Abstract
Osteosarcoma (OS) is the most common non-hematologic malignant tumor of bone in adults and children. As sarcomas are more common in adolescents and young adults than most other forms of cancer, there are a significant number of years of life lost secondary to these malignancies. OS is associated with a poor prognosis secondary to a high grade at presentation, resistance to chemotherapy and a propensity to metastasize to the lungs. Current OS management involves both chemotherapy and surgery. The incorporation of cytotoxic chemotherapy into therapeutic regimens escalated cure rates from <20% to current levels of 65-75%. Furthermore, limb-salvage surgery is now offered to the majority of OS patients. Despite advances in chemotherapy and surgical techniques over the past three decades, there has been stagnation in patient survival outcome improvement, especially in patients with metastatic OS. Thus, there is a critical need to identify novel and directed therapy for OS. Several Phase I trials for sarcoma therapies currently ongoing or recently completed have shown objective responses in OS. Novel drug delivery mechanisms are currently under phase II and III clinical trials. Furthermore, there is an abundance of preclinical research which holds great promise in the development of future OS-directed therapeutics. Our continuously improving knowledge of the molecular and cell-signaling pathways involved in OS will translate into more effective therapies for OS and ultimately improved patient survival. The present review will provide an overview of current therapies, ongoing clinical trials and therapeutic targets under investigation for OS.
Keywords: Bisphosphonates, BMP, IGF, IGFBP5, limb-salvage, osteosarcoma, OS99
INTRODUCTION
Osteosarcoma (OS) generally occurs in pediatric patients and young adults, most commonly during the adolescent years when bone growth is rapid. In fact, there exists a direct relationship between rapid bone growth and OS [1,2]. OS is a highly malignant bone tumor, the most common primary malignancy of bone in children and adolescents and the fifth most common malignancy overall in children and young adults [3]. The incidence of OS is slightly higher in males than females, which is attributable to a longer duration of skeletal growth in males [4]. OS most frequently occurs in the metaphyseal area of long bones adjacent to or involving the growth plate, with approximately 75% of all cases occurring in the distal femur and proximal tibia [1,5,6]. Approximately 20% of OS patients present with clinically detectable metastatic disease at diagnosis, and the most common site of metastases is the lung [7]. In a study by Kager et al., the presence of greater than five metastatic lesions was associated with a 5-year survival rate of only 19% [8]. Critical determinants of long-term survival in patients presenting with metastatic disease are location of disease and surgical resectability, number of metastases at diagnosis, location of metastases, surgical remission and tumor necrosis following neoadjuvant chemotherapy [9-12]. While there are a number of histologic subtypes, there are three major subtypes of OS classified by tumor matrix predominance, osteoblastic, chondroblastic and fibroblastic; although treatment and patient outcomes do not differ among subtypes [13,14]. Sub-types also differ depending on location; making up less than 5% of OS cases, parosteal, central low-grade and periosteal OS have distinct morphologies but portend an improved prognosis [14-19]. Pathological diagnosis of OS depends on interpretation of osteoid matrix type produced by cancer cells since no diagnostic immunohistochemical or genetic studies yet exist [13].
The current standard of care treatment for OS patients is comprised of surgery with wide excision of the primary tumor and cytotoxic chemotherapy, a treatment which often proves difficult for patients due to the systemic toxicities of these agents [20]. Currently, chemotherapy plays a major role in the treatment of OS. The benefit of early neoadjuvant chemotherapy is greater than surgery alone, and this is due to the frequent presence of subclinical micrometastatic disease [21-23]. Chemotherapy alone can eradicate these micrometastases if initiated when the micrometastatic disease burden is low [24], statistically improving survival even in patients diagnosed with localized high-grade OS. Prior to neoadjuvant chemotherapy regimens, amputation was the sole therapy for OS and resulted in a 5-year overall survival rate of only 10-20% [25-27]. Over the past half century the development of effective chemotherapeutic agents, improvement in local tumor control and the incorporation of these modalities into therapeutic regimens has increased OS cure rates from <20% prior to the 1970’s, even with full local tumor control, to 65-75% today [22,24,28,38]. While chemotherapy has revolutionized OS therapy, surgical and imaging techniques have also improved considerably over the past three decades, allowing for limb-salvage procedures in greater than 90% of patients compared to 1980s limb-salvage rates of 53%. Limb-salvage procedures benefit patients from a psychological and cosmetic standpoint while having local recurrence and survival rates similar to amputation [21,25, 30,39,]. In fact, improvements in surgical techniques permitting limb-salvage procedures have paralleled improvements in patient survival outcomes [21].
While modifications in chemotherapeutic regimens, surgical procedures and imaging have allowed limb-salvage procedures to become commonplace in treating OS and have improved overall patient survival, there have been no significant improvements in survival outcomes for patients with metastatic OS over the past three decades. Survival rates for patients with pulmonary metastases, even following aggressive multimodal treatment, is currently only 25-30% and has not improved over the past 25 years [11,28,47-55]. Extrapulmonary metastases developing in uncommon sites such as the kidneys, brain, heart, mediastinum and epidural space present especially difficult challenges [28,56]. Thus, there is a critical need to identify novel and directed therapy for OS. Several clinical trials are currently ongoing for various sarcoma therapies that have demonstrated objective responses in OS. Novel drug delivery mechanisms are also at the clinical trial phases. Furthermore, there is an abundance of preclincial research which holds great promise in the development of future OS-directed therapeutics. This review aims to discuss today’s standard therapies for OS, recently completed and current clinical trials that may soon be implemented into OS treatment regimens and preclinical studies that will hopefully improve OS patient outcomes in the coming years.
History of Chemotherapeutics in OS
The history of chemotherapeutic treatment for OS began in the early 1960s when Sutow et al. demonstrated anti-tumor activity against OS with L-phenylalanine mustard, resulting in a 14% disease-free survival rate [28,57]. In 1969, shortly after the combination of vincristine, dactinomycin and cyclophosphamide (VAC regimen) was shown to be effective in treating rhabdomyosarcoma, Sutow et al. achieved a 33% disease-free survival rate in patients treated with a pulse VAC regimen consisting of intermittent intensive pulses of cyclophosphamide with the traditional VAC regimen [28,58,59]. After doxorubicin demonstrated anti-tumor activity in OS, Sutow et al. substituted doxorubicin for dactinomycin in the pulse VAC regimen and also added L-phenylalanine mustard in a regimen named “Conpadri “ or Conpadri I [28,57,60], resulting in a 55% disease-free survival rate. Addition of methotrexate to the regimen, called “Compadri” or “Compadri II” (each successive number indicating an evolution in the regimen), delayed the development of pulmonary metastases [28,61,62]. Subsequently, the Compadri IV and V regimens increased the dosages of methotrexate and doxorubicin, using aggressive “front loading.” Together, the Compadri regimens comprise the first successful attempt at utilizing combination chemotherapy, with different mechanisms of action and minimal overlapping toxicities, as adjuvant OS therapy. Prior to the 1970s, survival rates for patients with metastatic OS were below 2% but improved to approximately 30% after 1970, demonstrating the profound impact of the incorporation of chemotherapy to OS treatment regimens [28,63]. The Compadri studies laid the foundation for clinical trials performed throughout the 1980s and 1990s that utilized multi-agent regimens consisting of doxorubicin, cisplatin, bleomycin, cyclophosphamide, ifosfamide, dactinomycin and high-dose methotrexate. In 1976, Rosen et al. introduced the concept of neoadjuvant chemotherapy for OS in an effort to provide ample time for the manufacturing of custom-made prostheses following limb-salvage procedures while reducing preoperative tumor volume [64].
Current Chemotherapeutics in OS
Today, standard chemotherapeutics include high-dose methotrexate with leucovorin rescue, doxorubicin (Adriamycin), cisplatin, cyclophosphamide and ifosfamide.
High-dose Methotrexate with Leucovorin Rescue
Methotrexate works by binding stoichiometrically and irreversibly to dihydrofolate reductase, inhibiting the formation of tetrahydrofolate and preventing the synthesis of thymidylate causing cell death [28]. Leucovorin is the antidote to methotrexate and serves to replenish tetrahydrofolate stores depleted by methotrexate. This regimen (MTX-L), a mainstay in OS chemotherapy, was established by Jaffe et al. and is comprised of IV doses of 10-12.5 g/m2 over 4-6h with leucovorin rescue starting 24h after the onset of methotrexate infusion [28,64-67]. When used in combination therapy, the interval between doses of MTX-L is generally 21-28 days. Doxorubicin is typically administered 8-10 days after the initial dose of MTX-L. When administered as a single adjuvant agent following surgical resection of the primary tumor, MTX-L resulted in a 40% disease-free survival. However, when combined with other agents as pre- and post-operative therapy, disease-free survival rates of 65-75% were achieved [28,64,66-72]. Methotrexate has been shown to potentiate the effects of radiation therapy [29].
Methotrexate blood levels of 700-1000 μmol/L at 4-6h following infusion are required for optimal results, and Jaffe et al. of the University of Texas MD Anderson Cancer Center find that concentrations of 1500 μmol/L or higher at 4-6h are desirable and more likely to be achieved by limiting pre- and intra-therapeutic intravenous (alkaline) hydration to 3L/m2/24h [28]. With optimal dosages administered over 4-6h along with appropriate hydration, peak MTX levels can be expected at the following time points after the initiation of infusion. Excess of the following values may result in toxicities [28,73-75]: 24h: 30-300 μmol/L; 48h: 3-30 μmol/L; 72h: <0.3 μmol/L.
Leucovorin is administered according to algorithms available in most institutions. Most protocols include an IV dose of 10 mg after completion of the methotrexate infusion followed by subsequent doses every 6 h until the MTX level falls below 0.1 μmol/L; this typically requires 72h and 12 doses.
Candidates for MTX-L therapy must have normal renal and hepatic function, a normal complete blood count (CBC) and the absence of infection. These prerequisites can be determined by a corrected creatinine clearance, basic metabolic panel, urinalysis, liver function tests and a complete blood count prior to each course of therapy. Although uncommon, toxicity may clinically manifest with gastrointestinal mucosal ulceration, myelosuppression and hepatorenal failure. Mucositis and nephrotoxicity are commonly associated with delayed MTX clearance and precipitation of methotrexate within the renal tubules, a process that can be adversely affected by administration of other nephrotoxic drugs such as cisplatin [76,77]. Treatment of toxicity includes increasing fluid intake to 4L/m2/24h, increasing leucovorin dose to 50-100 mg every 6h, administering carboxypeptidase G-2 in the event of oliguria/anuria and renal dialysis.
Doxorubicin
Doxorubicin is used in most OS combination chemotherapy regimens and has been called the single most effective agent [28,78]. Doxorubicin is an anthracyline chemotherapeutic which functions by intercalating into DNA, inducing single- and double-strand breaks. Initial studies using doxorubicin in patients with pulmonary metastases resulted in response rates of 35-40%, including the complete disappearance of lung lesions in some patients and an overall 40% reduction in tumor volume [28,79-81]. Similar to methotrexate, doxorubicin can potentiate the therapeutic effects of radiation therapy, albeit with occasional complications of erythema and ulceration of skin when used intra-arterially; these adverse effects can preclude limb-salvage procedures [28,82,83].
The major toxicity of doxorubicin is delayed congestive heart failure but can generally be prevented by limiting the cumulative dose to 300 mg/m2 in children below the age of six, 450-500 mg/m2 in adolescents and approximately 600 mg/m2 in adults [28,84]. Dexrazoxane, a derivative of the metal-chelating agent EDTA, has been administered in combination with doxorubicin to prevent cardiac failure [28,85]. This agent functions to chelate iron and reduce the number of metal ions that complex with doxorubicin, thus decreasing the production of cytotoxic superoxide radicals [86]. Newer liposomal formulations of doxorubicin are less cardiotoxic but not yet approved for the treatment of OS despite significant activity in some soft tissue sarcoma subtypes [87-90]. Candidates for doxorubicin treatment require a cardiac assessment consisting of an electrocardiogram and echocardiogram; these should be obtained prior to each course of treatment, with further assessments obtained at regular intervals for long-term survivors.
Cisplatin
Cisplatin (cis-diamminedichloroplatinum II) is a platinum-containing chemotherapeutic that, although lacking an alkyl group, is also classified as an alkylating agent. It is thought to function by binding and crosslinking DNA, interfering with transcription and DNA replication [91]. Cisplatin can be administered intravenously or intra-arterially. Initial studies in patients with unresectable or metastatic OS demonstrated responses of 30-50% when administered intravenously as a single agent or in combination with doxorubicin [28,92-94]. Surprisingly, response rates were as high as 60-90% when administered intra-arterially as a single agent for primary tumor treatment [28,95,96]. Intra-arterial administration increases local cytotoxic concentrations, thereby improving tumor penetration [28,96-100]. Studies at the University of Texas MD Anderson Cancer Center utilized a regimen consisting of 120 mg/m2 of cisplatin intra-arterially over 4h with 95 mg/m2 doxorubicin concurrently administered over 24h in a series of four preoperative courses administered at 4-week intervals. Although tumoricidal effects are achieved more rapidly with intra-arterial therapy, similar cytotoxic effects can be achieved with intravenous therapy administered in several courses [28]. Toxicities of cisplatin include auditory and renal dysfunction [28,101,102]. Hearing impairment may occur in approximately 40% of patients [87]. Patients can also develop peripheral neuropathies. Another platinum-containing chemotherapeutic agent, carboplatin, has been used in combination with other chemotherapeutics and will be discussed later in this review [9,20,28,103,104].
Oxazaphosphorines
Alkylating agents of the oxazaphosphorine family used in treating OS include cyclophosphamide and ifosfamide. Alkylating agents function by adding alkyl groups to guanine nucleotides, inducing DNA damage and resulting in cytotoxicity. Furthermore, the addition of etoposide, a topoisomerase inhibitor which interferes with DNA structural changes during the normal cell cycle, to cyclophosphamide may synergize in treating both primary and metastatic OS. The administration of regimens containing ifosfamide and etoposide (IE) to poor responders to chemotherapy demonstrated outcome improvements similar to those of good responders [13,54]. Intensification of IE chemotherapy in patients with limited tumor necrosis following first-line therapy is currently being evaluated in a clinical trial [13,105]. Alkylating agents are not cross-resistant, and patients who experience relapse after treatment with one alkylating agent may achieve responses when using another [28,106,107].
The major toxicity of the oxazaphosphorines is hemorrhagic cystitis, an effect caused directly by the metabolite acroline. Hemorrhagic cystitis can be prevented by administration of Mesna, an agent which absorbs acroline, along with generous administration of fluids [28]. Other less common toxicities include moderate myelosuppresion and renal dysfunction; renal dysfunction is less likely with the use of cyclophosphamide. In one study, investigators at the University of Texas MD Anderson Cancer Center used high-dose ifosfamide (17.5 g/m2) and recommend limiting this dose to four courses due to the development of moderate renal failure following the fifth course [28]. When there is evidence of renal dysfunction, it is recommended that cyclophosphamide is substituted for ifosfamide. Azospermia occurs in nearly all patients receiving a total ifosfamide dose great than 75 g/m2 [87].
Chemotherapy Regimens
Regimens consisting of neoadjuvant and adjuvant chemotherapy in addition to definitive surgical resection of the primary tumor have become the current standard of care for OS. Nearly all OS chemotherapy regimens consist of multiple agents with different mechanisms and minimal overlapping toxicities. The most commonly employed regimens use combinations of the chemotherapeutics cisplatin, doxorubicin, ifosfamide and high-dose methotrexate with leucovorin rescue [5,36,60,93,108-112]. Regimens containing methotrexate, doxorubicin (Adriamycin) and cisplatin are termed MAP [13]. Current multi-agent chemotherapy regimens combined with surgical resection result in 5-year survival rates of approximately 70% [87,113], and the quality of response to neoadjuvant chemotherapy is an important predictor of future survival. In fact, patients with greater than 90% necrosis in the resected tumor following neoadjuvant chemotherapy have an increased 5-year survival rate of 90% [87,114].
Different recommendations regarding adjuvant and neoadjuvant chemotherapy exist depending on the grade and stage of the tumor. Neoadjuvant chemotherapy is preferred for all patients with high-grade tumors prior to wide excision. Adjuvant chemotherapy is recommended for patients with low-grade or periosteal lesions with surgical pathological findings of high-grade disease. All patients with high-grade OS should receive both neoadjuvant and adjuvant chemotherapy following surgical resection. Despite National Comprehensive Cancer Network (NCCN) guidelines which offer the option of switching chemotherapeutic regimens in patients with poor histologic responses to neoadjuvant chemotherapy, there is no data yet to support this recommendation [87].
As one of the major challenges of OS therapy, the optimal treatment for patients with relapsed or metastatic OS remains to be defined. In the event of relapse, surgical resection if accessible and/or second-line chemotherapy are recommended [87,115]. Recommended regimens for relapsed or refractory metastatic OS include docetaxel and gemcitabine; cyclophosphamide and etoposide; cyclophosphamide and topotecan; gemcitabine, ifosfamide and etoposide; ifosfamide, carboplatin and etoposide; and high-dose methotrexate, etoposide and ifosfamide. There have been no reports to date identifying any markers detecting silent pulmonary metastases. Although a promising therapeutic avenue, molecular profiles of gene expression patterns and phenotypes of OS that may lead to personalized, less toxic regimens have not yet been well described. Man et al. set out to predict responses of OS patients to neoadjuvant chemotherapy based on genetic profiles by developing a multigene classifier. A total of 45 genes were identified, and overexpression of these genes was seen in poor responders [108,116,117]. Poor responders with overexpression of these specific genes were found to more frequently experience relapse and pulmonary metastases despite aggressive chemotherapy [108,118].
The Surgical Treatment of OS
Amputation was the primary modality of therapy of OS before the advent of chemotherapy and limb salvage. Prior to neoadjuvant chemotherapy regimens, amputation was the only surgical treatment and alone had a 5-year overall survival rate of 10-20% [25-27]. Today, surgery remains an essential component of OS treatment regimens. The primary objective of surgical therapy is complete excision of the primary tumor with conservation of the limb as a secondary goal and amputation a last resort [5,108]. Nonetheless, amputation is indicated if resection to disease-free margins results in a non-functional limb, tumor extent is beyond resectability or the patient desires the functionality of a prosthesis to the cosmesis of limb-salvage. In fact, advances in prosthetic joints which now utilize microprocessor technology have improved functional outcomes in patients undergoing amputation [113,119]. Amputation may also result in improved local control if extensive contamination of tumorous tissue is found at biopsy [21]. Young patients who are not candidates for limb-salvage procedures may sometimes undergo rotationplasty, an intercalary resection of a segment of bone followed by reconstruction of the limb by rotating 180 degrees cranio-caudally with the rotated ankle serving as a knee joint [13,120]. This alternative has outstanding oncologic, functional and psychosocial results for young patients [13,120-122].
Limb-salvage, a procedure involving allograft bone and/or prosthetic joint replacement, is often performed following complete, margin-free resection of the primary tumor and must result in reconstruction of a viable and functional limb [5,108,118]. In the 1980s and early 1990s, a 5-cm margin above or below the tumor or articular cartilage was considered adequate in preventing tumor spread [21,123,124]. Today, a 2-cm margin above or below the tumor is considered a safe margin for resection [21,40,125]. Current evidence supports the notion that closer resection margins are acceptable in patients with good responses to neoadjuvant chemotherapy and do not increase local recurrence rates [21,126,127]. Furthermore, smaller bone resection margins are more likely to allow for preservation of the growth plate as well as a larger area for endoprosthetic fixation [76].
Advances in imaging technique, neoadjuvant chemotherapy regimens and improvements in surgical methods have allowed limb-salvage surgery to become the predominant alternative to amputation [25,30,39,41-46]. Specifically, improvements in MRI have helped surgeons to better delineate the extent of the primary tumor and its relation to the neurovascular margin, precisely reducing margins and making limb-salvage more feasible [21,22,29]. By combining current chemotherapeutic regimens with limb-sparing operations, greater than two-thirds of patients with non-metastatic OS of the extremities are long-term survivors with an overall limb preservation rate of greater than 80% [21,38]. Limb-salvage surgery results in improved limb functionality, appearance and psychological outcomes while having local recurrence and survival rates similar to those undergoing amputation [25,21,39,40]. However, because of the removal of the primary tumor with adequate margins, limb-salvage procedures often result in large bone and soft tissues losses and associated long recovery times, poor joint stability and partial loss of function [25,39,44,45]. Nonetheless, since limb-salvage more successfully preserves limb function and provides improved psychological and physiological benefits, it is preferred to amputation in the vast majority of OS cases [21,25,40,44,128-131].
A retrospective study by Ayerza et al. studied 251 patients with high-grade OS and compared survival rates, limb-salvage surgery rates and rates of amputation following limb-sparing operations over three periods of time between 1980 and 2004 [21]. The 5-year survival rate during the first period in the 1980s was 36%, while it was 60% and 67% in the 1990s and early 2000s, respectively. Similarly, the limb-salvage surgery rate in the 1980s was 53%, whereas it was 91% and 97% in the 1990’s and early 2000’s, respectively. The overall limb-salvage rate, accounting for both primary and secondary amputation, was 36% in the 1980s, whereas it was 91% and 93% in the 1990s and 2000’s, respectively. This important study demonstrated that OS patients treated during the past 25 years had higher rates of limb-salvage operations and a parallel improved overall survival rate with lower rates of secondary amputation. Survival rates in this study were similar to other studies [21,30-32,126,132,133].
In order to evaluate the efficacy of limb-sparing surgery, rates of amputation following limb-sparing operations, or secondary amputation, must be considered [25,130]. In a retrospective study by Tan PX et al. 120 patients with OS of the knee underwent joint reconstruction using hinged knee prosthesis and had a secondary amputation rate of 10% (n=12). Secondary amputation resulted from local tumor recurrence in eight patients and deep infection in four patients, comparable to previous reports [21,25-27,30,40,128-131]. Aside from local recurrence and deep infection, long-term complications of prosthetic replacement include aseptic loosening with poor bone/implant interface contact, mechanical wear and peri-prosthetic fractures [21,25,42,109]. The Memorial Sloane-Kettering Cancer Center reports that 15.8% of prosthetic knee reconstructions require revision for aseptic loosening, with overall failure-free rates of 82%, 71% and 50% at 3, 5 and 10 years, respectively [109,134]. Tan et al. demonstrated a median long-term prosthesis survival of 6.2 years [25]. With this, young survivors of OS must anticipate multiple prosthetic revisions during their lifetimes.
