Abstract
Objective:
To investigate the utility of diffusion-weighted (DW) MRI using high b-value vs standard b-value for patients with medulloblastoma (MB). Minimum apparent diffusion coefficient (ADCMIN) values were also compared with tumour cellularity.
Methods:
High and standard b-value DW images were obtained for 17 patients with MB. The number and location of the lesions, signal intensities (SIs), signal-to-noise ratios (SNRs), contrast-to-noise ratios, contrast ratios (CRs) and ADCs of the lesions were compared. Tumour cellularity was also measured and compared with ADCMIN values.
Results:
All 20 lesions were hyperintense on the DW MR images with high and standard b-values. Four additional lesions were revealed on high b-value, and all 24 lesions were more conspicuous at high b-value. SI, SNR and ADC values for the lesions were lower in the high b-value images than in the standard b-value images. The ADCMIN value at b = 3000 s mm−2 was more significantly associated with tumour cellularity than that at b = 1000 s mm−2. CR values were significantly higher in the high b-value images than in the standard b-value images.
Conclusion:
DW imaging using high b-value may be beneficial for detecting additional, less prominent lesions and may improve the contrast between MB lesions and normal tissue. A stronger inverse correlation with tumour cellularity was identified using the ADCMIN values at high b-value.
Advances in knowledge:
This study demonstrates the superiority of high b-value DW imaging compared with standard b-value imaging for the detection of MB lesions, especially those with subtle foci.
INTRODUCTION
Medulloblastoma (MB) is a highly malignant neuroepithelial tumour of the posterior fossa that predominantly develops in children. However, it can also develop in adults. MB is thought to arise from primitive, undifferentiated, small round cells that are located in the superior medullary velum (at the roof the fourth ventricle) early in life, and later in life, these cells may migrate laterally.1 The cerebellum is the most common location for MB, and most cases arise in the midline cerebellar vermis. However, in older children, adolescents and adults, MB manifests in more lateral locations within the cerebellar hemisphere. MB consists predominantly of small cells with minimal cytoplasm and oval, round or wedge-shaped homogeneously dark nuclei, and MB exhibits a high signal intensity (SI) on diffusion-weighted (DW) imaging, possibly reflecting the small-cell histology of this tumour.2 In general, MBs exhibit either classic histological features or histological features of one of the following non-classic variant subtypes: desmoplastic MB, MB with extensive nodularity, or large cell or anaplastic MB.3
DW MRI detects the diffusion of water in biological tissues. However, water distribution is complicated by bulk flow within capillaries and the active transport of water. Water diffusion is also affected by tumour cellularity, with the motion of water in the interstitium being the main contributor to higher apparent diffusion coefficient (ADC) values.2 Highly cellular tumours incorporate less interstitial space and, therefore, are characterized by reduced water diffusion and lower ADC values. Accordingly, DW imaging may be useful for evaluating gliomas since cellularity is a key factor in determining the tumour grade. For lymphomas,4,5 meningiomas6 and other tumours with high cellularity, a correlation between ADC values and cellularity has been observed. However, DW imaging does have limitations. For example, the sensitivity of DW imaging is low for small lesions such as leptomeningeal metastatic MB nodules, for various regions of the brainstem, and for imaging performed in the early stages following acute ischaemic stroke.
Theoretically, high b-value DW imaging provides better contrast owing to its sensitivity to tissue diffusivity and its reduced T2 shine-through effect.7 Accordingly, DW imaging at 3.0 T enables images to be captured at higher b-values with greater sensitivity. Moreover, this has been found to improve pre-operative histological grading of cerebral gliomas7 and meningiomas.8 DW imaging using a higher b-value has also been used for the diagnosis of acute stroke9,10 and Creutzfeldt–Jakob disease,11,12 and has produced better contrast between lesions and normal tissue in neurodegenerative diseases13 and primary central nervous system lymphoma (PCNSL).14
To our knowledge, only standard DW imaging has previously been used to investigate MBs.1,2,15–20 The b-value that is commonly used in conventional DW imaging is 1000 s mm−2, while b-values ≥3000 s mm−2 are considered high. A disadvantage of using a high b-value for DW imaging is that the signal-to-noise ratio (SNR) is reduced owing to the application of larger diffusion gradients.21 As a result, the MR signal decays. Therefore, the aim of the present study was to compare the use of high b-value (b = 3000 s mm−2) vs standard b-value (b = 1000 s mm−2) 3.0-T DW MRI for patients with MB. A possible correlation between ADC values and the cellularity of MB was also investigated.
