Abstract
Estimates of the incidence of acute hepatitis C virus (HCV) infection are complicated by the lack of a specific laboratory test and its generally asymptomatic presentation. This study aimed to validate estimates of the incidence of acute HCV infection in Massachusetts. The authors found that acute HCV infection may be underreported when current methods of surveillance are used.
Background
In 2010, the incidence of hepatitis C virus (HCV) infection in the United States was estimated to be 17 000 cases annually, based on 850 acute HCV cases reported to the Centers for Disease Control and Prevention by local public health authorities. Absence of symptomatic disease and lack of a specific laboratory test for acute infection complicates diagnosis and surveillance.
Objective
To validate estimates of the incidence of acute HCV infection by determining the reporting rate of clinical diagnoses of acute infection to the Massachusetts Department of Public Health (MDPH) and Centers for Disease Control and Prevention.
Design
Case series and chart review.
Setting
Two hospitals and the state correctional health care system in Massachusetts.
Patients
183 patients clinically diagnosed with acute HCV infection from 2001 to 2011 and participating in a research study.
Measurements
Rate of electronic case reporting of acute HCV infection to the MDPH and rate of subsequent confirmation according to national case definitions.
Results
149 of 183 (81.4%) clinical cases of acute HCV infection were reported to the MDPH for surveillance classification. The MDPH investigated 43 of these reports as potential acute cases of HCV infection based on their surveillance requirements; ultimately, only 1 met the national case definition and was counted in nationwide statistics published by the Centers for Disease Control and Prevention. Discordance in clinical and surveillance classification was often related to missing clinical or laboratory data at the MDPH as well as restrictive definitions, including requirements for negative hepatitis A and B laboratory results.
Limitation
Findings may not apply to other jurisdictions because of differences in resources for surveillance.
Conclusion
Clinical diagnoses of acute HCV infection were grossly underascertained by formal surveillance reporting. Incomplete clinician reporting, problematic case definitions, limitations of diagnostic testing, and imperfect data capture remain major limitations to accurate case ascertainment despite automated electronic laboratory reporting. These findings may have implications for national estimates of the incidence of HCV infection.
At least 185 million persons worldwide are infected with hepatitis C virus (HCV), with an estimated 3 to 4 million new infections occurring each year (1, 2). In developed countries, persons who inject drugs are primarily at risk for HCV infection from bloodborne exposure by means of contaminated drug paraphernalia (3). After a sharp decrease in the incidence of HCV infection in the United States in the 1990s, estimates suggest a more moderate but steady decline over the past decade, with rates calculated at 0.3 to 0.7 cases per 100 000 persons (4, 5).
Accurate and current estimates of the incidence of HCV infection at the local, state, and national levels are critical for quantifying disease burden, guiding public health agency initiatives, and tracking the outcomes of preventive interventions. Unlike acute hepatitis A and hepatitis B infections, which are diagnosed with immunoglobulin M (IgM) antibody testing, there is no single diagnostic test for acute HCV infection. Without a definitive test, surveillance by local public health officials hinges on a complex composite of risk factors; symptom reporting; laboratory assessments, including antibodies to HCV (anti-HCVs), nucleic acid testing, and aminotransferase levels; and exclusion of alternative causes of hepatitis. During acute HCV infection, aminotransferase and HCV RNA levels can fluctuate and seroconversion from negative to positive anti-HCV status can occur over time. Most patients are asymptomatic and specific symptoms, such as jaundice, are uncommon in acute infections, which further complicates detection. Patients whose acute infections clear spontaneously may have low or normal aminotransferase levels at presentation and thereby escape detection.
Acute HCV infections are reportable in most jurisdictions in the United States, which subsequently report cases to the Centers for Disease Control and Prevention (CDC) through the National Notifiable Disease Surveillance System. In 2010, 850 acute cases of HCV infection were reported to the CDC, which applied a multiplier of 20 to arrive at an estimate of 17 000 new HCV infections per year in the United States (4, 6). This calculation assumes that for each reported acute infection there are 20 unreported cases because most patients are asymptomatic and persons who inject drugs—the group with highest incidence of infection—often do not seek medical care.
In Massachusetts, all laboratory evidence of HCV infection is reportable to its department of public health. Heroin use has increased markedly in Massachusetts and has been accompanied by a sharp increase in cases of HCV infection in patients younger than 30 years, with more than 2000 new reports of prevalent cases in this age group in 2012 (7, 8). To assess the contribution of acute cases of HCV infection in Massachusetts to national incidence estimates, we retrospectively reviewed 183 diagnoses of acute HCV infection in a local cohort. We aimed to determine the proportion of clinical cases of acute HCV infection classified as confirmed for surveillance purposes and to determine why clinical cases were not counted in national statistics.
Methods
Participants
BAHSTION (Boston Acute HCV Study: Transmission, Immunity, and Outcomes Network) is a longitudinal study that recruited patients with acute HCV infection (9). Recruitment began in 1998 and patients from 2 hospitals in Boston, Massachusetts (Massachusetts General Hospital, a tertiary care hospital that also provides primary care services in several communities, and Lemuel Shattuck Hospital, a facility that serves prisoners and patients referred by public agencies) were enrolled through referrals to infectious disease and gastrointestinal specialists. The cohort also included inmates who entered Massachusetts correctional facilities and were enrolled through referrals from health care providers (10) and through a systematic screening program that asked incoming inmates about specific injection practices. This resulted in a tripling of the rate of identification of acute HCV infection from that previously reported in those facilities (11).
