Abstract
Background
Cataract and glaucoma are leading causes of blindness worldwide, and their co‐existence is common in elderly people. Glaucoma surgery can accelerate cataract progression, and performing both surgeries may increase the rate of postoperative complications and compromise the success of either surgery. However, cataract surgery may independently lower intraocular pressure (IOP), which may allow for greater IOP control among patients with co‐existing cataract and glaucoma. The decision between undergoing combined glaucoma and cataract surgery versus cataract surgery alone is complex. Therefore, it is important to compare the effectiveness of these two interventions to aid clinicians and patients in choosing the better treatment approach.
Objectives
To assess the relative effectiveness and safety of combined surgery versus cataract surgery (phacoemulsification) alone for co‐existing cataract and glaucoma. The secondary objectives include cost analyses for different surgical techniques for co‐existing cataract and glaucoma.
Search methods
We searched CENTRAL (which contains the Cochrane Eyes and Vision Group Trials Register) (2014, Issue 10), Ovid MEDLINE, Ovid MEDLINE In‐Process and Other Non‐Indexed Citations, Ovid MEDLINE Daily, Ovid OLDMEDLINE (January 1946 to October 2014), EMBASE (January 1980 to October 2014), PubMed (January 1948 to October 2014), Latin American and Caribbean Health Sciences Literature Database (LILACS) (January 1982 to October 2014), the metaRegister of Controlled Trials (mRCT) (www.controlled‐trials.com), ClinicalTrials.gov (www.clinicaltrials.gov), and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp/search/en). We did not use any date or language restrictions in the electronic searches for trials. We last searched the electronic databases on 3 October 2014.
We checked the reference lists of the included trials to identify further relevant trials. We used the Science Citation Index to search for references to publications that cited the studies included in the review. We also contacted investigators and experts in the field to identify additional trials.
Selection criteria
We included randomized controlled trials (RCTs) of participants who had open‐angle, pseudoexfoliative, or pigmentary glaucoma and age‐related cataract. The comparison of interest was combined cataract surgery (phacoemulsification) and any type of glaucoma surgery versus cataract surgery (phacoemulsification) alone.
Data collection and analysis
Two review authors independently assessed study eligibility, collected data, and judged risk of bias for included studies. We used standard methodological procedures expected by the Cochrane Collaboration.
Main results
We included nine RCTs, with a total of 655 participants (657 eyes), and follow‐up periods ranging from 12 to 30 months. Seven trials were conducted in Europe, one in Canada and South Africa, and one in the United States. We graded the overall quality of the evidence as low due to observed inconsistency in study results, imprecision in effect estimates, and risks of bias in the included studies.
Glaucoma surgery type varied among the studies: three studies used trabeculectomy, three studies used iStent® implants, one study used trabeculotomy, and two studies used trabecular aspiration. All of these studies found a statistically significant greater decrease in mean IOP postoperatively in the combined surgery group compared with cataract surgery alone; the mean difference (MD) was ‐1.62 mmHg (95% confidence interval (CI) ‐2.61 to ‐0.64; 489 eyes) among six studies with data at one year follow‐up. No study reported the proportion of participants with a reduction in the number of medications used after surgery, but two studies found the mean number of medications used postoperatively at one year was about one less in the combined surgery group than the cataract surgery alone group (MD ‐0.69, 95% CI ‐1.28 to ‐0.10; 301 eyes). Five studies showed that participants in the combined surgery group were about 50% less likely compared with the cataract surgery alone group to use one or more IOP‐lowering medications one year postoperatively (risk ratio (RR) 0.47, 95% CI 0.28 to 0.80; 453 eyes). None of the studies reported the mean change in visual acuity or visual fields. However, six studies reported no significant differences in visual acuity and two studies reported no significant differences in visual fields between the two intervention groups postoperatively (data not analyzable). The effect of combined surgery versus cataract surgery alone on the need for reoperation to control IOP at one year was uncertain (RR 1.13, 95% CI 0.15 to 8.25; 382 eyes). Also uncertain was whether eyes in the combined surgery group required more interventions for surgical complications than those in the cataract surgery alone group (RR 1.06, 95% CI 0.34 to 3.35; 382 eyes). No study reported any vision‐related quality of life data or cost outcome. Complications were reported at 12 months (two studies), 12 to 18 months (one study), and two years (four studies) after surgery. Due to the small number of events reported across studies and treatment groups, the difference between groups was uncertain for all reported adverse events.
Authors' conclusions
There is low quality evidence that combined cataract and glaucoma surgery may result in better IOP control at one year compared with cataract surgery alone. The evidence was uncertain in terms of complications from the surgeries. Furthermore, this Cochrane review has highlighted the lack of data regarding important measures of the patient experience, such as visual field tests, quality of life measurements, and economic outcomes after surgery, and long‐term outcomes (five years or more). Additional high‐quality RCTs measuring clinically meaningful and patient‐important outcomes are required to provide evidence to support treatment recommendations.
Plain language summary
Combined glaucoma and cataract surgery versus cataract surgery alone for eyes with cataract and glaucoma
Review question The aim of this systematic review was to compare the effectiveness and safety of combined glaucoma and cataract surgery compared with cataract surgery alone.
Background Cataract and glaucoma are leading causes of blindness worldwide. Good vision requires a transparent lens in the eye. Cataract is a clouding of the lens that is increasingly common with age. The most common treatment for cataract is surgery, in which the cloudy lens of a person's eye is removed and, usually, replaced with an artificial lens. Glaucoma is a chronic progressive disease of the optic nerve which leads to irreversible vision loss. The most important risk factor associated with glaucoma is high pressure in the eye, known as intraocular pressure (IOP). Thus, glaucoma treatment aims to lower IOP and prevent loss of vision. When medications and laser treatment are no longer able to lower IOP, surgery is necessary. The most common glaucoma surgery is called trabeculectomy, which creates an opening in the wall of the eye to release fluid from within the eye and reduce the IOP.
Since many elderly people have both cataract and glaucoma, the decision to perform both surgeries at the same time or cataract surgery alone must be made. This decision is difficult to make because glaucoma surgery can accelerate cataract progression, cataract surgery can lower IOP independently, and performing both surgeries may increase the rate of complications.
Study characteristics We included nine studies in which a total of 655 people (657 eyes) were enrolled. Participants had glaucoma and age‐related cataract, and each study compared combined cataract and glaucoma surgery versus cataract surgery alone. Seven trials were conducted in Europe, one in Canada and South Africa, and one in the United States. Three trials were conducted at multiple centers, and the follow‐up period ranged from 12 to 30 months after surgery. The evidence is current to 3 October 2014.
Key results We concluded from the available evidence that combined glaucoma and cataract surgery may lead to slightly greater decreases in IOP one year after surgery compared with cataract surgery alone. However, due to differences in the effects among the individual studies and potential for bias in the study results, this conclusion is not definitive. The effect between combined surgery and cataract surgery alone on the rate of complications was uncertain. No information was available for long‐term outcomes (five or more years after surgery).
Quality of the evidence Overall, the quality of the evidence was very low to low due to differences in study characteristics (e.g., type of glaucoma surgery) and poor reporting of outcomes from included studies. These factors may influence the treatment effects when comparing combined glaucoma and cataract surgery versus cataract surgery alone.
Summary of findings
Summary of findings for the main comparison. Combined surgery versus phacoemulsification alone for eyes with cataract and glaucoma.
| Combined surgery versus phacoemulsification alone for eyes with cataract and glaucoma | ||||||
| Patient or population: patients with eyes with cataract and glaucoma Settings: eye clinic Intervention: combined surgery (phacoemulsification and any type of glaucoma surgery) Comparison: phacoemulsification alone | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk: Cataract surgeryalone | Corresponding risk: Combined surgery | |||||
| Mean change in IOP Measured by Goldmann applanation Follow‐up: one year after surgery | The mean change in IOP at one year after surgery ranged across cataract surgery alone groups from 5.8 mmHg to 1.0 mmHg lower | The mean change in IOP at one year after surgery in the combined surgery groups was on average 1.62 mmHg lower than cataract surgery alone (95% CI 2.61 mmHg to 0.64 mmHg lower) | MD ‐1.62 mmHg (‐2.61 to ‐0.64) | 489 (6 studies) | ⊕⊕⊝⊝ low1,2,3 | No evidence for a difference in effect according to type of glaucoma surgery (trabeculectomy, iStent®, and trabeculotomy); Of 3 studies not included in meta‐analysis due to insufficient reporting of data or reporting data at other follow‐up times, two studies reported more reduction in IOP in the combined surgery group compared with the cataract surgery alone group and one study reported no difference between groups |
| Mean change in medications Measured by number of bottles Follow‐up: one year after surgery | The mean change in medication at one year ranged across cataract surgery alone groups from 1 bottle to 0.5 bottles fewer | The mean change in medication at one year in the combined surgery groups was on average 0.69 bottles fewer (95% CI 1.28 to 0.1 fewer) |
MD ‐0.69 bottles (‐1.28 to ‐0.10) |
301 (2 studies) | ⊕⊕⊝⊝ low1,2,3 | |
| Proportion using one or more medications at one year after surgery Number of participants Follow‐up: one year after surgery | Study population | RR 0.47 (0.28 to 0.80) | 453 (5 studies) | ⊕⊕⊝⊝ low1,2,3 | ||
| 592 per 1000 | 278 per 1000 (166 to 474) | |||||
| Proportion who received re‐operation to control IOP within one year Number of participants Follow‐up: one year after surgery | Study population | RR 1.13 (0.15 to 8.25) | 382 (4 studies) | ⊕⊕⊝⊝ low2,3,4 | ||
| 15 per 1000 | 17 per 1000 (2 to 124) | |||||
| Proportion who received intervention for surgical complications within one year Number of participants Follow‐up: one year after surgery | Study population | RR 1.06 (0.34 to 3.35) | 382 (4 studies) | ⊕⊕⊝⊝ low2,3,4 | ||
| 245 per 1000 | 260 per 1000 (83 to 821) | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
CI: confidence interval; IOP: intraocular pressure; MD: mean difference; RR: risk ratio
*The basis for the assumed risk is the mean cataract surgery alone group risk across studies. The corresponding risk (and its 95% CI) is based on the assumed risk in the cataract surgery alone group and the relative effect of the combined surgery (and its 95% CI).
1Substantial statistical heterogeneity. 2Risk of bias assessed as high or unclear among studies. 3Studies measuring outcome did not report sufficient information for meta‐analysis. 4Wide CI (imprecision) and crosses the line of appreciable benefit.
Background
Description of the condition
Cataract and glaucoma are the most common causes of visual impairment worldwide (Resnikoff 2004). Projecting from the 2000 United States census data, the number of cataract patients is estimated to increase by 50% to 30.1 million patients by 2020 (Congdon 2004). In addition, the number of glaucoma patients worldwide is expected to increase from 60.5 million in 2010 to 79.6 million by 2020 (Quigley 2006). Co‐existence of cataract and glaucoma is a common problem in elderly people.
Cataract reduces vision when opacification (cloudiness) of the crystalline lens inside the eye interferes with the transmission of light to the retina. The definitive treatment for cataract is surgery. Non‐surgical treatment for cataract consists of changing prescription eyeglasses, but eyeglasses do not cure the disease.
Glaucoma is an optic neuropathy with a characteristic pattern of damage to the optic nerve that results in irreversible vision loss. Glaucoma is categorized into open‐angle and closed‐angle, based upon the configuration of the anterior chamber angle. The level of intraocular pressure (IOP) correlates with the risk of development of glaucoma and progression of glaucoma (Anderson 2003; Lee 2003; Leske 2003). Changes in the visual field, optic nerve, and nerve fiber layer are monitored for progression to glaucoma. Lowering IOP can prevent the development of glaucoma in eyes with elevated IOP, and slow the worsening of glaucoma in eyes with established glaucoma damage (Anderson 2003; Lee 2003; Leske 2003; Vass 2007). When elevated IOP is diagnosed, the ophthalmologist typically prescribes eyedrops. When medications and laser treatment are insufficient, glaucoma surgery is performed to lower the IOP (Burr 2012; Rolim de Moura 2007).
Description of the intervention
Surgical removal of the cataractous lens is the only way to cure the condition. Phacoemulsification is the most commonly performed surgery for cataract in high‐income countries (Leaming 2003) and produces the best visual outcomes (Riaz 2013; de Silva 2014). It uses the principle of ultrasound to emulsify the lens into small fragments and then aspirate it through the tip of the machine. After the cataractous lens has been removed, an artificial refractive correction lens is implanted in the lens capsule.
Among patients with severe glaucoma, surgery has been shown to reduce the rate of progression more effectively than medication (Burr 2012). Glaucoma surgery lowers the IOP either by increasing the outflow of aqueous humor from the eye or by decreasing the production of aqueous humor within the eye. Outflow procedures include trabeculectomy, trabeculotomy, trabecular aspiration, viscocanalostomy, aqueous drainage devices, trabectome, canaloplasty, and iStent® (Glaukos Corporation, USA) (Dietlein 2008; Godfrey 2009; Minckler 2006; Minckler 2009). Procedures that reduce aqueous humor production, and hence lower IOP, are endoscopic cyclophotocoagulation and transscleral cyclophotocoagulation. Both of these procedures use laser energy to damage the ciliary processes (Lin 2008). When eyes have co‐existing cataract and glaucoma, surgery for both conditions may be performed during one visit to the operating room or at different visits.
How the intervention might work
Although the primary goal of cataract surgery is to improve vision by replacing the opacified crystalline lens with a transparent intraocular lens (IOL) prosthesis, cataract surgery has been reported to reduce IOP in both open‐ and closed‐angle glaucoma (Shingleton 2006; Shrivastava 2010; Tham 2008; Tham 2009). The mechanism by which cataract surgery lowers IOP in open‐angle glaucoma is unclear, but may involve increasing the facility of aqueous outflow (Meyer 1997). In closed‐angle glaucoma, cataract surgery, specifically phacoemulsification, reduces IOP by widening the angle and moving the ciliary process backwards (Nonaka 2006; Tham 2010b). The ability to reduce IOP is related to the degree of angle closure. Lowering IOP reduces the risk of optic nerve damage related to the IOP, and reduces or prevents the irreversible vision loss that is the hallmark of glaucoma. Combined cataract and glaucoma surgery is another option to treat patients who have co‐existing glaucoma and cataracts. If the IOP‐lowering effects of the two procedures are additive, the combined procedure would be expected to lower IOP more than either cataract surgery alone or glaucoma surgery alone.
Why it is important to do this review
Co‐existing cataract and glaucoma is common in elderly people, and its rate increases significantly after the age of 60 (Quigley 2006). When a patient with advanced glaucoma (i.e., uncontrolled IOP or using maximal medical therapy) also requires surgical intervention for cataract, the ophthalmologist must decide whether to perform simultaneous cataract and glaucoma surgery or cataract surgery alone. This decision is complex for several reasons, including the fact that certain types of glaucoma surgery can accelerate the progression of cataract (Costa 1993; Edmunds 2002; Gedde 2009); cataract surgery has an IOP‐lowering effect of its own (Kim 2009; Lee 2009; Mathalone 2005; Poley 2009; Shingleton 2006); cataract surgery after glaucoma surgery may compromise IOP control (Halikiopoulos 2001; Wang 2009); and there are many surgical options available for glaucoma. On the other hand, performing glaucoma surgery after phacoemulsification may decrease the success rate of glaucoma surgery (Takihara 2014). In addition the rate of complications such as anterior chamber shallowing, conjunctival wound leak, and choroidal detachment in combined cataract and glaucoma surgery is higher than in cataract surgery alone (Tham 2010a), which may make some patients uncomfortable with undergoing combined surgery. Uncertainty about the better strategy is the reason why this Cochrane review is important.
Objectives
To assess the relative effectiveness and safety of combined surgery versus cataract surgery (phacoemulsification) alone for co‐existing cataract and glaucoma. The secondary objectives include cost analyses for different surgical techniques for co‐existing cataract and glaucoma.
Methods
Criteria for considering studies for this review
Types of studies
We included only randomized controlled trials (RCTs) in this review.
Types of participants
We included RCTs of participants with a history of open‐angle, pseudoexfoliative, or pigmentary glaucoma and age‐related cataract. We excluded trials in which participants had other types of secondary glaucoma, congenital glaucoma, angle‐closure glaucoma, or had earlier glaucoma surgery (not including iridectomy, iridoplasty, and trabeculoplasty), unless outcomes were reported separately by type of glaucoma.
Types of interventions
We included trials in which participants were randomized either to cataract surgery (phacoemulsification) alone or to combined cataract surgery (phacoemulsification) and any type of glaucoma surgery. We excluded RCTs of other types of cataract surgery or RCTs in which combined surgery had been compared with glaucoma surgery alone. We included trials in which adjunctive antimetabolites such as mitomycin C and 5‐Fluorouracil (5‐FU) were used during or after surgery.
Types of outcome measures
Primary outcomes
The primary outcome for comparison of treatments was the mean change in IOP from baseline, measured by Goldmann applanation or other validated measures as reported by studies, at one year after surgery. As we anticipated that time points for assessments of IOP would vary appreciably by trial, we also considered other time points as reported from included studies (e.g., two years).
Secondary outcomes
Secondary outcomes for comparison of treatments included:
proportion of participants with a reduction in the number of medications at one year after surgery (combination medications counted separately);
mean change in best corrected visual acuity (BCVA) in LogMAR units (in some cases converted from Snellen fractions) from baseline to one year after surgery;
mean change in visual field parameters at one year after surgery; visual field parameters include corrected pattern standard deviation, glaucoma hemifield test, statpac total deviation, and mean deviation change. We included all validated measures as reported in the included studies;
proportion of participants who received reoperation to control IOP within one year after surgery;
proportion of participants who received intervention for surgical complications within one year after surgery;
vision‐related quality of life (NEI‐VFQ or any other vision/glaucoma‐specific quality of life scores reported) at one year after surgery
We recorded and compared the proportion of participants reported to have experienced clinically important adverse outcomes, including hypotony, infection, suprachoroidal hemorrhage, and any others reported within one year after surgery and at other time points as reported from included studies.
