Abstract
Defects of articular cartilage present a unique clinical challenge due to its poor self-healing capacity and avascular nature. Current surgical treatment options do not ensure consistent regeneration of hyaline cartilage in favor of fibrous tissue. Here, we review the current understanding of the most important biological regulators of chondrogenesis and their interactions, to provide insight into potential applications for cartilage tissue engineering. These include various signaling pathways, including fibroblast growth factors (FGFs), transforming growth factor β (TGF-β)/bone morphogenic proteins (BMPs), Wnt/β-catenin, Hedgehog, Notch, hypoxia, and angiogenic signaling pathways. Transcriptional and epigenetic regulation of chondrogenesis will also be discussed. Advances in our understanding of these signaling pathways have led to promising advances in cartilage regeneration and tissue engineering.
Keywords: BMPs, Cartilage, Cell signaling, Chondrogenesis, FGF, Regenerative medicine, Sox9, TGFβ
Introduction
Articular cartilage injury and repair is an issue of growing importance around the world. Kurtz et al predict that the number of total knee replacements will jump from 700,000 to 3.48 million annually by the year 2030.1 Although common, articular cartilage defects continue to hold a unique clinical challenge due to its poor self-healing capacity, which is largely due to its avascular nature. Current surgical treatment options do not reliably produce hyaline cartilage tissue in favor of fibrous tissue, and therefore remain controversial. Deposition of fibrocartilage instead of hyaline cartilage can lead to poor durability and rapid decline in activity levels after 1 year. Therefore, there is a critical need for more effective cartilage regenerative therapies. The difficulty in obtaining a sufficiently large number of chondrocytes for implantation could be overcome by utilizing gene therapy to promote chondrogenesis from bone marrow-derived mesenchymal stem cells (MSCs). However, research regarding exogenous control over this multi-step process has yielded only limited clinically applicable results. For this reason, the ability to differentiate MSCs into chondrocytic cells, and subsequently implant these chondrocytes into cartilaginous defects is of significant therapeutic value.
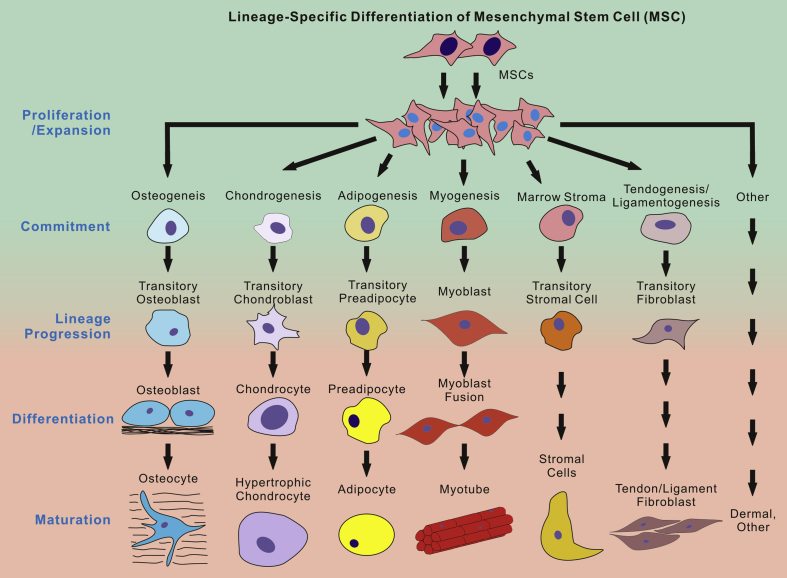
MSCs are multipotent progenitor cells that can differentiate into several lineages, including bone, cartilage, fat, and muscle (Fig. 1). The proliferation and differentiation of mesenchymal cells to chondrocytes, or chondrogenesis, is a complex process that is regulated by a host of factors. These factors include intracellular proteins, receptor ligands, and transcription factors; and a disruption in signaling can result in defective chondrocyte production. Here, we review the current understanding of the most important biological regulators of chondrogenesis and how these regulators interact to provide insight into their potential for cartilage tissue engineering.
Figure 1.
Currently hypothesized lineage-specific differentiation of mesenchymal stem cells (MSCs). Upon receiving appropriate differentiation cues, MSCs are multipotent stem cells and may undergo a cascade of differentiation stages, including progenitor proliferation and expansion, lineage commitment and progression, differentiation and maturation, and subsequently differentiate into multiple types of tissues, such as bone, cartilage, fat, msulce, tendon, etc. It remains a challenge to direct MSCs to differentiate into a specific type of cells/tissues. Furthermore, it is not well understood how early different lineages diverge from MSCs. Nonetheless, MSCs represent one of the most promising populations of progenitors for regenerative medicine and tissue engineering.
Fibroblast growth factor (FGF) signaling pathway
FGFs are a family of heparin-binding growth factors that are involved in the proliferation and differentiation of a variety of tissues. In general, FGFs are known to be positive regulators of chondrogenesis and they appear to be involved throughout the process. The most well studied and generally accepted function of the FGF family with regards to chondrogenesis is its role in the proper patterning of developing cartilage. The developing limb is divided into two components: the apical ectodermal ridge (AER) and the limb bud mesenchyme. The FGFs that are produced in the AER, zone of polarizing activity (ZPA), and dorsal ectoderm are necessary for early patterning events in the developing embryo.2 The AER, which serves as the primary source of FGF2, FGF4, FGF8, and FGF9 in the developing embryo, is maintained by Wnt3 and FGF10 signaling.3, 4 The effect of FGF10 on the apical ectodermal ridge is the result of a positive feedback loop with FGF8. FGF2, 4, and 8 serve as important regulators of proximal-distal growth, and FGF2 has the additional function of priming MSCs for chondrocytic differentiation.5, 6 The latter effect is mediated by an increase in basal expression of prochondrogenic transcription factor Sox9 and inactivation of IGF-I and TGF-β signaling.5, 7
Sequential expression of three FGFs has been shown to impact the progression of chondrocyte differentiation and maturation: FGF2, FGF9, and FGF18.8, 9, 10 As previously stated, FGF2 administration is known to be sufficient to enhance chondrocyte proliferation speed and prepare chondroprogenitor cells for terminal differentiation; however, the mechanism by which FGF2 exerts these effects has been largely unknown until recently.8 FGF2's ability to enhance chondrocyte proliferation speed is due to its ability to enhance TGFβ1 expression.11 Likewise, FGF2-induced chondrocyte differentiation is a consequence of FGF2-mediated downregulation of TGF-β2 expression.7 Given FGF2's interactions with TGF-β1 and TGF-β2, research sought to elucidate the impact of combining FGF2 with TGF-β3. It has now been determined that FGF2 is able to induce chondrocyte differentiation and enhance proliferation in the presence of TGF-β3, however these effects are accompanied by inhibition of chondrocyte hypertrophy.12 In addition to its interactions with the TGF-β family of proteins, combination of FGF2 with Wnt3a or a combination of platelet-derived growth factor (PDGF), insulin, and TGF-β1 has been shown to be sufficient to support long-term production of fully functional cartilage.13, 14
While FGF2 is known to have a major impact on priming chondroprogenitors for the chondrocyte differentiation program, FGF9 and FGF18 are regulators for early chondrogenic differentiation, augmentation of ECM production, and terminal chondrocyte hypertrophy.9 FGF9 can be both prochondrogenic and proosteogenic depending on its expression level and the cell population in which it is expressed.15 FGF9 has not only been found to promote chondrocyte hypertrophy at early stages but also to drive the vascularization of the growth plate prior to osteogenesis.16 The multifunctionality of FGF9 is believed to be the result of its ability to upregulate expression of other prochondrogenic proteins, such as Sox9, Indian hedgehog (Ihh), and Col2a1. FGF9 also upregulates proosteogenic proteins, such as Col10a1.17 Research regarding FGF18 has yielded conflicting results in the in vitro setting.18, 19 However, FGF18 has been shown to stimulate repair of damaged cartilage in the setting of osteoarthritis in rats so it is generally accepted that FGF18 is prochondrogenic.20
Transforming growth factor β (TGF-β)/bone morphogenic protein (BMP) signaling pathway
Members of the transforming growth factor β (TGF-β) superfamily, particularly TGF-βs and BMPs, are essential for multiple stages of embryonic chondrogenesis.21, 22 This pathway is often utilized to induce chondrocyte differentiation in MSCs and expanded chondrocyte populations.21, 23, 24, 25, 26, 27 TGF-β1 and TGF-β2 have long been known to serve as major regulators in chondrogenesis and osteogenesis.28, 29, 30, 31, 32, 33, 34, 35, 36 Members of the BMP family are required for condensation of chondroprogenitor cells and chondrocyte differentiation both in vivo and in vitro.37, 38, 39 TGF-β also mediates the end of proliferation and the subsequent initiation of chondrocyte differentiation.22 This shift from proliferation to differentiation has been extensively studied both in vivo during embryonic cartilage development and in vitro using MSCs.40 Recent investigation in tissue engineering has focused on developing suitable molecular scaffolds to deliver TGF-β to damaged articular cartilage in vivo in order to induce chondrogenic repair of the damaged cartilage.41 Proper development of joints—another process involving chondrogenesis—requires the activity of several BMPs, including BMP-2, BMP-4, and growth and differentiation factor-5 (GDF5), another member of the BMP family.38, 42, 43, 44, 45 However, exogenous addition of GDF5 to synovial joints during development results in fused joints and chondrocyte overgrowth, suggesting that BMP signaling effects are dose-dependent.38, 43, 44
TGF-β/BMP signaling primarily activates Smad-dependent signal transduction pathways in the target cell.22 There are two types of receptors for TGF-βs, types I and II; which, upon activation, phosphorylate type-specific receptor-Smads (R-Smads). Following BMP signaling, activated Smad1, Smad5, and Smad8 associate with Smad4 and translocate to the nucleus in order to regulate the expression of genes.37, 46, 47 In contrast, Smad6 and Smad7 are inhibitory (I-Smads). Smad6 selectively inhibits Smad1/5/8 signaling, whereas Smad7 inhibits all R-Smad signaling pathways. Thus, Smad7 can inhibit chondrocyte differentiation at several different stages.22, 48 TGF-β can also signal via the mitogen activated protein kinase (MAPK) proteins p38, ERK, and JNK in MSCs; which can contribute to the progression from condensation to differention.49, 50, 51
Though TGF-β is generally associated with differentiation, BMP signaling appears to play a key role in condensation. The coalescence of small aggregates of chondroprogenitor cells into a single distinct cluster in vitro, a prerequisite of differentiation, and the condensation of limb bud mesenchyme in culture both require BMP-mediated signaling.37, 52, 53 Prior to the differentiation of chondroprogenitor cells, TGF-β counteracts FGF-mediated cell proliferation.22 TGF-β2 and TGF-β3 are highly expressed in the AER of the developing chick embryo limb bud, which corresponds to a reduction in cell proliferation.54 Simultaneous downregulation of Gremlin (Grem), an inhibitor of BMP, was also observed, suggesting that TGF-β signaling might indirectly inhibit proliferation by inhibiting Grem; thus, the aforementioned downregulation enables BMPs to counteract the proliferative effects of FGF.55 BMP-2 has also been shown to have anti-proliferative effects in the mouse limb bud AER, opposing the effects of FGF-4.2, 56, 57 In addition to inhibiting Grem, TGF-β signaling has been found to activate the cyclin D kinase (CDK) inhibitors p16, p21, and p53, which arrest MSCs in the G1 phase of the cell cycle and induce cellular senescence.22 FGF2 promotes proliferation by inhibiting the expression of TGF-β2, preventing this senescence.58 TGF-βR1 and Smad3 expression increased steadily over time in a 3-month long MSC chondrocyte culture, corresponding directly to an observed decrease in proliferation and increase in differentiation.59
In addition to inhibiting proliferation, TGF-β has been shown to mediate the differentiation of chondroprogenitor cells into chondrocytes.28, 32, 60 TGF-β has long been used as the prototype for growth factor-induced chondrogenesis in MSCs in vitro.61 TGF-β-mediated Smad2/3 and Smad1/5/8 signaling is essential for the initiation of chondrogenic differentiation.22 TGF-β also activates MAPK proteins p38, ERK, and JNK in MSCs, which lead to downregulation of N-cadherin expression by blocking Wnt-mediated β-catenin nuclear translocation.49 Reducing N-cadherin expression is a necessary step to proceed from condensation to differentiation.62, 63, 64 Inhibiting TGF-β signaling during chondrogenesis by blocking TGF-βR2 expression alters differentiation and digital joint formation without affecting prior stages of chondrogenesis.65, 66, 67
BMPs also take part in the process of chondrocyte differentiation. Simultaneous removal of either BMPR1A and BMPR1B or SMAD1 and SMAD5 from chondrogenic cells blocks the formation of the endochondral skeleton in mice, suggesting that those cells are unable to form cartilage.37, 39, 68 Application of BMP-2, 4, 6, 7, 9, 13, or 15 can increase in vitro articular chondrocyte synthesis of type II collagen.22, 38, 69, 70 Although BMPs take part in the seemingly mutually exclusive condensation and differentiation stages, it is likely that the effects of BMP signaling are time-dependent and concentration-dependent.38
Axin, a protein best known for its role in the β-catenin degradation complex, also interacts with the TGF-β signaling pathways during chondrogenesis.22 During chondrocyte differentiation/maturation, Axin acts as an adaptor between TGF-βR and SMAD3. This adaptor interaction allows for greater phosphorylation and activation of SMAD3 by TGF-βR, which enhances the effect of TGF-β signaling. Axin further enhances this signal by a similar facilitation of TGF-βR phosphorylation of the inhibitor SMAD7, resulting in SMAD7 degradation.71, 72 Disrupting Axin2 signaling has also been shown to accelerate chondrocyte maturation, further supporting Axin's role in chondrocyte differentiation.73 Given that Axin also mediates the downregulation of Wnt/β-catenin signaling, this activity is further suggestive of the importance of the switch from Wnt-mediated signaling during condensation to TGF-β signaling during differentiation.
After chondrocytes have differentiated, continued TGF-β-mediated SMAD1/5/8 signaling leads to cartilage hypertrophy.22, 74 This finding is primarily relevant to efforts to use tissue engineering methods to medically repair damaged articular cartilage (e.g., in osteoarthritis), because TGF-β-induced chondrogenesis in MSCs produces hypertrophic hyaline cartilage.75
Wnt/β-catenin signaling pathway
It is well established that Wnt signaling is involved in chondrogenesis, but the exact nature of its involvement remains to be fully elucidated. Several studies have demonstrated that Wnt signaling is capable of both enhancing and inhibiting chondrogenic differentiation of adult and embryonic progenitor cells.75, 76, 77, 78 These antagonistic effects may be the product of a variety of factors including the phase of differentiation of target cells, level of Wnt activity, particular type of Wnt signal, and even crosstalk with other signaling pathways, such as the TGF-β/BMP signaling pathway.77, 79, 80 The canonical Wnt/β-catenin pathway is the most studied with regards to chondrogenesis and will be focused on herein.
Although Wnt signaling's role in the early phase of chondrogenesis has not been well characterized, functional analysis of a few specific Wnt proteins has revealed Wnt to be an important player in chondrogenic cell migration and condensation.81, 82 It has been demonstrated, for example, that Wnt5b plays a regulatory role in chondroprogenitor cell migration through activation of the planar cell polarity pathway (to be discussed further), and decreases adhesion by inhibiting expression of a destabilizing cadherin receptor as well as exerting regulatory effects on a variety of cadherins.94 Wnt5a has also been associated with cellular migration and focal adhesion dynamics in embryonic fibroblasts, but in contrast to Wnt5b, it has been shown to promote cellular adhesion.81, 83, 84
Progenitor cell migration and cell–cell adhesion regulation are crucial steps in early chondrogenesis and are, in part, mediated by Wnt signaling.52 During development of pharyngeal arches, cranial neural crest cells (CNCCs) undergo convergence and extension to form a cartilaginous framework for future ossification.85, 86, 87, 88 Wnt activation of the non-canonical planar cell polarity pathway is a fundamental regulatory process in these preliminary CNCC migration and condensation patterns.89, 90, 91 It has been well documented that the absence of Wnt signaling results in craniofacial deformities in vertebrates, confirming the importance of Wnt signaling in chondrogenic cell migration and condensation.92, 93, 94, 95 Wnt-mediated CNCC migration and condensation have been studied in zebrafish, in which it has been demonstrated that Wnt9a knockout results in severe craniofacial malformations.96, 97 These findings do not provide extensive insight into the mechanisms of Wnt's mediating effects on cell migration and condensation, but they do demonstrate the importance of Wnt signaling in these processes.
Several Wnt proteins, including Wnt1, Wnt3a, Wnt4, Wnt5b, and Wnt8 have been shown to signal via the canonical pathway in vitro.76, 77, 98, 99, 100 However, their effects on chondrogenesis are inconsistent. Wnt1, Wnt4, and Wnt8 have reportedly been shown to inhibit differentiation of chondrocytes whereas Wnt3a and Wnt5b stimulate it. Similarly, Wnt3a and Wnt5b have been shown to inhibit chondrocyte hypertrophy, an important process in the latter stages of chondrogenesis, while Wnt4 and Wnt8 stimulate it.89 Other Wnt proteins have similar contradictory effects: Wnt7a inhibits chondrocyte differentiation, Wnt9a inhibits both chondrocyte differentiation and hypertrophy, Wnt5a stimulates chondrocyte differentiation but delays hypertrophy, and Wnt11 seems to have no effect on chondrogenesis.101, 102, 103 This complicated network of seemingly contradicting effects, which can be found in Table 1, may be the result of Wnt activating two or more of the aforementioned intracellular cascades with effects that modulate each other. For example, Wnt5b has been shown to both stimulate and repress chondrocyte differentiation via the canonical β-catenin and non-canonical JNK pathways, respectively.78, 81 Similarly, Yan et al showed that Wnt3a signals via the canonical signal cascade as well as the JNK pathway.104 Additionally, as already mentioned, crosstalk between other signaling pathways such as TGF-β and BMP may be partially responsible for the opposing effects of different Wnt proteins.
Table 1.
