Abstract
Aims
Following ischemic injury, myocardial healing and remodeling occur with characteristic myofibroblast trans-differentiation and scar formation. The current study tests the hypothesis that hyperoxia and nitric oxide (NO) regulate TGF-β1 signaling in the post-ischemic myocardium.
Main methods
C57BL/6 wild-type (WT), endothelial and inducible nitric oxide synthase knockout (eNOS−/− and iNOS−/−) mice were subjected to 30-min left anterior descending coronary artery occlusion followed by reperfusion. Myocardial tissue oxygenation was monitored with electron paramagnetic resonance oximetry. Protein expressions of TGF-β1, receptor-activated small mothers against decapentaplegic homolog (Smad), p21 and α-smooth muscle actin (α-SMA) were measured with enzyme-linked immunosorbent assay (ELISA), Western immunoblotting, and immunohistochemical staining.
Key findings
There was a hyperoxic state in the post-ischemic myocardial tissue. Protein expressions of total and active TGF-β1, p-Smad2/3 over t-Smad2/3 ratio, p21, and α-SMA were significantly increased in WT mice compared to Sham control. Knockout of eNOS or iNOS further increased protein expression of these signals. The expression of α-SMA was more abundant in the infarct of eNOS−/− and iNOS−/− mice than WT mice. A protein band indicating nitration of TGF-β type-II receptor (TGFβRII) was observed from WT heart. Carbogen (95% O2 plus 5% CO2) treatment increased the ratio of p-Smad2/t-Smad2, which was inhibited by 10006329 EUK (EUK134) and sodium nitroprusside (SNP). In conclusion, hyperoxia up-regulated and NO/ ONOO− inhibited cardiac TGF-β1 signaling and myofibroblast trans-differentiation.
Significance
These findings may provide new insights in myocardial infarct healing and repair.
Keywords: Ischemia reperfusion injury, Hyperoxia, Nitric oxide, Fibrosis, Oximetry
Introduction
Acute myocardial ischemia and ultimately myocardial infarction claims more than 1.5 million American lives every year (Tavazzi, 1999; Weir et al., 2006). Reperfusion not only reduces the ischemic injury, but also induces “reperfusion injury” (Rapapaport, 1989; Prasad et al., 2009). The mechanisms underlying ischemia and reperfusion injury (I/R) are associated with reactive oxygen/nitrogen species (ROS/RNS), which causes oxidative and nitrative damages to the inflicted myocardium (Bolli, 1996; Ferdinandy and Schulz, 2003; Kin et al., 2008; Liu et al., 2009; Zweier et al., 1994, 1988).
Immediately following I/R injury, the necrosis of myocytes sets into motion a cascade of inflammatory signals leading to degradation of cellular debris and infarct healing and remodeling with fibrotic scar formation (Becker et al., 1999; Boyle and Weisman, 1993; Factor et al., 1987; Frangogiannis, 2006; Honan et al., 1990; Kim and Braunwald, 1993; Tyagi, 1997; Zhao et al., 1987). Adequate reparative fibrosis in the infarct is beneficial to preventing either adverse fibrotic remodeling or cardiac rupture (Hutchins and Bulkley, 1978; Jugdutt, 2003b; Weber et al., 1992;Weisman and Healy, 1987).
In the ischemic and reperfused myocardium, infiltrated fibroblasts produce most of the matrix macromolecules including collagen (Eghbali et al., 1988;Miller and Gay, 1987; Fan et al., 2012), and contribute to reactive fibrosis. However, α-smooth muscle actin (α-SMA)- expressing myofibroblast trans-differentiation mediates scar contraction (Dobaczewski et al., 2010; Eghbali et al., 1991; Marijianowski et al., 1997; Rhaleb et al., 2001; Sun and Weber, 2000). It has been demonstrated that transforming growth factor-β1 (TGF-β1) is a critical mediator of hyperoxia-induced remodeling in epithelial cells and fibroblasts (Corroyer et al., 1996; Jugdutt, 2003a). Under hyperoxia, cell-cycle inhibiting protein cyclin dependent kinase inhibitor p21 was up-regulated, resulting in the cessation of fibroblast proliferation and initiation of its trans-differentiation (Roy et al., 2007). However, the mechanisms responsible for TGF-β1 activation and myofibroblast trans-differentiation in the infarcted heart are poorly understood (Birdsall et al., 1997; Desmouliere et al., 1993; Frangogiannis, 2006; Frantz et al., 2008).
Ligand binding of TGF-β1 to its type-II and type-I receptors (TGFβRII and TGFβRI) leads to the phosphorylation and nuclear translocation of receptor-activated small mothers against decapentaplegic homolog (Smads), which modulate the transcription of a number of genes, including α smooth muscle actin (α-SMA) (Martin et al., 2007; Saura et al., 2005). Nitric oxide (NO) has been reported to induce the degradation of Smad2/3 resulting in the suppression of TGF-β1-induced signaling in endothelial cells (Lizarbe et al., 2008; Saura et al., 2005). NO has also been shown to increase the release of the active form of TGF-β1 in cultured myocytes (Mehta et al., 2002). However, the role of NO on ventricular infarct zone fibrosis and remodeling still remains undecided (Jugdutt, 2003b).
Recently, our laboratory has demonstrated that NO, superoxide, and their derivative peroxynitrite (ONOO−) suppressed oxygen consumption leading to myocardial tissue hyperoxia (Xu et al., 2008; Zhao et al., 2005; Zhu et al., 2007). NO/ONOO−-induced protein tyrosine nitration may be responsible for the reduced mitochondrial respiration (Liu et al., 2009). However, little is known about the role of tissue hyperoxia on myocardial healing and repair.
In the current study, an electron paramagnetic resonance (EPR) oximetry technique was employed to measure the in vivo myocardial tissue oxygenation (He et al., 2002). Enzyme-linked immunosorbent assay (ELISA), Western immunoblotting, and immunohistochemical staining were used to determine the expressions of TGF-β1, Smad2/3, p21 and α-SMA in the post-ischemic mouse heart. To determine the effect of post-ischemic hyperoxic stress on TGF-β1 signaling and the mechanistic role of ROS or NO/ONOO−, mice were also treated with Carbogen, EUK134, and SNP. With these measurements, the regulation of cardiac TGF-β1 signaling by hyperoxia and NO/ONOO− was examined. These results may provide new insights in myofibroblast trans-differentiation in the healing myocardial infarct.
Materials and methods
Animals and chemicals
Male C57BL/6wild-type (WT), endothelial and inducible NO synthase knockout (eNOS−/− and iNOS−/−) mice were purchased from Jackson Laboratory (Bar Harbor, ME). All mice were housed under a 12:12-h light–dark cycle and were provided with water and food ad libitum. All procedures were performed with the approval of the Institutional Animal Care and Use Committee at the Ohio State University, Columbus, Ohio, and conformed to the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. 10006329 EUK134 (EUK134, Cayman Chemical, Ann Arbor, MI) and sodium nitroprusside (SNP, Sigma-Aldrich, St. Louis, MO) solutions were prepared in phosphate buffered saline (PBS).
In vivo myocardial I/R model
In vivo myocardial I/R model was prepared similarly to the methods described previously (Xu et al., 2008; Zhao et al., 2005; Zhu et al., 2007). Briefly, mice were anesthetized with ketamine (55 mg/kg) and xylazine (15 mg/kg) through intraperitoneal (i.p.) injection. Atropine (0.05 mg subcutaneous (s.c.)) was administered to reduce airway secretions. Mice were orally intubated with polyethylene (PE)-90 tubing and connected to a mouse mini-ventilator (model 845; Harvard Apparatus) with a tidal volume of 250 µl and a respiratory rate of 120 breath/min. Isofluorane was used in all the experiments to maintain a stable anesthetic status. Core body temperature was maintained at 37±0.5 °C with a thermo heating lamp and monitored with a rectal thermometer. After median thoracotomy, the left anterior descending coronary artery (LAD)was visualized and ligated for 30 min by tightening a 7–0 silk suture over a length of PE-10 tubing beneath the LAD at points 1–2 mm inferior to the left auricle. The suture was similarly placed in the sham group but without LAD occlusion. At the end of 30-min ischemia, the ligature was removed, and reperfusion was visually confirmed.
