Abstract
INTRODUCTION
Damage control laparotomy (DCL) is a life-saving operation used in critically ill patients; however, interval primary fascial closure remains a challenge. We hypothesized that flaccid paralysis of the lateral abdominal wall musculature induced by Botulinum Toxin A (BTX), would improve of rates of primary fascial closure, decrease duration of hospital stay (LOS), and enhance pain control.
METHODS
Consenting adults who had undergone a DCL at two institutions were prospectively randomized to receive ultrasound-guided injections of their external oblique, internal oblique, and transversus abdominus muscles with either BTX (150cc, 2units/cc) or placebo (150cc 0.9%NaCl). Patients were excluded if they had a BMI>50, remained unstable or coagulopathic, were home O2 dependent or had an existing neuromuscular disorder. Outcomes were assessed in a double-blinded manner. Univariate and Kaplan Meier estimates of cumulative probability of abdominal closure were performed.
RESULTS
We randomized 46 patients (24 BTX, 22 placebo). There were no significant differences in demographics, comorbidities, and physiological status. Injections were performed on average 1.8 ± 2.8 days after DCL (range 0-14). The 10-day cumulative probability of primary fascial closure was similar between groups: 96% for BTX (95% CI 72%-99%) and 93% for placebo (95% CI 61%-99%); HR =1.0 (95% CI 0.5-1.8). No difference between BTX and placebo groups was observed for LOS (37 vs 26 days, p=0.30) or intensive care unit stay (17 vs 11 days, p=0.27). There was no difference in median morphine equivalents following DCL. The overall complication rate was similar (63% vs 68%, p=0.69), with 2 deaths in the placebo group and 0 in the BTX group. No BTX or injection procedure complications were observed.
CONCLUSION
Use of BTX after DCL was safe but did not appear to affect primary fascial closure, LOS, or pain modulation after DCL. Given higher than expected rates of primary fascial closure, type II error may have occurred.
Keywords: Open Abdomen, Damage Control Laparotomy, Trauma, Emergency Surgery
Introduction
Damage control laparotomy (DCL) is a lifesaving maneuver used successfully in critically ill trauma and emergency general surgery patients.1,2 This technique utilizes source control of hemorrhage and sepsis via an abbreviated laparotomy with temporary abdominal closure (TAC). Subsequent resuscitation in the surgical intensive care unit using Surviving Sepsis and damage control resuscitation techniques are performed until re-exploration once stabilized.3,4 At this point, injuries are definitively repaired and abdominal fascial re-approximation is attempted.5 Unfortunately, only 43% of patients will be closed at the first attempt with decreasing rates of closure thereafter. 6 In fact, 10-50% of patients will leave the hospital with a hernia. 9,7,8
For those patients in whom fascial closure is not achieved, complications including wound infections, enterocutaneous fistulae, and intra-abdominal abscesses occur at alarmingly high rates.9,10 Additionally, extensive abdominal wall reconstruction will likely be required in the long term. The key for preventing these complications is definitive closure of the abdominal fascia. The application of midline tension from temporary abdominal closure devices such as negative pressure dressings have been shown to improve rates of primary fascial closure; despite these advances, planned ventral hernia rates remain substantial. 11,12,13,14
The Food and Drug Administration has approved the clinical use of botulinum toxin a (BTX; Botox; Allergan, Inc.; Irvine, CA) for myriad conditions including cosmetic, motor and pain disorders.15,16 As a neuromodulating agent, BTX temporarily blocks the release of peptide-rich endosomes at the pre-synaptic cholinergic nerve terminal. These endosomes primarily contain acetylcholine in addition to pain and inflammatory mediators such as calcitonin gene related peptide and substance P. When the synaptic transmission of these agents is prevented, the injected skeletal muscle becomes flaccidly paralyzed with diminished pain sensation.17,18 Injection of BTX, therefore, into the lateral abdominal wall muscles should result in reduced lateral tension. When used in combination with known techniques that provide midline tension, a theoretical synergistic effect may improve the rates of primary fascial closure after TAC. In fact, we have previously published our retrospective results in a separate patient population with encouraging results.19 We aimed to determine the actual effect of BTX on the abdominal wall after DCL. We hypothesized that patients injected with BTX would have greater rates of primary fascial closure, lesser rates of complications, and correspondingly lower pain medication requirements.