Effect of Wnt signaling on chondrocyte differentiation and hypertrophy.
| Wnt | Differentiation | Hypertrophy | References |
|---|---|---|---|
| Wnt1 | Inhibits | – | 318 |
| Wnt3a | Stimulates | Inhibits | 77, 319 |
| Wnt4 | Inhibits | Stimulates | 76, 320 |
| Wnt5a | Stimulates | Inhibits | 76, 321 |
| Wnt5b | Stimulates | Inhibit | 76, 81 |
| Wnt8 | Inhibits | Stimulates | 3, 19, 322 |
| Wnt7a | Inhibits | – | 77 |
| Wnt9a | Inhibits | Stimulates | 96, 97, 124 |
| Wnt11 | No effect | No effect | 103 |
Furthermore, the expression patterns of Wnt proteins differ based on the proliferating cell type. Hartmann et al demonstrated that Wnt4, Wnt5a, Wnt5b, and various components of the Wnt cascade show remarkably different expression patterns in perichondrial cells versus chondrocytes (Fig. 2). Artificial induction of inappropriate Wnt signals in either cell type resulted in severe chick wing malformation.76 Other research has found that Wnt5a and Wnt11 are expressed preferentially in the perichondrium, Wnt5b in the prehypertrophic chondrocytes, and Wnt7a and Wnt8 in the mature chondrocytes.76, 98, 102, 105, 106 The highly localized and sensitive expression pattern of Wnt genes suggests that Wnt signaling is a tightly regulated and highly specific process critical for chondrogenesis.
Figure 2.
Marker/regulator gene expression in different zones of the growth plate and the perichondrium. The important and representative marker genes are listed.
The positive mediating effect of Wnt and Wnt agonists on chondrogenesis has been observed in pericytes, adult marrow stromal cells, chondroblast precursors, and other multipotent mesenchymal cell types.76, 77, 80 This has been documented to occur through the canonical pathway involving downstream stabilization of cytoplasmic β-catenin and resultant lymphoid enhancing factor (LEF)/T-cell factor (TCF)-induced gene transcription, which, among other functions, promotes chondrogenesis.107, 108, 109
Hedghog signaling pathways
Sonic hedgehog (Shh) signaling
Shh is a morphogenic protein that plays an important role in the developing limb and midline structures of the brain.110 While it is primarily known for its morphogenic properties in the developing limb cartilage, Shh remains an important regulator of chondrogenesis in adults and therefore warrants attention regarding its potential therapeutic impact. Shh signaling, along with the BMP pathway, is critically important in early chondrogenesis due to its role in determining cell fate. For example, the relative timing of Shh and BMP signaling controls whether presomitic mesoderm will adopt either a chondrogenic or lateral plate mesoderm fate. Shh, like other hedgehog proteins, signals through effector molecules known as Gli factors. These Gli factors can be silenced through a direct interaction with GATA factors, whose transcription is BMP-dependent.111 The strict regulation of this feedback loop explains why the timing of Shh and BMP signaling is crucial during the early stages of chondrogenesis.
In addition to its role in early chondrogenesis, Shh helps drive chondrogenesis through robust synergism with other prochondrogenic factors.112, 113, 114 Shh and BMPs work together to establish a positive regulatory loop with Sox9 and Nkx3.2, a transcription factor that increases Sox9 expression.115, 116, 117, 118 Along with establishing this regulatory loop, transient Shh expression increases target cellular response to BMP signaling.27, 119 In conjunction with Shh's synergistic ability, it has been shown to significantly enhance chondrogenesis in the presence of two members of the FGF family, FGF2 and FGF8.120, 121 Altogether, Shh is an important prochondrogenic factor that can have a substantial impact on cartilage tissue engineering because of its synergistic activity with many other chondrogenesis-mediating factors.
Indian hedgehog (Ihh) signaling
Ihh, another member of the mammalian hedgehog family of proteins, is encoded by a mechanosensitive gene and serves as a regulator for both osteogenesis and chondrogenesis.122 Ihh is synthesized in prehypertrophic and hypertrophic chondrocytes and couples chondrogenesis to osteogenesis through singaling mechanisms involving chondrocytes and preosteoblasts (Fig. 2).123 Expression of Ihh in prehypertrophic and hypertrophic chondrocytes is dependent on expression of Wnt9a, whose effector β-catenin/Lef1 complex interacts directly with an Ihh promoter.124 Due to its ability to drive both osteogenesis and chondrogenesis, precise homeostatic regulation of Ihh expression is crucial for promoting chondrogenesis over osteogenesis.
Although Ihh is not considered one of the master regulators of chondrogenesis, studies show that in primary MSCs it is an equally potent inducer of chondrogenesis in comparison to TGF-β1 and BMP-2. Solo Ihh expression is sufficient to drive chondrogenesis in an acute injury model, but its major prochondrogenic effects are carried out through interactions with other factors.125 For example, endogenously produced Ihh is thought to mediate TGF-β1-induced chondrogenesis in human bone marrow stromal cells (hBMSCs).126, 127 Similarly, synergistic interactions between Ihh and other central regulators of chondrogenesis have been characterized and include Runx proteins and FGFs. Runx2 and Runx3 are essential for chondrocyte maturation and regulation of limb growth, and these effects are carried out through Runx-induced expression of Ihh.128, 129 Similarly, Ihh works in conjunction with FGF8 during digit primordia elongation. Like its mammalian hedgehog family member Shh, Ihh promotes chondrocyte differentiation via expansion of Sox9 expression. Additionally, Ihh is known to upregulate the expression of FGF8 in MSCs. It is believed that these effects stem from FGF8-induced upregulation of Ihh in MSCs, creating a positive feedback loop that is antagonized by BMP signaling.130, 131
Along with interactions with major signaling pathways, Ihh expression is regulated by a variety of peptides. The most well-studied peptide interaction is the Ihh/PTHrP negative feedback loop. In the growth plate, Ihh produced by the prehypertrophic and hypertrophic chondrocytes increases expression of PTHrP, which antagonizes Ihh-mediated differentiation via a protein kinase A-mediated mechanism. This feedback loop helps to coordinate chondrocyte proliferation and differentiation, and this system is negatively regulated by retinoic acid.132, 133, 134, 135 Sirtuins (Sirts), a class of proteins with deacylase activity, have been found to enhance Ihh-mediated chondrocyte differentiation. Sirt6 knockdowns reveal markedly decreased expression of Ihh, and consequently depressed Ihh-mediated chondrocyte differentiation.136 In contrast, the oncogene delta-EF1 serves as a negative regulator of Ihh expression, implying that knockdown of delta-EF1 would actually enhance Ihh-mediated chondrocyte differentiation at the growth plate.137
Notch signaling pathway
The Notch signaling pathway is a cell signaling pathway involving one of four transmembrane notch receptors: NOTCH1-4. The Notch signaling pathway is highly conserved and it is known to play roles in many developmental and cell type specification processes.138 Previous studies have demonstrated differential expression of markers for Notch signaling during cartilage development, underscoring its importance in chondrogenesis and suggesting a role in cartilage tissue engineering.139, 140
The function of the Notch intracellular domain (NICD) is central to Notch-mediated regulation of chondrogenesis. The NICD, one of the end products of the Notch signaling pathway, translocates into the nucleus where it complexes with CSL-type DNA binding proteins CBF1(RBP-J), Su(H), and Lag-1. During this interaction, NICD converts the aforementioned DNA binding proteins from transcriptional repressors to transcriptional activators.141 These transcriptional activators go on to induce the transcription of members of the HES gene family. The HES gene family encodes for basic helix-loop-helix (bHLH) nuclear proteins that suppress transcription.142 Members of this family, HES-1 and HEY-1 act on the Sox9 binding site in the col2a1 enhancer and consequently prevent Sox9-mediated transcriptional activation of col2a1.143, 144 Thus, it is clear that Notch signaling has the ability to inhibit chondrocytic differentiation through the upregulation of HES-1 and HEY-1 activity.
Although a mechanism detailing one way Notch signaling can regulate chondrogenesis has been elucidated, there are no data which suggest a mechanism behind the differential expression of Notch receptors. NOTCH1 is the most well-studied receptor with regards to chondrogenesis and is highly expressed in mesenchymal condensations, prehypertrophic and hypertrophic zones in embryos, and the surface layer of joint cartilage in mice (Fig. 2). Likewise, NOTCH2, a modulator of NOTCH1 and NOTCH3 activity, is broadly distributed throughout proliferating, prehypertrophic, and hypertrophic chondrocytes in articular cartilage and the epiphyseal growth plate.139, 140, 145, 146 As a result of these findings, it is commonly accepted that Notch receptors can serve as markers for chondroprogenitor cells.147 This differential expression suggests a dual role for Notch signaling during the early and late stages of chondrogenesis.
Recent findings have determined that the impact of Notch signaling on chondrogenesis is conditional. This is evident by the finding that both overexpression of NICD and loss of NICD activity result in skeletal malformations due to defective endochondral ossification.148 These results suggest that Notch signaling is necessary for chondrogenesis to occur completely, although overactivity of the pathway creates inhibitory effects. We have previously discussed that increased Notch signaling can inhibit Sox9-induced chondrogenesis, but the fact that Notch receptors are highly expressed in chondroprogenitor cells implies that Notch signaling can maintain proliferating chondrocytes in their progenitor stage. Karlsson et al have confirmed this hypothesis by showing that there is high expression of HES-5 in undifferentiated chondroyctes and that downregulation of HES-5 is required for expression of genes with a hyaline phenotype.149, 150 In summary, current research suggests that transient Notch signaling is required to prime MSCs for chondrogenesis, but Notch signaling must be switched off for chondrogenesis to proceed to completion. As a result, finding ways to regulate Notch signaling activity may prove to have a significant impact on cartilage tissue engineering.
Hypoxia and angiogenic signaling pathways
Hypoxia signaling
Hypoxia-inducible factors (HIFs) are transcription factors that respond to decreases in oxygen, or hypoxia, in the cellular environment. It is well known that cartilage is a largely avascular tissue, and as a result, chondrocytes almost always reside in a state of mild hypoxia.151, 152 Severe hypoxia (1% O2 saturation) slows cell differentiation, including chondrogenesis, through decreases in gene expression secondary to cell damage.153 The link between mild hypoxia (5% O2 saturation) and the presence of chondrocytes is of tremendous importance for cartilage tissue engineering, and HIFs play a crucial role in establishing this link. Because chondrocytes primarily reside in a hypoxic environment, it is conceivable that HIFs are involved throughout the entirety of chondrogenesis. In early chondrogenesis, HIFs help maintain the chondrocyte phenotype and ensure survival of proliferating chondroprogenitor cells.154, 155, 156 HIFs also enhance chondrocyte secretion of ECM and alter gene expression in chondroprogenitor cells. Specifically, HIFs enhance expression of col2a1, Sox9, aggrecan, CD44, CD105, and PDGF receptor α; while inhibiting the expression of col1a1, col1a2, and col3a1.157, 158, 159, 160, 161, 162 There are 6 members of the HIF family, but HIF-1α and HIF-2α are the primary HIFs that have been shown to promote chondrogenesis.
There is a competing body of evidence regarding HIF-1α’s role in chondrogenesis, but many researchers have shown that HIF-1α is critical to the survival of the hypoxic chondrocyte.163, 164, 165, 166 HIF-1α accomplishes this function primarily through two mechanisms, the first of which involves interaction of HIF-1α with the cAMP response element-binding protein binding protein/p300. This complex serves as a coactivator for HIF-1α and enhances the transcription of HIF-1α’s target genes.167 The second mechanism by which HIF-1α promotes hypoxic chondrocyte survival is through upregulating expression of heat shock proteins Hsp90 and Hsp70. HIF-1α-mediated increases in Hsp90 and Hsp70 expression is also believed to be the mechanism of action of protection against chondral inflammation.168, 169 These important signaling pathways initiated by HIF-1α are stabilized in vivo by Runx2, which blocks the interaction between von Hippel-Lindau protein and HIF-1α.170
While it has been established that HIF-1α serves as the primary HIF expressed to maintain the chondrocyte phenotype, HIF-2α has emerged as the primary HIF involved in chondrocyte differentiation. HIF-2α induces chondrocyte differentiation by enhancing the transcription of many prochondrogenic genes, including the Sox trio, aggrecan, versican, Col2a1, Col9a1, Col10a1, and Col11a1.171 HIF-2α-mediated chondrogenesis is reinforced via increased activity of the PI3K-Akt signaling pathway.172
Vascular endothelial growth factor (VEGF) sigaling pathway
VEGF, which is secreted by hypertrophic chondrocytes, is a signaling protein that stimulates vasculogenesis and angiogenesis, thus making a proosteogenic factor.173, 174 As previously stated, angiogenesis reduces the severity of hypoxic states, which is needed to maintain the chondrocyte phenotype. Vascularization of cartilage tissue results in loss of phenotype, and subsequently, differentiation of chondrocytes to bone. Therefore, regulation of VEGF expression is important in maintenance of cartilage tissue.
Much of the research regarding VEGF's impact on chondrogenesis comes in the form of knockout studies. Here, we have attempted to summarize the most pertinent results. Antibodies against VEGF inhibit migration of chondroprogenitor cells in vitro. These results are also seen when chondroprogenitor cells are introduced to media containing antibodies against VEGF receptor 2, and are consistent with the findings that VEGF plays a key role in chondrocyte metabolism and accelerates the proliferation of chondroprogenitor cells in vitro.175, 176, 177, 178 In contrast, Flt-1, a soluble VEGF antagonist, has been shown to improve BMP-4- and TGF-β3-induced chondrogenesis.178, 179 Similar results were seen with administration of sFlk-1 or Bevacizumab.180
In addition to helping determine cell fate by disrupting hypoxia, VEGF is involved in different phases of cartilage tissue development. VEGF is important for the formation of blood vessels needed for limb development. In this capacity, VEGF serves as the effector molecule of Sphingosine-1-Phosphate receptor, a G-protein coupled receptor central to endothelial cell morphogenesis and vascular maturation.181 Exogenous administration of VEGF to the developing limb markedly increases blood vessel number and density, but it inhibits digit development due to repression of Sox9 expression.182 Alternatively, complete inactivation of VEGF-A in areas of col2a1 expression results in embryonic lethality.183 These results imply that a closely regulated level of VEGF expression is necessary for proper limb development, a process that includes extensive chondrogenesis.
Transcriptional regulation of chondrogenesis
SRY-box 9 (Sox9)
Sox9 is a prochondrogenic transcription factor that is arguably the single most important regulator of chondrogenesis because high levels of Sox9 are required for full expression of the chondrocyte phenotype.184 Sox9 expression is induced by compressive force: a variety of prochondrogenic factors, including RelA, Pref-1, Arib5b; and two other Sox transcription factors, Sox5 and Sox6.185, 186, 187, 188, 189, 190 As a transcription factor, Sox9's mechanism of action is directly related to the genes that it regulates. Akiyama et al have elucidated that Sox9 interacts with two binding sites for HMG-domain proteins and activates elements in the promoters of col2a1, col9a1, col11a2, Bbf2h7-sec23a, and aggrecan.191, 192, 193 Furthermore, Sox9 induces its own transcription in addition to the transcription of Sox5 and Sox6, forming the Sox Trio. The individual components of the Sox Trio act cooperatively to increase the expression of the previously discussed gene targets of Sox9.191, 194, 195, 196
Sox9 primarily regulates chondrocyte proliferation and differentiation through direct control of the expression of many chondrocyte-specific genes. In addition to this observation, many studies have elucidated Sox9's role in earlier phases of chondrogenesis, such as condensation. Numerous studies show that Sox9 expression levels are markedly elevated in condensing mesenchymal progenitors, both in vitro and in vivo, while Sox9 knockout mice are unable to condensate mesenchymal cells.197, 198, 199 As previously stated, FGF2 induces chondrocyte proliferation by upregulating basal expression levels of Sox9.200, 201 Sox9 then potentiates BMP2's prochondrogenic properties and inhibits BMP2-induced osteogenic differentiation and endochondral ossification.202 While this relationship has been well studied, it is only one of many Sox9 interactions that help regulate chondrogenesis.
Sox9's ability to promote chondrocyte differentiation and maturation is dependent upon the expression of coactivators of Sox9-mediated transcription and the silencing of factors that stimulate osteogenesis. Many coactivators of Sox9 expression have been identified in MSCs, including Paraspeckle regulatory protein 54-kDa nuclear RNA-binding protein (p54nrb), Smad proteins 2 and 3, peroxisome proliferator-activated receptor γ co-activator 1α (PGC-1α), Tip60, Lc-Maf, CypA, Scleraxis/E47, and p300.203, 204, 205, 206, 207, 208, 209 Having so many coactivators most likely plays a role in fine-tuning local expression levels of Sox9, which may prove advantageous within the field of cartilage tissue engineering. Sox9's major antagonist is Runx2, the master regulator of osteogenesis. Expression of Sox9 inhibits early and late osteogenic differentiation by depressing the expression of Runx2, as well as osterix, a transcription factor for osteogenic differentiation, col1a1, and col10a1.197, 202, 210 In addition to regulating the expression of proosteogenic transcription factors and genes, Sox9 also targets Wnt/β-catenin promoters for degradation by the ubiquitination/proteasome system.198, 211 Finally, Sox9 activity can be blunted by its two primary negative regulators, Runx2 and RhoA protein.211, 212
Runt-related transcription factors (Runx)
Runx proteins, including Runx1, Runx2, and Runx3, are a family of transcription factors that play significant roles in organogenesis and embryonic patterning. While all 3 members of the Runx protein family are expressed during chondrogenesis, Runx1 is believed to have a minimal effect on chondrogenesis and will not be discussed in this review.213
Runx2 and Runx3 are essential for chondrocyte maturation, but Runx2 serves a secondary role as a potent inducer of osteogenesis. The regulation of osteogenesis and chondrocyte proliferation by Runx2 is accomplished through an interaction with PI3K-Akt signaling.214 Regulatory control of Runx2 is handled primarily by Sox9, as stated previously, helping to explain Sox9's apparent dominance over Runx2 in MSCs destined for a chondrogenic lineage.215 This interaction is further complicated by interactions with CypA, a positive regulator of both Runx2 and Sox9 expression in chondroprogenitor cells. Predictably, CypA knockdowns have depressed chondrogenesis and endochondral ossification.208 Nkx3.2 serves as another method for regulatory control of Runx2. Nkx3.2 represses Runx2 activity through direct interaction with the Runx2 promoter, and this repression is required for the progression of BMP-induced chondrogenesis.216, 217
The regulation of Runx2 expression is of extreme importance with regards to chondrogenesis, given the variety of effects Runx2 expression can have on MSCs. Col10a1, a type of collagen expressed in hypertrophic chondrocytes destined for endochondral ossification, is a direct transcriptional target of Runx2.218 Runx2's ability to drive osteogenesis is most likely related to its ability to cooperate with HIFs to stimulate angiogenesis. As already discussed, angiogenesis decreases the severity of local hypoxia which inhibits chondrogenesis while promoting osteogenesis.219 In addition to driving osteogenesis, Runx2 has also been shown to induce adipogenic differentiation in vitro.220 Finally, Hinoi et al have shown that Runx2 enhances expression of FGF18, a negative regulator of chondrocyte maturation, in the perichondrium.221
Trico-rhino phalangeal syndrome type 1(Trps1)
Trps1 is a GATA-like transcription factor that serves as a novel regulator of chondrocyte differentiation and proliferation through regulation of a variety of genes. As a GATA-like transcription factor, Trps1 acts as a downstream effector of the Gdf5 signaling pathway to promote both differentiation and apoptosis.222 Trps1's versatility as a transcription factor is linked to its ability to effect expression levels of key regulators, like Sox9, as well as less researched gene targets, such as Stat3.223, 224 In addition to its function as a transcription factor, Trsp1 also interacts with many microRNAs (miRs) and other signaling pathways. Specifically, Trps1 is responsible for maintaining low levels of miR-221, which allows MSCs to commit toward the chondrocyte lineage.225 The interaction between Trps1 and miR-221 may be a part of a feedback loop, as it has been shown that Trps1 expression is regulated by seven Runx2-targeting miRs (miRs-23a, -30c, -34c, -133a, -135a, -205, and -217).226 Furthermore, Trps1 interacts with Ihh/Gli3 signaling, as evidenced by studies in which Trps1 knockout mice overexpress PTHrP and inhibit Ihh-mediated chondrogenesis via the Ihh/PTHrP negative feedback loop.227, 228
MIA/CD-RAP in chondrogenic differentiation
The MIA/CD-RAP gene is a gene locus that encodes for the melanoma inhibitory activity protein (MIA) and the cartilage-derived retinoic acid-sensitive protein (CD-RAP). Normal expression of this gene locus is limited to cartilage, but pathological expression does occur in melanoma and chondrosarcoma.229 Given the cartilage-selective expression of the MIA/CD-RAP gene, it makes sense that these proteins are noteworthy regulators of chondrogenesis.
