Abstract
Study objective
A clinical prediction tool, the Denver HIV Risk Score, was recently developed to help identify patients with increased probability of undiagnosed HIV infection. Our goal was to compare targeted rapid HIV screening using the Denver HIV Risk Score to nontargeted rapid HIV screening in an urban emergency department (ED) and urgent care.
Methods
We used a prospective, before-after design at an urban medical center with an approximate annual census of 110,000 visits. Patients aged 13 years or older were eligible for screening. Targeted HIV screening of patients identified as high-risk by nurses using the Denver HIV Risk Score during medical screening was compared to nontargeted HIV screening offered by medical screening nurses during 2 separate 4-month time periods. The primary outcome was newly diagnosed HIV-infected patients.
Results
28,506 patients presented during the targeted phase, 1,718 were identified as high-risk, and 551 completed HIV testing. Of these, 7 (1.3%, 95% confidence interval [CI] 0.5% to 2.6%) were newly diagnosed with HIV infection. 29,510 patients presented during the nontargeted phase and 3,591 completed HIV testing. Of these, 7 (0.2%, 95% CI 0.1% to 0.4%) were newly diagnosed with HIV infection. Targeted HIV screening was significantly associated with identification of newly diagnosed HIV infection when compared to nontargeted screening, adjusting for patient demographics and payer status (relative risk [RR] 10.4, 95% CI 3.4 to 32.0).
Conclusion
Targeted HIV screening using the Denver HIV Risk Score was strongly associated with new HIV diagnoses when compared to nontargeted screening. Although both HIV screening methods identified the same absolute number of newly diagnosed patients, significantly fewer tests were required during the targeted phase to achieve the same effect.
INTRODUCTION
In the United States, approximately 250,000 individuals are infected with HIV but their condition remains undiagnosed,1 whereas approximately 50,000 new infections occur annually.2 In 2006, to improve identification of HIV-infected persons, the Centers for Disease Control and Prevention (CDC) recommended routine (nontargeted) opt-out HIV screening in all health care settings,3 and in 2011 in response to the National HIV/AIDS Strategy proposed by the White House in 2010,4 the CDC introduced its High Impact HIV Prevention initiative.5 The goal of this new initiative is to “maximize the effectiveness of current HIV prevention methods … by combining scientifically proven, cost-effective, and scalable interventions targeted to the right populations in the right geographic areas.”5
Since 2006, 11 studies have evaluated nontargeted HIV screening in an emergency department (ED) setting.6 Although each study demonstrated the ability to identify patients with HIV infection using this approach, the effectiveness of such large-scale screening has been judged as modest,7 whereas others have raised concerns about the costs and inefficiency of this approach.8 Nontargeted HIV screening requires testing thousands of patients, many of whom are at low risk of acquiring HIV infection, to identify only a handful of newly diagnosed patients.
Targeted HIV screening serves as an alternative approach to nontargeted HIV screening.9 Although the concept of targeted HIV screening has existed for approximately 25 years10 and risk characteristics have been widely studied,11,12 specific targeted screening strategies remain largely undefined and have not been systematically evaluated in clinical practice.13–16 Recently, the Denver HIV Risk Score was derived and validated to help estimate a patient’s probability of being infected with HIV (Table 1).17 In doing so, it was argued that this clinical prediction instrument could be used to identify patients at increased risk and target testing resources to those most at risk.
Table 1.
The Denver HIV Risk Score.*
| Variable | Score |
|---|---|
| Age (years) | |
| <22 or >60 | 0 |
| 22–25 or 55–60 | +4 |
| 26–32 or 47–54 | + 10 |
| 33–46 | + 12 |
| Sex | |
| Female | 0 |
| Male | + 21 |
| Race/ethnicity | |
| Black | +9 |
| Hispanic | + 3 |
| Other† | 0 |
| White | 0 |
| Sexual practices | |
| Sex with a male | + 22 |
| Vaginal intercourse | −10 |
| Receptive anal intercourse | +8 |
| Other risks | |
| Injection drug use | +9 |
| Past HIV test | −4 |
The Denver HIV Risk Score ranges from −14 to +81 and a threshold of ≥30 was used in this study to define “high risk.”
Represents American or Alaskan Native, Native Hawaiian, or non–Hawaiian Pacific Islander.
The primary goal of the current study was to compare the effectiveness of targeted opt-in rapid HIV screening using the Denver HIV Risk Score to identify patients at increased risk to nontargeted opt-in rapid HIV screening in an ED and urgent care setting. Our hypothesis was that targeted screening would be more strongly associated with identification of patients with newly diagnosed HIV infection than nontargeted screening. Our secondary goal was to compare ED and urgent care operational process metrics (eg, waiting time, length of stay) between the 2 HIV screening approaches.
METHODS
Study Design
We used a prospective before–after design to evaluate the 2 HIV testing approaches. From June 1, 2010, through September 30, 2010, we implemented nontargeted opt-in rapid HIV screening using nurses to offer testing during medical screening, and from January 1, 2011, through April 30, 2011, we implemented targeted opt-in rapid HIV screening using nurses to offer testing to patients identified as at increased risk using the Denver HIV Risk Score during medical screening. October 1, 2010, through December 31, 2010 served as a 3-month washout period. This study was approved by the Colorado Multiple Institutional Review Board with a waiver of informed consent.
