Introduction
A variety of pathological conditions can increase risk for development of ventricular arrhythmias in humans including diabetes, obesity, myocardial infarction (MI), and heart failure. Many of these diseases involve global disruption of the autonomic nervous system, including increased sympathetic drive and parasympathetic withdrawal, but another common factor among these disorders is sympathetic dysfunction within the heart. Treatments that target cardiac sympathetic transmission, including beta blockers and ganglionectomy, prolong life and decrease arrhythmias 1–5.
Sympathetic control of the heart under normal conditions occurs primarily via norepinephrine (NE) acting on β adrenergic receptors (β-AR) to stimulate increases in heart rate (chronotropy), conduction velocity (dromotropy), and contractility (inotropy). These positive effects of sympathetic stimulation allow myocytes to meet increased cardiac demands during stress or exercise, serving to maintain homeostasis. The nervous system adapts to changing conditions, however, and sympathetic neurons undergo structural and functional alterations in response to injury and disease. There are at least four types of sympathetic remodeling that occur during conditions of increased arrhythmia susceptibility: hyperinnervation (increased nerve density), denervation (decreased nerve density), altered neurotransmitter or neuropeptide production, and increased neuronal excitability. Rubart and Zipes proposed a model to explain how these diverse changes in sympathetic transmission might contribute to arrhythmia generation 6, suggesting that inappropriate heterogeneity of NE release within the heart leads to differential electrical remodeling of cardiac myocytes and predisposes the heart to electrical instability. Many studies now support the hypothesis that heterogeneity of noradrenergic transmission increases the risk of arrhythmia, and have identified some of the mechanisms that underlie neuronal remodeling. This review will examine the mechanisms of sympathetic remodeling and will connect neural changes to increased arrhythmia susceptibility. We will focus on ventricular arrhythmias, as atrial arrhythmias were reviewed recently 7.
Hyperinnervation and excess NE release
Regional hyperinnervation was the first type of neural remodeling linked to arrhythmia generation in humans 8, 9. Areas of sympathetic hyperinnervation, defined as increased nerve fiber density compared to control tissue, have now been identified in many conditions with increased arrhythmia susceptibility including heart failure 9, myocardial infarction 10, 11, spinal cord injury 12, and diet induced obesity13, 14. Nerve growth factor (NGF) has been the focus of studies related to cardiac hyperinnervation since NGF stimulates the extension, or sprouting, of sympathetic nerve endings during development and after injury. Cardiac NGF is elevated in animal and human hearts during conditions associated with hyperinnervation 10, 12, 15, and is decreased in conditions that include a loss of functional sympathetic innervation such as late stage heart failure 16 and diabetic neuropathy 17.
Sympathetic axon outgrowth is triggered through activation of the TrkA (Tropomyosin receptor kinase A) receptor, which stimulates serine phosphorylation of STAT3 (signal transducer and activator of transcription 3) in addition to activating other signaling pathways. Although TrkA can be activated by both NGF and Neurotrophin 3 during development, NGF is the only ligand that activates TrkA in mature sympathetic neurons, and thus is crucial for maintaining sympathetic neuron health and stimulating axon regeneration. Recent studies indicate that cytokines like Leukemia Inhibitory Factor (LIF) and Cardiotrophin-1 (CT-1), which activate the gp130 receptor, do not stimulate axon growth on their own but are required for maximal NGF-induced sympathetic axon extension 18. These cytokines stimulate tyrosine phosphorylation of STAT3, and phosphorylation of STAT3 on both serine and tyrosine is required for maximal axon outgrowth 18. The related cytokine leptin also enhances sympathetic axon outgrowth 19, and may contribute to the cardiac hyperinnervation observed in diet-induced obesity.
Pathological conditions resulting in hyperinnervation within the heart are also associated with increased sympathetic drive from the CNS, and enhanced excitability of post-ganglionic neurons may also contribute to elevated adrenergic transmission in the heart. Neuronal cell size is increased significantly in stellate ganglia removed from humans with ischemic and non-ischemic cardiomyopathy compared to control ganglia 20, and similar changes identified in canines coincide with increased neuronal excitability 21. Similarly, increased dendrite field size and synapse number contribute to elevated cardiac sympathetic tone in rats following T5 spinal cord transection 22. Retrograde signaling by NGF regulates synapse formation during development 23 and high NGF likely contributes to increased cell size and synapse formation after injury. NGF may also increase sympathetic excitability by altering sensitivity to inflammatory mediators 24, and by altering neuronal firing properties 25. Interestingly, statins decrease sympathetic neuron excitability by stimulating dendrite retraction 26, raising the possibility that dampening sympathetic drive via peripheral actions contributes to their therapeutic value in patients with cardiovascular disease.
Functional noradrenergic transmission requires a balance between NE synthesis, release, and reuptake, each of which can be regulated independently 27. For example, after myocardial infarction newly sprouting sympathetic fibers have low NE content 28, but there is a paradoxical increase in extracellular NE 27. This can be explained in part by elevated NE synthesis and release in other regions of the heart that are not matched by a similar increase in NE removal 11. Such functional changes have been identified in patients with heart failure, where increased NE release as well as decreased NE reuptake contribute to a buildup of extracellular NE and excessive activation of β-AR 29.
Myocardial responses to acute hyperinnervation/excess NE
Norepinephrine released from sympathetic nerves activates cardiac β-AR to modulate myocyte repolarization and contractility. Sympathetic nerves are not distributed evenly across the heart, but are most dense near the base of the ventricles. Likewise, the epicardial to endocardial gradient in cardiac action potential duration (APD) 30–32 that is critical for normal activation and repolarization of the left ventricle is regulated by innervation, and disrupting the normal organization of sympathetic nerves in an otherwise healthy heart is arrhythmogenic 33, 34. Activation of cardiac β-AR modulates myocyte repolarization by altering transmembrane currents and Ca2+ homeostasis 35–37. β-AR stimulated cAMP leads to phosphorylation of proteins involved in excitation-contraction coupling including phospholamban, L-type Ca2+ channels, and ryanodine receptors (RyR), resulting in increased sarcoplasmic reticulum (SR) Ca2+-ATPase (SERCA) activity and an increase in SR Ca2+ content38. Thus, during sympathetic stimulation more Ca2+ is released from the SR39 to activate the myofilaments, increasing contractility, but spontaneous Ca2+ release from the SR also becomes more likely 40, 41. Therefore, the positive inotropic effects of sympathetic stimulation that allow myocytes to meet increased cardiac demands are accompanied by an increased risk for pathological arrhythmias via focal (triggered) mechanisms.
At the cellular level, focal activity during sympathetic activation is likely due to delayed afterdepolarizations (DADs),42 which are membrane depolarizations occurring during phase 4 of the action potential. The increased cytosolic and SR Ca2+ levels that occur during sympathetic activation can lead to SR Ca2+ overload (Figure 1), which may result in spontaneous opening of RyRs and Ca2+ release that is not in response to an action potential. This leads to Ca2+ extrusion from the cytosol via the Na+/Ca2+ exchanger (NCX).43 NCX is electrogenic, extruding one Ca2+ ion (2+) in exchange for 3 Na+ ions (3+), which produces a net inward current. If the inward current is large enough, the cell membrane depolarizes and a triggered action potential may occur.