METHODS AND MATERIALS
Patients
The present retrospective study was approved by our institutional review board. MRI and DW imaging were performed for patients diagnosed with MB between June 2009 and July 2013. The 17 patients included in this study (11 males and 6 females) with a mean age of 7 years were diagnosed with MB based on the pathological examinations of surgical specimens as defined by the World Health Organization (WHO). There was no more than 7 days between the imaging examinations and the surgeries performed. All of the human and animal studies performed were approved by the review board of the First Affiliated Hospital of Xiamen University and were performed in accordance with the ethical standards established in the 1964 Declaration of Helsinki and its subsequent amendments. In addition, all of the participating patients provided informed consent prior to inclusion in this study.
MRI
All MRI studies were performed using a 3.0-T superconducting MRI system (Magnetom® Verio Tim; Siemens Healthcare, Erlangen, Germany). Single-shot echoplanar DW imaging was performed with b-values of 1000 s mm−2 and 3000 s mm−2. The effective gradient was 40 mT m−1, and the slew rate was 150 mT m−1 ms−1. An eight-channel phased-array head coil was used with a repetition time (TR) of 8200 ms and an echo time (TE) of 91 ms. For standard and high b-value images, the TR and TE values were 8200 and 100 ms, respectively. The field of view (FOV) was 22 × 22 cm2 and the matrix was 128 × 128. The section thickness was 6 mm, and there were 24 sections and average of 2. The scan times were 2 min 5 s and 2 min 10 s to obtain standard b-value and high b-value images, respectively.
Conventional MRI (cMRI) studies were performed in three orthogonal planes, and they included non-enhanced T1 weighted images [250 ms TR, 2.48 ms TE, 22 × 22 cm2 FOV, 320 × 256 matrix size, 6-mm section thickness (average, one)], T2 weighted images [6000 ms TR, 96 ms TE, 22 × 22 cm2 FOV, 320 × 320 matrix size, 6-mm section thickness (average, one)] and fluid-attenuated inversion recovery (FLAIR) imaging [4000 ms TR, 94 ms TE, 22 × 22 cm2 FOV, 320 × 256 matrix size, 6-mm section thickness (average, one)]. Transverse, sagittal and coronal spin echo T1 weighted images and FLAIR images were acquired after an intravenous administration of 0.1-mmol kg−1 body weight gadopentetic acid, a gadolinium-based contrast medium (Magnevist®; Bayer Schering Pharma AG, Berlin, Germany).
Image analyses
Two neuroradiologists blinded to patient history and information evaluated the DW images, ADC maps and cMRI images to detect and localize the areas exhibiting restricted diffusion. Matching DW images and ADC maps were presented in a random order. In the case of a disagreement, the third neuroradiologist assessed the images in question, and a final decision was made. For each case, the number of lesions detected using b = 1000 s mm−2 and b = 3000 s mm−2 DW imaging were counted and compared with the number of lesions revealed by gadolinium-based, T1 weighted imaging and contrast-enhanced FLAIR. The latter is considered a reference standard and has been shown to improve the detection of leptomeningeal metastatic spread of MB and cerebral metastasis compared with routine contrast-enhanced T1 weighted imaging.22,23
Quantitative analyses
All DW images were measured and analysed using a Siemens workstation. Regions of interest (ROIs) in lesion sites and normal contralateral brain tissue were measured on both high b-value and standard b-value image sets and ADC maps. The pons was selected to represent normal brain tissue when MB lesions were vermis lesions, or in cases involving bilateral lesions present in both cerebellar hemispheres. As much as possible, these measurements were made on similarly sized ROIs of the same region. ROIs were also placed as centrally as possible within the enhanced lesions present on contrast-enhanced T1 weighted images, were selected according to the size of the lesion and were placed to avoid the lesion capsule and cystic, necrotic and hemorrhagic regions that might influence the ADC values. MRI SIs, SNRs, contrast-to-noise ratios (CNRs), contrast ratios (CRs) and ADC values for each of the b = 1000 s mm−2 and b = 3000 s mm−2 DW images were calculated. The ADC values for each lesion were calculated based on ADC maps at b = 1000 s mm−2 and b = 3000 s mm−2. Minimum ADC (ADCMIN), maximum ADC (ADCMAX) and mean ADC (ADCMEAN) absolute values were recorded. Image noise was measured from a large area outside the brain parenchyma, and this was defined as the background SI.
The SI and ADC values were measured for b = 1000 s mm−2 and b = 3000 s mm−2 DW images, and both mean and standard deviation (SD) values were calculated. SNRs, CNRs and CRs were also calculated using the following equations:9,14
where Sbrain is the average SI of the contralateral normal brain tissue, Slesion is the average of SI of the lesion and σnoise is the SD of the background noise.