Setting
The study was performed in the adult and pediatric EDs and adult urgent care center at Denver Health Medical Center in Denver, Colorado. Denver Health Medical Center is a 477-bed urban, public safety-net hospital with approximately 110,000 ED and urgent care patient visits per year. It is also a level 1 trauma center and a nationally recognized model for the integration of a public hospital, community health center clinics, and public health department.18 Denver Health serves a large number of underserved patients at risk for HIV infection.
Population
During the study period, all patients aged 13 years or older who presented to the ED or urgent care setting for care, were assessed as clinically stable, and were capable of providing consent for general medical care were eligible for participation. Patients were excluded if they were (1) unable to consent for care or HIV testing (eg, altered mentation, intoxication, critical illness); (2) a prisoner or detainee; (3) a victim of sexual assault; (4) sought care as a result of an occupational exposure to HIV; or (5) self-identified as being infected with HIV.
Nontargeted HIV Screening Phase
During the initial 4-month study phase, nontargeted rapid opt-in HIV screening was initiated by nurses during medical screening 24 hours per day, and in a completely integrated fashion using existing ED and hospital staff. Patients who presented to the ED or urgent care and who met criteria for inclusion were offered rapid HIV testing using an opt-in consent approach.
Rapid HIV testing was performed by the hospital’s laboratory and included a sequential algorithm to help improve the predictive value of testing.19 Whole blood was obtained from all patients who consented for testing and sent to the hospital laboratory. The specimen was first tested using a rapid HIV test (Uni-Gold Recombingen; Trinity Biotech, Wicklow, Ireland), and if this test was negative, no other rapid test was performed, the result was reported as nonreactive and the patient was considered HIV seronegative. Seronegative patients were notified of their result by their treating physician and no confirmatory testing or posttest counseling was provided. If the first test was reactive, a second rapid test (Oraquick Advance Rapid HIV-1/2 Antibody Test, OraSure Technologies, Inc., Bethlehem, PA) was immediately performed. Because the predictive value of this testing algorithm is unknown, a reactive HIV test (whether concordant positive or discordant positive) was considered a preliminary positive result in the ED, and all such patients had additional blood drawn for confirmatory Western blot testing. Patients who had preliminary positive test results were notified specifically by their physician and a social worker. Social workers provided client-centered HIV prevention counseling, coordinated having blood drawn for confirmatory testing, and linked patients into ongoing medical care.
Targeted HIV Screening Phase
During the second 4-month study phase, targeted rapid opt-in HIV screening was performed by nurses during medical screening using the Denver HIV Risk Score. The Denver HIV Risk Score was empirically derived and externally validated as a clinical prediction instrument to stratify patients into different HIV risk groups.17 The risk score includes 3 demographic characteristics (age, sex, and race/ethnicity) and 5 behavioral characteristics (sex with a male, vaginal intercourse, receptive anal intercourse, injection drug use, and past HIV test), each with different assigned points. The cumulative score ranges from −14 to +81 and was shown to categorize individuals into 5 unique risk strata (ie, <20 = very low risk; 20 to 29 = low risk; 30 to 39 = moderate risk; 40 to 49 = high risk; and ≥50 = very high risk).17 For the purposes of this study, in an effort to simplify its use by clinicians, we defined patients as high risk if they scored 30 or higher.
The Denver HIV Risk Score was incorporated into the electronic medical screening and patient tracking system (EMeSIS, Denver Health, Denver, CO) in the ED and urgent care. Nurses were able to electronically enter responses to each of the risk score questions during medical screening. The questions were ordered to present potentially less sensitive questions before more sensitive questions. In addition, the system was developed to calculate a risk score in real time and stop—regardless of the number of questions answered—when a patient was determined to be either low- or high-risk. All patients who were identified as high risk by this screening process were offered rapid HIV testing by the nurse using an opt-in consent approach. We did not use an opt-out consent approach as prior research in our setting demonstrated a high level of misunderstanding by patients with this method.20 During both study phases, diagnostic rapid HIV testing was also performed at the discretion of the treating physician in patients who were not screened, were identified as low risk during the targeted phase and therefore not offered HIV testing, or declined testing when offered during screening.21
Outcome Measures
Confirmed newly identified HIV infection was our primary outcome. In anticipation of testing patients with previously diagnosed HIV infection, our secondary outcome included all patients identified with HIV infection. Additional outcomes included CD4 counts (cells/μL) at the time of diagnosis, viral load (copies/mL) at the time of diagnosis, successful linkage into medical care (defined by completion of an initial HIV clinic visit after preliminary diagnosis in the ED or urgent care setting), and the following specific operational process metrics: (a) proportion of patients who left before completing evaluation in the ED or urgent care; (b) waiting time (minutes); (c) length of stay (hours); (d) boarding time (hours); and (e) laboratory turnaround time for HIV tests (minutes).
Data Management and Statistical Analyses
Data were transferred electronically or entered manually into a database (Microsoft Access, Microsoft Corporation, Inc., Redmond, WA) and transferred into native SAS format using translational software (dfPower DBMS/Copy, DataFlux Corporation, Cary, NC). Statistical analyses were performed using SAS, version 9.3 (SAS Institute, Inc., Cary, NC).
