Abstract
Background
Genetic influences contribute significantly to comorbidity between conduct disorder and substance use disorders. Estimating the extent of overlap can assist in the development of phenotypes for genomic analyses.
Methods
Multivariate quantitative genetic analyses were conducted using data from 9577 individuals, including 3982 complete twin pairs and 1613 individuals whose cotwin was not interviewed (aged 24–37 years) from two Australian twin samples. Analyses examined the genetic correlation between alcohol dependence, nicotine dependence and cannabis abuse/dependence and the extent to which the correlations were attributable to genetic influences shared with conduct disorder.
Results
Additive genetic (a2=0.48 to 0.65) and nonshared environmental factors explained variance in substance use disorders. Familial effects on conduct disorder were due to additive genetic (a2=0.39) and shared environmental (c2=0.15) factors. All substance use disorders were influenced by shared genetic factors (rg=0.38–0.56), with all genetic overlap between substances attributable to genetic influences shared with conduct disorder. Genes influencing individual substance use disorders were also significant, explaining 40–73% of the genetic variance per substance.
Conclusions
Among substance users in this sample, the well-documented clinical comorbidity between conduct disorder and substance use disorders is primarily attributable to shared genetic liability. Interventions targeted at generally reducing deviant behaviors may address the risk posed by this shared genetic liability. However, there is also evidence for genetic and environmental influences specific to each substance. The identification of these substance-specific risk factors (as well as potential protective factors) is critical to the future development of targeted treatment protocols.
Keywords: conduct disorder, substance use disorders, alcohol, nicotine, cannabis, genetic overlap, twins
INTRODUCTION
There is substantial evidence that similar genetic factors influence liability to multiple substance use disorders (e.g., (Kendler et al. 2003a; Kendler et al. 2007; Palmer et al. 2013; Rhee et al. 2006; Sartor et al. 2010; Xian et al. 2008; Young et al. 2006). Most notably, Kendler and colleagues (Kendler et al. 2007) identified two genetic factors, corresponding to the covariance across licit (alcohol, nicotine, caffeine) and illicit (cannabis, cocaine) drugs, however the genetic factors were highly correlated (r=0.82). Conduct disorder has been repeatedly implicated as a comorbid feature of substance use disorders and there is substantial evidence that genetic influences contribute to this comorbidity. For instance, another study by Kendler and colleagues found that alcohol and drug dependence shared a sizeable proportion of their genetic liability with conduct and antisocial personality disorder, and less so with internalizing disorders, such as depression (Kendler et al. 2003b). Button and colleagues (Button et al. 2006) also found significant genetic overlap between conduct disorder and both alcohol and illicit drug dependence in an adolescent sample, and that residual genetic overlap between alcohol and illicit drug dependence, after adjusting for conduct disorder, was non-significant. Similar results indicating the absence of a residual genetic correlation between alcohol and cannabis dependence when accounting for genetic overlap with antisocial personality disorder have also been noted in adult Vietnam Era males (Fu et al. 2002). Other studies suggest that this shared liability extends to additional aspects of externalizing behavior, including novelty seeking and non-substance related behavioral disinhibition (Hicks et al. 2011; Krueger et al. 2002).
Based on this extant literature, it remains unanswered the extent to which the genetic covariation across the three most common forms of substance use disorder (i.e. alcohol, nicotine and cannabis) is attributable to genes shared with conduct disorder. Two outcomes might be expected. One possibility is that all the genetic influences on the three substances are overlapping with each other and with conduct disorder, with limited evidence for substance-specific or conduct disorder-specific genetic factors. This is unlikely given prior evidence for significant substance-specific genetic influences on substance dependence symptoms and on conduct disorder (Button et al., 2006; Button et al., 2007; Kendler et al., 2003b). Alternatively, a moderate to high genetic correlation across the three substances might be expected, with evidence for substance-specific genes. Whether the entire proportion of this genetic correlation is due to genes shared with conduct disorder or whether some of this genetic correlation is independent of it (i.e. shared across the substances but not with conduct disorder) has not been examined. This study of the extent to which the genetics of conduct disorder contribute to genetic liability to substance use disorders is critical to gene-finding efforts where increasing sample sizes, often capitalizing on differing definitions of phenotypes with overlapping genetic underpinnings, is a necessity (Hicks et al. 2011). Equally important is the estimation of substance-specific genetic influences. For instance, Kendler and colleagues (2007) noted that substance-specific genetic variance ranged from 3% for cocaine to 91% for caffeine abuse/dependence symptoms.
To examine this question, we utilized data from 9577 adult male and female twins aged 24–37 years drawn from two samples derived from the Australian Twin Registry. Specifically we fit a quadrivariate model that examined the extent to which genetic and environmental influences were shared across conduct disorder and DSM-IV diagnoses of nicotine dependence (ND) and alcohol dependence (AD), and a modified cannabis abuse/dependence (CAD) diagnosis based on a subset of DSM-IV criteria.
METHODS AND MATERIALS
Sample
Twin pairs from two cohorts of the volunteer Australian Twin Registry were included in the present analyses (Maciejewski et al. 2014). As described in detail elsewhere, telephone diagnostic interviews were completed for one cohort in 1996–2000 (see(Knopik et al. 2004) for details) and for the other in 2005–2009 (Lynskey et al. 2012). The samples had comparable ages at the time of interview (M=29.94 and M=31.85 respectively). After removing individuals of unknown zygosity and those with no data for any of the four outcomes, the sample for the present analyses included 9577 individuals (6255 of 6257 interviewed individuals in the first cohort; 3322 of 3348 interviewed individuals in the second cohort). The 3982 complete twin pairs included: 1096 monozygotic (MZ, or identical) female twin pairs, 665 MZ male pairs, 817 dizygotic (DZ, or fraternal) female pairs, 513 DZ male pairs, and 891 male-female twin pairs; data from 1613 individuals without cotwin data, which contribute to estimates of prevalence (but not variance decomposition), were also included. Zygosity was determined using standard items about physical similarity which have been shown in a subset of the present respondents to have 95% agreement with DNA analysis (Medland et al. 2009). Verbal informed consent was obtained from all participants, and the procedures were approved by the Human Research Protection Office at Washington University and the Human Research Ethics Committee at the Queensland Institute of Medical Research.
Measures
Both cohorts completed a diagnostic telephone interview based on the Semi-structured Assessment of the Genetics of Alcoholism (Australian version, SSAGA-OZ (Bucholz et al. 1994; Heath et al. 1997)), which included DSM-IV (American Psychiatric Association, 1994) assessments of conduct disorder, and ND, AD, and CAD.