Histopathological studies
All pathological specimens were evaluated by a single experienced reviewer (SZ, a paediatric pathologist with 15 years' experience). Tumours were classified according to the latest WHO classification of tumours of the central nervous system.24 Briefly, following surgical resection, tumour specimens were fixed in 10% phosphate-buffered formalin, embedded in paraffin and representative slides were stained with haematoxylin–eosin reagent for standard histological diagnosis according to WHO criteria. Tumour cellularity was evaluated as described previously.25 Briefly, at least four separate fields containing high cellularity regions were selected from areas of each solid tumour sample. The cellularity of each field was calculated by manually counting the total number of cells present in each photomicrograph obtained at ×400 magnification. The mean number of cells observed was recorded for each case.
Statistical analyses
Statistical analyses were performed using a commercially available software package, Medcalc v. 9.3 (Medcalc Software, Mariakerke, Belgium). Student's t-test was used to compare mean SI, SNR, CNR and ADC values between b = 1000 s mm−2 and b = 3000 s mm−2 DW images. A simple linear regression analysis was used to evaluate the relationship between tumour cellularity and ADC values. A p-value <0.05 was considered statistically significant.
RESULTS
17 patients (11 males and 6 females) with a mean age of 7 years (range: 1/3–17 years) were included in this study. A total of 24 lesions were detected by cMRI. 14 patients had one lesion each (classic MB), 1 patient had two lesions (MB with extensive nodularity), another patient had three lesions (classic MB) and another patient had five lesions (desmoplastic MB). Of these lesions, 12 were located in the vermis, 8 were located in the cerebellar hemisphere and 4 small lesions (<0.5 cm in diameter) were located in the right central sulcus, left frontal lobe, right temporal lobe and left occipital lobe, respectively (Table 1).
Table 1.
Characteristics of the additional leptomeningeal medulloblastoma lesions that were detected
| Patient number | Tumour shape | Tumour location | Gadolinium-based T1 weighted imaging | Contrast-enhanced (gadolinium) fluid-attenuated inversion recovery | Standard b-value DW imaging | Higher b-value DW imaging |
|---|---|---|---|---|---|---|
| N3 | Round | Left frontal lobe | No | Yes | No | Yes |
| N6 | Oval | Right central sulcus | No | Yes | No | Yes |
| N11 | Dotted | Right temporal lobe | No | Yes | No | Yes |
| N14 | Dotted | Left occipital lobe | No | Yes | No | Yes |
DW, diffusion-weighted.
Qualitative analyses
Lesions were detected in DW images obtained using high and standard b-values, and areas of restricted diffusion were more conspicuous in the high b-value images (Figures 1 and 2). Correspondingly, the total number of lesions detected at b = 1000 s mm−2 and b = 3000 s mm−2 were 20 and 24, respectively. The two reviewers of these images (HH and CH, with 7 and 8 years' experience, respectively) agreed on the identification of 19 lesions in the standard b-value DW images and only had to resolve by consensus one disagreement regarding a single lesion. By contrast, both reviewers agreed on the presence of all 24 lesions that were identified in the high b-value images. Moreover, all of the lesions visualized in the standard b-value DW images were also detected in the high b-value DW images. For the four additional lesions that were identified in the high b-value DW images (Figure 3), these were not characterized by pathological examination, whereas the other 20 lesions were. Artefacts also appeared to be more pronounced in the high b-value images.
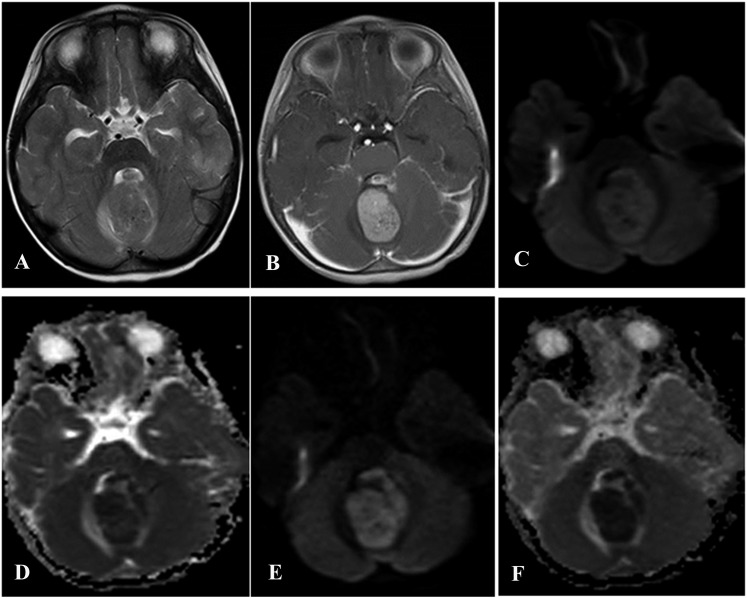
Figure 1.
