Abstract
Although sensory processing challenges have been noted since the first clinical descriptions of autism, it has taken until the release of the fifth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) in 2013 for sensory problems to be included as part of the core symptoms of autism spectrum disorder (ASD) in the diagnostic profile. Because sensory information forms the building blocks for higher-order social and cognitive functions, we argue that sensory processing is not only an additional piece of the puzzle, but rather a critical cornerstone for characterizing and understanding ASD. In this review we discuss what is currently known about sensory processing in ASD, how sensory function fits within contemporary models of ASD, and what is understood about the differences in the underlying neural processing of sensory and social communication observed between individuals with and without ASD. In addition to highlighting the sensory features associated with ASD, we also emphasize the importance of multisensory processing in building perceptual and cognitive representations, and how deficits in multisensory integration may also be a core characteristic of ASD.
Keywords: autism spectrum disorder, sensory processing deficits, sensory representations, psychophysics, neuroimaging
1 Introduction
Autism spectrum disorder (ASD) is a complex neurodevelopmental disorder of strikingly high incidence that represents a major public health challenge. Recent evidence suggests that the incidence of ASD in the general population is now 1 child in every 68, with that number being as high as 1 in 42 for boys (ADDM, 2014). This high incidence, coupled with the often-debilitating symptoms of ASD, result in substantial hardships at the individual, family, and societal levels.
The traditional core diagnostic and clinical features of ASD are weaknesses in social communicative abilities and the presence of restricted interests and repetitive behaviors. In addition, and as recently more formally recognized in the DSM-5, children with ASD also frequently suffer from disturbances in sensory function. Although these sensory deficits have only recently appeared in the diagnostic profile of ASD, they have been reported in the descriptions of autism dating back to the original writings of Kanner (Kanner, 1943). In fact, sensory abnormalities are one of the most prevalent symptoms of ASD, reported in up to 87% of individuals (Le Couteur et al., 1989; Lord, 1995).
The historical absence of sensory features in the diagnostic definition of ASD, despite widespread acknowledgement of their presence, is likely a result of several factors, including difficulties in characterizing sensory function in a strongly empirical manner and a greater focus on the more readily apparent social and cognitive symptoms. Anecdotal and caregiver reports, however, are rife with descriptions of sensory problems in children with ASD, and structured questionnaires invariably identify the presence of processing challenges in a number of sensory domains (Baranek et al., 2006; Rogers et al., 2003; Watling et al., 2001). Thus, the acknowledged high prevalence of sensory features in ASD, coupled with the emerging view that these “lower-level” sensory aspects may play an integral role in the better-characterized, “higher-order” differences (see below for more detail on this argument), demands a more empirical view into sensory contributions in ASD.
Although this examination of sensory processing in ASD must start with exploring differences in the processing of information within the different senses, it must also be extended to include the processing of information across the different senses. Indeed, it can be argued that such multisensory function is likely to be more strongly altered in ASD, given that many of the multisensory deficits observed in ASD go beyond what would be predicted by the individual unisensory performance. For example, deficits in multisensory integration are noted between children with ASD and their typically developing (TD) peers even when unisensory performance is unimpaired (Foxe et al., 2013; Stevenson et al., 2014c; Stevenson et al., 2014d, e). Furthermore, evidence for differences in connectivity between distant brain regions in children with ASD (Abrams et al., 2013; Assaf et al., 2010; Maximo et al., 2014; Plitt et al., 2015) would also suggest a propensity towards multisensory deficits. Such communication across regions of the cerebral cortex provides the substrate for multisensory processing and integration, given that it demands the coordination of information processing across different sensory domains (i.e., regions of visual, auditory and somatosensory cortex need to communicate and exchange information in order to accomplish multisensory integration).
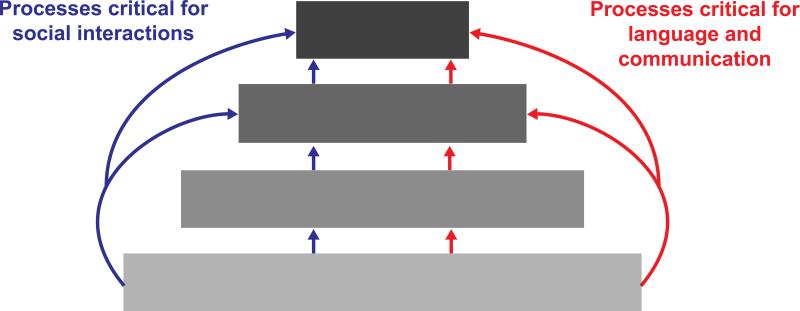
The integration of information across the different senses is an essential process in the construction of healthy perceptual representations, and can be argued to represent one of the basic building blocks for the construction of cognitive representations and abilities (see Figure 1). Given that we live in a world in which we are continually confronted with information conveyed via our different senses, an essential function of the nervous system is to combine and synthesize this information into a coherent perceptual whole. Whereas some of this information is reflective of a common source or event and needs to be integrated, much of it is unrelated and needs to be perceptually segregated. Failure to build an accurate and meaningful perceptual representation of the world around us is likely to cascade into higher order deficits, making it difficult to navigate and interact with our environment (Stevenson et al., 2014b).
Figure 1.
Conceptual view of the relationship between sensory processing and ‘higher-order’ perceptual and cognitive processes. Sensory representations form the building blocks for multisensory representations, which in turn are built upon for perceptual and cognitive representations. Social communication and language, both of which are impacted in ASD, rely on the convergence of these representations. Thus, examining sensory and multisensory representations in addition to cognitive representations will be necessary to fully tease apart the mechanisms behind social and language deficits in ASD.
Because of the importance of multisensory function for our perceptual abilities, the brain has a number of circuits and regions that are preferentially utilized for the combination and integration of information across the different senses. These multisensory regions are found throughout the spinal cord and brainstem and are widely elaborated within the cerebral cortex (Beauchamp et al., 2004; Perrault et al., 2005; Stein et al., 2014; Wallace and Stein, 2007). Within these regions, neurons and neuronal circuits are specialized for the integration of information from the different sensory modalities, and frequently show marked non-linearities in their response profiles that are associated with the active integration process (Beauchamp, 2005; Meredith and Stein, 1983, 1985; Stevenson et al., 2012; Stevenson et al., 2014a; Wallace et al., 1998; Wallace et al., 1996). Rather than combining this information in an indiscriminant manner, these neurons and circuits appear to be strongly sensitive to the statistical relationships of the stimuli to one another. Thus, stimuli from the different senses that are spatially (Carriere et al., 2008; Ghose and Wallace, 2014; Krueger et al., 2009; Meredith and Stein, 1986a, 1996; Rohe and Noppeney, 2015; Royal et al., 2009) and temporally (Cappe et al., 2012; Cappe et al., 2009; Diederich and Colonius, 2015; Meredith et al., 1987; Rowland and Stein, 2014; Stevenson and Wallace, 2013; van Eijk et al., 2008) proximate generally result in large enhancements of neuronal response, whereas those that are more disparate in these domains generally fail to elicit these large enhancements, and if sufficiently far apart in space and/or time, can often result in dramatic depressions in neuronal response (Sarko et al., 2012; Senkowski et al., 2011; Senkowski et al., 2007; Stevenson et al., 2010; Stevenson and Wallace, 2013; Teder-Salejarvi et al., 2005; Wallace et al., 1996). Such a coding strategy makes a great deal of intuitive sense if we think of the brain as a statistical machine that is using this information to make probabilistic judgments about which stimuli belong together (Beck et al., 2008; Kording et al., 2007; Magnotti and Beauchamp, 2014; Magnotti et al., 2013; Shams, 2012).
The behavioral and perceptual benefits of multisensory integration have been shown to be quite impressive and to confer a highly degree of adaptive benefit (Calvert et al., 2004; Fetsch et al., 2010; Murray and Wallace, 2012; Stein and Meredith, 1993; Stevenson et al., 2014a). One of the most straightforward ways to illustrate multisensory-mediated behavioral benefits is in the context of a simple reaction time task in which subjects are asked to press a button as quickly as they can when they detect a light, a sound, or a light-sound pairing. The speed of responses to the light-sound (i.e., multisensory) pairing is substantially faster than to either of the unisensory (i.e., visual alone or auditory alone) conditions, and is also faster than would be predicted based on pure probability summation (i.e., if one took the fastest response from each of the two distributions; Colonius and Diederich, 2004; Gondan et al., 2004; Martuzzi et al., 2007; Miller, 1982; Murray et al., 2012; Schroter et al., 2007; Sperdin et al., 2009; Todd, 1912). Such speeded responses that beat so-called “race model” predictions reinforce the concept that there is an active convergence and integration of the sensory information (Raab, 1962). Along with such behavioral advantages one can also see striking perceptual benefits under multisensory conditions. One of the most powerful and illustrative of these is the gain seen in speech intelligibility when the subject has the ability to see the lips of a speaker in a noisy environment (Fraser et al., 2010; MacLeod and Summerfield, 1987; Ross et al., 2007). Estimates suggest that the information gain in this situation is the equivalent of boosting the audible signal by up to 15 dB (Sumby and Pollack, 1954).
Taking into account the importance of such multisensory integration for normal behavioral and perceptual processes, as well as the central role that such integration plays in social and communicative function (given the highly multisensory nature of our social and language cues), it seems plausible that disruptions in sensory function that extend beyond the individual modalities may be important in ASD (Stevenson et al., 2014b). In the current review, we present evidence for changes in both unisensory and multisensory function in the context of ASD, and relate these sensory-based changes to the more classical phenotypic and clinical features of ASD. Furthermore, we review the evidence for changes in neurobiological networks associated with these differences in sensory and multisensory processing, and attempt to frame these studies in the context of several of the prevailing theories of ASD. Finally, we present preliminary evidence that suggests that perceptual plasticity based approaches grounded in improvements in (multi)sensory abilities may hold promise as a remediation tool to be used in ASD treatment, and suggest that investigation of individual differences in sensory and multisensory function may provide valuable insights that will be ultimately useful in more individualized or personalized approaches to ASD treatment.
2 Atypical sensory processing in ASD
Despite the fact that many sensory processing issues have only been qualitatively reported via sources such as questionnaires and parent/caregiver reports, they have provided an important view into the prevalence of sensory symptoms and the broad nature of these changes. These sensory changes are typically not seen in just a single sensory modality (when examined), but rather appear to extend to multiple sensory systems including vision, hearing, touch, proprioception, taste, and smell (Baranek et al., 2006; Dawson and Watling, 2000; Kasari and Sigman, 1997; Kern et al., 2007; Kientz and Dunn, 1997; O'Neill and Jones, 1997; Rogers et al., 2003; Talay-Ongan and Wood, 2000; Watling et al., 2001; Wing and Potter, 2002). Sensory processing issues in ASD can take many forms, and are generally divided into three main patterns: sensory hyper-sensitivity (negative reactions to low-level environmental stimuli generally considered to be innocuous), sensory hypo-sensitivity (diminished or absent responses to stimuli, including pain) and sensory seeking (strong desire for a particular kind of sensory experience) (Baranek et al., 2006). Furthermore, these sensory processing issues can be tied to the other core features of ASD, such that differences in sensory perception are often significantly correlated with symptom severity in other domains impacted in ASD such as social communication (Foss-Feig et al., 2012; Kern et al., 2007).
2.1 Sensory function within the individual modalities in ASD
2.1.1 Visual processing
In addition to the sensory processing issues noted with these more qualitative methods, a growing number of studies are using more laboratory-based measures, and have noted examples of both enhancements and deficits in behavioral or perceptual performance in individuals with ASD (de Jonge et al., 2006; Iarocci and McDonald, 2006). For example, those with ASD have been reported to show behavioral improvements when perceiving simple, non-social sensory stimuli, yet to show deficits when perceiving more complex stimuli (Bertone et al., 2003, 2005; Bonnel et al., 2003; Minshew and Hobson, 2008; Mottron et al., 2006; Ristic et al., 2005; Vlamings et al., 2005). The evidence for these diverging results between simple and complex stimulus processing has perhaps been best established within the visual system (Cavanagh and Mather, 1989; Chubb and Sperling, 1988; Schofield, 2000). For example, individuals with ASD have been shown to be better in discriminating between visual stimulus orientations that are defined on the basis of changes in luminance (“first-order” features, characterized at each point of an image independently), but to show difficulties in perceiving differences when these orientations are defined on the basis of contrast or textural cues (“second-order” features, which require detecting relationships between points of an image; Bertone et al., 2005). In the same study, similar differences were found for visual motion detection, with difficulties only seen for moving stimuli defined on the basis of second-order cues. When viewed from the perspective of the underlying neurobiology, these observations provide hints as to where within the brain sensory processing differences may arise for those with ASD. Whereas the processing of first-order visual features is believed to be subserved by regions of primary visual cortex, second- and higher-order processing is suggested to be mediated by regions further up the processing hierarchy (Dumoulin et al., 2003).
In a related set of observations to this simple/complex dichotomy, a great deal of evidence suggests that individuals with ASD have either normal or enhanced performance in tasks that rely on the analysis of stimulus detail, but may have difficulties when this detail needs to be integrated in order to form a holistic image. One of the most powerful and frequently used tools in teasing apart these differences is the Embedded Figures Test (EFT; Bertone et al., 2005; de Jonge et al., 2006; Happé, 1996; Jolliffe and Baron-Cohen, 1997; Joseph et al., 2009; Kemner et al., 2008; Minshew et al., 1997; O'Riordan and Plaisted, 2001; Shah and Frith, 1983; Shah and Frith, 1993). In the simplest example of this test, children are asked to report on the number of simple geometric objects (e.g., triangles) that are embedded within a holistic object (e.g., a clock). Children with ASD are often significantly better at counting the number of simple shapes within the larger image, but often show decreased performance in identifying the larger holistic image.
As is the case with much of ASD research, these results can be interpreted in a variety of ways and there is still significant debate surrounding these observations. Thus, although there is good consensus around the idea that individuals with ASD do indeed tend to focus on local visual detail, some suggest that attention may play an underappreciated role in contributing to the observed differences in sensory function. For example, when they are undirected, individuals with ASD tend to focus more on the local relative to the global aspects of a visual image when compared with their TD peers (Plaisted et al., 1999). This can be seen in tasks that utilize composite letters where small letters (local element) are grouped to form a larger letter (global element; Navon, 1977). However, when explicitly directed to use global focus (i.e., identify the large letters), individuals with ASD perform the same as their TD peers (Deruelle et al., 2006; Mottron et al., 2003; Mottron et al., 2006; Plaisted et al., 1999; Wang et al., 2004), supporting the idea that some of these performance differences may be a result of differences in attentional scope as opposed to sensory processing per se.
An additional distinction that can be drawn in assessing visual function in ASD is the classic dichotomy between so-called “where” versus “what” processes (Ungerleider and Haxby, 1994). In this traditional division, the dorsal visual stream (i.e., the where stream) begins within the magnocellular layers of the lateral geniculate nucleus (LGN) and continues through areas V1, V2, and then MT before projecting to regions of parietal cortex involved in spatial analyses. In contrast, the ventral visual stream (i.e., the what stream), which begins in the parvocellular layers of the LGN, projects to V1, V2 and then to V4 and finally areas of the inferotemporal cortex important in form and featural analyses.
In ASD, there is evidence for alterations in both dorsal and ventral stream functions, although there remains little consensus on the exact nature of these deficits. A great deal of research has focused on motion processing in ASD, a presumptive function of the dorsal stream. Several comprehensive reviews of visual function in autism (Dakin and Frith, 2005; Simmons et al., 2009) highlight the tremendous disparity in the results of studies designed to assess visual motion processing in ASD, with evidence ranging from no differences relative to TD controls (Bertone et al., 2003; Del Viva et al., 2006; Vandenbroucke et al., 2008), to significant changes on a series of motion coherence tasks (Milne et al., 2002; Pellicano et al., 2005; Spencer et al., 2000). One area of motion processing that appears to have the strongest evidence for differences is in the perception of biological motion. Although a number of these studies are suggestive of changes in motion processing networks (Blake et al., 2003; Freitag et al., 2008; Herrington et al., 2207), which likely link to the local versus global distinctions discussed earlier, it can extremely difficult to tease out the respective contributions of altered sensory function and elements associated with changes in social cognition. Furthermore, in a comparative analysis of visual motion versus form perception, it was found that although children with ASD indeed showed deficits in visual motion tasks (with the strongest changes being seen in biological motion processing), that a large portion of this could be accounted for by differences in higher-order cognitive factors (Koldewyn et al., 2010).
In a similar manner to that seen for the dorsal stream, the picture of ventral stream contributions to visual dysfunction in ASD remains complex and unresolved. The most notable focus of these studies has been in the realm of face processing (reviewed in Behrmann et al. (2006), Simmons et al. (2009)), where deficits in both patterns of gaze and face recognition have been well established. In order to establish these differences as a result of changes in ventral stream function, and not due to the high social valence of face stimuli, it is necessary to show that changes extend to stimuli beyond faces and that are processed by the ventral stream. Efforts to do this have focused on objects, and have indeed shown evidence for weaknesses in the processing of objects in children with ASD (Behrmann et al., 2006; Koldewyn et al., 2013). Once again, more work is needed in order to parse out whether these differences in ASD are a direct result of changes in visual sensory function, or whether they stem from differences in cognitive capacities such as attention and executive function and that consequently impact performance.
2.1.2 Auditory processing
Studies of auditory processing and perception in ASD show a pattern of results similar to those seen for visual perception, with difficulties appearing to manifest more as the complexity of the auditory information increases. Indeed, the pinnacle of this can be viewed as the processing of the most complex form of auditory information – social-communicative signals (O'Connor, 2012) – in which individuals with ASD are frequently impaired. In processing the simple features of an auditory signal, such as its loudness, children with ASD often show intact performance (Bonnel et al., 2010; Jones et al., 2009). Similar results (and perhaps a tendency toward enhanced performance relative to their TD counterparts) have been observed with other low-level auditory features such as frequency (Heaton et al., 1999; Jones et al., 2009; Mottron et al., 2000).
The dichotomy between the processing of simple and complex auditory information in ASD can perhaps best be seen in a trio of studies by Järvinen-Pasley and colleagues (Järvinen-Pasley and Heaton, 2007; Järvinen-Pasley et al., 2008a; Järvinen-Pasley et al., 2008c). In this work, participants were presented with spoken sentences and asked to make a judgment on either the pitch or semantic content of the sentence. Individuals with ASD outperformed their TD peers on pitch perception, but underperformed on the perception of sentence content. In addition, when low-level features that are built on pitch but that are socially and communicatively relevant (such as in prosody) are examined, a striking decrease in perception in individuals with ASD emerges (Golan et al., 2006; Golan et al., 2007; Järvinen-Pasley et al., 2008b; Kleinman et al., 2001; Lindner and Rosén, 2006; Mazefsky and Oswald, 2007; Peppé et al., 2007; Philip et al., 2010). These decreases associated with the processing of socio-communicative auditory information can even be seen in simple orienting tasks, where individuals with ASD (though showing reduced orienting abilities to all stimulus types) show an exaggerated difficulty in orienting to socially relevant auditory stimuli including, but not limited to, speech (Dawson et al., 1998; Dawson et al., 2004; Kuhl et al., 2005; Paul et al., 2007). These studies highlight one of the complications in interpreting the results on complex auditory processing in ASD, since speech and language cues are laden with social information (in addition to the basic auditory content).
2.1.3 Processing within other exteroreceptive sensory modalities
Although much of the sensory research that has been conducted in ASD focuses on auditory or visual processing alone (perhaps because the connection to social communication function is most easily apparent), tactile processing plays a prominent role in social development (Myers, 1984), suggesting that it may also be an important modality to examine for differences in ASD. Furthermore, abnormalities in tactile responsiveness are one of the most frequently reported sensory-processing challenges reported by parents of children with ASD (Rogers et al., 2003; Tomchek and Dunn, 2007). Therefore, studying and quantifying responses to tactile stimuli in a more empirical manner will provide important clues to understanding ASD neurobiology and symptomology.
As with the visual and auditory modalities, examination of low-level tactile tasks in ASD has revealed some evidence for enhanced performance, including decreased detection thresholds to high frequency (200Hz) vibrations (Blakemore et al., 2006) and low frequency (33Hz) vibrations (Cascio et al., 2008). Other studies report no difference in tactile thresholds in children with ASD compared with TD (O'Riordan and Passetti, 2006), but have found a strong correlation between scores from the touch and emotional subsets of the Sensory Profile questionnaire (Guclu et al., 2007), suggesting a possible emotional component to sensory processing. Still other studies have reported that children with ASD show increased thresholds when asked to localize a static (no change in amplitude over time) stimulus, but no differences in thresholds for the detection of a dynamic (amplitude increasing over time) stimulus (Puts et al., 2014). Collectively this work reveals a mix of findings in regards to tactile function in ASD, which is likely due to wide variations in the type of stimuli used and the site of stimulation on the body. Future work needs to employ a more systematic approach to better characterize basic elements of tactile processing and perception in ASD.
Other work has revealed that sensory disturbances and difficulties also extend to the chemical senses. Taste aversions are a frequently reported symptom of individuals with ASD (Cermak et al., 2010; Kral et al., 2013). Common factors for food refusal that are greater in children with ASD compared with TD include texture/consistency, taste/smell, mixtures, brand, and shape (Hubbard et al., 2014). Using the sweet taste test, Damiano and colleagues found that individuals with ASD show the same sensitivity and pleasurable effects of sweet taste as individuals with TD, suggesting that taste aversions may be specific to certain kinds of flavors as opposed to overall gustatory function (Damiano et al., 2014). In the olfactory domain, individuals with ASD rate odors as less pleasant compared with individuals with TD (Hrdlicka et al., 2011). However, within a group of individuals with ASD, detection thresholds and identification accuracy were not correlated with ASD severity (Dudova and Hrdlicka, 2013).
2.1.4 Interoceptive processing
Finally, recent work suggests that a focus on the interoreceptive senses may reveal intriguing differences between those with ASD and those considered TD. Differences in vestibular and proprioceptive processing are a robust observation in the ASD literature (Ornitz, 1974), and are a major focus of sensory integration therapy (Ayres and Tickle, 1980; Smoot Reinert et al., 2014). Individuals with ASD seem to show a greater reliance on proprioceptive information (Haswell et al., 2009; Masterton and Biederman, 1983) but poor postural control (Minshew et al., 2004; Molloy et al., 2003). Furthermore, it has been shown empirically that children with ASD show an enhanced ability to track their own heartbeat over long internals compared with children with TD (Schauder et al., 2015a). These results, coupled with the social deficits observed in ASD, suggest that individuals with ASD may preferentially attend to internal, as opposed to external, sensory cues. Clearly, much more work is needed to explore how sensory function differs across different stimuli and individuals differences in perceptual styles in ASD.