For study purposes, a clinical case of acute HCV infection was defined by both of the following criteria and classified as definite, probable, or possible (Figure 1): risk factor for HCV infection within the past year and consistent or supportive clinical or laboratory criteria, including compatible illness (especially jaundice), alanine aminotransferase (ALT) level greater than 7 times the upper limit of normal, seroconversion (defined as a newly positive anti-HCV test result in the context of a previous negative result), and HCV RNA characteristics (low-level viremia or fluctuations), as described in previous reports (11, 12).
Figure 1.
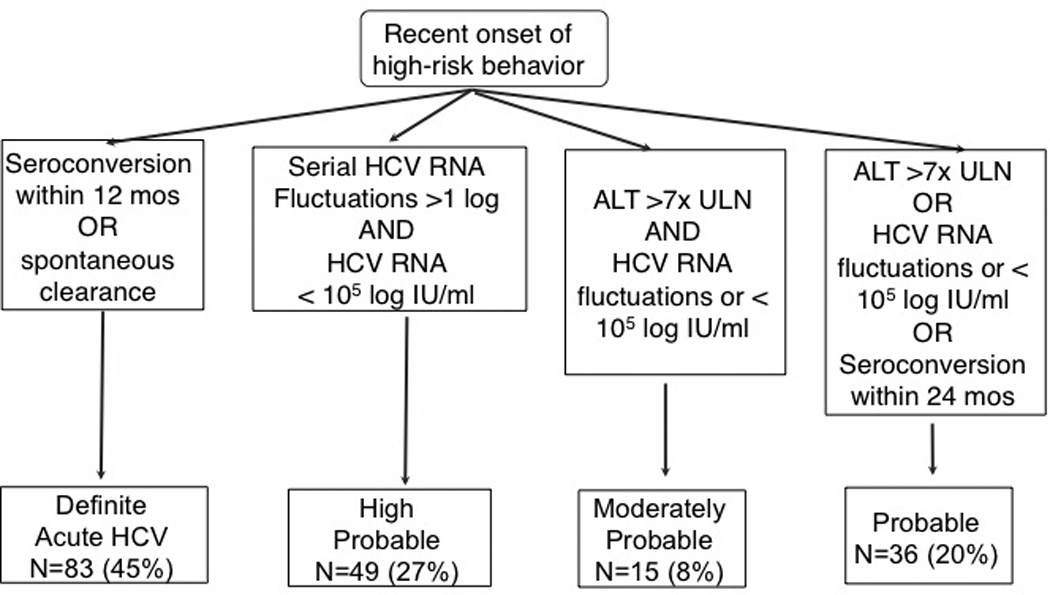
Definitions of acute HCV in BAHSTION. ALT = alanine aminotransferase; BAHSTION = Boston Acute HCV Study: Transmission, Immunity, and Outcomes Network; HCV = hepatitis C virus; ULN = upper limit of normal.
Surveillance Definitions of Past or Present HCV Infection and Acute HCV Infection
Since 2003, a confirmed case of “past or present HCV infection” for CDC surveillance purposes has been defined as having 1 or more of the following criteria: anti-HCVs with a signal–cutoff ratio predictive of a true positive result, positive results for HCV on a recombinant immunoblot assay, or positive results for HCV RNA on a nucleic acid test (including qualitative, quantitative, or genotype testing) (13). Details of specific requirements are found in the Appendix (available at www.annals.org).
Before 2012, the CDC defined a confirmed case of acute HCV infection for surveillance purposes as first meeting the previously mentioned definition of “past or present HCV infection,” plus meeting clinical criteria, including an acute illness with discrete onset of any sign or symptom consistent with acute viral hepatitis (that is, anorexia, abdominal discomfort, nausea, or vomiting) and either 1) jaundice or dark urine or 2) serum ALT levels greater than 400 IU/L. To meet the case definition, the test results must be negative for both IgM antibodies to hepatitis A virus and hepatitis B core antigen. The BAHSTION clinical case definition differs from the pre-2012 CDC surveillance definition of acute HCV infection by including the following criteria: detailed risk factor history, HCV RNA criteria that help to differentiate acute from chronic infection (low-level viremia or HCV RNA level fluctuations and spontaneous clearance of detectable viremia), use of a different threshold of ALT elevation (that is, 7 times the upper limit of normal [385 U/L vs. 400 U/L]), and inclusion of seroconversion (Appendix Table 1). In 2012, the CDC adopted changes in the case definition of acute HCV infection developed by the Council of State and Territorial Epidemiologists. These changes included seroconversion within 6 months as sufficient for diagnosis and removal of the requirement of documentation of the status of IgM antibodies to hepatitis A virus or hepatitis B core antigen (13).
Surveillance in Massachusetts for Acute HCV Infection
Hepatitis C virus infection in Massachusetts residents has been reportable to the Massachusetts Department of Public Health (MDPH) since 1992. In 2005, because of the high burden of new reports of HCV infection, the MDPH switched from case investigations done by local health departments to clinician-based reporting, in which the ordering provider completes a short, single-page HCV case report form (CRF) with patient demographic characteristics, clinical history, confirmatory laboratory results, and basic risk factors (Supplement 1, available at www.annals.org). Before 2007, if the submitted form indicated a case potentially meeting the surveillance definition for acute HCV infection (acute illness with jaundice or ALT levels >400 IU/L), follow-up was assigned to the local public health official who interviewed the patient using a more detailed acute HCV CRF (Supplement 2, available at www.annals.org). After recognition of an increase in cases of acute HCV infection identified in young patients in 2007 (7, 8), the MDPH also began sending the longer form directly to clinicians for patients aged 15 to 25 years. Epidemiologists from the MDPH review all completed acute HCV CRFs and reported laboratory results, and assign case status based on the current standard surveillance case definitions. Case classification may be modified and updated based on additional information. Those classified according to the national surveillance case definition are submitted to the CDC on a weekly basis. Reporting is the provider's responsibility, and enrollment in BAHSTION does not result in reporting to the MDPH.
