Abstract
Objective:
Incidence and mortality from ischaemic heart disease (IHD) was studied in an extended cohort of 22,377 workers first employed at the Mayak Production Association during 1948–82 and followed up to the end of 2008.
Methods:
Relative risks and excess relative risks per unit dose (ERR/Gy) were calculated based on the maximum likelihood using Epicure software (Hirosoft International Corporation, Seattle, WA). Dose estimates used in analyses were provided by an updated “Mayak Worker Dosimetry System—2008”.
Results:
A significant increasing linear trend in IHD incidence with total dose from external γ-rays was observed after having adjusted for non-radiation factors and dose from internal radiation {ERR/Gy = 0.10 [95% confidence interval (CI): 0.04 to 0.17]}. The pure quadratic model provided a better fit of the data than did the linear one. No significant association of IHD mortality with total dose from external γ-rays after having adjusted for non-radiation factors and dose from internal alpha radiation was observed in the study cohort [ERR/Gy = 0.06 (95% CI: <0 to 0.15)]. A significant increasing linear trend was observed in IHD mortality with total absorbed dose from internal alpha radiation to the liver after having adjusted for non-radiation factors and dose from external γ-rays in both the whole cohort [ERR/Gy = 0.21 (95% CI: 0.01 to 0.58)] and the subcohort of workers exposed at alpha dose <1.00 Gy [ERR/Gy = 1.08 (95% CI: 0.34 to 2.15)]. No association of IHD incidence with total dose from internal alpha radiation to the liver was found in the whole cohort after having adjusted for non-radiation factors and external gamma dose [ERR/Gy = 0.02 (95% CI: not available to 0.10)]. Statistically significant dose effect was revealed in the subcohort of workers exposed to internal alpha radiation at dose to the liver <1.00 Gy [ERR/Gy = 0.44 (95% CI: 0.09 to 0.85)].
Conclusion:
This study provides strong evidence of IHD incidence and mortality association with external γ-ray exposure and some evidence of IHD incidence and mortality association with internal alpha-radiation exposure.
Advances in knowledge:
It is the first time the validity of internal radiation dose estimates has been shown to affect the risk of IHD incidence.
INTRODUCTION
Cancer among workers of the first Russian nuclear facility Mayak Production Association (PA) has been studied for several decades.1–5 More recently, non-cancer effects of prolonged occupational radiation exposure in the Mayak Worker Cohort have been examined.6–12 Non-cancer effects, such as circulatory and respiratory diseases, were first investigated in the cohort of workers first employed during the first decade of the Mayak PA operation (1948–58). These workers were exposed at high doses and dose rates to both external and internal radiation.6,7,10 Subsequent analyses were carried out on an extended cohort additionally including workers employed during later years (1959–72) and occupationally exposed at lower radiation doses and dose rates.8,9 The substantial drop in occupational doses and dose rates after 1958 was the result of significant improvements of technologies used at the Mayak PA and the introduction of additional safety measures for its staff, such as individual protection devices.13
The previous studies9,14 showed an increased risk of ischaemic heart disease (IHD) incidence [410–414 ninth revision of International Classification of Diseases (ICD-9) codes] in workers exposed to external γ-rays at total doses above 1.00 Gy (for only 15- and 20-year lag periods) as compared with those workers who had been exposed at lower doses. A significant linear association of IHD incidence risk with dose from external γ-rays was found after adjusting for non-radiation factors and dose from internal alpha-radiation, excess relative risks per unit dose (ERR/Gy) = 0.09 (95% CI: 0.03 to 0.15)9 and ERR/Gy = 0.12 (95% CI: 0.05 to 0.18).14 A significant linear association of IHD mortality with total absorbed dose from internal alpha-radiation to the liver was also observed after adjusting for non-radiation factors only, ERR/Gy = 0.26 (95% CI: 0.07 to 0.46)9 and ERR/Gy = 0.34 (95% CI: 0.07 to 0.60).14
This article provides results of analyses of IHD incidence and mortality in the extended Mayak PA Worker Cohort (additionally includes 3521 workers first employed between 1973 and 1982 and exposed to radiation at low doses) with the follow-up period extended by 3 years (till 31 December 2008), providing a significant increase of the statistical power of the study. For the first time, quantitative data on smoking (smoking index) was used in the study of non-cancer effects in the Mayak Worker Cohort.
METHODS AND MATERIALS
The study cohort and follow-up
The study cohort includes 22,377 workers (of whom 25% are females) first employed at one of the main Mayak PA facilities (reactors, radiochemical and plutonium production plants) in 1948–82 and followed up till the end of 2008.
The age of first employment at the facility for the majority of workers (>80%) was below 30 years. The mean age at first employment at the Mayak PA was 24.11 ± 7.13 years for males and 27.32 ± 7.97 for females (hereinafter, data are given as mean values ± one standard deviation). Duration of employment at the Mayak PA ranged from 1 day to 60 years with a mean of 18.04 ± 14.28 years, but only 4.7% of workers were employed at the Mayak PA for less than 1 year. Main characteristics of the study cohort are presented in Table 1.
Table 1.
Characteristics of the study cohort
| Characteristics of the study cohort | Males | Females | Both |
|---|---|---|---|
| Number of workers included in the cohort | 16,687 | 5690 | 22,377 |
| Workers with acute radiation sickness excluded of them | 16,653 | 5681 | 22,334 |
| IHD cases | 5098 | 2127 | 7225 |
| IHD deaths | 2304 | 544 | 2848 |
| PYR (IHD incidence) | 318,157 | 129,124 | 447,281 |
| PYR (IHD mortality) | 600,589 | 235,459 | 836,048 |
| Migrated from Ozyorsk as of 31 December 2005 | 7190 | 2015 | 9205 |
| Vital status known as of 31 December 2008 | 15,831 | 5436 | 21,267 |
| Died | 8954 | 2417 | 11,371 |
| Cause of death known for workers known to have died | 8530 | 2337 | 10,867 |
| Incidence data available | 16,101 | 5430 | 21,531 |
| Smoking status available of them | 15,561 | 5170 | 20,731 |
| Known data on qualitative parameters of smoking | 9742 | 5011 | 14,753 |
| Data on alcohol consumption available | 14,590 | 4924 | 19,514 |
| Data on hypertension available | 15,055 | 4918 | 19,973 |
| Data on body mass index | 12,485 | 4209 | 16,694 |
| Mean age at first employment (years) (SD) | 24.11 (7.13) | 27.32 (7.97) | 24.97 (7.47) |
| Mean duration of employment at the Mayak PA (years) (SD) | 18.28 (14.76) | 17.36 (12.77) | 18.04 (14.28) |
| Mean age at death for workers known to have died (years) (SD) | 60.17 (13.58) | 68.47 (12.38) | 61.99 (13.76) |
| Mean age of workers known to be alive (years) (SD) | 66.49 (10.13) | 74.75 (9.26) | 68.76 (11.53) |
| Mean age as of migration date (years) (SD) | 31.15 (10.24) | 34.21 (11.91) | 31.62 (10.30) |
| Mean total gamma dose (Gy) (SD) | 0.54 (0.76) | 0.44 (0.65) | 0.51 (0.73) |
| Excluded workers with no plutonium doses | 4853 | 2098 | 6951 |
| Mean total absorbed alpha dose to the liver (Gy) (SD) | 0.23 (0.77) | 0.44 (2.11) | 0.29 (1.33) |
IHD, ischaemic heart disease; PYR, person-years at risk; SD, one standard deviation.
IHD incidence and mortality were the outcomes of interest. The follow-up of the study cohort was defined as starting from the date of the first employment at one of the main Mayak PA facilities and continued till the earliest of the following events:
date of IHD diagnosis (for the incidence analysis)
date of death
31 December 2008 for workers known to be alive and reside in Ozyorsk (residents)
31 December 2005 for workers known to be alive but who had migrated from Ozyorsk by that date (migrants)
date of migration from Ozyorsk for migrants with the unknown vital status
date of “the last medical information” for workers–residents with the unknown vital status.
A worker was considered as a resident prior to his/her migration from Ozyorsk, since that date the worker was considered as a migrant.
Inconsistent follow-up periods for residents and migrants stem from the fact that owing to new privacy protection legislation in the Russian Federation, it is not possible to obtain follow-up information on migrants from Ozyorsk after 31 December 2005. However, the level of migration in the cohort is generally decreasing and to date does not exceed 0.25% of the whole cohort between 2006 and 2008.
About 41% of the cohort members are known to have migrated from Ozyorsk by 31 December 2005. The mean age at migration was 31.6 ± 10.3 years.
Information on diseases registered during the whole follow-up period was collected for 96% of workers of the study cohort. The incidence data were available only for Ozyorsk residents. After a worker had left the city for another place of residence, there was no access to medical information on his health history. The collection of data on diseases was carried out in absolutely one worker and the same manner for each worker of the study cohort using the standard protocol. The sources providing these data were medical records and patients' medical histories for workers which are stored in the Southern Urals Biophysics Institute (SUBI) archive up to now. Those sources provide all the results of annual medical examinations performed for each of the Mayak workers in accordance with a uniform standard program on a mandatory basis as well as the results of meticulous health examinations performed in a hospital (every 5 years). After termination of the Mayak employment, the medical surveillance of a worker (annual medical examinations) was continued (until the date of death or the date of migration from the city). The primary morbidity data were unavailable for only 4% of the workers owing to loss of medical records.
At the end of the follow-up, vital status was known for 95% of the cohort members of whom 53.5% are known to have deceased and 46.5% are known to be alive. The mean age of workers known to be alive at the end of the follow-up was 66.49 ± 10.13 years in males and 74.75 ± 9.26 years in females.
Information on causes of death was collected for 96% of the cohort members (99% for Ozyorsk residents and 92% for migrants) known to have deceased by the end of the follow-up period. The mean age at death for workers known to have deceased by the end of the follow-up was 60.17 ± 13.58 years in males and 68.47 ± 12.38 in females.
All diseases and causes of death were coded according to ICD-9.15 Information on dates and causes of death for Ozyorsk residents and migrants were collected from different sources. For residents, the sources of the primary information on dates and causes of death were medical records, case histories, autopsy protocols, medical death certificates and death certificates issued by civil registry offices in Ozyorsk. Information on vital status, dates and causes of death for migrants was provided by the Medical and Dosimetry Registry for Mayak Workers and was collected from death certificates issued by civil registry offices in places of migration. Search and collection of these data was reported in details earlier.16,17
A number of non-radiation factors such as sex, age, smoking, hypertension, obesity etc. are known to play an important role in IHD occurrence. Information on various non-radiation factors such as smoking status (92.8%), alcohol consumption (87.4%), blood pressure (BP) (89.4%) and body mass index (BMI) (74.7%) was collected for the study cohort.
Information on smoking was considered over the entire follow-up and estimated as qualitative and quantitative parameters. Qualitative index possessed values “unknown”, “never smoker” and “ever-smoker”. Under “never smoker”, we considered workers who stated at >1 medical observation that he/she did not smoke. It is worth noting that quantitative information on smoking was also collected for approximately 71.2% of the cohort members with the known smoking status. This quantitative information consisted of data on individual's age at first smoking, number and type of cigarettes smoked, and age at smoking cessation (or resuming where breaks in smoking were identified). Quantitative measure was referred to as the smoking index calculated as the mean number of cigarette packs smoked in a day times years of smoking. The smoking index is measured by pack-years and for “never smokers” was equated with zero.
Information on alcohol consumption was also considered over the entire follow-up and estimated only as a qualitative parameter with values “unknown”, “never drinker” and “ever drinker”. Under “never drinker”, we considered a worker who stated at >1 medical observation that they did not drink alcohol.
For the present study, BMI and BP were considered at the date of pre-employment medical examination of an individual to avoid the possibility that BP or BMI which changed over time (which could correlate with accumulating dose) might affect the association of IHD incidence and mortality risk estimates with radiation exposure. And on the contrary, smoking and alcohol consumption factors were registered at the date when the last information was provided by an individual.
BMI is a measure used for assessing underweight, normal weight or overweight of an individual. BMI for a person is defined as body mass in kilograms divided by the square of their height in metres. The “normal BMI” lies within the range 18.5–24.99 kg m−2. For the study, BMI was considered as a qualitative index with values “below normal”, “normal”, “above normal” and “unknown”.
Hypertension was defined in cases when systolic BP was >140 mmHg and/or diastolic BP was >90 mmHg. Hypertension was considered in the study as a qualitative index with values “without hypertension”, “with hypertension” and “unknown”.
Quality control of diagnoses and underlying causes of death
As in the previous studies,6,7,16 quality control checks were carried out to:
check the completeness of diagnoses provided by the “Clinic” database via cross checking with hard copies of medical records
check the quality of IHD diagnoses (to verify the diagnoses) based on the standard criteria (clinical symptoms and signs of the disease)
identify the diagnoses missed within the lifetime via the random selection of workers older than 40 years free from IHD diagnosis registered in medical records and in the database but for whom findings from electrocardiogram testing performed regularly for all workers at the age above 40 years were available
check to what extent the update of IHD diagnosis in case of death was more precise for those workers for whom autopsy was performed than for workers for whom it was not and assess the inconsistency rate between the clinical diagnosis and autopsy report conclusion.
It should be noted that the error frequency revealed during each quality control check did not exceed 5% supporting an assertion that the data are of high quality.
Dosimetry
The estimation of individual doses from external and internal radiations for Mayak PA workers is carried out in the framework of the Russian-American collaboration which has been continuously developing and refining dose estimates for the past 15 years. The Mayak Worker Dosimetry System—2008 (MWDS-2008) dosimetry system is the latest product of this collaboration18,19 and provides the dose estimates for the present study.
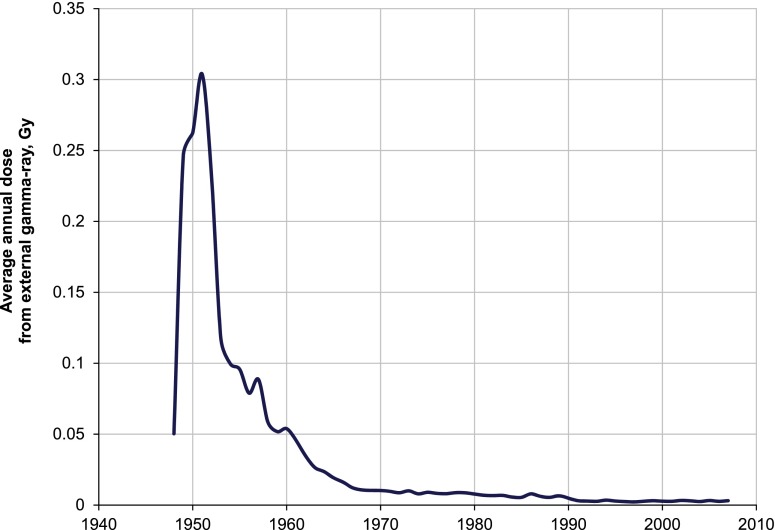
Annual individual external gamma-dose estimates were available for all members of the cohort. The improvement of external gamma doses in MWDS-2008 compared with Doses-2005, the previous dosimetry system, was owing to adjusting for energy and angular dependence of dosimeter responses.13,20 The mean total dose from external γ-rays was 0.54 ± 0.76 Gy (95% percentile 2.21 Gy) for males and 0.44 ± 0.65 Gy (95% percentile 1.87 Gy) for females. Mean annual doses from external γ-rays were the highest in early years of Mayak PA operation. In 1951, the mean annual gamma dose was 0.30 Gy per year, but it decreased sharply over the next decade to 0.05 Gy per year by 1960. The doses continued to fall at a lower rate until 1980 when the annual doses from external γ-rays reached about 0.01 Gy per year and remained stable at this level from that time onwards (Figure 1).
