Abstract
Psoriasis is present in all racial groups, but in varying frequencies and severity. Considering that small plaque psoriasis is specific to the Asian population and severe psoriasis is more predominant in the Western population, we defined Asian small and intermediate plaque psoriasis as psoriasis subtypes, and compared their molecular signatures with classic subtype of Western large plaque psoriasis. Two different characteristics of psoriatic spreading—vertical growth and radial expansion—were contrasted between subtypes, and genomic data were correlated to histologic and clinical measurements. Compared to Western large plaque psoriasis, Asian small plaque psoriasis revealed limited psoriasis spreading, but IL-17A and IL-17-regulated pro-inflammatory cytokines were highly expressed. Paradoxically, IL-17A and IL-17-regulated pro-inflammatory cytokines were lower in Western large plaque psoriasis, while T cells and dendritic cells in total psoriatic skin area were exponentially increased. Negative immune regulators, such as CD69 and FAS, were decreased in both Western large plaque psoriasis and psoriasis with accompanying arthritis or obesity, and their expression was correlated with psoriasis severity index. Based on the disease subtype comparisons, we propose that dysregulation of T cell expansion enabled by downregulation of immune negative regulators is the main mechanism for development of large plaque psoriasis subtypes.
Keywords: Psoriasis, Subtype, Genomic comparison, Molecular phenotyping
INTRODUCTION
Psoriasis is a common skin disease affecting 2 to 3 percent of the world population (Koo, 1996a; Stern et al., 2004; van de Kerkhof, 2008). It begins as a pinhead-sized papule on the skin that vertically grows to become raised, red, scaly patch, and radially expands to peripheral body surface area, progressing eventually to severe cutaneous disease in some individuals. Severe psoriasis is frequently associated with systemic inflammation and comorbidities, such as psoriatic arthritis, cardiovascular disease, diabetes, and depression (Boehncke et al., 2011; Cohen et al., 2008b; Devrimci-Ozguven et al., 2000; Gladman et al., 2005; Koo, 1996b; Neimann et al., 2006; Schon and Boehncke, 2005). Although high throughput technologies have enabled us to perform comprehensive molecular characterization of psoriasis, the molecular determinants that are proportional to disease severity and extent are not yet understood. This is, in part, because psoriasis is a heterogeneous disease caused by complex interactions between genetic, environmental, and immunological factors, and none of the preclinical murine models fully represent the cellular changes and molecular features of psoriasis (Christophers, 2008; Danilenko, 2008; Guttman-Yassky et al., 2011; Lowes et al., 2014; Suarez-Farinas et al., 2013; Swindell et al., 2011). To overcome this translational gap and explore potential mechanisms of disease progression in humans, we identified subtypes of psoriasis in which disease progression is limited, and contrasted their cellular and molecular signatures with a more severe form of psoriasis.
Psoriasis is present in all racial groups, but in varying frequencies and severity. The prevalence is 2.5% in Caucasians, 1.3% in African Americans, but significantly lower in Asians (0.8% in India, 0.4% in China, and 0.3% in Japan) (Campalani and Barker, 2005; Gelfand et al., 2005; Prashant, 2010). In Asians, less severe psoriasis is more predominant and a distinct phenotype has been particularly reported, known as “small plaque psoriasis” (Lew et al., 2004; Youn, 2012). Small plaque psoriasis is the typical form of chronic plaque psoriasis in adults living in Korea and other Asian countries. This form of psoriasis has some resemblance to guttate psoriasis, but differs in that it is a chronic, stable form of psoriasis in adults across many years of disease persistence and overall skin surface involvement is usually <10% body surface area (Supplementary Table S1 online).
Considering that small plaque psoriasis is specific to the Asian population and severe psoriasis is more predominant in the Western population, we compared plaque psoriasis subtypes between Asian and Western populations to explore possible disease progression mechanisms. We first subtyped Asian small and intermediate psoriasis based on the individual plaque size and PASI. Then, we confirmed Asian isoforms as true psoriasis subtypes by identifying a similar transcriptome and histology compared to moderate-to-severe Western large psoriasis. We next explored models of disease progression by contrasting different cellular and molecular signatures of the subtypes. The working models represented bi-dimensional features of disease progression by separating two different phases of psoriasis progression: vertical growth (epidermal hyperplasia) and radial expansion (the extension of overall psoriasis area and severity). By correlating the genomic data to the clinical and histologic measurements of disease progression, we propose that, rather than the increase in driver cytokines, dysregulation of T cell expansion enabled by downregulation of negative regulators is the main mechanism of disease progression to large plaque psoriasis subtypes and overall disease severity.
RESULTS
Clinical stratification of small and intermediate psoriasis in the Asian population
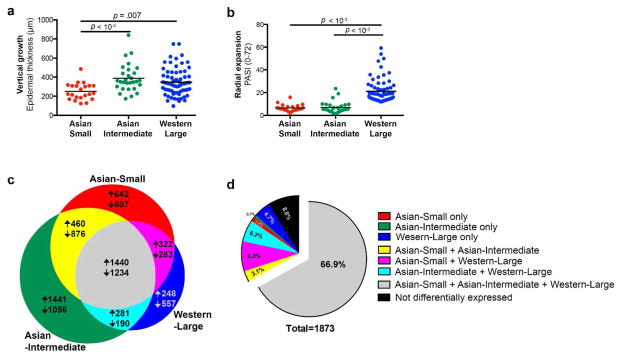
We studied 51 biopsy-confirmed psoriasis patients in Seoul, Korea, where small plaque psoriasis has been previously reported (Lew et al., 2004). Patients were clinically stratified into Asian small versus Asian intermediate psoriasis by considering individual plaque size and disease severity measured by PASI. Compared to Western large psoriasis, which has large individual plaque size and typical PASI > 12 (Feldman, 2004; Schmitt and Wozel, 2005), we defined Asian small and intermediate psoriasis as psoriasis subtypes with limited disease progression or “mild” disease (Supplementary Figure S1 online). 43% (22/51) of patients had Asian small psoriasis, in which individual plaque size (< 2 cm) and the extent of psoriasis area and severity (mean PASI 6.5 ± 0.64) were both limited (Table S1 online) (Lew et al., 2004). Smaller plaque size was consistent with thinner epidermal thickness, representing less epidermal hyperplasia in disease progression (Figure 1a). Asian small psoriasis also revealed low PASI (Figure 1b), even when psoriatic plaques were widely distributed on the trunk and/or extremities (Supplementary Figure S2 online).
Figure 1. Comparison of epidermal thickness, PASI, differentially expressed genes (DEGs), and meta-analysis-derived psoriasis transcriptome between Asian small, Asian intermediate, and Western large psoriasis.
