Abstract
Bullous pemphigoid (BP) is an autoimmune blistering disease characterized by antibodies (IgG and IgE) targeting cell-substrate adhesion proteins. A variety of BP models suggest that autoantibody-dependent neutrophil degranulation is essential for blister formation. However, lesional biopsies reveal a predominance of eosinophils and few neutrophils. Our goal was to evaluate the role of antibodies and complement in eosinophil localization, degranulation, and split formation at the dermo-epidermal junction (DEJ) utilizing a human skin cryosection model of BP paired with a human eosinophilic cell line, 15HL-60. Expression of receptors for IgG (FcγRII), IgE (FcεRI), and complement (CR1 and CR3) was confirmed on 15HL-60 cells using flow cytometry. 15HL-60 expression of granule protein (eosinophil derived neurotoxin (EDN) and eosinophil peroxidase (EPO)) mRNA and their degranulation in vitro was confirmed using RT-PCR and ELISA, respectively. For cryosection experiments, BP or control sera or IgG and IgE antibodies purified from BP sera were utilized in combination with 15HL-60 cells ± fresh complement. Both BP serum and fresh complement were required for localization of 15-HL60 cells to the DEJ. Interestingly, eosinophil localization to the DEJ was dependent on IgG, but not IgE, and complement. However, no subepidermal split was observed. Additionally, the 15HL-60 cells did not degranulate under any experimental conditions and direct application of cell lysate to cryosections did not result in a split. Our observation that eosinophil localization to the DEJ is dependent on IgG mediated complement fixation provides additional insight into the sequence of events during the development of BP lesions.
Keywords: bullous, autoantibody, eosinophil, human, skin
Introduction
Bullous pemphigoid (BP) is an autoimmune blistering disease that is clinically characterized by urticarial plaques and tense fluid-filled bullae. Microscopic examination of BP lesions reveals separation of skin at the dermal-epidermal junction (DEJ) and an inflammatory infiltrate comprised of primarily eosinophils, mast cells and, to a lesser extent, neutrophils (1–6). Deposition of autoantibodies (IgG and IgE) and complement (C3) at the DEJ is demonstrated using direct immunofluorescence (DIF) (7, 8). Additionally, the majority of BP patients exhibit elevated circulating levels of IgE and systemic eosinophilia (9, 10), which are correlated with disease activity (11–13). Despite these observations, the role of eosinophils in BP has not been established. In particular, it is not known how eosinophils are recruited specifically to the DEJ or if their degranulation contributes directly to loss of epidermal adhesion.
The severity of BP is correlated with levels of autoantibodies targeting the hemidesmosomal BP180 protein (type XVII collagen) (14–17). Approximately, 90% of BP sera contain both IgG and IgE class autoantibodies specific for the non-collagenous 16A region (NC16A) of the BP180 (12, 18–21). Experiments demonstrating the in vivo pathogenicity of these antibodies have been limited by the lack of conservation of the human BP180 protein in mice (22). Transfer of rabbit IgG specific for mouse BP180 demonstrated that complement activation (23), mast cell degranulation (6), and neutrophilic infiltration (24–26) were required for fragility of the DEJ. More recently, IgG antibodies purified from BP sera induced skin fragility in mice expressing human type XVII collagen (27–29). However, these IgG-based models failed to fully reproduce clinical BP. The early phase of lesion development, including urticaria, eosinophil infiltration and spontaneous blistering, were only observed in models utilizing IgE autoantibodies from patient sera or monocolonal IgE antibodies specific for BP180 (30, 31). Interestingly, circulating eosinophil numbers correlate with levels of both NC16A-specific IgG and IgE in BP sera (11), but it is unknown if these autoantibodies directly influence lesional eosinophils.
To eliminate species specific differences in the BP180 protein (28, 29) and Fc-receptor expression and function (22), a human cryosection model has been utilized to dissect the mechanisms of blister formation in BP. Experiments using this model have demonstrated that formation of a subepidermal split is dependent on Fcγ receptor-dependent neutrophil degranulation, which is triggered upon encouter with IgG bound to the DEJ (32–36). However, the prominent role of neutrophils in these studies poorly mimics the eosinophil-dominant inflammatory infiltrate observed in BP. In this report, we utilize the human cryosection model of BP to understand the role of IgG and IgE antibodies and complement in the localization of eosinophils to the DEJ and examine their influence on eosinophil degranulation and/or formation of a subepidermal split.
Materials and Methods
Patients and sample collection
Samples were collected from patients with clinical, histological, and immunofluorescent characteristics of BP (n=21) or age- and gender-matched controls (n=16) with no known history of autoimmunity or immunosuppression. Patients were recruited from the University of Iowa Hospitals and Clinics and written informed consent obtained prior to inclusion in the study. This study was approved by the University Institutional Review Board (IRB # 200701758) and was performed in adherence to the Declaration of Helsinki Guidelines. The Institutional Review Board waived the need for informed consent to obtain neonatal foreskins obtained at the time of routine circumcision.
ELISA and Total IgE
Commercially available ELISA kits were used to evaluate the following: NC16A and BP230 IgG, eosinophil-derived neurotoxin (EDN), eosinophil cationic protein (ECP) (MBL International, Japan). NC16A-specific IgE was quantified using a previously described protocol (37). Total IgE levels were quantitated using electrochemiluminescence performed by the pathology laboratory services at the University of Iowa.