Methods
Institutional Review Board approval at Mayo Clinic (Rochester, MN) and the University of Minnesota - Regions Hospital (St. Paul, MN) was obtained to perform a randomized, double blinded, placebo controlled, multi-institutional pilot study comparing the injection of BTX versus placebo (0.9% injectable normal saline) into the lateral abdominal wall muscles of patients who underwent TAC for trauma and emergency general surgery. Given the novelty of the intervention and lack of comparative data, the study was run as a pilot study to accrue data for potential further BTX studies in open abdomen (OA) patients. The study began in November 2011 and was completed May 2014. Patients were excluded if they were younger than 18 years of age, had a body mass index greater than 50, failed to achieve hemodynamic stability prior to injection (stable/decreasing lactate, base deficit, or vasopressor use), pregnancy, prisoners, pre-existing pareses (Amyotrophic Lateral Sclerosis, myopathies, motor polyneuropathies), impaired neuromuscular transmission (Myasthenia Gravis, Lambert-Eaton Syndrome), concurrent aminoglycoside use, complicated Chronic Obstructive Pulmonary Disease (COPD; chronic oxygen dependence, chronic hypercarbia 50 mm Hg or greater or steroid dependent), known metastatic malignancy, necrotizing fasciitis of the trunk, or International Normalized Ratio greater than 1.5. Data was collected in a prospective fashion and managed using REDCap electronic data capture tools hosted at Mayo Clinic. REDCap (Research Electronic Data Capture) is a secure, web-based application designed to support data capture for research studies, providing 1) an intuitive interface for validated data entry; 2) audit trails for tracking data manipulation and export procedures; 3) automated export procedures for seamless data downloads to common statistical packages; and 4) procedures for importing data from external sources.20 Informed consent was obtained from the patient or their legal authorized representative using trained study coordinators and/or the site Principal Investigators.
A DCL protocol was followed at both institutions to ensure standardization of DCL management. The treating surgeon’s discretion was used to determine the need for DCL. The ABThera system was used (KCI, Inc. San Antonio, TX) for TAC purposes in concert with fascial retention sutures. Planned re-exploration occurred at least every 48 hours to attempt fascial re-approximation. Patients who had uncontrolled sources of sepsis or hemorrhage were returned to the operating room as dictated by their clinical course.
The primary endpoint of interest was the rate of primary fascial closure by hospital dismissal. Secondary endpoints of interest included in-hospital mortality, complications (wound infection, fascial dehiscence, enterocutaneous fistula, acute renal failure, pneumonia), ventilator days, ICU LOS, hospital LOS, and narcotic pain medication utilization.
Injection Procedure
Mayo Clinic provided the blocked randomization scheme for 6 patients to Regions Hospital. BTX was prepared at each institution. Patients randomized to the BTX group underwent injection of 150 units BTX diluted 2:1 in 300 cc of 0.9% normal saline while patients randomized to the placebo arm had 300 cc of 0.9% normal saline injected. Both the clinicians and patients were blinded to the group assigned; both injection fluids are clear. Our technique of BTX injection has been previously published.19,21 Briefly, ultrasound guidance is used to identify the lateral abdominal wall muscles (external oblique, internal oblique, and transversus abdominus) to guide an 18 gauge needle into their respective muscular planes. All three muscles are injected bilaterally (6 muscles total) with a total of 50 cc per muscle. Each muscle had 3 separate injections points; right/left subcostal; right/left anterior axillary; right/left lower quadrants to ensure adequate distribution of the injected fluid.