Much of the data capture and management process of acute HCV infection by the MDPH has been automated through the Massachusetts Virtual Epidemiologic Network (MAVEN), an integrated surveillance and case management system that enables state and local public health professionals to share data efficiently and securely over the Internet (14). MAVEN was instituted in 2006 and houses historic surveillance data dating back to 1988. Automated electronic laboratory reporting (ELR) of HCV antibody and RNA test results to the MDPH was instituted at Massachusetts General Hospital in 2008 and the Lemuel Shattuck Hospital in 2010.
Statistical Analysis
BAHSTION and the MDPH independently identified and classified acute cases of HCV infection. Data from BAHSTION clinical cases between 2001 and 2011 were extracted and sent to the MDPH. Clinical cases were considered “reported” to the MDPH if a matching case was found in MAVEN by name or date of birth. For inexact matches, including varied spellings of names and transposed dates of birth, cases were considered a match if the address was the same. BAHSTION provided data to the MDPH on HCV antibody tests, HCV RNA and genotype test results, ALT levels, jaundice, risk history, and results of tests for hepatitis A and B virus.
The earliest reported positive HCV test result recorded in the BAHSTION data was compared with the earliest laboratory report in MAVEN to determine timeliness of reporting. Case report forms were considered “completed” if they were returned to the MDPH. Peak ALT levels during acute HCV infection and the level closest to the date that the provider reported to the MDPH from BAHSTION were compared with those in MAVEN. Assigned surveillance case status was reassessed with combined data to determine why some cases were not confirmed as “acute”. Data were analyzed with SAS, version 9.3 (SAS Institute).
All patients enrolled in BAHSTION gave informed consent to participate under protocols approved by local institutional review boards. The prison-based study was approved a priori by the Human Research Review Committee of Lemuel Shattuck Hospital, which included a prisoner advocate. Activities conducted by the MDPH were surveillance activities authorized by Massachusetts law and regulation.
Role of the Funding Source
This study was funded by the National Institutes of Health and Centers for Disease Control and Prevention. The funding sources played no role in the design, conduct, or reporting of the study.
Results
Acute Cases of HCV Infection and Rate of Reporting to the MDPH
Clinical characteristics of the 183 Massachusetts residents diagnosed with acute HCV infection by referring clinicians who were enrolled in BAHSTION between 2001 and 2011 are in the Table. Of the 183 clinical cases, 149 (81.4%) were reported to the MDPH and recorded in MAVEN on the basis of the matching algorithm (Figure 2). The Appendix Figure (available at www.annals.org) shows the number of clinical cases reported and missing by year. Reporting improved over time, particularly with the initiation of ELR, and reporting in MAVEN in 2010 and 2011 was complete. Timeliness of reporting allows efficient investigation of cases for classification; however, for 24 clinical cases, there was a substantial delay between the first recorded laboratory result with BAHSTION and the earliest HCV laboratory result in MAVEN (median, 554 days; range, 52 to 2631 days).
Table.
Demographic and Clinical Characteristics of Patients With Acute HCV Infectionin BAHSTION, 2001–2011 (n = 183)*
| Characteristic | Patients With Acute HCV Infection |
|---|---|
| Sex, % | |
| Male | 48 |
| Female | 52 |
| Race, % | |
| White | 94 |
| Black | 3 |
| Asian | 1 |
| >1 race | 1 |
| Ethnicity, % | |
| Non-Hispanic | 89 |
| Hispanic | 11 |
| Mean age (range), y | 29.8 (17.0–64.0) |
| Risk factor, % | |
| Injection drug use | 85 |
| Sexual/MSM | 5 |
| Health care–acquired | 1 |
| Other/unknown | 9 |
| Seroconversion documented, % | 45 |
| <6 mo | 23 |
| 6 to <12 mo | 14 |
| 12 to <24 mo | 8 |
| Median peak ALT level (IQR), U/L | 402 (243–988)** |
| ALT level >400 U/L, n (%) | 89 (48.6) |
| Symptoms, n (%) | |
| Jaundice | 43 (23.5) |
| Symptomatic without jaundice | 62 (33.9) |
| Asymptomatic | 73 (39.9) |
| Unknown | 5 (2.7) |
| CDC criteria, n (%) | |
| Met pre-2012 criteria | 33 (18.0) |
| Met 2012 criteria | 108 (59.0) |
ALT = alanine aminotransferase; BAHSTION = Boston Acute HCV Study: Transmission, Immunity, and Outcomes Network; CDC = Centers for Disease Control and Prevention; HCV = hepatitis C virus; IQR = interquartile range; MSM = men who have sex with men.
Percentages may not sum to 100 due to rounding.
Peak ALT values within the first 3 months of infection were missing for 17 individuals.
Figure 2.
