Abstract
Objective:
To evaluate breath-hold stability and constancy for a voluntary breath-hold (VBH) technique in a retrospective analysis.
Methods:
Movie loop sequences of electronic portal image data from multiple breath holds in a cohort of 19 patients were used to assess within and between breath-hold stability. In vivo dosimetry data based on electronic portal imaging (EPI) were analysed for 31 VBH patients plus a cohort of free-breathing (FB) patients to provide a reference. A phantom experiment simulated the impact on dose of FB, breath hold and unplanned release of breath hold.
Results:
165/174 (93%) movie loop data sets had no detectable displacement. For the remaining 12, median displacement = 1.5 mm and maximum displacement = 3 mm (one patient on one fraction). In vivo dosimetry data analysis showed a median dose difference measured to planned of −0.2% (VBH) and −0.1% (FB). Dose distribution evaluation (γ) pass rates were 84% (VBH) and 91% (FB) including the lung region; 93% and 96% with a lung override. Unplanned release of phantom breath-hold position changed median dose by ≤1% and degraded γ pass rates to 79–62%. Failing regions were mostly in the periphery of the treated volume.
Conclusion:
The data confirmed that multiple VBHs using visual monitoring are stable; in vivo dose verification via EPI was within expected and acceptable levels.
Advances in knowledge:
These data provide further reassurance that VBH is a safe technique for cardiac sparing breast radiotherapy and support its rapid, widespread implementation.
INTRODUCTION
Radiotherapy as a therapeutic agent for breast cancer reduces local recurrence and contributes to a decrease in mortality.1,2 With the number of breast cancer survivors in the UK population set to treble to 1.7 million by 2040; however, reducing the risks of long-term side-effects from radiotherapy is of increasing importance.3 The Early Breast Trialists' Collaborative Group meta-analysis of 2005 suggests that the biggest contributor to late non-breast cancer-related mortality is cardiovascular disease.2 Recent evidence suggests that there is no threshold dose below which the late cardiac effects of breast radiotherapy do not occur.4 As such, it is critical to use techniques which minimize cardiac doses without compromising breast tissue coverage.
Modifications to dosimetry such as the use of multileaf collimation (MLC) and intensity-modulated radiotherapy (IMRT) are potential solutions to reducing heart dose in radiotherapy for patients with breast cancer. However, the use of MLC has the disadvantage of shielding breast tissue whilst IMRT may increase low-dose radiation to non-target organs depending on the priorities set in the planning process.5–7 An approach which minimizes the compromise between target tissue coverage and heart sparing is the use of deep inspiration breath hold during the radiotherapy delivery. This moves the chest wall away from the heart thereby reducing the radiation dose to heart tissue. There are several breath-hold techniques available, most of which require additional equipment to a standard linear accelerator such as the Active Breathing Coordinator™ (ABC) (Elekta AB (Publ), Stockholm, Sweden) and Real-time Position Management™ systems (Varian® Medical Systems Inc., Palo Alto, CA). A lower technology alternative to these is to use a voluntary breath-hold (VBH) technique whereby the patient simply takes a breath in and holds it for around 20 s whilst the radiotherapy beam is on. The breath hold is monitored visually using the treatment unit field light indicator or the positioning lasers against reference skin marks. This VBH technique has been compared to and found equivalent to an ABC breath-hold technique in patients receiving left breast radiotherapy in terms of set-up reproducibility and cardiac dose reduction.8,9 The VBH technique has also been reported by Bartlett et al9 to be superior to ABC in terms of patient acceptability and ease of implementation. However, areas of potential concern which might inhibit the rapid roll out of VBH into routine practice are (i) the stability of each breath hold, (ii) the magnitude of breath hold-to-breath-hold variation for a number of repeat breath holds, and (iii) the impact on delivered dose of a failure to maintain breath hold for the planned duration.
This work presents:
A retrospective analysis of electronic portal imaging (EPI) data acquired during multiple breath holds for a cohort of patients treated within a clinical trial.
A retrospective in vivo dosimetry assessment of VBH and a matched cohort of free-breathing (FB) breast radiotherapy. These data were acquired using an EPI device (EPID).
A phantom study simulating the effect of unplanned loss of breath hold on the delivered dose.