In very young children with osteosarcoma involving the long bones, surgical resection poses challenges and often causes limb length discrepancies [13]. Measures taken by surgeons to minimize limb length discrepancies include lengthening following resection or extendible prostheses mediated by an externally applied magnetic field which lengthens the prosthesis as the patient grows [13,135]. If the joint surface is spared, intercalary allografts with or without vascularized fibular transfer have demonstrated excellent outcomes and may be considered in this group of patients [13,136,137]. Farfalli et al. recently investigated the relative performance of biologically fixed prostheses, methods which may improve endoprosthetic reconstruction fixation [13,138]. Bone distraction has been successfully used in patients with tumors not invading the physis to create newly formed bone distally and provide a margin for tumor excision followed by intercalary joint-sparing reconstruction; this method should not be used in poor responders to neoadjuvant chemotherapy [13,138-140].
While most forms of osteosarcoma require chemotherapy in addition to surgical resection, low-grade central, parosteal and periosteal OS regimens consist of surgery only and have significantly decreased risks of distant metastases [13,15-19,141,142]. To prevent the unnecessary administration of chemotherapy and its associated adverse effects, it is of the utmost importance to appropriately diagnose these subtypes.
The Role of Radiation Therapy in OS
Although not a cornerstone in OS therapy, a malignancy once considered radioresistant, radiation therapy does have a role in the treatment of certain patients. For unresectable, incompletely resectable and metastatic tumors especially within the axial skeleton, radiotherapy is recommended as part of a multimodal treatment program [13,108]. More specifically, radiation therapy can be used for the treatment of chemoresistant pulmonary metastases, inoperable primary tumors and the alleviation of cord compression and bone pain [28,111,143-145]. High-dose photon irradiation (50-70 Gy) in combination with chemotherapy can be used when tumors are located in inaccessible sites including the pelvis, vertebrae and the base of the skull as well as in patients who are not surgical candidates or refuse surgery [108,146,147]. To reduce recurrence risk and to increase the success rate in cases of limb amputation, radiotherapy may be considered as adjunct therapy [108,118].
A recent study by Ciemik et al. used proton radiotherapy with a median dose of 68.4 Gy in patients with unresectable or incompletely resected OS and demonstrated local control at 3 and 5 years of 82% and 72%, respectively [13,148]. Heavy ions may be advantageous in treating radioresistant tumors and have been investigated in OS: Utilization of high-dose carbon ions (70.4 Gy) demonstrated local control at 3 years of 92% in patients with unresectable head and neck OS [13, 149, 150]. As a palliative measure for patients with painful, inoperable bone metastases, radiotherapy with external beam radiation or radioisotopes including samarium or bisphosphonates can be considered [151,152]. Anacak et al. described an approach of intraoperative extracorporeal irradiation in which 15 patients underwent primary tumor resection with limb-salvage and single-dose extracorporeal irradiation at 50 Gy with re-implantation of involved bone segments. This method resulted in no local recurrence or graft failure in irradiated bone during a mean follow-up period of 22 months, however this novel method merits further investigation before its widespread incorporation into limb-salvage-containing regimens [108,153].
Recently Completed and Ongoing Clinical Trials for OS Therapy
While evolving regimens containing conventional cytotoxic chemotherapeutics have revolutionized OS treatment, there has been stagnation in the improvement of patient outcomes over the past three decades in patients with metastatic OS. Furthermore, many patients with metastatic and/or recurrent OS receive nearly all standard chemotherapeutic agents during the initial phase of treatment, limiting therapeutic options secondary to cumulative organ toxicity and/or resistance to previously used agents [154]. Thus, there is a critical need to develop novel and effective therapies for the treatment OS in order to improve patient survival. The following is a review of recently completed clinical trials as well as targets in current clinical trials for osteosarcoma (Table 1).
Table 1.
Recently Completed Clinical Trials for OS Therapies
| Clinical Trial | Phase Level |
Agent(s) Tested | Mechanism of Agent(s) | Study Results | Ref. |
|---|---|---|---|---|---|
| Carboplatin/Ifosfamide Window Therapy for Osteosarcoma: OS91 Trial |
II | Carboplatin, Ifos- famide |
Carboplatin: Platinum- containing chemotherapeu- tic, Ifosfamide: Alkylating chemotherapeutic |
Carboplatin/ifosfamide-containing regimen demonstrated similar re- sponse rates to cisplatin-containing therapies with lower toxicity |
Meyer et al.
2001 |
| Frontline treatment of localized osteosarcoma without methotrexate: OS99 Trial |
II | Carboplatin, Ifos- famide, Methotrexate |
Methotrexate: Dihydrofo- late reductase inhibitor |
Carboplatin/ifosfamide-containing regimen demonstrated similar re- sponse rates to both cisplatin- containing or methotrexate- containing regimens |
Daw et al.
2011 |
| Addition of pamidronate to chemo- therapy for the treatment of os- teosarcoma |
I | Pamidronate | Bisphosphonate | Pamidronate was safely incorporated into OS chemotherapy regimens without altering efficacy and may improve durability of reconstruction |
Meyers et al. 2011 |
| The Addition of Muramyl Tripep- tide to Chemotherapy Improves Overall Survival: Intergroup Study 0133 |
III | Liposomal mu- ramyl tripeptide phosphatidyl ethanolamine (L- MTP-PE) |
Non-specific immune modulator activates pul- monary inflammatory cells |
Addition of ifosfamide to cis- platin/doxorubicin and MTX did not improve EFS or overall survival; addition of L-MTP-PE significantly improved overall survival with a trend toward improved EFS |
Meyers et al. 2008 |
| Addition of muramyl tripeptide to chemotherapy for patients with newly diagnosed metastatic OS |
III | Liposomal mu- ramyl tripeptide phosphatidyl ethanolamine (L- MTP-PE) |
Non-specific immune modulator activates pul- monary inflammatory cells |
Addition of L-MTP-PE did not demonstrate improvement in out- comes among patients with metas- tatic OS |
Chou et al. 2009 |
| Inhaled GM-CSF for First Pulmo- nary Recurrence of Osteosarcoma: Effects on Disease-Free Survival and Immunomodulation |
I | Granulocyte- Macrophage Col- ony Stimulating Factor (GM-CSF) |
Immunostimulatory protein secreted by WBCs aug- ments proliferation and function of WBCs |
Inhalation of GM-CSF was feasible with low toxicity, but no detectable immunostimulatory effects or im- proved outcomes were demonstrated |
Arndt et al. 2010 |
| Phase II Trial of Trastuzumab in Combination With Cytotoxic Chemotherapy for Treatment of Metastatic Osteosarcoma With HER2R Overexpression |
II | Trastuzumab | Humanized monoclonal antibody to human epider- mal growth factor receptor 2 (HER2) |
Addition of trastuzumab to cytotoxic chemotherapy in patients with HER2-positive OS did not improve patient outcomes. Trastuzumab was safely administered without short- term cardiotoxicity |
Ebb et al. 2012 |
St. Jude Children’s Research Hospital OS99 and OS91Trials-HDMTX and Carboplatin
As discussed earlier, methotrexate has been a mainstay in OS chemotherapy for decades. However, the necessity of high-dose methotrexate (HDMTX) within a multi-agent regimen has come into question as many theorize that its toxicity interferes with the dose-intensive delivery of other agents [76,77,155-157]. Furthermore, HDMTX requires sophisticated pharmacokinetic monitoring and leucovorin rescue dose-adjusted to MTX levels, a process which can be difficult for institutions that cannot adequately provide this [76,77]. The recently completed St. Jude Children’s Research Hospital OS99 Trial investigated the efficacy of a regimen comprised of carboplatin, ifosfamide and doxorubicin without HDMTX in patients with newly diagnosed, localized, resectable OS [76]. The previously performed OS91 trial utilized a regimen comprised of carboplatin, ifosfamide, doxorubicin and HDMTX, yielding similar outcomes with comparably less toxicity to cisplatin-based regimens [76,103].
In the OS99 trial, treatment consisted of 12 cycles of chemotherapy administered over 35 weeks: 3 weeks of carboplatin (8 mg/mL × min on day 1) and ifosfamide (2.65 g/m2 × 3d) and one cycle of doxorubicin (25 mg/m2 × 3d) prior to surgical resection. Following resection, 2 cycles of carboplatin combined with ifosfamide and 3 cycles each of doxorubicin (25 mg/m2 daily × 2d) combined with ifosfamide or carboplatin were administered [76]. Out of 72 eligible patients with a median age of 13.4 years enrolled from 1999 to 2006, 40 of 66 (60.6%) evaluable patients demonstrated good histologic responses (>90% tumor necrosis) following neoadjuvant chemotherapy. Estimated 5-year event-free survival rate was 66.7%±7.0% for the OS99 trial compared to 66.0%±6.8% for the OS91 trial (p=.98), which contained HDMTX treatment. Estimated 5-year survival rate was 78.9±6.3% for the OS99 trial and 74.5%±6.3% for the OS91 trial (P=0.40).
The OS99 trial also investigated whether resection of the primary tumor with a 3-cm rather than 5-cm (as used in the OS91 trial) bone margin could be safely performed without increasing the rate of local recurrence [76]. The use of a 3-cm bone resection margin did not adversely affect the rate of local control as compared to the OS91 trial, findings that may also be partially attributable to improved surgical and imaging techniques over the past decade [76]. In fact, a local recurrence rate of 3% with a 3-cm margin in the OS99 trial is on the low end of the range cited by other studies [12,36,54,76,158,159].
The results of the OS99 trial demonstrate that the regimen tested produces outcomes comparable to those of both cisplatin-containing and HDMTX-containing regimens. HDMTX-free regimens may provide safe alternatives to patients who demonstrate intolerance to HDMTX, especially those with renal dysfunction, as well as institutions that cannot provide adequate pharmacokinetic monitoring for HDMTX [76]. Furthermore, use of the OS99 regimen may reduce the cost and treatment complexity associated with administration of HDMTX.
The OS91 trial demonstrated that carboplatin-containing regimens resulted in similar outcomes with less toxicity than cisplatin-containing regimens while the OS99 trial demonstrated that HDMTX-free regimens resulted in similar outcomes as HDMTX-containing compounds in patients with localized OS. As most OS chemotherapy regimens today utilize methotrexate and/or cisplatin, the findings of the OS91 and OS99 trials may provide less toxic and costly alternatives with equivalent efficacy to the current standard of care.
Bisphosphonates
Bisphosphonates including zoledronate, minodronate, risedronate and alendronate have recently demonstrated anti-tumor activity in OS [108,117,160-163]. Bisphosphonates are analogues of endogenous pyrophosphates, molecules delivered to sites of increased bone formation and resorption which strongly bind to hydroxyapatite on bone surfaces [109]. Bisphosphonates are frequently used for the treatment of hypercalcemia of malignancy, and it was originally thought that these drugs exclusively stabilized bone. However, bisphosphonates have shown direct effects on tumor cells, with in vitro studies demonstrating that bisphosphonate treatment of myeloma cells causes inhibition of proliferation and induction of apoptosis; these effects were also seen in breast cancer cell lines [109,164-166]. Bisphosphonates likely inhibit adhesion of tumor cells to bony matrix and may also inhibit matrix metalloproteinases, enzymes utilized by tumor cells to invade tissue and metastasize [109,167,168]. In addition to interfering with mechanisms used by tumor cells to establish metastases, several of the genes upregulated in OS cells may be inhibited by bisphosphonates [109]. Numerous in vitro and in vivo studies of both human and animal OS cell lines have demonstrated activity of the bisphosphonates alendronate, clodronate, minodronate, pamidronate and zoledronate against OS, both alone and in combination with standard chemotherapy [109,117,160-163,169-184].
In a study by Mintz et al., expression profiling demonstrated significantly different expression of many genes involved in osteoclastogenesis and bone resorption including osteoprotegerin, annexin 2, SMAD and TGFB-1 between groups of patients with favorable and non-favorable responses to chemotherapy, suggesting that the interaction of tumor and microenvironment is a critical determinant of chemotherapeutic response and may be altered by the therapeutic use of bisphosphonates [109,185].
A study by Horie et al. combined zoledronate with paclitaxel or gemcitabine and demonstrated antitumor effects in mouse OS cells. While zoledronate alone was found to have anti-OS activity, the agent acted synergistically when combined with paclitaxel or gemcitabine. Furthermore, pretreatment of OS cells prior to doxorubicin administration augmented OS cell sensitivity [108,176].
A Phase I clinical trial performed by Meyers et al. investigated the safety and feasibility of adding pamidronate, the most widely used bisphosphonate in children and young adults, to standard OS chemotherapy regimens [109,186-193]. Forty OS patients were enrolled in the study, 29 without clinically detectable metastatic disease and 11 with clinically detectable metastatic disease at presentation. They were treated with cisplatin, doxorubicin and methotrexate with the addition of pamidronate 2mg/kg/dose (max 90 mg) monthly for 12 doses. Endpoints included overall survival, event-free survival (EFS) and durability of prosthetic reconstruction. Patients with localized disease demonstrated 5-year EFS of 72% and overall survival of 93%. Patients with metastatic disease demonstrated 5-year EFS of 45% and overall survival of 64%. These survival rates are similar to previous studies [21,30-32,126,132,133]. Toxicity for patients treated with pamidronate was similar to those treated with chemotherapy alone. Allograft reconstruction resulted in 2 graft failures, 4 delayed unions and 6 successful grafts with 5 of 33 overall reconstruction failures and no stress fractures or growth disturbances.
The results of this clinical trial demonstrate that pamidronate can be safely incorporated into standard chemotherapeutic regimens for the treatment of OS while not impairing the efficacy of chemotherapy and possibly improving the durability of limb reconstruction in limb-salvage surgery. These promising results provide justification for a prospective randomized trial with the addition of bisphosphonates to chemotherapy for the treatment of OS. While pamidronate was selected for this clinical trial, newer and more potent bisphosphonates, such as zoledronate, may have more potent anti-tumor effects. In fact, more preclinical data exists supporting the anti-OS effects of zoledronate than any other bisphosphonate [109,117,160,170-172,175-178,182-184,194]. However, employing zoledronate in future trials may increase risk of osteonecrosis of the jaw or atypical fractures, necessitating more vigilant observation for toxicity during therapy [109,195]. Two OS clinical trials are currently under way using zoledronate [196,197].
With a multitude of promising preclinical studies, a successful phase I clinical trial and ongoing phase II/III clinical trials, bisphosphonates may soon be incorporated into OS treatment regimens. These drugs may not only improve patient survival but also the durability of reconstruction following limb-salvage surgery.
Liposomal Muramyl Tripeptide Phosphatidylethanolamine (L-MTP-PE)
Liposomal muramyl tripeptide phosphatidyl ethanolamine (L-MTP-PE) or MEPACT (Milennium: The Takeda Oncology Company, Cambridge, Massachusetts, USA) functions by causing infiltration of inflammatory macrophages into pulmonary metastases [28,113,198,199]. MTP-PE is a synthetic analog of a component of bacterial cell walls which acts as a non-specific immune modulator [200]. MTP-PE has been incorporated into liposomes, allowing targeted delivery of MTP-PE to monocytes and macrophages in locations such as the lungs, the most common site of OS metastasis [200,201]. MTP-PE is a ligand for the nucleotide-binding oligomerization domain 2 (NOD2) receptor, a receptor expressed by monocytes, macrophages, dendritic cells and granulocytes [202,203,204]. MTP-PE binding with NOD2 causes activation of monocytes, stimulating increased expression of adhesion molecules and pro-inflammatory mediators including TNF-α, IL-1, -6, and -8 and monocyte chemotactic protein-1 (MCP-1) [205,206]. Several preclinical studies have demonstrated and confirmed the anti-OS effects of L-MTP-PE in rodent and canine models [200,201,207-217]. Importantly, concurrent administration of standard cytotoxic chemotherapy does not interfere with the antitumor effects of L-MTP-PE [200,213].
In the 1990s, the Children’s Cancer Group and Pediatric Oncology Group performed a prospective, randomized phase III clinical trial of newly diagnosed OS patients named Intergroup Study 0133, the largest ever randomized trial in OS. The study was a 2x2 factorial design with one group of patients receiving either a 3-drug regimen containing doxorubicin, cisplatin and HDMTX or a 4-drug regimen containing the same three drugs as well as ifosfamide. The second group of patients was randomly assigned either the 3- or 4-drug regimen with L-MTP-PE. Patients received one of four randomized treatments and underwent complete resection of the primary tumor. There was a significant improvement in overall survival (p=0.03, 70% vs. 78%, hazard radio 0.71, 95% CI 0.52-0.96) and a trend toward improved event-free survival (p=0.08) in patients receiving L-MTP-PE, however no benefit for patients receiving the 4-drug regimen versus the 3-drug regimen [113,200]. Both chemotherapy regimens resulted in similar EFS and overall survival: EFS for all patients treated with the three-agent chemotherapy regimen was 65% and 63% at 4 and 6 years, respectively and 66% and 64% for all patients receiving the four-agent regimen (p=0.91).
In a prospective randomized phase III clinical trial for patients presenting with metastatic OS, Chou et al. of the Children’s Oncology Group (COG) investigated the efficacy of L-MTP-PE when added to a standard 3-agent chemotherapy regimen of cisplatin, doxorubicin and HDMTX in 91 patients. 46 patients received three-agent chemotherapy and L-MTP-PE, while 45 patients received only three-agent chemotherapy. There was a trend but non-significant improvement in 5-year EFS for patients receiving L-MTP-PE (42% vs. 26%, RR 0.72, p=0.23, 95% CI 0.42-1.2). There was also a trend but non-significant improvement in 5-year overall survival for patients receiving L-MTP-PE (53% vs. 40%, RR 0.72, p=0.27, CI 0.4-1.3). The results of this study do not strongly suggest that the addition of L-MTP-PE to standard three-agent chemotherapy can improve outcomes in patients with metastatic OS, however the outcome pattern is similar to that seen in the statistically significant non-metastatic cohort from the Intergroup Study 0133, the largest prospective randomized OS trial performed to date [200]; the comparatively small number of patients in the COG study likely precludes precise evaluation of treatment efficacy. L-MTP-PE was recently approved by the European Medicine Agency and currently is used in Europe for treatment of resectable, non-metastatic high-grade OS. It is also available through a Compassionate Investigational New Drug (CIND) application in some trials in the United States [198,199,200].
The L-MTP-PE clinical trials demonstrate that this novel drug may improve survival as part of a treatment regimen in patients presenting with non-metastatic OS. Furthermore, future larger clinical trials may demonstrate improved patient outcomes in patients presenting with metastatic OS. With recent approval for the treatment of non-metastatic high-grade OS in Europe, we may soon see benefit from the use of this drug that will facilitate its approval for use in the United States.
Inhaled Granulocyte-Macrophage Colony Stimulating Factor (GM-CSF)
Granulocyte-macrophage colony stimulating factor is a protein secreted by several types of WBCs that stimulates the proliferation and differentiation of hematopoietic cells and augments the function of WBCs including neutrophils, monocytes, macrophages and dendritic cells [218]. GM-CSF also has immunomodulatory and immunostimulatory effects that promote recruitment and increased cytotoxicity of activated macrophages, increased numbers of CD4 T-cells, and enhanced function of natural killer and dendritic cells [154,219-221]. As the lung is the most common location for OS metastasis, an immunostimulatory approach directly targeting the lungs may provide a therapeutic avenue for patients with pulmonary recurrence of OS. Preclinical data has demonstrated antitumor effects of GM-CSF [154,222,223]. Additionally, initial phase I data on inhalational GM-CSF in humans exists [154,218,224,225]. OS cells metastatic to the lung have shown decreased cell surface expression of Fas and Fas ligand (FasL) [154,226,227], and induction of Fas on lung metastases using aerosol therapy has been demonstrated to induce tumor regression in a mouse model [154,228].
Arndt et al. of the Children’s Oncology Group (COG) recently performed a phase I clinical trial using inhaled GM-CSF in patients with first isolated pulmonary recurrence of OS. Forty three eligible patients received inhaled GM-CSF at doses ranging from 250 to 1750 μg twice daily on alternating weeks. After two cycles, thoracotomy was performed to resect tumors and analyze pulmonary nodules for expression of Fas/Fas L and dendritic cells via immunostaining for CD1a, clusterin and S100. Patients then received 12 adjuvant cycles of therapy on alternating weeks or until disease progression.
Dose escalation to 1750 μg twice daily was found to be feasible without toxicity. 3-year EFS and 3-year overall survival were 7.8% and 35.4%, respectively, similar to multiple prior studies [35,38,51,53,154,229-232]. However, only 11 of 30 nodules following inhalation stained positive for CD1a, 4 of 30 for S100 and 6 of 30 for clusterin. While the results of this study demonstrate that the administered doses of inhaled GM-CSF do not result in toxicity and aerosolized delivery of a biological agent to patients is feasible, no detectable immunostimulatory effects in pulmonary metastases or improved patient outcomes occur following administration of this drug.
While exciting preclinical data has demonstrated the anti-OS effects of GM-CSF, this phase I clinical trial established safety at administered doses without any significant improvement in patient outcomes or immunostaining, a surrogate measure of the immunostimulatory effects of inhaled GM-CSF. With safety of this therapy established, a future larger prospective randomized trial may demonstrate improved outcomes in OS patients.
Human Epidermal Growth Factor Receptor 2
As a correlation has been established between overexpression of the human epidermal growth factor receptor 2 (HER2) and poor OS patient outcomes, this receptor has become a therapeutic target [233-237]. Trastuzumab, a humanized monoclonal antibody which specifically binds HER2, has demonstrated therapeutic responses when combined with cytotoxic chemotherapy in breast cancer patients with elevated expression levels of HER2 [238-244]. While a promising target, cardiotoxicity is an adverse effect associated with trastuzumab therapy and is particularly concerning when combined in regimens with doxorubicin or other anthracyclines [233].
Ebb et al. investigated the feasibility and safety of treating children newly diagnosed with metastatic OS with trastuzumab combined with cytotoxic chemotherapy [233]. Prior to enrollment, all patients underwent testing for HER2 expression using immunohistochemical staining and grading. Only patients with overexpression of HER2 were treated with trastuzumab. The chemotherapeutic agents used in the study were doxorubicin, methotrexate, cisplatin, ifosfamide and etoposide. To minimize the risk of cardiotoxicity, dexrazoxane was included in the treatment regimen. 96 evaluable patients were enrolled; 41 were HER2 positive and 55 were HER2 negative.
