Abstract
Neuroinflammation is a component of secondary injury following traumatic brain injury (TBI) that can persist beyond the acute phase. Leukotrienes are potent, pro-inflammatory lipid mediators generated from membrane phospholipids. In the absence of injury, leukotrienes are undetectable in brain, but after trauma they are rapidly synthesized by a transcellular event involving infiltrating neutrophils and endogenous brain cells. Here, we investigate the efficacy of MK-886, an inhibitor of 5-lipoxygenase activating protein (FLAP), in blocking leukotriene synthesis, secondary brain damage, synaptic dysfunction, and cognitive impairments after TBI. Male Sprague Dawley rats (9-11 weeks) received either MK-886 or vehicle after they were subjected to unilateral moderate fluid percussion injury (FPI) to assess the potential clinical use of FLAP inhibitors for TBI. MK-886 was also administered before FPI to determine the preventative potential of FLAP inhibitors. MK-886 given before or after injury significantly blocked the production of leukotrienes, measured by reverse-phase liquid chromatography coupled to tandem mass spectrometry (RP LC-MS/MS), and brain edema, measured by T2-weighted magnetic resonance imaging (MRI). MK-886 significantly attenuated blood-brain barrier disruption in the CA1 hippocampal region and deficits in long-term potentiation (LTP) at CA1 hippocampal synapses. The prevention of FPI-induced synaptic dysfunction by MK-886 was accompanied by fewer deficits in post-injury spatial learning and memory performance in the radial arms water maze (RAWM). These results indicate that leukotrienes contribute significantly to secondary brain injury and subsequent cognitive deficits. FLAP inhibitors represent a novel anti-inflammatory approach for treating human TBI that is feasible for both intervention and prevention of brain injury and neurologic deficits.
Keywords: traumatic brain injury, neuroinflammation, leukotrienes, FLAP inhibitor, hippocampus, long-term potentiation, memory and learning, blood-brain barrier, edema
Introduction
Accumulating evidence of neurodegenerative pathology and progressive neurological dysfunction following repetitive concussion in high-impact sports (Jordan, 2013; McKee et al., 2013; Smith et al., 2013) and the rising number of TBI cases in war veterans exposed to explosive blasts (Taber et al., 2006; Warden, 2006) has increased public awareness of TBI. An estimated 1.7 million people in the United States suffer a TBI each year, but this estimate only includes injuries for which medical care is sought (Langlois et al., 2006; Faul et al., 2010). Because of this, TBI is considered a ‘silent epidemic’ as many mild TBI cases are unrecognized and unreported, and the magnitude of morbidity and mortality associated with these injuries has been largely underestimated. Advances in life support procedures have decreased the mortality rate of TBI, but many patients still face life-long physical and cognitive disabilities (Selassie et al., 2008). In the past several years, there has been increased interest in the diagnosis of mild TBI through advanced neuroimaging techniques and the use of plasma biomarkers. However, the development of drugs for blocking the detrimental consequences of TBI is lagging behind.
The pathophysiology of TBI is complex and heterogeneous. The primary injury at the time of trauma activates multiple pathways that lead to secondary injury days to weeks later (Gennarelli, 1993; Kochanek et al., 2009). The primary injury can present as any combination of skull fractures, intracranial hematomas, lacerations, contusions, and/or penetrating wounds. Secondary injury results from the activation of multiple pathways that lead to altered ionic balance, BBB permeability, edema, increased intracranial pressure, oxidative stress, neuronal cell death, and eventual neurologic impairment (Barkhoudarian et al., 2011). At the time of BBB disruption a neuroinflammatory response is activated that can persist for several weeks following TBI (Morgantini-Kossman et al., 2007; Shlosberg et al., 2010). This disruption results from mechanical shearing of blood vessels at the time of injury and/or chemically-mediated signaling cascades resulting in increased BBB permeability (Schmidt et al., 2005; Morgantini-Kossman et al., 2007; Shlosberg et al., 2010). Infiltrating peripheral immune cells (i.e. leukocytes) activate resident astrocytes and microglia, which initiates pro-inflammatory signaling pathways that contribute to further BBB breakdown and brain edema (Streit et al., 2004; Schmidt et al., 2005; Morgantini-Kossman et al., 2007).
Leukotrienes are potent bioactive lipids that are important mediators of inflammation (Murphy et al., 1979). Leukotriene biosynthesis is initiated by mechanical injury to cells or by calcium entry, which releases arachidonic acid (AA) from membrane glycerolphospholipids (Folco et al., 2006). The enzymatic action of 5-LO and FLAP converts AA into leukotriene A4 (LTA4). LTA4 is quickly converted to LTB4 by LTA4-hydrolase or to LTC4 by LTC4-synthase. LTC4 can then be converted to LTD4 and LTE4, and these three LTs (LTC4, LTD4, LTE4) are collectively known as the cysteinyl-leukotrienes. The actions of cysteinyl leukotrienes have been studied primarily in the context of asthma where they are known to induce vascular permeability, extravasation of large molecules, stimulation of cytokine release, and contraction of bronchial smooth muscle (Boyce, 2007).
Leukotrienes are undetectable in the healthy brain (Farias et al., 2009). However, our laboratory has demonstrated that leukotrienes are rapidly produced after TBI by a transcellular mechanism involving infiltrating neutrophils and endogenous brain cells (Farias et al., 2007; Farias et al., 2009). To explore the role of leukotrienes in TBI and the clinical potential of using FLAP inhibitors, we investigated the efficacy of a commercially available FLAP inhibitor, MK-886, in reducing injury-induced leukotriene production, edema, BBB disruption, as well as hippocampal-related synaptic dysfunction and cognitive deficits. Our findings have important implications for treating human TBI and suggest that development of FLAP inhibitors for use in TBI is feasible for both intervention when administered shortly after TBI and prevention when administered to “at risk” individuals prior to potential brain trauma.