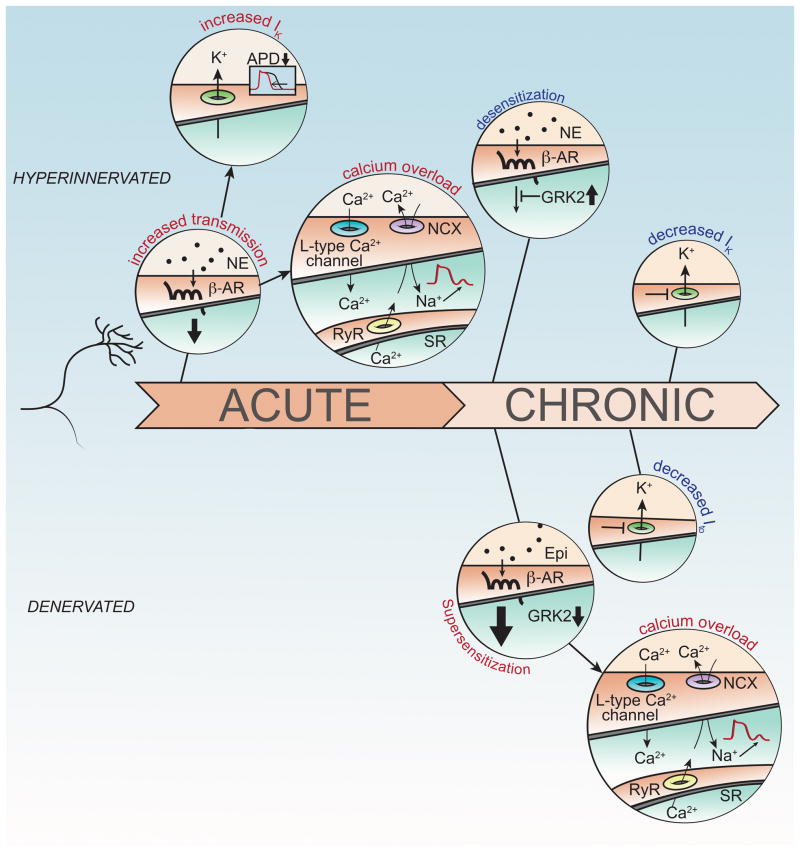
Figure 1.
Hyperinnervation and denervation can both contribute to arrhythmias. Acute hyperinnervation or excess NE (Top) leads to increased activation of β-ARs (beta adrenergic receptors) and subsequent changes in Ik and calcium overload. β-AR signaling increases Ik which shortens action potential duration (APD). At the same time, intracellular Ca2+ rises due to enhanced influx via the L-type Ca2+ channel and increased release from the sarcoplasmic reticulum (SR). Extrusion of Ca2+ from the cytosol via the Na+/Ca2+ exchanger (NCX) produces a net inward current leading to delayed afterdepolarizations (DADs). In contrast, chronic hyperinnervation leads to desensitization of β-AR signaling and decreased Ik. Chronic denervation (Bottom) results in β-AR supersensitivity and decreased Ito. Activation of super-sensitive β-AR signaling pathways by circulating epinephrine leads to calcium overload and DADs.
At the tissue level, several thousand cells must all experience DADs simultaneously in order to generate enough depolarizing current to produce a propagating action potential (called the ‘source-sink mismatch’).44, 45 Recent studies revealed that local application of β-AR agonists such as NE or isoproterenol can induce premature ventricular complexes (PVCs) and sustained focal VT in intact hearts,46–49 providing a mechanistic link between the numerous experimental and clinical investigations that have found regional hyperinnervation accompanied by increased arrhythmia risk. 8 Furthermore, electrophysiological remodeling of cardiac myocytes in response to myocardial infarction or heart failure can cause changes (e.g., increased expression of NCX,50 increased SR Ca2+ leak through RyR,51 decreased inward rectifying K+ current,43 decreased gap junction coupling,52 and fibrosis) that further increase the likelihood of DADs and arrhythmia generation in response to localized sympathetic stimulation. 45, 46
Sympathetic stimulation also has effects on ionic currents that impact the ventricular action potential and risk for reentrant arrhythmias. The effects of adrenergic activation on individual ion channels have been reviewed elsewhere53, 54, but one of the best-known features of β-AR stimulation is an increase in L-type Ca2+ current via phosphorylation of the channel (Cav1.2).55 This effect, by itself, is expected to increase APD, but is counterbalanced by the effect of β-AR on K+ currents, most notably an increase in IKs, although IKr may also be involved.56, 57 The net effect of NE typically results in a shortening of the APD, a requirement for the heart to beat at faster rates during sympathetic activity. Due to the base to apex gradient in cardiac sympathetic nerves, however, sympathetic activation results in non-uniform changes in APD throughout the ventricle. For example, sympathetic nerve stimulation in a normal rabbit heart led to increased dispersion of repolarization and reversed the direction of the repolarization wavefront.58 Administration of the β-AR agonist isoproterenol generated a different set of responses, suggesting that the dramatic changes in repolarization observed with sympathetic nerve stimulation were not due to differences in β-AR distribution or sensitivity, but rather due to the heterogeneous distribution of the nerves. 58 Similar results have been obtained in porcine ventricles, where dramatically different spatial patterns of activation-recovery intervals (ARIs, surrogate measure for APD) and repolarization were observed in response to sympathetic nerve stimulation vs. circulating NE59, 60. Thus, even under non-pathological conditions, considerable heterogeneity of sympathetic nerve distribution leads to increased dispersion of repolarization and potential for reentrant arrhythmias. Therefore, in conditions of maladaptive nerve remodeling, significantly greater heterogeneity of APD and repolarization may exist. Indeed, sympathetic stimulation after myocardial infarction not only caused increased dispersion of repolarization compared to controls, but activation and propagation patterns were also altered significantly 61. This was confirmed in patients with MI in whom reflex sympathetic stimulation caused a 230% increase in dispersion of repolarization compared to patients with structurally normal hearts.62
Myocardial responses to chronic hyperinnervation/excess NE
Acute effects of sympathetic activation often occur on a background of remodeled myocardial properties induced by heart failure or MI, but alterations to sympathetic transmission can also lead to chronic remodeling of the myocardium. Sympathetic hyperinnervation and elevated sympathetic tone are key features of many cardiovascular diseases. In these conditions the myocardium becomes less responsive to adrenergic stimulation over time, and simultaneously less capable of maintaining adequate cardiac output, which further increases sympathetic drive from the CNS. The loss of cardiomyocyte responsiveness to adrenergic stimulation is a hallmark of sustained adrenergic stimulation and hyperinnervation 63. Several factors contribute to this loss of sensitivity, including down-regulation of the receptor itself 64, but an especially important regulator of β-AR activity is the G-protein receptor kinase 2 (GRK2, also known as βARK1). Acutely, GRK2 is activated by PKA in response to adrenergic stimulation and acts to inhibit β-AR activity in a self-contained negative feedback loop. Sustained activation of β-AR in adult mice, however, leads to increased GRK2 expression 64. A similar increase in GRK2 is seen in canine heart failure, where it is reversed by sympathetic denervation, confirming regulation of GRK2 by sympathetic transmission 65. Long term activation of β-AR also leads to G-protein uncoupling and a reduction of Gαs protein 66, as well as a reduction of repolarizing K+ current 67, 68. Thus, sustained activation of adrenergic receptors leads to adaptations that limit myocyte sensitivity to adrenergic stimulation and alter ion channel expression. Long term treatment with beta blockers blunts many of these adaptations 63 and normalizes myocyte calcium handling 69, contributing to the well-established protective effects of sympathetic blockade 1–4.