Continuous data are reported as medians with interquartile ranges (IQRs) and categorical data are reported as percentages with 95% confidence intervals (CIs). Observations from each study phase were compared and reported as absolute differences with 95% CIs, where appropriate. Multivariable analyses were performed to estimate the associations between those offered HIV testing during enhanced targeted HIV screening and the primary and secondary outcomes. Whether patients were offered testing during the nontargeted screening phase was used as the reference, while adjusting for age, sex, race/ethnicity, and payer status. Generalized estimating equations were used to perform all multivariable analyses, and given the relatively rare outcomes, a binary Poisson distribution was used.22 Patients who were only diagnostically tested for HIV infection were excluded from the analyses as our goal was to estimate the associations between screen testing and HIV diagnoses and not diagnostic testing and HIV diagnoses.
In addition, because of the quasi-experimental design, we assessed the potential impact of secular trends by developing separate multivariable models where time of day, day of week, and week of the study were included as covariates in separate multivariable models with study phase as the independent variable and where screening, offering testing, and completion of testing were used as dependent variables, respectively. Finally, anticipating a relatively small number of outcomes, an additional confirmatory analysis using 1,000 bootstrapped datasets was used to estimate the distribution of the point estimate of the associations between enhanced targeted screening and the outcomes. Because recidivism is common in high-volume unscheduled ambulatory care settings and repeat screening is common in large prevention programs, analyses were performed using patient visit as the unit of analysis unless otherwise indicated.
RESULTS
During the 8-month cumulative study period, 78,784 patient visits occurred and similar numbers of patients were included in each study arm. During the 4-month nontargeted HIV screening phase, 29,510 eligible patient visits occurred, and of these, 19,634 (67%) were offered HIV testing, 3,591 (18%) agreed to and completed HIV testing, and 7 (0.2%, 95% CI 0.1% to 0.4%) were newly identified with HIV infection (Figure). During the 4-month targeted screening phase, 28,506 eligible patient visits occurred, and of these, 17,726 (62%) completed the Denver HIV Risk Score and 1,718 (10%, 95% CI 9% to 11%) were identified as high risk. Of those identified as high risk, 1,584 (92%) were offered HIV testing, 551 (35%) accepted and completed HIV testing, and 7 (1.3%, 95% CI 0.5% to 2.6%) were newly identified with HIV infection (Figure). The prevalence of newly diagnosed HIV infection was higher among those tested during the targeted screening phase when compared to the nontargeted screening phase (difference 1.1%, 95% CI 0.1% to 2.0%). In addition, acceptance and completion of HIV testing was higher during the targeted phase (difference 16.4%, 95% CI 14.1% to 18.9%) when compared to the nontargeted phase.
Figure.
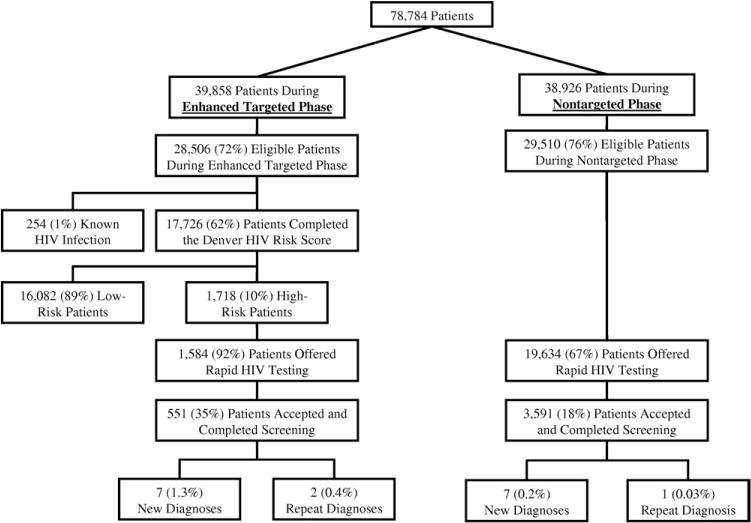
Patient flow diagram. Eligible patients were defined as those aged 13 years or older who completed medical screening and were not prisoners or detainees. The unit of analysis is patient visit.
The median CD4 counts for patients newly diagnosed during the targeted phase and during the nontargeted phase were 244 cells/μL (IQR 101 to 434) and 272 cells/μL (IQR 254 to 285), respectively. The median viral loads for patients newly diagnosed during the targeted and nontargeted phases were 42,435 copies/mL (IQR 17,275 to 844,498) and 192,551 copies/mL (IQR 110,681 to 301,223), respectively. In addition, of the 14 newly diagnosed patients, 4 (28.6%) had CD4 counts higher than 350 cells/μL, of which 3 (75.0%) were identified during the targeted phase. Finally, all 14 newly diagnosed patients were successfully linked into medical care.
Table 2 shows patient demographics for those offered, agreed to, and completed HIV testing by study phase. During the targeted phase, larger proportions of males were offered (difference 14.4%, 95% CI 11.4% to 17.3%) and completed (difference 16.2%, 95% CI 11.4% to 21.1%) HIV testing. In addition, during the targeted phase, larger proportions of black patients were offered (difference 14.9%, 95% CI 12.2% to 17.6%) and completed (difference 9.5%, 95% CI 5.3% to 13.8%) HIV testing, whereas smaller proportions of Hispanic patients were offered (difference 11.9%, 95% CI 9.2% to 14.7%) and completed (difference 14.0%, 95% CI 9.3% to 18.6%) HIV testing.
Table 2.
