Abstract
In vivo cell tracking has emerged as a much sought after tool for design and monitoring of cell-based treatment strategies. Various techniques are available for pre-clinical animal studies, from which much has been learned and still can be learned. However, there is also a need for clinically translatable techniques. Central to in vivo cell imaging is labelling of cells with agents that can give rise to signals in vivo, that can be detected and measured non-invasively. The current imaging technology of choice for clinical translation is MRI in combination with labelling of cells with magnetic agents. The main challenge encountered during the cell labelling procedure is to efficiently incorporate the label into the cell, such that the labelled cells can be imaged at high sensitivity for prolonged periods of time, without the labelling process affecting the functionality of the cells. In this respect, nanoparticles offer attractive features since their structure and chemical properties can be modified to facilitate cellular incorporation and because they can carry a high payload of the relevant label into cells. While these technologies have already been applied in clinical trials and have increased the understanding of cell-based therapy mechanism, many challenges are still faced.
INTRODUCTION
Cell-based therapies have, in recent years, been recognized as an important therapeutic option in healthcare.1 Based on the plasticity and migratory capacity of cells, cell-based therapeutics offer unique possibilities in regenerative medicine, cancer treatment and metabolic diseases.2–5 For these applications, the ability of cells to repair damaged tissue, act as drug carriers or modulate or enhance natural cellular processes is used as a treatment strategy. Crucial issues for guaranteeing safe and effective use of cell transplants are in determining the most optimal cell type, the route, dose, accuracy and timing of administration, and the persistence and functionality of the transplanted cells. To effectively address these issues, non-invasive visualization of the in vivo fate of the transplanted cells may be crucial.6
In the past decade, various in vivo cell imaging techniques have been developed that enable researchers to track transplanted cells in real-time in vivo by optical imaging (OI), MRI single photon emission tomography (SPECT) or positron emission tomography (PET).7,8 Central to these techniques is the labelling or tagging of the cells prior to transplantation. The most commonly used and the easiest way to achieve this is by introducing a labelling agent into the cells by exposing the cells to the labelling agent in culture.9–11 The cells then actively incorporate the particles through endocytotic pathways where they generally end up in endosomal compartments.12 The now cell-associated labelling agent then serves as the signalling beacon by which transplanted cells can be identified in imaging studies (Figure 1). An alternative way of labelling cells is an indirect approach by introducing a reporter gene into the cells of interest. This technology offers various advantages regarding the in vivo monitoring of cell fate and function but while widely used in animal models, this approach is currently far from clinical translation and beyond the scope of this review. Interested readers are referred to other reviews dealing with this technology.13,14
Figure 1.
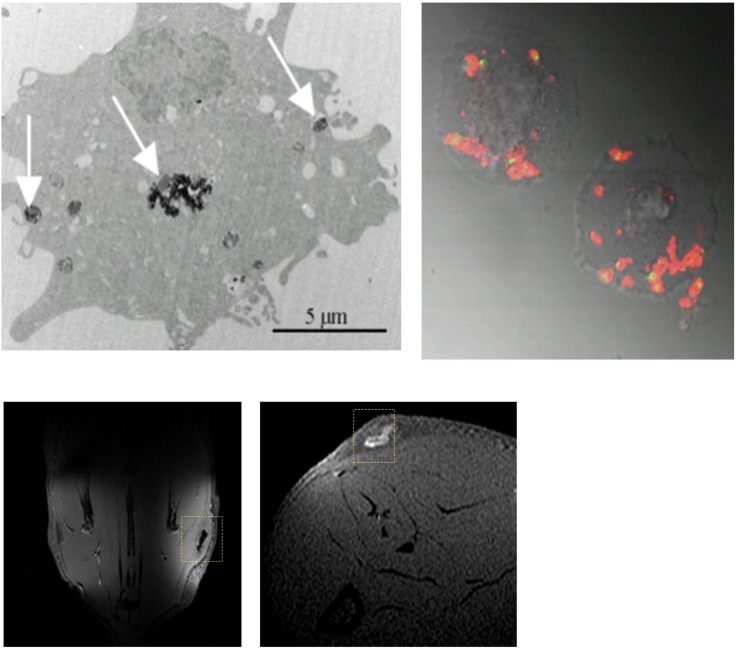
Nanoparticle labelling and imaging of cells. Top panels: an electron microscopy (left) and fluorescent microscopy (right) image of human umbilical vein cells labelled with iron oxide nanoparticles and fluorescent Gd–liposomes, respectively, showing intracellular presence of the nanoparticles after labelling procedure. Arrows indicate intracellular deposits of iron oxide nanoparticles. Bottom panels: magnetic resonance images obtained from rats injected subcutaneously with cells labelled with iron oxide particles or Gd–liposomes (liposomes containing gadopentetate dimeglumine in the water phase).
The main challenge encountered during the cell labelling procedure is to efficiently incorporate the label into the cell, such that the labelled cells can be imaged at high sensitivity for prolonged periods of time, without the labelling process affecting the functionality of the cells. In this respect, nanoparticles offer attractive features since their structure and chemical properties can be modified to facilitate cellular incorporation and because they can carry a high payload of the relevant label into cells.15
The various imaging techniques each have their own advantages and disadvantages regarding their use in cell tracking studies. OI techniques offer various advantages and have been widely used in pre-clinical studies. The limited tissue penetration capability of light, however, limits the use of these techniques to a large extent to small laboratory animals.16 Studies aimed at clinical translatability, have therefore focused on MRI, PET or SPECT, which are not limited by signal penetration depths in tissue.8,17 However, despite the fact that, as of yet, the only FDA-approved cell tracking agent is Indium-111 (111In)-oxine, the use of nuclear imaging techniques for in vivo cell tracking beyond lymphocyte scintigraphy, has been limited by concerns regarding radiation damage to cells and the generally short half-life of suitable radioisotopes (in the order of 2 h–6 days). In addition, issues regarding limited intracellular retention of the most commonly used agents are considered an important disadvantage of nuclear imaging approaches for cell tracking.8,17 Currently, MRI is regarded as the imaging technique of choice for clinically applicable cell tracking. The main advantages of MRI over other techniques are its excellent three-dimensional anatomical imaging capabilities at high resolution together with functional imaging capabilities. The fact that no ionizing radiation is needed makes it therefore suitable for non-invasive and repeatable imaging.
Imaging agents for MRI-based cell tracking can be subdivided into the following categories: superparamagnetic contrast agents (typically containing iron oxide), paramagnetic contrast agents (typically containing gadolinium or manganese), chemical exchange saturation transfer (CEST) agents and non-proton contrast agents (typically containing fluorine). Each of these categories of contrast agents have specific properties with associated advantages and limitations. In many cases, these agents have been used in the form of nanoparticles in order to increase their biocompatibility, delivery efficiency and/or signalling properties. In the following sections, we will discuss the role of the most commonly used nanoparticles and in in vivo cell tracking, including recent developments. Some of the key features of these cell labelling agents are summarized in Table 1.
Table 1.
Most commonly used types of contrast agents (probes) for MRI-based cell tracking
| Probe | Basic imaging principle | Main advantage | Main disadvantage | Detection sensitivity (number of cells)f | Used for cell tracking in human subjects |
|---|---|---|---|---|---|
| Iron oxide nanoparticles | Shortening T2 relaxation of surrounding water protons | High sensitivity | Lack of specific signal; i.e. signal loss | 1 cella,18,19 | Yes20–29 |
| Gd-based nanoparticles | Shortening T1 relaxation of surrounding water molecules | Giving rise to signal enhancement | Issues regarding toxicity | 300–3000 cellsb,30–32 | No |
| Manganese-based nanoparticles | Shortening T1 relaxation of surrounding water molecules | Natural body mineral | Issues regarding toxicity | 1000–100,000 cellsc,33–35 | No |
| Chemical exchange saturation transfer agents | Transfer of selectively saturated, exchangeable spins to surrounding bulk water via chemical exchange | Multispectral imaging | Requires specialized imaging techniques | 10,000 cellsd,36 | No |
| 19F-based nanoparticles | Magnetic spin of 19F nuclei | Not naturally present in body, therefore providing unique signal | Requires specialized imaging techniques | 2000–9000 cellse,37,38 | Yes39 |
19F, fluorine-19.
at intracellular concentrations of 9–50 pg/cell; voxel size 0.26–1 × 10−3 mm3.
at intracellular Gd concentrations of 0.05–70 pg/cell; voxel size 2.24–10.3 × 10−3 mm3.
at intracellular Mn concentrations of 0.35–0.7 pg/cell; voxel size 160 × 10−-3 mm3 (only specified by Letourneau et al.35).
at intracellular CEST agent concentrations of 3–4 mM/cell; voxel size 24–73 × 10−3 mm3.
at intracellular 19F concentrations of 0.35–0.7 pg/cell; voxel size 660–2000 × 10−3 mm3.
Detection sensitivity is highly dependent of various conditions, such as the cell type, intracellular loading, imaging parameters including voxel size, and magnetic field strength.
SUPERPARAMAGNETIC IRON OXIDE NANOPARTICLES
Superparamagnetic iron oxide (SPIO) particles typically consist of a crystalline iron oxide (Fe3O4 or Fe2O3) core coated with a hydrophilic shell of dextran, citrate, polymers or lipids. The iron oxide crystals have a strong magnetic moment, causing a disturbance of the local magnetic field by which they affect the T2 relaxation of surrounding water protons resulting in local signal loss in MR images. After some initial reports in the early nineties, showing the feasibility of tracking iron oxide-labelled cells by MRI,40,41 many studies followed in which a large variety of iron oxide nanoparticles was studied for their use as cell tracking agents.42,43 This field of research was especially boosted by the major advances made in stem cell biology and the still increasing interest in cell-based therapies,1,2 and the realization that in vivo cell tracking technology could help in the development of safe and effective cell-based treatment strategies. Rapid clinical implementation appeared feasible when it was shown that cells could be labelled efficiently with commercially available SPIO particles that were FDA-approved as a liver contrast agent, i.e. ferumoxides and ferucarbotran.44,45 These agents were indeed used in early clinical studies (Table 2), but some practical and technical limitations ultimately prevented more widespread clinical use of SPIO-based cell tracking. Possibly, the first main reason for the limited number of clinical studies with SPIO particles is the fact that manufacturing of the aforementioned preparations was discontinued in the late 90s because of lack of sales for its FDA-approved application for the detection of liver tumours.21,46 Besides this lack of availability of clinically approved SPIO particles, a number of technical limitations in the use of SPIO particles for cell tracking was also revealed, probably contributing to reduced interest in this technology. The main limitations of SPIO particles are a lack of specificity of the signal,47 persistence of extracellular deposits48,49 and very complex quantifiability (Figure 2). Even though in some studies a linear relationship between iron oxide concentration and R2/R2* values50,51 has been demonstrated, the reliability of such methods is limited since the R2/R2* values are also dependent on intravoxel distribution.52–54
Table 2.