(a) Asian small psoriasis with limited epidermal thickness in comparison to Asian intermediate or Western large psoriasis. (b) Western large psoriasis with higher PASI in comparison to Asian small or intermediate psoriasis. (c) Area-proportional Venn diagram comparing DEGs. 27.7% of all DEGs were shared by the three comparison subtypes. (d) DEGs among meta-analysis-derived psoriasis transcriptome for Affymetrix Human Genome U133 Plus 2.0 Array (MAD3)(Suarez-Farinas et al., 2012). 66.9% of the meta-analysis-derived psoriasis transcriptome was differentially expressed in all subtypes (> 2 FCH and < 0.05 FDR, PASI: Psoriasis Area and Severity Index, FCH: fold change, FDR: false discovery rate)
Asian intermediate psoriasis comprised 57% (29/51), in which individual plaque size (> 5 cm) was larger than Asian small psoriasis (< 2 cm) but the extent of psoriasis area and severity (mean PASI 7.0 ± 0.93) was less than large psoriasis (PASI > 12). Psoriatic plaques were localized to the knees, elbows, scalp, and lower backs/buttocks. Otherwise, the plaques were distributed to limited areas of the trunk or extremities. Overall, the clinical features of Asian intermediate psoriasis were similar to mild cases of psoriasis in the Western population. Clinical photos and immunohistochemical images of 10 representative Asian plaque psoriasis patients are presented in Supplementary Figure S3 online.
Genomic and histologic confirmation of Asian small and intermediate psoriasis as true psoriasis subtypes
We obtained gene expression profiles with the Affymetrix Human Genome U133 Plus 2.0 Array, and established the psoriatic transcriptomes of Asian small, Asian intermediate, and Western large psoriasis by defining differentially expressed genes (DEGs) between lesional versus non-lesional skin within each subtype (> 2 fold change (FCH), < 0.05 false discovery rate (FDR)). Among 9637 DEGs, 2674 (27.7%) were common to the three subtypes (Figure 1c). When the gene sets were narrowed down to a meta-analysis-derived transcriptome (MAD3) of Western large psoriasis for the identical array (Tian et al., 2012), there was 66.9% commonality in DEGs (Figure 1d).
We next validated if the dysregulation of established psoriasis gene sets (Bowcock et al., 2001; Gudjonsson et al., 2009; Jabbari et al., 2011; Suarez-Farinas et al., 2012; Suarez-Farinas et al., 2010; Tian et al., 2012; Yao et al., 2008) was equivalently observed in Asian small and intermediate psoriasis in comparison to Western large psoriasis. We performed Gene Set Variation Analysis (GSVA) with the combined z-score method and generated pathway enrichment scores from the observed gene expression levels. The analysis revealed that the enrichment scores of Asian small and intermediate psoriasis were not different from, or were even higher than the scores of Western large psoriasis (p < 0.01 and FDR < 0.01; Supplementary Figure S4 online).
Histological findings in Asian small and intermediate psoriasis also revealed hallmarks of histological findings in Western large psoriasis. In the psoriatic lesional skin of both Asian small and intermediate psoriasis, the epidermis revealed hyperplasia with focal parakeratosis (Supplementary Figure S3 online, immunohistochemical images). Key cellular subsets of psoriasis immunopathogenesis, CD3+ T cells and CD11c+ myeloid dendritic cells, accumulated in both subtypes. Numbers of CD3+ T cells and CD11c+ dendritic cells in Asian small psoriasis were not different from Western large psoriasis in slide sections of lesional skin (Supplementary Figure S5 online). Number of CD3+ T cells in Asian intermediate psoriasis was also not different from Western large psoriasis, while CD11c+ dendritic cells were more abundant in Asian intermediate psoriasis compared to Western large psoriasis.
Taken together, Asian small and intermediate psoriasis phenotypes were validated as psoriasis variants, sharing a common psoriasis transcriptome and histologic findings with Western large psoriasis (psoriasis vulgaris).
Models of disease progression emerge from subtype comparisons
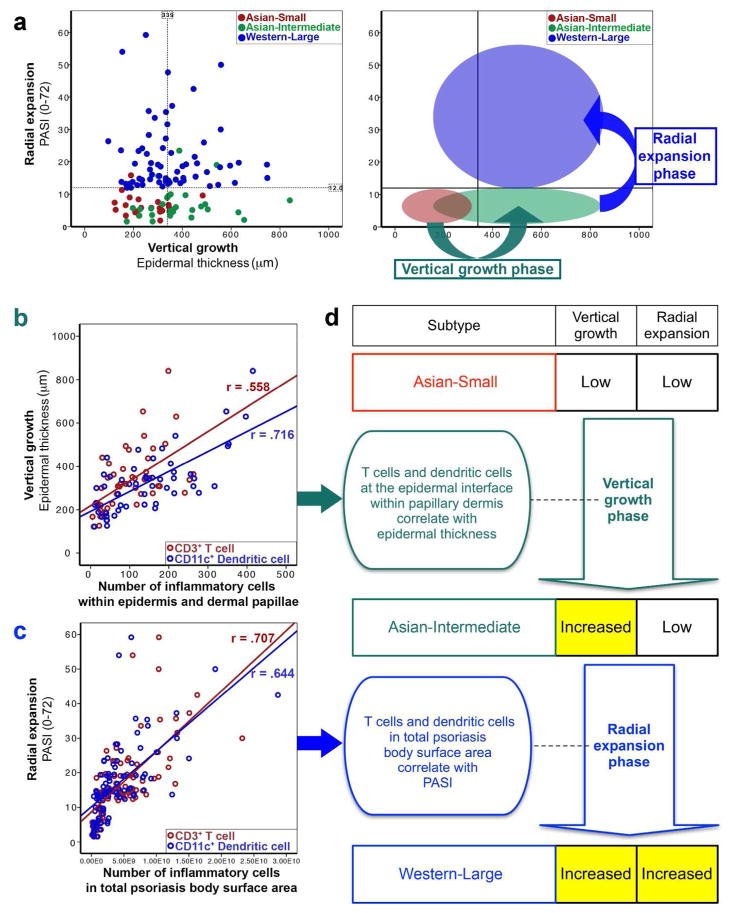
We next explored models of disease progression by correlating two different phases of disease progression: vertical growth (epidermal hyperplasia) measured by epidermal thickness of lesional skin and radial expansion (the extension of overall psoriasis area and severity) measured by PASI (Figure 2). Since Asian small psoriasis was limited in both epidermal thickness and PASI, we considered it as a model of the initial stage of disease progression.
Figure 2. Exploratory models of disease progression.
(a) Vertical growth and radial expansion models emerged from contrasting epidermal thickness and PASI between Asian small, Asian intermediate, and Western large psoriasis. (b) In the model of vertical growth phase, epidermal thickness was correlated with the numbers of CD3+ T cells and CD11c+ dendritic cells within the epidermis and dermal papillae (method of counting cells in epidermis and dermal papillae: Supplementary Figure S6 online). (c) In the model of radial expansion phase, PASI was correlated with CD3+ T cells and CD11c+ dendritic cells in total psoriasis body surface area (d) Summary of exploratory models (r = Pearson correlation, p < 0.0001, PASI: Psoriasis Area and Severity Index, Number of inflammatory cells in total psoriasis body surface area = cell count in the slide section × body surface area × proportion of psoriasis involvement).