A classic medulloblastoma in a 4-year-old male with a 2-month history of ataxic gait. (a) An axial T2 weighted MR image reveals slight hyperintensity in the cerebellar vermis. (b) A contrast-enhanced axial T1 weighted MR image demonstrates a slightly heterogeneous, yet intense enhancement of the lesion. (c) A diffusion-weighted (DW) image that was obtained using a b-value of 1000 s mm−2. (d) An apparent diffusion coefficient (ADC) map that shows the slightly restricted diffusion values that were observed in the lesion. (e) A DW image that was obtained using a b-value of 3000 s mm−2. (f) An ADC map that shows a slight increase in signal intensities in the cerebellar vermis.
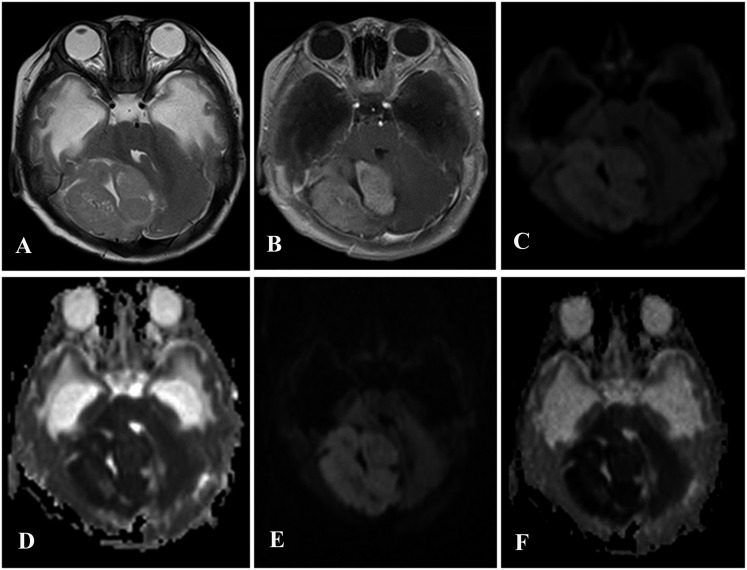
Figure 2.
A 4-month-old male diagnosed with medulloblastoma with extensive nodularity. (a) An axial T2 weighted MR image demonstrates an eccentric posterior fossa tumour present in the right cerebellar hemisphere. (b) In a post-contrast T1 weighted image, marked heterogeneous enhancement was observed. (c) A diffusion-weighted (DW) image obtained using a b-value of 1000 s mm−2. (d) An apparent diffusion coefficient (ADC) map that shows slightly restricted diffusion values for the lesion. (e) A DW image obtained using a b-value of 3000 s mm−2. (f) An ADC map that shows increased signal intensities in the right cerebellar.
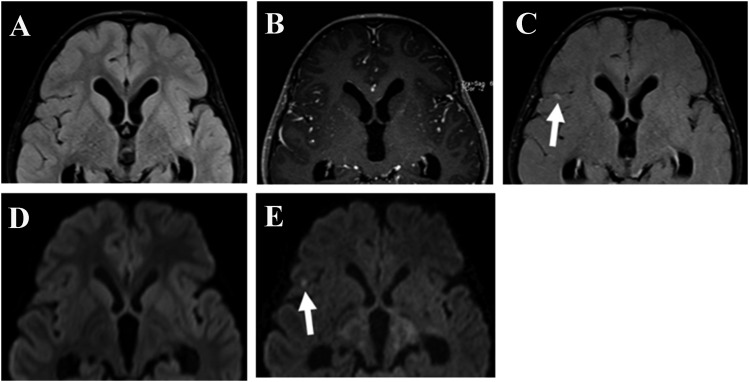
Figure 3.
A 6-year-old male diagnosed with classic medulloblastoma in the cerebellar vermis. There was no lesion detected on an axial fluid-attenuated inversion recovery (FLAIR) MR image (a), a post-contrast T1 weighted image (b) or with standard diffusion-weighted (DW) imaging (d). However, DW imaging using a high b-value (e) and contrast-enhanced FLAIR (c) showed that restricted diffusion and nodular enhancement was present in the right central sulcus, thereby indicating the presence of a small metastatic lesion (arrows).
