progenitor cells for neovascularization. Nature medicine. 1999;5:434–8. doi: 10.1038/7434. [DOI] [PubMed] [Google Scholar]
- 22.Khatami S, Brummer E, Stevens DA. Effects of granulocyte-macrophage colony stimulating factor (GM-CSF) in vivo on cytokine production and proliferation by spleen cells. Clinical and experimental immunology. 2001;125:198–201. doi: 10.1046/j.1365-2249.2001.01600.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marcus GM, Smith LM, Glidden DV, Wilson E, McCabe JM, Whiteman D, et al. Markers of inflammation before and after curative ablation of atrial flutter. Heart rhythm : the official journal of the Heart Rhythm Society. 2008;5:215–21. doi: 10.1016/j.hrthm.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.