Statistics
As a pilot study, a power analysis was not performed. A blocked randomization code was assigned to each patient to help ensure a relatively equal distribution of patients who received BTX or placebo. The statisticians were blinded to group allocation until the point of study completion and data analysis. Associations of primary fascial closure, morbidity, mortality, and duration of hospital and ICU stay were compared using the chi-square, Fisher exact, and Wilcoxon rank sum tests. The magnitude of the association of each feature studied was evaluated with logistic regression and summarized with an odds ratio and 95% confidence interval. All tests were two-sided with p-values <0.05 considered statistically significant.
Results
A total of 155 patients underwent DCL during the study period and were screened for eligibility, of whom 46 were enrolled (24 BTX, 22 placebo; Figure 1). Six patients were injected at Regions Hospital (3 BTX, 3 placebo) and 39 (21 BTX, 19 placebo) were injected at Mayo Clinic. All patients received the intended treatment and were therefore included in analysis of the primary endpoint. As expected from randomization, no differences were noted in baseline clinical characteristics or demographics save for an elevated BMI in the placebo group (Table 1).
Figure 1.
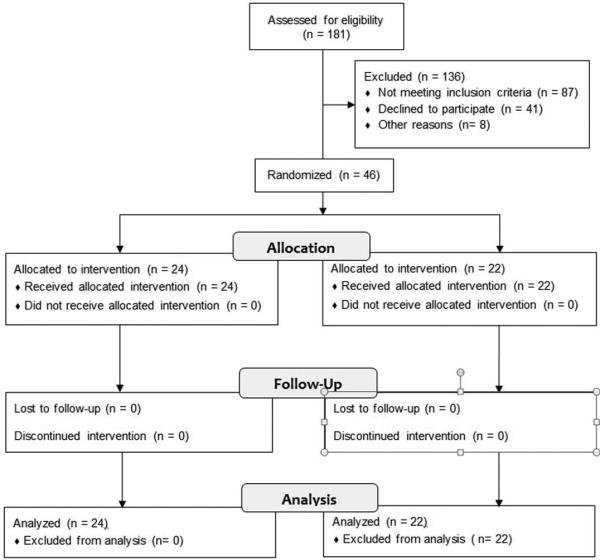
CONSORT Flow Diagram for screening and eligibility criteria.
Table 1.
Baseline Preoperative and Intraoperative Patient Characteristics. Continuous variables are presented as median (interquartile range) while nominal variables are presented as n (percentages).
| Patient Characteristic/Comorbidity |
BTX
(n = 24) |
Placebo
(n = 22) |
P
value |
|---|---|---|---|
| Age | 58.3 (52.6-67.9) | 61.9 (48.4-67.3) | .88 |
| Male gender | 13% (54%) | 17 (77%) | .13 |
| BMI | 26.3 (19.9-30.7) | 30.0 (26.9-32.9) | .032 |
| Lowest systolic blood pressure | 81 (67-91) | 76 (70-87) | .71 |
| Highest heart rate | 114 (104-129) | 130 (100-147) | .45 |
| Highest temperature (C) | 38.1 (37.3-39.2) | 37.8 (37.0-38.7) | .36 |
| Greatest base deficit | −6 (−10—4) | −5 (−14—2) | >.99 |
| Highest lactate (mmol/L) | 3.5 (1.8-4.2) | 4.4 (2.1-7.0) | .93 |
| Highest white blood cell count | 15.4 (12.0-23.5) | 15.3 (11.9-20.6) | .53 |
| Lowest pre-op hemoglobin | 9.2 (7.9-11.2) | 9.3 (7.9-13.2) | >.99 |
| Highest pre-op INR | 1.3 (1.1-1.5) | 1.4 (1.3-2.0) | .18 |
| CAD or CHF | 7 (29) | 9 (41) | .54 |
| Diabetes mellitus | 5 (21) | 3 (14) | .70 |
| Hypertension | 13 (54) | 12 (55) | >.99 |
| Chronic Renal Insufficiency | 5 (21) | 4 (18) | >.99 |
| Tobacco use within 30 days | 6 (26) | 7 (35) | .74 |
| Anticoagulated* | 6 (25) | 8 (40) | .34 |
| Prior Abdominal Operation | 14 (61) | 10 (48) | .55 |
| History of steroid use | 3 (13) | 2 (9) | >.99 |
| ASA score ≥ 3 | 21 (88) | 19 (86) | .43 |
| Intra-op packed Red Blood Cells (units) | 8.0 (1.0-16.9) | 4.8 (0-4.3) | .18 |
| Emergency laparotomy | 20 (83) | 16 (76) | .71 |
| Bowel viable | 10 (42) | 11 (50) | .77 |
| Contamination | 6 (25) | 4 (18) | .73 |