For HER2-positive patients, 30-month EFS was 32% with a 30-month overall survival of 59%. Among HER2-negative patients, 30-month EFS was 32% with a 30-month overall survival of 50%, with differences between HER2-positive and negative groups not significant (p=0.54 for EFS, p=0.58 for overall survival). Regarding cardiotoxicity, no patient developed clinical evidence of congestive heart failure, significant differences in blood pressures or left ventricular fractional shortening (LVFS). While these results demonstrate that trastuzumab can be safely delivered in combination with anthracycline-containing regimens and dexrazoxane, the therapeutic benefit in HER2-positive patients remains uncertain. However, while HER2-negaitve patients and HER2-positive patients demonstrated similar EFS and overall survival outcomes, it is possible that the addition of trastuzumab to the regimen of HER2-positive patients may have diminished the adverse prognostic impact of HER2 overexpression in this cohort of patients.
While the safety of trastuzumab was demonstrated in this phase II clinical trial, administration of this monoclonal antibody against HER2 was not randomized and thus its survival benefits cannot be concluded from this study alone. A prospective randomized study will be essential in determining this agent’s true efficacy. A recently completed study by the Children’s Oncology Group describing the impact of HER2 status and response/survival in non-metastatic OS patients will likely be the basis of future prospective randomized trials with trastuzumab.
Insulin-like Growth Factor Pathway
The insulin-like growth factor pathway has wide implications in a variety of different cancer types, and overexpression of IGF-1, IGF-2 and IGF-1 receptor (IGF-1R) has been demonstrated in OS [13,245-247]. Therapies targeting the IGF pathway are currently being explored in clinical trials for Ewing’s Sarcoma and OS [13,248-250], including inhibitors of the IGF-1 receptor (IGF-1R) [251,252]. Ganitumab, a fully human monoclonal antibody to IGF-1R, has been shown to decrease proliferation in OS cell lines and inhibit OS tumor growth in vivo [253,254]. Recently, a phase II clinical trial investigated the effects of ganitumab in patients with metastatic Ewing family tumors (EFT) or desmoplastic small round cell tumors (DSRCT) [252]. Patients received ganitumab monotherapy every 2 weeks which demonstrated significant anti-tumor activity in these inevitably fatal and aggressive tumors. Although tolerated, adverse effects included fatigue, thrombocytopenia, hyperglycemia and infusion reactions, findings also reported in the first in-human study with ganitumab [255]; the deaths in the study were all due to disease progression.
While ganitumab monotherapy was found to demonstrate modest clinical activity in patients with EFT/DSRT, there have been no published clinical trials to date demonstrating anti-tumor activity in OS. The results of these clinical trials in EFT/DRST are the foundation on which current clinical trials targeting the IGF pathway in EFT and OS are based. The promising results from these initial trials will hopefully demonstrate anti-tumor activity in metastatic OS as well.
Angiogenesis
Targets of angiogenesis as potential OS therapies include inhibitors of vascular endothelial growth factor (VEGF) and mammalian target of rapamycin (mTOR) with agents such as bevacizumab and ridaforolimus, respectively. These agents are being studied at both the preclinical and clinical level [13,256-259]. Angiogenesis is critical for tumor development, invasion and metastasis and is regulated by proangiogenic factors including vascular endothelial growth factor (VEGF), a factor expressed in nearly all human tumors and highly associated with increased tumor vascularity [260]. Antibodies of VEGF have demonstrated antitumor activity in both preclinical and early phase I/II trials in adults [261]. However, there is a concern for toxicity when monoclonal VEGF-neutralizing antibodies are used in the pediatric population which is commonly affected by OS. A recent study demonstrated a severe adverse events (SAEs) rate of 17% in pediatric patients with recurrent or poor-prognosis malignancies including Ewing Sarcoma and rhabdomyosarcoma, rates higher than previous reports [262,263]. Further investigation with larger cohorts will be needed to assess the safety profile and benefits of bevacizumab in pediatric cancer patients.
A recent clinical phase II trial with ridaforolimus assessed its antitumor activity in patients with advanced sarcomas including OS [256]. Of 212 patients treated, four patients demonstrated partial responses including two patients with OS. All patients reported adverse effects, although generally mild, including fatigue, stomatitis and hypertriglyceridemia. While demonstrating promising progression-free survival rates among patients with advanced sarcoma, the true clinical benefit would be best addressed by a randomized clinical trial. This is currently being evaluated in patients with advanced bone and soft tissue sarcoma in the ongoing Sarcoma mUlti-Center Clinical Evaluation of the Efficacy of riDaforolmus (SUCCEED) trial.
While these recently completed clinical trials do not specifically demonstrate anti-OS activity, ongoing clinical trials will hopefully better describe the efficacy and safety of these drugs and their potential role in OS therapy.
Other Clinical Trials
Recombinant human apoptosis ligand 2/tumor necrosis factor-related apoptosis-inducing ligand (rhu Apo2L/TRAIL) (ligand for the death receptor), also called dulamnermin, is a proapoptotic receptor agonist which binds death receptor 4 and death receptor 5 (also called TRAIL 1 and 2, respectively) and triggers cell death independently of the p53 pathway via activation of the intrinsic pathway [198,264]. A recent phase I study of Apo2L/TRAIL demonstrated significant partial responses (PRs) in chondrosarcoma patients and is now being used in phase I trials for OS patients. The underlying mechanisms of response and resistance of this drug in sarcoma patients remains to be determined [198].
A bone-seeking radiopharmaceutical Samarium-153 ethylene diamine tetramethylene phosphonate (153Sm-EDTMP) was found in one study to be effective in treating metastatic OS, but follow-up studies have not confirmed these findings [87,265]. As earlier, symptomatic bone metastases have also been treated with high-dose 153Sm-EDTMP, resulting in significant relief but also resulting in severe myelosuppression [28,266].
Cediranib (Recentin, AZD2171, AstraZeneca Pharmaceuticals, Wilmington, Delaware, USA) is an orally available small molecule which inhibits the tyrosine kinase activity of vascular endothelial growth factor receptor 1 (VEGFR-1), VEGFR-2 and VEGFR-3, receptors which mediate both angiogenesis and lymphangiogenesis. In a pediatric phase I study in OS patients with pulmonary metastases, objective responses were demonstrated in patients with Ewing sarcoma, synovial sarcoma and osteosarcoma [198,267,268]. Phase I studies using a combination of cediranib with bevacizumab (Avastin, Genentech/Roche, and CA) are in progress (ClinicalTrials.gov number NCT00458731) [198]. A phase II study is currently in development [267].
Rexin-G (Epeius Biotechnologies, San Marino, California, USA) is designed as a tumor-targeted retrovector nanoparticle consisting of a collagen matrix binding motif on its surface and a dominant negative cyclin G1 construct which functions by inhibiting the G1 phase of the cell cycle [198,269]. A recently completed independent phase I/II and phase II study in chemotherapy-resistant OS patients receiving escalating doses of IV Rexin-G demonstrated median progression-free survival of 3.7 months and median overall survival of 7.8 months at high doses, showing a dose-response relationship in the Phase I/II trial. Results of the phase II study were similar. The results of this phase study demonstrate that Rexin-G therapy is safe with no dose-limiting toxicity at the doses administered and may improve survival in chemotherapy-resistant sarcoma and osteosarcoma [211].
Other specific targets of phase I/II clinical trials for which OS patients may be enrolled include p53/mouse double minute (MDM2) inhibitor, MET, histone deacetylase (HDAC) and notch/gamma secretase inhibition [198].
Molecular Basis of Osteosarcoma
OS tumors arise during times of high bone turnover, most commonly during the adolescent growth spurt [146,270]. Mutations involving oncogenes and tumor suppressors arising from chromosomal amplifications, deletions, rearrangements or translocations may explain some of the molecular underpinnings of OS [146,270,271]. Aberrations in various signaling pathways have also been associated with OS tumor growth and metastasis. Furthermore, recent studies have suggested that OS may arise from differentiation defects in which affected cells are arrested as undifferentiated precursors [272]. Therefore, current research in the development of novel OS therapies seeks to address molecular targets and differentiation defects in order to reduce disease severity, improving patient prognoses.
Tumor Suppressors
Mutations in Rb and p53 tumor suppressor pathways are associated with OS pathogenesis. An important regulator of the G1/S transition, hypophosphorylated Rb binds to and inactivates the transcription factor E2F, leading to cell cycle arrest. Rb itself is inactivated through phosphorylation by the Cyclin D1/CDK4 complex, liberating E2F to promote cell entry into S phase. Nearly 70% of sporadic OS cases have been associated with an abnormality in the Rb1 locus [146,271-276]. Furthermore, in addition to being linked to a poor prognosis following OS diagnosis, individuals heterozygous for an inactivation of Rb1 have a 1,000-times greater risk of developing OS
Another tumor suppressor in the Rb pathway, p16INK4A, is associated with development of OS [277]. Normally, p16INK4A inactivates CDK4 and therefore prevents the G1 to S-phase transition. Mutations in p16INK4A can mimic Rb inactivation, resulting in loss of tumor suppressor ability and thus uninhibited cell cycle progression. Also similar to Rb, p16INK4A alterations are associated with a poor OS prognosis, especially in pediatric patients [271,278].
Also implicated in OS, the p53 gene is involved in regulating cell cycle progression within the context of apoptosis and DNA repair mechanisms [279-282]. Point mutations, gene rearrangements and loss of the p53 allele at the 17p13 region are frequently demonstrated in OS patients [271,283]. In such cases, inactivation of this important tumor suppressor allows cells to bypass repair checkpoints and proliferate without adequate regulation.
Oncogenes
Several oncogenes have been associated with the development of OS. The c-Myc gene, encoding a transcription factor involved in cell proliferation and tumor growth, appears to be amplified in nearly 12% of OS cases; it has been suggested that patients with overexpression of c-Myc have a higher rate of tumor recurrence [284-289]. MDM2 is involved in inactivating and marking the tumor suppressor p53 for degradation and is amplified at the 12q13 locus in about 10% of OS patients [280-283,290-292]. CDK4, which forms a complex with Cyclin D1 and promotes cell cycle progression, also appears to have higher levels of expression in OS, resulting in increased cell proliferation and tumor growth [293]. Additional oncogenes for which activating mutations have been associated with OS include CCND1, FOS and ERBB2 [270].
Signaling Pathway Dysregulation
Aberrant signaling in a variety of pathways has been implicated in osteosarcoma development. As one of the most extensively studied pathways in OS, variations in the TGF-β signaling pathway, consisting of three proteins (TGF-β1-3) involved in cellular differentiation, growth and apoptosis, have been demonstrated to cause tumor progression [294-297]. Increased expression levels of TGF-β1 and TGF-β3 have been demonstrated in OS patients, with tumor progression strongly correlated to increased expression [298].
Defects in the insulin-like growth factor (IGF) signaling axis also have been implicated in tumor growth and the metastatic propensity of OS. IGF-1 and IGF-2 are growth hormones critical in the development of long bone, particularly during the adolescent growth spurt [299]. By binding to the IGF-1 receptor (IGF-1R), either ligand can promote cell proliferation while also weakening cell-to-cell and cell-to-extracellular matrix interactions [300]. When overexpressed, IGF hormones can lead to tumor growth. Similarly, overexpression of IGF-1R can lead to a malignant OS phenotype [301]. Furthermore, insulin-like growth factor binding protein 5 (IGFBP5), one of six proteins that modulates the activity of IGF hormones, is significantly underexpressed in OS and may explain both the highly proliferative and metastatic potential of these tumors [299].
Other signaling pathways also implicated in OS include Shh, FGFR2 and MET/HGF, though their molecular underpinnings are not well understood [302-304]. Finally, pathways associated with Wnt proteins and Runx2, which will be discussed in detail later in this review, are associated with defects in osteogenic differentiation and seem to be involved in OS tumor development.
OS-Associated Syndromes
There are several medical conditions that have been implicated in patients who also develop OS. Rothmund-Thomson syndrome leads to photosensitivity, cataracts and skeletal dysplasias due to a mutation in a RECQ helicase [305]. Patients afflicted with this autosomal recessive condition have a higher tendency to develop OS, with rates suggested as high as 32%, and at a younger age than typical OS patients. Approximately 1% of patients with Paget’s disease develop OS. Resulting from an imbalance in osteoblast and osteoclast activity, Paget’s contributes to large deformations in bone which may play a critical role in the development of OS, especially among older patients developing this malignancy [306]. Neurofibromatosis 2 (NF2) is thought to contribute to development of OS through destabilization of p53. Patients with NF2 have decreased expression of merlin, an ERM-related tumor suppressor that inhibits MDM2-mediated degradation of p53 [307,308]. Mice heterozygous for NF2 have been shown to have a higher propensity for poorly differentiated and highly metastatic OS tumors.
Osteosarcoma Arises From Defects in Mesenchymal Stem Cell Differentiation
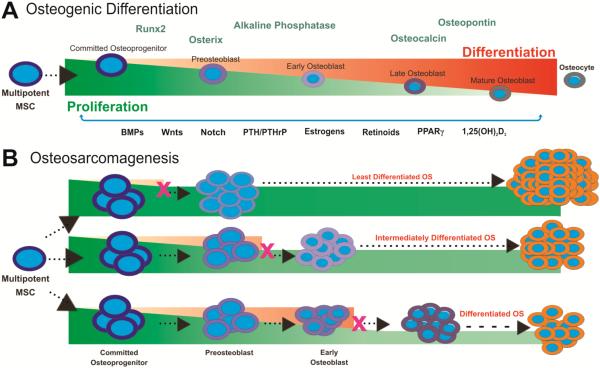
Stem cells are undifferentiated precursor cells defined by their ability to self-renew and proliferate which can give rise to many tissue types [309]. Specifically, mesenchymal stem cells (MSCs) are bone marrow stromal cells with the capacity to differentiate into bone, muscle, tendon and adipose tissue [310-313]. Various endogenous and environmental factors play a critical role in tightly regulating osteogenic differentiation, leading to proper bone formation [272]. There are also several markers of osteoblastic differentiation indicating specific time points along the cascade, including connective tissue growth factor (CTGF), alkaline phosphatase (ALP), Osterix, Runx2, osteopontin (OPN), osteocalcin (OCN) and collagen [270,272,294,309,313-318]. Dysregulation in the balance between proliferation and terminal differentiation of MSCs, indicated by the overexpression or absence of these markers, may lead to the development of tumors (Fig. 1).
Fig. (1). Osteosarcoma is a differentiation disease.
(A) Osteogenic differentiation of mesenchymal stem cells (MSCs). Multipotent bone marrow stromal cells have the capacity to differentiate into bone, muscle, tendon and adipose tissue, and are shown progressing along the osteogenic differentiation cascade. Osteogenic differentiation is a tightly regulated process which can be monitored by using early, middle and late markers. Early markers of the osteogenic differentiation cascade include the essential osteogenic transcription factor Runx2. Early/middle osteogenic markers include alkaline phosphatase (ALP) and Osterix. Osteocalcin and osteopontin are late markers of bone formation. MSCs can be direted along the osteogenic pathway by stimulation from osteogenic regulators including BMPs, Wnts, Notch, PTH/PTHrP, Estrogens, Retinoids, PPARυ and 1,25(OH)2D3. (B) Defects in osteogenic differentiation may lead to OS development. Alterations in MSC differentiation include activation of oncogenes (eg, MDM2, c-Myc, CDK4), inactivation of tumor suppressor genes (eg, p53, RB, p16INK4A) and epigenetic changes. If these genetic or epigenetic changes block progression to terminally differentiated osteoblasts or osteocytes, tumorigenic precursors may be formed, maintaining proliferative capacity and increasing susceptibility to OS development. Alterations disrupting the tightly regulated balance between proliferation and differentiation may lead to a tumorigenic phenotype, with defects at early stages leading to the development of more aggressive and undifferentiated OS.
Undifferentiated osteoprogenitors and OS cells are similar in that they are both highly proliferative, resistant to apoptosis and express many of the same osteogenic markers. Normally, gradual telomere shortening over many replication cycles leads to senescence as a cell approaches terminal differentiation. However, in the case of OS, an alternative lengthening of telomere (ALT) pathway can prevent the loss of telomeres with cells instead remaining in a highly proliferative, stem cell-like state [319]. This ability to evade senescence allows OS cells to self-renew and continually respond to growth factors in the same manner as early osteoprogenitors [320]. Furthermore, aggressive OS phenotypes more closely resemble early progenitors, while less aggressive phenotypes share similarities with more differentiated MSCs [272,312]. The expression of ALP, an early/middle marker of osteogenesis, is decreased in OS cells when compared to a committed osteoblastic line [317,321]. Conversely, human OS cells demonstrate increased basal levels of CTGF, a marker upregulated in the earliest stages of osteogenic differentiation [322]. Late markers such as OPN and OCN are highly expressed in mature osteoblasts but only expressed at minimal levels in OS cell lines and tumors [294,313,323,324]. These findings strongly suggest that OS cells arise from MSCs that are unable to undergo terminal differentiation, with increasing degress of differentiation associated with an improved prognosis.
To better understand defects in osteogenesis that can promote OS tumor growth, Runx2 and Wnt can be investigated. Runx2, a transcription factor of the runt family, is a master regulator of osteoblastic differentiation and thought to be linked to various human cancers including OS when its expression is altered [325-327]. When associated with p27KIP1 and hypophosphorylated Rb, Runx2 normally induces exit from the cell cycle at the G1 checkpoint, leading to terminal osteoblastic differentiation [327]. Furthermore, Runx2 regulates bone morphogenetic protein (BMP)-induced differentiation along the osteogenic cascade. In OS, Runx2 is significantly underexpressed and therefore leads to uncontrolled proliferation and poor differentiation, contributing to a malignant phenotype [270]. Additionally, decreased p27KIP1 expression levels are associated with poor differentiation and high-grade tumors [327]. Therefore, a thorough understanding of the Runx2 pathway and aberrations may provide valuable insight into the development of OS, its prognosis and the development of novel therapies.
The Wnt pathway has also been studied in the context of various cancers. Typically, the Wnt glycoprotein binds to the Frizzled receptor and LRP5/6 co-receptors [318,328,329] which inhibits downstream phosphorylation and degradation of β-catenin, facilitating nuclear translocation, proliferation and differentiation [316]. During osteoblastic differentiation, Wnt3a suppresses osteogenic differentiation and promotes proliferation in adult MSCs [330]. Certain variations in this pathway have been implicated in OS [331,332]. Overexpression of β-catenin, for example, is associated with osteoprogenitor proliferation and an increased metastatic potential of OS cells. Furthermore, LRP5 overexpression by OS cells is correlated with poorer prognoses and decreased survival [333]. Similar to the Runx2 pathway, it is evident that aberrations in the Wnt signaling pathway can lead to increased proliferation and decreased differentiation of immature osteoblast progenitors.
Potential Therapeutic Agents for MSC Differentiation Defects
Since defects in differentiation may lead to the development of OS, there is great promise in identifying therapeutic targets with the ability to induce terminal osteoprogenitor differentiation, decrease aberrant cell proliferation and promote apoptosis. Such therapies may also circumvent the need for highly toxic and often resistance-inducing chemotherapy regimens. Recent studies have investigated a variety of therapeutic approaches to promote osteogenic differentiation including the use of nuclear receptor agonists and growth factors [272,312,334-339].
Super proteins of the nuclear receptor family, including PPARυ, retinoids and estrogens, have been investigated as potential OS differentiation agents. PPARυ agonists have been shown to prevent proliferation, increase susceptibility to apoptosis and induce differentiation with in OS cells (1:15,153,59)[272,302,313,315]. 9-cis retinoic acid and all-trans retinoic acid also inhibited growth while promoting differentiation of OS cells, demonstrating a strong synergistic effect when combined with PPARυ agonists [336,337]. Furthermore, various estrogen receptor antagonists are able to inhibit proliferation, increase osteoblast differentiation and promote apoptosis in OS cells [340].
1,25-dihydroxyvitamin D3 (1,25(OH)2D3) is another nuclear receptor agonist that has shown promise in promoting differentiation along the osteogenic cascade [339]. 1,25(OH)2D3 induces expression of p21, a downstream target of the p53 tumor suppressor, which in turn induces differentiation of osteoprogenitors and also triggers apoptosis. Since p53 is often absent or mutated in OS cells, 1,25(OH)2D3 may be used as an exogenous promoter of p21 activity and cellular differentiation, preventing tumorigenesis and promoting apoptosis [338].
Parathyroid hormone (PTH) and parathyroid hormone-related peptide (PTHrP) have demonstrated the ability to promote differentiation in MG63 OS cells [334]. After forming heterodimers, these molecules bind to a G-protein trans-membrane receptor. Acting through the MAPK/PKA/PKC pathway, these ligands have been shown to elevate expression of markers of osteogenic differentiation including ALP and type I collagen [334,341]. Furthermore, transfection of MG63 OS cells with an inhibitor of the PTH/PTHrP pathway resulted in decreased levels of ALP and type I collagen, indicating incomplete differentiation of the OS cells [334]. PTH also appears to regulate c-fos, an oncoprotein whose overexpression can lead to poor differentiation and aggressive malignancy, further demonstrating the therapeutic potential of this hormone [342-344].
Finally, bone morphogenetic proteins (BMPs) appear to be involved in osteogenic differentiation, and may prove to be valuable therapeutic agents. The highly osteogenic BMPs, BMP2, 4, 6 and 9 induce the expression of ALP, OCN and OPN differentiation markers in MSCs [294,311,313,323, 345-348]. However, in OS cells, these osteogenic BMPs alone are unable to promote terminal differentiation with no increase on ALP, OCN or OPN levels and conversely may promote tumor growth [317]. Since OS cells likely contain differentiation defects regulated by upstream BMPs, exogenous administration of these proteins may promote cell proliferation rather than overcoming the underlying aberrations, facilitating tumor growth [270]. However, when combined with Runx2 overexpression, BMPs appear to induce osteogenic differentiation and inhibit tumor growth, bypassing downstream defects [270,317].
Potential Therapeutic Agents for OS Signaling Pathway Defects
Because of the cytotoxicity and serious adverse effects associated with current standard-of-care chemotherapy regimens, there is a critical need to develop novel OS therapies that specifically target tumor cells without harming normal, healthy tissue. Several recent studies have sought to identify molecular targets in OS cells (Table 2), which hopefully will lead to the development of new treatments leading to improved survival and quality of life in OS patients.
Table 2.