Clinical trials with MRI-based cell tracking of magnetic nanoparticle-labelled cells
| Study subjects | Cell type | Number of cells and route of administration | Cell labelling agent | Follow-up time with imaging | Main reported finding(s) | Publication year | |
|---|---|---|---|---|---|---|---|
| Patients with melanoma | Autologous dendritic cells | 7.5 × 106 cells/intranodal | Ferumoxide (Endorem; Guerbet) | 2 days after transplantation | MRI provided the ability to assess the accuracy of dendritic cell delivery and of internodal and intranodal cell migration patterns | 200520 | |
| Patients with traumatic brain injury | Autologous neural stem cells | Cell number not specified/intracerebral around the area of brain damage | Ferumoxidea (Feridex®; AMAG pharmaceuticals) | 24 h and every 7 days for 10 weeks after transplantation | Visualization of neural stem cell proliferation and migration from injection site to perilesional areas based on dynamic signal changes that were similar to patterns of migration observed in a subsequent study performed in rats | 200628 | |
| Patients with chronic spinal cord injury patients | Autologous bone marrow-derived CD34+ cells | 0.45–1.22 × 106 cells/intrathecal into the spinal cord | CD34 monoclonal antibody-coated, micrometre-sized magnetic beads (DynalBeads®; Dynal Biotech) | 20 days and 35 days after transplantation | Visualization of hypointense signal areas at the site of injection that redistribute over time suggesting migration of the transplanted cells from the injection site to the lesion site | 200729 | |
| Patients with Type 1 diabetes | Pancreatic islets | 28–58 × 104 pancreatic islet equivalents through multiple time-spaced injections/intraportal | Ferucarbotran (Resovist, Schering) | 5 days, 6 weeks and 6 months after transplantation | Visualization of transplant-associated hypointense spots in the liver after transplantation that persisted over a period of up to 6 months. Labelling of pancreatic islets did not affect their functionality as evidenced by their retained insulin-producing capacity. Iron overload in the liver as often seen in patients with diabetes interferes with detection of labelled islets | 200827 | |
| Patients with Type 1 diabetes | Pancreatic islets | 7–68 × 104 pancreatic islet equivalents/intraportal | Ferucarbotran (Resovist®, Schering) | 1, 4 and 24 weeks after transplantation | Visualization of pancreatic islets grafts in the liver via hypointense signal spots. Gradual decrease of the number of hypointense spot over time to 40% of original number. Sensitivity of spot detection was related to the length of labelling time of the pancreatic islets with iron oxide particles. No indications of adverse effects were found | 201026 | |
| Patients with Multiple sclerosis (MS) and amyotrophic lateral sclerosis (ALS) | Autologous bone marrow-derived mesenchymal stem cells | 60–100 × 106 cells/intrathecally and intravenously at a ratio of 2 : 1 of total dose | Ferumoxideb (Feridex; AMAG pharmaceuticals) | 4 to 48 hours and 1, 3 and 6 months after MSC infusion | MRI indicated possible dissemination of the MSCs from the lumbar site of inoculation to the occipital horns, meninges, spinal roots and spinal cord parenchyma. Observed data support the feasibility for intrathecal injection of cells for treatment of MS and ALS. No cell labelling-associated effects were observed | 201024 | |
| Severe global brain ischaemic injury patient (infant)e | Autologous cord blood-derived neural progenitors | 12 × 106 cells (25% labelled with iron oxide)/intraventricular in the brain | Ferumoxidec (Endorem; Guerbet) | 1 day, 1 week, and 1, 2 and 4 months after transplantation. | MRI revealed persistent (4 months) alignment of cell graft (hypointense) along lateral ventricle wall, without evidence of migration into the brain parenchyma. No cell labelling-associated adverse effects were observed. | 201023 | |
| Healthy volunteers | Peripheral blood mononuclear cells | 1–10 × 108 cells/intramuscular or intravenous | Ferumoxide d(Endorem; Guerbet) | 7 days after transplantation | MRI allowed for clear visualization of cell graft. Cell dose-dependent reduction of signal intensity in liver and spleen following intravenous cell injection, indicating dose-dependent accumulation of injected cells in these organs. Migration of labelled cells to an induced site of inflammation (performed in one volunteer). No cell labelling-associated adverse effects were observed. | 201225 | |
| Patients with Type 1 diabetes | Pancreatic islets | 32 × 104 pancreatic islet equivalents/intraportal | Ferucarbotran (Resovist, Schering) | 6 months after transplantation | Feasibility of using positive MRI techniques of iron oxide-labelled cells in a clinical setting with potentially improved transplanted cell identification capabilities. | 201421 | |
| Severe global brain ischaemic injury patient (infant)e | Autologous cord blood-derived neural progenitors | 12 × 106 cells (25% labelled with iron oxide)/intraventricular | Ferumoxidec(Endorem; Guerbet) | 1 day, 1 week, and 1, 2, 4, 33 months after transplantation. | Total dissipation of the labelled cell-associated hypointense spots over prolonged period of time. Demonstrated in vitro feasibility of forced migration of iron-labelled cells by an external magnet. | 201422 | |
| Patients with colorectal adenocarcinoma | Autologous dendritic cells | 1–10 × 106 cells/intradermal | Clinical grade perfluorocarbon nanoparticles (CS-1000, Celsense, Inc) | 4 and 24 h after transplantation | Visualization of cell graft at site of injection at 4 h post transplantation with a approximately 50% reduction of signal at 24 h using a scan time of 10 minutes. No imaging evidence of migration of labelled cells was found possibly due to limited detection sensitivity. No cell labelling-associated adverse effects were observed | 201439 |
Endoorem, Guerbet, Sulzbach, Germany; Feridex, AMAG Pharmaceuticals, Cambridge, WA; Dynal beads, Dynal Biotech, Osio, Norway; Resovist, Schering, Berlin, Germany; CS-1000, Celsense Inc., Pittsburgh, PA.
A non-clinical grade transfection agent (Effectene®; Qiagen, Hilden, Germany) was used to promote cellular uptake of the iron oxide nanoparticles.
The transfection agent poly-l-lysine was used to promote cellular uptake of the iron oxide nanoparticles.
The transfection agent poly-l-lysine (Sigma, St Louis, MI) was used to promote cellular uptake of the iron oxide nanoparticles.
Clinical grade protamine sulphate was used as a transfection agent to promote cellular uptake of the iron oxide nanoparticles.
Figure 2.
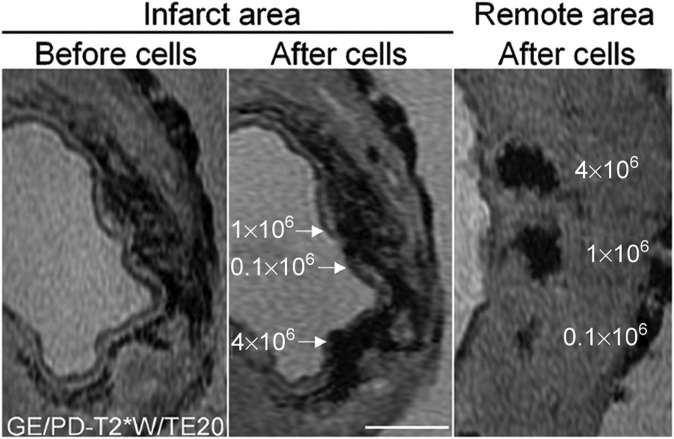
Limited signal specificity of the iron oxide-labelled cells injected intramyocardially in a porcine myocardial infarction model. The left panel shows gradient echo scan, before injection of iron oxide-labelled cells. The middle panel shows the same slice after injection with 0.1, 1 or 4 × 106 iron oxide-labelled cells. The right panel shows a similar series of injections in remote, non-infarcted myocardium. Although the cell injections create larger areas of signal voids in the middle panel, their precise location cannot be determined because of the signal voids induced by the presence of haemoglobin degradation products. Bar indicates 0.5 cm. Reprinted from van den Bos et al47 with permission from Oxford University Press.
However, despite these limitations, SPIO particles are still considered of interest for cell tracking in clinical studies.21,22,55,56 Main incentives for this continued interest in SPIO particles are the fact that as of yet, they appear to provide the highest detection sensitivity (Table 1), and the vast amount of available data on the safety of the use of SPIO. From the FDA-approved use of ferumoxide and ferucarbotran for liver tumour imaging, systemic safety of SPIO was established. In addition, many studies using these agents for labelling of a variety of cells demonstrated safety of the use of SPIOs for intracellular labelling. In general, no adverse effects on cell survival or cell functionality in terms of differentiation capacity, cytokine release profiles or migratory capacity have been observed. Also in a recently published article in which healthy volunteers were injected with SPIO-labelled peripheral blood mononuclear cells, in vivo safety of SPIO cell injections was demonstrated.25 Therefore, there are continued efforts in generating new, optimized SPIO particles for cell labelling and imaging.57–59 However, major challenges are faced in going through the regulatory requirements55,56 and the costs involved may be prohibitive in bringing such particles to the clinic.56,60 Current strategies seem to be focused on off-label use of already FDA-approved components in generating a SPIO-based intracellular labelling agent.56,61
PARAMAGNETIC GADOLINIUM-BASED NANOPARTICLES
Gadolinium(III) chelates are the most commonly used contrast agents in clinical MRI and are generally characterized as T1 or positive contrast agents. The seven unpaired electrons of the Gd3+ ion create a magnetic moment that accelerate the relaxation of surrounding water protons typically resulting in signal enhancement on MR images. The possibility of obtaining positive contrast from labelled cells instead of negative contrast as obtained with SPIOs has been considered a major advantage and stimulated the use of Gd-based nanoparticles for cell labelling. Particulate Gd contrast agents were shown to be far more effective than regular Gd chelates in terms of the amount of Gd initially incorporated and the retention of Gd in the cell over time.31,62,63 Various particulate formulations of Gd-based cell tracking agents have been developed over the past few years including liposomal31,64 and micellar63,65 nanoparticles, polymer-coated Gd–oxide particles32,66,67 and carbon nanostructures68 in order to increase cellular loading with Gd or to increase the signalling capacity by increasing the T1 relaxivity.
A remarkable finding with Gd-based cell labelling has been that Gd-labelled cells showed different contrast behaviour depending on the labelling strategy used and/or the specific environmental circumstances. In contrast to an expected signal enhancement, clusters of Gd-labelled cells were found to give rise to signal loss under both in vivo and in vitro conditions.31,69,70 Explanations for this phenomenon include aspects regarding the local concentration of Gadolinium and the effect of compartmentalization of the Gd-based agents influencing the rate of exchange and the availability of free water protons. Capitalizing on the compartmentalization effect, Guenoun et al71 proposed using the changing contrast behaviour of cells labelled with gadolinium liposomes as a read out method for assessing the functional status of injected cells (Figure 3). They showed that at an identical Gd concentration, Gd incorporated inside the cell gave rise to signal loss while Gd released from non-viable cells resulted in signal gain which quickly dissipated.31,71
Figure 3.
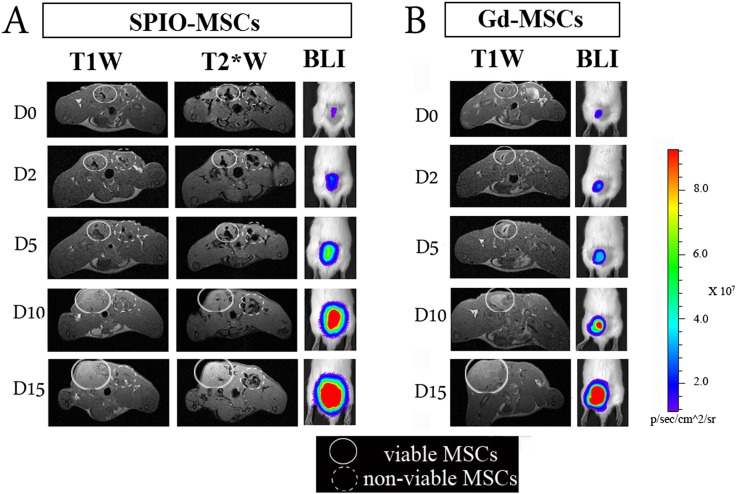
Monitoring the cellular status of cells by situation-dependent contrast behaviour of Gd. MSCs were labelled with either Gd–liposomes or iron oxide particles and an optical reporter gene (firefly luciferase). Following labelling, cell populations were split in two identical samples. One part was then submitted to repeated freeze-thawing to generate non-viable intact cells. Dual-labelled cells were injected intramuscularly into the lower back of rats, i.e. viable labelled cells on the left side and non-viable cells on the right side. Rats were imaged by MRI (3.0 T) and bioluminescent imaging at several time points over a period of 2 weeks. (a) SPIO–MSCs caused a signal void (hypointensity), regardless of the cell viability. In the acute post-transplantation stage, no substantial differences in visual appearance were detected between viable and non-viable SPIO-MSCs. (b) Viable Gd–MSCs showed a different dynamic signal behaviour compared with non-viable MSCs. Immediately post-transplantation, viable MSCs were consistently detected as a hypointense area on T1 weighted scans (“quenched signal intensity”), whereas a similar density of non-viable Gd–MSCs resulted in increased signal intensity (hyperintensity) at the injection site. In contrast to SPIO–MSCs, hyperintense signal from non-viable Gd–MSCs had already resolved after 2 h post-transplantation. An increased signal intensity on BLI images reflects the cell proliferation that contributed to the tracer dilution observed by MRI.T1W, T1 weighted; T2*W, T2* weighted; BLI, bioluminescence imaging; MSCs, mesenchymal stem cells. Reprinted from Guenoun et al71 with permission from John Wiley and Sons.