2.1.5 Shared differences across sensory systems in autism
One of the outstanding questions in sensory research into autism is the specificity of the observed deficits to a single sensory modality. Stated a bit differently, we can ask the question of whether the sensory deficits and/or processing alterations transcend modality distinctions. Such a perspective has critical mechanistic connotations, as a more multisensory account of ASD would shift the neurobiological focus away from the processing architecture of a particular sense and on to neurobiological networks regulating more global functions such as executive control, attention, and temporal processes.
Unfortunately, one of the major limitations of ASD research to date has been a traditional focus upon a single sense (reviewed in this sections above), limiting our ability to generalize across the different modalities. Indeed, such a pansensory perspective on ASD has been much of the driving force in studies that are increasingly focusing on examinations of multisensory function, and by extension, that are attempting to tease out the respective contributions of changes within and across the different sensory systems.
2.2 Differences in the integration of information across sensory modalities
The ubiquity of these sensory-processing differences in ASD, coupled with the evidence that these differences frequently span multiple modalities, suggest that we may want to conceptualize sensory issues in ASD from a broader and more “multisensory” perspective. In fact, emerging evidence strongly supports the presence of specific multisensory processing deficits in ASD that extend beyond those predicted on the basis of changes within the individual sensory modalities (Brandwein et al., 2012; Dunn et al., 2002; Foss-Feig et al., 2010; Kern et al., 2006; Kientz and Dunn, 1997; Kwakye et al., 2011; Leekam et al., 2007; Ornitz et al., 1977; Ornitz et al., 1978; Rogers et al., 2003; Russo et al., 2010; Stevenson et al., 2014b; Stevenson et al., 2014c; Stevenson et al., 2014d, e; Watling et al., 2001). For example, children with ASD do not benefit as much as children with TD from seeing the additional visual information provided by a speaker's face while performing a speech-in-noise task (Foxe et al., 2013). These differences in multisensory performance appear to be related to a reduced ability to perceptually bind individual pieces of sensory information into a coherent unified percept. Furthermore, Foxe and colleagues’ finding suggests that this difference in integration between individuals with and without ASD is largest in conditions where there is a low signal-to-noise ratio, the very conditions in which multisensory integration is most beneficial (Meredith and Stein, 1986b; Ross et al., 2011; Stevenson and James, 2009; Stevenson et al., 2015). Recent work in the realm of visual-vestibular integration, however, suggests that individuals with ASD may be able to integrate information in an optimal fashion but have a poor tolerance for noise (Zaidel et al., 2015). Whether this is a more general feature of multisensory perception in ASD or unique to self-motion perception is a matter of ongoing work.
Like the unisensory processing differences described above and in which individuals with ASD exhibit intact or even enhanced processing of simple stimuli and difficulties processing of more complex stimuli (Bertone et al., 2005; Minshew and Hobson, 2008), differences in processing capabilities in multisensory perception also vary according to stimulus type and complexity. One example of multisensory integration of simple stimuli can be seen in the sound-induced flash illusion (SIFI), where an individual is presented with a single flash of light paired with multiple beeps in rapid succession. The participant is asked to count the number of flashes while ignoring the beeps, yet the ignored beeps frequently induce the illusory perception of multiple flashes (Shams et al., 2000). Importantly, this illusion uses simple, non-speech stimuli, and appears to be diminished in children with ASD (Stevenson et al., 2014e).
Speech, which is an inherently complex multisensory signal, shows a much stronger tendency to be differentially affected in ASD when compared with the processing of non-speech stimuli (Bebko et al., 2006; Mongillo et al., 2008; Stevenson et al., 2014c). This difference in perceptual integration or binding can be readily demonstrated using the McGurk effect (McGurk and MacDonald, 1976), an audiovisual illusion in which an individual is presented with an auditory stimulus of a speaker uttering one syllable (e.g. “ba”) paired with a visual stimulus of the speaker articulating another syllable (e.g. “ga”). Subjects frequently perceive the speaker to be saying an entirely different syllable (e.g. “da” or “tha”), reflecting a perceptual synthesis of the auditory and visual channels. Most studies have concluded that individuals with ASD are less likely to report this perceptual fusion, with their choices typically reflecting the auditory stimulus (i.e., “ba” (de Gelder et al., 1991; Irwin et al., 2011; Mongillo et al., 2008; Stevenson et al., 2014c; Stevenson et al., 2014d; Williams et al., 2004), though this finding has not been universal (Iarocci et al., 2010; Woynaroski et al., 2013).
Outside of the audiovisual domain, the rubber hand illusion is another multisensory illusion (relying on the integration of visual and tactile information), to which children with ASD show differential susceptibility, requiring a longer exposure to synchronous brushing of the real and rubber limb in order to perceive the illusory percept (Cascio et al., 2012a). In the spatio-temporal domain, children with ASD also show reduced illusory reversal of temporal order judgments in the crossed hand illusion (Wada et al., 2014). Thus, across both a number of both simple and more complex sensory illusions that rely on integration for the illusory percept, individuals with ASD seem to show multisensory integration to a lesser extent than their TD peers based on their reduced susceptibility to these illusions.
The temporal effect observed in the crossed-hand illusion reflects one of the core findings in regards to altered multisensory function in ASD. The processing of the temporal relations of sensory inputs across modalities, which is a strong cue as to which inputs should be integrated or bound, has reliably been shown to differ in individuals with ASD. Individuals with ASD are less likely than their TD peers to detect an asynchrony between an auditory and visual stimulus (Kwakye et al., 2011; Stevenson et al., 2014c) and less accurate at making audiovisual temporal-order judgments (de Boer-Schellekens et al., 2013). The perception of multisensory illusions such as the McGurk effect (Woynaroski et al., 2013) and the sound-induced flash illusion (Foss-Feig et al., 2010) are less influenced by temporal discrepancies between the auditory and visual presentations in ASD children when compared with their TD peers. Using eye tracking, researchers have shown that individuals with ASD do not show the typical gaze preference for synchronous over asynchronous audiovisual presentations (Bebko et al., 2006). Indeed, differences in eye movement patterns between individuals with ASD and TD present an important potential confound in interpreting sensory studies in ASD (Papagiannopoulou et al., 2014), as eye gaze patterns are known to differ in certain contexts (Guillon et al., 2014).
Similar to the results from unisensory studies and studies of more general multisensory function, multisensory temporal acuity differences in ASD also appear to be shaped by the nature of the stimuli that are being combined. All studies save one (Grossman et al., 2009) agree that multisensory temporal processing differences are seen with speech stimuli (Bebko et al., 2006; de Boer-Schellekens et al., 2013; Grossman et al., 2015; Stevenson et al., 2014c; Woynaroski et al., 2013). Results are in less agreement with non-speech stimuli, where some have found no difference between individuals with and without ASD (Bebko et al., 2006; Stevenson et al., 2014c) while others have found differences (de Boer-Schellekens et al., 2013; Foss-Feig et al., 2010; Kwakye et al., 2011). Perhaps most importantly, regardless of the stimuli used, studies have shown that individuals with ASD who show better multisensory temporal acuity show more typical patterns of speech perception (Stevenson et al., 2014c) and higher levels of receptive language functioning (Patten et al., 2014), suggesting a strong link between low-level sensory processing and higher-level symptomology in ASD.
One of the key aspects of the multisensory work conducted to date in ASD have been efforts to parse whether changes in unisensory function are sufficient to account for the observed multisensory changes. Indeed, processing deficits within a sensory modality will undoubtedly result in processing changes when indexing multisensory function, and consequently can call into question claims of any degree of multisensory specificity regarding the documented changes. In large measure, most of the studies detailed above not only measure multisensory function, but also entail a battery of unisensory “control” experiments in an effort to describe both unisensory and multisensory deficits. Although some of these studies have shown little change in unisensory function despite substantial changes in multisensory performance (Foxe et al., 2013; Stevenson et al., 2014c; Stevenson et al., 2014d, e), others have illustrated a complex pattern of differences that are ultimately difficult to interpret (Brandwein et al., 2012; Kwakye et al., 2011; Russo et al., 2010).
Part of the challenge in this regard is determining the proper combinatorial model to predict multisensory function from the respective unisensory correlates. For example, if there is a 10% difference in auditory performance and a 10% difference in visual performance between groups, what is the expected resulting audiovisual performance? This question is still largely undetermined and likely affected by both task constraints and stimulus properties, but the problem is being made increasingly tractable with the development of sophisticated models of multisensory function which help tease apart the relative contributions of sensory inputs and integration processes.
3 Theories of altered sensory and cognitive functioning in ASD
Since the first clinical descriptions of autism in the 1940's, much work has been done in an effort to provide an overarching theory that would link the multifaceted set of symptoms that accompany ASD. Given the heterogeneity of ASD symptomology, a cogent model should explain how such diverse symptomology can be encompassed under a single mechanism or set of mechanisms, how ASD differs from other developmental disabilities, and, for the purpose of the current review, how the increasingly recognized sensory and multisensory changes can be captured by the model. Highlighting the complexity of the disorder and perhaps the changes in diagnostic criteria, though a number of theories have been put forth to date, no strong consensus yet exists as to a “unifying” theory or view on ASD. Nonetheless, each of these theories (several of the most popular of which are detailed below) has interesting components that provide insight into ASD neurobiology. Although these theories are often espoused as distinct theoretical and mechanistic viewpoints, many of these views have marked commonalities with one another.
In the following sections we provide a brief description of several of the prevailing and best-established theories of ASD, and try to frame these theories through the lens of sensory function. As highlighted earlier, the benefit of viewing ASD through such a filter is that sensory differences are one of, and to date the most common, issue observed in ASD, and much of our cognitive and social representations are built upon sensory input. For example, to infer sarcasm (which is often difficult for individuals with ASD), one must not only analyze the incoming auditory information to understand the literal meaning of the words, but also be able to process the shifts in frequency and prosodic cues that are layered with linguistic content. Visual information in the form of facial movements and body “language” such as gesture and posture provide additional information. In many circumstances, touch adds additional information to the signal. Thus, the integration of information across the different senses serves as a bridge between sensory processing and the resultant sensory representations and the higher-order social and cognitive abilities and representations.
3.1 Theory of mind
One of the most highly espoused theories of ASD revolves around the concept of Theory of Mind (ToM) (ToM, Baron-Cohen, 1989). This theory suggests that those with ASD have a diminished ability to imagine motives and feelings outside of their own mind, and thus have profound deficits in social communicative interactions. Within such a framework, those with ASD have great difficulty inferring information about the feelings and thoughts of those with whom they should be interacting. The initial evidence for this theory came from the “transfer of false belief test,” in which a story is told about a doll who believes that an item is in a location that the participant knows is false. The vast majority (i.e., 80%) of children with ASD were unable to correctly assess what the doll knew about the location of the item. While the results of the original study may have been accounted for partially by differences in verbal mental age (Happé, 1995), additional research has shown that this finding holds even when accounting for such individual differences (Happé, 1994).
Neuroimaging studies that have examined the neuronal correlates of ToM tasks frequently reveal active regions of the upper bank of the superior temporal sulcus (STS, associated with biological motion perception; Blake et al., 2003), as well as inferior frontal regions (including the putative ‘mirror neuron’ system), and the anterior cingulate cortex/medial prefrontal cortex (Frith and Frith, 1999; van Veluw and Chance, 2014). One area that has been strongly implicated in ToM abilities is the temporoparietal junction (TPJ). For example, in TD adults Gallagher and colleagues found significant activation of TPJ bilaterally when participants were viewing stories and cartoons that required ToM abilities compared to control cartoons and stories that did not require ToM abilities (Gallagher et al., 2000). In comparison, the evoked neural response in the medial prefrontal cortex and right TPJ to ToM cartoons is reduced in children ASD, and these differences in activation patterns also correlate with ASD symptom severity (O'Nions et al., 2014). Both reduced functional connectivity and reduced white matter integrity has been observed in individuals with ASD in multiple ToM associated areas (Kana et al., 2014), further supporting the connection between weakened ToM abilities and the observed neuronal processing of such ToM tasks in individuals with ASD.
When framed in the context of sensory function, compromised ToM abilities could be a result, at least in part, of changes in sensory and multisensory function. The ability to infer the actions of others is a result of the collective processing and integration of sensory information from a number of sensory modalities. Thus, if the integrity of the early sensory processing streams, or the later integration across these different streams is altered in ASD, then such alterations could weaken ToM processes. Two of the presumptive brain regions mediating ToM function, the STS and TPJ (see above), receive convergent input from a variety of sensory domains and have been shown to play important roles in the integration of this information (Beauchamp et al., 2012; Downar et al., 2000, 2001; James et al., 2011; Shulman et al., 2003; Stevenson et al., 2007; Stevenson and James, 2009). Failure to register and appropriately integrate incoming sensory inputs, particularly inputs conveying social content, would make inferring the feelings and thoughts of others very difficult.
3.2 Weak central coherence
An alternative hypothesis regarding the neurobiology of ASD is a concept known as weak central coherence (WCC; Burnette et al., 2005; Frith and Happé, 1994). Across both lower-level sensory and higher-level cognitive and perceptual processing, meaning is built through the active integration of information across wide swaths of the processing hierarchy. For example, understanding the meaning of a complex visual scene is based not on the individual components but rather on the holistic processing of these components into a meaningful image (Oliva and Torralba, 2006). The theory of WCC is built on observations that individuals with ASD often focus on local details rather than the global picture (for a review see Happé, 1999). Harkening back to some of the sensory findings presented earlier in this review, evidence for this theory comes from performance on tasks like the Embedded Figures Test (EFT), in which those with ASD often outperformed neurotypical subjects when focused on counting the number of figures that make up the larger image, but in which these individuals are impaired in the holistic level processing (Witkin et al., 1971). Furthermore, initial work suggested that individuals with ASD might be less susceptible to visual illusions, which also generally occur as the result of Gestalt processing (Bolte et al., 2007; Ropar and Mitchell, 2001). However, work by Brosnan and colleagues have shown that individuals with ASD may actually be just as susceptible to visual illusions, but that the key in revealing this is in how the question is framed in the task. For example, on the Müller-Lyer illusion, individuals with ASD report the illusory percept when asked which line looks longer, but are not susceptible when asked which line is longer (Brosnan et al., 2004) .
Neuroimaging evidence in support of WCC has come in several different forms. In functional magnetic resonance imaging (fMRI) study, Lee and colleagues found that children with ASD performing the EFT showed activation in only a subset of the cortical network observed in children with TD (Lee et al., 2007b). Among the inactive regions in children with ASD was the left dorsolateral and medial prefrontal cortex, as well as the bilateral ventral temporal cortex, which was interpreted as supporting a model of weak top-down control in perception. A later fMRI study of the EFT found not only reduced activation of the same visuospatial network, but also reduced connectivity between frontal areas and visuospatial areas (Damarla et al., 2010). A consistent finding in neuroimaging work in ASD research is indeed changes in connectivity, with many studies showing reduced long range connectivity in individuals with ASD (Glazebrook and Wallace, 2015; Kikuchi et al., 2015). This further supports the role of connectivity in atypical perceptual processing in ASD.
As for ToM, the WCC model can also be seen as partly reflective of changes in sensory function, most notably in the context of multisensory integration. Thus, at its core, WCC suggests limited communication and connectivity between regions of the brain that should be strongly linked and whose coherence is necessary for more holistic level processing.
3.3 The predictive-coding hypothesis
A theory of ASD that is rapidly gaining credibility is the notion that individuals with ASD do not have a robust historical representation of the world. This lack of a probabilistic “map” of the world makes it difficult for those with ASD to predict upcoming events, and this lack of predictive coding thus limits interactions with the external environment (Pellicano and Burr, 2012; Sinha et al., 2014; van Boxtel and Lu, 2013; Van de Cruys et al., 2014).
A framework for understanding how sensory inputs and past experience interact has been formally defined using approaches grounded in Bayesian statistics, where a generative model is built that specifies inputs, a mechanism for internal probabilistic perception, and a behavioral (or neural) output (Pizlo, 2001; Pouget et al., 2003). In a Bayesian framework, poor predictive coding can be thought of as having a weak (flat) prior probability distribution (Lawson et al., 2014) – that is, the individual does not know how likely an event is to occur in the real world. In this view, sensory-based performance differences may arise not from changes in the fidelity of the incoming sensory information, but rather from weaknesses in the ability to compare the incoming sensory stream with a statistical model of the world.
Although the predictive coding theory is a fairly new idea in ASD research, existing behavioral studies can be readily interpreted with this model in a way that provides a unifying mechanism across domains. For example, a common observation of children with ASD is an insistence on sameness and repetitive behaviors. Within a predictive coding framework, these behavioral patterns can be interpreted as a way of limiting contact with an endlessly novel world. Repetitive behaviors like stimming could be explained as an intentional act to limit novel sensory input. Furthermore, difficulties in inferring mental states can similarly be traced to errors in predicting current states based on past experience. Pellicano and colleagues utilized an adaptation paradigm called the face-identity aftereffect where exposure to a particular face identity biases subsequent perception towards a different face identity (Pellicano et al., 2007). This can be seen as evidence of updating our world representation with past experience, which is then compared to incoming sensory information. In this study, children with ASD showed a reduced face-identity aftereffect, indicating that they were less affected by previous sensory experience. Additional evidence for this reduced impact of past sensory experience can be found in studies of mismatch negativity (MMN), where the enhanced response to the deviant stimulus in an oddball paradigm is absent or weaker in children with ASD, and has been observed with tone bursts (Abdeltawwab and Baz, 2015), emotional voices (Fan and Cheng, 2014), and words and pseudowords (Ludlow et al., 2014). In an MEG study of resting state activity in individuals with ASD and TD, analysis of the active information storage (AIS), defined as mutual information in successive time points in a signal, found reduced AIS in the hippocampus in individuals with ASD compared with TD (Gomez et al., 2014), indicating that neural information was less predictable in individuals with ASD.
Within the context of alterations in predictive coding, it is easy to extend this concept to sensory and multisensory function. Indeed, some of the most characteristic symptoms of ASD include hyper- (Gomot et al., 2002) and hypo-sensitivity to sensory cues, which could readily result because of a constantly unpredictable world. In such a scenario, even if the incoming sensory information is relatively normal, it is being combined with central representations that are poorly matched to the incoming signals, and thus that may result in either an extreme or absent response.
3.4 Reduced sensory precision and reliability
A related model to the predictive coding concept is grounded in reliability, and suggests that those with ASD may have greater variability (i.e., less reliability) in their behaviors and perceptions, and that these changes in reliability are related to changes in the variability of neural response patterns (Perez Velazquez and Galan, 2013). Thus, this model posits that differences in sensory processing occur in the actual representation of the sensory inputs themselves instead of a weak statistical representation of the world, as in the predictive coding model. While much of the neuroimaging work in ASD has focused on relative differences of activation (over or under) in individuals with ASD across different brain networks, more recent work has also investigated the possibility that differences in ASD may not be necessarily found in only differences in activation, but also may be associated with differences in the reliability of behavioral performance (Geurts et al., 2008) or evoked neural responses (Coskun et al., 2009; Dinstein et al., 2012; Haigh et al., 2014; Milne, 2011). This work suggests the importance of not only characterizing response magnitudes across the brain to different tasks, but also the distribution of responses across trials as an important facet of neuronal and behavioral responses.
Although not necessarily restricted to sensory systems, these changes in reliability may begin as early as the initial sensory processing streams, and may actually be amplified as one adds additional noise at each level of the processing hierarchy. One possible mechanism that may underlie such internal noise is an imbalance of excitatory and inhibitory neural responses, which is in itself an account of ASD.
3.4 Imbalance in excitatory and inhibitory processes
As in all homeostatic systems, within the brain excitation and inhibition exist in a competitive balance that confers varying degrees of adaptive plasticity and stability on the organism. One suggestion in ASD is that the normal balance between excitation and inhibition is changed, with the prevailing view that excitation may be increased (greater glutamateric signaling) and inhibition may be reduced (less GABAergic signaling; Rubenstein and Merzenich, 2003). Because glutamatergic and GABAergic signaling are involved in a wide array of cortical functioning (including sensory, social, and emotional systems), these global changes in excitation and inhibition could help explain the wide array of deficits observed in ASD. In general, hyper-excitability in the cortex would lead to poor differentiation of cortical maps, which would impair processing across a number of neural systems. Furthermore, abnormal excitability may help explain hypersensitivity in sensory processing—if a sensory input evokes an abnormally large cortical response, the experience may be overwhelming for individuals with ASD. Using simple sensory (auditory and visual) stimuli, Green et al. (2013) found increased activation in individuals with ASD in both primary sensory cortices as well as in the amygdala, hippocampus, and orbital-frontal cortex, suggesting that changes in sensory function have a cascading impact on brain regions involved in social, emotional and cognitive processing. Furthermore, the level of activity in these areas was positively correlated with sensory over-responsivity scores and behavioral anxiety ratings.
Techniques such as magnetic resonance spectroscopy (MRS) now allow for the in vivo measurement of various biochemical compounds like neurotransmitters and their metabolites in human subjects. These MRS studies in children with ASD have found reduced GABA (the major inhibitory neurotransmitter) levels in auditory and motor cortex (Gaetz et al., 2014) and frontal cortex (Kubas et al., 2012). However, these studies have yet to link these changes in neurotransmitter levels to phenotypic characteristics such as social communication.
Consistent with the concept of a shift in the excitation/inhibition balance toward greater excitability, there is a high degree of comorbidity between ASD and epilepsy. Thus, studies have shown that approximately 30% of individuals with ASD reporting seizures (Gillberg and Billstedt, 2000), and that 50-70% of children with ASD show abnormal electrical activity during sleep (Lewine et al., 1999).
Because of the fundamental importance of excitation and inhibition for normal sensory function, it is easy to reconcile changes in this balance with changes in sensory function. Indeed, excitation and inhibition work in concert in order to build the filter sets that are critical in not only shaping the nature of the incoming sensory streams, but also in creating the features that are best represented within specific cortical regions. Even a small imbalance in the excitatory/inhibitory ratio could have dramatic impact on these filters and the representations built from them (Foss-Feig et al., 2013; Snijders et al., 2013).