Patient-level demographics for those screened and completed rapid testing for HIV infection according to study phase.*
| Enhanced Targeted Screening Phase No. (%) | Nontargeted Screening Phase No. (%) | |
|---|---|---|
| Offered HIV testing | 1,131 | 13,910 |
| Median age (IQR) | 40 (31–49) | 38 (27–51) |
| Male sex | 694 (61) | 6,538 (47) |
| Race/ethnicity | ||
| Asian | 6 (1) | 274 (2) |
| Black | 337 (30) | 2,074 (15) |
| Hispanic | 330 (29) | 5,720 (41) |
| White | 379 (33) | 5,050 (36) |
| Other | 11 (1) | 159 (1) |
| Unknown/missing | 68 (6) | 633 (5) |
| Completed HIV testing | 451 | 3,083 |
| Median age (IQR) | 39 (30–47) | 35 (26–47) |
| Male sex | 270 (60) | 1,345 (44) |
| Race/ethnicity | ||
| Asian | 1 (0) | 42 (1) |
| Black | 118 (26) | 513 (17) |
| Hispanic | 146 (32) | 1,429 (46) |
| White | 159 (35) | 973 (31) |
| Other | 5 (1) | 40 (1) |
| Unknown/missing | 22 (5) | 86 (3) |
Demographics calculated at the individual patient level. For patients who left without being seen (approximately 7% for both study phases), complete registration information (ie, medical record number) was not available; therefore it was not possible to determine recidivism among these patients and, as a result, they were not included in the denominators of this table.
When compared to those in the nontargeted phase, the association between patients determined to be high risk using the Denver HIV Risk Score and offered HIV testing and newly diagnosed HIV infection was significantly higher (adjusted relative risk [RR] 10.4, 95% CI 3.4 to 32.0) (Table 3). In addition, when compared to those during the nontargeted phase, the association between patients determined to be at high risk using the Denver HIV Risk Score and offered HIV testing and all HIV diagnoses was significantly higher (adjusted RR 11.4, 95% CI 4.0 to 32.4) (Table 3).
Table 3.
Associations between enhanced targeted HIV screening and HIV diagnoses.*
| Newly Diagnosed HIV Infection RR (95% CI) | All-Diagnosed HIV Infection RR (95% CI) | |
|---|---|---|
| Enhanced targeted screening | 10.4† (3.4–32.0) | 11.4† (4.0–32.4) |
| Nontargeted screening | ref | ref |
| Age‡ | 1.0 (0.9–1.0) | 1.0 (0.9–1.0) |
| Male sex | 9.9 (1.5–67.6) | 5.3 (1.2–23.2) |
| Race/ethnicity | ||
| Black | 0.4 (0.05–3.2) | 0.7 (0.2–3.2) |
| Hispanic | 1.1 (0.3–3.3) | 1.7 (0.4–3.5) |
| White/other§ | ref | ref |
| Payer status | ||
| State sponsored | 1.4 (0.5–4.3) | 0.9 (0.3–2.7) |
| Self-pay | 0.5 (0.07–5.6) | 0.8 (0.2–4.5) |
| Medicare/Medicaid | 0.6 (0.05–5.6) | 0.3 (0.03–3.3) |
| Commercial insurance | ref | ref |
ref, reference.
Each model was performed using Generalized Estimating Equations and accounting for repeated visits.
To verify the stability of the results given the relatively small number of outcomes, additional confirmatory bootstrap analyses using 1,000 resampled data sets were performed and included a RR range for enhanced targeted screening from 1.8 to 42.1 for newly diagnosed HIV infection and 3.7 to 52.5 for all-diagnosed HIV infection.
Age was included as a continuous variable.
Defined as Asian, American or Alaskan Native, Native Hawaiian, or non–Hawaian Pacific Islander.
When secular trends were assessed in terms of screening, offering testing, or completing testing, no significant trends were identified (results not shown). Confirmatory bootstrap analyses using 1,000 resampled data sets were also performed and resulted in an RR range for enhanced targeted screening from 1.8 to 42.1 for newly diagnosed HIV infection and 3.7 to 52.5 for all-diagnosed HIV infection.
Table 4 shows distributions of responses to the Denver HIV Risk Score items by patients included in the targeted phase. The median total risk scores for all respondents was 20 (IQR 15 to 26), for those identified as high risk was 34 (IQR 31 to 39), for those offered HIV testing was 35 (IQR 31 to 39), for those who completed testing was 35 (IQR 31 to 39), and those newly diagnosed with HIV infection was 49 (IQR 44 to 52). In addition, of the 7 patients newly identified with HIV infection during the nontargeted phase, all would have been classified as “high risk” based on available demographic and behavioral characteristics (median Denver HIV Risk Score 49; range 33 to 55).
Table 4.