Purification of IgG and IgE from BP sera
Autoantibodies were purified from sera of two well-characterized BP patients known to have high levels of NC16A-specific IgG and IgE using two-step affinity chromatography as previously described (19). Evaluation of IgG subclass via immunoblot revealed that IgG2 and IgG4 were primarily responsible for NC16A reactivity in these samples (not shown).
Human Eosinophils
A naturally transformed human meyloid leukemia 15HL-60 (ATCC®, CRL-1964) was differentiated and maintained in an eosinophilic state through culture (RPMI, 10% FBS, 1% pen-strep) under alkaline conditions (pH 7.6) and treatment with butyric acid (38). These cells are reported to express Fc, complement and IL-5 receptors and secrete eosinophil granule proteins (38). Maintenance of the eosinophilic lineage was confirmed periodically staining for major basic protein (MBP; antibody clone BMK3, EMD Millipore, Germany).
Indirect immunofluorescence (IIF) and complement fixation
IIF was conducted on cryosections (7 μm) of human foreskin to confirm specificity of purified IgG and IgE as described (37) with the addition of 3rd-step secondary-specific antibody to increase sensitivity of IgE staining (Life Technologies, Madison, WI). Control experiments were conducted to ensure isotype specificity of secondary antibodies (not shown). For complement (C′) fixation, cryosections were incubated with affinity-purified IgG (2.5 mg/mL), IgE (900 ng/mL), or heat-inactivated serum (diluted 1:10) from the same patient, or a matched control. Sections were washed and incubated with a fresh source of human complement. Fixed complement was detected using goat-anti-human C3-FITC (Bethyl Laboratories, Montgomery, TX). Nuclei were counterstained with DAPI (Life Technologies, Madison, WI) and images were captured with a Nikon epifluorescent microscope and CCD camera.
Evaluation of eosinophil surface markers and degranulation
15HL-60 eosinophils (CD16−CD49d+) were evaluated by flow cytometry for their expression of antibody and complement receptors (CD18/CR3, CD32, CD35/CR1, FcεRI-α and FcγRII) or LAMP-1/CD107a, as an indicator of degranulation (all antibodies from eBiosciences)(11). For degranulation studies, 105 cells were treated (0.5 – 4 hrs) with increasing amounts of ionomycin (0 – 50 ug/ml) and supernatants were collected for evaluation of granule proteins by ELISA. Cells lysed with Triton X-100 or sonicated (20 × 5 second cycles, 30% max) were used as a positive control. For surface detection of LAMP-1/CD107a, ionomycin-treated cells were washed in ice cold PBS, and fixed in 0.2% paraformaldehyde prior to staining. For flow cytometry, ≥104 cells were collected and degranulation was evaluated based on the % of viable cells expressing LAMP-1/CD107a.
RNA Isolation, Reverse Transcription and PCR
RNA was extracted and one-step PCR was performed as previously described (13). Conditions were as follows: RT at 55°C for 30 minutes and inactivation at 94°C for 2 minutes; 30 cycles of PCR utilized 94°C denaturation, 15 sec, template-specific annealing (57.5°C for β-actin and EPO; 60°C for EDN), 30 sec, and 68°C extension for 1 minute. Final extension was at 68°C for 7 minutes. PCR products were run on a 1.5% agarose gel. Primers for eosinophil derived neurotoxin (EDN) and eosinophil peroxidase (EPO) were: EDN F5′–GTG AAC TGG AAC CAC CGG ATA-3′ and EDN R5′-CCA GCA CAT CAA TAT GAC CTC; EPO F5′-CAA AGT GAG AGG GGA GCA GAG-3′ and R5′-GAC CGC TGG GGT TGT AAC TT-3′ and β-actin F5′-GGA CTT CGA GCA AGA GAT GG-3′ and R5′-AGC ACT GTG TTG GCG TAC AG-3′.
Cryosection model
Thin (7 μm) cryosections of human foreskins, approximately 10mm × 5mm, were placed in duplicate on glass microscope slides and stored at −80°C for up to one week. Cryosections were incubated (90 min, room temperature) with heat-inactivated (HI) serum (diluted 1:10) from a BP patient, or a matched control or purified IgG (2.5 mg/mL) or IgE (900 ng/mL). Slides were washed extensively and sections were overlaid with 5 × 106 peripheral blood granulocytes or 4 × 106 15HL60 cells, diluted in culture media supplemented with 10% fresh control serum (untreated or HI) for 6 hours. In separate experiments, 25 × 106 15HL-60 cells/mL were lysed by sonication and the supernatant was applied to cryosections for 1, 2, 4, or 18 hours at 37°C. Supernatants were collected off sections for ELISA and slides were washed gently, stained with H & E, and images were captured via light microscopy. Cell localization to the DEJ was determined using NIH ImageJ as follows; 1) by selecting and counting nuclei of overlaid 15HL-60 cells localized to the DEJ/total number of 15HL-60 cells on entire section = % total cells localized to the DEJ; or 2) the % split was calculated by measuring the total area of separation at the DEJ/the total length of the DEJ.
Statistical Analysis
Experiments were conducted with the number of individual patient samples indicated as N and results were expressed as mean ± SD. Assays utilizing human cells or tissues were conducted with duplicate samples from the same patient being averaged and represented as n = 1. Statistical analysis was performed using GraphPad Prism software, version 5.0 (GraphPad Software, San Diego, CA). A non-parametric unpaired T-test (Mann-Whitney U-test) or ANOVA (Kruskal-Wallis) was used to determine statistical significance between groups. Spearman’s rank correlation coefficient (r) was used to determine the statistical dependence between variables. A P-value of ≤0.05 was considered to be statistically significant.
