Abstract
Competition theory states that multiple species should not be able to occupy the same niche indefinitely. Morphologically, similar species are expected to be ecologically alike and exhibit little niche differentiation, which makes it difficult to explain the co‐occurrence of cryptic species. Here, we investigated interspecific niche differentiation within a complex of cryptic bumblebee species that co‐occur extensively in the United Kingdom. We compared the interspecific variation along different niche dimensions, to determine how they partition a niche to avoid competitive exclusion. We studied the species B. cryptarum, B. lucorum, and B. magnus at a single location in the northwest of Scotland throughout the flight season. Using mitochondrial DNA for species identification, we investigated differences in phenology, response to weather variables and forage use. We also estimated niche region and niche overlap between different castes of the three species. Our results show varying levels of niche partitioning between the bumblebee species along three niche dimensions. The species had contrasting phenologies: The phenology of B. magnus was delayed relative to the other two species, while B. cryptarum had a relatively extended phenology, with workers and males more common than B. lucorum early and late in the season. We found divergent thermal specialisation: In contrast to B. cryptarum and B. magnus, B. lucorum worker activity was skewed toward warmer, sunnier conditions, leading to interspecific temporal variation. Furthermore, the three species differentially exploited the available forage plants: In particular, unlike the other two species, B. magnus fed predominantly on species of heather. The results suggest that ecological divergence in different niche dimensions and spatio‐temporal heterogeneity in the environment may contribute to the persistence of cryptic species in sympatry. Furthermore, our study suggests that cryptic species provide distinct and unique ecosystem services, demonstrating that morphological similarity does not necessarily equate to ecological equivalence.
Keywords: Bombus, coexistence, community, diet, ecological divergence, niche overlap, niche region, PCR‐RFLP, pollinators, specialisation
Introduction
According to competition theory, ecologically similar coexisting species must partition resources (Hardin 1960). Multiple species should not be able to occupy the same niche indefinitely, as the best adapted species should eventually exclude inferior species from a given location (Gause 1932; Holt et al. 1994). Closely related species are often morphologically, physiologically, and behaviorally similar. Morphologically, similar species are expected to be ecologically alike and exhibit little niche differentiation (Violle et al. 2011; Cothran et al. 2013). This makes it difficult to explain the co‐occurrence of cryptic species, which are distinct species with similar or identical morphology, often historically “hidden” under a single species name and thus wrongly classified (Bickford et al. 2007; Williams et al. 2012b). Indeed, some studies have found that cryptic species are less likely to co‐occur than congeneric noncryptic species (Vodă et al. 2015a,b). Yet, cryptic species are common in nature and often do co‐occur at local scales (Ortells et al. 2003; Feulner et al. 2006; Stuart et al. 2006; Gabaldón et al. 2013; Van Campenhout et al. 2014); this makes them important test cases for studying the mechanisms that facilitate species coexistence. In this study, we investigated interspecific niche differentiation within a bumblebee cryptic species complex, comparing the degree to which species vary in different niche dimensions.
Approximately 250 species of bumblebees exist worldwide, distributed across the temperate, alpine, and arctic regions of the Northern Hemisphere and also South America. In much of this range, it is common for multiple species to occur in sympatry despite high niche overlap. Morphologically, most bumblebee species are very similar, with obvious differences only in size, tongue length, and coloration (Goulson and Darvill 2004; Goulson 2010). As they also all rely exclusively on pollen and nectar for food, theory would predict that bumblebee communities should be shaped by high levels of interspecific competition for these resources (Heinrich 1976; Inouye 1978).
Bombus species are notorious for possessing convergent color patterns between species, but also for displaying high intraspecific variation (Ellis et al. 2006; Williams 2007; Williams et al. 2012a). The subgenus Bombus sensu stricto is a widespread and commercially exploited taxon of bumblebee, comprising 17 species worldwide (Williams et al. 2012b), of which five are found in Europe: Bombus (Bombus) cryptarum, (Fabricius), B. (B.) lucorum (Linnaeus), B. (B.) magnus (Vogt), B. (B.) sporadicus (Nylander), and B. (B.) terrestris (Linnaeus). The taxonomic status of the latter two species is well established, but B. lucorum, B. magnus and B. cryptarum are morphologically indistinguishable in much of their range (Fig. 1), which has triggered considerable debate about their species status. B. magnus and B. cryptarum have previously been regarded by some as subspecies of B. lucorum and are often referred to collectively as the “lucorum complex,” or simply synonymized to B. lucorum (Benton 2006; Edwards & Jenner 2005). However, these three species are now recognised as a cryptic species complex: Studies on labial gland secretions have shown discrete genetic differences between the three species (Bertsch et al. 2005), as have studies of the CO1 gene (Carolan et al. 2012; Murray et al. 2007; Williams et al. 2012b), which suggest that B. magnus and B. cryptarum are more closely related to each other than to B. lucorum (Bertsch et al. 2005; Murray et al. 2008; Williams et al. 2012b). Morphological diagnostic characters have been proposed for queens, but some of these vary along a continuum, overlapping considerably between species, making them unreliable for identification (Carolan et al. 2012).
Figure 1.

One of the lucorum complex species, which are morphologically indistinguishable in the field. This individual was feeding on heather in Glencoe. Photograph credit: Jessica Scriven.
With a history of identification difficulties, relatively little is known about the field ecology of these cryptic lucorum complex species; in particular, the details of how they differentially exploit their general niche remain unclear. The three species are morphologically and ecologically similar; they are all short‐tongued species, meaning they have potential foraging access to the same floral resources. They occur sympatrically with broadly overlapping ranges in the UK and Ireland (Murray et al. 2007; Waters et al. 2011; Williams et al. 2012b; Stanley et al. 2013; Scriven et al. 2015). All three species co‐occur at many locations in Great Britain and Ireland; additionally, Scriven et al. (2015), Murray et al. (2007), and Stanley et al. (2013) found B. lucorum to be present at every site surveyed, suggesting that it is a relative ecological generalist. The only study in which B. lucorum was found to be absent from some locations was carried out in the far north‐west of Great Britain (Waters et al. 2011). Studies of geographic distributions have suggested that the lucorum complex species may be adapted to exploit different climatic conditions (Waters et al. 2011; Scriven et al. 2015): Unlike B. lucorum, both B. cryptarum and B. magnus occurred most commonly at sites with lower summer temperatures (Scriven et al. 2015). Furthermore, Scriven et al. (2015) and Waters et al. (2011) found that B. magnus was strongly associated with the forage plants Calluna vulgaris and Erica spp. and consequently with heathland habitats where these ericaceous plants were present.
Bumblebees and some other pollinators have recently suffered declines in abundance and range contractions across much of Western Europe and North America (Williams 1982, 2005; Goulson et al. 2008; Goulson 2010; Cameron et al. 2011). In the UK, seven of the 27 species are listed as priority species in the UK post‐2010 Biodiversity Framework (previously Biodiversity Action Plan), a higher proportion than for any other invertebrate group (Goulson 2010). Thus, as well as enabling us to test fundamental ecological theories, a thorough understanding of niche use in bumblebees has important conservation implications. This is especially critical for B. magnus, which is the rarest of the lucorum complex species and is tightly associated with threatened heathland habitat (Waters et al. 2011; Scriven et al. 2015).