Conduct disorder
Respondents were asked about 15 behaviors associated with DSM-IV conduct disorder (e.g., bullying, threatening, stealing, rule-breaking). Individuals who indicated that 3 or more behaviors (without requiring impairment) occurred within a 12-month period prior to age 18 were coded as having conduct disorder. None of the items used in the conduct disorder section was related to substance use or misuse.
Nicotine
Respondents who had smoked at least 100 cigarettes lifetime or who had smoked 21–99 cigarettes and had smoked at least 2 or more days a week for three or more weeks, completed a detailed DSM-IV nicotine dependence (ND) assessment. Individuals who endorsed 3 or more dependence symptoms (out of 7) within a 12-month period were coded as having a history of ND. Because individuals who do not initiate using a substance (i.e. never user) should be considered unmeasured for their liability to developing substance use disorders (Neale et al. 2006), individuals who had never tried cigarettes were coded as missing for ND (n=1001). Individuals with less than 3 dependence symptoms, those whose symptoms did not cluster within a 12-month period, and individuals who had tried cigarettes but had only experimented with nicotine (i.e., had smoked fewer than 100 cigarettes lifetime and had not smoked at least 2 or more days per week, n=3900) were coded as unaffected for ND.
Alcohol
Respondents were asked about their lifetime alcohol consumption. Individuals who had consumed at least 5 drinks in a 24-hour period at least once completed a detailed DSM-IV alcohol dependence (AD) assessment and were included in analyses of AD. Those individuals who endorsed 3 or more AD symptoms (out of 7) within a 12-month period were coded as having a history of AD. Non-drinkers (n=105) and those who had not consumed at least five drinks in a 24 hour period (n=908) were coded as missing for AD analyses.
Cannabis
Individuals who had used cannabis 11 or more times or who had used cannabis at least monthly during the period of heaviest use were asked questions tapping DSM-IV cannabis abuse and dependence (CAD). The first cohort was only asked about 2 abuse symptoms (hazardous use and role interference) and 4 dependence symptoms (tolerance, using more than intended, continued use despite emotional/psychological problems caused/exacerbated by use, and repeated desire or inability to cut down on use). Although the second cohort was asked additional abuse and dependence questions, the present analyses used only the symptoms assessed in both cohorts. Respondents were identified as having CAD if they endorsed any abuse symptom or they endorsed 2 or more dependence symptoms (symptoms were not required to have clustered within a 12 month period). Because individuals who do not initiate using a substance (i.e. never user) should be considered unmeasured for their liability to developing substance use disorders (Neale et al. 2006), individuals who had never tried cannabis were coded as missing for CAD (n=3499). Individuals who completed the CAD section but did not meet the criteria for CAD listed above as well as those who had minimal cannabis exposure (i.e., had tried cannabis but used it less than 11 times and had not used it at least monthly during their period of heaviest use; n=3114) were coded as unaffected for CAD.
Statistical Analyses
Quantitative genetic analyses were used to estimate the relative contributions of genetic and environmental factors to outcomes, and the overlap between diagnoses. As detailed elsewhere (Neale and Cardon, 1992), twin models allow for the estimation of additive genetic factors (A), non-additive genetic factors (D), shared environmental factors (C; environmental influences that make twins similar to each other), and nonshared environmental factors (E; environmental influences not shared by twins, as well as error variance). Genetic influences are indicated when the identical twin (MZ) correlation is greater than the fraternal twin (DZ) correlation. If all twin pair similarity were attributable to A, the MZ correlation would be about twice the DZ correlation, because MZ twins share all of their genes and DZ twins share half of their segregating genes (on average). D is indicated when the DZ correlation is less than ½ the MZ correlation (because MZ twins again share all non-additive influences but DZ twins only share ¼ of such influences, on average), and C is indicated when the DZ correlation is more than ½ the MZ correlation. Models containing only twins reared together cannot estimate both C and D simultaneously, and a decision as to which should be estimated is made based on correlations between identical and fraternal twins.
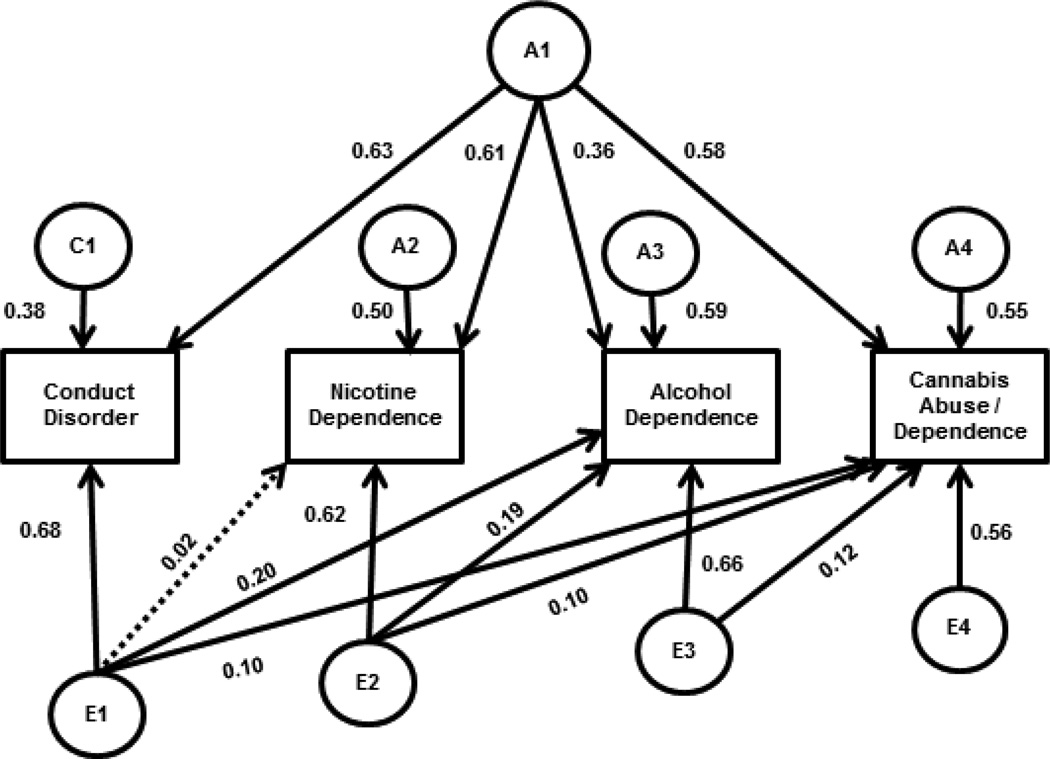
A quadrivariate Cholesky (lower triangular) decomposition was used to assess the degree of genetic and environmental influence on each measure, as well as the overlap across conduct disorder, ND, AD, and CAD. A full Cholesky model allows for influences on the first variable to also load on all subsequent variables, and for novel influences on each subsequent variable to load on all remaining variables. The E parameters in Figure 1 show a full Cholesky parameterization, and the comparable Cholesky parameterizations for the familial components are shown in Supplemental Figure 1 (see also work by Neale and colleagues (Neale, 2004; Neale and Cardon, 1992)). Conduct disorder was entered into the Cholesky model first. Given that individuals become at risk for developing dependence when they initiate substance use and that not all users develop dependence, ordering of the substance diagnosis variables in the Cholesky model (ND, AD, CAD) was determined by the most typical pattern of substance initiation. Of those who had tried all three substances, 60.0% tried cigarettes before both alcohol and cannabis, and an additional 18.3% initiated multiple substances in the same year, with cigarettes being one of those substances; 20.0% initiated alcohol first; 0.8% initiated cannabis first. Of individuals who had tried two of the three substances, 94.6% had used cigarettes and alcohol; 60.7% of those who had used two substances initiated cigarettes before alcohol, 21.1% initiated alcohol before cigarettes, and 12.9% initiated cigarettes and alcohol within the same year. This ordering of variables in the Cholesky model (CD, ND, AD, CAD) was also supported by the mean ages of initiation (see Table 1).