Despite the fact that various studies showed limited or no adverse effects of the Gd-based labelling agents on the functionality of labelled cells at relevant labelling concentrations,31,62,63,66,68,72 major concerns regarding the toxicity of the long-term presence of ionic Gd exist,70,73,74 which may limit introduction of such agents into the clinic. Gadolinium contrast agents have been associated with the occurrence of nefrogenic systemic fibrosis in patients with impaired kidney functions, and Modo et al74 reported on a negative effect on disease pathology of implanted neural stem cells labelled with the Gd-based contrast agent GRID (Gadolinium-Rhodamine Dextran) in a rat stroke model.
PARAMAGNETIC MANGANESE-BASED NANOPARTICLES
Similar to Gd, manganese is also known as a T1 contrast agent, shortening the relaxation time of surrounding water protons. Because of the concerns regarding the cellular toxicity of Gd ions, manganese has been studied as an alternative positive contrast agent for cell labelling. Manganese is a natural cellular component and functions, for instance, as a cofactor for enzymes and receptors. Manganese as a contrast agent has been used in the form of manganese chloride (FDA-approved). Initial studies on the use of manganese-based agents for cell labelling and imaging also used MnCl2.75 Moreover, because of the fact that manganese is transported by calcium channels, MnCl2 has been proposed as a cell labelling agent by which a direct read out of the viability of cells would be possible.76 Because of the low relaxivity properties of MnCl2, nanoparticle formulations of manganese have been explored as T1 agents for cell labelling. This involves manganese oxide particles with variations in coating with the goal to improve biocompatibility, stability and/or relaxivity.34,35,77 Most recently silica-coated MnO particles were shown to have excellent relaxivity properties, also at magnetic field strengths >3.0 T. This allowed for highly sensitive, positive contrast detection of MnO-labelled cells; in the order of several thousand cells (Table 1). Unfortunately, however, in various reports significant effects of MnO particles on cell functionality have also been reported, such as reduced cell survival33,76,78 and impaired multipotent differentiation capacity.34,79 These findings may significantly reduce interest for clinical translation of such approaches.
CHEMICAL EXCHANGE SATURATION TRANSFER -AGENT NANOPARTICLES
CEST agents are a special class of contrast agents for MRI. CEST agents contain slow exchangeable protons that can be selectively saturated by an off-resonance pulse upon which the saturation is transferred to surrounding bulk water via chemical exchange.80 The possibility to generate multiple contrast signatures by choosing agents with unique and different resonance frequencies, offered the attractive feature of imaging different cell populations in the same anatomical site, by labelling each cell population with a different CEST contrast agent.36 Like with T1 agents, the use of CEST agents also has been limited by sensitivity issues. Therefore, much effort has been put into the generation of macromolecular and nanoparticle-based CEST agents for molecular imaging and cell tracking purposes.80–82 A type of nanoformulation that has been receiving most interest in this respect is liposomal-based CEST agents.82,83 While these nanoformulations of CEST agents were shown to have unique features and the potential for interesting molecular imaging applications, their use as efficient intracellular labels was negated by the finding that upon internalization of liposomal CEST agents, image contrast of these agents is significantly reduced.84 Nonetheless, particulate CEST agents are still considered of interest in the monitoring of cell-based treatment strategies.8 In a recent study, Chan et al85 demonstrated the use of a liposomal CEST agent as a nanosensor for monitoring survival of transplanted cells. In this study, the liposomal CEST agent was encapsulated together with hepatocytes in an alginate hydrogel. Due to the pH-sensitive characteristics of the CEST agent, changes in pH within the hydrogel resulting from cell death could be monitored and used as a measure for cell survival (Figure 4). The authors concluded that since all components used were clinical grade, this approach lends itself for clinical translation. Currently there are, to our knowledge, no reports on severe adverse effects of CEST agents on cell functionality.
Figure 4.
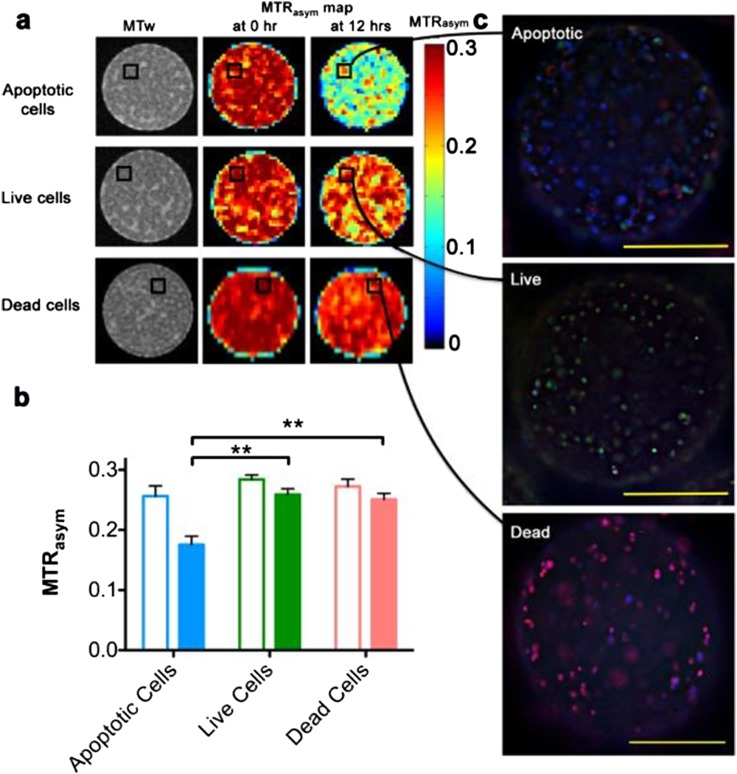
Imaging the functional status of cells by lipoCEST nanosensors. (a) MR images of LipoCEST capsules containing hepatocytes. Shown are magnetization transfer-weighted (MTw) images and magnetization transfer ratio (MTR) asymmetry (MTRasym) maps at 2 ppm of various cell samples. “Apoptotic cells”: LipoCEST capsules containing hepatocytes before (0 h) and after (12 hrs) addition of 50 μM staurosporine. “Live cells”: LipoCEST capsules containing hepatocytes without the addition of staurosporine imaged at time points 0 and 12 h. “Dead cells”: LipoCEST capsules containing hepatocytes treated with STS before encapsulation imaged at time points 0 and 12 h. (b) MTRasym for the three groups at 0 h (open bars) and 12 h (solid bars). (c) Fluorescence overlay images of capsules from the STS and control phantoms shown in (a). Samples are stained for live cells (fluorescein diacetate, green), dead cells (propridium iodide, red) and apoptotic cells (Annexin V, blue). Scale bar = 200 μm. ** indicates statistical significance for the difference in measured values. Reprinted from Chan et al85 with permission from Nature Publishing Group.
Fluorine-19-BASED NANOPARTICLES
A cell labelling strategy that has been gaining increasing interest in recent years lies within the use of fluorine-19 (19F)-based contrast agents. This non-proton-based imaging strategy offers an important advantage from the fact that 19F is not naturally present in the body, consequently endowing 19F-labelled cells with a highly specific signal.86 Additionally, 19F MRI allows for robust quantification of labelled cells.87 These characteristics, together with the fact that biologically inert and stable 19F formulations exist and have already been used in clinical settings as oxygen carriers88 or ultrasound contrast agent (http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm418509.htm) have further promoted this interest for 19F-based cell imaging. However, 19F imaging does require some dedicated hardware on a MRI system, which is usually not routinely available in most MRI centres.
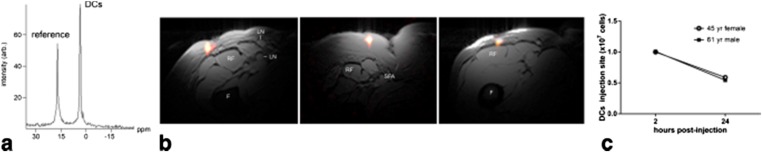
For cell labelling, studies have mainly focused on the use on perfluorocarbons (PFCs).10 A main advantage of PFCs is that each molecule contains a high number of 19F nuclei, increasing the signalling capacity per molecule. In order to promote biocompatibility and cell loading efficiency, formulations of PFCs used for cell labelling and imaging generally consist of coated nanoemulsions or polymer-based nanoparticles.10,89,90 A nanoemulsion-based formulation of PFCs was also used in the very recently published first report on 19F-based cell imaging in humans.39 In this study, dendritic cells (DCs) were labelled with a PFC nanoemulsion formulation, with the purpose of visualizing the DCs after intradermal administration as part of a Phase-I trial for DC-based immunotherapy of Stage-4 colorectal cancer (Figure 5). While through this study basic clinical feasibility of 19F-based cell tracking was demonstrated, the current main concern of 19F-based cell imaging, i.e. limited sensitivity, was also highlighted. In this study, three patients received a DC injection dose of 1 × 107 cells which could be reliably detected and quantified 4 h after injection. In contrast, in two patients injected with a DC dose of 1 × 106 cells no reliable detection of the cell transplant was possible. For the patients who received 1 × 107, the calculated average intravoxel cell concentration was between 4 × 105 and 6.25 × 105 cells/voxel. In comparison, in the first reported human application of iron oxide-based cell imaging a significantly lower detection sensitivity of labelled cells was demonstrated.20 In this study, a detection sensitivity of 2 × 103 cells/voxel was demonstrated. Nonetheless, the report by Ahrens et al39 is encouraging and will certainly further inspire already ongoing efforts into the development of improved 19F-based cell imaging techniques. These involve efforts to increase cellular uptake by probe modifications or the use of transfection agents that promote cellular uptake of particles,91–93 probe modifications that increase the signalling capacity per molecule,94,95 and hardware and software developments.37
Figure 5.
In vivo imaging of dendritic cells (DCs) labelled with fluorine-19 (19F) nanoparticles injected intradermally into quadriceps of patients with colorectal cancer. In these patients, approximately 1 × 107 labelled cells were injected. (a) A representative 19F MRS spectrum of patient at 4 h post inoculation. The DCs appear as a single narrow peak. “Reference” is from an external tube containing triflouroacetic acid placed alongside the patient. (b) Axial composite 19F/1H images of the right thigh at 4 h post inoculation in three patients, a 53-year-old female (left), a 45-year-old female (middle) and a 61-year-old male (right), where the DCs are rendered in “hot-iron” pseudocolor and the 1H anatomy is displayed in greyscale (F, femur; RF, rectus femoris; SFA, superficial femoral artery; LN, inguinal lymph node). (c) The results of the in vivo quantification of apparent cell numbers using the 19F MRI data, measured in two patients. By approximately 24 h post inoculation, roughly half of the injected DCs were still present at the injection site. Reprinted from Bonetto et al39 with permission from John Wiley and Sons.
NANOPARTICLE CHARACTERISTICS THAT MAY AFFECT CELL LABELLING EFFICIENCY AND TOXICITY
For nanoparticles to be used for cell labelling purposes, main requirements that should be met are biocompatibility, stability and high contrast generating properties. In the past years various advances in nanotechnology have increased the possibilities in producing a large variety of nanoparticles towards this end. However, various studies have also demonstrated that in practice, limitations regarding these issues are still encountered. In the next paragraphs, we will highlight some of these findings in which specific nanoparticle characteristics are responsible for, sometimes unexpected, effects on cell labelling and/or imaging efficiency and cellular toxicity. In some cases, however, these effects seem to be strongly cell type dependent.
Effects of nanoparticle size
The effect of nanoparticle size on its performance as an intracellular label has been extensively studied and described for iron oxide particles.96–101 In general, with increasing particle size (up to several microns) more efficient cellular uptake and signalling capacity have been observed. However, also some contradictory results have been reported, where smaller particles showed higher labelling efficiency.102 Effects of particle size have also been related to some adverse consequences, i.e. reduced migration capacity of DCs.103 In this study, it was shown that migration of DCs labelled with iron oxide nanoparticles was reduced compared with unlabelled cells. The reduction in migration capacity was stronger for larger particles (0.9 µm) compared with smaller particles (80–120 nm). Reduced migratory capacity or cell motility has also been described for iron oxide-labelled neural stem cells and mesenchymal stem cells.49,104 However, migratory capacity was restored following active exocytosis of the iron oxide nanoparticles. The speed of exocytosis has also been reported to be dependent on nanoparticle size.105 In a study by Xu et al106 larger PLGA-encapsulated iron oxide nanoparticles had a 3-fold longer retention in mesenchymal stem cells compared with smaller iron oxide nanoparticles. Active exocytosis has also been reported for various other types of nanoparticles107–109 and can occur via lysosome secretion, vesicle-related secretion and non-vesicle-related secretion.105 Release of nanoparticles either by active exocytosis or following cell death is considered one of the main disadvantages of the use of nanoparticles for cell labelling and imaging. The released nanoparticles can reside for a long time or be taken up by other cells and thus create a contrast agent-related signal that is not related to the presence of the transplanted cell itself.51,71,110–113 In the study by Guenoun et al,71 it was shown that prolonged retention of released cell label is dependent on nanoparticle characteristics. In this study, a comparison was made between iron oxide nanoparticles and Gd–liposomes (liposomes containing gadopentetate dimeglumine in the water phase) as cell labelling agents for monitoring the in vivo fate of transplanted cells. Both viable and non-viable cells were injected subcutaneously and monitored over time by MRI. For cells labelled with Gd–liposomes, the cell-associated signalling effect disappeared rapidly when non-viable cells were injected, while the signalling effect of non-viable cells labelled with iron oxide persisted for a long time (Figure 3).