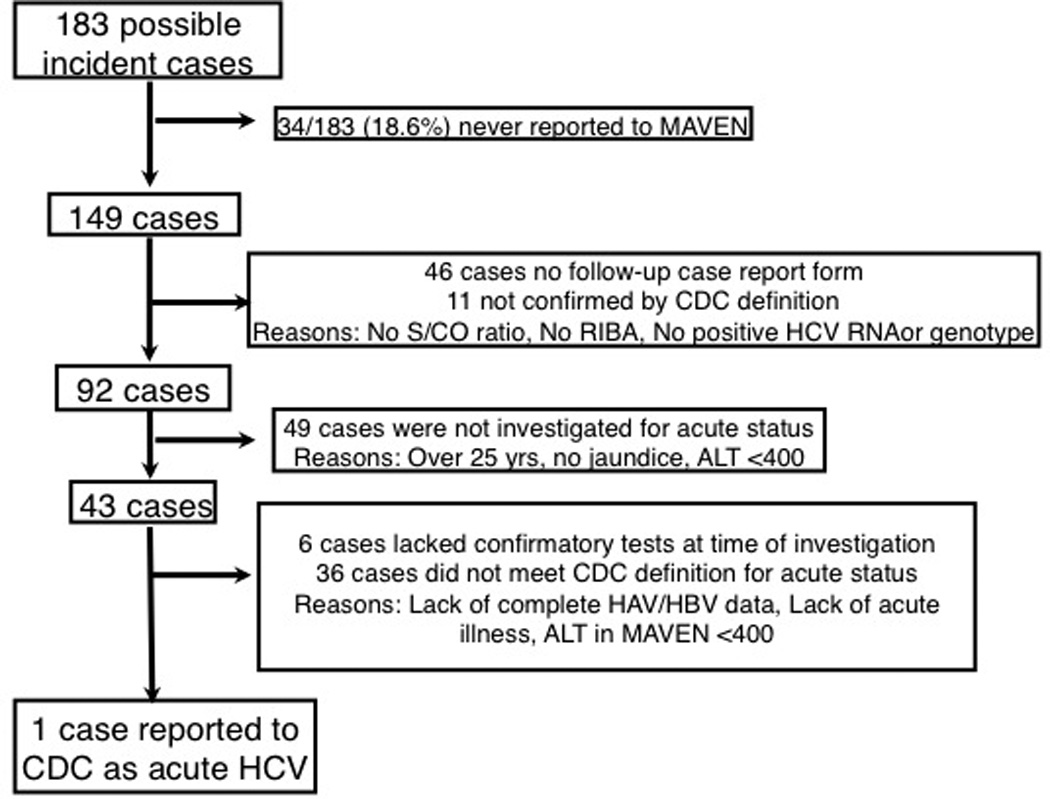
Study flow diagram. Bottlenecks in acute HCV case classification are indicated. Cases that were never reported were ineligible for further investigation. Further investigation was impaired further by lack of completed case report forms, lack of complete ALT data, and restrictive surveillance definitions. ALT = alanine aminotransferase; BAHSTION = Boston Acute HCV Study: Transmission, Immunity, and Outcomes Network; CDC = Centers for Disease Control and Prevention; HAV = hepatitis A virus; HBV = hepatitis V virus; HCV = hepatitis C virus; MAVEN = Massachusetts Virtual Epidemiologic Network; RIBA = recombinant immunoblot assay; ULN = upper limit of normal.
Thirteen clinical cases that were reported to the MDPH had laboratory results in MAVEN more than 30 days before the earliest result on record in the BAHSTION data (median, 111 days; range, 33 to 1340 days). A review of 5 clinical cases with evidence of HCV infection in MAVEN greater than 180 days before enrollment in BAHSTION indicated that 3 patients were most likely acutely reinfected with HCV between being reported in MAVEN and being recorded in BASHTION, and the other 2 were chronically infected due to previous evidence of viremia and misdiagnosis of acute HCV infection.
Assignment of Acute HCV Infection Case Definition to Reported Cases
BAHSTION had laboratory results that confirmed active HCV infection (that is, detectable HCV RNA or genotype) in 141 of the 149 clinical cases reported, with the remaining 8 cases diagnosed with acute HCV infection via seroconversion. The MDPH classified 125 of those cases as confirmed past or present HCV infection (surveillance case definition) based on the information reported and the case definition at the time of review. Of 8 cases for which BAHSTION did not have detectable RNA or genotype, 5 were classified as confirmed past or present HCV infection in MAVEN. Overall, 130 of the 149 cases were classified as “confirmed” past or present HCV infection in MAVEN.
To ascertain the likelihood of acute HCV infection, attempts to collect additional data about symptoms and laboratory results were made through completed CRFs. However, the forms were not returned for 46 (31%) of the 149 reported cases, precluding further classification (Figure 2). In addition, ALT levels were only available for 91 (61%) cases.
The MDPH evaluated cases for acute status if the CRF included an ALT result greater than 400 U/L, jaundice, or—starting in 2007—younger age (15 to 25 years). Forty-three cases met at least 1 criterion and were evaluated for reasons described in Appendix Table 2 (available at www.annals.org). Only 1 met the CDC confirmed acute surveillance case definition and was reported to the National Notifiable Disease Surveillance System (Figure 2). Of the cases that were not reported, 17 met the pre-2012 CDC surveillance case definition except for documentation of testing for hepatitis A and B virus infection. The remaining cases did not meet the surveillance case definition because acute symptoms, jaundice, or ALT levels greater than 400 U/L were not reported (n = 16) or tests considered confirmatory for HCV infection according to the surveillance case definition were missing at the time of review (n = 9).
Incomplete ALT Data and Restrictive Case Definitions
The MDPH CRFs included a request for 1 measurement each of ALT and aspartate aminotransferase levels. These measurements may have also been automatically collected through ELR if ordered simultaneously with an HCV test. Initial and peak ALT levels reported to the MDPH were compared with those on record with BAHSTION and on the CRFs (Figure 3). When an ALT level greater than 400 U/L was used as an indicator, only 25 cases were identified as potentially acute by levels reported to MAVEN despite about one half of patients exceeding this threshold during their clinical course (Table). No cases were identified as acute when only the earliest ALT level reported to MAVEN was used. If the peak ALT level from the BAHSTION records had been available to the MDPH, 48 additional cases would have potentially been reviewed for acute status; otherwise, only 6 would have been reviewed because of reported jaundice or young age at the time of reporting. Five other cases were not eligible for review because of data entry error, missing ALT level, or co-infection with other hepatitis viruses.
