Abstract
To optimize drug candidates, modern medicinal chemists are increasingly turning to an unconventional structural motif: small, strained ring systems. However, the difficulty of introducing substituents such as bicyclo[1.1.1]pentanes, azetidines, or cyclobutanes often outweighs the challenge of synthesizing the parent scaffold itself. Thus, there is an urgent need for general methods to rapidly and directly append such groups onto core scaffolds. Here we report a general strategy to harness the embedded potential energy of effectively spring-loaded C–C and C–N bonds with the most oft-encountered nucleophiles in pharmaceutical chemistry, amines. Strain release amination can diversify a range of substrates with a multitude of desirable bioisosteres at both the early and late-stages of a synthesis. The technique has also been applied to peptide labeling and bioconjugation.
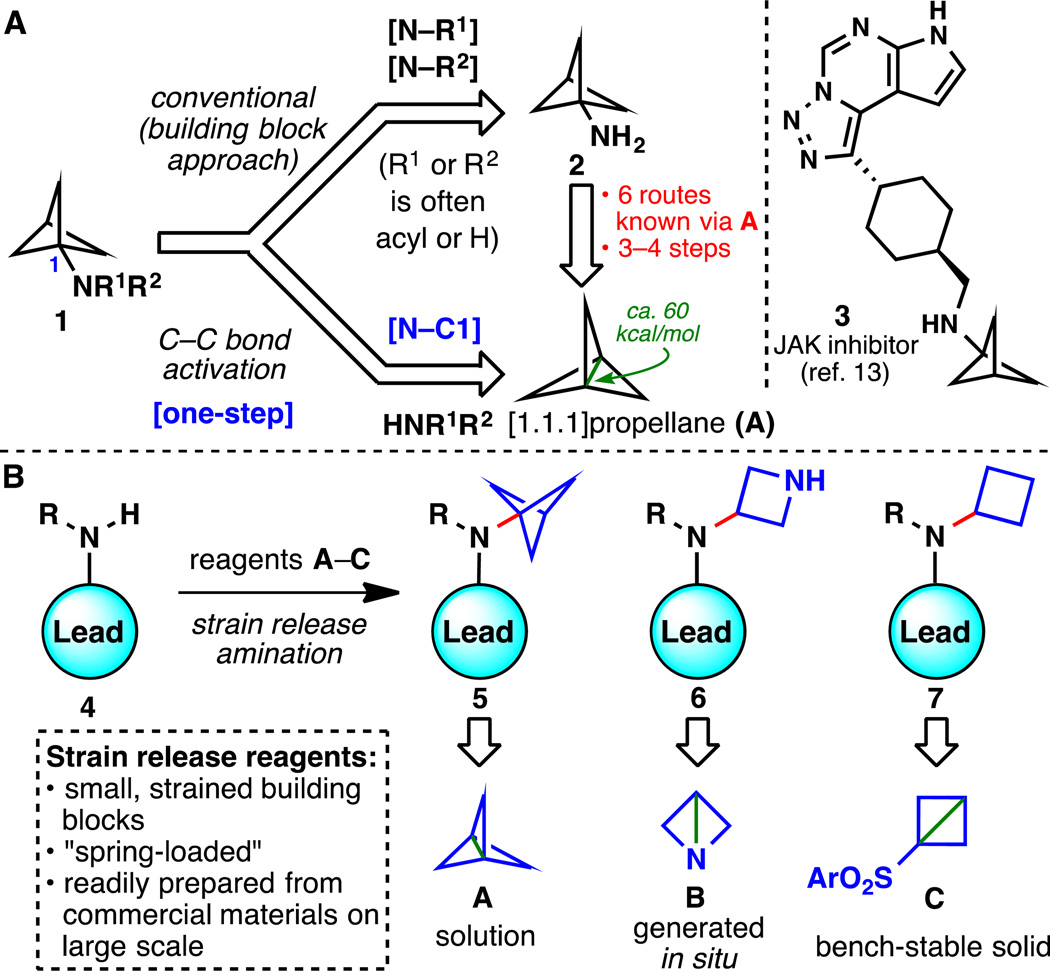
Systematic structural tuning of drug candidates, or leads, is an essential feature of medicinal chemistry research. Exchanging substituents that exhibit similar yet distinct properties in biological environments, termed bioisosteres, can address a myriad of structural liabilities, circumventing issues such as unwanted metabolic clearance. Such structures also serve to combat the continued challenge of narrowing intellectual property (IP) space (1). These motifs can be rather unusual in that they are often not found in natural products: fluoroalkyl groups (2, 3) and strained ring systems including small spirocycles and bicycles are examples (4). Interest in the latter area was fueled by an ongoing program at Pfizer (5), where difficulties in the synthesis of bicyclo[1.1.1]pentan-1-amine (2, Fig. 1A) led to the abandonment of a lead oncology clinical candidate. (6). Developments in the synthesis of this strained motif date back to 1970 with Wiberg's classic synthesis of 2 from bicyclo[1.1.1]pentane in 4 steps via the intermediacy of bicyclo[1.1.1]pentan-1-carboxylic acid (see Fig S1) (7). Although this pioneering work allowed synthetic access to 2 and subsequent studies pointed to the counterintuitive stability of A, many improvements were carried out over the ensuing 45 years (8–10). All of these reports required ≥3 steps to form amine 2 due to the need for multiple functional group interconversions, rendering Pfizer’s current in-house approach unsustainable (10). More globally, conventional preparations of substituted bicyclo[1.1.1]pentan-1-amine 1 have required the synthesis of the parent amine 2, followed by amide formation (11) or substitution chemistry (12), limiting the retrosynthetic analysis of lead compounds such as 3 (13). The goal of this work was to