3.5 Temporal binding hypothesis
Another brain-based explanation of ASD, and which has many unifying features with other theories such as ToM and WCC is the temporal binding hypothesis (Brock et al., 2002). This hypothesis proposes that the processes used to synchronize activity within neural networks, specifically synchronization of high-frequency gamma oscillations, are impacted in ASD. Disruption in such neural integration across brain regions may account for many of the experimental findings in ASD with the specific prediction that cognitive abilities that require integrated action across brain regions will be impacted. This theory is particularly germane in the context of multisensory function, which by definition entails the communication and synchronization of information transfer across broad regions of the cerebral cortex. One hypothesized mechanism for this information transfer across broad regions is the phase reset and synchronization of ongoing oscillatory activity (Diederich et al., 2012; Fries, 2005; Lakatos et al., 2007; Mercier et al., 2013; Varela et al., 2001), which may be impacted in ASD (Buard et al., 2013; Khan et al., 2013; Milne et al., 2009). Perturbations in these communication processes, particularly within the temporal domain, could have strong negative impact on the network functions that serve to bind the features of the sensory world into a unified and coherent perceptual construct.
4 Evidence for alterations in neural processing relevant to sensory function in ASD
4.1 Using functional MRI to probe altered neuronal circuits in ASD
In an effort to understand the brain basis of ASD, a number of neuroimaging approaches have been employed to investigate the underlying neuronal differences in individuals with ASD compared with TD, and to link those findings to the different behavioral patterns represented in ASD symptomology. One of the most powerful of these is magnetic resonance imaging (fMRI), which has been used to understand some of the structural and functional differences between the autistic brain and the neurotypical brain, and to relate these differences to the observed symptoms in ASD. A benefit of MRI studies is the excellent spatial localization of the signal, allowing a high-resolution view into the impacted brain areas. Because of the number of MRI studies that have been done in ASD, and because of the nature of the current review, we will focus the following on MRI studies with a strong sensory emphasis or that relate in some way to differences in sensory function.
4.1.1 fMRI studies of visual processing in ASD
A number of neuroimaging efforts have focused on examining vision and visual cortical processing in ASD. In terms of basic organization of visual space (ratio of central vs. peripheral representation) in the visual cortex, individuals with ASD appear very similar to individuals with TD (Hadjikhani et al., 2004). However, receptive fields in extrastriate cortex (V2, V3, and V4) appear to be larger in individuals with ASD (Schwarzkopf et al., 2014). Intriguingly, in this study the size of the receptive fields were positively correlated with autistic traits (as measured by the Autism Quotient questionnaire), but not with behavioral measures like orientation and direction discrimination.
Echoing behavioral findings of visual performance in individuals with ASD, evidence for deficits in both the “where” and “what” pathways of visual processing can also be found in neuroimaging data. In terms of simple visual processing like motion coherence, Robertson and colleagues found reduced activation in two areas of the dorsal visual stream, primary visual cortex as well as the middle temporal area, in individuals with ASD despite similar performance to individuals with TD (Robertson et al., 2014), which was interpreted as reflective as global differences in the neural circuitry underlying visual perception. Whether these global changes are indicative of more or less efficient processing is not immediately clear. In a visual change detection task, adults with ASD performed at the same level as adults with TD, but showed greater activity in bilateral occipital cortex, but decreased activity in frontal (superior and middle frontal gyri bilaterally) cortex (Clery et al., 2013). In a meta-analysis of ASD visual processing fMRI studies, Samson and colleagues found overall greater activity across a number of occipital, temporal, and parietal regions in individuals with ASD in face, object, and word perception studies (Samson et al., 2012).
Perhaps the greatest area of focus in neuroimaging (and perceptual) work in the domain of vision has been on face processing. In addition to the well-established deficits in behavioral and perceptual studies of face processing (Boucher and Lewis, 1992; Harms et al., 2010; Papagiannopoulou et al., 2014), numerous imaging studies have shown reduced activation in the fusiform face area (FFA) in individuals with ASD (Corbett et al., 2009; Hubl et al., 2003; Pierce et al., 2001), indicating changes to the ventral visual stream. Indeed, individual differences in the severity of autistic symptoms have been shown to correlate with activation of the FFA, with greater symptom severity negatively correlated with right FFA activity (Scherf et al., 2015). A robust finding in these studies is a reduction in the amount of repetition suppression seen in response to the repeated presentation of faces, again suggestive of altered activation in visual cortical regions involved in face processing (Ewbank et al., 2014; Fiorentini et al., 2012). Additional work has extended these results to show that altered neural processing of faces is also related to individual differences in social communication (Pellicano et al., 2007).
In addition to the FFA, the posterior STS (pSTS) is also an integral node in the face-processing network, and in ASD has been shown to have atypical functional responses to faces (Pierce et al., 2001), including faces conveying social information (Zilbovicius et al., 2006). This functional difference in pSTS response to visual faces in ASD is further backed by findings of differential functional connectivity (Just et al., 2004; Koshino et al., 2005; Koshino et al., 2008), anatomical structure (Boddaert et al., 2004; Boddaert and Zilbovicius, 2002; Boddaert et al., 2009; Brunelle et al., 2009; Levitt et al., 2003), and structural connectivity (Boddaert et al., 2004; Conturo et al., 2008; Lange et al., 2010; Lee et al., 2007a). Because the pSTS is also a key node in the multisensory integration network, future studies should examine both multisensory and social stimulus processing in the pSTS.
4.1.2 fMRI studies of auditory processing in ASD
Functional MRI studies of auditory function in ASD have revealed striking differences in activation patterns across a wide range of auditory stimuli. In an auditory detection task using simple tone stimuli, individuals with ASD performed equivalently to their TD peers, and fMRI showed similar activation in temporal cortex but greater activation of right prefrontal and premotor cortex as well as left inferior parietal cortex in individuals with ASD during the detection task (Gomot et al., 2008). In another study, evoked responses to complex (i.e., frequency modulated) non-social sounds were diminished in non-primary auditory cortical regions (anterolateral and posterior superior temporal gyrus (STG)) in individuals with ASD (Samson et al., 2011). Moving up the complexity hierarchy, children with ASD showed reduced responses in the STS (an area of robust activation voices in TD individuals) in response to a social auditory stimulus (voice), but similar responses to their TD counterparts with non-vocal (environmental) sounds (Gervais et al., 2004). Finally, in a study comparing the perception of language versus songs in children with ASD, reduced activation was observed in the left IFG and STG for speech stimuli, but greater activation was observed during song perception relative to TD controls (Sharda et al., 2014).
4.1.3 fMRI studies of tactile processing in ASD
In a study focused on tactile perception, individuals with ASD were shown to exhibit reduced responses for pleasant or neutral textures presented to the hand, but displayed a greater response to stimulation with an unpleasant texture in the posterior cingulate and insula (Cascio et al., 2012b). Together, these sensory studies suggest that different types of stimuli may utilize neural networks differently, prompting the need to explore multiple stimulus types to fully understand and characterize sensory processing in ASD.
4.1.4. fMRI studies of social stimuli in ASD
In addition to these studies that have focused on the processing of simple sensory stimuli, the processing of more complex social stimuli has also been of great interest, given the social communication deficits observed in ASD. As previously highlighted, much of this work has focused on speech and the processing of faces, but additional studies have also shed important light on differences in cortical activation patterns in response to social stimuli. For example, in response to socially-awkward situations, reduced activation of the right TPJ and pSTS , areas implicated in ToM processes (see above), has been observed in individuals with ASD (Pantelis et al., 2015). While children with ASD are less accurate in detecting sincerity or irony, they exhibit greater activation of the right IFG, and many temporal regions bilaterally, interpreted as reflecting the additional effort required for individuals with ASD to intuit mental states (Wang et al., 2006).
4.1.5 fMRI studies of multisensory processing in ASD
Surprisingly little work has been done to examine multisensory networks and multisensory integration using fMRI in individuals with ASD. Doyle-Thomas and colleagues used an emotion-labeling task and found that teens with ASD were impaired at correctly identifying emotional faces (visual only condition), but showed intact performance for emotional voices (auditory only condition) and face/voice pairs (audiovisual condition; Doyle-Thomas et al., 2013). Although both teens with ASD and their TD peers showed greater activation in the multisensory condition compared to the unisensory condition, individuals with ASD seemed to activate a different network than the individuals with TD, suggesting alternate processing networks are utilized for certain tasks despite similar performance. Future work will need to systematically test differences in activation in network function for multisensory compared to unisensory input, and to relate these differences back to differences in performance at the behavioral level.
4.1.6 Functional and structural connectivity studies in ASD
As highlighted in the earlier section on theories concerning the brain basis of ASD, one of ideas strongly based in neuroimaging data is that it is a connectivity disorder. That is, the observed deficits in ASD arise from poorly connected neural networks (Hughes, 2007), with nodes unable to effectively transfer and integrate information. Some of this work has come from task-based functional connectivity studies, where task evoked activity is examined and the correlation of response between regions is examined. The reduced network connections observed in individuals with ASD include those between the FFA and inferior frontal and ventral and middle temporal area during visuospatial and face processing. (Kleinhans et al., 2008), Studies have also found reduced connectivity between the pSTS and regions implicated in reward circuitry (including ventral tegmental areas, nucleus accumbens, insula, and orbitofrontal cortex) suggesting that some kinds of social stimuli do not evoke the same rewarding response in individuals with ASD as they do in individuals with typical development (Abrams et al., 2013). In addition to task-specific connectivity, further work has shown that the default mode network (DMN), which is active when the brain is not performing an explicit task and is a commonly associated with intact connectivity (Greicius et al., 2003), may be impacted in ASD. For example, individuals with ASD fail to fully deactivate the DMN, a sign of weak neural coherence between disparate brain regions, during speech processing (Hesling et al., 2010).
In addition to studies of functional connectivity, structural connectivity of white matter tracts can also be imaged using an MRI technique known as diffusion tensor imaging (DTI). In these studies, fractional anisotropy, a measure of the organization of fiber bundles, has been shown to be reduced in ASD compared to TD in regions including the STG (Lee et al., 2007a), fronto-striatal tracts (Langen et al., 2012), and parieto-occipital tracts (Chang et al., 2014). These changes on the structural level mirror the findings of reduced functional connectivity, suggesting that decreases in connectivity are a core and consistent feature of ASD.
4.1.7 Resting-state studies of ASD
One of the imaging methods of choice in ASD has been resting state fMRI (i.e., spontaneous, non task-evoked activity; Biswal et al., 1997), because it does not entail a task and hence is not fraught with some of the practical problems seen with more traditional task-based fMRI studies (most notable task compliance). Many of these studies focus on the DMN. In individuals with ASD, functional connectivity between regions in the DMN network show reduced connectivity (Cherkassky et al., 2006), and the magnitude of connection strength between DMN nodes is inversely correlated with the severity of social and communication difficulties (Assaf et al., 2010). Studies of resting state connectivity consistently show reliable differences between ASD and TD, but have yet to surpass behavioral measures as a diagnostic tool (Plitt et al., 2015).
Unfortunately, many resting state fMRI studies are plagued with interpretational confounds. Motion can spuriously increase correlations between areas because movement across the head is correlated. Therefore, much work has been done to attempt to ameliorate the effects of motion in resting state fMRI that analyze functional connectivity. However, these efforts to reduce the effects of motion can actually introduce artifacts into the data. In the case of resting state fMRI, certain kinds of data scrubbing have been shown to artificially increase short-range connectivity and decrease long range connectivity (Gotts et al., 2013). Because these findings align nicely with some theories of ASD, special caution must be taking when interpreting resting state studies of functional connectivity with individuals with ASD.
4.2 EEG studies of ASD
EEG studies of sensory processing have also provided a good deal of insight into how sensory processes differ between individuals with and without ASD. Furthermore, EEG studies are relatively practical in this population; unlike fMRI, EEG does not require the same levels of stillness or exposure to loud scanning noises, which can be quite a challenge, particularly in children with ASD. EEG studies also have the benefit of high temporal fidelity, allowing an experimenter to assess at which processing stage atypical sensory processing takes place.
4.2.1 EEG studies of visual processing in ASD
Studies of visual processing in ASD using EEG reveal a series of varied and occasionally divergent results. Individuals with ASD show stronger responses to high spatial frequency stimuli, but weaker responses to low spatial frequency stimuli (Vlamings et al., 2010). Those with ASD also show atypical N170 responses to faces, specifically when the faces are attended (Churches et al., 2010). Also, individuals with ASD exhibit increased inter-trial variability in visual evoked potentials (Haigh et al., 2014), suggesting that not only the amplitude but also the reliability of the evoked neural response to sensory stimuli may contribute to sensory issues.
Because of the high temporal resolution of EEG, in addition to traditional event related potential (ERP) analyses, time-frequency analyses can also shed light on differences in neural processing between groups. In general, oscillations at different frequency bands observed in EEG are thought to reflect the ability of the brain to coordinate the communication of information across separate populations of neurons. This synchronization of activity through oscillatory activity has been proposed to facilitate the binding of information across a number of perceptual and cognitive processes (Basar et al., 1999; Herrmann et al., 2015; Klimesch, 1999; Pulvermuller et al., 1997). Thus, in addition to changes in visual evoked potentials, the overall coherence of neural activity between early visual areas is also reduced in ASD (Isler et al., 2010), and abnormal gamma-band oscillations, thought to be involved in perceptual binding of sensory information, is atypical in individuals with ASD in response to both face (Grice et al., 2001) and non-face stimuli (Brown et al., 2005). In addition to atypical gamma-band power, differences in the alpha band, thought to be important in cognitive processing, have also been observed, including decreased inter-trial alpha-band phase coherence during a visual spatial discrimination task (Milne, 2011), and decreased alpha-band power in a cued intersensory attention task (Murphy et al., 2014). Overall, these findings support the view that individuals with ASD are less able to bind information, a crucial function for not only sensory but also perceptual and cognitive processing.
4.2.1 EEG studies of auditory processing in ASD
Studies using EEG have been most heavily applied in the auditory domain (for a review see Marco et al. (2011)). However, much as what has been seen in behavioral, perceptual and neuroimaging work, these studies have provided conflicting results. At the level of the auditory brainstem response (ABR), a derivative method of EEG that focuses on the ascending auditory pathway, a number of studies have found differences between children with and without ASD. Some of the reported findings include longer III–V interpeak latency (Kwon et al., 2007; Rosenhall et al., 2003) and decreased wave III amplitude (Källstrand et al., 2010) in individuals with ASD, suggestive of changes in the auditory brainstem and midbrain. However, a number of studies have failed to replicate these differences (Courchesne et al., 1984; Courchesne et al., 1985; Dunn et al., 2008). Surprisingly, even at the level of the brainstem, individuals with ASD show atypical patterns of activity in response to complex stimuli when compared with simple stimuli (Russo et al., 2009; Russo et al., 2008), reflecting the behavioral performance differences discussed earlier.
Studies of auditory evoked potentials (AEP), reflecting the processing of sound in auditory cortical regions, have also produced disparate results. Whereas several of these studies have shown faster AEP latencies in those with ASD (Ferri et al., 2003; Martineau et al., 1984), others have shown the opposite pattern (Bruneau et al., 2003; Cardy et al., 2008). Preliminary evidence also suggests that AEP differences may be predictors of language function (Bruneau et al., 2003; Cardy et al., 2008). Greater AEP amplitudes originating from the right temporal cortices (N1c) are correlated with increased verbal and non-verbal communicative skills (Bruneau et al., 2003), and latency of the right hemispheres AEP (particularly the M50 component) are accurate predictors of language impairment. These results further support the idea that sensory processing, even at subcortical and early cortical levels, may be significantly related to higher-order impariments in ASD.
4.2.3 EEG studies of tactile processing in ASD
Very little work has been done to examine evoked responses to touch with EEG. Using median nerve stimulation, Miyazaki and colleagues found that some children with ASD showed abnormal somatosensory evoked potentials (Miyazaki et al., 2007). Furthermore, they observed a greater disturbance in the response when stimulating the left vs. right median nerve, suggesting increased hyperactivity in the right vs. left hemisphere. With an oddball paradigm, a greater P3 response to novel somatosensory stimuli was observed in children with ASD compared to children with typical development, ADHD, or dyslexia (Kemner et al., 1994). Future work should utilize both traditional psychophysics as well as EEG to examine the neural correlates of potentially heightened evoked sensory responses in ASD.
4.2.4 EEG studies of multisensory processing in ASD
In addition to this EEG work focused on the processing of information within specific senses, several studies have also used EEG methods to focus on the integration of information across the different senses. Given that ERPs have such high temporal fidelity, one of the most relevant questions posed in EEG studies is where in the stages of sensory integration deficits emerge. Children with ASD show decreased multisensory integration as early as 100 ms post stimulus presentation, and appear to recruit different neural networks to accomplish this integration (Brandwein et al., 2012). Through contrasting semantically congruent and incongruent audiovisual presentations, it was determined that the differences in integration in ASD were the result of differences in early perceptual processing as opposed to later semantic operations (Russo et al., 2012). These perceptual differences were linked to changes in latency as well as in the networks recruited to support the perceptual processing. Indeed, this concept of individuals with ASD using a different network of brain regions than their TD peers to perform auditory and visual tasks, known as differential recruitment, has been a common one (Samson et al., 2012). Furthermore, changes in early multisensory integration as measured through EEG (between 100-130 ms post stimulus) have been significantly correlated with clinical severity of ASD, suggesting that integrative abilities may be intrinsically linked to core symptomology (Brandwein et al., 2015). While differences in integration measured with EEG have primarily focused on audiovisual integration, similar results have also been found in auditory-somatosensory responses, with individuals with ASD showing weaker and delayed neural signatures of integration around 175 ms post stimulus (Russo et al., 2010).
5 Plasticity and remediation therapies in ASD
Although little consensus exists as to the precise neurobiological substrates impacted in ASD (likely a result, at least in part, of the marked heterogeneity of the disorder), it should be clear from the above that sensory features frequently accompany ASD, and that these sensory issues may play an important and underappreciated role in domains of dysfunction such as social communication. With this as a backdrop, it is interesting to consider how efforts to change sensory performance might impact higher-order functional domains. Stated a bit differently, if diminished sensory abilities contribute to higher-order differences in social communication, could improvements in sensory abilities result in improvements in these domains?
A great deal of work has been done within this area of sensory or perceptual plasticity in neurotypical populations, and has successfully shown that marked performance improvements can be achieved through training. The vast majority of this work has been carried out within individual sensory systems, and has shown that very simple sensory-based judgments and abilities, such as Vernier acuity (vision), frequency discrimination (auditory) and two-point discrimination (tactile), can be greatly improved using training approaches in which subjects are receiving feedback on the basis of their ongoing judgments (Kaas et al., 2013; Lev et al., 2014; Zaltz et al., 2010; Zhou et al., 2006). Although fascinating, these studies have also reinforced that these training-based improvements rarely extend beyond the trained task. For example, improvements in Vernier acuity do not transfer to improvements in any other tasks of visual spatial acuity (Mckee and Westhe, 1978). These findings have greatly limited the larger applicability of these sensory training regimens, since their ability to transfer or generalize to more real-world functions are generally absent.
Recently however, we have found that such lack of generalization is not the case following multisensory training and feedback. Thus, we found that when subjects (typically developed young adults) are asked to perform a simultaneity judgment on paired audiovisual stimuli, but in the presence of feedback, marked improvements in temporal acuity were seen, not only in the trained task and stimuli (i.e., simultaneity judgment regarding the timing of flashes and beeps), but also in different temporally-constrained tasks using different stimuli (e.g., the McGurk effect and the sound-induced flash illusion). Furthermore, multisensory performance also improved following training within an individual sensory system (i.e., vision), suggesting that unisensory changes can cascade into multisensory representations (Stevenson et al., 2013). Such results suggest that the limitations of transfer may not apply to multisensory systems, possibly because multisensory tasks are deliberately indexing communication across wide regions of the cortical network. Indeed, follow up neuroimaging studies have identified a network of brain regions, that include regions of auditory and visual cortices, and that appears to be orchestrated by the pSTS, an area commonly implicated in audiovisual integration (Beauchamp et al., 2008; Hein and Knight, 2008; Hocking and Price, 2008; James et al., 2009; James and Stevenson, 2012; James et al., 2012), as the key nodes whose activity is altered following such multisensory training (Powers et al., 2012).
The presence of such generalization provides hope for the application of multisensory methods toward improving sensory function in ASD, for if we can train children with ASD to integrate audiovisual stimuli over shorter temporal intervals, these improvements in temporal integration may cascade up into higher-order networks. If we think about the practical applications of such a set of results, audiovisual temporal training might result in the construction of stronger and more accurate speech representations. Ongoing work is examining the feasibility of such multisensory-based training approaches. If this work proves promising, a future goal would be to add (multi)sensory training regimens into the behavioral toolbox that is used for skill building in children with ASD.
It is vitally important that these plasticity-based conceptual approaches toward ASD treatment be grounded in a rigorous empirical foundation. Thus, although sensory integration therapy (SIT) is one of the most commonly employed approaches used in ASD treatment and therapy, it is strongly founded in the subjective judgments of the occupational therapists administering the training. Furthermore, SIT training is based on a somewhat different conceptual foundation than that presented above, with the major emphasis being exposure to a wide array of sensory information in an effort to improve how this information is integrated with cognitive representations. Although such an approach makes a great deal of practical sense, it also often precludes the ability to draw strong empirical conclusions because of the highly individualized nature of the caregiver judgments and consequent treatment decisions. Here we argue for a more empirically-based approach in which children are administered a battery of sensory and multisensory tasks to characterize performance, and which will provide the clearest possible picture as to how sensory and multisensory factors contribute to the puzzle of ASD. We believe that such a base characterization can then be used to tailor individual treatment approaches, as the task battery will specifically identify areas of weakness that can then be targeted for more directed improvement. Such a personalized or precision approach to ASD treatment would mimic contemporary advances that are being made in cardiovascular health and cancer therapy (Morini et al., 2015; Santos et al., 2015).
6 Conclusions and future directions
As should be clear from the contents of this review, much more needs to be done to further our views as to the neurobiological underpinnings of ASD. The purpose of this review is to place an emphasis on better understanding the sensory contributions to this complex and heterogeneous disorder, which to date have been both poorly recognized and poorly characterized. Although there is now increased recognition and appreciation for a role for sensory dysfunction in the context of ASD, this work is still in its earliest stages, and better empirical characterization of sensory function and dysfunction in ASD is sorely needed.