Search methods for identification of studies
Electronic searches
We searched CENTRAL (which contains the Cochrane Eyes and Vision Group (CEVG) Trials Register) (2014, Issue 10), Ovid MEDLINE, Ovid MEDLINE In‐Process and Other Non‐Indexed Citations, Ovid MEDLINE Daily Update, Ovid OLDMEDLINE (January 1946 to October 2014), EMBASE (January 1980 to October 2014), PubMed (January 1948 to October 2014), Latin American and Caribbean Health Sciences Literature Database (LILACS) (January 1982 to October 2014), the metaRegister of Controlled Trials (mRCT) (www.controlled‐trials.com), ClinicalTrials.gov (www.clinicaltrial.gov), and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp/search/en). We did not use any date or language restrictions in the electronic searches for trials. We last searched the electronic databases on 3 October 2014.
See the appendices for details of search strategies for CENTRAL (Appendix 1), MEDLINE (Appendix 2), EMBASE (Appendix 3), PubMed (Appendix 4), LILACS (Appendix 5), mRCT (Appendix 6), ClinicalTrials.gov (Appendix 7), and ICTRP (Appendix 8).
Searching other resources
We searched the reference lists of included studies to identify any additional trials for inclusion, and used the Science Citation Index‐Expanded database to identify additional studies that may have cited trials included in this review. We did not handsearch conference proceedings or journals specifically for the purpose of this review.
Data collection and analysis
Selection of studies
Two review authors independently reviewed titles and abstracts of records identified from searches according to the Criteria for considering studies for this review stated above. We classified each record as 'definitely relevant', 'possibly relevant', or 'definitely not relevant'. We resolved any disagreement through discussion and retrieved the full‐text reports for records classified as 'definitely relevant' or 'possibly relevant'. Two review authors independently assessed the full‐text reports and classified each as either 'include', 'unsure', or 'exclude'. We contacted study investigators whose reports we classified as 'unsure' for further information to determine eligibility when needed after examining the available study reports. After two attempts with no response after two or more weeks, we assessed the studies based on the information provided by the study reports. We resolved any disagreement in study eligibility through discussion.
We reported studies excluded after full review and documented the reasons for exclusion in the 'Characteristics of excluded studies' table. We classified some included studies as 'ongoing' when the study was eligible but not yet completed, or study results were not yet available, and listed these in the 'Characteristics of ongoing studies' table.
Data extraction and management
Two review authors independently extracted data related to study methods, participant characteristics, and outcomes using forms developed by the CEVG. One review author entered data into RevMan 2014 and a second author verified all values. We resolved any discrepancies through discussion.
Assessment of risk of bias in included studies
Two review authors independently assessed the included studies for sources of potential bias according to the guidelines in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a). We evaluated the studies for the following criteria: selection bias (sequence generation and allocation concealment before randomization), detection bias (masking (i.e., blinding) of outcome assessors), attrition bias (incomplete outcome data), reporting bias (selective outcome reporting), and other sources of bias. We judged each included study to be at 'low', 'high', or 'unclear' (information insufficient to assess) risk for each potential source of bias. We did not assess individual studies for performance bias (masking of participants) because we judge the main outcomes of this review (IOP, visual acuity, etc.) unlikely to be influenced by lack of masking of participants.
We resolved disagreements through discussion. We contacted the study investigators for additional information on issues that were unclear after reviewing the study reports. Whenever the primary investigator did not respond within two weeks, we assessed the risk of bias on the basis of the available information. One review author entered data into the 'Characteristics of included studies' table and a second review author verified the data.
Measures of treatment effect
For continuous outcomes, we considered the normality of distributions and calculated mean differences (MDs) with 95% confidence intervals (CIs) for measurements judged to be normally or nearly normally distributed. Continuous outcomes for this review included the primary outcome (mean change of IOP at one year from baseline) and some of the vision‐related secondary outcomes (mean change in visual acuity, visual field parameters, and vision‐related quality of life scores). We chose the mean difference approach to estimate effects because all the included studies reported data on the same scale so that the standardized mean difference approach, as outlined in Chapter 9 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b), was unnecessary.
For dichotomous outcomes, we calculated summary risk ratios (RRs) with 95% CIs. Dichotomous outcomes for this review included the proportion of participants with a reduction in the number of medications after surgery, the proportion of participants who received reoperation to control IOP, the proportion of participants who had received interventions for surgical complications, and the proportion of participants who had experienced one or more adverse outcomes.
We planned to provide a narrative summary of any available economic data when data were available. However, none of the included studies reported economic data.
Unit of analysis issues
The unit of analysis was mostly the participant (one eye per participant); one study included both eyes of two participants. For analyses in which both eyes of these two participants were included, the unit of analysis was the eye.
Dealing with missing data
We contacted the investigators of included studies to request details regarding study methods, outcome data, standard deviations for means, and any other desired information that had not been reported or had been reported unclearly. When an investigator did not respond within two weeks, we used information available in the study reports. We did not impute any data.
Assessment of heterogeneity
We assessed clinical and methodological heterogeneity by examining potential variations in participant characteristics, inclusion and exclusion criteria, and assessments of primary and secondary outcomes. We used the I2 statistic (expressed as a percentage) to estimate the proportion of variation due to statistical heterogeneity and considered a value above 50% as suggesting substantial statistical heterogeneity. We also examined the result of the Chi2 test for heterogeneity and the degree of overlap in CIs on effect estimates from included studies. We considered poor overlap of CIs to suggest heterogeneity.
Assessment of reporting biases
We planned to assess selective outcome reporting by comparing the outcomes reported in the included studies versus the outcomes listed in the study protocols; however, we were unable to obtain the protocol for any included study. To assess for selective outcome reporting, we compared outcome measures described in the Methods section of study reports with outcomes reported in the Results section. We did not have a sufficient number of trials (n ≥ 10) to examine the funnel plots of the intervention effect estimates for evidence of asymmetry. A symmetric funnel plot is expected in the absence of publication bias. An asymmetric funnel plot may imply possible selection/publication bias, poor reporting of small trials, true heterogeneity, or chance.
Data synthesis
When clinical or methodological heterogeneity was observed, we did not combine studies in a meta‐analysis. When the I2 statistic and an inspection of the forest plot did not suggest substantial heterogeneity, we combined the results of included trials in a meta‐analysis using a random‐effects model. If the I2 statistic was suggestive of substantial heterogeneity (above 50%), we combined the results of included trials if the direction of estimates across studies were in agreement. We used a fixed‐effect model whenever the number of trials included in a meta‐analysis was fewer than three.
Subgroup analysis and investigation of heterogeneity
We conducted subgroup analyses by glaucoma surgery type for the primary outcome. We planned to analyze included studies by glaucoma diagnosis type and those in which antimetabolites were used in both treatment groups separately from those in which antimetabolites were used in only one treatment group, and those in which antimetabolites were not used in either treatment group. However, none of the included studies provided the data for such subgroup analyses.
When there was substantial statistical heterogeneity and evidence of potential clinical or methodological heterogeneity and sufficient data were available, we summarized the possible reasons for heterogeneity from comparisons of participant characteristics, follow‐up duration, method of accounting for losses to follow‐up, number of withdrawals, and other clinical or methodological characteristics as appropriate.
Sensitivity analysis
We did not conduct planned sensitivity analyses to determine the impact of excluding studies at higher risk of bias (specifically for incomplete outcome data and selective outcome reporting), industry‐funded studies, and those studies unpublished at the time of this review due to insufficient differences in these criteria among studies.
Summary of findings
We presented a summary of findings table of relative and absolute risks based on the risks across intervention groups from included studies. Two authors independently graded the overall quality of the evidence for each outcome using the GRADE classification (www.gradeworkinggroup.org/).
Results
Description of studies
Results of the search
The searches of electronic databases performed on 3 October 2014 resulted in a total of 8748 records (Figure 1). After eliminating duplicate records and conducting manual searches, we screened 5049 unique records and excluded 5025 references that were not relevant to the review. We retrieved full‐text reports for 24 potentially relevant records and two review authors independently assessed the eligibility of each study. We excluded eight full‐text reports from seven studies, included 10 reports from nine studies, and identified five reports from five ongoing trials. The one remaining report is awaiting classification until we can confirm whether the study is a RCT. We attempted to contact investigators of six trials for additional data via their email addresses as the corresponding authors of reports (Fea 2010;Georgopoulos 2000;Jacobi 1999;Liaska 2014;Samuelson 2011;Storr‐Paulsen 1998). Only Storr‐Paulsen 1998 responded. The email addresses of the investigators of two trials were no longer valid (Georgopoulos 2000; Jacobi 1999); we received no response from three other trial investigators (Fea 2010;Liaska 2014;Samuelson 2011).
1.
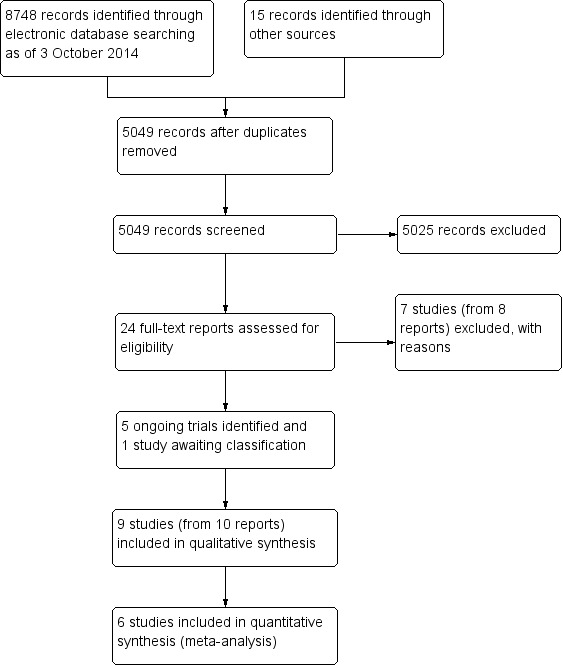
Study flow diagram.
Included studies
We included nine studies (total of 657 eyes of 655 participants) in our review (Anders 1997; Fea 2010; Fernández‐Barrientos 2010; Georgopoulos 2000; Gimbel 1995; Jacobi 1999; Liaska 2014; Samuelson 2011; Storr‐Paulsen 1998). Here we provide a summary of the study characteristics; additional study details are shown in Table 2 and the Characteristics of included studies section.
1. Comparison of included studies' characteristics.
| Studies | RCT type | Country | Participants (men/women) | Age in years for combined surgery group* | Age in years for cataract surgery alone group* | Diagnosis | Glaucoma intervention | Follow‐up time in months | Main outcome |
| Anders 1997 | Single center | Germany | 21/64 | 78.2 ± 7.9 | 74.9 ± 9.6 | POAG | Filtering surgery | 12 | Postoperative mean IOP |
| Fea 2010 | Single center | Italy | 13/23 | 64.5 ± 3.4 | 64.9 ± 3.1 | POAG | iStent® | 15 | Postoperative mean IOP |
| Fernández‐Barrientos 2010 | Multicenter | Spain | 15/18 | 75.2 ± 7.2 | 76.7 ± 5.8 | POAG or OHT | 2 iStents® | 12 | Aqueous flow change |
| Georgopoulos 2000 | Single center | Greece | NR | 67.4 ± 4.8 | 65.8 ± 4.4 | PEXG | Trabecular aspiration | Mean 15.7 (range 12 to 18) | Postoperative mean IOP |
| Gimbel 1995 | Multicenter | Canada | NR | 75.5 | 77.5 | POAG, OHT PG, PEXG | Trabeculotomy | 24 | Postoperative mean IOP |
| Jacobi 1999 | Single center | Germany | 28/20 | 69.4 ± 5.6 | 71.3 ± 6.1 | PEXG | Trabecular aspiration | 30 | Postoperative mean IOP |
| Liaska 2014 | Single center | Greece | 32/28 | 77 ± 6.7 | 78.1 ± 7.26 | POAG | Filtering surgery | 24 | Postoperative IOP, visual field mean deviation, visual acuity, number of glaucoma medications |
| Samuelson 2011 | Multicenter | USA | 98/142 | 74 ± 8 | 73 ± 9 | POAG, PG, PEXG | iStent® | 12 | Proportion of participants with IOP ≤ 21 mmHg without ocular medication |
| Storr‐Paulsen 1998 | Single center | Denmark | 4/16 | Median 79 (57 to 83) | Median 81 (77 to 88) | POAG | Filtering surgery | 12 | Postoperative median IOP |
*values are mean ± standard deviation unless otherwise noted.
IOP: intraocular pressure; NR: not reported; OHT: ocular hypertension; PEXG: pseudoexfoliation glaucoma; PG: pigmentary glaucoma; POAG: primary open‐angle glaucoma; RCT: randomized controlled trial.
Settings
Seven of the nine studies were conducted in Europe: two in Germany (Anders 1997; Jacobi 1999), two in Greece (Georgopoulos 2000; Liaska 2014), and one each in Italy (Fea 2010), Spain (Fernández‐Barrientos 2010), and Denmark (Storr‐Paulsen 1998). The remaining two studies were conducted in Canada and South Africa (Gimbel 1995), and the United States (Samuelson 2011). Three studies were multicenter trials: Samuelson 2011 (29 sites), Fernández‐Barrientos 2010 (three sites), and Gimbel 1995 (three sites); all other studies were conducted at a single site.
The longest length of follow‐up was 12 months in three studies (Anders 1997; Fernández‐Barrientos 2010; Storr‐Paulsen 1998), 15 months in Fea 2010, 18 months in Georgopoulos 2000, 24 months in three studies (Gimbel 1995; Liaska 2014; Samuelson 2011), and 30 months in one study (Jacobi 1999).
Participants
The diagnoses of participants included co‐existing cataract with: primary open‐angle glaucoma (POAG) (Anders 1997; Fea 2010; Liaska 2014; Storr‐Paulsen 1998), POAG/ocular hypertension (Fernández‐Barrientos 2010), pseudoexfoliative glaucoma (Georgopoulos 2000; Jacobi 1999), POAG/ocular hypertension/pseudoexfoliative/pigmentary glaucoma (Gimbel 1995), and POAG/pseudoexfoliative/pigmentary glaucoma (Samuelson 2011). Seven studies reported the gender of participants; most participants in each study and overall were women (311/522, 59%). Furthermore, since glaucoma is a disease of aging, and on average women live longer than men, women predominated among older glaucoma patients. The age range of participants was 48 to 88 years old (as reported from six studies), with an overall mean age of 73 years in both intervention groups among all nine studies. Data from a total of 596 eyes of 594 participants (91% of randomized participants) were analyzed among the nine studies.
Investigators of four of the nine studies reported sample size calculations (Fea 2010; Fernández‐Barrientos 2010; Liaska 2014; Samuelson 2011); each estimate was based on a different outcome to be detected with 80% power. Fea 2010 selected a sample size of 12 eyes in the combined surgery group and 24 eyes in the cataract surgery alone group to detect a difference in IOP of 3 mmHg between the two intervention groups. Samuelson 2011 calculated a sample size of 90 eyes in each group to detect a 19.5% difference in the proportion of eyes with IOP < 21 between the two intervention groups with a 1‐sided significance level of P = 0.05. In order to detect a 0.3 µL/min/mmHg difference in the outflow facility, Fernández‐Barrientos 2010 calculated a sample size of 12 participants in each intervention group. Liaska 2014 calculated a sample size of 32 participants per intervention group to detect a reduction in IOP of 2 mmHg (between‐patient SD = 2.5 mmHg, within‐patient SD = 2 mmHg) with a two‐sided significance level of P = 0.05 and an anticipated dropout rate of 10%.
Interventions
The nine included studies compared four types of glaucoma surgeries combined with cataract surgery versus cataract surgery alone (Table 2). Three studies used trabeculectomy (Anders 1997; Liaska 2014; Storr‐Paulsen 1998), three studies used iStents® (Fea 2010; Fernández‐Barrientos 2010; Samuelson 2011), two studies used trabecular aspiration (Georgopoulos 2000; Jacobi 1999), and one study used trabeculotomy (Gimbel 1995). In the iStent® group, Fernández‐Barrientos 2010 used two implanted iStents® in the same eye as the intervention. Three studies specifically reported implanting IOLs at the time of cataract surgery (Fea 2010; Gimbel 1995; Jacobi 1999); the remaining studies did not report IOL implantation.
Primary outcome
Investigators of all but one study (Fernández‐Barrientos 2010) reported postoperative IOP levels as the main outcome. Six of the nine studies reported postoperative mean IOP as the primary outcome while the other three studies reported median IOP (Storr‐Paulsen 1998), proportion of eyes with unmedicated IOP ≤ 21 (Samuelson 2011), and aqueous flow and trabecular outflow facility (Fernández‐Barrientos 2010). Four studies used the a priori criteria exactly as stated in the Types of outcome measures section above and reported the mean change in IOP from baseline at one year follow‐up as an outcome. Fea 2010 reported mean change in IOP from baseline at 15 months follow‐up, which we analyzed as one year outcomes, and Storr‐Paulsen 1998 provided the mean change in IOP in response to our emailed query. Thus, we included six studies in the meta‐analysis of mean change in IOP.
Secondary outcomes
Investigators of no included study reported the proportion of participants with a reduction in the number of medications at one year after surgery. All but two studies (Gimbel 1995; Liaska 2014) reported the mean number of medications used postoperatively. Two studies reported the mean change from baseline in the number of medications in use at one year after surgery (Anders 1997; Samuelson 2011).