RESULTS
BP patients
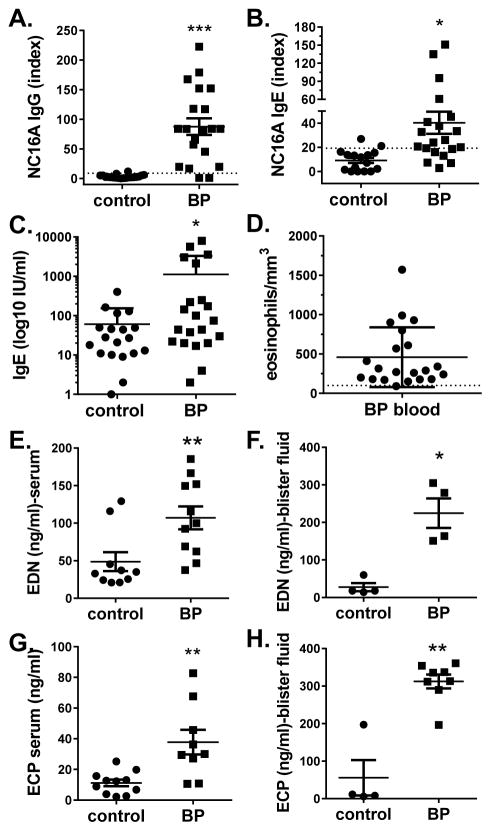
Autoantibody levels, total IgE and eosinophil numbers were evaluated in the study subjects (Fig. 1). As expected, levels of NC16A-specific IgG and IgE, and total IgE, were significantly elevated in BP sera (n = 20) compared to control sera (n = 16). BP patients also exhibited elevated circulating eosinophil counts (mean 694.4/mm3 ± 1110, range 100–5830) compared to the normal range (40–390/mm3). As previously reported by others (39, 40), EDN and ECP were significantly elevated in sera and blister fluid of BP patients, and these levels were higher in the blister fluid then the circulation (Fig. 1, panels E–H).
Figure 1. Immunologic characterization of BP patients.
Sera from BP patients or age-matched controls were examined for levels of NC16A-specific IgG, NC16A-specific IgE, total IgE and eosinophil granule proteins, EDN and ECP, by ELISA. Circulating eosinophil counts were also obtained. Each point represents an individual patient sample. A non-parametric t-test was used to determine significant differences between groups. (*p<0.05, **p<.005).
To better understand the relationship between autoantibody levels and eosinophils in BP, their degree of association was evaluated using a Spearman rank correlation. A strong correlation (r = 0.441, p = 0.040) between EDN concentrations and NC16A- specific IgG was observed in BP sera. A similar trend was observed for circulating ECP levels, but this was not significant (r = 0.476, p = 0.088). Interestingly, no correlation was observed between circulating granule proteins and NC16A-specific IgE concentrations. However, circulating ECP levels correlated very strongly (r = 0.821, p = 0.034) with disease activity (BPDAI). Finally, the concentrations of granule proteins in blister fluid did not correlate with concentrations NC16A-specific IgG or IgE or total IgE. It should be noted, however, it is possible that circulating granule protein concentrations do not directly reflect eosinophil degranulation since ECP, EDN and MBP are variably expressed (~10–1000 fold less) by other immune cells and tissues (41).
Characterization of IgG and IgE autoantibodies purified from BP sera
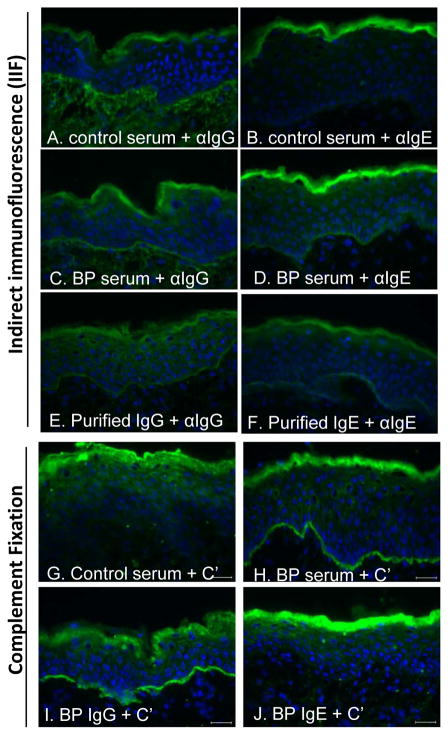
To evaluate the independent effects of IgG and IgE, these autoantibody isotypes were purified from BP serum using established protocols (19, 30). Prior to purification, IIF studies revealed linear binding of both IgG and IgE at the DEJ of human skin (Fig 2, C, D) that was not evident using control serum (Fig 2, A, B). After purification, both IgG and IgE isotypes retained their ability to bind the DEJ (Fig 2, E, F). As expected, complement fixation was mediated by IgG, but not IgE (Fig 2, G–J). Similar results were obtained using autoantibodies purified from a second BP serum (not shown).
Figure 2. BP autoantibody purification and complement fixation.
IgG and IgE antibodies were purifed via two-step affinity chromatography from a well-characterized BP serum known to have high NC16A-specific autoantibody titers. The serum and the purified IgG and IgE were evaluated by IIF on human skin for specific binding (A–F) and complement (C′) fixation (G–J) at the DEJ using Alexa-488-isotype- or C′-specific antibodies. Nuclei were stained with DAPI.