In this study, we determine how these three cryptic species, B. lucorum, B. cryptarum, and B. magnus, partition a niche to avoid competitive exclusion. For the first time, we characterise the niches of these species at a single site across the duration of their flight season. We assess niche differentiation in three different ecological dimensions: Patterns of temporal activity, weather sensitivity, and forage resource use. In doing so, we aim to test which of these niche‐use phenotypes has most flexibly responded to the selection pressures generated by interspecific competition to facilitate niche differentiation and species co‐occurrence.
Methods
Sampling
The study site was the area in and around Glencoe village in the Highlands of Scotland, UK. A previous study found all three lucorum complex species in good numbers at this site (Scriven et al. 2015). Sampling was carried out below 150 m altitude within a 3 km radius of 56.68° N and ‐5.09° W, which included two villages and the bottom of Glencoe valley. The site was visited repeatedly between 30 April and 2 October 2014 on average every 11 days (interval: max. 13 days, min. 9 days). Sampling was carried out over approximately 2 days per visit (max. 3 days and min. 1 day). Road verges, paths, and any other accessible areas were searched continuously on foot throughout the day, from early morning until the evening; the exact times changed according to daylight hours throughout the season. Routes walked were varied so that all areas were visited at different times of day. Bumblebees resembling the lucorum complex species were caught and placed in a queen marking cage. For each individual captured, we recorded the following: date, time of day, forage plant, temperature (°C) using a TES Dual K‐type Thermometer (model 1312A), wind speed (m/sec) using an Airflow Developments anemometer (model LCA6000), amount of sun (scale 0–4, Table S1) and amount of rain (scale 0–5, Table S2). A single tarsus was removed from each individual and stored in absolute ethanol for subsequent DNA extraction, after which bees were released. All bees were checked for missing tarsi to prevent sampling the same individuals twice. Bees were predominantly captured when foraging on flowers. Early in the season queens were observed foraging high in the canopy of Acer pseudoplatanus and Salix sp. trees, it was not possible to catch these individuals; therefore, they are not included in this study.
Species identification
DNA was extracted from tarsal samples using Chelex® 100 (Walsh et al. 1991). For species identification, we used a PCR‐RFLP method, digesting an amplified fragment of the cytochrome oxidase I (COI) gene following Murray et al. (2008): This yields a diagnostic digestion pattern for each of the cryptic lucorum complex species and B. terrestris. Samples that did not produce unambiguous results after two attempts were discarded. Of the 519 bees sampled, 4.2% were identified as B. terrestris, some workers of which are morphologically similar to B. lucorum workers (Wolf et al. 2010). These B. terrestris individuals were excluded from further analyses.
Analyses
All analyses were carried out using R version 3.0.2 (R Core Team 2014). To reduce the number of weather‐related explanatory variables, we employed principal component analysis (PCA) using the FactoMineR package (ver. 1.28, Lê et al. 2008). All variables were scaled to unit variance prior to analysis. PCA scores for axis 1 were associated with the level of sunshine and temperature (Figure S4B); this PCA variable (PCA 1) was used in some subsequent analyses.
To compare the seasonal and daily activity of the three bumblebee species and determine whether they were differentially affected by weather variables, we performed pairwise analyses between the species. We used generalised linear models with binary error distributions to test the association between these variables and the relative probability a sampled bumblebee belonged to a particular species within each pair. This analysis was performed separately for each caste (overwintered queens, workers, males); B. magnus was excluded from the analysis of males due to low sample size. Optimal models were selected to minimise AICc using the function dredge in the MuMIn package (ver. 1.9.5; Barton 2013) to run a complete set of models with all combinations of fixed effects and their two‐way interactions.
To define the niche of each species, we used the nicheROVER package (ver. 1.0; Swanson et al. 2015); we calculated the niche region (NR) for each of the three bumblebee species and the degree of niche overlap, based on phenology, time of day and sensitivity to weather variables. NR is defined as a specific region of parameter space in which a randomly chosen individual has the probability α of being found (Swanson et al. 2015). For these analyses, α = 95%. Niche overlap was calculated as the probability that a sampled individual from species A was found in the NR of species B (Swanson et al. 2015). Analyses were carried out separately for queens and workers; sample sizes for males were too small.
We determined diet differences between bumblebee species in three separate comparisons: for queens, workers, and males. We took the records of the flower species that each captured bee was visiting and used rarefaction to account for differences in sample size in our calculations of diet breadth (Williams et al. 2007). We noted the number of observations for the bee species with the fewest samples, rounded this down to the nearest multiple of five, and then drew this number of random samples of foraging observations without replacement for each bumblebee species. We drew 100 replicate subsamples per bee species to estimate the mean number of plant taxa each bee species would be expected to visit during a comparable number of flower visits, then determined the number of forage plant species in these subsamples. The preference of each bumblebee species for the ericaceous plants Calluna vulgaris or Erica spp. was examined using generalised linear models with individual bee as the unit of replication. The binary response represented whether the bee was recorded foraging on a C. vulgaris/Erica spp. flower (1) or any other plant species (0) and bumblebee species was used as the explanatory variable. This analysis was performed separately for queens and workers. We also calculated dissimilarity between the diets of the three species as Bray–Curtis distance measures (Bray and Curtis 1957) employing the Vegan package (ver. 2.3‐0, Oksanen et al. 2015) using relative abundances.
Results
Species identification
Of the 497 bees that belonged to the lucorum complex, 51.7% were B. cryptarum, 40.4% were B. lucorum, and 7.8% were B. magnus. Queens of the three species were similarly abundant; however, in comparison with B. lucorum and B. cryptarum, workers and males of B. magnus were relatively rare (Table 1).
Table 1.
The total number of queens, workers, and males sampled for each species
| Species | Queens | Workers | Males | Total |
|---|---|---|---|---|
| B. lucorum | 21 | 153 | 27 | 201 |
| B. cryptarum | 26 | 174 | 57 | 257 |
| B. magnus | 23 | 14 | 2 | 39 |
| Total | 70 | 341 | 86 | 497 |
Interspecific differences in phenology and diurnal activity
We found interspecific phenological differences for each of the separate castes. For overwintered queens, B. magnus was scarcer than the other two species early in the season but became relatively more common as the season progressed (Fig. 2B–D & Table S3). In contrast, there was no significant difference in the dates when B. cryptarum and B. lucorum queens were on the wing (Fig. 2A,D & Table S3).
Figure 2.