METHODS AND MATERIALS
Patient population
Data for this study were obtained via a cohort of 31 patients who received radiotherapy for breast cancer with a VBH technique within a clinical trial between January 2013 and April 2014.10 The total cohort size for the trial was 34, data for 3 patients could not be dearchived; hence, 31 data sets were available for this study. Patients were eligible if they had left breast cancer, had undergone breast-conserving surgery and required radiotherapy to the breast (±tumour bed boost) but no nodal irradiation and a breast size ≥750 cm3. A cohort of 31 non-trial patients, with similar characteristics, was selected sequentially from the main treatment planning database to provide a reference against which to assess the VBH data. These patients were treated in FB. Treatment plans for both cohorts consisted of right anterior (RAO) (medial tangent) and left posterior oblique (LPO) (lateral tangent) fields. A simple field-in-field method was used with the open tangential component of each field contributing 80–90% of the beam weight with 1–4 shaped sub fields providing modulation to improve the dose homogeneity.
Data acquisition
EPI data for each patient were collected as part of the trial protocol and to conform to local treatment verification practice. The data consisted of images of the RAO and LPO fields. Interfraction set-up reproducibility for the VBH method has been previously reported to be similar to equipment-assisted breath hold and is not considered further in this work.8–10 The work presented is a retrospective analysis of the data.
EPI data were collected with Elekta iView® systems integrated with Elekta Synergy® linear accelerators. Local policy for on-treatment dose verification in routine practice requires an in vivo dosimetry procedure on three fractions within the first week. This was performed for both trial and non-trial cohorts. The in vivo dosimetry procedure used an Elekta iView EPI with the system set to integrate the images using the IMRT Dosimetric Weighting mode. For the remaining fractions of treatment, the mode settings were changed to acquire portal images for set-up verification only. In addition, for 19 patients of the VBH cohort, frames acquired every 3–4 s (movie loop mode) were collected within individual breath holds. The movie loop data were used to obtain reproducibility data within individual breath holds (intrabreath hold) and from breath hold to breath hold (interbreath hold) within a treatment fraction. Ideally, movie loop data for all VBH patients would have been collected. Image acquisition settings for the in vivo dosimetry and the movie loops are incompatible and several software changes are required to change modes. At the time of the trial, resource priority was given to the rapid implementation of in vivo dosimetry for all patients whether in trials or not. The importance of acquiring these data meant movie loop data were not collected for all VBH trial patients.
Data analysis
Intrabreath-hold stability and interbreath-hold consistency
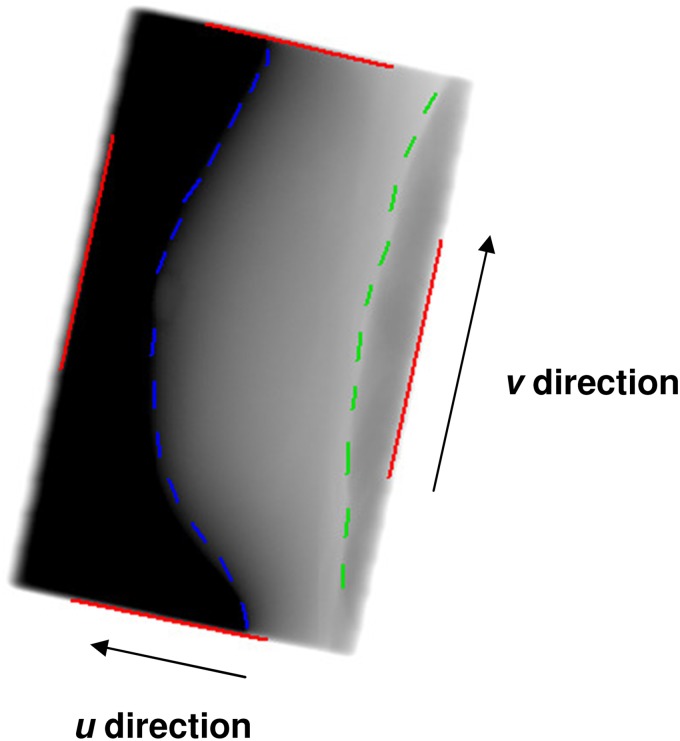
The imaging data sets available for this analysis included the acquired movie loops consisting of 3–5 frames within each breath hold. The number of frames acquired in each movie loop was limited by breath-hold length which varied between 12 and 20 s depending on the treatment plan parameters. For this patient cohort 2 to 3 breath holds for each of the right anterior and left posterior fields were typical. Images were matched by one trained operator using manual matching to a reference template based on the breast/chest wall interface and the breast outline (Figure 1). Intrabreath-hold data stability data were obtained using the first frame of the movie loop as a reference and matching subsequent frames to this image. The first frame of each breath hold was used as a reference so that the variation from the start time in breath hold could be assessed. Data on interbreath-hold variation were obtained using the first frame of the first breath hold for each field and matching subsequent breath-hold frames to this reference. Outputs from all verification image matching were two-dimensional displacements in the imager (u–v) plane of patient anatomy with respect to the reference image. The u and v directions are shown on Figure 1. A detectable displacement was defined as a displacement consistently ≥1 mm on repeat matching.