| Septic shock | 2 (8) | 1 (5) | >.99 |
| Hemorrhagic shock | 8 (33) | 8 (36) | >.99 |
| Abdominal compartment syndrome | 6 (25) | 1 (5) | .10 |
| Bowel resection | 13 (57) | 15 (68) | .54 |
| Prior Abdominal Operation | 14 (61) | 10 (48) | .55 |
BMI – body mass index; INR – International Normalized Ratio; ASA – American Society of Anesthesiologists; CAD – coronary artery disease; CHF – congestive heart failure; INR – international normalized ratio
Anticoagulated with clopidogrel, warfarin, or heparin
The primary endpoint of primary fascial closure was equivalent whether patients received BTX or placebo (96%; 95% CI 72%-99% vs. 93%; 95% CI 61%-99%; HR =1.0 95% CI 0.5-1.8). The average post-DCL day of injection was 1.8 ± 2.8 days (range 0-14). There was no difference in outcomes in regards to durations of hospital (LOS; 37 vs 26 days, p=0.30) or intensive care unit stay (17 vs 11 days, p=0.27; Table 2). The overall complication rate was similar (63% vs 68%, p=0.69), with 2 deaths in the placebo group and 0 in the BTX group. No difference was noted in the amount of morphine equivalents throughout the first 5 days following DCL (Table 3). No BTX or injection procedure complications were observed.
Table 2.
Comparison of outcomes between groups. Continuous variables are presented as median (interquartile range) while nominal variables are presented as n (percentages).
| Outcomes and Complications |
BTX
(n = 24) |
Placebo
(n = 22) |
P
value |
|---|---|---|---|
| Number of index abdominal procedures | 2 (1-3) | 2.0 (1-3) | .14 |
| Number of days of open abdomen | 5.0 (2.0-6.5) | 2.0 (2.0-5.0) | .15 |
| Duration of hospital stay | 23.5 (13.5-36.5) | 19.0 (11.0-23.0) | .19 |
| ICU Duration of stay (median) | 8.0 (4.0-25.0) | 6.0 (3.0-13.5) | .32 |
| Ventilator days | 3.0 (1.0-9.0) | 4.0 (1.0-9.0) | .44 |
| 30 day mortality | 0 (0) | 2 (9) | .22 |
| Wound infection | 2 (8) | 0(0) | >.99 |
| Intraabdominal abscess | 1 (4) | 1 (5) | >.99 |
| Anastomotic leak | 1 (4) | 0 (0) | >.99 |
| Dehiscence | 2 (8) | 0 (0) | .49 |
| Enterocutaneous fistula | 1 (4) | 1 (5) | >0.99 |
| Bleeding | 5 (21) | 4 (18) | >.99 |
| Reoperation after closure | 0 (0) | 1 (5) | .48 |
| Acute renal failure | 6 (25) | 7 (32) | .75 |
ICU – intensive care unit
Table 3.
Postoperative morphine equivalents of BTX vs Placebo groups. Results are presented as median (interquartile ranges).
| Day |
BTX
(MSO4 mg) |
Placebo
(MSO4 mg) |
P |
|---|---|---|---|
| 1 | 112 (63.4-147.3) | 90.8 (44.6-144.8) | .13 |
| 2 | 105 (67.3-157.8) | 86.2 (40-155.5) | .21 |
| 3 | 88.9 (52.3-153.6) | 95.3 (38.8-120.1) | .37 |
| 4 | 55.5 (30.7-148.7) | 80.9 (33.8-99.4) | .40 |
| 5 | 53.8 (23.0-136.2) | 45.5 (25.6-64.5) | .19 |
Discussion
The ability to close the OA remains a significant limitation to the use of DCL. Despite the historically low rates of primary fascial closure in the literature, our study demonstrated a very high rate no matter if BTX or placebo was injected. Additionally, there was no difference in the other secondary endpoints under study.