Characterization of human eosinophil line, 15HL-60
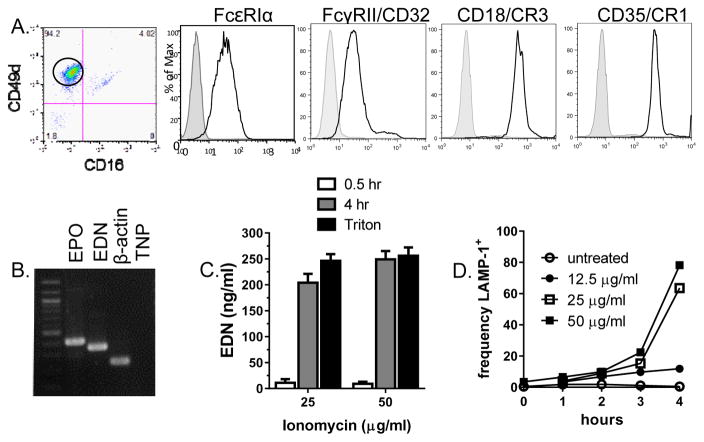
Studies of human eosinophils are limited by their rarity in vivo and lack of a lineage-specific surface marker to aid in purification of live cells. Thus, a human eosinophilic cell line, 15HL-60 (38), was evaluated for use in cryosection experiments. Flow cytometry (Fig 3, panel A) confirmed the eosinophilic phenotype (CD49d+CD16−) and surface expression of receptors for IgG (FcγRII), IgE (FcεRI) and complement (CD18, CD35). RT-PCR analysis confirmed that the15HL-60 cells expressed mRNA for eosinophil granule proteins, EPO and EDN (Fig. 3B). Furthermore, incubation of 15HL-60 cells with ionomycin resulted in release of EDN into culture supernatants and increased surface expression of LAMP-1/CD107a (Fig 3C, D).
Figure 3. Characterization of 15HL60 human eosinophil line.
Surface expression of receptors for IgE (FcεRI), IgG (FcγRII) and complement (CD18/CR3 and CD35/CR1) was evaluated by flow cytometry on CD16−CD49d+ cells (A). Histograms indicate positive staining (open histogram) compared to isotype controls (shaded histogram). Expression of granule protein mRNA was evaluated by RT-PCR (B). Lanes show a distinct band of appropriate size using primers for eosinophil peroxidase (EPO), eosinophil-derived neurotoxin (EDN), β-actin and template/no primer control (TNP). Degranulation of 15HL-60 cells was detected by ELISA for the release of EDN into supernatants of cells stimulated with Ionomycin, at doses and times indicated, or lysed with 1% Triton X-100 (C) and expression of LAMP-1 on the surface of stimulated cells by flow cytometry (D).
Cryosection experiments
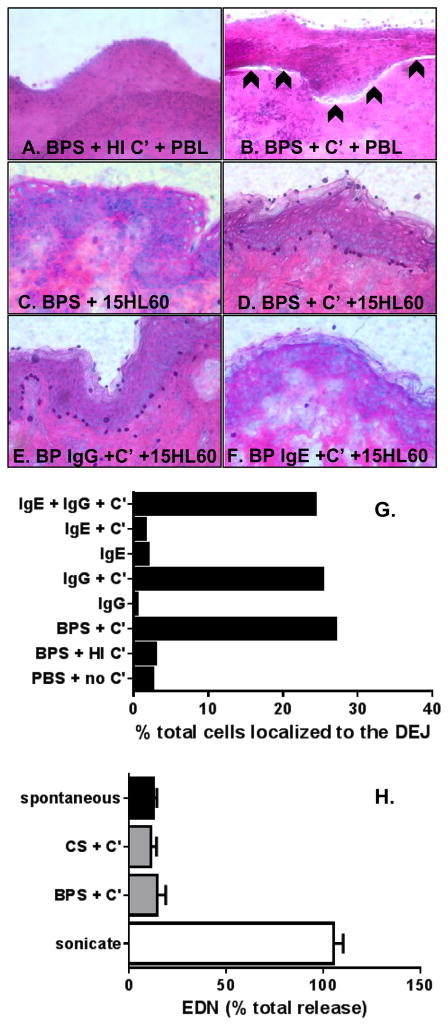
The cryosection model has been used by others to demonstrate pathogenicity of BP180-specific IgG antibodies (32, 34, 36), where autoantibodies bound to the DEJ triggered Fc-receptor-mediated activation neutrophil degranulation and formation of a subepidermal split. We adapted this model for use with eosinophils and validated our protocol using freshly isolated granulocytes and complement. Using this method, a subepidermal split was observed when human skin cryosections were incubated with PBL and fresh complement, but not when heat inactivated (HI) complement was used (Fig. 4A, B) or control serum with or without complement (data not shown). Since lesional biopsies from BP patients reveal a predominance of eosinophils, our goal was to determine the role of IgG, IgE and complement in eosinophil localization to the DEJ, and to evaluate eosinophil degranulation and subepidermal split formation under these conditions. In the first set of experiments, 15HL-60 eosinophils localized to the DEJ of human skin in the presence of BP serum and fresh complement (Fig. 4D). No localization was observed when HI complement was used (Fig. 4C) or in the presence of control serum (not shown) or PBS (Fig. 4G).
Figure 4. IgG and complement are required for eosinophil localization to the BMZ.