solve both of these issues by i) the invention of a process-friendly synthesis of amine 2; and ii) development of a route to 1 that does not even require the intermediacy of 2, bypassing conventional retrosynthetic logic. Our strategy to address these challenges was to embrace the innate reactivity of the most strained C–C bond (ca. 60 kcal/mol are stored in this bond) (14) present in propellane A by engaging it directly with an amine. This concept was then extended to other systems containing “spring-loaded” bonds (15, 16) as a general tool to append small, cyclic bioisoteric motifs (4 → 5–7, Fig. 1B). We describe the preparation and use of convenient “strain release reagents” (such as A–C) to expand chemical space for drug discovery: specifically, the introduction of motifs such as bicyclo[1.1.1.]pentanes, azetidines and cyclobutanes is delineated. The exquisite chemoselectivity of this approach has also been established and initial applications to the areas of peptide labeling and bioconjugation are reported (vide infra). This work lays a foundation for the synthesis of new chemical entities, probe molecules, and click-like connections that rely upon the native activation of strained C–C bonds.
Fig. 1. Synthetic methods for incorporating small, strained ring systems.
(A) Revisiting the retrosynthetic disconnection of an important scaffold in medicinal chemistry, bicyclo[1.1.1]pentan-1-amine. (B) Strain release amination: any-stage functionalization of lead compounds in drug discovery.
Application of propellane strain-release amination
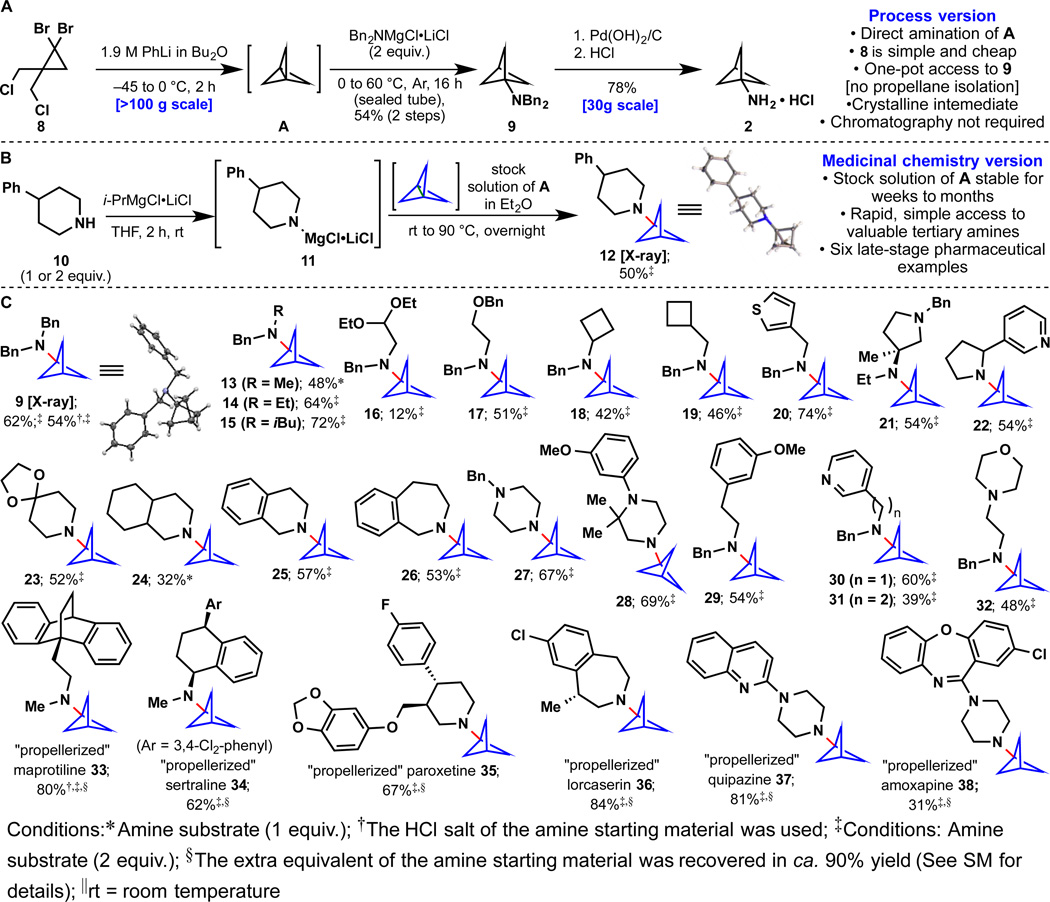
As mentioned above, efforts in this area were initiated due to the practical difficulties encountered at Pfizer for procuring large quantities of bicyclo[1.1.1]pentan-1-amine 2 (Fig. 2A). The tendency of propellane A to react with strong nucleophiles such as t-BuLi and aryl Grignard reagents inspired our approach (17, 18). Extensive exploration (see Table S1) identified Davies-type amine nucleophiles (Bn2N-Li) (19) as a good starting point, furnishing 9 in ca. 20% yield. The key breakthrough was the finding that the corresponding Turbo-amide (20) (Bn2NMgCl•LiCl) led to clean formation of 9 even on >100 gram scale. The use of PhLi leads to reproducible, scalable, and clean formation of propellane A. The dibenzyl group was then easily removed and the HCl salt of 2 was precipitated (30 gram scale). This protocol was successfully scaled up at an outsourcing vendor and can now be used in a process setting to deliver bicyclo[1.1.1]pentan-1-amine-containing clinical candidates economically on scale.
Fig. 2. “Propellerization” of amine-containing substrates. Isolated yields are reported.