BTX, as a neuromodulating agent, decreases intra-abdominal pressure thereby increasing intra-abdominal volume in rats.22 The “chemical component separation” provided by BTX has also been shown to achieve 79% of the tension of a mechanical components separation in pigs.23 Whether this translates into a beneficial clinical effect in humans, however, is uncertain. In the only previously existing study of BTX in the OA, we were able to demonstrate a significant rate of improvement in primary fascial closure from 66% to 83% after BTX; however, while BTX injection into the abdominal wall in the OA setting was demonstrated, it was the inherent limitations from this flawed retrospective study which prompted us to perform the current clinical trial.19 We did not, however, expect such a dramatic improvement in the rate primary fascial closure in the new study which was a significant limitation. Previous reports show a rate of primary fascial closure ranging from 52% to 83%.2,7,19 Even a recent multi-institutional, prospective study of Level I trauma centers had a primary fascial closure rate of 66%.14 The reasons for the improvement we documented are unclear but likely multifactorial. With the advent of damage control resuscitation for hemorrhage, significantly less crystalloid is administered, likely improving the pliability of the abdominal wall.24 In addition, the recognition of the short- and long-term complications associated with planned ventral hernia has resulted in our increased aggressiveness in pursuing primary fascial closure. Another potential bias to the study are the inclusion criteria we utilized. Given the novelty of the procedure and the need to ensure safety, we incorporated multiple exclusion criteria. It is entirely possible that the patients who were excluded may be the very ones that could have benefited from BTX the most (e.g. obesity, COPD, anticoagulation, and hemodynamic instability requiring increased fluid for resuscitation). Lastly, in anticipation of this study, we protocolized OA management inclusive of re-exploration every 48 hours as well as the use of abdominal retention sutures.8 The combination of these factors likely improved the rate of primary fascial closure thereby diminishing our ability to statistically discern a difference when perhaps a true difference existed.
The use of BTX injection has better studied for ventral hernias. Similar to DCL, lateral tension against the hernia repair is a risk factor for recurrence.25 Minimization of this tension in the short term, perhaps through the injection of BTX, may lead to decreased rates of recurrence in the long term.26,27 In addition, given the analgesic effects of BTX, there may be a benefit to injection in terms of pain control and limited use of narcotic medications.21 This analgesic benefit, however, was not demonstrated in our study.
Despite the theoretical benefits to reduction of lateral abdominal wall tension for OA patients, no benefit was noted. While there are many potential reasons for this, we believe the primary reason may be due to the mechanism of action of BTX. By blocking neurotransmitter release into the synaptic cleft, flaccid paralysis of the injected muscle will result. This effect, however, takes at least 2-3 days to demonstrate any effect and up to three weeks to reach maximum effect. While this can be anticipated for elective repairs of ventral hernias, the unpredictability of trauma does not provide the luxury of injection prior to DCL. As a result, only the initial effects of BTX were present for the majority of the patients included in this study. Knowing the limitations to the mechanism of action of BTX, persistence in closure of the abdominal wall after injection may have biased the study towards a higher rate of primary fascial closure in both arms. This detriment in the short term, however, may prove to be a benefit in the long term as flaccid paralysis can last up to 6 months. During this time, the midline fascial closure is healing. Reduced lateral abdominal wall tension at this stage may limit rates of long term incisional ventral hernias.
The ability to perform primary fascial closure after DCL seems to be improving; nevertheless, significant complications endure. While long term follow-up is required, BTX injection during the setting of DCL did not have any appreciable clinical benefits in the short term in this pilot study. Nevertheless, its use appears to be safe as the observed outcomes and complications were similar whether BTX or placebo was injected. Aggressive pursuit of primary fascial closure should continue in order to reach 100% primary fascial closure.