While a growing number of studies have begun to examine sensory processing in ASD, they often continue to focus on a single task or small subset of tasks within a specific sensory modality. Future work needs to expand this paradigm such that we are measuring a number of facets of unisensory (i.e., vision alone, audition alone, touch alone) and multisensory function within the same children. Only with such an approach will we be able to dissect out the sensory-specific changes from the more amodal (domain general), supramodal (cross domain), or multisensory changes. In addition, only with a rigorous characterization of the changes within the senses will we be able to tease out the specificity of the changes for integration across the different sensory systems.
This work to understand the respective contributions of unisensory and multisensory function in ASD will also greatly benefit from the application of sophisticated computational and modeling approaches. Indeed, using modeling frameworks such as Bayesian approaches to cue combination (Seilheimer et al., 2014), the unique impact on multisensory function can begin to be assessed by building models based on changes in unisensory performance. The benefit of using such an approach would allow for the ability to model hidden, internal states of an observer, as opposed to response probabilities alone, which are often unable to distinguish between multiple possible differences in sensory processing mechanisms. For example, the predictive coding model of ASD proposes a dramatically different sensory processing and integration strategy (flat prior, high precision in sensory representation) compared to the variability model of ASD (same prior distribution as individuals with TD, poor precision in sensory representation). Here Bayesian approaches can begin to tease apart differences in prior distributions vs. high noise in representations of sensory inputs. Furthermore, possible changes in the combination of cues (i.e. suboptimal integration) can also be tested.
In a similar fashion, neuroimaging-based research into ASD needs to acknowledge the contribution of sensory networks to the broader patterns of change in functional activation and connectivity patterns. Future work therefore should not only focus upon activity changes in individual brain regions and networks that are known to be central for the higher-order areas of weakness seen in ASD (i.e., areas instrumental in social communication such as the amygdala, pSTS, and TPJ), but also attempt to better elucidate how sensory brain regions are communicating with these regions. These functional (and structural) connectivity-based approaches are being increasingly employed in the study of ASD, but still often focus on higher-order networks
In addition to focusing upon changes in sensory and multisensory function, future work also needs to be more expansive in regards to relating changes in sensory perception and performance to changes in domains of acknowledged clinical weakness such as social communication and repetitive behaviors. Thus, as described earlier, although some work has shown intriguing relationships between changes in multisensory temporal function and changes in speech and language abilities, these types of relational studies need to be greatly expanded. Such work can be very powerful when viewed from the perspective of relating performance on a battery of tasks to evaluate sensory function with a battery of tasks for evaluating clinical and cognitive performance. The correlational analyses derived from such comparisons across these tasks will likely shed important light on how various facets of sensory performance underpin higher cognitive abilities.
In addition to these correlational analyses within the various phenotypic domains and the neuroimaging work to understand differences in brain function and connectivity, future work needs to attempt to link between these large areas of research emphasis, and also to relate these findings to the genetic diversity that characterizes ASD. Thus, although efforts have been made to look at genotype-phenotype relations in ASD (Bruining et al., 2014; Chang et al., 2015; Schauder et al., 2015b; Veatch et al., 2014), and to relate structural and functional imaging features to either genotype or phenotype (Hedrick et al., 2012; Raznahan et al., 2012; Wiggins et al., 2012; Wiggins et al., 2014), little has been done to bridge across these three areas, and even less has been done to incorporate sensory features and sensory networks into these analyses. Although such work will be laborious and resource intensive, it will only be with such an integrated approach that we will be able to reveal new and important relationships that provide key insights into the marked heterogeneity that characterizes ASD. Given the complex grouping of symptoms observed in ASD, the most powerful characterization of this disorder will likely come from a multifaceted profile of performance across a wide variety of tasks within individual subjects. Deconstructing this heterogeneity using individual differences across a wide range of domains will also likely be crucial for advancing treatment and therapeutic strategies in ASD.
Finally, much more work is necessary to examine the shared characteristics of autism with other neuropsychiatric conditions. Indeed, recent emphases in psychiatric research have advocated a shift away from a slavish adherence to strict diagnostic criteria and have instead suggested a more nuanced dimensional-based perspective toward elucidating the mechanistic underpinnings of psychiatric disease. This Research Domain Criteria (RDoC) framework, advocated by the National Institutes of Mental Health (Insel et al., 2010), focuses on specific characteristics or phenotypic traits that may be shared across conditions but that may be grounded in a common neurobiological process.
Examples of conditions that share common characteristics with autism range from dyslexia to schizophrenia. In the context of dyslexia, a profound reading disability, there appear to be shared deficits in the temporal acuity within which audiovisual stimuli are integrated, a finding that may map on to common circuit changes across these conditions (Wallace and Stevenson, 2014). For schizophrenia, the distinctions are more complex, but each disorder has elements of a disturbed sense of self, which may relate to differences in multisensory constructs such as the representation of peripersonal space. Indeed, autism and schizophrenia may represent opposing ends of a continuum of self-representation, and in which the former is characterized by greater weighting of self (versus other) and the latter is characterized by a greater weighting of factors beyond the self (and thus the propensity to hallucinations, etc.). Such examples serve to illustrate the power of the dimensional approach, as these opposing extremes may represent the ends of a continuum spectrum linked to a common set of neurobiological processes (Eicher and Gruen, 2015; Wible, 2012).
Ultimately, application of these more dimensional approaches, in addition to revealing mechanistic commonalities that cross diagnostic borders, offers great promise in advancing our understanding of the neurobiology of autism, given that such an emphasis greatly expands the palate of experimental approaches that bear on the pathophysiology of autism.
Table 1.
Summary of proposed theoretical models of ASD.
| Model | Description | References |
|---|---|---|
| Theory of Mind (ToM) | This theory proposes that individuals with ASD are less able to infer and understand the motives and feelings of others. | Baron-Cohen (1989); Blake et al. (2003); Frith and Frith (1999); Gallagher et al. (2000); Happé (1994, 1995); Kana et al. (2014); O'Nions et al. (2014); van Veluw and Chance (2014) |
| Weak Central Coherence (WCC) | The theory of WCC postulates that social and cognitive deficits observed in individuals with ASD is the result of excessive focus on local details rather than the global meaning that is constructed through the integration of information across wide brain regions and networks. | Bolte et al. (2007); Brosnan et al. (2004); Burnette et al. (2005); Damarla et al. (2010); Frith and Happé (1994); Happé (1999); Lee et al. (2007b); Ropar and Mitchell (2001); Witkin et al. (1971) |
| Predictive Coding | This theory proposes that individuals with ASD do not have a strong predictive representation (in terms of a Bayesian framework, a set of weak “priors”) of the world, which makes it difficult to predict upcoming events and therefore limits interactions with the external environment. | Gomez et al. (2014); Lawson et al. (2014); Pellicano and Burr (2012); (Pellicano et al., 2007); Sinha et al. (2014); van Boxtel and Lu (2013); Van de Cruys et al. (2014) |
| Reduced Sensory Precision | This theory suggests that those with ASD may have greater variability (i.e., less reliability) in behaviors and perceptions obtained in response to sensory stimuli, as well as in the neural response patterns that form the representation of the sensory inputs. | Coskun et al. (2009); Dinstein et al. (2012); Geurts et al. (2008); Haigh et al. (2014); Milne (2011); Perez Velazquez and Galan (2013) |
| Excitatory/inhibitory imbalance | In this view the normal balance between excitation and inhibition might be changed in individuals with ASD, with the prevailing view that excitation may be increased and inhibition may be reduced. Global changes in excitation and inhibition would affect a wide array of cortical functioning, which could help explain the extensive array of deficits observed in ASD. | Gaetz et al. (2014); Gillberg and Billstedt (2000); Green et al. (2013); Kubas et al. (2012); Lewine et al. (1999); Rubenstein and Merzenich (2003) |
Box 1: Animal models of ASD.
As highlighted by the name, Autism Spectrum Disorder represents a complex and heterogeneous developmental disability of multifactorial etiology. A great deal of work has focused on identifying genetic risk factors for ASD, and the list of susceptibility genes now numbers well over 100, with many of these coding for proteins critical in developmental processes such as synapse formation and maintenance (De Rubeis et al., 2014). Familial and twin studies have illustrated the complex heritability patterns associated with ASD. Thus, in a recent study of over 2 million children in Sweden, the cumulative probability at age 20 of being diagnosed with ASD ranged from 12.9% for individuals with a full sibling who also had a diagnosis of ASD to 59.2% for monozygotic twins (Sandin et al., 2014). These numbers were in stark contrast to an incidence rate of 1.2% for individuals without a family history of ASD.
Despite this emphasis on autism genetics, it is almost certain that environmental factors play an important role in the pathophysiology of autism. Although little consensus exists as to these factors, it is generally accepted that only with an understanding of the complex interplay between genes and environment that we will be able to make great strides in elucidating the pathophysiology of ASD (Hallmayer et al., 2011; Hertz-Picciotto et al., 2006).
The complexity of autism and the mysteries in regards to its pathophysiological bases has spurred tremendous interest in the development of animal models that recapitulate various aspects of ASD. Many of these models are structured so as to examine the contribution of specific genes to phenotypic behavioral features, such as repetitive behaviors, social interactions and communicative function. Because of the powerful molecular genetic tools now available, much of this work has been carried out in rodent models (Esclassan et al., 2015; Oguchi-Katayama et al., 2013; Wohr and Scattoni, 2013).
One example of these animal-model directed studies has focused on the transporter for the neurotransmitter serotonin. Hyperserotonemia (elevated levels of circulating serotonin) is found in 30% of children with ASD, and rare variants of the serotonin transporter (SERT) have been associated with autism (Sutcliffe et al., 2005). The most common of these variants, known as SERT Ala56, was found to be overtransmitted in autism protobands, and was associated with both rigid-compulsive behavior and sensory aversions (Veenstra-VanderWeele et al., 2012). A mouse model of this SERT variant has been created, and has served as powerful tool in teasing out the biochemical and behavioral impacts of altered serotonin signaling and homeostasis. Most strikingly, these SERT Ala56 mice exhibit changes in social interactions, stereotypic behaviors, and communication signaling that map back on to the major clinical categories of dysfunction in ASD. Such work establishes an animal model with both construct and face validity and that will allow mechanistic insights into ASD pathophysiology that lay the groundwork for the development of novel treatments.
In an effort to evaluate sensory contributions in the context of this model, our laboratory has recently developed a multisensory behavioral assay for mouse that seeks to explore sensory and multisensory function (Siemann et al., 2014). In this work, mice are trained to detect and approach auditory and visual targets, and the pairing of audiovisual stimuli results in marked improvements in task accuracy. Ongoing work is now examining how visual, auditory and audiovisual function is altered in SERT Ala56 mice. If changes are seen in the multisensory behavior of these animals, this will further amplify the validity of this model, and creates the opportunity for neurophysiological experiments structured to identify circuit-based differences in multisensory function.
Box 2: Sensory Integration Therapy- lack of empirical evidence.
One of the most frequently employed clinical treatment approaches for ASD is sensory integration therapy (SIT). Based on the historical foundation of theory and work of Jean Ayres and her colleagues (Ayres and Mailloux, 1983; Ayres and Tickle, 1980; Slavik et al., 1984), the approach is firmly grounded in Ayres model of sensory integration, which highlights the importance of combining information from the different senses for activities of daily life, including learning, motoric behavior and emotional regulation. The theory has become a practical foundation for the field of occupational therapy (OT), whose practitioners are important caregivers and treatment providers in those with ASD.
In SIT protocols, children are often placed in rich sensory environments, where treatment regimens are structured in a highly individualized manner in order to appropriately target the child's sensory processing challenges. A frequent emphasis of these treatments is on the domains of vestibular and proprioceptive function, with exercises structured to improve balance, coordination and sensorimotor function. In addition, touch is a frequently targeted sensory system, and SIT protocols often involve deep pressure in an effort to ameliorate sensory and regulatory dysfunction.
Although increasingly practiced in children with ASD, SIT approaches continue to sit on a weak empirical foundation. Part of this is a function of the highly individualized treatment approaches and protocols, which are tailored toward the specific challenges of the child and which are frequently adapted based on the judgments and clinical observations of the occupational therapist. Although such customized treatments make a great deal of sense within the framework of the child's specific problems, they also greatly limit the ability to draw broader conclusions about the efficacy of SIT in a larger context and the generalizability of the results across children. Furthermore, the choice of treatment options in generally made on the basis of clinical observations, with limited use of more objective measures.
Future efforts within this arena should seek to combine the clinical expertise of the OT community with more empirically based evaluative processes and treatment protocols derived from our psychophysical and neurobiological knowledge bases in ASD. Efforts to manualize SIT treatment approaches are currently underway, and should seek to incorporate, wherever possible, psychophysical and neuroimaging assessment. This synthetic approach could not only better tailor treatment regimens based on objective sensory measures, but could also provide a powerful tool to evaluate the efficacy of these therapeutic endeavors.
Highlights.
-Although sensory processing problems are common in ASD, they are poorly characterized.
– There is increasing recognition of the prevalence and importance of multisensory processing changes in ASD.
-Sensory and multisensory representations form the building blocks of higher-order cognitive representations.
- Hence low-level sensory changes in ASD are likely to impact domains such as social communication.
- Remediation approaches that focus on sensory function may hold promise in ASD treatment.
Abbreviation list
- ASD
Autism Spectrum Disorder
- DSM-5
Diagnostic and Statistical Manual of Mental Disorders
- EFT
Embedded Figures Test
- TD
Typically developing
- SIFI
Sound Induced Flash Illusion
- ToM
Theory of Mind
- WCC
Weak Central Coherence
- STS
Superior temporal sulcus
- TPJ
Temporal parietal junction
- fMRI
Functional magnetic resonance imaging
- AIS
Active Information Storage
- GABA
γ-Aminobutyric acid
- MRS
Magnetic resonance spectroscopy
- FFA
Fusiform face area
- STG
Superior temporal gyrus
- IFG
Inferior frontal gyrus
- DMN
Default mode network
- DTI
Diffusion tensor imaging
- EEG
Electroencephalography
- AEP
Auditory evoked potentials
- SIT
Sensory Integration Therapy
- SERT
Serotonin transporter
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdeltawwab MM, Baz H. Automatic Pre-Attentive Auditory Responses: MMN to Tone Burst Frequency Changes in Autistic School-Age Children. J Int Adv Otol. 2015;11:36–41. doi: 10.5152/iao.2014.438. [DOI] [PubMed] [Google Scholar]
- Abrams DA, Lynch CJ, Cheng KM, Phillips J, Supekar K, Ryali S, Uddin LQ, Menon V. Underconnectivity between voice-selective cortex and reward circuitry in children with autism. Proc Natl Acad Sci U S A. 2013;110:12060–12065. doi: 10.1073/pnas.1302982110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ADDM Prevalence of autism spectrum disorder among children aged 8 years - autism and developmental disabilities monitoring network, 11 sites, United States, 2010. Morbidity and mortality weekly report. Surveillance summaries. 2014;63:1–21. [PubMed] [Google Scholar]
- Assaf M, Jagannathan K, Calhoun VD, Miller L, Stevens MC, Sahl R, O'Boyle JG, Schultz RT, Pearlson GD. Abnormal functional connectivity of default mode sub-networks in autism spectrum disorder patients. Neuroimage. 2010;53:247–256. doi: 10.1016/j.neuroimage.2010.05.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayres AJ, Mailloux ZK. Possible pubertal effect on therapeutic gains in an autistic girl. The American journal of occupational therapy : official publication of the American Occupational Therapy Association. 1983;37:535–540. doi: 10.5014/ajot.37.8.535. [DOI] [PubMed] [Google Scholar]
- Ayres AJ, Tickle LS. Hyper-responsivity to touch and vestibular stimuli as a predictor of positive response to sensory integration procedures by autistic children. The American journal of occupational therapy : official publication of the American Occupational Therapy Association. 1980;34:375–381. doi: 10.5014/ajot.34.6.375. [DOI] [PubMed] [Google Scholar]
- Baranek GT, David FJ, Poe MD, Stone WL, Watson LR. Sensory Experiences Questionnaire: discriminating sensory features in young children with autism, developmental delays, and typical development. Journal of child psychology and psychiatry, and allied disciplines. 2006;47:591–601. doi: 10.1111/j.1469-7610.2005.01546.x. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S. The theory of mind hypothesis of autism: a reply to Boucher. The British journal of disorders of communication. 1989;24:199–200. doi: 10.3109/13682828909011956. [DOI] [PubMed] [Google Scholar]
- Basar E, Basar-Eroglu C, Karakas S, Schurmann M. Are cognitive processes manifested in event-related gamma, alpha, theta and delta oscillations in the EEG? Neurosci Lett. 1999;259:165–168. doi: 10.1016/s0304-3940(98)00934-3. [DOI] [PubMed] [Google Scholar]
- Beauchamp MS. Statistical Criteria in fMRI Studies of Multisensory Integration. Neuroinformatics. 2005;3:93–113. doi: 10.1385/NI:3:2:093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchamp MS, Lee KE, Argall BD, Martin A. Integration of auditory and visual information about objects in superior temporal sulcus. Neuron. 2004;41:809–823. doi: 10.1016/s0896-6273(04)00070-4. [DOI] [PubMed] [Google Scholar]
- Beauchamp MS, Sun P, Baum SH, Tolias AS, Yoshor D. Electrocorticography links human temporoparietal junction to visual perception. Nature Neuroscience. 2012 doi: 10.1038/nn.3131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchamp MS, Yasar NE, Frye RE, Ro T. Touch, sound and vision in human superior temporal sulcus. NeuroImage. 2008;41:1011–1020. doi: 10.1016/j.neuroimage.2008.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bebko JM, Weiss JA, Demark JL, Gomez P. Discrimination of temporal synchrony in intermodal events by children with autism and children with developmental disabilities without autism. Journal of child psychology and psychiatry, and allied disciplines. 2006;47:88–98. doi: 10.1111/j.1469-7610.2005.01443.x. [DOI] [PubMed] [Google Scholar]