In vivo EPR oximetry
For the in vivo measurement of myocardial tissue oxygenation (Po2), EPR oximetry was used as described previously (Xu et al., 2008; Zhao et al., 2005). Specifically, about 10 µg of lithium phthalocyanine (LiPc), an oxygen-sensitive probe, was loaded in a 27-gauge needle and implanted in the area at risk (AAR) after the heart was exposed. The location of the probe was confirmed by histology in the mid-myocardium. Then the mouse was transferred to an L-band EPR spectrometer (Magnettech GmbH, Germany). After 30 min equilibration of the probe with the surrounding tissue, EPR spectra were collected before and during the 30-min ischemia, and during the 60-min reperfusion. Then the chest was closed and the mouse was allowed to recover. Additional EPR oximetry was performed at days 1, 3, 5, 7, and 14 after reperfusion with the mouse re-anesthetized. The following are the EPR parameters: frequency, 1.1 GHz; microwave power, 16 mW; and modulation amplitude, 0.045 G. The sensitivity of the probe to oxygen is 5.8 mG/mm Hg.
ELISA and Western immunoblotting analyses
To measure protein expression levels, frozen tissue from the AAR was thawed, finely minced and homogenized. After centrifugation, the supernatant was collected. Protein concentration was determined with the bicinchoninic acid (BCA) kit (PIERCE). Total and active TGF-β1 was measured using a commercially available ELISA kit (Quantikine mouse/rat/canine TGF-β1, R&D Systems). For Western immunoblotting assay, tissue homogenate was boiled in the NuPAGE®LDS sample buffer (Invitrogen) at 70 °C for 10 min. Proteins of the homogenate were subjected to electrophoresis on NuPAGE® Novex 4–12% Bis-Tris gels (Invitrogen) and transferred onto nitrocellulose membranes (Amersham Biosciences). After blocking with 5% dry milk in Tween-20- and Tris-buffered saline (TTBS) for 1 h at room temperature, the membranes were incubated with rabbit anti-p-Smad2/3 (Ser 423/425) polyclonal antibody, rabbit anti-Smad2/3 polyclonal antibody, rabbit anti-p21 polyclonal antibody, goat anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH) polyclonal antibody (1:1000, Santa Cruz) and mouse anti- α-SMA monoclonal antibody (1:1000, Sigma). After incubation, the membranes were washed with TTBS and exposed to the antibodies conjugated with horseradish peroxidase for 1 h at room temperature. Proteins were detected by use of chemiluminescence Western immunoblotting detection reagents (Amersham Biosciences). Densitometric analyses of the immunoblots were performed using an Alpha Imager 3300 system (Alpha Innotech, San Leandro, CA).
IP for 3-NT formation
Heart tissue from the AAR was collected and homogenized in RIPA buffer supplemented with protease inhibitor cocktail (1:40). The suspension was collected with centrifugation at 13,000 g for 10 min. Concentration of the sample was adjusted to 8 mg/ml, and 0.5 ml of the solution was incubated with anti-TGFβRII and anti-TGFβRI polyclonal antibodies (1:100; rabbit immunoglobulin G (IgG)). Then, 60 µl of protein A agarose beads was added. After centrifugation at 1000 g for 5 min at 4 °C, the collected immunoprecipitates were washed with TTBS. The supernatant was pre-cleared using the Preclearing Matrix B-rabbit (Exacta Cruz B, Santa Cruz Biotechnology) for 30 min at 4 °C and the matrix was removed later by brief centrifugation. An immunoprecipitation (IP) antibody-IP matrix complex was prepared using primary anti-TGFβRII (10 µl) antibody (Cell Signaling Technology). The complex and the pre-cleared supernatant were incubated at 4 °C overnight. After centrifugation, the matrix was washed three times with PBS. The washed pellets were boiled in the Laemmli sample buffer at 70 °C for 5 min to dissociate the antigen-antibody complex. Then the boiled sample was subjected to sodium dodecyl sulfate-polyacrylamide-gel electrophoresis (SDS-PAGE) and transferred to nitrocellulose membranes. After blocking with 5% dry milk in TTBS, the membranes were probed with an anti-3-nitrotyrosine (3-NT) monoclonal antibody (1:1000; mouse IgG). After incubation, the membranes were washed and exposed to the peroxidase-linked species-specific anti-mouse IgG (1:2000) for 1 h at room temperature. The membranes were washed again with TTBS and the 3-NT-positive proteins were visualized using chemiluminescence Western immuno-blotting detection reagents.
Immunohistochemical staining of α-SMA
After 14 days of reperfusion, mouse hearts were excised, fixed in paraformaldehyde, sliced, and stained with anti-α-SMA antibody for visualizing α-SMA-positive cells indicating the trans-differentiation of cardiac myofibroblast.
Carbogen, EUK134, and SNP treatment
For I/R-independent hyperoxia treatment, mice were housed in a gas-tight chamber purged with carbogen (95% O2 plus 5% CO2) at a constant flow rate of 1.5 l/min. Food, water, temperature, humidity, and normal light cycles were maintained. At the end of 3-day treatment, mice were anesthetized and the left ventricle (LV) tissue was collected. Protein expressions of Smad2 and p21 were measured with Western immunoblotting analyses. In a second group of carbogen-treated mice, myocardial tissue oxygenation was monitored with EPR oximetry. To determine whether ROS or NO played a role on the regulation of TGF-β1 signaling, two additional groups of carbogen-treated mice were given a bolus injection of EUK134 (10 mg/kg intravenous (i.v.) which has both SOD and catalase activity to eliminate superoxide and hydrogen peroxide generation) and SNP (5 mg/kg i.v. which is a potent NO donor) immediately before the treatment.
Statistical analysis
A two-way ANOVA was used for data analyses of Po2. A one-way ANOVA was used for protein expressions; these were followed by Student–Newman–Keuls multiple-comparison test among the groups. Generally, the number of animals was 4–6/group, except that in the Po2 measurement, where N=7/group. Data were represented as means±SE. A value of p<0.05 was considered significant.
Results
Myocardial tissue hyperoxia in the reperfused mouse heart
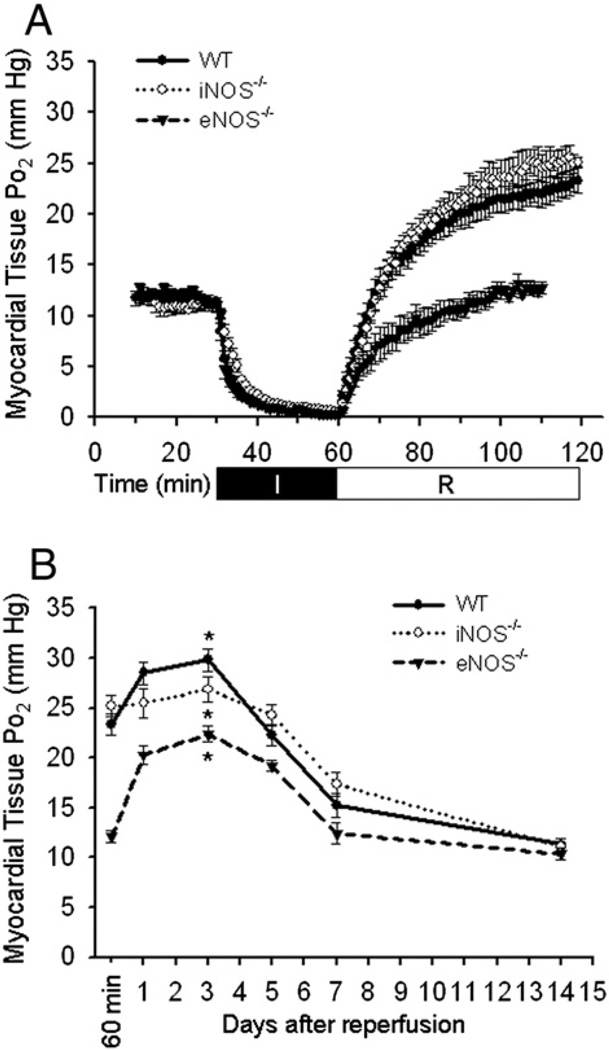
In vivo myocardial tissue oxygenation was measured with EPR oximetry before and during the 30-min ischemia, during the 60-min reperfusion, and at days 1, 3, 5, 7, and 14 after reperfusion. As shown in Fig. 1A, there is an overshoot of tissue Po2 in the acutely reperfused WT mouse heart, which is attenuated in the heart of eNOS−/− but not iNOS−/− mice. As shown in Fig. 1B, myocardial tissue Po2 levels continue to rise with a peak at day 3 post reperfusion in all three groups (WT: 29.8±1.13 mm Hg, iNOS−/−: 26.9±1.3 mm Hg, eNOS−/−: 22.4±0.8 mm Hg), which are significantly higher than that before ischemia (11.2±0.4 mm Hg on average, *p<0.05).