The Denver HIV Risk Score stratified by those who completed screening, identified as high risk (defined as a score ≥30), offered and completed HIV testing, and newly diagnosed with HIV infection from the targeted phase.
| Item | Score | All Respondents (n = 17,980) No. (%) |
High-Risk Patients (n = 1,898) No. (%) |
Patients Offered HIV Testing (n = 1,584) No. (%) |
Patients Accepted and Completed Testing (n = 552) No. (%) |
New Diagnoses (n = 7) No. (%) |
|---|---|---|---|---|---|---|
| Age (years) | ||||||
| <22 or >60 | 0 | 3,229 (18) | 100 (5) | 90 (6) | 33 (6) | 1 (14) |
| 22–25 or 55–60 | +4 | 3,385 (19) | 151 (8) | 132 (8) | 53 (10) | 1 (14) |
| 26–32 or 47–54 | + 10 | 6,113 (34) | 876 (46) | 749 (47) | 227 (41) | 4 (57) |
| 33–46 | + 12 | 5,183 (29) | 771 (41) | 613 (39) | 239 (43) | 1 (17) |
| Male sex | + 21 | 8,964 (50) | 1,276 (67) | 990 (63) | 336 (61) | 7 (100) |
| Race/ethnicity | ||||||
| Black | +9 | 2,727 (15) | 563 (30) | 492 (31) | 151 (27) | 0 (0) |
| Hispanic | + 3 | 6,891 (38) | 562 (30) | 447 (28) | 185 (34) | 4 (57) |
| Other | 0 | 966 (5) | 60 (3) | 50 (3) | 15 (3) | 1 (14) |
| White | 0 | 7,065 (39) | 677 (36) | 543 (34) | 194 (35) | 2 (29) |
| Sexual practices | ||||||
| Sex with a male | + 22 | 8,722 (49) | 1,086 (57) | 1,031 (65) | 393 (63) | 7 (100) |
| Vaginal intercourse | −10 | 16,262 (91) | 1,202 (63) | 1,146 (72) | 424 (77) | 2 (29) |
| Receptive anal intercourse | +8 | 559 (3) | 482 (25) | 468 (64) | 195 (35) | 3 (43) |
| Other risks | ||||||
| Injection drug use | +9 | 1,484 (8) | 545 (29) | 516 (33) | 204 (37) | 0 (0) |
| Past HIV test | −4 | 11,519 (64) | 1,118 (59) | 952 (60) | 357 (65) | 6 (86) |
Table 5 shows results of operational processes stratified by the 2 screening phases. No differences existed in patients who left before being placed in a treatment room or before completing evaluation. In addition, no significant differences existed in length of stay for those not admitted to the hospital, boarding times for those admitted to the hospital, or laboratory turnaround times for HIV tests. Waiting times were statistically significantly longer for patients included in the targeted phase, and total length of stay among those admitted to the hospital (ie, ED or urgent care length of stay in addition to boarding time) was significantly longer in the nontargeted phase.
Table 5.
Emergency department and urgent care center processes of care related to enhanced targeted HIV screening and nontargeted HIV screening.*
| Enhanced Targeted Screening Phase No. (%) |
Nontargeted Screening Phase No. (%) |
Difference (95% CI) | |
|---|---|---|---|
| Total patient visits† | 39,858 | 38,926 | |
| Left before being placed in a treatment room | 3,258 (8.2) | 3,242 (8.3) | −0.1 (−0.5, 0.2) |
| Left before completing evaluation | 307 (0.8) | 340 (0.9) | −0.1 (−0.2, 0.0) |
| Total patient visits who completed evaluation† | 36,293 | 35,344 | |
| Waiting time (minutes) | 23 (1–76) | 16 (0–79) | 7 (6, 8) |
| Length of stay (not admitted) (hours) | 2.4 (1.4–4.0) | 2.4 (1.4–3.9) | 0 (0, 0) |
| Length of stay (admitted) (hours) | 5.4 (3.9–8.1) | 5.9 (4.0–8.6) | −0.5 (−0.4, −0.6) |
| Boarding time (hours) | 2.6 (1.8–4.0) | 2.5 (1.7–4.1) | 0.1 (0, 0.2) |
| Total patient visits with an HIV test | 552 | 3,591 | |
| Laboratory turnaround time (minutes) | 18 (14–24) | 19 (15–25) | −1 (−2, 0) |
Categorical data are reported as percentages and continuous data are reported as medians with interquartile ranges.
Includes eligible and noneligible patients in order to evaluate the impact of each HIV testing program on all patients, rather than just those patients who were eligible for screening.
LIMITATIONS
Although this was a prospective interventional study, threats to the validity of the “before-after” design include, in part, lack of random allocation and secular differences between screening methods, evolution of the HIV epidemic in the population as a whole, or ED or urgent care operations. Randomization was not possible because the screening programs were fully integrated into ED and urgent care and because of the complexity of providing these services 24 hours per day in a high-volume setting; however, we used multivariable regression analyses to adjust for potential imbalances between the study groups in an effort to maximize the validity of our estimates. We also performed analyses to assess for secular trends.
This study was also conducted at a single institution with a track record of conducting HIV screening, and at an institution where the Denver HIV Risk Score was developed. Thus, the generalizability of our results may be limited when applied to other clinical sites or settings, including those where HIV testing has not routinely been performed. In addition, because “low risk” patients were not offered HIV testing and specific data regarding reasons for why patients were not screened, offered testing, or completed testing, it is impossible to know to what extent such patients with HIV infection were missed or specific reasons why screening may have missed patients with HIV infection. Finally, our assessment of the operational impact of both screening approaches may not have accounted for secular trends in staffing, crowding, or other operational changes.