- Beck JM, Ma WJ, Kiani R, Hanks T, Churchland AK, Roitman J, Shadlen MN, Latham PE, Pouget A. Probabilistic population codes for Bayesian decision making. Neuron. 2008;60:1142–1152. doi: 10.1016/j.neuron.2008.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrmann M, Avidan G, Leonard GL, Kimchi R, Luna B, Humphreys K, Minshew N. Configural processing in autism and its relationship to face processing. Neuropsychologia. 2006;44:110–129. doi: 10.1016/j.neuropsychologia.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Bertone A, Mottron L, Jelenic P, Faubert J. Motion perception in autism: a “complex” issue. J Cogn Neurosci. 2003;15:218–225. doi: 10.1162/089892903321208150. [DOI] [PubMed] [Google Scholar]
- Bertone A, Mottron L, Jelenic P, Faubert J. Enhanced and diminished visuospatial information processing in autism depends on stimulus complexity. Brain. 2005;128:2430–2441. doi: 10.1093/brain/awh561. [DOI] [PubMed] [Google Scholar]
- Biswal BB, Van Kylen J, Hyde JS. Simultaneous assessment of flow and BOLD signals in resting-state functional connectivity maps. NMR Biomed. 1997;10:165–170. doi: 10.1002/(sici)1099-1492(199706/08)10:4/5<165::aid-nbm454>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Blake R, Turner LM, Smoski MJ, Pozdol SL, Stone WL. Visual recognition of biological motion is impaired in children with autism. Psychol Sci. 2003;14:151–157. doi: 10.1111/1467-9280.01434. [DOI] [PubMed] [Google Scholar]
- Blakemore SJ, Tavassoli T, Calo S, Thomas RM, Catmur C, Frith U, Haggard P. Tactile sensitivity in Asperger syndrome. Brain Cogn. 2006;61:5–13. doi: 10.1016/j.bandc.2005.12.013. [DOI] [PubMed] [Google Scholar]
- Boddaert N, Chabane N, Gervais H, Good CD, Bourgeois M, Plumet MH, Barthelemy C, Mouren MC, Artiges E, Samson Y, Brunelle F, Frackowiak RS, Zilbovicius M. Superior temporal sulcus anatomical abnormalities in childhood autism: a voxel-based morphometry MRI study. NeuroImage. 2004;23:364–369. doi: 10.1016/j.neuroimage.2004.06.016. [DOI] [PubMed] [Google Scholar]
- Boddaert N, Zilbovicius M. Functional neuroimaging and childhood autism. Pediatr Radiol. 2002;32:1–7. doi: 10.1007/s00247-001-0570-x. [DOI] [PubMed] [Google Scholar]
- Boddaert N, Zilbovicius M, Philipe A, Robel L, Bourgeois M, Barthelemy C, Seidenwurm D, Meresse I, Laurier L, Desguerre I, Bahi-Buisson N, Brunelle F, Munnich A, Samson Y, Mouren MC, Chabane N. MRI findings in 77 children with non-syndromic autistic disorder. PLoS One. 2009;4:e4415. doi: 10.1371/journal.pone.0004415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolte S, Holtmann M, Poustka F, Scheurich A, Schmidt L. Gestalt perception and local-global processing in high-functioning autism. J Autism Dev Disord. 2007;37:1493–1504. doi: 10.1007/s10803-006-0231-x. [DOI] [PubMed] [Google Scholar]
- Bonnel A, McAdams S, Smith B, Berthiaume C, Bertone A, Ciocca V, Burack JA, Mottron L. Enhanced pure-tone pitch discrimination among persons with autism but not Asperger syndrome. Neuropsychologia. 2010;48:2465–2475. doi: 10.1016/j.neuropsychologia.2010.04.020. [DOI] [PubMed] [Google Scholar]
- Bonnel A, Mottron L, Peretz I, Trudel M, Gallun E, Bonnel AM. Enhanced pitch sensitivity in individuals with autism: a signal detection analysis. J Cogn Neurosci. 2003;15:226–235. doi: 10.1162/089892903321208169. [DOI] [PubMed] [Google Scholar]
- Boucher J, Lewis V. Unfamiliar face recognition in relatively able autistic children. Journal of child psychology and psychiatry, and allied disciplines. 1992;33:843–859. doi: 10.1111/j.1469-7610.1992.tb01960.x. [DOI] [PubMed] [Google Scholar]
- Brandwein AB, Foxe JJ, Butler JS, Frey H-P, Bates JC, Shulman LH, Molholm S. Neurophysiological Indices of Atypical Auditory Processing and Multisensory Integration are Associated with Symptom Severity in Autism. Journal of autism and developmental disorders. 2015;45:230–244. doi: 10.1007/s10803-014-2212-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandwein AB, Foxe JJ, Butler JS, Russo NN, Altschuler TS, Gomes H, Molholm S. The Development of Multisensory Integration in High-Functioning Autism: High-Density Electrical Mapping and Psychophysical Measures Reveal Impairments in the Processing of Audiovisual Inputs. Cereb Cortex. 2012 doi: 10.1093/cercor/bhs109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brock J, Brown CC, Boucher J, Rippon G. The temporal binding deficit hypothesis of autism. Dev Psychopathol. 2002;14:209–224. doi: 10.1017/s0954579402002018. [DOI] [PubMed] [Google Scholar]
- Brosnan MJ, Scott FJ, Fox S, Pye J. Gestalt processing in autism: failure to process perceptual relationships and the implications for contextual understanding. Journal of child psychology and psychiatry, and allied disciplines. 2004;45:459–469. doi: 10.1111/j.1469-7610.2004.00237.x. [DOI] [PubMed] [Google Scholar]
- Brown C, Gruber T, Boucher J, Rippon G, Brock J. Gamma abnormalities during perception of illusory figures in autism. Cortex. 2005;41:364–376. doi: 10.1016/s0010-9452(08)70273-9. [DOI] [PubMed] [Google Scholar]
- Bruining H, Eijkemans MJ, Kas MJ, Curran SR, Vorstman JA, Bolton PF. Behavioral signatures related to genetic disorders in autism. Molecular autism. 2014;5:11. doi: 10.1186/2040-2392-5-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruneau N, Bonnet-Brilhault F, Gomot M, Adrien J-L, Barthélémy C. Cortical auditory processing and communication in children with autism: electrophysiological/behavioral relations. International Journal of Psychophysiology. 2003;51:17–25. doi: 10.1016/s0167-8760(03)00149-1. [DOI] [PubMed] [Google Scholar]
- Brunelle F, Boddaert N, Zilbovicius M. [Autism and brain imaging] Bull Acad Natl Med. 2009;193:287–297. discussion 297-288. [PubMed] [Google Scholar]
- Buard I, Rogers SJ, Hepburn S, Kronberg E, Rojas DC. Altered oscillation patterns and connectivity during picture naming in autism. Front Hum Neurosci. 2013;7:742. doi: 10.3389/fnhum.2013.00742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnette CP, Mundy PC, Meyer JA, Sutton SK, Vaughan AE, Charak D. Weak central coherence and its relations to theory of mind and anxiety in autism. J Autism Dev Disord. 2005;35:63–73. doi: 10.1007/s10803-004-1035-5. [DOI] [PubMed] [Google Scholar]
- Calvert GA, Spence C, Stein BE. Handbook of Multisensory Processes. MIT Press; Cambridge, MS: 2004. [Google Scholar]
- Cappe C, Thelen A, Romei V, Thut G, Murray MM. Looming signals reveal synergistic principles of multisensory integration. J Neurosci. 2012;32:1171–1182. doi: 10.1523/JNEUROSCI.5517-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappe C, Thut G, Romei V, Murray MM. Selective integration of auditory-visual looming cues by humans. Neuropsychologia. 2009;47:1045–1052. doi: 10.1016/j.neuropsychologia.2008.11.003. [DOI] [PubMed] [Google Scholar]
- Cardy JEO, Flagg EJ, Roberts W, Roberts TP. Auditory evoked fields predict language ability and impairment in children. International Journal of Psychophysiology. 2008;68:170–175. doi: 10.1016/j.ijpsycho.2007.10.015. [DOI] [PubMed] [Google Scholar]
- Carriere BN, Royal DW, Wallace MT. Spatial heterogeneity of cortical receptive fields and its impact on multisensory interactions. J Neurophysiol. 2008;99:2357–2368. doi: 10.1152/jn.01386.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cascio C, McGlone F, Folger S, Tannan V, Baranek G, Pelphrey KA, Essick G. Tactile perception in adults with autism: a multidimensional psychophysical study. J Autism Dev Disord. 2008;38:127–137. doi: 10.1007/s10803-007-0370-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cascio CJ, Foss-Feig JH, Burnette CP, Heacock JL, Cosby AA. The rubber hand illusion in children with autism spectrum disorders: delayed influence of combined tactile and visual input on proprioception. Autism : the international journal of research and practice. 2012a;16:406–419. doi: 10.1177/1362361311430404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cascio CJ, Moana-Filho EJ, Guest S, Nebel MB, Weisner J, Baranek GT, Essick GK. Perceptual and neural response to affective tactile texture stimulation in adults with autism spectrum disorders. Autism research : official journal of the International Society for Autism Research. 2012b;5:231–244. doi: 10.1002/aur.1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh P, Mather G. Motion: the long and short of it. Spatial vision. 1989;4:103–129. doi: 10.1163/156856889x00077. [DOI] [PubMed] [Google Scholar]
- Cermak SA, Curtin C, Bandini LG. Food selectivity and sensory sensitivity in children with autism spectrum disorders. Journal of the American Dietetic Association. 2010;110:238–246. doi: 10.1016/j.jada.2009.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang J, Gilman SR, Chiang AH, Sanders SJ, Vitkup D. Genotype to phenotype relationships in autism spectrum disorders. Nat Neurosci. 2015;18:191–198. doi: 10.1038/nn.3907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang YS, Owen JP, Desai SS, Hill SS, Arnett AB, Harris J, Marco EJ, Mukherjee P. Autism and sensory processing disorders: shared white matter disruption in sensory pathways but divergent connectivity in social-emotional pathways. PLoS One. 2014;9:e103038. doi: 10.1371/journal.pone.0103038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherkassky VL, Kana RK, Keller TA, Just MA. Functional connectivity in a baseline resting-state network in autism. Neuroreport. 2006;17:1687–1690. doi: 10.1097/01.wnr.0000239956.45448.4c. [DOI] [PubMed] [Google Scholar]
- Chubb C, Sperling G. Drift-balanced random stimuli: a general basis for studying non-Fourier motion perception. Journal of the Optical Society of America. A, Optics and image science. 1988;5:1986–2007. doi: 10.1364/josaa.5.001986. [DOI] [PubMed] [Google Scholar]
- Churches O, Wheelwright S, Baron-Cohen S, Ring H. The N170 is not modulated by attention in autism spectrum conditions. Neuroreport. 2010;21:399–403. doi: 10.1097/wnr.0b013e328334311b. [DOI] [PubMed] [Google Scholar]
- Clery H, Andersson F, Bonnet-Brilhault F, Philippe A, Wicker B, Gomot M. fMRI investigation of visual change detection in adults with autism. NeuroImage. Clinical. 2013;2:303–312. doi: 10.1016/j.nicl.2013.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colonius H, Diederich A. Multisensory interaction in saccadic reaction time: a time-window-of-integration model. Journal of cognitive neuroscience. 2004;16:1000–1009. doi: 10.1162/0898929041502733. [DOI] [PubMed] [Google Scholar]
- Conturo TE, Williams DL, Smith CD, Gultepe E, Akbudak E, Minshew NJ. Neuronal fiber pathway abnormalities in autism: an initial MRI diffusion tensor tracking study of hippocampo-fusiform and amygdalo-fusiform pathways. J Int Neuropsychol Soc. 2008;14:933–946. doi: 10.1017/S1355617708081381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbett BA, Carmean V, Ravizza S, Wendelken C, Henry ML, Carter C, Rivera SM. A functional and structural study of emotion and face processing in children with autism. Psychiatry research. 2009;173:196–205. doi: 10.1016/j.pscychresns.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coskun MA, Varghese L, Reddoch S, Castillo EM, Pearson DA, Loveland KA, Papanicolaou AC, Sheth BR. Increased response variability in autistic brains? Neuroreport. 2009;20:1543–1548. doi: 10.1097/WNR.0b013e32833246b5. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Kilman BA, Galambos R, Lincoln AJ. Autism: processing of novel auditory information assessed by event-related brain potentials. Electroencephalography and Clinical Neurophysiology/Evoked Potentials Section. 1984;59:238–248. doi: 10.1016/0168-5597(84)90063-7. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Lincoln AJ, Kilman BA, Galambos R. Event-related brain potential correlates of the processing of novel visual and auditory information in autism. Journal of autism and developmental disorders. 1985;15:55–76. doi: 10.1007/BF01837899. [DOI] [PubMed] [Google Scholar]
- Dakin S, Frith U. Vagaries of visual perception in autism. Neuron. 2005;48:497–507. doi: 10.1016/j.neuron.2005.10.018. [DOI] [PubMed] [Google Scholar]
- Damarla SR, Keller TA, Kana RK, Cherkassky VL, Williams DL, Minshew NJ, Just MA. Cortical underconnectivity coupled with preserved visuospatial cognition in autism: Evidence from an fMRI study of an embedded figures task. Autism research : official journal of the International Society for Autism Research. 2010;3:273–279. doi: 10.1002/aur.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damiano CR, Aloi J, Burrus C, Garbutt JC, Kampov-Polevoy AB, Dichter GS. Intact Hedonic Responses to Sweet Tastes in Autism Spectrum Disorder. Research in autism spectrum disorders. 2014;8:230–236. doi: 10.1016/j.rasd.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson G, Meltzoff AN, Osterling J, Rinaldi J, Brown E. Children with autism fail to orient to naturally occurring social stimuli. Journal of autism and developmental disorders. 1998;28:479–485. doi: 10.1023/a:1026043926488. [DOI] [PubMed] [Google Scholar]
- Dawson G, Toth K, Abbott R, Osterling J, Munson J, Estes A, Liaw J. Early social attention impairments in autism: social orienting, joint attention, and attention to distress. Developmental psychology. 2004;40:271. doi: 10.1037/0012-1649.40.2.271. [DOI] [PubMed] [Google Scholar]
- Dawson G, Watling R. Interventions to facilitate auditory, visual, and motor integration in autism: a review of the evidence. J Autism Dev Disord. 2000;30:415–421. doi: 10.1023/a:1005547422749. [DOI] [PubMed] [Google Scholar]
- de Boer-Schellekens L, Eussen M, Vroomen J. Diminished sensitivity of audiovisual temporal order in autism spectrum disorder. Front Integr Neurosci. 2013;7:8. doi: 10.3389/fnint.2013.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Gelder B, Vroomen J, Van der Heide L. Face recognition and lip-reading in autism. European Journal of Cognitive Psychology. 1991;3:69–86. [Google Scholar]
- de Jonge MV, Kemner C, van Engeland H. Superior disembedding performance of high-functioning individuals with autism spectrum disorders and their parents: The need for subtle measures. Journal of autism and developmental disorders. 2006;36:677–683. doi: 10.1007/s10803-006-0113-2. [DOI] [PubMed] [Google Scholar]
- De Rubeis S, He X, Goldberg AP, Poultney CS, Samocha K, Cicek AE, Kou Y, Liu L, Fromer M, Walker S, Singh T, Klei L, Kosmicki J, Shih-Chen F, Aleksic B, Biscaldi M, Bolton PF, Brownfeld JM, Cai J, Campbell NG, Carracedo A, Chahrour MH, Chiocchetti AG, Coon H, Crawford EL, Curran SR, Dawson G, Duketis E, Fernandez BA, Gallagher L, Geller E, Guter SJ, Hill RS, Ionita-Laza J, Jimenz Gonzalez P, Kilpinen H, Klauck SM, Kolevzon A, Lee I, Lei I, Lei J, Lehtimaki T, Lin CF, Ma'ayan A, Marshall CR, McInnes AL, Neale B, Owen MJ, Ozaki N, Parellada M, Parr JR, Purcell S, Puura K, Rajagopalan D, Rehnstrom K, Reichenberg A, Sabo A, Sachse M, Sanders SJ, Schafer C, Schulte-Ruther M, Skuse D, Stevens C, Szatmari P, Tammimies K, Valladares O, Voran A, Li-San W, Weiss LA, Willsey AJ, Yu TW, Yuen RK, Study DDD, Homozygosity Mapping Collaborative for, A. Consortium, U.K. Cook EH, Freitag CM, Gill M, Hultman CM, Lehner T, Palotie A, Schellenberg GD, Sklar P, State MW, Sutcliffe JS, Walsh CA, Scherer SW, Zwick ME, Barett JC, Cutler DJ, Roeder K, Devlin B, Daly MJ, Buxbaum JD. Synaptic, transcriptional and chromatin genes disrupted in autism. Nature. 2014;515:209–215. doi: 10.1038/nature13772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Viva MM, Igliozzi R, Tancredi R, Brizzolara D. Spatial and motion integration in children with autism. Vision Res. 2006;46:1242–1252. doi: 10.1016/j.visres.2005.10.018. [DOI] [PubMed] [Google Scholar]
- Deruelle C, Rondan C, Gepner B, Fagot J. Processing of compound visual stimuli by children with autism and Asperger syndrome. International Journal of Psychology. 2006;41:97–106. [Google Scholar]
- Diederich A, Colonius H. The Time Window of Multisensory Integration: Relating Reaction Times and Judgments of Temporal Order. Psychological review. 2015 doi: 10.1037/a0038696. [DOI] [PubMed] [Google Scholar]
- Diederich A, Schomburg A, Colonius H. Saccadic reaction times to audiovisual stimuli show effects of oscillatory phase reset. PLoS One. 2012;7:e44910. doi: 10.1371/journal.pone.0044910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinstein I, Heeger DJ, Lorenzi L, Minshew NJ, Malach R, Behrmann M. Unreliable Evoked Responses in Autism. Neuron. 2012;75:981–991. doi: 10.1016/j.neuron.2012.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downar J, Crawley AP, Mikulis DJ, Davis KD. A multimodal cortical network for the detection of changes in the sensory environment. Nat Neurosci. 2000;3:277–283. doi: 10.1038/72991. [DOI] [PubMed] [Google Scholar]
- Downar J, Crawley AP, Mikulis DJ, Davis KD. The effect of task relevance on the cortical response to changes in visual and auditory stimuli: an event-related fMRI study. Neuroimage. 2001;14:1256–1267. doi: 10.1006/nimg.2001.0946. [DOI] [PubMed] [Google Scholar]
- Doyle-Thomas KA, Goldberg J, Szatmari P, Hall GB. Neurofunctional underpinnings of audiovisual emotion processing in teens with autism spectrum disorders. Frontiers in psychiatry. 2013;4:48. doi: 10.3389/fpsyt.2013.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudova I, Hrdlicka M. Olfactory functions are not associated with autism severity in autism spectrum disorders. Neuropsychiatr Dis Treat. 2013;9:1847–1851. doi: 10.2147/NDT.S54893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumoulin SO, Baker CL, Jr., Hess RF, Evans AC. Cortical specialization for processing first- and second-order motion. Cereb Cortex. 2003;13:1375–1385. doi: 10.1093/cercor/bhg085. [DOI] [PubMed] [Google Scholar]
- Dunn MA, Gomes H, Gravel J. Mismatch negativity in children with autism and typical development. Journal of autism and developmental disorders. 2008;38:52–71. doi: 10.1007/s10803-007-0359-3. [DOI] [PubMed] [Google Scholar]
- Dunn W, Myles BS, Orr S. Sensory processing issues associated with Asperger syndrome: a preliminary investigation. American Journal of Occupational Therapy. 2002;56:97–102. doi: 10.5014/ajot.56.1.97. [DOI] [PubMed] [Google Scholar]
- Eicher JD, Gruen JR. Language impairment and dyslexia genes influence language skills in children with autism spectrum disorders. Autism research : official journal of the International Society for Autism Research. 2015;8:229–234. doi: 10.1002/aur.1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esclassan F, Francois J, Phillips KG, Loomis S, Gilmour G. Phenotypic characterization of nonsocial behavioral impairment in neurexin 1alpha knockout rats. Behavioral neuroscience. 2015;129:74–85. doi: 10.1037/bne0000024. [DOI] [PubMed] [Google Scholar]
- Ewbank MP, Rhodes G, von dem Hagen EA, Powell TE, Bright N, Stoyanova RS, Baron-Cohen S, Calder AJ. Repetition Suppression in Ventral Visual Cortex Is Diminished as a Function of Increasing Autistic Traits. Cereb Cortex. 2014 doi: 10.1093/cercor/bhu149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan YT, Cheng Y. Atypical mismatch negativity in response to emotional voices in people with autism spectrum conditions. PLoS One. 2014;9:e102471. doi: 10.1371/journal.pone.0102471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferri R, Elia M, Agarwal N, Lanuzza B, Musumeci SA, Pennisi G. The mismatch negativity and the P3a components of the auditory event-related potentials in autistic low-functioning subjects. Clinical neurophysiology. 2003;114:1671–1680. doi: 10.1016/s1388-2457(03)00153-6. [DOI] [PubMed] [Google Scholar]
- Fetsch CR, Deangelis GC, Angelaki DE. Visual-vestibular cue integration for heading perception: applications of optimal cue integration theory. Eur J Neurosci. 2010;31:1721–1729. doi: 10.1111/j.1460-9568.2010.07207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorentini C, Gray L, Rhodes G, Jeffery L, Pellicano E. Reduced face identity aftereffects in relatives of children with autism. Neuropsychologia. 2012;50:2926–2932. doi: 10.1016/j.neuropsychologia.2012.08.019. [DOI] [PubMed] [Google Scholar]
- Foss-Feig JH, Heacock JL, Cascio CJ. Tactile Responsiveness Patterns and Their Association with Core Features in Autism Spectrum Disorders. Research in autism spectrum disorders. 2012;6:337–344. doi: 10.1016/j.rasd.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foss-Feig JH, Kwakye LD, Cascio CJ, Burnette CP, Kadivar H, Stone WL, Wallace MT. An extended multisensory temporal binding window in autism spectrum disorders. Experimental brain research. Experimentelle Hirnforschung. 2010;203:381–389. doi: 10.1007/s00221-010-2240-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foss-Feig JH, Tadin D, Schauder KB, Cascio CJ. A substantial and unexpected enhancement of motion perception in autism. J Neurosci. 2013;33:8243–8249. doi: 10.1523/JNEUROSCI.1608-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foxe JJ, Molholm S, Del Bene VA, Frey H-P, Russo NN, Blanco D, Saint-Amour D, Ross LA. Severe multisensory speech integration deficits in high-functioning school-aged children with autism spectrum disorder (ASD) and their resolution during early adolescence. Cerebral Cortex. 2013:bht213. doi: 10.1093/cercor/bht213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser S, Gagne JP, Alepins M, Dubois P. Evaluating the effort expended to understand speech in noise using a dual-task paradigm: the effects of providing visual speech cues. Journal of speech, language, and hearing research : JSLHR. 2010;53:18–33. doi: 10.1044/1092-4388(2009/08-0140). [DOI] [PubMed] [Google Scholar]
- Freitag CM, Konrad C, Haberlen M, Kleser C, von Gontard A, Reith W, Troje NF, Krick C. Perception of biological motion in autism spectrum disorders. Neuropsychologia. 2008;46:1480–1494. doi: 10.1016/j.neuropsychologia.2007.12.025. [DOI] [PubMed] [Google Scholar]
- Fries P. A mechanism for cognitive dynamics: neuronal communication through neuronal coherence. Trends Cogn Sci. 2005;9:474–480. doi: 10.1016/j.tics.2005.08.011. [DOI] [PubMed] [Google Scholar]
- Frith CD, Frith U. Interacting minds--a biological basis. Science. 1999;286:1692–1695. doi: 10.1126/science.286.5445.1692. [DOI] [PubMed] [Google Scholar]