For MnO particles similar observations regarding the effect of particle size on cellular uptake efficiency and signalling capacity were made in a study by Létourneau et al.35 In this study, small and ultrasmall MnO particles were generated and used for cell labelling. Analogous to observations with iron oxide particles, the larger MnO particles exhibit more efficient cellular uptake. In suspension, the larger particles also exhibited higher relaxivity per Mn atom. Upon internalization into cells, however, the relaxation effect produced by Mn was not anymore different between these two formulations. The likely explanation for this phenomenon is that during endocytosis of the ultrasmall particles, clustering of these particles in endosomes occurred, which apparently resulted in the same net relaxation effect obtained with the larger particles. However, because cellular uptake of the larger particles was more efficient resulting in a higher total cell load on a per cell basis, the larger particles showed superior signalling capacity. A similar effect of intracellular clustering on relaxation properties was found for iron oxide-based nanoparticles.114 As a negative effect of larger particle size, the authors found a higher toxicity level, reflected in reduced cell viability, for the larger particles than for the smaller particles.35 The maximal tolerated dose for labelling, that is without affecting cell viability, was approximately two-fold higher for the smaller particles.
To our knowledge, particle size in and of itself has in general not been reported as a major factor affecting cellular function. In general, iron oxide particles are considered as safe for cell labelling with little to no cellular toxicity, at doses relevant for cell labelling. Generally, adverse effects have only been observed at doses exceeding 100 µg ml−1.115 However, several studies did show negative effects on cellular function following labelling with iron oxide particles at lower doses, including negative effects on multilineage differentiation capacity,116 migratory capacity,104 altered cytokine production117 or cell survival.118 In a review by Singh et al,114 the various mechanisms by which iron oxide agents can exert adverse effects on cell function have been discussed, and include effects on membrane integrity, mitochondrial function, generation of reactive oxygen species and DNA damage. In many cases, the toxic effects are dose dependent and related to specific nanoparticle composition aspects.119 Composition has been shown to be clearly related to potential adverse effects and in general for their performance as cell labelling agent for a large variety of nanoparticle as also illustrated in the section on nanoparticle composition.
Effects of nanoparticle composition
A key aspect of nanoparticles is that they offer a large variability regarding their composition, both in terms of core composition and shell composition. Therefore, nanoparticles can theoretically be generated with a specific purpose in mind, for instance with the goal to be used as a cell labelling agent. The ideal cell labelling agent would have to fulfil the following main requirements: excellent dispersion and stability in physiological fluids and environments, efficient incorporation into cells, high signalling capabilities and excellent biocompatibility. Various studies on the use of nanoparticles as a cell labelling agent have demonstrated that in practice the generation of a nanoparticle that fulfils all these requirements is apparently not so straightforward.
Coating of nanoparticles must provide a good stability to its dispersion and containment of the metal ions that generate the signalling effect. This is important to maintain the signalling properties and to prevent adverse effects caused by free metal atoms, which have been shown to cause cytotoxic effects. For loosely bound coatings such as with the much used dextrans, particle degradation in the presence of an acidic environment, such as in endosomes and lysosomes, has been reported.120 For iron oxide particles, this was shown to result in rapid modification of the magnetic properties and hampering of long-term follow-up by MRI. Additionally, following degradation of the particle, metal atoms can subsequently leach out into the cytosol causing unwanted cellular effects. Negative effects of leached metal atoms have been shown for iron, gadolinium and manganese.73,78,115,120 Generally, the observed effects were strongly dose dependent and also connected to uptake efficiency. In various studies, it was also shown that next to uptake efficiency of particles, the extent of the adverse effects could also be strongly cell type dependent.78,99,121,122 Because of these findings there is a continued effort in trying to generate more stable particles and/or particles with an improved balance between core composition and coating.77,114,123–125 In our group, we made the observation that for a liposomal encapsulated gadolinium particle, adverse effects on cellular functionality was more directly associated with the liposomal capsule than with the chelated gadolinium molecules.31 The maximal tolerated dose of the cell label was identical for Gd-encapsulated liposomes as for empty liposomes. In order to promote efficient cellular uptake, we had chosen to generate the liposomes in such a way that they would have a net positive surface charge. As demonstrated in the article, such a formulation did indeed allow for highly efficient uptake of the particles. However, the aspect that promoted cellular uptake was most likely also responsible for the observed dose-dependent adverse effect. Cationic lipids have been described for their potential to negatively affect cellular function.126 As an additional positive feature, we did find excellent stability of the intracellular cell label at a non-toxic labelling dose of the Gd–liposomes. Total intracellular Gd content, i.e. within the total daughter cell population, did not majorly decline over a culture period of 21 days.
Next to stability of the particle, Kim et al34 demonstrated that particle coating can also directly influence the signalling efficacy of metal-based probes. In this study, on a new type of MnO particle, they showed that coating with mesoporous silica was superior to coating with PEG–phospholipids and dense silica in terms of T1 relaxivity (>10-fold higher). The superior relaxivity of the mesoporous silica-coated nanoparticles was explained by the facilitated access of surrounding water molecules to the core of the particle containing the manganese ions leading to efficient relaxation of the surrounding water molecules. Their study also demonstrated the effect of coating on cellular uptake efficiency. The mesoporous silica coating endowed the MnO particles with a net negative surface charge. With cell membranes typically having a net negative surface charge, interaction between cells and mesoporous particles is less efficient. Therefore, to promote cell uptake of the negatively charged MnO particles additional manipulation of the cells by electroporation was necessary to obtain efficient labelling. Once internalized, however, the label was well retained in mesenchymal stem cells, allowing for in vivo tracking of cells transplanted into the brain of mice for more than 2 weeks.
In a study by Kasten et al127 effects of nanoparticle composition on signalling efficacy and toxicity were demonstrated using human adipose tissue-derived stem cells. In this study, two types of newly synthetized iron oxide nanoparticles were used that were similar regarding particle size and surface charge but differed regarding iron crystal composition and particle coating. Specifically, these particles consisted of a single iron core particle surrounded by a dextran matrix, called nanomag-D-spio, or a multicrystalline iron core surrounded by a starch shell, called bionized nanoferrite (BNF) starch particles. While both particles showed similar cellular uptake efficiency, a clear difference in signalling capacity and cellular toxicity was found. Unfortunately, the BNF particles that had a more beneficial relaxivity also showed more extensive adverse effects on cellular function in terms of multilineage differentiation capability. Similar effects of iron core composition on signalling capacity were also reported by other groups101 without reporting any significant differences of core composition on cellular function. In a study by van Tilborg et al,128 effects of particle core composition on signalling properties, incorporation efficiency and cell functionality were shown. In this study, various biocompatible components were used to generate iron oxide-based nanoparticles for cell labelling. The various components used to generate the particles were chosen with the goal to obtain particles for efficient cell labelling and imaging. The main surprising finding in this study was that a combination of by itself highly biocompatible components, such as soybean oil, iron oxide phospholipids and polyethylene glycol, resulted in a cytotoxic product. In addition, shelf life of the end product was also significantly reduced following chemical interactions of the components used. In this study, the authors concluded on a negative effect of oxidizing properties of the iron oxide crystals on lipid components of the particle. Furthermore, this study also demonstrated that not only size and surface properties of nanoparticles can strongly influence cellular uptake but also core composition.
In the generation of nanoparticles as a cell labelling agent, apparently various challenges exist in creating an end product that has high signalling capacity, shows efficient cellular uptake and does not negatively affect cell functionality. Therefore, careful testing of all these aspects is required during the evaluation of newly generated products.
CLINICALLY RELEVANT INSIGHTS GAINED FROM PRE-CLINICAL STUDIES AND CLINICAL EXPERIENCE WITH CELL TRACKING
Despite the various challenges met, as described in the previous sections, the value of MRI-based cell tracking for the development and use of cell-based treatment strategies was, amongst others, clearly demonstrated with the first published article on the use of cell tracking by MRI in human patients.20 In this study, tumour antigen-containing DCs were labelled with iron oxide nanoparticles or 111In-oxine and coinjected into lymph nodes of 10 patients with melanoma under ultrasound guidance. The patients subsequently underwent MRI and scintigraphy, by which the labelled DC vaccine could be visualized. Not only did the study show feasibility of such an approach, the study also demonstrated, owing to the high level of anatomical information obtained with MRI, that in four out of eight evaluable patients, the injection of the DC vaccine did not occur in the right place. In these four patients, the vaccine was injected into either the surrounding muscle or fat tissue instead of into the target lymph node. Furthermore, imaging of the labelled cells also demonstrated that depending on the patient, higher or larger numbers (1–40%) of the injected cells migrated from the initial target lymph node to surrounding draining lymph nodes. Since this type of therapy is based on direct cell-to-cell interactions between the DC vaccine and T cells in lymph nodes, information such as obtained in this study may be of crucial importance for effective design, execution and monitoring of these kinds of treatment strategies. In the years following this first report on MRI-based cell tracking in humans, a number of other reports on clinical trials were published, in which in vivo monitoring of cells labelled with iron oxide nanoparticles was performed.27–29 These studies in patients with traumatic brain injury, spinal cord injury or diabetes, mainly demonstrated the feasibility of MRI-based cell tracking techniques to monitor delivery and migration of labelled cells. They also provided additional proof on the lack of adverse effects of cell functionality by the labelling procedure, in that cell migration was not impaired or in the case of transplanted pancreatic islets27 their insulin-producing ability was not impaired. Following these initial four clinical studies, another seven reports on clinical studies with iron oxide-labelled cells appeared. In addition, recently two articles were published describing initial evaluation or preparation of MRI-based cell tracking using PFC nanoparticles39 and ferumoxytol56 as cell labelling agents, respectively. Some specifics of these studies and the main findings are summarized in Table 2.