Figure 1.
Average annual dose from external γ-rays based on Mayak Worker Dosimetry System—2008.
In addition to dose estimates for occupational exposure, MWDS-2008 provides estimates for medical diagnostic X-ray exposure for 85% of workers over their period of residence in Ozyorsk. The most relevant to IHD development diagnostic external X-ray dose was judged to be that to the lung; however, this dose was found to be several orders of magnitude lower than the occupational external gamma dose in the study cohort [the mean total lung dose from external diagnostic exposure was 0.05 ± 0.05 Gy (95% percentile 0.14) for males and 0.05 ± 0.04 Gy (95% percentile 0.12) for females for the whole follow-up period]; therefore, it was decided not to consider this information for the present analysis.
A routine individual neutron radiation dose monitoring was carried out for Mayak PA workers from 1983. To reconstruct neutron doses for the period before 1983, a special technique was introduced within a framework of Russian and American collaborative research.
We should note that considerable neutron fluxes were registered only in central reactor halls and in some areas of radiochemical and plutonium plants. The mean effective energy of neutron spectrum was estimated to range between 300 and 500 keV in central reactor halls and between 100 and 300 keV in some areas of radiochemical and plutonium plants. 4749 workers of the study cohort were exposed to neutron radiation with the mean neutron dose 0.07 Gy; however, we did not take this dose into account during the current analyses since it was an order of magnitude lower than occupational external gamma dose.21
Alpha activity of plutonium in urine bioassay was measured in 31% of the whole cohort members (or in 41% of workers employed at radiochemical and plutonium production facilities who could potentially be exposed to plutonium aerosols). Plutonium alpha-activity in urine bioassays was measured more than once for 87% of the monitored workers; average number of measurements was 7.51 ± 7.47. Generally, we revealed a trend for males to have a higher plutonium body burden than that in females; however, a number of females accumulated very high levels of plutonium body burden in first years of the facility operation when the radiation protection system was ineffective.
Changes in methods of estimating internal alpha-particle exposure owing to incorporated plutonium between MWDS-2008 and Doses-2005 (the dosimetry system implemented for the previous analysis) mainly related to the modification of the model describing the transfer of plutonium from the lungs into the lung lymph nodes and further to the systemic circulation and its deposition in extrapulmonary organs.19 In MWDS-2008, measurements of plutonium activity in urine bioassays below the limit of detection (LOD) were used to calculate doses from internal alpha particles. According to the dosimetry protocol, each of the below LOD measurements was assigned to a point estimate equated with half value of the threshold of detectability at the time the biophysical examination was carried out. However, the plutonium concentration in urine bioassay, in fact, could vary from zero to the threshold estimate. The MWDS-2008 includes a validity measure for each member of the cohort for whom internal alpha doses were estimated. The validity measure denotes the proportion of measurements of plutonium activity in urine bioassays that exceeded the LOD threshold out of the total number of plutonium activity measurements and ranges from 0 to 1. This measure allows the impact of the assumptions about the LOD measurements to be evaluated. Moreover, the approach for considering the effect of smoking on the retention of different plutonium compounds in the lungs was also revised for MWDS-2008. In particular, the estimates of dose from internal alpha radiation based on Doses-2005 assumed that all workers were lifelong non-smokers, whereas the estimates based on MWDS-2008 took account of individual smoking status when setting the parameters for the modelling of plutonium retention in the lungs.
As in our previous studies, the analyses of IHD risk in relation to internal alpha-particle exposure due to plutonium used the absorbed dose to liver. This is primarily owing to the fact that the dosimetry system for Mayak PA workers does not provide estimates of internal radiation dose to blood vessels, heart or brain, i.e. for circulatory organs. Meanwhile, we should note that the biokinetic model within MWDS-2008 consists of three main parts: a systemic model, a gastrointestinal tract model and a respiratory tract model. The systemic model describes plutonium metabolism within the liver and other organs excluding the respiratory and gastrointestinal tracts. All the dose estimates to organs based on the systemic biokinetic model are highly correlated (Spearman rank correlation coefficient is 0.99). The mean total absorbed alpha-particle dose to the liver owing to incorporated plutonium was 0.23 ± 0.77 Gy (95% percentile 0.89 Gy) in males and 0.44 ± 2.11 Gy (95% percentile 1.25 Gy) in females.
The intakes of radionuclides other than plutonium were registered at the Mayak PA, but the major contribution to the dose from internal radiation in the study cohort of Mayak workers was owing to incorporated plutonium (>90%).
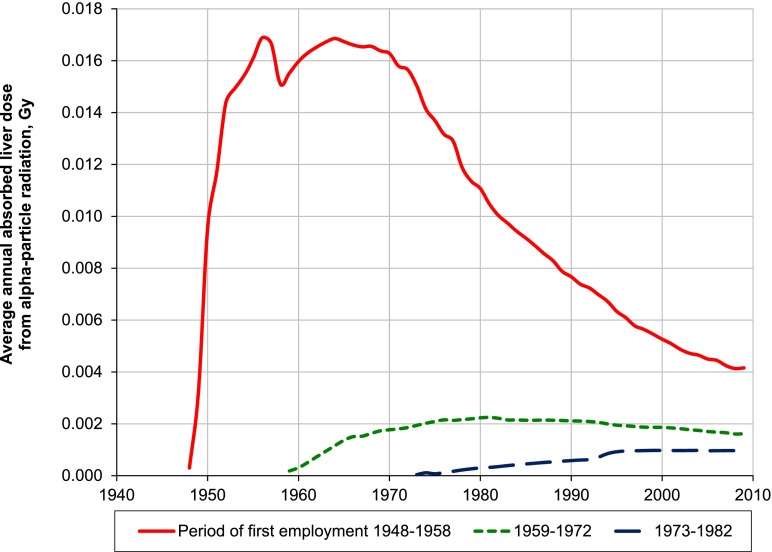
Figure 2 demonstrates the downward trend in liver dose from internal radiation for the original cohort of workers first employed during 1948–58 and for the subcohorts of the previous extension (individuals employed in 1959–72) and of the current extension (individuals employed in 1973–82). The dip in the annual average dose for the original cohort (1948–58) in 1958 is owing to a large number of newly employed workers in that year. The figure clearly demonstrates the decrease of internal exposure over the decades and emphasizes that the dose in any year does not only depend on the exposure experienced by a worker in that year but also on the exposures in previous years.
Figure 2.
Average annual absorbed liver dose from internal alpha-particle radiation.
Statistical analysis
A consistent approach has been used across the previous analyses of non-cancer diseases in the cohort,9,14 and this approach has again been employed. The analysis excluded workers who had experienced acute external γ-ray or gamma-neutron radiation exposure during nuclear accidents (43 individuals).
Analyses of non-radiation factors were first performed to confirm which factors influenced IHD incidence and mortality rates.
The radiation risk analyses consisted of the categorical analysis in which relative risks (RRs) were calculated for categories of total dose from external γ-rays (<0.10, 0.10–, 0.20–, 0.50–, 0.75–, 1.00–, 1.50–, 2.00–, 3.00–, ≥4.00 Gy) and total absorbed dose from internal alpha radiation to the liver (<0.025, 0.025–, 0.05–, 0.1–, 0.25–, 0.50–, ≥1.00 Gy) relative to baseline groups.
RR estimates were calculated using Poisson regression methods by using Epicure software (Hirosoft International Corporation, Seattle, WA).22 95% confidence intervals (CIs) for the RRs and p-values from tests of statistical significance were obtained via likelihood-based methods, using Epicure software. Differences were assumed statistically significant if p < 0.05. Radiation doses and smoking index were considered as time-dependant variables.
The analysis of risk associated with dose from internal alpha radiation was restricted to workers monitored for internal exposure and excluded unmonitored workers (i.e. those workers for whom no measurements of plutonium-particle activity in urine samples had been taken). Workers who had experienced acute accidental radionuclide intake (their doses from internal alpha radiation failed the dosimetry quality control check) were categorized as “unmonitored” workers (66 workers).
In RRs, analysis adjustments were made via stratification for the following non-radiation factors: sex, attained age (<20, 20–25, …, 80–85, >85 years), calendar period (1948–50, 1951–55, 1956–60, …, 2006–08), period of first employment at one of the main facilities (1948–53, 1954–58, 1959–63, 1964–68, 1969–72, 1973–78, 1979–82), facility type (reactor, radiochemical, plutonium production) (The category “Plutonium Production” consisted of workers ever employed at the Plutonium production facility, “Radiochemical” consisted of workers ever employed at the Radiochemical facility but never at the Plutonium production facility and “Reactors” consisted of workers employed at Reactors facility but never at either of the two other plants), smoking status (ever smoker, never smoker and unknown), alcohol consumption (ever drinker, never drinker and unknown), residence status (resident or migrant) in the mortality analysis, dose from internal alpha-radiation exposure when analysing external γ-ray exposure and vice versa. Workers unmonitored for internal exposure were included into a separate category when stratifying for internal alpha-particle radiation dose. Calendar period and, in particular, period of first employment correlate with radiation dose, but these variables are included into stratification to take into account indirectly factors which cannot be adequately measured (either their quality or their magnitude) at present. Among them are chemical exposures, particularly during early years of Mayak operation, different criteria for occupational selection to the production facility, particularly, for females, various means of radiation protection, different social and economic environments etc.
The incidence analysis was restricted to the period of residence in Ozyorsk since data on IHD incidence and non-radiation factors could not be collected for migrants once they had left the city.
To assess how the quality of causes of death affected risk estimates, the analysis of RRs for IHD mortality was performed for the whole cohort and for a subcohort of Ozyorsk residents.
Dose–response models were fitted to estimate the linear trend in IHD rates with external and internal exposures also using Poisson regression methods (using Epicure software). These analyses provide estimates of excess relative risk (ERR) per unit dose with 95% CIs and p-values from tests of statistical significance. Deviations from the linear model were estimated also when fitting alternative models: quadratic (Q) dose–response model, linear-quadratic (LQ) dose–response model, linear-exponential (LE) dose–response model, linear threshold model and linear piecewise model. We used differences in maximum likelihood to compare nested models or the Akaike23 information criterion for non-nested models.
The risk in relation to external γ-ray exposure was analysed for the whole cohort (full data set) and for a subcohort restricted to workers exposed to γ-rays at total absorbed dose <4.00 Gy (dose-restricted data set). Similarly, the risk in relation to internal alpha exposure was analysed for the whole cohort and for a subcohort restricted to workers exposed to alpha radiation at total absorbed liver dose <1.00 Gy. These additional analyses were performed to assess effects of high doses that potentially could induce deterministic effects in terms of prolonged exposure (chronic radiation sickness and plutonium-associated lung fibrosis) and their contribution to CD mortality risks.24
ERR analyses included adjustments (via stratification) for the same non-radiation factors for which adjustments were included during RR analyses. In addition, sensitivity analyses were conducted for linear trend analyses to investigate the effects of additional stratification by: hypertension (without hypertension, hypertension, unknown), BMI (<normal value, normal, >normal value and unknown), duration of employment (<1, 1–, 5–, 10–, 20–, ≥30 years), smoking index (unknown, 0, >0, 10–, ≥20 pack-years) instead of smoking status, dose from internal alpha-radiation exposure when analysing external γ-ray exposure and vice versa; unadjusted for smoking and alcohol consumption; restricted employment duration >1 year; restriction of follow-up by residence in Ozyorsk while analysing mortality from IHD; different lag periods (0, 5, 10, 15, 20, 25 and 30) for dose from both internal and external occupational exposures. For analyses with imposed lag periods, person-years were included since the start of employment and the first x years after that date were assigned “zero doses” when lagging external/internal dose by x years.
The sensitivity analysis adjusted for smoking index was performed only for the subcohort of Ozyorsk residents because quantitative information on smoking was available for Ozyorsk residents only.
The regular monitoring of internal alpha radiation was started after 1970 when the major part of migrants (83%) had already migrated from the city. Of migrants, plutonium activity in urine samples was measured in 4% only. For this reason, the sensitivity analysis which included the adjustment for internal alpha radiation when analysing external γ-ray exposure and vice versa was performed for the whole cohort and the subcohort of Ozyorsk residents.
The RR and ERR analyses of IHD incidence were repeated separately for males and females. To determine how the “validity” of dose from internal alpha radiation affected IHD incidence risk, two additional analyses including adjustments for non-radiation factors and dose from external γ-rays were performed for the following subcohorts of Mayak workers (only males): one containing only those workers with no below the LOD bioassay measurements (validity = 1) and the other containing only the workers with at least one bioassay measurement above the LOD (validity > 0). These additional analyses were conducted for IHD incidence risk but not mortality since the number of deaths was low compared with the number of cases implying the low statistical power.
Modifications of radiation risk with sex, facility type and attained age were also investigated (tests for heterogeneity). In addition, the risk association with attained age was studied. In this analysis, we compared ERRs/Gy with/without an adjustment for the attained age centred to 50 years (test for a log-linear trend in the ERR/Gy with attained age). All significant tests were two-sided.
Ethics
This record-based epidemiological study did not require any contact with the cohort members. The project was reviewed and approved by the Institutional Review Board of the SUBI.
RESULTS
By the end of follow-up, 7225 cases and 2848 deaths from IHD (as the underlying cause of death) were registered during 447,281 and 836,048 person-years, respectively. Chronic types of IHD (414.0–414.1 ICD-9 codes) made the greatest contribution into IHD incidence and mortality patterns (98%). Acute IHD cases include acute myocardial infarction (ICD-9 code 410.0) and acute coronary failure (ICD-9 code 411); typically they were registered as an immediate cause of death and were not analysed within the present study. All other types of IHD (codes 412, 413, 414.9) are chronic.
Non-radiation factors
RRs of IHD incidence and mortality in the study cohort associated with non-radiation factors are presented online (Supplementary Table A).