Cardiac denervation and axon degeneration
The mechanisms by which too much sympathetic transmission can be toxic for the heart are well-characterized, but the local loss of sympathetic transmission within the heart also contributes to rhythm instability. Regional deficits in sympathetic transmission, identified in patients by imaging the uptake of labeled NE transporter substrates, have been observed in several pathological conditions including myocardial infarction 70, 71, heart failure 72, and Parkinson’s Disease 73. Several recent clinical studies suggest that sympathetic denervation after MI predicts the probability of serious ventricular arrhythmias 74–76, and a detailed electrical mapping study in human hearts revealed that sympathetic denervation of the normal myocardium adjacent to the scar resulted in β-AR agonist super-sensitivity and increased dispersion of repolarization that was arrhythmogenic 62.
Paradoxically, members of the neurotrophin family of growth factors can be involved in the destruction of sympathetic nerves following cardiac injury. While the neurotrophin NGF stimulates TrkA in sympathetic neurons to promote axon maintenance and process outgrowth, its precursor protein ProNGF, which is elevated in the human heart after MI 77, activates the p75 neurotrophin receptor (p75NTR; also called TNF receptor super family 16, TNFRS16), to trigger axon degeneration 78, 79 (Figure 2). Similarly, ProBDNF (Pro Brain Derived Neurotrophic Factor) and BDNF selectively activate p75NTR on sympathetic neurons to stimulate axon degeneration 80. The Trk tyrosine kinase receptors and p75NTR have opposing actions not just in cardiac sympathetic nerves81, but also in the coronary vasculature 77 and cardiac myocytes 15, 82. Thus, ProNGF activation of p75NTR after MI leads to the loss of nerve fibers in viable myocardium 81 as well as microvascular damage and scar extension 77.
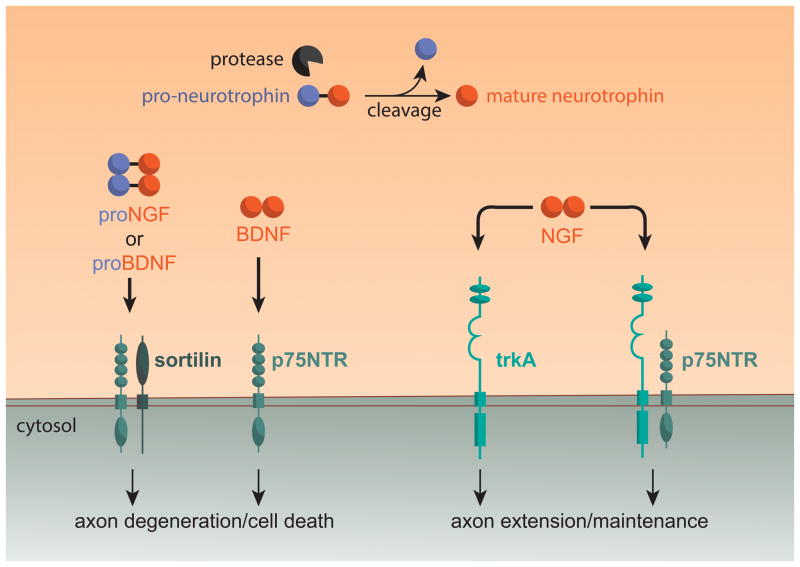
Figure 2.
Neurotrophins stimulate different effects in sympathetic neurons via activation of p75NTR and/or TrkA. Pro-Neurotrophins like ProNGF and ProBDNF are processed to mature neurotrophins (NGF, BDNF) by intra- and extra-cellular proteases. Activation of a p75NTR/Sortilin receptor complex by ProNGF or ProBDNF, or activation of p75NTR by BDNF, stimulates axon degeneration in sympathetic neurons. In contrast, NGF signaling via TrkA or a TrkA/p75NTR receptor complex stimulates sympathetic axon maintenance and growth.
While activation of p75NTR can contribute to the loss of cardiac nerves, other factors are involved in sustaining denervation. Chondroitin sulfate proteoglycans (CSPGs) are produced in the cardiac scar after ischemia-reperfusion, where they prevent reinnervation of the border zone and scar 83. This contrasts with the scar that forms after sustained ischemia, which is devoid of CSPGs 83 and receives sympathetic hyperinnervation 10, 27. Removing or inhibiting the CSPG receptor protein tyrosine phosphatase receptor sigma (PTPσ) in mice leads to reinnervation of the scar and border zone, restoring normal NE content and β-AR responsiveness to that region of the damaged left ventricle 84. Consistent with the human studies linking post-MI denervation to arrhythmia risk, restoring innervation throughout the scar and border zone in mouse heart normalizes post-MI calcium handling and decreases arrhythmia susceptibility 84.
Myocardial responses to chronic denervation
Just as sympathetic hyperinnervation can alter the molecular makeup of myocytes, sustained sympathetic denervation has similarly profound effects. One of the best characterized changes is a loss of the transient outward K+ current Ito, which is responsible for the initial repolarization in phase 1 of the action potential. Sympathetic denervation in rat decreases Ito by lowering expression of several different K+ channel subunits, and increases susceptibility to ventricular fibrillation 85, 86. Decreased Ito is also observed in disease states characterized by sympathetic denervation including Chagas disease, diabetic neuropathy, and myocardial infarction 84, 87, 88. Restoring adrenergic transmission in Chagas animals with NE infusion 89, or promoting sympathetic re-innervation of denervated infarct and border zone tissue 84 reverses the loss of Ito.
The consequences of sympathetic denervation are not limited to the transient outward K+ current. While hyperinnervation increases GRK2, sustained treatment with the beta blocker atenolol in mice90 and surgical sympathectomy in dogs65 leads to GRK2 down-regulation. This reduction in GRK2 may play an important role in the β-AR supersensitivity observed following sympathetic denervation, as GRK2 knock out mice exhibit a similar supersensitivity91. The absence of GRK2 also alters Ca2+ homeostasis by reducing SERCA activity, which leads to reduced SR Ca2+ load and increased cytosolic Ca2+ levels, thus increasing NCX activity91. Increased activity of the electrogenic NCX can initiate DADs, and the adrenergic supersensitivity that accompanies decreased GRK2 increases the likelihood that β-AR stimulation will be sufficient to overcome source-sink mismatch and generate focal arrhythmia47. Consistent with this possibility, isoproterenol stimulation of hearts after MI triggers focal arrhythmias that arise from denervated tissue near the infarct, while release of NE from sympathetic nerves in the same hearts does not trigger arrhythmias 84. Restoration of sympathetic innervation to the scar and border zone of infarcted hearts prevents isoproterenol-induced arrhythmias and abnormal Ca2+ handling, confirming a role for denervation induced β-AR super-sensitivity in arrhythmia generation 84. Sudden cardiac death is most common in the morning 92 when circulating catecholamines are rising rapidly 93, suggesting that high circulating NE and epinephrine trigger arrhythmias in denervated myocardium via activating super-sensitive β-AR signaling pathways. Thus, denervation and hyperinnervation may trigger arrhythmias via similar mechanisms within cardiac myocytes.