Mean ± SD values for SIs, SNRs, CNRs, CRs and all ADC values are summarized in Tables 2 and 3. All of the results were statistically significant (p < 0.05), except for the CNRs. The SNRs calculated from the high b-value images were lower than those for the standard b-value images. Moreover, the ADCMIN, ADCMAX and ADCMEAN values were all significantly lower for the high b-value images compared with the standard b-value images.
Table 2.
Signal intensity (SI), signal-to-noise (SNR), contrast-to-noise (CNR) and contrast ratio (CR) values for b = 1000 s mm−2 and b = 3000 s mm−2 diffusion-weighted images
| Parameters | b = 1000 s mm−2 | b = 3000 s mm−2 | p-value |
|---|---|---|---|
| SI | 333.54 ± 80.32 | 185.33 ± 69.59 | <0.0001 |
| SNR | 363.11 ± 95.16 | 237.19 ± 103.10 | 0.0002 |
| CNR | 120.36 ± 43.49 | 127.73 ± 78.43 | 0.7216 |
| CR | 0.204 ± 0.067 | 0.374 ± 0.134 | <0.0004 |
Table 3.
Apparent diffusion coefficient (ADC) values from b = 1000 s mm−2 and b = 3000 s mm−2 diffusion-weighted images
| ADC values | b = 1000 s mm−2 | b = 3000 s mm−2 | p-value |
|---|---|---|---|
| Minimum ADC | 0.549 ± 0.058 | 0.383 ± 0.044 | <0.0001 |
| Maximum ADC | 0.665 ± 0.072 | 0.457 ± 0.037 | <0.0001 |
| Mean ADC | 0.596 ± 0.043 | 0.426 ± 0.033 | <0.0001 |
In the high b-value images, the SI decreased by 44.4% (185.33 ± 69.59 vs 333.54 ± 80.32 at b = 3000 s mm−2 and b = 1000 s mm−2, respectively). The SNR also decreased by 34.6% (237.19 ± 103.10 vs 363.11 ± 95.16, respectively). By contrast, the CR was significantly higher in the high b-value images (0.374 ± 0.134 vs 0.204 ± 0.067, respectively; p < 0.004). The ADCMIN, ADCMAX and ADCMEAN values calculated from the high b-value images were also lower by 30.2%, 31.2% and 28.5%, respectively, compared with the standard b-value images.
Cellularity and minimum apparent diffusion coefficient values of the tumour tissues examined
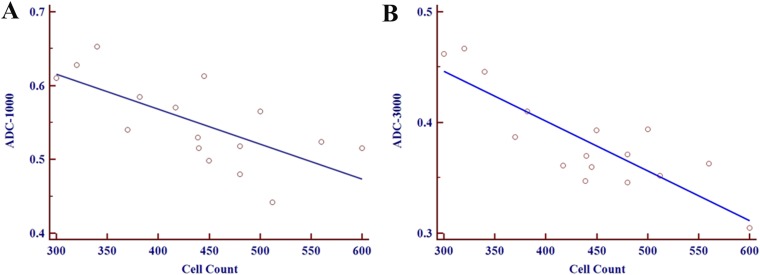
The mean cell density per field was 439 cells (range: 300–612 cells), and a negative correlation between tumour cellularity and ADCMIN values was observed for the MBs examined. Moreover, a stronger negative correlation was associated with the high b-value images (r = −0.846, p < 0.001) compared with the low b-value images (r = −0.686, p = 0.003) (Figure 4).
Figure 4.
A negative correlation between minimum apparent diffusion coefficient (ADC) values and tumour cellularity in a medulloblastoma [r = −0.686, p = 0.003 vs r = −0.846, p < 0.001 at b = 1000 s mm−2 (a) and b = 3000 s mm−2 (b), respectively].
DISCUSSION
DW imaging is a technique which uses phase-defocusing and phase-refocusing gradients to determine the rates of water diffusion within tissues. Other MRI techniques cannot measure diffusion, and often water diffusion is altered in various diseases, including MB. The b-value of an MR image depends on the strength, duration and timing of the applied diffusion-sensitizing gradients. While DW imaging using a higher b-value can be achieved using a 1.5-T MR system, the SNR is very low for the images obtained. Consequently, the use of higher b-values for DW imaging is limited when a 1.5-T MR system is used. However, a 3.0-T MR system can achieve a higher SNR, and thus, higher b-values for DW imaging can be used with a 3.0-T MR system.26 Moreover, DW imaging with a 3.0-T MR system provides greater diffusion sensitivity.