Figure 1.
Final quadrivariate genetic model with standardized parameter estimates.
Footnotes.
NOTE: Paths are only shown for one twin; paths for the second twin are equated to the first twin, and connected to each other by fixed values in keeping with quantitative genetic theory (1.0 for genetic paths between MZ twins; 0.5 for genetic paths between DZ twins; 1.0 for shared environmental paths between MZ and DZ twins). Latent variables are indicated by circles; observed variables by rectangles. A=additive genetic influences, C=shared environmental influences, E=nonshared environmental influences. The subscript 1 indicates factors influencing conduct disorder; 2 is additional factors for nicotine dependence; 3 is additional factors for alcohol dependence; 4 is additional factors for cannabis abuse/dependence; A1 is a genetic factor common to all measures. Diagonal lines indicate paths from one measure that load on other measures. Solid lines indicate significant paths. Parameter estimates can be squared to determine the proportion of variance in the observed construct attributable to that path.
Table 1.
Demographic and substance use characteristics of 9577 members of Australian twin pairs aged 24–37 years.
| Mean in years (sd) | |
|---|---|
| Mean age at interview (SD) | 30.60 (2.6) |
| Mean age at conduct disorder onset | 13.84 (2.4) |
| Mean age at cigarette initiation | 14.06 (3.4) |
| Mean age at nicotine dependence onset | 21.96 (4.1) |
| Mean age at alcohol initiation | 15.83 (2.6) |
| Mean age at alcohol dependence onset | 21.87 (3.9) |
| Mean age at cannabis initiation | 18.50 (3.4) |
| Mean age at cannabis dependence onseta | 21.36 (3.9) |
| No. (%) | |
| Male | 3972 (41.5) |
| MZ | 4102 (42.8) |
| DSM-IV Conduct Disorder | 1100 (11.6) |
| Nicotine use and dependence: | |
| Ever smoked a cigarette | 8470 (88.5) |
| DSM-IV Nicotine Dependence | |
| Population prevalence | 2770 (28.9) |
| Of ever smokers | 2770 (32.7) |
| Alcohol use and dependence: | |
| Ever had a full drink of alcohol | 9456 (98.9) |
| Ever had 5+ drinks in a 24-hour period | 8544 (89.2) |
| DSM-IV Alcohol Dependence | |
| Population prevalence | 2219 (23.2) |
| Of those having consumed at least 5 drinks in a 24 hour period | 2219 (26.0) |
| Cannabis use and abuse/dependence: | |
| Ever used cannabis | 6017 (63.2) |
| Cannabis Abuse/Dependence (modified DSM-IV) | |
| Population prevalence | 1683 (17.7) |
| Of ever users | 1683 (28.0) |
Note: data from n=9577 individuals; n=9501 individuals for conduct disorder, n=n=8470 for nicotine dependence, 8544 for alcohol dependence, and n=6017 for cannabis abuse/dependence.
age at clustering for n=311 individuals in the second cohort who met the modified DSM-IV cannabis abuse/dependence diagnosis (CAD). The n=1683 with CAD included n=1122 from the first cohort (age at diagnosis not assessed); of the n=561 cohort 2 individuals with the modified CAD diagnosis, age was not assessed for the n=177 with abuse only or the n=73 with the modified 2+ symptom dependence diagnosis but not the full DSM-IV dependence diagnosis with 3+ symptoms and clustering
The fit of the full Cholesky model was compared to a saturated model, which allowed us to test whether the assumptions of twin modeling were met. Sub-models dropping specific paths were used to test the significance of familial influences, and the overlap in influences across measures. To minimize the large number of tests that would need to be conducted to examine combinations of paths that could be dropped from the model, omnibus tests that constrained a family of paths (e.g. simultaneously constraining all C paths to zero) were first fit to the data. If these omnibus tests resulted in a significant deterioration in model fit, the significance of individual paths of potential importance were tested. Fit of the sub-models was determined by calculating the difference in −2 times the log-likelihood of the full model and the sub-model, which is interpreted as a Chi-square test for the given degrees of freedom. Models were fitted using the statistical package Mx (Neale, 2004) using full information maximum likelihood estimation with raw data. Analyzing raw data in Mx (Neale, 2004) allowed us to utilize data from conduct disorder for everyone, and substance dependence data from each individual for the substances he/she had used more times than the cutoff established. All quadrivariate models included gender and cohort as covariates.
RESULTS
Demographic and substance use characteristics are shown in Table 1. Alcohol initiation was endorsed by almost 99% of the respondents, with 89% of the sample reporting having consumed at least 5 drinks in a 24-hour period and 26% of those having consumed 5 or more drinks in a 24-hour period reporting a lifetime history of AD. Over 88% of respondents had tried cigarettes, with 33% of initiators having a history of ND. Cannabis initiation was endorsed by 63% of respondents, with 28% of initiators having a history of CAD. Mean age of onset of conduct disorder was 13.84 years, which predated the average age of initiation for cigarettes (M=14.06), alcohol (M=15.83), and cannabis (M=18.50).