(A) An improved synthesis of the known bicyclo[1.1.1]pentan-1-amine. (B) A general “propellerization” of amines enabled by strain release reagents. (C) Substrate scope of amine-containing substrates.
With a reliable route to stock solutions of propellane A (after co-distillation with Et2O, solution is stable for weeks to months at −20 °C or −78 °C, respectively), the scope of this direct "propellerization" was explored (Fig. 2B). Strain release amination of A using a variety of in situ derived Turbo amides delivered a wide range of tertiary amines containing the valuable bicyclo[1.1.1]pentane bioisosteric motif. Figure 2 illustrates twenty-nine different amines varying in complexity that can be easily accessed. In cases when the reaction did not go to completion the starting amine could be recovered (e.g. 16, 24, 38). The method tolerates a variety of functional groups including acetals (16), benzyl ethers (17), ketals (23), and Lewis-basic groups (21, 22, 27, 28, 30–32, 37, 38). Late-stage incorporation of these challenging bioisosteres onto six different commercial drugs (Fig. 2C, 33–38) obviated otherwise laborious multi-step sequences to access these analogs. The use of turbo amides is key to enabling the “any-stage” functionalization of both simple and complex amines with A. We anticipate that the path to these bioisosteres will find immediate and widespread use in medicinal chemistry. Indeed, this chemistry has already been field-tested at Pfizer (for example, compounds 14, 15, 17, 19, 21, and 30 were prepared at Pfizer for use in ongoing programs).
Introduction of azetidine via strain-release
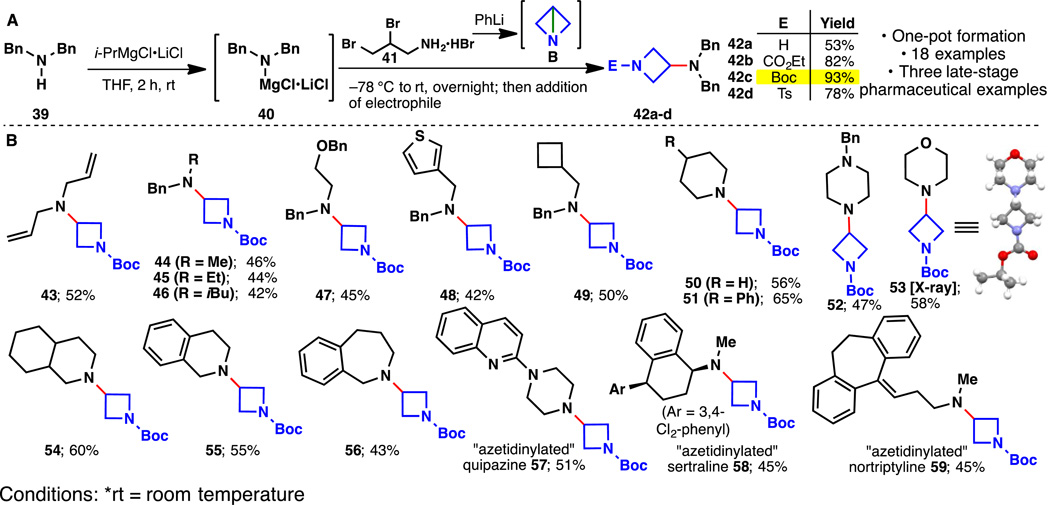
The documented use of azetidines as a tactic to both rigidify amine backbones and serve as phenyl bioisosteres inspired the evaluation of a similar approach (1, 20). Like the propellane systems, access to amino-azetidines is largely limited to a building-block approach that relies on the multistep synthesis of protected azetidinones (21). Strain-release amination of B was therefore evaluated as a means to simplify the preparation of such compounds. Isolated examples of the addition of nucleophiles to B are known but require superstoichiometric amounts of Lewis acids and only work with dibenzyl amine, anilines and thiols (22). As depicted in Fig. 3, the addition of in situ generated Turbo-amides to a solution of in situ generated B leads cleanly to azetidinylated products (42–59) that are subsequently trapped with a variety of acylating agents to simplify isolation and handling (free azetidines can be generated if desired). Using this protocol, azetidines were directly appended to eighteen different amines varying in complexity, including three pharmaceutical agents.