DISCUSSION
The Denver HIV Risk Score was originally derived using data from a sexually transmitted diseases clinic based at a county public health department and externally validated in 2 ED settings.17,23 This is the first study to evaluate its use as a screening tool integrated into clinical ED or urgent care processes of care on a 24-hour basis in order to facilitate identification of patients at increased risk for HIV infection. Although our results demonstrate large proportions of patients not screened or tested for HIV infection as a principal result of practical limitations of implementing large preventive interventions in a busy ED setting, our results nonetheless demonstrate a large and significant association between an enhanced targeted HIV screening approach, where the Denver HIV Risk Score was used to identify patients at increased risk for HIV infection during medical screening, and identification of newly diagnosed HIV infection when compared to a nontargeted HIV screening approach, as recommended by the CDC.3 Our results also demonstrate that use of the Denver HIV Risk Score to facilitate HIV screening results in comparable absolute numbers of patients identified with newly diagnosed HIV infection, while focusing limited HIV testing resources on those most at risk, and also not dramatically affecting clinical processes of care.
To our knowledge, this study represents the first comparative effectiveness evaluation of targeted versus nontargeted HIV screening in an ED or urgent care setting. The results represent a substantial shift in terms of our understanding of the relative effectiveness of these 2 screening approaches. An important underlying premise of our study was that targeted screening may be as or more effective than nontargeted screening. As such, we utilized an empirically derived and validated instrument to help risk stratify patients into groups. We did not, however, assess more conventional targeted methods, including those previously recommended by the CDC.10–12 Although it is possible that those methods would provide a simpler approach, we believe the Denver HIV Risk Score allows for a more granular differentiation of risk while including many of the commonly known risk characteristics (eg, men who have sex with men, injection drug use) and some that are not as widely used. The Denver HIV Risk Score also includes risk characteristics that mirror the epidemiology of HIV infection on a national level (ie, age-specific groups and racial/ethnic minority populations), thereby likely improving its ability to identify patients with HIV infection.
In 2006, the CDC recommended nontargeted HIV screening in health care settings where the undiagnosed prevalence meets or exceeds 0.1%.3 In 2007, the World Health Organization (WHO) released its guidance on provider-initiated HIV testing in health care facilities, and in contrast to the CDC recommendations, recommended more selective HIV testing (ie, diagnostic testing or targeted screening) in settings where the HIV epidemic was concentrated (defined as an HIV prevalence consistently higher than 5% in at least one defined subpopulation).24 According to WHO definitions, even the highest prevalence areas in the United States (eg, Washington, DC) are considered concentrated. Similarly, in 2007, the United States Preventive Services Task Force recommended targeted HIV screening as the principal approach to HIV testing, based in part on the assessment that little empiric evidence existed to support a broader HIV screening initiative.25
Nontargeted HIV screening in an ED setting does not appear to reach the expected numbers of patients tested and identifies only a modest number of newly diagnosed, even when fully integrated into ED operations on a 24-hour basis.6,7,26 Our findings demonstrate that a novel, enhanced targeted HIV screening approach may be as, or more, effective as non-risk-based screening while focusing scarce testing resources on those most at risk. Patients enrolled in the nontargeted screening phase of our study compare similarly to those reported previously, especially in terms of the proportions of eligible patients offered HIV testing, tested for HIV infection, and confirmed infected with HIV.26 Consistent with prior research, the relatively small proportion of eligible patients who completed HIV testing likely related to operational limitations of HIV screening that was fully integrated into ED and urgent care operations, the relatively large number of patients unable to consent for HIV testing due to acuity of illness or altered mentation, or the relatively large number of patients who declined testing because they believed they were not at risk.
Substantial public health initiatives have been developed to mitigate the impact of the HIV epidemic, including the 2006 CDC recommendations,3 its 2007 “Expanded Testing Initiative,” and in 2011, its “High Impact HIV Prevention” initiative in response to the “National HIV/AIDS Strategy” released by the White House in 2010.4,5 In the United States, the HIV epidemic primarily exists among men who have sex with men, blacks, and Latinos, and EDs serve as integral venues for HIV testing. The focus on EDs results from the fact that more than 120 million ED visits occur annually in the United States,27 a large proportion of patients at risk of acquiring HIV infection use EDs as their primary source of care, and because EDs are considered the most common site of missed opportunities for diagnosing HIV infection.28
Federal initiatives, such as the recent CDC response to the National HIV/AIDS Strategy, should serve as an important framework for prevention practices. However, public health recommendations, including those on HIV screening, must be rooted in the results of rigorous, large-scale, effectiveness research. The goal of the High Impact HIV Prevention Initiative is to “advance the prevention goals of National HIV/AIDS Strategy and … maximize the effectiveness of current HIV prevention methods … by combining scientifically proven, cost-effective, and scalable interventions targeted to the right populations.”5 A growing body of literature suggests that nontargeted HIV screening is resource intensive while only identifying a modest number of newly diagnosed individuals, and the results of this study confirm these conclusions while supporting an alternative targeted approach that may be more resource friendly.
Although previous research has demonstrated the cost effectiveness of performing routine HIV screening from a societal perspective,29,30 others have argued that targeted HIV screening may be superior.8 Our results may require confirmation from larger-scale prospective trials and cost-effectiveness analyses using applied results. In addition, evaluation of the Denver HIV Risk Score as a part of HIV screening in other clinical venues (eg, primary care clinics, sexually transmitted disease clinics) should be undertaken and compared to other methods of HIV identification.
In conclusion, targeted HIV screening enhanced by use of the Denver HIV Risk Score was strongly associated with new HIV diagnoses when compared to nontargeted screening in an ED and urgent care setting. Although both HIV screening methods identified the same absolute number of newly diagnosed patients, significantly fewer tests were required during the targeted phase.