To explore mechanisms of vertical growth, we compared Asian small and intermediate psoriasis, since epidermal thickness was significantly different between the two subtypes, without a difference in PASI (Figure 2a). In this model, CD3+ T cell and CD11c+ dendritic cell infiltrates within the epidermis and dermal papillary area were significantly different (Supplementary Figure S6 online). In addition, CD3+ T cells and CD11c+ dendritic cells within the epidermis and dermal papillary area were linearly correlated with the epidermal thickness (Figure 2b and 2d; Supplementary Table S2 online).
To explore mechanisms of radial expansion, we compared Asian intermediate and Western large psoriasis, since PASI was significantly different between the two subtypes, without a difference in epidermal thickness (Figure 2a). In this model, the accumulated T cell and dendritic cell numbers in total psoriasis body surface area of Western large psoriasis (CD3+ T cells: 6.24×109 ± 4.68×109, CD11c+ dendritic cells: 5.13×109 ± 4.74×109) were exponentially higher than the numbers for Asian intermediate psoriasis (CD3+ T cells: 1.18×109 ± 9.76×108, CD11c+ dendritic cells: 1.45×109 ± 1.43×109) (Supplementary Figure S5 online). In addition, CD3+ T cells and CD11c+ dendritic cells in total psoriasis body surface area were highly correlated to PASI (Figure 2c and 2d; Supplementary Table S2 online).
Genomic exploration of disease progression models
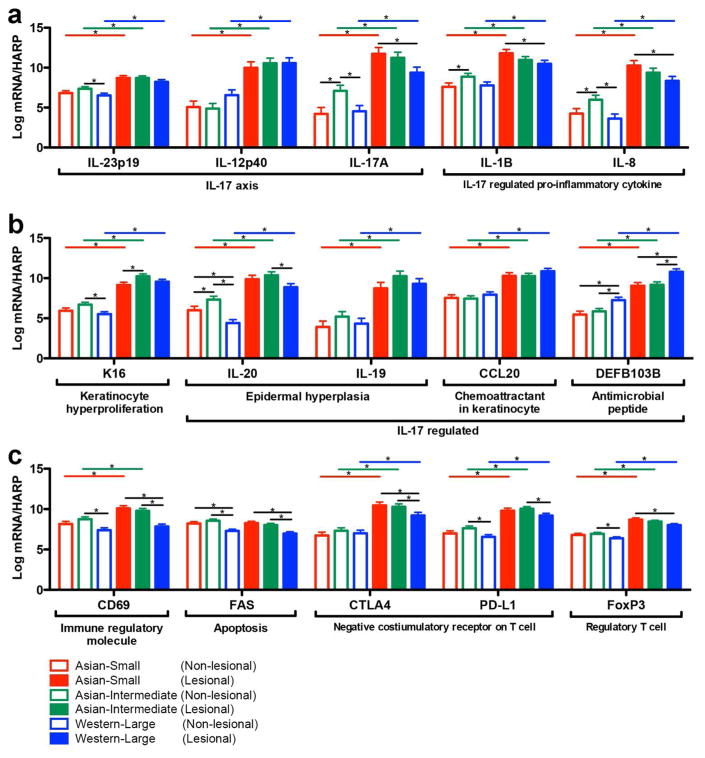
To explore molecular correlates of disease progression, we simultaneously measured expression levels of 35 genes in both lesional and non-lesional skin of Asian small (N=16), Asian intermediate (N=21), and Western large (N=20) psoriasis by RT-PCR (Figure 3 and Supplementary Figure S7 online). In the model of the initial stage of disease progression, IL-17A and IL-17-regulated pro-inflammatory cytokines (IL-1B and IL-8) were highly expressed even before vertical growth and radial expansion. The expression levels of IL-17A, IL-1B, and IL-8 in RT-PCR were highest in lesional skin of Asian small psoriasis, and were significantly higher than in Western large psoriasis (Figure 3a).
Figure 3. Quantitative comparison of gene expression in psoriatic lesional and non-lesional skin between Asian small (N=16), Asian intermediate (N=21), and Western large (N=20) psoriasis.
Expression levels of genes involved in psoriasis disease progression were quantified by RT-PCR (Gene expression: Log2 conversion of mRNA expression normalized to human acidic ribosomal protein (HARP), *p < 0.05).
In our disease progression model of the vertical growth phase phase (Asian small versus Asian intermediate psoriasis), epidermal hyperplasia was confirmed by high lesional expression of K16 in RT-PCR (Figure 3b) and Ki67 in microarray data (FCH 3.9, p < 0.05 and FDR < 0.05). IL-20, a key molecule for epidermal hyperplasia response, was expressed higher in Asian intermediate psoriasis in non-lesional skin (Figure 3b). In the multiple linear regression model predicting epidermal thickness from the RT-PCR expression levels of 35 psoriatic genes in lesional and non-lesional skin (expressions of Asian small and intermediate psoriasis in Figure 3 and Supplementary Figure S7 online), epidermal thickness of lesional skin increased in accordance with IL-17A expression in non-lesional skin (Epidermal thicknessLesional = 230.6 + 34.8 × DEFB103Lesional + 12.4 × IL-17ANon-lesional + 11.7 × IFN-αLesional – 35.9 × FasLesional, R2 = 0.47, adjusted R2 = 0.40).