Because all univariate genetic analyses indicated that the parameter estimates could be equated for men and women (Δχ2(3) range: 2.30 – 6.38, all p > 0.05), quadrivariate analyses were collapsed across gender and gender was included as a covariate (see Supplemental Table 1 for the correlations for all five zygosity groups). MZ and DZ twin-pair correlations, tested using SAS software version 9.2 (SAS Institute, Inc., 2002–2008), indicated significant familiality for all measures (see Table 2). Univariate genetic models confirmed that additive genetic influences and nonshared environmental influences were significant for all measures, with shared environmental influences not reaching significance for any measure, but reaching p=0.06 for conduct disorder (see Table 2).
Table 2.
Twin-pair correlations and final univariate genetic models (and 95% confidence intervals) for conduct disorder, nicotine dependence, alcohol dependence, and cannabis abuse/dependence.
| rMZ | rDZ | A | C | E | |
|---|---|---|---|---|---|
| Conduct Disorder | 0.55* (0.46 – 0.64) |
0.39* (0.30 – 0.48) |
0.32* (0.05 – 0.59) |
0.20a (0.00 – 0.41) |
0.48* (0.38 – 0.58) |
| Nicotine Dependence | 0.63* (0.56 – 0.69) |
0.31* (0.24 – 0.38) |
0.62* (0.57 – 0.68) |
--- | 0.38* (0.32 – 0.44) |
| Alcohol Dependence | 0.51* (0.43 – 0.59) |
0.22* (0.14 – 0.30) |
0.47* (0.40 – 0.54) |
--- | 0.53* (0.46 – 0.60) |
| Cannabis Abuse/Dep. | 0.63* (0.55 – 0.71) |
0.40* (0.30 – 0.49) |
0.63* (0.56 – 0.70) |
--- | 0.37* (0.30 – 0.44) |
NOTE: A=additive genetic influences, C=shared environmental influences, E=nonshared environmental influences.
Data from n=9501 individuals for conduct disorder, n=8470 for nicotine dependence, n=8544 for alcohol dependence, and n=6017 for cannabis abuse/dependence.
significant at p < 0.05;
Δχ2 = 3.59 when removed from the model, p = 0.06
A saturated model was used to estimate the tetrachoric correlations in Mx (Neale, 2004), and served as a baseline from which to test the assumptions of the genetic models (−2 times the log − likelihood = 31827.135 with 72 estimated parameters). The initial genetic model, which included additive genetic, shared environmental, and nonshared environmental paths in a Cholesky parameterization, indicated that the Cholesky parameterization was reasonable for the current data (Δχ2 = 19.023, Δdf = 30,p = 0.94 compared to the saturated model). A sub-model specifying that all genetic overlap between the substances could be explained by genetic influences shared with conduct disorder fit the data well (i.e., deleting paths a32, a42, and a43 from Supplemental Figure 1; Δχ2 = 2.196, Δdf = 3,p = 0.53). Eliminating shared environmental influences on the substance dependence measures resulted in a more parsimonious model without a significant decrement in fit (i.e., deleting all C paths except c11 from Supplemental Figure 1; Δχ2 = 4.241, Δdf = 9,p = 0.89). All remaining genetic influences were statistically significant, as indicated by a significant decrement in fit when any path was deleted (Δχ2 range:85.22 – 262.989 with 1 df, all p < 0.001). Although modest in magnitude, nonshared environmental influences overlapped across measures, with 5 of 6 paths being statistically significant (Δχ2 range:5.846 – 28.275 with 1 df, all p < 0.05; the nonshared environmental path from CD to ND, which could be dropped without a significant decrement in fit Δχ2 = 0.169 with 1 df,p > 0.05, was retained in the final model but is indicated by the dashed line in Figure 1). The significance of the 3 substance-to-substance nonshared environmental parameters (e32, e42, and e43 from Supplemental Figure 1) indicates that individual-specific effects were shared across substances independent of the effects each substance shared with conduct disorder.
The standardized parameter estimates from the best-fitting model are shown in Figure 1, with the proportions of variance presented in Table 3 and the genetic and environmental correlations in Table 4. As shown in Table 3, familial influences accounted for substantial variance in all measures, with genetic factors explaining 39% of the variance in conduct disorder, and 62%, 48%, and 65% of the variance in ND, AD, and CAD respectively. Shared environmental influences accounted for an additional 15% of the variance in conduct disorder. Genetic correlations were significant and substantial (ranging from 0.38 – 0.77; above the diagonal in Table 4), especially between conduct disorder and ND and CAD, where over 50% of the genetic influences were overlapping. Importantly, the genetic correlation across substances was entirely attributable to genes shared with conduct disorder. Nonshared environmental influences were predominantly measure specific, as shown by the modest correlations between individual-specific environmental factors (range: 0.03 – 0.28; below the diagonal in Table 4).
Table 3.
Standardized proportions of variance [95% Confidence Intervals] attributable to additive genetic, shared environmental, and nonshared environmental influences in the best-fitting quadrivariate model examining the genetic and environmental contributions to the comorbidity between conduct disorder, nicotine dependence alcohol dependence, and cannabis abuse/dependence in 9577 Australian twins.
| Additive Genetic | Shared Environment | Nonshared Environment |
|
|---|---|---|---|
| Conduct Disorder | 0.39 (0. 31– 0. 48) | 0.15 (0.07– 0.22) | 0.46 (0.39 – 0.53) |
| Nicotine Dependence | 0.62 (0.56 – 0.67) | --- | 0.38 (0.34– 0.44) |
| Alcohol Dependence | 0.48 (0.41 – 0.55) | --- | 0.52 (0.45 – 0.59) |
| Cannabis Abuse/Dependence | 0.65 (0. 58– 0.71) | --- | 0.35 (0.29– 0.43) |
Note: All variance components significant at p < 0.05
Table 4.