No study investigator reported the mean change in BCVA, although six studies reported postoperative visual acuity at various time points (Anders 1997; Georgopoulos 2000; Jacobi 1999; Liaska 2014; Samuelson 2011; Storr‐Paulsen 1998). Investigators of three studies reported preoperative visual acuity and postoperative visual acuity at one year (Anders 1997; Samuelson 2011) or two years (Liaska 2014) using the logMAR scale, but did not provide details regarding the type of chart used to measure visual acuity. Georgopoulos 2000 measured preoperative visual acuity and postoperative (between 12 to 18 months) visual acuity using a Snellen chart and presented the data in a scattergram. Jacobi 1999 measured BCVA using Snellen chart preoperatively and at two years after surgery. Storr‐Paulsen 1998 used Snellen charts, but reported only the postoperative proportion of eyes with BCVA better than 6/12 at one year.
No study investigator reported the mean change in any visual field parameter. Investigators of only two studies measured visual fields. Liaska 2014 tested visual fields with automated static perimetry (Dikon, 80/30) and reported visual field mean deviation (dB) at baseline and at two years postoperatively. Storr‐Paulsen 1998 performed automatic visual field testing using the Competer 750 and reported postoperative visual field data as performance value graphs at one year.
Reports from five studies gave the proportion of participants who were reoperated to control IOP and who received interventions for surgical complications within the first postoperative year (Anders 1997; Fea 2010; Fernández‐Barrientos 2010; Liaska 2014; Samuelson 2011). No included study report included vision‐related quality of life data.
Complications were reported variably among six studies (Anders 1997; Georgopoulos 2000; Gimbel 1995; Jacobi 1999; Liaska 2014; Samuelson 2011). We recorded the most clinically important and surgery‐related adverse events of interest in Table 3, which included intraoperative complications (capsular tear, zonular tear, and vitreous loss) and postoperative complications (hyphema, shifted IOL, ocular hypotony, choroidal detachment, anterior chamber flattening, and stent obstruction).
2. Complications reported by included studies at 1 and 2 years.
| Adverse eventa | Within 1 year after surgeryb | Within 2 years after surgery | ||||||
| Study ID | Combined surgery | Cataract surgery alone | RR (95% CI) | Study ID | Combined surgery | Cataract surgery alone | RR (95% CI) | |
| Intraoperative | ||||||||
| Capsular tear | Georgopoulos 2000 | 0/14 (0%) | 1/13 (8%) | 0.31 (0.01, 7.02) | Jacobi 1999 | 0/26 (0%) | 1/13 (8%) | 0.17 (0.01, 3.97) |
| Zonular tear | Georgopoulos 2000 | 2/14 (14%) | 1/13 (8%) | 1.86 (0.19, 18.13) | Jacobi 1999 | 3/26 (12%) | 4/13 (31%) | 0.38 (0.10, 1.43) |
| Vitreous loss | Georgopoulos 2000 | 0/14 (0%) | 1/13 (8%) | 0.31 (0.01, 7.02) | Jacobi 1999 | 0/26 (0%) | 1/13 (8%) | 0.17 (0.01, 3.97) |
| Postoperative | ||||||||
| Hyphema | Anders 1997 | 2/42 (5%) | 0/41 (0%) | 4.88 (0.24, 98.72) | Gimbel 1995 | 1/53 (2%) | 0/53 (0%) | 3.00 (0.12, 72.02) |
| Jacobi 1999 | 0/26 (0%) | 0/13 (0%) | ‐ | |||||
| IOL shift | ‐ | ‐ | ‐ | ‐ | Jacobi 1999 | 0/26 (0%) | 0/13 (0%) | ‐ |
| Ocular hypotony | Anders 1997 | 0/42 (0%) | 0/41 (0%) | ‐ | Jacobi 1999 | 0/26 (0%) | 0/13 (0%) | ‐ |
| Liaska 2014 | 5/29 (17%) | 0/31 (0%) | 11.73 (0.68, 203.23) | |||||
| Choroidal detachment | Anders 1997 | 0/42 (0%) | 0/41 (0%) | ‐ | Jacobi 1999 | 0/26 (0%) | 0/13 (0%) | ‐ |
| Liaska 2014 | 2/29 (7%) | 0/31 (0%) | 5.33 (0.27, 106.61) | |||||
| Anterior chamber flattening | Anders 1997 | 0/42 (0%) | 0/41 (0%) | ‐ | Jacobi 1999 | 0/26 (0%) | 0/13 (0%) | ‐ |
| Stent obstruction | Samuelson 2011 | 4/111 (4%) | Not applicable | ‐ | Samuelson 2011 | 5/116 (4%) | Not applicable | ‐ |
avalues represent the number of participants with event/total number of participants assessed for event (%). bwe analyzed data measured at 12 to 18 months as 1 year outcomes.
95% CI: 95% confidence interval; RR: risk ratio; ‐ : no data available.
Excluded studies
We excluded seven studies, of which five studies did not evaluate the intervention of interest (Bobrow 1998; Cillino 2004; D'Eliseo 2003; Ghirelli 1995; Shin 2001) and reports from two studies did not provide sufficient information to confirm eligibility criteria (Pillunat 2001; Tanaka 1998). We detailed the specific reasons for exclusion in the 'Characteristics of excluded studies' table.
Risk of bias in included studies
We summarized our judgements regarding the risk of bias for individual studies in the 'Characteristics of included studies' tables and in Figure 2.
2.
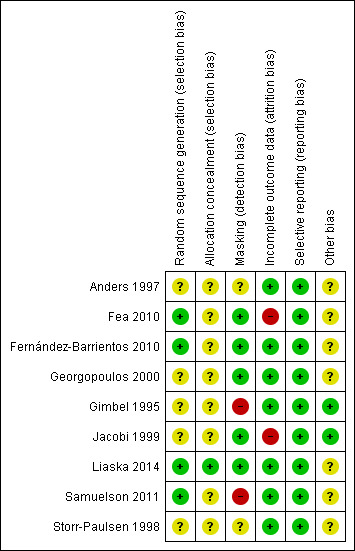
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
With respect to random sequence generation, we judged four studies in which a computer‐based random number generator was used to be at low risk of bias (Fea 2010; Fernández‐Barrientos 2010; Liaska 2014; Samuelson 2011). However, only one of these four studies used adequate allocation concealment methods (Liaska 2014); the other three studies did not report allocation concealment before randomization. We judged five studies to be at unclear risk of selection bias due to inadequate reporting of allocation methods (Anders 1997; Georgopoulos 2000; Gimbel 1995; Jacobi 1999; Storr‐Paulsen 1998).
Masking (detection bias)
Investigators of five studies stated that outcome assessors were masked to treatment assignments; thus, we judged these studies to be at low risk of detection bias (Fea 2010; Fernández‐Barrientos 2010; Georgopoulos 2000; Jacobi 1999; Liaska 2014). We judged two studies to be at high risk of detection bias (Gimbel 1995; Samuelson 2011). In Gimbel 1995, the outcome assessor (physician) was not masked. Samuelson 2011 was designated as an open‐label trial in which physicians were unmasked. We judged two studies to be at unclear risk of detection bias because they did not report masking (Anders 1997; Storr‐Paulsen 1998).
Incomplete outcome data
We judged two studies to be at high risk of bias due to incomplete outcome data (Fea 2010; Jacobi 1999). Fea 2010 excluded three of 24 participants in the cataract surgery alone group from final analysis. In Jacobi 1999, data were missing for over 50% of participants at the study's primary outcome assessment time of 30 months. We judged the remaining seven studies to be at low risk of attrition bias.
Selective reporting
Based on full‐text reports, all nine studies reported results for all outcomes specified in the methods sections of the report. However, because no protocol was available for any included study, we could not evaluate reporting bias based on prespecified outcomes.
Other potential sources of bias
We did not identify other potential sources of bias for two studies (Gimbel 1995; Jacobi 1999), but we judged seven studies to be at unclear risk of bias. Among each of three studies within the iStent® subgroup of the combined surgery group (Fea 2010; Fernández‐Barrientos 2010; Samuelson 2011), at least one study author had a financial affiliation with the manufacturer of the drainage devices. Investigators for four studies did not report either sources of funding or possible disclosures of interest (Anders 1997; Georgopoulos 2000; Liaska 2014; Storr‐Paulsen 1998). Additionally, in Storr‐Paulsen 1998, the unit of randomization (18 participants) differed from the unit of analysis (20 eyes).
Effects of interventions
See: Table 1
The comparison of interest in this Cochrane review was combined cataract (phacoemulsification) and glaucoma surgery versus cataract surgery (phacoemulsification) alone. We obtained all data presented in this review from published reports with the exception of the mean IOP change from Storr‐Paulsen 1998 which was obtained via correspondence. A total of 596 eyes of 594 participants were analyzed among the nine studies. Our findings are summarized in Table 1.
Intraocular pressure
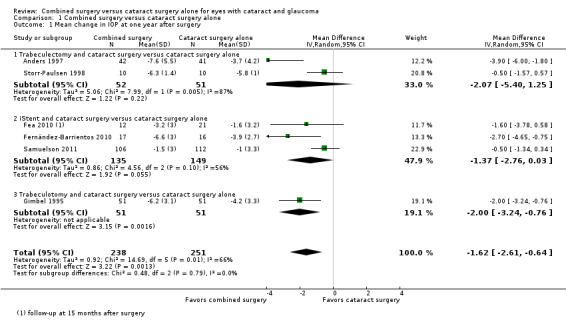
Six of nine studies provided sufficient data to include in meta‐analysis of mean change in IOP from baseline at one year after surgery (Figure 3). Overall, combined surgery provided a 1.62 mmHg greater reduction in IOP compared with cataract surgery alone at one year after surgery (MD ‐1.62 mmHg, 95% CI ‐2.61 to ‐0.64; Analysis 1.1).
3.

Forest plot of comparison: 1 Combined surgery versus cataract surgery alone, outcome: 1.1 Mean change in IOP at one year after surgery.
1.1. Analysis.
Comparison 1 Combined surgery versus cataract surgery alone, Outcome 1 Mean change in IOP at one year after surgery.
Given the significant technical differences in the types of glaucoma surgeries performed among the included studies, we also analyzed this outcome separately within four subgroups based on type of glaucoma surgery: 1) trabeculectomy, 2) iStent®, 3) trabeculotomy, and 4) trabecular aspiration. Due to the small number of studies in each subgroup, the power to test for subgroup differences is low, but the effect sizes appear similar across subgroups. There were 163 eyes of 161 participants analyzed in trabeculectomy studies, 284 participants analyzed in iStent® studies, 102 participants analyzed in trabeculotomy studies, and 47 participants analyzed in the trabecular aspiration study.
Among three trabeculectomy studies, two studies contributed data for IOP outcomes at one year after surgery (Anders 1997; Storr‐Paulsen 1998). Although the estimates of effect size for both studies favored the combined surgery group, there was poor overlap of CIs (I2 = 87%) and the pooled estimate suggests uncertainty of effectiveness (MD ‐2.07 mmHg, 95% CI ‐5.40 to 1.25; Analysis 1.1). Liaska 2014, which was not included in the meta‐analysis due to follow‐up at only two years postoperatively, found a ‐1.7 mmHg (95% CI ‐3.1 to ‐0.2) greater reduction in IOP in the combined surgery group compared with the cataract surgery alone group.
Among three iStent® studies, two studies reported data at one year after surgery (Fernández‐Barrientos 2010; Samuelson 2011) and the third study reported data at 15 months after surgery (Fea 2010), which we analyzed as one‐year data. At one year postoperatively, the summary estimate suggested a 1.37 mmHg greater reduction in IOP for the combined surgery group compared with cataract surgery alone (MD ‐1.37 mmHg, 95% CI ‐2.76 to 0.03; I2 = 56%; Analysis 1.1). Results most favorable for the combined surgery group were reported by Fernández‐Barrientos 2010, in which two iStents® were used in each eye, while other studies used only one iStent®. At 24 months after surgery, Samuelson 2011 reported that the mean IOP in the combined surgery group was 8.4 mmHg lower than the baseline IOP versus 7.5 mmHg lower in the cataract surgery alone group.
The one trabeculotomy study (Gimbel 1995) showed a 2 mmHg greater decrease in IOP in the combined surgery group compared with the cataract surgery alone group at one year after surgery (MD ‐2.00 mmHg, 95% CI ‐3.24 to ‐0.76; Analysis 1.1).
Among two trabecular aspiration studies, data reported were insufficient to include in meta‐analysis (standard deviations not available for analysis). Georgopoulos 2000 reported statistically significantly lower postoperative IOP in the combined group versus the cataract surgery alone group at 12 months (‐4.1 mmHg in the combined surgery group versus ‐2.0 mmHg in the cataract surgery alone group) and 15 months (‐4.6 mmHg in the combined surgery group versus ‐2.0 mmHg in the cataract surgery alone group). However, Jacobi 1999 reported similar mean changes in IOP for both groups at 12 months (‐13.6 mmHg in both the combined surgery and cataract surgery alone groups), 24 months (‐13.5 mmHg in the combined surgery group versus ‐14.0 mmHg in the cataract surgery alone group), and 30 months (‐13.1 mmHg in the combined surgery group versus ‐12.9 mmHg in the cataract surgery alone group) postoperatively.
Medications after surgery
No study investigators reported the proportion of participants with a reduction from baseline in the number of medications used after surgery; however, five studies reported the proportion of participants who were using one or more medications at one year after surgery (Anders 1997; Fea 2010; Georgopoulos 2000; Gimbel 1995; Samuelson 2011), two studies reported the mean reduction in the number of medications at one year after surgery (Anders 1997; Samuelson 2011), and one study reported the mean reduction in the number of medications at two years after surgery (Liaska 2014). Three studies did not report any outcome related to IOP‐lowering medications used after surgery (Fernández‐Barrientos 2010; Jacobi 1999; Storr‐Paulsen 1998)
Data from five studies showed that participants in the combined surgery group were about 50% less likely to use one or more medications compared with participants in the cataract surgery alone group one year postoperatively (RR 0.47, 95% CI 0.28 to 0.80; Analysis 1.2; Figure 4). Gimbel 1995, the most precise of the five studies, also had the smallest estimated treatment effect contributing to substantial statistical heterogeneity as indicated by the I2 statistic (78%).
1.2. Analysis.
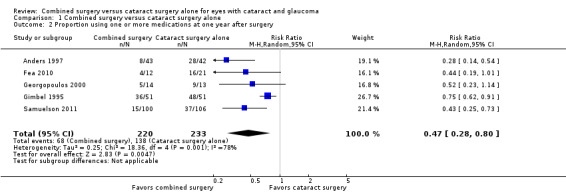
Comparison 1 Combined surgery versus cataract surgery alone, Outcome 2 Proportion using one or more medications at one year after surgery.
4.
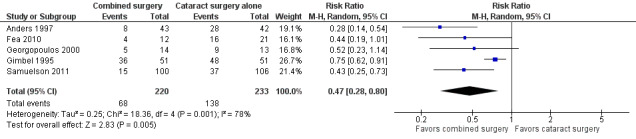
Forest plot of comparison: 1 Combined surgery versus cataract surgery alone, outcome: 1.2 Proportion using one or more medications at one year after surgery.
At one year postoperatively, combined surgery reduced the mean number of medications from none to one compared with cataract surgery alone (MD ‐0.69, 95% CI ‐1.28 to ‐0.10; I2 = 56%; Analysis 1.3). At two years postoperatively, Liaska 2014 reported a similar effect (MD ‐0.6, 95% CI ‐1.2 to ‐0.05).
1.3. Analysis.
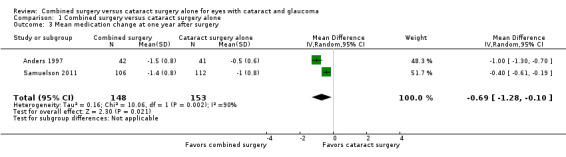
Comparison 1 Combined surgery versus cataract surgery alone, Outcome 3 Mean medication change at one year after surgery.
Visual acuity
No study reported the mean change in BCVA at one year after surgery; however, six studies reported postoperative visual acuity values. In Anders 1997, mean visual acuity (logMAR) in the combined surgery group changed from 0.26 ± 0.14 at baseline to 0.53 ± 0.24 at one year and in the cataract surgery alone group from 0.25 ± 0.11 at baseline to 0.47 ± 0.19 at one year. Neither baseline (MD 0.01, 95% CI ‐0.04 to 0.06; P = 0.72) nor postoperative (MD 0.06; 95% CI ‐0.03 to 0.15; P = 0.21) visual acuity differed significantly between the two groups. Georgopoulos 2000 reported no significant difference in visual acuity between the two groups (P > 0.1) at 12 to 18 months postoperatively (no other data reported). Jacobi 1999 reported that BCVA improved from 20/100 (0.70 logMAR) to 20/35 (0.24 logMAR) in the combined surgery group and from 20/200 (1.00 logMAR) to 20/45 (0.35 logMAR) in the cataract surgery alone group at two years after surgery. As the statistical analysis in this study compared three groups simultaneously (two randomized groups plus a third group that was not randomized), we could not determine the difference in BCVA between our interventions of interest. Samuelson 2011 reported that the proportion of eyes with BCVA equal to or better than 20/40 increased from 45% preoperatively to 94% at 12 months and 93% at 24 months in the combined surgery group, and from 44% to 90% at 12 months and 91% at 24 months in the cataract surgery alone group. At 12 months, 97% in the combined surgery group versus 95% in the cataract surgery alone group had improved BCVA. Storr‐Paulsen 1998 reported similar proportions of eyes with BCVA ≥ 6/12 in both groups at one year after surgery (8/10 eyes in the combined surgery group and 7/10 eyes in the cataract surgery alone group). In Liaska 2014, mean logMAR BCVA in the combined surgery group was 0.7 (95% CI 0.6 to 0.8) and in the cataract surgery alone group was 0.63 (95% CI 0.52 to 0.75) at two years after surgery. Thus, the difference between the two interventions with respect to the effect on BCVA was small or none.