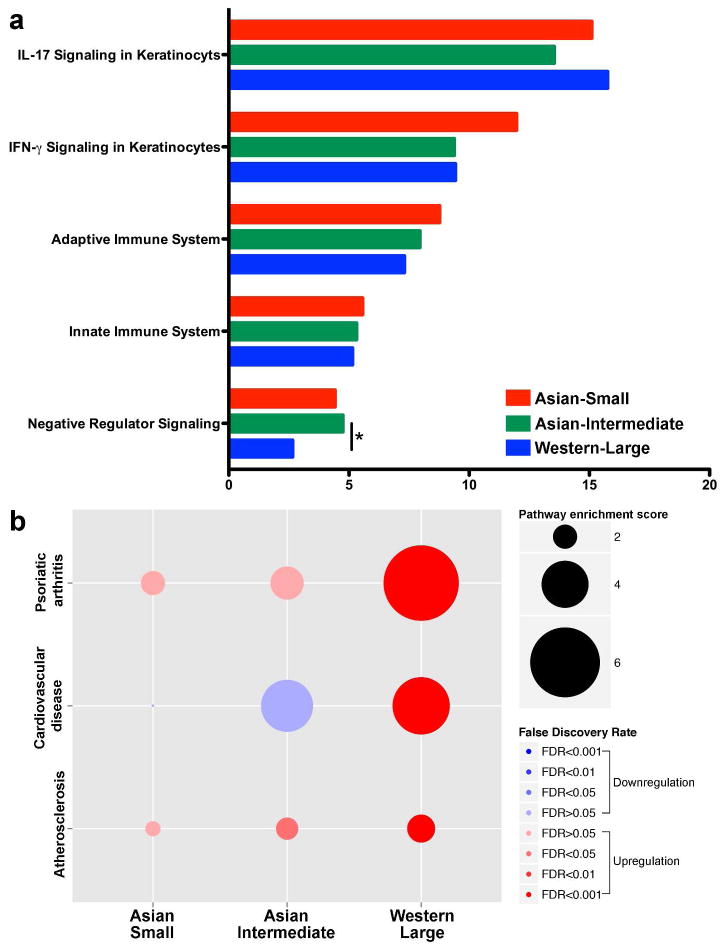
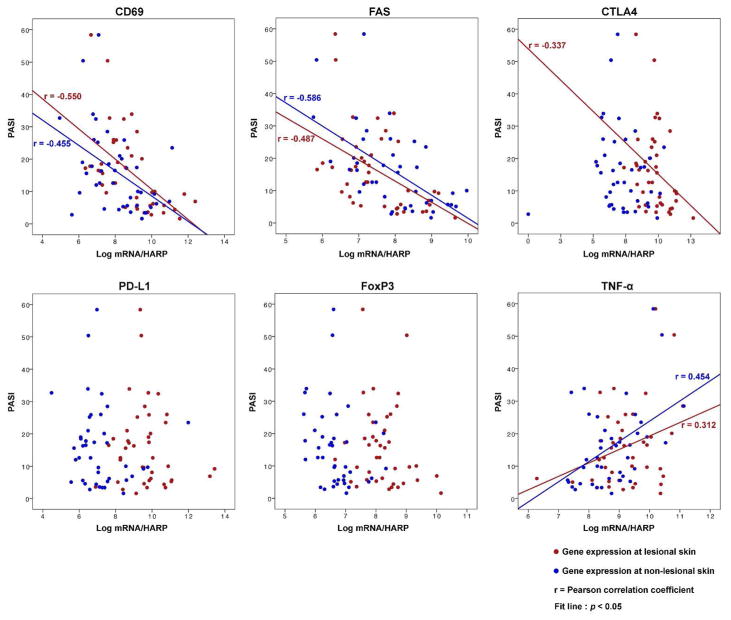
In our disease progression model of the radial expansion phase (Asian intermediate versus Western large psoriasis), we observed an increase in CD3+ T cells (Figure 2c) and a paradoxical decrease in driver cytokines (IL-17A, IL-1B, IL-8, and IL-20) (Figure 3a and 3b) in psoriatic lesional skin. We hypothesized that rather than the increase in driver cytokines, dysregulation of T cell expansion is the main mechanism of the radial expansion phase. To explore this hypothesis, we first identified significant downregulation of negative regulator signaling in Western large psoriasis in comparison to Asian intermediate psoriasis (Figure 4a). Among the immune molecules whose function as a negative regulator have been described (Abrams et al., 1999; Bovenschen et al., 2011; Chen et al., 2008; Cortes et al., 2014; Gorbachev and Fairchild, 2010; Kagen et al., 2006; Sancho et al., 2005; Stranges et al., 2007; Sugiyama et al., 2005), downregulation of CD69, Fas, CTLA4, PD-L1, and FoxP3 was confirmed by RT-PCR in lesional skin of Western large psoriasis in comparison to Asian small or intermediate psoriasis (Figure 3c). In addition, the expressions of CD69 (lesional and non-lesional), Fas (lesional and non-lesional), and CTLA4 (lesional) were negatively correlated with PASI (Figure 5 and Supplementary Table S2 online). Using a multiple regression model to predict PASI from the RT-PCR expression levels of 35 psoriatic genes in lesional and non-lesional skin (expressions of Asian intermediate and Western large psoriasis in Figure 3 and Supplementary Figure S7 online), PASI increased in accordance with the reduction of CD69 expression in non-lesional skin (PASI = −18.2 + 5.5 × TNF-αNon-lesional + 3.3 × DEFB103Non-lesional – 4.1 × CD69Non-lesional – 1.2 × IL-9Lesional, R2 = .67, adjusted R2 = .63).
Figure 4. Psoriasis pathway enrichment scores.
(a) The enrichment of pivotal immune pathways of IL-17 and IFN-γ signaling was not different between Asian small, Asian intermediate, and Western large psoriasis. Negative regulator signaling in Western large psoriasis was downregulated compared to Asian intermediate psoriasis. (*p < 0.01 and FDR < 0.01) (b) Pathways involved in psoriasis comorbidities (psoriatic arthritis, cardiovascular disease, and atherosclerosis) were significantly enriched only in Western large psoriasis (p < 0.001 and FDR < 0.001). The scores were generated by GSVA with the combined z-score method. Gene sets for pathway analysis were curated from published papers (Belasco et al., 2014; Chiricozzi et al., 2014), Molecular signatures database (http://www.broadinstitute.org/gsea/msigdb), and Gene ontology consortium (http://geneontology.org).
Figure 5. Correlation between PASI and expression levels of negative regulators in lesional and non-lesional skin.
Overlay scatter plot to display the correlation between PASI and expression levels of negative regulators (CD69, FAS, CTLA4, PD-L1, and FoxP3) in lesional (red) and non-lesional (blue) skin. The expression of TNF-α in lesional and non-lesional skin is presented as a control (Gene expression: Log2 conversion of mRNA expression normalized to human acidic ribosomal protein (HARP), PASI: Psoriasis Area and Severity Index).
Disease progression beyond cutaneous lesions
When comparing comorbid diseases across the psoriasis variants of Asian small, Asian intermediate and Western large psoriasis, there was much more frequent detection of comorbid conditions with Western large psoriasis. The difference was statistically significant for Body Mass Index (BMI), psoriatic arthritis, obesity, hypertension, and depression (p < 0.05, Supplementary Table S3 online). In a subsequent pairwise comparison with Bonferroni correction, Western large psoriasis had a higher prevalence of psoriatic arthritis and obesity than both Asian small and intermediate psoriasis (p < 0.017). To understand the significant differences in comorbidities between Asian small or intermediate psoriasis versus Western large psoriasis, we tested for a difference in the activation of comorbidity-associated pathways between the subtypes (Figure 4b). As conditions comorbid with psoriasis are often associated with atherosclerosis (Cohen et al., 2008a; Gladman et al., 2005; Gonzalez-Juanatey et al., 2007; Neimann et al., 2006; Pyorala et al., 1987), we selected gene sets of psoriatic arthritis, cardiovascular disease, and atherosclerosis for comparison. The pathway analysis revealed that psoriasis comorbidity-related gene sets were enriched only in Western large psoriasis, while there was no significant enrichment of the same gene sets in Asian small or intermediate psoriasis (p < 0.001 and FDR < 0.001) (Lee et al., 2008).