Next to the above mentioned studies, other in vivo cell tracking studies (mainly pre-clinical) have provided some valuable insights into the mechanistic aspects of cell-based therapies. For instance, in order for transplanted cells to fulfil their intended function, a first prerequisite is that the cells reach the target site. Connected to this the question arises on what the most efficient route of administration is for a given situation. For stem cell-based therapy of cardiovascular disease, various delivery routes have been contemplated and used, e.g. intravenous, intracoronary and intramyocardial injection. In vivo tracking studies have revealed that only a limited number of cells reach the infarcted myocardium after intravenous and intracoronary injection.129,130 Direct intramyocardial injection was shown to result in higher cell delivery efficiency to the infarcted myocardium, however, being a technically quite challenging technique to execute.131
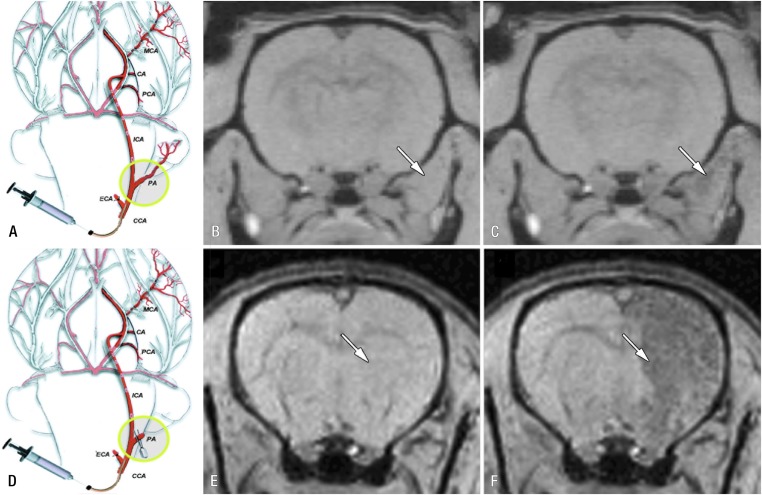
Another example of how in vivo cell tracking may help in defining the optimal delivery route was demonstrated in a study in rats by Gorelik et al.132 In this study, homing of glial precursor cell delivery to inflamed brain after intra-arterial injection was studied. To this end, the cells were labelled with iron oxide particles and injected into the carotid artery and their homing to the brain was monitored by MRI. Initial studies showed that without ligation of side-branches of the internal carotid artery, the bulk of injected cells ended up in tissue areas outside the brain. Only after ligation of the side-branches, successful targeting of the injected cells to the brain was achieved (Figure 6). In addition, the investigators also showed that activation of precursor glial cells prior to injection resulted in higher targeting efficiency compared with non-activated cells. This aspect touches upon another important aspect where cell tracking may help in designing effective cell-based treatment strategies. Currently, many cell types are being considered for cell-based therapy; here, the challenge lies in determining which types are most suitable in trying to fulfil criteria regarding therapeutic efficacy, ease of access and practical use.133 Using fluorine-18 fludeoxyglucose as cell labelling agent and PET imaging, Hofmann et al129 could demonstrate differences in biodistribution and homing to infarcted myocardium between selected CD34+ bone marrow cells and unselected bone marrow cells when injected intracoronary in patients with myocardial infarction. In another approach, van der Bogt et al134,135 used bioluminescent imaging of firefly luciferase transduced cells, to compare the efficacy of various cell types for the treatment of myocardial infarction using a murine myocardial infarction model. Most disappointingly, however, the main finding from these studies was the general poor survival in the myocardium of the various cell types tested, including bone marrow mononuclear cells, adipose stromal cells, mesenchymal stem cells, skeletal myoblasts and fibroblasts. Using the same imaging technology, Janowski et al136 recently demonstrated the site-dependent survival of allogeneic neural progenitor grafts in the brain of mice with a clear involvement of the immune system. Poor graft survival has now been generally accepted as a major hurdle for successful cell therapy in regenerative medicine approaches, therefore much effort is currently put into strategies to improve graft survival.137–141
Figure 6.
Real-time monitoring of injection accuracy with MRI. (a) Diagram of procedure with pterygopalatine artery left intact. After ligation of external carotid and occipital arteries, common carotid artery was cannulated and SPIO-labelled cells were infused. (b, c) T2* weighted MR images of rat brain and surrounding muscles obtained immediately before (b) and after (c) injection demonstrate that vast majority of cells are localized into extracerebral tissue (arrows), with negligible binding within brain. (d) Diagram of procedure with ligation of pterygopalatine artery. All infused cells were perfused into internal carotid artery and localized successfully into ipsilateral hemisphere. (e, f) MR images obtained immediately before (e) and after (f) injection. Arrows indicate area of cell docking. CA, choroidal anterior artery; CCA, common carotid artery; ECA, external carotid artery; ICA, internal carotid artery; MCA, middle cerebral artery; PA, pterygopalatine artery; PCA, posterior cerebral artery. Reprinted from Gorelik et al132 with permission from the Radiological Society of North America.
Clearly, in vivo cell tracking can provide valuable information for the design and use of cell-based treatment strategies. Unfortunately, current limitations imposed by practical, technical and regulatory issues still prevent widespread clinical use of available imaging technology. Currently no FDA-approved, nanoparticle-based cell labelling agents are available for clinical use. Ferumoxytol, a FDA-approved iron oxide nanoparticle for treatment of anaemia, is now being considered for off-label use as a cell labelling agent in clinical trials.55,56 To limit the number of regulatory requirements that would have to be fulfilled for this off-label use, an alternative strategy for labelling of mesenchymal stem cells was recently proposed.55,61 This encompasses labelling of cells in vivo instead of ex vivo by intravenous injection of ferumoxytol, which is then endocytosed by mesenchymal stromal cells (MSC) in bone marrow. Surprisingly, this technique proved to be highly efficient and superior to ex vivo labelling. While this technique was used in an osteochondral defect model, it may be suitable for various other applications and may also be suitable for various other types of nanoparticle-based labels.142 Of course, for new particles basic safety and signalling efficacy will still have to be proven.
PERSPECTIVES IN CELL TRACKING
Widespread clinical application of cell tracking techniques is at the least, still many years away. However, in coming years much can already be learned from small and large animal models where recent technological developments can further help in elucidating the mechanisms underlying the success of cell-based therapies. In such pre-clinical studies on cell-based therapies, not only questions regarding accurate delivery of the cell graft can be addressed, but also how the cell grafts function and how therapeutic effect is mediated. These insights can then be used for the design of clinical trials. Next to that, current technology can already be used to address questions regarding the accurate delivery of the graft and migratory behaviour of the cells in specific situations, as has been demonstrated by studies in patients performed so far. The challenge for clinical cell tracking still lies in the development of the ideal technique. No available technique will be able to address all relevant questions. Therefore, depending on the question(s) addressed, the most suitable technique or combination of techniques i.e. using multiple modalities, will have to be selected. This will also include the most suitable label for a specific question.
Contributor Information
Monique R Bernsen, Email: m.bernsen@erasmusmc.nl.
Jamal Guenoun, Email: j.genoun@erasmusmc.nl.
Sandra T van Tiel, Email: s.vantiel@erasmusmc.nl.
Gabriel P Krestin, Email: g.p.krestin@erasmusmc.nl.
REFERENCES
- 1.Fischbach MA, Bluestone JA, Lim WA. Cell-based therapeutics: the next pillar of medicine. Sci Transl Med 2013; 5: 179ps7. doi: 10.1126/scitranslmed.3005568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Culme-Seymour EJ, Davie NL, Brindley DA, Edwards-Parton S, Mason C. A decade of cell therapy clinical trials (2000-2010). Regen Med 2012; 7: 455–62. doi: 10.2217/rme.12.45 [DOI] [PubMed] [Google Scholar]
- 3.Sánchez A, Schimmang T, Garcia-Sancho J. Cell and tissue therapy in regenerative medicine. Adv Exp Med Biol 2012; 741: 89–102. doi: 10.1007/978-1-4614-2098-9_7 [DOI] [PubMed] [Google Scholar]
- 4.Hawkins RE, Gilham DE, Debets R, Eshhar Z, Taylor N, Abken H, et al. Development of adoptive cell therapy for cancer: a clinical perspective. Hum Gene Ther 2010; 21: 665–72. doi: 10.1089/hum.2010.086 [DOI] [PubMed] [Google Scholar]
- 5.Gioviale MC, Bellavia M, Damiano G, Lo Monte AI. Beyond islet transplantation in diabetes cell therapy: from embryonic stem cells to transdifferentiation of adult cells. Transplant Proc 2013; 45: 2019–24. doi: 10.1016/j.transproceed.2013.01.076 [DOI] [PubMed] [Google Scholar]
- 6.Au P, Hursh DA, Lim A, Moos MC, Jr, Oh SS, Schneider BS, et al. FDA oversight of cell therapy clinical trials. Sci Transl Med 2012; 4: 149fs31. doi: 10.1126/scitranslmed.3004131 [DOI] [PubMed] [Google Scholar]
- 7.Ruggiero A, Thorek DL, Guenoun J, Krestin GP, Bernsen MR. Cell tracking in cardiac repair: what to image and how to image. Eur Radiol 2012; 22: 189–204. doi: 10.1007/s00330-011-2190-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Srivastava AK, Bulte JW. Seeing stem cells at work in vivo. Stem Cell Rev 2014; 10: 127–44. doi: 10.1007/s12015-013-9468-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Castaneda RT, Khurana A, Khan R, Daldrup-Link HE. Labeling stem cells with ferumoxytol, an FDA-approved iron oxide nanoparticle. J Vis Exp 2011; 4: e3482. doi: 10.3791/3482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Srinivas M, Boehm-Sturm P, Aswendt M, Pracht ED, Figdor CG, de Vries IJ, et al. In vivo 19F MRI for cell tracking. J Vis Exp 2013; (81): e50802. doi: 10.3791/50802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bernsen MR, Moelker AD, Wielopolski PA, van Tiel ST, Krestin GP. Labelling of mammalian cells for visualisation by MRI. Eur Radiol 2010; 20: 255–74. doi: 10.1007/s00330-009-1540-1 [DOI] [PubMed] [Google Scholar]
- 12.Arbab AS, Liu W, Frank JA. Cellular magnetic resonance imaging: current status and future prospects. Expert Rev Med Devices 2006; 3: 427–39. doi: 10.1586/17434440.3.4.427 [DOI] [PubMed] [Google Scholar]
- 13.Brader P, Serganova I, Blasberg RG. Noninvasive molecular imaging using reporter genes. J Nucl Med 2013; 54: 167–72. doi: 10.2967/jnumed.111.099788 [DOI] [PubMed] [Google Scholar]
- 14.Vandsburger MH, Radoul M, Cohen B, Neeman M. MRI reporter genes: applications for imaging of cell survival, proliferation, migration and differentiation. NMR Biomed 2013; 26: 872–84. doi: 10.1002/nbm.2869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang Y, Xu C, Ow H. Commercial nanoparticles for stem cell labeling and tracking. Theranostics 2013; 3: 544–60. doi: 10.7150/thno.5634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sutton EJ, Henning TD, Pichler BJ, Bremer C, Daldrup-Link HE. Cell tracking with optical imaging. Eur Radiol 2008; 18: 2021–32. doi: 10.1007/s00330-008-0984-z [DOI] [PubMed] [Google Scholar]
- 17.Aarntzen EH, Srinivas M, Radu CG, Punt CJ, Boerman OC, Figdor CG, et al. In vivo imaging of therapy-induced anti-cancer immune responses in humans. Cell Mol Life Sci 2013; 70: 2237–57. doi: 10.1007/s00018-012-1159-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shapiro EM, Sharer K, Skrtic S, Koretsky AP. In vivo detection of single cells by MRI. Magn Reson Med 2006; 55: 242–9. doi: 10.1002/mrm.20718 [DOI] [PubMed] [Google Scholar]