Genetic (above diagonal) and nonshared environmental correlationsa between conduct disorder, nicotine dependence, alcohol dependence, and cannabis abuse/dependence from the best-fitting model examining the genetic and environmental contributions to the comorbidity between conduct disorder, alcohol dependence, nicotine dependence and cannabis abuse/dependence in 9577 Australian twins.
| Conduct Disorder |
Nicotine Dependence |
Alcohol Dependence |
Cannabis Abuse/Dependence |
|
|---|---|---|---|---|
| Conduct Disorder | --- | 0.77* (0.69 – 0.87) |
0.52* (0. 44– 0.61) |
0.72* (0.64– 0.81) |
| Nicotine Dependence | 0.03 (−0.10 – 0.14) |
--- | 0.40* (0.33 – 0.48) |
0.56* (0.48 – 0.64) |
| Alcohol Dependence | 0.28* (0.19 – 0.38) |
0.27* (0.17 – 0.35) |
--- | 0.38* (0.31– 0.45) |
| Cannabis Abuse/Dependence | 0.17* (0.05 – 0.31) |
0.18* (0.06 – 0.31) |
0.28* (0.17 – 0.39) |
--- |
Note: A=additive genetic influences, C=shared environmental influences, E=nonshared environmental influences.
Genetic correlations are above the diagonal; nonshared environmental correlations are below the diagonal.
indicates p < 0.05
DISCUSSION
In a large cohort of adult Australian twins, we show that the previously well-documented shared genetic etiology across nicotine dependence (ND), alcohol dependence (AD), and cannabis abuse/dependence (CAD) was entirely attributable to genes contributed by a shared liability to conduct disorder. Our study extends previous research in that we: (a) examined conduct disorder, ND, AD, and CAD simultaneously in an adult sample (researchers have examined these substances together [Kendler et al., 2007, 2008], and have examined conduct disorder with AD and general substance use disorders [Kendler et al.,2003; Krueger et al., 2002], but not all in the same analysis); (b) extended Hicks and colleagues analysis of adolescents (16–19 years old; Hicks et al., 2011), which could yield different patterns of overlap; (c) set to missing the data from individuals who had not initiated a substance and hence had unknown genetic liability. Our results are consistent with previous reports (Button et al. 2006; Fu et al. 2002; Hicks et al. 2011; Kendler et al. 2003b) showing that alcohol and drug dependence tend to share an appreciable proportion of their genetic liability with conduct and antisocial personality disorder, and further demonstrating that similar effects extend to ND and to substance use disorders contingent on initiation. The overall structure of genetic overlap (see Figure 1) is also broadly consistent with numerous twin studies of adolescent and adult populations indexing a general liability factor underlying a host of externalizing disorders, including conduct disorder, substance use disorders and also impulsivity and non-substance related disinhibition (Hicks et al. 2011; Krueger et al. 2002).
Such results have key implications for gene-finding efforts such as genomewide association studies (GWAS). In particular, they imply that, as least when examining initiators, phenotypes that aggregate across various substance use disorders (e.g. (Wetherill et al. 2014) or examine genetic variants that influence multiple substances (McGue et al. 2013; Vrieze et al. 2014) may effectively be capturing genetic variation shared with liability to conduct disorder. Thus, approaches of aggregating across substances or of using conduct disorder and other aspects of behavioral disinhibition as proxies may be particularly useful in increasing power to detect genetic variants shared across substances, particularly in adolescent cohorts where subjects may not have fully passed through the period of greatest risk for onset of substance use disorders (Button et al. 2006; Hicks et al. 2011; Iacono et al. 1999; Rose et al. 2004). However, at least one study reports that polygenic scores created for individual substance-related measures and behavioral disinhibition are only modestly (r=0.03–0.07) correlated with each other (Vrieze et al. 2013). Developmental changes in the degree to which genetic factors contribute to substance involvement itself also should be considered, although there is disagreement about the pattern of change. For instance, research by Vrieze and colleagues (Vrieze et al. 2012) suggests that the genetic contribution to the overlap across substances may decline from adolescence into adulthood, while research by Kendler and colleagues suggests that the genetic contribution to overlap across substance use remains steady, even increases modestly, across that time span (Kendler et al. 2008). There is also evidence that the correlation between CD and substance involvement is more attributable to shared environmental factors during adolescence (Rose et al., 2004). Thus, our finding of strong genetic overlap may be specific to the age of our cohort (young adults) and may not generalize to other developmental settings.
Despite genetic overlap, the statistically significant role of substance-specific genetic influences cannot be ignored. In fact, a significant proportion of the genetic variance in ND (40%), AD (73%), and CAD (47%) was substance-specific. The finding of substance-specific genetic influences corroborates previous research, although the relatively higher proportion of specific genetic influences on AD versus ND is in contrast to one prior study that found ND to be most significantly influenced by specific genetic factors (Kendler et al. 2007). The magnitude of these specific genetic factors underscores that when studying the genomic underpinnings of individual substance use disorders, variants are not exclusively expected to reflect a general liability to conduct disorder or externalizing problems. Most notably, for AD and ND, the most robustly validated single nucleotide polymorphisms identified via GWAS have been substance specific. For alcohol, rs1229984 in the alcohol dehydrogenase (ADH1B) gene is associated with accelerated conversion of ethanol to acetaldehyde, thus protecting against alcoholism via a specific metabolic pathway (Thomasson et al. 1993). This functional polymorphism has been identified at genomewide significant levels in two independent studies (Bierut et al. 2011; Gelernter et al. 2014). For tobacco smoking, a similar metabolic variant in the cytochrome P450 A6 (CYP2A6) gene has been identified in one large meta-analysis (Thorgeirsson et al. 2010). However, the most widely replicated findings are for tobacco smoking and rs16969968 in the cholinergic nicotinic receptor alpha 5 subunit (CHRNA5) (Liu et al. 2010; Thorgeirsson et al. 2010; Tobacco and Genetics Consortium, 2010) and other variants in the family of genes encoding nicotinic receptor subunits, which have been repeatedly identified at genomewide significant levels. It is worth noting that the large GWAS meta-analyses of smoking that identified rs16969968 relied on nicotine consumption measures such as cigarettes per day (not dependence) and primarily included samples in which the comorbid effects of conduct disorder are likely to be minimal. These substance-specific findings have also arisen in a sample with a high degree of drug-related comorbidity (e.g.(Rice et al. 2012)) but in that study, when studying one substance, diagnoses of other substance use disorders were accounted for, thus likely refining the ability to home in on substance-specific results. Future studies may also wish to control for conduct disorder to further enhance chances of identifying drug-specific variants.