Molecular Targets of OS Therapies Under Investigation
| Target | Therapy | Outcome | Mechanism | Ref. |
|---|---|---|---|---|
| IGF-1R | Ganitumab (mono- clonal antibody in- hibitor of IGF-1R) |
Decreased OS cell proliferation in vitro |
Prevents binding of IGF-1 and IGF-2 ligands to IGF-1R |
Beltran et al. 2009 |
| Decreased OS tumor growth in vivo | Beltran et al. 2011 |
|||
| IGF-1 (and IGF-2) | Overexpression of IGFBP5 |
Decreased OS cell proliferation, migration and invasion in vitro |
(i) N-terminal IGFBP5 binds to IGF mole- cules and prevents interaction with IGF-1R |
Su et al. 2011 |
| Decreased tumor growth and pulmo- nary metastases in vivo |
(ii) C-terminal IGFBP5 binds to ECM and decreases metastatic potential |
Luther et al. 2012 |
||
| mTOR (in AKT signaling pathway) |
Rapamycin | Decreased OS tumor growth in vivo | (i) Increases length of G1-phase and de- creases S-phase |
Gazitt et al. 2009 |
| (ii) Leads to decreased expression of Cy- clin D1 and mTOR | ||||
| VEGF | AZD2171 (VEGF inhibitor) |
Decreased tumor growth in vivo | Inhibits angiogenesis | Maris et al. 2008 |
| PEDF | Decreased OS differentiation, prolif- eration and metastasis in vitro |
(i) Inhibits angiogenesis by suppressing VEGF |
Takenaka et al. 2005 |
|
| Decreased OS tumor growth and pulmonary metastases in vivo |
(ii) Promotes Fas apoptotic cascade | Ek et al. 2007 |
As mentioned earlier, defects within the insulin-like growth factor (IGF) signaling axis have been observed in OS cells [299]. Insulin-like growth factor binding protein 5 (IGFBP5), which is significantly underexpressed in OS cells, is also being investigated as a potential therapy [299]. Adenoviral-mediated overexpression of IGFBP5 leads to decreased cell proliferation, migration and invasion while promoting apoptosis in vitro, and furthermore inhibits tumor growth and pulmonary metastases in vivo in mice. Moreover, our lab has recently demonstrated that the N-terminal domain of IGFBP5 has anti-proliferative and pro-apoptotic effects, whereas the C-terminal domain inhibits cell migration and invasion. Similar results were demonstrated in an orthotopic xenograft model. The effects of the N-terminal domain may be explained by a hydrophobic stretch of amino acids that binds directly to IGF molecules and prevents ligand interaction with IGF-1R, thereby preventing proliferation and promoting apoptosis [300]. The C-terminal domain, on the other hand, appears to bind directly to the extracellular matrix (ECM), modulating cell-to-cell and cell-to-ECM interactions as a result. Overall, there appears to be great promise in identifying molecular targets within the IGF signaling axis in the development of more effective and less toxic OS therapies.
Various studies have investigated the binding of various growth factors. Downstream of receptor activation, the AKT signaling pathway is thought to induce cell proliferation and possibly tumor growth in osteosarcoma [349,350]. mTOR is a critical element of this signaling cascade, and rapamycin inhibitors of this protein have shown promising results [302]. By increasing the length of the G1-phase and decreasing the S-phase, rapamycin leads to lower expression levels of cyclin D1 and mTOR [351]. These effects have demonstrated diminished OS tumor growth in a mouse model as well as in pediatric clinical trials [258].
There is strong evidence supporting the significant role of angiogenesis in supporting tumor growth, and this has led researchers to investigate the notion that inhibition of vascular growth factors may be an effective therapeutic approach for OS. Vascular endothelial growth factor (VEGF) inhibits apoptosis and promotes angiogenesis [352]. An inhibitor of the VEGF receptor, AZD2171, has shown promising results by inhibiting OS tumor growth in an in vivo model [352]. In contrast to VEGF, pigment epithelium-derived growth factor (PEDF) inhibits angiogenesis and is expressed at much lower levels in many tumors [353]. By suppressing the activity of VEGF and promoting the Fas apoptotic cascade, recombinant PEDF overexpression leads to OS antitumor effects through decreased differentiation, proliferation and metastases [354].
Osteoclast activity has also been demonstrated as an important component of OS tumorigenesis. Receptor activator of the nuclear factor kappa-B ligand (RANKL) is a key promoter of osteoclast development and activity [355]. Inhibitors of RANKL may serve as potential therapeutic agents with antitumor properties. Denosumab is a monoclonal antibody to RANKL has been studied in breast cancer patients to prevent bone metastases [356]. Future studies using Denosumab or similar RANK pathway inhibitors may reveal potential applications in OS.
Augmenting Chemotherapy and Improving Drug Delivery
There are several molecules under investigation that may improve OS patient outcomes when administered in conjunction with chemotherapy. One such agent is interferon-υ, which has been demonstrated to augment the effect of chemotherapy on OS tumors [357]. Interferon- υ can upregulate Fas receptors and caspase 8, both of which are involved in the extrinsic apoptotic cascade. The sensitization induced by interferon-υ may therefore be advantageous when administering apoptosis-inducing drugs such as doxorubicin or cisplatin to OS cells with an intact p53 pathway.
Interleukins (ILs) have also been studied as potential agents to augment the immune response during highly toxic chemotherapy regimens [118]. Specifically, IL-2 has been shown to induce differentiation of natural killer (NK) cells, with elevated numbers of NK cells strongly correlated with improved patient outcomes [118,358]. Following surgical resection of the primary tumor and also during administration of aggressive chemotherapy, administration of IL-2 has demonstrated the ability to maintain sufficiently high levels of NK cells, potentially leading to improved patient prognoses [358].
New drug delivery techniques are currently being investigated to improve the efficacy of standard-of-care therapeutic agents. For patients with pulmonary metastases, researchers have experimented with aerosolized forms of chemotherapy agents, such as cisplatin. This route of delivery has great promise for delivering maximum local concentrations of drug while limiting systemic side effects. Aerosolized cisplatin is currently in early clinical trials [359]. In order to control and optimize timing of drug release, achieve maximum potency and minimize harm to surrounding tissue, substances such as liposomes, lipoproteins, microcapsules and other synthetic polymers are being studied. Currently undergoing clinical testing, these agents may likely prove to be effective in treating drug-resistant or inaccessible tumors that normally require high doses of toxic chemotherapy with devastating effects on healthy tissue and quality of life [359]. Finally there have been limited studies investigating combination stem cell therapy and chemotherapy [360,361].
CONCLUDING REMARKS AND FUTURE DIRECTIONS
While tremendous progress has been made in the treatment of osteosarcoma over the past half century, there is a critical need for the development of novel therapies to improve patient survival. Patients with localized osteosarcoma have benefitted greatly from improvements in medical treatments as well as surgical and imaging techniques, but therapies for metastatic OS have not improved patient outcomes significantly over the past few decades. Limb-salvage surgery is now performed in the vast majority of OS patients, improving patient satisfaction from a functional, psychological and cosmetic standpoint. Nonetheless, pediatric patients often undergo multiple revisions throughout their lifetimes, and improvements in prosthetic construction and biologically fixed prostheses as well as the use of bisphosphonates may improve endoprosthetic fixation and ultimately patient satisfaction. The various clinical trials described hold great promise in improving patient survival and limiting the toxicity of standard-of-care cytotoxic chemotherapy. Cisplatin-free regimens containing carboplatin and methotrexate-free regimens may soon become the standard of care based upon the results of recently completed clinical trials. L-MTP-PE, now approved for use in Europe for non-metastatic high-grade OS, may soon be incorporated into regimens in the US. Other agents, including inhaled GM-CSF, HER-2 inhibitors, IGF pathway inhibitors, VEGF-inhibitors and mTOR inhibitors are currently under investigation in clinical trials and warrant further investigation before their incorporation into OS regimens. Finally, promising preclincial studies involving the IGF pathway, the mTOR/AKT signaling pathway and targets of MSC differentiation such as PPARυ, retinoids, 1,25(OH)2D3 PTH/PTHrP and the BMPs will be the basis on which future OS clinical trials are based.
ACKNOWLEDGEMENTS
The reported work was supported in part by research grants from the OREF and the National Institutes of Health (RCH, TCH and HHL) and the 973 Program of the Ministry of Science and Technology of China (#2011CB707906 to JL and TCH).
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
REFERENCES
- [1].Longhi A, Errani C, De Paolis M, Mercuri M, Bacci G. Primary bone osteosarcoma in the pediatric age: state of the art. Cancer Treat Rev. 2006;32(6):423–36. doi: 10.1016/j.ctrv.2006.05.005. [DOI] [PubMed] [Google Scholar]
- [2].Cotterill SJ, Wright CM, Pearce MS, Craft AW. Stature of young people with malignant bone tumors. Pediatr Blood Cancer. 2004;42(1):59–63. doi: 10.1002/pbc.10437. [DOI] [PubMed] [Google Scholar]
- [3].Huvos A. Diagnosis, Treatment and Prognosis. 2nd WB Saunders; Philadelphia, PA: 1991. Osteogenic Sarcoma. Bone Tumors. [Google Scholar]
- [4].Rytting M, Pearson P, Raymond AK, et al. Osteosarcoma in preadolescent patients. Clin Orthop Relat Res. 2000;(373):39–50. doi: 10.1097/00003086-200004000-00007. [DOI] [PubMed] [Google Scholar]
- [5].Wittig JC, Bickels J, Priebat D, et al. Osteosarcoma: a multidisciplinary approach to diagnosis and treatment. Am Fam Physician. 2002;65(6):1123–32. [PubMed] [Google Scholar]
- [6].Link MPGM, Meyers PA. Osteosarcoma. In: Pizzo PAPD, editor. Principles and Practice of Pediatric Oncology. Lippincott Williams and Wilkins; Philadelphia: 2002. pp. 1051–80. [Google Scholar]
- [7].Wesolowski R, Budd GT. Use of chemotherapy for patients with bone and soft-tissue sarcomas. Cleve Clin J Med. 77(Suppl 1):S23–6. doi: 10.3949/ccjm.77.s1.05. [DOI] [PubMed] [Google Scholar]
- [8].Kager L, Zoubek A, Potschger U, et al. Primary metastatic osteosarcoma: presentation and outcome of patients treated on neoadjuvant Cooperative Osteosarcoma Study Group protocols. J Clin Oncol. 2003;21(10):2011–8. doi: 10.1200/JCO.2003.08.132. [DOI] [PubMed] [Google Scholar]
- [9].Daw NC, Billups CA, Rodriguez-Galindo C, et al. Metastatic osteosarcoma. Cancer. 2006;106(2):403–12. doi: 10.1002/cncr.21626. [DOI] [PubMed] [Google Scholar]
- [10].Mialou V, Philip T, Kalifa C, et al. Metastatic osteosarcoma at diagnosis: prognostic factors and long-term outcome--the French pediatric experience. Cancer. 2005;104(5):1100–9. doi: 10.1002/cncr.21263. [DOI] [PubMed] [Google Scholar]
- [11].Meyers PA, Heller G, Healey JH, et al. Osteogenic sarcoma with clinically detectable metastasis at initial presentation. J Clin Oncol. 1993;11(3):449–53. doi: 10.1200/JCO.1993.11.3.449. [DOI] [PubMed] [Google Scholar]
- [12].Bielack SS, Kempf-Bielack B, Delling G, et al. Prognostic factors in high-grade osteosarcoma of the extremities or trunk: an analysis of 1,702 patients treated on neoadjuvant cooperative osteosarcoma study group protocols. J Clin Oncol. 2002;20(3):776–90. doi: 10.1200/JCO.2002.20.3.776. [DOI] [PubMed] [Google Scholar]
- [13].Arndt CA, Rose PS, Folpe AL, Laack NN. Common musculoskeletal tumors of childhood and adolescence. Mayo Clin Proc. 87(5):475–87. doi: 10.1016/j.mayocp.2012.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Folpe A, Inwards CY. Bone and Soft Tissue Pathology. Saunders/Elsevier; Philadelphia, PA: 2010. [Google Scholar]
- [15].Cesari M, Alberghini M, Vanel D, et al. Periosteal osteosarcoma: a single-institution experience. Cancer. 117(8):1731–5. doi: 10.1002/cncr.25718. [DOI] [PubMed] [Google Scholar]
- [16].Han I, Oh JH, Na YG, Moon KC, Kim HS. Clinical outcome of parosteal osteosarcoma. J Surg Oncol. 2008;97(2):146–9. doi: 10.1002/jso.20902. [DOI] [PubMed] [Google Scholar]
- [17].Kurt AM, Unni KK, McLeod RA, Pritchard DJ. Low-grade intraosseous osteosarcoma. Cancer. 1990;65(6):1418–28. doi: 10.1002/1097-0142(19900315)65:6<1418::aid-cncr2820650629>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- [18].Okada K, Frassica FJ, Sim FH, Beabout JW, Bond JR, Unni KK. Parosteal osteosarcoma. A clinicopathological study. J Bone Joint Surg Am. 1994;76(3):366–78. doi: 10.2106/00004623-199403000-00007. [DOI] [PubMed] [Google Scholar]
- [19].Raymond AK. Surface osteosarcoma. Clinical orthopaedics and related research. 1991(270):140–8. [PubMed] [Google Scholar]
- [20].Ferguson WS, Goorin AM. Current treatment of osteosarcoma. Cancer investigation. 2001;19(3):292–315. doi: 10.1081/cnv-100102557. [DOI] [PubMed] [Google Scholar]
- [21].Ayerza MA, Farfalli GL, Aponte-Tinao L, Muscolo DL. Does increased rate of limb-sparing surgery affect survival in osteosarcoma? Clin Orthop Relat Res. 468(11):2854–9. doi: 10.1007/s11999-010-1423-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Eilber F, Giuliano A, Eckardt J, Patterson K, Moseley S, Goodnight J. Adjuvant chemotherapy for osteosarcoma: a randomized prospective trial. J Clin Oncol. 1987;5(1):21–6. doi: 10.1200/JCO.1987.5.1.21. [DOI] [PubMed] [Google Scholar]
- [23].Link MP, Goorin AM, Horowitz M, et al. Adjuvant chemotherapy of high-grade osteosarcoma of the extremity. Updated results of the Multi-Institutional Osteosarcoma Study. Clin Orthop Relat Res. 1991(270):8–14. [PubMed] [Google Scholar]
- [24].Bruland OS, Hoifodt H, Saeter G, Smeland S, Fodstad O. Hematogenous micrometastases in osteosarcoma patients. Clin Cancer Res. 2005;11(13):4666–73. doi: 10.1158/1078-0432.CCR-05-0165. [DOI] [PubMed] [Google Scholar]
- [25].Tan PX, Yong BC, Wang J, et al. Analysis of the efficacy and prognosis of limb-salvage surgery for osteosarcoma around the knee. Eur J Surg Oncol. doi: 10.1016/j.ejso.2012.07.003. [DOI] [PubMed] [Google Scholar]
- [26].Ferrari S, Palmerini E, Staals EL, et al. The treatment of nonmetastatic high grade osteosarcoma of the extremity: review of the Italian Rizzoli experience. Impact on the future. Cancer Treat Res. 2009;152:275–87. doi: 10.1007/978-1-4419-0284-9_14. [DOI] [PubMed] [Google Scholar]
- [27].Jeon DG, Song WS. How can survival be improved in localized osteosarcoma? Expert Rev Anticancer Ther. 10(8):1313–25. doi: 10.1586/era.10.79. [DOI] [PubMed] [Google Scholar]
- [28].Jaffe N. Osteosarcoma: review of the past, impact on the future. The American experience. Cancer Treat Res. 2009;152:239–62. doi: 10.1007/978-1-4419-0284-9_12. [DOI] [PubMed] [Google Scholar]
- [29].Bacci G, Ferrari S, Lari S, et al. Osteosarcoma of the limb. Amputation or limb salvage in patients treated by neoadjuvant chemotherapy. J Bone Joint Surg Br. 2002;84(1):88–92. doi: 10.1302/0301-620x.84b1.12211. [DOI] [PubMed] [Google Scholar]
- [30].Bacci G, Ferrari S, Longhi A, et al. Neoadjuvant chemotherapy for high grade osteosarcoma of the extremities: long-term results for patients treated according to the Rizzoli IOR/OS-3b protocol. J Chemother (Florence, Italy) 2001;13(1):93–9. doi: 10.1179/joc.2001.13.1.93. [DOI] [PubMed] [Google Scholar]
- [31].Bacci G, Forni C, Longhi A, et al. Local recurrence and local control of non-metastatic osteosarcoma of the extremities: a 27-year experience in a single institution. J Surg Oncol. 2007;96(2):118–23. doi: 10.1002/jso.20628. [DOI] [PubMed] [Google Scholar]
- [32].Bacci G, Longhi A, Versari M, Mercuri M, Briccoli A, Picci P. Prognostic factors for osteosarcoma of the extremity treated with neoadjuvant chemotherapy: 15-year experience in 789 patients treated at a single institution. Cancer. 2006;106(5):1154–61. doi: 10.1002/cncr.21724. [DOI] [PubMed] [Google Scholar]
- [33].Dahlin DC, Coventry MB. Osteogenic sarcoma. A study of six hundred cases. J Bone Joint Surg Am. 1967;49(1):101–10. [PubMed] [Google Scholar]
- [34].Enneking WF. A system of staging musculoskeletal neoplasms. Clin Orthop Relat Res. 1986(204):9–24. [PubMed] [Google Scholar]
- [35].Ferrari S, Briccoli A, Mercuri M, et al. Postrelapse survival in osteosarcoma of the extremities: prognostic factors for long-term survival. J Clin Oncol. 2003;21(4):710–5. doi: 10.1200/JCO.2003.03.141. [DOI] [PubMed] [Google Scholar]
- [36].Ferrari S, Smeland S, Mercuri M, et al. Neoadjuvant chemotherapy with high-dose Ifosfamide, high-dose methotrexate, cisplatin, and doxorubicin for patients with localized osteosarcoma of the extremity: a joint study by the Italian and Scandinavian Sarcoma Groups. J Clin Oncol. 2005;23(34):8845–52. doi: 10.1200/JCO.2004.00.5785. [DOI] [PubMed] [Google Scholar]
- [37].Goorin AM, Schwartzentruber DJ, Devidas M, et al. Presurgical chemotherapy compared with immediate surgery and adjuvant chemotherapy for nonmetastatic osteosarcoma: Pediatric Oncology Group Study POG-8651. J Clin Oncol. 2003;21(8):1574–80. doi: 10.1200/JCO.2003.08.165. [DOI] [PubMed] [Google Scholar]
- [38].Hawkins DS, Arndt CA. Pattern of disease recurrence and prognostic factors in patients with osteosarcoma treated with contemporary chemotherapy. Cancer. 2003;98(11):2447–56. doi: 10.1002/cncr.11799. [DOI] [PubMed] [Google Scholar]
- [39].Wallace RD, Davoudi MM, Neel MD, Lachica RD. The role of the pediatric plastic surgeon in limb salvage surgery for osteosarcoma of the lower extremity. J Craniofac Surg. 2003;14(5):680–6. doi: 10.1097/00001665-200309000-00014. [DOI] [PubMed] [Google Scholar]
- [40].Aksnes LH, Bauer HC, Jebsen NL, et al. Limb-sparing surgery preserves more function than amputation: a Scandinavian sarcoma group study of 118 patients. J Bone Joint Surg Br. 2008;90(6):786–94. doi: 10.1302/0301-620X.90B6.19805. [DOI] [PubMed] [Google Scholar]