Figure 3.
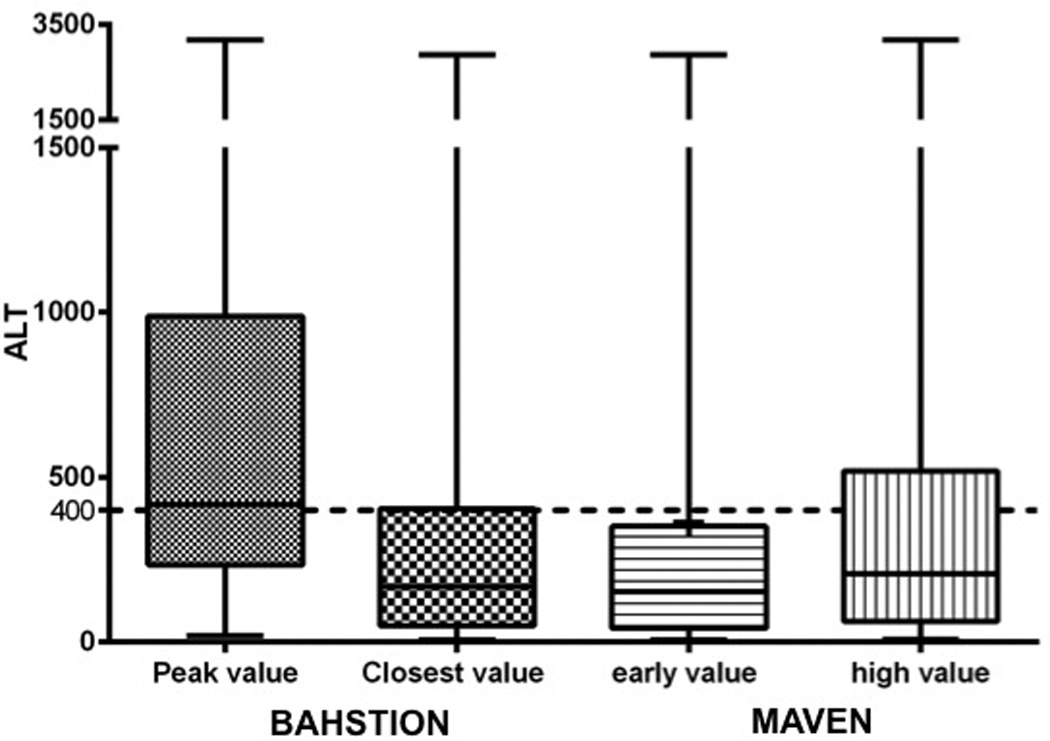
Comparison of peak ALT level documented in BAHSTION, closest level to time case reported, and earliest/highest levels in MAVEN. ALT = alanine aminotransferase; BAHSTION = Boston Acute HCV Study: Transmission, Immunity, and Outcomes Network; MAVEN = Massachusetts Virtual Epidemiologic Network.
Cases were rereviewed with all available data from MAVEN and BAHSTION. According to the surveillance case definition of acute HCV infection used before 2012, 33 cases would have been reported to the CDC as acute if full data were available to the MDPH. An additional 75 cases met the surveillance case definition in 2012, which no longer required negative hepatitis A and B laboratory results and accepted a documented negative anti-HCV laboratory test result followed within 6 months by a positive result regardless of clinical presentation.
We then explored additional potential criteria for defining acute HCV infection. Expanding the seroconversion window to 12 months would have added 16 confirmed acute cases and expanding to 24 months would include 7 more. After 2 patients were reclassified as chronic (based on data in MAVEN), 50 clinical cases still did not meet an expanded surveillance case definition with seroconversion extended to 24 months. Six had a negative antibody test result more than 24 months before their first positive test result and still represented incident cases based on other diagnostic criteria. Fluctuating HCV RNA titers greater than 1 log, a rare occurrence in the chronic phase of HCV infection (12), were noted in 19 cases. The remaining 23 did not meet the criteria but had recent risk history compatible with acute HCV infection, especially initiation of injection drug use in the past year with new exposures by means of shared injection equipment.
Discussion
This investigation showed that fewer than 1% of clinically diagnosed acute cases of HCV infection between 2001 and 2011 in Massachusetts ultimately were reported to the CDC as confirmed acute surveillance cases of HCV infection and included in national estimates of incidence of HCV infection. This low rate of reporting to national authorities is problematic because it is already recognized that clinical diagnoses of acute HCV infection underestimate the true burden of new infections due to the difficulty in making a diagnosis, minimal symptoms, and fragmented care of patients at highest risk. The primary reason was not lack of reporting to the MDPH because approximately 80% of cases of acute HCV infection in this cohort were reported and eligible for further case investigation by the state. This rate of reporting is high compared with the relatively low rates of capture by surveillance in other jurisdictions (15–17). Massachusetts currently has more than 95% of clinical laboratories reporting results electronically to the MDPH’s surveillance system, which allows for automated triage of data and centralized case management to prompt follow-up of potential acute cases of HCV infection (7, 8). Moreover, surveillance for acute infection was enhanced in 2007 by more intensive requests for information in patients aged 15 to 25 years who were likely to have been infected recently. Despite these advantages, we found a low rate of confirmed surveillance case classification of acute HCV infection, resulting in gross underreporting to the CDC.