Figure 1.
Electronic portal image of a left posterior oblique (lateral tangent) field showing the “u” and “v” directions of displacements in the plane of the imager. Field edges are indicated by solid lines, breast and lung outlines are indicated by dashed lines.
In vivo dosimetry
The iView EPID systems have been calibrated so that dosimetric parameters may be obtained from the images when acquired in IMRT dosimetric mode. These data are analysed within a software package based on the work of Wendling et al.11 This software reports a reference point dose value plus an evaluation of the measured dose distribution for each beam using a parameter known as γ.12 The purpose of the γ analysis is to compare a treatment planning system prediction of the dose distribution (the reference) to a measured one. It combines the value of the dose difference at a point and the spatial distance of a measured dose level from that predicted. If the calculated γ < 1, then the agreement between planned and measured dose is within the specified limits. These limits are expressed as an allowed dose deviation and distance variations (distance to agreement). Criteria of 5% dose deviation and 5 mm distance to agreement were used to calculate the γ for breast patients in routine practice and in this study. One of the parameters reported by the software was the percentage of measured dose points with a γ value <1. If there is good agreement between measured and expected doses, then this value is high as most of the measured points are within the set criteria (5% and 5 mm).
The data from the 31 non-trial FB whole left breast radiotherapy cases were analysed in the same way to provide a reference against which to assess the VBH results.
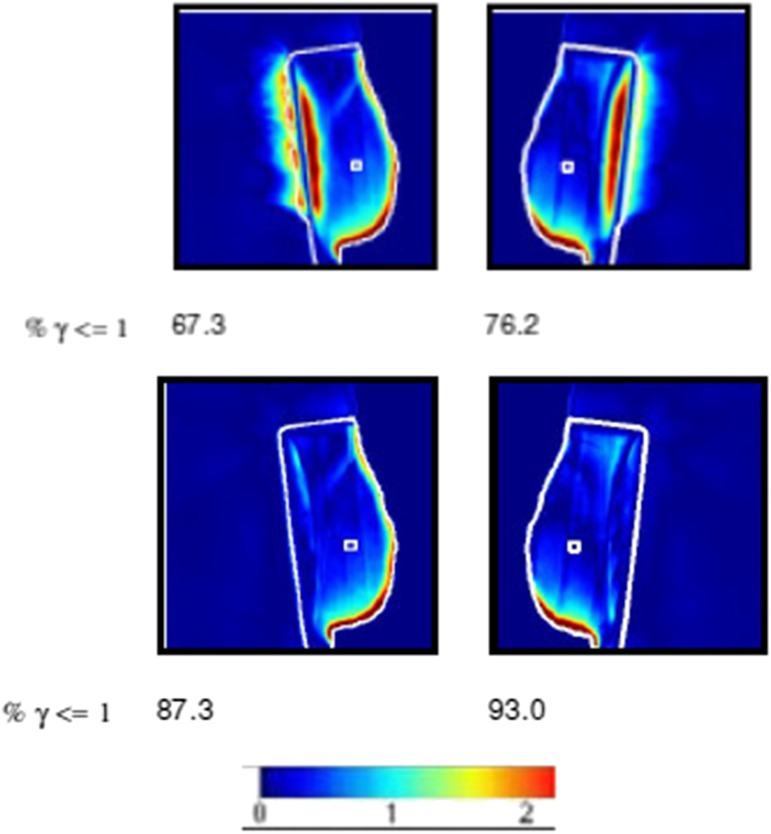
The EPID in vivo dosimetry software has a limitation as it is unable to analyse dose correctly in low-density tissue. Results may appear poor because of this limitation rather than an issue with the treatment delivery or patient set-up. There is a facility with the software to recalculate predicted and measured data with low-density tissue such as lung, replaced by the density of water.13 Figure 2 shows an example of the effect for a VBH patient. Ten VBH and ten FB cases were recalculated using this facility.
Figure 2.
The upper panel shows regions of high-gamma values in the lung area. These contribute to the low values of the % γ ≤ 1 metric (67.3% and 76.2%). The lower panel shows the lung override option activated. It removes these regions and the % γ ≤ 1 values increase to 87.3% and 93.0%. The colour bar shows how the colours related to the γ value.