Altered diffusion within a tumour can demarcate tumour tissue from normal tissue. Specifically, when MB tumours block water diffusion, they appear hyperintense on DW images and appear hypointense on ADC maps. In the low b-value DW images of the present study, most of the lesions exhibited slightly and moderately restricted diffusion (n = 20), although a few lesions (n = 4) were not hyperintense relative to the surrounding cortical grey matter. Furthermore, the SI, SNR and ADC values were lower in the high b-value images, while the CR values were significantly higher. As a result, the lesions present were more conspicuous. Additional lesions were also revealed in the high b-value images, indicating that the use of a higher b-value for DW imaging may be better for detecting small lesions. All of the additional lesions detected in the present study were leptomeningeal MB lesions, and they were surrounded by the grey matter or CSF. The contrast between the lesions with adjacent CSF and the lesions with adjacent grey matter was greater when the grey matter signals were suppressed, except when the CSF signals were nullified with the higher b-value DW imaging. These findings are in concordance with those from previous studies where high b-value DW imaging was applied to patients with ischaemic stroke9,10 and patients with PCNSL.14 In both studies, high b-value images were also better at visualizing small lesions and multiple lesions in patients with stroke and PCNSL. To our knowledge, this is the first study to compare standard b-value and high b-value DW imaging in the detection of MB lesions.
In the present study, the ADCMIN, ADCMAX and ADCMEAN values obtained from the high b-value DW images were consistently lower than those obtained from the standard b-value images. In previous studies,15–19 the ADCMEAN values obtained at a b-value of 1000 s mm−2 ranged from 0.47 × 10−3 to 0.75 × 10−3 mm2 s−1 in patients with MB, and these data are consistent with the present results.
Comparisons between the ADCMIN value at b = 1000 s mm−2 and cellularity in the MBs examined showed that ADC values are inversely associated with tumour cellularity. Thus, high cellularity leads to more restricted diffusion. Other studies have also reported an inverse correlation between tumour cellularity and the ADC values for meningiomas6 and gliomas,27 and the results of the present study are in concordance with these. However, the results of the present study further demonstrate that the inverse correlation is stronger when the b-value is 3000 s mm−2 rather than 1000 s mm−2 for DW imaging.
In general, ADC values decrease when b-values increase above 1000 s mm−2. If the relationship between the MR signal and the b-value is monoexponential, then the ADC values would be constant for any two-point calculation as the b-values increased. However, ADC values have been found to decrease as b-values increase, thereby suggesting that bi-exponential SI decay may occur.28,29 Fast and slow diffusion components have been described in brain models, and at low and high b-values, SI is dominated by fast and slow diffusion, respectively. In future studies, the use of different b-values during DW imaging could be used to explore whether bi-exponential SI decay occurs in MB lesions.
To our knowledge, the present study is the first to evaluate the use of standard vs high b-values for MRI of patients with MB. However, the sample size in the present study was small since cases of MB are relatively rare. Therefore, in order to confirm the present results, additional DW images and ADC values from a larger prospective study are needed. In addition, most of the MBs examined exhibited a classical histology, while only two non-classic variant subtypes of MB were available. Correspondingly, differences between classic MB and MB subtypes were not explored. The present study also did not address contributions from bulk capillary flow, active transport mechanisms and partial volume effects on ADC values.30 Future large-scale studies should also include these types and considerations.
In conclusion, although a quantitative analysis demonstrated that higher SI, SNR and ADC values were associated with standard b-value DW imaging, the use of a higher b-value (b = 3000 s mm−2) facilitated the detection of more subtle lesions, and it also improved the contrast between lesions and normal tissue. In particular, a stronger inverse correlation between ADCMIN at a b-value of 3000 s mm−2 from 3.0-T MRI and tumour cellularity was identified.
Contributor Information
Chengkun Han, Email: flytianyu@sina.com.
Long Zhao, Email: flytianyu@sina.com.
Shan Zhong, Email: flytianyu@sina.com.
Xiurong Wu, Email: flytianyu@sina.com.
Jianfeng Guo, Email: flytianyu@sina.com.
Xiongjie Zhuang, Email: zhuangxiongjievip@163.com, flytianyu@sina.com.
Haiwei Han, Email: hanminghui360@163.com.