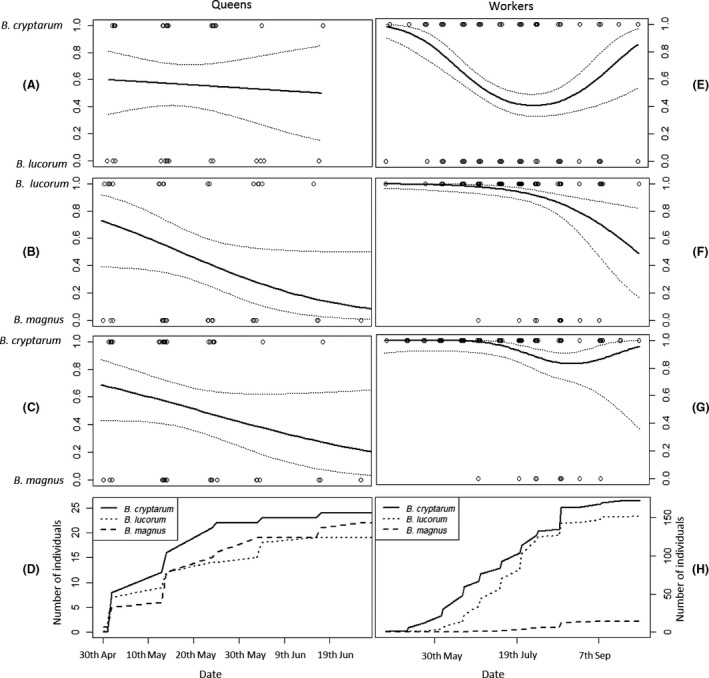
Interspecific variation in phenology of queens (A–D) and workers (E–H) of the lucorum complex species. Panels show the changes in the probability of an individuals belonging to the following: B. cryptarum compared to B. lucorum (A & E), B. lucorum compared to B. magnus (B & F), and B. cryptarum compared to B. magnus (C & G) as a function of date. The relative abundance of species pairs changed significantly through the season for all comparisons except for between B. cryptarum and B. lucorum queens (A; see S. 7). Trend lines are model fits from generalised linear models representing quadratic relationships in (E & G) and linear relationships in (B, C & F); 95% confidence intervals are shown around these relationships. Panels D & H show how the cumulative abundance of overwintered queens (D) and workers (H) shifted through the season for each bumblebee species.
The relative abundance of foraging workers of the three species also varied throughout the season, reflecting distinct phenologies. Relative to either B. lucorum or B. cryptarum, B. magnus workers were significantly more common later in the season than they were at the beginning (Fig. 2F–H & Table S5). This meant that the period during which B. magnus workers were active coincided with the flowering of Erica cinerea and C. vulgaris. All B. magnus workers (n = 14) were on the wing when heather was flowering, whereas a lower percentage of all B. cryptarum (81%, n = 174) and B. lucorum (95%, n = 153) workers were flying at this time; nevertheless, this interspecific difference in the degree of activity bias toward the heather flowering season was not significant (Fisher Exact test P > 0.1). Comparing B. cryptarum and B. lucorum phenology, we found that at the beginning and end of the season, B. cryptarum workers were more common than B. lucorum, but in between, both species were equally abundant (Fig. 2E & Table S5). The strength of this seasonal shift in relative abundance varied according to the weather conditions (see below; Figs. S5 & S6).
Considering new reproductives, when males were first encountered (21 July), they were mostly B. lucorum but this trend reversed significantly later in the season so that B. cryptarum males became more common (Fig. 3 & Table S7). Only two B. magnus males were found in the entire study, but the first of these was found over a month later (27 August) than when the first B. cryptarum and B. lucorum males appeared (Fig. 3B). Only five new queens were captured (two B. cryptarum, two B. lucorum, and one B. magnus), too few to draw conclusions about their phenology.
Figure 3.
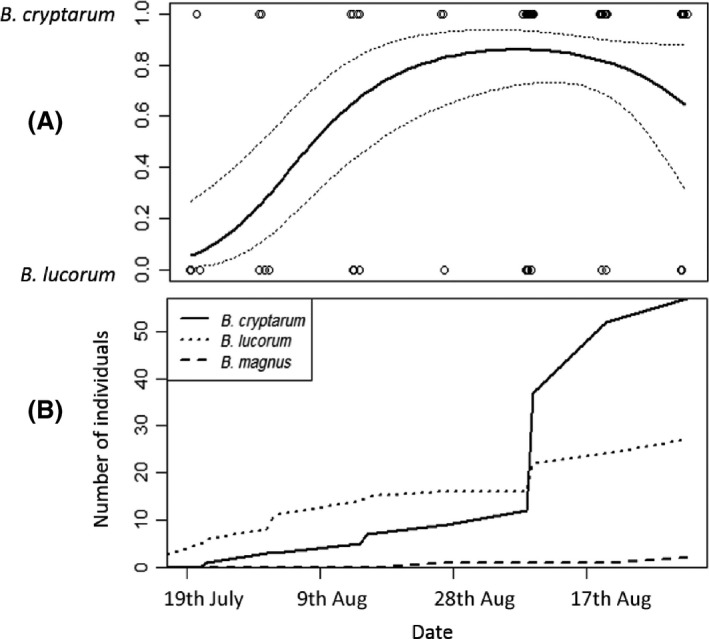
Interspecific variation in phenology of males of B. cryptarum, B. lucorum and B. magnus. (A) the probability that a male belonged to either B. cryptarum or B. lucorum changed significantly through the season. The trend line shows a quadratic relationship fit from a generalised linear model with 95% confidence intervals. The numbers of B. magnus males were too low to perform this analysis. (B) Changes in the cumulative number of males of each of the three bumblebee species over the season.
Effects of weather on activity of the three species
We used PCA to reduce the number of weather variables. The first PCA axis, which accounted for 40.28% of the total variation, exhibited a positive correlation with temperature and amount of sunshine: Increasingly positive values represent generally warmer and sunnier conditions (Figure S4B). The three species showed variation along this axis: The average observations for B. magnus were lower (negative values) than for B. lucorum (positive values), observations for B. cryptarum were intermediate (Figure S4A). The second PCA axis was negatively associated with the wind speed; however, there was little variation between the species in this metric (Figure S4A). Therefore, we substituted values from the first PCA axis (PCA 1) for the explanatory variables “temperature” and “level of sunshine” in subsequent analyses for worker and males. For overwintered queens, we found that using PCA 1, which represents a combined measure of warmth and sunniness, instead of the separate sunshine and temperature variables did not improve the best model; therefore, we retained both weather variables. Controlling for phenological variation (by retaining date as a fixed effect explanatory variable), B. magnus queens were relatively less active than B. lucorum in sunny conditions and relatively more active when it was overcast (Fig. 4A & Table S3). We did not detect any significant effect of weather on the relative abundance of B. cryptarum queens in comparison with queens of either of the other species (Table S3).
Figure 4.