Fig. 1.
EPR oximetry in the ischemic and reperfused mouse heart. A, tissue Po2 in the acute phase of ischemia and reperfusion. B, tissue Po2 in the late reperfused mouse heart. In the acute phase, eNOS but not iNOS knockout attenuated the post-ischemic tissue hyperoxia. In the late phase however, all three groups exhibited hyperoxia with Po2 values peaked at day 3 of reperfusion. *p<0.05, day 3 vs. pre-ischemia in all three groups, N=7/group.
Active and total TGF-β1 in the reperfused mouse heart
Since the peak of myocardial tissue hyperoxia occurred at day 3 post-ischemia, to determine how TGF-β1 signaling is regulated by hyperoxia in the reperfused myocardium, heart tissue from WT, eNOS−/− and iNOS−/− mice was excised at day 3 of reperfusion and TGF-β1 protein expression was measured.
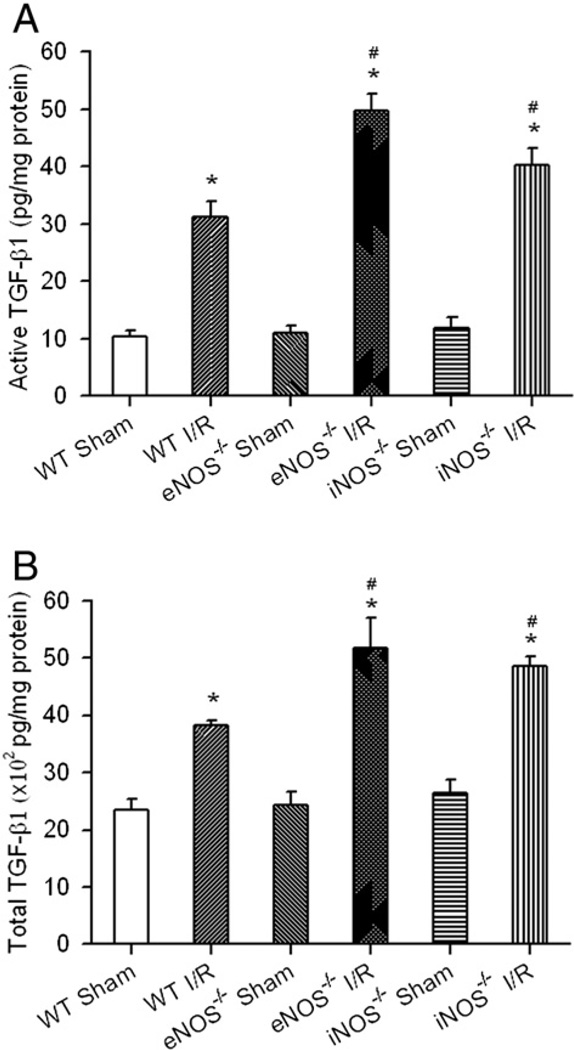
As shown in Fig. 2, active and total TGF-β1 protein expressions were up-regulated in the reperfused WT mouse heart (active: WT I/R 31.2±2.7 vs. WT Sham 10.5±0.9 pg/mg protein; total: WT I/R 3819.6±85.4 vs. WT Sham 2355.1±176.6 pg/mg protein; *p<0.05). The up-regulation of TGF-β1 protein expression correlates with that of the hyperoxia state in the reperfused WT mouse heart as shown in Fig. 1B. Interestingly, the active and total TGF-β1 protein expressions were even higher in the eNOS−/− (active 49.7±3.0 and total 5170.9±521.0 pg/mg protein) and iNOS−/− (active 40.3±2.8 and total 4847.1±184.1 pg/mg protein) I/R mice than that in the WT (*p<0.05 vs. respective Sham controls; # p<0.05 vs. WT I/R) I/R mice with no significant difference among all the sham control groups.
Fig. 2.
TGF-β1 protein expression in the reperfused myocardium. Mouse hearts of WT, eNOS−/− and iNOS−/− were subjected to 30-min coronary ligation followed by 3 days of reperfusion. Active TGF-β1 (A) and total TGF-β1 (B) from the supernatants of tissue homogenate were measured with Quantikine Mouse/Rat/Porcine/Canine TGF-β1 ELISA kit (R&D Systems). Significant increases of active and total TGF-β1 was observed. The levels of active and total TGF-β1 were even higher in the eNOS−/− and iNOS−/− I/R mouse hearts compared to WT I/R mice. *p<0.05 vs. Sham controls; #p<0.05 vs. WT I/R. N=6/group.
Smad2/3 phosphorylation, p21 and α-SMA protein expressions in the reperfused mouse heart
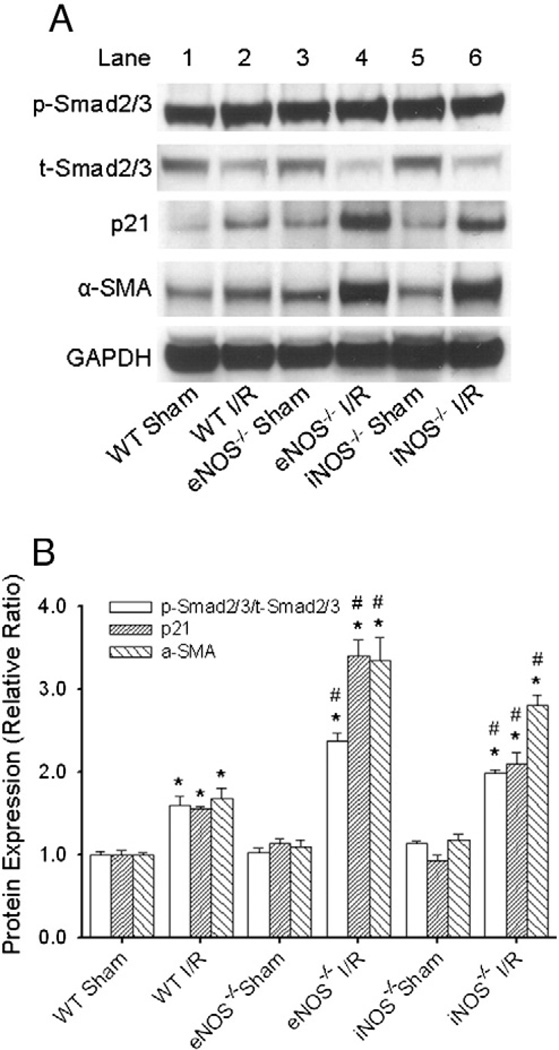
To further determine the regulation of the downstream signaling of TGF-β1 in the reperfused mouse heart, protein expressions of p-Smad2/3, t-Smad2/3, p21, and α-SMA (which is a further downstream signal of Smad2/3 and p21) were measured with Western immunoblotting as described in the Materials and methods section. Fig. 3A displays the representative Western blots from WT, eNOS−/−, and iNOS−/− mouse hearts after 3 days (for p-Smad2/3, t-Smad2/3, and p21) or 14 days (for α-SMA) of reperfusion. As summarized in Fig. 3B, p-Smad2/3/t-Smad2/3 ratio, p21, and α-SMA protein expressions were all up-regulated in the reperfused WT mouse heart (1.60±0.11, 1.55±0.03, and 1.68±0.12; *p<0.05 vs. WT Sham). Knockout of eNOS or iNOS further increased the expression of these proteins (eNOS: 2.37± 0.09, 3.39±0.19, and 3.34±0.27; iNOS: 1.98±0.05, 2.10±0.14, and 2.81±0.12; *p<0.05 vs. respective Sham controls, # p<0.05 vs. WT I/R).