- [41].Bacci G, Balladelli A, Palmerini E, et al. Neoadjuvant chemotherapy for osteosarcoma of the extremities in preadolescent patients: the Rizzoli Institute experience. J Pediatr Hematol Oncol. 2008;30(12):908–12. doi: 10.1097/MPH.0b013e31817e4aee. [DOI] [PubMed] [Google Scholar]
- [42].Federman N, Bernthal N, Eilber FC, Tap WD. The multidisciplinary management of osteosarcoma. Curr Treat Options Oncol. 2009;10(1-2):82–93. doi: 10.1007/s11864-009-0087-3. [DOI] [PubMed] [Google Scholar]
- [43].Sakamoto A, Iwamoto Y. Current status and perspectives regarding the treatment of osteo-sarcoma: chemotherapy. Rev Recent Clin Trials. 2008;3(3):228–31. doi: 10.2174/157488708785700267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].DiCaprio MR, Friedlaender GE. Malignant bone tumors: limb sparing versus amputation. J Am Acad Orthop Surg. 2003;11(1):25–37. [PubMed] [Google Scholar]
- [45].Malawer MM, Chou LB. Prosthetic survival and clinical results with use of large-segment replacements in the treatment of high-grade bone sarcomas. J Bone Joint Surg Am. 1995;77(8):1154–65. doi: 10.2106/00004623-199508000-00003. [DOI] [PubMed] [Google Scholar]
- [46].Enneking WF, Spanier SS, Goodman MA. A system for the surgical staging of musculoskeletal sarcoma. Clin Orthop Relat Res. 1980(153):106–20. [PubMed] [Google Scholar]
- [47].Kaste SC, Pratt CB, Cain AM, Jones-Wallace DJ, Rao BN. Metastases detected at the time of diagnosis of primary pediatric extremity osteosarcoma at diagnosis: imaging features. Cancer. 1999;86(8):1602–8. doi: 10.1002/(sici)1097-0142(19991015)86:8<1602::aid-cncr31>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- [48].Su WT, Chewning J, Abramson S, et al. Surgical management and outcome of osteosarcoma patients with unilateral pulmonary metastases. J Pediatr Surg. 2004;39(3):418–23. doi: 10.1016/j.jpedsurg.2003.11.030. discussion -23. [DOI] [PubMed] [Google Scholar]
- [49].Meyer WH, Schell MJ, Kumar AP, et al. Thoracotomy for pulmonary metastatic osteosarcoma. An analysis of prognostic indicators of survival. Cancer. 1987;59(2):374–9. doi: 10.1002/1097-0142(19870115)59:2<374::aid-cncr2820590235>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- [50].Skinner KA, Eilber FR, Holmes EC, Eckardt J, Rosen G. Surgical treatment and chemotherapy for pulmonary metastases from osteosarcoma. Arch Surg. 1992;127(9):1065–70. doi: 10.1001/archsurg.1992.01420090073010. discussion 70-1. [DOI] [PubMed] [Google Scholar]
- [51].Chou AJ, Merola PR, Wexler LH, et al. Treatment of osteosarcoma at first recurrence after contemporary therapy: the Memorial Sloan-Kettering Cancer Center experience. Cancer. 2005;104(10):2214–21. doi: 10.1002/cncr.21417. [DOI] [PubMed] [Google Scholar]
- [52].Harting MT, Blakely ML, Jaffe N, et al. Long-term survival after aggressive resection of pulmonary metastases among children and adolescents with osteosarcoma. J Pediatr Surg. 2006;41(1):194–9. doi: 10.1016/j.jpedsurg.2005.10.089. [DOI] [PubMed] [Google Scholar]
- [53].Ward WG, Mikaelian K, Dorey F, et al. Pulmonary metastases of stage IIB extremity osteosarcoma and subsequent pulmonary metastases. J Clin Oncol. 1994;12(9):1849–58. doi: 10.1200/JCO.1994.12.9.1849. [DOI] [PubMed] [Google Scholar]
- [54].Bacci G, Ferrari S, Bertoni F, et al. Long-term outcome for patients with nonmetastatic osteosarcoma of the extremity treated at the istituto ortopedico rizzoli according to the istituto ortopedico rizzoli/osteosarcoma-2 protocol: an updated report. J Clin Oncol. 2000;18(24):4016–27. doi: 10.1200/JCO.2000.18.24.4016. [DOI] [PubMed] [Google Scholar]
- [55].Nathan SS, Gorlick R, Bukata S, et al. Treatment algorithm for locally recurrent osteosarcoma based on local disease-free interval and the presence of lung metastasis. Cancer. 2006;107(7):1607–16. doi: 10.1002/cncr.22197. [DOI] [PubMed] [Google Scholar]
- [56].Baram TZ, van Tassel P, Jaffe NA. Brain metastases in osteosarcoma: incidence, clinical and neuroradiological findings and management options. J Neurooncol. 1988;6(1):47–52. doi: 10.1007/BF00163540. [DOI] [PubMed] [Google Scholar]
- [57].Sutow WW, Sullivan MP, Fernbach DJ, Cangir A, George SL. Adjuvant chemotherapy in primary treatment of osteogenic sarcoma. A Southwest Oncology Group study. Cancer. 1975;36(5):1598–602. doi: 10.1002/1097-0142(197511)36:5<1598::aid-cncr2820360511>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- [58].Finklestein JZ, Hittle RE, Hammond GD. Evaluation of a high dose cyclophosphamide regimen in childhood tumors. Cancer. 1969;23(5):1239–42. doi: 10.1002/1097-0142(196905)23:5<1239::aid-cncr2820230535>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- [59].Sutow WW. Chemotherapeutic management of childhood rhabdomyosarcoma. In: Taylor GSW, editor. Neoplasia in Childhood. Year Book Medical Publishers; Chicago: 1969. pp. 201–8. [Google Scholar]
- [60].Cortes EP, Holland JF, Wang JJ, Sinks LF. Doxorubicin in disseminated osteosarcoma. Jama. 1972;221(10):1132–8. doi: 10.1001/jama.221.10.1132. [DOI] [PubMed] [Google Scholar]
- [61].Sutow WW, Gehan EA, Vietti TJ, Frias AE, Dyment PG. Multidrug chemotherapy in pulmonary treatment of osteosarcoma. J Bone Joint Surg Am. 1976;58(5):629–33. [PubMed] [Google Scholar]
- [62].Sutow WWGE, Dyment PC, et al. Multi-drug adjuvant chemotherapy in osteosarcoma: Interim report of the Southwest Oncology Group studies. Cancer Treat Rep. 1978(62):265–9. [PubMed] [Google Scholar]
- [63].Sutow WW, Herson J, Perez C. Survival after metastasis in osteosarcoma. Natl Cancer Inst Monogr. 1981(56):227–31. [PubMed] [Google Scholar]
- [64].Rosen G, Murphy ML, Huvos AG, Gutierrez M, Marcove RC. Chemotherapy, en bloc resection, and prosthetic bone replacement in the treatment of osteogenic sarcoma. Cancer. 1976;37(1):1–11. doi: 10.1002/1097-0142(197601)37:1<1::aid-cncr2820370102>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- [65].Jaffe N, Link MP, Cohen D, et al. High-dose methotrexate in osteogenic sarcoma. Natl Cancer Inst Monogr. 1981(56):201–6. [PubMed] [Google Scholar]
- [66].Goorin AMDM, Gelber RD, et al. The Dana-Farber Cancer Institute/The Children’s Hospital adjuvant chemotherapy trials for osteosarcoma: Three sequential studies. Cancer Treat Symp. 1986;3:155–9. [Google Scholar]
- [67].Rosen G. Role of chemotherapy in the treatment of primary osteogenic sarcoma. A five-year follow-up of T-10 neoadjuvant chemotherapy. In: Kimura KYY-M, editor. Methotrexate in Cancer Therapy. Raven Press; New York: 1986. pp. 227–38. [Google Scholar]
- [68].Delepine N, Delepine G, Jasmin C, Desbois JC, Cornille H, Mathe G. Importance of age and methotrexate dosage: prognosis in children and young adults with high-grade osteosarcomas. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie. 1988;42(4):257–62. [PubMed] [Google Scholar]
- [69].Winkler K, Beron G, Delling G, et al. Neoadjuvant chemotherapy of osteosarcoma: results of a randomized cooperative trial (COSS-82) with salvage chemotherapy based on histological tumor response. J Clin Oncol. 1988;6(2):329–37. doi: 10.1200/JCO.1988.6.2.329. [DOI] [PubMed] [Google Scholar]
- [70].Bacci G, Picci P, Avella M, et al. The importance of dose-intensity in neoadjuvant chemotherapy of osteosarcoma: a retrospective analysis of high-dose methotrexate, cisplatinum and adriamycin used preoperatively. J Chemother (Florence, Italy) 1990;2(2):127–35. doi: 10.1080/1120009x.1990.11738996. [DOI] [PubMed] [Google Scholar]
- [71].Provisor AJ, Ettinger LJ, Nachman JB, et al. Treatment of nonmetastatic osteosarcoma of the extremity with preoperative and post-operative chemotherapy: a report from the Children's Cancer Group. J Clin Oncol. 1997;15(1):76–84. doi: 10.1200/JCO.1997.15.1.76. [DOI] [PubMed] [Google Scholar]
- [72].Meyers PA, Gorlick R, Heller G, et al. Intensification of preoperative chemotherapy for osteogenic sarcoma: results of the Memorial Sloan-Kettering (T12) protocol. J Clin Oncol. 1998;16(7):2452–8. doi: 10.1200/JCO.1998.16.7.2452. [DOI] [PubMed] [Google Scholar]
- [73].Cohen HJ, Jaffe N. Pharmacokinetic and clinical studies of 24-h infusions of high-dose methotrexate. Cancer Chemother Pharmacol. 1978;1(1):61–4. doi: 10.1007/BF00253148. [DOI] [PubMed] [Google Scholar]
- [74].Wang YM, Kim PY, Lantin E, van Eys DC, Romsdahl MM, Sutow WW. Degradation and clearance of methotrexate in children with osteosarcoma receiving high-dose infusion. Med Pediatr Oncol. 1978;4(3):221–9. doi: 10.1002/mpo.2950040305. [DOI] [PubMed] [Google Scholar]
- [75].Perez C, Wang YM, Sutow WW, Herson J. Significance of the 48-hour plasma level in high-dose methotrexate regimens. Cancer clinical trials. 1978;1(2):107–11. [PubMed] [Google Scholar]
- [76].Daw NC, Neel MD, Rao BN, et al. Frontline treatment of localized osteosarcoma without methotrexate: results of the St. Jude Children's Research Hospital OS99 trial. Cancer. 117(12):2770–8. doi: 10.1002/cncr.25715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Crews KR, Liu T, Rodriguez-Galindo C, et al. High-dose methotrexate pharmacokinetics and outcome of children and young adults with osteosarcoma. Cancer. 2004;100(8):1724–33. doi: 10.1002/cncr.20152. [DOI] [PubMed] [Google Scholar]
- [78].Smith MAUR, Horowitz ME, et al. Influence of doxorubicin dose intensity on response and outcome for patients with osteogenic sarcoma and Ewing’s sarcoma. J Natl Cancer Inst. 1991;83:1460–70. doi: 10.1093/jnci/83.20.1460. [DOI] [PubMed] [Google Scholar]
- [79].Bonnadonna GMS, De Lena M, et al. Phase I and preliminary phase II evaluation of Adriamycin (NSC/123127) Cancer Res. 1970;30:2527–82. [PubMed] [Google Scholar]
- [80].Middleman E, Luce J, Frei E., 3rd Clinical trials with adriamycin. Cancer. 1971;28(4):844–50. doi: 10.1002/1097-0142(1971)28:4<844::aid-cncr2820280407>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- [81].O'Bryan RM, Luce JK, Talley RW, Gottlieb JA, Baker LH, Bonadonna G. Phase II evaluation of adriamycin in human neoplasia. Cancer. 1973;32(1):1–8. doi: 10.1002/1097-0142(197307)32:1<1::aid-cncr2820320101>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- [82].Cassady JR, Richter MP, Piro AJ, Jaffe N. Radiation-adriamycin interactions: preliminary clinical observations. Cancer. 1975;36(3):946–9. doi: 10.1002/1097-0142(197509)36:3<946::aid-cncr2820360316>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- [83].Eilber FR, Grant T, Morton DL. Adjuvant therapy for osteosarcoma: preoperative and postoperative treatment. Cancer treatment reports. 1978;62(2):213–6. [PubMed] [Google Scholar]
- [84].Basser RL, Green MD. Strategies for prevention of anthracycline cardiotoxicity. Cancer treatment reviews. 1993;19(1):57–77. doi: 10.1016/0305-7372(93)90027-o. [DOI] [PubMed] [Google Scholar]
- [85].Seifert CF, Nesser ME, Thompson DF. Dexrazoxane in the prevention of doxorubicin-induced cardiotoxicity. Ann Pharmacother. 1994;28(9):1063–72. doi: 10.1177/106002809402800912. [DOI] [PubMed] [Google Scholar]
- [86].Jones RL. Utility of dexrazoxane for the reduction of anthracycline-induced cardiotoxicity. Expert Rev Cardiovasc Ther. 2008;6(10):1311–7. doi: 10.1586/14779072.6.10.1311. [DOI] [PubMed] [Google Scholar]
- [87].D'Adamo DR. Appraising the current role of chemotherapy for the treatment of sarcoma. Semin Oncol. 38(Suppl 3):S19–29. doi: 10.1053/j.seminoncol.2011.09.004. [DOI] [PubMed] [Google Scholar]
- [88].Northfelt DW, Martin FJ, Working P, et al. Doxorubicin encapsulated in liposomes containing surface-bound polyethylene glycol: pharmacokinetics, tumor localization, and safety in patients with AIDS-related Kaposi's sarcoma. J Clin Pharmacol. 1996;36(1):55–63. doi: 10.1002/j.1552-4604.1996.tb04152.x. [DOI] [PubMed] [Google Scholar]
- [89].Judson I, Radford JA, Harris M, et al. Randomised phase II trial of pegylated liposomal doxorubicin (DOXIL/CAELYX) versus doxorubicin in the treatment of advanced or metastatic soft tissue sarcoma: a study by the EORTC Soft Tissue and Bone Sarcoma Group. Eur J Cancer. 2001;37(7):870–7. doi: 10.1016/s0959-8049(01)00050-8. [DOI] [PubMed] [Google Scholar]
- [90].Toma STA, Villani G, Carteni G, Spadini N, Palumbo R. Liposomal doxorubicin (Caelyx) in advanced pretreated soft tissue sarcomas: a phase II study of the Italian Sarcoma Group (ISG) Anticancer Res. 2002;20:485–91. [PubMed] [Google Scholar]
- [91].Fuertes MA, Castilla J, Alonso C, Perez JM. Cisplatin biochemical mechanism of action: from cytotoxicity to induction of cell death through interconnections between apoptotic and necrotic pathways. Curr Med Chem. 2003;10(3):257–66. doi: 10.2174/0929867033368484. [DOI] [PubMed] [Google Scholar]
- [92].Baum EGL, Ganyon P, et al. Use of Cis-diamminedichloroplatinum-II (CPDD) in Osteogenic Sarcoma (OS) in Children (Abstract C-315) Proc AACR-ASCO. 1978;19:385. [Google Scholar]
- [93].Nitschke R, Starling KA, Vats T, Bryan H. Cis-diamminedichloroplatinum (NSC-119875) in childhood malignancies: a Southwest Oncology Group study. Med Pediatr Oncol. 1978;4(2):127–32. doi: 10.1002/mpo.2950040208. [DOI] [PubMed] [Google Scholar]
- [94].Ochs JJ, Freeman AI, Douglass HO, Jr., Higby DS, Mindell ER, Sinks LF. cis-Dichlorodiammineplatinum (II) in advanced osteogenic sarcoma. Cancer treatment reports. 1978;62(2):239–45. [PubMed] [Google Scholar]
- [95].Jaffe N, Knapp J, Chuang VP, et al. Osteosarcoma: intra-arterial treatment of the primary tumor with cis-diamminedichloroplatinum II (CDP). Angiographic, pathologic, and pharmacologic studies. Cancer. 1983;51(3):402–7. doi: 10.1002/1097-0142(19830201)51:3<402::aid-cncr2820510308>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- [96].Jaffe N, Raymond AK, Ayala A, et al. Effect of cumulative courses of intraarterial cis-diamminedichloroplatin-II on the primary tumor in osteosarcoma. Cancer. 1989;63(1):63–7. doi: 10.1002/1097-0142(19890101)63:1<63::aid-cncr2820630110>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- [97].Wright JD, Hagemann A, Rader JS, et al. Bevacizumab combination therapy in recurrent, platinum-refractory, epithelial ovarian carcinoma: A retrospective analysis. Cancer. 2006;107(1):83–9. doi: 10.1002/cncr.21969. [DOI] [PubMed] [Google Scholar]
- [98].Chuang VP, Benjamin R, Jaffe N, et al. Radiographic and angiographic changes in osteosarcoma after intraarterial chemotherapy. Ajr. 1982;139(6):1065–9. doi: 10.2214/ajr.139.6.1065. [DOI] [PubMed] [Google Scholar]
- [99].Shirkhoda A, Jaffe N, Wallace S, Ayala A, Lindell MM, Zornoza J. Computed tomography of osteosarcoma after intraarterial chemotherapy. Ajr. 1985;144(1):95–9. doi: 10.2214/ajr.144.1.95. [DOI] [PubMed] [Google Scholar]
- [100].Pan G, Raymond AK, Carrasco CH, et al. Osteosarcoma: MR imaging after preoperative chemotherapy. Radiology. 1990;174(2):517–26. doi: 10.1148/radiology.174.2.2296660. [DOI] [PubMed] [Google Scholar]
- [101].Ruiz L, Gilden J, Jaffe N, Robertson R, Wang YM. Auditory function in pediatric osteosarcoma patients treated with multiple doses of cis-diamminedichloroplatinum(II) Cancer research. 1989;49(3):742–4. [PubMed] [Google Scholar]
- [102].Jaffe N, Keifer R, 3rd, Robertson R, Cangir A, Wang A. Renal toxicity with cumulative doses of cis-diamminedichloroplatinum-II in pediatric patients with osteosarcoma. Effect on creatinine clearance and methotrexate excretion. Cancer. 1987;59(9):1577–81. doi: 10.1002/1097-0142(19870501)59:9<1577::aid-cncr2820590908>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- [103].Meyer WH, Pratt CB, Poquette CA, et al. Carboplatin/ifosfamide window therapy for osteosarcoma: results of the St Jude Children's Research Hospital OS-91 trial. J Clin Oncol. 2001;19(1):171–82. doi: 10.1200/JCO.2001.19.1.171. [DOI] [PubMed] [Google Scholar]
- [104].Petrilli AS, de Camargo B, Filho VO, et al. Results of the Brazilian Osteosarcoma Treatment Group Studies III and IV: prognostic factors and impact on survival. J Clin Oncol. 2006;24(7):1161–8. doi: 10.1200/JCO.2005.03.5352. [DOI] [PubMed] [Google Scholar]
- [105].Euramos I Trial. Accessed August 28, 2012, at http://www.ctu.mrc.ac.uk/euramos/euramos_i_trial.asp.
- [106].Grana NG-PJ, Cassano W, et al. Etoposide (VP-16) infusion plus Cyclophosphamide (CY) pulses: An effective combination for refractory cancer. Proc ASCO. 1989;8:300. [Google Scholar]
- [107].Saleh RA, Graham-Pole J, Cassano W, et al. Response of osteogenic sarcoma to the combination of etoposide and cyclophosphamide as neoadjuvant chemotherapy. Cancer. 1990;65(4):861–5. doi: 10.1002/1097-0142(19900215)65:4<861::aid-cncr2820650405>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- [108].Dai X, Ma W, He X, Jha RK. Review of therapeutic strategies for osteosarcoma, chondrosarcoma, and Ewing's sarcoma. Med Sci Monit. 17(8):RA177–90. doi: 10.12659/MSM.881893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Meyers PA, Healey JH, Chou AJ, et al. Addition of pamidronate to chemotherapy for the treatment of osteosarcoma. Cancer. 117(8):1736–44. doi: 10.1002/cncr.25744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Baum ES, Gaynon P, Greenberg L, Krivit W, Hammond D. Phase II study of cis-dichlorodiammineplatinum(II) in childhood osteosarcoma: Children's Cancer Study Group Report. Cancer treatment reports. 1979;63(9-10):1621–7. [PubMed] [Google Scholar]
- [111].Jaffe N, Paed D, Farber S, et al. Favorable response of metastatic osteogenic sarcoma to pulse high-dose methotrexate with citrovorum rescue and radiation therapy. Cancer. 1973;31(6):1367–73. doi: 10.1002/1097-0142(197306)31:6<1367::aid-cncr2820310611>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- [112].Marti C, Kroner T, Remagen W, Berchtold W, Cserhati M, Varini M. High-dose ifosfamide in advanced osteosarcoma. Cancer treatment reports. 1985;69(1):115–7. [PubMed] [Google Scholar]
- [113].Meyers PA, Schwartz CL, Krailo M, et al. Osteosarcoma: a randomized, prospective trial of the addition of ifosfamide and/or muramyl tripeptide to cisplatin, doxorubicin, and high-dose methotrexate. J Clin Oncol. 2005;23(9):2004–11. doi: 10.1200/JCO.2005.06.031. [DOI] [PubMed] [Google Scholar]
- [114].Bramwell VH, Steward WP, Nooij M, et al. Neoadjuvant chemotherapy with doxorubicin and cisplatin in malignant fibrous histiocytoma of bone: A European Osteosarcoma Intergroup study. J Clin Oncol. 1999;17(10):3260–9. doi: 10.1200/JCO.1999.17.10.3260. [DOI] [PubMed] [Google Scholar]
- [115].The NCCN Clinical Practice Guidences in Oncology (NCCN Guidelines): Bone Cancer. 2011 Version 2.2011. Accessed August 15, 2012, at NCCN.org.