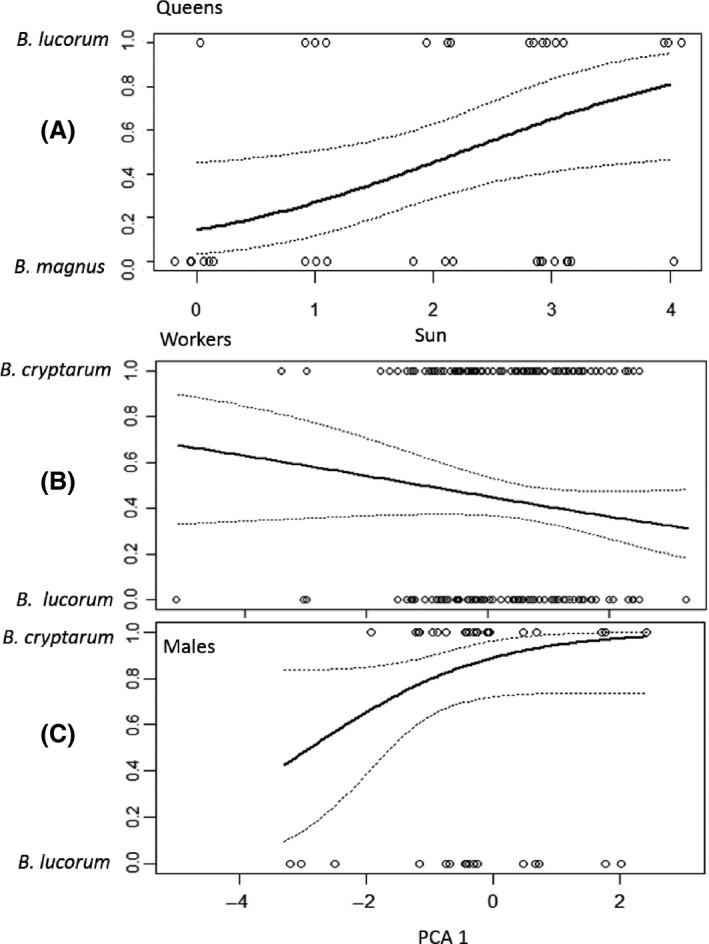
Differences in the effects of weather variables on the activity of lucorum complex species. (A) The level of sunshine differentially affected the abundance of queens of B. lucorum and B. magnus: The sun axis represents a scale ranging from 0 (heavy complete cloud cover) to 4 (<25% cloud cover; see Table S1). In (B & C), PCA 1 represents a scale where low values indicate cool cloudy conditions and higher values indicate warmer, sunnier conditions (see Fig. S4): Figures display the relative impact of changes in this weather axis on the activity of workers (B) and males (C) of B. cryptarum compared to B. lucorum. Trend lines are model fits from a generalised linear model with 95% confidence intervals.
Weather differentially affected worker activity for B. cryptarum and B. lucorum. Averaging across the whole season, B. cryptarum workers were relatively more common than B. lucorum workers when conditions were cooler and cloudier (although this effect was not significant: Fig. 4B & Table S5). Nevertheless, there was a significant effect of the interaction between the date quadratic term and PCA 1 (χ 2 2 = 7.57, P = 0.02, Table S5). This interaction demonstrated that while B. lucorum workers were rare early and late in the season, becoming relatively more common around midsummer, the midsummer increase in relative abundance was more pronounced when it was warm and sunny, compared to cooler cloudy conditions (Figure S6). Weather did not significantly affect the relative abundance of B. magnus workers compared to either of the other species. Considering males, there were fewer B. cryptarum males encountered compared to B. lucorum in the coolest, cloudiest conditions (Fig. 4C & Table S7).
Temperature varies throughout the day, so we tested whether the differential temperature effects on the probability of activity in B. cryptarum and B. lucorum led to temporal separation of foraging across the day. Model selection using AICc incorporating time of day instead of PCA 1, favored models incorporating time (436.7 vs. 432.8 AICc points), but the pattern was the same. The significant interaction between the quadratic terms, date, and time of day (χ 2 4 = 16.1, P < 0.005, Table S8) showed that the mid‐season peak in the relative abundance of B. lucorum workers was strongest early in the morning compared to later in the day. We did not detect differences in temporal activity in any other interspecific comparisons.
Niche overlap between the bumblebee species
We defined the niche region (NR) exploited by each bumblebee species based on the date, weather conditions, and time of day when each individual was out foraging. Among queens, there was little difference in NR and the probabilities of overlap were quite similar for all interspecific comparisons (overlap probability: 0.76–0.88; Fig. 5A & Table S10). Among workers, B. magnus had the smallest NR (Fig. 5B), which is due in large part to a narrower range of dates (later in the season) when they were on the wing. B. cryptarum workers displayed the largest NR, partly driven by the fact they were on the wing for the longest period (Fig. 5B). We therefore found strong asymmetry in the degree of interspecific niche overlap for workers. While B. magnus workers were highly likely to exploit the niche of B. cryptarum and B. lucorum workers (overlap probability: 0.94 and 0.95, respectively), the probability that B. cryptarum and B. lucorum workers were found in the niche of B. magnus workers was much lower (overlap probability: 0.47 and 0.50, respectively, Table S10).
Figure 5.
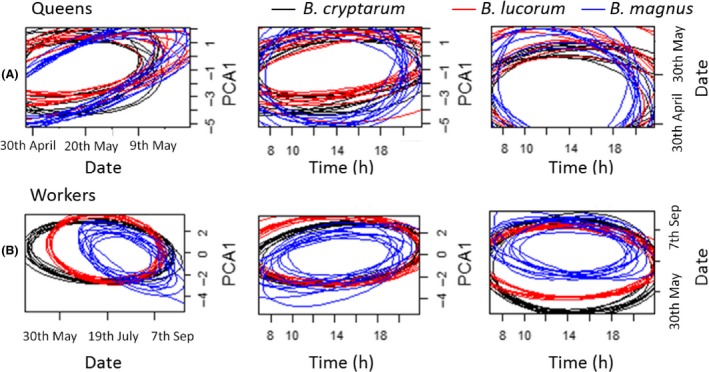
Comparisons of the niche size for the three lucorum complex species. Panels show ten random elliptical projections of niche region (NR) for each bumblebee species defined by pairs of variables for (A) queens and (B) workers. Niche regions were estimated based on the modeled influence of seasonal, weather and daily activity variables on occurrence. Each plot illustrates the projected niche region for a different combination of the three variables. PCA 1 represents a weather axis where low values indicate cool cloudy conditions and higher values indicate warmer, sunnier conditions (Fig. S4). Black lines represent B. cryptarum, red represent B. lucorum and blue represent B. magnus.
Forage use
Queens of all three bumblebee species were found feeding on a similar number of plant taxa (range 7–8). However, 52.7% of B. magnus queens (n = 14) were recorded visiting Erica spp., whereas only a single individual of both B. cryptarum (n = 13) and B. lucorum (n = 10) foraged on heather plants (Table S11). Previous studies (Waters et al. 2011; Scriven et al. 2015) suggest a tight association between B. magnus and heather (C. vulgaris and Erica spp.). We tested this explicitly and found that queens of the three bumblebee species differed significantly in their probability of foraging on heather, compared to all other plant species (χ 2 2 = 6.79, P < 0.05); parameter estimates show this was due to B. magnus queens foraging on this taxon more often than B. cryptarum, but post hoc tests did not detect any individually significant differences between pairs of species (P > 0.05).