Visual field
Liaska 2014 reported no significant difference in mean deviation improvement in visual field (1.4 dB, 95% CI ‐1.2 to 2.96) between the combined surgery group compared with the cataract surgery alone group. Storr‐Paulsen 1998 reported an equal but not statistically significant increase in visual field performance in both intervention groups compared with preoperative values (P > 0.05), as well as no significant difference between the two groups preoperatively and at 12 months. However, no data on the effect estimate or effect size was reported. Investigators of the remaining seven studies did not report visual field outcomes.
Reoperation to control IOP
Reports from four of nine studies, representing 64% (382/596) of potentially available eyes, provided the proportion of participants who received reoperation to control IOP within one year after surgery (Anders 1997; Fea 2010; Fernández‐Barrientos 2010; Samuelson 2011). No participants in Fea 2010 received reoperation to control IOP; the combined effect for the other three studies between the combined surgery and cataract surgery alone groups was uncertain (RR 1.13; 95% CI 0.15 to 8.25; Analysis 1.4; Figure 5). Although the confidence intervals for all three studies crossed the line of no effect, the direction of effect favored cataract surgery alone for Anders 1997, which used trabeculectomy, and combined surgery for Fernández‐Barrientos 2010 and Samuelson 2011, which used iStent®.
1.4. Analysis.

Comparison 1 Combined surgery versus cataract surgery alone, Outcome 4 Proportion receiving reoperation to control IOP within one year after surgery.
5.
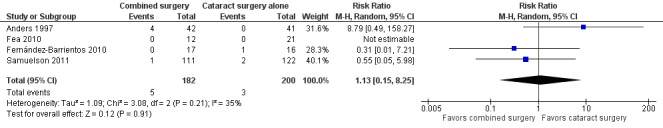
Forest plot of comparison: 1 Combined surgery versus cataract surgery alone, outcome: 1.4 Proportion receiving reoperation to control IOP within one year after surgery.
At two years after surgery, Liaska 2014 reported that one participant in the cataract surgery alone group needed a trabeculectomy for IOP control within two years after surgery; no reoperation to control IOP was required in the combined surgery group.
Interventions for surgical complications
Investigators of the same four of nine studies reported the proportion of participants who received at least one intervention for surgical complications within one year after surgery (Anders 1997; Fea 2010; Fernández‐Barrientos 2010; Samuelson 2011). Both Fea 2010 and Fernández‐Barrientos 2010 reported no event in either intervention group and thus had non‐estimable individual RRs. There was uncertainty in the effect between the combined surgery and cataract surgery alone groups estimated from Anders 1997 and Samuelson 2011 (RR 1.06; 95% CI 0.34 to 3.35; Analysis 1.5; Figure 6). The overall effect estimate depended primarily on Samuelson 2011 in which the proportion of eyes with this outcome was substantially larger than for the other three studies combined.
1.5. Analysis.
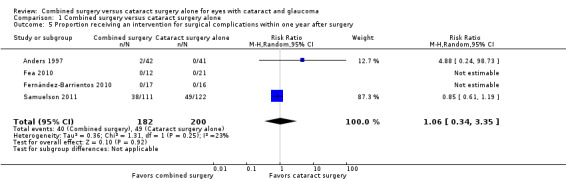
Comparison 1 Combined surgery versus cataract surgery alone, Outcome 5 Proportion receiving an intervention for surgical complications within one year after surgery.
6.

Forest plot of comparison: 1 Combined surgery versus phacoemulsification alone, outcome: 1.5 Proportion who received intervention for surgical complications within one year.
At two years, Jacobi 1999 reported posterior capsule opacification requiring Nd:YAG laser capsulotomy in 15 (53%) eyes in the combined surgery group and 9 (40%) eyes in the cataract surgery alone group. Samuelson 2011 reported 3 (3%) and 7 (6%) additional eyes required surgical intervention between one and two years after surgery in the combined surgery group and cataract surgery alone group, respectively.
Vision‐related quality of life and economic outcomes
No included study reported data for these outcomes at any time point.
Adverse outcomes
We have summarized all adverse outcome results and calculable RRs in Table 3. Two of nine studies reported adverse outcomes within one year after surgery (Anders 1997; Samuelson 2011) and one study reported adverse outcomes within 18 months after surgery (Georgopoulos 2000); however, the three studies did not report any of the same adverse outcomes. Anders 1997 reported two of 42 participants in the combined surgery group compared with none of 41 participants in the cataract surgery alone group had postoperative hyphema; no participant in either group was reported to have had postoperative ocular hypotony, choroidal detachment, or anterior chamber flattening. Samuelson 2011 observed complications of stent obstruction in four of 111 participants in the combined surgery group; this event was not applicable to the 122 participants in the cataract surgery alone group. Neither Anders 1997 nor Samuelson 2011 reported any intraoperative complications. Georgopoulos 2000 reported only intraoperative complications (capsular tear, zonular tear, vitreous loss): two of 14 participants in the combined surgery group and three of 13 participants in the cataract surgery alone group.
Four of the nine studies reported adverse outcomes within two years after surgery (Gimbel 1995; Jacobi 1999; Liaska 2014; Samuelson 2011). Types of adverse events varied among studies. Hyphema occurred in one participant in the combined surgery group across two studies (Gimbel 1995; Jacobi 1999) as compared with none in the cataract surgery alone group. In two studies (Jacobi 1999;Liaska 2014), five participants had ocular hypotony and two had choroidal detachment in the combined surgery group compared with no event of either complication in the cataract surgery alone group. In Jacobi 1999, neither intervention group reported any instances of postoperative IOL shifting or anterior chamber flattening; there were no instances of intraoperative capsular tear or vitreous loss in the combined surgery group, but one instance of each of these events in the cataract surgery alone group; and three of 26 participants in the combined surgery group compared with four of 13 participants in the cataract surgery alone group experienced intraoperative zonular tear. Samuelson 2011 reported complications of stent obstruction in five of 116 participants in the combined surgery group, but this event was not applicable to the 117 participants in the cataract surgery alone group.
Discussion
Summary of main results
Co‐existing cataract and glaucoma commonly affects the aging population. The decision to perform simultaneous cataract and glaucoma surgery versus cataract surgery alone is complex. The effect of combined surgery on IOP control and postoperative complications has been an issue of concern. We have summarized our findings from reviewing the available evidence in Table 1. Our analyses show that combined glaucoma and cataract surgery resulted in a greater decrease in IOP and fewer glaucoma medications used at one year after surgery in eight out of nine included studies. However, there was substantial heterogeneity among studies for both outcomes and the size of effects were small. Even the trabecular aspiration subgroup of the combined surgery group of one study (Jacobi 1999) reported no difference in IOP reduction between the two interventions, the combined surgery group still used significantly fewer glaucoma medications postoperatively compared with the cataract surgery alone group. The estimated effect was similar to the overall effect in all subgroups based on type of glaucoma surgery; however, in the subgroup of trabeculectomy the effect with respect to IOP lowering was not significantly different between the two interventions. Among other secondary outcomes, there were similar improvements in visual acuity in both intervention groups. There was no statistically significant difference between combined surgery and cataract surgery alone with respect to the proportions of participants who received reoperation to control IOP, who received intervention for surgical complications, or who experienced clinically important complications. None of the included studies reported quality of life outcomes or economic data. The overall risk of bias among individual studies was low or unknown.
Overall completeness and applicability of evidence
When choosing between combined glaucoma and cataract surgery and cataract surgery alone, the amount of IOP lowering and rate of complications after surgery are the most important considerations.
We included a total of nine studies that randomized 657 eyes of 655 participants in this Cochrane review. A major issue in this review is the variety of glaucoma surgeries that can be combined with cataract surgery. To address this issue, we examined outcomes in four subgroups; however, the resulting number of eyes per subgroup was small and estimated effects from individual studies were substantially heterogeneous. All included studies reported more IOP lowering in the combined surgery group, although in different ways, including mean postoperative IOP, mean IOP change, and number of medications needed for IOP control. In the trabeculectomy studies, the surgical technique differed among the three studies: Storr‐Paulsen 1998 used postoperative 5‐FU (fluorouracil) injections, Liaska 2014 used mitomycin C intraoperatively, augmented as needed with postoperative 5‐FU injections, and Anders 1997 did not use an anti‐fibrosis agent. The findings from Anders 1997 and Storr‐Paulsen 1998 may be of little interest at present, when the use of mitomycin C is more common. The single drainage device study that showed significant IOP reduction in the combined surgery group used two iStents® (Fernández‐Barrientos 2010). The trabeculotomy group included only one study (Gimbel 1995), which showed a statistically significantly greater reduction in IOP in the combined surgery group. Trabecular aspiration was designed only for pseudoexfoliative glaucoma patients, so this procedure does not apply to all types of open‐angle glaucoma. With regard to complications, the frequency of important adverse effects are difficult to estimate given the small total number of events reported, the number of eyes in this review, and variations in reporting.
Most of the studies were conducted in Europe or in populations of largely European ancestry. Surgical techniques and populations in these countries are relatively similar (Table 2). Hence, the findings should be applicable to patients with glaucoma in Europe, North America, and other similar populations. Data on racial differences in outcomes were not reported in the studies, limiting the generalizability of our conclusions.
Quality of the evidence
Overall, we graded the quality of the evidence as very low to low because of substantial heterogeneity among studies, wide CIs in quantitative results, and high or unclear risk of bias assessments for some domains. (Table 1). Eight of nine studies did not report methods of allocation concealment prior to randomization and were judged to be at unclear risk of bias for this domain. Two studies did not mask outcome assessors; we judged them to be at high risk of detection bias. Two studies had incomplete outcome data, with a substantial proportion of participants lost to follow‐up and excluded from analyses of outcomes. In addition, all report authors from the three iStent® studies were affiliated with the device manufacturer.
Potential biases in the review process
There are potential limitations to the present review. Firstly, data from some relevant studies may not have been included as we excluded two potentially relevant reports due to unavailability of information needed from study investigators to confirm eligibility. Secondly, with the exception of Storr‐Paulsen 1998, report authors from five of six studies did not respond to our queries for additional relevant data. As a result, we were unable to obtain all relevant data and could perform meta‐analyses with data from only six of the nine included studies. Thirdly, we were unable to determine whether there was active or passive ascertainment of safety outcomes, or whether adverse events were predefined.
Nevertheless, we rigorously adhered to our inclusion criteria when selecting relevant studies, abstracting data, and analyzing outcome data. Two review authors independently screened reports and abstracted data; we resolved all disagreements using an adjudication process. We screened reports from studies in languages other than English and assessed them for eligibility.
Agreements and disagreements with other studies or reviews
In their systematic literature review and analysis, Friedman 2002 assessed long‐term (> 24 months) IOP control in combined glaucoma and cataract surgery versus cataract surgery alone. In contrast to the present review, Friedman 2002 included non‐RCTs, cohort studies, and case series with at least 100 patients in addition to RCTs. Furthermore, studies of angle‐closure glaucoma patients were eligible for that review and, while the only type of glaucoma surgery considered was trabeculectomy, two types of cataract surgery were included: phacoemulsification and extracapsular cataract extraction (ECCE). Of the 39 studies included in their analysis, three studies also met our inclusion criteria (Anders 1997; Gimbel 1995; Storr‐Paulsen 1998). Despite the methodological differences, Friedman 2002 found good evidence that long‐term IOP on average was lowered 3 to 4 mmHg more by combined surgery than cataract surgery alone and that fewer medications were required after combined surgery. This conclusion was based on the three RCTs and one retrospective chart review of 21 eyes in the combined surgery group and 35 eyes in the phacoemulsification alone group (Yalvac 1997). Although Yalvac 1997 found a similar benefit to combined surgery, the differences between groups were not statistically significant for IOP reduction (MD ‐3.40 mmHg; 95% CI ‐6.83 to 0.03), number of glaucoma medications, or visual acuity at six months. Friedman 2002 also observed weak but consistent evidence that either form of cataract surgery alone lowered long‐term IOP by 2 to 4 mmHg. Interestingly, but not addressed by our review, they concluded that combined surgery resulted in slightly poorer long‐term IOP control when compared to trabeculectomy alone.
Authors' conclusions
Implications for practice.
This review identified only low quality evidence that combined cataract and glaucoma surgery, including both trabeculectomy and non‐penetrating surgery (iStent®, trabecular aspiration, trabeculotomy), may provide a small benefit in terms of controlling IOP than cataract surgery (phacoemulsification) alone. However, given the observed inconsistency in study results, imprecision in effect estimates and risk of bias in the included studies, we cannot definitively rely on this conclusion. Results regarding adverse events showed uncertainty in the effect between the two groups, given the rarity of events reported from most trials and the wide CIs of estimates of intervention effects. This review has highlighted the lack of use or reporting of visual field testing, quality of life measurements, and economic outcomes after surgery. Visual field testing is the main measurement of symptomatic vision loss experienced by patients with glaucoma and is a clinically important indicator of disease progression, whereas lowering IOP in and of itself is not a direct determinant of the disease process. Quality of life and economic measurements are important patient‐centered assessments of operative success and burden, respectively. In particular, a significant difference in either of these two measurements between the combined surgery and cataract surgery alone groups could influence the choice of one option over the other, given the small differences in outcome rates estimated for clinical outcomes for which evidence was available.
Implications for research.
There is need for future high‐quality RCTs to address these issues. Several such studies involving the so‐called "Minimally Invasive Glaucoma Surgery (MIGS)" procedures are currently underway (NCT00326066; NCT01052558; NCT01085357; NCT01539239; NCT01818115). We also need more studies in the United States and developing countries since the current trials are mainly limited to Europe, Canada, and South Africa. These trials should ensure adequate representation of both men and women and include diverse ethnicities. Furthermore, an international standard of reported outcomes for such trials (e.g., mean change in visual fields parameters, and IOP) would allow for better comparisons among studies and stronger evidence of effects. Intraoperative and postoperative complications should be well‐defined with rigorous reporting standards and methods. Designers of future trials should define the intensity and frequency of ascertainment of complications and adverse events, state whether the ascertainment is active or passive, and consider carefully whether events measured are pre‐specified or spontaneously reported. Patient‐important outcomes, including quality of life and economic outcomes, should be incorporated in the trial design. Rigorously conducted prospective observational studies also can inform the harms related to various surgical techniques. We are also in need of clinical trials that compare combined cataract and glaucoma surgery with staged surgery (cataract surgery first, then glaucoma surgery or vice versa) because in clinical practice, the decision is more often between combined versus staged surgery. As additional high‐quality, methodologically rigorous, and outcome‐standardized RCTs are conducted, the quality of evidence in future updates of this review can be significantly improved and increasingly meaningful.
History
Protocol first published: Issue 9, 2010 Review first published: Issue 7, 2015
| Date | Event | Description |
|---|---|---|
| 7 April 2014 | New citation required and minor changes | New author ML Zhang added; title amended and protocol revised and republished. |
Acknowledgements
We thank Iris Gordon and Lori Rosman, Trials Search Co‐ordinators of the CEVG, for designing the search strategy and conducting the electronic searches. We also acknowledge the support of the CEVG methodologist Xue Wang, Sueko Ng, and the editorial team during the preparation of this review, and thank the peer reviewers for their comments.
Richard Wormald (Coordinating Editor for CEVG) acknowledges financial support for his CEVG research sessions from the Department of Health through the award made by the National Institute for Health Research to Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology for a Specialist Biomedical Research Centre for Ophthalmology. The views expressed in this publication are those of the review authors and not necessarily those of the NIHR, NHS, or the Department of Health.