Regulation of chondrogenesis by MIA/CD-RAP occurs through two primary mechanisms. First, transcription factor YBX1 has been shown to mediate MIA/CD-RAP-dependent activation of p54nrb transcription.230 Second, consistent data support the concept of MIA/CD-RAP-mediated inhibition of ERK signaling. ERK is known to block chondrogenic differentiation, and its inhibition helps drive chondrogenesis.231 Through these two mechanisms, it has become clear that MIA/CD-RAP proteins are required for chondrocyte differentiation. This is confirmed by the finding that MIA knockout mice show enhanced chondrocyte proliferation but delayed and defective differentiation, which can be rescued by decreasing AP1 and CRE activity.230, 232 Given the close proximity of the MIA and CD-RAP genes, alterations in transcription often change the expression of both proteins. Therefore, interactions involving the promoter region of MIA/CD-RAP have a significant impact on the regulation of chondrogenesis. Known regulators of MIA/CD-RAP expression include delta-EF1, USF1/USF2, Sox9, retinoic acid, AP-2, and HMGA. Sox9 serves as a positive regulator, while the others serve as negative regulators.233, 234, 235, 236
Epigenetic regulations of chrondrogenesis
Histone deacetylase superfamily
Histone deacetylases (HDACs) are a class of enzymes that remove acetyl groups from histone proteins, allowing higher affinity DNA binding and consequent inhibition of transcription. Therefore, it is no surprise that HDACs primarily function as repressors of the transcription of prochondrogenic genes. For example, HDAC1 and HDAC2 repress the expression of cartilage-specific genes in human chondrocytes.237 Furthermore, LRF-mediated repression of COMP gene expression and chondrocyte differentiation requires HDAC1 activity.238 HDACs are also known negative regulators of osteogenesis. Specifically, inhibition of HDAC activity potentiates BMP9-induced osteogenic signaling in mouse MSCs and attenuates chondrocyte proliferation and differentiation.239, 240, 241, 242 Similarly, HDAC4 overexpression promotes chondrogenesis while inhibiting chondrocyte hypertrophy via modulating TGF-β1 activity.243 Negative regulation of osteogenesis by HDACs may help explain these findings.
microRNAs (miRs)
MicroRNAs are small, non-coding RNA fragments whose function involves mRNA silencing and post-transcriptional gene regulation. Since their discovery in the early 1990's, it has been well known that microRNAs are important for developmental timing in both vertebrates and invertabrates.244, 245 More recent findings have confirmed this notion, showing that universal disruption of Dicer, a RNA endonuclease required for microRNA production, results in embryonic lethality and depletion of pluripotent stem cells.246, 247 These findings, among others, have sparked significant research interest in the role of microRNAs in chondrogenesis and their potential therapeutic effects.
MicroRNAs have well characterized effects on both pre- and post-natal chondrogenesis. Pre-natally, microRNA expression profiles are extremely important for the development of cartilaginous tissues, such as the pharyngeal arches. For example, Weinholds et al have shown that 10 microRNAs (miRs-23a, -27a, -27b, -140, -140*, -145, -146, -199a, -199a*, and -214) were specifically expressed in the developing pharyngeal arches of zebrafish embryos.248 In addition, post-natal expression profiles of microRNAs and their effects on chondrogenesis are currently large areas of research. Studies of many cell types have been conducted, and the results are much too expansive to be detailed in this review. With this in mind, we have organized what we believe to be the most compelling results into Table 2.
Table 2.
List of known microRNA expression in human and mouse MSCs undergoing chondrogenesis.
| microRNA | Expression | References |
|---|---|---|
| miR-26b | Upregulated | 323 |
| miR-28 | Upregulated | 323 |
| miR-130b | Upregulated | 323 |
| miR-152 | Upregulated | 323 |
| miR-193b | Upregulated | 323 |
| miR-15b | Upregulated | 324 |
| miR-16 | Upregulated | 324 |
| miR-23b | Upregulated | 3, 25, 325 |
| miR-27b | Upregulated | 324 |
| miR-140 | Upregulated | 324 |
| miR-148 | Upregulated | 324 |
| miR-197 | Upregulated | 324 |
| miR-222 | Upregulated | 324 |
| miR-328 | Upregulated | 324 |
| miR-505 | Upregulated | 324 |
| miR-127 | Upregulated | 326 |
| miR-140 | Upregulated | 326 |
| miR-125b* | Upregulated | 326 |
| miR-99a | Upregulated | 326 |
| miR-140* | Upregulated | 326 |
| miR-181a-1 | Upregulated | 326 |
| let-7f | Upregulated | 326 |
| miR-30a | Upregulated | 326 |
| miR-24 | Upregulated | 327 |
| miR-101 | Upregulated | 327 |
| miR-124a | Upregulated | 327 |
| miR-199a | Upregulated | 3, 28, 328 |
| miR-199b | Upregulated | 327 |
| miR-130b | Upregulated | 323 |
| miR-193b | Upregulated | 323 |
| miR-455 | Upregulated | 329 |
| miR-337 | Upregulated | 330 |
| miR-574-3p | Upregulated | 331 |
| miR-212 | Downregulated | 326 |
| miR-132 | Downregulated | 326 |
| miR-143 | Downregulated | 326 |
| miR-125b-3p | Downregulated | 326 |
| miR-18 | Downregulated | 326 |
| miR-96 | Downregulated | 326 |
| miR-145 | Downregulated | 332 |
| miR-490-5p | Downregulated | 333 |
| miR-124 | Downregulated | 328 |
| miR-199* | Downregulated | 334, 335 |
A large number of microRNAs have been implicated in regulating chondrogenesis, but three major target genes have been implicated in mediating their effects: C/EBPb, master regulator Sox9, and Adam9. C/EBPb is believed to protect articular cartilage from degenerative diseases such as osteoarthritis, and it has been shown to be negatively regulated by several miRNAs during chondrogenic differentiation of hADSCs.249 Sox9 and Adam9 expressivity are similarly regulated by a variety of microRNAs. Adam9 is a negative regulator of chondrogenesis, and its upregulation could lead to apoptosis of chondrogenic progenitors and inhibition of MSC migration.250
Implications in cartilage regeneration and tissue engineering
Cartilage tissue engineering is an area of significant research amongst engineers and physicians. This research is of significant therapeutic importances given the high prevalence of osteochondrodysplasias, including but not limited to achondroplasia, cleidocranial dysostosis, and ovine chondrodysplasia. Over the past decade, cartilage tissue engineering has become dependent upon the selection of an appropriate cell line, subsequent chondrogenic differentiation of the selected cell line, and the fabrication of a biocompatible and mechanically suitable scaffold.251 Given these challenges, current progress has been unable to show significant improvement in tissue restoration over proven surgical techniques. Here, we review the most recent advances in cartilage tissue engineering regarding overcoming these challenges.
An appropriate cell line for cartilage tissue engineering must have the ability to differentiate into mature, fully functional cartilage and maintain a chondrocyte phenotype long-term. A clear starting point was chondrocytes extracted from the patient, which have the ability to maintain a chondrocyte phenotype and remodel. Unfortunately, these cells are limited in number, and their expansion leads to dedifferentiation with resultant phenotypic loss.251 Other cell types explored as candidates for cartilage tissue engineering include human MSCs (hMSCs), adipose-derived stem cells, and embryonic stem cells. Adipose-derived stem cells and embryonic stem cells have the ability to undergo chondrogenic differentiation in response to certain signals, but they are limited by specific growth requirements and ethical controversy, respectively.252, 253 hMSCs are the most promising candidate, and use of combinations of growth factors in hMSCs is the current gold standard for scaffold-based cartilage tissue engineering.251, 254, 255
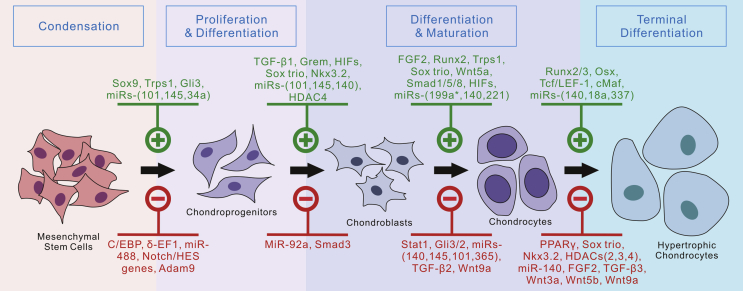
The many biological regulators of chondrogenesis, many of which are detailed in this review, provide various ways to induce chondrogenic differentiation in progenitor cells (Fig. 3). Current research suggests that among the many growth factors implicated to have a regulatory effect on chondrogenesis, TGF-β proteins are the most potent inducers of chondrogenesis in hMSCs. In contrast, other growth factors, including insulin-like growth factors (IGFs), FGFs, and PDGFs, appear to mediate chondrocytic physiology to a greater degree than they promote chondrogenesis in hMSCs.19, 61, 254, 255, 256, 257, 258, 259, 260, 261, 262, 263, 264, 265, 266, 267, 268, 269, 270, 271 In addition to members of the TGF-β family of proteins, VEGF, hypoxia, and Sox9 have been shown to induce chondrocytic differentiation in a variety of 3D scaffolds.272, 273, 274, 275, 276 The challenge in using signaling molecules to optimize cartilage regeneration is that their regulatory effects depend on a large number of factors that must be tightly controlled: the dosing and timing of administration, the cell type, and the medium in which they act. This phenomenon could help explain the variability in the results regarding the effects of different growth factors in cartilage regeneration. Researchers circumvent this inconsistency by infecting target cells with adenoviruses expressing a gene of interest and through the use of gene therapy.277, 278, 279, 280, 281
Figure 3.
Currently identified regulators of chondrogenic differentiation from MSCs. Currently reported signaling molecules, transcription factors, microRNAs and other regulators are listed. Positive regulators are shown on the top panel in green, while the inhibitory regulators are shown in red at the bottom panel.
While current research suggests that administration of biologic agents can delay progression of articular cartilage degeneration in an experimental setting, few are available clinically, necessitating consideration for surgical treatment. Although total joint arthroplasty may be the ultimate end point in many cases, early surgical treatment options include microfracture, osteochondral auto- or allograft transfer, and autologous chondrocyte implantation (ACI). The determination of which method provides the ideal solution depends on the size and location of the lesion, patient age and activity level, and concomitant pathology. In ACI, a full-thickness sample from a low-weight-bearing region of the joint is collected by punch biopsy and then implanted into the debrided cartilage defect and covered by a membrane. When successful, this technique can produce a greater amount of hyaline-like cartilage at the repair site. Limitations of the technique include the need for two separate surgical procedures, a prolonged recovery time, and increased procedural complexity.282 In addition, the procedure has a comparatively high reoperation rate and cost is prohibitive for many patients. Other early surgical interventions, including matrix-induced autologous chondrocyte implantation (MACI), have had promising histological results, but superiority over ACI remains unproven.283, 284
The function of a tissue engineering scaffold is to provide a temporary structure on which cells seeded within the biodegradable matrix synthesize new, natural tissue. Scaffold-based techniques are preferred over scaffold-free approaches because they increase control over filling defects, eliminate the complications associated with a surgical donor site, shorten recovery time, and are associated with a reduced risk of dedifferentiation.285 Ex vivo studies have shown that successful cartilage regeneration is dependent on both the chondrocyte proliferation rate and on the differentiation capacity of stem cells within a 3D scaffold. Taken together with evidence that cartilage production varies with scaffold architecture, the importance of choosing the appropriate scaffold for cartilage tissue engineering is clear.286, 287, 288 The two most well-studied types of scaffolds with regards to cartilage tissue engineering are poly(α-hydroxy esters) and hydrogels. Poly(α-hydroxy esters) are the most commonly used synthetic polymers for cartilage tissue engineering, while poly(lactic acid) (PLA) and poly(lactic-co-glycolic acid) (PLGA) are the most investigated. Both in vitro and in vivo studies have demonstrated that progenitor cells cultured on PLA or PLGA scaffolds retain chondrocyte phenotype and produce cartilage-specific ECM.289, 290, 291, 292, 293, 294, 295, 296, 297 In contrast, hydrogels are cross-linked polymer networks that have the ability to absorb large amounts of water, making them attractive scaffolds for engineering tissues with high water content, such as cartilage.251 Despite theoretical strengths in the design, use of hydrogel scaffolds has failed to improve clinical outcomes when compared to the less expensive poly(α-hydroxy esters).
Recently, there has been a growing interest in the understanding of the mechanobiology of MSCs, given that mechanical factors are known to influence the structure and function of musculoskeletal tissues at all stages of life.298, 299 A significant proportion of the current research in cartilage tissue engineering is directed at discerning how mechanical stimulation induces chondrogenesis in chondroprogenitor cells, and how scientists can use these signals to drive cartilage tissue production. However, the molecular mechanisms of mechanical signal transduction in chondrocytes and chondroprogenitor cells are still unclear. Current theories implicate ion channels, primary cilia, the nucleus, and the cytoskeleton as potential transducers of mechanical signals in these cells.300, 301, 302, 303 Regardless of the molecular mechanism, it has been well established that a variety of forms of mechanical stimulation in MSCs can produce a prochondrogenic state that is favorable for cartilage regeneration and repair. For example, Choi et al showed that low-intensity ultrasound enhanced chondrogenesis in MSCs cultured in fibrin-hyaluronic acid.304 Likewise, Kupcsik et al showed that mechanical stimulation of multipotent stem cells enhanced collagen type X and lubricin expression.305
Another method employed by researchers to overcome the previously described challenges of cartilage tissue engineering is the use of bioreactor systems to enhance the growth of cartilaginous constructs. Bioreactor systems have emerged as valuable enhancers of cartilage tissue engineering because they accelerate chondrocyte growth by supplying nutrients or mechanical stimulation.251 Bioreactor systems come in two forms: loading reactors and hydrodynamic reactors. Loading reactors, hydrodynamic reactors, and combinations of the two have all been shown to expedite the rate and quality of cartilaginous tissue growth.263, 297, 306, 307, 308, 309, 310, 311, 312 Promising results in cartilage tissue engineering have been achieved through the utilization of self-assembling MSCs in a bioreactor model. Self-assembling MSCs were directed to form condensed mesenchymal bodies (CMBs) and were fused together with the aid of press molding.313, 314, 315, 316, 317 This novel approach has enabled a breakthrough in the ability to engineer human cartilage from MSCs with the same physiological Young modulus and friction coefficient.313
Concluding remarks and future directions
Regulation of chondrogenesis and cartilage tissue engineering remain areas of significant research given the increasing prevalence of osteodegenerative disease and orthopedic trauma. Cartilage tissue engineering has been complicated by three main challenges: production of a chondrogenic progenitor cell line that can proliferate and differentiate into mature chondrocytes, finding biological factors that can facilitate the proliferation and differentiation of chondrogenic seed cells, and finding biomaterial scaffolds that provide a 3D architecture resembling the cartilaginous matrix. While many biological regulators have been identified, implantation of chondrocytes grown in vitro has failed to yield significant clinical results. The application of bioreactor systems, which allows scientists and physicians to deliver nutrients to a largely avascular tissue, has yielded the most promising results and appears to be the future of cartilage tissue engineering.
Conflict of interest
The authors declare no conflict of interest.
Acknowledgments
The authors' laboratories were supported in part by research grants from the National Institutes of Health (AR50142, AR054381, and AT004418 to RCH, HHL, and TCH) and Scoliosis Research Society (MJL). JDG and VT were recipients of the Pritzker Summer Research Fellowship funded through a NIH T-35 training grant (NIDDK). MKM was a recipient of Howard Hughes Medical Institute Medical Research Fellowship. Funding sources were not involved in the study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Footnotes
Peer review under responsibility of Chongqing Medical University.