Concerning workers, the plants most commonly visited by B. cryptarum were C. vulgaris (17.1%) and Erica spp. (15.3%). However, the proportional representation of these species in the B. cryptarum diet was markedly lower than for B. magnus, of which 84.6% foraged on C. vulgaris (61.5%) or Erica spp. (23.1%, Table S12). Only 26% of B. lucorum workers were foraging on these plant taxa. We found that, like queens, workers of the three bumblebee species also differed significantly in their probability of foraging on heather compared to all other plant species (χ 2 2 = 15.15, P < 0.001): Erica spp. and C. vulgaris made a significantly larger contribution to the diet of B. magnus workers than to the diets of B. cryptarum or B. lucorum (P < 0.01). This may in part be because B. magnus workers are only on the wing during the flowering period of Erica spp. and C. vulgaris; whereas workers of the other two species were also on the wing when these plant species were not available as food sources. However, considering just the period when Erica spp. and C. vulgaris were in flower, the bumblebee species still foraged on these plant species to differing extents (χ 2 2 = 16.92, P < 0.001): Comparisons were individually significant for both B. magnus ‐ B. cryptarum (P < 0.05) and B. magnus ‐ B. lucorum (P < 0.005). Moreover, during this heather flowering period, the probability of B. cryptarum foraging on Erica spp. and C. vulgaris was significantly greater than for B. lucorum (P < 0.05). The forage plant that made the greatest contribution to the diet of B. lucorum workers was Rubus sp. (27.6%), a species that contributed significantly less (12.9%) to the diet of B. cryptarum (χ 2 1 = 9.97, P = 0.002, Table S12).
Males of B. cryptarum and B. lucorum had a similar diet breadth; however, their diets only overlapped on four plant taxa (total taxa = 9 and 7, respectively, Table S13). The plant most frequently visited by B. cryptarum males (66.1%) was Succisa pratensis, which contributed significantly less (29.6%) to the diet of B. lucorum males (χ 2 1 = 8.3, P = 0.003). The other most often utilised forage plant for B. lucorum males was Chamerion angustifolium (29.6%), which was not used at all by B. cryptarum (Fisher's exact test P < 0.001). Only two B. magnus males were found preventing assessment of diet breadth for males of this species.
We calculated between‐species dissimilarity in diet composition using the Bray–Curtis coefficient (Bray and Curtis 1957). A Bray–Curtis distance value of zero indicates that the two forage assemblages are identical for both species, whereas a value of one means they are completely dissimilar. The degree of dietary dissimilarity between B. cryptarum and B. lucorum was almost identical for queens and workers (0.30 and 0.31, respectively), whereas for males, it was greater (0.69, Table 2). Among queens, the greatest difference in diet composition was between B. cryptarum and B. magnus. For workers, the degree of dissimilarity was greatest between B. magnus and the other two species (>0.6); the diets of B. lucorum and B. cryptarum workers were more similar (0.31, Table 2).
Table 2.
Differences in the plant species assemblages used as forage resources by the three lucorum complex species. Bray–Curtis distance measures showing the dissimilarity between the diets of each caste of each of the three bumblebee species. A value of 0 indicates that the two assemblages were identical, whereas a value of 1 indicates that they were completely different. B. magnus males are not included because the sample size was too low: Only overwintered queens are included
| Caste | B. cryptarum | B. lucorum | |
|---|---|---|---|
| B. lucorum | Queens | 0.30 | – |
| Workers | 0.31 | – | |
| Males | 0.69 | – | |
| B. magnus | Queens | 0.51 | 0.35 |
| Workers | 0.64 | 0.72 |
Discussion
The sympatric occurrence of cryptic species challenges ecological theory because their strong biological similarity should generate intense interspecific competition and potential competitive exclusion (Gause 1932; Hardin 1960; Cothran et al. 2013; Van Campenhout et al. 2014). Nevertheless, the lucorum complex contains three cryptic bumblebee species with near‐identical morphology, which co‐occur across large parts of the UK (Bertsch et al. 2005; Waters et al. 2011; Carolan et al. 2012; Scriven et al. 2015) and elsewhere (Murray et al. 2008; Stanley et al. 2013; Williams et al. 2012b). In this study of B. lucorum, B. cryptarum, and B. magnus, we demonstrate clearly that although the niches of these three cryptic species overlap considerably, they do have distinct ecologies. We reveal niche utilisation differences that may be sufficient to prevent competitive exclusion by reducing the intensity of interspecific competition. We also provide the first reliable evidence for differences in their phenology.
We focussed on interspecific variation in three fundamental biotic and abiotic niche‐use dimensions at a single site: differences in responses to weather conditions, different forage use, and different temporal activity patterns. B. magnus had the most distinct niche. It has a narrow, highly specialised diet, feeding predominantly on species of heather plant (Ericacae). The phenology of all three B. magnus castes was delayed relative to the other two species; furthermore, queens of B. magnus were more active in overcast conditions, compared to those of B. lucorum. In contrast, although all castes of B. cryptarum and B. lucorum were on the wing for the same period of time, B. lucorum workers showed a strong peak in abundance around midsummer, followed by an earlier peak in the production of male reproductives. B. lucorum worker activity was skewed toward warmer, sunnier conditions compared to B. cryptarum, and the elevated abundance of B. lucorum in midsummer was strongest in warm conditions. B. cryptarum had a different phenology: Worker numbers increased faster, and decreased more slowly as the season progressed, compared to B. lucorum. This may in part be because B. cryptarum is better adapted for activity in colder conditions: its workers foraged more in cooler cloudier conditions than did B. lucorum (though, conversely, we captured fewer B. cryptarum males in cool cloudy conditions). There was some subtle diurnal temporal separation of foraging behavior between B. lucorum and B. cryptarum: In midsummer, when B. lucorum was relatively most common, its workers were more numerous early in the morning and least active in the afternoon.
The broad conclusions that B. lucorum is adapted for activity in warmer sunnier conditions, whereas B. magnus and B. cryptarum are adapted to forage in cooler cloudier conditions, recapitulates previous species distribution analysis. Scriven et al. (2015) showed that across Great Britain, B. magnus and B. cryptarum were more commonly found at sites with lower summer temperatures. Our current findings may help explain why B. lucorum is ecologically dominant throughout much of lowland southern and eastern England where it is warmer and sunnier, whereas B. cryptarum and B. magnus tend to occur in upland and northerly locations (Scriven et al. 2015). Similarly, our demonstration that the colony cycle of B. magnus is delayed in the season relative to the other two species is supported by previous work at our fieldwork location in August 2013, when both workers and males of B. lucorum and B. cryptarum were present, whereas only B. magnus workers were detected (Scriven et al. 2015). Such variation in the timing of male production in these three species may reduce the likelihood of hybridisation, thereby reinforcing reproductive isolation.