Cyrosections of human skin were incubated with BP sera (BPS) + heat inactivated (HI) or fresh complement (C′) + PBL from a healthy donor, as negative (A) and positive controls (B), respectively. Slides were stained with H & E, images were captured via light microscopy. A subepidermal split is indicated by the arrowheads (B). Next, cryosections were incubated with BPS + 15HL60 eosinophils ± complement (C, D). Incubation of cryosections with purified IgG or IgE + C′ + 15HL60 cells revealed that eosinophil localization to the BMZ is dependent on IgG and C′. The %of the total overlaid cells that localized to the DEJ was determined using NIH Image J (G). Collection of supernatants from cryosection experiments revealed that 15HL60 eosinophils do not degranulate under these conditions (H). Likewise, application of 15HL60 lysates did not cause a subepidermal split (data not shown). Bars represent the average value from two cryosections treated identically (G) or as mean ± SD of supernatants collected from triplicate samples treated identically (H).
Since human eosinophils express receptors for both IgG and IgE, the contribution of each autoantibody isotype to their localization to the DEJ was examined. Antibodies were purified from BP sera (n = 2) and used at physiologic concentrations − 2.5 mg/ml IgG and 900 ng/ml IgE. Eosinophil localization to the DEJ was dependent on both IgG autoantibodies and fresh complement (Fig. 4E, F, G). When sections were incubated with fresh complement, additional cells are also observed decorating the epidermis. In studies utilizing neutrophils (32), this non-specific attachment of leukocytes to the stratum corneum was attributed to corneocyte-activation of the alternative complement pathway resulting in antibody-dependent cellular adhesion (42, 43). IgE antibodies did not affect the distribution of overlaid eosinophils. Finally, eosinophils did not mediate dermal-epidermal separation under any conditions.
The absence of a subepidermal split in these experiments could result from i) lack of eosinophil degranulation under these conditions, or ii) an inability of eosinophil-derived factors to mediate split formation. To understand which scenario might be at play, supernatants were collected after incubation of cryosections under various conditions and assayed for EDN by ELISA. In parallel, supernatants were collected from equal numbers of eosinophils incubated in media alone (spontaneous release), treated with 1% Triton X-100 (total release) or eosinophils lysed by sonication (Fig. 4H). Overall, the conditions of the cryosection experiments did not trigger eosinophil degranulation. Furthermore, no difference in EDN levels was observed when 15HL-60 cells were exposed to purified IgG or IgE in the presence of fresh complement. Finally, to determine whether eosinophil granule proteins are capable of mediating separation at the DEJ in a cryosection model, 15HL-60 cell lysates were applied directly to cryosections alone or in the presence of BP serum for 1, 2, 4 or 18 hrs. No subepidermal split was observed (not shown).
Discussion
Several lines of evidence support a role for eosinophils in disease pathogenesis. First, degranulated eosinophils and free (intact) eosinophil granules are observed in developing BP lesions (3). Second, eosinophils and eosinophil granule proteins are present in the fluid of intact blisters (1, 2, 39, 44, 45) and, at lower concentrations, in the circulation of BP patients (39, 40, 46). Finally, BP disease activity is closely paralleled by peripheral eosinophil numbers (47). In this report, we demonstrate using a cryosection model of BP that eosinophil localization to the DEJ is dependent on IgG-mediated complement fixation. Extension of these findings to human disease suggests that eosinophil localization to the DEJ occurs only after autoantibody deposition in the skin. These findings provide additional insight into the sequence of events during the development of BP lesions.
The cryosection model has been used to demonstrate pathogenicity of IgG antibodies specific for BP180 (32, 34, 36). In Sitaru (2002), formation of a subepidermal split was dependent on the presence of NC16A specific antibodies and human neutrophils, but not complement (32). Neutrophil attachment to the DEJ and split formation peaked after 1 and 3 hr of incubation, respectively, and was dependent on autoantibody concentration. This delay in split formation is likely due dependence on the destructive action of neutrophil-derived proteases at the DEJ (36). Optimal split formation was also dependent on autoantibody concentration. In our experiments utilizing granulocytes, we observe a subepidermal split only in the presence of fresh complement. This is most likely because our experiments were optimized using a lower cell number (1–5 × 106) than was required to produce a split in previous studies (>107 cells/section), based on the goal of eventually utilizing freshly isolated eosinophils for these experiments. Thus, the requirement for complement in our studies may reflect the fact that there is a lower limit to the number of leukocytes capable of inducing a split. Indeed, it was reported that no split was observed (with or without complement) when fewer leukocytes were used (32). Activation of cells through both the Fcγ- and complement receptors would trigger an increase in the number of cells activated and, accordingly, higher concentrations of destructive proteases at the DEJ. This is in agreement with the observation that potential for split formation is higher with antibody isotypes capable of complement fixation (34) and the fact that a longer incubation (6 hrs) was required for split formation in our experiments.
These experiments were conducted using physiologic concentrations of IgG (~2–3 mg/ml) and IgE (900 ng/ml = 375 U/ml). Ideally, IgE and IgG concentrations would be titrated equally for direct comparison; however, this is not technically possible due to limitations in starting concentrations and loss of IgE during purification. For this reason, the experiments were designed using circulating antibody concentrations, although it is possible that higher concentrations are present in lesional skin. Although eosinophils in BP lesions express both the low and high affinity IgE receptors, these antibodies may not play a direct role in eosinophil activation or localization at the BMZ. Rather, IgE may play an indirect role via activation of mast cells whose production of histamine, platelet-activating factor (PAF), vascular endothelial growth factor (VEGF), and others, is known to trigger eosinophil migration. Once in the dermis, eosinophil localization to the BMZ is mediated via expression of complement receptors, CR1 and CR3, known to be expressed by eosinophils (48)).