Generally, the results of the current analyses of IHD incidence and mortality in relation to non-radiation factors are consistent with those of the previous analyses.14 IHD incidence and mortality RRs were significantly lower in females than in males and increased with attained age in both sexes. IHD incidence and mortality risks increased with age at first employment for both sexes (but not RR of mortality among females) likely owing to the increasing attained age. IHD incidence risk was significantly lower in workers first employed at Mayak PA after 1953 than those first employed in the earliest period of the Mayak operation (1948–53) (but not in females first employed between 1954 and 58). These results were obtained without radiation dose taken into account. An additional analysis of IHD incidence and mortality risks in relation to period of first employment including adjustments for dose from external γ-rays and internal alpha radiation demonstrated that the results obtained for IHD mortality (in males and females) and IHD incidence (among females) changed just modestly. Meanwhile, in male workers, adjustments for external and internal doses resulted in insignificant modification of the risk estimates obtained for the period of first employment 1954–68 and loss of statistical significance within the category of first employment “after 1969”. IHD incidence risk rose from a low level in 1948 to its highest level during 1956–60 periods and then smoothly decreased to the end of the follow-up both in males and females. Incidence and mortality from IHD in relation to calendar period is presented online (Supplementary Figure 1). The increase of incidence in this period was due to diagnoses of chronic IHD types (made during regular and meticulous medical examinations) missed before employment at the Mayak PA and during a pre-employment medical examination. By contrast, the RR for IHD mortality was significantly decreased in males during 1961–75 and, on the contrary, during 1991–95, IHD mortality risk was increasing both in males and females; however, it was significant only for females. Comparison of risks among workers in relation to the facility type found no significant variation for either IHD incidence or mortality (for both sexes) except that IHD incidence risk in females employed at the plutonium production plant was significantly higher.
Again as in the previous studies14 the IHD incidence and mortality risks were significantly higher among smokers (but not incidence risk for females). Risks of IHD incidence and mortality in male workers increased with the smoking index. No similar association was observed for females, likely owing to the low number of female smokers in the study cohort and, as a result, owing to the low statistical power of the study. Alcohol consumption had different effects on the IHD mortality (but not incidence): the risk in males who consumed alcohol increased significantly, whereas in females, we observed a significant reduction of the risk. This difference was owing to both quantity and quality of alcohol (males tend to consume a lot of strong spirits, while females drink wine moderately). Some studies have shown that low doses of alcohol have cardioprotective effects, while high doses of alcohol increase the risk of circulatory diseases.25
The IHD incidence and mortality risks were significantly higher in workers who had been diagnosed with hypertension (but not incidence risk in females). RRs of IHD incidence and mortality were significantly higher in workers with the above normal BMI than those with the normal BMI.
IHD mortality risk among migrants was significantly lower than in Ozyorsk residents owing to differing quality of data on cause of death.
External gamma radiation: mortality
The results of the analysis of the RRs of mortality from IHD (RRs) by categories of the total external gamma dose as well as ERRs/Gy for the entire cohort (full data set) and a subcohort restricted to workers exposed at total external gamma doses <4.00 Gy (dose-restricted data set) are presented in Tables 2 and 3.
Table 2.
Relative risks (RRs) of ischaemic heart disease mortality for various categories of total dose from external γ-rays (0-year lag period)
| External gamma dose (Gy) | Mean total external gamma dose (Gy) |
Person-years at risk |
Cases |
RR (95% confidence interval) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Restricting follow-up to Ozyorsk | Migrants | Whole cohort | Restricting follow-up to Ozyorsk | Migrants | Whole cohort | Restricting follow-up to Ozyorsk | Migrants | Whole cohort | Restricting follow-up to Ozyorsk | Migrants | Whole cohort | |
| 0–0.1 | 0.037 | 0.033 | 0.035 | 192,795 | 94,048 | 291,625 | 408 | 344 | 748 | 1 | 1 | 1 |
| 0.1–0.2 | 0.144 | 0.146 | 0.144 | 75,750 | 32,855 | 110,108 | 214 | 139 | 353 | 0.98 (0.79 to 1.21) | 0.95 (0.76 to 1.19) | 0.96 (0.83 to 1.12) |
| 0.2–0.5 | 0.326 | 0.328 | 0.326 | 106,661 | 53,412 | 162,230 | 355 | 204 | 560 | 0.93 (0.76 to 1.14) | 0.78 (0.63 to 0.95) | 0.84 (0.73 to 0.96) |
| 0.5–0.75 | 0.615 | 0.614 | 0.615 | 41,417 | 23,486 | 65,904 | 180 | 88 | 268 | 1.08 (0.84 to 1.38) | 0.79 (0.60 to 1.02) | 0.91 (0.76 to 1.08) |
| 0.75–1.00 | 0.869 | 0.871 | 0.870 | 30,491 | 15,571 | 46,568 | 115 | 70 | 187 | 0.99 (0.76 to 1.30) | 0.87 (0.64 to 1.17) | 0.92 (0.76 to 1.12) |
| 1.00–1.50 | 1.234 | 1.230 | 1.234 | 38,869 | 20,199 | 59,677 | 172 | 97 | 270 | 1.18 (0.92 to 1.53) | 0.97 (0.73 to 1.27) | 1.05 (0.87 to 1.26) |
| 1.50–2.00 | 1.729 | 1.725 | 1.728 | 23,831 | 12,236 | 36,499 | 134 | 52 | 186 | 1.41 (1.07 to 1.87) | 0.72 (0.51 to 1.01) | 1.06 (0.76 to 1.30) |
| 2.00–3.00 | 2.395 | 2.367 | 2.388 | 24,350 | 10,077 | 34,769 | 158 | 63 | 221 | 1.52 (1.15 to 2.02) | 1.01 (0.71 to 1.40) | 1.24 (1.00 to 1.53) |
| 3.00–4.00 | 3.409 | 3.457 | 3.425 | 4612 | 2348 | 7098 | 23 | 18 | 41 | 1.27 (0.75 to 2.09) | 0.87 (0.46 to 1.54) | 1.00 (0.67 to 1.45) |
| ≥4.00 | 4.740 | 4.932 | 4.840 | 1386 | 1785 | 3210 | 6 | 8 | 14 | 1.28 (0.49 to 2.78) | 0.57 (0.22 to 1.19) | 0.80 (0.42 to 1.39) |
Table 3.
Ischaemic heart disease mortality: analysis in relation to dose from external γ-rays, excess relative risk per unit dose (95% confidence interval)
| Analyses | Whole dose range | Doses < 4.00 Gy |
|---|---|---|
| Main analysis, 0-year lag | 0.05 (−0.02 to 0.12) | 0.07 (−0.01 to 0.15) |
| Main analyses, in which the first x years following the start of radiation work were assigned to a “zero-dose” category when lagging doses by x years | ||
| Main analysis, 5-year lag | 0.05 (−0.01 to 0.13) | 0.07 (<0 to 0.16) |
| Main analysis, 10-year lag | 0.05 (−0.01 to 0.13) | 0.07 (>0 to 0.16) |
| Main analysis, 15-year lag | 0.05 (−0.01 to 0.13) | 0.07 (<0 to 0.16) |
| Main analysis, 20-year lag | 0.05 (−0.02 to 0.13) | 0.07 (−0.01 to 0.16) |
| Main analysis, 25-year lag | 0.05 (−0.02 to 0.14) | 0.06 (−0.01 to 0.16) |
| Main analysis, 30-year lag | 0.06 (−0.02 to 0.15) | 0.06 (−0.02 to 0.16) |
| Main analysis but unadjusted for smoking and alcohol consumption, 0-year lag | 0.06 (<0 to 0.14) | 0.07 (>0 to 0.16) |
| Adding to stratification (0-year lag) | ||
| Hypertension | 0.04 (−0.02 to 0.13) | 0.06 (−0.01 to 0.15) |
| Body mass index | 0.08 (0.01 to 0.18) | 0.11 (0.02 to 0.21) |
| Employment duration | 0.06 (<0 to 0.15) | 0.09 (0.01 to 0.18) |
| Internal dose to the liver | 0.06 (<0 to 0.15) | 0.09 (0.01 to 0.19) |
| Restricting follow-up to Ozyorsk | 0.09 (<0 to 0.21) | 0.1 (>0 to 0.23) |
| Also adjusting for internal dose to the liver | 0.19 (0.07 to 0.36) | 0.21 (0.08 to 0.39) |
| Also adjusting for smoking index | 0.08 (−0.01 to 0.20) | 0.08 (−0.02 to 0.21) |
| Migrants | >0 (−0.08 to 0.10) | 0.02 (−0.07 to 0.14) |
| Also adjusting for internal dose to the liver | −0.03 (−0.10 to 0.07) | −0.01 (−0.11 to 0.11) |
| Main analysis, restricted employment duration >1 year | 0.05 (−0.02 to 0.13) | 0.07 (−0.01 to 0.16) |
| Analyses (0-year lag) restricted to workers at | ||
| Males (0-year lag) | 0.05 (−0.02 to 0.13) | 0.06 (−0.01 to 0.16) |
| Females (0-year lag) | 0.05 (−0.10 to 0.30) | 0.07 (−0.10 to 0.33) |
| P1 > 0.50 | P1 = 0.38 | |
| Reactors | 0.03 (−0.09 to 0.19) | 0.04 (−0.09 to 0.22) |
| Radiochemical plant | 0.06 (−0.07 to 0.25) | 0.03 (−0.11 to 0.21) |
| Plutonium plant | 0.05 (−0.03 to 0.16) | 0.09 (−0.01 to 0.22) |
| P2 > 0.50 | P2 > 0.50 | |
| Attained age (0-year lag) (years) | ||
| <50 | −0.12 (NA to 0.11) | −0.08 (−0.23 to 0.21) |
| 50–59 | −0.07 (NA to 0.10) | >0 (−0.13 to 0.20) |
| 60–69 | 0.10 (−0.01 to 0.26) | 0.10 (−0.03 to 0.26) |
| ≥70 | 0.08 (−0.02 to 0.23) | 0.10 (−0.02 to 0.25) |
| P3 = 0.13 | P3 > 0.50 | |
| P4 < 0.01 | P4 = 0.01 | |
NA, not available; P1, test for heterogeneity between males and females; P2, test for heterogeneity between radiochemical and plutonium plant workers; P3, test for heterogeneity between groups of workers of different attained age; P4, test for a log-linear trend in the ERR/Gy with attained age.
The categorical analysis did not reveal any significant differences among IHD mortality RRs across various dose categories either in the whole cohort or in a subcohort of workers restricted to Ozyorsk residents except for a significant decrease of RR in dose category 0.20–0.50 Gy for the whole cohort and a significant increase of RRs in dose categories 1.50–2.00 and 2.00–3.00 Gy for the subcohort of residents and in category 2.00–3.00 Gy for the whole cohort compared with the reference category (0–0.10 Gy).
No significant associations were revealed for IHD mortality with total dose from external γ-rays in either full or dose-restricted data sets (the linear model) [ERR/Gy = 0.05 (95% CI: −0.02 to 0.12) and ERR/Gy = 0.07 (95% CI: −0.01 to 0.15), respectively]. Applying various lag periods almost did not affect the results, but the risk became significant only after having applied a 10-year lag period to the dose-restricted data set. The exclusion of the smoking and alcohol consumption adjustments had no effect on the risk estimate for the full data set, but the risk for the dose-restricted data set became significant. Additional adjustment for hypertension did not change the result while the BMI adjustment caused the increase of the risk estimates for the both data sets and the risk became significant.
Inclusion of an adjustment for dose from internal alpha radiation did not affect the result observed for the full data set; however, it provided a modest but significant increase of ERR/Gy in the dose-restricted data set. However, when the analysis was restricted to workers who were Ozyorsk residents and an adjustment for dose from internal alpha radiation was included, the ERR/Gy significantly increased (approximately by a factor of 2) and the risk was significant in both data sets. Risk in migrants was much lower and insignificant than that in residents. But, this result is to be interpreted with caution as statistical power of this analysis was significantly lower than that of residents.
No significant differences were observed in risk estimates between the sexes (p > 0.5 and pc0.38), among facility types (p > 0.5 and p > 0.5) and attained age categories (p = 0.13 and p > 0.5) in either the full or the dose-restricted data set. ERR/Gy increased with attained age in both data sets (p = 0.004 and p = 0.012, respectively).
Non-linear dose–response models did not provide better fit of IHD mortality association with dose from external γ-rays. The test for non-linearity based on a comparison between the linear and non-linear dose–response models was not statistically significant (p > 0.5 for the LQ and LE models, the difference in Akaike information criterion statistics between the linear and pure quadratic models was 3.12). The best estimated threshold dose was 0 Gy.
External gamma radiation: incidence
The results of the analysis of the RRs of IHD incidence by categories of the total external gamma dose as well as ERRs/Gy for the entire cohort (full data set) and a subcohort restricted to workers exposed at total external gamma doses <4.00 Gy (dose-restricted data set) are presented in Tables 4 and 5. The most important finding of these analyses was a highly significant difference in RRs and ERRs/Gy between males and females of the study cohort. For this reason, all the analyses were repeated separately for males and females.
Table 4.
Relative risks (RRs) of ischaemic heart disease incidence for various categories of total dose from external γ-rays (0-year lag period)
| External gamma dose (Gy) | Mean total external gamma dose (Gy) |
Person-years at risk |
Cases |
RR (95% confidence interval) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Males | Females | Both | Males | Females | Both | Males | Females | Both | Males | Females | Both | |
| 0–0.1 | 0.039 | 0.032 | 0.037 | 108,594 | 52,481 | 161,076 | 990 | 758 | 1748 | 1 | 1 | 1 |
| 0.1–0.2 | 0.144 | 0.142 | 0.144 | 46,286 | 16,104 | 62,389 | 640 | 305 | 945 | 1.18 (1.04 to 1.33) | 0.91 (0.75 to 1.10) | 1.09 (0.99 to 1.20) |
| 0.2–0.5 | 0.324 | 0.331 | 0.325 | 65,838 | 19,560 | 85,398 | 1110 | 385 | 1495 | 1.08 (0.96 to 1.22) | 0.90 (0.74 to 1.10) | 1.01 (0.91 to 1.11) |
| 0.5–0.75 | 0.614 | 0.615 | 0.614 | 23,310 | 8463 | 31,772 | 529 | 156 | 685 | 1.22 (1.06 to 1.40) | 0.82 (0.63 to 1.06) | 1.10 (0.98 to 1.22) |
| 0.75–1.00 | 0.870 | 0.871 | 0.869 | 15,782 | 7302 | 23,084 | 368 | 129 | 497 | 1.10 (0.94 to 1.29) | 0.68 (0.51 to 0.89) | 0.97 (0.85 to 1.11) |
| 1.00–1.50 | 1.233 | 1.229 | 1.231 | 19,936 | 8752 | 28,688 | 537 | 180 | 717 | 1.25 (1.08 to 1.46) | 0.78 (0.59 to 1.01) | 1.10 (0.97 to 1.26) |
| 1.50–2.00 | 1.731 | 1.720 | 1.732 | 12,053 | 4800 | 16,853 | 368 | 93 | 461 | 1.32 (1.12 to 1.57) | 0.77 (0.55 to 1.06) | 1.16 (1.00 to 1.30) |
| 2.00–3.00 | 2.402 | 2.362 | 2.390 | 11,924 | 4033 | 15,957 | 417 | 94 | 511 | 1.52 (1.28 to 1.80) | 0.82 (0.58 to 1.13) | 1.31 (1.13 to 1.52) |
| 3.00–4.00 | 3.422 | 3.634 | 3.404 | 2397 | 837 | 3234 | 78 | 18 | 96 | 1.45 (1.09 to 1.92) | 1.25 (0.66 to 2.22) | 1.34 (1.04 to 1.71) |
| ≥4.00 | 4.724 | 4.717 | 4.736 | 762 | 157 | 919 | 28 | 3 | 31 | 1.30 (0.83 to 1.97) | 0.58 (0.09 to 2.03) | 1.12 (0.72 to 1.67) |
Table 5.