The observation that B. magnus preferentially chose to forage on heather plants supports observations that B. magnus is more commonly found on heather moorland (Scriven et al. 2015). Previous studies also revealed differences in the diet of these three species; however, these studies have combined data from multiple sites. For example, Scriven et al. (2015) found that across the UK, B. lucorum had the broadest diet and B. magnus had the narrowest diet breadth. However, it is not clear whether this resulted from different forage preferences, because bee species with restricted geographic ranges may have access to a restricted range of forage plants. In the present study, all the bumblebees had the same forage plants to choose from; despite this, clear differences in their diets remained.
Cryptic species provide important case studies to investigate the types of niche‐utilisation traits that diverge most readily after speciation events. The lucorum complex species seem to have diverged relatively recently (<100,000 years ago; based on COI divergence and diversity reported by Carolan et al. 2012 and Murray et al. 2008, using the approach of Jiggins and Tinsley 2005, and the standard insect molecular clock of Brower 1994) and previous work suggests that B. magnus and B. cryptarum are the most closely related of the three (Bertsch et al. 2005; Murray et al. 2008; Williams et al. 2012b). The interspecific niche differentiation we have observed may have underlain this speciation process. Evolution in metabolic pathways or morphology may be responsible for the thermal specialisation for activity in cooler conditions exhibited by B. cryptarum and B. magnus. Bumblebees are facultatively endothermic, requiring preflight metabolic warm‐up, large body size, and thoracic insulation for flight. Thermal specialisation is an important mechanism that may reduce the strength of interspecific competition; it may also mean that the members of a community of bee species can offer complementary pollination services to plants (Herrera 1997; Peat et al. 2005; Lye et al. 2010; Frund et al. 2013). However, in our dataset, air temperature and time of day covaried (after accounting for seasonal changes); therefore, it is not possible to definitively rule out divergent circadian rhythms as an explanation for interspecific differences in the association between activity and temperature. The most dramatic aspect of niche divergence within this cryptic species complex is the strong preference in B. magnus to forage on the heathers, C. vulgaris, E. cinerea, and E. tetralix to the exclusion of other potentially suitable species that were common in the area.
We have shown significant differences along three niche dimensions of three cryptic species that are likely to facilitate their coexistence. However, there is also considerable niche overlap, which must lead to competition. Direct interference competition between these bumblebee species is unlikely, but there is the potential for exploitative competition for resources. An important resource for which both inter‐ and intraspecific competition may occur is pollen and nectar. Therefore, as all three lucorum complex species are present at this site and draw on similar resources, the differences found in their use of forage plants may possibly be driven by competition and reflect differences in their realised niches, rather than fundamental niches. In contrast, the other niche dimensions investigated, phenology and response to weather, are less likely to be influenced by competition, and may thus represent interspecific differences in the fundamental niche. Patterns of bumblebee visitation to the same plant species can vary through space and time, potentially as a response to variation in pollen abundance and quality (Vaudo et al. 2014) and also to avoid interspecific competition (Lye et al. 2010). We found that interspecific niche overlap was higher for queens than it was for either workers or males. However, seasonal changes in the abundance of forage plants relative to bumblebees mean that it is hard to determine the impact of this shift in niche overlap on the strength of interspecific competition acting on the different castes. In terms of the temporal and weather niche, B. magnus workers were most differentiated, with worker production delayed compared to the other species, potentially in order to coincide with the flowering of their principal forage plants. Consequently, despite being most differentiated, the niche of B. magnus workers was situated mostly within the niche region of the other two species. This creates an asymmetry in niche overlap, with B. magnus potentially suffering more strongly from competition than either of the species it interacts with. However, more specialised species are presumed to be more efficient in their preferred conditions than generalists (Pianka 1994). In the UK, C. vulgaris forms an important, and often dominant, component of both upland and lowland heaths (Thompson et al. 1995; Groves et al. 2012). As a specialist on heather species, B. magnus may be an optimal forager on this resource, exploiting it more efficiently than the other species. Furthermore, by delaying worker production until C. vulgaris and Erica spp. flower, B. magnus could be able to profit from this extremely abundant resource, limiting the impact of overlap along the other niche dimensions, while avoiding worker competition with the other two bumblebee species earlier in the season when resources are more limited.
The niche differences that we have observed in this study may assist co‐occurrence of these cryptic species by causing variation in the responses of each species to spatio‐temporal heterogeneity in seasonally changing foraging sites. When the resources available for colony growth are continuously changing, the competitive relations between colonies of different species can be reversed, leading to the maintenance of a larger number of species in a region (Westphal et al. 2006). The composition and abundance of bee populations have been shown to undergo considerable variation between years (Minckley et al. 1999; Oertli et al. 2005; Iserbyt and Rasmont 2012). Iserbyt and Rasmont (2012) found that in one mountainous region, the dominant bumblebee species one year was seldom dominant another year, some species disappeared totally for several years and the proportion of permanent species was low. We observed clear abundance differences in the bumblebee species: B. cryptarum was the most common species and B. magnus was by far the least abundant. Yet previous sampling in 2011 found the most common species to be B. magnus (50.5%), whereas B. cryptarum was the least common (19.4%, Scriven et al. 2015). Clearly, the relative proportions of B. magnus and B. cryptarum can vary strongly between years, suggesting that the two species do not respond to environmental fluctuations in the same way. This could therefore represent a system where ecological divergence, niche partitioning, and spatio‐temporal heterogeneity in the environment mean that none of the three species is able to consistently exclude another to the point of local extinction. This has considerable implications for conservation, as small alterations to any of these dimensions could modify interspecific interactions putting one or more species at risk. B. magnus relies heavily on threatened and declining heathland habitat; further losses could therefore shift the balance and seriously affect populations of this species. Studying these species over several consecutive years may reveal trends in the population composition linked to annual climatic variations and allow us to understand in more detail what climatic factors affect the success of these three species. Similarly, broadening the study to include other sites would demonstrate whether these patterns are consistent across areas.
The discovery of co‐occurring cryptic species presents problems for several areas of ecological theory: The limits of ecological differentiation required for species coexistence, phylogenetic limiting similarity, and competitive exclusion (Violle et al. 2011; Gabaldón et al. 2013; Van Campenhout et al. 2014). We show that a combination of varying levels of ecological divergence in different niche dimensions and spatio‐temporal heterogeneity in the environment may contribute to the persistence of cryptic species in sympatry. Furthermore, our study suggests that cryptic species provide distinct and unique ecosystem services, clearly demonstrating that morphological similarity between species does not necessarily equate to ecological equivalence.
Conflict of Interest
None declared.
Data accessibility
Data available from the Dryad Digital Repository: doi:10.5061/dryad.js1gm.
Supporting information
Table S1. Scale used for categorising amount of sun.
Table S2. Scale used for categorising rainfall.
Table S3. Phenological variation and differences in responses to weather conditions between over‐wintered queens of each bumblebee species.
Figure S4. Results of principal component analysis (PCA) on the variation in weather condition metrics (sun, wind speed, rain and air temperature) when each individual bumblebee was encountered. Axis 1 (PCA 1) and Axis 2 (PCA 2) describe 40.3% and 26.4% of the total variation respectively.