Acknowledgments
Funding and support: By Annals policy, all authors are required to disclose any and all commercial, financial, and other relationships in any way related to the subject of this article as per ICMJE conflict of interest guidelines (see www.icmje.org). The authors have stated that no such relationships exist. This study was funded by a programmatic grant from the Colorado Department of Public Health and Environment (CDPHE) and supported, in part, by grant K02 HS017526 from the Agency for Healthcare Research and Quality (AHRQ) and R01 AI106057 from the National Institute of Allergy and Infectious Diseases (NIAID) to JSH.
CDPHE, AHRQ, or NIAID had no role in the design or conduct of the study, or collection, management, analysis, interpretation of the data, or drafting/revising of the manuscript.
Footnotes
The authors are indebted to the following individuals: Katherine Bakes, MD (Denver Health, Denver, CO); Jeremy Long, MD (Denver Health, Denver, CO), members of the Denver Emergency Department HIV Testing Research Consortium, and all emergency department and laboratory staff at Denver Health Medical Center.
Supervising editor: Melissa L. McCarthy, ScD
Author contributions: JSH, EH, and BB conceived and designed the study. JSH, EH, and BB acquired the data. JSH, EH, BB, CS, AAAT, and MWT analyzed and interpreted the data. JSH and EH drafted the manuscript. JSH, EH, BB, CS, AAAT, and MWT undertook critical revision of the manuscript for important intellectual content. JSH and EH performed statistical analysis. JSH, EH, and BB obtained funding. JSH, EH, and BB provided administrative, technical, or material support. JSH supervised the study. JSH takes responsibility for the paper as a whole.
Members of the Denver Emergency Department HIV Testing Research Consortium, other than those listed as authors, include: Lucy Bradley-Springer, PhD, RN (Aurora, CO); Sheri Eisert, PhD (Denver, CO); Allison Sabel, MD, MPH, PhD (Denver, CO); Kimberly Bender, PhD (Denver, CO); Janell Bezdek (Denver, CO); Bob Bongiovanni, MA (Denver, CO); Steve Cantrill, MD (Denver, CO); Eric Christensen, RN, BSN (Denver, CO); Jessica Forsyth, MSW (Aurora, CO); Michael Fuhriman (Denver, CO); Edward Gardner, MD (Denver, CO); David Ginosar, MD (Denver, CO); Jennifer Guess, MT (Denver, CO); Timothy Jenkins, MD (Denver, CO); Steve Johnson, MD (Denver, CO); Linda Kaufman (Denver, CO); Jeremy Long, MD (Denver, CO); Michael Lyons, MD, MPH (Cincinnati, OH); Wendy McDermott, MSW (Denver, CO); Yesenia Mendez (Denver, CO); Richard Rothman, MD, PhD (Baltimore, MD); Briana Sprague (Denver, CO); Mark Tartletsky, MT, MCIS (Denver, CO); Diane Weed, MA, MT (Denver, CO); Julia Weise, LCSW, MSW (Denver, CO); Douglas White, MD (Oakland, CA); Ralph Wilmoth, MD (Denver, CO); and Melody Zwakenberg, ANP-BC (Denver, CO).
None of these individuals received compensation as part of this study and none have declared existing competing interests.
Presented, in part, at the 19th Conference on Retroviruses and Opportunistic Infections, Seattle, Washington, March 7 to 8, 2012.
References
- 1.Centers for Disease Control and Prevention. HIV surveillance report. 2009 http://www.cdc.gov/hiv/surveillance/resources/reports/2009report/index.htm. Accessed September 27, 2011.
- 2.Prejean J, Song R, Hernandez A, et al. Estimated HIV incidence in the United States, 2006–2009. PLoS One. 2011;6:e17502. doi: 10.1371/journal.pone.0017502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Branson BM, Handsfield HH, Lampe MA, et al. Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. MMWR Recomm Rep. 2006;55:1–17. [PubMed] [Google Scholar]
- 4.White House Office of National AIDS Policy. National HIV/AIDS strategy for the United States. 2010 http://www.whitehouse.gov/sites/default/files/uploads/NHAS.pdf. Accessed September 27, 2011.
- 5.Centers for Disease Control and Prevention. High-impact HIV prevention: CDC’s approach to reducing HIV infection in the United States. 2011 http://www.cdc.gov/hiv/nhas/dhap/pdf/nhas_booklet.pdf. Accessed September 27, 2011.