Ischaemic heart disease incidence: analysis in relation to dose from external γ-rays, excess relative risk per unit dose (ERR/Gy) (95% confidence interval)
| Analyses | Whole dose range |
Doses < 4.00 Gy |
||||
|---|---|---|---|---|---|---|
| Males | Females | Both | Males | Females | Both | |
| Main analysis, 0-year lag | 0.18 (0.11 to 0.27) | 0.03 (−0.06 to 0.14) | 0.14 (0.09 to 0.21) | 0.19 (0.12 to 0.28) | 0.03 (−0.06 to 0.15) | 0.15 (0.09 to 0.21) |
| P1 = 0.022 |
P1 = 0.024 |
|||||
| Main analyses, in which the first x years following the start of radiation work were assigned to a “zero-dose” category when lagging doses by x years | ||||||
| Main analysis, 5-year lag | 0.18 (0.11 to 0.26) | 0.04 (−0.05 to 0.15) | 0.14 (0.08 to 0.21) | 0.18 (0.11 to 0.27) | 0.04 (−0.05 to 0.16) | 0.14 (0.08 to 0.21) |
| Main analysis, 10-year lag | 0.18 (0.10 to 0.26) | 0.05 (−0.04 to 0.17) | 0.14 (0.08 to 0.21) | 0.19 (0.11 to 0.28) | 0.05 (−0.04 to 0.18) | 0.15 (0.09 to 0.22) |
| Main analysis, 15-year lag | 0.20 (0.12 to 0.30) | 0.07 (−0.03 to 0.19) | 0.16 (0.10 to 0.24) | 0.22 (0.13 to 0.32) | 0.07 (−0.03 to 0.20) | 0.17 (0.10 to 0.25) |
| Main analysis, 20-year lag | 0.17 (0.08 to 0.27) | 0.07 (−0.03 to 0.20) | 0.13 (0.07 to 0.21) | 0.18 (0.10 to 0.29) | 0.08 (−0.03 to 0.21) | 0.15 (0.08 to 0.23) |
| Main analysis, 25-year lag | 0.16 (0.07 to 0.27) | 0.09 (−0.02 to 0.23) | 0.14 (0.06 to 0.22) | 0.18 (0.08 to 0.30) | 0.10 (−0.02 to 0.25) | 0.15 (0.07 to 0.24) |
| Main analysis, 30-year lag | 0.22 (0.10 to 0.36) | 0.12 (<0 to 0.28) | 0.18 (0.09 to 0.28) | 0.24 (0.11 to 0.39) | 0.13 (>0 to 0.30) | 0.19 (0.10 to 0.30) |
| Main analysis but unadjusted for smoking and alcohol consumption, 0-year lag | 0.20 (0.13 to 0.28) | 0.04 (−0.05 to 0.14) | 0.15 (0.10 to 0.22) | 0.2 (0.13 to 0.29) | 0.04 (−0.05 to 0.15) | 0.16 (0.10 to 0.22) |
| Adding to stratification (0-year lag) | ||||||
| Hypertension | 0.17 (0.10 to 0.25) | 0.04 (−0.05 to 0.17) | 0.14 (0.08 to 0.20) | 0.18 (0.10 to 0.27) | 0.05 (−0.05 to 0.17) | 0.14 (0.08 to 0.21) |
| Body mass index | 0.18 (0.11 to 0.27) | 0.06 (−0.04 to 0.19) | 0.15 (0.09 to 0.22) | 0.19 (0.11 to 0.28) | 0.07 (−0.04 to 0.20) | 0.16 (0.09 to 0.23) |
| Employment duration | 0.18 (0.11 to 0.27) | 0.04 (−0.05 to 0.15) | 0.14 (0.08 to 0.21) | 0.19 (0.11 to 0.28) | 0.04 (−0.05 to 0.16) | 0.15 (0.09 to 0.22) |
| Also adjusting for internal dose to the liver | 0.14 (0.07 to 0.23) | −0.03 (−0.12 to 0.08) | 0.10 (0.04 to 0.17) | 0.16 (0.08 to 0.25) | −0.03 (−0.12 to 0.10) | 0.11 (0.05 to 0.19) |
| Also adjusting for smoking index | 0.17 (0.10 to 0.26) | 0.02 (−0.06 to 0.13) | 0.13 (0.07 to 0.20) | 0.18 (0.10 to 0.27) | 0.03 (−0.06 to 0.14) | 0.13 (0.07 to 0.20) |
| Main analysis, restricted employment duration >1 year | 0.18 (0.11 to 0.27) | 0.03 (−0.05 to 0.15) | 0.14 (0.09 to 0.21) | 0.19 (0.12 to 0.28) | 0.03 (−0.05 to 0.16) | 0.15 (0.09 to 0.22) |
| Analyses (0-year lag) restricted to workers at | ||||||
| Reactors | 0.21 (0.07 to 0.39) | −0.29 (−0.47 to 0.01) | 0.15 (0.03 to 0.29) | 0.24 (0.09 to 0.43) | −0.29 (−0.47 to 0.01) | 0.16 (0.04 to 0.31) |
| Radiochemical plant | 0.26 (0.11 to 0.44) | 0.11 (−0.08 to 0.38) | 0.21 (0.09 to 0.36) | 0.26 (0.11 to 0.45) | 0.11 (−0.08 to 0.38) | 0.22 (0.09 to 0.37) |
| Plutonium plant | 0.14 (0.06 to 0.25) | 0.04 (−0.06 to 0.19) | 0.11 (0.04 to 0.20) | 0.14 (0.05 to 0.25) | 0.05 (−0.06 to 0.21) | 0.11 (0.04 to 0.20) |
| P2 = 0.22 | P2 ≥ 0.50 | P2 = 0.19 | P2 = 0.22 | P2 > 0.50 | P2 = 0.02 | |
| Attained age (0-year lag) (years) | ||||||
| <50 | 0.19 (0.08 to 0.33) | −0.12 (not available to 0.08) | 0.14 (0.05 to 0.26) | 0.20 (0.09 to 0.35) | −0.11 (−0.23 to 0.10) | 0.14 (0.05 to 0.27) |
| 50–59 | 0.16 (0.06 to 0.29) | −0.06 (−0.16 to 0.09) | 0.10 (0.02 to 0.20) | 0.17 (0.06 to 0.31) | −0.06 (−0.17 to 0.09) | 0.10 (0.02 to 0.21) |
| 60–69 | 0.22 (0.06 to 0.45) | 0.21 (0.00 to 0.54) | 0.22 (0.08 to 0.40) | 0.23 (0.06 to 0.46) | 0.22 (0.01 to 0.57) | 0.23 (0.09 to 0.41) |
| ≥70 | 0.13 (−0.09 to 0.58) | 0.25 (−0.03 to 0.77) | 0.19 (−0.01 to 0.50) | 0.10 (−0.12 to 0.54) | 0.24 (−0.05 to 0.77) | 0.17 (−0.03 to 0.48) |
| P3 > 0.50 | P3 = 0.03 | P3 > 0.50 | P3 > 0.50 | P3 = 0.04 | P3 > 0.50 | |
| P4 > 0.50 | P4 = 0.10 | P4 = 0.28 | P4 > 0.50 | P4 = 0.11 | P4 = 0.34 | |
| Calendar period | ||||||
| ≤1960 | 0.28 (0.10 to 0.53) | — | 0.22 (0.06 to 0.43) | 0.26 (0.08 to 0.51) | — | 0.20 (0.04 to 0.41) |
| 1961–2008 | 0.16 (0.09 to 0.25) | 0.05 (−0.04 to 0.16) | 0.13 (0.07 to 0.20) | 0.18 (0.10 to 0.27) | 0.05 (−0.04 to 0.17) | 0.14 (0.08 to 0.21) |
| P5 = 0.29 | — | P5 = 0.34 | P5 = 0.47 | — | P5 > 0.50 | |
P1, test for heterogeneity between males and females; P2, test for heterogeneity between radiochemical and plutonium plant workers; P3, test for heterogeneity between groups of workers of different attained age; P4, test for a log-linear trend in the ERR/Gy with attained age; P5, test for heterogeneity between groups of calendar period.
The categorical analysis based on the whole cohort showed significantly raised RRs of IHD incidence in dose categories 1.50–2.00, 2.00–3.00, 3.00–4.00 Gy compared with the reference category (0–0.10 Gy). Significantly raised RRs of IHD incidence among male (but not female) workers were observed in all dose categories except for categories 0.20–0.50, 0.75–1.00 and >4.00 Gy.
Significantly raised ERRs/Gy were found in both the full and dose-restricted data sets [ERR/Gy = 0.14 (95% CI: 0.09 to 0.21) and ERR/Gy = 0.15 (95% CI: 0.09 to 0.21), respectively]. The analysis of IHD incidence for females showed no raised ERRs/Gy in either of the data sets except for the significantly increased ERRs/Gy with 30-year lag periods imposed in the dose-restricted data set. A significantly raised ERR/Gy was shown for IHD incidence among females of attained age of 60–69 years in both data sets. Moreover, it should be noted that differences in risk estimates for females among categories of attained age were significant in both data sets (p = 0.03 and p = 0.04, respectively). ERR/Gy increase with the attained age was insignificant (p = 0.09 and p = 0.11, respectively).
The main dose–response analysis for males revealed significantly raised ERRs/Gy of IHD incidence for both the full and dose-restricted data sets [ERR/Gy = 0.18 (95% CI: 0.11 to 0.27) and ERR/Gy = 0.19 (95% CI: 0.12 to 0.28), respectively]. There was little effect on these results when various lag periods were imposed or adjustments for additional non-radiation factors were included and adjustments for smoking and alcohol consumption were excluded. The ERR/Gy for IHD incidence did reduce marginally when adjustment for dose from internal alpha radiation to the liver was added for both the full and dose-restricted data sets, but the risks remained significant [ERR/Gy = 0.14 (95% CI: 0.7 to 0.23) and ERR/Gy = 0.16 (95% CI: 0.08 to 0.20), respectively].
Significantly raised ERRs/Gy were revealed for workers employed at each of the facilities while analysing both data sets; however, no significant differences were observed among these groups (p = 0.22 and p = 0.22, respectively). No significant changes in ERRs/Gy were observed among categories of attained age (p > 0.5 and p > 0.5, respectively). ERRs/Gy were significantly raised for both pre- and post-1960 calendar periods, and differences between these estimates were insignificant for both data sets (p = 0.29 and p = 0.47, respectively).
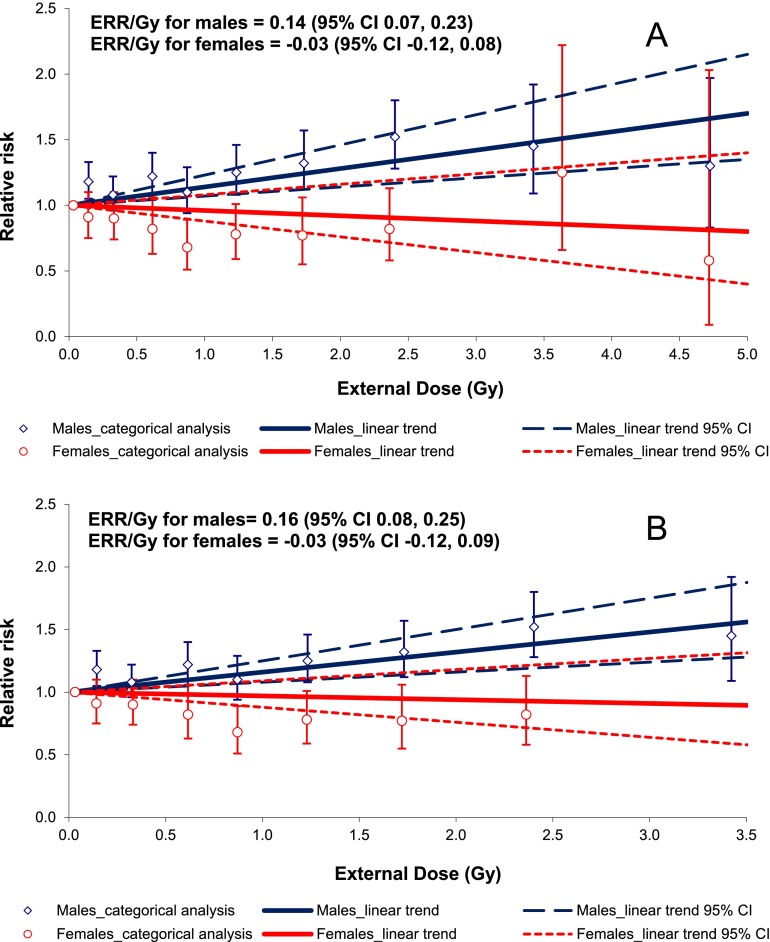
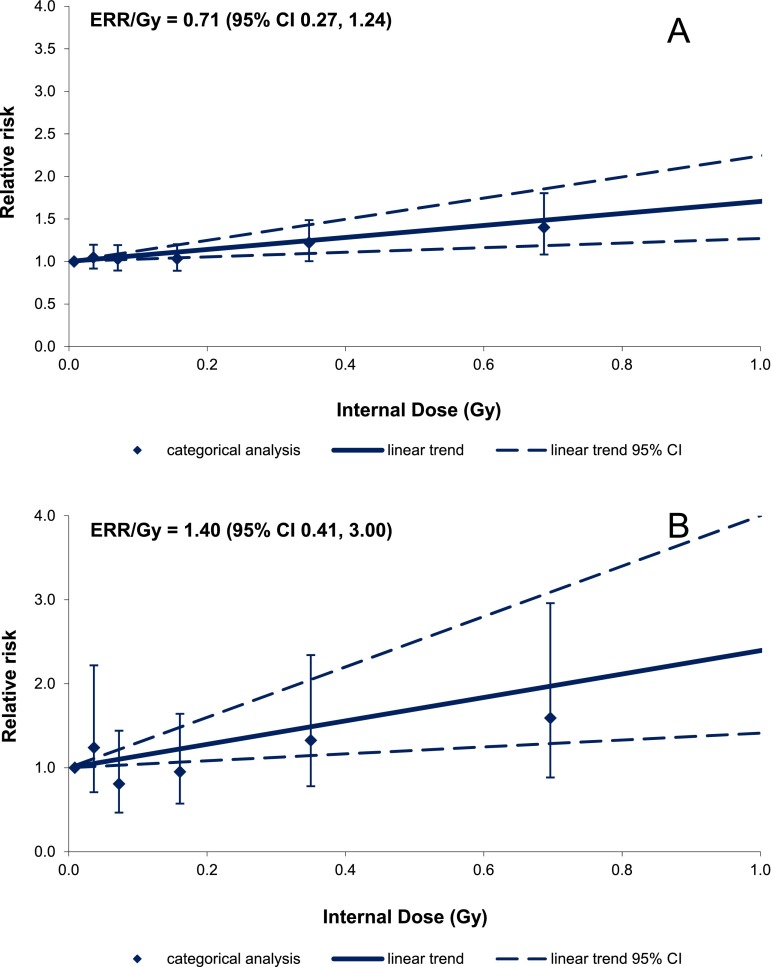
Figure 3 demonstrates the comparison between results of the dose–response analyses for males and females. As may be inferred from Figure 3, categorical analysis results are in good agreement with the linear trend for females (but not for males) using both full and dose-restricted data sets. The test for non-linearity based on the comparison between the linear and non-linear dose–response models was not statistically significant for males (p = 0.22 for LQ and p = 0.20 for LE models); however, the pure quadratic model provided a better fit of the data than the linear one (the difference in Akaike information criterion statistics between the linear and pure quadratic models was 12.19). The test for non-linearity based on the comparison between linear and non-linear dose–response models was not statistically significant for females (p = 0.38 for LQ and p > 0.5 for LE models, the difference in Akaike information criterion statistics between the linear and pure quadratic models was 0.51). The best estimated threshold dose for both sexes was 0 Gy.