Table S5. Differences in phenology and responses to weather conditions of workers for each bumblebee species.
Figure S6. The effect of seasonality and changing weather conditions on the abundance of B. cryptarum and B. lucorum workers on the wing.
Table S7. Differences in phenology and responses to weather conditions between males of B. cryptarum and B. lucorum.
Table S8. Changes in seasonal and daily activity in workers of B. cryptarum and B. lucorum.
Figure S9. The effect of date and time of day on the abundance of B. cryptarum and B. lucorum workers on the wing.
Table S10. Differences in niche overlap between queens and workers of each of the three lucorum complex species.
Table S11. Forage use and measures of diet breadth for lucorum complex over‐wintered queens.
Table S12. Forage use and measures of diet breadth for B. lucorum complex workers.
Table S13. Forage use and measures of diet breadth for B. lucorum complex males.
Acknowledgments
We thank the National Trust for Scotland for allowing us to use their land and the Glencoe Visitor Centre staff and rangers in particular, for their help and advice. The project was funded by the University of Stirling.
References
- Barton, K. 2013. MuMIn: Multi‐model inference. R package version 1.9. 5.
- Bertsch, A. , Schweer H., Titze A., and Tanaka H.. 2005. Male labial gland secretions and mitochondrial DNA markers support species status of Bombus cryptarum and B. magnus (Hymenoptera, Apidae). Insectes Soc. 52:45–54. [Google Scholar]
- Bickford, D. , Lohman D. J., Sodhi N. S., Ng P. K. L., Meier R., Winker K., et al. 2007. Cryptic species as a window on diversity and conservation. Trends Ecol. Evol. 22:148–155. [DOI] [PubMed] [Google Scholar]
- Bray, J. R. , and Curtis J. T.. 1957. An Ordination of the upland forest community of southern Wisconsin.pdf. Ecol. Monogr. 27:325–349. [Google Scholar]
- Brower, A. 1994. Rapid morphological radiation and convergence among races of the butterfly Heliconius erato inferred from patterns of mitochondrial DNA evolution. Proc Natl Acad Sci USA 91:6491–6495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron, S. A. , Lozier J. D., Strange J. P., Koch J. B., Cordes N., Solter L. F., and Griswold T.. 2011. Patterns of widespread decline in North American bumble bees. Proc. Natl Acad. Sci. USA 108:662–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carolan, J. C. , Murray T. E., Fitzpatrick Ú., Crossley J., Schmidt H., Cederberg B., et al. 2012. Colour patterns do not diagnose species: quantitative evaluation of a DNA barcoded cryptic bumblebee complex. PLoS One 7:e29251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cothran, R. D. , Henderson K. A., Schmidenberg D., and Relyea R. A.. 2013. Phenotypically similar but ecologically distinct: differences in competitive ability and predation risk among amphipods. Oikos, 122:1429–1440. [Google Scholar]
- Ellis, J. S. , Knight M. E., Carvell C., and Goulson D.. 2006. Cryptic species identification: a simple diagnostic tool for discriminating between two problematic bumblebee species. Mol. Ecol. Notes 6:540–542. [Google Scholar]
- Feulner, P. G. D. , Kirschbaum F., Schugardt C., Ketmaier V., and Tiedemann R.. 2006. Electrophysiological and molecular genetic evidence for sympatrically occuring cryptic species in African weakly electric fishes (Teleostei: Mormyridae: Campylomormyrus). Mol. Phylogenet. Evol. 39:198–208. [DOI] [PubMed] [Google Scholar]
- Frund, J. , Dormann C. F., Holzschuh A., and Tscharntke T.. 2013. Bee diversity effects on pollination depend on functional complementarity and niche shifts. Ecology 94:2042–2054. [DOI] [PubMed] [Google Scholar]
- Gabaldón, C. , Montero‐Pau J., Serra M., and Carmona M. J.. 2013. Morphological similarity and ecological overlap in two rotifer species. PLoS One 8:23–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gause, G. 1932. Experimental studies on the struggle for existence I. Mixed population of two species of yeast. J. Exp. Biol. 9:389–402. [Google Scholar]
- Goulson, D. . 2010. Bumblebees: behaviour, ecology, and conservation. OUP, Oxford. [Google Scholar]
- Goulson, D. , and Darvill B.. 2004. Niche overlap and diet breadth in bumblebees; are rare species more specialized in their choice of flowers? Apidologie 35:55–63. [Google Scholar]
- Goulson, D. , Lye G. C., and Darvill B.. 2008. Decline and conservation of bumble bees. Annu. Rev. Entomol. 53:191–208. [DOI] [PubMed] [Google Scholar]
- Groves, J. A. , Waller M. P., Grant M. J., and Schofield J. E.. 2012. Long‐term development of a cultural landscape: the origins and dynamics of lowland heathland in southern England. Veg. Hist. Archaeobot., 21:453–470. [Google Scholar]
- Hardin, G. 1960. The competitive exclusion principle. Science 131:1292–1297. [DOI] [PubMed] [Google Scholar]
- Heinrich, B. 1976. Resource partitioning among some eusocial insects: bumblebees. Ecology 57:874–889. [Google Scholar]
- Herrera, C. M. 1997. Thermal biology and foraging responses of insect pollinators to the forest floor irradiance mosaic. Oikos 78:601–611. [Google Scholar]
- Holt, R. D. , Grover J., and Tilman D.. 1994. Simple rules for interspecific dominance in systems. Am. Nat. 144:741–771. [Google Scholar]
- Inouye, D. W. 1978. Resource partitioning in bumblebees: experimental studies of foraging behavior. Ecology 59:672–678. [Google Scholar]
- Iserbyt, S. , and Rasmont P.. 2012. The effect of climatic variation on abundance and diversity of bumblebees: a ten years survey in a mountain hotspot. nn. Soc. Entomol. Fr. 48:261–273. [Google Scholar]
- Jiggins, F. , and Tinsley M. C.. 2005. An ancient mitochondrial polymorphism in Adalia bipunctata linked to a sex‐ratio‐distorting bacterium. Genetics 171: 1115–1124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le, S. , Josse J., and Husson F.. 2008. FactoMineR: An R package for multivariate analysis. J Stat Softw 25: 1–18. [Google Scholar]
- Lye, G. C. , Kaden J. C., Park K. J., and Goulson D.. 2010. Forage use and niche partitioning by non‐native bumblebees in New Zealand: implications for the conservation of their populations of origin. J. Insect Conserv. 14:607–615. [Google Scholar]
- Minckley, R. L. , Cane J. H., Kervin L., and Roulston T. H.. 1999. Spatial predictability and resource specialization of bees (Hymenoptera: Apoidea) at a superabundant, widespread resource. Biol. J. Linn. Soc. 67:119–147. [Google Scholar]
- Murray, T. , Fitzpatrick U., Brown M., and Paxton R.. 2007. Cryptic species diversity in a widespread bumble bee complex revealed using mitochondrial DNA RFLPs. Conserv. Genet. 9:653–666. [Google Scholar]
- Oertli, S. , Müller A., and Dorn S.. 2005. Ecological and seasonal patterns in the diversity of a species‐rich bee assemblage (Hymenoptera: Apoidea: Apiformes. Eur. J. Entomol. 102:53–63. [Google Scholar]
- Oksanen, J. , Blanchet F. G., Kindt R., Legendre P., Minchin P. R., O'Hara R. B., et al. 2015. The vegan Package: Community Ecology Package.