Appendices
Appendix 1. CENTRAL search strategy
#1 MeSH descriptor: [Cataract] explode all trees #2 MeSH descriptor: [Cataract Extraction] explode all trees #3 MeSH descriptor: [Lens, Crystalline] explode all trees #4 MeSH descriptor: [Lenses, Intraocular] explode all trees #5 MeSH descriptor: [Lens Implantation, Intraocular] explode all trees #6 intraocular lens* or intra‐ocular lens* or intra ocular lens* or IOL* #7 MeSH descriptor: [Phacoemulsification] this term only #8 phaco* or phako* #9 extracapsular near/2 cataract* #10 extra capsular near/2 cataract* #11 ECCE #12 manual near/3 small near/3 incision near/3 cataract* #13 MISICS or SICS #14 MeSH descriptor: [Capsulorhexis] this term only #15 continuous near/3 curvilinear near/3 capsulorhexis #16 continuous near/3 curvilinear near/3 capsulor?hexis #17 continuous near/3 circular near/3 capsulorhexis #18 continuous near/3 circular near/3 capsulor?hexis #19 CCC or CCS #20 endocapsular #21 (#1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20) #22 MeSH descriptor: [Glaucoma] explode all trees #23 glaucoma* #24 MeSH descriptor: [Intraocular Pressure] explode all trees #25 (ocular or intraocular or intra‐ocular) near/1 (pressure*) #26 MeSH descriptor: [Ocular Hypertension] this term only #27 ocular hypertension #28 IOP or OHT #29 MeSH descriptor: [Filtering Surgery] explode all trees #30 trabeculectom* or trabeculotom* #31 Trabectome #32 Canaloplasty #33 sclerostom* or sclerectom* #34 Endoscopic cyclophotocoagulation or endoscopic ciliary photocoagulation or ECP #35 MeSH descriptor: [Glaucoma Drainage Implants] explode all trees #36 implant* or shunt* or valve* or tube* #37 (Filtering or filtration) near/2 surger* #38 (#22 or #23 or #24 or #25 or #26 or #27 or #28 or #29 or #30 or #31 or #32 or #33 or #34 or #35 or #36 or #37) #39 combin* #40 coexist* or co exist* #41 versus #42 alone #43 without #44 Compare or comparison #45 "same time" #46 coincidental or "co incidental" #47 Separate #48 "Single site" or "Two site" #49 #39 or #40 or #41 or #42 or #43 or #44 or #45 or #46 or #47 or #48 #50 #21 and #38 and #49
Appendix 2. MEDLINE (OvidSP) search strategy
1. Randomized Controlled Trial.pt. 2. Controlled Clinical Trial.pt. 3. (randomized or randomised).ab,ti. 4. placebo.ab,ti. 5. drug therapy.fs. 6. randomly.ab,ti. 7. trial.ab,ti. 8. groups.ab,ti. 9. 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 10. exp animals/ not humans.sh. 11. 9 not 10 12. exp cataract/ 13. cataract extraction/ 14. exp lens crystalline/ 15. exp lenses intraocular/ 16. lens implantation intraocular/ 17. (intraocular lens* or intra‐ocular lens* or intra ocular lens* or IOLS).tw. 18. phacoemulsification/ 19. (phaco* or phako*).tw. 20. (extracapsular adj2 cataract*).tw. 21. (extra capsular adj2 cataract*).tw. 22. ECCE.tw. 23. (manual adj3 small adj3 incision adj3 cataract*).tw. 24. (MISICS or SICS).tw. 25. capsulorhexis/ 26. (continuous adj3 curvilinear adj3 capsulorhexis).tw. 27. (continuous adj3 curvilinear adj3 capsulor?hexis).tw. 28. (continuous adj3 circular adj3 capsulorhexis).tw. 29. (continuous adj3 circular adj3 capsulor?hexis).tw. 30. (CCC or CCS).tw. 31. endocapsular.tw. 32. or/12‐31 33. exp glaucoma/ 34. glaucoma*.tw. 35. exp intraocular pressure/ 36. ((ocular or intraocular or intra‐ocular) adj1 pressure*).tw. 37. Ocular Hypertension/ 38. ocular hypertension.tw. 39. (IOP or OHT).tw. 40. exp filtering surgery/ 41. (trabeculectom* or trabeculotom*).tw. 42. Trabectome.tw. 43. Canaloplasty.tw. 44. (sclerostom* or sclerectom*).tw. 45. (Endoscopic cyclophotocoagulation or endoscopic ciliary photocoagulation or ECP).tw. 46. exp Glaucoma Drainage Implants/ 47. (implant* or shunt* or valve* or tube*).tw. 48. ((Filtering or filtration) adj2 surger*).tw. 49. or/33‐48 50. 11 and 32 and 49 51. combin*.tw. 52. (coexist* or co exist*).tw. 53. versus.tw. 54. alone.tw. 55. without.tw. 56. (Compare or comparison).tw. 57. same time.tw. 58. (coincidental or co incidental).tw. 59. Separate.tw. 60. (Single site or Two site).tw. 61. or/51‐60 62. 50 and 61
The search filter for trials at the beginning of the MEDLINE strategy is from Glanville 2006.
Appendix 3. EMBASE.com search strategy
#1 'randomized controlled trial'/exp #2 'randomization'/exp #3 'double blind procedure'/exp #4 'single blind procedure'/exp #5 random*:ab,ti #6 #1 OR #2 OR #3 OR #4 OR #5 #7 'animal'/exp OR 'animal experiment'/exp #8 'human'/exp #9 #7 AND #8 #10 #7 NOT #9 #11 #6 NOT #10 #12 'clinical trial'/exp #13 (clin* NEAR/3 trial*):ab,ti #14 ((singl* OR doubl* OR trebl* OR tripl*) NEAR/3 (blind* OR mask*)):ab,ti #15 'placebo'/exp #16 placebo*:ab,ti #17 random*:ab,ti #18 'experimental design'/exp #19 'crossover procedure'/exp #20 'control group'/exp #21 'latin square design'/exp #22 #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 #23 #22 NOT #10 #24 #23 NOT #11 #25 'comparative study'/exp #26 'evaluation'/exp #27 'prospective study'/exp #28 control*:ab,ti OR prospectiv*:ab,ti OR volunteer*:ab,ti #29 #25 OR #26 OR #27 OR #28 #30 #29 NOT #10 #31 #30 NOT (#11 OR #23) #32 #11 OR #24 OR #31 #33 'cataract'/exp #34 'cataract extraction'/exp #35 'lens'/exp #36 'lens implant'/exp #37 'lens implantation'/exp #38 (intraocular NEAR/1 lens*):ab,ti OR ('intra ocular' NEAR/1 lens*):ab,ti AND ior AND iols:ab,ti #39 'phacoemulsification'/exp #40 phaco*:ab,ti OR phako*:ab,ti #41 'extracapsular cataract extraction'/exp #42 (extracapsular NEAR/2 cataract*):ab,ti #43 extra:ab,ti AND (capsular NEAR/2 cataract*):ab,ti #44 ecce:ab,ti #45 (manual NEAR/3 small):ab,ti AND (small NEAR/3 incision):ab,ti AND (incision NEAR/3 cataract*):ab,ti #46 misics:ab,ti OR sics:ab,ti #47 'capsulorhexis'/exp #48 (continuous NEAR/3 curvilinear):ab,ti AND (curvilinear NEAR/3 capsulorhexis):ab,ti #49 (continuous NEAR/3 curvilinear):ab,ti AND (curvilinear NEAR/3 capsulor?hexis):ab,ti #50 (continuous NEAR/3 circular):ab,ti AND (circular NEAR/3 capsulorhexis):ab,ti #51 (continuous NEAR/3 circular):ab,ti AND (circular NEAR/3 capsulor?hexis):ab,ti #52 ccc:ab,ti OR ccs:ab,ti #53 endocapsular:ab,ti #54 #33 OR #34 OR #35 OR #36 OR #37 OR #38 OR #39 OR #40 OR #41 OR #42 OR #43 OR #44 OR #45 OR #46 OR #47 OR #48 OR #49 OR #50 OR #51 OR #52 OR #53 #55 'glaucoma'/exp #56 glaucoma*:ab,ti #57 'intraocular pressure'/exp #58 ((ocular OR intraocular OR 'intra ocular') NEAR/1 pressure*):ab,ti #59 'intraocular hypertension'/exp #60 'ocular hypertension':ab,ti #61 iop:ab,ti OR oht:ab,ti #62 'filtering operation'/exp #63 trabeculectom*:ab,ti OR trabeculotom*:ab,ti #64 trabectome:ab,ti #65 canaloplasty:ab,ti #66 sclerostom*:ab,ti OR sclerectom*:ab,ti #67 'endoscopic cyclophotocoagulation':ab,ti OR 'endoscopic ciliary photocoagulation':ab,ti OR ecp:ab,ti #68 'glaucoma drainage implant'/exp #69 implant*:ab,ti OR shunt*:ab,ti OR valve*:ab,ti OR tube*:ab,ti #70 ((filtering OR filtration) NEAR/2 surger*):ab,ti #71 #55 OR #56 OR #57 OR #58 OR #59 OR #60 OR #61 OR #62 OR #63 OR #64 OR #65 OR #66 OR #67 OR #68 OR #69 OR #70 #72 combin*:ab,ti #73 coexist*:ab,ti OR (co near/1exist*):ab,ti #74 versus:ab,ti #75 alone:ab,ti #76 without:ab,ti #77 compare:ab,ti OR comparison:ab,ti #78 'same time':ab,ti #79 coincidental:ab,ti OR 'co incidental':ab,ti #80 separate:ab,ti #81 'single site':ab,ti OR 'two site':ab,ti #82 #72 OR #73 OR #74 OR #75 OR #76 OR #77 OR #78 OR #79 OR #80 OR #81 #83 #32 AND #54 AND #71 AND #82
Appendix 4. PubMed search strategy
#1 ((randomized controlled trial[pt]) OR (controlled clinical trial[pt]) OR (randomised[tiab] OR randomized[tiab]) OR (placebo[tiab]) OR (drug therapy[sh]) OR (randomly[tiab]) OR (trial[tiab]) OR (groups[tiab])) NOT (animals[mh] NOT humans[mh]) #2 ((intraocular lens*[tiab] OR intra‐ocular lens*[tiab] OR intra ocular lens*[tiab] OR IOLS[tiab]) NOT Medline[sb]) #3 (phaco*[tiab] OR phako*[tiab]) NOT Medline[sb] #4 (extracapsular[tiab] AND cataract*[tiab]) NOT Medline[sb] #5 ("extra capsular"[tiab] AND cataract*[tiab]) NOT Medline[sb] #6 ECCE[tiab] NOT Medline[sb] #7 (manual[tiab] AND small[tiab] AND incision[tiab] AND cataract*[tiab]) NOT Medline[sb] #8 (MISICS[tiab] OR SICS[tiab]) NOT Medline[sb] #9 (continuous[tiab] AND curvilinear[tiab] AND capsulorhexis[tiab]) NOT Medline[sb] #10 (continuous[tiab] AND curvilinear[tiab] AND capsulorrhexis[tiab]) NOT Medline[sb] #11 (continuous[tiab] AND circular[tiab] AND capsulorhexis[tiab]) NOT Medline[sb] #12 (continuous[tiab] AND circular[tiab] AND capsulorrhexis[tiab]) NOT Medline[sb] #13 (CCC[tiab] OR CCS[tiab]) NOT Medline[sb] #14 endocapsular[tiab] NOT Medline[sb] #15 (#2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14) #16 (glaucoma*[tiab] NOT Medline[sb]) #17 ((ocular[tiab] OR intraocular[tiab] OR intra‐ocular[tiab]) AND pressure*[tiab]) NOT Medline[sb] #18 "ocular hypertension"[tiab] NOT Medline[sb] #19 (IOP[tiab] OR OHT[tiab]) NOT Medline[sb] #20 (trabeculectom*[tiab] OR trabeculotom*[tiab]) NOT Medline[sb] #21 Trabectome[tiab] NOT Medline[sb] #22 Canaloplasty[tiab] NOT Medline[sb] #23 (sclerostom*[tiab] OR sclerectom*[tiab]) NOT Medline[sb] #24 ("Endoscopic cyclophotocoagulation"[tiab] OR "endoscopic ciliary photocoagulation"[tiab] OR ECP[tiab]) NOT Medline[sb] #25 (implant*[tiab] OR shunt*[tiab] OR valve*[tiab] OR tube*[tiab]) NOT Medline[sb] #26 ((Filtering[tiab] OR filtration[tiab]) AND surger*[tiab]) NOT Medline[sb] #27 (#16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26) #28 combin*[tiab] NOT Medline[sb] #29 (coexist*[tiab] OR co exist*[tiab]) NOT Medline[sb] #30 versus[tiab] NOT Medline[sb] #31 alone[tiab] NOT Medline[sb] #32 without[tiab] NOT Medline[sb] #33 (Compare[tiab] OR comparison[tiab]) NOT Medline[sb] #34 same time[tiab] NOT Medline[sb] #35 (coincidental[tiab] OR "co incidental"[tiab]) NOT Medline[sb] #36 Separate[tiab] NOT Medline[sb] #37 ("Single site"[tiab] OR "Two site"[tiab]) NOT Medline[sb] #38 (#28 OR #29 OR #30 OR #31 OR #32 OR #33 OR #34 OR #35 OR #36 OR #37) #39 (#1 AND #15 AND #27 AND #38)
Appendix 5. LILACS search strategy
(Cataract$ or Catarata or MH:C11.510.245$ or Cataract Extraction$ or "Extracción de Catarata" or "Extração de Catarata" or MH:E04.540.825.249$ or "Crystalline Lens" or Cristalino or MH:A09.371.060.500$ or Intraocular Lens$ or "Lentes Intraoculares" or MH:E07.632.500.460$ or MH:E07.695.460$ or MH:VS2.006.001.009.003$ or "Intraocular Lens Implantation" or "Implantación de Lentes Intraoculares" or "Implante de Lente Intraocular" or MH:E04.540.825.600$ or IOLS or Phaco$ or Phako$ or Faco$ or MH:E04.540.825.249.704$ or MH:E04.943.875$ or extracapsular cataract$ or ECCE or "manual small incision cataract" or MISICS or SICS or Capsulorhexis or Capsulorrexis or Capsulorrexe or MH:E04.540.825.249.352$ or CCC or CCS or endocapsular) and (Glaucoma$ or MH:C11.525.381$ or "Intraocular Pressure" or "Presión Intraocular" or "Pressão Intraocular" or MH:G14.440$ or "ocular pressure" or "Ocular Hypertension" or "Hipertensión Ocular" or "Hipertensão Ocular" or MH:C11.525$ or IOP or OHT or "Filtering Surgery" or "Cirugía Filtrante" or "Cirurgia Filtrante" or "Filtration Surgery" or MH:E04.540.450$ or trabeculectom$ or trabeculotom$ or trabectome or canaloplasty or sclerostom$ or sclerectom$ or "endoscopic cyclophotocoagulation" or "endoscopic ciliary photocoagulation" or ecp or "Glaucoma Drainage Implants" or "Implantes de Drenaje de Glaucoma" or "Implantes para Drenagem de Glaucoma" or MH:E07.695.250$ or implant$ or shunt$ or valve$ or tube$) and (combin$ or coexist$ or versus or alone or without or compare or comparison or "same time" or coincidental or "co incidental" or separate or "single site" or "two site")
Appendix 6. metaRegister of Controlled Trials search strategy
cataract AND glaucoma
Appendix 7. ClinicalTrials.gov search strategy
cataract AND glaucoma
Appendix 8. WHO ICTRP search strategy
cataract AND glaucoma
Data and analyses
Comparison 1. Combined surgery versus cataract surgery alone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mean change in IOP at one year after surgery | 6 | 489 | Mean Difference (IV, Random, 95% CI) | ‐1.62 [‐2.61, ‐0.64] |
| 1.1 Trabeculectomy and cataract surgery versus cataract surgery alone | 2 | 103 | Mean Difference (IV, Random, 95% CI) | ‐2.07 [‐5.40, 1.25] |
| 1.2 iStent and cataract surgery versus cataract surgery alone | 3 | 284 | Mean Difference (IV, Random, 95% CI) | ‐1.37 [‐2.76, 0.03] |
| 1.3 Trabeculotomy and cataract surgery versus cataract surgery alone | 1 | 102 | Mean Difference (IV, Random, 95% CI) | ‐2.0 [‐3.24, ‐0.76] |
| 2 Proportion using one or more medications at one year after surgery | 5 | 453 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.28, 0.80] |
| 3 Mean medication change at one year after surgery | 2 | 301 | Mean Difference (IV, Random, 95% CI) | ‐0.69 [‐1.28, ‐0.10] |
| 4 Proportion receiving reoperation to control IOP within one year after surgery | 4 | 382 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.15, 8.25] |
| 5 Proportion receiving an intervention for surgical complications within one year after surgery | 4 | 382 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.34, 3.35] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Anders 1997.
| Methods |
Study design: parallel‐group RCT Unit of randomization: the individual (one eye per participant) Number randomized: 85 total; 43 eyes of 43 participants in combined surgery group and 42 eyes of 42 participants in cataract surgery alone group Unit of analysis: the individual (one eye per participant) Number analyzed: 83 total; 42 eyes of 42 participants in combined surgery group and 41 eyes of 41 participants in cataract surgery alone group Exclusions and losses to follow‐up: one participant in each group did not complete follow‐up exam at one year and was excluded from the analysis Power calculation: none reported |
|
| Participants |
Country: Germany (single site) Age (mean ± SD): 78.2 ± 7.9 years in combined surgery group; 74.9 ± 9.6 years in cataract surgery alone group Gender: 9 men and 34 women in combined surgery group; 12 men and 30 women in cataract surgery alone group Ethnicity: not reported Inclusion criteria:
Exclusion criteria: not specified Equivalence of baseline characteristics: yes (IOP, amount of glaucoma medications, visual acuity) Diagnoses in participants: advanced chronic POAG and cataract |
|
| Interventions |
Intervention 1: combined cataract surgery (phacoemulsification) and filtering surgery (trabeculectomy) Intervention 2: cataract surgery (phacoemulsification) alone Study follow‐up: 12 months |
|
| Outcomes |
Primary outcome: mean postoperative IOP Secondary outcomes:
Adverse events reported: yes Intervals at which outcomes assessed: 1 day, 1 week, 4 weeks, and 3, 6, and 12 months after surgery |
|
| Notes |
Type of study: published Study period: not reported Funding source: not reported Disclosures of interest: not reported Publication language: English Subgroup analysis: none reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomization not reported |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported |
| Masking (detection bias) | Unclear risk | Masking not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2 of 85 (2%) participants excluded from 12 months analysis: 1/43 in combined surgery group and 1/42 in cataract surgery alone group |
| Selective reporting (reporting bias) | Low risk | Results for outcomes described in Methods section of study publication reported |
| Other bias | Unclear risk | Neither source of funding or possible disclosures of interest reported |
Fea 2010.