Fig. 3.
Western immunoblotting analyses of protein expressions of p-Smad2/3, (total) t-Smad2/3, p21, and α-SMA in the reperfused mouse heart. A, representative Western blots from WT, eNOS−/− and iNOS−/− mouse hearts after 3 days (for p-Smad2/3, t-Smad2/3, and p21) or 14 days (for α-SMA) of reperfusion. B, statistic analyses of these proteins in the I/R vs. Sham control groups. The levels of p-Smad2/3/t-Smad2/3 ratio, p21 and α-SMA were all increased in the WT I/R mice. Knockout of eNOS or iNOS further increased the expression of these proteins in the eNOS−/− and iNOS−/− I/R mice. *p<0.05 vs. Sham controls; # p<0.05 vs. WT I/R. N=6/group.
Immunohistochemical staining of α-SMA in the reperfused myocardium
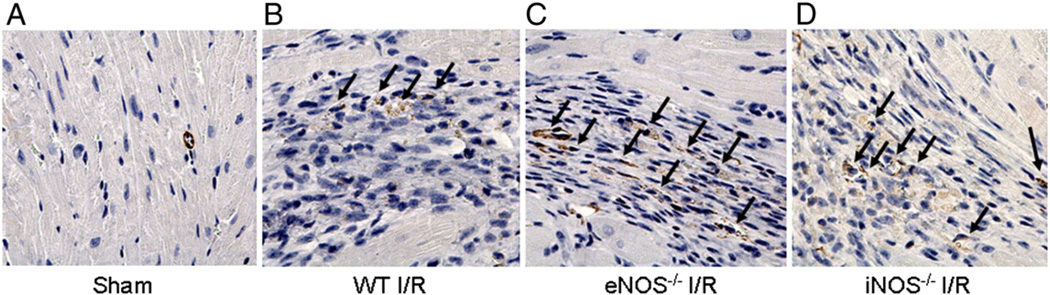
Since formation of α-SMA is a hallmark of the trans-differentiation of cardiac myofibroblast, to further demonstrate if myofibroblasts are formed in the reperfused myocardium, immunohistochemical staining was performed at day 14 post-reperfusion. As shown in Fig. 4, very few staining was evident in the WT Sham control heart slice (A). After I/R (B), increased α-SMA staining was present in the infarcted area in the WT mouse heart (as pointed by black arrows, indicating possible spindle-shaped and brown-stained cardiac myofibroblasts). In slices C and D, more α-SMA staining was observed in the eNOS−/− and iNOS−/− I/R hearts.
Fig. 4.
Immunohistochemical staining of α-SMA (400×). WT, eNOS−/− and iNOS−/− mice were subjected to 30-min coronary ligation followed by 14 days of reperfusion. Hearts were excised, sliced and stained with anti-α-SMA antibody. A,W T Sham control, very little stained α-SMA is shown. B, increased α-SMA staining in the infarcted region of the WT I/R heart (brown color with spindle shape). C and D, more α-SMA staining is evident in the infarcted regions of the eNOS−/− and iNOS−/− I/R hearts. Arrows point to positive staining.
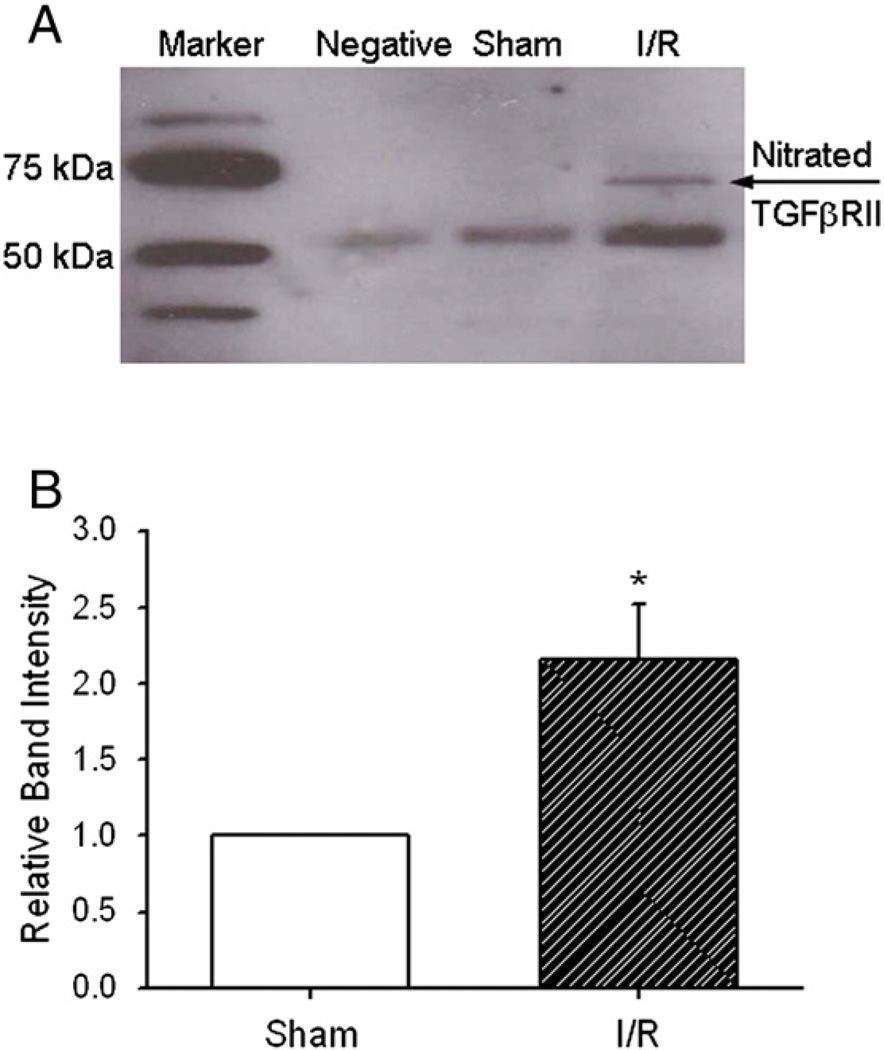
Protein tyrosine nitration on TGFβRII
Protein tyrosine nitration has been recognized as a biomarker of ONOO−, the derivative of NO and superoxide, to modify protein structure and activities (Liu et al., 2009; MacMillan-Crow et al., 1998; Murray et al., 2003). To determine possible mechanisms on NO-related down-regulation of TGF-β1 signaling, 3-NT formation on two TGF-β1 receptors, TGFβRII and TGFβRI, were measured with immunoprecipitation and Western immunoblotting. As shown in Fig. 5A, there is a clear protein band at 70 kDa that indicates the nitration of TGFβRII in the reperfused WT mouse heart. However, when TGFβRI was probed, no nitrated bands were observed (negative data not shown). Fig. 5B shows the relative intensity of the nitrated band (*p<0.05 vs. Sham control). There is a two-fold increase of protein tyrosine nitration in the reperfused mouse heart.
Fig. 5.