- 19.Zhang Z, van den Bos EJ, Wielopolski PA, de Jong-Popijus M, Bernsen MR, Duncker DJ, et al. In vitro imaging of single living human umbilical vein endothelial cells with a clinical 3.0-T MRI scanner. MAGMA 2005; 18: 175–85. doi: 10.1007/s10334-005-0108-6 [DOI] [PubMed] [Google Scholar]
- 20.de Vries IJ, Lesterhuis WJ, Barentsz JO, Verdijk P, van Krieken JH, Boerman OC, et al. Magnetic resonance tracking of dendritic cells in melanoma patients for monitoring of cellular therapy. Nat Biotechnol 2005; 23: 1407–13. doi: 10.1038/nbt1154 [DOI] [PubMed] [Google Scholar]
- 21.Deligianni X, Jirák D, Berková Z, Hájek M, Scheffler K, Bieri O. In vivo visualization of cells labeled with superparamagnetic iron oxides by a sub-millisecond gradient echo sequence. MAGMA 2014; 27: 329–37. doi: 10.1007/s10334-013-0422-3 [DOI] [PubMed] [Google Scholar]
- 22.Janowski M, Walczak P, Kropiwnicki T, Jurkiewicz E, Domanska-Janik K, Bulte JW, et al. Long-term MRI cell tracking after intraventricular delivery in a patient with global cerebral ischemia and prospects for magnetic navigation of stem cells within the CSF. PLoS One 2014; 9: e97631. doi: 10.1371/journal.pone.0097631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jozwiak S, Habich A, Kotulska K, Sarnowska A, Kropiwnicki T, Janowski M, et al. Intracerebroventricular transplantation of cord blood-derived neural progenitors in a child with severe global brain ischemic injury. Cell Med 2010; 1: 71–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karussis D, Karageorgiou C, Vaknin-Dembinsky A, Gowda-Kurkalli B, Gomori JM, Kassis I, et al. Safety and immunological effects of mesenchymal stem cell transplantation in patients with multiple sclerosis and amyotrophic lateral sclerosis. Arch Neurol 2010; 67: 1187–94. doi: 10.1001/archneurol.2010.248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Richards JM, Shaw CA, Lang NN, Williams MC, Semple SI, MacGillivray TJ, et al. In vivo mononuclear cell tracking using superparamagnetic particles of iron oxide: feasibility and safety in humans. Circ Cardiovasc Imaging 2012; 5: 509–17. doi: 10.1161/CIRCIMAGING.112.972596 [DOI] [PubMed] [Google Scholar]
- 26.Saudek F, Jirák D, Girman P, Herynek V, Dezortová M, Kríz J, et al. Magnetic resonance imaging of pancreatic islets transplanted into the liver in humans. Transplantation 2010; 90: 1602–6. doi: 10.1097/TP.0b013e3181ffba5e [DOI] [PubMed] [Google Scholar]
- 27.Toso C, Vallee JP, Morel P, Ris F, Demuylder-Mischler S, Lepetit-Coiffe M, et al. Clinical magnetic resonance imaging of pancreatic islet grafts after iron nanoparticle labeling. Am J Transpl 2008; 8: 701–6. doi: 10.1111/j.1600-6143.2007.02120.x [DOI] [PubMed] [Google Scholar]
- 28.Zhu J, Zhou L, XingWu F. Tracking neural stem cells in patients with brain trauma. N Engl J Med 2006; 355: 2376–8. doi: 10.1056/NEJMc055304 [DOI] [PubMed] [Google Scholar]
- 29.Callera F, de Melo CM. Magnetic resonance tracking of magnetically labeled autologous bone marrow CD34+ cells transplanted into the spinal cord via lumbar puncture technique in patients with chronic spinal cord injury: CD34+ cells' migration into the injured site. Stem Cells Dev 2007; 16: 461–6. doi: 10.1089/scd.2007.0083 [DOI] [PubMed] [Google Scholar]
- 30.Figueiredo S, Cutrin JC, Rizzitelli S, De Luca E, Moreira JN, Geraldes CF, et al. MRI tracking of macrophages labeled with glucan particles entrapping a water insoluble paramagnetic Gd-based agent. Mol Imaging Biol 2013; 15: 307–15. doi: 10.1007/s11307-012-0603-x [DOI] [PubMed] [Google Scholar]
- 31.Guenoun J, Koning GA, Doeswijk G, Bosman L, Wielopolski PA, Krestin GP, et al. Cationic Gd-DTPA liposomes for highly efficient labeling of mesenchymal stem cells and cell tracking with MRI. Cell Transpl 2012; 21: 191–205. doi: 10.3727/096368911X593118 [DOI] [PubMed] [Google Scholar]
- 32.Aspord C, Laurin D, Janier MF, Mandon CA, Thivolet C, Villiers C, et al. Paramagnetic nanoparticles to track and quantify in vivo immune human therapeutic cells. Nanoscale 2013; 5: 11409–15. doi: 10.1039/c3nr34240a [DOI] [PubMed] [Google Scholar]
- 33.Sterenczak KA, Meier M, Glage S, Meyer M, Willenbrock S, Wefstaedt P, et al. Longitudinal MRI contrast enhanced monitoring of early tumour development with manganese chloride (MnCl2) and superparamagnetic iron oxide nanoparticles (SPIOs) in a CT1258 based in vivo model of prostate cancer. BMC Cancer 2012; 12: 284. doi: 10.1186/1471-2407-12-284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim T, Momin E, Choi J, Yuan K, Zaidi H, Kim J, et al. Mesoporous silica-coated hollow manganese oxide nanoparticles as positive T1 contrast agents for labeling and MRI tracking of adipose-derived mesenchymal stem cells. J Am Chem Soc 2011; 133: 2955–61. doi: 10.1021/ja1084095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Létourneau M, Tremblay M, Faucher L, Rojas D, Chevallier P, Gossuin Y, et al. MnO-labeled cells: positive contrast enhancement in MRI. J Phys Chem B 2012; 116: 13228–38. doi: 10.1021/jp3032918 [DOI] [PubMed] [Google Scholar]
- 36.Ferrauto G, Delli Castelli D, Terreno E, Aime S. In vivo MRI visualization of different cell populations labeled with PARACEST agents. Magn Reson Med 2013; 69: 1703–11. doi: 10.1002/mrm.24411 [DOI] [PubMed] [Google Scholar]
- 37.Amiri H, Srinivas M, Veltien A, van Uden MJ, de Vries IJ, Heerschap A. Cell tracking using (19)F magnetic resonance imaging: technical aspects and challenges towards clinical applications. Eur Radiol 2015; 25: 726–35. doi: 10.1007/s00330-014-3474-5 [DOI] [PubMed] [Google Scholar]
- 38.Bonetto F, Srinivas M, Heerschap A, Mailliard R, Ahrens ET, Figdor CG, et al. A novel (19)F agent for detection and quantification of human dendritic cells using magnetic resonance imaging. Int J Cancer 2011; 129: 365–73. doi: 10.1002/ijc.25672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ahrens ET, Helfer BM, O'Hanlon CF, Schirda C. Clinical cell therapy imaging using a perfluorocarbon tracer and fluorine-19 MRI. Magn Reson Med 2014; 72: 1696–701. doi: 10.1002/mrm.25454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bulte JW, Ma LD, Magin RL, Kamman RL, Hulstaert CE, Go KG, et al. Selective MR imaging of labeled human peripheral blood mononuclear cells by liposome mediated incorporation of dextran-magnetite particles. Magn Reson Med 1993; 29: 32–7. doi: 10.1002/mrm.1910290108 [DOI] [PubMed] [Google Scholar]
- 41.Norman AB, Thomas SR, Pratt RG, Lu SY, Norgren RB. Magnetic resonance imaging of neural transplants in rat brain using a superparamagnetic contrast agent. Brain Res 1992; 594: 279–83. doi: 10.1016/0006-8993(92)91135-2 [DOI] [PubMed] [Google Scholar]
- 42.Bulte JW, Kraitchman DL. Iron oxide MR contrast agents for molecular and cellular imaging. NMR Biomed 2004; 17: 484–99. doi: 10.1002/nbm.924 [DOI] [PubMed] [Google Scholar]
- 43.Li L, Jiang W, Luo K, Song H, Lan F, Wu Y, et al. Superparamagnetic iron oxide nanoparticles as MRI contrast agents for non-invasive stem cell labeling and tracking. Theranostics 2013; 3: 595–615. doi: 10.7150/thno.5366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Daldrup-Link HE, Meier R, Rudelius M, Piontek G, Piert M, Metz S, et al. In vivo tracking of genetically engineered, anti-HER2/neu directed natural killer cells to HER2/neu positive mammary tumors with magnetic resonance imaging. Eur Radiol 2005; 15: 4–13. doi: 10.1007/s00330-004-2526-7 [DOI] [PubMed] [Google Scholar]
- 45.Frank JA, Zywicke H, Jordan EK, Mitchell J, Lewis BK, Miller B, et al. Magnetic intracellular labeling of mammalian cells by combining (FDA-approved) superparamagnetic iron oxide MR contrast agents and commonly used transfection agents. Acad Radiol 2002; 9 (Suppl 2): S484–7. [DOI] [PubMed] [Google Scholar]
- 46.Bulte JW. In vivo MRI cell tracking: clinical studies. AJR Am J Roentgenol 2009; 193: 314–25. doi: 10.2214/AJR.09.3107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.van den Bos EJ, Baks T, Moelker AD, Kerver W, van Geuns RJ, van der Giessen WJ, et al. Magnetic resonance imaging of haemorrhage within reperfused myocardial infarcts: possible interference with iron oxide-labelled cell tracking? Eur Heart J 2006; 27: 1620–6. doi: 10.1093/eurheartj/ehl059 [DOI] [PubMed] [Google Scholar]
- 48.Terrovitis J, Stuber M, Youssef A, Preece S, Leppo M, Kizana E, et al. Magnetic resonance imaging overestimates ferumoxide-labeled stem cell survival after transplantation in the heart. Circulation 2008; 117: 1555–62. doi: 10.1161/CIRCULATIONAHA.107.732073 [DOI] [PubMed] [Google Scholar]
- 49.Cromer Berman SM, Kshitiz, Wang CJ, Orukari I, Levchenko A, Bulte JW, et al. Cell motility of neural stem cells is reduced after SPIO-labeling, which is mitigated after exocytosis. Magn Reson Med 2013; 69: 255–62. doi: 10.1002/mrm.24216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang Q, Li K, Quan Q, Zhang G. R2* and R2 mapping for quantifying recruitment of superparamagnetic iron oxide-tagged endothelial progenitor cells to injured liver: tracking in vitro and in vivo. Int J Nanomedicine 2014; 9: 1815–22. doi: 10.2147/IJN.S58269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.van Buul GM, Kotek G, Wielopolski PA, Farrell E, Bos PK, Weinans H, et al. Clinically translatable cell tracking and quantification by MRI in cartilage repair using superparamagnetic iron oxides. PLoS One 2011; 6: e17001. doi: 10.1371/journal.pone.0017001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Girard OM, Ramirez R, McCarty S, Mattrey RF. Toward absolute quantification of iron oxide nanoparticles as well as cell internalized fraction using multiparametric MRI. Contrast Media Mol Imaging. 2012; 7: 411–17. doi: 10.1002/cmmi.1467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kotek G, van Tiel ST, Wielopolski PA, Houston GC, Krestin GP, Bernsen MR. Cell quantification: evolution of compartmentalization and distribution of iron-oxide particles and labeled cells. Contrast Media Mol Imaging 2012; 7: 195–203. doi: 10.1002/cmmi.481 [DOI] [PubMed] [Google Scholar]
- 54.Ruggiero A, Guenoun J, Smit H, Doeswijk GN, Klein S, Krestin GP, et al. In vivo MRI mapping of iron oxide-labeled stem cells transplanted in the heart. Contrast Media Mol Imaging 2013; 8: 487–94. doi: 10.1002/cmmi.1582 [DOI] [PubMed] [Google Scholar]
- 55.Daldrup-Link HE, Nejadnik H. MR imaging of stem cell transplants in arthritic joints. J Stem Cell Res Ther 2014; 4: 165. doi: 10.4172/2157-7633.1000165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gutova M, Frank JA, D'Apuzzo M, Khankaldyyan V, Gilchrist MM, Annala AJ, et al. Magnetic resonance imaging tracking of ferumoxytol-labeled human neural stem cells: studies leading to clinical use. Stem Cells Transl Med 2013; 2: 766–75. doi: 10.5966/sctm.2013-0049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bridot JL, Stanicki D, Laurent S, Boutry S, Gossuin Y, Leclère P, et al. New carboxysilane-coated iron oxide nanoparticles for nonspecific cell labelling. Contrast Media Mol Imaging 2013; 8: 466–74. doi: 10.1002/cmmi.1552 [DOI] [PubMed] [Google Scholar]
- 58.Liu J, Wang L, Cao J, Huang Y, Lin Y, Wu X, et al. Functional investigations on embryonic stem cells labeled with clinically translatable iron oxide nanoparticles. Nanoscale 2014; 6: 9025–33. doi: 10.1039/c4nr01004c [DOI] [PubMed] [Google Scholar]