Neurotransmitter and neuropeptide production
In addition to the loss or gain of nerve fibers, sympathetic neurons innervating the heart can undergo changes in neurotransmitter and peptide production and release following injury. Sympathetic nerves in the heart produce the peptide co-transmitter neuropeptide Y (NPY), which inhibits release of ACh from cardiac parasympathetic nerves 94 and causes vasoconstriction on the cardiac vasculature 95. NPY is elevated after MI 96, and high plasma NPY levels in patients with acute ST elevation MI correlate with increased microvascular resistance following reperfusion 97. NPY is released during periods of high sympathetic drive, and in the context of myocardial infarction high levels of sympathetic activation resulting in NPY release appears to be detrimental for the heart. Over a longer time frame, cardiac damage can lead to changes in neuropeptide and neurotransmitter expression in sympathetic neurons. The best characterized change in sympathetic transmission is a developmental transition from production of NE to ACh due to the actions of gp130 cytokines 98. Recent studies revealed a similar change in phenotype triggered by cytokines like LIF and CT-1 during heart failure 99. Stellate ganglia obtained from humans with heart failure also exhibited expression of proteins associated with cholinergic transmission 99, suggesting that cholinergic sympathetic transmission can occur in the human heart. Although the functional consequences of ACh release from sympathetic nerves are unclear, NE and ACh have opposing effects on ventricular action potential duration (NE shortens whereas ACh lengthens). Thus, cholinergic sympathetic transmission may indeed be arrhythmogenic by limiting the adaptation of the action potential duration to increased heart rates during sympathetic activity. Therefore, the functional impact of changes in neurotransmitter phenotype represents an important area for future investigation.
Summary
Interactions between sympathetic neurons and cardiac myocytes can become destructive in pathophysiological conditions, giving rise to electrical instability and increased arrhythmia susceptibility. We have summarized the most common changes that occur in cardiac sympathetic neurons during pathologies associated with increased ventricular arrhythmia risk, and how altered neurotransmission might contribute to arrhythmia generation. Many relevant studies were excluded due to reference limits, but we have tried to cite work from different laboratories who have contributed to our understanding. Interventions that target the sympathetic innervation of the heart have been successful in treating arrhythmias, and our hope is that this review will stimulate the development of new interventions aimed at normalizing sympathetic dysfunction.
Acknowledgments
The authors thank O’Reilly Science Art, LLC for developing the figures.
Funding Sources: Work in the author’s laboratories is supported by grants from the NIH including HL068231, HL093056 (B.A.H.), HL111600 (C.J.R.), and T32HL094294 (R.T.G.), an American Heart Association Scientist Development Grant (C.J.R.), the British Heart Foundation (R.C.M.), and the Wellcome Trust (R.C.M.).
Footnotes
Conflict of Interest Disclosures: None
References
- 1.Freemantle N, Cleland J, Young P, Mason J, Harrison J. beta Blockade after myocardial infarction: systematic review and meta regression analysis. BMJ. 1999;318:1730–1737. doi: 10.1136/bmj.318.7200.1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Effect of carvedilol on outcome after myocardial infarction in patients with left-ventricular dysfunction: the CAPRICORN randomised trial. Lancet. 2001;357:1385–1390. doi: 10.1016/s0140-6736(00)04560-8. [DOI] [PubMed] [Google Scholar]
- 3.Poole-Wilson PA, Swedberg K, Cleland JG, Di Lenarda A, Hanrath P, Komajda M, Lubsen J, Lutiger B, Metra M, Remme WJ, Torp-Pedersen C, Scherhag A, Skene A. Comparison of carvedilol and metoprolol on clinical outcomes in patients with chronic heart failure in the Carvedilol Or Metoprolol European Trial (COMET): randomised controlled trial. Lancet. 2003;362:7–13. doi: 10.1016/S0140-6736(03)13800-7. [DOI] [PubMed] [Google Scholar]
- 4.Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in-Congestive Heart Failure (MERIT-HF) Lancet. 1999;353:2001–2007. [PubMed] [Google Scholar]
- 5.Schwartz PJ. Cardiac sympathetic denervation to prevent life-threatening arrhythmias. Nat Rev Cardiol. 2014;11:346–353. doi: 10.1038/nrcardio.2014.19. [DOI] [PubMed] [Google Scholar]
- 6.Rubart M, Zipes DP. Mechanisms of sudden cardiac death. J Clin Invest. 2005;115:2305–2315. doi: 10.1172/JCI26381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shen MJ, Choi EK, Tan AY, Lin SF, Fishbein MC, Chen LS, Chen PS. Neural mechanisms of atrial arrhythmias. Nat Rev Cardiol. 2012;9:30–39. doi: 10.1038/nrcardio.2011.139. [DOI] [PubMed] [Google Scholar]
- 8.Chen PS, Chen LS, Cao JM, Sharifi B, Karagueuzian HS, Fishbein MC. Sympathetic nerve sprouting, electrical remodeling and the mechanisms of sudden cardiac death. Cardiovasc Res. 2001;50:409–416. doi: 10.1016/s0008-6363(00)00308-4. [DOI] [PubMed] [Google Scholar]
- 9.Cao JM, Fishbein MC, Han JB, Lai WW, Lai AC, Wu TJ, Czer L, Wolf PL, Denton TA, Shintaku IP, Chen PS, Chen LS. Relationship between regional cardiac hyperinnervation and ventricular arrhythmia. Circulation. 2000;101:1960–1969. doi: 10.1161/01.cir.101.16.1960. [DOI] [PubMed] [Google Scholar]
- 10.Zhou S, Chen LS, Miyauchi Y, Miyauchi M, Kar S, Kangavari S, Fishbein MC, Sharifi B, Chen PS. Mechanisms of cardiac nerve sprouting after myocardial infarction in dogs. Circ Res. 2004;95:76–83. doi: 10.1161/01.RES.0000133678.22968.e3. [DOI] [PubMed] [Google Scholar]
- 11.Li W, Knowlton D, Van Winkle DM, Habecker BA. Infarction alters both the distribution and noradrenergic properties of cardiac sympathetic neurons. Am J Physiol Heart Circ Physiol. 2004;286:H2229–H2236. doi: 10.1152/ajpheart.00768.2003. [DOI] [PubMed] [Google Scholar]