Downstream reporting of acute HCV infection to the CDC was incomplete for various reasons, including imperfect or delayed data capture from clinicians and laboratories and restrictive surveillance case definitions for acute HCV infection. Although a physician synthesizes information from several data sources that incorporate risk factor history, presenting signs and symptoms, and laboratory results to make a clinical diagnosis (18), a stricter case definition for acute HCV infection has recently been applied for surveillance purposes (19, 20) to create consistency in classification across jurisdictions and enhance specificity.
To assist public health agencies in improving surveillance of acute HCV infection, several recommendations can be made based on our analysis. First, we agree with the decision to add seroconversion to the CDC’s surveillance case definition of acute HCV infection in late 2012 to account for incident cases without need for an illness compatible with HCV infection, a criterion that is often absent, and to remove the requirement for negative test results for hepatitis A and B virus. Successful application of seroconversion as a criterion requires regular interval testing of high-risk patients (21). More detailed risk behavior history about specific injection practices and history of onset was extremely useful in a systematic screening for HCV infection in the Massachusetts state prison system, tripling the rate of identification (10). Second, because any seroconversion represents, at a minimum, incident infection, comprehensive capture of negative test results by surveillance systems would help to identify acute cases of HCV infection. Because only annual testing of high-risk populations is recommended (22), not every case will be captured reported by use of only a 6-month period; therefore, the time frame could be made consistent with current testing guidelines. Third, peak ALT levels are incompletely captured by current systems; improved ascertainment may be achieved by capturing more ALT values by automated means or chart review or by asking providers to report peak level instead of any level, which is likely to capture levels close to the case report completion. Finally, report of any single quantitative viral titer less than 100 000 IU/mL or fluctuations in HCV RNA levels greater than 1 log raises clinical suspicion for recent acquisition of HCV infection (12, 23) and could be incorporated to trigger investigation for potential cases. These HCV RNA criteria would be particularly helpful when the peak ALT level was not obtained. Electronic laboratory reporting and integrated surveillance systems, such as MAVEN, that allow synthesis of data from several facilities may be more comprehensive than data from a single clinical entity and may reduce reliance on the clinician’s return of a cumbersome, detailed form. This approach would be particularly useful to capture seroconversions; however, the level of data management may exceed the current capacity of public health agencies.
An estimate of incidence using a multiplication factor relies on the base number of acute cases of HCV infection, which seems to be grossly underreported. The dearth of reported surveillance cases of acute infection was discordant with a burgeoning epidemic of HCV infection among adolescents and younger adults in Massachusetts (7, 8). The CDC uses a multiplication factor of 20 to account for unreported cases when estimating incidence; for each case, they estimate 3.3 symptomatic and 16.7 asymptomatic cases (6). When applied to the 115 confirmed cases of acute HCV infection in Massachusetts reported to the CDC between 2001 and 2011, this correction factor results in 2300 total new cases. This estimate is substantially lower than the 16 622 confirmed cases of past or present HCV infection reported in Massachusetts residents aged 15 to 30 years during this same period. Moreover, confirmed cases may represent a fraction of true cases among at-risk persons because several studies suggest that most infected persons are unlikely to receive medical attention at all, let alone be reported to health departments (15, 24–27).
The main limitation of our study is generalizability from our jurisdiction to others, in which surveillance activities may be more limited or absent as detailed by the Institute of Medicine’s comprehensive report on viral hepatitis (17). Strengthening HCV surveillance systems in the United States will allow a more accurate ascertainment of acute cases of HCV infection and estimates of incidence and burden. This augmentation is a key recommendation of the Institute of Medicine’s report (17), and enhanced surveillance projects have demonstrated the capacity to acquire higher-quality data on acute hepatitis cases (19, 28, 29). Another limitation is that our study was not a population-based survey of acute HCV infection; without the overall denominator, we cannot provide more information about the true multiplier that should be applied to reported acute cases of HCV infection to calculate incidence. Nonetheless, we show that a multiplier of 20 applied between 2001 and 2011 did not fully account for incident infections in our state.
Our analysis suggests that national incidence of HCV infection during this time frame may be greater than previously estimated. In the context of a staggering increase in opiate use (2), with estimates of new heroin injectors reaching 178 000 in 2011 (30) and related outbreaks of HCV infection in other geographic locations (8, 31–33), a reexamination of the methods for measuring the burden of incident HCV infection in the United States is necessary.
Supplementary Material
Case Definition Comparison*
Criteria by Which 43 Cases of Acute HCV Infection Were Identified for Follow-up*
Acknowledgments
Grant Support: Dr. Kim is supported by the National Institutes of Health, National Institute on Drug Abuse, National Institute of Allergy and Infectious Diseases (grants U19 AI066345, U19 AI082630, R01 DA033541, and R01 DA031056). Dr. Lauer is supported by the National Institutes of Health, National Institute of Allergy and Infectious Diseases (grants U19 AI066345, U19 AI082630, and R01 AI105035). The MDPH was also supported by the Centers for Disease Control and Prevention (grant CDC-RFA-PS13-130302CONT14).
Dr. McGovern reports personal fees from AbbVie Pharmaceuticals outside the submitted work. Mr. Church reports grants from the Centers for Disease Control and Prevention during the conduct of the study. Dr. Kim reports grants from the National Institutes of Health, personal fees from Bristol-Myers Squibb, and grants and personal fees from AbbVie Pharmaceuticals and Gilead Sciences during the conduct of the study.
Footnotes
Primary Funding Source: National Institutes of Health.
Disclaimer: Views expressed in this article are solely those of the authors and do not represent and should not be construed to representation a determination of policy of the State of Massachusetts.
Disclosures: Authors not named here have disclosed no conflicts of interest. Forms can be viewed at www.acponline.org/authors/icmje/ConflictOfInterestForms.do?msNum=M14-2939.