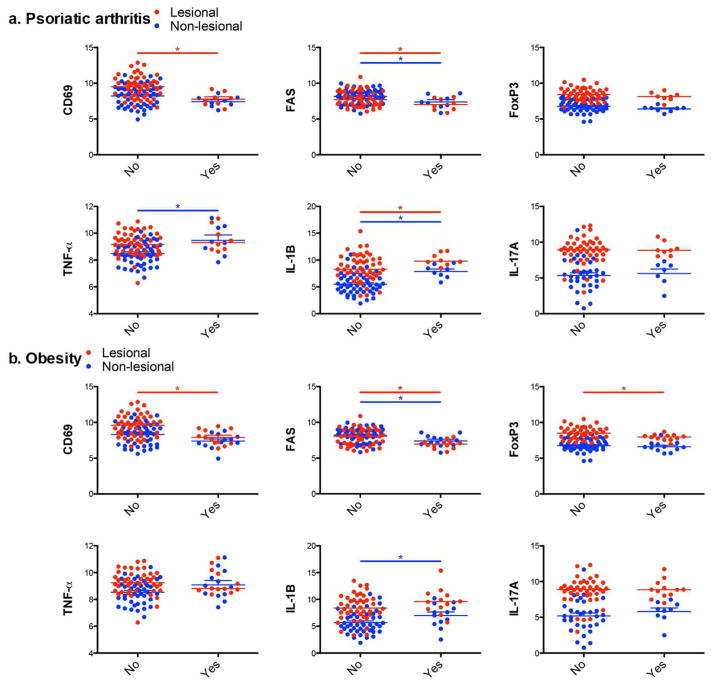
Next, we confirmed clinically the downregulation of negative regulators contrary to the upregulation of driver cytokines in the skin of psoriasis patients with accompanying comorbidities (Figure 6). Compared to psoriasis patients without psoriatic arthritis, patients with psoriatic arthritis revealed lower expression levels of negative regulators (CD69 in lesional skin, FAS in both lesional and non-lesional skin) in contrast to higher expression levels of driver cytokines (TNF-α in non-lesional skin, IL-1B in both lesional and non-lesional skin) (Figure 6a). Similarly, obese psoriasis patients revealed lower expression levels of negative regulators (CD69 in lesional skin, FAS in both lesional and non-lesional skin, and FoxP3 in lesional skin) and a higher expression level of driver cytokine (IL-1B in non-lesional skin) (Figure 6b).
Figure 6. Downregulation of negative regulators and upregulation of driver cytokines in the skin of psoriasis patients with psoriatic arthritis/obesity.
Overlay scatter plot to display different expression levels of negative regulators (CD69, FAS, and FoxP3) and driver cytokines (TNF-α, IL-1B, and IL-17A) in lesional (red) and non-lesional (blue) skin between psoriasis without (No) and with (Yes) comorbidities (Psoriatic arthritis (a), Obesity (b)). The data include total study population of Asian small, Asian intermediate, and Western large psoriasis. Gene expression: Log2 conversion of mRNA expression normalized to human acidic ribosomal protein.
DISCUSSION
Recent trans-ethnic genome-wide meta-analysis of Asian and Western psoriasis populations observed the population-specific effects of the AA positions 114 and 144 of HLA-A as well as another 10 non-MHC psoriasis susceptibility loci (Yin et al., 2015). These population-specific risk factors might lead to ethnically different expression patterns of psoriasis-associated transcriptomes, resulting in different psoriasis prevalence and clinical phenotypes. In this study, we compared psoriasis transcriptomes of clinically different Asian and Western psoriasis cohorts by two independent measures of clinical phenotyping – epidermal thickness (vertical growth) and PASI (radial expansion). To understand functional disease status by molecular characterization, quantitative expression analysis of RT-PCR (Figure 3 and Supplementary Figure S7 online) and pathway analysis of microarray (Figure 4) were combined to compare against the mRNA transcriptome that has been established in previous studies (Jabbari et al., 2011; Loscalzo, 2007; Perera et al., 2014; Suarez-Farinas et al., 2012; Suarez-Farinas et al., 2010). Serving as a reference list of genes for the core pathogenesis of psoriasis, we utilized a meta-analysis-derived transcriptome built upon the same microarray platform (Figure 1d) (Tian et al., 2012). This approach revealed that IL-17A and IL-17-regulated pro-inflammatory cytokines drive disease progression from the early stage of psoriatic plaque development, particularly as IL-17 expression was highest in Asian small psoriasis (Figure 3a). Currently, IL-17-targeted therapies that neutralize IL-17 (i.e., secukinumab and ixekizumab) have been studied in Phase I to III clinical trials and receive FDA approval only for “moderate-to-severe” psoriasis (typical PASI > 12) (Chiricozzi and Krueger, 2013). However, Asian forms of psoriasis, which are classified as “mild” disease (mean PASI < 7) in spite of significant disease extent, might also benefit from anti-IL-17 agents.
Our study also revealed that non-lesional skin around the psoriatic plaques is not normal but actively reflects disease progression status: the epidermal thickness and PASI increase in accordance with IL-17 and TNF-α in non-lesional skin, respectively. Also, there was a strong and significant correlation between PASI and negative immune regulatory gene expressions (CD69 and FAS) in non-lesional skin (Figure 5 and Supplementary Table S2 online). Given that excessive immune activation may be present in non-lesional skin, the expression of negative immune regulatory pathways may be important in restraining activation to below-thresholds of producing clinically detectable disease.
The function of negative regulators within lesional and non-lesional skin is likely important in preventing the disease progression of psoriasis. In accordance with PASI, CD3+ T cells were increased in psoriasis body surface area (Figure 2c and Supplementary Table S2 online), while the expression of negative regulators decreased proportionally (Figure 5 and Supplementary Table S2 online). Therefore, we hypothesized that the lack of negative regulator signals may allow for extensive expansion of disease-related T cell clones. In our study, the expression of FoxP3 in non-lesional skin increased as PASI increased, but the correlation was not linear (Figure 5 and Supplementary Table S2 online). However, the expression of CTLA4, the inhibitory co-receptor of Tregs, revealed significant linear correlation with PASI in lesional skin. The expression of the Fas receptor, which is a component of the Fas-FasL interactions employed by CD4+ CD25+ Tregs to restrict CD8+ T cell-mediated immune response in the skin (Gorbachev and Fairchild, 2010; Stranges et al., 2007), also revealed significant correlation with PASI in both lesional and non-lesional skin. Fas is an important regulator of migratory dendritic cell function and was recently identified as central to human skin dendritic cell programing (Anandasabapathy et al., 2014; Baratin et al., 2015). Potentially, negative immune regulators such as CTLA4 and Fas could be more important than classical Tregs in regulating T cell expression in psoriasis.