Although studies utilizing the cryosection model are essential for determining the requirements for localization to the DEJ or creation of a subepidermal split, these studies do not represent a complete replication of the in vivo mechanisms of disease. In our in vivo studies, injection of IgE into the dermis led to mast cell degranulation, eosinophil infiltration and histologic separation at the DEJ, but not gross blistering. Further, the in vivo nature of these studies precludes the conclusion that IgE’s mechanism of action is through direct interaction with eosinophils rather than indirectly via other innate or inflammatory mediators. Rather, immunofluorescent and electron microscopy of graft tissue from these studies suggest that IgE triggers degranulation of resident mast cells before eosinophil degranulation is observed. When taken with our cryosection studies, we hypothesize that eosinophil infiltration into lesional skin is mediated via the direct effects of IgE on tissue mast cells; however, once in the skin, eosinophil localization to the DEJ is complement dependent. Furthermore, it is likely that proteases or additional factors provided by the inflammatory environment augment IgE’s ability to produce a subepidermal split in vivo whereas in the non-metabolic state of the cryosection experiments, we show that eosinophils alone cannot produce a split. Future studies will be aimed at understanding the influence of the inflammatory environment of BP lesions on eosinophil degranulation.
Acknowledgments
This material is based upon work supported in part by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Biomedical Laboratory Research and Development 1BX001680-01 (JAF). We gratefully acknowledge Julie McKillip, RN, for her assistance with IRB submissions, patient enrollment and sample collection. Some data were obtained at the Flow Cytometry Facility, which is a Carver College of Medicine/Holden Comprehensive Cancer Center core research facility at the University of Iowa. The Facility is funded through user fees and the generous financial support of the Carver College of Medicine, Holden Comprehensive Cancer Center, and Iowa City Veteran’s Administration Medical Center.
Footnotes
Author contribution
KM, JW, HH RS and SA performed the research; KM and JF designed the research study; KM analyzed the data and wrote the paper.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
References
- 1.Dvorak AM, Mihm MC, Jr, Osage JE, et al. Bullous pemphigoid, an ultrastructural study of the inflammatory response: eosinophil, basophil and mast cell granule changes in multiple biopsies from one patient. Journal of Investigative Dermatology. 1982;78:91–101. doi: 10.1111/1523-1747.ep12505711. [DOI] [PubMed] [Google Scholar]
- 2.Schaumburg-Lever G, Orfanos CE, Lever WP. Electron microscopic study of bullous pemphigoid. Archives of Dermatology. 1972;106:662–667. [PubMed] [Google Scholar]
- 3.Borrego B, Maynard B, Peterson EA, et al. Deposition of Eosinophil Granule Proteins Precedes Blister Formation in Bullous Pemphigoid. Comparison with Neutrophil and Mast Cell Granule Proteins. American Journal of Pathology. 1996;148:13. [PMC free article] [PubMed] [Google Scholar]
- 4.Chen R, Fairley JA, Zhao ML, et al. Macrophages, but not T and B lymphocytes, are critical for subepidermal blister formation in experimental bullous pemphigoid: macrophage-mediated neutrophil infiltration depends on mast cell activation. Journal of Immunology. 2002;169:3987–3992. doi: 10.4049/jimmunol.169.7.3987. [DOI] [PubMed] [Google Scholar]
- 5.Zhao W, Kepley CL, Morel PA, et al. Fc gamma RIIa, not Fc gamma RIIb, is constitutively and functionally expressed on skin-derived human mast cells. Journal of Immunology. 2006;177:694–701. doi: 10.4049/jimmunol.177.1.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen R, Ning G, Zhao ML, et al. Mast cells play a key role in neutrophil recruitment in experimental bullous pemphigoid. J Clin Invest. 2001;108:1151–1158. doi: 10.1172/JCI11494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Provost TT, Tomasi TB., Jr Immunopathology of bullous pemphigoid. Basement membrane deposition of IgE, alternate pathway components and fibrin. Clinical & Experimental Immunology. 1974;18:193–200. [PMC free article] [PubMed] [Google Scholar]
- 8.Jordon RE, Beutner EH, Witebsky E, et al. Basement zone antibodies in bullous pemphigoid. Journal of the American Medical Association. 1967;200:751–756. [PubMed] [Google Scholar]
- 9.Arbesman CE, Wypych JI, Reisman RE, et al. IgE levels in sera of patients with pemphigus or bullous pemphigoid. Archives of Dermatology. 1974;110:378–381. [PubMed] [Google Scholar]
- 10.Wakugawa M, Nakamura K, Hino H, et al. Elevated levels of eotaxin and interleukin-5 in blister fluid of bullous pemphigoid: correlation with tissue eosinophilia. British Journal of Dermatology. 2000;143:112–116. doi: 10.1046/j.1365-2133.2000.03599.x. [DOI] [PubMed] [Google Scholar]