Figure 3.
Ischaemic heart disease incidence in relation to total external gamma dose (a) for the full data set with 0 lag period (b) for the dose-restricted data set (external dose <4.00 Gy) with 0 lag period. CI, confidence interval; ERR/Gy, excess relative risk per unit dose.
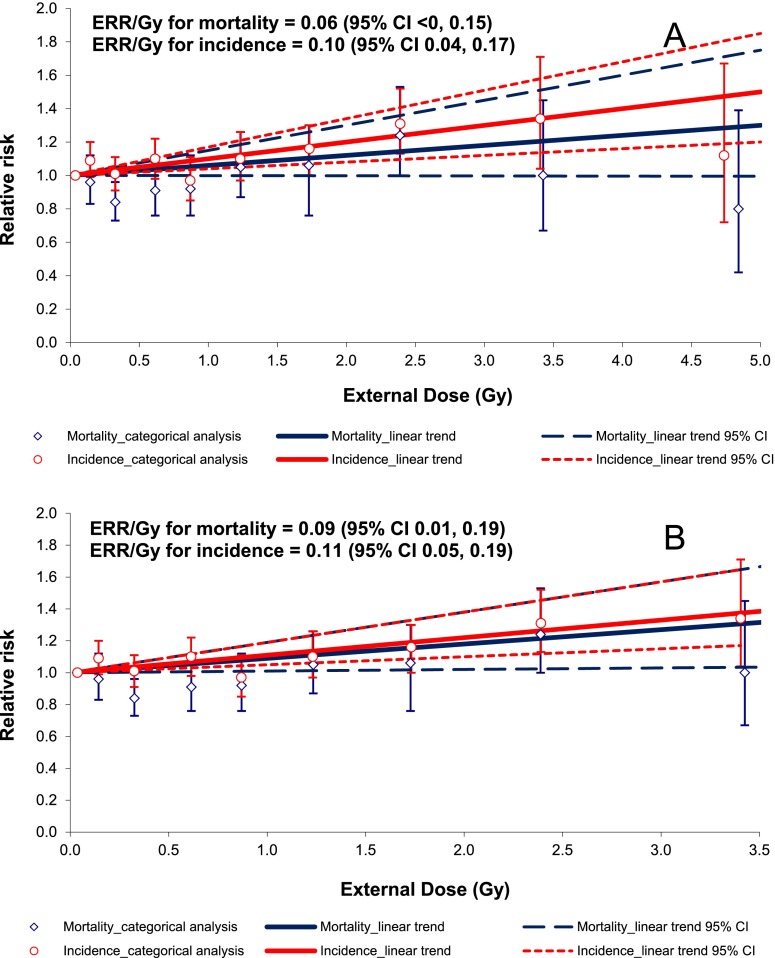
Figure 4 demonstrates a comparison between IHD incidence and mortality in relation to total dose from external γ-rays.
Figure 4.
Ischaemic heart disease mortality and incidence in relation to total external gamma dose (a) for the full data set with 0 lag period (b) for the dose-restricted data set (external dose <4.00 Gy) with 0 lag period. CI, confidence interval; ERR/Gy, excess relative risk per unit dose.
Internal alpha radiation: mortality
The results of the analysis of the RRs of IHD mortality by categories of the total absorbed internal alpha dose to the liver as well as ERRs/Gy for the entire cohort (full data set) and a subcohort restricted to workers exposed at alpha doses <1.00 Gy (dose-restricted data set) are presented in Tables 6 and 7.
Table 6.
Relative risks (RRs) of ischaemic heart disease mortality for various categories of total absorbed dose from internal alpha radiation to the liver (0-year lag period)
| Total absorbed alpha dose in the liver (Gy) | Mean total absorbed alpha dose in the liver (Gy) |
Person-years at risk |
Cases |
RR (95% confidence interval) |
||||
|---|---|---|---|---|---|---|---|---|
| Restricting follow-up to Ozyorsk | Whole cohort | Restricting follow-up to Ozyorsk | Whole cohort | Restricting follow-up to Ozyorsk | Whole cohort | Restricting follow-up to Ozyorsk | Whole cohort | |
| 0–0.025 | 0.008 | 0.008 | 151,743 | 153,420 | 174 | 177 | 1 | 1 |
| 0.025–0.05 | 0.036 | 0.036 | 37,961 | 39,292 | 175 | 180 | 1.41 (1.09 to 1.82) | 1.43 (1.12 to 1.83) |
| 0.05–0.1 | 0.071 | 0.071 | 29,699 | 31,119 | 178 | 182 | 1.90 (1.46 to 2.50) | 1.80 (1.39 to 2.34) |
| 0.1–0.25 | 0.159 | 0.159 | 29,734 | 31,241 | 173 | 186 | 1.46 (1.10 to 1.94) | 1.43 (1.09 to 1.89) |
| 0.25–0.50 | 0.347 | 0.348 | 12,656 | 13,618 | 89 | 94 | 1.75 (1.25 to 2.45) | 1.68 (1.21 to 2.34) |
| 0.50–1.00 | 0.692 | 0.692 | 6183 | 6796 | 57 | 62 | 2.28 (1.53 to 3.39) | 2.20 (1.50 to 3.25) |
| ≥1.00 | 2.854 | 3.085 | 5859 | 7164 | 44 | 56 | 1.86 (1.16 to 2.95) | 1.93 (1.24 to 2.99) |
Table 7.
Ischaemic heart disease mortality: analyses in relation to total absorbed dose from internal alpha radiation to the liver, excess relative risk per unit dose (ERR/Gy) (95% confidence interval)
| Analyses | Whole dose range | Doses < 1.00 Gy |
|---|---|---|
| Main analysis, 0-year lag | 0.30 (0.10 to 0.59) | 1.22 (0.51 to 2.19) |
| Main analyses, in which the first x years following the start of radiation work were assigned to a “zero-dose” category when lagging doses by x years | ||
| Main analysis, 5-year lag | 0.36 (0.13 to 0.69) | 1.54 (0.69 to 2.71) |
| Main analysis, 10-year lag | 0.38 (0.12 to 0.75) | 1.59 (0.65 to 2.89) |
| Main analysis, 15-year lag | 0.40 (0.12 to 0.81) | 1.64 (0.58 to 3.11) |
| Main analysis, 20-year lag | 0.42 (0.11 to 0.89) | 1.58 (0.38 to 3.27) |
| Main analysis, 25-year lag | 0.45 (0.09 to 1.00) | 1.30 (−0.04 to 3.20) |
| Main analysis, 30-year lag | 0.49 (0.07 to 1.16) | 0.82 (−0.67 to 3.00) |
| Main analysis but unadjusted for smoking and alcohol consumption, 0-year lag | 0.27 (0.09 to 0.54) | 1.19 (0.50 to 2.10) |
| Adding to stratification (0-year lag) | ||
| Hypertension | 0.38 (0.14 to 0.75) | 1.31 (0.54 to 2.37) |
| Body mass index | 0.41 (0.15 to 0.80) | 1.31 (0.53 to 2.41) |
| Employment duration | 0.31 (0.10 to 0.62) | 1.22 (0.50 to 2.22) |
| External gamma dose | 0.21 (0.01 to 0.58) | 1.08 (0.34 to 2.15) |
| Restricting follow-up to Ozyorsk | 0.28 (0.06 to 0.59) | 1.18 (0.46 to 2.15) |
| Also adjusting for external gamma dose | 0.20 (NA to 0.59) | 1.05 (0.30 to 2.15) |
| Also adjusting for smoking index | 0.23 (0.03 to 0.53) | 1.11 (0.40 to 2.08) |
| Main analysis, restricted employment duration >1 year | 0.27 (0.08 to 0.55) | 1.08 (0.40 to 2.00) |
| Analyses (0-year lag) restricted to workers at | ||
| Males (0-year lag) | 0.30 (0.05 to 0.68) | 1.76 (0.84 to 3.05) |
| Females (0-year lag) | 0.29 (0.02 to 0.83) | −0.30 (−0.92 to 0.95) |
| P1 > 0.50 | P1 = 0.01 | |
| Radiochemical plant | 0.32 (0.06 to 0.74) | 1.58 (0.59 to 3.05) |
| Plutonium plant | 0.25 (NA to 0.70) | 0.59 (−0.23 to 1.89) |
| P2 > 0.50 | P2 = 0.22 | |
| Attained age (0-year lag) (years) | ||
| <50 | 0.50 (NA to 8.53) | 4.26 (NA to 34.84) |
| 50–59 | 3.88 (1.25 to 10.06) | 8.45 (3.51 to 19.62) |
| 60–69 | 0.15 (NA to 0.59) | 0.84 (−0.11 to 2.41) |
| ≥70 | 0.20 (>0 to 0.55) | 0.30 (−0.34 to 1.29) |
| P3 = 0.01 | P3 < 0.01 | |
| P4 = 0.02 | P4 < 0.01 | |
NA, not available; P1, test for heterogeneity between males and females; P2, test for heterogeneity between radiochemical and plutonium plant workers; P3, test for heterogeneity between groups of workers of different attained age; P4, test for a log-linear trend in the ERR/Gy with attained age.
The categorical analysis showed significantly raised RRs of IHD mortality in all internal alpha-dose categories compared with the reference category (<0.01 Gy) for both the whole cohort and the subcohort restricted to Ozyorsk residents.
We observed a significant linear association of IHD mortality with total absorbed dose from internal alpha radiation to the liver for both data sets with ERRs/Gy 0.30 (95% CI: 0.10 to 0.59) and 1.22 (95% CI: 0.51 to 2.19). ERR/Gy for the full data set increased with the increasing lag period, whereas for the dose-restricted data set, the risk increased with the increasing lag period up to 15 years and decreased with the further increasing lags and lost its statistical significance with 25- and 30-year lag periods imposed.
The finding was unaffected either by exclusion of adjustments for smoking and alcohol consumption or by inclusion of an adjustment for duration of employment. After having adjusted for hypertension and BMI, increased ERRs/Gy were observed in both data sets while the adjustment for smoking index (rather than smoking status) produced lower risk estimates in both data sets; however, the risk was still statistically significant. Restriction of the data set to workers employed at the Mayak PA longer than 1 year had no effect on ERR/Gy estimate for IHD incidence.
Inclusion of an adjustment for dose from external γ-rays resulted in decrease of ERRs/Gy for IHD mortality in both data sets [ERR/Gy = 0.21 (95% CI: 0.01 to 0.58) and ERR/Gy = 1.08 (95% CI: 0.34 to 2.15)]. The similar result was observed for the subcohort restricted to Ozyorsk residents, but the risk remained significant when estimated for the dose-restricted data set and when the full data set was used the risk could not be defined [ERR/Gy = 1.05 (95% CI: 0.30 to 2.15) and ERR/Gy = 0.20 (95% CI: NA to 0.59)].
The ERR/Gy for IHD mortality was higher for workers of the radiochemical plant than for those employed at the plutonium production plant, but tests for heterogeneity did not reveal significant differences with both analysed data sets (p > 0.5 and p=0.22). Sex-specific analysis of IHD mortality risk using the full data set showed that ERR/Gy values were approximately equal (p > 0.5) and significant for both sexes. However, with the dose-restricted data set used in the analysis, a significantly raised ERR/Gy was observed only in males (but not in females); these differences were statistically significant (p = 0.013). ERR/Gy changed significantly with the attained age category in both data sets (p = 0.016 and p = 0.001, respectively).
The test for non-linearity based on the comparison between the linear and LQ dose–response models was not statistically significant (p > 0.5); moreover, the linear model provided the better fit of the data than the pure quadratic one (the difference in Akaike information criterion statistics was 2.18). However, the LE model fitted the data better than the pure linear one (p = 0.003). The linear piecewise model provided the better data fit than the linear model with the knot below 0.25 Gy.
Internal alpha radiation: incidence
The results of the analysis of the RRs of IHD incidence by categories of the total absorbed internal alpha dose to the liver as well as ERRs/Gy for the entire cohort (full data set) and a subcohort restricted to workers exposed at total internal alpha doses <1.00 Gy (dose-restricted data set) are presented in Tables 8 and 9.
Table 8.
Relative risks (RRs) of ischaemic heart disease incidence for various categories of total absorbed dose from internal alpha radiation to the liver (0-year lag period)
| Total absorbed alpha dose in the liver (Gy) | Mean total absorbed alpha dose in the liver (Gy) |
Person-years at risk |
Cases |
RR (95% confidence interval) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Males | Females | Both | Males | Females | Both | Males | Females | Both | Males | Females | Both | |
| 0–0.025 | 0.007 | 0.007 | 0.007 | 94,719 | 40,737 | 135,456 | 1003 | 449 | 1452 | 1 | 1 | 1 |
| 0.025–0.05 | 0.035 | 0.036 | 0.036 | 18,587 | 8646 | 27,233 | 481 | 205 | 686 | 1.04 (0.91 to 1.18) | 1.03 (0.82 to 1.29) | 1.03 (0.92 to 1.16) |
| 0.05–0.1 | 0.071 | 0.072 | 0.071 | 13,427 | 6929 | 20,356 | 400 | 192 | 592 | 1.00 (0.87 to 1.15) | 1.24 (0.97 to 1.59) | 1.06 (0.94 to 1.20) |
| 0.1–0.25 | 0.156 | 0.158 | 0.157 | 11,599 | 6837 | 18,436 | 429 | 241 | 670 | 1.02 (0.88 to 1.18) | 1.38 (1.09 to 1.77) | 1.11 (0.98 to 1.26) |
| 0.25–0.50 | 0.347 | 0.342 | 0.345 | 4459 | 2471 | 6930 | 204 | 107 | 311 | 1.21 (0.99 to 1.47) | 1.14 (0.83 to 1.56) | 1.18 (0.99 to 1.38) |
| 0.50–1.00 | 0.687 | 0.699 | 0.691 | 2166 | 1149 | 3314 | 104 | 36 | 140 | 1.38 (1.06 to 1.77) | 0.90 (0.56 to 1.42) | 1.23 (0.98 to 1.53) |
| ≥1.00 | 2.350 | 4.176 | 3.234 | 1666 | 1570 | 3237 | 96 | 51 | 147 | 1.53 (1.16 to 2.02) | 1.08 (0.68 to 1.68) | 1.37 (1.08 to 1.74) |
Table 9.