C-type lectin receptor, CD69, exerts a proinflammatory function in vitro, but recent in vivo studies revealed its function as an immunoregulatory molecule induced following activation (Sancho et al., 2005). Since CD69 induction can block sphingosine 1-phosphate receptor-1-mediated T cell egress from lymphoid tissues through interaction with membrane helix 4, CD69 is demonstrated to be a critical determinant of prolonged T cell retention (Bankovich et al., 2010; Mackay et al., 2015; Shiow et al., 2006). In this context, downregulation of CD69 in Western large psoriasis despite an exponentially higher number of CD3+ T cells in total psoriasis body surface area may represent how T cell expansion is dysregulated in psoriasis. Also, it is feasible that CD69 could be persistently expressed by a subset of regulatory T cells (Sancho et al., 2005). Although CD69+ and CD69− FoxP3+ Tregs exist in homoestatsis, only CD69-expressing Tregs express high levels of CTLA4 and have potent suppressor activity secreting high amounts of TGF-β (Cortes et al., 2014). Considering that TGF participates in the differentiation of both Tregs and TH17 cells, and that Tregs in psoriasis patients can easily differentiate into IL-17A-producing cells, CD69 might be the key determinant of disease progression in psoriasis (Bettelli et al., 2006; Bovenschen et al., 2011; Cortes et al., 2014; Veldhoen et al., 2006). In our study, the expression of CD69 in both lesional and non-lesional skin was highly correlated with PASI in a negative direction (Figure 5 and Supplementary Table S2 online).
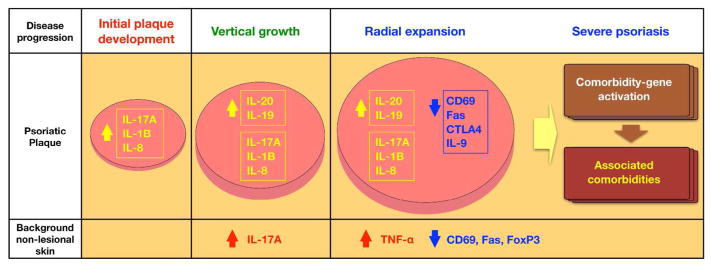
Taken together, we showed the utility of disease subtype comparisons with detailed cellular and molecular signatures to advance new hypothesis generation and future testing of concepts in clinical trials (Figure 7). A limitation of study is comparing subtypes between distinct ethnic populations under the assumption that psoriasis transcriptome profiles represent functional disease status regardless of ethnicity. Ethnicity may confound the correlation between RNA expression of genes involved in psoriasis pathogenesis and clinical phenotypes that might be influenced by other factors, such as genetic/environmental differences. To overcome this limitation, the concepts should be further tested in different subtyping strategies, and ultimately in clinical trials blocking driver cytokines and/or boosting negative regulators in an attempt to prevent disease progression.
Figure 7. Molecular determinants of disease progression in psoriasis.
Potential molecular determinants of disease progression at different stages were explored by correlating cellular and molecular signatures to histologic and clinical measurements of disease progression.
MATERIALS & METHODS
A detailed Materials and Methods description is available in Supplementary Materials and Methods online.
Study design
This study was designed to conduct ex vivo human observation with human skin biopsy tissues for the purpose of subtype comparisons (Supplementary Figure S8 online). Psoriasis lesional and non-lesional skin biopsy tissues were obtained in accordance with the Helsinki Declaration and approved by the Institutional Review Board of Korea University Guro Hospital, Seoul, South Korea and the Rockefeller University, New York, NY, USA. Written informed consent was obtained from all patients. 59 psoriasis patients were enrolled at Korea University Guro Hospital, Seoul, South Korea and 21 patients were enrolled at the Rockefeller University Hospital, New York, NY, USA (ClinicalTrials.gov; NCT01920906). 8 patients enrolled in Korea were excluded because the pathology report from independent dermatopathologists did not support the diagnosis of psoriasis. Asian small psoriasis was diagnosed according to the criteria described in Supplementary Table S1 online (Lew et al., 2004). As widely used in randomized clinical trials, Western large psoriasis was defined as moderate-to-severe psoriasis vulgaris with PASI greater than 12 (Feldman, 2004; Schmitt and Wozel, 2005).
Three different subtypes (Asian small psoriasis, Asian intermediate psoriasis, and Western large psoriasis) were compared by clinical data (demographics and comorbidities), immunohistochemical analysis, gene expression profiling, and quantitative analysis of gene expression. For immunohistochemical analysis and gene expression profiling of Western large psoriasis, we utilized de-identified repository data from established cohorts: 1) Immunohistochemistry repository data from tissue bank in the Laboratory for Investigative Dermatology at the Rockefeller University, New York (N=69), 2) NCBI GEO repository data (GSE30999, N=65).
Supplementary Material
Acknowledgments
We honor Dr. Wook Lew, who first described small plaque psoriasis in the Asian population (2004). We thank psoriasis patients attending Korea University Guro Hospital, Seoul, Korea and the Rockefeller University Hospital, New York, NY, USA for donating tissue. Korea University Guro Hospital Biobank facilitated international collaboration between Korea University Guro Hospital, Seoul, Korea and the Rockefeller University, New York, NY, USA. We thank research support from the Translational Technology Core Laboratory (Clinical Translational Science Award, Rockefeller University Center for Clinical and Translational Science, Grant#8 UL1 TR000043) from the National Center for Advancing Translational Sciences (NCATS, NIH).
Jaehwan Kim, Dong Joo Kim, Joel Correa da Rosa, and Mayte Suarez-Farinas are supported by the Rockefeller University Center for Clinical and Translational Science grant #UL1 TR000043 from the National Center for Advancing Translational Sciences (NCATS), National Institutes of Health (NIH) Clinical and Translational Science Award (CTSA) program. Jaehwan Kim, Mayte Suarez-Farinas, Michelle A. Lowes and James G. Krueger are supported by the National Psoriasis Foundation Discovery Grant Program. Jaehwan Kim is supported by the Vilcek Foundation. Mayte Suarez-Farinas is supported by Irma T. Hirschl/Monique Weill-Caulier Career Scientist Award.
Abbreviations
- CTLA4
Cytotoxic T-Lymphocyte-Associated Protein 4
- DEFB103
Defensin, Beta 103B
- FasL
Fas ligand
- FOXP3
forkhead box P3
- IFN
Interferon
- K16
Keratin 16
- PASI
Psoriasis Area and Severity Index
- PD-L1
Programmed death-ligand 1
- TGF
Transforming growth factor
- TH17
T helper 17
- TNF
Tumor necrosis factor
- Treg
Regulator T cell
Footnotes
Availability of supporting data: Raw microarray data have been deposited in NCBI’s Gene Expression Omnibus (GSE67853) and are accessible with the following link: http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?token=srinkyqcnpupbcf&acc=GSE67853
Conflict of Interest: The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this article. J.G.K. has been a consultant to and receives research support from companies developing therapeutics for psoriasis, including Amgen, Boehringer, Centocor/Janssen, Merck, Pfizer, Idera, and Astellas.