- [116].Man TK, Chintagumpala M, Visvanathan J, et al. Expression profiles of osteosarcoma that can predict response to chemotherapy. Cancer research. 2005;65(18):8142–50. doi: 10.1158/0008-5472.CAN-05-0985. [DOI] [PubMed] [Google Scholar]
- [117].Ory B, Heymann MF, Kamijo A, Gouin F, Heymann D, Redini F. Zoledronic acid suppresses lung metastases and prolongs overall survival of osteosarcoma-bearing mice. Cancer. 2005;104(11):2522–9. doi: 10.1002/cncr.21530. [DOI] [PubMed] [Google Scholar]
- [118].Ta HT, Dass CR, Choong PF, Dunstan DE. Osteosarcoma treatment: state of the art. Cancer Metastasis Rev. 2009;28(1-2):247–63. doi: 10.1007/s10555-009-9186-7. [DOI] [PubMed] [Google Scholar]
- [119].Highsmith MJ, Kahle JT, Bongiorni DR, Sutton BS, Groer S, Kaufman KR. Safety, energy efficiency, and cost efficacy of the C-Leg for transfemoral amputees: A review of the literature. Prosthet Orthot Int. 34(4):362–77. doi: 10.3109/03093646.2010.520054. [DOI] [PubMed] [Google Scholar]
- [120].Fuchs B, Sim FH. Rotationplasty about the knee: surgical technique and anatomical considerations. Clinical anatomy (New York, NY. 2004;17(4):345–53. doi: 10.1002/ca.10211. [DOI] [PubMed] [Google Scholar]
- [121].Fuchs B, Kotajarvi BR, Kaufman KR, Sim FH. Functional outcome of patients with rotationplasty about the knee. Clin Orthop Relat Res. 2003(415):52–8. doi: 10.1097/01.blo.0000093896.12372.c1. [DOI] [PubMed] [Google Scholar]
- [122].Rodl RW, Pohlmann U, Gosheger G, Lindner NJ, Winkelmann W. Rotationplasty--quality of life after 10 years in 22 patients. Acta Orthop Scand. 2002;73(1):85–8. doi: 10.1080/000164702317281468. [DOI] [PubMed] [Google Scholar]
- [123].Mercuri M, Capanna R, Manfrini M, et al. The management of malignant bone tumors in children and adolescents. Clin Orthop Relat Res. 1991(264):156–68. [PubMed] [Google Scholar]
- [124].Uchida A, Myoui A, Araki N, Yoshikawa H, Shinto Y, Ueda T. Neoadjuvant chemotherapy for pediatric osteosarcoma patients. Cancer. 1997;79(2):411–5. [PubMed] [Google Scholar]
- [125].Stiller CA, Bielack SS, Jundt G, Steliarova-Foucher E. Bone tumours in European children and adolescents, 1978-1997. Report from the Automated Childhood Cancer Information System project. Eur J Cancer. 2006;42(13):2124–35. doi: 10.1016/j.ejca.2006.05.015. [DOI] [PubMed] [Google Scholar]
- [126].Simon MA, Aschliman MA, Thomas N, Mankin HJ. Limb-salvage treatment versus amputation for osteosarcoma of the distal end of the femur. J Bone Joint Surg Am. 1986;68(9):1331–7. [PubMed] [Google Scholar]
- [127].Sluga M, Windhager R, Lang S, Heinzl H, Bielack S, Kotz R. Local and systemic control after ablative and limb sparing surgery in patients with osteosarcoma. Clin Orthop Relat Res. 1999(358):120–7. [PubMed] [Google Scholar]
- [128].Akahane T, Shimizu T, Isobe K, Yoshimura Y, Fujioka F, Kato H. Evaluation of postoperative general quality of life for patients with osteosarcoma around the knee joint. J Pediatr Orthop. 2007;16(4):269–72. doi: 10.1097/BPB.0b013e3280925670. [DOI] [PubMed] [Google Scholar]
- [129].Clerici CA, Ferrari A, Luksch R, et al. Clinical experience with psychological aspects in pediatric patients amputated for malignancies. Tumori. 2004;90(4):399–404. doi: 10.1177/030089160409000407. [DOI] [PubMed] [Google Scholar]
- [130].Ottaviani G, Robert RS, Huh WW, Jaffe N. Functional, psychosocial and professional outcomes in long-term survivors of lower-extremity osteosarcomas: amputation versus limb salvage. Cancer Treat Res. 2009;152:421–36. doi: 10.1007/978-1-4419-0284-9_23. [DOI] [PubMed] [Google Scholar]
- [131].Davis AM, Devlin M, Griffin AM, Wunder JS, Bell RS. Functional outcome in amputation versus limb sparing of patients with lower extremity sarcoma: a matched case-control study. Arch Phys Med Rehabil. 1999;80(6):615–8. doi: 10.1016/s0003-9993(99)90161-2. [DOI] [PubMed] [Google Scholar]
- [132].Ferrari S, Bertoni F, Mercuri M, et al. Predictive factors of disease-free survival for non-metastatic osteosarcoma of the extremity: an analysis of 300 patients treated at the Rizzoli Institute. Ann Oncol. 2001;12(8):1145–50. doi: 10.1023/a:1011636912674. [DOI] [PubMed] [Google Scholar]
- [133].Honegger HP, Cserhati MD, Exner GU, von Hochstetter A, Groscurth P. Zurich experience with preoperative, high dose methotrexate-containing chemotherapy in patients with extremity osteosarcomas (OSA) Ann Oncol. 1991;2(7):489–94. doi: 10.1093/oxfordjournals.annonc.a057998. [DOI] [PubMed] [Google Scholar]
- [134].Kawai A, Lin PP, Boland PJ, Athanasian EA, Healey JH. Relationship between magnitude of resection, complication, and prosthetic survival after prosthetic knee reconstructions for distal femoral tumors. J Surg Oncol. 1999;70(2):109–15. doi: 10.1002/(sici)1096-9098(199902)70:2<109::aid-jso9>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- [135].Neel MD, Wilkins RM, Rao BN, Kelly CM. Early multicenter experience with a noninvasive expandable prosthesis. Clin Orthop Relat Res. 2003(415):72–81. doi: 10.1097/01.blo.0000093899.12372.25. [DOI] [PubMed] [Google Scholar]
- [136].Abed YY, Beltrami G, Campanacci DA, Innocenti M, Scoccianti G, Capanna R. Biological reconstruction after resection of bone tumours around the knee: long-term follow-up. J Bone Joint Surg Br. 2009;91(10):1366–72. doi: 10.1302/0301-620X.91B10.22212. [DOI] [PubMed] [Google Scholar]
- [137].Muscolo DL, Ayerza MA, Aponte-Tinao L, Farfalli G. Allograft reconstruction after sarcoma resection in children younger than 10 years old. Clin Orthop Relat Res. 2008;466(8):1856–62. doi: 10.1007/s11999-008-0303-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Farfalli GL, Boland PJ, Morris CD, Athanasian EA, Healey JH. Early equivalence of uncemented press-fit and Compress femoral fixation. Clin Orthop Relat Res. 2009;467(11):2792–9. doi: 10.1007/s11999-009-0912-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Teplyakov VSJ, Aliev M. Transosseous osteosynthesis by Ilizarov in the treatment of patients with primary malignant tumours of long bones; 17th Annual Meeting of the European Musculoskeletal Oncology Society; Oslo, Norway. Jun 9-11, 2004. 2004. 2004. [Google Scholar]
- [140].Canadell J, Forriol F, Cara JA. Removal of metaphyseal bone tumours with preservation of the epiphysis. Physeal distraction before excision. J Bone Joint Surg Br. 1994;76(1):127–32. [PubMed] [Google Scholar]
- [141].Rose PS, Dickey ID, Wenger DE, Unni KK, Sim FH. Periosteal osteosarcoma: long-term outcome and risk of late recurrence. Clin Orthop Relat Res. 2006;453:314–7. doi: 10.1097/01.blo.0000229341.18974.95. [DOI] [PubMed] [Google Scholar]
- [142].Sheth DS, Yasko AW, Raymond AK, et al. Conventional and dedifferentiated parosteal osteosarcoma. Diagnosis, treatment, and outcome. Cancer. 1996;78(10):2136–45. [PubMed] [Google Scholar]
- [143].Rosen G, Tefft M, Martinez A, Cham W, Murphy ML. Combination chemotherapy and radiation therapy in the treatment of metastatic osteogenic sarcoma. Cancer. 1975;35(3):622–30. doi: 10.1002/1097-0142(197503)35:3<622::aid-cncr2820350313>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- [144].Machak GN, Tkachev SI, Solovyev YN, et al. Neoadjuvant chemotherapy and local radiotherapy for high-grade osteosarcoma of the extremities. Mayo Clinic proceedings. 2003;78(2):147–55. doi: 10.4065/78.2.147. [DOI] [PubMed] [Google Scholar]
- [145].Ozaki T, Flege S, Kevric M, et al. Osteosarcoma of the pelvis: experience of the Cooperative Osteosarcoma Study Group. J Clin Oncol. 2003;21(2):334–41. doi: 10.1200/JCO.2003.01.142. [DOI] [PubMed] [Google Scholar]
- [146].Marina N, Gebhardt M, Teot L, Gorlick R. Biology and therapeutic advances for pediatric osteosarcoma. Oncologist. 2004;9(4):422–41. doi: 10.1634/theoncologist.9-4-422. [DOI] [PubMed] [Google Scholar]
- [147].Halperin E. Osteosarcoma. In: Halperin ECCL, Tarbell N, editors. Pediatric Radiation Oncology. Lippincott Williams & Wilkins; Philadelphia: 2005. pp. 291–318. [Google Scholar]
- [148].Ciernik IF, Niemierko A, Harmon DC, et al. Proton-based radiotherapy for unresectable or incompletely resected osteosarcoma. Cancer. 117(19):4522–30. doi: 10.1002/cncr.26037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Blattmann C, Oertel S, Schulz-Ertner D, et al. Non-randomized therapy trial to determine the safety and efficacy of heavy ion radiotherapy in patients with non-resectable osteosarcoma. BMC cancer. 10:96. doi: 10.1186/1471-2407-10-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Jingu K, Tsujii H, Mizoe JE, et al. Carbon ion radiation therapy improves the prognosis of unresectable adult bone and soft-tissue sarcoma of the head and neck. Int J Radiat Oncol Biol Phys. 82(5):2125–31. doi: 10.1016/j.ijrobp.2010.08.043. [DOI] [PubMed] [Google Scholar]
- [151].Hillegonds DJ, Franklin S, Shelton DK, Vijayakumar S, Vijayakumar V. The management of painful bone metastases with an emphasis on radionuclide therapy. J Natl Med Assoc. 2007;99(7):785–94. [PMC free article] [PubMed] [Google Scholar]
- [152].Schwarz R, Bruland O, Cassoni A, Schomberg P, Bielack S. The role of radiotherapy in oseosarcoma. Cancer Treat Res. 2009;152:147–64. doi: 10.1007/978-1-4419-0284-9_7. [DOI] [PubMed] [Google Scholar]
- [153].Anacak Y, Sabah D, Demirci S, Kamer S. Intraoperative extracorporeal irradiation and re-implantation of involved bone for the treatment of musculoskeletal tumors. J Exp Clin Cancer Res. 2007;26(4):571–4. [PubMed] [Google Scholar]
- [154].Arndt CA, Koshkina NV, Inwards CY, et al. Inhaled granulocyte-macrophage colony stimulating factor for first pulmonary recurrence of osteosarcoma: effects on disease-free survival and immunomodulation. a report from the Children's Oncology Group. Clin Cancer Res. 16(15):4024–30. doi: 10.1158/1078-0432.CCR-10-0662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Bramwell VH, Burgers M, Sneath R, et al. A comparison of two short intensive adjuvant chemotherapy regimens in operable osteosarcoma of limbs in children and young adults: the first study of the European Osteosarcoma Intergroup. J Clin Oncol. 1992;10(10):1579–91. doi: 10.1200/JCO.1992.10.10.1579. [DOI] [PubMed] [Google Scholar]
- [156].Souhami RL, Craft AW, Van der Eijken JW, et al. Randomised trial of two regimens of chemotherapy in operable osteosarcoma: a study of the European Osteosarcoma Intergroup. Lancet. 1997;350(9082):911–7. doi: 10.1016/S0140-6736(97)02307-6. [DOI] [PubMed] [Google Scholar]
- [157].Epelman SSN, Melaragno R, et al. Treatment of newly diagnosed high grade osteosarcoma (OS) with ifosfamide (IFOS), adriamycin (ADR) and cisplatin (CDP) without high dose methotrexate [abstract] Med Pediatr Oncol. 1996;27(226) [Google Scholar]
- [158].Picci P, Sangiorgi L, Rougraff BT, Neff JR, Casadei R, Campanacci M. Relationship of chemotherapy-induced necrosis and surgical margins to local recurrence in osteosarcoma. J Clin Oncol. 1994;12(12):2699–705. doi: 10.1200/JCO.1994.12.12.2699. [DOI] [PubMed] [Google Scholar]
- [159].Rodriguez-Galindo C, Shah N, McCarville MB, et al. Outcome after local recurrence of osteosarcoma: the St. Jude Children's Research Hospital experience (1970-2000) Cancer. 2004;100(9):1928–35. doi: 10.1002/cncr.20214. [DOI] [PubMed] [Google Scholar]
- [160].Kubista B, Trieb K, Sevelda F, et al. Anticancer effects of zoledronic acid against human osteosarcoma cells. J Orthop Res. 2006;24(6):1145–52. doi: 10.1002/jor.20129. [DOI] [PubMed] [Google Scholar]
- [161].Kubo T, Shimose S, Matsuo T, et al. Inhibitory effects of a new bisphosphonate, minodronate, on proliferation and invasion of a variety of malignant bone tumor cells. J Orthop Res. 2006;24(6):1138–44. doi: 10.1002/jor.20177. [DOI] [PubMed] [Google Scholar]
- [162].Murayama T, Kawasoe Y, Yamashita Y, et al. 2008;28(4B):2147–54. [PubMed] [Google Scholar]
- [163].Cheng YY, Huang L, Lee KM, Li K, Kumta SM. Alendronate regulates cell invasion and MMP-2 secretion in human osteosarcoma cell lines. Pediatr Blood Cancer. 2004;42(5):410–5. doi: 10.1002/pbc.20019. [DOI] [PubMed] [Google Scholar]
- [164].Senaratne SG, Colston KW. Direct effects of bisphosphonates on breast cancer cells. Breast Cancer Res. 2002;4(1):18–23. doi: 10.1186/bcr412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Shipman CM, Rogers MJ, Apperley JF, Russell RG, Croucher PI. Bisphosphonates induce apoptosis in human myeloma cell lines: a novel anti-tumour activity. Br J Haematol. 1997;98(3):665–72. doi: 10.1046/j.1365-2141.1997.2713086.x. [DOI] [PubMed] [Google Scholar]
- [166].Senaratne SG, Pirianov G, Mansi JL, Arnett TR, Colston KW. Bisphosphonates induce apoptosis in human breast cancer cell lines. Br J Haematol. 2000;82(8):1459–68. doi: 10.1054/bjoc.1999.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Boissier S, Ferreras M, Peyruchaud O, et al. Bisphosphonates inhibit breast and prostate carcinoma cell invasion, an early event in the formation of bone metastases. Cancer research. 2000;60(11):2949–54. [PubMed] [Google Scholar]
- [168].Boissier S, Magnetto S, Frappart L, et al. Bisphosphonates inhibit prostate and breast carcinoma cell adhesion to unmineralized and mineralized bone extracellular matrices. Cancer research. 1997;57(18):3890–4. [PubMed] [Google Scholar]
- [169].Ashton JA, Farese JP, Milner RJ, Lee-Ambrose LM, van Gilder JM. Investigation of the effect of pamidronate disodium on the in vitro viability of osteosarcoma cells from dogs. Am J Vet Res. 2005;66(5):885–91. doi: 10.2460/ajvr.2005.66.885. [DOI] [PubMed] [Google Scholar]
- [170].Benassi MS, Chiechi A, Ponticelli F, et al. Growth inhibition and sensitization to cisplatin by zoledronic acid in osteosarcoma cells. Cancer letters. 2007;250(2):194–205. doi: 10.1016/j.canlet.2006.10.004. [DOI] [PubMed] [Google Scholar]
- [171].Dass CR, Choong PF. Zoledronic acid inhibits osteosarcoma growth in an orthotopic model. Mol Cancer Ther. 2007;6(12 Pt 1):3263–70. doi: 10.1158/1535-7163.MCT-07-0546. [DOI] [PubMed] [Google Scholar]
- [172].Evdokiou A, Labrinidis A, Bouralexis S, Hay S, Findlay DM. Induction of cell death of human osteogenic sarcoma cells by zoledronic acid resembles anoikis. Bone. 2003;33(2):216–28. doi: 10.1016/s8756-3282(03)00223-0. [DOI] [PubMed] [Google Scholar]
- [173].Farese JP, Ashton J, Milner R, Ambrose LL, Van Gilder J. The effect of the bisphosphonate alendronate on viability of canine osteosarcoma cells in vitro. In vitro cellular & developmental biology. 2004;40(3-4):113–7. doi: 10.1290/1543-706x(2004)040<0113:teotba>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- [174].Heikkila P, Teronen O, Hirn MY, et al. Inhibition of matrix metalloproteinase-14 in osteosarcoma cells by clodronate. J Surg Res. 2003;111(1):45–52. doi: 10.1016/s0022-4804(03)00086-6. [DOI] [PubMed] [Google Scholar]
- [175].Heymann D, Ory B, Blanchard F, et al. Enhanced tumor regression and tissue repair when zoledronic acid is combined with ifosfamide in rat osteosarcoma. Bone. 2005;37(1):74–86. doi: 10.1016/j.bone.2005.02.020. [DOI] [PubMed] [Google Scholar]
- [176].Horie N, Murata H, Kimura S, et al. Combined effects of a third-generation bisphosphonate, zoledronic acid with other anticancer agents against murine osteosarcoma. Br J Cancer. 2007;96(2):255–61. doi: 10.1038/sj.bjc.6603548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Horie N, Murata H, Nishigaki Y, et al. The third-generation bisphosphonates inhibit proliferation of murine osteosarcoma cells with induction of apoptosis. Cancer letters. 2006;238(1):111–8. doi: 10.1016/j.canlet.2005.06.041. [DOI] [PubMed] [Google Scholar]
- [178].Iguchi T, Miyakawa Y, Saito K, et al. Zoledronate-induced S phase arrest and apoptosis accompanied by DNA damage and activation of the ATM/Chk1/cdc25 pathway in human osteosarcoma cells. Int J Oncol. 2007;31(2):285–91. [PubMed] [Google Scholar]
- [179].Kubo T, Shimose S, Matsuo T, Sakai A, Ochi M. Efficacy of a nitrogen-containing bisphosphonate, minodronate, in conjunction with a p38 mitogen activated protein kinase inhibitor or doxorubicin against malignant bone tumor cells. Cancer Chemother Pharmacol. 2008;62(1):111–6. doi: 10.1007/s00280-007-0580-y. [DOI] [PubMed] [Google Scholar]
- [180].Mackie PS, Fisher JL, Zhou H, Choong PF. Bisphosphonates regulate cell growth and gene expression in the UMR 106-01 clonal rat osteosarcoma cell line. Br J Cancer. 2001;84(7):951–8. doi: 10.1054/bjoc.2000.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181].Molinuevo MS, Bruzzone L, Cortizo AM. Alendronate induces anti-migratory effects and inhibition of neutral phosphatases in UMR106 osteosarcoma cells. Eur J Pharmacol. 2007;562(1-2):28–33. doi: 10.1016/j.ejphar.2007.01.054. [DOI] [PubMed] [Google Scholar]
- [182].Muraro M, Mereuta OM, Carraro F, Madon E, Fagioli F. Osteosarcoma cell line growth inhibition by zoledronate-stimulated effector cells. Cellular immunology. 2007;249(2):63–72. doi: 10.1016/j.cellimm.2007.11.005. [DOI] [PubMed] [Google Scholar]
- [183].Ory B, Blanchard F, Battaglia S, Gouin F, Redini F, Heymann D. Zoledronic acid activates the DNA S-phase checkpoint and induces osteosarcoma cell death characterized by apoptosis-inducing factor and endonuclease-G translocation independently of p53 and retinoblastoma status. Mol Pharmacol. 2007;71(1):333–43. doi: 10.1124/mol.106.028837. [DOI] [PubMed] [Google Scholar]
- [184].Ory B, Moriceau G, Trichet V, et al. Farnesyl diphosphate synthase is involved in the resistance to zoledronic acid of osteosarcoma cells. Journal of cellular and molecular medicine. 2008;12(3):928–41. doi: 10.1111/j.1582-4934.2008.00141.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [185].Mintz MB, Sowers R, Brown KM, et al. An expression signature classifies chemotherapy-resistant pediatric osteosarcoma. Cancer research. 2005;65(5):1748–54. doi: 10.1158/0008-5472.CAN-04-2463. [DOI] [PubMed] [Google Scholar]
- [186].Barr RD, Guo CY, Wiernikowski J, Webber C, Wright M, Atkinson S. Osteopenia in children with acute lymphoblastic leukemia: a pilot study of amelioration with Pamidronate. Med Pediatr Oncol. 2002;39(1):44–6. doi: 10.1002/mpo.10057. [DOI] [PubMed] [Google Scholar]
- [187].Black DM, Kelly MP, Genant HK, et al. Bisphosphonates and fractures of the subtrochanteric or diaphyseal femur. N Engl J Med. 362(19):1761–71. doi: 10.1056/NEJMoa1001086. [DOI] [PubMed] [Google Scholar]
- [188].Lala R, Matarazzo P, Bertelloni S, Buzi F, Rigon F, de Sanctis C. Pamidronate treatment of bone fibrous dysplasia in nine children with McCune-Albright syndrome. Acta Paediatr. 2000;89(2):188–93. doi: 10.1080/080352500750028816. [DOI] [PubMed] [Google Scholar]
- [189].Rauch F, Travers R, Plotkin H, Glorieux FH. The effects of intravenous pamidronate on the bone tissue of children and adolescents with osteogenesis imperfecta. J Clin Invest. 2002;110(9):1293–9. doi: 10.1172/JCI15952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [190].Schmid I, Stachel D, Schon C, Bauer M, Haas RJ. Pamidronate and calcitonin as therapy of acute cancer-related hypercalcemia in children. Klin Padiatr. 2001;213(1):30–4. doi: 10.1055/s-2001-11271. [DOI] [PubMed] [Google Scholar]
- [191].Shaw NJ, Boivin CM, Crabtree NJ. Intravenous pamidronate in juvenile osteoporosis. Arch Dis Child. 2000;83(2):143–5. doi: 10.1136/adc.83.2.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [192].Young G, Shende A. Use of pamidronate in the management of acute cancer-related hypercalcemia in children. Med Pediatr Oncol. 1998;30(2):117–21. doi: 10.1002/(sici)1096-911x(199802)30:2<117::aid-mpo9>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- [193].Zelcer S, Kellick M, Wexler LH, Gorlick R, Meyers PA. The Memorial Sloan Kettering Cancer Center experience with outpatient administration of high dose methotrexate with leucovorin rescue. Pediatr Blood Cancer. 2008;50(6):1176–80. doi: 10.1002/pbc.21419. [DOI] [PubMed] [Google Scholar]
- [194].Kutluk MT, Hazar V, Akyuz C, Varan A, Buyukpamukcu M. Childhood cancer and hypercalcemia: report of a case treated with pamidronate. J Pediatr. 1997;130(5):828–31. doi: 10.1016/s0022-3476(97)80030-3. [DOI] [PubMed] [Google Scholar]
- [195].Wasilewski-Masker K, Kaste SC, Hudson MM, Esiashvili N, Mattano LA, Meacham LR. Bone mineral density deficits in survivors of childhood cancer: long-term follow-up guidelines and review of the literature. Pediatrics. 2008;121(3):e705–13. doi: 10.1542/peds.2007-1396. [DOI] [PubMed] [Google Scholar]
- [196].Evaluation of Zoledronic Acid as a Single Agent or as an Adjuvant to Chemotherapy in High Grade Osteosarcoma. 2012 Accessed August 28, 2012, at http://www.cancer.gov/clinicaltrials/search/view?cdrid=598171&version=HealthProfessional&protocolsearchid=9768045.
- [197].Combination Chemotherapy With or Without Zoledronic Acid in Treating Patients With Osteosarcoma. 2012 Accessed August 28, 2012, at http://www.cancer.gov/clinicaltrials/search/view?cdrid=543987&version=HealthProfessional&protocolsearchid=10809449.