References
- 1.Kurtz S., Ong K., Lau E. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89:780–785. doi: 10.2106/JBJS.F.00222. [DOI] [PubMed] [Google Scholar]
- 2.Martin G.R. The roles of FGFs in the early development of vertebrate limbs. Genes Dev. 1998;12:1571–1586. doi: 10.1101/gad.12.11.1571. [DOI] [PubMed] [Google Scholar]
- 3.Barrow J.R., Thomas K.R., Boussadia-Zahui O. Ectodermal Wnt3/beta-catenin signaling is required for the establishment and maintenance of the apical ectodermal ridge. Genes Dev. 2003;17:394–409. doi: 10.1101/gad.1044903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ohuchi H., Nakagawa T., Yamamoto A. The mesenchymal factor, FGF10, initiates and maintains the outgrowth of the chick limb bud through interaction with FGF8, an apical ectodermal factor. Development. 1997;124:2235–2244. doi: 10.1242/dev.124.11.2235. [DOI] [PubMed] [Google Scholar]
- 5.Handorf A.M., Li W.J. Fibroblast growth factor-2 primes human mesenchymal stem cells for enhanced chondrogenesis. PLoS One. 2011;6:e22887. doi: 10.1371/journal.pone.0022887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Solchaga L.A., Penick K., Porter J.D. FGF-2 enhances the mitotic and chondrogenic potentials of human adult bone marrow-derived mesenchymal stem cells. J Cell Physiol. 2005;203:398–409. doi: 10.1002/jcp.20238. [DOI] [PubMed] [Google Scholar]
- 7.Ito T., Sawada R., Fujiwara Y., Tsuchiya T. FGF-2 increases osteogenic and chondrogenic differentiation potentials of human mesenchymal stem cells by inactivation of TGF-beta signaling. Cytotechnology. 2008;56:1–7. doi: 10.1007/s10616-007-9092-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oseni AO, Crowley C, Boland MZ, et al. Cartilage tissue engineering: the application of nanomaterials and stem cell technology. Tissue Eng Regen Med. 201:doi:10.5772/22453.
- 9.Correa D., Somoza R.A., Lin P. Sequential exposure to fibroblast growth factors (FGF) 2, 9 and 18 enhances hMSC chondrogenic differentiation. Osteoarthr Cartil. 2015;23:443–453. doi: 10.1016/j.joca.2014.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hellingman C.A., Koevoet W., Kops N. Fibroblast growth factor receptors in in vitro and in vivo chondrogenesis: relating tissue engineering using adult mesenchymal stem cells to embryonic development. Tissue Eng Part A. 2010;16:545–556. doi: 10.1089/ten.TEA.2008.0551. [DOI] [PubMed] [Google Scholar]
- 11.Stevens M.M., Marini R.P., Martin I. FGF-2 enhances TGF-β1-induced periosteal chondrogenesis. J Orthop Res. 2004;25:1114–1119. doi: 10.1016/j.orthres.2003.12.021. [DOI] [PubMed] [Google Scholar]
- 12.Richter W., Bock R., Hennig T., Weiss S. Influence of FGF-2 and PTHrP on chondrogenic differentiation of human mesenchymal stem cells. J Bone Jt Surg Br. 2009;91-B(suppl III):444. [Google Scholar]
- 13.Narcisi R., Cleary M.A., Brama P.A. Long-term expansion, enhanced chondrogenic potential, and suppression of endochondral ossification of adult human MSCs via WNT signaling modulation. Stem Cell Rep. 2015;4:459–472. doi: 10.1016/j.stemcr.2015.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ng F., Boucher S., Koh S. PDGF, TGF-beta, and FGF signaling is important for differentiation and growth of mesenchymal stem cells (MSCs): transcriptional profiling can identify markers and signaling pathways important in differentiation of MSCs into adipogenic, chondrogenic, and osteogenic lineages. Blood. 2008;112:295–307. doi: 10.1182/blood-2007-07-103697. [DOI] [PubMed] [Google Scholar]
- 15.Dai J., Wang J., Lu J. The effect of co-culturing costal chondrocytes and dental pulp stem cells combined with exogenous FGF9 protein on chondrogenesis and ossification in engineered cartilage. Biomaterials. 2012;33:7699–7711. doi: 10.1016/j.biomaterials.2012.07.020. [DOI] [PubMed] [Google Scholar]
- 16.Hung I.H., Yu K., Lavine K.J., Ornitz D.M. FGF9 regulates early hypertrophic chondrocyte differentiation and skeletal vascularization in the developing stylopod. Dev Biol. 2007;307:300–313. doi: 10.1016/j.ydbio.2007.04.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Govindarajan V., Overbeek P.A. FGF9 can induce endochondral ossification in cranial mesenchyme. BMC Dev Biol. 2006;6:7. doi: 10.1186/1471-213X-6-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu Z., Xu J., Colvin J.S., Ornitz D.M. Coordination of chondrogenesis and osteogenesis by fibroblast growth factor 18. Genes Dev. 2002;16:859–869. doi: 10.1101/gad.965602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Davidson D., Blanc A., Filion D. Fibroblast growth factor (FGF) 18 signals through FGF receptor 3 to promote chondrogenesis. J Biol Chem. 2005;280:20509–20515. doi: 10.1074/jbc.M410148200. [DOI] [PubMed] [Google Scholar]
- 20.Moore E.E., Bendele A.M., Thompson D.L. Fibroblast growth factor-18 stimulates chondrogenesis and cartilage repair in a rat model of injury-induced osteoarthritis. Osteoarthr Cartil. 2005;13:623–631. doi: 10.1016/j.joca.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 21.Goldring M., Tsuchimochi K., Ijiri K. The control of chondrogenesis. J Cell Biochem. 2006;97:33–44. doi: 10.1002/jcb.20652. [DOI] [PubMed] [Google Scholar]
- 22.Cleary M.A., van Osch G.J., Brama P.A. FGF, TGFβ and Wnt crosstalk: embryonic to in vitro cartilage development from mesenchymal stem cells. J Tissue Eng Regen. 2015;9:332–342. doi: 10.1002/term.1744. [DOI] [PubMed] [Google Scholar]
- 23.Carrington J.L., Chen P., Yanagishita M., Reddi A.H. Osteogenin (bone morphogenetic protein-3) stimulates cartilage formation by chick limb bud cells in vitro. Dev Biol. 1991;146:406–415. doi: 10.1016/0012-1606(91)90242-u. [DOI] [PubMed] [Google Scholar]
- 24.Leonard C.M., Fuld H.M., Frenz D.A. Role of transforming growth factor-β in chondrogenic pattern formation in the embryonic limb: stimulation of mesenchymal condensation and fibronectin gene expression by exogenenous TGF-β and evidence for endogenous TGF-β-like activity. Dev Biol. 1991;145:99–109. doi: 10.1016/0012-1606(91)90216-p. [DOI] [PubMed] [Google Scholar]
- 25.Chimal-Monroy J., Díaz de León L. Differential effects of transforming growth factors beta 1, beta 2, beta 3 and beta 5 on chondrogenesis in mouse limb bud mesenchymal cells. Int J Dev Biol. 1997;41:91–102. [PubMed] [Google Scholar]
- 26.Merino R., Ganan Y., Macias D. Morphogenesis of digits in the avian limb is controlled by FGFs, TGFβs, and Noggin through BMP signaling. Dev Biol. 1998;200:35–45. doi: 10.1006/dbio.1998.8946. [DOI] [PubMed] [Google Scholar]
- 27.Karamboulas K., Dranse H.J., Underhill T.M. Regulation of BMP-dependent chondrogenesis in early limb mesenchyme by TGFβ signals. J Cell Sci. 2010;123:2068–2076. doi: 10.1242/jcs.062901. [DOI] [PubMed] [Google Scholar]
- 28.Joyce M.E., Roberts A.B., Sporn M.B., Bolander M.E. Transforming growth factor-beta and the initiation of chondrogenesis and osteogenesis in the rat femur. J Cell Biol. 1990;110:2195–2207. doi: 10.1083/jcb.110.6.2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Canalis E., McCarthy T., Centrella M. Growth factors and the regulation of bone remodeling. J Clin Invest. 1988;81:277–281. doi: 10.1172/JCI113318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Centrella M., McCarthy T.L., Canalis E. Transforming growth factor beta is a bifunctional regulator of replication and collagen synthesis in osteoblast-enriched cell cultures from fetal rat bone. J Biol Chem. 1987;262:2869–2874. [PubMed] [Google Scholar]
- 31.Robey P.G., Young M.F., Flanders K.C. Osteoblasts synthesize and respond to transforming growth factor-type beta (TGF-beta) in vitro. J Cell Biol. 1987;105:457–463. doi: 10.1083/jcb.105.1.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Seyedin S.M., Thomas T.C., Thompson A.Y. Purification and characterization of two cartilage-inducing factors from bovine demineralized bone. Proc Natl Acad Sci U. S. A. 1985;82:2267–2271. doi: 10.1073/pnas.82.8.2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Heine U., Munoz E.F., Flanders K.C. Role of transforming growth factor-beta in the development of the mouse embryo. J Cell Biol. 1987;105:2861–2876. doi: 10.1083/jcb.105.6.2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Joyce M.E. Expression and localization of transforming growth factor-beta in a model of fracture healing. Orthop Trans. 1989;13:460–461. [Google Scholar]
- 35.Sandberg M., Autio-Harmainen H., Vuorio E. Localization of the expression of types I, III, and IV collagen, TGF-beta 1 and c-fos genes in developing human calvarial bones. Dev Biol. 1998;130:324–334. doi: 10.1016/0012-1606(88)90438-1. [DOI] [PubMed] [Google Scholar]
- 36.Carrington J.L., Roberts A.B., Flanders K.C. Accumulation, localization, and compartmentation of transforming growth factor beta during endochondral bone development. J Cell Biol. 1988;107:1969–1975. doi: 10.1083/jcb.107.5.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Long F., Ornitz D.M. Development of the endochondral skeleton. Cold Spring Harb Perspect Biol. 2013;5:a008334. doi: 10.1101/cshperspect.a008334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hidaka C., Goldring M.B. Regulatory mechanisms of chondrogenesis and implications for understanding articular cartilage homeostasis. Curr Rheumatol Rev. 2008;4:136–147. [Google Scholar]
- 39.Yoon B.S., Ovchinnikov D.A., Yoshii I. Bmpr1a and Bmpr1b have overlapping functions and are essential for chondrogenesis in vivo. Proc Natl Acad Sci U. S. A. 2005;102:5062–5067. doi: 10.1073/pnas.0500031102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Augustyniak E., Trzeciak T., Richter M. The role of growth factors in stem cell-directed chondrogenesis: a real hope for damaged cartilage regeneration. Int Orthop. 2015;39:995–1003. doi: 10.1007/s00264-014-2619-0. [DOI] [PubMed] [Google Scholar]
- 41.Madry H., Rey-Rico A., Venkatesan J.K. Transforming growth factor Beta-releasing scaffolds for cartilage tissue engineering. Tissue Eng Part B Rev. 2014;20:106–125. doi: 10.1089/ten.TEB.2013.0271. [DOI] [PubMed] [Google Scholar]
- 42.Coleman C.M. Tuan RS. Growth/differentiation factor 5 enhances chondrocyte maturation. Dev Dyn Off Publ Am Assoc Anat. 2003;228:208–216. doi: 10.1002/dvdy.10369. [DOI] [PubMed] [Google Scholar]
- 43.Francis-West P.H., Richardson M.K., Bell E. The effect of overexpression of BMPs and GDF-5 on the development of chick limb skeletal elements. Ann N. Y Acad Sci. 1996;785:254–255. doi: 10.1111/j.1749-6632.1996.tb56276.x. [DOI] [PubMed] [Google Scholar]
- 44.Francis-West P.H., Parish J., Lee K., Archer C.W. BMP/GDF-signalling interactions during synovial joint development. Cell Tissue Res. 1999;296:111–119. doi: 10.1007/s004410051272. [DOI] [PubMed] [Google Scholar]
- 45.Storm E.E., Kingsley D.M. GDF5 coordinates bone and joint formation during digit development. Dev Biol. 1999;209:11–27. doi: 10.1006/dbio.1999.9241. [DOI] [PubMed] [Google Scholar]
- 46.Feng X.H., Derynck R. Specificity and versatility in tgf-beta signaling through Smads. Annu Rev Cell Dev Biol. 2005;21:659–693. doi: 10.1146/annurev.cellbio.21.022404.142018. [DOI] [PubMed] [Google Scholar]
- 47.Massagué J., Seoane J., Wotton D. Smad transcription factors. Genes Dev. 2005;19:2783–2810. doi: 10.1101/gad.1350705. [DOI] [PubMed] [Google Scholar]
- 48.Iwai T., Murai J., Yoshikawa H., Tsumaki N. Smad7 Inhibits chondrocyte differentiation at multiple steps during endochondral bone formation and down-regulates p38 MAPK pathways. J Biol Chem. 2008;283:27154–27164. doi: 10.1074/jbc.M801175200. [DOI] [PubMed] [Google Scholar]
- 49.Tuli R., Tuli S., Nandi S. Transforming growth factor-beta-mediated chondrogenesis of human mesenchymal progenitor cells involves N-cadherin and mitogen-activated protein kinase and Wnt signaling cross-talk. J Biol Chem. 2003;278:41227–41236. doi: 10.1074/jbc.M305312200. [DOI] [PubMed] [Google Scholar]
- 50.Zhang Y.E. Non-Smad pathways in TGF-beta signaling. Cell Res. 2009;19:128–139. doi: 10.1038/cr.2008.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mu Y., Gudey S.K., Landström M. Non-Smad signaling pathways. Cell Tissue Res. 2012;347:11–20. doi: 10.1007/s00441-011-1201-y. [DOI] [PubMed] [Google Scholar]
- 52.Barna M., Niswander L. Visualization of cartilage formation: insight into cellular properties of skeletal progenitors and chondrodysplasia syndromes. Dev Cell. 2007;12:931–941. doi: 10.1016/j.devcel.2007.04.016. [DOI] [PubMed] [Google Scholar]
- 53.Pizette S., Niswander L. BMPs are required at two steps of limb chondrogenesis: formation of prechondrogenic condensations and their differentiation into chondrocytes. Dev Biol. 2000;219:237–249. doi: 10.1006/dbio.2000.9610. [DOI] [PubMed] [Google Scholar]
- 54.Lorda-Diez C.I., Montero J.A., Garcia-Porrero J.A., Hurle J.M. Tgfbeta2 and 3 are coexpressed with their extracellular regulator Ltbp1 in the early limb bud and modulate mesodermal outgrowth and BMP signaling in chicken embryos. BMC Dev Biol. 2010;10:69. doi: 10.1186/1471-213X-10-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Merino R., Rodriguez-Leon J., Macias D. The BMP antagonist Gremlin regulates outgrowth, chondrogenesis and programmed cell death in the developing limb. Dev Camb Engl. 1999;126:5515–5522. doi: 10.1242/dev.126.23.5515. [DOI] [PubMed] [Google Scholar]
- 56.Hofmann C., Luo G., Balling R., Karsenty G. Analysis of limb patterning in BMP-7-deficient mice. Dev Genet. 1996;19:43–50. doi: 10.1002/(SICI)1520-6408(1996)19:1<43::AID-DVG5>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 57.Niswander L., Tickle C., Vogel A. FGF-4 replaces the apical ectodermal ridge and directs outgrowth and patterning of the limb. Cell. 1993;75:579–587. doi: 10.1016/0092-8674(93)90391-3. [DOI] [PubMed] [Google Scholar]
- 58.Ito T., Sawada R., Fujiwara Y., Seyama Y., Tsuchiya T. FGF-2 suppresses cellular senescence of human mesenchymal stem cells by down-regulation of TGF-beta2. Biochem Biophys Res Commun. 2007;359:108–114. doi: 10.1016/j.bbrc.2007.05.067. [DOI] [PubMed] [Google Scholar]
- 59.Sawada R., Ito T., Tsuchiya T. Changes in expression of genes related to cell proliferation in human mesenchymal stem cells during in vitro culture in comparison with cancer cells. J Artif Organs Off J Jpn Soc Artif Organs. 2006;9:179–184. doi: 10.1007/s10047-006-0338-z. [DOI] [PubMed] [Google Scholar]
- 60.Rosen D.M., Stempien S.A., Thompson A.Y. Differentiation of rat mesenchymal cells by cartilage-inducing factor. Enhanced phenotypic expression by dihydrocytochalasin B. Exp Cell Res. 1986;165:127–138. doi: 10.1016/0014-4827(86)90538-0. [DOI] [PubMed] [Google Scholar]
- 61.Johnstone B., Hering T.M., Caplan A.I. In vitro chondrogenesis of bone marrow-derived mesenchymal progenitor cells. Exp Cell Res. 1998;238:265–272. doi: 10.1006/excr.1997.3858. [DOI] [PubMed] [Google Scholar]
- 62.Tufan A.C., Tuan R.S. Wnt regulation of limb mesenchymal chondrogenesis is accompanied by altered N-cadherin-related functions. FASEB J Off Publ Fed Am Soc Exp Biol. 2001;15:1436–1438. doi: 10.1096/fj.00-0784fje. [DOI] [PubMed] [Google Scholar]
- 63.Tufan A.C., Daumer K.M., DeLise A.M., Tuan R.S. AP-1 transcription factor complex is a target of signals from both WnT-7a and N-cadherin-dependent cell-cell adhesion complex during the regulation of limb mesenchymal chondrogenesis. Exp Cell Res. 2002;273:197–203. doi: 10.1006/excr.2001.5448. [DOI] [PubMed] [Google Scholar]
- 64.Oh C.D., Chang S.H., Yoon Y.M. Opposing role of mitogen-activated protein kinase subtypes, erk-1/2 and p38, in the regulation of chondrogenesis of mesenchymes. J Biol Chem. 2000;275:5613–5619. doi: 10.1074/jbc.275.8.5613. [DOI] [PubMed] [Google Scholar]
- 65.Kozhemyakina E., Lassar A.B., Zelzer E. A pathway to bone: signaling molecules and transcription factors involved in chondrocyte development and maturation. Dev Camb Engl. 2015;142:817–831. doi: 10.1242/dev.105536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Seo H.S., Serra R. Deletion of Tgfbr2 in Prx1-cre expressing mesenchyme results in defects in development of the long bones and joints. Dev Biol. 2007;310:304–316. doi: 10.1016/j.ydbio.2007.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Spagnoli A., O'Rear L., Chandler R.L. TGF-β signaling is essential for joint morphogenesis. J Cell Biol. 2007;177:1105–1117. doi: 10.1083/jcb.200611031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Retting K.N., Song B., Yoon B.S., Lyons K.M. BMP canonical Smad signaling through Smad1 and Smad5 is required for endochondral bone formation. Dev Camb Engl. 2009;136:1093–1104. doi: 10.1242/dev.029926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chubinskaya S., Kuettner K.E. Regulation of osteogenic proteins by chondrocytes. Int J Biochem Cell Biol. 2003;35:1323–1340. doi: 10.1016/s1357-2725(03)00035-9. [DOI] [PubMed] [Google Scholar]