References
- Abrams JR, Lebwohl MG, Guzzo CA, et al. CTLA4Ig-mediated blockade of T-cell costimulation in patients with psoriasis vulgaris. The Journal of clinical investigation. 1999;103:1243–52. doi: 10.1172/JCI5857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anandasabapathy N, Feder R, Mollah S, et al. Classical Flt3L-dependent dendritic cells control immunity to protein vaccine. J Exp Med. 2014;211:1875–91. doi: 10.1084/jem.20131397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bankovich AJ, Shiow LR, Cyster JG. CD69 suppresses sphingosine 1-phosophate receptor-1 (S1P1) function through interaction with membrane helix 4. The Journal of biological chemistry. 2010;285:22328–37. doi: 10.1074/jbc.M110.123299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baratin M, Foray C, Demaria O, et al. Homeostatic NF-kappaB Signaling in Steady-State Migratory Dendritic Cells Regulates Immune Homeostasis and Tolerance. Immunity. 2015;42:627–39. doi: 10.1016/j.immuni.2015.03.003. [DOI] [PubMed] [Google Scholar]
- Belasco J, Louie JS, Gulati N, et al. Comparative genomic profiling of psoriatic arthritis synovium versus skin lesions. Arthritis & Rheumatology. 2014 doi: 10.1002/art.38995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettelli E, Carrier Y, Gao W, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–8. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- Boehncke WH, Boehncke S, Tobin AM, et al. The ‘psoriatic march’: a concept of how severe psoriasis may drive cardiovascular comorbidity. Experimental dermatology. 2011;20:303–7. doi: 10.1111/j.1600-0625.2011.01261.x. [DOI] [PubMed] [Google Scholar]
- Bovenschen HJ, van de Kerkhof PC, van Erp PE, et al. Foxp3+ regulatory T cells of psoriasis patients easily differentiate into IL-17A-producing cells and are found in lesional skin. The Journal of investigative dermatology. 2011;131:1853–60. doi: 10.1038/jid.2011.139. [DOI] [PubMed] [Google Scholar]
- Bowcock AM, Shannon W, Du F, et al. Insights into psoriasis and other inflammatory diseases from large-scale gene expression studies. Human molecular genetics. 2001;10:1793–805. doi: 10.1093/hmg/10.17.1793. [DOI] [PubMed] [Google Scholar]
- Campalani E, Barker J. The clinical genetics of psoriasis. Current genomics. 2005;6:51–60. [Google Scholar]
- Chen L, Shen Z, Wang G, et al. Dynamic frequency of CD4+CD25+Foxp3+ Treg cells in psoriasis vulgaris. Journal of dermatological science. 2008;51:200–3. doi: 10.1016/j.jdermsci.2008.04.015. [DOI] [PubMed] [Google Scholar]
- Chiricozzi A, Krueger JG. IL-17 targeted therapies for psoriasis. Expert opinion on investigational drugs. 2013;22:993–1005. doi: 10.1517/13543784.2013.806483. [DOI] [PubMed] [Google Scholar]
- Chiricozzi A, Nograles KE, Johnson-Huang LM, et al. IL-17 induces an expanded range of downstream genes in reconstituted human epidermis model. PloS one. 2014;9:e90284. doi: 10.1371/journal.pone.0090284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christophers E. Explaining phenotype heterogeneity in patients with psoriasis. Br J Dermatol. 2008;158:437–41. doi: 10.1111/j.1365-2133.2007.08307.x. [DOI] [PubMed] [Google Scholar]
- Cohen AD, Sherf M, Vidavsky L, et al. Association between psoriasis and the metabolic syndrome. A cross-sectional study. Dermatology. 2008a;216:152–5. doi: 10.1159/000111512. [DOI] [PubMed] [Google Scholar]
- Cohen AD, Van-Dijk D, Naggan L, et al. Factor analysis of the Beer Sheva Psoriasis Severity Score (BPSS) The Israel Medical Association journal : IMAJ. 2008b;10:419–23. [PubMed] [Google Scholar]
- Cortes JR, Sanchez-Diaz R, Bovolenta ER, et al. Maintenance of immune tolerance by Foxp3+ regulatory T cells requires CD69 expression. Journal of autoimmunity. 2014;55:51–62. doi: 10.1016/j.jaut.2014.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danilenko DM. Review paper: preclinical models of psoriasis. Veterinary pathology. 2008;45:563–75. doi: 10.1354/vp.45-4-563. [DOI] [PubMed] [Google Scholar]
- Devrimci-Ozguven H, Kundakci TN, Kumbasar H, et al. The depression, anxiety, life satisfaction and affective expression levels in psoriasis patients. Journal of the European Academy of Dermatology and Venereology : JEADV. 2000;14:267–71. doi: 10.1046/j.1468-3083.2000.00085.x. [DOI] [PubMed] [Google Scholar]
- Feldman S. A quantitative definition of severe psoriasis for use in clinical trials. Journal of dermatological treatment. 2004;15:27–9. doi: 10.1080/09546630310019382. [DOI] [PubMed] [Google Scholar]
- Gelfand JM, Stern RS, Nijsten T, et al. The prevalence of psoriasis in African Americans: results from a population-based study. Journal of the American Academy of Dermatology. 2005;52:23–6. doi: 10.1016/j.jaad.2004.07.045. [DOI] [PubMed] [Google Scholar]
- Gladman D, Antoni C, Mease P, et al. Psoriatic arthritis: epidemiology, clinical features, course, and outcome. Annals of the Rheumatic Diseases. 2005;64:ii14–ii7. doi: 10.1136/ard.2004.032482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Juanatey C, Llorca J, Amigo-Diaz E, et al. High prevalence of subclinical atherosclerosis in psoriatic arthritis patients without clinically evident cardiovascular disease or classic atherosclerosis risk factors. Arthritis and rheumatism. 2007;57:1074–80. doi: 10.1002/art.22884. [DOI] [PubMed] [Google Scholar]
- Gorbachev AV, Fairchild RL. CD4+CD25+ regulatory T cells utilize FasL as a mechanism to restrict DC priming functions in cutaneous immune responses. European journal of immunology. 2010;40:2006–15. doi: 10.1002/eji.200939387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudjonsson JE, Ding J, Li X, et al. Global gene expression analysis reveals evidence for decreased lipid biosynthesis and increased innate immunity in uninvolved psoriatic skin. Journal of Investigative Dermatology. 2009;129:2795–804. doi: 10.1038/jid.2009.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guttman-Yassky E, Nograles KE, Krueger JG. Contrasting pathogenesis of atopic dermatitis and psoriasis—part I: clinical and pathologic concepts. Journal of Allergy and Clinical Immunology. 2011;127:1110–8. doi: 10.1016/j.jaci.2011.01.053. [DOI] [PubMed] [Google Scholar]
- Jabbari A, Suárez-Fariñas M, Dewell S, et al. Transcriptional profiling of psoriasis using RNA-seq reveals previously unidentified differentially expressed genes. Journal of Investigative Dermatology. 2011;132:246–9. doi: 10.1038/jid.2011.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagen M, McCormick T, Cooper K. Cytokines as Potential Therapeutic Targets for Inflammatory Skin Diseases. Springer; 2006. Regulatory T cells in psoriasis; pp. 193–209. [Google Scholar]