- [198].Subbiah V, Kurzrock R. Phase 1 clinical trials for sarcomas: the cutting edge. Curr Opin Oncol. 23(4):352–60. doi: 10.1097/CCO.0b013e3283477a94. [DOI] [PubMed] [Google Scholar]
- [199].Anderson PM, Tomaras M, McConnell K. Mifamurtide in osteosarcoma--a practical review. Drugs Today (Barc) 46(5):327–37. doi: 10.1358/dot.2010.46.5.1500076. [DOI] [PubMed] [Google Scholar]
- [200].Chou AJ, Kleinerman ES, Krailo MD, et al. Addition of muramyl tripeptide to chemotherapy for patients with newly diagnosed metastatic osteosarcoma: a report from the Children's Oncology Group. Cancer. 2009;115(22):5339–48. doi: 10.1002/cncr.24566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [201].Asano T, Kleinerman ES. Liposome-encapsulated MTP-PE: a novel biologic agent for cancer therapy. J Immunother Emphasis Tumor Immunol. 1993;14(4):286–92. doi: 10.1097/00002371-199311000-00006. [DOI] [PubMed] [Google Scholar]
- [202].Girardin SE, Boneca IG, Viala J, et al. Nod2 is a general sensor of peptidoglycan through muramyl dipeptide (MDP) detection. J Biol Chem. 2003;278(11):8869–72. doi: 10.1074/jbc.C200651200. [DOI] [PubMed] [Google Scholar]
- [203].Ogura Y, Inohara N, Benito A, Chen FF, Yamaoka S, Nunez G. Nod2, a Nod1/Apaf-1 family member that is restricted to monocytes and activates NF-kappaB. J Biol Chem. 2001;276(7):4812–8. doi: 10.1074/jbc.M008072200. [DOI] [PubMed] [Google Scholar]
- [204].Gutierrez O, Pipaon C, Inohara N, et al. Induction of Nod2 in myelomonocytic and intestinal epithelial cells via nuclear factor-kappa B activation. J Biol Chem. 2002;277(44):41701–5. doi: 10.1074/jbc.M206473200. [DOI] [PubMed] [Google Scholar]
- [205].Asano T, Matsushima K, Kleinerman ES. Liposome-encapsulated muramyl tripeptide up-regulates monocyte chemotactic and activating factor gene expression in human monocytes at the transcriptional and post-transcriptional levels. Cancer Immunol Immunother. 1994;38(1):16–22. doi: 10.1007/BF01517165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [206].Asano T, McIntyre BW, Bednarczyk JL, Wygant JN, Kleinerman ES. Liposomal muramyl tripeptide upregulates adhesion molecules on the surface of human monocytes. Oncology research. 1995;7(5):253–7. [PubMed] [Google Scholar]
- [207].MacEwen EG, Kurzman ID, Helfand S, et al. Current studies of liposome muramyl tripeptide (CGP 19835A lipid) therapy for metastasis in spontaneous tumors: a progress review. J Drug Target. 1994;2(5):391–6. doi: 10.3109/10611869408996814. [DOI] [PubMed] [Google Scholar]
- [208].Kleinerman ES, Jia SF, Griffin J, Seibel NL, Benjamin RS, Jaffe N. Phase II study of liposomal muramyl tripeptide in osteosarcoma: the cytokine cascade and monocyte activation following administration. J Clin Oncol. 1992;10(8):1310–6. doi: 10.1200/JCO.1992.10.8.1310. [DOI] [PubMed] [Google Scholar]
- [209].Kleinerman ES, Maeda M, Jaffe N. Liposome-encapsulated muramyl tripeptide: a new biologic response modifier for the treatment of osteosarcoma. Cancer Treat Res. 1993;62:101–7. doi: 10.1007/978-1-4615-3518-8_14. [DOI] [PubMed] [Google Scholar]
- [210].MacEwen EG, Kurzman ID, Rosenthal RC, et al. Therapy for osteosarcoma in dogs with intravenous injection of liposome-encapsulated muramyl tripeptide. J Natl Cancer Inst. 1989;81(12):935–8. doi: 10.1093/jnci/81.12.935. [DOI] [PubMed] [Google Scholar]
- [211].Kleinerman ES, Gano JB, Johnston DA, Benjamin RS, Jaffe N. Efficacy of liposomal muramyl tripeptide (CGP 19835A) in the treatment of relapsed osteosarcoma. Am J Clin Oncol. 1995;18(2):93–9. doi: 10.1097/00000421-199504000-00001. [DOI] [PubMed] [Google Scholar]
- [212].Kleinerman ES. Biologic therapy for osteosarcoma using liposome-encapsulated muramyl tripeptide. Hematol Oncol Clin North Am. 1995;9(4):927–38. [PubMed] [Google Scholar]
- [213].Kleinerman ES, Meyers PA, Raymond AK, Gano JB, Jia SF, Jaffe N. Combination therapy with ifosfamide and liposome-encapsulated muramyl tripeptide: tolerability, toxicity, and immune stimulation. J Immunother Emphasis Tumor Immunol. 1995;17(3):181–93. doi: 10.1097/00002371-199504000-00007. [DOI] [PubMed] [Google Scholar]
- [214].Kurzman ID, Shi F, Vail DM, MacEwen EG. In vitro and in vivo enhancement of canine pulmonary alveolar macrophage cytotoxic activity against canine osteosarcoma cells. Cancer Biother Radiopharm. 1999;14(2):121–8. doi: 10.1089/cbr.1999.14.121. [DOI] [PubMed] [Google Scholar]
- [215].Anderson P. Liposomal muramyl tripeptide phosphatidyl ethanolamine: ifosfamide-containing chemotherapy in osteosarcoma. Future oncology (London, England) 2006;2(3):333–43. doi: 10.2217/14796694.2.3.333. [DOI] [PubMed] [Google Scholar]
- [216].Anderson PM, Pearson M. Novel therapeutic approaches in pediatric and young adult sarcomas. Current oncology reports. 2006;8(4):310–5. doi: 10.1007/s11912-006-0038-0. [DOI] [PubMed] [Google Scholar]
- [217].Nardin A, Lefebvre ML, Labroquere K, Faure O, Abastado JP. Liposomal muramyl tripeptide phosphatidylethanolamine: Targeting and activating macrophages for adjuvant treatment of osteosarcoma. Curr Cancer Drug Targets. 2006;6(2):123–33. doi: 10.2174/156800906776056473. [DOI] [PubMed] [Google Scholar]
- [218].Armitage JO. Emerging applications of recombinant human granulocyte-macrophage colony-stimulating factor. Blood. 1998;92(12):4491–508. [PubMed] [Google Scholar]
- [219].Dunussi-Joannopoulos K, Dranoff G, Weinstein HJ, Ferrara JL, Bierer BE, Croop JM. Gene immunotherapy in murine acute myeloid leukemia: granulocyte-macrophage colony-stimulating factor tumor cell vaccines elicit more potent antitumor immunity compared with B7 family and other cytokine vaccines. Blood. 1998;91(1):222–30. [PubMed] [Google Scholar]
- [220].Baxevanis CN, Tsavaris NB, Papadhimitriou SI, et al. Granulocyte-macrophage colony-stimulating factor improves immunological parameters in patients with refractory solid tumours receiving second-line chemotherapy: correlation with clinical responses. Eur J Cancer. 1997;33(8):1202–8. doi: 10.1016/s0959-8049(97)00053-1. [DOI] [PubMed] [Google Scholar]
- [221].Hart DN. Dendritic cells: unique leukocyte populations which control the primary immune response. Blood. 1997;90(9):3245–87. [PubMed] [Google Scholar]
- [222].Dranoff G, Jaffee E, Lazenby A, et al. Vaccination with irradiated tumor cells engineered to secrete murine granulocyte-macrophage colony-stimulating factor stimulates potent, specific, and long-lasting anti-tumor immunity. Proceedings of the National Academy of Sciences of the United States of America. 1993;90(8):3539–43. doi: 10.1073/pnas.90.8.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [223].Kumar R, Yoneda J, Fidler IJ, Dong Z. GM-CSF-transduced B16 melanoma cells are highly susceptible to lysis by normal murine macrophages and poorly tumorigenic in immune-compromised mice. J Leukoc Biol. 1999;65(1):102–8. doi: 10.1002/jlb.65.1.102. [DOI] [PubMed] [Google Scholar]
- [224].Anderson PM, Markovic SN, Sloan JA, et al. Aerosol granulocyte macrophage-colony stimulating factor: a low toxicity, lung-specific biological therapy in patients with lung metastases. Clin Cancer Res. 1999;5(9):2316–23. [PubMed] [Google Scholar]
- [225].Di Carlo E, Forni G, Lollini P, Colombo MP, Modesti A, Musiani P. The intriguing role of polymorphonuclear neutrophils in antitumor reactions. Blood. 2001;97(2):339–45. doi: 10.1182/blood.v97.2.339. [DOI] [PubMed] [Google Scholar]
- [226].Gordon N, Arndt CA, Hawkins DS, et al. Fas expression in lung metastasis from osteosarcoma patients. J Pediatr Hematol Oncol. 2005;27(11):611–5. doi: 10.1097/01.mph.0000188112.42576.df. [DOI] [PubMed] [Google Scholar]
- [227].Worth LL, Lafleur EA, Jia SF, Kleinerman ES. Fas expression inversely correlates with metastatic potential in osteosarcoma cells. Oncology reports. 2002;9(4):823–7. [PubMed] [Google Scholar]
- [228].Gordon N, Koshkina NV, Jia SF, et al. 2007;13(15 Pt 1):4503–10. doi: 10.1158/1078-0432.CCR-07-0313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [229].Duffaud F, Digue L, Mercier C, et al. Recurrences following primary osteosarcoma in adolescents and adults previously treated with chemotherapy. Eur J Cancer. 2003;39(14):2050–7. doi: 10.1016/s0959-8049(03)00435-0. [DOI] [PubMed] [Google Scholar]
- [230].Kempf-Bielack B, Bielack SS, Jurgens H, et al. Osteosarcoma relapse after combined modality therapy: an analysis of unselected patients in the Cooperative Osteosarcoma Study Group (COSS) J Clin Oncol. 2005;23(3):559–68. doi: 10.1200/JCO.2005.04.063. [DOI] [PubMed] [Google Scholar]
- [231].Pastorino U, Valente M, Gasparini M, et al. Median sternotomy and multiple lung resections for metastatic sarcomas. Eur J Cardiothorac Surg. 1990;4(9):477–81. doi: 10.1016/1010-7940(90)90169-z. [DOI] [PubMed] [Google Scholar]
- [232].Tabone MD, Kalifa C, Rodary C, Raquin M, Valteau-Couanet D, Lemerle J. Osteosarcoma recurrences in pediatric patients previously treated with intensive chemotherapy. J Clin Oncol. 1994;12(12):2614–20. doi: 10.1200/JCO.1994.12.12.2614. [DOI] [PubMed] [Google Scholar]
- [233].Ebb D, Meyers P, Grier H, et al. Phase II Trial of Trastuzumab in Combination With Cytotoxic Chemotherapy for Treatment of Metastatic Osteosarcoma With Human Epidermal Growth Factor Receptor 2 Overexpression: A Report From the Children's Oncology Group. J Clin Oncol. 30(20):2545–51. doi: 10.1200/JCO.2011.37.4546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [234].Gorlick R, Huvos AG, Heller G, et al. Expression of HER2/erbB-2 correlates with survival in osteosarcoma. J Clin Oncol. 1999;17(9):2781–8. doi: 10.1200/JCO.1999.17.9.2781. [DOI] [PubMed] [Google Scholar]
- [235].Hynes NE. Amplification and overexpression of the erbB-2 gene in human tumors: its involvement in tumor development, significance as a prognostic factor, and potential as a target for cancer therapy. Semin Cancer Biol. 1993;4(1):19–26. [PubMed] [Google Scholar]
- [236].Onda M, Matsuda S, Higaki S, et al. ErbB-2 expression is correlated with poor prognosis for patients with osteosarcoma. Cancer. 1996;77(1):71–8. doi: 10.1002/(SICI)1097-0142(19960101)77:1<71::AID-CNCR13>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- [237].Scotlandi K, Manara MC, Hattinger CM, et al. Prognostic and therapeutic relevance of HER2 expression in osteosarcoma and Ewing's sarcoma. Eur J Cancer. 2005;41(9):1349–61. doi: 10.1016/j.ejca.2005.03.015. [DOI] [PubMed] [Google Scholar]
- [238].Hancock MC, Langton BC, Chan T, et al. A monoclonal antibody against the c-erbB-2 protein enhances the cytotoxicity of cisdiamminedichloroplatinum against human breast and ovarian tumor cell lines. Cancer research. 1991;51(17):4575–80. [PubMed] [Google Scholar]
- [239].Mariani G, Fasolo A, De Benedictis E, Gianni L. Trastuzumab as adjuvant systemic therapy for HER2-positive breast cancer. Nature clinical practice. 2009;6(2):93–104. doi: 10.1038/ncponc1298. [DOI] [PubMed] [Google Scholar]
- [240].Pegram MD, Lipton A, Hayes DF, et al. Phase II study of receptor-enhanced chemosensitivity using recombinant humanized anti-p185HER2/neu monoclonal antibody plus cisplatin in patients with HER2/neu-overexpressing metastatic breast cancer refractory to chemotherapy treatment. J Clin Oncol. 1998;16(8):2659–71. doi: 10.1200/JCO.1998.16.8.2659. [DOI] [PubMed] [Google Scholar]
- [241].Pietras RJ, Fendly BM, Chazin VR, Pegram MD, Howell SB, Slamon DJ. Antibody to HER-2/neu receptor blocks DNA repair after cisplatin in human breast and ovarian cancer cells. Oncogene. 1994;9(7):1829–38. [PubMed] [Google Scholar]
- [242].Romond EH, Perez EA, Bryant J, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353(16):1673–84. doi: 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- [243].Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science (New York, NY. 1987;235(4785):177–82. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- [244].Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344(11):783–92. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- [245].Kolb EA, Gorlick R, Houghton PJ, et al. Initial testing (stage 1) of a monoclonal antibody (SCH 717454) against the IGF-1 receptor by the pediatric preclinical testing program. Pediatr Blood Cancer. 2008;50(6):1190–7. doi: 10.1002/pbc.21450. [DOI] [PubMed] [Google Scholar]
- [246].Maloney EK, McLaughlin JL, Dagdigian NE, et al. An anti-insulin-like growth factor I receptor antibody that is a potent inhibitor of cancer cell proliferation. Cancer research. 2003;63(16):5073–83. [PubMed] [Google Scholar]
- [247].Scotlandi K, Manara MC, Nicoletti G, et al. Antitumor activity of the insulin-like growth factor-I receptor kinase inhibitor NVP-AEW541 in musculoskeletal tumors. Cancer research. 2005;65(9):3868–76. doi: 10.1158/0008-5472.CAN-04-3192. [DOI] [PubMed] [Google Scholar]
- [248].Juergens H, Daw NC, Geoerger B, et al. Preliminary efficacy of the anti-insulin-like growth factor type 1 receptor antibody figitumumab in patients with refractory Ewing sarcoma. J Clin Oncol. 29(34):4534–40. doi: 10.1200/JCO.2010.33.0670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [249].Maki RG. Small is beautiful: insulin-like growth factors and their role in growth, development, and cancer. J Clin Oncol. 28(33):4985–95. doi: 10.1200/JCO.2009.27.5040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [250].Pappo AS, Patel SR, Crowley J, et al. R1507, a monoclonal antibody to the insulin-like growth factor 1 receptor, in patients with recurrent or refractory Ewing sarcoma family of tumors: results of a phase II Sarcoma Alliance for Research through Collaboration study. J Clin Oncol. 29(34):4541–7. doi: 10.1200/JCO.2010.34.0000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [251].Weroha SJ, Haluska P. IGF-1 receptor inhibitors in clinical trials--early lessons. J Mammary Gland Biol Neoplasia. 2008;13(4):471–83. doi: 10.1007/s10911-008-9104-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [252].Tap WD, Demetri G, Barnette P, et al. Phase II study of ganitumab, a fully human anti-type-1 insulin-like growth factor receptor antibody, in patients with metastatic Ewing family tumors or desmoplastic small round cell tumors. J Clin Oncol. 30(15):1849–56. doi: 10.1200/JCO.2011.37.2359. [DOI] [PubMed] [Google Scholar]
- [253].Beltran PJ, Chung YA, Moody G, et al. Efficacy of ganitumab (AMG 479), alone and in combination with rapamycin, in Ewing's and osteogenic sarcoma models. J Pharmacol Exp Ther. 2011;337(3):644–54. doi: 10.1124/jpet.110.178400. [DOI] [PubMed] [Google Scholar]
- [254].Beltran PJ, Mitchell P, Chung YA, et al. AMG 479, a fully human anti-insulin-like growth factor receptor type I monoclonal antibody, inhibits the growth and survival of pancreatic carcinoma cells. Mol Cancer Ther. 2009;8(5):1095–105. doi: 10.1158/1535-7163.MCT-08-1171. [DOI] [PubMed] [Google Scholar]
- [255].Tolcher AW, Sarantopoulos J, Patnaik A, et al. Phase I, pharmacokinetic, and pharmacodynamic study of AMG 479, a fully human monoclonal antibody to insulin-like growth factor receptor 1. J Clin Oncol. 2009;27(34):5800–7. doi: 10.1200/JCO.2009.23.6745. [DOI] [PubMed] [Google Scholar]
- [256].Chawla SP, Staddon AP, Baker LH, et al. Phase II study of the mammalian target of rapamycin inhibitor ridaforolimus in patients with advanced bone and soft tissue sarcomas. J Clin Oncol. 30(1):78–84. doi: 10.1200/JCO.2011.35.6329. [DOI] [PubMed] [Google Scholar]
- [257].Ferrara N, Hillan KJ, Gerber HP, Novotny W. Discovery and development of bevacizumab, an anti-VEGF antibody for treating cancer. Nature reviews. 2004;3(5):391–400. doi: 10.1038/nrd1381. [DOI] [PubMed] [Google Scholar]
- [258].Houghton PJ, Morton CL, Kolb EA, et al. Initial testing (stage 1) of the mTOR inhibitor rapamycin by the pediatric preclinical testing program. Pediatr Blood Cancer. 2008;50(4):799–805. doi: 10.1002/pbc.21296. [DOI] [PubMed] [Google Scholar]
- [259].Kurmasheva RT, Dudkin L, Billups C, Debelenko LV, Morton CL, Houghton PJ. The insulin-like growth factor-1 receptor-targeting antibody, CP-751,871, suppresses tumor-derived VEGF and synergizes with rapamycin in models of childhood sarcoma. Cancer research. 2009;69(19):7662–71. doi: 10.1158/0008-5472.CAN-09-1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [260].de Pasquale MD, Castellano A, de Sio L, et al. Bevacizumab in pediatric patients: how safe is it? Anticancer Res. 31(11):3953–7. [PubMed] [Google Scholar]
- [261].Glade-Bender J, Kandel JJ, Yamashiro DJ. VEGF blocking therapy in the treatment of cancer. Expert Opin Biol Ther. 2003;3(2):263–76. doi: 10.1517/14712598.3.2.263. [DOI] [PubMed] [Google Scholar]
- [262].Benesch M, Windelberg M, Sauseng W, et al. Compassionate use of bevacizumab (Avastin) in children and young adults with refractory or recurrent solid tumors. Ann Oncol. 2008;19(4):807–13. doi: 10.1093/annonc/mdm510. [DOI] [PubMed] [Google Scholar]
- [263].Glade Bender JL, Adamson PC, Reid JM, et al. Phase I trial and pharmacokinetic study of bevacizumab in pediatric patients with refractory solid tumors: a Children's Oncology Group Study. J Clin Oncol. 2008;26(3):399–405. doi: 10.1200/JCO.2007.11.9230. [DOI] [PubMed] [Google Scholar]
- [264].Herbst RS, Eckhardt SG, Kurzrock R, et al. Phase I dose-escalation study of recombinant human Apo2L/TRAIL, a dual proapoptotic receptor agonist, in patients with advanced cancer. J Clin Oncol. 28(17):2839–46. doi: 10.1200/JCO.2009.25.1991. [DOI] [PubMed] [Google Scholar]
- [265].Massimo BGG, Giostra A, et al. Phase II study of 153-samarium-EDTMP followed by haematopoietic stem cell for patients with osteosarcoma with bone metasases. J Clin Oncol. 2011;29:2873–6. [Google Scholar]
- [266].Anderson PM, Wiseman GA, Dispenzieri A, et al. High-dose samarium-153 ethylene diamine tetramethylene phosphonate: low toxicity of skeletal irradiation in patients with osteosarcoma and bone metastases. J Clin Oncol. 2002;20(1):189–96. doi: 10.1200/JCO.2002.20.1.189. [DOI] [PubMed] [Google Scholar]
- [267].Fox E, Aplenc R, Bagatell R, et al. A phase 1 trial and pharmacokinetic study of cediranib, an orally bioavailable pan-vascular endothelial growth factor receptor inhibitor, in children and adolescents with refractory solid tumors. J Clin Oncol. 28(35):5174–81. doi: 10.1200/JCO.2010.30.9674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [268].Wall MA, Wohl ME, Jaffe N, Strieder DJ. Lung function in adolescents receiving high-dose methotrexate. Pediatrics. 1979;63(5):741–6. [PubMed] [Google Scholar]
- [269].Chawla SP, Chua VS, Fernandez L, et al. Phase I/II and phase II studies of targeted gene delivery in vivo: intravenous Rexin-G for chemotherapy-resistant sarcoma and osteosarcoma. Mol Ther. 2009;17(9):1651–7. doi: 10.1038/mt.2009.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [270].Wagner ER, Luther G, Zhu G, et al. Defective osteogenic differentiation in the development of osteosarcoma. Sarcoma. 2011;2011:325238. doi: 10.1155/2011/325238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [271].Sandberg AA, Bridge JA. Updates on the cytogenetics and molecular genetics of bone and soft tissue tumors: osteosarcoma and related tumors. Cancer Genet Cytogenet. 2003;145(1):1–30. [PubMed] [Google Scholar]
- [272].Wagner ER, He BC, Chen L, et al. Therapeutic Implications of PPARgamma in Human Osteosarcoma. PPAR Res. 2010;2010:956427. doi: 10.1155/2010/956427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [273].Alonso J, Garcia-Miguel P, Abelairas J, Mendiola M, Pestana A. A microsatellite fluorescent method for linkage analysis in familial retinoblastoma and deletion detection at the RB1 locus in retinoblastoma and osteosarcoma. Diagn Mol Pathol. 2001;10(1):9–14. doi: 10.1097/00019606-200103000-00003. [DOI] [PubMed] [Google Scholar]
- [274].Araki N, Uchida A, Kimura T, et al. Involvement of the retinoblastoma gene in primary osteosarcomas and other bone and soft-tissue tumors. Clin Orthop Relat Res. 1991(270):271–7. [PubMed] [Google Scholar]