Fig. 3. “Azetidinylation” of amine-containing substrates.
(A) A general “azetidinylation” of amines enabled by strain release reagents. (B) Scope of amine-containing substrates.
Introduction of cyclobutane via strain-release
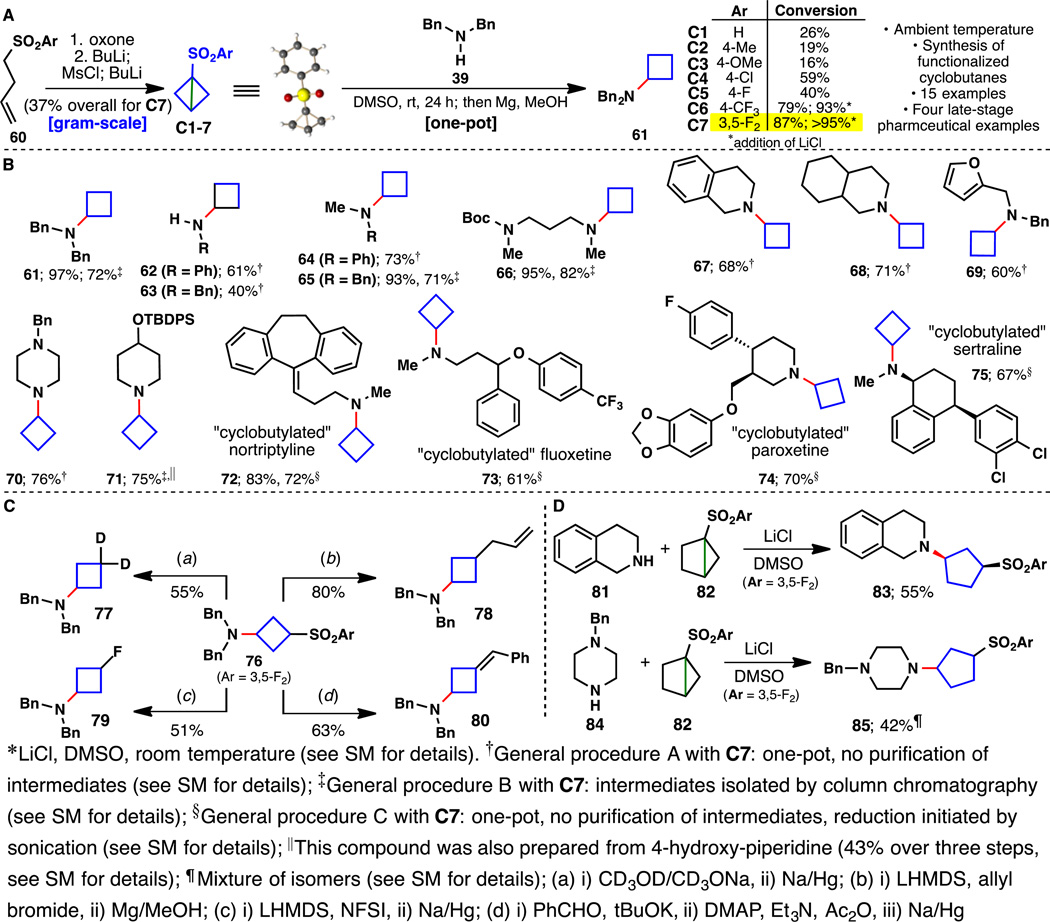
Given the variety of medicinal contexts in which cyclobutane derivatives have been enlisted (23), we next explored a strain release approach for this motif. The goal was to generate a stable reagent that would enable both rapid and mild "cyclobutylation" of amines but also permit further functionalization of intermediate adducts. Bicyclobutane and its substituted derivatives, since their first preparation in 1959 (24), have been the subject of many synthetic studies, the majority of which either engaged the strained system as a nucleophile or cleaved the center bond via a transition metal-mediated process (25, 26). Rather than pursuing the parent bicyclobutane (a gas at room temperature) (27), we appended an arylsulfonyl group as a means to both activate the strained C–C bond and render the reagent bench stable. Encouragingly, a few examples have been reported wherein benzylamine, when employed as solvent, could be added to phenylsulfonyl-substituted bicyclobutanes at 140 °C (28, 29). In seeking a reagent that would allow for more mild reaction conditions and the use of the amine as a limiting reagent, we synthesized a variety of substituted phenylsulfonylated bicyclobutanes (C2-C7, Fig. 4) and evaluated them in a strain release amination with amine 39. Not surprisingly, arylsulfones containing electron-withdrawing substituents were the most reactive and the addition of LiCl further accelerated the amination. Removal of the arylsulfonyl group could be easily achieved in the same pot using mild reductive conditions (Mg, MeOH). This protocol was applied to sixteen diverse amines using reagent C7, including four commercial drugs, to append the cyclobutyl group (Fig. 4B). The reaction of C7 is chemoselective for amines in the presence of free hydroxyl groups; 71 could be prepared from 4-hydroxypiperidine in 43% yield over three steps (see SM for details). The arylsulfonyl group could also be used as a handle to generate