- 12.Lujan HL, Palani G, Dicarlo SE. Structural neuroplasticity following T5 spinal cord transection: increased cardiac sympathetic innervation density and SPN arborization. Am J Physiol Regul Integr Comp Physiol. 2010;299:R985–R995. doi: 10.1152/ajpregu.00329.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aubin MC, Cardin S, Comtois P, Clement R, Gosselin H, Gillis MA, Le QK, Nattel S, Perrault LP, Calderone A. A high-fat diet increases risk of ventricular arrhythmia in female rats: enhanced arrhythmic risk in the absence of obesity or hyperlipidemia. J Appl Physiol (1985) 2010;108:933–940. doi: 10.1152/japplphysiol.01281.2009. [DOI] [PubMed] [Google Scholar]
- 14.McCully BH, Hasan W, Streiff CT, Houle JC, Woodward WR, Giraud GD, Brooks VL, Habecker BA. Sympathetic cardiac hyperinnervation and atrial autonomic imbalance in diet-induced obesity promote cardiac arrhythmias. Am J Physiol Heart Circ Physiol. 2013;305:H1530–H1537. doi: 10.1152/ajpheart.00196.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meloni M, Caporali A, Graiani G, Lagrasta C, Katare R, Van LS, Spillmann F, Campesi I, Madeddu P, Quaini F, Emanueli C. Nerve Growth Factor Promotes Cardiac Repair following Myocardial Infarction. Circ Res. 2010;106:1275–1284. doi: 10.1161/CIRCRESAHA.109.210088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qin F, Vulapalli RS, Stevens SY, Liang CS. Loss of cardiac sympathetic neurotransmitters in heart failure and NE infusion is associated with reduced NGF. Am J Physiol Heart Circ Physiol. 2002;282:H363–H371. doi: 10.1152/ajpheart.00319.2001. [DOI] [PubMed] [Google Scholar]
- 17.Ieda M, Kanazawa H, Ieda Y, Kimura K, Matsumura K, Tomita Y, Yagi T, Onizuka T, Shimoji K, Ogawa S, Makino S, Sano M, Fukuda K. Nerve growth factor is critical for cardiac sensory innervation and rescues neuropathy in diabetic hearts. Circulation. 2006;114:2351–2363. doi: 10.1161/CIRCULATIONAHA.106.627588. [DOI] [PubMed] [Google Scholar]
- 18.Pellegrino MJ, Habecker BA. STAT3 integrates cytokine and neurotrophin signals to promote sympathetic axon regeneration. Mol Cell Neurosci. 2013;56:272–282. doi: 10.1016/j.mcn.2013.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pellegrino MJ, McCully BH, Habecker BA. Leptin stimulates sympathetic axon outgrowth. Neurosci Lett. 2014;566:1–5. doi: 10.1016/j.neulet.2014.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ajijola OA, Wisco JJ, Lambert HW, Mahajan A, Stark E, Fishbein MC, Shivkumar K. Extra-Cardiac Neural Remodeling in Humans with Cardiomyopathy. Circ Arrhythm Electrophysiol. 2012;5:1010–1016. doi: 10.1161/CIRCEP.112.972836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Han S, Kobayashi K, Joung B, Piccirillo G, Maruyama M, Vinters HV, March K, Lin SF, Shen C, Fishbein MC, Chen PS, Chen LS. Electroanatomic remodeling of the left stellate ganglion after myocardial infarction. J Am Coll Cardiol. 2012;59:954–961. doi: 10.1016/j.jacc.2011.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lujan HL, Janbaih H, DiCarlo SE. Dynamic interaction between the heart and its sympathetic innervation following T5 spinal cord transection. J Appl Physiol. 2012;113:1332–1341. doi: 10.1152/japplphysiol.00522.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sharma N, Deppmann CD, Harrington AW, St HC, Chen ZY, Lee FS, Ginty DD. Long-Distance Control of Synapse Assembly by Target-Derived NGF. Neuron. 2010;67:422–434. doi: 10.1016/j.neuron.2010.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vivas O, Kruse M, Hille B. Nerve Growth Factor Sensitizes Adult Sympathetic Neurons to the Proinflammatory Peptide Bradykinin. J Neurosci. 2014;34:11959–11971. doi: 10.1523/JNEUROSCI.1536-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Luther JA, Birren SJ. p75 and TrkA signaling regulates sympathetic neuronal firing patterns via differential modulation of voltage-gated currents. J Neurosci. 2009;29:5411–5424. doi: 10.1523/JNEUROSCI.3503-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim WY, Gonsiorek EA, Barnhart C, Davare MA, Engebose AJ, Lauridsen H, Bruun D, Lesiak A, Wayman G, Bucelli R, Higgins D, Lein PJ. Statins decrease dendritic arborization in rat sympathetic neurons by blocking RhoA activation. J Neurochem. 2009;108:1057–1071. doi: 10.1111/j.1471-4159.2008.05854.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kimura K, Ieda M, Fukuda K. Development, Maturation, and Transdifferentiation of Cardiac Sympathetic Nerves. Circ Res. 2012;110:325–336. doi: 10.1161/CIRCRESAHA.111.257253. [DOI] [PubMed] [Google Scholar]
- 28.Kimura K, Ieda M, Kanazawa H, Yagi T, Tsunoda M, Ninomiya S, Kurosawa H, Yoshimi K, Mochizuki H, Yamazaki K, Ogawa S, Fukuda K. Cardiac sympathetic rejuvenation: a link between nerve function and cardiac hypertrophy. Circ Res. 2007;100:1755–1764. doi: 10.1161/01.RES.0000269828.62250.ab. [DOI] [PubMed] [Google Scholar]
- 29.Eisenhofer G, Friberg P, Rundqvist B, Quyyumi AA, Lambert G, Kaye DM, Kopin IJ, Goldstein DS, Esler MD. Cardiac Sympathetic Nerve Function in Congestive Heart Failure. Circulation. 1996;93:1667–1676. doi: 10.1161/01.cir.93.9.1667. [DOI] [PubMed] [Google Scholar]
- 30.Antzelevitch C, Sicouri S, Litovsky SH, Lukas A, Krishnan SC, Di Diego JM, Gintant GA, Liu DW. Heterogeneity within the ventricular wall. Electrophysiology and pharmacology of epicardial, endocardial, and M cells. Circ Res. 1991;69:1427–1449. doi: 10.1161/01.res.69.6.1427. [DOI] [PubMed] [Google Scholar]
- 31.Nabauer M, Beuckelmann DJ, Uberfuhr P, Steinbeck G. Regional Differences in Current Density and Rate-Dependent Properties of the Transient Outward Current in Subepicardial and Subendocardial Myocytes of Human Left Ventricle. Circulation. 1996;93:168–177. doi: 10.1161/01.cir.93.1.168. [DOI] [PubMed] [Google Scholar]
- 32.Brunet S, Aimond F, Li H, Guo W, Eldstrom J, Fedida D, Yamada KA, Nerbonne JM. Heterogeneous expression of repolarizing, voltage-gated K+ currents in adult mouse ventricles. J Physiol. 2004;559:103–120. doi: 10.1113/jphysiol.2004.063347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ieda M, Kanazawa H, Kimura K, Hattori F, Ieda Y, Taniguchi M, Lee JK, Matsumura K, Tomita Y, Miyoshi S, Shimoda K, Makino S, Sano M, Kodama I, Ogawa S, Fukuda K. Sema3a maintains normal heart rhythm through sympathetic innervation patterning. Nat Med. 2007;13:604–612. doi: 10.1038/nm1570. [DOI] [PubMed] [Google Scholar]