- Ortells, R. , Gómez A., and Serra M.. 2003. Coexistence of cryptic rotifer species: ecological and genetic characterisation of Brachionus plicatilis . Freshw. Biol. 48:2194–2202. [Google Scholar]
- Peat, J. , Darvill B., Ellis J., and Goulson D.. 2005. Effects of climate on intra‐ and interspecific size variation in bumble‐bees. Funct. Ecol. 19:145–151. [Google Scholar]
- Pianka, E. 1994. Evolutionary ecology. HarperCollins College Publishers, New York, NY. [Google Scholar]
- R Core Team . 2014. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. [Google Scholar]
- Scriven, J. J. , Woodall L. C., Tinsley M. C., Knight M. E., Williams P. H., Carolan J. C., et al. 2015. Revealing the hidden niches of cryptic bumblebees in Great Britain: implications for conservation. Biol. Conserv. 182:126–133. [Google Scholar]
- Stanley, D. A. , Knight M. E., and Stout J. C.. 2013. Ecological variation in response to mass‐flowering oilseed rape and surrounding landscape composition by members of a cryptic bumblebee complex. PLoS One 8:e65516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart, B. L. , Inger R. F., and Voris H. K.. 2006. High level of cryptic species diversity revealed by sympatric lineages of Southeast Asian forest frogs. Biol. Lett. 2:470–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson, H. , Lysy M., and Power M.. 2015. A new probabilistic method for quantifying n‐dimensional ecological niches and niche overlap. Ecology 96:318–324. [DOI] [PubMed] [Google Scholar]
- Thompson, D. B. A. , Macdonald A. J., Marsden J. H., and Galbraith C. A.. 1995. Upland heather moorland in Great Britain: nature conservation. Biol. Conserv. 71:163–178. [Google Scholar]
- Van Campenhout, J. , Derycke S., Moens T., and Vanreusel A.. 2014. Differences in life‐histories refute ecological equivalence of cryptic species and provide clues to the origin of Bathyal Halomonhystera (Nematoda) (D Fontaneto, Ed). PLoS One 9:e111889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaudo, A. D. , Patch H. M., Mortensen D. A., Grozinger C. M., and Tooker J. F.. 2014. Bumble bees exhibit daily behavioral patterns in pollen foraging. Arthropod‐Plant Interact., 8:273–283. [Google Scholar]
- Violle, C. , Nemergut D. R., Pu Z., and Jiang L.. 2011. Phylogenetic limiting similarity and competitive exclusion. Ecol. Lett. 14:782–787. [DOI] [PubMed] [Google Scholar]
- Vodă, R. , Dapporto L., Dincă V., and Vila R.. 2015a. Cryptic matters: overlooked species generate most butterfly beta‐diversity. Ecography 38:405–409. [Google Scholar]
- Vodă, R. , Dapporto L., Dincă V., and Vila R.. 2015b. Why do cryptic species tend not to co‐occur? A case study on two cryptic pairs of butterflies. PLoS One 10:e0117802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh, P. S. , Metzger D. A., and Higuchi R.. 1991. Chelex 100 as a medium for simple extraction of DNA for PCR‐based typing from forensic material. Biotechniques 10:506–513. [PubMed] [Google Scholar]
- Waters, J. , Darvill B., Lye G. C., and Goulson D.. 2011. Niche differentiation of a cryptic bumblebee complex in the Western Isles of Scotland. Insect Conserv. Divers. 4:46–52. [Google Scholar]
- Westphal, C. , Steffan‐Dewenter I., and Tscharntke T.. 2006. Bumblebees experience landscapes at different spatial scales: possible implications for coexistence. Oecologia 149:289–300. [DOI] [PubMed] [Google Scholar]
- Williams, P. 1982. The distribution and decline of British bumble bees (Bombus Latr.). J. Apic. Res. 21:236–245. [Google Scholar]
- Williams, P . 2005. Does specialization explain rarity and decline among British bumblebees? A response to Goulson et al. Biol. Conserv., 122:33–43. [Google Scholar]
- Williams, P. 2007. The distribution of bumblebee colour patterns worldwide: possible significance for thermoregulation, crypsis, and warning mimicry. Biol. J. Linn. Soc. 92:97–118. [Google Scholar]
- Williams, P. H. , Araújo M. B., and Rasmont P.. 2007. Can vulnerability among British bumblebee (Bombus) species be explained by niche position and breadth? Biol. Conserv. 138:493–505. [Google Scholar]
- Williams, P. H. , An J., Brown M. J. F., Carolan J. C., Goulson D., Huang J., et al. 2012a. Cryptic bumblebee species: consequences for conservation and the trade in greenhouse pollinators. PLoS One 7:e32992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams, P. H. , Brown M. J. F., Carolan J. C., An J., Goulson D., Aytekin A. M., et al. 2012b. Unveiling cryptic species of the bumblebee subgenus Bombus s. str. worldwide with COI barcodes (Hymenoptera: Apidae). Syst. Biodivers. 10:21–56. [Google Scholar]
- Wolf, S. , Rhode M., and Moritz R. F. A.. 2010. The reliability of morphological traits in the differentiation of Bombus terrestris and B. lucorum (Hymenoptera : Apidae). Apidologie, 41:45–53. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Scale used for categorising amount of sun.
Table S2. Scale used for categorising rainfall.
Table S3. Phenological variation and differences in responses to weather conditions between over‐wintered queens of each bumblebee species.
Figure S4. Results of principal component analysis (PCA) on the variation in weather condition metrics (sun, wind speed, rain and air temperature) when each individual bumblebee was encountered. Axis 1 (PCA 1) and Axis 2 (PCA 2) describe 40.3% and 26.4% of the total variation respectively.
Table S5. Differences in phenology and responses to weather conditions of workers for each bumblebee species.
Figure S6. The effect of seasonality and changing weather conditions on the abundance of B. cryptarum and B. lucorum workers on the wing.
Table S7. Differences in phenology and responses to weather conditions between males of B. cryptarum and B. lucorum.
Table S8. Changes in seasonal and daily activity in workers of B. cryptarum and B. lucorum.
Figure S9. The effect of date and time of day on the abundance of B. cryptarum and B. lucorum workers on the wing.
Table S10. Differences in niche overlap between queens and workers of each of the three lucorum complex species.
Table S11. Forage use and measures of diet breadth for lucorum complex over‐wintered queens.
Table S12. Forage use and measures of diet breadth for B. lucorum complex workers.
Table S13. Forage use and measures of diet breadth for B. lucorum complex males.
Data Availability Statement
Data available from the Dryad Digital Repository: doi:10.5061/dryad.js1gm.