- 11.Messingham KN, Holahan HM, Frydman AS, et al. Human Eosinophils Express the High Affinity IgE Receptor, FcepsilonRI, in Bullous Pemphigoid. PLoS One. 2014;9:e107725. doi: 10.1371/journal.pone.0107725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Delaporte E, Dubost-Brama A, Ghohestani R, et al. IgE autoantibodies directed against the major bullous pemphigoid antigen in patients with a severe form of pemphigoid. Journal of Immunology. 1996;157:3642–3647. [PubMed] [Google Scholar]
- 13.Messingham KA, Holahan HM, Fairley JA. Unraveling the significance of IgE autoantibodies in organ-specific autoimmunity: lessons learned from bullous pemphigoid. Immunologic research. 2014;59:273–278. doi: 10.1007/s12026-014-8547-7. [DOI] [PubMed] [Google Scholar]
- 14.Schmidt E, Obe K, Brocker EB, et al. Serum levels of autoantibodies to BP180 correlate with disease activity in patients with bullous pemphigoid. [see comment] Archives of Dermatology. 2000;136:174–178. doi: 10.1001/archderm.136.2.174. [DOI] [PubMed] [Google Scholar]
- 15.Tsuji-Abe Y, Akiyama M, Yamanaka Y, et al. Correlation of clinical severity and ELISA indices for the NC16A domain of BP180 measured using BP180 ELISA kit in bullous pemphigoid. Journal of Dermatological Science. 2005;37:145–149. doi: 10.1016/j.jdermsci.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 16.Hofmann S, Thoma-Uszynski S, Hunziker T, et al. Severity and phenotype of bullous pemphigoid relate to autoantibody profile against the NH2- and COOH-terminal regions of the BP180 ectodomain. Journal of Investigative Dermatology. 2002;119:1065–1073. doi: 10.1046/j.1523-1747.2002.19529.x. [DOI] [PubMed] [Google Scholar]
- 17.Amo Y, Ohkawa T, Tatsuta M, et al. Clinical significance of enzyme-linked immunosorbent assay for the detection of circulating anti-BP180 autoantibodies in patients with bullous pemphigoid. Journal of Dermatological Science. 2001;26:14–18. doi: 10.1016/s0923-1811(00)00149-3. [DOI] [PubMed] [Google Scholar]
- 18.Giudice GJ, Emery DJ, Zelickson BD, et al. Bullous pemphigoid and herpes gestationis autoantibodies recognize a common non-collagenous site on the BP180 ectodomain. Journal of Immunology. 1993;151:5742–5750. [PubMed] [Google Scholar]
- 19.Fairley JA, Fu CL, Giudice GJ. Mapping the binding sites of anti-BP180 immunoglobulin E autoantibodies in bullous pemphigoid. Journal of Investigative Dermatology. 2005;125:467–472. doi: 10.1111/j.0022-202X.2005.23853.x. [DOI] [PubMed] [Google Scholar]
- 20.Zillikens D, Rose PA, Balding SD, et al. Tight clustering of extracellular BP180 epitopes recognized by bullous pemphigoid autoantibodies. Journal of Investigative Dermatology. 1997;109:573–579. doi: 10.1111/1523-1747.ep12337492. [DOI] [PubMed] [Google Scholar]
- 21.Dopp R, Schmidt E, Chimanovitch I, et al. IgG4 and IgE are the major immunoglobulins targeting the NC16A domain of BP180 in Bullous pemphigoid: serum levels of these immunoglobulins reflect disease activity. Journal of the American Academy of Dermatology. 2000;42:577–583. [PubMed] [Google Scholar]
- 22.Sesarman A, Oswald E, Chiriac MT, et al. Why human pemphigoid autoantibodies do not trigger disease by the passive transfer into mice? Immunol Lett. 2012;143:92–100. doi: 10.1016/j.imlet.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 23.Liu Z, Giudice GJ, Swartz SJ, et al. The role of complement in experimental bullous pemphigoid. Journal of Clinical Investigation. 1995;95:1539–1544. doi: 10.1172/JCI117826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu Z, Giudice GJ, Zhou X, et al. A major role for neutrophils in experimental bullous pemphigoid. Journal of Clinical Investigation. 1997;100:1256–1263. doi: 10.1172/JCI119639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin L, Betsuyaku T, Heimbach L, et al. Neutrophil elastase cleaves the murine hemidesmosomal protein BP180/type XVII collagen and generates degradation products that modulate experimental bullous pemphigoid. Matrix Biology. 2012;31:38–44. doi: 10.1016/j.matbio.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu Z, Shapiro SD, Zhou X, et al. A critical role for neutrophil elastase in experimental bullous pemphigoid. Journal of Clinical Investigation. 2000;105:113–123. doi: 10.1172/JCI3693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Natsuga K, Nishie W, Shinkuma S, et al. Antibodies to Pathogenic Epitopes on Type XVII Collagen Cause Skin Fragility in a Complement-Dependent and -Independent Manner. The Journal of Immunology. 2012;188:5792–5799. doi: 10.4049/jimmunol.1003402. [DOI] [PubMed] [Google Scholar]
- 28.Liu Z, Sui W, Zhao M, et al. Subepidermal blistering induced by human autoantibodies to BP180 requires innate immune players in a humanized bullous pemphigoid mouse model. Journal of Autoimmunity. 2008;31:331–338. doi: 10.1016/j.jaut.2008.08.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nishie W, Sawamura D, Goto M, et al. Humanization of autoantigen. Nature Medicine. 2007;13:378–383. doi: 10.1038/nm1496. [DOI] [PubMed] [Google Scholar]