| Methods |
Study design: parallel‐group RCT Unit of randomization: the individual (one eye per participant) Number randomized: 36 total; 12 eyes of 12 participants in combined surgery group and 24 eyes of 24 participants in cataract surgery alone group Unit of analysis: the individual (one eye per participant) Number analyzed: 33 total; 12 eyes of 12 participants in combined surgery group and 21 eyes of 21 participants in cataract surgery alone group Exclusions and losses to follow‐up: 3 participants in the cataract surgery alone group: 1 capsule rupture, 1 did not present for the 6 months follow‐up, 1 died Power calculation: sample size of 12 eyes in combined surgery group and 24 eyes in cataract surgery alone group needed for 80% power to detect a difference in IOP of around 3 mmHg between the two intervention groups |
|
| Participants |
Country: Torino, Italy (single site) Age (mean ± SD): 64.5 ± 3.4 years (range: 60 to 70) in combined surgery group; 64.9 ± 3.1 years (range: 59 to 71) in cataract surgery alone group Gender: 4 men and 8 women in combined surgery group; 9 men and 15 women in the cataract surgery alone group Ethnicity: not reported Inclusion criteria:
Exclusion criteria:
Equivalence of baseline characteristics: yes (IOP, number of glaucoma medications) Diagnoses in participants: POAG and cataract |
|
| Interventions |
Intervention 1: combined cataract surgery (phacoemulsification) with IOL implantation and iStent® implantation Intervention 2: cataract surgery (phacoemulsification) with IOL implantation alone Study follow‐up: 15 months |
|
| Outcomes |
Primary outcome: mean IOP Secondary outcomes: number and type of glaucoma medications Adverse events reported: no Intervals at which outcomes assessed: 24 hours, 1 week, and 1, 2, 3, 6, 9, 12, and 15 months after surgery |
|
| Notes |
Type of study: published Study period: not reported Funding source: study devices provided by Glaukos Corp., Laguna Hills, California, USA; supported by Ricerca Finalizzata Della Regione Piemonte 2007 Disclosures of interest: "The author has no financial or proprietary interest in any material or method mentioned." Publication language: English Subgroup analysis: none reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer random number generator used: "Patient randomization was generated with a 2:1 ratio using Stata data analysis and statistical software" |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported |
| Masking (detection bias) | Low risk | "Patients were masked to their assignment, as were staff members who measured IOP throughout the study" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 3 of 36 (8%) participants excluded from final analysis: all in cataract surgery alone group (12%) |
| Selective reporting (reporting bias) | Low risk | Results for outcomes described in Methods section of study publication reported |
| Other bias | Unclear risk | Study devices provided by device manufacturer |
Fernández‐Barrientos 2010.
| Methods |
Study design: parallel‐group RCT Unit of randomization: the individual (one eye per participant) Number randomized: 33 total; 17 eyes of 17 participants in combined surgery group and 16 eyes of 16 participants in cataract surgery alone group Unit of analysis: the individual (one eye per participant) Number analyzed: 33 total; 17 eyes of 17 participants in combined surgery group and 16 eyes of 16 participants in cataract surgery alone group Exclusions and losses to follow‐up: none for IOP study Power calculation: sample size of 12 eyes in each group needed for 80% power to detect a 0.3 µL/min/mmHg difference in the outflow facility |
|
| Participants |
Country: Madrid, Spain (3 sites) Age (mean ± SD): 75.2 ± 7.2 years (range: 63 to 86) in combined surgery group; 76.7 ± 5.8 years (range: 64 to 89) in cataract surgery alone group Gender: 6 men and 11 women in combined surgery group; 9 men and 7 women in cataract surgery alone group Ethnicity: not reported Inclusion criteria:
Exclusion criteria:
Equivalence of baseline characteristics: yes (age, sex, stage of glaucoma) Diagnoses in participants: open‐angle glaucoma or ocular hypertension and cataract |
|
| Interventions |
Intervention 1: combined cataract surgery (phacoemulsification) and 2 iStent® implantations Intervention 2: cataract surgery (phacoemulsification) alone Study follow‐up: 12 months |
|
| Outcomes |
Primary outcomes: aqueous flow and trabecular outflow facility Secondary outcomes:
Adverse events reported: no Intervals at which outcomes assessed: 1, 2 and 7 to 14 days, and 1, 3, 6, and 12 months after surgery |
|
| Notes |
Type of study: published Study period: January to June 2006 enrolment Funding source: supported by Glaukos Corporation Disclosures of interest: authors reported receiving financial support, consulting, and/or being the recipient of gifts from the Glaukos Corporation Publication language: English Subgroup analysis: none reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer random number generator used |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported |
| Masking (detection bias) | Low risk | "All postoperative evaluations… were performed by the same examiner (YFB), who was masked to the type of surgery performed" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete outcome data for IOP and participants analyzed by group to which they were randomized |
| Selective reporting (reporting bias) | Low risk | Results for outcomes described in Methods section of study publication reported |
| Other bias | Unclear risk | Industry support and all authors affiliated with industry |
Georgopoulos 2000.
| Methods |
Study design: parallel‐group RCT Unit of randomization: the individual (one eye per participant) Number randomized: 28 total; 14 eyes of 14 participants in combined surgery group and 14 eyes of 14 participants in cataract surgery alone group Unit of analysis: the individual (one eye per participant) Number analyzed: 27 total; 14 eyes of 14 participants in combined surgery group and 13 eyes of 13 participants in cataract surgery alone group Exclusions and losses to follow‐up: 1 participant in cataract surgery alone group excluded due to vitreous loss during surgery Power calculation: none reported |
|
| Participants |
Country: Athens, Greece (one site) Age (mean ± SD): 67.4 ± 4.8 years in combined surgery group; 65.8 ± 4.4 years in cataract surgery alone group Gender: not reported Ethnicity: not reported Inclusion criteria:
Exclusion criteria: previous surgery or laser trabeculoplasty treatment Equivalence of baseline characteristics: yes (age, IOP, number of anti‐glaucoma medications, visual acuity) Diagnoses in participants: PEXG and cataract |
|
| Interventions |
Intervention 1: combined cataract surgery (phacoemulsification) and trabecular aspiration Intervention 2: cataract surgery (phacoemulsification) alone Study follow‐up: 12 to 18 months (mean of 15.7 months) |
|
| Outcomes |
Primary outcome: mean IOP Secondary outcomes:
Adverse events reported: yes Intervals at which outcomes assessed: 24 hours, 3 days, 1 month after surgery, and thereafter every 3 months |
|
| Notes |
Type of study: published Study period: referrals for surgery between March to September 1998 Funding source: not reported Disclosures of interest: not reported Publication language: English Subgroup analysis: none reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomization not reported |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported |
| Masking (detection bias) | Low risk | "A masked observer made all measurements" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 of 28 (4%) participants excluded from final analysis: participant in cataract surgery alone group |
| Selective reporting (reporting bias) | Low risk | Results for outcomes described in Methods section of study publication reported |
| Other bias | Unclear risk | Neither source of funding or possible disclosures of interest reported |
Gimbel 1995.
| Methods |
Study design: parallel‐group RCT Unit of randomization: the individual (one eye per participant) Number randomized: 106 total; 53 eyes of 53 participants in combined surgery group and 53 eyes of 53 participants in cataract surgery alone group Unit of analysis: the individual (one eye per participant) Number analyzed: 102 total; 51 eyes of 51 participants in combined surgery group and 51 eyes of 51 participants in cataract surgery alone group Exclusions and losses to follow‐up: 4 participants; 2 in each group Power calculation: none reported |
|
| Participants |
Country: Canada (one site), South Africa (two sites) Age (mean ± SD): 75.5 years in combined surgery group; 77.5 years in cataract surgery alone group (SDs not reported) Gender: not reported Ethnicity: not reported Inclusion criteria:
Exclusion criteria:
Equivalence of baseline characteristics: yes (preoperative IOP), no (percentage of participants who used beta blockers) Diagnoses in participants: POAG or ocular hypertension and cataract; participants with pigment dispersion or pseudoexfoliation glaucoma also included |
|
| Interventions |
Intervention 1: combined cataract surgery (phacoemulsification) with IOL implantation and trabecular aspiration (trabeculotomy ab externo) Intervention 2: cataract surgery (phacoemulsification) with IOL implantation alone Study follow‐up: 24 months |
|
| Outcomes |
Primary outcomes: mean IOP and mean change in IOP Secondary outcomes: complications, number of glaucoma medications Adverse events reported: yes Intervals at which outcomes assessed: 1 day, and 3, 6, 12, and 24 months after surgery |
|
| Notes |
Type of study: published Study period: not reported Funding source: not reported Disclosures of interest: "None of the authors has a proprietary interest in the development or marketing of this technique or in any of the instruments or drugs described in this paper." Publication language: English Subgroup analysis: none reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomization not reported |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported |
| Masking (detection bias) | High risk | "the physician following the patient was not masked as to whether the patient was in the study or control group" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 4 of 106 (4%) participants excluded from analysis: 2/53 in combined surgery group and 2/53 in cataract surgery alone group |
| Selective reporting (reporting bias) | Low risk | Results for outcomes described in Methods section of study publication reported |
| Other bias | Low risk | None identified |
Jacobi 1999.
| Methods |
Study design: parallel‐group RCT Unit of randomization: the individual (one eye per participant) Number randomized: 48 total; 26 eyes of 26 participants in combined surgery group and 22 eyes of 22 participants in cataract surgery alone group Unit of analysis: the individual (one eye per participant) Number analyzed: 20 total; 13 eyes of 13 participants in combined surgery group and 7 eyes of 7 participants in cataract surgery alone group at 30 months Exclusions and losses to follow‐up: At one year: none in combined surgery group and 6 eyes of 6 participants in cataract surgery alone group At 30 months: 13 eyes of 13 participants in combined surgery group and 15 eyes of 15 participants in cataract surgery alone group Power calculation: none reported |
|
| Participants |
Country: Cologne, Germany (one site) Age (mean ± SD): 69.4 ± 5.6 years (range: 52 to 77) in combined surgery group; 71.3 ± 6.1 years (range: 48 to 78) in cataract surgery alone group Gender: 10 men and 16 women in combined surgery group; 10 men and 12 women in cataract surgery alone group Ethnicity: not reported Inclusion criteria:
Exclusion criteria:
Equivalence of baseline characteristics: yes (age, sex, severity of glaucoma (vertical cup‐to‐disc ratio), number of glaucoma medications, IOP, visual severity of cataract, presence of diabetes, hypertension, or age‐related maculopathy) Diagnoses in participants: PEXG and cataract |
|
| Interventions |
Intervention 1: combined cataract surgery (phacoemulsification) with IOL implantation and trabecular aspiration Intervention 2: cataract surgery (phacoemulsification) with IOL implantation alone (Intervention 3: triple procedure (standard trabeculectomy, phacoemulsification, and IOL implantation); this group excluded from this report because it consisted of patients who opted out of the trial and therefore were not randomized) Study follow‐up: 30 months |
|
| Outcomes |
Primary outcome: mean change in IOP Secondary outcomes:
Adverse events reported: yes Intervals at which outcomes assessed: 1 and 2 weeks, and 1, 3, 6, 12, 24, and 30 months after surgery |
|
| Notes |
Type of study: published Study period: not reported Funding source: Deutsche Forschungsgemeinschaft, Bonn, Germany Disclosures of interest: "None of the authors has any proprietary interest in the development or marketing of equipment used in this study or any competing piece of equipment." Publication language: English Subgroup analysis: none reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomization not reported |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported |
| Masking (detection bias) | Low risk | "Goldmann applanation tonometry was done at the slitlamp in a double‐masked fashion by the same examiner" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 28 of 48 (53%) participants excluded from 30 months analysis: 13/26 in combined surgery group and 15/22 in cataract surgery alone group |
| Selective reporting (reporting bias) | Low risk | Results for outcomes described in Methods section of study publication reported |
| Other bias | Low risk | None identified |
Liaska 2014.
| Methods |
Study design: parallel‐group RCT Unit of randomization: the individual (one eye per participant) Number randomized: 61 total; 29 eyes of 29 participants in combined surgery group and 32 eyes of 32 participants in cataract surgery alone group Unit of analysis: the individual (one eye per participant) Number analyzed: 60 total; 29 eyes of 29 participants in combined surgery group and 31 eyes of 31 participants in cataract surgery alone group Exclusions and losses to follow‐up: 1 eye of 1 participant in cataract surgery alone group was "recruited in error" and was not included in the analysis Power calculation: sample size of 32 participants in each group needed for 80% power to detect a reduction in IOP of 2 mmHg (between‐patient SD = 2.5 mmHg, within‐patient SD = 2 mmHg) between groups given an anticipated dropout rate of 10% |
|
| Participants |
Country: Greece (one site) Age (mean ± SD): 77.0 ± 6.7 years in combined surgery group; 78.1 ± 7.26 years in cataract surgery alone group Gender: 13 men and 16 women in combined surgery group; 19 men and 12 women in cataract surgery alone group Ethnicity: not reported Inclusion criteria:
Exclusion criteria: not specified Equivalence of baseline characteristics: yes (IOP, visual field, amount of glaucoma medications, visual acuity, age, central corneal thickness) Diagnoses in participants: advanced chronic POAG and cataract |
|
| Interventions |
Intervention 1: combined cataract surgery (phacoemulsification) and trabeculectomy Intervention 2: cataract surgery (phacoemulsification) alone Study follow‐up: 24 months |
|
| Outcomes |
Primary outcomes:
Secondary outcomes: none reported Adverse events reported: no Intervals at which outcomes assessed: Combined surgery group: 1 and 2 days, 1 week and weekly until the end of the second month, twice a month until the end of the fourth month, 4, 5, 6 , 9, 12, 18, and 24 months Cataract surgery alone group: 1, 4, 7, and 20 days, 1 month and monthly until achieving visual acuity 20/20 and controlled IOP, then every three months thereafter to 24 months |
|
| Notes |
Type of study: published Study period: April 2005 to August 2010 Funding source: not reported Disclosures of interest: not reported Publication language: English Subgroup analysis: none reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer random number generator used: "Participants were randomly assigned following simple randomization procedures, with a 1:1 allocation to one of two treatment groups. For allocation of the participants, a computer‐generated list of random numbers was used." |
| Allocation concealment (selection bias) | Low risk | "The allocation sequence was concealed from the researcher (AL), enrolling and assessing participants in sequentially numbered, opaque and sealed envelopes. Envelopes were opened only after the enrolled participants completed all baseline assessments and it was time to allocate the intervention." |
| Masking (detection bias) | Low risk | "data collectors, outcome assessors, and data analysts were kept uninformed of the allocation" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "The primary analysis was intention‐to‐treat (ITT) and involved all patients who were randomly assigned, except for one woman in the phacoemulsification‐alone group who consistently performed visual fields with MD better than ‐12dB postoperatively. After consultation with the trial’s steering committee, it was determined that she had been "recruited in error" and therefore she was not included in the analysis." |
| Selective reporting (reporting bias) | Low risk | Results for outcomes described in Methods section of study publication reported |
| Other bias | Unclear risk | Neither source of funding or possible disclosures of interest reported |
Samuelson 2011.
| Methods |
Study design: parallel‐group RCT Unit of randomization: the individual (one eye per participant) Number randomized: 240 total; 117 eyes of 117 participants in combined surgery group and 123 eyes of 123 participants in cataract surgery alone group Unit of analysis: the individual (one eye per participant) Number analyzed: 218 total; 106 eyes of 106 participants in combined surgery group and 112 eyes of 112 participants in cataract surgery alone group Exclusions and losses to follow‐up: 22 total; 15 eyes of 15 participants excluded (6 in combined surgery group and 9 in cataract surgery alone group) and 7 eyes of 7 participants lost to follow‐up (5 in combined surgery group and 2 in cataract surgery alone group) Power calculation: sample size of 90 eyes in each group needed for 80% power to detect a 19.5% difference in the primary efficacy outcome between groups (i.e., 55% responder rate in the combined surgery group versus 35.5% in the cataract surgery alone group) |
|
| Participants |
Country: USA (29 sites) Age (mean ± SD): 74 ± 8 years (range: 53 to 88) in combined surgery group; 73 ± 9 years (range: 48 to 88) in cataract surgery alone group Gender: 46 men and 71 women in combined group; 52 men and 71 women in cataract surgery alone group Ethnicity: (American Indian or Alaska Native: Asian: Black or African American: Native Hawaiian or Pacific Islander: Hispanic or Latino: White): 1:1:15:1:16:83 in combined surgery group; 1:0:19:0:15:88 in cataract surgery alone group Inclusion criteria:
Exclusion criteria:
Equivalence of baseline characteristics: yes (age, gender, race, study eye, visual field, additional glaucoma diagnosis, IOP at screening, IOP at baseline, BCVA) Diagnoses in participants: open‐angle glaucoma (includes PEXG, pigmentary glaucoma) and cataract |
|
| Interventions |
Intervention 1: combined cataract surgery (phacoemulsification) and iStent® implantation Intervention 2: cataract surgery (phacoemulsification) alone Study follow‐up: 12 months and 24 months |
|
| Outcomes |
Primary outcome: proportion of participants with IOP ≤ 21 mmHg without ocular hypotensive medication 1 year postoperatively Secondary outcomes:
Adverse events reported: yes Intervals at which outcomes assessed: 3 to 7 hours, 1 day, 1 to 2 weeks, and 3, 6, 12, 18, and 24 months after surgery |
|
| Notes |
Type of study: published Study period: enrolment from April 2005 to June 2007 Funding source: Glaukos Disclosures of interest: authors reported receiving financial support from or consulting Alcon, Allergan, AMO, AqueSys, Glaukos, iScience, Ivantis, Lumenis, Pfizer, QLT, and Santen; all trial investigators were consultants to Glaukos for the conduct of this study; four trial investigators are equity owners of Glaukos Publication language: English Subgroup analysis: none reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer random number generator used: "Computer‐generated randomization was performed (PROC PLAN, PC‐SAS, SAS Inc., Cary NC)" |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported |
| Masking (detection bias) | High risk | "The study was, by design, open‐label, given that there was no way to mask the treatment to the surgeon during the surgical intervention, or to the observer at the time of gonioscopy." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 22 of 240 (9%) participants excluded from 12 months analysis: 11/117 in combined surgery group and 11/123 in cataract surgery alone group |
| Selective reporting (reporting bias) | Low risk | Results for outcomes described in Methods section of study publication reported |
| Other bias | Unclear risk | Funded by device manufacturer; all trial investigators were consultants to Glaukos for the conduct of this study; four of the investigators are equity owners of Glaukos |
Storr‐Paulsen 1998.