- 34.Lorentz CU, Alston EN, Belcik JT, Lindner JR, Giraud GD, Habecker BA. Heterogeneous ventricular sympathetic innervation, altered beta adrenergic receptor expression, and rhythm instability in mice lacking p75 neurotrophin receptor. Am J Physiol Heart Circ Physiol. 2010;298:H1652–H1660. doi: 10.1152/ajpheart.01128.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thomas D, Kiehn J, Katus HA, Karle CA. Adrenergic regulation of the rapid component of the cardiac delayed rectifier potassium current, I(Kr), and the underlying hERG ion channel. Basic Res Cardiol. 2004;99:279–287. doi: 10.1007/s00395-004-0474-7. [DOI] [PubMed] [Google Scholar]
- 36.Cutler MJ, Jeyaraj D, Rosenbaum DS. Cardiac electrical remodeling in health and disease. Trends Pharmacol Sci. 2011;32:174–180. doi: 10.1016/j.tips.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bers DM. Calcium Cycling and Signaling in Cardiac Myocytes. Annu Rev Physiol. 2008;70:23–49. doi: 10.1146/annurev.physiol.70.113006.100455. [DOI] [PubMed] [Google Scholar]
- 38.Bers DM. Cardiac excitation-contraction coupling. Nature. 2002;415:198–205. doi: 10.1038/415198a. [DOI] [PubMed] [Google Scholar]
- 39.Shannon TR, Ginsburg KS, Bers DM. Reverse mode of the sarcoplasmic reticulum calcium pump and load-dependent cytosolic calcium decline in voltage-clamped cardiac ventricular myocytes. Biophys J. 2000;78:322–333. doi: 10.1016/S0006-3495(00)76595-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Valdivia HH, Kaplan JH, Ellis-Davies GC, Lederer WJ. Rapid adaptation of cardiac ryanodine receptors: modulation by Mg2+ and phosphorylation. Science. 1995;267:1997–2000. doi: 10.1126/science.7701323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marx SO, Reiken S, Hisamatsu Y, Jayaraman T, Burkhoff D, Rosemblit N, Marks AR. PKA phosphorylation dissociates FKBP12.6 from the calcium release channel (ryanodine receptor): defective regulation in failing hearts. Cell. 2000;101:365–376. doi: 10.1016/s0092-8674(00)80847-8. [DOI] [PubMed] [Google Scholar]
- 42.Pogwizd SM, Bers DM. Cellular basis of triggered arrhythmias in heart failure. Trends Cardiovasc Med. 2004;14:61–66. doi: 10.1016/j.tcm.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 43.Pogwizd SM, Schlotthauer K, Li L, Yuan W, Bers DM. Arrhythmogenesis and contractile dysfunction in heart failure: Roles of sodium-calcium exchange, inward rectifier potassium current, and residual beta-adrenergic responsiveness. Circ Res. 2001;88:1159–1167. doi: 10.1161/hh1101.091193. [DOI] [PubMed] [Google Scholar]
- 44.Kumar R, Wilders R, Joyner RW, Jongsma HJ, Verheijck EE, Golod DA, van Ginneken AC, Goolsby WN. Experimental model for an ectopic focus coupled to ventricular cells. Circulation. 1996;94:833–841. doi: 10.1161/01.cir.94.4.833. [DOI] [PubMed] [Google Scholar]
- 45.Xie Y, Sato D, Garfinkel A, Qu Z, Weiss JN. So little source, so much sink: requirements for afterdepolarizations to propagate in tissue. Biophys J. 2010;99:1408–1415. doi: 10.1016/j.bpj.2010.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Myles RC, Wang L, Bers DM, Ripplinger CM. Decreased inward rectifying K(+) current and increased ryanodine receptor sensitivity synergistically contribute to sustained focal arrhythmia in the intact rabbit heart. J Physiol. 2015;593:1479–1493. doi: 10.1113/jphysiol.2014.279638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Myles RC, Wang L, Kang C, Bers DM, Ripplinger CM. Local beta-adrenergic stimulation overcomes source-sink mismatch to generate focal arrhythmia. Circ Res. 2012;110:1454–1464. doi: 10.1161/CIRCRESAHA.111.262345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nash MP, Thornton JM, Sears CE, Varghese A, O’Neill M, Paterson DJ. Ventricular activation during sympathetic imbalance and its computational reconstruction. J Appl Physiol. 2001;90:287–298. doi: 10.1152/jappl.2001.90.1.287. [DOI] [PubMed] [Google Scholar]
- 49.Doppalapudi H, Jin Q, Dosdall DJ, Qin H, Walcott GP, Killingsworth CR, Smith WM, Ideker RE, Huang J. Intracoronary infusion of catecholamines causes focal arrhythmias in pigs. J Cardiovasc Electrophysiol. 2008;19:963–970. doi: 10.1111/j.1540-8167.2008.01199.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pogwizd SM, Qi M, Yuan W, Samarel AM, Bers DM. Upregulation of Na(+)/Ca(2+) exchanger expression and function in an arrhythmogenic rabbit model of heart failure. Circ Res. 1999;85:1009–1019. doi: 10.1161/01.res.85.11.1009. [DOI] [PubMed] [Google Scholar]
- 51.Shannon TR, Pogwizd SM, Bers DM. Elevated sarcoplasmic reticulum Ca2+ leak in intact ventricular myocytes from rabbits in heart failure. Circ Res. 2003;93:592–594. doi: 10.1161/01.RES.0000093399.11734.B3. [DOI] [PubMed] [Google Scholar]
- 52.Poelzing S, Rosenbaum DS. Altered connexin43 expression produces arrhythmia substrate in heart failure. Am J Physiol Heart Circ Physiol. 2004;287:H1762–H1770. doi: 10.1152/ajpheart.00346.2004. [DOI] [PubMed] [Google Scholar]
- 53.Gallego M, Alday A, Alonso H, Casis O. Adrenergic regulation of cardiac ionic channels: role of membrane microdomains in the regulation of kv4 channels. Biochim Biophys Acta. 2014;1838:692–699. doi: 10.1016/j.bbamem.2013.06.025. [DOI] [PubMed] [Google Scholar]
- 54.Hartzell HC. Regulation of cardiac ion channels by catecholamines, acetylcholine and second messenger systems. Prog Biophys Mol Biol. 1988;52:165–247. doi: 10.1016/0079-6107(88)90014-4. [DOI] [PubMed] [Google Scholar]
- 55.Sperelakis N. Regulation of calcium slow channels of heart by cyclic nucleotides and effects of ischemia. Adv Pharmacol. 1994;31:1–24. doi: 10.1016/s1054-3589(08)60605-5. [DOI] [PubMed] [Google Scholar]
- 56.Marx SO, Kurokawa J, Reiken S, Motoike H, D’Armiento J, Marks AR, Kass RS. Requirement of a macromolecular signaling complex for beta adrenergic receptor modulation of the KCNQ1-KCNE1 potassium channel. Science. 2002;295:496–499. doi: 10.1126/science.1066843. [DOI] [PubMed] [Google Scholar]