All data were tested for normality using the Shapiro–Wilks test and the non-parametric independent samples Mann–Whitney U test was used to determine the statistical significance of any differences between the in vivo dosimetry data sets. The significance value was set to p = 0.01 to provide a stringent measure of statistical significance. The analysis was carried out using IBM SPSS® Statistics v. 22 (IBM Corporation, Armonk, NY; formerly SPSS Inc., Chicago, IL).
Phantom experiment
An experiment to obtain quantification of the effect on the dose distribution of a failure of breath hold during a radiation delivery was performed using a Scandidos Delta4® dosimetry phantom (Scandidos AB, Uppsala, Sweden) and an in-house high precision, programmable motion platform gated to an Elekta linear accelerator. The Delta4 phantom is a verification tool to confirm dose delivery in IMRT and VMAT treatments.14 It consists of two planes of semi-conductor diodes within a cylindrical plastic phantom. These planes are at 45° to the vertical and cross at the centre of the phantom.
A typical treatment plan from one patient in the trial was transferred onto a CT image of the cylindrical Delta4 phantom using the quality assurance (QA) tools within the treatment planning system. No changes were made to the plan and the dose distribution was recalculated using the monitor units from the patient plan. This dose distribution was transferred to the Delta4 system and became the reference. The Delta4 software calculates the expected dose at the detectors from the reference data and records the measured dose. These two sets of data were compared in a variety of ways e.g. absolute dose in Gy; relative dose (isodose levels) or as a γ index at each diode position. The γ index criteria were set to 3% and 3 mm. These are used commonly for this type of phantom verification and were tighter than that used for the in vivo dosimetry. The EPID dosimetry measures the effect of the patient and the radiation delivery together; the phantom experiment was set to a tighter tolerance as the phantom was a rigid structure which could be positioned more accurately and its motion was well defined and controlled. Both tolerances are typical for the respective situations.
The Delta4 phantom was set up on the programmable motion platform which itself was placed on the couch of an Elekta linear accelerator. The couch was displaced laterally and vertically such that the platform and Delta4 phantom were in a position typical of that for a breast cancer treatment. The whole treatment plan for one fraction was delivered for four situations. The motion platform was programmed to (i) move with a sinusoidal motion to mimic FB (ii) move into a position simulating VBH (iii) move into the breath-hold position for 10 s then into the FB position with sinusoidal motion for the remainder of the fraction (iv) move into the breath-hold position for 1 s then into the FB position with sinusoidal motion for the remainder of the fraction. In the FB case, the phantom was in the correct position for FB; in the VBH case, the phantom was displaced from this FB position to the VBH position (1 cm superior and 1 cm anterior to the FB zero). In the other two cases, the phantom started in the correct VBH position but moved to the FB position after 10 and 1 s, respectively, aiming to simulate a failure of a breath hold and the inferior and posterior movement of a patient's chest, albeit on a cylindrical phantom. The motion platform and the Delta4 system were gated to the linear accelerator pulse repetition frequency circuit, so that radiation delivery, platform motion and data acquisition were synchronised.
RESULTS
Intrafraction/intrabreath-hold reproducibility
The total number of breath holds with movie loop data available for analysis was 174 from 19 patients. The intrabreath data analysis showed no detectable displacement in 162 (93%) breath holds. Of the 12 in which a displacement was seen, 10 were during the RAO field. The median magnitudes of these displacements were 1.5 mm (u—direction, 12 breath holds) and 1.5 mm (v—direction, 2 breath holds). A 3 mm maximum value (u—direction) was from one patient on one fraction for the RAO field only. The largest magnitude variations were between the first frame and subsequent frames. The comparison of the first frame in the movie loop data sets across breath holds (interbreath) within a treatment fraction gave displacements of zero in all cases.
In vivo dosimetry
Table 1 summarises the median data for the VBH and FB cohorts. None of the data were normally distributed. Mann–Whitney U tests showed a statistically significant difference between the distributions of the VBH and FB data for both LPO and RAO fields (p = 0.001 for both). There were no statistically significant differences between the point dose data (p = 0.947). The amount of lung within a treatment beam is increased slightly with VBH; this seemed a possible reason for the lower % γ <1 in the VBH in vivo dosimetry data, given the limitations of the software. Where the lung override facility of the software was used on subsets of both cohorts, the resulting median values of % γ <1 were close in magnitude: 93% and 96% for VBH and FB cases, respectively. Any remaining regions of high γ were mostly at the breast/air interface. These might be real e.g. changes in the breast shape/position, or a result of algorithm limitations either from the planning system or the EPID software. (Any issues seen for any patient were investigated at the time of treatment.) Data for the LPO field did not show a statistically significant difference between the cohorts (null hypothesis was retained; p = 0.861, Mann–Whitney U test). This was not the case for the RAO field data (92.3% c.f. 95.7%) and the dose point data (−0.8% c.f. −1.8%) where there were statistically significant but small magnitude differences (p = 0.002 for both).