- Frith U, Happé FG. Autism: beyond “theory of mind”. Cognition. 1994;50:115–132. doi: 10.1016/0010-0277(94)90024-8. [DOI] [PubMed] [Google Scholar]
- Gaetz W, Bloy L, Wang DJ, Port RG, Blaskey L, Levy SE, Roberts TP. GABA estimation in the brains of children on the autism spectrum: measurement precision and regional cortical variation. Neuroimage. 2014;86:1–9. doi: 10.1016/j.neuroimage.2013.05.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher HL, Happé FG, Brunswick N, Fletcher PC, Frith U, Frith CD. Reading the mind in cartoons and stories: an fMRI study of ‘theory of mind’ in verbal and nonverbal tasks. Neuropsychologia. 2000;38:11–21. doi: 10.1016/s0028-3932(99)00053-6. [DOI] [PubMed] [Google Scholar]
- Gervais H, Belin P, Boddaert N, Leboyer M, Coez A, Sfaello I, Barthelemy C, Brunelle F, Samson Y, Zilbovicius M. Abnormal cortical voice processing in autism. Nat Neurosci. 2004;7:801–802. doi: 10.1038/nn1291. [DOI] [PubMed] [Google Scholar]
- Geurts HM, Grasman RP, Verte S, Oosterlaan J, Roeyers H, van Kammen SM, Sergeant JA. Intra-individual variability in ADHD, autism spectrum disorders and Tourette's syndrome. Neuropsychologia. 2008;46:3030–3041. doi: 10.1016/j.neuropsychologia.2008.06.013. [DOI] [PubMed] [Google Scholar]
- Ghose D, Wallace MT. Heterogeneity in the spatial receptive field architecture of multisensory neurons of the superior colliculus and its effects on multisensory integration. Neuroscience. 2014;256:147–162. doi: 10.1016/j.neuroscience.2013.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillberg C, Billstedt E. Autism and Asperger syndrome: coexistence with other clinical disorders. Acta psychiatrica Scandinavica. 2000;102:321–330. doi: 10.1034/j.1600-0447.2000.102005321.x. [DOI] [PubMed] [Google Scholar]
- Glazebrook JF, Wallace R. Pathologies in functional connectivity, feedback control and robustness: a global workspace perspective on autism spectrum disorders. Cognitive processing. 2015;16:1–16. doi: 10.1007/s10339-014-0636-y. [DOI] [PubMed] [Google Scholar]
- Golan O, Baron-Cohen S, Hill J. The Cambridge mindreading (CAM) face-voice battery: Testing complex emotion recognition in adults with and without Asperger syndrome. Journal of autism and developmental disorders. 2006;36:169–183. doi: 10.1007/s10803-005-0057-y. [DOI] [PubMed] [Google Scholar]
- Golan O, Baron-Cohen S, Hill JJ, Rutherford M. The ‘Reading the Mind in the Voice'test-revised: a study of complex emotion recognition in adults with and without autism spectrum conditions. Journal of autism and developmental disorders. 2007;37:1096–1106. doi: 10.1007/s10803-006-0252-5. [DOI] [PubMed] [Google Scholar]
- Gomez C, Lizier JT, Schaum M, Wollstadt P, Grutzner C, Uhlhaas P, Freitag CM, Schlitt S, Bolte S, Hornero R, Wibral M. Reduced predictable information in brain signals in autism spectrum disorder. Frontiers in neuroinformatics. 2014;8:9. doi: 10.3389/fninf.2014.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomot M, Belmonte MK, Bullmore ET, Bernard FA, Baron-Cohen S. Brain hyper-reactivity to auditory novel targets in children with high-functioning autism. Brain. 2008;131:2479–2488. doi: 10.1093/brain/awn172. [DOI] [PubMed] [Google Scholar]
- Gomot M, Giard MH, Adrien JL, Barthelemy C, Bruneau N. Hypersensitivity to acoustic change in children with autism: electrophysiological evidence of left frontal cortex dysfunctioning. Psychophysiology. 2002;39:577–584. doi: 10.1017.S0048577202394058. [DOI] [PubMed] [Google Scholar]
- Gondan M, Lange K, Rosler F, Roder B. The redundant target effect is affected by modality switch costs. Psychon Bull Rev. 2004;11:307–313. doi: 10.3758/bf03196575. [DOI] [PubMed] [Google Scholar]
- Gotts SJ, Saad ZS, Jo HJ, Wallace GL, Cox RW, Martin A. The perils of global signal regression for group comparisons: a case study of Autism Spectrum Disorders. Front Hum Neurosci. 2013;7:356. doi: 10.3389/fnhum.2013.00356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green SA, Rudie JD, Colich NL, Wood JJ, Shirinyan D, Hernandez L, Tottenham N, Dapretto M, Bookheimer SY. Overreactive brain responses to sensory stimuli in youth with autism spectrum disorders. J Am Acad Child Adolesc Psychiatry. 2013;52:1158–1172. doi: 10.1016/j.jaac.2013.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci U S A. 2003;100:253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grice SJ, Spratling MW, Karmiloff-Smith A, Halit H, Csibra G, de Haan M, Johnson MH. Disordered visual processing and oscillatory brain activity in autism and Williams syndrome. Neuroreport. 2001;12:2697–2700. doi: 10.1097/00001756-200108280-00021. [DOI] [PubMed] [Google Scholar]
- Grossman RB, Schneps MH, Tager-Flusberg H. Slipped lips: onset asynchrony detection of auditory-visual language in autism. J Child Psychol Psychiatry. 2009;50:491–497. doi: 10.1111/j.1469-7610.2008.02002.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman RB, Steinhart E, Mitchell T, McIlvane W. “Look who's talking!” Gaze Patterns for Implicit and Explicit Audio-Visual Speech Synchrony Detection in Children With High-Functioning Autism. Autism research : official journal of the International Society for Autism Research. 2015;8:307–316. doi: 10.1002/aur.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guclu B, Tanidir C, Mukaddes NM, Unal F. Tactile sensitivity of normal and autistic children. Somatosensory & motor research. 2007;24:21–33. doi: 10.1080/08990220601179418. [DOI] [PubMed] [Google Scholar]
- Guillon Q, Hadjikhani N, Baduel S, Roge B. Visual social attention in autism spectrum disorder: insights from eye tracking studies. Neurosci Biobehav Rev. 2014;42:279–297. doi: 10.1016/j.neubiorev.2014.03.013. [DOI] [PubMed] [Google Scholar]
- Hadjikhani N, Chabris CF, Joseph RM, Clark J, McGrath L, Aharon I, Feczko E, Tager-Flusberg H, Harris GJ. Early visual cortex organization in autism: an fMRI study. Neuroreport. 2004;15:267–270. doi: 10.1097/00001756-200402090-00011. [DOI] [PubMed] [Google Scholar]
- Haigh SM, Heeger DJ, Dinstein I, Minshew N, Behrmann M. Cortical Variability in the Sensory-Evoked Response in Autism. J Autism Dev Disord. 2014 doi: 10.1007/s10803-014-2276-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallmayer J, Cleveland S, Torres A, Phillips J, Cohen B, Torigoe T, Miller J, Fedele A, Collins J, Smith K, Lotspeich L, Croen LA, Ozonoff S, Lajonchere C, Grether JK, Risch N. Genetic heritability and shared environmental factors among twin pairs with autism. Archives of general psychiatry. 2011;68:1095–1102. doi: 10.1001/archgenpsychiatry.2011.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Happé FG. An advanced test of theory of mind: understanding of story characters’ thoughts and feelings by able autistic, mentally handicapped, and normal children and adults. J Autism Dev Disord. 1994;24:129–154. doi: 10.1007/BF02172093. [DOI] [PubMed] [Google Scholar]
- Happé FG. The role of age and verbal ability in the theory of mind task performance of subjects with autism. Child development. 1995;66:843–855. [PubMed] [Google Scholar]
- Happé FG. Studying weak central coherence at low levels: children with autism do not succumb to visual illusions. A research note. Journal of Child Psychology and Psychiatry. 1996;37:873–877. doi: 10.1111/j.1469-7610.1996.tb01483.x. [DOI] [PubMed] [Google Scholar]
- Happé FG. Autism: cognitive deficit or cognitive style? Trends Cogn Sci. 1999;3:216–222. doi: 10.1016/s1364-6613(99)01318-2. [DOI] [PubMed] [Google Scholar]
- Harms MB, Martin A, Wallace GL. Facial emotion recognition in autism spectrum disorders: a review of behavioral and neuroimaging studies. Neuropsychol Rev. 2010;20:290–322. doi: 10.1007/s11065-010-9138-6. [DOI] [PubMed] [Google Scholar]
- Haswell CC, Izawa J, Dowell LR, Mostofsky SH, Shadmehr R. Representation of internal models of action in the autistic brain. Nat Neurosci. 2009;12:970–972. doi: 10.1038/nn.2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton P, Pring L, Hermelin B. A pseudo-savant: A case of exceptional musical splinter skills. Neurocase. 1999;5:503–509. [Google Scholar]
- Hedrick A, Lee Y, Wallace GL, Greenstein D, Clasen L, Giedd JN, Raznahan A. Autism risk gene MET variation and cortical thickness in typically developing children and adolescents. Autism research : official journal of the International Society for Autism Research. 2012;5:434–439. doi: 10.1002/aur.1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hein G, Knight RT. Superior temporal sulcus--It's my area: or is it? Journal of cognitive neuroscience. 2008;20:2125–2136. doi: 10.1162/jocn.2008.20148. [DOI] [PubMed] [Google Scholar]
- Herrington JD, Baron-Cohen S, Wheelwright S, Singh KD, Bullmore ET, Brammer M. The role of MT+/V5 during biological motion perception in Asperger syndrome: An fMRI study. Research in autism spectrum disorders. 2207;1:14–27. [Google Scholar]
- Herrmann CS, Struber D, Helfrich RF, Engel AK. EEG oscillations: From correlation to causality. International journal of psychophysiology : official journal of the International Organization of Psychophysiology. 2015 doi: 10.1016/j.ijpsycho.2015.02.003. [DOI] [PubMed] [Google Scholar]
- Hertz-Picciotto I, Croen LA, Hansen R, Jones CR, van de Water J, Pessah IN. The CHARGE study: an epidemiologic investigation of genetic and environmental factors contributing to autism. Environmental health perspectives. 2006;114:1119–1125. doi: 10.1289/ehp.8483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesling I, Dilharreguy B, Peppe S, Amirault M, Bouvard M, Allard M. The integration of prosodic speech in high functioning autism: a preliminary FMRI study. PLoS One. 2010;5:e11571. doi: 10.1371/journal.pone.0011571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hocking J, Price CJ. The role of the posterior superior temporal sulcus in audiovisual processing. Cerebral Cortex. 2008;18:2439–2449. doi: 10.1093/cercor/bhn007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hrdlicka M, Vodicka J, Havlovicova M, Urbanek T, Blatny M, Dudova I. Brief report: significant differences in perceived odor pleasantness found in children with ASD. J Autism Dev Disord. 2011;41:524–527. doi: 10.1007/s10803-010-1084-x. [DOI] [PubMed] [Google Scholar]
- Hubbard KL, Anderson SE, Curtin C, Must A, Bandini LG. A comparison of food refusal related to characteristics of food in children with autism spectrum disorder and typically developing children. Journal of the Academy of Nutrition and Dietetics. 2014;114:1981–1987. doi: 10.1016/j.jand.2014.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubl D, Bolte S, Feineis-Matthews S, Lanfermann H, Federspiel A, Strik W, Poustka F, Dierks T. Functional imbalance of visual pathways indicates alternative face processing strategies in autism. Neurology. 2003;61:1232–1237. doi: 10.1212/01.wnl.0000091862.22033.1a. [DOI] [PubMed] [Google Scholar]
- Hughes JR. Autism: the first firm finding = underconnectivity? Epilepsy & behavior : E&B. 2007;11:20–24. doi: 10.1016/j.yebeh.2007.03.010. [DOI] [PubMed] [Google Scholar]
- Iarocci G, McDonald J. Sensory integration and the perceptual experience of persons with autism. J Autism Dev Disord. 2006;36:77–90. doi: 10.1007/s10803-005-0044-3. [DOI] [PubMed] [Google Scholar]
- Iarocci G, Rombough A, Yager J, Weeks DJ, Chua R. Visual influences on speech perception in children with autism. Autism : the international journal of research and practice. 2010;14:305–320. doi: 10.1177/1362361309353615. [DOI] [PubMed] [Google Scholar]
- Insel T, Cuthbert B, Garvey M, Heinssen R, Pine DS, Quinn K, Sanislow C, Wang P. Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. The American journal of psychiatry. 2010;167:748–751. doi: 10.1176/appi.ajp.2010.09091379. [DOI] [PubMed] [Google Scholar]
- Irwin JR, Tornatore LA, Brancazio L, Whalen DH. Can children with autism spectrum disorders “hear” a speaking face? Child Development. 2011;82:1397–1403. doi: 10.1111/j.1467-8624.2011.01619.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isler J, Martien K, Grieve P, Stark R, Herbert M. Reduced functional connectivity in visual evoked potentials in children with autism spectrum disorder. Clinical Neurophysiology. 2010;121:2035–2043. doi: 10.1016/j.clinph.2010.05.004. [DOI] [PubMed] [Google Scholar]
- James TW, Kim S, Stevenson RA. The International Society for Psychophysics. Galway, Ireland: 2009. Assessing multisensory interaction with additive factors and functional MRI. [Google Scholar]
- James TW, Stevenson RA. The Use of fMRI to Assess Multisensory Integration. In: Murray MM, Wallace MT, editors. The Neural Bases of Multisensory Processes. Boca Raton (FL): 2012. [PubMed] [Google Scholar]
- James TW, Stevenson RA, Kim S. Inverse effectiveness in multisensory processing. In: Stein BE, editor. The New Handbook of Multisensory Processes. MIT Press; Cambridge, MA: 2012. [Google Scholar]
- James TW, VanDerKlok RM, Stevenson RA, James KH. Multisensory perception of action in posterior temporal and parietal cortices. Neuropsychologia. 2011;49:108–114. doi: 10.1016/j.neuropsychologia.2010.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Järvinen-Pasley A, Heaton P. Evidence for reduced domain - specificity in auditory processing in autism. Developmental science. 2007;10:786–793. doi: 10.1111/j.1467-7687.2007.00637.x. [DOI] [PubMed] [Google Scholar]
- Järvinen-Pasley A, Pasley J, Heaton P. Is the linguistic content of speech less salient than its perceptual features in autism? Journal of autism and developmental disorders. 2008a;38:239–248. doi: 10.1007/s10803-007-0386-0. [DOI] [PubMed] [Google Scholar]
- Järvinen-Pasley A, Peppé S, King-Smith G, Heaton P. The relationship between form and function level receptive prosodic abilities in autism. Journal of Autism and Developmental Disorders. 2008b;38:1328–1340. doi: 10.1007/s10803-007-0520-z. [DOI] [PubMed] [Google Scholar]
- Järvinen-Pasley A, Wallace GL, Ramus F, Happé F, Heaton P. Enhanced perceptual processing of speech in autism. Developmental science. 2008c;11:109–121. doi: 10.1111/j.1467-7687.2007.00644.x. [DOI] [PubMed] [Google Scholar]
- Jolliffe T, Baron-Cohen S. Are people with autism and Asperger syndrome faster than normal on the Embedded Figures Test? Journal of child psychology and psychiatry, and allied disciplines. 1997;38:527–534. doi: 10.1111/j.1469-7610.1997.tb01539.x. [DOI] [PubMed] [Google Scholar]
- Jones CR, Happé F, Baird G, Simonoff E, Marsden AJ, Tregay J, Phillips RJ, Goswami U, Thomson JM, Charman T. Auditory discrimination and auditory sensory behaviours in autism spectrum disorders. Neuropsychologia. 2009;47:2850–2858. doi: 10.1016/j.neuropsychologia.2009.06.015. [DOI] [PubMed] [Google Scholar]
- Joseph RM, Keehn B, Connolly C, Wolfe JM, Horowitz TS. Why is visual search superior in autism spectrum disorder? Developmental science. 2009;12:1083–1096. doi: 10.1111/j.1467-7687.2009.00855.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Just MA, Cherkassky VL, Keller TA, Minshew NJ. Cortical activation and synchronization during sentence comprehension in high-functioning autism: evidence of underconnectivity. Brain. 2004;127:1811–1821. doi: 10.1093/brain/awh199. [DOI] [PubMed] [Google Scholar]
- Kaas AL, van de Ven V, Reithler J, Goebel R. Tactile perceptual learning: learning curves and transfer to the contralateral finger. Exp Brain Res. 2013;224:477–488. doi: 10.1007/s00221-012-3329-8. [DOI] [PubMed] [Google Scholar]
- Källstrand J, Olsson O, Nehlstedt SF, Sköld ML, Nielzén S. Abnormal auditory forward masking pattern in the brainstem response of individuals with Asperger syndrome. Neuropsychiatric disease and treatment. 2010;6:289. doi: 10.2147/ndt.s10593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kana RK, Libero LE, Hu CP, Deshpande HD, Colburn JS. Functional brain networks and white matter underlying theory-of-mind in autism. Soc Cogn Affect Neurosci. 2014;9:98–105. doi: 10.1093/scan/nss106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanner L. Autistic disturbances of affective contact. Nervous Child. 1943;2:217–250. [PubMed] [Google Scholar]
- Kasari C, Sigman M. Linking parental perceptions to interactions in young children with autism. J Autism Dev Disord. 1997;27:39–57. doi: 10.1023/a:1025869105208. [DOI] [PubMed] [Google Scholar]
- Kemner C, Van Ewijk L, Van Engeland H, Hooge I. Brief report: Eye movements during visual search tasks indicate enhanced stimulus discriminability in subjects with PDD. Journal of Autism and Developmental Disorders. 2008;38:553–557. doi: 10.1007/s10803-007-0406-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemner C, Verbaten MN, Cuperus JM, Camfferman G, Van Engeland H. Visual and somatosensory event-related brain potentials in autistic children and three different control groups. Electroencephalogr Clin Neurophysiol. 1994;92:225–237. doi: 10.1016/0168-5597(94)90066-3. [DOI] [PubMed] [Google Scholar]
- Kern JK, Trivedi MH, Garver CR, Grannemann BD, Andrews AA, Savla JS, Johnson DG, Mehta JA, Schroeder JL. The pattern of sensory processing abnormalities in autism. Autism : the international journal of research and practice. 2006;10:480–494. doi: 10.1177/1362361306066564. [DOI] [PubMed] [Google Scholar]
- Kern JK, Trivedi MH, Grannemann BD, Garver CR, Johnson DG, Andrews AA, Savla JS, Mehta JA, Schroeder JL. Sensory correlations in autism. Autism : the international journal of research and practice. 2007;11:123–134. doi: 10.1177/1362361307075702. [DOI] [PubMed] [Google Scholar]
- Khan S, Gramfort A, Shetty NR, Kitzbichler MG, Ganesan S, Moran JM, Lee SM, Gabrieli JD, Tager-Flusberg HB, Joseph RM, Herbert MR, Hamalainen MS, Kenet T. Local and long-range functional connectivity is reduced in concert in autism spectrum disorders. Proc Natl Acad Sci U S A. 2013;110:3107–3112. doi: 10.1073/pnas.1214533110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kientz MA, Dunn W. A comparison of the performance of children with and without autism on the Sensory Profile. The American journal of occupational therapy : official publication of the American Occupational Therapy Association. 1997;51:530–537. doi: 10.5014/ajot.51.7.530. [DOI] [PubMed] [Google Scholar]
- Kikuchi M, Yoshimura Y, Hiraishi H, Munesue T, Hashimoto T, Tsubokawa T, Takahashi T, Suzuki M, Higashida H, Minabe Y. Reduced long-range functional connectivity in young children with autism spectrum disorder. Soc Cogn Affect Neurosci. 2015;10:248–254. doi: 10.1093/scan/nsu049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinhans NM, Richards T, Sterling L, Stegbauer KC, Mahurin R, Johnson LC, Greenson J, Dawson G, Aylward E. Abnormal functional connectivity in autism spectrum disorders during face processing. Brain. 2008;131:1000–1012. doi: 10.1093/brain/awm334. [DOI] [PubMed] [Google Scholar]
- Kleinman J, Marciano PL, Ault RL. Advanced theory of mind in high-functioning adults with autism. Journal of autism and developmental disorders. 2001;31:29–36. doi: 10.1023/a:1005657512379. [DOI] [PubMed] [Google Scholar]
- Klimesch W. EEG alpha and theta oscillations reflect cognitive and memory performance: a review and analysis. Brain research. Brain research reviews. 1999;29:169–195. doi: 10.1016/s0165-0173(98)00056-3. [DOI] [PubMed] [Google Scholar]
- Koldewyn K, Weigelt S, Kanwisher N, Jiang Y. Multiple object tracking in autism spectrum disorders. J Autism Dev Disord. 2013;43:1394–1405. doi: 10.1007/s10803-012-1694-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koldewyn K, Whitney D, Rivera SM. The psychophysics of visual motion and global form processing in autism. Brain. 2010;133:599–610. doi: 10.1093/brain/awp272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kording KP, Beierholm U, Ma WJ, Quartz S, Tenenbaum JB, Shams L. Causal inference in multisensory perception. PloS one. 2007;2:e943. doi: 10.1371/journal.pone.0000943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshino H, Carpenter PA, Minshew NJ, Cherkassky VL, Keller TA, Just MA. Functional connectivity in an fMRI working memory task in high-functioning autism. NeuroImage. 2005;24:810–821. doi: 10.1016/j.neuroimage.2004.09.028. [DOI] [PubMed] [Google Scholar]
- Koshino H, Kana RK, Keller TA, Cherkassky VL, Minshew NJ, Just MA. fMRI investigation of working memory for faces in autism: visual coding and underconnectivity with frontal areas. Cereb Cortex. 2008;18:289–300. doi: 10.1093/cercor/bhm054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kral TV, Eriksen WT, Souders MC, Pinto-Martin JA. Eating behaviors, diet quality, and gastrointestinal symptoms in children with autism spectrum disorders: a brief review. Journal of pediatric nursing. 2013;28:548–556. doi: 10.1016/j.pedn.2013.01.008. [DOI] [PubMed] [Google Scholar]
- Krueger J, Royal DW, Fister MC, Wallace MT. Spatial receptive field organization of multisensory neurons and its impact on multisensory interactions. Hear Res. 2009;258:47–54. doi: 10.1016/j.heares.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubas B, Kulak W, Sobaniec W, Tarasow E, Lebkowska U, Walecki J. Metabolite alterations in autistic children: a 1H MR spectroscopy study. Advances in medical sciences. 2012;57:152–156. doi: 10.2478/v10039-012-0014-x. [DOI] [PubMed] [Google Scholar]
- Kuhl PK, Coffey - Corina S, Padden D, Dawson G. Links between social and linguistic processing of speech in preschool children with autism: behavioral and electrophysiological measures. Developmental science. 2005;8:F1–F12. doi: 10.1111/j.1467-7687.2004.00384.x. [DOI] [PubMed] [Google Scholar]