Ischaemic heart disease incidence: analyses in relation to total absorbed dose from internal alpha radiation to the liver, excess relative risk per unit dose (ERR/Gy) (95% confidence interval)
| Analyses | Whole dose range |
Doses < 1.00 Gy |
||||
|---|---|---|---|---|---|---|
| Males | Females | Both | Males | Females | Both | |
| Main analysis, 0-year lag | 0.20 (0.07 to 0.36) | 0.02 (−0.02 to 0.09) | 0.07 (0.01 to 0.14) | 0.57 (0.21 to 0.98) | 0.05 (−0.39 to 0.63) | 0.42 (0.13 to 0.75) |
| P1 = 0.015 |
P1 = 0.129 |
|||||
| Main analyses, in which the first x years following the start of radiation work were assigned to a “zero-dose” category when lagging doses by x years | ||||||
| Main analysis, 5-year lag | 0.23 (0.08 to 0.43) | 0.03 (−0.02 to 0.11) | 0.08 (0.01 to 0.17) | 0.70 (0.26 to 1.22) | 0.09 (−0.44 to 0.78) | 0.51 (0.16 to 0.92) |
| Main analysis, 10-year lag | 0.27 (0.08 to 0.52) | 0.04 (−0.03 to 0.15) | 0.10 (0.01 to 0.22) | 0.85 (0.29 to 1.52) | 0.12 (−0.51 to 0.97) | 0.62 (0.17 to 1.13) |
| Main analysis, 15-year lag | 0.35 (0.09 to 0.67) | 0.05 (NA to 0.22) | 0.14 (0.02 to 0.30) | 1.05 (0.32 to 1.95) | 0.22 (−0.58 to 1.29) | 0.76 (0.19 to 1.43) |
| Main analysis, 20-year lag | 0.46 (0.10 to 0.92) | 0.06 (NA to 0.29) | 0.18 (0.01 to 0.40) | 1.24 (0.23 to 2.47) | 0.40 (−0.63 to 1.84) | 0.91 (0.15 to 1.83) |
| Main analysis, 25-year lag | 0.66 (0.13 to 1.41) | 0.06 (NA to 0.42) | 0.24 (−0.01 to 0.59) | 1.28 (−0.11 to 3.06) | 0.81 (−0.64 to 2.88) | 1.09 (0.03 to 2.39) |
| Main analysis, 30-year lag | 0.94 (0.10 to 2.20) | −0.09 (NA to 0.42) | 0.20 (NA to 0.72) | 1.00 (−0.88 to 3.61) | 1.47 (−0.69 to 4.68) | 1.21 (−0.28 to 3.15) |
| Main analysis but unadjusted for smoking and alcohol consumption, 0-year lag | 0.19 (0.07 to 0.35) | 0.02 (−0.02 to 0.08) | 0.06 (0.01 to 0.13) | 0.53 (0.19 to 0.93) | 0.04 (−0.38 to 0.58) | 0.38 (0.11 to 0.70) |
| Adding to stratification (0-year lag) | ||||||
| Hypertension | 0.22 (0.08 to 0.39) | 0.02 (−0.02 to 0.10) | 0.07 (0.01 to 0.16) | 0.60 (0.23 to 1.03) | 0.14 (−0.35 to 0.80) | 0.47 (0.17 to 0.83) |
| Body mass index | 0.20 (0.06 to 0.37) | 0.02 (−0.02 to 0.10) | 0.07 (0.01 to 0.15) | 0.55 (0.18 to 0.98) | 0.13 (−0.36 to 0.78) | 0.43 (0.13 to 0.79) |
| Employment duration | 0.21 (0.07 to 0.38) | 0.03 (−0.02 to 0.11) | 0.08 (0.02 to 0.17) | 0.57 (0.20 to 1.00) | 0.01 (−0.44 to 0.61) | 0.41 (0.11 to 0.75) |
| Also adjusting for external gamma dose | 0.14 (<0 to 0.33) | <0 (NA to 0.06) | 0.02 (NA to 0.10) | 0.71 (0.27 to 1.24) | −0.23 (−0.67 to 0.40) | 0.44 (0.09 to 0.85) |
| Also adjusting for smoking index | 0.21 (0.07 to 0.38) | 0.02 (−0.02 to 0.09) | 0.07 (0.01 to 0.15) | 0.53 (0.17 to 0.96) | 0.05 (−0.39 to 0.64) | 0.39 (0.10 to 0.73) |
| Main analysis, restricted employment duration >1 year | 0.19 (0.06 to 0.35) | 0.02 (−0.02 to 0.09) | 0.07 (0.01 to 0.14) | 0.54 (0.19 to 0.95) | 0.06 (−0.38 to 0.65) | 0.40 (0.12 to 0.73) |
| Analyses (0-year lag) restricted to workers at | ||||||
| Radiochemical plant | 0.11 (−0.02 to 0.29) | −0.12 (NA to 0.06) | 0.04 (−0.04 to 0.16) | 0.51 (0.08 to 1.05) | 0.28 (−0.38 to 1.22) | 0.46 (0.08 to 0.91) |
| Plutonium plant | 0.38 (0.13 to 0.70) | 0.04 (−0.01 to 0.13) | 0.08 (0.01 to 0.19) | 0.66 (0.10 to 1.38) | −0.18 (−0.69 to 0.59) | 0.36 (−0.06 to 0.89) |
| P2 = 0.08 | P2 = 0.09 | P2 > 0.50 | P2 ≥ 0.50 | P2 = 0.36 | P2 > 0.50 | |
| Attained age (0-year lag) (years) | ||||||
| <50 | 0.1 (NA to 0.40) | >0 (NA to 0.11) | 0.01 (NA to 0.13) | 0.73 (0.04 to 1.66) | 0.23 (NA to 2.53) | 0.66 (0.02 to 1.48) |
| 50–59 | 0.22 (0.04 to 0.49) | 0.07 (−0.01 to 0.22) | 0.12 (0.02 to 0.26) | 0.73 (0.17 to 1.46) | −0.34 (−0.88 to 0.55) | 0.44 (−0.01 to 1.01) |
| 60–69 | 0.32 (0.04 to 0.74) | −0.05 (NA to 0.18) | 0.11 (−0.03 to 0.33) | 0.36 (−0.21 to 1.13) | 0.61 (−0.27 to 1.97) | 0.43 (−0.07 to 1.08) |
| ≥70 | −0.18 (NA to 0.83) | −0.02 (NA to 0.31) | −0.04 (NA to 0.26) | −0.06 to 1.48) | −0.16 (NA to 1.11) | −0.12 (NA to 0.77) |
| P3 > 0.50 | P3 > 0.50 | P3 = 0.39 | P3 > 0.50 | P3 = 0.47 | P3 > 0.50 | |
| P4 > 0.50 | P4 > 0.50 | P4 > 0.50 | P4 = 0.19 | P4 > 0.50 | P4 = 0.19 | |
| Calendar period | ||||||
| ≤1960 | 0.72 (NA to 2.42) | — | 0.27 (NA to 1.53) | 1.35 (−0.57 to 5.17) | — | 0.88 (−0.76 to 4.17) |
| 1961–2008 | 0.19 (0.06 to 0.35) | 0.02 (−0.02 to 0.10) | 0.07 (0.01 to 0.14) | 0.54 (0.19 to 0.96) | 0.07 (−0.38 to 0.66) | 0.41 (0.12 to 0.74) |
| P5 = 0.29 | P5 = NA | P5 > 0.50 | P5 > 0.50 | P5 = NA | P5 > 0.50 | |
| Validity > 0 | 0.20 (0.03 to 0.43) | <0 (NA to 0.07) | 0.02 (NA to 0.12) | 0.66 (0.20 to 1.24) | −0.23 (−0.71 to 0.53) | 0.40 (0.04 to 0.86) |
| Validity = 1 | 0.39 (0.07 to 0.95) | −0.02 (NA to 0.04) | <0 (NA to 0.25) | 1.40 (0.41 to 3.00) | — | 1.80 (0.70 to 3.59) |
NA, not available; P1, test for heterogeneity between males and females; P2, test for heterogeneity between radiochemical and plutonium plant workers; P3, test for heterogeneity between groups of workers of different attained age; P4, test for a log-linear trend in the ERR/Gy with attained age; P5, test for heterogeneity between groups of calendar period.
The categorical analysis revealed significantly raised RRs of IHD incidence in males exposed to internal alpha radiation at total absorbed doses to the liver 0.50–1.00 Gy and >1.00 Gy and in females exposed at doses 0.10–0.25 Gy compared with the reference category (0–0.025 Gy).
A large significant difference was found in ERR/Gy estimates for IHD incidence between males and females for the full data set. Hence, the full set of analyses was repeated for each sex separately.
In females, significant ERRs/Gy for IHD incidence in relation to internal alpha radiation were revealed in neither the full nor the dose-restricted data sets.
In males, the main dose–response analysis showed strongly significant differences in ERR/Gy values for IHD incidence between the full and dose-restricted data sets [ERR/Gy = 0.20 (95% CI: 0.07 to 0.36) and ERR/Gy = 0.57 (95% CI: 0.21 to 0.98), respectively, p < 0.001]. ERRs/Gy increased with the increasing lag period (except for 25- and 30-year lags with the dose-restricted data set analysed) but were unaffected by additional adjustments for smoking index (rather than smoking status), BMI, hypertension or employment duration. However, when an additional adjustment for dose from external γ-rays was included, the ERR/Gy for IHD incidence in male workers decreased in the full data set [ERR/Gy = 0.14 (95% CI: <0 to 0.33)] and lost its statistical significance whereas in the restricted data set this estimate significantly increased [ERR/Gy = 0.71 (95% CI: 0.27 to 1.24)]. The IHD incidence risk estimates for males did not vary significantly among the facilities (p = 0.08 and p > 0.5), categories of attained age (p > 0.5 and p > 0.5) or calendar periods of diagnosis (p = 0.29 and p > 0.5) in either of the data sets.
The test for non-linearity based on the comparison between the linear and non-linear dose–response models was statistically significant for males (p = 0.01 for LQ and p < 0.001 for LE models, the difference in Akaike information criterion statistics between the linear and pure quadratic models was 10.50). In the previous study,11 it was shown that the non-linear dose–response could be associated with uncertainties in internal alpha-radiation doses, in particular, with the use of below LOD measurements of plutonium activity in urine bioassays for dose estimation. For this reason to assess the effect of alpha-dose validity on IHD incidence, a set of additional analyses was performed (including the adjustment for external γ-ray dose). Exclusion of workers with all measurements of plutonium activity in the urine bioassays below LOD (analysis restricted to workers with validity >0) modified the results only slightly. But, the restriction of the analysis to only include workers with no measurements that were below LOD (validity = 1) resulted in a two-fold increase (as compared with the main analysis) of the ERR/Gy estimates for IHD incidence in males in both the full and dose-restricted data sets [ERR/Gy = 0.39 (95% CI: 0.07 to 0.95) and ERR/Gy = 1.40 (95% CI 0.41 to 3.00), respectively] (Table 9).
The results of two more detailed categorical analyses based on the subcohort of workers exposed to internal alpha radiation to the liver at total absorbed dose <1.00 Gy and incorporating additional adjustment for external γ-ray dose are presented in Table 10. For the first one, no validity of internal dose was taken into account; the second one was based on a subcohort of workers with validity = 1.0. Figure 5 demonstrates how the results of the categorical analysis agree with the results of the dose–response analysis based on the linear model (Table 9). As Figure 5 shows the validity of the internal alpha dose to the liver had a strong effect on the dose response for IHD incidence in males.
Table 10.
Relative risks (RRs) of ischaemic heart disease incidence in males for various categories of total absorbed liver dose from internal alpha radiation accounting for the validity of dose estimates
| Total absorbed alpha dose in the liver (Gy) | Mean total absorbed alpha in the liver (Gy) |
Person-years at risk |
Cases |
RR (95% confidence interval) |
||||
|---|---|---|---|---|---|---|---|---|
| 1a | 2b | 1 | 2 | 1 | 2 | 1 | 2 | |
| 0–0.025 | 0.007 | 0.009 | 94,716 | 9761 | 1003 | 61 | 1 | 1 |
| 0.025–0.05 | 0.035 | 0.036 | 18,586 | 3865 | 482 | 50 | 1.05 (0.92 to 1.20) | 1.24 (0.71 to 2.22) |
| 0.05–0.1 | 0.071 | 0.073 | 13,427 | 4299 | 400 | 71 | 1.03 (0.89 to 1.19) | 0.81 (0.47 to 1.44) |
| 0.1–0.25 | 0.156 | 0.161 | 11,598 | 5577 | 428 | 157 | 1.03 (0.89 to 1.20) | 0.95 (0.57 to 1.64) |
| 0.25–0.5 | 0.347 | 0.350 | 4459 | 2864 | 204 | 118 | 1.22 (1.00 to 1.49) | 1.33 (0.78 to 2.34) |
| 0.5–1.0 | 0.687 | 0.696 | 2165 | 1584 | 104 | 62 | 1.40 (1.08 to 1.80) | 1.59 (0.88 to 2.96) |
Workers monitored for internal alpha radiation without taking into account the validity of dose estimates.
Workers monitored for internal alpha radiation with valid dose estimates (validity = 1).
Figure 5.
Ischaemic heart disease (IHD) incidence for males in relation to total absorbed alpha dose in the liver in workers exposed to internal alpha radiation at doses <1.00 Gy without taking into account (a) and taking into account (b) the validity of internal dose estimates. CI, confidence interval; ERR/Gy, excess relative risk per unit dose.
DISCUSSION
This study of IHD incidence and mortality was carried out for the extended cohort of Mayak PA workers. The previous cohort14 was extended via the inclusion of workers first employed at the Mayak PA between 1973 and 1982 (3521 individuals) who had been exposed over a prolonged period at lower radiation dose rates than workers first employed earlier, particularly in 1948–58.7 This in addition to the extension of the follow-up (for 3 years) enabled the statistical power of the study to be increased in the low-dose region (100–200 mGy). The overall number of IHD cases in the extended cohort increased by 1006 and IHD deaths increased by 291; however, the mortality study was still characterized with lower statistical power than the incidence analyses (7225 cases vs 2848 deaths).
Other studies of circulatory diseases including IHD and cerebrovascular diseases showed that atherosclerosis which is the underlying health condition for their development is a multifactorial disease and may develop owing to a number of internal and external factors. As it is expected, non-radiation factors (sex, attained age, smoking, alcohol consumption, hypertension, BMI etc.) are important factors influencing IHD incidence and mortality in the study cohort. The results of risk analysis in relation to non-radiation factors were similar to result of the previous study.14 However, this was the first analysis of IHD in this cohort for which qualitative smoking information (smoking index) was used. The findings revealed a clear pattern of significantly increasing IHD incidence and mortality risks with increasing smoking index.