- 70.Gründer T., Gaissmaier C., Fritz J. Bone morphogenetic protein (BMP)-2 enhances the expression of type II collagen and aggrecan in chondrocytes embedded in alginate beads. Osteoarthr Cartil. 2004;12:559–567. doi: 10.1016/j.joca.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 71.Furuhashi M., Yagi K., Yamamoto H. Axin facilitates Smad3 activation in the transforming growth factor beta signaling pathway. Mol Cell Biol. 2001;21:5132–5141. doi: 10.1128/MCB.21.15.5132-5141.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Liu W., Rui H., Wang J. Axin is a scaffold protein in TGF-beta signaling that promotes degradation of Smad7 by Arkadia. EMBO J. 2006;25:1646–1658. doi: 10.1038/sj.emboj.7601057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dao D.Y., Yang X., Flick L.M. Axin2 regulates chondrocyte maturation and axial skeletal development. J Orthop Res. 2010;28:89–95. doi: 10.1002/jor.20954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hellingman C.A., Davidson E.N., Koevoet W. Smad signaling determines chondrogenic differentiation of bone-marrow-derived mesenchymal stem cells: inhibition of Smad1/5/8P prevents terminal differentiation and calcification. Tissue Eng Part A. 2011;17:1157–1167. doi: 10.1089/ten.TEA.2010.0043. [DOI] [PubMed] [Google Scholar]
- 75.Leijten J.C., Emons J., Sticht C. Gremlin 1, frizzled-related protein, and Dkk-1 are key regulators of human articular cartilage homeostasis. Arthritis Rheum. 2012;64:3302–3312. doi: 10.1002/art.34535. [DOI] [PubMed] [Google Scholar]
- 76.Hartmann C., Tabin C.J. Dual roles of Wnt signaling during chondrogenesis in the chicken limb. Development. 2000;127:3141–3159. doi: 10.1242/dev.127.14.3141. [DOI] [PubMed] [Google Scholar]
- 77.Fischer L., Boland G., Tuan R.S. Wnt-3A enhances bone morphogenetic protein-2-mediated chondrogenesis of murine C3H10T1/2 mesenchymal cells. J Biol Chem. 2002;277:30870–30878. doi: 10.1074/jbc.M109330200. [DOI] [PubMed] [Google Scholar]
- 78.Yano F., Kugimiya F., Ohba S. The canonical Wnt signalling pathway promotes chondrocyte differentiation in a Sox9- dependent manner. Biochem Biophys Res Commun. 2005;333:1300–1308. doi: 10.1016/j.bbrc.2005.06.041. [DOI] [PubMed] [Google Scholar]
- 79.Hartmann C.A. Wnt canon orchestrating osteoblastogenesis. Trends Cell Biol. 2006;16:151–158. doi: 10.1016/j.tcb.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 80.Zhou S., Eid K., Glowacki J. Cooperation between TGF-β and Wnt pathways during chondrocyte and adipocyte differentiation of human marrow stromal cells. J Bone Min Res. 2004;19:463–470. doi: 10.1359/JBMR.0301239. [DOI] [PubMed] [Google Scholar]
- 81.Bradley E.W., Drissi M.H. Wnt5b regulates mesenchymal cell aggregation and chondrocyte differentiation through the planar cell polarity pathway. J Cell Physiol. 2011;226:1683–1693. doi: 10.1002/jcp.22499. [DOI] [PubMed] [Google Scholar]
- 82.Kamel G., Hoyos T., Rochard L. Requirements for frzb and fzd7a in cranial neural crest convergence and extension mechanisms during zebrafish palate and jaw morphogenesis. Dev Biol. 2013;381:423–433. doi: 10.1016/j.ydbio.2013.06.012. [DOI] [PubMed] [Google Scholar]
- 83.Nishita M., Yoo S.K., Nomachi A. Filopodia formation mediated by receptor tyrosine kinase Ror2 is required for Wnt5a-induced cell migration. J Cell Biol. 2006;175:555–562. doi: 10.1083/jcb.200607127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Matsumoto S., Fumoto K., Okamoto T. Binding of APC and dishevelled mediates Wnt5a-regulated focal adhesion dynamics in migrating cells. EMBO J. 2010;29:1192–1204. doi: 10.1038/emboj.2010.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Geetha-Loganathan P., Nimmagadda S., Antoni L. Expression of WNT signalling pathway genes during chicken craniofacial development. Dev Dyn. 2009;238:1150–1165. doi: 10.1002/dvdy.21934. [DOI] [PubMed] [Google Scholar]
- 86.Knight R.D., Schilling T.F. Cranial neural crest and development of the head skeleton. Adv Exp Med Biol. 2006;589:120–133. doi: 10.1007/978-0-387-46954-6_7. [DOI] [PubMed] [Google Scholar]
- 87.Schilling T.F., Kimmel C.B. Musculoskeletal patterning in the pharyngeal segments of the zebrafish embryo. Development. 1997;124:2945–2960. doi: 10.1242/dev.124.15.2945. [DOI] [PubMed] [Google Scholar]
- 88.Topczewski J., Sepich D.S., Myers D.C. The zebrafish glypican knypek controls cell polarity during gastrulation movements of convergent extension. Dev Cell. 2001;1:251–264. doi: 10.1016/s1534-5807(01)00005-3. [DOI] [PubMed] [Google Scholar]
- 89.Veeman M.T., Axelrod J.D., Moon R.T. A second canon. Functions and mechanisms of beta-catenin-independent Wnt signaling. Dev Cell. 2003;5:367–377. doi: 10.1016/s1534-5807(03)00266-1. [DOI] [PubMed] [Google Scholar]
- 90.Wallingford J.B., Fraser S.E., Harland R.M. Convergent extension: the molecular control of polarized cell movement during embryonic development. Dev Cell. 2002;2:695–706. doi: 10.1016/s1534-5807(02)00197-1. [DOI] [PubMed] [Google Scholar]
- 91.Yin C., Ciruna B., Solnica-Krezel L. Convergence and extension movements during vertebrate gastrulation. Curr Top Dev Biol. 2009;89:163–192. doi: 10.1016/S0070-2153(09)89007-8. [DOI] [PubMed] [Google Scholar]
- 92.Brugmann S.A., Goodnough L.H., Gregorieff A. Wnt signaling mediates regional specification in the vertebrate face. Development. 2007;134:3283–3295. doi: 10.1242/dev.005132. [DOI] [PubMed] [Google Scholar]
- 93.Garcia-Castro M.I., Marcelle C., Bronner-Fraser M. Ectodermal Wnt function as a neural crest inducer. Science. 2002;297:848–851. doi: 10.1126/science.1070824. [DOI] [PubMed] [Google Scholar]
- 94.Lee J.M., Kim J.Y., Cho K.W. Wnt11/Fgfr1b cross-talk modulates the fate of cells in palate development. Dev Biol. 2008;314:341–350. doi: 10.1016/j.ydbio.2007.11.033. [DOI] [PubMed] [Google Scholar]
- 95.Wodarz A., Nusse R. Mechanisms of Wnt signaling in development. Annu Rev Cell Dev Biol. 1998;14:59–88. doi: 10.1146/annurev.cellbio.14.1.59. [DOI] [PubMed] [Google Scholar]
- 96.Curtin E., Hickey G., Kamel G. Zebrafish wnt9a is expressed in pharyngeal ectoderm and is required for palate and lower jaw development. Mech Dev. 2011;128:104–115. doi: 10.1016/j.mod.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 97.Dougherty M., Kamel G., Grimaldi M. Distinct requirements for wnt9a and irf6 in extension and integration mechanisms during zebrafish palate morphogenesis. Development. 2013;140:76–81. doi: 10.1242/dev.080473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Enomoto-Iwamoto M., Kitagaki J., Koyama E. The Wnt antagonist Frzb-1 regulates chondrocyte maturation and long bone development during limb skeletogenesis. Dev Biol. 2002;251:142–156. doi: 10.1006/dbio.2002.0802. [DOI] [PubMed] [Google Scholar]
- 99.Filali M., Cheng N., Abbott D. Wnt-3A/beta-catenin signaling induces transcription from the LEF-1 promoter. J Biol Chem. 2002;277:33398–33410. doi: 10.1074/jbc.M107977200. [DOI] [PubMed] [Google Scholar]
- 100.Sen M., Reifert J., Lauterbach K. Regulation of fibronectin and metalloproteinase expression by Wnt signaling in rheumatoid arthritis synoviocytes. Arthritis Rheum. 2002;46:2867–2877. doi: 10.1002/art.10593. [DOI] [PubMed] [Google Scholar]
- 101.Rudnicki J.A., Brown A.M. Inhibition of chondrogenesis by Wnt gene expression in vivo and in vitro. Dev Biol. 1997;185:104–118. doi: 10.1006/dbio.1997.8536. [DOI] [PubMed] [Google Scholar]
- 102.Hartmann C., Tabin J. Wnt-14 plays a pivotal role in inducing synovial joint formation in the developing appendicular skeleton. Cell. 2001;104:341–351. doi: 10.1016/s0092-8674(01)00222-7. [DOI] [PubMed] [Google Scholar]
- 103.Church V., Nohno T., Linker C. Wnt regulation of chondrocyte differentiation. J Cell Sci. 2002;115:4809–4818. doi: 10.1242/jcs.00152. [DOI] [PubMed] [Google Scholar]
- 104.Yan D., Wallingford J.B., Sun T.Q. Cell autonomous regulation of multiple Dishevelled-dependent path-ways by mammalian Nkd. Proc Natl Acad Sci U. S. A. 2001;98:3802–3807. doi: 10.1073/pnas.071041898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kawakami Y., Wada N., Nishimatsu S.I. Involvement of Wnt-5a in chondrogenic pattern formation in the chick limb bud. Dev Growth Differ. 1999;41:29–40. doi: 10.1046/j.1440-169x.1999.00402.x. [DOI] [PubMed] [Google Scholar]
- 106.Lako M., Lindsay S., Bullen P. A novel mammalian wnt gene, WNT8B, shows brain- restricted expression in early development, with sharply delimited expression boundaries in the developing forebrain. Hum Mol Genet. 1998;7:813–822. doi: 10.1093/hmg/7.5.813. [DOI] [PubMed] [Google Scholar]
- 107.Kirton J.P., Crofts N.J., Brennan S.J., Canfield A.E. Wnt/β-catenin signaling stimulates chondrogenic and inhibits adipogenic differentiation of pericytes. Circ Res. 2007;101:581–589. doi: 10.1161/CIRCRESAHA.107.156372. [DOI] [PubMed] [Google Scholar]
- 108.Seidensticker M.J., Behrens J. Biochemical interactions in the wnt pathway. Biochim Biophys Acta. 2000;1495:168–182. doi: 10.1016/s0167-4889(99)00158-5. [DOI] [PubMed] [Google Scholar]
- 109.Moon R.T., Kohn A.D., De Ferrari G.V., Kaykas A. WNT and beta-catenin signalling: diseases and therapies. Nat Rev Genet. 2004;5:691–701. doi: 10.1038/nrg1427. [DOI] [PubMed] [Google Scholar]
- 110.Wada N., Javidan Y., Nelson S., Carney T.J., Kelsh R.N., Schilling T.F. Hedgehog signaling is required for cranial neural crest morphogenesis and chondrogenesis at the midline in the zebrafish skull. Development. 2005;132:3977–3988. doi: 10.1242/dev.01943. [DOI] [PubMed] [Google Scholar]
- 111.Daoud G., Kempf H., Kumar D. BMP-mediated induction of GATA4/5/6 blocks somitic responsiveness to SHH. Development. 2014;141:3978–3987. doi: 10.1242/dev.111906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Enomoto-Iwamoto M., Nakamura T., Aikawa T. Hedgehog proteins stimulate chondrogenic cell differentiation and cartilage formation. J Bone Min Res. 2000;15:1659–1668. doi: 10.1359/jbmr.2000.15.9.1659. [DOI] [PubMed] [Google Scholar]
- 113.Warzecha J., Göttig S., Brüning C. Sonic hedgehog protein promotes proliferation and chondrogenic differentiation of bone marrow-derived mesenchymal stem cells in vitro. J Orthop Sci. 2006;11:491–496. doi: 10.1007/s00776-006-1058-1. [DOI] [PubMed] [Google Scholar]
- 114.Wu X., Cai Z.D., Lou L.M., Chen Z.R. The effects of inhibiting hedgehog signaling pathways by using specific antagonist cyclopamine on the chondrogenic differentiation of mesenchymal stem cells. Int J Mol Sci. 2013;14:5966–5977. doi: 10.3390/ijms14035966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zeng L., Kempf H., Murtaugh L.C. Shh establishes an Nkx3.2/Sox9 autoregulatory loop that is maintained by BMP signals to induce somitic chondrogenesis. Genes Dev. 2002;16:1990–2005. doi: 10.1101/gad.1008002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Murtaugh L.C., Zeng L., Chyung J.H., Lassar A.B. The chick transcriptional repressor Nkx3.2 acts downstream of Shh to promote BMP-dependent axial chondrogenesis. Dev Cell. 2001;1:411–422. doi: 10.1016/s1534-5807(01)00039-9. [DOI] [PubMed] [Google Scholar]
- 117.Cairns D.M., Sato M.E., Lee P.G. A gradient of Shh establishes mutually repressing somitic cell fates induced by Nkx3.2 and Pax3. Dev Biol. 2008;323:152–165. doi: 10.1016/j.ydbio.2008.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Provot S., Kempf H., Murtaugh L.C. Nkx3.2/Bapx1 acts as a negative regulator of chondrocyte maturation. Development. 2006;133:651–662. doi: 10.1242/dev.02258. [DOI] [PubMed] [Google Scholar]
- 119.Murtaugh L.C., Chyung J.H., Lassar A.B. Sonic hedgehog promotes somitic chondrogenesis by altering the cellular response to BMP signaling. Genes Dev. 1999;13:225–237. doi: 10.1101/gad.13.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Liu W., Li G., Chien J.S. Sonic hedgehog regulates otic capsule chondrogenesis and inner ear development in the mouse embryo. Dev Biol. 2002;248:240–250. doi: 10.1006/dbio.2002.0733. [DOI] [PubMed] [Google Scholar]
- 121.Abzhanov A., Tabin C.J. Shh and Fgf8 act synergistically to drive cartilage outgrowth during cranial development. Dev Biol. 2004;273:134–148. doi: 10.1016/j.ydbio.2004.05.028. [DOI] [PubMed] [Google Scholar]
- 122.Wu Q., Zhang Y., Chen Q. Indian hedgehog is an essential component of mechanotransduction complex to stimulate chondrocyte proliferation. J Biol Chem. 2001;276:35290–35296. doi: 10.1074/jbc.M101055200. [DOI] [PubMed] [Google Scholar]
- 123.Chung U.I., Schipani E., McMahon A.P., Kronenberg H.M. Indian hedgehog couples chondrogenesis to osteogenesis in endochondral bone development. J Clin Invest. 2001;107:295–304. doi: 10.1172/JCI11706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Später D., Hill T.P., O'sullivan R.J. Wnt9a signaling is required for joint integrity and regulation of Ihh during chondrogenesis. Development. 2006;133:3039–3049. doi: 10.1242/dev.02471. [DOI] [PubMed] [Google Scholar]
- 125.Zou S., Chen T., Wang Y. Mesenchymal stem cells overexpressing Ihh promote bone repair. J Orthop Surg Res. 2014;9:102. doi: 10.1186/s13018-014-0102-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Steinert A.F., Weissenberger M., Kunz M. Indian hedgehog gene transfer is a chondrogenic inducer of human mesenchymal stem cells. Arthritis Res Ther. 2012;14:R168. doi: 10.1186/ar3921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Handorf A.M., Chamberlain C.S., Li W.J. Endogenously produced Indian hedgehog regulates TGFβ-driven chondrogenesis of human bone marrow stromal/stem cells. Stem Cells Dev. 2015;24:995–1007. doi: 10.1089/scd.2014.0266. [DOI] [PubMed] [Google Scholar]
- 128.Kim E.J., Cho S.W., Shin J.O. Ihh and Runx2/Runx3 signaling interact to coordinate early chondrogenesis: a mouse model. PLoS One. 2013;8:e55296. doi: 10.1371/journal.pone.0055296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Yoshida C.A., Yamamoto H., Fujita T. Runx2 and Runx3 are essential for chondrocyte maturation, and Runx2 regulates limb growth through induction of Indian hedgehog. Genes Dev. 2004;18:952–963. doi: 10.1101/gad.1174704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zhou J., Meng J., Guo S. IHH and FGF8 coregulate elongation of digit primordia. Biochem Biophys Res Commun. 2007;363:513–518. doi: 10.1016/j.bbrc.2007.08.198. [DOI] [PubMed] [Google Scholar]
- 131.Minina E., Kreschel C., Naski M.C. Interaction of FGF, Ihh/Pthlh, and BMP signaling integrates chondrocyte proliferation and hypertrophic differentiation. Dev Cell. 2002;3:439–449. doi: 10.1016/s1534-5807(02)00261-7. [DOI] [PubMed] [Google Scholar]
- 132.St-Jacques B., Hammerschmidt M., McMahon A.P. Indian hedgehog signaling regulates proliferation and differentiation of chondrocytes and is essential for bone formation. Genes Dev. 1999;13:2072–2086. doi: 10.1101/gad.13.16.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kronenberg H.M. Developmental regulation of the growth plate. Nature. 2003;423:332–336. doi: 10.1038/nature01657. [DOI] [PubMed] [Google Scholar]
- 134.Deckelbaum R.A., Chan G., Miao D. Ihh enhances differentiation of CFK-2 chondrocytic cells and antagonizes PTHrP-mediated activation of PKA. J Cell Sci. 2002;115:3015–3025. [Google Scholar]
- 135.Kirimoto A., Takagi Y., Ohya K., Shimokawa H. Effects of retinoic acid on the differentiation of chondrogenic progenitor cells, ATDC5. J Med Dent Sci. 2005;52:153–162. [PubMed] [Google Scholar]