- 6.Haukoos J. Impact of non-targeted HIV screening in emergency departments and the ongoing need for targeted strategies. Arch Intern Med. 2012;172:20–22. doi: 10.1001/archinternmed.2011.538. [DOI] [PubMed] [Google Scholar]
- 7.d’Almeida KW, Kierzek G, de Truchis P, et al. Modest public health impact of nontargeted human immunodeficiency virus screening in 29 emergency departments. Arch Intern Med. 2012;172:12–20. doi: 10.1001/archinternmed.2011.535. [DOI] [PubMed] [Google Scholar]
- 8.Holtgrave DR. Cost and consequences of the US Centers for Diease Control and Prevention’s recommendations for opt-out HIV testing. PLoS Med. 2007;4:e194. doi: 10.1371/journal.pmed.0040194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rothman RE, Lyons MS, Haukoos JS. Uncovering HIV infection in the emergency department: a broader perspective. Acad Emerg Med. 2007;14:653–657. doi: 10.1197/j.aem.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention. Additional recommendations to reduce sexual and drug abuse-related transmission of human T-lymphotropic virus type III/lymphadenopathy-associated virus. MMWR Morb Mortal Wkly Rep. 1986;35:152–155. [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention. Recommendations for HIV testing services for inpatients and outpatients in acute-care hospital settings. MMWR Recomm Rep. 1993;42:1–6. [PubMed] [Google Scholar]
- 12.Centers for Disease Control and Prevention. Revised guidelines for HIV counseling, testing, and referral. MMWR Recomm Rep. 2001;50:1–57. [PubMed] [Google Scholar]
- 13.Chen Z, Branson B, Ballenger A, et al. Risk assessment to improve targeting of HIV counseling and testing services for STD clinical patients. Sex Transm Dis. 1998;25:539–543. doi: 10.1097/00007435-199811000-00008. [DOI] [PubMed] [Google Scholar]
- 14.Peterman TA, Todd KA, Mupanduki I. Opportunities for targeting publicly funding human immundeficiency virus counseling and testing. J Acquir Immune Defic Syndr. 1996;12:69–74. doi: 10.1097/00042560-199605010-00010. [DOI] [PubMed] [Google Scholar]
- 15.Gerbert B, Bronstone A, McPhee S, et al. Development and testing of an HIV-risk screening instrument for use in health care settings. Am J Prev Med. 1998;15:103–13. doi: 10.1016/s0749-3797(98)00025-7. [DOI] [PubMed] [Google Scholar]
- 16.Menza TW, Hughes JP, Celum CL, et al. Prediction of HIV acquisition among men who have sex with men. Sex Transm Dis. 2009;36:547–55. doi: 10.1097/OLQ.0b013e3181a9cc41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haukoos JS, Lyons MS, Lindsell CJ, et al. Derivation and validation of the Denver HIV Risk Score for targeted HIV screening. Am J Epidemiol. 2012;175:838–846. doi: 10.1093/aje/kwr389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gabow P, Eisert S, Wright R. Denver Health: a model for the integration of a public hospital and community health centers. Ann Intern Med. 2003;138:143–149. doi: 10.7326/0003-4819-138-2-200301210-00016. [DOI] [PubMed] [Google Scholar]
- 19.Delaney KP, Heffelfinger JD, Wesolowski LG, et al. Performance of an alternative laboratory-based algorithm for HIV diagnosis in a high-risk population. J Clin Virol. 2011;52(Suppl 1):S5–S10. doi: 10.1016/j.jcv.2011.09.013. [DOI] [PubMed] [Google Scholar]
- 20.Haukoos JS, Hopkins E, Bender B, et al. Use of kiosks and patient understanding of opt-out and opt-in consent for routine rapid HIV screening in the emergency department. Acad Emerg Med. 2012;19:469–480. doi: 10.1111/j.1553-2712.2012.01290.x. [DOI] [PubMed] [Google Scholar]
- 21.Haukoos JS, Hopkins E, Eliopoulos VT, et al. Development and implementation of a model to improve identification of patients infected with HIV using diagnostic rapid testing in the emergency department. Acad Emerg Med. 2007;14:1149–1157. doi: 10.1197/j.aem.2007.07.015. [DOI] [PubMed] [Google Scholar]
- 22.Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159:702–706. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 23.Hsieh YH, Haukoos J, Rothman R. External validation of an abbreviated version of the Denver HIV Risk Score. Acad Emerg Med. 2011;18:S122. doi: 10.1016/j.ajem.2014.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.World Health Organization. Guidance on Provider-Initiated HIV Testing and Counselling in Health Facilities. Geneva: World Health Organization; May, 2007. [Google Scholar]
- 25.Chou R, Huffman L. Screening for Human Immunodeficiency Virus: Focused Update of a 2005 Systematic Review for the U.S. Preventive Services Task Force. Rockville, MD: Agency for Healthcare Research and Quality; Apr, 2007. [PubMed] [Google Scholar]
- 26.Haukoos JS, Hopkins E, Conroy AA, et al. Routine opt-out rapid HIV screening and detection of HIV infection in emergency department patients. JAMA. 2010;304:284–292. doi: 10.1001/jama.2010.953. [DOI] [PubMed] [Google Scholar]
- 27.Pitts SR, Niska RW, Xu J, et al. National Hospital Ambulatory Medical Care Survey: 2006 emergency department summary. Natl Health Stat Report. 2008:1–38. [PubMed] [Google Scholar]
- 28.Jenkins TC, Gardner EM, Thrun MW, et al. Risk-based human immunodeficiency virus (HIV) testing fails to detect the majority of HIV-infected persons in medical care settings. Sex Transm Dis. 2006;33:329–333. doi: 10.1097/01.olq.0000194617.91454.3f. [DOI] [PubMed] [Google Scholar]
- 29.Paltiel AD, Weinstein MC, Kimmel AD, et al. Expanded screening for HIV in the United States—an analysis of cost-effectiveness. N Engl J Med. 2005;352:586–595. doi: 10.1056/NEJMsa042088. [DOI] [PubMed] [Google Scholar]
- 30.Sanders GD, Bayoumi AM, Sundarem V, et al. Cost-effectiveness of screening for HIV in the era of highly active antiretroviral therapy. N Engl J Med. 2005;352:570–585. doi: 10.1056/NEJMsa042657. [DOI] [PubMed] [Google Scholar]