Comparison with previous studies of the Mayak Worker cohort
Comparison between the present study findings and those from previous studies9,14 of IHD incidence and mortality are presented in Table 11.
Table 11.
Comparison of the results of the current and previous studies of the Mayak worker cohort, excess relative risk per unit dose (95% confidence interval)
| Effect | Cohort: period of first employment and dosimetry system | Cohort (full/dose-restricted) | External dose |
Internal dose |
||
|---|---|---|---|---|---|---|
| 1a | 2b | 1 | 2 | |||
| Incidence | 1948–1972, D-20059 | Full | 0.10 (0.05 to 0.15) | 0.09 (0.03 to 0.15) | 0.01 (−0.01 to 0.03) | 0.02 (−0.02 to 0.05) |
| 1948–1972, MWDS-200814 | Full | 0.15 (0.08 to 0.21) | 0.12 (0.05 to 0.18) | 0.03 (−0.02 to 0.08) | −0.001 (−0.05 to 0.05) | |
| 1948–1982, MWDS-2008 (this study) | Full | 0.14 (0.09 to 0.21) | 0.10 (0.04 to 0.17) | 0.07 (0.01 to 0.14) | 0.02 (NA to 0.10) | |
| Restricted | 0.15 (0.09 to 0.21) | 0.11 (0.05 to 0.19) | 0.42 (0.13 to 0.75) | 0.44 (0.09 to 0.85) | ||
| Mortality | 1948–1972, D-20059 | Full | 0.06 (−0.01 to 0.13) | 0.10 (−0.02 to 0.21) | 0.26 (0.07 to 0.46) | 0.10 (−0.09 to 0.29) |
| 1948–1972, MWDS-200814 | Full | 0.03 (−0.04 to 0.10) | 0.17 (0.02 to 0.31) | 0.34 (0.07 to 0.60) | 0.29 (−0.05 to 0.63) | |
| 1948–1982, MWDS-2008 (this study) | Full | 0.05 (−0.02 to 0.12) | 0.19 (0.07 to 0.36) | 0.30 (0.10 to 0.59) | 0.2 (NA to 0.59) | |
| Restricted | 0.07 (−0.01 to 0.15) | 0.21 (0.08 to 0.39) | 1.22 (0.51 to 2.19) | 1.05 (0.30 to 2.15) | ||
D-2005, dosimetry system “Doses-2005”; MWDS-2008, “Mayak Worker Dosimetry System—2008”; NA, not available.
Without adjustment for dose from internal alpha radiation when analysing external γ-ray and vice versa.
After having adjusted for dose from internal alpha radiation when analysing external γ-ray and vice versa (for mortality analysis the cohort was restricted to Ozyorsk residents).
As in previous studies,9,14 a significant increasing linear trend in IHD incidence with dose from external γ-rays was observed after having adjusted for non-radiation factors and dose from internal radiation.
With regard to IHD mortality, the findings of the present study agree well with results of the previous study14 based on MWDS-2008 dose estimates; in particular, the increasing linear trend in IHD mortality with increasing dose from external γ-rays was observed after inclusion of adjustments for non-radiation factors and dose from internal alpha radiation in the subcohort restricted to Ozyorsk residents. When an adjustment for dose from internal alpha radiation was excluded, the ERR/Gy decreased and lost statistical significance. However, the findings of both the present and the previous studies based on MWDS-200814 differed from those obtained in a study based on Doses-2005 estimates9 which did not reveal any association of IHD mortality with external gamma dose either including an adjustment for internal dose or not.
Results of the current analysis of IHD incidence associated with internal alpha radiation agree well with results of previous studies9,14 (including adjustments for non-radiation factors and dose from external γ-rays). When the adjustment for the external gamma dose was excluded, the results did not agree with those from previous studies9,14 (after exclusion of the adjustment for gamma dose, ERR/Gy increased and became significant (in the present study).
ERRs/Gy for IHD mortality in relation to internal alpha radiation estimated during this study are in good agreement with the corresponding estimates from previous studies.9,14
The differences in results between the current and previous studies9,14 of the Mayak worker cohort are likely owing to the following reasons: changes in biokinetic and dosimetry models used for the new dosimetry system MWDS-2008; taking into account below LOD measurements of plutonium activity in urine bioassays for estimation of doses from internal alpha radiation and statistical power of the study.
The analysis of IHD incidence associated with both external and internal radiations in the extended cohort showed significant differences in RRs and ERRs/Gy between males and females. ERRs/Gy for IHD incidence estimated separately for males and females in the current and previous studies are almost similar. ERRs/Gy were considerably higher in males than in females and made a prevalent contribution to risk estimates for the whole study cohort. Presumably, these differences were caused mostly by different statistical power of sex-specific analyses (since females comprise 25% of the cohort members).
Differences between incidence and mortality from IHD revealed in the extended Mayak worker cohort in terms of external exposure are primarily owing to the fact that not each of the IHD cases is followed by fatal sequelae and death. An analysis of fatal IHD cases (acute myocardial infarction and acute coronary failure) is planned for future after extension of the follow-up period and increase of statistical power of the study. Analysis of IHD risk in relation to internal alpha radiation in the extended cohort demonstrated that ERR/Gy was higher for mortality than for incidence as for the full cohort as the subcohort restricted was to the Ozyorsk residents. There are likely some other reasons for differences between these risks about which we know nothing at present and which are to be investigated in future.
The present study was the first one to analyse IHD incidence and mortality associated with internal radiation using a subcohort of workers exposed at total absorbed doses to the liver <1.00 Gy. These additional analyses were carried out to assess the contribution of high doses of radiation which could potentially induce deterministic effects of the prolonged exposure (chronic radiation syndrome and plutonium-induced lung fibrosis) to IHD mortality risks.24 This analysis both with and without the adjustment for dose from external γ-rays revealed a significant increasing linear trend in both incidence and mortality from IHD with dose from internal alpha radiation. But, ERR/Gy for the subcohort of workers exposed to internal alpha radiation at dose <1.00 Gy was much higher than for the entire cohort, which is apparently primarily owing to significant uncertainties of internal alpha doses, particularly within the low-dose range.
As noted above, the validity of dose estimates for internal alpha radiation had a significant effect on the IHD incidence associated with internal radiation using both the full and dose-restricted data sets. Since plutonium activity in urine bioassays was measured using alpha-radiometric methods during early years of Mayak PA operation, the LOD was rather high, and as a result, the considerable proportion of plutonium activity was impossible to measure. Later, alpha spectrometry came into use and the LOD remarkably decreased. Internal alpha doses based on plutonium activity measurements in urine bioassays below LOD to which point estimates equal to half value of the LOD had been assigned could underestimate or overestimate the true radiation dose and subsequently affect the risk estimate.
Also, it should be noted that results of the present study showed that additional adjustments for non-radiation factors such as smoking, alcohol consumption, hypertension and increased body weight affected the ERR/Gy estimate for the Mayak workers cohort.
Previously, analysis of chronic bronchitis (CB) in the Mayak worker cohort10 demonstrated increased incidence rates in the first decade of the follow-up period. Establishment of CB diagnosis is a complicated process; therefore, long-lasting and thorough monitoring is required to register a final diagnosis, that is why during the first years, in fact, pre-existing CB cases were registered (actually CB prevalence was fixed). With regard to IHD, the results of the analysis demonstrated that ERRs/Gy estimated for pre and post 1960 periods did not differ significantly, indicating that this was not an issue of concerns for the results of this study.
Comparison with other studies
Table 12 presents a comparison of the results of the present study of IHD incidence and mortality in the Mayak cohort with those from other studies which report about ERR estimates per unit dose for IHD.
Table 12.
Estimates of the excess relative risk per unit dose (ERR/Gy) of ischaemic heart disease following exposure to external low-linear energy transfer radiation
| Cohort | Mean total dose (Gy) | Mortality or incidence | Lag period (years) | No. of deaths or cases | ERR/Gy |
|---|---|---|---|---|---|
| Japanese A-bomb survivors: Life Span Study26,a | 0.20b | Mortality | 5 | 4477 | 0.17 (90% CI: 0.08 to 0.26) |
| Japanese A-bomb survivors: Life Span Study27,c | 0.20 | Mortality | 5 | 3252 | 0.02d (95% CI: −0.10 to 0.15) |
| Japanese A-bomb survivors: Adult Health Study28 | 0.57e | Incidence | 13 | 1546 | 0.05f (95% CI: −0.05 to 0.16) |
| Nuclear workers (international)29 | 0.018g | Mortality | 10 | 5821 | −0.01 (95% CI: −0.59 to 0.69) |
| BNFL workers (United Kingdom)30 | 0.053 | Mortality | 15 | 3567 | 0.70h (90% CI: 0.33 to 1.11) |
| UK National Registry for Radiation Workers31 | 0.025 | Mortality | 10 | 7168 | 0.26 (95% CI: −0.05 to 0.61) |
| Chernobyl recovery operations workers32 | 0.109 | Incidence | —i | 10,942 | 0.41(95% CI: 0.05 to 0.78) |
| 0.20j (95% CI: −0.23 to 0.63) | |||||
| Mayak Workers Cohort12 | 0.62k | Incidence | 0 | 3888 | 0.10 (95% CI: 0.04 to 0.18) |
| Mayak Workers Cohort12 | 0.62k | Mortality | 0 | 2083 | 0.08 (95% CI: 0.02 to 0.15) |
| French combined cohort of nuclear workers33 | 0.0225 | Mortality | 10 | 583 | 0.71 (95% CI: −1.20 to 3.18)l |
| Canadian Fluoroscopy Cohort34 | 0.79m | Mortality | 10 | 5818 | 0.007 (95% CI: −0.044 to 0.072) |
BNFL, British Nuclear Fuels; CI, confidence interval.
Values given are for deaths from ninth revision of International Classification of Diseases (ICD-9) codes 390–429.
Weighted sum of gamma and neutron doses to the colon among survivors with doses of ≥0.005 Sv.
Values given are for deaths from ICD-9 codes 410–414 during 1950–2003.
Not adjusted for smoking or alcohol.
Weighted sum of gamma and neutron shielded kerma doses. Doses to the Japanese A-bomb survivors arose predominantly from γ-radiation.
Adjusted for smoking and alcohol.
Dose to the lung.
Based on underlying cause of death. Results based on underlying and contributory causes combined are similar to these.
Not cited in article.
Values given are for deaths from ICD-9 codes 414.0.
For males with adjustment for dose from internal alpha radiation.
Adjusted for sex, age, calendar year, duration of employment and social economic status, 90% CI.
Cumulative person-time-weighted lung dose.
ERRs/Gy for IHD mortality in relation to external γ-rays adjusted for dose from internal alpha radiation found in the present study are in good agreement with results from the Life Span Study (LSS) cohort.26 Meanwhile, risks estimated in the present study without inclusion of the adjustment for dose from internal radiation were markedly lower showing an agreement with results of another LSS study.27 A significant increased ERR/Gy for IHD mortality was found in the cohort of British Nuclear Fuels (BNFL) workers,30 but the risk estimate was several times higher than the risk estimated in the present study of Mayak workers. No significant associations of IHD mortality with dose from external γ-rays were revealed in other studies.29,31,33,34
ERR/Gy for IHD incidence in relation to external γ-rays found in the present study does not agree with the results from the LSS cohort.28 Meanwhile, ERR/Gy for IHD incidence in males [ERR/Gy = 0.14 (95% CI: 0.07 to 0.23)] is in good agreement with the results from the Chernobyl clean-up worker cohort (males);32 IHD incidence risk for Mayak workers was about three times smaller than ERR/Gy shown in this study but closer to ERR/Gy for chronic IHD incidence (ICD-9 code: 414.0) [ERR/Gy = 0.20 (95% CI: –0.23 to 0.63)]; however, the latter estimate was statistically insignificant.
Comparison between the results of the present study and those reported by Simonetto et al12 who used another methodology to perform analyses demonstrated an agreement of ERR/Gy dose for both IHD incidence and mortality [ERR/Gy = 0.14 (95% CI: 0.07 to 0.23) and ERR/Gy = 0.10 (95% CI: 0.04 to 0.18), respectively] associated with dose from external γ-rays among males (with the adjustment for dose from internal alpha radiation) [ERR/Gy = 0.16 (95% CI: 0.03 to 0.30) for residents and ERR/Gy = 0.08 (95% CI: 0.02 to 0.15), respectively].
No studies of other populations exposed to internal alpha radiation are available at present to perform the comparison between risks of IHD.
Strengths and weaknesses of the study
The main advantage of the present study of Mayak workers is available information on both incidence (for 96% of cohort members) and mortality (96% of the deceased). All workers of the study cohort underwent a pre-employment medical examination providing an assessment of the initial health status. Regular health surveillance of the cohort members continued during subsequent years (>60) until a date of death or migration from Ozyorsk. Information on main non-radiation factors affecting IHD incidence and mortality was collected for the majority of the cohort members (93%).
Furthermore, the primary data and the medical and dosimetry information in the “Clinic” database are regularly verified,16 and additional quality assurance (QA) checks were performed on the data selected for this analysis. The error rate for all types of QA checks on the cohort was <5% indicating the high quality of the data. Availability of all the primary medical records, detailed disease histories, autopsy protocols stored in the uniform archive enables the verification of the diseases and causes of deaths of interest. In particular, owing to the high proportion (53%) of autopsies performed on workers who had died in Ozyorsk, it was possible to verify the clinical diagnoses based on the pathological examinations. A low level of disagreement between the two was found (<2%).
One of the advantages of the Mayak worker cohort is availability of individually measured annual doses from external γ-rays for almost each of the cohort member (99.5%).
On the other hand, the study has some weaknesses arising from the lack of measurements of plutonium alpha activity in bioassays for the majority of workers (70%) and from uncertainties of the available measurements as well as those parameters which were considered by biokinetic and dosimetry models definitely causing large uncertainties of estimates of absorbed doses from internal alpha radiation owing to incorporated plutonium. However, the Mayak worker dosimetry system is being updated and improved regularly. As planned, in case new doses from internal alpha radiation appear in the future (MWDS-2013), risk estimates are to be clarified.
Also, owing to new Russian data privacy laws, there is now no facility to effectively follow up workers who leave Ozyorsk (migrants), although this has only had a minor effect on the results of these analyses since the loss of mortality follow-up among migrants was only 3 years and the cohort migration rate is generally declining, and between 2006 and 2008, it did not exceed 0.25%.
Mechanisms of circulatory diseases
The overwhelming majority of IHD included in the current analysis were owing to coronary atherosclerosis (ICD-9: 414.0). Atherosclerosis is a systemic process with a long development time. It is a multifactorial disease, which appears as a result of interrelation between genetic, biological and environmental factors. Ionizing radiation can be considered as one of the environmental factors leading to and/or accelerating development of atherosclerosis in different blood vessels (coronary, cerebral, kidney, extremities arteries etc.)