other useful cyclobutane building blocks containing deuterium (77), alkyl (78), fluorine (79), and olefin (80) substitutents. Strain release amination is not limited to the three ring systems described here as illustrated in Fig. 4D wherein cyclopentane (30) could be easily appended to 1,2,3,4-tetrahydroisoquinoline (81 → 83) and N-benzylpiperazine (84 → 85). Given these collective findings, we anticipate that a wide range of strained C–C bonds will be amenable to amination and further functionalization.
Fig. 4. Cyclobutylation of amine-containing substrates.
(A) A general cyclobutylation of amines with C7 enabled by strain release reagents. (B) Substrate scope of amine-containing substrates. (C) Diversification of intermediate cyclobutylsulfone 76. (D) Installation of cyclopentane onto primary and secondary amines by strain release amination.
Applications to peptide labeling
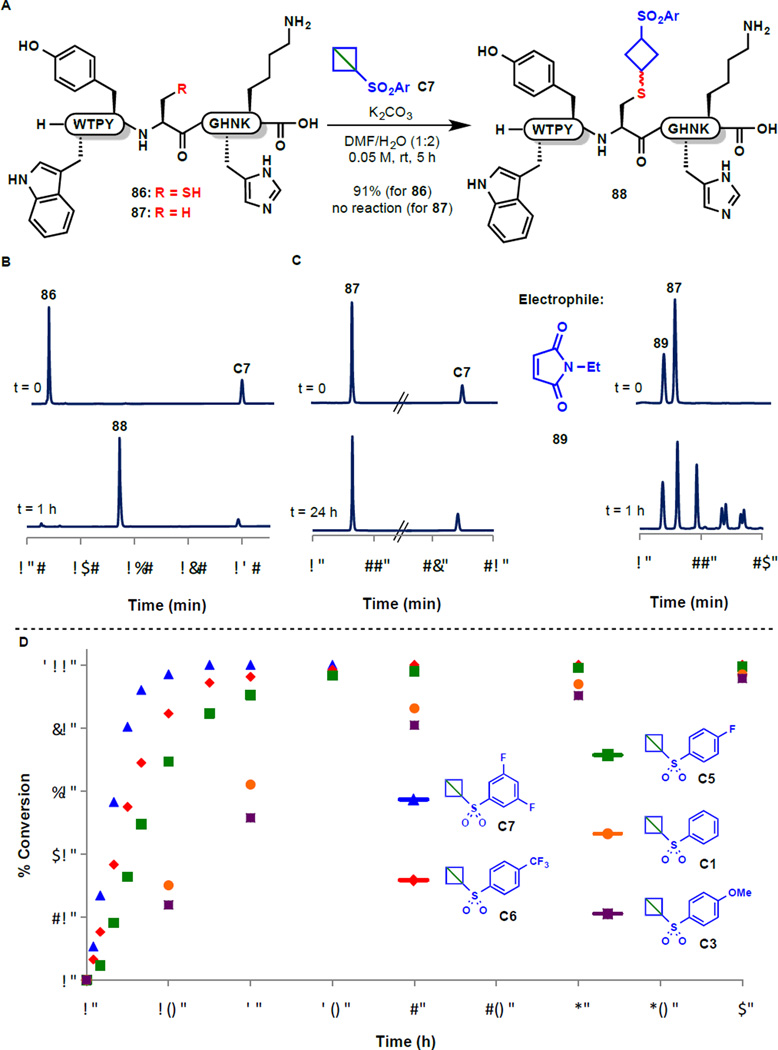
The "spring-loaded" electrophiles described herein exhibit a broad substrate scope for amination and inspired exploration of this platform in a more biologically relevant context. A model peptide (86, Fig. 5) was therefore prepared containing an assortment of proteinogenic nucleophilic functional groups and exposed to strain release reagent C7 in a mixed organic/aqueous solvent system. Remarkably, complete selectivity was observed for labeling of the cysteine thiol (91% isolated yield of 88 after 5 h, see HPLC trace in Fig. 5B). In the presence of cysteine-free peptide 87, no background reaction was observed (Fig. 5C) after 24 hours. In striking contrast, the commonly employed maleimide electrophile led to multiple adducts with 87 after only one hour of exposure. The complete chemoselectivity observed for cysteine bodes well for the use of strain-release functionalization in a variety of contexts such as site-selective bioconjugation (31–34) and peptide stapling (35–38). The efficient tagging of other thiols, including glutathione and cysteine methyl ester, attests to the generality of the approach (see SM for details). Further, by modifying the electronic character of the aryl sulfone group we could adjust the temporal parameters of the functionalization (Fig. 5D). This tunable click reaction may facilitate the strategic design of electrophilic covalent warheads for enzyme inhibition and activity-based protein profiling.