Immunoprecipitation (TGFβRII) and immunoblotting (3-NT) of protein tyrosine nitration on TGFβRII. A, Negative control: antibodies only; Sham control: tissue without I/R; I/R: tissue with I/R. There is a clear nitrated band at about 70 kDa: the nitrated TGFβRII. B, relative intensity of the nitrated band. *p<0.05 vs. Sham control. N=4/ group.
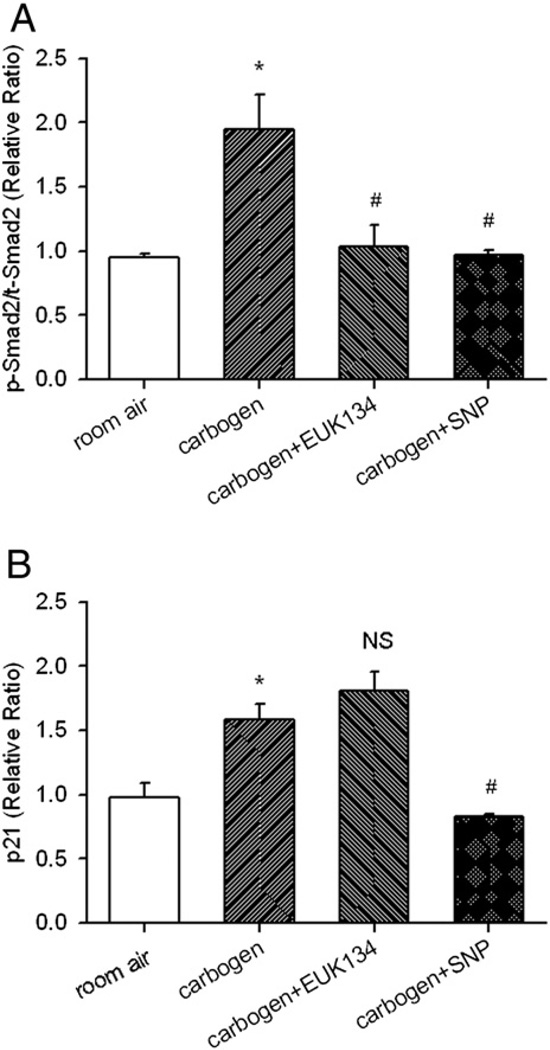
Carbogen, EUK134, and SNP treatment and the regulation of Smad2 phosphorylation and p21 in non-ischemic mouse heart
As shown in previous sections, TGF-β1 signaling was up-regulated in the reperfused mouse heart, which correlates with a hyperoxia state in the myocardial tissue. Knockout of eNOS or iNOS did not eliminate the hyperoxic state but further increased the TGF-β1 signaling. This may imply a stimulatory role of tissue hyperoxia and an inhibitory role of NO or its derivative ONOO− on the TGF-β1 signaling. To test this hypothesis, independent of other signaling pathways during ischemia and reperfusion, WT mice without subjecting to I/R surgery were treated with carbogen (95% O2 plus 5% CO2) for 3 days to mimic the hyperoxic status induced in the reperfused I/R mouse heart. Then, Smad2 phosphorylation (representing the activity of TGF-β1) and p21 were determined with Western immunoblotting analyses. First as shown in Fig. 6, carbogen treatment significantly increased myocardial tissue Po2 (28.8±0.9 vs. 12.1±0.1 mm Hg in room air control, *p<0.05) and induced myocardial tissue hyperoxia to a similar level as in the WT I/R mouse heart 3 days post-reperfusion (Fig. 1B). As further shown in Fig. 7, carbogen treatment significantly increased the ratio of p-Smad2/t-Smad2 and p21 expressions (1.9±0.3 and 1.6±0.1 vs. 0.9±0.1 and 1.0±0.1 in room air controls; *p<0.05). Treatment of EUK134 (a bolus i.v. injection at 10 mg/kg) prior to carbogen breathing blunted carbogen-induced up-regulation of p-Smad2/t-Smad2 ratio (1.0±0.2, # p<0.05 vs. carbogen alone), but not p21 expression (1.8±0.1, NS vs. carbogen alone). However, treatment of SNP (a bolus i.v. injection at 5 mg/kg) prior to carbogen breathing completely blunted the carbogen-induced up-regulation of both p-Smad2/t-Smad2 (1.0±0.1, # p<0.05 vs. carbogen alone) and p21 (0.8±0.1, # p<0.05 vs. carbogen alone).
Fig. 6.
Myocardial tissue Po2 in the carbogen-treated non-ischemic mouse heart. WT mice were subjected to carbogen (95% O2 plus 5% CO2) treatment and in vivo EPR oximetry was performed. After 3-day carbogen breathing, myocardial tissue Po2 increased significantly compared to room air-treated mouse heart. *p<0.05 vs. room air control. N=6/group.
Fig. 7.
Western immunoblotting analyses of protein expression in the carbogen-treated mouse heart. WT mice were subjected to room air or carbogen treatment (95% O2 plus 5% CO2) for three days. In the carbogen+EUK134 and carbogen+SNP groups, a bolus i.v. injection of EUK134 and SNP was given to the mice before carbogen treatment. At the end of 3-day treatment, hearts were excised and proteins of p-Smad2/t-Smad2 (A) and p21 (B) were analyzed. Carbogen treatment significantly increased myocardial p-Smad2/t-Smad2 and p21 levels. EUK134 treatment suppressed the carbogen-induced increase in p-Smad2/t-Smad2 but not p21 expression. SNP treatment suppressed both carbogen-induced p-Smad2/t-Smad2 and p21 up-regulation. *p<0.05 vs. room air controls; # p<0.05 vs. carbogen alone; NS, none significant. N=6/group.
Discussion
Immediately following ischemia and reperfusion injury, infarct healing and repair set into motion. Reperfusion is critical as it reduces ischemic injury and results in less infarct zone remodeling and accelerated healing (Boyle and Weisman, 1993; Kim and Braunwald, 1993). However, reperfusion is also associated with decreased infarct zone collagen and persistent left ventricle dysfunction (Honan et al., 1990), an irreconcilable effect to its benefits (Jugdutt, 2003a, 2003b). Therefore, understanding the underlying mechanisms of myocardial infarct healing and repair and developing appropriate strategies to maximize the beneficial effects of reperfusion is of paramount significance.
There are several new findings in the current study. Firstly, a sustained tissue hyperoxia in the reperfused mouse heart was observed, which is consistent with our previous observation of an overshoot of tissue Po2 in the acutely reperfused heart (Xu et al., 2008; Zhao et al., 2005; Zhu et al., 2007). Knockout of eNOS or iNOS did not eliminate the persistent hyperoxic status in the late reperfused myocardium, indicating that the I/R-induced hyperoxia depends partially on both eNOS- or iNOS-derived NO as also suggested by a previous report (Zhao et al., 2007).
Secondly, tissue hyperoxia induced by I/R up-regulates the TGF-β1/ Smad2/3/p21 signaling, which was further confirmed in carbogen-treated non-ischemic mouse heart. These in vivo observations are consistent with our recent report on the role of oxygen on TGF-β signaling in cardiac fibroblasts in vitro (Roy et al., 2010). It is also known that TGF-β1/Smad2/3 signaling is pivotal in the trans-differentiation of cardiac myofibroblast (Eghbali et al., 1991; Miller and Gay, 1987; Rhaleb et al., 2001) and p21 is critical in fibroblast proliferation arrest (Roy et al., 2007). Our results demonstrate that there were myofibroblast trans-differentiation in the reperfused mouse heart, and inhibition of NO or its derivatives further increased the formation of myofibroblasts. The carbogen treatment experiments indicated that hyperoxia-induced up-regulation of p-Smad2/t-Smad2 was ROS-dependent but not that of p21, suggesting TGF-β1 activation and p21 up-regulation are two independent pathways downstream of tissue hyperoxia. In normal cardiac tissue, collagen deposition may increase stress and cause adverse remodeling (Weber et al., 1992). However, following I/R injury with void space cleared of necrotic cellular debris, myofibroblast trans-differentiation and timely collagen deposition are critical to the prevention of cardiac rupture (Becker et al., 1999). Therefore, the I/R-induced tissue hyperoxia and the induction of the TGF-β1/Smad2/3/p21 signaling may represent an intrinsic mechanism for the healing of the infarcted myocardium.