REFERENCES
- 1.Koeller KK, Rushing EJ. From the archives of the AFIP: medulloblastoma: a comprehensive review with radiologic-pathologic correlation. Radiographics 2003; 23: 1613–37. doi: 10.1148/rg.236035168 [DOI] [PubMed] [Google Scholar]
- 2.Yamashita Y, Kumabe T, Higano S, Watanabe M, Tominaga T. Minimum apparent diffusion coefficient is significantly correlated with cellularity in medulloblastomas. Neurol Res 2009; 31: 940–6. doi: 10.1179/174313209X382520 [DOI] [PubMed] [Google Scholar]
- 3.Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, eds. WHO classification of tumours of the central nervous system. Lyon, France: IARC Press; 2007. pp. 132–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guo AC, Cummings TJ, Dash RC, Provenzale JM. Lymphomas and high-grade astrocytomas: comparison of water diffusibility and histologic characteristics. Radiology 2002; 224: 177–83. doi: 10.1148/radiol.2241010637 [DOI] [PubMed] [Google Scholar]
- 5.Doskaliyev A, Yamasaki F, Ohtaki M, Kajiwara Y, Takeshima Y, Watanabe Y, et al. Lymphomas and glioblastomas: differences in the apparent diffusion coefficient evaluated with high b-value diffusion-weighted magnetic resonance imaging at 3T. Eur J Radiol 2012; 81: 339–44. doi: 10.1016/j.ejrad.2010.11.005 [DOI] [PubMed] [Google Scholar]
- 6.Filippi CG, Edgar MA, Uluğ AM, Prowda JC, Heier LA, Zimmerman RD. Appearance of meningiomas on diffusion-weighted images: correlating diffusion constants with histopathologic findings. AJNR Am J Neuroradiol 2001; 22: 65–72. [PMC free article] [PubMed] [Google Scholar]
- 7.Seo HS, Chang KH, Na DG, Kwon BJ, Lee DH. High b-value diffusion (b = 3000 s/mm2) MR imaging in cerebral gliomas at 3T: visual and quantitative comparisons with b = 1000 s/mm2. AJNR Am J Neuroradiol 2008; 29: 458–63. doi: 10.3174/ajnr.A0842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Watanabe Y, Yamasaki F, Kajiwara Y, Takayasu T, Nosaka R, Akiyama Y, et al. Preoperative histological grading of meningiomas using apparent diffusion coefficient at 3T MRI. Eur J Radiol 2013; 82: 658–63. doi: 10.1016/j.ejrad.2012.11.037 [DOI] [PubMed] [Google Scholar]
- 9.Cihangiroglu M, Citci B, Kilickesmez O, Firat Z, Karlikaya G, Uluğ AM, et al. The utility of high b-value DWI in evaluation of ischemic stroke at 3T. Eur J Radiol 2011; 78: 75–81. doi: 10.1016/j.ejrad.2009.10.011 [DOI] [PubMed] [Google Scholar]
- 10.Purroy F, Begue R, Quílez A, Sanahuja J, Gil MI. Contribution of high-b-value diffusion-weighted imaging in determination of brain ischemia in transient ischemic attack patients. J Neuroimaging 2013; 23: 33–8. doi: 10.1111/j.1552-6569.2011.00696.x [DOI] [PubMed] [Google Scholar]
- 11.Hyare H, Thornton J, Stevens J, Mead S, Rudge P, Collinge J, et al. High-b-value diffusion MR imaging and basal nuclei apparent diffusion coefficient measurements in variant and sporadic Creutzfeldt-Jakob disease. AJNR Am J Neuroradiol 2010; 31: 521–6. doi: 10.3174/ajnr.A1860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Riva-Amarante E, Jiménez-Huete A, Toledano R, Calero M, Alvarez-Linera J, Escribano J, et al. Usefulness of high b-value diffusion-weighted MRI in the diagnosis of Creutzfeldt-Jakob disease. [In Spanish.] Neurologia 2011; 26: 331–6. doi: 10.1016/j.nrl.2010.12.003 [DOI] [PubMed] [Google Scholar]
- 13.Yoshiura T, Mihara F, Tanaka A, Ogomori K, Ohyagi Y, Taniwaki T, et al. High b value diffusion-weighted imaging is more sensitive to white matter degeneration in Alzheimer's disease. Neuroimage 2003; 20: 413–19. doi: 10.1016/S1053-8119(03)00342-2 [DOI] [PubMed] [Google Scholar]
- 14.Han H, Han C, Huang S, Guo J, Zhuang X. Comparison of diffusion-weighted imaging between high and standard b-values for primary central nervous system lymphoma. Clin Radiol 2014; 69: 974–9. doi: 10.1016/j.crad.2014.05.002 [DOI] [PubMed] [Google Scholar]