- 57.Kagan A, Melman YF, Krumerman A, McDonald TV. 14-3-3 amplifies and prolongs adrenergic stimulation of HERG K+ channel activity. EMBO J. 2002;21:1889–1898. doi: 10.1093/emboj/21.8.1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mantravadi R, Gabris B, Liu T, Choi BR, de Groat WC, Ng GA, Salama G. Autonomic nerve stimulation reverses ventricular repolarization sequence in rabbit hearts. Circ Res. 2007;100:e72–e80. doi: 10.1161/01.RES.0000264101.06417.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yagishita D, Chui RW, Yamakawa K, Rajendran PS, Ajijola OA, Nakamura K, So EL, Mahajan A, Shivkumar K, Vaseghi M. Sympathetic nerve stimulation, not circulating norepinephrine, modulates T-peak to T-end interval by increasing global dispersion of repolarization. Circ Arrhythm Electrophysiol. 2015;8:174–185. doi: 10.1161/CIRCEP.114.002195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ajijola OA, Vaseghi M, Zhou W, Yamakawa K, Benharash P, Hadaya J, Lux RL, Mahajan A, Shivkumar K. Functional differences between junctional and extrajunctional adrenergic receptor activation in mammalian ventricle. Am J Physiol Heart Circ Physiol. 2013;304:H579–H588. doi: 10.1152/ajpheart.00754.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ajijola OA, Yagishita D, Patel KJ, Vaseghi M, Zhou W, Yamakawa K, So E, Lux RL, Mahajan A, Shivkumar K. Focal myocardial infarction induces global remodeling of cardiac sympathetic innervation: neural remodeling in a spatial context. Am J Physiol Heart Circ Physiol. 2013;305:H1031–H1040. doi: 10.1152/ajpheart.00434.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vaseghi M, Lux RL, Mahajan A, Shivkumar K. Sympathetic stimulation increases dispersion of repolarization in humans with myocardial infarction. Am J Physiol Heart Circ Physiol. 2012;302:H1838–H1846. doi: 10.1152/ajpheart.01106.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bristow MR. β-Adrenergic Receptor Blockade in Chronic Heart Failure. Circulation. 2000;101:558–569. doi: 10.1161/01.cir.101.5.558. [DOI] [PubMed] [Google Scholar]
- 64.Lefkowitz RJ, Rockman HA, Koch WJ. Catecholamines, cardiac beta-adrenergic receptors, and heart failure. Circulation. 2000;101:1634–1637. doi: 10.1161/01.cir.101.14.1634. [DOI] [PubMed] [Google Scholar]
- 65.Yatani A, Shen YT, Yan L, Chen W, Kim SJ, Sano K, Irie K, Vatner SF, Vatner DE. Down regulation of the L-type Ca2+ channel, GRK2, and phosphorylated phospholamban: protective mechanisms for the denervated failing heart. J Mol Cell Cardiol. 2006;40:619–628. doi: 10.1016/j.yjmcc.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 66.Soltysinska E, Thiele S, Olesen SP, Osadchii OE. Chronic sympathetic activation promotes downregulation of beta-adrenoceptor-mediated effects in the guinea pig heart independently of structural remodeling and systolic dysfunction. Pflugers Arch. 2011;462:529–543. doi: 10.1007/s00424-011-1005-7. [DOI] [PubMed] [Google Scholar]
- 67.Zhang LM, Wang Z, Nattel S. Effects of sustained beta-adrenergic stimulation on ionic currents of cultured adult guinea pig cardiomyocytes. Am J Physiol Heart Circ Physiol. 2002;282:H880–H889. doi: 10.1152/ajpheart.01138.2000. [DOI] [PubMed] [Google Scholar]
- 68.Aflaki M, Qi XY, Xiao L, Ordog B, Tadevosyan A, Luo X, Maguy A, Shi Y, Tardif JC, Nattel S. Exchange protein directly activated by cAMP mediates slow delayed-rectifier current remodeling by sustained beta-adrenergic activation in guinea pig hearts. Circ Res. 2014;114:993–1003. doi: 10.1161/CIRCRESAHA.113.302982. [DOI] [PubMed] [Google Scholar]
- 69.Reiken S, Wehrens XH, Vest JA, Barbone A, Klotz S, Mancini D, Burkhoff D, Marks AR. Beta-blockers restore calcium release channel function and improve cardiac muscle performance in human heart failure. Circulation. 2003;107:2459–2466. doi: 10.1161/01.CIR.0000068316.53218.49. [DOI] [PubMed] [Google Scholar]
- 70.Stanton MS, Tuli MM, Radtke NL, Heger JJ, Miles WM, Mock BH, Burt RW, Wellman HN, Zipes DP. Regional sympathetic denervation after myocardial infarction in humans detected noninvasively using I-123-metaiodobenzylguanidine. J Am Coll Cardiol. 1989;14:1519–1526. doi: 10.1016/0735-1097(89)90391-4. [DOI] [PubMed] [Google Scholar]
- 71.Hartikainen J, Kuikka J, Mantysaari M, Lansimies E, Pyorala K. Sympathetic reinnervation after acute myocardial infarction. Am J Cardiol. 1996;77:5–9. doi: 10.1016/s0002-9149(97)89125-4. [DOI] [PubMed] [Google Scholar]
- 72.Jacobson AF, Senior R, Cerqueira MD, Wong ND, Thomas GS, Lopez VA, Agostini D, Weiland F, Chandna H, Narula J. Myocardial Iodine-123 Meta-Iodobenzylguanidine Imaging and Cardiac Events in Heart Failure: Results of the Prospective ADMIRE-HF (AdreView Myocardial Imaging for Risk Evaluation in Heart Failure) Study. J Am Coll Cardiol. 2010;55:2212–2221. doi: 10.1016/j.jacc.2010.01.014. [DOI] [PubMed] [Google Scholar]
- 73.Goldstein DS. Cardiac denervation in patients with Parkinson disease. Cleve Clin J Med. 2007;74 (Suppl 1):S91–94. doi: 10.3949/ccjm.74.suppl_1.s91. [DOI] [PubMed] [Google Scholar]
- 74.Boogers MJ, Borleffs CJ, Henneman MM, van Bommel RJ, van RJ, Boersma E, Dibbets-Schneider P, Stokkel MP, van der Wall EE, Schalij MJ, Bax JJ. Cardiac sympathetic denervation assessed with 123-iodine metaiodobenzylguanidine imaging predicts ventricular arrhythmias in implantable cardioverter-defibrillator patients. J Am Coll Cardiol. 2010;55:2769–2777. doi: 10.1016/j.jacc.2009.12.066. [DOI] [PubMed] [Google Scholar]
- 75.Nishisato K, Hashimoto A, Nakata T, Doi T, Yamamoto H, Nagahara D, Shimoshige S, Yuda S, Tsuchihashi K, Shimamoto K. Impaired Cardiac Sympathetic Innervation and Myocardial Perfusion Are Related to Lethal Arrhythmia: Quantification of Cardiac Tracers in Patients with ICDs. J Nucl Med. 2010;51:1241–1249. doi: 10.2967/jnumed.110.074971. [DOI] [PubMed] [Google Scholar]
- 76.Fallavollita JA, Heavey BM, Luisi AJ, Jr, Michalek SM, Baldwa S, Mashtare TL, Jr, Hutson AD, Dekemp RA, Haka MS, Sajjad M, Cimato TR, Curtis AB, Cain ME, Canty JM., Jr Regional myocardial sympathetic denervation predicts the risk of sudden cardiac arrest in ischemic cardiomyopathy. J Am Coll Cardiol. 2014;63:141–149. doi: 10.1016/j.jacc.2013.07.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Siao CJ, Lorentz CU, Kermani P, Marinic T, Carter J, McGrath K, Padow VA, Mark W, Falcone DJ, Cohen-Gould L, Parrish DC, Habecker BA, Nykjaer A, Ellenson LH, Tessarollo L, Hempstead BL. ProNGF, a cytokine induced after myocardial infarction in humans, targets pericytes to promote microvascular damage and activation. J Exp Med. 2012;209:2291–2305. doi: 10.1084/jem.20111749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kohn J, Aloyz RS, Toma JG, Haak-Frendscho M, Miller FD. Functionally antagonistic interactions between the TrkA and p75 neurotrophin receptors regulate sympathetic neuron growth and target innervation. J Neurosci. 1999;19:5393–5408. doi: 10.1523/JNEUROSCI.19-13-05393.