- 59.Leder A, Raschzok N, Schmidt C, Arabacioglu D, Butter A, Kolano S, et al. Micron-sized iron oxide-containing particles for microRNA-targeted manipulation and MRI-based tracking of transplanted cells. Biomaterials 2015; 51: 129–37. doi: 10.1016/j.biomaterials.2015.01.065 [DOI] [Google Scholar]
- 60.Chapman S, Dobrovolskaia M, Farahani K, Goodwin A, Joshi A, Lee H, et al. Nanoparticles for cancer imaging: the good, the bad, and the promise. Nano Today 2013; 8: 454–60. doi: 10.1016/j.nantod.2013.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Khurana A, Nejadnik H, Chapelin F, Lenkov O, Gawande R, Lee S, et al. Ferumoxytol: a new, clinically applicable label for stem-cell tracking in arthritic joints with MRI. Nanomedicine (Lond) 2013; 8: 1969–83. doi: 10.2217/nnm.12.198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rudelius M, Daldrup-Link HE, Heinzmann U, Piontek G, Settles M, Link TM, et al. Highly efficient paramagnetic labelling of embryonic and neuronal stem cells. Eur J Nucl Med Mol Imaging 2003; 30: 1038–44. doi: 10.1007/s00259-002-1110-0 [DOI] [PubMed] [Google Scholar]
- 63.Tseng CL, Shih IL, Stobinski L, Lin FH. Gadolinium hexanedione nanoparticles for stem cell labeling and tracking via magnetic resonance imaging. Biomaterials 2010; 31: 5427–35. doi: 10.1016/j.biomaterials.2010.03.049 [DOI] [PubMed] [Google Scholar]
- 64.Ghaghada KB, Ravoori M, Sabapathy D, Bankson J, Kundra V, Annapragada A. New dual mode gadolinium nanoparticle contrast agent for magnetic resonance imaging. PLoS One 2009; 4: e7628. doi: 10.1371/journal.pone.0007628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nejadnik H, Henning TD, Do T, Sutton EJ, Baehner F, Horvai A, et al. MR imaging features of gadofluorine-labeled matrix-associated stem cell implants in cartilage defects. PLoS One 2012; 7: e49971. doi: 10.1371/journal.pone.0049971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Di Corato R, Gazeau F, Le Visage C, Fayol D, Levitz P, Lux F, et al. High-resolution cellular MRI: gadolinium and iron oxide nanoparticles for in-depth dual-cell imaging of engineered tissue constructs. ACS Nano 2013; 7: 7500–12. doi: 10.1021/nn401095p [DOI] [PubMed] [Google Scholar]
- 67.Vivero-Escoto JL, Rieter WJ, Lau H, Huxford-Phillips RC, Lin W. Biodegradable polysilsesquioxane nanoparticles as efficient contrast agents for magnetic resonance imaging. Small 2013; 9: 3523–31. doi: 10.1002/smll.201300198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tran LA, Hernández-Rivera M, Berlin AN, Zheng Y, Sampaio L, Bové C, et al. The use of gadolinium-carbon nanostructures to magnetically enhance stem cell retention for cellular cardiomyoplasty. Biomaterials 2014; 35: 720–6. doi: 10.1016/j.biomaterials.2013.10.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Terreno E, Geninatti Crich S, Belfiore S, Biancone L, Cabella C, Esposito G, et al. Effect of the intracellular localization of a Gd-based imaging probe on the relaxation enhancement of water protons. Magn Reson Med 2006; 55: 491–7. doi: 10.1002/mrm.20793 [DOI] [PubMed] [Google Scholar]
- 70.Brekke C, Morgan SC, Lowe AS, Meade TJ, Price J, Williams SC, et al. The in vitro effects of a bimodal contrast agent on cellular functions and relaxometry. NMR Biomed 2007; 20: 77–89. doi: 10.1002/nbm.1077 [DOI] [PubMed] [Google Scholar]
- 71.Guenoun J, Ruggiero A, Doeswijk G, Janssens RC, Koning GA, Kotek G, et al. In vivo quantitative assessment of cell viability of gadolinium or iron-labeled cells using MRI and bioluminescence imaging. Contrast Media Mol Imaging 2013; 8: 165–74.doi: 10.1002/cmmi.1513 [DOI] [PubMed] [Google Scholar]
- 72.Nejadnik H, Castillo R, Daldrup-Link HE. Magnetic resonance imaging and tracking of stem cells. Methods Mol Biol 2013; 1052: 167–76. doi: 10.1007/7651_2013_16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang S, Jiang Z, Liu X, Zhou L, Peng W. Possible gadolinium ions leaching and MR sensitivity over-estimation in mesoporous silica-coated upconversion nanocrystals. Nanoscale 2013; 5: 8146–55. doi: 10.1039/c3nr01902k [DOI] [PubMed] [Google Scholar]
- 74.Modo M, Beech JS, Meade TJ, Williams SC, Price J. A chronic 1 year assessment of MRI contrast agent-labelled neural stem cell transplants in stroke. Neuroimage 2009; 47 (Suppl 2): T133–42. doi: 10.1016/j.neuroimage.2008.06.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gilad AA, Walczak P, McMahon MT, Na HB, Lee JH, An K, et al. MR tracking of transplanted cells with “positive contrast” using manganese oxide nanoparticles. Magn Reson Med 2008; 60: 1–7. doi: 10.1002/mrm.21622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yamada M, Gurney PT, Chung J, Kundu P, Drukker M, Smith AK, et al. Manganese-guided cellular MRI of human embryonic stem cell and human bone marrow stromal cell viability. Magn Reson Med 2009; 62: 1047–54. doi: 10.1002/mrm.22071 [DOI] [PubMed] [Google Scholar]
- 77.Xiao J, Tian XM, Yang C, Liu P, Luo NQ, Liang Y, et al. Ultrahigh relaxivity and safe probes of manganese oxide nanoparticles for in vivo imaging. Sci Rep 2013; 3: 3424. doi: 10.1038/srep03424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yu C, Zhou Z, Wang J, Sun J, Liu W, Sun Y, et al. In depth analysis of apoptosis induced by silica coated manganese oxide nanoparticles in vitro. J Hazard Mater 2015; 283: 519–28. doi: 10.1016/j.jhazmat.2014.09.060 [DOI] [PubMed] [Google Scholar]
- 79.Bellusci M, La Barbera A, Padella F, Mancuso M, Pasquo A, Grollino MG, et al. Biodistribution and acute toxicity of a nanofluid containing manganese iron oxide nanoparticles produced by a mechanochemical process. Int J Nanomedicine 2014; 9: 1919–29. doi: 10.2147/IJN.S56394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Vinogradov E, Sherry AD, Lenkinski RE. CEST: from basic principles to applications, challenges and opportunities. J Magn Reson 2013; 229: 155–72. doi: 10.1016/j.jmr.2012.11.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ferrauto G, Carniato F, Tei L, Hu H, Aime S, Botta M. MRI nanoprobes based on chemical exchange saturation transfer: Ln(III) chelates anchored on the surface of mesoporous silica nanoparticles. Nanoscale 2014; 6: 9604–7. doi: 10.1039/c4nr02753a [DOI] [PubMed] [Google Scholar]
- 82.Castelli DD, Terreno E, Longo D, Aime S. Nanoparticle-based chemical exchange saturation transfer (CEST) agents. NMR Biomed 2013; 26: 839–49. doi: 10.1002/nbm.2974 [DOI] [PubMed] [Google Scholar]
- 83.Chan KW, Bulte JW, McMahon MT. Diamagnetic chemical exchange saturation transfer (diaCEST) liposomes: physicochemical properties and imaging applications. Wiley Interdiscip Rev Nanomed Nanobiotechnol 2014; 6: 111–24. doi: 10.1002/wnan.1246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kok MB, Hak S, Mulder WJ, van der Schaft DW, Strijkers GJ, Nicolay K. Cellular compartmentalization of internalized paramagnetic liposomes strongly influences both T1 and T2 relaxivity. Magn Reson Med 2009; 61: 1022–32. doi: 10.1002/mrm.21910 [DOI] [PubMed] [Google Scholar]
- 85.Chan KW, Liu G, Song X, Kim H, Yu T, Arifin DR, et al. MRI-detectable pH nanosensors incorporated into hydrogels for in vivo sensing of transplanted-cell viability. Nat Mater 2013; 12: 268–75. doi: 10.1038/nmat3525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ruiz-Cabello J, Barnett BP, Bottomley PA, Bulte JW. Fluorine (19F) MRS and MRI in biomedicine. NMR Biomed 2011; 24: 114–29. doi: 10.1002/nbm.1570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Srinivas M, Heerschap A, Ahrens ET, Figdor CG, de Vries IJ. (19)F MRI for quantitative in vivo cell tracking. Trends Biotechnol 2010; 28: 363–70. doi: 10.1016/j.tibtech.2010.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zarif L, Postel M, Trevino L, Riess JG, Valla A, Follana R. Biodistribution and excretion of a mixed fluorocarbon-hydrocarbon “dowel” emulsion as determined by 19F NMR. Artif Cells Blood Substit Immobil Biotechnol 1994; 22: 1193–8. doi: 10.3109/10731199409138815 [DOI] [PubMed] [Google Scholar]
- 89.Helfer BM, Balducci A, Nelson AD, Janjic JM, Gil RR, Kalinski P, et al. Functional assessment of human dendritic cells labeled for in vivo (19)F magnetic resonance imaging cell tracking. Cytotherapy 2010; 12: 238–50. doi: 10.3109/14653240903446902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Srinivas M, Cruz LJ, Bonetto F, Heerschap A, Figdor CG, de Vries IJ. Customizable, multi-functional fluorocarbon nanoparticles for quantitative in vivo imaging using 19F MRI and optical imaging. Biomaterials 2010; 31: 7070–7. doi: 10.1016/j.biomaterials.2010.05.069 [DOI] [PubMed] [Google Scholar]
- 91.Srinivas M, Boehm-Sturm P, Figdor CG, de Vries IJ, Hoehn M. Labeling cells for in vivo tracking using (19)F MRI. Biomaterials 2012; 33: 8830–40. doi: 10.1016/j.biomaterials.2012.08.048 [DOI] [PubMed] [Google Scholar]
- 92.Ahrens ET, Zhong J. In vivo MRI cell tracking using perfluorocarbon probes and fluorine-19 detection. NMR Biomed 2013; 26: 860–71. doi: 10.1002/nbm.2948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Waiczies S, Lepore S, Sydow K, Drechsler S, Ku MC, Martin C, et al. Anchoring dipalmitoyl phosphoethanolamine to nanoparticles boosts cellular uptake and fluorine-19 magnetic resonance signal. Sci Rep 2015; 5: 8427. doi: 10.1038/srep08427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chalmers KH, Kenwright AM, Parker D, Blamire AM. 19F-lanthanide complexes with increased sensitivity for 19F-MRI: optimization of the MR acquisition. Magn Reson Med 2011; 66: 931–6. doi: 10.1002/mrm.22881 [DOI] [PubMed] [Google Scholar]
- 95.de Vries A, Moonen R, Yildirim M, Langereis S, Lamerichs R, Pikkemaat JA, et al. Relaxometric studies of gadolinium-functionalized perfluorocarbon nanoparticles for MR imaging. Contrast Media Mol Imaging 2014; 9: 83–91. doi: 10.1002/cmmi.1541 [DOI] [PubMed] [Google Scholar]
- 96.Shapiro EM, Skrtic S, Koretsky AP. Sizing it up: cellular MRI using micron-sized iron oxide particles. Magn Reson Med 2005; 53: 329–38. doi: 10.1002/mrm.20342 [DOI] [PubMed] [Google Scholar]
- 97.Matuszewski L, Persigehl T, Wall A, Schwindt W, Tombach B, Fobker M, et al. Cell tagging with clinically approved iron oxides: feasibility and effect of lipofection, particle size, and surface coating on labeling efficiency. Radiology 2005; 235: 155–61. doi: 10.1148/radiol.2351040094 [DOI] [PubMed] [Google Scholar]
- 98.Chen CL, Zhang H, Ye Q, Hsieh WY, Hitchens TK, Shen HH, et al. A new nano-sized iron oxide particle with high sensitivity for cellular magnetic resonance imaging. Mol Imaging Biol 2011; 13: 825–39. doi: 10.1007/s11307-010-0430-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.van Tiel ST, Wielopolski PA, Houston GC, Krestin GP, Bernsen MR. Variations in labeling protocol influence incorporation, distribution and retention of iron oxide nanoparticles into human umbilical vein endothelial cells. Contrast Media Mol Imaging. 2010; 5: 247–57. doi: 10.1002/cmmi.379 [DOI] [PubMed] [Google Scholar]
- 100.Saito S, Tsugeno M, Koto D, Mori Y, Yoshioka Y, Nohara S, et al. Impact of surface coating and particle size on the uptake of small and ultrasmall superparamagnetic iron oxide nanoparticles by macrophages. Int J Nanomedicine 2012; 7: 5415–21. doi: 10.2147/IJN.S33709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Trekker J, Leten C, Struys T, Lazenka VV, Argibay B, Micholt L, et al. Sensitive in vivo cell detection using size-optimized superparamagnetic nanoparticles. Biomaterials 2014; 35: 1627–35. doi: 10.1016/j.biomaterials.2013.11.006 [DOI] [PubMed] [Google Scholar]