Fig. 5. Use of reagent C as a chemoselective cysteine tag for peptide and protein labeling.
(A) Reaction of C7 with functionalized peptides 86 and 87. (B) HPLC chromatogram depicting rapid and clean conversion of 86 to cysteine-labeled product 88 after 1 h. (C) Superior chemoselectivity of reagent C7 relative to maleimide 89 in the presence of cysteine-free peptide 87. D Reaction kinetics demonstrating the tunable functionalization of 86 with substituted arylsulfonyl bicyclobutane reagents.
Outlook
The operational simplicity, mild reaction conditions, inexpensive preparation, and chemoselectivity exhibited by strain release reagents A-C will facilitate their rapid adoption. More globally, an enormous variety of reagents based on this concept can be envisaged. For the task of procuring a specific target, this approach to bond formation will enable practitioners to refocus on the challenge of synthesizing a molecular scaffold rather than on the difficulty posed by small ring systems. We anticipate that this approach will also enable formation of distinct connections in the materials, polymer, and bioconjugation arena.
Supplementary Material
Acknowledgments
Financial support for this work was provided by Pfizer, Inc. The National Institutes of Health provided a postdoctoral fellowship to J. M. L., the Shanghai Institute of Organic Chemistry, Zhejiang Medicine Co., and Pharmaron provided a postdoctoral fellowship to J. W., and the University of the Basque Country provided a predoctoral fellowship to L. P. We would like to thank Dr. Y. Ishihara for assistance with the initial preparation of the manuscript. We are grateful to Dr. D.-H. Huang and Dr. L. Pasternack (TSRI) for assistance with NMR spectroscopy and Professor A. L. Rheingold and Dr. C. E. Moore (UCSD) for X-ray crystallographic analysis. “Crystallographic data for compounds 9, 12, 53, C1, and S23 are available free of charge from the Cambridge Crystallographic Data Centre under accession numbers CCDC 1431179, 1438966, 1431180, 1431182, 1431183 respectively.”