Current knowledge on how the biological mechanisms of atherosclerosis could be affected by exposure to high doses of radiation is based on experimental studies and studies in cohorts of patients exposed for diagnostic and therapeutic purposes. It has been shown that mechanisms such as endothelial dysfunction, inflammation, oxidative stress, changes in coagulation and platelet activity, DNA damages, cell senescence and cell killing may play a role in development of radiation-induced cardiovascular effects at doses >2 Sv. The effect of low-to-moderate doses of radiation on the mechanisms of atherosclerosis is still unclear, although, the recent scientific literature35,36 contains numerous studies of the mechanisms of atherosclerosis at these dose levels. In reviews of these studies, Little et al35,36 and Borghini et al37 have hypothesized that the biological mechanisms of radiation-induced effects at moderate and low doses differ from those occurring at high doses (e.g., radiation therapy). In addition, recently published results37,38 showed that radiation exposure may cause premature cell senescence.
CONCLUSION
Risk analysis in the extended Mayak PA worker cohort demonstrated a significant increasing linear trend in IHD incidence with total dose from external γ-rays after having adjusted for non-radiation factors and dose from internal radiation [ERR/Gy = 0.10 (95% CI: 0.04 to 0.17)]. ERR/Gy for IHD incidence in males was 6 times higher than in females (p = 0.02). The pure quadratic model provided a better fit of the data than the linear one.
No significant association of IHD mortality with total dose from external γ-rays after having adjusted for non-radiation factors and dose from internal alpha radiation was observed in the study cohort [ERR/Gy = 0.06 (95% CI: <0 to 0.15)]. A significant increasing linear trend in IHD mortality with dose from external γ-rays was observed after having adjusted for non-radiation factors and dose from internal alpha radiation only in the subcohort of workers restricted to Ozyorsk residents [ERR/Gy = 0.19 (95% CI: 0.07 to 0.36)].
No association of IHD incidence with total absorbed dose from internal alpha radiation to the liver was found in the whole cohort (full data set) after having adjusted for non-radiation factors and external gamma dose [ERR/Gy = 0.02 (95% CI: NA to 0.10)]. Statistically significant dose effect was revealed in the subcohort of workers exposed to internal alpha radiation at dose to the liver <1.00 Gy [ERR/Gy = 0.44 (95% CI; 0.09 to 0.85)]. The association of IHD incidence with total absorbed dose from internal alpha radiation to the liver was found to be highly sensitive to “validity” of dose from internal alpha radiation to the liver.
A significant increasing linear trend was observed in IHD mortality with total absorbed dose from internal alpha radiation to the liver after having adjusted for non-radiation factors and dose from external γ-rays in both the whole cohort [ERR/Gy = 0.21 (95% CI: 0.01 to 0.58)] and the subcohort of workers exposed at alpha dose <1.00 Gy [ERR/Gy = 1.08 (95% CI: 0.34 to 2.15)].
The results of the present study agree with findings of the previous studies of the Mayak PA worker and LSS cohorts as well as with the results of other studies.9,14
Acknowledgments
ACKNOWLEDGMENTS
The authors thank the Federal State Unitary Enterprise Mayak PA and SUBI Internal Dosimetry Department for providing access to MWDS-2008 database created in the framework of Russian-American collaboration; SUBI Laboratory of Epidemiology for providing access to Medical and Dosimetry Register of Mayak.
Contributor Information
Tamara V Azizova, Email: clinic@subi.su.
Evgeniya S Grigoryeva, Email: es_grig@mail.ru.
Richard G E Haylock, Email: Richard.Haylock@phe.gov.uk.
Maria V Pikulina, Email: clinic@subi.su.
Maria B Moseeva, Email: clinic@subi.su.
FUNDING
The research leading to these results has received funding from the European Union Seventh Framework Program and Federal Medical Biological Agency of Russia under Grant Agreement No. 249675 “Epidemiological Studies of Exposed Southern Urals Populations (SOLO)”.
REFERENCES
- 1.Koshurnikova NA, Buldakov LA, Bysogolov GD, Bolotnikova MG, Komleva NS, Peternikova VS. Mortality from malignancies of the hematopoietic and lymphatic tissues among personnel of the first nuclear plant in the USSR. Sci Total Environ 1994; 142: 19−23. [DOI] [PubMed] [Google Scholar]
- 2.Tokarskaya ZB, Okladnikova ND, Belyaeva ZD, Drozhko EG. Multifactorial analysis of lung cancer dose-response relationship for workers at the Mayak nuclear enterprise. Health Phys 1997; 69: 356−66. [DOI] [PubMed] [Google Scholar]
- 3.Shilnikova NS, Preston DL, Ron E, Gilbert ES, Vasilenko EK, Romanov SA, et al. Cancer mortality risk among workers at the Mayak nuclear complex. Radiat Res 2003; 159: 787−98. [DOI] [PubMed] [Google Scholar]
- 4.Sokolnikov ME, Gilbert ES, Preston DL, Ron E, Shilnikova NS, Khokhryakov VV, et al. Lung, liver and bone cancer mortality in Mayak workers. Int J Cancer 2008; 123: 905−11. doi: 10.1002/ijc.23581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gilbert ES, Sokolnikov ME, Preston DL, Schonfeld SJ, Schadilov AE, Vasilenko EK, et al. Lung cancer risks from plutonium: an updated analysis of data from the Mayak worker cohort. Radiat Res 2013; 179: 332−42. doi: 10.1667/RR3054.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Azizova TV, Muirhead CR, Druzhinina MB, Grigoryeva ES, Vlasenko EV, Sumina MV, et al. Cerebrovascular diseases in the cohort of workers first employed at Mayak PA in 1948–1958. Radiat Res 2010; 174: 851–64. [DOI] [PubMed] [Google Scholar]
- 7.Azizova TV, Muirhead CR, Druzhinina MB, Grigoryeva ES, Vlasenko EV, Sumina MV, et al. Cardiovascular diseases in the cohort of workers first employed at Mayak PA in 1948–1958. Radiat Res 2010; 174: 155–68. [DOI] [PubMed] [Google Scholar]
- 8.Azizova TV, Muirhead CR, Moseeva MB, Grigoryeva ES, Sumina MV, O'Hagan J, et al. Cerebrovascular diseases in nuclear workers first employed at the Mayak PA in 1948–1972. Radiat Environ Biophys 2011; 50: 539−52. [DOI] [PubMed] [Google Scholar]
- 9.Azizova TV, Muirhead CR, Moseeva MB, Grigoryeva ES, Vlasenko EV, Hunter N, et al. Ischemic heart disease in nuclear workers first employed at the Mayak PA in 1948–1972. Health Phys 2012; 103: 3–14. [DOI] [PubMed] [Google Scholar]
- 10.Azizova TV, Zhuntova GV, Haylock RGE, Moseeva MB, Grigoryeva ES, Hunter N, et al. Chronic bronchitis in the cohort of Mayak workers first employed 1948–1958. Radiat Res 2013; 180: 610–21. doi: 10.1667/RR13228.1 [DOI] [PubMed] [Google Scholar]
- 11.Azizova TV, Haylock RGE, Moseeva MB, Bannikova MV, Grigoryeva ES. Cerebrovascular diseases incidence and mortality in an extended Mayak Worker Cohort 1948–1982. Radiat Res 2014; 182: 529–44. doi: 10.1667/RR13680.1 [DOI] [PubMed] [Google Scholar]
- 12.Simonetto C, Azizova TV, Grigoryeva ES, Kaiser JC, Schöllnberger H, Eidemüller M. Ischemic heart disease in workers at Mayak PA: latency of incidence risk after radiation exposure. PLoS One 2014; 9: e96309. doi: 10.1371/journal.pone.0096309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vasilenko EK, Khokhryakov VF, Miller SC, Fix JJ, Eckerman K, Choe DO, et al. Mayak worker dosimetry study: an overview. Health Phys 2007; 93: 190−206. doi: 10.1097/01.HP.0000266071.43137.0e [DOI] [PubMed] [Google Scholar]
- 14.Moseeva MB, Azizova TV, Grigorieva ES, Haylock R. Risk of circulatory diseases among Mayak PA workers with radiation doses estimated using the improved Mayak Workers Dosimetry System 2008. Radiat Environ Biophys 2014; 53: 469−77. [DOI] [PubMed] [Google Scholar]
- 15.ICD-9 guidelines for coding diseases, injures and causes of death/revision 1975. Geneva, Switzerland: WHO; 1980. [Google Scholar]
- 16.Azizova TV, Day RD, Wald N, Muirhead CR, O'Hagan JA, Sumina MV, et al. The “clinic” medical-dosimetric database of Mayak production association workers: structure, characteristics and prospects of utilization. Health Phys 2008; 94: 449–58. doi: 10.1097/01.HP.0000300757.00912.a2 [DOI] [PubMed] [Google Scholar]
- 17.Koshurnikova NA, Shilnikova NS, Okatenko PV. Characteristics of the cohort of workers at the Mayak nuclear complex. Radiat Res 1999; 152: 352–63. [PubMed] [Google Scholar]
- 18.Vasilenko EK, Scherpelz RI, Gorelov MV, Stram DJ, Smetanin MY. External dosimetry reconstruction for Mayak workers. 2010. AAHP Special Session Health Physics Society Annual Meeting. Available from: http://www.hps1.org/aahp/public/AAHP_Special_Sessions/2010_Salt_Lake_City/pm-1.pdf [Google Scholar]
- 19.Khokhryakov VV, Khokhryakov VF, Suslova KG, Vostrotin VV, Vvedensky VE, Sokolova AB, et al. Mayak Worker Dosimetry System 2008 (MWDS-2008): assessment of internal alpha-dose from measurement results of plutonium activity in urine. Health Phys 2013; 104: 366–78. doi: 10.1097/HP.0b013e31827dbf60 [DOI] [PubMed] [Google Scholar]
- 20.Wieser A, Vasilenko EK, Fattibene P, Bayankin S, El-Faramawy N, Ivanov D, et al. Comparison of EPR occupational lifetime external dose assessments for Mayak nuclear workers and film badge dose data. Radiat Environ Biophys 2006; 44: 279–88. doi: 10.1007/s00411-005-0024-1 [DOI] [PubMed] [Google Scholar]
- 21.Vasilenko EK, Smetanin MU, Aleksandrova ON, Gorelov MV, Knyazev VA, Teplyakov II, et al. Verification of individual doses from external radiation exposure to workers of PA “Mayak” (methods and results). [In Russian.] Med Radiol Radiol Saf 2001; 46: 37–57. [Google Scholar]
- 22.Preston D, Lubin J, Pierce D, McConney M. Epicure users guide. Seattle, WA: Hirosoft; 1993. [Google Scholar]
- 23.Akaike H. A new look at statistical model identification. IEEE Trans Automat Control 1974; 19: 716−23. [Google Scholar]
- 24.Claycamp HG, Okladnikova ND, Azizova TV, Beljaeva ZD, Boecker BB, Pesternikova VS, et al. Deterministic effects from occupational radiation exposures in a cohort of Majak PA workers: date base description. Health Phys 2000; 79: 48–54. [DOI] [PubMed] [Google Scholar]
- 25.Nemtsov AV, Terekhin AT. Cardiovascular mortality and alcohol consumption in Russia. [In Russian.] Dis Prev Health Promot 2008; 3: 25–30. [Google Scholar]
- 26.Preston DL, Shimizu Y, Pierce DA, Suyama A, Mabuchi K. Studies of mortality of atomic bomb survivors. Report 13: solid cancer and noncancer disease motality: 1950–1997. Radiat Res 2012; 178: AV146–72. [DOI] [PubMed] [Google Scholar]
- 27.Shimizu Y, Kodama K, Nishi N, Kasagi F, Suyama A, Soda M. Radiation exposure and circulatory disease risk: Hiroshima and Nagasaki atomic bomb survivor data, 1950–2003. BMJ 2010; 340: b5349. doi: 10.1136/bmj.b5349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yamada M, Wong FL, Fujiwara S, Akahoshi M, Suzuki G. Non-cancer disease incidence in atomic bomb survivors, 1958–1998. Radiat Res 2004; 161: 622–32. [DOI] [PubMed] [Google Scholar]
- 29.Vrijheid M, Cardis E, Ashmore P, Auvinen A, Bae JM, Engels H, et al. Mortality from diseases other than cancer following low doses of ionizing radiation: results from the 15-country study of nuclear industry workers. Int J Epidemiol 2007; 36: 1126–35. doi: 10.1093/ije/dym138 [DOI] [PubMed] [Google Scholar]
- 30.McGeoghegan D, Binks K, Gillies M, Jones S, Whaley S. The non-cancer mortality experience of male workers at British Nuclear Fuels plc, 1946–2005. Int J Epidemiol 2008; 37: 506–18. doi: 10.1093/ije/dyn018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Muirhead CR, O'Hagan JA, Haylock RGE, Phillipson MA, Willcock T, Berridge GLC, et al. Mortality and cancer incidence following occupational radiation exposure: third analysis of the National Registry for Radiation Workers. Br J Cancer 2009; 100: 206–12. doi: 10.1038/sj.bjc.6604825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ivanov V, Maksioutov MA, Chekin SY, Petrov AV, Biryukov AP, Kruglova ZG, et al. The risk of radiation-induced cerebrovascular disease in Chernobyl emergency workers. Health Phys 2006; 90: 199–207. doi: 10.1097/01.HP.0000175835.31663.ea [DOI] [PubMed] [Google Scholar]
- 33.Metz-Flamant C, Laurent O, Samson E, Caer-Lorho S, Acker A, Hubert D, et al. Mortality associated with chronic external radiation exposure in the French combined cohort of nuclear workers. Occup Environ Med 2013; 70: 630–8. doi: 10.1136/oemed-2012-101149 [DOI] [PubMed] [Google Scholar]
- 34.Zablotska LB, Little MP, Cornett RJ. Potential increased risk of ischemic heart disease mortality with significant dose fractionation in the Canadian Fluoroscopy cohort study. Am J Epidemiol 2014; 179: 120–31. doi: 10.1093/aje/kwt244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Little MP, Tawn EJ, Tzoulaki I, Wakeford R, Hildebrandt G, Paris F. Review and meta-analysis of epidemiological associations between low/moderate doses of ionizing radiation and circulatory disease risks and their possible mechanisms. Radiat Environ Biophys 2010; 49: 139–53. doi: 10.1007/s00411-009-0250-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Little MP. A review of non-cancer effects, especially circulatory and ocular diseases. Radiat Environ Biophys 2013; 52: 435–49. doi: 10.1007/s00411-013-0484-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Borghini A, Gianicolo EAL, Picano E, Andreassi MG. Ionizing radiation and atherosclerosis: current knowledge and future challenges. Atherosclerosis 2013; 230: 40–7. doi: 10.1016/j.atherosclerosis.2013.06.010 [DOI] [PubMed] [Google Scholar]
- 38.Lowe D, Raj K. Premature aging induced by radiation exhibits pro-atherosclerotic effects mediated by epigenetic activation of CD44 expression. Aging Cell 2014; 13: 900–10. doi: 10.1111/acel.12253 [DOI] [PMC free article] [PubMed] [Google Scholar]