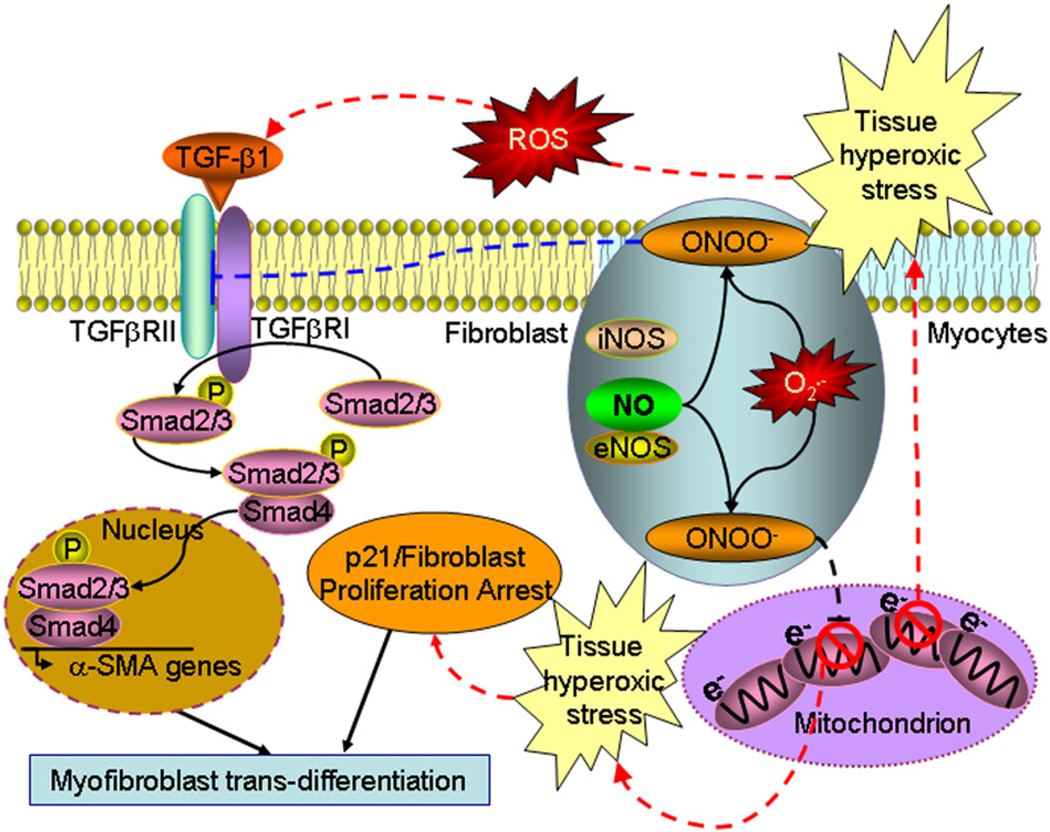
Thirdly, our results indicate that elimination of eNOS- or iNOS-derived NO or its derivatives further up-regulates TGF-β1, suggesting a suppressive role of NO on the TGF-β1 signaling in the reperfused mouse heart, which was further confirmed in non-ischemic mouse heart treated with carbogen and SNP. These results are consistent with some previous reports (Saura et al., 2005), but contrary to others (Lizarbe et al., 2008; Mehta et al., 2002), indicating that the role of NO on ventricular fibrosis and remodeling in the infarct still remains an unresolved issue. Although our current study didn't further investigate the underlying mechanisms on the regulation of TGF-β1/Smad2/3/p21 signaling by NO, our results on protein tyrosine nitration imply at least one of the possible mechanisms, i.e., ONOO− nitrates TGFβRII, inhibits the phosphorylation of Smad2/3, a previously unrecognized mechanism, which is complementary to a previous report that NO/cGMP/PKG signaling induces the degradation of p-Smad2/3/4 and suppression ofα-SMA gene transcription (Saura et al., 2005). Our in vivo data also demonstrated that knockout of eNOS or iNOS up-regulated and SNP treatment blunted the TGF-β1/Smad2/3/p21 signaling, suggesting that NO played a counter regulatory role in the formation of α-SMA and trans-differentiation of myofibroblast with respect to that of tissue hyperoxia. These two counter regulatory mechanisms may represent an intrinsic synergic response of an injured heart to keep fibrotic remodeling on check. Fig. 8 summarizes some possible signaling pathways involved in the hyperoxia-induced and NO-inhibited TGF-β1/ Smad2/3/p21 signaling as suggested in our results.
Fig. 8.
A schematic diagram of the speculated signaling pathways from the current study (the dashed lines). eNOS- or iNOS-derived NO/ONOO− suppresses mitochondrial oxygen consumption and induces tissue hyperoxia (see references Zhao et al., 2007, 2005). The I/R-induced tissue hyperoxic stress activates the TGF-β1 signaling in a ROS-dependent manner. The activated TGF-β1 activates Smad2/3, and α-SMA signaling. Tissue hyperoxic stress also up-regulates p21 leading to fibroblast proliferation arrest and myofibroblast trans-differentiation. On the other hand, NO derivative ONOO− nitrates TGFβRII and, possibly with other NO-associated mechanisms (see reference Saura et al., 2005), results in inhibition of the TGF-β1 signaling. Therefore, tissue hyperoxia and NO counter regulate the trans-differentiation of myofibroblast and keep the post-ischemic myocardial fibrotic remodeling on check.
Lastly, carbogen-induced tissue hyperoxia up-regulated Smad2 phosphorylation, e.g. the activation of TGF-β1, in the non-ischemic mouse heart, which is consistent with the correlation of tissue hyperoxia and up-regulation of TGF-β1 signaling in the WT I/R mouse heart. To minimize the vasoconstrictive effect of pure O2 and maintain regional blood flow due to the vasodilatory effect mediated by CO2 to achieve high local tissue oxygenation, carbogen (with 5% CO2) was used to treat the mice. Since EUK134 has both catalase and superoxide dismutase activities, our data further demonstrate that hyperoxia-induced up-regulation of TGF-β1 signaling but not p21 is ROS-dependent. The results from SNP treatment suggest that NO down-regulates the hyperoxia-induced TGF-β1 signaling and p21, which is consistent with the role of NO/ONOO− on the down-regulation of TGF-β1 signaling and p21 in the WT I/R mouse heart.
It is worth noting that there are also some limitations in this study. First of all, since knockout of iNOS or eNOS did not eliminate tissue hyperoxia in the reperfused heart, the complete mechanism responsible for the I/R-induced hyperoxic response warrants further study. We speculate that the eNOS-independent tissue hyperoxia in the eNOS−/− I/R mouse heart may result from both the loss of myocytes and the reduced induction of iNOS (Zhao et al., 2007). Secondly, since eNOS- or iNOS-derived NO induces tissue hyperoxia which in turn activates the TGF-β1/Smad2/3/p21 signaling, and NO or its derivative also inhibits the same signaling pathway, it is almost impossible to separate the dual effects of NO in the I/R mouse heart model. It seems reasonably unnecessary to use L-NAME or eNOS and iNOS double knockout model to inhibit both eNOS- and iNOS-derived NO since it would have blunted both the up- and down-regulatory effects of NO on the same signaling pathway. By using a non-ischemic mouse heart model with carbogen and SNP treatment, the direct effect of tissue hyperoxia and NO on the regulation of the TGF-β1/Smad2/3/p21 signaling in the myocardium can be dissected, although the effect of carbogen-induced hypercapnia is not further determined in the current study. Lastly, the limited amount of tissue from a mouse heart prevented further study on the specific site of the nitration on TGFβRII, a membrane-bound protein. Therefore, it warrants future investigation on how protein tyrosine nitration would affect the activity of this specific receptor.