Footnotes
Supplementary Materials:
Materials and Methods
Figures S1-S68
Tables S1-S5
References (39–68)
NMR Spectra
References and Notes
- 1.Meanwell NA. J. Med. Chem. 2011;54:2529–2591. doi: 10.1021/jm1013693. [DOI] [PubMed] [Google Scholar]
- 2.Ni C, Hu M, Hu J. Chem. Rev. 2015;115:765–825. doi: 10.1021/cr5002386. [DOI] [PubMed] [Google Scholar]
- 3.Fujiwara Y, et al. Nature. 2012;492:95–99. doi: 10.1038/nature11680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Westphal MV, Wolfstadter BT, Plancher JM, Gatfield J, Carreira EM. ChemMedChem. 2015;10:461–469. doi: 10.1002/cmdc.201402502. [DOI] [PubMed] [Google Scholar]
- 5.Michaudel Q, Ishihara Y, Baran PS. Acc. Chem. Res. 2015;48:712–721. doi: 10.1021/ar500424a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stepan AF, et al. J. Med. Chem. 2012;55:3414–3424. doi: 10.1021/jm300094u. [DOI] [PubMed] [Google Scholar]
- 7.Wiberg KB, Williams VZ. J. Org. Chem. 1970;35:369–373. [Google Scholar]
- 8.Levin MD, Kaszynski P, Michl J. Chem. Rev. 2000;100:169–234. doi: 10.1021/cr990094z. [DOI] [PubMed] [Google Scholar]
- 9.Wiberg KB. Chem. Rev. 1989;89:975–983. [Google Scholar]
- 10.Bunker KD, Sach NW, Huang QH, Richardson PF. Org. Lett. 2011;13:4746–4748. doi: 10.1021/ol201883z. [DOI] [PubMed] [Google Scholar]
- 11.Zehnder L, et al. J. Med. Chem. 2011;54:3368–3385. doi: 10.1021/jm200128m. [DOI] [PubMed] [Google Scholar]
- 12.Bennett BL, et al. PCT Int. Appl. WO 2012145569. 2012 [Google Scholar]
- 13.Hayashi K, et al. PCT Int. Appl. WO 2013024895. 2013 [Google Scholar]
- 14.Wiberg KB, Walker FH. J. Am. Chem. Soc. 1982;104:5239–5240. [Google Scholar]
- 15.Wiberg KB. Angew. Chem. Int. Ed. Engl. 1986;25:312–322. [Google Scholar]
- 16.Kolb HC, Finn MG, Sharpless KB. Angew. Chem. Int. Ed. 2001;40:2004. doi: 10.1002/1521-3773(20010601)40:11<2004::AID-ANIE2004>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 17.Della EW, Taylor DK, Tsanaktsidis J. Tetrahedron Lett. 1990;31:5219–5220. [Google Scholar]
- 18.Messner M, Kozhushkov SI, de Meijere A. Eur. J. Org. Chem. 2000:1137–1155. [Google Scholar]
- 19.Davies SG, Fletcher AM, Roberts PM, Thomson JE. Tetrahedron Asymmetry. 2012;23:1111–1153. [Google Scholar]
- 20.Rohbogner CJ, Closki GC, Knochel P. Angew. Chem. Int. Ed. Engl. 2008;47:1503–1507. doi: 10.1002/anie.200703382. [DOI] [PubMed] [Google Scholar]
- 21.Brandi A, Cicchi S, Cordero FM. Chem. Rev. 2008;108:3988–4035. doi: 10.1021/cr800325e. [DOI] [PubMed] [Google Scholar]
- 22.Ikee Y, et al. Chem. Pharm. Bull. 2008;56:346–356. doi: 10.1248/cpb.56.346. [DOI] [PubMed] [Google Scholar]
- 23.Wrobleski ML, et al. Bioorg. Med. Chem. Lett. 2006;16:3859–3863. doi: 10.1016/j.bmcl.2006.04.031. [DOI] [PubMed] [Google Scholar]
- 24.Wiberg KB, Ciula RP. J. Am. Chem. Soc. 1959;81:5261–5262. [Google Scholar]
- 25.Wiberg KB. Advances in Alicyclic Chemistry. Vol. 2. Academic Press; 1968. pp. 185–254. [Google Scholar]
- 26.Walczak MAA, Krainz T, Wipf P. Acc. Chem. Res. 2015;48:1149–1158. doi: 10.1021/ar500437h. [DOI] [PubMed] [Google Scholar]
- 27.Lampman GM, Aumiller JC. Org. Synth. 1971;51:55–59. [Google Scholar]
- 28.Gaoni Y. Tetrahedron Lett. 1988;29:1591–1594. [Google Scholar]
- 29.Gaoni Y. Org. Prep. Proc Intl. 1995;27:185–212. [Google Scholar]
- 30.Jeffery SM, Stirling CJM. J. Chem. Soc. Perkin Trans. 2. 1993:1617–1624. [Google Scholar]
- 31.Boutureira O, Bernardes GJL. Chem. Rev. 2015;115:2174–2195. doi: 10.1021/cr500399p. [DOI] [PubMed] [Google Scholar]
- 32.McKay CS, Finn MG. Chemistry & Biology. 2014;21:1075–1101. doi: 10.1016/j.chembiol.2014.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stephanopoulos N, Francis MB. Nat. Chem. Biol. 2011;7:876–884. doi: 10.1038/nchembio.720. [DOI] [PubMed] [Google Scholar]