- 30.Fairley JA, Burnett CT, Fu C-L, et al. A Pathogenic Role for IgE in Autoimmunity: Bullous Pemphigoid IgE Reproduces the Early Phase of Lesion Development in Human Skin Grafted to nu//nu Mice. J Invest Dermatol. 2007;127:2605–2611. doi: 10.1038/sj.jid.5700958. [DOI] [PubMed] [Google Scholar]
- 31.Zone JJ, Taylor T, Hull C, et al. IgE basement membrane zone antibodies induce eosinophil infiltration and histological blisters in engrafted human skin on SCID mice. Journal of Investigative Dermatology. 2007;127:1167–1174. doi: 10.1038/sj.jid.5700681. [DOI] [PubMed] [Google Scholar]
- 32.Sitaru C, Schmidt E, Petermann S, et al. Autoantibodies to bullous pemphigoid antigen 180 induce dermal-epidermal separation in cryosections of human skin. Journal of Investigative Dermatology. 2002;118:664–671. doi: 10.1046/j.1523-1747.2002.01720.x. [DOI] [PubMed] [Google Scholar]
- 33.Sesarman A, Oswald E, Chiriac MT, et al. Why human pemphigoid autoantibodies do not trigger disease by the passive transfer into mice? Immunology Letters. 2012;143:92–100. doi: 10.1016/j.imlet.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 34.Mihai S, Chiriac MT, Herrero-Gonzalez JE, et al. IgG4 autoantibodies induce dermal-epidermal separation. Journal of cellular and molecular medicine. 2007;11:1117–1128. doi: 10.1111/j.1582-4934.2007.00081.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sitaru C, Kromminga A, Hashimoto T, et al. Autoantibodies to type VII collagen mediate Fcgamma-dependent neutrophil activation and induce dermal-epidermal separation in cryosections of human skin. Am J Pathol. 2002;161:301–311. doi: 10.1016/s0002-9440(10)64182-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shimanovich I, Mihai S, Oostingh GJ, et al. Granulocyte-derived elastase and gelatinase B are required for dermal-epidermal separation induced by autoantibodies from patients with epidermolysis bullosa acquisita and bullous pemphigoid. The Journal of pathology. 2004;204:519–527. doi: 10.1002/path.1674. [DOI] [PubMed] [Google Scholar]
- 37.Messingham KA, Noe MH, Chapman MA, et al. A novel ELISA reveals high frequencies of BP180-specific IgE production in bullous pemphigoid. J Immunol Methods. 2009;346:18–25. doi: 10.1016/j.jim.2009.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fischkoff SA. Graded increase in probability of eosinophilic differentiation of HL-60 promyelocytic leukemia cells induced by culture under alkaline conditions. Leukemia Research. 1988;12:679–686. doi: 10.1016/0145-2126(88)90103-8. [DOI] [PubMed] [Google Scholar]
- 39.Czech W, Schaller J, Schöpf E, et al. Granulocyte activation in bullous diseases: Release of granular proteins in bullous pemphigoid and pemphigus vulgaris. Journal of the American Academy of Dermatology. 1993;29:210–215. doi: 10.1016/0190-9622(93)70170-x. [DOI] [PubMed] [Google Scholar]
- 40.Caproni M, Palleschi GM, Falcos D, et al. Serum eosinophil cationic protein (ECP) in bullous pemphigoid. Int J Dermatol. 1995;34:177–180. doi: 10.1111/j.1365-4362.1995.tb01562.x. [DOI] [PubMed] [Google Scholar]
- 41.Acharya KR, Ackerman SJ. Eosinophil granule proteins: form and function. J Biol Chem. 2014;289:17406–17415. doi: 10.1074/jbc.R113.546218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Terui T, Kato T, Tagami H. Stratum corneum activation of complement through the antibody-independent alternative pathway. J Invest Dermatol. 1989;92:593–597. doi: 10.1111/1523-1747.ep12709634. [DOI] [PubMed] [Google Scholar]
- 43.Terui T, Zhen YX, Kato T, et al. Mechanism of human polymorphonuclear leukocyte adhesion to serum-treated corneocytes. J Invest Dermatol. 1995;104:297–301. doi: 10.1111/1523-1747.ep12612833. [DOI] [PubMed] [Google Scholar]
- 44.Dubertret L, Bertaux B, Fosse M, et al. Cellular events leading to blister formation in bullous pemphigoid. British Journal of Dermatology. 1980;103:615–624. doi: 10.1111/j.1365-2133.1980.tb01683.x. [DOI] [PubMed] [Google Scholar]
- 45.Wintroub BU, Wasserman SI. The molecular pathogenesis of bullous pemphigoid. Clin Dermatol. 1987;5:126–134. doi: 10.1016/0738-081x(87)90057-5. [DOI] [PubMed] [Google Scholar]
- 46.D’Auria L, Pietravalle M, Mastroianni A, et al. IL-5 levels in the serum and blister fluid of patients with bullous pemphigoid: correlations with eosinophil cationic protein, RANTES, IgE and disease severity. Archives of Dermatological Research. 1998;290:25–27. doi: 10.1007/s004030050272. [DOI] [PubMed] [Google Scholar]
- 47.Yu KK, Crew AB, Messingham KA, et al. Omalizumab therapy for bullous pemphigoid. J Am Acad Dermatol. 2014;71:468–474. doi: 10.1016/j.jaad.2014.04.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fischer E, Capron M, Prin L, et al. Human eosinophils express CR1 and CR3 complement receptors for cleavage fragments of C3. Cellular immunology. 1986;97:297–306. doi: 10.1016/0008-8749(86)90400-4. [DOI] [PubMed] [Google Scholar]