- [275].Belchis DA, Meece CA, Benko FA, Rogan PK, Williams RA, Gocke CD. Loss of heterozygosity and microsatellite instability at the retinoblastoma locus in osteosarcomas. Diagn Mol Pathol. 1996;5(3):214–9. doi: 10.1097/00019606-199609000-00011. [DOI] [PubMed] [Google Scholar]
- [276].Benassi MS, Molendini L, Gamberi G, et al. Alteration of pRb/p16/cdk4 regulation in human osteosarcoma. Int J Cancer. 1999;84(5):489–93. doi: 10.1002/(sici)1097-0215(19991022)84:5<489::aid-ijc7>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- [277].Quelle DE, Zindy F, Ashmun RA, Sherr CJ. Alternative reading frames of the INK4a tumor suppressor gene encode two unrelated proteins capable of inducing cell cycle arrest. Cell. 1995;83(6):993–1000. doi: 10.1016/0092-8674(95)90214-7. [DOI] [PubMed] [Google Scholar]
- [278].Kansara M, Thomas DM. Molecular pathogenesis of osteosarcoma. DNA Cell Biol. 2007;26(1):1–18. doi: 10.1089/dna.2006.0505. [DOI] [PubMed] [Google Scholar]
- [279].el-Deiry WS. Regulation of p53 downstream genes. Semin Cancer Biol. 1998;8(5):345–57. doi: 10.1006/scbi.1998.0097. [DOI] [PubMed] [Google Scholar]
- [280].Hansen R, Oren M. p53; from inductive signal to cellular effect. Curr Opin Genet Dev. 1997;7(1):46–51. doi: 10.1016/s0959-437x(97)80108-6. [DOI] [PubMed] [Google Scholar]
- [281].Hung J, Anderson R. p53: functions, mutations and sarcomas. Acta Orthop Scand Suppl. 1997;273:68–73. doi: 10.1080/17453674.1997.11744705. [DOI] [PubMed] [Google Scholar]
- [282].Levine AJ. p53, the cellular gatekeeper for growth and division. Cell. 1997;88(3):323–31. doi: 10.1016/s0092-8674(00)81871-1. [DOI] [PubMed] [Google Scholar]
- [283].Chandar N, Billig B, McMaster J, Novak J. Inactivation of p53 gene in human and murine osteosarcoma cells. Br J Cancer. 1992;65(2):208–14. doi: 10.1038/bjc.1992.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [284].Barrios C, Castresana JS, Kreicbergs A. Clinicopathologic correlations and short-term prognosis in musculoskeletal sarcoma with c-myc oncogene amplification. Am J Clin Oncol. 1994;17(3):273–6. doi: 10.1097/00000421-199406000-00019. [DOI] [PubMed] [Google Scholar]
- [285].Barrios C, Castresana JS, Ruiz J, Kreicbergs A. Amplification of c-myc oncogene and absence of c-Ha-ras point mutation in human bone sarcoma. J Orthop Res. 1993;11(4):556–63. doi: 10.1002/jor.1100110410. [DOI] [PubMed] [Google Scholar]
- [286].Cole MD, McMahon SB. The Myc oncoprotein: a critical evaluation of transactivation and target gene regulation. Oncogene. 1999;18(19):2916–24. doi: 10.1038/sj.onc.1202748. [DOI] [PubMed] [Google Scholar]
- [287].Gamberi G, Benassi MS, Bohling T, et al. C-myc and c-fos in human osteosarcoma: prognostic value of mRNA and protein expression. Oncology. 1998;55(6):556–63. doi: 10.1159/000011912. [DOI] [PubMed] [Google Scholar]
- [288].Nesbit CE, Tersak JM, Prochownik EV. MYC oncogenes and human neoplastic disease. Oncogene. 1999;18(19):3004–16. doi: 10.1038/sj.onc.1202746. [DOI] [PubMed] [Google Scholar]
- [289].Pompetti F, Rizzo P, Simon RM, et al. Oncogene alterations in primary, recurrent, and metastatic human bone tumors. J Cell Biochem. 1996;63(1):37–50. doi: 10.1002/(SICI)1097-4644(199610)63:1%3C37::AID-JCB3%3E3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- [290].Lonardo F, Ueda T, Huvos AG, Healey J, Ladanyi M. p53 and MDM2 alterations in osteosarcomas: correlation with clinicopathologic features and proliferative rate. Cancer. 1997;79(8):1541–7. [PubMed] [Google Scholar]
- [291].Momand J, Jung D, Wilczynski S, Niland J. The MDM2 gene amplification database. Nucleic Acids Res. 1998;26(15):3453–9. doi: 10.1093/nar/26.15.3453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [292].Oliner JD, Kinzler KW, Meltzer PS, George DL, Vogelstein B. Amplification of a gene encoding a p53-associated protein in human sarcomas. Nature. 1992;358(6381):80–3. doi: 10.1038/358080a0. [DOI] [PubMed] [Google Scholar]
- [293].Ragazzini P, Gamberi G, Benassi MS, et al. Analysis of SAS gene and CDK4 and MDM2 proteins in low-grade osteosarcoma. Cancer Detect Prev. 1999;23(2):129–36. doi: 10.1046/j.1525-1500.1999.09907.x. [DOI] [PubMed] [Google Scholar]
- [294].Luo J, Sun MH, Kang Q, et al. Gene therapy for bone regeneration. Curr Gene Ther. 2005;5(2):167–79. doi: 10.2174/1566523053544218. [DOI] [PubMed] [Google Scholar]
- [295].Massague J. TGF-beta signal transduction. Annu Rev Biochem. 1998;67:753–91. doi: 10.1146/annurev.biochem.67.1.753. [DOI] [PubMed] [Google Scholar]
- [296].Massague J, Chen YG. Controlling TGF-beta signaling. Genes Dev. 2000;14(6):627–44. [PubMed] [Google Scholar]
- [297].Massague J, Wotton D. Transcriptional control by the TGF-beta/Smad signaling system. EMBO J. 2000;19(8):1745–54. doi: 10.1093/emboj/19.8.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [298].Kloen P, Gebhardt MC, Perez-Atayde A, et al. Expression of transforming growth factor-beta (TGF-beta) isoforms in osteosarcomas: TGF-beta3 is related to disease progression. Cancer. 1997;80(12):2230–9. [PubMed] [Google Scholar]
- [299].Su Y, Wagner ER, Luo Q, et al. Insulin-like growth factor binding protein 5 suppresses tumor growth and metastasis of human osteosarcoma. Oncogene. 2011;30(37):3907–17. doi: 10.1038/onc.2011.97. [DOI] [PubMed] [Google Scholar]
- [300].Mukherjee A, Rotwein P. Insulin-like growth factor binding protein-5 in osteogenesis: facilitator or inhibitor? Growth Horm IGF Res. 2007;17(3):179–85. doi: 10.1016/j.ghir.2007.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [301].Burrow S, Andrulis IL, Pollak M, Bell RS. Expression of insulin-like growth factor receptor, IGF-1, and IGF-2 in primary and metastatic osteosarcoma. J Surg Oncol. 1998;69(1):21–7. doi: 10.1002/(sici)1096-9098(199809)69:1<21::aid-jso5>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- [302].Ferrari C, Benassi S, Ponticelli F, et al. Role of MMP-9 and its tissue inhibitor TIMP-1 in human osteosarcoma: findings in 42 patients followed for 1-16 years. Acta Orthop Scand. 2004;75(4):487–91. doi: 10.1080/00016470410001295-1. [DOI] [PubMed] [Google Scholar]
- [303].Scotlandi K, Baldini N, Oliviero M, et al. Expression of Met/hepatocyte growth factor receptor gene and malignant behavior of musculoskeletal tumors. Am J Pathol. 1996;149(4):1209–19. [PMC free article] [PubMed] [Google Scholar]
- [304].Wilkie AO, Patey SJ, Kan SH, van den Ouweland AM, Hamel BC. FGFs, their receptors, and human limb malformations: clinical and molecular correlations. Am J Med Genet. 2002;112(3):266–78. doi: 10.1002/ajmg.10775. [DOI] [PubMed] [Google Scholar]
- [305].Wang LL, Levy ML, Lewis RA, et al. Clinical manifestations in a cohort of 41 Rothmund-Thomson syndrome patients. Am J Med Genet. 2001;102(1):11–7. doi: 10.1002/1096-8628(20010722)102:1<11::aid-ajmg1413>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- [306].McNairn JD, Damron TA, Landas SK, Ambrose JL, Shrimpton AE. Inheritance of osteosarcoma and Paget's disease of bone: a familial loss of heterozygosity study. J Mol Diagn. 2001;3(4):171–7. doi: 10.1016/S1525-1578(10)60669-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [307].McClatchey AI. Neurofibromatosis type II: mouse models reveal broad roles in tumorigenesis and metastasis. Mol Med Today. 2000;6(6):252–3. doi: 10.1016/s1357-4310(00)01696-8. [DOI] [PubMed] [Google Scholar]
- [308].McClatchey AI, Saotome I, Mercer K, et al. Mice heterozygous for a mutation at the Nf2 tumor suppressor locus develop a range of highly metastatic tumors. Genes Dev. 1998;12(8):1121–33. doi: 10.1101/gad.12.8.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [309].Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414(6859):105–11. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- [310].Aubin JE. Regulation of osteoblast formation and function. Rev Endocr Metab Disord. 2001;2(1):81–94. doi: 10.1023/a:1010011209064. [DOI] [PubMed] [Google Scholar]
- [311].Deng ZL, Sharff KA, Tang N, et al. Regulation of osteogenic differentiation during skeletal development. Front Biosci. 2008;13:2001–21. doi: 10.2741/2819. [DOI] [PubMed] [Google Scholar]
- [312].He BC, Chen L, Zuo GW, et al. Synergistic antitumor effect of the activated PPARgamma and retinoid receptors on human osteosarcoma. Clin Cancer Res. 2010;16(8):2235–45. doi: 10.1158/1078-0432.CCR-09-2499. [DOI] [PubMed] [Google Scholar]
- [313].Luu HH, Song WX, Luo X, et al. Distinct roles of bone morphogenetic proteins in osteogenic differentiation of mesenchymal stem cells. J Orthop Res. 2007;25(5):665–77. doi: 10.1002/jor.20359. [DOI] [PubMed] [Google Scholar]
- [314].Glass DA, 2nd, Karsenty G. In vivo analysis of Wnt signaling in bone. Endocrinology. 2007;148(6):2630–4. doi: 10.1210/en.2006-1372. [DOI] [PubMed] [Google Scholar]
- [315].Haydon RC, Luu HH, He TC. Osteosarcoma and osteoblastic differentiation: a new perspective on oncogenesis. Clin Orthop Relat Res. 2007;454:237–46. doi: 10.1097/BLO.0b013e31802b683c. [DOI] [PubMed] [Google Scholar]
- [316].Luo J, Chen J, Deng ZL, et al. Wnt signaling and human diseases: what are the therapeutic implications? Lab Invest. 2007;87(2):97–103. doi: 10.1038/labinvest.3700509. [DOI] [PubMed] [Google Scholar]
- [317].Luo X, Chen J, Song WX, et al. Osteogenic BMPs promote tumor growth of human osteosarcomas that harbor differentiation defects. Lab Invest. 2008;88(12):1264–77. doi: 10.1038/labinvest.2008.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [318].Reya T, Clevers H. Wnt signalling in stem cells and cancer. Nature. 2005;434(7035):843–50. doi: 10.1038/nature03319. [DOI] [PubMed] [Google Scholar]
- [319].Wang LL. Biology of osteogenic sarcoma. Cancer J. 2005;11(4):294–305. doi: 10.1097/00130404-200507000-00005. [DOI] [PubMed] [Google Scholar]
- [320].Scott RE. Differentiation, differentiation/gene therapy and cancer. Pharmacol Ther. 1997;73(1):51–65. doi: 10.1016/s0163-7258(96)00120-9. [DOI] [PubMed] [Google Scholar]
- [321].Harris SA, Enger RJ, Riggs BL, Spelsberg TC. Development and characterization of a conditionally immortalized human fetal osteoblastic cell line. J Bone Miner Res. 1995;10(2):178–86. doi: 10.1002/jbmr.5650100203. [DOI] [PubMed] [Google Scholar]
- [322].Luo Q, Kang Q, Si W, et al. Connective tissue growth factor (CTGF) is regulated by Wnt and bone morphogenetic proteins signaling in osteoblast differentiation of mesenchymal stem cells. J Biol Chem. 2004;279(53):55958–68. doi: 10.1074/jbc.M407810200. [DOI] [PubMed] [Google Scholar]
- [323].Kang Q, Sun MH, Cheng H, et al. Characterization of the distinct orthotopic bone-forming activity of 14 BMPs using recombinant adenovirus-mediated gene delivery. Gene Ther. 2004;11(17):1312–20. doi: 10.1038/sj.gt.3302298. [DOI] [PubMed] [Google Scholar]
- [324].Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- [325].Li QL, Ito K, Sakakura C, et al. Causal relationship between the loss of RUNX3 expression and gastric cancer. Cell. 2002;109(1):113–24. doi: 10.1016/s0092-8674(02)00690-6. [DOI] [PubMed] [Google Scholar]
- [326].Lund AH, van Lohuizen M. RUNX: a trilogy of cancer genes. Cancer Cell. 2002;1(3):213–5. doi: 10.1016/s1535-6108(02)00049-1. [DOI] [PubMed] [Google Scholar]
- [327].Thomas DM, Johnson SA, Sims NA, et al. Terminal osteoblast differentiation, mediated by runx2 and p27KIP1, is disrupted in osteosarcoma. J Cell Biol. 2004;167(5):925–34. doi: 10.1083/jcb.200409187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [328].Clevers H. Wnt/beta-catenin signaling in development and disease. Cell. 2006;127(3):469–80. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- [329].Luu HH, Zhang R, Haydon RC, et al. Wnt/beta-catenin signaling pathway as a novel cancer drug target. Curr Cancer Drug Targets. 2004;4(8):653–71. doi: 10.2174/1568009043332709. [DOI] [PubMed] [Google Scholar]
- [330].Boland GM, Perkins G, Hall DJ, Tuan RS. Wnt 3a promotes proliferation and suppresses osteogenic differentiation of adult human mesenchymal stem cells. J Cell Biochem. 2004;93(6):1210–30. doi: 10.1002/jcb.20284. [DOI] [PubMed] [Google Scholar]
- [331].Haydon RC, Deyrup A, Ishikawa A, et al. Cytoplasmic and/or nuclear accumulation of the beta-catenin protein is a frequent event in human osteosarcoma. Int J Cancer. 2002;102(4):338–42. doi: 10.1002/ijc.10719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [332].Iwaya K, Ogawa H, Kuroda M, Izumi M, Ishida T, Mukai K. Cytoplasmic and/or nuclear staining of beta-catenin is associated with lung metastasis. Clin Exp Metastasis. 2003;20(6):525–9. doi: 10.1023/a:1025821229013. [DOI] [PubMed] [Google Scholar]
- [333].Hoang BH, Kubo T, Healey JH, et al. Expression of LDL receptor-related protein 5 (LRP5) as a novel marker for disease progression in high-grade osteosarcoma. Int J Cancer. 2004;109(1):106–11. doi: 10.1002/ijc.11677. [DOI] [PubMed] [Google Scholar]
- [334].Carpio L, Gladu J, Goltzman D, Rabbani SA. Induction of osteoblast differentiation indexes by PTHrP in MG-63 cells involves multiple signaling pathways. Am J Physiol Endocrinol Metab. 2001;281(3):E489–99. doi: 10.1152/ajpendo.2001.281.3.E489. [DOI] [PubMed] [Google Scholar]
- [335].Fukushima H, Jimi E, Kajiya H, Motokawa W, Okabe K. Parathyroid-hormone-related protein induces expression of receptor activator of NF-{kappa}B ligand in human periodontal ligament cells via a cAMP/protein kinase A-independent pathway. J Dent Res. 2005;84(4):329–34. doi: 10.1177/154405910508400407. [DOI] [PubMed] [Google Scholar]
- [336].Haydon RC, Zhou L, Feng T, et al. Nuclear receptor agonists as potential differentiation therapy agents for human osteosarcoma. Clin Cancer Res. 2002;8(5):1288–94. [PMC free article] [PubMed] [Google Scholar]
- [337].Hong SH, Kadosawa T, Nozaki K, et al. In vitro retinoid-induced growth inhibition and morphologic differentiation of canine osteosarcoma cells. Am J Vet Res. 2000;61(1):69–73. doi: 10.2460/ajvr.2000.61.69. [DOI] [PubMed] [Google Scholar]
- [338].Nozaki K, Kadosawa T, Nishimura R, Mochizuki M, Takahashi K, Sasaki N. 1,25-Dihydroxyvitamin D3, recombinant human transforming growth factor-beta 1, and recombinant human bone morphogenetic protein-2 induce in vitro differentiation of canine osteosarcoma cells. J Vet Med Sci. 1999;61(6):649–56. doi: 10.1292/jvms.61.649. [DOI] [PubMed] [Google Scholar]
- [339].Zenmyo M, Komiya S, Hamada T, et al. Transcriptional activation of p21 by vitamin D(3) or vitamin K(2) leads to differentiation of p53-deficient MG-63 osteosarcoma cells. Hum Pathol. 2001;32(4):410–6. doi: 10.1053/hupa.2001.23524. [DOI] [PubMed] [Google Scholar]
- [340].Kallio A, Guo T, Lamminen E, et al. Estrogen and the selective estrogen receptor modulator (SERM) protection against cell death in estrogen receptor alpha and beta expressing U2OS cells. Mol Cell Endocrinol. 2008;289(1-2):38–48. doi: 10.1016/j.mce.2008.03.005. [DOI] [PubMed] [Google Scholar]
- [341].Partridge NC, Bloch SR, Pearman AT. Signal transduction pathways mediating parathyroid hormone regulation of osteoblastic gene expression. J Cell Biochem. 1994;55(3):321–7. doi: 10.1002/jcb.240550308. [DOI] [PubMed] [Google Scholar]
- [342].Eferl R, Wagner EF. AP-1: a double-edged sword in tumorigenesis. Nat Rev Cancer. 2003;3(11):859–68. doi: 10.1038/nrc1209. [DOI] [PubMed] [Google Scholar]
- [343].Grigoriadis AE, Schellander K, Wang ZQ, Wagner EF. Osteoblasts are target cells for transformation in c-fos transgenic mice. J Cell Biol. 1993;122(3):685–701. doi: 10.1083/jcb.122.3.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [344].McCauley LK, Koh AJ, Beecher CA, Rosol TJ. Proto-oncogene c-fos is transcriptionally regulated by parathyroid hormone (PTH) and PTH-related protein in a cyclic adenosine monophosphate-dependent manner in osteoblastic cells. Endocrinology. 1997;138(12):5427–33. doi: 10.1210/endo.138.12.5587. [DOI] [PubMed] [Google Scholar]
- [345].Cheng H, Jiang W, Phillips FM, et al. Osteogenic activity of the fourteen types of human bone morphogenetic proteins (BMPs) J Bone Joint Surg Am. 2003;85-A(8):1544–52. doi: 10.2106/00004623-200308000-00017. [DOI] [PubMed] [Google Scholar]
- [346].He TC. Distinct osteogenic activity of BMPs and their orthopaedic applications. J Musculoskelet Neuronal Interact. 2005;5(4):363–6. [PubMed] [Google Scholar]
- [347].Urist MR. Bone: formation by autoinduction. Science. 1965;150(3698):893–9. doi: 10.1126/science.150.3698.893. [DOI] [PubMed] [Google Scholar]
- [348].Wozney JM, Rosen V, Celeste AJ, et al. Novel regulators of bone formation: molecular clones and activities. Science. 1988;242(4885):1528–34. doi: 10.1126/science.3201241. [DOI] [PubMed] [Google Scholar]
- [349].Dai X, Ma W, He X, Jha RK. Review of therapeutic strategies for osteosarcoma, chondrosarcoma, and Ewing's sarcoma. Med Sci Monit. 2011;17(8):RA177–90. doi: 10.12659/MSM.881893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [350].Wan X, Mendoza A, Khanna C, Helman LJ. Rapamycin inhibits ezrin-mediated metastatic behavior in a murine model of osteosarcoma. Cancer Res. 2005;65(6):2406–11. doi: 10.1158/0008-5472.CAN-04-3135. [DOI] [PubMed] [Google Scholar]
- [351].Gazitt Y, Kolaparthi V, Moncada K, Thomas C, Freeman J. Targeted therapy of human osteosarcoma with 17AAG or rapamycin: characterization of induced apoptosis and inhibition of mTOR and Akt/MAPK/Wnt pathways. Int J Oncol. 2009;34(2):551–61. [PubMed] [Google Scholar]
- [352].Maris JM, Courtright J, Houghton PJ, et al. Initial testing of the VEGFR inhibitor AZD2171 by the pediatric preclinical testing program. Pediatr Blood Cancer. 2008;50(3):581–7. doi: 10.1002/pbc.21232. [DOI] [PubMed] [Google Scholar]
- [353].Clark JC, Dass CR, Choong PF. A review of clinical and molecular prognostic factors in osteosarcoma. J Cancer Res Clin Oncol. 2008;134(3):281–97. doi: 10.1007/s00432-007-0330-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [354].Ek ET, Dass CR, Contreras KG, Choong PF. Pigment epithelium-derived factor overexpression inhibits orthotopic osteosarcoma growth, angiogenesis and metastasis. Cancer Gene Ther. 2007;14(7):616–26. doi: 10.1038/sj.cgt.7701044. [DOI] [PubMed] [Google Scholar]
- [355].Akiyama T, Dass CR, Choong PF. Novel therapeutic strategy for osteosarcoma targeting osteoclast differentiation, bone-resorbing activity, and apoptosis pathway. Mol Cancer Ther. 2008;7(11):3461–9. doi: 10.1158/1535-7163.MCT-08-0530. [DOI] [PubMed] [Google Scholar]
- [356].Nakase M, Inui M, Okumura K, Kamei T, Nakamura S, Tagawa T. p53 gene therapy of human osteosarcoma using a transferrin-modified cationic liposome. Mol Cancer Ther. 2005;4(4):625–31. doi: 10.1158/1535-7163.MCT-04-0196. [DOI] [PubMed] [Google Scholar]
- [357].Inaba H, Glibetic M, Buck S, Ravindranath Y, Kaplan J. Interferon-gamma sensitizes osteosarcoma cells to Fas-induced apoptosis by up-regulating Fas receptors and caspase-8. Pediatr Blood Cancer. 2004;43(7):729–36. doi: 10.1002/pbc.20151. [DOI] [PubMed] [Google Scholar]
- [358].Luksch R, Perotti D, Cefalo G, et al. Immunomodulation in a treatment program including pre- and post-operative interleukin-2 and chemotherapy for childhood osteosarcoma. Tumori. 2003;89(3):263–8. doi: 10.1177/030089160308900306. [DOI] [PubMed] [Google Scholar]
- [359].Chou AJBM, Mackinson C, et al. Phase Ib/IIa study of sustained release lipid inhalation targeting cisplatin by inhalation in the treatment of patients with relapsed/progressive osteosarcoma metastatic to the lung-ASCO Abstract 18S 9525. Journal of Clinical Oncology; ASCO Annual Meeting Proceedings Part I.2007. [Google Scholar]
- [360].Fagioli F, Aglietta M, Tienghi A, et al. High-dose chemotherapy in the treatment of relapsed osteosarcoma: an Italian sarcoma group study. J Clin Oncol. 2002;20(8):2150–6. doi: 10.1200/JCO.2002.08.081. [DOI] [PubMed] [Google Scholar]
- [361].Sauerbrey A, Bielack S, Kempf-Bielack B, Zoubek A, Paulussen M, Zintl F. High-dose chemotherapy (HDC) and autologous hematopoietic stem cell transplantation (ASCT) as salvage therapy for relapsed osteosarcoma. Bone Marrow Transplant. 2001;27(9):933–7. doi: 10.1038/sj.bmt.1703023. [DOI] [PubMed] [Google Scholar]