- 136.Piao J., Tsuji K., Ochi H. Sirt6 regulates postnatal growth plate differentiation and proliferation via Ihh signaling. Sci Rep. 2013;3:3022. doi: 10.1038/srep03022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Bellon E., Luyten F.P., Tylzanowski P. delta-EF1 is a negative regulator of Ihh in the developing growth plate. J Cell Biol. 2009;187:685–699. doi: 10.1083/jcb.200904034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Austin J., Kimble J. glp-1 is required in the germ line for regulation of the decision between mitosis and meiosis in C. elegans. Cell. 1987;51:589–599. doi: 10.1016/0092-8674(87)90128-0. [DOI] [PubMed] [Google Scholar]
- 139.Watanabe N., Tezuka Y., Matsuno K. Suppression of differentiation and proliferation of early chondrogenic cells by Notch. J Bone Min Metab. 2003;21:344–352. doi: 10.1007/s00774-003-0428-4. [DOI] [PubMed] [Google Scholar]
- 140.Crowe R., Zikherman J., Niswander L. Delta-1 negatively regulates the transition from prehypertrophic to hypertrophic chondrocytes during cartilage formation. Development. 1999;126:987–998. doi: 10.1242/dev.126.5.987. [DOI] [PubMed] [Google Scholar]
- 141.Honjo T. The shortest path from the surface to the nucleus: RBP-J kappa/Su(H) transcription factor. Genes Cells. 1996;1:1–9. doi: 10.1046/j.1365-2443.1996.10010.x. [DOI] [PubMed] [Google Scholar]
- 142.Kageyama R., Ohtsuka T., Kobayashi T. The Hes gene family: repressors and oscillators that orchestrate embryogenesis. Development. 2007;134:1243–1251. doi: 10.1242/dev.000786. [DOI] [PubMed] [Google Scholar]
- 143.Grogan S.P., Olee T., Hiraoka K., Lotz M.K. Repression of chondrogenesis through binding of notch signaling proteins HES-1 and HEY-1 to N-box domains in the COL2A1 enhancer site. Arthritis Rheum. 2008;58:2754–2763. doi: 10.1002/art.23730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Haller R., Schwanbeck R., Martini S. Notch1 signaling regulates chondrogenic lineage determination through Sox9 activation. Cell Death Differ. 2012;19:461–469. doi: 10.1038/cdd.2011.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Hayes A.J., Dowthwaite G.P., Webster S.V., Archer C.W. The distribution of Notch receptors and their ligands during articular cartilage development. J Anat. 2003;202:495–502. doi: 10.1046/j.1469-7580.2003.00185.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Shimizu K., Chiba S., Saito T. Functional diversity among Notch1, Notch2, and Notch3 receptors. Biochem Biophys Res Commun. 2002;291:775–779. doi: 10.1006/bbrc.2002.6528. [DOI] [PubMed] [Google Scholar]
- 147.Karlsson C., Stenhamre H., Sandstedt J., Lindahl A. Neither Notch1 expression nor cellular size correlate with mesenchymal stem cell properties of adult articular chondrocytes. Cells Tissues Organs. 2008;187:275–285. doi: 10.1159/000113409. [DOI] [PubMed] [Google Scholar]
- 148.Mead T.J., Yutzey K.E. Notch pathway regulation of chondrocyte differentiation and proliferation during appendicular and axial skeleton development. Proc Natl Acad Sci U. S. A. 2009;106:14420–14425. doi: 10.1073/pnas.0902306106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Karlsson C., Jonsson M., Asp J., Brantsing C., Kageyama R., Lindahl A. Notch and HES5 are regulated during human cartilage differentiation. Cell Tissue Res. 2007;327:539–551. doi: 10.1007/s00441-006-0307-0. [DOI] [PubMed] [Google Scholar]
- 150.Oldershaw R.A., Murdoch A., Brennan K., Hardingham T.E. The putative role of the notch ligand, jagged 1, in the mediation of the early events of human mesenchymal stem cell chondrogenesis. Int J Exp Pathol. 2005;86:A47–A48. [Google Scholar]
- 151.Brighton C.T., Krebs A.G. Oxygen tension of healing fractures in the rabbit. J Bone Joint Surg Am. 1972;54:323–332. [PubMed] [Google Scholar]
- 152.Brighton C.T., Heppenstall R.B. Oxygen tension of the epiphyseal plate distal to an arteriovenous fistula. Clin Orthop Relat Res. 1971;80:167–173. doi: 10.1097/00003086-197110000-00024. [DOI] [PubMed] [Google Scholar]
- 153.Cicione C., Muiños-López E., Hermida-Gómez T. Effects of severe hypoxia on bone marrow mesenchymal stem cells differentiation potential. Stem Cells Int. 2013;2013:232896. doi: 10.1155/2013/232896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Zuscik M.J., Hilton M.J., Zhang X. Regulation of chondrogenesis and chondrocyte differentiation by stress. J Clin Invest. 2008;118:429–438. doi: 10.1172/JCI34174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Komatsu D.E., Bosch-Marce M., Semenza G.L., Hadjiargyrou M. Enhanced bone regeneration associated with decreased apoptosis in mice with partial HIF-1alpha deficiency. J Bone Min Res. 2007;22:366–374. doi: 10.1359/JBMR.061207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Coyle C.H., Izzo N.J., Chu C.R. Sustained hypoxia enhances chondrocyte matrix synthesis. J Orthop Res. 2009;27:793–799. doi: 10.1002/jor.20816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Peansukmanee S., Vaughan-Thomas A., Carter S.D. Effects of hypoxia on glucose transport in primary equine chondrocytes in vitro and evidence of reduced GLUT1 gene expression in pathologic cartilage in vivo. J Orthop Res. 2008;27:529–535. doi: 10.1002/jor.20772. [DOI] [PubMed] [Google Scholar]
- 158.Lafont J.E., Talma S., Murphy C.L. Hypoxia-inducible factor 2alpha is essential for hypoxic induction of the human articular chondrocyte phenotype. Arthritis Rheum. 2007;56:3297–3306. doi: 10.1002/art.22878. [DOI] [PubMed] [Google Scholar]
- 159.Koay E.J., Athanasiou K.A. Hypoxic chondrogenic differentiation of human embryonic stem cells enhances cartilage protein synthesis and biomechanical functionality. Osteoarthr Cartil. 2008;16:1450–1456. doi: 10.1016/j.joca.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 160.Adesida A.B., Mulet-Sierra A., Jomha N.M. Hypoxia mediated isolation and expansion enhances the chondrogenic capacity of bone marrow mesenchymal stromal cells. Stem Cell Res Ther. 2012;3:9. doi: 10.1186/scrt100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Duval E., Leclercq S., Elissalde J.M., Demoor M., Galéra P., Boumédiene K. Hypoxia-inducible factor 1alpha inhibits the fibroblast-like markers type I and type III collagen during hypoxia-induced chondrocyte redifferentiation: hypoxia not only induces type II collagen and aggrecan, but it also inhibits type I and type III collagen in the hypoxia-inducible factor 1alpha-dependent redifferentiation of chondrocytes. Arthritis Rheum. 2009;60:3038–3048. doi: 10.1002/art.24851. [DOI] [PubMed] [Google Scholar]
- 162.Schipani E. Hypoxia and HIF-1 alpha in chondrogenesis. Semin Cell Dev Biol. 2005;16:539–546. doi: 10.1016/j.semcdb.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 163.Malladi P., Xu Y., Chiou M. Hypoxia inducible factor-1α deficiency affects chondrogenesis of adipose-derived adult stromal cells. Tissue Eng. 2007;13:1159–1171. doi: 10.1089/ten.2006.0265. [DOI] [PubMed] [Google Scholar]
- 164.Kanichai M., Ferguson D., Prendergast P.J., Campbell V.A. Hypoxia promotes chondrogenesis in rat mesenchymal stem cells: a role for AKT and hypoxia-inducible factor (HIF)-1alpha. J Cell Physiol. 2008;216:708–715. doi: 10.1002/jcp.21446. [DOI] [PubMed] [Google Scholar]
- 165.Schipani E., Ryan H.E., Didrickson S. Hypoxia in cartilage: HIF-1alpha is essential for chondrocyte growth arrest and survival. Genes Dev. 2001;15:2865–2876. doi: 10.1101/gad.934301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Sun X., Wei Y. The role of hypoxia-inducible factor in osteogenesis and chondrogenesis. Cytotherapy. 2009;11:261–267. doi: 10.1080/14653240902824765. [DOI] [PubMed] [Google Scholar]
- 167.Ke Q., Costa M. Hypoxia-inducible factor-1 (HIF-1) Mol Pharmacol. 2006;70:1469–1480. doi: 10.1124/mol.106.027029. [DOI] [PubMed] [Google Scholar]
- 168.Genin O., Hasdai A., Shinder D., Pines M. Hypoxia, hypoxia-inducible factor-1α (HIF-1α), and heat-shock proteins in tibial dyschondroplasia. Poult Sci. 2008;87:1556–1564. doi: 10.3382/ps.2008-00124. [DOI] [PubMed] [Google Scholar]
- 169.Yudoh K., Nakamura H., Masuko-Hongo K. Catabolic stress induces expression of hypoxia-inducible factor (HIF)-1 alpha in articular chondrocytes: involvement of HIF-1 alpha in the pathogenesis of osteoarthritis. Arthritis Res Ther. 2005;7:R904–R914. doi: 10.1186/ar1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Lee S.H., Che X., Jeong J.H. Runx2 protein stabilizes hypoxia-inducible factor-1α through competition with von Hippel-Lindau protein (pVHL) and stimulates angiogenesis in growth plate hypertrophic chondrocytes. J Biol Chem. 2012;287:14760–14771. doi: 10.1074/jbc.M112.340232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Khan W.S., Adesida A.B., Hardingham T.E. Hypoxic conditions increase hypoxia-inducible transcription factor 2alpha and enhance chondrogenesis in stem cells from the infrapatellar fat pad of osteoarthritis patients. Arthritis Res Ther. 2007;9:R55. doi: 10.1186/ar2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Lee H.H., Chang C.C., Shieh M.J. Hypoxia enhances chondrogenesis and prevents terminal differentiation through PI3K/Akt/FoxO dependent anti-apoptotic effect. Sci Rep. 2013;3:2683. doi: 10.1038/srep02683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Gerber H.P., Vu T.H., Ryan A.M. VEGF couples hypertrophic cartilage remodeling, ossification and angiogenesis during endochondral bone formation. Nat Med. 1999;5:623e8. doi: 10.1038/9467. [DOI] [PubMed] [Google Scholar]
- 174.Bian L., Zhai D.Y., Tous E. Enhanced MSC chondrogenesis following delivery of TGF-β3 from alginate microspheres within hyaluronic acid hydrogels in vitro and in vivo. Biomaterials. 2011;32:6425–6434. doi: 10.1016/j.biomaterials.2011.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Carlevaro M.F., Cermelli S., Cancedda R., Descalzi Cancedda F. Vascular endothelial growth factor (VEGF) in cartilage neovascularization and chondrocyte differentiation: auto-paracrine role during endochondral bone formation. J Cell Sci. 2000;113:59–69. doi: 10.1242/jcs.113.1.59. [DOI] [PubMed] [Google Scholar]
- 176.Chen G., Shi X., Sun C. VEGF-mediated proliferation of human adipose tissue-derived stem cells. PLoS One. 2013;8:e73673. doi: 10.1371/journal.pone.0073673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Murata M., Yudoh K., Masuko K. The potential role of vascular endothelial growth factor (VEGF) in cartilage: how the angiogenic factor could be involved in the pathogenesis of osteoarthritis? Osteoarthr Cartil. 2008;16:279–286. doi: 10.1016/j.joca.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 178.Kubo S., Cooper G.M., Matsumoto T. Blocking vascular endothelial growth factor with soluble Flt-1 improves the chondrogenic potential of mouse skeletal muscle-derived stem cells. Arthritis Rheum. 2009;60:155–165. doi: 10.1002/art.24153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Matsumoto T., Cooper G.M., Gharaibeh B. Blocking VEGF as a potential approach to improve cartilage healing after osteoarthritis. J Musculoskelet Neuronal Interact. 2008;8:316–317. [PubMed] [Google Scholar]
- 180.Centola M., Abbruzzese F., Scotti C. Scaffold-based delivery of a clinically relevant anti-angiogenic drug promotes the formation of in vivo stable cartilage. Tissue Eng Part A. 2013;19:1960–1971. doi: 10.1089/ten.tea.2012.0455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Chae S.S., Paik J.H., Allende M.L. Regulation of limb development by the sphingosine 1-phosphate receptor S1p1/EDG-1 occurs via the hypoxia/VEGF axis. Dev Biol. 2004;268:441–447. doi: 10.1016/j.ydbio.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 182.Yin M., Pacifici M. Vascular regression is required for mesenchymal condensation and chondrogenesis in the developing limb. Dev Dyn. 2001;222:522–533. doi: 10.1002/dvdy.1212. [DOI] [PubMed] [Google Scholar]
- 183.Haigh J.J., Gerber H.P., Ferrara N., Wagner E.F. Conditional inactivation of VEGF-A in areas of collagen2a1 expression results in embryonic lethality in the heterozygous state. Development. 2000;127:1445–1453. doi: 10.1242/dev.127.7.1445. [DOI] [PubMed] [Google Scholar]
- 184.Zhao Q., Eberspaecher H., Lefebvre V., de Crombrugghe B. Parallel expression of Sox9 and Col2a1 in cells undergoing chondrogenesis. Dev Dyn. 1997;209:377–386. doi: 10.1002/(SICI)1097-0177(199708)209:4<377::AID-AJA5>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 185.Ushita M., Saito T., Ikeda T. Transcriptional induction of SOX9 by NF-kappaB family member RelA in chondrogenic cells. Osteoarthr Cartil. 2009;17:1065–1075. doi: 10.1016/j.joca.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 186.Wang Y. Sul HS. Pref-1 regulates mesenchymal cell commitment and differentiation through Sox9. Cell Metab. 2009;9:287–302. doi: 10.1016/j.cmet.2009.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Hata K., Takashima R., Amano K. Arid5b facilitates chondrogenesis by recruiting the histone demethylase Phf2 to Sox9-regulated genes. Nat Commun. 2013;4:2850. doi: 10.1038/ncomms3850. [DOI] [PubMed] [Google Scholar]
- 188.Takahashi I., Nuckolls G.H., Takahashi K. Compressive force promotes sox9, type II collagen and aggrecan and inhibits IL-1beta expression resulting in chondrogenesis in mouse embryonic limb bud mesenchymal cells. J Cell Sci. 1998;111:2067–2076. doi: 10.1242/jcs.111.14.2067. [DOI] [PubMed] [Google Scholar]
- 189.Juhász T., Matta C., Somogyi C. Mechanical loading stimulates chondrogenesis via the PKA/CREB-Sox9 and PP2A pathways in chicken micromass cultures. Cell Signal. 2014;26:468–482. doi: 10.1016/j.cellsig.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 190.Yamashita S., Miyaki S., Kato Y. L-Sox5 and Sox6 proteins enhance chondrogenic miR-140 microRNA expression by strengthening dimeric Sox9 activity. J Biol Chem. 2012;287:22206–22215. doi: 10.1074/jbc.M112.343194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Akiyama H., Chaboissier M.C., Martin J.F. The transcription factor Sox9 has essential roles in successive steps of the chondrocyte differentiation pathway and is required for expression of Sox5 and Sox6. Genes Dev. 2002;16:2813–2828. doi: 10.1101/gad.1017802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Hino K., Saito A., Kido M. Master regulator for chondrogenesis, Sox9, regulates transcriptional activation of the endoplasmic reticulum stress transducer BBF2H7/CREB3L2 in chondrocytes. J Biol Chem. 2014;289:13810–13820. doi: 10.1074/jbc.M113.543322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Akiyama H. Control of chondrogenesis by the transcription factor Sox9. Mod Rheumatol. 2008;18:213–219. doi: 10.1007/s10165-008-0048-x. [DOI] [PubMed] [Google Scholar]
- 194.Ikeda T., Kamekura S., Mabuchi A. The combination of SOX5, SOX6, and SOX9 (the SOX trio) provides signals sufficient for induction of permanent cartilage. Arthritis Rheumatism. 2004;50:3561–3573. doi: 10.1002/art.20611. [DOI] [PubMed] [Google Scholar]
- 195.Smits P., Li P., Mandel J. The transcription factors L-Sox5 and Sox6 are essential for cartilage formation. Dev Cell. 2001;1:277–290. doi: 10.1016/s1534-5807(01)00003-x. [DOI] [PubMed] [Google Scholar]
- 196.Lefebvre V., Behringer R.R., de Crombrugghe B. L-Sox5, Sox6 and SOx9 control essential steps of the chondrocyte differentiation pathway. Osteoarthr Cartil. 2001;9:S69–S75. doi: 10.1053/joca.2001.0447. [DOI] [PubMed] [Google Scholar]
- 197.Mori-Akiyama Y., Akiyama H., Rowitch D.H., de Crombrugghe B. Sox9 is required for determination of the chondrogenic cell lineage in the cranial neural crest. Proc Natl Acad Sci U. S. A. 2003;100:9360–9365. doi: 10.1073/pnas.1631288100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Akiyama H., Lyons J.P., Mori-Akiyama Y. Interactions between Sox9 and beta-catenin control chondrocyte differentiation. Genes Dev. 2004;18:1072–1087. doi: 10.1101/gad.1171104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Quintana L., Zur Nieden N.I., Semino C.E. Morphogenetic and regulatory mechanisms during developmental chondrogenesis: new paradigms for cartilage tissue engineering. Tissue Eng B. 2009;15:29–41. doi: 10.1089/ten.teb.2008.0329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Shi S., Wang C., Acton A.J. Role of Sox9 in growth factor regulation of articular chondrocytes. J Cell Biochem. 2015;116:1391–1400. doi: 10.1002/jcb.25099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Pan Q., Yu Y., Chen Q. Sox9, a key transcription factor of bone morphogenetic protein-2-induced chondrogenesis, is activated through BMP pathway and a CCAAT box in the proximal promoter. J Cell Physiol. 2008;217:228–241. doi: 10.1002/jcp.21496. [DOI] [PubMed] [Google Scholar]