Acknowledgments
NIH Registration #NCT01495962. The Biomechanical Effects of Flaccid Paralysis Induced by Botulinum Toxin a After Damage Control Laparotomy. Accessible at clinicaltrials.gov. Funded by the Eastern Association for the Surgery of Trauma.
Mayo IRB# 10-001672
Footnotes
Presented in part at the 28th annual meeting of the Eastern Association for the Surgery of Trauma, January 13–17, 2014, in Orlando, Florida
Level of Evidence: II (RCT with one negative criterion – inadequate statistical power)
Study Type: Therapeutic/Care Management Study
Disclosure: Off-label use of Botulinum Toxin A
Author Contribution
Martin D. Zielinski, MD – grant writing, study and site PI, trial design, subject consent, data review, manuscript writing, injection procedure concept genesis
Melissa Kuntz – lead study coordinator, subject consent, IRB liaison, manuscript editing, data collection
Xiaoming Zhang, PhD – surface wave elastography performance and data analysis and technology developer, manuscript writing and editing
Abigail E. Zagar – Regions lead study coordinator, subject consent, data collection
Mohammad A. Khasawneh, MBBS – data collection, injection procedure, consent, manuscript editing,
Benjamin Zendejas, MD - data collection, injection procedure, consent, manuscript editing,
Stephanie F. Polites, MD - manuscript editing, figures and tables creation, data review
Michael Ferrara – consent, injection procedure
William S. Harmsen, MS – data analysis, manuscript editing
Karla S. Ballman, PhD – data analysis, manuscript editing, overall statistics plan developer
Myung S. Park, MD - manuscript editing, data review, injection procedure,
Henry J. Schiller, MD - manuscript editing, data review, injection procedure,
David Dries, MD – Regions site PI, manuscript editing, data review, injection procedure,
Donald H. Jenkins, MD - manuscript editing, data review, injection procedure, injection procedure concept genesis
References
- 1.Waibel BH, Rotondo MF. Damage control in trauma and abdominal sepsis. Crit Care Med. 2010;38(9 Suppl):S421–30. doi: 10.1097/CCM.0b013e3181ec5cbe. [DOI] [PubMed] [Google Scholar]
- 2.Goussous N, Kim BD, Jenkins DH, Zielinski MD. Factors affecting primary fascial closure of the open abdomen in the nontrauma patient. Surgery. 2012;152(4):777–83. doi: 10.1016/j.surg.2012.07.015. discussion 783-4. [DOI] [PubMed] [Google Scholar]
- 3.Dellinger RP, Carlet JM, Masur H, Gerlach H, Calandra T, Cohen J, Gea-Banacloche J, Keh D, Marshall JC, Parker MM, et al. Surviving Sepsis Campaign Management Guidelines Committee. Surviving Sepsis Campaign guidelines for management of severe sepsis and septic shock. Crit Care Med. 2004;32(3):858–73. doi: 10.1097/01.ccm.0000117317.18092.e4. [DOI] [PubMed] [Google Scholar]
- 4.Cotton BA, Reddy N, Hatch QM, LeFebvre E, Wade CE, Kozar RA, Gill BS, Albarado R, McNutt MK, Holcomb JB. Damage control resuscitation is associated with a reduction in resuscitation volumes and improvement in survival in 390 damage control laparotomy patients. Ann Surg. 2011;254(4):598–605. doi: 10.1097/SLA.0b013e318230089e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rotondo MF1, Schwab CW, McGonigal MD, Phillips GR, 3rd, Fruchterman TM, Kauder DR, Latenser BA, Angood PA. ‘Damage control': an approach for improved survival in exsanguinating penetrating abdominal injury. J Trauma. 1993 Sep;35(3):375–82. discussion 382-3. [PubMed] [Google Scholar]