Conclusions
In conclusion, I/R-induced tissue hyperoxia activates and NO, or its derivative ONOO−, inhibits the TGF-β1/Smad2/3/p21 signaling in the transcription of α-SMA and trans-differentiation of cardiac myofibroblasts. Understanding the effect of tissue hyperoxia and the dual role of NO on the healing and repair of the infarcted myocardium may shed lights on the beneficial effects of reperfusion and prevention of post-ischemic myocardial adverse healing and remodeling.
Acknowledgment
This work was supported by the National Institutes of Health [Grant HL081630 to G.H., ES016588 and ES018900 to Q.S., HL094650 to Z.L., and HL081734 to A.J.C.]; Diabetic Action Research and Education Foundation Grant to G.H.; and the University of Texas at Austin [UTA11-000297 to A.J.C.]. The LiPc was made with the support of PO1 EB2180 (H.M.S).
Footnotes
Conflict of interest statement None.
References
- Becker RC, Hochman JS, Cannon CP, Spencer FA, Ball SP, Rizzo MJ, et al. Fatal cardiac rupture among patients treated with thrombolytic agents and adjunctive thrombin antagonists: observations from the thrombolysis and thrombin inhibition in myocardial infarction 9 study. J Am Coll Cardiol. 1999;33:479–487. doi: 10.1016/s0735-1097(98)00582-8. [DOI] [PubMed] [Google Scholar]
- Birdsall HH, Green DM, Trial J, Youker KA, Burns AR, MacKay CR, et al. Complement C5a, TGF-beta 1, and MCP-1, in sequence, induce migration of monocytes into ischemic canine myocardium within the first one to five hours after reperfusion. Circulation. 1997;95:684–692. doi: 10.1161/01.cir.95.3.684. [DOI] [PubMed] [Google Scholar]
- Bolli R. The early and late phases of preconditioning against myocardial stunning and the essential role of oxyradicals in the late phase: an overview. Basic Res Cardiol. 1996;91:57–63. doi: 10.1007/BF00788866. [DOI] [PubMed] [Google Scholar]
- Boyle MP, Weisman HF. Limitation of infarct expansion and ventricular remodeling by late reperfusion. Study of time course and mechanism in a rat model. Circulation. 1993;88:2872–2883. doi: 10.1161/01.cir.88.6.2872. [DOI] [PubMed] [Google Scholar]
- Corroyer S, Maitre B, Cazals V, Clement A. Altered regulation of G1 cyclins in oxidant-induced growth arrest of lung alveolar epithelial cells. Accumulation of inactive cyclin E-DCK2 complexes. J Biol Chem. 1996;271:25117–25125. doi: 10.1074/jbc.271.41.25117. [DOI] [PubMed] [Google Scholar]
- Desmouliere A, Geinoz A, Gabbiani F, Gabbiani G. Transforming growth factor-beta 1 induces alpha-smooth muscle actin expression in granulation tissue myofibroblasts and in quiescent and growing cultured fibroblasts. J Cell Biol. 1993;122:103–111. doi: 10.1083/jcb.122.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobaczewski M, Bujak M, Li N, Gonzalez-Quesada C, Mendoza LH, Wang XF, et al. Smad3 signaling critically regulates fibroblast phenotype and function in healing myocardial infarction. Circ Res. 2010;107:418–428. doi: 10.1161/CIRCRESAHA.109.216101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eghbali M, Czaja MJ, Zeydel M, Weiner FR, Zern MA, Seifter S, et al. Collagen chain mRNAs in isolated heart cells from young and adult rats. J Mol Cell Cardiol. 1988;20:267–276. doi: 10.1016/s0022-2828(88)80059-2. [DOI] [PubMed] [Google Scholar]
- Eghbali M, Tomek R, Woods C, Bhambi B. Cardiac fibroblasts are predisposed to convert into myocyte phenotype: specific effect of transforming growth factor beta. Proc Natl Acad Sci U S A. 1991;88:795–799. doi: 10.1073/pnas.88.3.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Factor SM, Robinson TF, Dominitz R, Cho SH. Alterations of the myocardial skeletal framework in acute myocardial infarction with and without ventricular rupture. A preliminary report. Am J Cardiovasc Pathol. 1987;1:91–97. [PubMed] [Google Scholar]
- Fan D, Takawale A, Lee J, Kassiri Z. Cardiac fibroblasts, fibrosis and extracellular matrix remodeling in heart disease. Fibrogenesis Tissue Repair. 2012;5:15–28. doi: 10.1186/1755-1536-5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferdinandy P, Schulz R. Nitric oxide, superoxide, and peroxynitrite in myocardial ischaemia-reperfusion injury and preconditioning. Br J Pharmacol. 2003;138:532–543. doi: 10.1038/sj.bjp.0705080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frangogiannis NG. The mechanistic basis of infarct healing. Antioxid Redox Signal. 2006;8:1907–1939. doi: 10.1089/ars.2006.8.1907. [DOI] [PubMed] [Google Scholar]
- Frantz S, Hu K, Adamek A, Wolf J, Sallam A, Maier SK, et al. Transforming growth factor beta inhibition increases mortality and left ventricular dilatation after myocardial infarction. Basic Res Cardiol. 2008;103:485–492. doi: 10.1007/s00395-008-0739-7. [DOI] [PubMed] [Google Scholar]
- He G, Evalappan SP, Hirata H, Deng Y, Petryakov S, Kuppusamy P, et al. Mapping of the B1 field distribution of a surface coil resonator using EPR imaging. Magn Reson Med. 2002;48:1057–1062. doi: 10.1002/mrm.10302. [DOI] [PubMed] [Google Scholar]
- Honan MB, Harrell FE, Jr, Reimer KA, Califf RM, Mark DB, Pryor DB, et al. Cardiac rupture, mortality and the timing of thrombolytic therapy: a meta-analysis. J Am Coll Cardiol. 1990;16:359–367. doi: 10.1016/0735-1097(90)90586-e. [DOI] [PubMed] [Google Scholar]
- Hutchins GM, Bulkley BH. Infarct expansion versus extension: two different complications of acute myocardial infarction. Am J Cardiol. 1978;41:1127–1132. doi: 10.1016/0002-9149(78)90869-x. [DOI] [PubMed] [Google Scholar]
- Jugdutt BI. Remodeling of the myocardium and potential targets in the collagen degradation and synthesis pathways. Curr Drug Targets Cardiovasc Haematol Disord. 2003a;3:1–30. doi: 10.2174/1568006033337276. [DOI] [PubMed] [Google Scholar]
- Jugdutt BI. Ventricular remodeling after infarction and the extracellular collagen matrix: when is enough enough? Circulation. 2003b;108:1395–1403. doi: 10.1161/01.CIR.0000085658.98621.49. [DOI] [PubMed] [Google Scholar]
- Kim CB, Braunwald E. Potential benefits of late reperfusion of infarcted myocardium. The open artery hypothesis. Circulation. 1993;88:2426–2436. doi: 10.1161/01.cir.88.5.2426. [DOI] [PubMed] [Google Scholar]
- Kin H, Wang NP, Mykytenko J, Reeves J, Deneve J, Jiang R, et al. Inhibition of myocardial apoptosis by postconditioning is associated with attenuation of oxidative stress-mediated nuclear factor-kappa B translocation and TNF alpha release. Shock. 2008;29:761–768. doi: 10.1097/SHK.0b013e31815cfd5a. [DOI] [PubMed] [Google Scholar]
- Liu B, Tewari AK, Zhang L, Green-Church KB, Zweier JL, Chen YR, et al. Proteomic analysis of protein tyrosine nitration after ischemia reperfusion injury: mitochondria as the major target. Biochim Biophys Acta. 2009;1794:476–485. doi: 10.1016/j.bbapap.2008.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lizarbe TR, Garcia-Rama C, Tarin C, Saura M, Calvo E, Lopez JA, et al. Nitric oxide elicits functional MMP-13 protein-tyrosine nitration during wound repair. FASEB J. 2008;22:3207–3215. doi: 10.1096/fj.07-103804. [DOI] [PubMed] [Google Scholar]