- 202.Liao J., Hu N., Zhou N. Sox9 potentiates BMP2-induced chondrogenic differentiation and inhibits BMP2-induced osteogenic differentiation. PLoS One. 2014;9:e89025. doi: 10.1371/journal.pone.0089025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Hata K., Nishimura R., Muramatsu S. Paraspeckle protein p54nrb links Sox9-mediated transcription with RNA processing during chondrogenesis in mice. J Clin Invest. 2008;118:3098–3108. doi: 10.1172/JCI31373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Furumatsu T., Tsuda M., Taniguchi N. Smad3 induces chondrogenesis through the activation of SOX9 via CREB-binding protein/p300 recruitment. J Biol Chem. 2005;280:8343–8350. doi: 10.1074/jbc.M413913200. [DOI] [PubMed] [Google Scholar]
- 205.Kawakami Y., Tsuda M., Takahashi S. Transcriptional coactivator PGC-1alpha regulates chondrogenesis via association with Sox9. Proc Natl Acad Sci U. S. A. 2005;102:2414–2419. doi: 10.1073/pnas.0407510102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Hattori T., Coustry F., Stephens S. Transcriptional regulation of chondrogenesis by coactivator Tip60 via chromatin association with Sox9 and Sox5. Nucleic Acids Res. 2008;36:3011–3024. doi: 10.1093/nar/gkn150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Huang W., Lu N., Eberspaecher H., de Crombrugghe B. A new long form of c-Maf cooperates with Sox9 to activate the type II collagen gene. J Biol Chem. 2002;277:50668–50675. doi: 10.1074/jbc.M206544200. [DOI] [PubMed] [Google Scholar]
- 208.Guo M., Shen J., Kwak J.H. A novel role of Cyclophilin A in regulation of chondrogenic commitment and endochondral ossification. Mol Cell Biol. 2015 doi: 10.1128/MCB.01414-14. pii:MCB.01414–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Furumatsu T., Asahara H. Histone acetylation influences the activity of Sox9-related transcriptional complex. Acta Med Okayama. 2015;64:351–357. doi: 10.18926/AMO/41320. [DOI] [PubMed] [Google Scholar]
- 210.Leung V.Y., Gao B., Leung K.K. SOX9 governs differentiation stage-specific gene expression in growth plate chondrocytes via direct concomitant transactivation and repression. PLoS Genet. 2011;7:e1002356. doi: 10.1371/journal.pgen.1002356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Cheng A., Genever P.G. SOX9 determines RUNX2 transactivity by directing intracellular degradation. J Bone Min Res. 2010;25:2680–2689. doi: 10.1002/jbmr.174. [DOI] [PubMed] [Google Scholar]
- 212.Kumar D., Lassar A.B. The transcriptional activity of Sox9 in chondrocytes is regulated by RhoA signaling and actin polymerization. Mol Cell Biol. 2009 Aug;29:4262–4273. doi: 10.1128/MCB.01779-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Yoshida C.A., Komori T. Role of Runx proteins in chondrogenesis. Crit Rev Eukaryot Gene Expr. 2005;15:243–254. doi: 10.1615/critreveukargeneexpr.v15.i3.60. [DOI] [PubMed] [Google Scholar]
- 214.Fujita T., Azuma Y., Fukuyama R. Runx2 induces osteoblast and chondrocyte differentiation and enhances their migration by coupling with PI3K-Akt signaling. J Cell Biol. 2004;166:85–95. doi: 10.1083/jcb.200401138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Zhou G., Zheng Q., Engin F. Dominance of SOX9 function over RUNX2 during skeletogenesis. Proc Natl Acad Sci U. S. A. 2006;103:19004–19009. doi: 10.1073/pnas.0605170103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Lengner C.J., Hassan M.Q., Serra R.W. Nkx3.2-mediated repression of Runx2 promotes chondrogenic differentiation. J Biol Chem. 2005;280:15872–15879. doi: 10.1074/jbc.M411144200. [DOI] [PubMed] [Google Scholar]
- 217.Woodburry B.W., Rodova M., Lu Q. March 6-9, 2010. uRunx2 and Wnt/β-catenin Signaling Cooperatively Suppress Chondrogenic Differentiation of Adult Dural Cells During BMP-induced Cranial Bone Repair. 56th Annual Meeting of the Orthopaedic Research Society, New Orleans, LA. [Google Scholar]
- 218.Zheng Q., Zhou G., Morello R. Type X collagen gene regulation by Runx2 contributes directly to its hypertrophic chondrocyte-specific expression in vivo. J Cell Biol. 2003;162:833–842. doi: 10.1083/jcb.200211089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Kwon T.G., Zhao X., Yang Q. Physical and functional interactions between Runx2 and HIF-1α induce vascular endothelial growth factor gene expression. J Cell Biochem. 2011;112:3582–3593. doi: 10.1002/jcb.23289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Enomoto H., Furuichi T., Zanma A. Runx2 deficiency in chondrocytes causes adipogenic changes in vitro. J Cell Sci. 2004;117:417–425. doi: 10.1242/jcs.00866. [DOI] [PubMed] [Google Scholar]
- 221.Hinoi E., Bialek P., Chen Y.T. Runx2 inhibits chondrocyte proliferation and hypertrophy through its expression in the perichondrium. Genes Dev. 2006;20:2937–2942. doi: 10.1101/gad.1482906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Itoh S., Kanno S., Gai Z. Trps1 plays a pivotal role downstream of Gdf5 signaling in promoting chondrogenesis and apoptosis of ATDC5 cells. Genes Cells. 2008;13:355–363. doi: 10.1111/j.1365-2443.2008.01170.x. [DOI] [PubMed] [Google Scholar]
- 223.Fantauzzo K.A., Kurban M., Levy B., Christiano A.M. Trps1 and its target gene Sox9 regulate epithelial proliferation in the developing hair follicle and are associated with hypertrichosis. PLoS Genet. 2012;8:e1003002. doi: 10.1371/journal.pgen.1003002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Suemoto H., Muragaki Y., Nishioka K. Trps1 regulates proliferation and apoptosis of chondrocytes through Stat3 signaling. Dev Biol. 2007;312:572–581. doi: 10.1016/j.ydbio.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 225.Lolli A., Lambertini E., Penolazzi L. Pro-chondrogenic effect of miR-221 and slug depletion in human MSCs. Stem Cell Rev. 2014;10:841–855. doi: 10.1007/s12015-014-9532-1. [DOI] [PubMed] [Google Scholar]
- 226.Zhang Y., Xie R.L., Gordon J. Control of mesenchymal lineage progression by microRNAs targeting skeletal gene regulators Trps1 and Runx2. J Biol Chem. 2012;287:21926–21935. doi: 10.1074/jbc.M112.340398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Nishioka K., Itoh S., Suemoto H. Trps1 deficiency enlarges the proliferative zone of growth plate cartilage by upregulation of Pthrp. Bone. 2008;43:64–71. doi: 10.1016/j.bone.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 228.Wuelling M., Kaiser F.J., Buelens L.A. Trps1, a regulator of chondrocyte proliferation and differentiation, interacts with the activator form of Gli3. Dev Biol. 2009;328:40–53. doi: 10.1016/j.ydbio.2009.01.012. [DOI] [PubMed] [Google Scholar]
- 229.Bosserhoff A.K., Kondo S., Moser M. Mouse CD-RAP/MIA gene: structure, chromosomal localization, and expression in cartilage and chondrosarcoma. Dev Dyn. 1997;208:516–525. doi: 10.1002/(SICI)1097-0177(199704)208:4<516::AID-AJA7>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 230.Schmid R., Meyer K., Spang R. YBX1 is a modulator of MIA/CD-RAP-dependent chondrogenesis. PLoS One. 2013;8:e82166. doi: 10.1371/journal.pone.0082166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Schubert T., Schlegel J., Schmid R. Modulation of cartilage differentiation by melanoma inhibiting activity/cartilage-derived retinoic acid-sensitive protein (MIA/CD-RAP) Exp Mol Med. 2010;42:166–174. doi: 10.3858/emm.2010.42.3.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Schmid R., Bosserhoff A.K. Redundancy in regulation of chondrogenesis in MIA/CD-RAP-deficient mice. Mech Dev. 2014;131:24–34. doi: 10.1016/j.mod.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 233.Li J, Sandell LJ. E-box-related transcription factors regulate CD-RAP gene expression by alternate formation of activating and repressing nucleoprotein complexes. 48th Annual Meeting of the Orthopaedic Research Society, Dallas, TX, February 10–13, 2002.
- 234.Xie W.F., Zhang X., Sakano S. Trans-activation of the mouse cartilage-derived retinoic acid-sensitive protein gene by Sox9. J Bone Min Res. 1999;14:757–763. doi: 10.1359/jbmr.1999.14.5.757. [DOI] [PubMed] [Google Scholar]
- 235.Xie W.F., Kondo S., Sandell L.J. Regulation of the mouse cartilage-derived retinoic acid-sensitive protein gene by the transcription factor AP-2. J Biol Chem. 1998;273:5026–5032. doi: 10.1074/jbc.273.9.5026. [DOI] [PubMed] [Google Scholar]
- 236.De Martino I., Visone R., Palmieri D. The Mia/Cd-rap gene expression is downregulated by the high-mobility group A proteins in mouse pituitary adenomas. Endocr Relat Cancer. 2007;14:875–886. doi: 10.1677/ERC-07-0036. [DOI] [PubMed] [Google Scholar]
- 237.Hong S., Derfoul A., Pereira-Mouries L., Hall D.J. A novel domain in histone deacetylase 1 and 2 mediates repression of cartilage-specific genes in human chondrocytes. FASEB J. 2009;23:3539–3552. doi: 10.1096/fj.09-133215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Liu C.J., Prazak L., Fajardo M. Leukemia/lymphoma-related factor, a POZ domain-containing transcriptional repressor, interacts with histone deacetylase-1 and inhibits cartilage oligomeric matrix protein gene expression and chondrogenesis. J Biol Chem. 2004;279:47081–47091. doi: 10.1074/jbc.M405288200. [DOI] [PubMed] [Google Scholar]
- 239.Hu N., Wang C., Liang X. Inhibition of histone deacetylases potentiates BMP9-induced osteogenic signaling in mouse mesenchymal stem cells. Cell Physiol Biochem. 2013;32:486–498. doi: 10.1159/000354453. [DOI] [PubMed] [Google Scholar]
- 240.Lee H.W., Suh J.H., Kim A.Y. Histone deacetylase 1-mediated histone modification regulates osteoblast differentiation. Mol Endocrinol. 2006 Oct;20:2432–2443. doi: 10.1210/me.2006-0061. [DOI] [PubMed] [Google Scholar]
- 241.Weston A.D., Chandraratna R.A., Torchia J., Underhill T.M. Requirement for RAR-mediated gene repression in skeletal progenitor differentiation. J Cell Biol. 2002;158:39–51. doi: 10.1083/jcb.200112029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Sakimura R., Tanaka K., Yamamoto S. The effects of histone deacetylase inhibitors on the induction of differentiation in chondrosarcoma cells. Clin Cancer Res. 2007;13:275. doi: 10.1158/1078-0432.CCR-06-1696. [DOI] [PubMed] [Google Scholar]
- 243.Pei M., Chen D., Li J., Wei L. Histone deacetylase 4 promotes TGF-beta1-induced synovium-derived stem cell chondrogenesis but inhibits chondrogenically differentiated stem cell hypertrophy. Differentiation. 2009;78:260–268. doi: 10.1016/j.diff.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 244.Lee R.C., Feinbaum R.L., Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell. 1993;75:843–854. doi: 10.1016/0092-8674(93)90529-y. [DOI] [PubMed] [Google Scholar]
- 245.Lagos-Quintana M., Rauhut R., Lendeckel W., Tuschl T. Identification of novel genes coding for small expressed RNAs. Science. 2001;294:853–858. doi: 10.1126/science.1064921. [DOI] [PubMed] [Google Scholar]
- 246.Bernstein E., Kim S.Y., Carmell M.A. Dicer is essential for mouse development. NatGenet. 2003;35:215–217. doi: 10.1038/ng1253. [DOI] [PubMed] [Google Scholar]
- 247.Liu J., Carmell M.A., Rivas F.V. Argonaute2 is the catalytic engine of mammalian RNAi. Science. 2004;305:1437–1441. doi: 10.1126/science.1102513. [DOI] [PubMed] [Google Scholar]
- 248.Wienholds E., Kloosterman W.P., Miska E. MicroRNA expression in zebrafish embryonic development. Science. 2005;309:310–311. doi: 10.1126/science.1114519. [DOI] [PubMed] [Google Scholar]
- 249.Zhang Z., Kang Y., Zhang Z. Expression of microRNAs during chondrogenesis of human adipose-derived stem cells. Osteoarthr Cartil. 2012;20:1638–1646. doi: 10.1016/j.joca.2012.08.024. [DOI] [PubMed] [Google Scholar]
- 250.Kim D., Song J., Kim S. MicroRNA-142-3p regulates TGF-beta3-mediated region-dependent chondrogenesis by regulating ADAM9. Biochem Biophys Res Commun. 2011;414:653–659. doi: 10.1016/j.bbrc.2011.09.104. [DOI] [PubMed] [Google Scholar]
- 251.Kuo C.K., Li W.J., Mauck R.L., Tuan R.S. Cartilage tissue engineering: its potential and uses. Curr Opin Rheumatol. 2006;18:64–73. doi: 10.1097/01.bor.0000198005.88568.df. [DOI] [PubMed] [Google Scholar]
- 252.Betre H., Ong S.R., Guilak F. Chondrocytic differentiation of human adipose-derived adult stem cells in elastin-like polypeptide. Biomaterials. 2005;27:91–99. doi: 10.1016/j.biomaterials.2005.05.071. [DOI] [PubMed] [Google Scholar]
- 253.Kim M.S., Hwang N.S., Lee J. Musculoskeletal differentiation of cells derived from human embryonic germ cells. Stem Cells. 2005;23:113–123. doi: 10.1634/stemcells.2004-0110. [DOI] [PubMed] [Google Scholar]
- 254.Indrawattana N., Chen G., Tadokoro M. Growth factor combination for chondrogenic induction from human mesenchymal stem cell. Biochem Biophys Res Commun. 2004;320:914–919. doi: 10.1016/j.bbrc.2004.06.029. [DOI] [PubMed] [Google Scholar]
- 255.Chua K.H., Aminuddin B.S., Fuzina N.H., Ruszymah B.H. Interaction between insulin-like growth factor-1 with other growth factors in serum depleted culture medium for human cartilage engineering. Med J Malays. 2004;59(suppl B):7–8. [PubMed] [Google Scholar]
- 256.Li W.J., Tuli R., Okafor C. A three-dimensional nanofibrous scaffold for cartilage tissue engineering using human mesenchymal stem cells. Biomaterials. 2005;26:599–609. doi: 10.1016/j.biomaterials.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 257.Stevens M.M., Marini R.P., Martin I. FGF-2 enhances TGF-beta1-induced periosteal chondrogenesis. J Orthop Res. 2004;22:1114–1119. doi: 10.1016/j.orthres.2003.12.021. [DOI] [PubMed] [Google Scholar]
- 258.Lee J.E., Kim K.E., Kwon I.C. Effects of the controlled-released TGF-beta 1 from chitosan microspheres on chondrocytes cultured in a collagen/chitosan/glycosaminoglycan scaffold. Biomaterials. 2004;25:4163–4173. doi: 10.1016/j.biomaterials.2003.10.057. [DOI] [PubMed] [Google Scholar]
- 259.Barbero A., Grogan S., Schafer D. Age related changes in human articular chondrocyte yield, proliferation and post-expansion chondrogenic capacity. Osteoarthr Cartil. 2004;12:476–484. doi: 10.1016/j.joca.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 260.Tay A.G., Farhadi J., Suetterlin R. Cell yield, proliferation, and postexpansion differentiation capacity of human ear, nasal, and rib chondrocytes. Tissue Eng. 2004;10:762–770. doi: 10.1089/1076327041348572. [DOI] [PubMed] [Google Scholar]
- 261.Hegewald A.A., Ringe J., Bartel J. Hyaluronic acid and autologous synovial fluid induce chondrogenic differentiation of equine mesenchymal stem cells: a preliminary study. Tissue Cell. 2004;36:431–438. doi: 10.1016/j.tice.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 262.Glowacki J., Yates K., Maclean R., Mizuno S. In vitro engineering of cartilage: effects of serum substitutes, TGF-beta, and IL-1alpha. Orthod Craniofac Res. 2005;8:200–208. doi: 10.1111/j.1601-6343.2005.00333.x. [DOI] [PubMed] [Google Scholar]
- 263.Vunjak-Novakovic G., Meinel L., Altman G., Kaplan D. Bioreactor cultivation of osteochondral grafts. Orthod Craniofac Res. 2005;8:209–218. doi: 10.1111/j.1601-6343.2005.00334.x. [DOI] [PubMed] [Google Scholar]
- 264.Wang Y., Kim U.J., Blasioli D.J. In vitro cartilage tissue engineering with 3D porous aqueous-derived silk scaffolds and mesenchymal stem cells. Biomaterials. 2005;26:7082–7094. doi: 10.1016/j.biomaterials.2005.05.022. [DOI] [PubMed] [Google Scholar]
- 265.Park Y., Sugimoto M., Watrin A. BMP-2 induces the expression of chondrocyte-specific genes in bovine synovium-derived progenitor cells cultured in three-dimensional alginate hydrogel. Osteoarthr Cartil. 2005;13:527–536. doi: 10.1016/j.joca.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 266.Majumdar M.K., Wang E., Morris E.A. BMP-2 and BMP-9 promotes chondrogenic differentiation of human multipotential mesenchymal cells and overcomes the inhibitory effect of IL-1. J Cell Physiol. 2001;189:275–284. doi: 10.1002/jcp.10025. [DOI] [PubMed] [Google Scholar]
- 267.Worster A.A., Brower-Toland B.D., Fortier L.A. Chondrocytic differentiation of mesenchymal stem cells sequentially exposed to transforming growth factor-beta1 in monolayer and insulin-like growth factor-I in a three-dimensional matrix. J Orthop Res. 2001;19:738–749. doi: 10.1016/S0736-0266(00)00054-1. [DOI] [PubMed] [Google Scholar]