Reproducible Research Statement: Study protocol: Available from akim1@mgh.harvard.edu… (e-mail, …). Statistical code: Available from shauna.onofrey@state.ma.us… (e-mail). Data set: Available from shauna.onofrey@state.ma.us… (e-mail).
Author Contributions: Conception and design: J. Aneja, E.H. Nagami, A. DeMaria, G.M. Lauer, D.R. Church, A.Y. Kim.
Analysis and interpretation of the data: J. Aneja, G.A. Haney, E.H. Nagami, A. DeMaria, K. Barton, N. Cocoros, D.R. Church, A.Y. Kim.
Drafting of the article: J. Aneja, G.A. Haney, E.H. Nagami, A. DeMaria, K. Barton, N. Cocoros, D.R. Church, A.Y. Kim.
Critical revision of the article for important intellectual content: A. DeMaria, N. Cocoros, D.R. Church, A.Y. Kim.
Final approval of the article: E.H. Nagami, A. DeMaria, G.M. Lauer, K. Barton, S. Kulaga, N. Cocoros, A.Y. Kim.
Provision of study materials or patients: A.Y. Kim.
Statistical expertise: J. Aneja, A.Y. Kim.
Obtaining of funding: G.M. Lauer, A.Y. Kim.
Administrative, technical, or logistic support: J. Aneja, A. DeMaria, S. Kulaga, M. Bowen.
Collection and assembly of data: J. Aneja, G.A. Haney, K. Barton, S. Kulaga, M. Bowen, A.Y. Kim.
References
- 1.Mohd Hanafiah K, Groeger J, Flaxman AD, Wiersma ST. Global epidemiology of hepatitis C virus infection: new estimates of age-specific antibody to HCV seroprevalence. Hepatology. 2013;57:1333–1342. doi: 10.1002/hep.26141. [PMID: 23172780] [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention (CDC) Vital signs: overdoses of prescription opioid pain relievers----United States, 1999–2008. MMWR Morb Mortal Wkly Rep. 2011;60:1487–1492. [PMID: 22048730] [PubMed] [Google Scholar]
- 3.Kim AY, Onofrey S, Church DR. An epidemiologic update on hepatitis C infection in persons living with or at risk of HIV infection. J Infect Dis. 2013;207(Suppl 1):S1–S6. doi: 10.1093/infdis/jis927. [PMID: 23390299] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klevens RM, Hu DJ, Jiles R, Holmberg SD. Evolving epidemiology of hepatitis C virus in the United States. Clin Infect Dis. 2012;55(Suppl 1):S3–S9. doi: 10.1093/cid/cis393. [PMID: 22715211] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Williams IT, Bell BP, Kuhnert W, Alter MJ. Incidence and transmission patterns of acute hepatitis C in the United States, 1982–2006. Arch Intern Med. 2011;171:242–248. doi: 10.1001/archinternmed.2010.511. [PMID: 21325115] [DOI] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention. Viral Hepatitis – Statistics & Surveillance. [3 March 2015]; Accessed at www.cdc.gov/hepatitis/Statistics/2010Surveillance/index.htm on.
- 7.Centers for Disease Control and Prevention (CDC) Notes from the field : hepatitis C virus infections among young adults---rural Wisconsin, 2010. MMWR Morb Mortal Wkly Rep. 2012;61:358. [PMID: 22592276] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention (CDC) Hepatitis C virus infection among adolescents and young adults: Massachusetts, 2002–2009. MMWR Morb Mortal Wkly Rep. 2011;60:537–541. [PMID: 21544042] [PubMed] [Google Scholar]
- 9.Cox AL, Page K, Bruneau J, Shoukry NH, Lauer GM, Kim AY, et al. Rare birds in North America: acute hepatitis C cohorts [Editorial] Gastroenterology. 2009;136:26–31. doi: 10.1053/j.gastro.2008.11.049. [PMID: 19059257] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McGovern BH, Wurcel A, Kim AY, Schulze zur Wiesch J, Bica I, Zaman MT, et al. Acute hepatitis C virus infection in incarcerated injection drug users. Clin Infect Dis. 2006;42:1663–1670. doi: 10.1086/504327. [PMID: 16705568] [DOI] [PubMed] [Google Scholar]
- 11.Kim AY, Nagami EH, Birch CE, Bowen MJ, Lauer GM, McGovern BH. A simple strategy to identify acute hepatitis C virus infection among newly incarcerated injection drug users. Hepatology. 2013;57:944–952. doi: 10.1002/hep.26113. [PMID: 23111904] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McGovern BH, Birch CE, Bowen MJ, Reyor LL, Nagami EH, Chung RT, et al. Improving the diagnosis of acute hepatitis C virus infection with expanded viral load criteria. Clin Infect Dis. 2009;49:1051–1060. doi: 10.1086/605561. [PMID: 19725787] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Centers for Disease Control and Prevention. Viral Hepatitis – Hepatitis C Information. [3 March 2015]; Accessed at www.cdc.gov/hepatitis/hcv/hcvfaq.htm on.