- 34.Kalia J, Raines RT. Curr. Org. Chem. 2010;14:138–147. doi: 10.2174/138527210790069839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lau YH, de Andrade P, Wu Y, Spring DR. Chem. Soc. Rev. 2015;44:91–102. doi: 10.1039/c4cs00246f. [DOI] [PubMed] [Google Scholar]
- 36.Spokoyny AM, et al. J. Am. Chem. Soc. 2013;135:5946–5949. doi: 10.1021/ja400119t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang Y, Chou DH-C. Angew. Chem. Int. Ed. 2015;54:10931–10934. doi: 10.1002/anie.201503975. [DOI] [PubMed] [Google Scholar]
- 38.Brown SP, Smith AB., III J. Am. Chem. Soc. 2015;137:4034–4037. doi: 10.1021/ja512880g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Toops DS, Barbachyn MR. J. Org. Chem. 1993;58:6505–6508. [Google Scholar]
- 40.Hossain MT, Timberlake JW. J. Org. Chem. 2001;66:6282–6285. doi: 10.1021/jo010212u. [DOI] [PubMed] [Google Scholar]
- 41.Goh YL, et al. Org. Lett. 2014;16:1884–1887. doi: 10.1021/ol500635p. [DOI] [PubMed] [Google Scholar]
- 42.Bunker KD. PCT Int. Appl. WO 201589170. 2015 [Google Scholar]
- 43.Shtarev AB, Pinkhassik E, Levin MD, Stibor I, Michl J. J. Am. Chem. Soc. 2001;123:3484–3492. doi: 10.1021/ja0000495. [DOI] [PubMed] [Google Scholar]
- 44.Eli Lilly and Company. PCT Int. Appl. WO 2006044454. 2006 [Google Scholar]
- 45.Bomann MD, Guch IC, DiMare M. J. Org. Chem. 1995;60:5995–5996. [Google Scholar]
- 46.Yang Ji Chemical Company Ltd. US Pat. Appl. 20050075345. 2005
- 47.Chen Z, Chen Z, Jiang Y, Hu W. Tetrahedron. 2005;61:1579–1586. [Google Scholar]
- 48.Bumgardner CL, Lawton EL, Carver JG. J. Org. Chem. 1972;37:407–409. [Google Scholar]
- 49.Satake K, Imai T, Kimura M, Morosawa S. Heterocycles. 1981;16:1271–1274. [Google Scholar]
- 50.Prokopcová H, et al. Chem. Eur. J. 2010;16:13063–13067. doi: 10.1002/chem.201001887. [DOI] [PubMed] [Google Scholar]
- 51.Meyers AI, Hutchings RH. Tetrahedron. 1993;49:1807–1820. [Google Scholar]
- 52.Bergbreiter DE, Osburn PL, Li C. Org. Lett. 2002;4:737–740. doi: 10.1021/ol017198s. [DOI] [PubMed] [Google Scholar]
- 53.Largeron M, Fleury M-B. Org. Lett. 2009;11:883–886. doi: 10.1021/ol802885b. [DOI] [PubMed] [Google Scholar]
- 54.Hayashi K, Kumagai T, Nagao Y. Heterocycles. 2000;53:447–452. [Google Scholar]
- 55.Barré B, et al. Org. Lett. 2014;16:6160–6163. doi: 10.1021/ol503043r. [DOI] [PubMed] [Google Scholar]
- 56.Hodgson DM, Mortimer CL, McKenna JM. Org. Lett. 2015;17:330–333. doi: 10.1021/ol503441d. [DOI] [PubMed] [Google Scholar]
- 57.Han M, Song C, Jeong N, Hahn H-G. ACS Med. Chem. Lett. 2014;5:999–1004. doi: 10.1021/ml500187a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Salituro FG, Saunders JO, Yan S. PCT Int. Appl. WO 201283246. 2012 [Google Scholar]
- 59.Pauls HW, et al. PCT Int. Appl. WO 201353051. 2013 [Google Scholar]
- 60.Gaoni Y. J. Org. Chem. 1982;47:2564–2571. [Google Scholar]
- 61.Gaoni Y, Tomazic A. J. Org. Chem. 1985;50:2948–2957. [Google Scholar]
- 62.Brown AC, Carpino LA. J. Org. Chem. 1985;50:1749–1750. [Google Scholar]
- 63.Oh HK, Kwon YB, Cho IH, Lee I. J. Chem. Soc., Perkin Trans. 2. 1994:1697–1701. [Google Scholar]
- 64.Satoh T, et al. Synthesis. 2011:397–408. [Google Scholar]
- 65.Marugan JJ, et al. J. Med. Chem. 2011;54:1033–1058. doi: 10.1021/jm1008902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cheng C, Brookhart M. J. Am. Chem. Soc. 2012;134:11304–11307. doi: 10.1021/ja304547s. [DOI] [PubMed] [Google Scholar]
- 67.Härter M, et al. US Pat. Appl. US 2013150325. 2013
- 68.Butov GM, Mokhov VM. Russ. J. Org. Chem. 2014;50:447–448. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.