- Kwakye LD, Foss-Feig JH, Cascio CJ, Stone WL, Wallace MT. Altered auditory and multisensory temporal processing in autism spectrum disorders. Frontiers in integrative neuroscience. 2011;4:129. doi: 10.3389/fnint.2010.00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon S, Kim J, Choe B-H, Ko C, Park S. Electrophysiologic assessment of central auditory processing by auditory brainstem responses in children with autism spectrum disorders. Journal of Korean medical science. 2007;22:656–659. doi: 10.3346/jkms.2007.22.4.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakatos P, Chen CM, O'Connell MN, Mills A, Schroeder CE. Neuronal oscillations and multisensory interaction in primary auditory cortex. Neuron. 2007;53:279–292. doi: 10.1016/j.neuron.2006.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange N, Dubray MB, Lee JE, Froimowitz MP, Froehlich A, Adluru N, Wright B, Ravichandran C, Fletcher PT, Bigler ED, Alexander AL, Lainhart JE. Atypical diffusion tensor hemispheric asymmetry in autism. Autism Res. 2010 doi: 10.1002/aur.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langen M, Leemans A, Johnston P, Ecker C, Daly E, Murphy CM, Dell'acqua F, Durston S, Consortium A, Murphy DG. Fronto-striatal circuitry and inhibitory control in autism: findings from diffusion tensor imaging tractography. Cortex. 2012;48:183–193. doi: 10.1016/j.cortex.2011.05.018. [DOI] [PubMed] [Google Scholar]
- Lawson RP, Rees G, Friston KJ. An aberrant precision account of autism. Front Hum Neurosci. 2014;8:302. doi: 10.3389/fnhum.2014.00302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Couteur A, Rutter M, Lord C, Rios P, Robertson S, Holdgrafer M, McLennan J. Autism diagnostic interview: a standardized investigator-based instrument. Journal of autism and developmental disorders. 1989;19:363–387. doi: 10.1007/BF02212936. [DOI] [PubMed] [Google Scholar]
- Lee JE, Bigler ED, Alexander AL, Lazar M, DuBray MB, Chung MK, Johnson M, Morgan J, Miller JN, McMahon WM, Lu J, Jeong EK, Lainhart JE. Diffusion tensor imaging of white matter in the superior temporal gyrus and temporal stem in autism. Neurosci Lett. 2007a;424:127–132. doi: 10.1016/j.neulet.2007.07.042. [DOI] [PubMed] [Google Scholar]
- Lee PS, Foss-Feig J, Henderson JG, Kenworthy LE, Gilotty L, Gaillard WD, Vaidya CJ. Atypical neural substrates of Embedded Figures Task performance in children with Autism Spectrum Disorder. Neuroimage. 2007b;38:184–193. doi: 10.1016/j.neuroimage.2007.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leekam SR, Nieto C, Libby SJ, Wing L, Gould J. Describing the sensory abnormalities of children and adults with autism. J Autism Dev Disord. 2007;37:894–910. doi: 10.1007/s10803-006-0218-7. [DOI] [PubMed] [Google Scholar]
- Lev M, Ludwig K, Gilaie-Dotan S, Voss S, Sterzer P, Hesselmann G, Polat U. Training improves visual processing speed and generalizes to untrained functions. Scientific reports. 2014;4:7251. doi: 10.1038/srep07251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitt JG, Blanton RE, Smalley S, Thompson PM, Guthrie D, McCracken JT, Sadoun T, Heinichen L, Toga AW. Cortical sulcal maps in autism. Cereb Cortex. 2003;13:728–735. doi: 10.1093/cercor/13.7.728. [DOI] [PubMed] [Google Scholar]
- Lewine JD, Andrews R, Chez M, Patil AA, Devinsky O, Smith M, Kanner A, Davis JT, Funke M, Jones G, Chong B, Provencal S, Weisend M, Lee RR, Orrison WW., Jr. Magnetoencephalographic patterns of epileptiform activity in children with regressive autism spectrum disorders. Pediatrics. 1999;104:405–418. doi: 10.1542/peds.104.3.405. [DOI] [PubMed] [Google Scholar]
- Lindner JL, Rosén LA. Decoding of emotion through facial expression, prosody and verbal content in children and adolescents with Asperger's syndrome. Journal of autism and developmental disorders. 2006;36:769–777. doi: 10.1007/s10803-006-0105-2. [DOI] [PubMed] [Google Scholar]
- Lord C. Follow - up of two - year - olds referred for possible autism. Journal of Child Psychology and Psychiatry. 1995;36:1365–1382. doi: 10.1111/j.1469-7610.1995.tb01669.x. [DOI] [PubMed] [Google Scholar]
- Ludlow A, Mohr B, Whitmore A, Garagnani M, Pulvermuller F, Gutierrez R. Auditory processing and sensory behaviours in children with autism spectrum disorders as revealed by mismatch negativity. Brain Cogn. 2014;86:55–63. doi: 10.1016/j.bandc.2014.01.016. [DOI] [PubMed] [Google Scholar]
- MacLeod A, Summerfield Q. Quantifying the contribution of vision to speech perception in noise. Br J Audiol. 1987;21:131–141. doi: 10.3109/03005368709077786. [DOI] [PubMed] [Google Scholar]
- Magnotti JF, Beauchamp MS. The noisy encoding of disparity model of the McGurk effect. Psychon Bull Rev. 2014 doi: 10.3758/s13423-014-0722-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnotti JF, Ma WJ, Beauchamp MS. Causal inference of asynchronous audiovisual speech. Front Psychol. 2013;4:798. doi: 10.3389/fpsyg.2013.00798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marco EJ, Hinkley LB, Hill SS, Nagarajan SS. Sensory processing in autism: a review of neurophysiologic findings. Pediatric Research. 2011;69:48R–54R. doi: 10.1203/PDR.0b013e3182130c54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martineau J, Garreau B, Barthelemy C, Lelord G. Evoked potentials and P300 during sensory conditioning in autistic children. Annals of the New York Academy of Sciences. 1984;425:362–369. doi: 10.1111/j.1749-6632.1984.tb23557.x. [DOI] [PubMed] [Google Scholar]
- Martuzzi R, Murray MM, Michel CM, Thiran JP, Maeder PP, Clarke S, Meuli RA. Multisensory interactions within human primary cortices revealed by BOLD dynamics. Cereb Cortex. 2007;17:1672–1679. doi: 10.1093/cercor/bhl077. [DOI] [PubMed] [Google Scholar]
- Masterton BA, Biederman GB. Proprioceptive versus visual control in autistic children. J Autism Dev Disord. 1983;13:141–152. doi: 10.1007/BF01531815. [DOI] [PubMed] [Google Scholar]
- Maximo JO, Cadena EJ, Kana RK. The implications of brain connectivity in the neuropsychology of autism. Neuropsychol Rev. 2014;24:16–31. doi: 10.1007/s11065-014-9250-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazefsky CA, Oswald DP. Emotion perception in Asperger's syndrome and high-functioning autism: The importance of diagnostic criteria and cue intensity. Journal of Autism and Developmental Disorders. 2007;37:1086–1095. doi: 10.1007/s10803-006-0251-6. [DOI] [PubMed] [Google Scholar]
- McGurk H, MacDonald J. Hearing lips and seeing voices. Nature. 1976;264:746–748. doi: 10.1038/264746a0. [DOI] [PubMed] [Google Scholar]
- Mckee SP, Westhe G. Improvement in vernier acuity with practice. Perception & psychophysics. 1978;24:258–262. doi: 10.3758/bf03206097. [DOI] [PubMed] [Google Scholar]
- Mercier MR, Foxe JJ, Fiebelkorn IC, Butler JS, Schwartz TH, Molholm S. Auditory-driven phase reset in visual cortex: human electrocorticography reveals mechanisms of early multisensory integration. Neuroimage. 2013;79:19–29. doi: 10.1016/j.neuroimage.2013.04.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith MA, Nemitz JW, Stein BE. Determinants of multisensory integration in superior colliculus neurons. I. Temporal factors. J Neurosci. 1987;7:3215–3229. doi: 10.1523/JNEUROSCI.07-10-03215.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith MA, Stein BE. Interactions among converging sensory inputs in the superior colliculus. Science. 1983;221:389–391. doi: 10.1126/science.6867718. [DOI] [PubMed] [Google Scholar]
- Meredith MA, Stein BE. Descending efferents from the superior colliculus relay integrated multisensory information. Science. 1985;227:657–659. doi: 10.1126/science.3969558. [DOI] [PubMed] [Google Scholar]
- Meredith MA, Stein BE. Spatial factors determine the activity of multisensory neurons in cat superior colliculus. Brain Res. 1986a;365:350–354. doi: 10.1016/0006-8993(86)91648-3. [DOI] [PubMed] [Google Scholar]
- Meredith MA, Stein BE. Visual, auditory, and somatosensory convergence on cells in superior colliculus results in multisensory integration. Journal of neurophysiology. 1986b;56:640–662. doi: 10.1152/jn.1986.56.3.640. [DOI] [PubMed] [Google Scholar]
- Meredith MA, Stein BE. Spatial determinants of multisensory integration in cat superior colliculus neurons. J Neurophysiol. 1996;75:1843–1857. doi: 10.1152/jn.1996.75.5.1843. [DOI] [PubMed] [Google Scholar]
- Miller J. Divided attention: evidence for coactivation with redundant signals. Cogn Psychol. 1982;14:247–279. doi: 10.1016/0010-0285(82)90010-x. [DOI] [PubMed] [Google Scholar]
- Milne E. Increased intra-participant variability in children with autistic spectrum disorders: evidence from single-trial analysis of evoked EEG. Frontiers in psychology. 2011;2:51. doi: 10.3389/fpsyg.2011.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milne E, Scope A, Pascalis O, Buckley D, Makeig S. Independent component analysis reveals atypical electroencephalographic activity during visual perception in individuals with autism. Biol Psychiatry. 2009;65:22–30. doi: 10.1016/j.biopsych.2008.07.017. [DOI] [PubMed] [Google Scholar]
- Milne E, Swettenham J, Hansen P, Campbell R, Jeffries H, Plaisted K. High motion coherence thresholds in children with autism. Journal of child psychology and psychiatry, and allied disciplines. 2002;43:255–263. doi: 10.1111/1469-7610.00018. [DOI] [PubMed] [Google Scholar]
- Minshew NJ, Goldstein G, Siegel DJ. Neuropsychologic functioning in autism: Profile of a complex information processing disorder. Journal of the International Neuropsychological Society. 1997;3:303–316. [PubMed] [Google Scholar]
- Minshew NJ, Hobson JA. Sensory sensitivities and performance on sensory perceptual tasks in high-functioning individuals with autism. J Autism Dev Disord. 2008;38:1485–1498. doi: 10.1007/s10803-007-0528-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minshew NJ, Sung K, Jones BL, Furman JM. Underdevelopment of the postural control system in autism. Neurology. 2004;63:2056–2061. doi: 10.1212/01.wnl.0000145771.98657.62. [DOI] [PubMed] [Google Scholar]
- Miyazaki M, Fujii E, Saijo T, Mori K, Hashimoto T, Kagami S, Kuroda Y. Short-latency somatosensory evoked potentials in infantile autism: evidence of hyperactivity in the right primary somatosensory area. Developmental medicine and child neurology. 2007;49:13–17. doi: 10.1017/s0012162207000059.x. [DOI] [PubMed] [Google Scholar]
- Molloy CA, Dietrich KN, Bhattacharya A. Postural stability in children with autism spectrum disorder. J Autism Dev Disord. 2003;33:643–652. doi: 10.1023/b:jadd.0000006001.00667.4c. [DOI] [PubMed] [Google Scholar]
- Mongillo E, Irwin J, Whalen D, Klaiman C, Carter A, Schultz R. Audiovisual processing in children with and without autism spectrum disorders. Journal of Autism and Developmental Disorders. 2008;38:1349–1358. doi: 10.1007/s10803-007-0521-y. [DOI] [PubMed] [Google Scholar]
- Morini E, Sangiuolo F, Caporossi D, Novelli G, Amati F. Application of Next Generation Sequencing for personalized medicine for sudden cardiac death. Frontiers in genetics. 2015;6:55. doi: 10.3389/fgene.2015.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mottron L, Burack JA, Iarocci G, Belleville S, Enns JT. Locally oriented perception with intact global processing among adolescents with high-functioning autism: evidence from multiple paradigms. Journal of child psychology and psychiatry, and allied disciplines. 2003;44:904–913. doi: 10.1111/1469-7610.00174. [DOI] [PubMed] [Google Scholar]
- Mottron L, Dawson M, Soulieres I, Hubert B, Burack J. Enhanced perceptual functioning in autism: an update, and eight principles of autistic perception. J Autism Dev Disord. 2006;36:27–43. doi: 10.1007/s10803-005-0040-7. [DOI] [PubMed] [Google Scholar]
- Mottron L, Peretz I, Menard E. Local and Global Processing of Music in High - functioning Persons with Autism: Beyond Central Coherence? Journal of Child Psychology and Psychiatry. 2000;41:1057–1065. [PubMed] [Google Scholar]
- Murphy JW, Foxe JJ, Peters JB, Molholm S. Susceptibility to distraction in autism spectrum disorder: probing the integrity of oscillatory alpha-band suppression mechanisms. Autism research : official journal of the International Society for Autism Research. 2014;7:442–458. doi: 10.1002/aur.1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray MM, Cappe C, Romei V, Martuzzi R, Thut G. Auditory-visual multisensory interactions in humans: a synthesis of findings from behavior, ERPs, fMRI, and TMS. In: Stein BE, editor. The New Handbook of Multisensory Processes. MIT Press; 2012. [Google Scholar]
- Murray MM, Wallace MT. The Neural Bases of Multisensory Processes. CRC Press; Boca Raton, FL: 2012. [PubMed] [Google Scholar]
- Myers BJ. Mother-infant bonding: The status of this critical-period hypothesis. Developmental Review. 1984;4:240–274. [Google Scholar]
- Navon D. Forest before trees: The precedence of global features in visual perception. Cognitive psychology. 1977;9:353–383. [Google Scholar]
- O'Neill M, Jones RS. Sensory-perceptual abnormalities in autism: a case for more research? J Autism Dev Disord. 1997;27:283–293. doi: 10.1023/a:1025850431170. [DOI] [PubMed] [Google Scholar]
- O'Nions E, Sebastian CL, McCrory E, Chantiluke K, Happé FG, Viding E. Neural bases of Theory of Mind in children with autism spectrum disorders and children with conduct problems and callous-unemotional traits. Developmental science. 2014;17:786–796. doi: 10.1111/desc.12167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Riordan M, Passetti F. Discrimination in autism within different sensory modalities. J Autism Dev Disord. 2006;36:665–675. doi: 10.1007/s10803-006-0106-1. [DOI] [PubMed] [Google Scholar]
- O'Riordan M, Plaisted K. Enhanced discrimination in autism. The Quarterly journal of experimental psychology. A, Human experimental psychology. 2001;54:961–979. doi: 10.1080/713756000. [DOI] [PubMed] [Google Scholar]
- O'Connor K. Auditory processing in autism spectrum disorder: a review. Neuroscience & Biobehavioral Reviews. 2012;36:836–854. doi: 10.1016/j.neubiorev.2011.11.008. [DOI] [PubMed] [Google Scholar]
- Oguchi-Katayama A, Monma A, Sekino Y, Moriguchi T, Sato K. Comparative gene expression analysis of the amygdala in autistic rat models produced by pre- and post-natal exposures to valproic acid. The Journal of toxicological sciences. 2013;38:391–402. doi: 10.2131/jts.38.391. [DOI] [PubMed] [Google Scholar]
- Oliva A, Torralba A. Building the gist of a scene: the role of global image features in recognition. Progress in brain research. 2006;155:23–36. doi: 10.1016/S0079-6123(06)55002-2. [DOI] [PubMed] [Google Scholar]
- Ornitz EM. The modulation of sensory input and motor output in autistic children. Journal of autism and childhood schizophrenia. 1974;4:197–215. doi: 10.1007/BF02115226. [DOI] [PubMed] [Google Scholar]
- Ornitz EM, Guthrie D, Farley AJ. The early development of autistic children. Journal of autism and childhood schizophrenia. 1977;7:207–209. doi: 10.1007/BF01538999. [DOI] [PubMed] [Google Scholar]
- Ornitz EM, Guthrie D, Farley AJ. The early symptoms of childhood autism. In: Serban G, editor. Cognitive defects in the development of mental illness. Brunner/Mazel; New York: 1978. pp. 24–42. [Google Scholar]
- Pallett PM, Cohen SJ, Dobkins KR. Face and object discrimination in autism, and relationship to IQ and age. J Autism Dev Disord. 2014;44:1039–1054. doi: 10.1007/s10803-013-1955-z. [DOI] [PubMed] [Google Scholar]
- Pantelis PC, Byrge L, Tyszka JM, Adolphs R, Kennedy DP. A specific hypoactivation of right temporo-parietal junction/posterior superior temporal sulcus in response to socially awkward situations in autism. Soc Cogn Affect Neurosci. 2015 doi: 10.1093/scan/nsv021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papagiannopoulou EA, Chitty KM, Hermens DF, Hickie IB, Lagopoulos J. A systematic review and meta-analysis of eye-tracking studies in children with autism spectrum disorders. Soc Neurosci. 2014;9:610–632. doi: 10.1080/17470919.2014.934966. [DOI] [PubMed] [Google Scholar]
- Patten E, Watson LR, Baranek GT. Temporal Synchrony Detection and Associations with Language in Young Children with ASD. Autism Research and Treatment. 2014 doi: 10.1155/2014/678346. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul R, Chawarska K, Fowler C, Cicchetti D, Volkmar F. “Listen my children and you shall hear”: Auditory preferences in toddlers with autism spectrum disorders. Journal of Speech, Language, and Hearing Research. 2007;50:1350–1364. doi: 10.1044/1092-4388(2007/094). [DOI] [PubMed] [Google Scholar]
- Pellicano E, Burr D. When the world becomes ‘too real’: a Bayesian explanation of autistic perception. Trends Cogn Sci. 2012;16:504–510. doi: 10.1016/j.tics.2012.08.009. [DOI] [PubMed] [Google Scholar]
- Pellicano E, Gibson L, Maybery M, Durkin K, Badcock DR. Abnormal global processing along the dorsal visual pathway in autism: a possible mechanism for weak visuospatial coherence? Neuropsychologia. 2005;43:1044–1053. doi: 10.1016/j.neuropsychologia.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Pellicano E, Jeffery L, Burr D, Rhodes G. Abnormal adaptive face-coding mechanisms in children with autism spectrum disorder. Curr Biol. 2007;17:1508–1512. doi: 10.1016/j.cub.2007.07.065. [DOI] [PubMed] [Google Scholar]
- Peppé S, McCann J, Gibbon F, O'Hare A, Rutherford M. Receptive and expressive prosodic ability in children with high-functioning autism. Journal of Speech, Language, and Hearing Research. 2007;50:1015–1028. doi: 10.1044/1092-4388(2007/071). [DOI] [PubMed] [Google Scholar]
- Perez Velazquez JL, Galan RF. Information gain in the brain's resting state: A new perspective on autism. Frontiers in neuroinformatics. 2013;7:37. doi: 10.3389/fninf.2013.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrault TJ, Vaughan JW, Stein BE, Wallace MT, Jr TJ. Superior Colliculus Neurons Use Distinct Operational Modes in the Integration of Multisensory Stimuli. Journal of neurophysiology. 2005;93:2575–2586. doi: 10.1152/jn.00926.2004. [DOI] [PubMed] [Google Scholar]
- Philip R, Whalley H, Stanfield A, Sprengelmeyer R, Santos I, Young A, Atkinson A, Calder A, Johnstone E, Lawrie S. Deficits in facial, body movement and vocal emotional processing in autism spectrum disorders. Psychological medicine. 2010;40:1919–1929. doi: 10.1017/S0033291709992364. [DOI] [PubMed] [Google Scholar]
- Pierce K, Muller RA, Ambrose J, Allen G, Courchesne E. Face processing occurs outside the fusiform ‘face area’ in autism: evidence from functional MRI. Brain. 2001;124:2059–2073. doi: 10.1093/brain/124.10.2059. [DOI] [PubMed] [Google Scholar]
- Pizlo Z. Perception viewed as an inverse problem. Vision Res. 2001;41:3145–3161. doi: 10.1016/s0042-6989(01)00173-0. [DOI] [PubMed] [Google Scholar]
- Plaisted K, Swettenham J, Rees L. Children with autism show local precedence in a divided attention task and global precedence in a selective attention task. Journal of child psychology and psychiatry. 1999;40:733–742. [PubMed] [Google Scholar]
- Plitt M, Barnes KA, Martin A. Functional connectivity classification of autism identifies highly predictive brain features but falls short of biomarker standards. NeuroImage. Clinical. 2015;7:359–366. doi: 10.1016/j.nicl.2014.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouget A, Dayan P, Zemel RS. Inference and computation with population codes. Annu Rev Neurosci. 2003;26:381–410. doi: 10.1146/annurev.neuro.26.041002.131112. [DOI] [PubMed] [Google Scholar]
- Powers AR, 3rd, Hevey MA, Wallace MT. Neural correlates of multisensory perceptual learning. J Neurosci. 2012;32:6263–6274. doi: 10.1523/JNEUROSCI.6138-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulvermuller F, Birbaumer N, Lutzenberger W, Mohr B. High-frequency brain activity: its possible role in attention, perception and language processing. Prog Neurobiol. 1997;52:427–445. doi: 10.1016/s0301-0082(97)00023-3. [DOI] [PubMed] [Google Scholar]
- Puts NA, Wodka EL, Tommerdahl M, Mostofsky SH, Edden RA. Impaired tactile processing in children with autism spectrum disorder. J Neurophysiol. 2014;111:1803–1811. doi: 10.1152/jn.00890.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raab DH. Statistical facilitation of simple reaction times. Transactions of the New York Academy of Sciences. 1962;24:574–590. doi: 10.1111/j.2164-0947.1962.tb01433.x. [DOI] [PubMed] [Google Scholar]
- Raznahan A, Lee Y, Vaituzis C, Tran L, Mackie S, Tiemeier H, Clasen L, Lalonde F, Greenstein D, Pierson R, Giedd JN. Allelic variation within the putative autism spectrum disorder risk gene homeobox A1 and cerebellar maturation in typically developing children and adolescents. Autism research : official journal of the International Society for Autism Research. 2012;5:93–100. doi: 10.1002/aur.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ristic J, Mottron L, Friesen CK, Iarocci G, Burack JA, Kingstone A. Eyes are special but not for everyone: the case of autism. Brain Res Cogn Brain Res. 2005;24:715–718. doi: 10.1016/j.cogbrainres.2005.02.007. [DOI] [PubMed] [Google Scholar]
- Robertson CE, Thomas C, Kravitz DJ, Wallace GL, Baron-Cohen S, Martin A, Baker CI. Global motion perception deficits in autism are reflected as early as primary visual cortex. Brain. 2014;137:2588–2599. doi: 10.1093/brain/awu189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers SJ, Hepburn S, Wehner E. Parent reports of sensory symptoms in toddlers with autism and those with other developmental disorders. J Autism Dev Disord. 2003;33:631–642. doi: 10.1023/b:jadd.0000006000.38991.a7. [DOI] [PubMed] [Google Scholar]
- Rohe T, Noppeney U. Cortical hierarchies perform bayesian causal inference in multisensory perception. PLoS Biol. 2015;13:e1002073. doi: 10.1371/journal.pbio.1002073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ropar D, Mitchell P. Susceptibility to illusions and performance on visuospatial tasks in individuals with autism. Journal of child psychology and psychiatry, and allied disciplines. 2001;42:539–549. [PubMed] [Google Scholar]
- Rosenhall U, Nordin V, Brantberg K, Gillberg C. Autism and auditory brain stem responses. Ear and hearing. 2003;24:206–214. doi: 10.1097/01.AUD.0000069326.11466.7E. [DOI] [PubMed] [Google Scholar]