- 14.Troppy S, Haney G, Cocoros N, Cranston K, DeMaria A., Jr Infectious disease surveillance in the 21st century: an integrated web-based surveillance and case management system. Public Health Rep. 2014;129:132–138. doi: 10.1177/003335491412900206. [PMID: 24587547] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hagan H, Snyder N, Hough E, Yu T, McKeirnan S, Boase J, et al. Case-reporting of acute hepatitis B and C among injection drug users. J Urban Health. 2002;79:579–585. doi: 10.1093/jurban/79.4.579. [PMID: 12468677] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hart-Malloy R, Carrascal A, Dirienzo AG, Flanigan C, McClamroch K, Smith L. Estimating HCV prevalence at the state level: a call to increase and strengthen current surveillance systems. Am J Public Health. 2013;103:1402–1405. doi: 10.2105/AJPH.2013.301231. [PMID: 23763407] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Colvin HM, Mitchell AE, editors. Committee on the Prevention and Control of Viral Hepatitis Infections; Institute of Medicine. Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C. Washington, DC: National Academies Pr; 2010. [PubMed] [Google Scholar]
- 18.Boesecke C, Wedemeyer H, Rockstroh JK. Diagnosis and treatment of acute hepatitis C virus infection. Infect Dis Clin North Am. 2012;26:995–1010. doi: 10.1016/j.idc.2012.08.011. [PMID: 23083829] [DOI] [PubMed] [Google Scholar]
- 19.Sacks-Davis R, VAN Gemert C, Bergeri I, Stoove M, Hellard M. Identifying newly acquired cases of hepatitis C using surveillance: a literature review. Epidemiol Infect. 2012;140:1925–1934. doi: 10.1017/S0950268812001033. [PMID: 22651915] [DOI] [PubMed] [Google Scholar]
- 20.Hajarizadeh B, Grebely J, Dore GJ. Case definitions for acute hepatitis C virus infection: a systematic review. J Hepatol. 2012;57:1349–1360. doi: 10.1016/j.jhep.2012.07.007. [PMID: 22796896] [DOI] [PubMed] [Google Scholar]
- 21.Deacon RM, Wand H, Stelzer-Braid S, Treloar C, Maher L. Improving surveillance for acute hepatitis C. Commun Dis Intell. 2011;35:16–20. [PubMed] [Google Scholar]
- 22.American Association for the Study of Liver Diseases, Infectious Diseases Society of America. [3 March 2015];Recommendations for Testing, Managing, and Treating Hepatitis C. doi: 10.1002/hep.31060. Accessed at http://hcvguidelines.org on. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ticehurst JR, Hamzeh FM, Thomas DL. Factors affecting serum concentrations of hepatitis C virus (HCV) RNA in HCV genotype 1–infected patients with chronic hepatitis. J Clin Microbiol. 2007;45:2426–2433. doi: 10.1128/JCM.02448-06. [PMID: 17537941] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alter MJ, Mares A, Hadler SC, Maynard JE. The effect of underreporting on the apparent incidence and epidemiology of acute viral hepatitis. Am J Epidemiol. 1987;125:133–139. doi: 10.1093/oxfordjournals.aje.a114496. [PMID: 3098091] [DOI] [PubMed] [Google Scholar]
- 25.Villano SA, Vlahov D, Nelson KE, Cohn S, Thomas DL. Persistence of viremia and the importance of long-term follow-up after acute hepatitis C infection. Hepatology. 1999;29:908–914. doi: 10.1002/hep.510290311. [PMID: 10051497] [DOI] [PubMed] [Google Scholar]
- 26.Edlin BR. Perspective: test and treat this silent killer. Nature. 2011;474:S18–S19. doi: 10.1038/474S18a. [PMID: 21613999] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Klevens RM, Liu S, Roberts H, Jiles RB, Holmberg SD. Estimating acute viral hepatitis infections from nationally reported cases. Am J Public Health. 2014;104:482–487. doi: 10.2105/AJPH.2013.301601. [PMID: 24432918] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iqbal K, Klevens RM, Jiles R. Comparison of acute viral hepatitis data quality using two methodologies, 2005–2007. Public Health Rep. 2012;127:591–597. doi: 10.1177/003335491212700609. [PMID: 23115384] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Centers for Disease Control and Prevention (CDC) Evaluation of acute hepatitis C infection surveillance ---- United States, 2008. MMWR Morb Mortal Wkly Rep. 2010;59:1407–1410. [PMID: 21048562] [PubMed] [Google Scholar]
- 30.CBHSQ. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2012. [10 June 2015]. Results from the 2011 National Survey on Drug Use and Health: Summary of National Findings (Publication no. SMA 12-4713, NSDUH Series H-44) Accessed at www.samhsa.gov/data/sites/default/files/Revised2k11NSDUHSummNatFindings/Revised2k11NSDUHSummNatFindings/NSDUHresults2011.htm on. [Google Scholar]
- 31.Centers for Disease Control and Prevention (CDC) Use of enhanced surveillance for hepatitis C virus infection to detect a cluster among young injection-drug users---New York, November 2004–April 2007. MMWR Morb Mortal Wkly Rep. 2008;57:517–521. [PMID: 18480744] [PubMed] [Google Scholar]
- 32.Centers for Disease Control and Prevention (CDC) Notes from the field : hepatitis C virus infections among young adults---rural Wisconsin, 2010. MMWR Morb Mortal Wkly Rep. 2012;61:358. [PMID: 22592276] [PubMed] [Google Scholar]
- 33.Suryaprasad AG, White JZ, Xu F, Eichler BA, Hamilton J, Patel A, et al. Emerging epidemic of hepatitis C virus infections among young nonurban persons who inject drugs in the United States, 2006–2012. Clin Infect Dis. 2014;59:1411–1419. doi: 10.1093/cid/ciu643. [PMID: 25114031] [DOI] [PubMed] [Google Scholar]
WEB ONLY
- 34.Centers for Disease Control and Prevention. Viral Hepatitis – Hepatitis C Information: Laboratory Testing. [3 March 2015]; Accessed at www.cdc.gov/hepatitis/HCV/LabTesting.htm on.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Case Definition Comparison*
Criteria by Which 43 Cases of Acute HCV Infection Were Identified for Follow-up*