- MacMillan-Crow LA, Crow JP, Thompson JA. Peroxynitrite-mediated inactivation of manganese superoxide dismutase involves nitration and oxidation of critical tyrosine residues. Biochemistry. 1998;37:1613–1622. doi: 10.1021/bi971894b. [DOI] [PubMed] [Google Scholar]
- Marijianowski MM, Teeling P, Becker AE. Remodeling after myocardial infarction in humans is not associated with interstitial fibrosis of noninfarcted myocardium. J Am Coll Cardiol. 1997;30:76–82. doi: 10.1016/s0735-1097(97)00100-9. [DOI] [PubMed] [Google Scholar]
- Martin MM, Buckenberger JA, Jiang J, Malana GE, Knoell DL, Feldman DS, et al. TGF-beta1 stimulates human AT1 receptor expression in lung fibroblasts by cross talk between the Smad, p38 MAPK JNK, PI3K signaling pathways. Am J Physiol Lung Cell Mol Physiol. 2007;293:L790–L799. doi: 10.1152/ajplung.00099.2007. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Mehta JL, Chen HJ, Li DY. Protection of myocytes from hypoxia-reoxygenation injury by nitric oxide is mediated by modulation of transforming growth factor-beta1. Circulation. 2002;105:2206–2211. doi: 10.1161/01.cir.0000015602.94990.3d. [DOI] [PubMed] [Google Scholar]
- Miller EJ, Gay S. The collagens: an overview and update. Methods Enzymol. 1987;144:3–41. doi: 10.1016/0076-6879(87)44170-0. [DOI] [PubMed] [Google Scholar]
- Murray J, Taylor SW, Zhang B, Ghosh SS, Capaldi RA. Oxidative damage to mitochondrial complex I due to peroxynitrite: identification of reactive tyrosines by mass spectrometry. J Biol Chem. 2003;278:37223–37230. doi: 10.1074/jbc.M305694200. [DOI] [PubMed] [Google Scholar]
- Prasad A, Stone GW, Holmes DR, Gersh B. Reperfusion injury, microvascular dysfunction, and cardioprotection: the “dark side” of reperfusion. Circulation. 2009;120:2105–2112. doi: 10.1161/CIRCULATIONAHA.108.814640. [DOI] [PubMed] [Google Scholar]
- Rapapaport E. Early interventions in acute myocardial infarction. Kluwer Academic Publishers; 1989. [Google Scholar]
- Rhaleb NE, Peng H, Harding P, Tayeh M, LaPointe MC, Carretero OA. Effect of N-acetyl-seryl-aspartyl-lysyl-proline on DNA and collagen synthesis in rat cardiac fibroblasts. Hypertension. 2001;37:827–832. doi: 10.1161/01.hyp.37.3.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy S, Khanna S, Rink T, Radtke J, Williams WT, Biswas S, et al. P21waf1/cip1/sdi1 as a central regulator of inducible smooth muscle actin expression and differentiation of cardiac fibroblasts to myofibroblasts. Mol Biol Cell. 2007;18:4837–4846. doi: 10.1091/mbc.E07-03-0270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy S, Khanna S, Azad A, Schnitt R, He G, Weigert C, et al. Fra-2 mediates oxygen-sensitive induction of transforming growth factor beta in cardiac fibroblasts. Cardiovasc Res. 2010;87:647–655. doi: 10.1093/cvr/cvq123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saura M, Zaragoza C, Herranz B, Griera M, Diez-Marques L, Rodriguez-Puyol D, et al. Nitric oxide regulates transforming growth factor-beta signaling in endothelial cells. Circ Res. 2005;97:1115–1123. doi: 10.1161/01.RES.0000191538.76771.66. [DOI] [PubMed] [Google Scholar]
- Sun Y, Weber KT. Infarct scar: a dynamic tissue. Cardiovasc Res. 2000;46:250–256. doi: 10.1016/s0008-6363(00)00032-8. [DOI] [PubMed] [Google Scholar]
- Tavazzi L. Clinical epidemiology of acute myocardial infarction. Am Heart J. 1999;138:S48–S54. doi: 10.1016/s0002-8703(99)70320-0. [DOI] [PubMed] [Google Scholar]
- Tyagi SC. Proteinases and myocardial extracellular matrix turnover. Mol Cell Biochem. 1997;168:1–12. doi: 10.1023/a:1006850903242. [DOI] [PubMed] [Google Scholar]
- Weber KT, Anversa P, Armstrong PW, Brilla CG, Burnett JC, Jr, Cruickshank JM, et al. Remodeling and reparation of the cardiovascular system. J Am Coll Cardiol. 1992;20:3–16. doi: 10.1016/0735-1097(92)90130-f. [DOI] [PubMed] [Google Scholar]
- Weir RA, McMurray JJ, Velazquez EJ. Epidemiology of heart failure and left ventricular systolic dysfunction after acute myocardial infarction: prevalence, clinical characteristics, and prognostic importance. Am J Cardiol. 2006;97:13F–25F. doi: 10.1016/j.amjcard.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Weisman HF, Healy B. Myocardial infarct expansion, infarct extension, and reinfarction: pathophysiologic concepts. Prog Cardiovasc Dis. 1987;30:73–110. doi: 10.1016/0033-0620(87)90004-1. [DOI] [PubMed] [Google Scholar]
- Xu Y, Liu B, Zweier JL, He G. Formation of hydrogen peroxide and reduction of peroxynitrite via dismutation of superoxide at reperfusion enhances myocardial blood flow and oxygen consumption in postischemic mouse heart. J Pharmacol Exp Ther. 2008;327:402–410. doi: 10.1124/jpet.108.142372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao MJ, Zhang H, Robinson TF, Factor SM, Sonnenblick EH, Eng C. Profound structural alterations of the extracellular collagen matrix in postischemic dysfunctional (“stunned”) but viable myocardium. J Am Coll Cardiol. 1987;10:1322–1334. doi: 10.1016/s0735-1097(87)80137-7. [DOI] [PubMed] [Google Scholar]
- Zhao X, He G, Chen YR, Pandian RP, Kuppusamy P, Zweier JL. Endothelium-derived nitric oxide regulates postischemic myocardial oxygenation and oxygen consumption by modulation of mitochondrial electron transport. Circulation. 2005;111:2966–2972. doi: 10.1161/CIRCULATIONAHA.104.527226. [DOI] [PubMed] [Google Scholar]
- Zhao X, Chen YR, He G, Zhang A, Druhan LJ, Strauch AR, et al. Endothelial nitric oxide synthase (NOS3) knockout decreases NOS2 induction, limiting hyperoxygenation and conferring protection in the postischemic heart. Am J Physiol Heart Circ Physiol. 2007;292:H1541–H1550. doi: 10.1152/ajpheart.00264.2006. [DOI] [PubMed] [Google Scholar]
- Zhu X, Liu B, Zhou S, Chen YR, Deng Y, Zweier JL, et al. Ischemic preconditioning prevents in vivo hyperoxygenation in postischemic myocardium with preservation of mitochondrial oxygen consumption. Am J Physiol Heart Circ Physiol. 2007;293:H1442–H1450. doi: 10.1152/ajpheart.00256.2007. [DOI] [PubMed] [Google Scholar]
- Zweier JL, Kuppusamy P, Lutty GA. Measurement of endothelial cell free radical generation: evidence for a central mechanism of free radical injury in postischemic tissues. Proc Natl Acad Sci U S A. 1988;85:4046–4050. doi: 10.1073/pnas.85.11.4046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweier JL, Broderick R, Kuppusamy P, Thompson-Gorman S, Lutty GA. Determination of the mechanism of free radical generation in human aortic endothelial cells exposed to anoxia and reoxygenation. J Biol Chem. 1994;269:24156–24162. [PubMed] [Google Scholar]