- 15.Koral K, Gargan L, Bowers DC, Gimi B, Timmons CF, Weprin B, et al. Imaging characteristics of atypical teratoid-rhabdoid tumor in children compared with medulloblastoma. AJR Am J Roentgenol 2008; 190: 809–14. doi: 10.2214/AJR.07.3069 [DOI] [PubMed] [Google Scholar]
- 16.Koral K, Mathis D, Gimi B, Gargan L, Weprin B, Bowers DC, et al. Common pediatric cerebellar tumors: correlation between cell densities and apparent diffusion coefficient metrics. Radiology 2013; 268: 532–7. doi: 10.1148/radiol.13121362 [DOI] [PubMed] [Google Scholar]
- 17.Yeom KW, Mobley BC, Lober RM, Andre JB, Partap S, Vogel H, et al. Distinctive MRI features of pediatric medulloblastoma subtypes. AJR Am J Roentgenol 2013; 200: 895–903. doi: 10.2214/AJR.12.9249 [DOI] [PubMed] [Google Scholar]
- 18.Liu HQ, Yin X, Li Y, Zhang J, Wang Y, Tchoyoson Lim CC, et al. MRI features in children with desmoplastic medulloblastoma. J Clin Neurosci 2012; 19: 281–5. doi: 10.1016/j.jocn.2011.04.029 [DOI] [PubMed] [Google Scholar]
- 19.Eran A, Ozturk A, Aygun N, Izbudak I. Medulloblastoma: atypical CT and MRI findings in children. Pediatr Radiol 2010; 40: 1254–62. doi: 10.1007/s00247-009-1429-9 [DOI] [PubMed] [Google Scholar]
- 20.Fruehwald-Pallamar J, Puchner SB, Rossi A, Garre ML, Cama A, Koelblinger C, et al. Magnetic resonance imaging spectrum of medulloblastoma. Neuroradiology 2011; 53: 387–96. doi: 10.1007/s00234-010-0829-8 [DOI] [PubMed] [Google Scholar]
- 21.García Santos JM, Ordóñez C, Torres del Río S. ADC measurements at low and high b values: insight into normal brain structure with clinical DWI. Magn Reson Imaging 2008; 26: 35–44. [DOI] [PubMed] [Google Scholar]
- 22.Griffiths PD, Coley SC, Romanowski CA, Hodgson T, Wilkinson ID. Contrast-enhanced fluid-attenuated inversion recovery imaging for leptomeningeal disease in children. AJNR Am J Neuroradiol 2003; 24: 719–23. [PMC free article] [PubMed] [Google Scholar]
- 23.Chen W, Wang L, Zhu W, Xia L, Qi J, Feng D, et al. Multicontrast single-slab 3D MRI to detect cerebral metastasis. AJR Am J Roentgenol 2012; 198: 27–32. doi: 10.2214/AJR.11.7030 [DOI] [PubMed] [Google Scholar]
- 24.Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol 2007; 114: 97–109. doi: 10.1007/s00401-007-0243-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barajas RF, Jr, Rubenstein JL, Chang JS, Hwang J, Cha S. Diffusion-weighted MR imaging derived apparent diffusion coefficient is predictive of clinical outcome in primary central nervous system lymphoma. AJNR Am J Neuroradiol 2010; 31: 60–6. doi: 10.3174/ajnr.A1750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu Z, Xiao X. The use of multi b values diffusion-weighted imaging in patients with acute stroke. Neuroradiology 2013; 55: 371–6. doi: 10.1007/s00234-012-1129-2 [DOI] [PubMed] [Google Scholar]
- 27.Kono K, Inoue Y, Nakayama K, Shakudo M, Morino M, Ohata K, et al. The role of diffusion-weighted imaging in patients with brain tumors. AJNR Am J Neuroradiol 2001; 22: 1081–8. [PMC free article] [PubMed] [Google Scholar]
- 28.DeLano MC, Cooper TG, Siebert JE, Potchen MJ, Kuppusamy K. High-b-value diffusion-weighted MR imaging of adult brain: image contrast and apparent diffusion coefficient map features. AJNR Am J Neuroradiol 2000; 21: 1830–6. [PMC free article] [PubMed] [Google Scholar]
- 29.Niendorf T, Dijkhuizen RM, Norris DG, van Lookeren Campagne M, Nicolay K. Biexponential diffusion attenuation in various states of brain tissue: implications for diffusion-weighted imaging. Magn Reson Med 1996; 36: 847–57. doi: 10.1002/mrm.1910360607 [DOI] [PubMed] [Google Scholar]
- 30.Le Bihan D. Apparent diffusion coefficient and beyond: what diffusion MR imaging can tell us about tissue structure. Radiology 2013; 268: 318–22. doi: 10.1148/radiol.13130420 [DOI] [PubMed] [Google Scholar]