Some limitations of the current study are worth noting. First, this is a sample of adult Caucasian Australians and findings may not generalize to other ethnic groups. Second, in order to test for the role of substance initiation on the genetic overlap with substance use disorder, we set to missing the substance-specific data for individuals who had never tried a given substance (conduct disorder was analyzed for everyone, as was substance dependence data from each individual for the substances he/she had used more times than the cutoff established). An alternate approach would have been to use two-stage twin models where the relationship between substance use and use disorders is expressly modeled (e.g. (Heath et al. 2002; Kendler et al. 1999). However, this would have resulted in a 7-variable multivariate structure which our sample was underpowered to support. Third, a related caveat is that we included nicotine and cannabis experimenters as unaffected for dependence; an alternative would have been to restrict the analyses to those with more substantial exposure, as we did with alcohol. It is possible that the genetic overlap of CD with ND and CAD might change with a different substance use threshold (e.g., if CD is associated more with the transition to “regular use” than with the transition to “dependence”). Fourth, the ordering of variables in our Cholesky model reflects the most commonly reported order of onset, but there are individuals who had a different order of onset. Although our variance components are applicable regardless of the order of onset, some of the parameter estimates in the Cholesky model would differ if a different variable order was used. Fifth, there is some evidence that the genetic structure of alcohol problems is not attributable to a single factor; in twin analyses examining the 7 individual symptoms of DSM-IV AD, Kendler and colleagues (2012) found that the individual symptoms were best explained using three distinct genetic factors. However, analyzing the individual symptoms for AD, ND and CAD was beyond the scope of our analysis. Sixth, our CAD diagnoses included only a subset of DSM-IV symptoms (1+ of 2 abuse symptoms or 2+ of 4 dependence symptoms), which may have affected our prevalence and/or heritability. This was done because only this subset of items was included in the 1996–2000 cohort assessment. However, Lynskey and colleagues (Lynskey et al. 2002) have reported that a diagnosis of CAD based on these 6 items reasonably approximates the population prevalence and does not attenuate estimates of heritability. Finally, all of our diagnoses are drawn from adult retrospective reports which may have been subject to recall bias.
In this era of big data, several efforts are underway to mega- and meta-analyze genomewide data for substance use disorders. Our study indicates that looking across substances may provide a unique set of genetic variants that relate to a general vulnerability to conduct problems (at least among substance initiators), whereas covarying for comorbid conduct disorder may isolate substance specific findings. Importantly, our study documents the enormous value of twin studies in providing a paradigm for development of substance-related phenotypes for gene-identification efforts.
Supplementary Material
Acknowledgments
The authors thank the Australian Twin Registry and the twins themselves for participating in this research.
Financial support
This study was supported by grant funding from the National Institute on Drug Abuse (NIDA) grants DA23668 (AA), DA32573 (AA), DA18267 (MTL) and T32DA007313 (LF) as well as from the National Institute of Alcohol Abuse and Alcoholism (NIAAA) including AA11998, AA07728 and AA13221 to ACH. Data collection for sample 2 was also facilitated through access to the Australian Twin Registry, a national resource supported by an Enabling Grant (ID 628911) from the National Health & Medical Research Council. Funding agencies did not participate in design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Footnotes
Conflicts of Interest
All authors report no potential conflicts of interest.
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008. JDG and AA had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Reference List
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- Bierut LJ, Goate AM, Breslau N, Johnson EO, Bertelsen S, Fox L, Agrawal A, Bucholz KK, Grucza R, Hesselbrock V, Kramer J, Kuperman S, Nurnberger J, Porjesz B, Saccone NL, Schuckit M, Tischfield J, Wang JC, Foroud T, Rice JP, Edenberg HJ. ADH1B is associated with alcohol dependence and alcohol consumption in populations of European and African ancestry. Mol. Psychiatry. 2011 doi: 10.1038/mp.2011.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucholz KK, Cadoret R, Cloninger CR, Dinwiddie SH, Hesselbrock VM, Nurnberger JI, Jr, Reich T, Schmidt I, Schuckit MA. A new, semi-structured psychiatric interview for use in genetic linkage studies: a report on the reliability of the SSAGA. J Stud Alcohol. 1994;55:149–158. doi: 10.15288/jsa.1994.55.149. [DOI] [PubMed] [Google Scholar]
- Button TM, Hewitt JK, Rhee SH, Young SE, Corley RP, Stallings MC. Examination of the causes of covariation between conduct disorder symptoms and vulnerability to drug dependence. Twin Res Hum Genet. 2006;9:38–45. doi: 10.1375/183242706776402993. [DOI] [PubMed] [Google Scholar]
- Button TM, Rhee SH, Hewitt JK, Young SE, Corley RP, Stallings MC. The role of conduct disorder in ex;laining the comorbidity between alcohol and illicit drug dependence in adolescence. Drug Alcohol Depend. 2007;87:46–53. doi: 10.1016/j.drugalcdep.2006.07.012. [DOI] [PubMed] [Google Scholar]
- Fu Q, Heath AC, Bucholz KK, Nelson E, Goldberg J, Lyons MJ, True WR, Jacob T, Tsuang MT, Eisen SA. Shared genetic risk of major depression, alcohol dependence, and marijuana dependence: contribution of antisocial personality disorder in men. Archives of General Psychiatry. 2002;59:1125–1132. doi: 10.1001/archpsyc.59.12.1125. [DOI] [PubMed] [Google Scholar]