- Ross LA, Molholm S, Blanco D, Gomez-Ramirez M, Saint-Amour D, Foxe JJ. The development of multisensory speech perception continues into the late childhood years. Eur J Neurosci. 2011;33:2329–2337. doi: 10.1111/j.1460-9568.2011.07685.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross LA, Saint-Amour D, Leavitt VM, Javitt DC, Foxe JJ. Do you see what I am saying? Exploring visual enhancement of speech comprehension in noisy environments. Cerebral Cortex. 2007;17:1147–1153. doi: 10.1093/cercor/bhl024. [DOI] [PubMed] [Google Scholar]
- Rowland BA, Stein BE. A model of the temporal dynamics of multisensory enhancement. Neurosci Biobehav Rev. 2014;41:78–84. doi: 10.1016/j.neubiorev.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royal DW, Carriere BN, Wallace MT. Spatiotemporal architecture of cortical receptive fields and its impact on multisensory interactions. Exp Brain Res. 2009;198:127–136. doi: 10.1007/s00221-009-1772-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubenstein JL, Merzenich MM. Model of autism: increased ratio of excitation/inhibition in key neural systems. Genes, brain, and behavior. 2003;2:255–267. doi: 10.1034/j.1601-183x.2003.00037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo N, Foxe JJ, Brandwein AB, Altschuler T, Gomes H, Molholm S. Multisensory processing in children with autism: high-density electrical mapping of auditory-somatosensory integration. Autism research : official journal of the International Society for Autism Research. 2010;3:253–267. doi: 10.1002/aur.152. [DOI] [PubMed] [Google Scholar]
- Russo N, Mottron L, Burack J, Jemel B. Parameters of semantic multisensory integration depend on timing and modality order among people on the autism spectrum: Evidence from event-related potentials. Neuropsychologia. 2012;50:2131–2141. doi: 10.1016/j.neuropsychologia.2012.05.003. [DOI] [PubMed] [Google Scholar]
- Russo N, Nicol T, Trommer B, Zecker S, Kraus N. Brainstem transcription of speech is disrupted in children with autism spectrum disorders. Developmental science. 2009;12:557–567. doi: 10.1111/j.1467-7687.2008.00790.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo N, Skoe E, Trommer B, Nicol T, Zecker S, Bradlow A, Kraus N. Deficient brainstem encoding of pitch in children with autism spectrum disorders. Clinical Neurophysiology. 2008;119:1720–1731. doi: 10.1016/j.clinph.2008.01.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samson F, Hyde KL, Bertone A, Soulieres I, Mendrek A, Ahad P, Mottron L, Zeffiro TA. Atypical processing of auditory temporal complexity in autistics. Neuropsychologia. 2011;49:546–555. doi: 10.1016/j.neuropsychologia.2010.12.033. [DOI] [PubMed] [Google Scholar]
- Samson F, Mottron L, Soulières I, Zeffiro TA. Enhanced visual functioning in autism: An ALE meta - analysis. Human brain mapping. 2012;33:1553–1581. doi: 10.1002/hbm.21307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandin S, Lichtenstein P, Kuja-Halkola R, Larsson H, Hultman CM, Reichenberg A. The familial risk of autism. JAMA : the journal of the American Medical Association. 2014;311:1770–1777. doi: 10.1001/jama.2014.4144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos C, Sanz-Pamplona R, Nadal E, Grasselli J, Pernas S, Dienstmann R, Moreno V, Tabernero J, Salazar R. Intrinsic cancer subtypes-next steps into personalized medicine. Cellular oncology. 2015;38:3–16. doi: 10.1007/s13402-014-0203-7. [DOI] [PubMed] [Google Scholar]
- Sarko DK, Nidiffer AR, Powers IA, Ghose D, Hillock-Dunn A, Fister MC, Krueger J, Wallace MT. Spatial and Temporal Features of Multisensory Processes: Bridging Animal and Human Studies. In: Murray MM, Wallace MT, editors. The Neural Bases of Multisensory Processes. Boca Raton (FL): 2012. [PubMed] [Google Scholar]
- Schauder KB, Mash LE, Bryant LK, Cascio CJ. Interoceptive ability and body awareness in autism spectrum disorder. Journal of experimental child psychology. 2015a;131:193–200. doi: 10.1016/j.jecp.2014.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauder KB, Muller CL, Veenstra-VanderWeele J, Cascio CJ. Genetic Variation in Serotonin Transporter Modulates Tactile Hyperresponsiveness in ASD. Research in autism spectrum disorders. 2015b;10:93–100. doi: 10.1016/j.rasd.2014.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherf KS, Elbich D, Minshew N, Behrmann M. Individual differences in symptom severity and behavior predict neural activation during face processing in adolescents with autism. NeuroImage. Clinical. 2015;7:53–67. doi: 10.1016/j.nicl.2014.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schofield AJ. What does second-order vision see in an image? Perception. 2000;29:1071–1086. doi: 10.1068/p2913. [DOI] [PubMed] [Google Scholar]
- Schroter H, Ulich R, Miller J. Effects of redundant auditory stimuli on reaction time. Psychon Bull Rev. 2007;14:39–44. doi: 10.3758/bf03194025. [DOI] [PubMed] [Google Scholar]
- Schwarzkopf DS, Anderson EJ, de Haas B, White SJ, Rees G. Larger extrastriate population receptive fields in autism spectrum disorders. J Neurosci. 2014;34:2713–2724. doi: 10.1523/JNEUROSCI.4416-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seilheimer RL, Rosenberg A, Angelaki DE. Models and processes of multisensory cue combination. Curr Opin Neurobiol. 2014;25:38–46. doi: 10.1016/j.conb.2013.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senkowski D, Saint-Amour D, Hofle M, Foxe JJ. Multisensory interactions in early evoked brain activity follow the principle of inverse effectiveness. Neuroimage. 2011;56:2200–2208. doi: 10.1016/j.neuroimage.2011.03.075. [DOI] [PubMed] [Google Scholar]
- Senkowski D, Talsma D, Grigutsch M, Herrmann CS, Woldorff MG. Good times for multisensory integration: Effects of the precision of temporal synchrony as revealed by gamma-band oscillations. Neuropsychologia. 2007;45:561–571. doi: 10.1016/j.neuropsychologia.2006.01.013. [DOI] [PubMed] [Google Scholar]
- Shah A, Frith U. An islet of ability in autistic children: a research note. Journal of child psychology and psychiatry, and allied disciplines. 1983;24:613–620. doi: 10.1111/j.1469-7610.1983.tb00137.x. [DOI] [PubMed] [Google Scholar]
- Shah A, Frith U. Why do autistic individuals show superior performance on the block design task? Journal of Child Psychology and Psychiatry. 1993;34:1351–1364. doi: 10.1111/j.1469-7610.1993.tb02095.x. [DOI] [PubMed] [Google Scholar]
- Shams L. Early Integration and Bayesian Causal Inference in Multisensory Perception. In: Murray MM, Wallace MT, editors. The Neural Bases of Multisensory Processes. Boca Raton (FL): 2012. [PubMed] [Google Scholar]
- Shams L, Kamitani Y, Shimojo S. Illusions. What you see is what you hear. Nature. 2000;408:788. doi: 10.1038/35048669. [DOI] [PubMed] [Google Scholar]
- Sharda M, Midha R, Malik S, Mukerji S, Singh NC. Fronto-Temporal Connectivity is Preserved During Sung but Not Spoken Word Listening, Across the Autism Spectrum. Autism research : official journal of the International Society for Autism Research. 2014 doi: 10.1002/aur.1437. [DOI] [PubMed] [Google Scholar]
- Shulman GL, McAvoy MP, Cowan MC, Astafiev SV, Tansy AP, d'Avossa G, Corbetta M. Quantitative analysis of attention and detection signals during visual search. J Neurophysiol. 2003;90:3384–3397. doi: 10.1152/jn.00343.2003. [DOI] [PubMed] [Google Scholar]
- Siemann JK, Muller CL, Bamberger G, Allison JD, Veenstra-VanderWeele J, Wallace MT. A novel behavioral paradigm to assess multisensory processing in mice. Frontiers in behavioral neuroscience. 2014;8:456. doi: 10.3389/fnbeh.2014.00456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons DR, Robertson AE, McKay LS, Toal E, McAleer P, Pollick FE. Vision in autism spectrum disorders. Vision Res. 2009;49:2705–2739. doi: 10.1016/j.visres.2009.08.005. [DOI] [PubMed] [Google Scholar]
- Sinha P, Kjelgaard MM, Gandhi TK, Tsourides K, Cardinaux AL, Pantazis D, Diamond SP, Held RM. Autism as a disorder of prediction. Proc Natl Acad Sci U S A. 2014;111:15220–15225. doi: 10.1073/pnas.1416797111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavik BA, Kitsuwa-Lowe J, Danner PT, Green J, Ayres AJ. Vestibular stimulation and eye contact in autistic children. Neuropediatrics. 1984;15:33–36. doi: 10.1055/s-2008-1052337. [DOI] [PubMed] [Google Scholar]
- Smoot Reinert S, Jackson K, Bigelow K. Using Posturography to Examine the Immediate Effects of Vestibular Therapy for Children with Autism Spectrum Disorders: A Feasibility Study. Physical & occupational therapy in pediatrics. 2014 doi: 10.3109/01942638.2014.975313. [DOI] [PubMed] [Google Scholar]
- Snijders TM, Milivojevic B, Kemner C. Atypical excitation-inhibition balance in autism captured by the gamma response to contextual modulation. NeuroImage. Clinical. 2013;3:65–72. doi: 10.1016/j.nicl.2013.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer J, O'Brien J, Riggs K, Braddick O, Atkinson J, Wattam-Bell J. Motion processing in autism: evidence for a dorsal stream deficiency. Neuroreport. 2000;11:2765–2767. doi: 10.1097/00001756-200008210-00031. [DOI] [PubMed] [Google Scholar]
- Sperdin HF, Cappe C, Foxe JJ, Murray MM. Early, low-level auditory-somatosensory multisensory interactions impact reaction time speed. Front Integr Neurosci. 2009;3:2. doi: 10.3389/neuro.07.002.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein BE, Meredith MA. The Merging of the Senses. 1993:224. [Google Scholar]
- Stein BE, Stanford TR, Rowland BA. Development of multisensory integration from the perspective of the individual neuron. Nat Rev Neurosci. 2014;15:520–535. doi: 10.1038/nrn3742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson RA, Altieri NA, Kim S, Pisoni DB, James TW. Neural processing of asynchronous audiovisual speech perception. Neuroimage. 2010;49:3308–3318. doi: 10.1016/j.neuroimage.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson RA, Fister JK, Barnett ZP, Nidiffer AR, Wallace MT. Interactions between the spatial and temporal stimulus factors that influence multisensory integration in human performance. Experimental Brain Research. 2012;219:121–137. doi: 10.1007/s00221-012-3072-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson RA, Geoghegan ML, James TW. Superadditive BOLD activation in superior temporal sulcus with threshold non-speech objects. Experimental Brain Research. 2007;179:85–95. doi: 10.1007/s00221-006-0770-6. [DOI] [PubMed] [Google Scholar]
- Stevenson RA, Ghose D, Fister JK, Sarko DK, Altieri NA, Nidiffer AR, Kurela LR, Siemann JK, James TW, Wallace MT. Identifying and Quantifying Multisensory Integration: A Tutorial Review. Brain Topography. 2014a doi: 10.1007/s10548-014-0365-7. [DOI] [PubMed] [Google Scholar]
- Stevenson RA, James TW. Audiovisual integration in human superior temporal sulcus: Inverse effectiveness and the neural processing of speech and object recognition. NeuroImage. 2009;44:1210–1223. doi: 10.1016/j.neuroimage.2008.09.034. [DOI] [PubMed] [Google Scholar]
- Stevenson RA, Nelms CE, Baum SH, Zurkovsky L, Barense MD, Newhouse PA, Wallace MT. Deficits in audiovisual speech perception in normal aging emerge at the level of whole-word recognition. Neurobiology of aging. 2015;36:283–291. doi: 10.1016/j.neurobiolaging.2014.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson RA, Segers M, Ferber S, Barense MD, Wallace MT. The impact of multisensory integration deficits on speech perception in children with autism spectrum disorders. Front Psychol. 2014b;5:379. doi: 10.3389/fpsyg.2014.00379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson RA, Siemann JK, Schneider BC, Eberly HE, Woynaroski TG, Camarata SM, Wallace MT. Multisensory temporal integration in autism spectrum disorders. J Neurosci. 2014c;34:691–697. doi: 10.1523/JNEUROSCI.3615-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson RA, Siemann JK, Woynaroski TG, Schneider BC, Eberly HE, Camarata SM, Wallace MT. Brief report: Arrested development of audiovisual speech perception in autism spectrum disorders. J Autism Dev Disord. 2014d;44:1470–1477. doi: 10.1007/s10803-013-1992-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson RA, Siemann JK, Woynaroski TG, Schneider BC, Eberly HE, Camarata SM, Wallace MT. Evidence for Diminished Multisensory Integration in Autism Spectrum Disorders. J Autism Dev Disord. 2014e doi: 10.1007/s10803-014-2179-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson RA, Wallace MT. Multisensory temporal integration: task and stimulus dependencies. Experimental brain research. Experimentelle Hirnforschung. Experimentation cerebrale. 2013 doi: 10.1007/s00221-013-3507-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson RA, Wilson MM, Powers AR, Wallace MT. The effects of visual training on multisensory temporal processing. Experimental brain research. Experimentelle Hirnforschung. 2013 doi: 10.1007/s00221-012-3387-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumby WH, Pollack I. Visual contribution to speech intelligibility in noise. Journal of the Acoustical Society of America. 1954;26:212–215. [Google Scholar]
- Sutcliffe JS, Delahanty RJ, Prasad HC, McCauley JL, Han Q, Jiang L, Li C, Folstein SE, Blakely RD. Allelic heterogeneity at the serotonin transporter locus (SLC6A4) confers susceptibility to autism and rigid-compulsive behaviors. American journal of human genetics. 2005;77:265–279. doi: 10.1086/432648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talay-Ongan A, Wood K. Unusual sensory sensitivities in autism: a possible crossroads. International Journal of Disability and Developmental Education. 2000;47:201–212. [Google Scholar]
- Teder-Salejarvi WA, Di Russo F, McDonald JJ, Hillyard SA. Effects of spatial congruity on audio-visual multimodal integration. J Cogn Neurosci. 2005;17:1396–1409. doi: 10.1162/0898929054985383. [DOI] [PubMed] [Google Scholar]
- Todd J. Reaction to multiple stimuli. Science Press; New York: 1912. [Google Scholar]
- Tomchek SD, Dunn W. Sensory processing in children with and without autism: a comparative study using the short sensory profile. The American journal of occupational therapy : official publication of the American Occupational Therapy Association. 2007;61:190–200. doi: 10.5014/ajot.61.2.190. [DOI] [PubMed] [Google Scholar]
- Ungerleider LG, Haxby JV. ‘What’ and ‘where’ in the human brain. Curr Opin Neurobiol. 1994;4:157–165. doi: 10.1016/0959-4388(94)90066-3. [DOI] [PubMed] [Google Scholar]
- van Boxtel JJ, Lu H. A predictive coding perspective on autism spectrum disorders. Front Psychol. 2013;4:19. doi: 10.3389/fpsyg.2013.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van de Cruys S, Evers K, Van der Hallen R, Van Eylen L, Boets B, de-Wit L, Wagemans J. Precise minds in uncertain worlds: predictive coding in autism. Psychological review. 2014;121:649–675. doi: 10.1037/a0037665. [DOI] [PubMed] [Google Scholar]
- van Eijk RLJ, Kohlrauch A, Juola JF, Van De Par S. Audiovisual synchrony and temporal order judgments: Effects of exerpimental method and stimulus type. Perception & psychophysics. 2008;70:955–968. doi: 10.3758/pp.70.6.955. [DOI] [PubMed] [Google Scholar]
- van Veluw SJ, Chance SA. Differentiating between self and others: an ALE meta-analysis of fMRI studies of self-recognition and theory of mind. Brain imaging and behavior. 2014;8:24–38. doi: 10.1007/s11682-013-9266-8. [DOI] [PubMed] [Google Scholar]
- Vandenbroucke MW, Scholte HS, van Engeland H, Lamme VA, Kemner C. Coherent versus component motion perception in autism spectrum disorder. J Autism Dev Disord. 2008;38:941–949. doi: 10.1007/s10803-007-0467-0. [DOI] [PubMed] [Google Scholar]
- Varela F, Lachaux JP, Rodriguez E, Martinerie J. The brainweb: phase synchronization and large-scale integration. Nat Rev Neurosci. 2001;2:229–239. doi: 10.1038/35067550. [DOI] [PubMed] [Google Scholar]
- Veatch OJ, Veenstra-Vanderweele J, Potter M, Pericak-Vance MA, Haines JL. Genetically meaningful phenotypic subgroups in autism spectrum disorders. Genes, brain, and behavior. 2014;13:276–285. doi: 10.1111/gbb.12117. [DOI] [PubMed] [Google Scholar]
- Veenstra-VanderWeele J, Muller CL, Iwamoto H, Sauer JE, Owens WA, Shah CR, Cohen J, Mannangatti P, Jessen T, Thompson BJ, Ye R, Kerr TM, Carneiro AM, Crawley JN, Sanders-Bush E, McMahon DG, Ramamoorthy S, Daws LC, Sutcliffe JS, Blakely RD. Autism gene variant causes hyperserotonemia, serotonin receptor hypersensitivity, social impairment and repetitive behavior. Proc Natl Acad Sci U S A. 2012;109:5469–5474. doi: 10.1073/pnas.1112345109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlamings PH, Stauder JE, van Son IA, Mottron L. Atypical visual orienting to gaze- and arrow-cues in adults with high functioning autism. J Autism Dev Disord. 2005;35:267–277. doi: 10.1007/s10803-005-3289-y. [DOI] [PubMed] [Google Scholar]
- Vlamings PHJM, Jonkman LM, van Daalen E, van der Gaag RJ, Kemner C. Basic abnormalities in visual processing affect face processing at an early age in autism spectrum disorder. Biological psychiatry. 2010;68:1107–1113. doi: 10.1016/j.biopsych.2010.06.024. [DOI] [PubMed] [Google Scholar]
- Wada M, Suzuki M, Takaki A, Miyao M, Spence C, Kansaku K. Spatio-temporal processing of tactile stimuli in autistic children. Scientific reports. 2014;4:5985. doi: 10.1038/srep05985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace MT, Meredith MA, Stein BE. Multisensory integration in the superior colliculus of the alert cat. J Neurophysiol. 1998;80:1006–1010. doi: 10.1152/jn.1998.80.2.1006. [DOI] [PubMed] [Google Scholar]
- Wallace MT, Stein BE. Early experience determines how the senses will interact. J Neurophysiol. 2007;97:921–926. doi: 10.1152/jn.00497.2006. [DOI] [PubMed] [Google Scholar]
- Wallace MT, Stevenson RA. The construct of the multisensory temporal binding window and its dysregulation in developmental disabilities. Neuropsychologia. 2014 doi: 10.1016/j.neuropsychologia.2014.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace MT, Wilkinson LK, Stein BE. Representation and integration of multiple sensory inputs in primate superior colliculus. J Neurophysiol. 1996;76:1246–1266. doi: 10.1152/jn.1996.76.2.1246. [DOI] [PubMed] [Google Scholar]
- Wang AT, Dapretto M, Hariri AR, Sigman M, Bookheimer SY. Neural correlates of facial affect processing in children and adolescents with autism spectrum disorder. Journal of the American Academy of Child & Adolescent Psychiatry. 2004;43:481–490. doi: 10.1097/00004583-200404000-00015. [DOI] [PubMed] [Google Scholar]
- Wang AT, Lee SS, Sigman M, Dapretto M. Neural basis of irony comprehension in children with autism: the role of prosody and context. Brain. 2006;129:932–943. doi: 10.1093/brain/awl032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watling RL, Deitz J, White O. Comparison of Sensory Profile scores of young children with and without autism spectrum disorders. The American journal of occupational therapy : official publication of the American Occupational Therapy Association. 2001;55:416–423. doi: 10.5014/ajot.55.4.416. [DOI] [PubMed] [Google Scholar]
- Wible CG. Hippocampal temporal-parietal junction interaction in the production of psychotic symptoms: a framework for understanding the schizophrenic syndrome. Front Hum Neurosci. 2012;6:180. doi: 10.3389/fnhum.2012.00180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiggins JL, Peltier SJ, Bedoyan JK, Carrasco M, Welsh RC, Martin DM, Lord C, Monk CS. The impact of serotonin transporter genotype on default network connectivity in children and adolescents with autism spectrum disorders. NeuroImage. Clinical. 2012;2:17–24. doi: 10.1016/j.nicl.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiggins JL, Swartz JR, Martin DM, Lord C, Monk CS. Serotonin transporter genotype impacts amygdala habituation in youth with autism spectrum disorders. Soc Cogn Affect Neurosci. 2014;9:832–838. doi: 10.1093/scan/nst039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J, Massaro DW, Peel NJ, Bosseler A, Suddendorf T. Visual-auditory integration during speech imitation in autism. Research in developmental disabilities. 2004;25:559–575. doi: 10.1016/j.ridd.2004.01.008. [DOI] [PubMed] [Google Scholar]
- Wing L, Potter D. The epidemiology of autistic spectrum disorders: is the prevalence rising? Mental retardation and developmental disabilities research reviews. 2002;8:151–161. doi: 10.1002/mrdd.10029. [DOI] [PubMed] [Google Scholar]
- Witkin H, Oltman P, Raskin E, Karp S. A manual for the embedded figures test. Consulting Psychologists Press; Palo Alto, CA: 1971. [Google Scholar]
- Wohr M, Scattoni ML. Behavioural methods used in rodent models of autism spectrum disorders: current standards and new developments. Behav Brain Res. 2013;251:5–17. doi: 10.1016/j.bbr.2013.05.047. [DOI] [PubMed] [Google Scholar]
- Woynaroski TG, Kwakye LD, Foss-Feig JH, Stevenson RA, Stone WL, Wallace MT. Multisensory Speech Perception in Children with Autism Spectrum Disorders. J Autism Dev Disord. 2013 doi: 10.1007/s10803-013-1836-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaidel A, Goin-Kochel RP, Angelaki DE. Self-motion perception in autism is compromised by visual noise but integrated optimally across multiple senses. Proc Natl Acad Sci U S A. 2015;112:6461–6466. doi: 10.1073/pnas.1506582112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaltz Y, Roth DA, Kishon-Rabin L. Does feedback matter in an auditory frequency discrimination learning task? Journal of basic and clinical physiology and pharmacology. 2010;21:241–254. doi: 10.1515/jbcpp.2010.21.3.241. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Huang C, Xu P, Tao L, Qiu Z, Li X, Lu ZL. Perceptual learning improves contrast sensitivity and visual acuity in adults with anisometropic amblyopia. Vision Res. 2006;46:739–750. doi: 10.1016/j.visres.2005.07.031. [DOI] [PubMed] [Google Scholar]
- Zilbovicius M, Meresse I, Chabane N, Brunelle F, Samson Y, Boddaert N. Autism, the superior temporal sulcus and social perception. Trends in neurosciences. 2006;29:359–366. doi: 10.1016/j.tins.2006.06.004. [DOI] [PubMed] [Google Scholar]