- 268.Awad H.A., Wickham M.Q., Leddy H.A. Chondrogenic differentiation of adipose-derived adult stem cells in agarose, alginate, and gelatin scaffolds. Biomaterials. 2004;25:3211–3222. doi: 10.1016/j.biomaterials.2003.10.045. [DOI] [PubMed] [Google Scholar]
- 269.Mauck R.L., Yuan X., Tuan R.S. Chondrogenic differentiation and maturation of MSC-laden hydrogel constructs. Trans Orthop Res Soc. 2005;30:262. [Google Scholar]
- 270.Angele P., Kujat R., Nerlich M. Engineering of osteochondral tissue with bone marrow mesenchymal progenitor cells in a derivatized hyaluronan-gelatin composite sponge. Tissue Eng. 1999;5:545–554. doi: 10.1089/ten.1999.5.545. [DOI] [PubMed] [Google Scholar]
- 271.Caterson E.J., Li W.J., Nesti L.J. Polymer/alginate amalgam for cartilage-tissue engineering. Ann N. Y Acad Sci. 2002;961:134–138. doi: 10.1111/j.1749-6632.2002.tb03066.x. [DOI] [PubMed] [Google Scholar]
- 272.Mentlein R., Pufe T. New functions of angiogenic peptides in osteoarthritic cartilage. Curr Rheumatol Rev. 2015;1:37–43. [Google Scholar]
- 273.Portron S., Hivernaud V., Merceron C. Inverse regulation of early and late chondrogenic differentiation by oxygen tension provides cues for stem cell-based cartilage tissue engineering. Cell Physiol Biochem. 2015;35:841–857. doi: 10.1159/000369742. [DOI] [PubMed] [Google Scholar]
- 274.Zhou N., Hu N., Liao J.Y. HIF-1α as a regulator of BMP2-induced chondrogenic differentiation, osteogenic differentiation, and endochondral ossification in stem cells. Cell Physiol Biochem. 2015;36:44–60. doi: 10.1159/000374052. [DOI] [PubMed] [Google Scholar]
- 275.Duval E., Baugé C., Andriamanalijaona R. Molecular mechanism of hypoxia-induced chondrogenesis and its application in in vivo cartilage tissue engineering. Biomaterials. 2012;33:6042–6051. doi: 10.1016/j.biomaterials.2012.04.061. [DOI] [PubMed] [Google Scholar]
- 276.Wang Z.H., Li X.L., He X.J. Delivery of the Sox9 gene promotes chondrogenic differentiation of human umbilical cord blood-derived mesenchymal stem cells in an in vitro model. Braz J Med Biol Res. 2014;47:279–286. doi: 10.1590/1414-431X20133539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Madry H., Cucchiarini M., Terwilliger E.F., Trippel S.B. Recombinant adeno-associated virus vectors efficiently and persistently transduce chondrocytes in normal and osteoarthritic human articular cartilage. Hum Gene Ther. 2003;14:393–402. doi: 10.1089/104303403321208998. [DOI] [PubMed] [Google Scholar]
- 278.Zhang X.L., Mao Z.B., Yu C.L. Suppression of early experimental osteoarthritis by gene transfer of interleukin-1 receptor antagonist and interleukin-10. J Orthop Res. 2004;22:742–750. doi: 10.1016/j.orthres.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 279.Arai M., Anderson D., Kurdi Y. Effect of adenovirus-mediated overexpression of bovine ADAMTS-4 and human ADAMTS-5 in primary bovine articular chondrocyte pellet culture system. Osteoarthr Cartil. 2004;12:599–613. doi: 10.1016/j.joca.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 280.Venkatesan N., Barre L., Benani A. Stimulation of proteoglycan synthesis by glucuronosyltransferase-1 gene delivery: a strategy to promote cartilage repair. Proc Natl Acad Sci U. S. A. 2004;101:18087–18092. doi: 10.1073/pnas.0404504102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Li Y., Tew S.R., Russell A.M. Transduction of passaged human articular chondrocytes with adenoviral, retroviral, and lentiviral vectors and the effects of enhanced expression of SOX9. Tissue Eng. 2004;10:575–584. doi: 10.1089/107632704323061933. [DOI] [PubMed] [Google Scholar]
- 282.Peterson L., Minas T., Brittberg M. Two- to 9-year outcome after autologous chondrocyte transplantation of the knee. Clin Orthop Relat Res. 2000;374:212–234. doi: 10.1097/00003086-200005000-00020. [DOI] [PubMed] [Google Scholar]
- 283.Marlovits S., Aldrian S., Wondrasch B. Clinical and radiological outcomes 5 years after matrix-induced autologous chondrocyte implantation in patients with symptomatic, traumatic chondral defects. Am J Sports Med. 2012;40:2273–2280. doi: 10.1177/0363546512457008. [DOI] [PubMed] [Google Scholar]
- 284.Zheng M.H., Willers C., Kirilak L. Matrix-induced autologous chondrocyte implantation (MACI): biological and histological assessment. Tissue Eng. 2007;13:737–746. doi: 10.1089/ten.2006.0246. [DOI] [PubMed] [Google Scholar]
- 285.Caron M.M., Emans P.J., Coolsen M.M. Redifferentiation of dedifferentiated human articular chondrocytes: comparison of 2D and 3D cultures. Osteoarthr Cartil. 2012;20:1170–1178. doi: 10.1016/j.joca.2012.06.016. [DOI] [PubMed] [Google Scholar]
- 286.Grigolo B., Lisignoli G., Piacentini A. Evidence for redifferentiation of human chondrocytes grown on a hyaluronan-based biomaterial (HYAff 11): molecular, immunohistochemical and ultrastructural analysis. Biomaterials. 2002;23:1187–1195. doi: 10.1016/s0142-9612(01)00236-8. [DOI] [PubMed] [Google Scholar]
- 287.Malda J., Woodfield T.B., van der Vloodt F. The effect of PEGT/PBT scaffold architecture on the composition of tissue engineered cartilage. Biomaterials. 2005;26:63–72. doi: 10.1016/j.biomaterials.2004.02.046. [DOI] [PubMed] [Google Scholar]
- 288.Miot S., Woodfield T., Daniels A.U. Effects of scaffold composition and architecture on human nasal chondrocyte redifferentiation and cartilaginous matrix deposition. Biomaterials. 2005;26:2479–2489. doi: 10.1016/j.biomaterials.2004.06.048. [DOI] [PubMed] [Google Scholar]
- 289.Vacanti C.A., Langer R., Schloo B., Vacanti J.P. Synthetic polymers seeded with chondrocytes provide a template for new cartilage formation. Plast Reconstr Surg. 1991;88:753–759. doi: 10.1097/00006534-199111000-00001. [DOI] [PubMed] [Google Scholar]
- 290.Cima L.G., Vacanti J.P., Vacanti C. Tissue engineering by cell transplantation using degradable polymer substrates. J Biomech Eng. 1991;113:143–151. doi: 10.1115/1.2891228. [DOI] [PubMed] [Google Scholar]
- 291.Mahmoudifar N., Doran P.M. Tissue engineering of human cartilage in bioreactors using single and composite cell-seeded scaffolds. Biotechnol Bioeng. 2005;91:338–355. doi: 10.1002/bit.20490. [DOI] [PubMed] [Google Scholar]
- 292.Mouw J.K., Case N.D., Guldberg R.E. Variations in matrix composition and GAG fine structure among scaffolds for cartilage tissue engineering. Osteoarthr Cartil. 2005;13:828–836. doi: 10.1016/j.joca.2005.04.020. [DOI] [PubMed] [Google Scholar]
- 293.Almarza A.J., Athanasiou K.A. Effects of initial cell seeding density for the tissue engineering of the temporomandibular joint disc. Ann Biomed Eng. 2005;33:943–950. doi: 10.1007/s10439-005-3311-8. [DOI] [PubMed] [Google Scholar]
- 294.Griffon D.J., Sedighi M.R., Sendemir-Urkmez A. Evaluation of vacuum and dynamic cell seeding of polyglycolic acid and chitosan scaffolds for cartilage engineering. Am J Vet Res. 2005;66:599–605. doi: 10.2460/ajvr.2005.66.599. [DOI] [PubMed] [Google Scholar]
- 295.Fuchs J.R., Hannouche D., Terada S. Cartilage engineering from ovine umbilical cord blood mesenchymal progenitor cells. Stem Cells. 2005;23:958–964. doi: 10.1634/stemcells.2004-0310. [DOI] [PubMed] [Google Scholar]
- 296.Hunter C.J., Levenston M.E. Maturation and integration of tissue-engineered cartilages within an in vitro defect repair model. Tissue Eng. 2004;10:736–746. doi: 10.1089/1076327041348310. [DOI] [PubMed] [Google Scholar]
- 297.Seidel J.O., Pei M., Gray M.L. Long-term culture of tissue engineered cartilage in a perfused chamber with mechanical stimulation. Biorheology. 2004;41:445–458. [PubMed] [Google Scholar]
- 298.Estes B.T., Gimble J.M., Guilak F. Mechanical signals as regulators of stem cell fate. Curr Top Dev Biol. 2004;60:91–126. doi: 10.1016/S0070-2153(04)60004-4. [DOI] [PubMed] [Google Scholar]
- 299.Archer C.W., Buxton P., Hall B.K., Francis-West P. Mechanical regulation of secondary chondrogenesis. Biorheology. 2006;43:355–370. [PubMed] [Google Scholar]
- 300.Barrett-Jolley R., Lewis R., Fallman R., Mobasheri A. The emerging chondrocyte channelome. Front Physiol. 2010;1:135. doi: 10.3389/fphys.2010.00135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Hoey D.A., Downs M.E., Jacobs C.R. The mechanics of the primary cilium: an intricate structure with complex function. J Biomech. 2012;45:17–26. doi: 10.1016/j.jbiomech.2011.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Martins R.P., Finan J.D., Guilak F., Lee D.A. Mechanical regulation of nuclear structure and function. Annu Rev Biomed Eng. 2012;14:431–455. doi: 10.1146/annurev-bioeng-071910-124638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Blain E.J. Involvement of the cytoskeletal elements in articular cartilage homeostasis and pathology. Int J Exp Pathol. 2009;90:1–15. doi: 10.1111/j.1365-2613.2008.00625.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Choi J.W., Choi B.H., Park S.H. Mechanical stimulation by ultrasound enhances chondrogenic differentiation of mesenchymal stem cells in a fibrin-hyaluronic acid hydrogel. Artif Organs. 2013;37:648–655. doi: 10.1111/aor.12041. [DOI] [PubMed] [Google Scholar]
- 305.Kupcsik L., Stoddart M.J., Li Z. Improving chondrogenesis: potential and limitations of SOX9 gene transfer and mechanical stimulation for cartilage tissue engineering. Tissue Eng Part A. 2010;16:1845–1855. doi: 10.1089/ten.TEA.2009.0531. [DOI] [PubMed] [Google Scholar]
- 306.Buschmann M.D., Gluzband Y.A., Grodzinsky A.J., Hunziker E.B. Mechanical compression modulates matrix biosynthesis in chondrocyte/agarose culture. J Cell Sci. 1995;108:1497–1508. doi: 10.1242/jcs.108.4.1497. [DOI] [PubMed] [Google Scholar]
- 307.Smith R.L., Carter D.R., Schurman D.J. Pressure and shear differentially alter human articular chondrocyte metabolism: a review. Clin Orthop Relat Res. 2004;(427 suppl):S89–S95. [PubMed] [Google Scholar]
- 308.Carver S.E., Heath C.A. Increasing extracellular matrix production in regenerating cartilage with intermittent physiological pressure. Biotechnol Bioeng. 1999;62:166–174. [PubMed] [Google Scholar]
- 309.Carver S.E., Heath C.A. Semi-continuous perfusion system for delivering intermittent physiological pressure to regenerating cartilage. Tissue Eng. 1999;5:1–11. doi: 10.1089/ten.1999.5.1. [DOI] [PubMed] [Google Scholar]
- 310.Carver S.E., Heath C.A. Influence of intermittent pressure, fluid flow, and mixing on the regenerative properties of articular chondrocytes. Biotechnol Bioeng. 1999;65:274–281. [PubMed] [Google Scholar]
- 311.Elder S.H., Fulzele K.S., McCulley W.R. Cyclic hydrostatic compression stimulates chondroinduction of C3H/10T1/2 cells. Biomech Model Mechanobiol. 2005;3:141–146. doi: 10.1007/s10237-004-0058-3. [DOI] [PubMed] [Google Scholar]
- 312.Neidlinger-Wilke C., Wurtz K., Liedert A. A three-dimensional collagen matrix as a suitable culture system for the comparison of cyclic strain and hydrostatic pressure effects on intervertebral disc cells. J Neurosurg Spine. 2005;2:457–465. doi: 10.3171/spi.2005.2.4.0457. [DOI] [PubMed] [Google Scholar]
- 313.Bhumiratana S., Vunjak-Novakovic G. Engineering physiologically stiff and stratified human cartilage by fusing condensed mesenchymal stem cells. Methods. 2015;84:109–114. doi: 10.1016/j.ymeth.2015.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Bhumiratana S., Eton R.E., Oungoulian S.R. Large, stratified, and mechanically functional human cartilage grown in vitro by mesenchymal condensation. PNAS. 2014;11:6940–6945. doi: 10.1073/pnas.1324050111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Hu J.C., Athanasiou K.A. A self-assembling process in articular cartilage tissue engineering. Tissue Eng. 2006;12:969–979. doi: 10.1089/ten.2006.12.969. [DOI] [PubMed] [Google Scholar]
- 316.Mackay A.M., Beck S.C., Murphy J.M. Chondrogenic differentiation of cultured human mesenchymal stem cells from marrow. Tissue Eng. 1998;4:415–428. doi: 10.1089/ten.1998.4.415. [DOI] [PubMed] [Google Scholar]
- 317.Murdoch A.D., Grady L.M., Ablett M.P. Chondrogenic differentiation of human bone marrow stem cells in transwell cultures: generation of scaffold-free cartilage. Stem Cells. 2007;25:2786–2796. doi: 10.1634/stemcells.2007-0374. [DOI] [PubMed] [Google Scholar]
- 318.Chun J.S., Oh H., Yang S., Park M. Wnt signaling in cartilage development and degeneration. BMB Rep. 2008;41:485–494. doi: 10.5483/bmbrep.2008.41.7.485. [DOI] [PubMed] [Google Scholar]
- 319.Qu F., Wang J., Xu N. WNT3A modulates chondrogenesis via canonical and non-canonical Wnt pathways in MSCs. Front Biosci (Landmark Ed) 2013;18:493–503. doi: 10.2741/4116. [DOI] [PubMed] [Google Scholar]
- 320.Lee H.H., Behringer R.R. Conditional expression of Wnt4 during chondrogenesis leads to Dwarfism in mice. PLoS One. 2007;2:e450. doi: 10.1371/journal.pone.0000450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Bradley E.W., Drissi M.H. WNT5A regulates chondrocyte differentiation through differential use of the CaN/NFAT and IKK/NF-kappaB pathways. Mol Endocrinol. 2010;24:1581–1593. doi: 10.1210/me.2010-0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Shapiro I.M., Boyan B., Anderson H.C. 2002;1. The Growth Plate; pp. 1–280. [Google Scholar]
- 323.Han J., Yang T., Gao J. Specific microRNA expression during chondrogenesis of human mesenchymal stem cells. Int J Mol Med. 2010;25:377–384. doi: 10.3892/ijmm_00000355. [DOI] [PubMed] [Google Scholar]
- 324.Miyaki S., Nakasa T., Otsuki S. MicroRNA-140 is expressed in differentiated human articular chondrocytes and modulates interleukin-1 responses. Arthritis Rheum. 2009;60:2723–2730. doi: 10.1002/art.24745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Ham O., Song B.W., Lee S.Y. The role of microRNA-23b in the differentiation of MSC into chondrocyte by targeting protein kinase A signaling. Biomaterials. 2012;33:4500–4507. doi: 10.1016/j.biomaterials.2012.03.025. [DOI] [PubMed] [Google Scholar]
- 326.Yang B., Guo H., Zhang Y. The microRNA expression profiles of mouse mesenchymal stem cell during chondrogenic differentiation. BMB Rep. 2011;44:28–33. doi: 10.5483/BMBRep.2011.44.1.28. [DOI] [PubMed] [Google Scholar]
- 327.Suomi S., Taipaleenmaki H., Seppanen A. MicroRNAs regulate osteogenesis and chondrogenesis of mouse bone marrow stromal cells. Gene Regul Syst Bio. 2008;2:177–191. doi: 10.4137/grsb.s662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Laine S.K., Alm J.J., Virtanen S.P. MicroRNAs miR-96, miR-124, and miR-199a regulate gene expression in human bone marrow-derived mesenchymal stem cells. J Cell Biochem. 2012;113:2687–2695. doi: 10.1002/jcb.24144. [DOI] [PubMed] [Google Scholar]
- 329.Swingler T.E., Wheeler G., Carmont V. The expression and function of microRNAs in chondrogenesis and osteoarthritis. Arthritis Rheum. 2012;64:1909–1919. doi: 10.1002/art.34314. [DOI] [PubMed] [Google Scholar]
- 330.Zhong N., Sun J., Min Z. MicroRNA-337 is associated with chondrogenesis through regulating TGFBR2 expression. Osteoarthr Cartil. 2012;20:593–602. doi: 10.1016/j.joca.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 331.Guérit D., Philipot D., Chuchana P. Sox9-regulated miRNA-574-3p inhibits chondrogenic differentiation of mesenchymal stem cells. PLoS One. 2013;8:e62582. doi: 10.1371/journal.pone.0062582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Yang B., Guo H., Zhang Y. MicroRNA-145 regulates chondrogenic differentiation of mesenchymal stem cells by targeting Sox9. PLoS One. 2011;6:e21679. doi: 10.1371/journal.pone.0021679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Yang Z., Hao J., Hu Z.M. MicroRNA expression profiles in human adipose-derived stem cells during chondrogenic differentiation. Int J Mol Med. 2015;35:579–586. doi: 10.3892/ijmm.2014.2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Dudek K.A., Lafont J.E., Martinez-Sanchez A., Murphy C.L. Type II collagen expression is regulated by tissue-specific MIR-675 in human articular chondrocytes. J Biol Chem. 2010;285:24381–24387. doi: 10.1074/jbc.M110.111328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Lin E.A., Kong L., Bai X.H. miR-199a, a bone morphogenic protein 2- responsive MicroRNA, regulates chondrogenesis via direct targeting to Smad1. J Biol Chem. 2009;284:11326–11335. doi: 10.1074/jbc.M807709200. [DOI] [PMC free article] [PubMed] [Google Scholar]