Table 1.
Electronic portal imaging in vivo dosimetry data: free breathing and breath hold
| Category of in vivo data | Median (interquartile range) |
|||
|---|---|---|---|---|
| % Gamma index <1 |
Dose difference (%) |
|||
| LPO field | RAO field | Both | Reference point | |
| VBH—whole cohort | 86.6 (12.4) | 81.7 (9.9) | 83.8 (11.5) | −0.2 (1.8) |
| FB—whole cohort | 90.5 (8.5) | 89.2 (12.5) | 90.5 (10.3) | 0.1 (2.7) |
| VBH—lung override | 92.5 (7.0) | 92.3 (7.7) | 92.5 (7.3) | −0.8 (1.9) |
| FB—lung override | 93.5 (6.7) | 95.7 (4.9) | 95.9 (5.5) | −1.8 (1.7) |
FB, free breathing; LPO, left posterior oblique; RAO, right anterior oblique; VBH, voluntary breath hold.
Phantom experiment
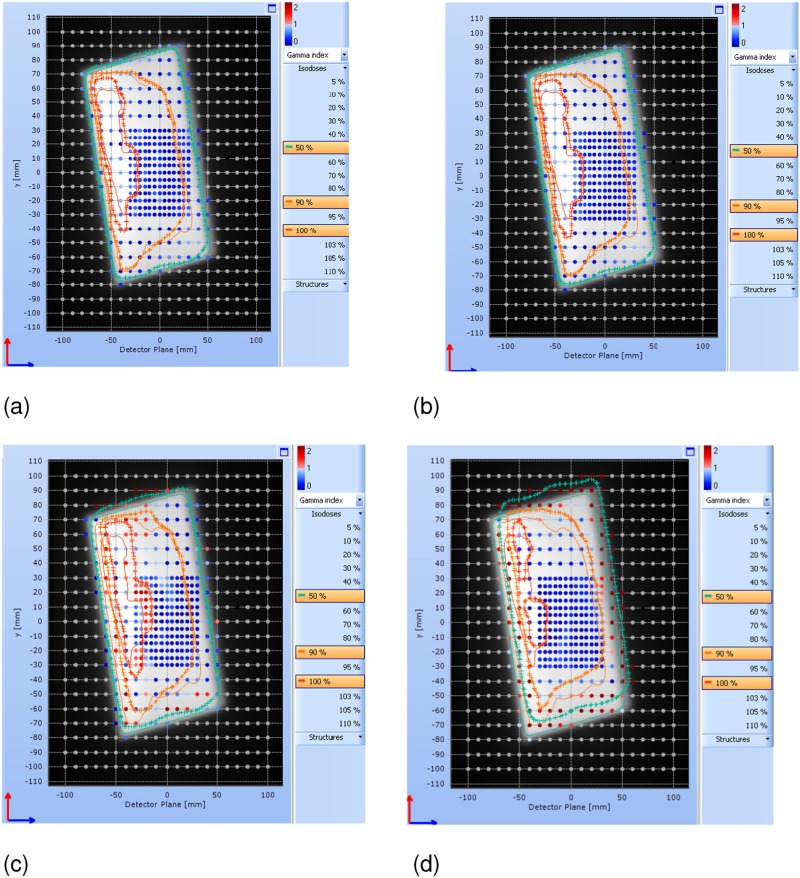
Table 2 and Figure 3a,b show that the VBH and FB data are similar and met the criteria of acceptable deviation between measured and expected dose (>90% of detectors with a γ <1 using 3% dose deviation and 3 mm distance to agreement criteria). In Figure 3a,b, the planned and measured isodose lines match, and the diode positions show blue/dark which indicates agreement within 3% and 3 mm. In the two situations where the breath hold is released early and the treatment continues (a realistic worst case situation), the median dose changes little but the dose distributions shift and the agreement with the planned dose worsens. In Figure 3c,d, the diode positions showing as red/pale indicate a measurement outside of the tolerance criteria. The isodose lines do not match well, most clearly seen in Figure 3d where the 50% isodose line extends beyond the planned treatment area in the superior direction and does not cover in the inferior direction. A similar effect of smaller magnitude is seen across the left–right of the image.
Table 2.