- 6.Pommerening MJ, DuBose JJ, Zielinski MD, Phelan HA, Scalea TM, Inaba K, Velmahos GC, Whelan JF, Wade CE, Holcomb JB, Cotton BA, et al. AAST Open Abdomen Study Group. Time to first take-back operation predicts successful primary fascial closure in patients undergoing damage control laparotomy. Surgery. 2014 Aug;156(2):431–8. doi: 10.1016/j.surg.2014.04.019. [DOI] [PubMed] [Google Scholar]
- 7.Arhinful E, Jenkins D, Schiller HJ, Cullinane DC, Smoot DL, Zielinski MD. Outcomes of Damage Control Laparotomy with Open Abdomen Management in the Octogenarian Population. J Trauma. 2011 Mar;70(3):616–21. doi: 10.1097/TA.0b013e31820d19ed. J Trauma 2010. [DOI] [PubMed] [Google Scholar]
- 8.Burlew CC, Moore EE, Biffl WL, Bensard DD, Johnson JL, Barnett CC. One hundred percent fascial approximation can be achieved in the postinjury open abdomen with a sequential closure protocol. J Trauma Acute Care Surg. 2012 Jan;72(1):235–41. doi: 10.1097/TA.0b013e318236b319. [DOI] [PubMed] [Google Scholar]
- 9.van Ruler O, Mahler CW, Boer KR, Reuland EA, Gooszen HG, Opmeer BC, de Graaf PW, Lamme B, Gerhards MF, Steller EP, van Till JVO, de Borgie CJAM, Gouma DJ, Reitsma JB, Boermeester MA. Comparison of On-Demand vs Planned Relaparotomy Strategy in Patients With Severe Peritonitis. A Randomized Trial. JAMA 2007. 298(8):865–873. doi: 10.1001/jama.298.8.865. [DOI] [PubMed] [Google Scholar]
- 10.Bradley MJ, Dubose JJ, Scalea TM, Holcomb JB, Shrestha B, Okoye O, Inaba K, Bee TK, Fabian TC, Whelan JF, Ivatury RR, et al. AAST Open Abdomen Study Group. Independent predictors of enteric fistula and abdominal sepsis after damage control laparotomy: results from the prospective AAST Open Abdomen registry. JAMA Surg. 2013;148(10):947–54. doi: 10.1001/jamasurg.2013.2514. [DOI] [PubMed] [Google Scholar]
- 11.Aprahamian C, Wittman DH, Bergstein JM, Quebbeman EJ. Temporary Abdominal Closure (TAC) for Planned Relaparotomy (Etappenlavage) in Trauma. J Trauma. 1990;30(6):719–723. doi: 10.1097/00005373-199006000-00011. [DOI] [PubMed] [Google Scholar]
- 12.Brock WB, Barker DE, Burns RP. Temporary closure of open abdominal wounds: The vacuum pack. Am Surg. 1995;61:30–5. [PubMed] [Google Scholar]
- 13.Barker DE, Kaufman HJ, Smith LA, et al. Vacuum pack technique of temporary abdominal closure: A 7-year experience with 112 patients. J Trauma. 2000;48:201–6. doi: 10.1097/00005373-200002000-00001. [DOI] [PubMed] [Google Scholar]
- 14.Dubose JJ, Scalea TM, Holcomb JB, Shrestha B, Okoye O, Inaba K, Bee TK, Fabian TC, Whelan J, Ivatury RR, et al. AAST Open Abdomen Study Group. Open abdominal management after damage-control laparotomy for trauma: a prospective observational American Association for the Surgery of Trauma multicenter study. J Trauma Acute Care Surg. 2013;74(1):113–20. doi: 10.1097/TA.0b013e31827891ce. discussion 1120-2. [DOI] [PubMed] [Google Scholar]
- 15.Truong D, Dressler D, Hallett M, editors. Manual of Botulinum Toxin Therapy. Cambridge University Press2009; [Google Scholar]
- 16.Jankovic J, Albanese A, Atassi MZ, Dolly JO, Hallett M, Mayer NH, editors. Botulinum Toxin Therapeutic Clinical Practice and Science. Saunders2009. [Google Scholar]