- 102.Hansen L, Hansen AB, Mathiasen AB, Ng M, Bhakoo K, Ekblond A, et al. Ultrastructural characterization of mesenchymal stromal cells labeled with ultrasmall superparamagnetic iron-oxide nanoparticles for clinical tracking studies. Scand J Clin Lab Invest 2014; 74: 437–46. doi: 10.3109/00365513.2014.900698 [DOI] [PubMed] [Google Scholar]
- 103.de Chickera SN, Snir J, Willert C, Rohani R, Foley R, Foster PJ, et al. Labelling dendritic cells with SPIO has implications for their subsequent in vivo migration as assessed with cellular MRI. Contrast Media Mol Imaging 2011; 6: 314–27. doi: 10.1002/cmmi.433 [DOI] [PubMed] [Google Scholar]
- 104.Schafer R, Kehlbach R, Müller M, Bantleon R, Kluba T, Ayturan M, et al. Labeling of human mesenchymal stromal cells with superparamagnetic iron oxide leads to a decrease in migration capacity and colony formation ability. Cytotherapy 2009; 11: 68–78. doi: 10.1080/14653240802666043 [DOI] [PubMed] [Google Scholar]
- 105.Oh N, Park JH. Endocytosis and exocytosis of nanoparticles in mammalian cells. Int J Nanomedicine 2014; 9 (Suppl 1): 51–63. doi: 10.2147/IJN.S26592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Xu C, Miranda-Nieves D, Ankrum JA, Matthiesen ME, Phillips JA, Roes I, et al. Tracking mesenchymal stem cells with iron oxide nanoparticle loaded poly(lactide-co-glycolide) microparticles. Nano Lett 2012; 12: 4131–9. doi: 10.1021/nl301658q [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bible E, Dell'Acqua F, Solanky B, Balducci A, Crapo PM, Badylak SF, et al. Non-invasive imaging of transplanted human neural stem cells and ECM scaffold remodeling in the stroke-damaged rat brain by (19)F- and diffusion-MRI. Biomaterials 2012; 33: 2858–71. doi: 10.1016/j.biomaterials.2011.12.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chu Z, Huang Y, Tao Q, Li Q. Cellular uptake, evolution, and excretion of silica nanoparticles in human cells. Nanoscale 2011; 3: 3291–9. doi: 10.1039/c1nr10499c [DOI] [PubMed] [Google Scholar]
- 109.Luciani N, Wilhelm C, Gazeau F. The role of cell-released microvesicles in the intercellular transfer of magnetic nanoparticles in the monocyte/macrophage system. Biomaterials 2010; 31: 7061–9. doi: 10.1016/j.biomaterials.2010.05.062 [DOI] [PubMed] [Google Scholar]
- 110.Huang Z, Li C, Yang S, Xu J, Shen Y, Xie X, et al. Magnetic resonance hypointensive signal primarily originates from extracellular iron particles in the long-term tracking of mesenchymal stem cells transplanted in the infarcted myocardium. Int J Nanomedicine 2015; 10: 1679–90. doi: 10.2147/IJN.S77858 [DOI] [Google Scholar]
- 111.Winter EM, Hogers B, van der Graaf LM, Gittenberger-de Groot AC, Poelmann RE, van der Weerd L. Cell tracking using iron oxide fails to distinguish dead from living transplanted cells in the infarcted heart. Magn Reson Med 2010; 63: 817–21. doi: 10.1002/mrm.22094 [DOI] [PubMed] [Google Scholar]
- 112.Pawelczyk E, Jordan EK, Balakumaran A, Chaudhry A, Gormley N, Smith M, et al. In vivo transfer of intracellular labels from locally implanted bone marrow stromal cells to resident tissue macrophages. PLoS One 2009; 4: e6712. doi: 10.1371/journal.pone.0006712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Gaudet JM, Ribot EJ, Chen Y, Gilbert KM, Foster PJ. Tracking the fate of stem cell implants with fluorine-19 MRI. PLoS One 2015; 10: e0118544. doi: 10.1371/journal.pone.0118544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Weis C, Blank F, West A, Black G, Woodward RC, Carroll MR, et al. Labeling of cancer cells with magnetic nanoparticles for magnetic resonance imaging. Magn Reson Med 2014; 71: 1896–905. doi: 10.1002/mrm.24832 [DOI] [PubMed] [Google Scholar]
- 115.Singh N, Jenkins GJ, Asadi R, Doak SH. Potential toxicity of superparamagnetic iron oxide nanoparticles (SPION). Nano Rev 2010; 1. doi: 10.3402/nano.v1i0.5358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kostura L, Kraitchman DL, Mackay AM, Pittenger MF, Bulte JW. Feridex labeling of mesenchymal stem cells inhibits chondrogenesis but not adipogenesis or osteogenesis. NMR Biomed 2004; 17: 513–17. doi: 10.1002/nbm.925 [DOI] [PubMed] [Google Scholar]
- 117.Siglienti I, Bendszus M, Kleinschnitz C, Stoll G. Cytokine profile of iron-laden macrophages: implications for cellular magnetic resonance imaging. J Neuroimmunol 2006; 173: 166–73. doi: 10.1016/j.jneuroim.2005.11.011 [DOI] [PubMed] [Google Scholar]
- 118.Arbab AS, Yocum GT, Wilson LB, Parwana A, Jordan EK, Kalish H, et al. Comparison of transfection agents in forming complexes with ferumoxides, cell labeling efficiency, and cellular viability. Mol Imaging 2004; 3: 24–32. doi: 10.1162/153535004773861697 [DOI] [PubMed] [Google Scholar]
- 119.Kunzmann A, Andersson B, Vogt C, Feliu N, Ye F, Gabrielsson S, et al. Efficient internalization of silica-coated iron oxide nanoparticles of different sizes by primary human macrophages and dendritic cells. Toxicol Appl Pharmacol 2011; 253: 81–93. doi: 10.1016/j.taap.2011.03.011 [DOI] [PubMed] [Google Scholar]
- 120.Soenen SJ, Himmelreich U, Nuytten N, Pisanic TR, 2nd, Ferrari A, De Cuyper M. Intracellular nanoparticle coating stability determines nanoparticle diagnostics efficacy and cell functionality. Small 2010; 6: 2136–45. doi: 10.1002/smll.201000763 [DOI] [PubMed] [Google Scholar]
- 121.Ketkar-Atre A, Struys T, Soenen SJ, Lambrichts I, Verfaillie CM, De Cuyper M, et al. Variability in contrast agent uptake by different but similar stem cell types. Int J Nanomedicine 2013; 8: 4577–91. doi: 10.2147/IJN.S51588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lojk J, Bregar VB, Rajh M, Miš K, Kreft ME, Pirkmajer S, et al. Cell type-specific response to high intracellular loading of polyacrylic acid-coated magnetic nanoparticles. Int J Nanomedicine 2015; 10: 1449–62. doi: 10.2147/IJN.S76134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Eamegdool SS, Weible MW, 2nd, Pham BT, Hawkett BS, Grieve SM, Chan-ling T. Ultrasmall superparamagnetic iron oxide nanoparticle prelabelling of human neural precursor cells. Biomaterials 2014; 35: 5549–64. doi: 10.1016/j.biomaterials.2014.03.061 [DOI] [PubMed] [Google Scholar]
- 124.Tian F, Chen G, Yi P, Zhang J, Li A, Zhang J, et al. Fates of Fe3O4 and Fe3O4@SiO2 nanoparticles in human mesenchymal stem cells assessed by synchrotron radiation-based techniques. Biomaterials 2014; 35: 6412–21. doi: 10.1016/j.biomaterials.2014.04.052 [DOI] [PubMed] [Google Scholar]
- 125.Matsushita H, Mizukami S, Sugihara F, Nakanishi Y, Yoshioka Y, Kikuchi K. Multifunctional core-shell silica nanoparticles for highly sensitive (19)F magnetic resonance imaging. Angew Chem Int Ed Engl 2014; 53: 1008–11. doi: 10.1002/anie.201308500 [DOI] [PubMed] [Google Scholar]
- 126.Soenen SJ, Brisson AR, De Cuyper M. Addressing the problem of cationic lipid-mediated toxicity: the magnetoliposome model. Biomaterials 2009; 30: 3691–701. doi: 10.1016/j.biomaterials.2009.03.040 [DOI] [PubMed] [Google Scholar]
- 127.Kasten A, Grüttner C, Kühn JP, Bader R, Pasold J, Frerich B. Comparative in vitro study on magnetic iron oxide nanoparticles for MRI tracking of adipose tissue-derived progenitor cells. PLoS One 2014; 9: e108055. doi: 10.1371/journal.pone.0108055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.van Tilborg GA, Cormode DP, Jarzyna PA, van der Toorn A, van der Pol SM, van Bloois L, et al. Nanoclusters of iron oxide: effect of core composition on structure, biocompatibility, and cell labeling efficacy. Bioconjug Chem 2012; 23: 941–50. doi: 10.1021/bc200543k [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hofmann M, Wollert KC, Meyer GP, Menke A, Arseniev L, Hertenstein B, et al. Monitoring of bone marrow cell homing into the infarcted human myocardium. Circulation 2005; 111: 2198–202. doi: 10.1161/01.CIR.0000163546.27639.AA [DOI] [PubMed] [Google Scholar]
- 130.Hou D, Youssef EA, Brinton TJ, Zhang P, Rogers P, Price ET, et al. Radiolabeled cell distribution after intramyocardial, intracoronary, and interstitial retrograde coronary venous delivery: implications for current clinical trials. Circulation 2005; 112 (Suppl 9): I150–6. [DOI] [PubMed] [Google Scholar]
- 131.Nguyen PK, Lan F, Wang Y, Wu JC. Imaging: guiding the clinical translation of cardiac stem cell therapy. Circ Res 2011; 109: 962–79. doi: 10.1161/CIRCRESAHA.111.242909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Gorelik M, Orukari I, Wang J, Galpoththawela S, Kim H, Levy M, et al. Use of MR cell tracking to evaluate targeting of glial precursor cells to inflammatory tissue by exploiting the very late antigen-4 docking receptor. Radiology 2012; 265: 175–85. doi: 10.1148/radiol.12112212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Reisman M, Adams KT. Stem cell therapy: a look at current research, regulations, and remaining hurdles. P T 2014; 39: 846–57. [PMC free article] [PubMed] [Google Scholar]
- 134.van der Bogt KE, Schrepfer S, Yu J, Sheikh AY, Hoyt G, Govaert JA, et al. Comparison of transplantation of adipose tissue- and bone marrow-derived mesenchymal stem cells in the infarcted heart. Transplantation 2009; 87: 642–52. doi: 10.1097/TP.0b013e31819609d9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.van der Bogt KE, Sheikh AY, Schrepfer S, Hoyt G, Cao F, Ransohoff KJ, et al. Comparison of different adult stem cell types for treatment of myocardial ischemia. Circulation 2008; 118(Suppl 14): S121–9. doi: 10.1161/CIRCULATIONAHA.107.759480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Janowski M, Engels C, Gorelik M, Lyczek A, Bernard S, Bulte JW, et al. Survival of neural progenitors allografted into the CNS of immunocompetent recipients is highly dependent on transplantation site. Cell Transpl 2014; 23: 253–62. doi: 10.3727/096368912X661328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.van Buul GM, Siebelt M, Leijs MJ, Bos PK, Waarsing JH, Kops N, et al. Mesenchymal stem cells reduce pain but not degenerative changes in a mono-iodoacetate rat model of osteoarthritis. J Orthop Res 2014; 32: 1167–74. doi: 10.1002/jor.22650 [DOI] [PubMed] [Google Scholar]
- 138.Fan W, Cheng K, Qin X, Narsinh KH, Wang S, Hu S, et al. mTORC1 and mTORC2 play different roles in the functional survival of transplanted adipose-derived stromal cells in hind limb ischemic mice via regulating inflammation in vivo. Stem Cells 2013; 31: 203–14. doi: 10.1002/stem.1265 [DOI] [PubMed] [Google Scholar]
- 139.Liang Y, Walczak P, Bulte JW. The survival of engrafted neural stem cells within hyaluronic acid hydrogels. Biomaterials 2013; 34: 5521–9. doi: 10.1016/j.biomaterials.2013.03.095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Hwang do W, Jin Y, Lee do H, Kim HY, Cho HN, Chung HJ, et al. In vivo bioluminescence imaging for prolonged survival of transplanted human neural stem cells using 3D biocompatible scaffold in corticectomized rat model. PLoS One 2014; 9: e105129. doi: 10.1371/journal.pone.0105129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Thomas DC, Wong FS, Zaccone P, Green EA, Wållberg M. Protection of islet grafts through transforming growth factor-β-induced tolerogenic dendritic cells. Diabetes 2013; 62: 3132–42. doi: 10.2337/db12-1740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Bulte JW. Science to practice: can stem cells be labeled inside the body instead of outside? Radiology 2013; 269: 1–3. doi: 10.1148/radiol.13131753 [DOI] [PMC free article] [PubMed] [Google Scholar]