- Gelernter J, Kranzler HR, Sherva R, Almasy L, Koesterer R, Smith AH, Anton R, Preuss UW, Ridinger M, Rujescu D, Wodarz N, Zill P, Zhao H, Farrer LA. Genome-wide association study of alcohol dependence:significant findings in African- and European-Americans including novel risk loci. Mol. Psychiatry. 2014;19:41–49. doi: 10.1038/mp.2013.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath AC, Bucholz KK, Madden PA, Dinwiddie SH, Slutske WS, Bierut LJ, Statham DJ, Dunne MP, Whitfield JB, Martin NG. Genetic and environmental contributions to alcohol dependence risk in a national twin sample: consistency of findings in women and men. Psychological Medicine. 1997;27:1381–1396. doi: 10.1017/s0033291797005643. [DOI] [PubMed] [Google Scholar]
- Heath AC, Martin NG, Lynskey MT, Todorov AA, Madden PA. Estimating two-stage models for genetic influences on alcohol, tobacco or drug use initiation and dependence vulnerability in twin and family data. Twin Research. 2002;5:113–124. doi: 10.1375/1369052022983. [DOI] [PubMed] [Google Scholar]
- Hicks BM, Schalet BD, Malone SM, Iacono WG, McGue M. Psychometric and genetic architecture of substance use disorder and behavioral disinhibition measures for gene association studies. Behav Genet. 2011;41:459–475. doi: 10.1007/s10519-010-9417-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacono WG, Carlson SR, Taylor J, Elkins IJ, McGue M. Behavioral disinhibition and the development of substance-use disorders: findings from the Minnesota Twin Family Study. Dev Psychopathol. 1999;11:869–900. doi: 10.1017/s0954579499002369. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Jacobson KC, Prescott CA, Neale MC. Specificity of genetic and environmental risk factors for use and abuse/dependence of cannabis, cocaine, hallucinogens, sedatives, stimulants, and opiates in male twins. American Journl of Psychiatry. 2003a;160:687–695. doi: 10.1176/appi.ajp.160.4.687. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Myers J, Prescott CA. Specificity of genetic and environmental risk factors for symptoms of cannabis, cocaine, alcohol, caffeine, and nicotine dependence 1. Arch Gen Psychiatry. 2007;64:1313–1320. doi: 10.1001/archpsyc.64.11.1313. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Neale MC, Sullivan P, Corey LA, Gardner CO, Prescott CA. A population-based twin study in women of smoking initiation and nicotine dependence. Psychological Medicines. 1999;29:299–308. doi: 10.1017/s0033291798008022. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Prescott CA, Myers J, Neale MC. The structure of genetic and environmental risk factors for common psychiatric and substance use disorders in men and women. Archives of General Psychiatry. 2003b;60:929–937. doi: 10.1001/archpsyc.60.9.929. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Schmitt E, Aggen SH, Prescott CA. Genetic and environmental influences on alcohol, caffeine, cannabis, and nicotine use from early adolescence to middle adulthood. Arch Gen Psychiatry. 2008;65:674–682. doi: 10.1001/archpsyc.65.6.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knopik VS, Heath AC, Madden PA, Bucholz KK, Slutske WS, Nelson EC, Statham D, Whitfield JB, Martin NG. Genetic effects on alcohol dependence risk: re-evaluating the importance of psychiatric and other heritable risk factors. Psychol Med. 2004;34:1519–1530. doi: 10.1017/s0033291704002922. [DOI] [PubMed] [Google Scholar]
- Krueger RF, Hicks BM, Patrick CJ, Carlson SR, Iacono WG, McGue M. Etiologic connections among substance dependence, antisocial behavior, and personality: modeling the externalizing spectrum. J Abnorm Psychol. 2002;111:411–424. [PubMed] [Google Scholar]
- Liu JZ, Tozzi F, Waterworth DM, Pillai SG, Muglia P, Middleton L, Berrettini W, Knouff CW, Yuan X, Waeber G, Vollenweider P, Preisig M, Wareham NJ, Zhao JH, Loos RJ, Barroso I, Khaw KT, Grundy S, Barter P, Mahley R, Kesaniemi A, McPherson R, Vincent JB, Strauss J, Kennedy JL, Farmer A, McGuffin P, Day R, Matthews K, Bakke P, Gulsvik A, Lucae S, Ising M, Brueckl T, Horstmann S, Wichmann HE, Rawal R, Dahmen N, Lamina C, Polasek O, Zgaga L, Huffman J, Campbell S, Kooner J, Chambers JC, Burnett MS, Devaney JM, Pichard AD, Kent KM, Satler L, Lindsay JM, Waksman R, Epstein S, Wilson JF, Wild SH, Campbell H, Vitart V, Reilly MP, Li M, Qu L, Wilensky R, Matthai W, Hakonarson HH, Rader DJ, Franke A, Wittig M, Schafer A, Uda M, Terracciano A, Xiao X, Busonero F, Scheet P, Schlessinger D, St CD, Rujescu D, Abecasis GR, Grabe HJ, Teumer A, Volzke H, Petersmann A, John U, Rudan I, Hayward C, Wright AF, Kolcic I, Wright BJ, Thompson JR, Balmforth AJ, Hall AS, Samani NJ, Anderson CA, Ahmad T, Mathew CG, Parkes M, Satsangi J, Caulfield M, Munroe PB, Farrall M, Dominiczak A, Worthington J, Thomson W, Eyre S, Barton A, Mooser V, Francks C, Marchini J. Meta-analysis and imputation refines the association of 15q25 with smoking quantity. Nat Genet. 2010;42:436–440. doi: 10.1038/ng.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynskey M, Agrawal A, Henders AK, Nelson EC, Madden P, Martin NG. An Australian Twin Study of Cannabis and Other Illicit Drug Use and Misuse, and other psychopathology. Twin Res Hum Genet. 2012;15:631–641. doi: 10.1017/thg.2012.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynskey MT, Heath AC, Nelson EC, Bucholz KK, Madden PA, Slutske WS, Statham DJ, Martin NG. Genetic and environmental contributions to cannabis dependence in a national young adult twin sample. Psychol Med. 2002;32:195–207. doi: 10.1017/s0033291701005062. [DOI] [PubMed] [Google Scholar]
- Maciejewski DF, Creemers HE, Lynskey MT, Madden PA, Heath AC, Statham DJ, Martin NG, Verweij KJ. Overlapping genetic and environmental influences on nonsuicidal self-injury and suicidal ideation: different outcomes, same etiology? JAMA Psychiatry. 2014;71:699–705. doi: 10.1001/jamapsychiatry.2014.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGue M, Zhang Y, Miller MB, Basu S, Vrieze S, Hicks B, Malone S, Oetting WS, Iacono WG. A genome-wide association study of behavioral disinhibition. Behav Genet. 2013;43:363–373. doi: 10.1007/s10519-013-9606-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medland SE, Nyholt DR, Painter JN, McEvoy BP, McRae AF, Zhu G, Gordon SD, Ferreira MA, Wright MJ, Henders AK, Campbell MJ, Duffy DL, Hansell NK, Macgregor S, Slutske WS, Heath AC, Montgomery GW, Martin NG. Common variants in the trichohyalin gene are associated with straight hair in Europeans. Am J Hum Genet. 2009;85:750–755. doi: 10.1016/j.ajhg.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neale MC. Dept. of Psychiatry. Richmond VA: 2004. Statistical Modeling with Mx; p. 23298. Box # 980710. [Google Scholar]