- 17.Göbel H, Heinze A, Reichel G, Hefter H, Benecke R. Dysport myofascial pain study group. Efficacy and safety of a single botulinum type A toxin complex treatment (Dysport) for the relief of upper back myofascial pain syndrome: results from a randomized double-blind placebocontrolled multicentre study. Pain. 2006 Nov;125(1-2):82–8. doi: 10.1016/j.pain.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 18.Smoot D, Zielinski M, Jenkins D, Schiller H. Botox A injection for pain after laparoscopic ventral hernia: a case report. Pain Med. 2011 Jul;12(7):1121–3. doi: 10.1111/j.1526-4637.2011.01147.x. [DOI] [PubMed] [Google Scholar]
- 19.Zielinski MD, Goussous N, Schiller HJ, Jenkins D. Chemical components separation with botulinum toxin A: a novel technique to improve primary fascial closure rates of the open abdomen. Hernia. 2013 Feb;17(1):101–7. doi: 10.1007/s10029-012-0995-1. [DOI] [PubMed] [Google Scholar]
- 20.Harris Paul A., Taylor Robert, Thielke Robert, Payne Jonathon, Gonzalez Nathaniel, Conde Jose G. Research electronic data capture (REDCap) - A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009 Apr;42(2):377–81. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zendejas B, Khasawneh MA, Srvantstyan B, Jenkins DH, Schiller HJ, Zielinski MD. Outcomes of chemical component paralysis using botulinum toxin for incisional hernia repairs. World J Surg. 2013 Dec;37(12):2830–7. doi: 10.1007/s00268-013-2211-6. [DOI] [PubMed] [Google Scholar]
- 22.Cakmak M, Caglayan F, Somuncu S, Leventoglu A, Ulusoy S, Akman H, Kaya M. Effect of paralysis of the abdominal wall muscles by botulinum A toxin to intraabdominal pressure: an experimental study. J Ped Surg. 2006;41:821–825. doi: 10.1016/j.jpedsurg.2005.12.023. [DOI] [PubMed] [Google Scholar]
- 23.Harth K, Rosen M, Blatnik J, Schomisch S, Cash A, Soltanian H. Chemical Myotomy With Botulinum Toxin for Abdominal Wall Reconstruction: results of a porcine pilot study. Abstract presented at Abdominal Wall Reconstruction 2010; Washington D.C. Jun 11-16, 2010. [Google Scholar]
- 24.Duchesne JC, >McSwain NE, Jr, Cotton BA, Hunt JP, Dellavolpe J, Lafaro K, Marr AB, Gonzalez EA, Phelan HA, Bilski T, Greiffenstein P, Barbeau JM, Rennie KV, Baker CC, Brohi K, Jenkins DH, Rotondo M. Damage control resuscitation: the new face of damage control. J Trauma. 2010 Oct;69(4):976–90. doi: 10.1097/TA.0b013e3181f2abc9. [DOI] [PubMed] [Google Scholar]
- 25.Ventral Hernia Working Group1. Breuing K, Butler CE, Ferzoco S, Franz M, Hultman CS, Kilbridge JF, Rosen M, Silverman RP, Vargo D. Incisional ventral hernias: review of the literature and recommendations regarding the grading and technique of repair. Surgery. 2010 Sep;148(3):544–58. doi: 10.1016/j.surg.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 26.Ibarra-Hurtado TR, Nuno-Guzman CM, Echeagaray-Herrara JE, Robles-Velez ER, Gonzalez-Jaime JJ. Use of botulinum toxin type A before abdominal wall hernia reconstruction. World J Surg. 2009;33:2553–2556. doi: 10.1007/s00268-009-0203-3. [DOI] [PubMed] [Google Scholar]
- 27.Ibarra-Hurtado TR1, Nuño-Guzmán CM, Miranda-Díaz AG, Troyo-Sanromán R, Navarro-Ibarra R, Bravo-Cuéllar L. Effect of botulinum toxin type A in lateral abdominal wall muscles thickness and length of patients with midline incisional hernia secondary to open abdomen management. Hernia. 2014 Oct;18(5):647–52. doi: 10.1007/s10029-014-1280-2. [DOI] [PubMed] [Google Scholar]