| Methods |
Study design: parallel‐group RCT Unit of randomization: the individual (one eye per participant; except for two participants in which both eyes were included) Number randomized: 20 eyes of 18 participants; 10 eyes in each group Unit of analysis: the eye Number analyzed: 20 eyes of 18 participants; 10 eyes in each group Exclusions and losses to follow‐up: none reported Power calculation: none reported |
|
| Participants |
Country: Denmark Age (median): 79 years (range: 57 to 83) in combined surgery group; 81 years (range: 77 to 88) in cataract surgery alone group Gender: 3 men and 7 women in combined surgery group; 1 man and 9 women in cataract surgery alone group Ethnicity: not reported Inclusion criteria: visually significant cataract, open‐angle glaucoma and one or more of the following characteristics: uncontrolled IOP (> 21 mmHg) on maximum acceptable medication, poor compliance, or progressing glaucomatous visual field defects despite medication Exclusion criteria:
Equivalence of baseline characteristics: yes (age, gender, IOP, medication, visual acuity, visual field) Diagnoses in participants: POAG and cataract |
|
| Interventions |
Intervention 1: combined cataract surgery (phacoemulsification) and trabeculectomy Intervention 2: cataract surgery (phacoemulsification) alone Study follow‐up: 12 months |
|
| Outcomes |
Primary outcome: IOP Secondary outcomes:
Adverse events reported: no Intervals at which outcomes assessed: 4 to 6 hours, 1 and 5 days, and 3 and 12 months after surgery |
|
| Notes |
Type of study: published Study period: enrolment from August 1994 to January 1996 Funding source: not reported Disclosures of interest: not reported Publication language: English Subgroup analysis: none reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomization not reported |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported |
| Masking (detection bias) | Unclear risk | Masking not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete outcome data and participants analyzed by group to which they were randomized |
| Selective reporting (reporting bias) | Low risk | Results for outcomes described in Methods section of study publication reported |
| Other bias | Unclear risk | The unit of randomization (the individual) was different from the unit of analysis (the eye): "Twenty eyes of 18 patients were enrolled in the study"; "The patients were then randomly assigned…" Neither source of funding nor possible disclosures of interest reported |
dB: decibel; IOL: intraocular lens; IOP: intraocular pressure; mmHg: millimeter of mercury; PEXG: pseudoexfoliation glaucoma; POAG: primary open‐angle glaucoma; SD: standard deviation; µL/min/mmHg: microliter per minute per millimeter of mercury
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bobrow 1998 | Not intervention of interest, cataract surgery not phacoemulsification (ECCE with posterior chamber IOL) |
| Cillino 2004 | Not intervention of interest, random assignment to two types of glaucoma surgery and cataract surgery was performed depending on the participant's diagnosis |
| D'Eliseo 2003 | Not intervention of interest, comparison between deep sclerectomy alone versus combined sclerectomy and phacoemulsification |
| Ghirelli 1995 | Not intervention of interest, cataract surgery performed depending on the participant's diagnosis |
| Pillunat 2001 | Potentially intervention of interest, but insufficient information to confirm eligibility (conference abstract only) |
| Shin 2001 | Not intervention of interest, compared verapamil versus placebo |
| Tanaka 1998 | Potentially intervention of interest, but insufficient information to confirm eligibility (conference abstract only) |
Characteristics of studies awaiting assessment [ordered by study ID]
Wang 2011.
| Methods |
Study design: "clinical comparative observation" using randomization Unit of randomization: the individual (one eye per participant) Number randomized: 96 participants; 32 participants in each of three groups Unit of analysis: the individual (one eye per participant) Number analyzed: 96 participants; 32 participants in each of three groups Exclusions and losses to follow‐up: none reported Power calculation: none reported |
| Participants |
Country: China Age (mean): 66 year (range: 38 to 76); not reported by group Gender: 60 men and 36 women; not reported by group Ethnicity: not reported Inclusion criteria:
Exclusion criteria: not reported Equivalence of baseline characteristics: yes Diagnoses in participants: not reported |
| Interventions |
Intervention 1: combined cataract surgery (phacoemulsification) with IOL implantation and trabeculectomy Intervention 2: cataract surgery (phacoemulsification) with IOL implantation alone Intervention 3: trabeculectomy alone Study follow‐up: 6 months |
| Outcomes |
Primary outcome: change in IOP Secondary outcomes:
Adverse events reported: no Intervals at which outcomes assessed: 2 weeks and 6 months |
| Notes |
Type of study: published Study period: enrolment from January 2007 to June 2010 Funding source: not reported Disclosures of interest: not reported Publication language: Chinese Subgroup analysis: not reported |
IOL: intraocular lens; IOP: intraocular pressure; mmHg: millimeter of mercury.
Characteristics of ongoing studies [ordered by study ID]
NCT00326066.
| Trial name or title | A Study of the Trabecular Micro‐bypass Stent in Combination With Cataract Surgery in Subjects With Newly Diagnosed Open Angle Glaucoma and Subjects Diagnosed With Ocular Hypertension |
| Methods |
Study design: parallel‐group RCT Unit of randomization: not reported Number randomized: 47 total; per group not reported Unit of analysis: not reported Number analyzed: not reported Exclusions and losses to follow‐up: not reported Power calculation: none reported |
| Participants |
Country: Vienna, Austria (Vienna Medical Hospital); Germany (Mainz University, Mainz; Augenklinik der Technischen Universitat, Munich; Universitats‐ Augenklinik, Wurzburg); Netherlands (The Netherlands Ophthalmic Research Institute, Amsterdam; Ophthalmic Clinic, Rotterdam); Spain (Clinico San Carlos, Madrid; Instituto Oftalmologico de Aragon, Zaragoza); Turkey (Beyoglu Eye Research and Education Hospital, Istanbul) Age: not reported; 18 years and older eligible Gender: not reported, both genders eligible Ethnicity: not reported Inclusion criteria:
Exclusion criteria:
Equivalence of baseline characteristics: not reported Diagnoses in participants: open‐angle glaucoma or ocular hypertension and cataract |
| Interventions |
Intervention 1: combined cataract surgery (phacoemulsification) and 2 iStent® implantations Intervention 2: cataract surgery (phacoemulsification) alone Study follow‐up: 24 months |
| Outcomes |
Primary outcome: efficacy (reduction in IOP) Secondary outcomes: not reported Adverse events assessed: not reported Intervals at which outcomes assessed: not reported |
| Starting date | February 2005 |
| Contact information | Study Director: Head of Clinical Affairs (Glaukos Corporation) |
| Notes |
Type of study: ongoing Study period: enrolment from February 2005 to May 2013 Funding source: Glaukos Corporation Disclosures of interest: affiliation with Glaukos Corporation Publication language: English Subgroup analysis: none reported |
NCT01052558.
| Trial name or title | A Prospective, Randomized, Controlled, Parallel Groups, Multicenter Clinical Investigation of the Glaukos Trabecular Micro‐bypass Stent Model GTS400 in Conjunction With Cataract Surgery |
| Methods |
Study design: parallel‐group RCT Unit of randomization: not reported Number randomized: estimated 500; per group not reported Unit of analysis: not reported Number analyzed: not reported Exclusions and losses to follow‐up: not reported Power calculation: none reported |
| Participants |
Country: USA (22 sites) Age: not reported; 18 to 90 years eligible Gender: not reported, both genders eligible Ethnicity: not reported Inclusion criteria:
Exclusion criteria:
Equivalence of baseline characteristics: not reported Diagnoses in participants: open‐angle glaucoma and cataract |
| Interventions |
Intervention 1: combined cataract surgery (phacoemulsification) and iStent® implantation Intervention 2: cataract surgery (phacoemulsification) alone Study follow‐up: 12 months |
| Outcomes |
Primary outcome: proportion of participants with 12 month diurnal IOP ≤ 21 mmHg without use of ocular hypotensive medications for 4 weeks or more prior to 12 month visit Secondary outcomes: not reported Adverse events assessed: not reported Intervals at which outcomes assessed: not reported |
| Starting date | January 2010 |
| Contact information |
Study Director: Jeff Wells, PharmD, MBA (Glaukos Corporation) Study Chair: Jay Katz, MD (Wills Eye Institute; Thomas Jefferson University) |
| Notes |
Type of study: ongoing Study period: enrolment from January 2010 to September 2013 Funding source: Glaukos Corporation Disclosures of interest: affiliation with Glaukos Corporation Publication language: English Subgroup analysis: none reported |
NCT01085357.
| Trial name or title | A Prospective, Randomized, Comparative, MultiCenter Clinical Study to Assess the Safety and Effectiveness of the Transcend CyPass Glaucoma Implant in Patients With Open Angle Glaucoma Undergoing Cataract Surgery |
| Methods |
Study design: parallel‐group RCT Unit of randomization: not reported Number randomized: 897 total; per group not reported Unit of analysis: not reported Number analyzed: not reported Exclusions and losses to follow‐up: not reported Power calculation: none reported |
| Participants |
Country: USA (22 sites) Age: not reported; 45 years and older eligible Gender: not reported, both genders eligible Ethnicity: not reported Inclusion criteria:
Exclusion criteria:
Equivalence of baseline characteristics: not reported Diagnoses in participants: POAG and cataract |
| Interventions |
Intervention 1: combined cataract surgery (phacoemulsification) and CyPass Micro‐Stent implantation Intervention 2: cataract surgery (phacoemulsification) alone Study follow‐up: 24 months |
| Outcomes |
Primary outcome: proportion of eyes with ≥ 20% decrease in IOP from baseline to the hypotensive medication‐free 24‐month postoperative examination Secondary outcomes:
Adverse events assessed: not reported Intervals at which outcomes assessed: not reported |
| Starting date | September 2009 |
| Contact information | None provided |
| Notes |
Type of study: ongoing Study period: September 2009 to March 2015 Funding source: Transcend Medical, Inc. Disclosures of interest: affiliation with Transcend Medical, Inc. Publication language: English Subgroup analysis: none reported |
NCT01539239.
| Trial name or title | The Safety and Effectiveness of the Hydrus Aqueous Implant for Lowering Intraocular Pressure in Glaucoma Patients Undergoing Cataract Surgery, A Prospective, Multicenter, Randomized, Controlled Clinical Trial |
| Methods |
Study design: parallel‐group RCT Unit of randomization: not reported Number randomized: estimated 800; per group not reported Unit of analysis: not reported Number analyzed: not reported Exclusions and losses to follow‐up: not reported Power calculation: none reported |
| Participants |
Country: USA, Canada, Germany, Italy, Mexico, Phillipines, Poland, Singapore, Spain, United Kingdom (37 sites) Age: not reported; 45 years and older eligible Gender: not reported, both genders eligible Ethnicity: not reported Inclusion criteria:
Exclusion criteria:
Equivalence of baseline characteristics: not reported Diagnoses in participants: POAG and cataract |
| Interventions |
Intervention 1: combined cataract surgery (phacoemulsification) and Hydrus Aqueous Implant implantation Intervention 2: cataract surgery (phacoemulsification) alone Study follow‐up: 24 months |
| Outcomes |
Primary outcome: reduction in mean diurnal IOP from baseline at 24 months following medication washout Secondary outcomes: mean diurnal washed out IOP change from baseline at 24 months compared between treatment and control groups Adverse events assessed: not reported Intervals at which outcomes assessed: annual follow‐up up to 5 years |
| Starting date | January 2012 |
| Contact information | Principal Investigator: Alan Crandall, MD (The Eye Institute of Utah) |
| Notes |
Type of study: ongoing Study period: January 2012 to January 2018 Funding source: Ivantis, Inc. Disclosures of interest: affiliation with Ivantis, Inc. Publication language: English Subgroup analysis: none reported |
NCT01818115.
| Trial name or title | A Prospective, Multi‐Center, Randomized Controlled Trial to Evaluate the Safety and Effectiveness of the Hydrus Implant for Lowering Intraocular Pressure in Glaucoma Patients Undergoing Cataract Surgery |
| Methods |
Study design: parallel‐group RCT Unit of randomization: not reported Number randomized: 100 total; per group not reported Unit of analysis: not reported Number analyzed: not reported Exclusions and losses to follow‐up: not reported Power calculation: none reported |
| Participants |
Country: Germany (2 sites), Italy (2 sites), Netherlands, Spain (2 sites) Age: not reported; 21 to 80 years eligible Gender: not reported, both genders eligible Ethnicity: not reported Inclusion criteria:
Exclusion criteria:
Equivalence of baseline characteristics: not reported Diagnoses in participants: POAG or PEXG and cataract |
| Interventions |
Intervention 1: combined cataract surgery (phacoemulsification) with IOL implantation and Hydrus Implant implantation Intervention 2: cataract surgery (phacoemulsification) with IOL implantation alone Study follow‐up: 24 months |
| Outcomes |
Primary outcome: difference in proportion of participants with 20% reduction in mean diurnal IOP at 24 months following the wash‐out of all glaucoma medications Secondary outcomes:
Adverse events assessed: not reported Intervals at which outcomes assessed: annual follow‐up up to 5 years |
| Starting date | January 2011 |
| Contact information | Principal Investigator: Norbert Pfeiffer, MD (Universitatsmedizin Mainz) |
| Notes |
Type of study: ongoing Study period: January 2011 to November 2014 Funding source: Ivantis, Inc. Disclosures of interest: affiliation with Ivantis, Inc. Publication language: English Subgroup analysis: none reported |
IOL: intraocular lens; IOP: intraocular pressure; PEXG: pseudoexfoliation glaucoma; POAG: primary open‐angle glaucoma.
Differences between protocol and review
We did not perform some methods described in the protocol for this review due to insufficient data being available for analysis (e.g., subgroup analyses, sensitivity analysis). If sufficient data become available when we update the review, we will follow the methods as described in the protocol (Hirunyachote 2014).
Based on the various types of devices used in the included studies, we analyzed subgroups for the outcome of mean IOP based on the type of devices used to assess for potential effect modification.
Contributions of authors
Conceived the review: PH and HJ Designed the review: MLZ, PH, and HJ Co‐ordinated the review: HJ Data collection for the review: Designed electronic search strategies: PH, HJ, and CEVG Trials Search Co‐ordinator Undertook electronic searches: CEVG Trials Search Co‐ordinator Screened search results: MLZ, PH, and HJ Organized retrieval of papers: PH and CEVG US Project (CEVG@US) Screened retrieved papers against inclusion criteria: MLZ, PH, and HJ Appraised quality of papers: MLZ and PH Extracted data from papers: MLZ and PH Wrote to authors of papers for additional information: MLZ and PH Obtained and screened data on unpublished studies: MLZ and PH Data management for the review: Entered data into RevMan 2014: MLZ and PH Analyzed data: MLZ, PH, and HJ Interpretation of data: Provided a methodological perspective: MLZ, PH, and HJ Provided a clinical perspective: MLZ, PH, and HJ Provided a policy perspective: MLZ, PH, and HJ Writing the review: MLZ, PH, and HJ Performed previous work that was the foundation of the current study: HJ
Sources of support
Internal sources
No sources of support supplied
External sources
-
National Eye Institute, National Institutes of Health, USA.
Methodological support provided by the CEVG US Project, funded by grant 1 U01 EY020522
-
National Institutute of Health Research (NIHR), UK.
Richard Wormald, Co‐ordinating Editor for Cochrane Eyes and Vision Group (CEVG) acknowledges financial support for his CEV research sessions from the Department of Health through the award made by the National Institute for Health Research to Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology for a Specialist Biomedical Research Centre for Ophthalmology.
- The NIHR also funds the CEVG Editorial Base in London.
The views expressed in this publication are those of the authors and not necessarily those of the NIHR, NHS, or the Department of Health.
Declarations of interest
PH and MLZ have no known conflicts of interest.
HJ has been a consultant within the past three years for Endo Optiks, which makes a device used in the treatment of glaucoma; for Ivantis and Transcend, companies that are developing new technologies for the surgical treatment of glaucoma; for Allergan, a company that manufactures pharmaceuticals for the treatment of glaucoma; and for ForSight, a company developing a controlled release device for IOP lowering medications.