- Koo J. Population-based epidemiologic study of psoriasis with emphasis on quality of life assessment. Dermatologic clinics. 1996a;14:485–96. doi: 10.1016/s0733-8635(05)70376-4. [DOI] [PubMed] [Google Scholar]
- Koo J. Population-based epidemiologic study of psoriasis with emphasis on quality of life assessment. Dermatologic clinics. 1996b;14:485–96. doi: 10.1016/s0733-8635(05)70376-4. [DOI] [PubMed] [Google Scholar]
- Lee E, Chuang HY, Kim JW, et al. Inferring pathway activity toward precise disease classification. PLoS computational biology. 2008;4:e1000217. doi: 10.1371/journal.pcbi.1000217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lew W, Lee E, Krueger J. Psoriasis genomics: analysis of proinflammatory (type 1) gene expression in large plaque (Western) and small plaque (Asian) psoriasis vulgaris. British Journal of Dermatology. 2004;150:668–76. doi: 10.1111/j.0007-0963.2004.05891.x. [DOI] [PubMed] [Google Scholar]
- Loscalzo J. Association studies in an era of too much information: clinical analysis of new biomarker and genetic data. Circulation. 2007;116:1866–70. doi: 10.1161/CIRCULATIONAHA.107.741611. [DOI] [PubMed] [Google Scholar]
- Lowes MA, Suarez-Farinas M, Krueger JG. Immunology of psoriasis. Annual review of immunology. 2014;32:227–55. doi: 10.1146/annurev-immunol-032713-120225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay LK, Braun A, Macleod BL, et al. Cutting edge: CD69 interference with sphingosine-1-phosphate receptor function regulates peripheral T cell retention. Journal of immunology. 2015;194:2059–63. doi: 10.4049/jimmunol.1402256. [DOI] [PubMed] [Google Scholar]
- Neimann AL, Shin DB, Wang X, et al. Prevalence of cardiovascular risk factors in patients with psoriasis. Journal of the American Academy of Dermatology. 2006;55:829–35. doi: 10.1016/j.jaad.2006.08.040. [DOI] [PubMed] [Google Scholar]
- Perera GK, Ainali C, Semenova E, et al. Integrative biology approach identifies cytokine targeting strategies for psoriasis. Sci Transl Med. 2014;6:223ra22. doi: 10.1126/scitranslmed.3007217. [DOI] [PubMed] [Google Scholar]
- Prashant DDB. Epidemiology of psoriasis in Malaysia: a hospital based study. Med J Malaysia. 2010;65:112. [PubMed] [Google Scholar]
- Pyorala K, Laakso M, Uusitupa M. Diabetes and atherosclerosis: an epidemiologic view. Diabetes/metabolism reviews. 1987;3:463–524. doi: 10.1002/dmr.5610030206. [DOI] [PubMed] [Google Scholar]
- Sancho D, Gomez M, Sanchez-Madrid F. CD69 is an immunoregulatory molecule induced following activation. Trends in immunology. 2005;26:136–40. doi: 10.1016/j.it.2004.12.006. [DOI] [PubMed] [Google Scholar]
- Schmitt J, Wozel G. The psoriasis area and severity index is the adequate criterion to define severity in chronic plaque-type psoriasis. Dermatology. 2005;210:194–9. doi: 10.1159/000083509. [DOI] [PubMed] [Google Scholar]
- Schon MP, Boehncke WH. Psoriasis. The New England journal of medicine. 2005;352:1899–912. doi: 10.1056/NEJMra041320. [DOI] [PubMed] [Google Scholar]
- Shiow LR, Rosen DB, Brdickova N, et al. CD69 acts downstream of interferon-alpha/beta to inhibit S1P1 and lymphocyte egress from lymphoid organs. Nature. 2006;440:540–4. doi: 10.1038/nature04606. [DOI] [PubMed] [Google Scholar]
- Stern RS, Nijsten T, Feldman SR, et al. Psoriasis is common, carries a substantial burden even when not extensive, and is associated with widespread treatment dissatisfaction. Proceedings of the Conference Psoriasis is common, carries a substantial burden even when not extensive, and is associated with widespread treatment dissatisfaction; Nature Publishing Group; 2004. [DOI] [PubMed] [Google Scholar]
- Stranges PB, Watson J, Cooper CJ, et al. Elimination of antigen-presenting cells and autoreactive T cells by Fas contributes to prevention of autoimmunity. Immunity. 2007;26:629–41. doi: 10.1016/j.immuni.2007.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suárez-Fariñas M, Arbeit R, Jiang W, et al. Suppression of molecular inflammatory pathways by Toll-like receptor 7, 8, and 9 antagonists in a model of IL-23-induced skin inflammation. PloS one. 2013;8:e84634. doi: 10.1371/journal.pone.0084634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suárez-Fariñas M, Li K, Fuentes-Duculan J, et al. Expanding the psoriasis disease profile: interrogation of the skin and serum of patients with moderate-to-severe psoriasis. Journal of Investigative Dermatology. 2012 doi: 10.1038/jid.2012.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suárez-Fariñas M, Lowes MA, Zaba LC, et al. Evaluation of the Psoriasis Transcriptome across Different Studies by Gene Set Enrichment Analysis (GSEA) PLoS ONE. 2010;5:e10247. doi: 10.1371/journal.pone.0010247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama H, Gyulai R, Toichi E, et al. Dysfunctional Blood and Target Tissue CD4+CD25high Regulatory T Cells in Psoriasis: Mechanism Underlying Unrestrained Pathogenic Effector T Cell Proliferation. The Journal of Immunology. 2005;174:164–73. doi: 10.4049/jimmunol.174.1.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swindell WR, Johnston A, Carbajal S, et al. Genome-wide expression profiling of five mouse models identifies similarities and differences with human psoriasis. PloS one. 2011;6:e18266. doi: 10.1371/journal.pone.0018266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian S, Krueger JG, Li K, et al. Meta-analysis derived (MAD) transcriptome of psoriasis defines the “core” pathogenesis of disease. PLoS One. 2012;7:e44274. doi: 10.1371/journal.pone.0044274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Kerkhof PC. Textbook of psoriasis. John Wiley & Sons; 2008. [Google Scholar]
- Veldhoen M, Hocking RJ, Atkins CJ, et al. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–89. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Yao Y, Richman L, Morehouse C, et al. Type I interferon: potential therapeutic target for psoriasis? PLoS One. 2008;3:e2737. doi: 10.1371/journal.pone.0002737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin X, Low HQ, Wang L, et al. Genome-wide meta-analysis identifies multiple novel associations and ethnic heterogeneity of psoriasis susceptibility. Nat Commun. 2015;6:6916. doi: 10.1038/ncomms7916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youn JI. Psoriasis in Korean. Korean Journal of Dermatology. 2012;50:387–402. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.