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Majdan M, Walsh GS, Aloyz R, Miller FD. TrkA mediates developmental sympathetic neuron survival in vivo by silencing an ongoing p75NTR-mediated death signal. J Cell Biol. 2001;155:1275–1286. doi: 10.1083/jcb.200110017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Teng KK, Felice S, Kim T, Hempstead BL. Understanding proneurotrophin actions: Recent advances and challenges. Dev Neurobiol. 2010;70:350–359. doi: 10.1002/dneu.20768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lorentz CU, Parrish DC, Alston EN, Pellegrino MJ, Woodward WR, Hempstead BL, Habecker BA. Sympathetic denervation of peri-infarct myocardium requires the p75 neurotrophin receptor. Exp Neurol. 2013;249:111–119. doi: 10.1016/j.expneurol.2013.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Feng N, Huke S, Zhu G, Tocchetti CG, Shi S, Aiba T, Kaludercic N, Hoover DB, Beck SE, Mankowski JL, Tomaselli GF, Bers DM, Kass DA, Paolocci N. Constitutive BDNF/TrkB signaling is required for normal cardiac contraction and relaxation. Proc Natl Acad Sci U S A. 2015;112:1880–1885. doi: 10.1073/pnas.1417949112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gardner RT, Habecker BA. Infarct-derived chondroitin sulfate proteoglycans prevent sympathetic reinnervation after cardiac ischemia-reperfusion injury. J Neurosci. 2013;33:7175–7183. doi: 10.1523/JNEUROSCI.5866-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gardner RT, Wang L, Lang BT, Cregg JM, Dunbar CL, Woodward WR, Silver J, Ripplinger CM, Habecker BA. Targeting protein tyrosine phosphatase sigma after myocardial infarction restores cardiac sympathetic innervation and prevents arrhythmias. Nature Comm. 2015;6:6235. doi: 10.1038/ncomms7235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bru-Mercier G, Deroubaix E, Capuano V, Ruchon Y, Rucker-Martin C, Coulombe A, Renaud JF. Expression of heart K+ channels in adrenalectomized and catecholamine-depleted reserpine-treated rats. J Mol Cell Cardiol. 2003;35:153–163. doi: 10.1016/s0022-2828(02)00290-0. [DOI] [PubMed] [Google Scholar]
- 86.Bai J, Ren C, Hao W, Wang R, Cao JM. Chemical sympathetic denervation, suppression of myocardial transient outward potassium current, and ventricular fibrillation in the rat. Can J Physiol Pharmacol. 2008;86:700–709. doi: 10.1139/y08-075. [DOI] [PubMed] [Google Scholar]
- 87.Lue WM, Boyden PA. Abnormal electrical properties of myocytes from chronically infarcted canine heart. Alterations in Vmax and the transient outward current. Circulation. 1992;85:1175–1188. doi: 10.1161/01.cir.85.3.1175. [DOI] [PubMed] [Google Scholar]
- 88.Jourdon P, Feuvray D. Calcium and potassium currents in ventricular myocytes isolated from diabetic rats. J Physiol. 1993;470:411–429. doi: 10.1113/jphysiol.1993.sp019866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Han W, Barr SC, Pacioretty LM, Gilmour RF., Jr Restoration of the transient outward potassium current by noradrenaline in chagasic canine epicardium. J Physiol. 1997;500:75–83. doi: 10.1113/jphysiol.1997.sp022000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Iaccarino G, Tomhave ED, Lefkowitz RJ, Koch WJ. Reciprocal in vivo regulation of myocardial G protein-coupled receptor kinase expression by beta-adrenergic receptor stimulation and blockade. Circulation. 1998;98:1783–1789. doi: 10.1161/01.cir.98.17.1783. [DOI] [PubMed] [Google Scholar]
- 91.Raake PW, Zhang X, Vinge LE, Brinks H, Gao E, Jaleel N, Li Y, Tang M, Most P, Dorn GW, 2nd, Houser SR, Katus HA, Chen X, Koch WJ. Cardiac G-protein-coupled receptor kinase 2 ablation induces a novel Ca2+ handling phenotype resistant to adverse alterations and remodeling after myocardial infarction. Circulation. 2012;125:2108–2118. doi: 10.1161/CIRCULATIONAHA.111.044255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Muller JE, Ludmer PL, Willich SN, Tofler GH, Aylmer G, Klangos I, Stone PH. Circadian variation in the frequency of sudden cardiac death. Circulation. 1987;75:131–138. doi: 10.1161/01.cir.75.1.131. [DOI] [PubMed] [Google Scholar]
- 93.Scheer FA, Hu K, Evoniuk H, Kelly EE, Malhotra A, Hilton MF, Shea SA. Impact of the human circadian system, exercise, and their interaction on cardiovascular function. Proc Natl Acad Sci USA. 2010;107:20541–20546. doi: 10.1073/pnas.1006749107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Herring N, Lokale MN, Danson EJ, Heaton DA, Paterson DJ. Neuropeptide Y reduces acetylcholine release and vagal bradycardia via a Y2 receptor-mediated, protein kinase C-dependent pathway. J Mol Cell Cardiol. 2008;44:477–485. doi: 10.1016/j.yjmcc.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 95.Herring N. Autonomic control of the heart: going beyond the classical neurotransmitters. Exp Physiol. 2015;100:354–358. doi: 10.1113/expphysiol.2014.080184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ajijola OA, Yagishita D, Reddy NK, Yamakawa K, Vaseghi M, Downs AM, Hoover DB, Ardell JL, Shivkumar K. Remodeling of stellate ganglion neurons after spatially targeted myocardial infarction: Neuropeptide and morphologic changes. Heart Rhythm. 2015;12:1027–1035. doi: 10.1016/j.hrthm.2015.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cuculi F, Herring N, De Caterina AR, Banning AP, Prendergast BD, Forfar JC, Choudhury RP, Channon KM, Kharbanda RK. Relationship of plasma neuropeptide Y with angiographic, electrocardiographic and coronary physiology indices of reperfusion during ST elevation myocardial infarction. Heart. 2013;99:1198–1203. doi: 10.1136/heartjnl-2012-303443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Stanke M, Duong CV, Pape M, Geissen M, Burbach G, Deller T, Gascan H, Otto C, Parlato R, Schutz G, Rohrer H. Target-dependent specification of the neurotransmitter phenotype: cholinergic differentiation of sympathetic neurons is mediated in vivo by gp 130 signaling. Development. 2006;133:141–150. doi: 10.1242/dev.02189. [DOI] [PubMed] [Google Scholar]
- 99.Kanazawa H, Ieda M, Kimura K, Arai T, Kawaguchi-Manabe H, Matsuhashi T, Endo J, Sano M, Kawakami T, Kimura T, Monkawa T, Hayashi M, Iwanami A, Okano H, Okada Y, Ishibashi-Ueda H, Ogawa S, Fukuda K. Heart failure causes cholinergic transdifferentiation of cardiac sympathetic nerves via gp130-signaling cytokines in rodents. J Clin Invest. 2010;120:408–421. doi: 10.1172/JCI39778. [DOI] [PMC free article] [PubMed] [Google Scholar]