New
References
References to studies included in this review
Anders 1997 {published data only}
- Anders N, Pham T, Holschbach A, Wollensak J. Combined phacoemulsification and filtering surgery with the 'no‐stitch' technique. Archives of Ophthalmology 1997;115(10):1245‐9. [DOI] [PubMed] [Google Scholar]
Fea 2010 {published data only}
- Fea AM. Phacoemulsification versus phacoemulsification with micro‐bypass stent implantation in primary open‐angle glaucoma: randomized double‐masked clinical trial. Journal of Cataract and Refractive Surgery 2010;36(3):407‐12. [DOI] [PubMed] [Google Scholar]
Fernández‐Barrientos 2010 {published data only}
- Fernández‐Barrientos Y, García‐Feijoó J, Martínez‐de‐la‐Casa JM, Pablo LE, Fernández‐Pérez C, García Sánchez J. Fluorophotometric study of the effect of the Glaukos trabecular microbypass stent on aqueous humor dynamics. Investigative Ophthalmology and Visual Science 2010;51(7):3327‐32. [DOI] [PubMed] [Google Scholar]
Georgopoulos 2000 {published data only}
- Georgopoulos GT, Chalkiadakis J, Livir‐Rallatos G, Theodossiadis PG, Theodossiadis GP. Combined clear cornea phacoemulsification and trabecular aspiration in the treatment of pseudoexfoliative glaucoma associated with cataract. Graefe's Archive for Clinical and Experimental Ophthalmology 2000;238(10):816‐21. [DOI] [PubMed] [Google Scholar]
Gimbel 1995 {published data only}
- Gimbel HV, Meyer D, DeBroff BM, Roux CW, Ferensowicz M. Intraocular pressure response to combined phacoemulsification and trabeculotomy ab externo versus phacoemulsification alone in primary open‐angle glaucoma. Journal of Cataract and Refractive Surgery 1995;21(6):653‐60. [DOI] [PubMed] [Google Scholar]
Jacobi 1999 {published data only}
- Jacobi PC, Dietlein TS, Krieglstein GK. Comparative study of trabecular aspiration vs trabeculectomy in glaucoma triple procedure to treat pseudoexfoliation glaucoma. Archives of Ophthalmology 1999;117(10):1311‐8. [DOI] [PubMed] [Google Scholar]
Liaska 2014 {published data only}
- Liaska A, Papaconstantinou D, Georgalas I, Koutsandrea C, Theodosiadis P, Chatzistefanou K. Phaco‐trabeculectomy in controlled, advanced, open‐angle glaucoma and cataract: parallel, randomized clinical study of efficacy and safety. Seminars in Ophthalmology 2014;29(4):226‐35. [DOI] [PubMed] [Google Scholar]
Samuelson 2011 {published data only}
- Craven ER, Katz LJ, Wells JM, Giamporcaro JE, iStent Study Group. Cataract surgery with trabecular micro‐bypass stent implantation in patients with mild‐to‐moderate open‐angle glaucoma and cataract: two‐year follow‐up. Journal of Cataract and Refractive Surgery 2012;38(8):1339‐45. [DOI] [PubMed] [Google Scholar]
- Samuelson TW, Katz LJ, Wells JM, Duh YJ, Giamporcaro JE, US iStent Study Group. Randomized evaluation of the trabecular micro‐bypass stent with phacoemulsification in patients with glaucoma and cataract. Ophthalmology 2011;118(3):459‐67. [DOI] [PubMed] [Google Scholar]
Storr‐Paulsen 1998 {published data only}
- Storr‐Paulsen A, Pedersen JH, Laugesen C. A prospective study of combined phacoemulsification‐trabeculectomy versus conventional phacoemulsification in cataract patients with coexisting open angle glaucoma. Acta Ophthalmologica Scandinavica 1998;76(6):696‐9. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Bobrow 1998 {published data only}
- Bobrow JC. Cataract extraction and lens implantation with and without trabeculectomy: an intrapatient comparison. Transactions of the American Ophthalmological Society 1998;96:521‐56. [PMC free article] [PubMed] [Google Scholar]
- Bobrow JC. Prospective intrapatient comparison of extracapsular cataract extraction and lens implantation with and without trabeculectomy. American Journal of Ophthalmology 2000;129(3):291‐6. [DOI] [PubMed] [Google Scholar]
Cillino 2004 {published data only}
- Cillino S, Pace F, Casuccio A, Calvaruso L, Morreale D, Vadalà M, et al. Deep sclerectomy versus punch trabeculectomy with or without phacoemulsification: a randomized clinical trial. Journal of Glaucoma 2004;13(6):500‐6. [DOI] [PubMed] [Google Scholar]
D'Eliseo 2003 {published data only}
- D'Eliseo D, Pastena B, Longanesi L, Grisanti F, Negrini V. Comparison of deep sclerectomy with implant and combined glaucoma surgery. Ophthalmologica 2003;217(3):208‐11. [DOI] [PubMed] [Google Scholar]
Ghirelli 1995 {published data only}
- Ghirelli G, Ciucci F, Leonori J, Bardocci A, Cocco F, Navarra M. Intraocular pressure after trabeculectomy and after combined procedure. Annali Di Ottalmologia E Clinica Oculistica 1995;121(1):13‐7. [Google Scholar]
Pillunat 2001 {published data only}
- Pillunat LE, Kamman J, Kohlhaas M. Clear cornea phacoemulsification as an intraocular pressure lowering procedure in glaucoma. Investigative Ophthalmology and Visual Science 2001; Vol. 42:ARVO E‐Abstract 579.
Shin 2001 {published data only}
- Shin DH, Kardasis CT, Kim C, Bsee, Juzych MS, Hughes BA, et al. Topical verapamil in glaucoma filtration surgery. Journal of Glaucoma 2001;10(3):211‐4. [DOI] [PubMed] [Google Scholar]
Tanaka 1998 {published data only}
- Tanaka GH, Vold SD, Ruderman JM. Primary combined phacoemulsification and trabeculectomy versus phacoemulsification alone in patients with well‐controlled glaucoma: early outcomes. Investigative Ophthalmology and Visual Science 1998; Vol. 39:ARVO E‐Abstract 1137.
References to studies awaiting assessment
Wang 2011 {published data only}
- Wang Y, Du JF. Clinical comparative observation of three methods for treating cataract complicated with glaucoma. International Journal of Ophthalmology 2011;11(10):1816‐8. [Google Scholar]
References to ongoing studies
NCT00326066 {unpublished data only}
- NCT00326066. A study of the trabecular micro‐bypass stent in combination with cataract surgery in subjects with newly diagnosed open angle glaucoma and subjects diagnosed with ocular hypertension. clinicaltrials.gov/ct2/show/NCT00326066 (accessed 7 August 2014).
NCT01052558 {unpublished data only}
- NCT01052558. A prospective, randomized, controlled, parallel groups, multicenter clinical investigation of the Glaukos trabecular micro‐bypass stent model GTS400 in conjunction with cataract surgery. clinicaltrials.gov/ct2/show/NCT01052558 (accessed 7 August 2014).
NCT01085357 {unpublished data only}
- NCT01085357. A prospective, randomized, comparative, multicenter clinical study to assess the safety and effectiveness of the Transcend CyPass glaucoma implant in patients with open angle glaucoma undergoing cataract surgery. clinicaltrials.gov/ct2/show/NCT01085357 (accessed 13 September 2014).
NCT01539239 {unpublished data only}
- NCT01539239. The safety and effectiveness of the Hydrus aqueous implant for lowering intraocular pressure in glaucoma patients undergoing cataract surgery, a prospective, multicenter, randomized, controlled clinical trial. clinicaltrials.gov/ct2/show/NCT01539239 (accessed 15 September 2014).
NCT01818115 {unpublished data only}
- NCT01818115. A prospective, multi‐center, randomized controlled trial to evaluate the safety and effectiveness of the Hydrus implant for lowering intraocular pressure in glaucoma patients undergoing cataract surgery. clinicaltrials.gov/ct2/show/NCT01818115 (accessed 13 September 2014).
Additional references
Anderson 2003
- Anderson DR, Normal Tension Glaucoma Study. Collaborative normal tension glaucoma study. Current Opinion in Ophthalmology 2003;14(2):86‐90. [DOI] [PubMed] [Google Scholar]
Burr 2012
- Burr J, Azuara‐Blanco A, Avenell A, Tuulonen A. Medical versus surgical interventions for open angle glaucoma. Cochrane Database of Systematic Reviews 2012, Issue 9. [DOI: 10.1002/14651858.CD004399.pub3] [DOI] [PubMed] [Google Scholar]
Congdon 2004
- Congdon N, Vingerling JR, Klein BE, West S, Friedman DS, Kempen J, et al. Prevalence of cataract and pseudophakia/aphakia among adults in the United States. Archives of Ophthalmology 2004;122(4):487‐94. [DOI] [PubMed] [Google Scholar]
Costa 1993
- Costa VP, Smith M, Spaeth GL, Gandham S, Markovitz B. Loss of visual acuity after trabeculectomy. Ophthalmology 1993;100(5):599‐612. [DOI] [PubMed] [Google Scholar]
de Silva 2014
- Silva SR, Riaz Y, Evans JR. Phacoemulsification with posterior chamber intraocular lens versus extracapsular cataract extraction (ECCE) with posterior chamber intraocular lens for age‐related cataract. Cochrane Database of Systematic Reviews 2014, Issue 1. [DOI: 10.1002/14651858.CD008812.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dietlein 2008
- Dietlein TS, Jordan J, Lueke C, Krieglstein GK. Modern concepts in antiglaucomatous implant surgery. Graefe's Archive for Clinical and Experimental Ophthalmology 2008;246(12):1653‐64. [DOI] [PubMed] [Google Scholar]
Edmunds 2002
- Edmunds B, Thompson JR, Salmon JF, Wormald RP. The National Survey of Trabeculectomy. III. Early and late complications. Eye 2002;16(3):297‐303. [DOI] [PubMed] [Google Scholar]
Friedman 2002
- Friedman DS, Jampel HD, Lubomski LH, Kempen JH, Quigley H, Congdon N, et al. Surgical strategies for coexisting glaucoma and cataract: an evidence‐based update. Ophthalmology 2002;109(10):1902‐13. [DOI] [PubMed] [Google Scholar]
Gedde 2009
- Gedde SJ, Schiffman JC, Feuer WJ, Herndon LW, Brandt JD, Budenz DL, et al. Three‐year follow‐up of the tube versus trabeculectomy study. American Journal of Ophthalmology 2009;148(5):670‐84. [DOI] [PubMed] [Google Scholar]
Glanville 2006
- Glanville JM, Lefebvre C, Miles JN, Camosso‐Stefinovic J. How to identify randomized controlled trials in MEDLINE: ten years on. Journal of the Medical Library Association 2006;94(2):130‐6. [PMC free article] [PubMed] [Google Scholar]
Godfrey 2009
- Godfrey DG, Fellman RL, Neelakantan A. Canal surgery in adult glaucomas. Current Opinion in Ophthalmology 2009;20(2):116‐21. [DOI] [PubMed] [Google Scholar]
Halikiopoulos 2001
- Halikiopoulos D, Moster MR, Azuara‐Blanco A, Wilson RP, Schmidt CM, Spaeth GL, et al. The outcome of the functioning filter after subsequent cataract extraction. Ophthalmic Surgery and Lasers 2001;32(2):108‐17. [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JPT, Altman DG (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Higgins 2011b
- Higgins JPT, Deeks JJ, Altman DG (editors). Chapter 16: Special topics in statistics. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Kim 2009
- Kim KS, Kim JM, Park KH, Choi CY, Chang HR. The effect of cataract surgery on diurnal intraocular pressure fluctuation. Journal of Glaucoma 2009;18(5):399‐402. [DOI] [PubMed] [Google Scholar]
Leaming 2003
- Leaming DV. Practice styles and preferences of ASCRS members‐‐2002 survey. Journal of Cataract and Refractive Surgery 2003;29(7):1412‐20. [DOI] [PubMed] [Google Scholar]
Lee 2003
- Lee BL, Wilson MR, Ocular Hypertension Treatment Study (OHTS). Ocular Hypertension Treatment Study (OHTS) commentary. Current Opinion in Ophthalmology 2003;14(2):74‐7. [DOI] [PubMed] [Google Scholar]
Lee 2009
- Lee YH, Yun YM, Kim SH, Lee EK, Lee JE, Kim CS. Factors that influence intraocular pressure after cataract surgery in primary glaucoma. Canadian Journal of Ophthalmology 2009;44(6):705‐10. [DOI] [PubMed] [Google Scholar]
Leske 2003
- Leske MC, Heijl A, Hussein M, Bengtsson B, Hyman L, Komaroff E, et al. Factors for glaucoma progression and the effect of treatment: the early manifest glaucoma trial. Archives of Ophthalmology 2003;121(1):48‐56. [DOI] [PubMed] [Google Scholar]
Lin 2008
- Lin SC. Endoscopic and transscleral cyclophotocoagulation for the treatment of refractory glaucoma. Journal of Glaucoma 2008;17(3):238‐47. [DOI] [PubMed] [Google Scholar]
Mathalone 2005
- Mathalone N, Hyams M, Neiman S, Buckman G, Hod Y, Geyer O. Long‐term intraocular pressure control after clear corneal phacoemulsification in glaucoma patients. Journal of Cataract and Refractive Surgery 2005;31(3):479‐83. [DOI] [PubMed] [Google Scholar]
Meyer 1997
- Meyer MA, Savitt ML, Kopitas E. The effect of phacoemulsification on aqueous outflow facility. Ophthalmology 1997;104(8):1221‐7. [DOI] [PubMed] [Google Scholar]
Minckler 2006
- Minckler D, Vedula SS, Li T, Mathew M, Ayyala R, Francis B. Aqueous shunts for glaucoma. Cochrane Database of Systematic Reviews 2006, Issue 2. [DOI: 10.1002/14651858.CD004918.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Minckler 2009
- Minckler DS, Hill RA. Use of novel devices for control of intraocular pressure. Experimental Eye Research 2009;88(4):792‐8. [DOI] [PubMed] [Google Scholar]
Nonaka 2006
- Nonaka A, Kondo T, Kikuchi M, Yamashiro K, Fujihara M, Iwawaki T, et al. Angle widening and alteration of ciliary process configuration after cataract surgery for primary angle closure. Ophthalmology 2006;113(3):437‐41. [DOI] [PubMed] [Google Scholar]
Poley 2009
- Poley BJ, Lindstrom RL, Samuelson TW, Schulze R Jr. Intraocular pressure reduction after phacoemulsification with intraocular lens implantation in glaucomatous and nonglaucomatous eyes: evaluation of a causal relationship between the natural lens and open‐angle glaucoma. Journal of Cataract and Refractive Surgery 2009;35(11):1946‐55. [DOI] [PubMed] [Google Scholar]
Quigley 2006
- Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. British Journal of Ophthalmology 2006;90(3):262‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Resnikoff 2004
- Resnikoff S, Pascolini D, Etya'ale D, Kocur I, Pararajasegaram R, Pokharel GP, et al. Global data on visual impairment in the year 2002. Bulletin of the World Health Organization 2004;82(11):844‐51. [PMC free article] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Riaz 2013
- Riaz Y, Silva SR, Evans JR. Manual small incision cataract surgery (MSICS) with posterior chamber intraocular lens versus phacoemulsification with posterior chamber intraocular lens for age‐related cataract. Cochrane Database of Systematic Reviews 2013, Issue 10. [DOI: 10.1002/14651858.CD008813.pub2] [DOI] [PubMed] [Google Scholar]
Rolim de Moura 2007
- Rolim de Moura CR, Paranhos A Jr, Wormald R. Laser trabeculoplasty for open angle glaucoma. Cochrane Database of Systematic Reviews 2007, Issue 4. [DOI: 10.1002/14651858.CD003919.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Shingleton 2006
- Shingleton BJ, Pasternack JJ, Hung JW, O'Donoghue MW. Three and five year changes in intraocular pressures after clear corneal phacoemulsification in open angle glaucoma patients, glaucoma suspects, and normal patients. Journal of Glaucoma 2006;15(6):494‐8. [DOI] [PubMed] [Google Scholar]
Shrivastava 2010
- Shrivastava A, Singh K. The effect of cataract extraction on intraocular pressure. Current Opinion in Ophthalmology 2010;21(2):118‐22. [DOI] [PubMed] [Google Scholar]
Takihara 2014
- Takihara Y, Inatani M, Ogata‐Iwao M, Kawai M, Inoue T, Iwao K, et al. Trabeculectomy for open‐angle glaucoma in phakic eyes vs in pseudophakic eyes after phacoemulsification: a prospective clinical cohort study. JAMA Ophthalmology 2014;132(1):69‐76. [DOI] [PubMed] [Google Scholar]
Tham 2008
- Tham CC, Kwong YY, Leung DY, Lam SW, Li FC, Chiu TY, et al. Phacoemulsification versus combined phacotrabeculectomy in medically controlled chronic angle closure glaucoma with cataract. Ophthalmology 2008;115(12):2167‐73. [DOI] [PubMed] [Google Scholar]
Tham 2009
- Tham CC, Kwong YY, Leung DY, Lam SW, Li FC, Chiu TY, et al. Phacoemulsification versus combined phacotrabeculectomy in medically uncontrolled chronic angle closure glaucoma with cataracts. Ophthalmology 2009;116(4):725‐31. [DOI] [PubMed] [Google Scholar]
Tham 2010a
- Tham CC, Kwong YY, Leung DY, Lam SW, Li FC, Chiu TY, et al. Phacoemulsification vs phacotrabeculectomy in chronic angle‐closure glaucoma with cataract: complications [corrected]. Archives of Ophthalmology 2010;128(3):303‐11. [DOI] [PubMed] [Google Scholar]
Tham 2010b
- Tham CC, Leung DY, Kwong YY, Li FC, Lai JS, Lam DS. Effects of phacoemulsification versus combined phaco‐trabeculectomy on drainage angle status in primary angle closure glaucoma (PACG). Journal of Glaucoma 2010;19(2):119‐23. [DOI] [PubMed] [Google Scholar]
Vass 2007
- Vass C, Hirn C, Sycha T, Findl O, Bauer P, Schmetterer L, et al. Medical interventions for primary open angle glaucoma and ocular hypertension. Cochrane Database of Systematic Reviews 2007, Issue 4. [DOI: 10.1002/14651858.CD003167.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wang 2009
- Wang X, Zhang H, Li S, Wang N. The effects of phacoemulsification on intraocular pressure and ultrasound biomicroscopic image of filtering bleb in eyes with cataract and functioning filtering blebs. Eye 2009;23(1):112‐6. [DOI] [PubMed] [Google Scholar]
Yalvac 1997
- Yalvac I, Airaksinen PJ, Tuulonen A. Phacoemulsification with and without trabeculectomy in patients with glaucoma. Ophthalmic Surgery and Lasers 1997;28(6):469‐75. [PubMed] [Google Scholar]
References to other published versions of this review
Hirunyachote 2010
- Hirunyachote P, Jampel H. Combined surgery versus staged surgery for eyes with cataract and glaucoma. Cochrane Database of Systematic Reviews 2010, Issue 9. [DOI: 10.1002/14651858.CD008671] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hirunyachote 2014
- Hirunyachote P, Zhang ML, Jampel H. Combined surgery versus cataract surgery alone for eyes with cataract and glaucoma. Cochrane Database of Systematic Reviews 2014, Issue 5. [DOI: 10.1002/14651858.CD008671.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]