Results of the phantom measurements, simulating the effect of an early release of breath-hold
| Type of phantom motion | 2D % γ < 1 (3% dose difference and 3 mm distance to agreement) | 2D % γ < 1 (5% dose difference and 5 mm distance to agreement) | Median dose difference (%) |
|---|---|---|---|
| Free breathing | 94.1 | 100 | −0.3 |
| VBH | 94.4 | 100 | −0.3 |
| Lose VBH after 10 s | 78.7 | 94.2 | 0.7 |
| Lose VBH after 1 s | 61.9 | 77.5 | 0.1 |
2D, two dimension; VBH, voluntary breath hold.
Figure 3.
The four panels (a–d) show a detector plane display from the Delta4 software (Scandidos AB, Uppsala, Sweden) for each of the measured phantom experiments using the 3% and 3 mm acceptance criteria. The detector plane is perpendicular to the posterior edges of the two treatment beams for a left breast treatment (in a patient this would be the chest wall/lung interface); the top of the image is the superior direction in a patient. Figure (a) shows reference free breathing (FB) for 20 s; Figure (b) shows breath hold for 20 s; Figure (c) shows a 10-s breath hold followed by FB motion to the end of the fraction; Figure (d) shows 1 s breath hold followed by FB motion to the end of the fraction. The dots show the positions of the measurement diodes in the phantom. The colour of the dots gives the gamma index calculated at each diode position; the colour bar scale is in the top right of each panel. The grey background is the plan to which the data are compared with three isodose levels displayed: 50%, 90% and 100%. Planned data are thin lines; the crossed lines represent isodoses constructed from the measured diode data. For colour image see online.
DISCUSSION
VBH for breast radiotherapy has been shown to be as reproducible as equipment-assisted methods, more comfortable for patients, and most importantly, as effective in reducing the radiation dose to cardiac tissue.9 Issues which may delay the rapid implementation of VBH include concerns about the stability of each breath hold, the magnitude of breath hold-to-breath hold variation and the impact on delivered dose of a failure to maintain breath hold especially as these might be considered less likely to occur with equipment-assisted methods. We present an analysis of data from a clinical trial using VBH which quantifies these effects.
Whilst there are some published data on patient set-up reproducibility with VBH techniques, there are few published data on the stability within, and between, breath holds.9,10,15 Lu et al16 measured the reproducibility of position with a VBH method using repeat CT scans and showed surface variation between breath holds of 2–5 mm in all but one case which was >5 mm. The body–lung interface data given by Lu et al16 were 2–5 mm with an average of 2.6 mm. For the 19 cases and 174 breath holds assessed in this study, no discernible motion was apparent in the movie loop images in 93% of the breath holds. The maximum variation within a breath hold of 3 mm occurred for one patient in one breath hold in the final frame of the movie loop. Observable displacements were from the RAO field mainly, given the small number of breath holds with measureable displacements found in this study, it is not possible to comment on whether this is a real effect. The median of all measured displacements was 1.5 mm. Given that interobserver and intraobserver errors from electronic portal image matching have been estimated as approximately 1 mm, these results demonstrate that multiple VBHs are stable and reproducible.17,18
Brouwers et al15 published a comprehensive set of data from an in vivo dosimetry system using EPI for 15 FB and 28 VBH cases. Our in vivo dosimetry data are of similar magnitude. The median percentage γ <1 values are 84% (VBH) and 91% (FB) in our cohorts of 31 cases each, compared with 89% (VBH) and 92% (FB) from Brouwers et al.15 We agreed with Brouwers et al that there may be an effect from the tissue density variation from lung to chest wall/breast on the in vivo dosimetry results. In addition, we considered that the increased amount of lung in the VBH cases might explain the lower values compared with the FB cohort in our study. We tested this using the override facility of the software and the results give credence to this hypothesis and reassurance that measured VBH deliveries are similar to those in FB. There is no statistically significant difference in the distribution of the γ values of the LPO fields between VBH and FB cohorts with the lung override; the difference in the RAO median γ data is only 3%.
EPID dosimetry was performed only in the first week of treatment. The purpose was to act as an independent check of the whole planning and delivery process so that any error (particularly a gross error) was found and corrected at an early stage. It is possible a form of continuous change could occur during the remaining weeks of treatment (e.g. a learning curve for breath hold for a patient) which would mean EPID dosimetry data for the beginning of treatment were not representative of the rest of the treatment. The magnitude of such a change would need to be greater than the 2–3 mm population systematic and random errors of breast patient set-up to be detected. There is no evidence of this type of change with time seen in the portal images.