- Neale MC, Cardon LR. Methodology for Genetic studies of Twins and Families. Netherlands: Kluwer Academic Publishers; 1992. [Google Scholar]
- Neale MC, Harvey E, Maes HH, Sullivan PF, Kendler KS. Extensions to the modeling of initiation and progression: applications to substance use and abuse. Behav Genet. 2006;36:507–524. doi: 10.1007/s10519-006-9063-x. [DOI] [PubMed] [Google Scholar]
- Palmer RH, Young SE, Corley RP, Hopfer CJ, Stallings MC, Hewitt JK. Stability and change of genetic and environmental effects on the common liability to alcohol, tobacco, and cannabis DSM-IV dependence symptoms. Behav Genet. 2013;43:374–385. doi: 10.1007/s10519-013-9599-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee SH, Hewitt JK, Young SE, Corley RP, Crowley TJ, Neale MC, Stallings MC. Comorbidity between alcohol dependence and illicit drug dependence in adolescents with antisocial behavior and matched controls. Drug Alcohol Depend. 2006;84:85–92. doi: 10.1016/j.drugalcdep.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Rice JP, Hartz SM, Agrawal A, Almasy L, Bennett S, Breslau N, Bucholz KK, Doheny KF, Edenberg HJ, Goate AM, Hesselbrock V, Howells WB, Johnson EO, Kramer J, Krueger RF, Kuperman S, Laurie C, Manolio TA, Neuman RJ, Nurnberger JI, Porjesz B, Pugh E, Ramos EM, Saccone N, Saccone S, Schuckit M, Bierut LJ. CHRNB3 is more strongly associated with Fagerstrom test for cigarette dependence-based nicotine dependence than cigarettes per day: phenotype definition changes genome-wide association studies results. Addiction. 2012;107:2019–2028. doi: 10.1111/j.1360-0443.2012.03922.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose RJ, Dick DM, Viken RJ, Pulkkinen L, Kaprio J. Genetic and environmental effects on conduct disorder and alcohol dependence symptoms and their covariation at age 14. Alcohol Clin. Exp. Res. 2004;28:1541–1548. doi: 10.1097/01.alc.0000141822.36776.55. [DOI] [PubMed] [Google Scholar]
- SAS Institute, Inc. SAS User’s Guide, Version 9.2. Cary, NC: SAS Institute, Inc; 2002–2008. [Google Scholar]
- Sartor CE, Grant JD, Bucholz KK, Madden PA, Heath AC, Agrawal A, Whitfield JB, Statham DJ, Martin NG, Lynskey MT. Common genetic contributions to alcohol and cannabis use and dependence symptomatology. Alcohol Clin Exp. Res. 2010;34:545–554. doi: 10.1111/j.1530-0277.2009.01120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomasson HR, Crabb DW, Edenberg HJ, Li TK. Alcohol and aldehyde dehydrogenase polymorphisms and alcoholism. Behav Genet. 1993;23:131–136. doi: 10.1007/BF01067417. [DOI] [PubMed] [Google Scholar]
- Thorgeirsson TE, Gudbjartsson DF, Surakka I, Vink JM, Amin N, Geller F, Sulem P, Rafnar T, Esko T, Walter S, Gieger C, Rawal R, Mangino M, Prokopenko I, Magi R, Keskitalo K, Gudjonsdottir IH, Gretarsdottir S, Stefansson H, Thompson JR, Aulchenko YS, Nelis M, Aben KK, den HM, Dirksen A, Ashraf H, Soranzo N, Valdes AM, Steves C, Uitterlinden AG, Hofman A, Tonjes A, Kovacs P, Hottenga JJ, Willemsen G, Vogelzangs N, Doring A, Dahmen N, Nitz B, Pergadia ML, Saez B, De D, V Lezcano V, Garcia-Prats MD, Ripatti S, Perola M, Kettunen J, Hartikainen AL, Pouta A, Laitinen J, Isohanni M, Huei-Yi S, Allen M, Krestyaninova M, Hall AS, Jones GT, van Rij AM, Mueller T, Dieplinger B, Haltmayer M, Jonsson S, Matthiasson SE, Oskarsson H, Tyrfingsson T, Kiemeney LA, Mayordomo JI, Lindholt JS, Pedersen JH, Franklin WA, Wolf H, Montgomery GW, Heath AC, Martin NG, Madden PA, Giegling I, Rujescu D, Jarvelin MR, Salomaa V, Stumvoll M, Spector TD, Wichmann HE, Metspalu A, Samani NJ, Penninx BW, Oostra BA, Boomsma DI, Tiemeier H, van Duijn CM, Kaprio J, Gulcher JR, McCarthy MI, Peltonen L, Thorsteinsdottir U, Stefansson K. Sequence variants at CHRNB3-CHRNA6 and CYP2A6 affect smoking behavior. Nat Genet. 2010;42:448–453. doi: 10.1038/ng.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobacco and Genetics Consortium. Genome-wide meta-analyses identify multiple loci associated with smoking behavior. Nat Genet. 2010;42:441–447. doi: 10.1038/ng.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrieze SI, Feng S, Miller MB, Hicks BM, Pankratz N, Abecasis GR, Iacono WG, McGue M. Rare nonsynonymous exonic variants in addiction and behavioral disinhibition. Biol. Psychiatry. 2014;75:783–789. doi: 10.1016/j.biopsych.2013.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrieze SI, Hicks BM, Iacono WG, McGue M. Decline in genetic influence on the co-occurrence of alcohol, marijuana, and nicotine dependence symptoms from age 14 to 29. Am J Psychiatry. 2012;169:1073–1081. doi: 10.1176/appi.ajp.2012.11081268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrieze SI, McGue M, Miller MB, Hicks BM, Iacono WG. Three mutually informative ways to understand the genetic relationships among behavioral disinhibition, alcohol use, drug use, nicotine use/dependence, and their co-occurrence: twin biometry, GCTA, and genome-wide scoring. Behav Genet. 2013;43:97–107. doi: 10.1007/s10519-013-9584-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetherill L, Agrawal A, Kapoor M, Bertelsen S, Bierut LJ, Brooks A, Dick D, Hesselbrock M, Hesselbrock V, Koller DL, Le N, Nurnberger JI, Jr, Salvatore JE, Schuckit M, Tischfield JA, Wang JC, Xuei X, Edenberg HJ, Porjesz B, Bucholz K, Goate AM, Foroud T. Association of substance dependence phenotypes in the COGA sample. Addict. Biol. 2014:10. doi: 10.1111/adb.12153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xian H, Scherrer JF, Grant JD, Eisen SA, True WR, Jacob T, Bucholz KK. Genetic and environmental contributions to nicotine, alcohol and cannabis dependence in male twins. Addiction. 2008;103:1391–1398. doi: 10.1111/j.1360-0443.2008.02243.x. [DOI] [PubMed] [Google Scholar]
- Young SE, Rhee SH, Stallings MC, Corley RP, Hewitt JK. Genetic and environmental vulnerabilities underlying adolescent substance use and problem use: general or specific? Behav Genet. 2006;36:603–615. doi: 10.1007/s10519-006-9066-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.