The phantom experiment demonstrated the effect of a failure to maintain a breath hold. The γ index acts as a surrogate for the delivered dose to the patient and degrades as expected if the breath hold is not maintained in the initial breath hold of the fraction. The realistic worst case experiments were for a situation where the treatment beam was not terminated when the breath hold failed very early and the phantom remained in the incorrect (non-breath hold) position for the remainder of the fraction.
In the two cases of breath-hold failure tested in the phantom experiment, the change in median dose was <1%. The main impact was a shift in the dose distribution, particularly in the superior and inferior direction. The change in dose is seen mostly around the edge of the treated volume (Figure 3d for the worse example in this study). The magnitude of this region will be related to the shift between FB and VBH positions for a particular patient. In our experience this is of the order of 1–1.5 cm for both the superoinferior and anteroposterior directions. It is unusual for tissue at highest risk of recurrence (i.e. tissue closest to the tumour bed) to be in the periphery of a standard treatment volume hence even where breath-hold failure occurs throughout a fraction, the clinical impact is likely to be low. No patients in the clinical trial received irradiation of the nodes. Should a breath-hold failure happen in such a patient, there would be a risk of a volume (approximately 1 cm width) at the inferior of the nodal fields receiving additional dose. This would occur only if no compensating change in position were made to the nodal fields.
The reason for breathing manoeuvres is to reduce heart dose. If it is assumed that VBH will have a mean FB heart dose then for an example change from 3 to 1.5 Gy, the effect of a breath-hold failure for one complete fraction of a 15 fraction treatment is a 7% increase in dose compared with the planning system prediction.19 If only one breath hold fails from four in the fraction, the increase in mean heart dose would be <2%. In practice, of course, the close visual monitoring of the patient by skilled therapeutic radiographers means that the probability of this situation occurring is very low.
We have shown that multiple VBHs were stable and reproducible and in vivo dosimetry data were comparable to those from FB. Delivery degradation from a breath-hold failure during one fraction of treatment is unlikely to have a negative clinical impact over the whole treatment course. This study provides further evidence of the efficacy and safety of VBH for breast cancer radiotherapy.
CONCLUSIONS
The data presented demonstrate a VBH technique with visual monitoring of breath hold is safe. The level of stability of individual breath holds, breath hold-to-breath hold variation, and the results of the dosimetric verification of the radiotherapy delivery are acceptable and are not a barrier to the widespread implementation of VBH in radiotherapy for patients with breast cancer.
FUNDING
National Institute for Health Research (NIHR) under its Research for Patient Benefit (RfPB) Programme (Grant Reference Number PB-PG-1010-23003). Ellen M Donovan is funded by an NIHR Career Development Fellowship (CDF-2013-06-005). The work was undertaken in The Royal Marsden NHS Foundation Trust which receives a proportion of its funding from the NHS Executive. NHS funding to the NIHR Biomedical Research Centre at the Royal Marsden and the ICR
Contributor Information
Ruth Colgan, Email: ruth.colgan@rmh.nhs.uk.
Matthew James, Email: Matthew.James@rmh.nhs.uk.
Frederick R Bartlett, Email: freddiebartlett@doctors.org.uk.
Anna M Kirby, Email: Anna.Kirby@rmh.nhs.uk.
Ellen M Donovan, Email: ellen.donovan@icr.ac.uk.
REFERENCES
- 1.Early Breast Cancer Trialists' Collaborative Group. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet 2011; 378: 1707–16. doi: 10.1016/S0140-6736(11)61629-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Early Breast Cancer Trialists' Collaborative Group. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet 2005; 366: 2087–106. doi: 10.1016/S0140-6736(05)67887-7 [DOI] [PubMed] [Google Scholar]
- 3.Maddams J, Utley M, Møller H. Projections of cancer prevalence in the United Kingdom, 2010–2040. Br J Cancer 2012; 107: 1195–202. doi: 10.1038/bjc.2012.366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Darby SC, Ewertz M, McGale P, Bennett AM, Blom-Goldman U, Brønnum D, et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med 2013; 368: 987–98. doi: 10.1056/NEJMoa1209825 [DOI] [PubMed] [Google Scholar]
- 5.Bartlett FR, Yarnold JR, Donovan EM, Evans PM, Locke I, Kirby AM. Multileaf collimation cardiac shielding in breast radiotherapy: cardiac doses are reduced, but at what cost? Clin Oncol (R Coll Radiol) 2013; 25: 690–6. doi: 10.1016/j.clon.2013.09.002 [DOI] [PubMed] [Google Scholar]
- 6.Zhang F, Zheng M. Dosimetric evaluation of conventional radiotherapy, 3-D conformal radiotherapy and direct machine parameter optimisation intensity-modulated radiotherapy for breast cancer after conservative surgery. J Med Imaging Radiat Oncol 2011; 55: 595–602. doi: 10.1111/j.1754-9485.2011.02313.x [DOI] [PubMed] [Google Scholar]
- 7.Schubert LK, Gondi V, Sengbusch E, Westerly DC, Siosson ET, Paliwal BR, et al. Dosimetric comparison of left-sided whole breast irradiation with 3DCRT forward-planned IMRT, inverse-planned IMRT, helical tomotherapy, and topotherapy. Radiother Oncol 2011; 100: 241–6. doi: 10.1016/j.radonc.2011.01.004 [DOI] [PubMed] [Google Scholar]
- 8.Bartlett FR, Colgan RM, Donovan EM, Carr K, Landeg S, Clements N, et al. Voluntary breath-hold technique for reducing heart dose in left breast radiotherapy. J Vis Exp 2014; 89: e51578. doi: 10.3791/51578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bartlett FR, Colgan RM, Carr K, Donovan EM, McNair HA, Locke I, et al. The UK HeartSpare Study: randomised evaluation of voluntary deep-inspiratory breath-hold in women undergoing breast radiotherapy. Radiother Oncol 2013; 108: 242–7. doi: 10.1016/j.radonc.2013.04.021 [DOI] [PubMed] [Google Scholar]
- 10.Bartlett FR, Colgan RM, Donovan EM, McNair HA, Carr K, Evans PM, et al. The UK HeartSpare Study (Stage 1B): randomised comparison of a voluntary breath-hold technique and prone radiotherapy after breast conserving surgery. Radiother Oncol 2015; 114: 66–72. doi: 10.1016/j.radonc.2014.11.018 [DOI] [PubMed] [Google Scholar]
- 11.Wendling M, Louwe RJ, McDermott LN, Sonke JJ, van Herk M, Mijnheer BJ. Accurate two-dimensional IMRT verification using a back-projection EPID dosimetry method. Med Phys 2006; 33: 259–73. doi: 10.1118/1.2147744 [DOI] [PubMed] [Google Scholar]
- 12.Low DA, Harms WB, Mutic S, Purdy JA. A technique for the quantitative evaluation of dose distributions. Med Phys 1998; 25: 656–61. doi: 10.1118/1.598248 [DOI] [PubMed] [Google Scholar]
- 13.Wendling M, McDermott LN, Mans A, Olaciregui-Ruiz Í, Pecharronmán-Gallego R, Sonke JJ, et al. In aqua vivo EPID dosimetry. Med Phys 2012; 39: 367–77. doi: 10.1118/1.3665709 [DOI] [PubMed] [Google Scholar]
- 14.Bedford JL, Lee YK, Wai P, South CP, Warrington AP. Evaluation of the Delta4 phantom for IMRT and VMAT verification. Phys Med Biol 2009; 54: N167–76. doi: 10.1088/0031-9155/54/9/N04 [DOI] [PubMed] [Google Scholar]
- 15.Brouwers PJ, Lustberg T, Borger JH, van Baardwijk AA, Jager JJ, Murrer LH, et al. Set-up verification and 2-dimensional electronic portal imaging device dosimetry during breath hold compared with free breathing in breast cancer radiation therapy. Pract Radiat Oncol 2015; 5: e135–41. [DOI] [PubMed] [Google Scholar]
- 16.Lu HM, Cash E, Chen MH, Chin L, Manning WJ, Harris J, et al. Reduction of cardiac volume in left-breast treatment fields by respiratory maneuvers: a CT study. Int J Radiat Oncol Biol Phys 2000; 47: 895–904. doi: 10.1016/S0360-3016(00)00512-5 [DOI] [PubMed] [Google Scholar]
- 17.Court LE, Allen A, Tishler R. Evaluation of the precision of portal-image-guided head-and-neck localization: an intra- and interobserver study. Med Phys 2007; 34: 2704–7. doi: 10.1118/1.2747050 [DOI] [PubMed] [Google Scholar]
- 18.Lewis DG, Ryan KR, Smith CW. Observer variability when evaluating patient movement from electronic portal images for pelvic radiotherapy fields. Radiother Oncol 2005; 74: 275–81. [DOI] [PubMed] [Google Scholar]
- 19.Vikström J, Hjelstuen MH, Mjaaland I, Dybvik KI. Cardiac and pulmonary dose reduction for tangentially irradiated breast cancer, utilising deep inspiration breath-hold with audio-visual guidance, without compromising target coverage. Acta Oncol 2011; 50: 42–50. doi: 10.3109/0284186X.2010.512923 [DOI] [PubMed] [Google Scholar]