Materials and Methods
Animals
Adult male Sprague Dawley rats (9-11 weeks old, 250-300g; Harlan Laboratories) were housed individually in temperature- and light-controlled housing with free access to food and water ad libitum. All procedures as described were performed under protocols approved by the University of Colorado Institutional Animal Care and Use Committee and in compliance with National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals. A total of 134 animals were used in this study.
Lateral fluid percussion injury
Craniotomy and FPI were performed using a previously validated and published procedure (Farias et al., 2009; Frey et al., 2009). Briefly, animals were anesthetized with 3-5% isoflurane (Isosol, VEDCO Inc., St. Joseph, MO) via nose cone and mounted in a stereotaxic head frame. A 3 mm craniotomy was created and centered at 3 mm caudal to bregma and 3.5 mm left of the sagittal suture, keeping the exposed dura intact. One steel support screw was embedded in the skull on the contralateral side. A Luer-Lock hub (inside diameter 3.5 mm) was centered over the craniotomy and bonded to the skull with cyanoacrylate adhesive and capped. Dental acrylic (Snap, Parkell, Inc., Edgewood, NJ) was poured around the hub and screw. After the acrylic hardened, antibiotic ointment was applied around the cap, and animals were returned to their cages. The next day (15-20 hr later) animals were anesthetized with isoflurane in an induction chamber, immediately connected to the FPI apparatus, and received a 20 msec pulse of pressurized sterile saline (2.7 atm, moderate severity impact) on the intact dural surface before awakening from anesthesia. Sham-injured animals underwent craniotomy and were anesthetized and connected to the FPI apparatus, but they did not receive the fluid pulse. All animals received a subcutaneous injection of the analgesic, buprenorphine (0.05 mg/kg; Buprenex), prior to craniotomy, and subsequent injections every 12 hours for two days. Moistened food pellets were provided after injury, and all animals were monitored daily for well-being and weight changes.
Administration of MK-886 and vehicle
MK-886 was prepared at a concentration of 2.5 mg/ml, dissolved in DMSO and then diluted with 0.9% saline to 10% DMSO. Animals were briefly anesthetized with 3-3.5% isoflurane and either MK-886 (6 mg/kg) or vehicle was administered intravenously (IV) by tail vein. All animals were allowed to wake before undergoing additional procedures.
Measurement of leukotrienes in brain
Extraction of rat brain lipids
Cortical and hippocampal regions from ipsilateral and contralateral hemispheres were collected in 4 ml of 80% methanol, homogenized with a Dounce homogenizer, and internal standards were added to the homogenates. Protein content was measured using BCA protein assay to normalize lipid levels to the amount of tissue. Samples were centrifuged and the supernatant was collected. Samples were diluted to a final methanol concentration of lower than 15% and then the lipids were extracted using a solid phase extraction cartridge (Strata C18-E, 100 mg/1 ml, Phenomenex, Torrence CA). The eluate (1 ml of methanol) was dried down and reconstituted in 70 μl of HPLC solvent A (8.3 mM acetic acid buffered to pH 5.7 with NH4OH) + 20 ml of solvent B (acetonitrile/methanol, 65/35, v/v).
RP LC-MS/MS
An aliquot of each sample (35 μL) was injected into an HPLC system and subjected to reverse-phase chromatography using a C18 (Columbus 150 × 1 mm, 5 μm, Phenomenex) column eluted at a flow rate of 50 μL/min with a linear gradient from 25% to 100% of mobile phase B. Solvent B was increased from 25% to 85% by 24 min, to 100% by 26 min, and held at 100% for a further 12 min. The HPLC effluent was directly connected to the electrospray source of a triple quadrupole mass spectrometer (Sciex API 2000, PE- Sciex, Thornhill, Ontario, Canada) and mass spectrometric analyses were performed in the negative ion mode using multiple reaction monitoring (MRM) of the specific transitions, m/z 624 → 272 for LTC4, m/z 495 → 177 for LTD4, m/z 335 → 195 for LTB4, m/z 339 → 197 for d4-LTB4, and m/z 629 → 277 for d5-LTC4. Quantitation was performed using a standard isotope dilution curve as previously described (Farias et al., 2007) with reference leukotriene standards and stable isotope analogs (Cayman Chemical, Ann Arbor, MI).
MRI
Acquisition
All MRI studies were performed in the University of Colorado Animal Imaging Shared Resource (AISR) facility. Animals underwent MRI imaging at 72 hours after injury, using T2-weighted sequences. For all MRIs, the rats were anesthetized with 2.5% isoflurane. Scans were done using a 4.7 Tesla Bruker PharmaScan, and a quadrature birdcage coil (inner diameter 38 mm), tuned to the 1H frequency of 200.27 MHz, was used for RF transmission and reception. T2-weighted axial MR scans were acquired using a RARE (rapid acquisition with relaxation enhancement) sequence with the following parameters: FOV: 4.6cm; TE/TR: 32/5000 msec; slice thickness= 1.20 mm; interslice distance 1.20 mm (no gap); number of slices= 20; number of averages = 4 per phase encode step; matrix size= 128×256.
T2-weighted MRI analysis
For each rat, five slices (1.2 mm) spanning the entire area of injury were used to calculate FPI-related brain swelling. The diameter of the injured, ipsilateral hemisphere was measured from midline to the widest point of the cortex (Fiji/ImageJ, NIH). The difference between the ipsilateral (ipsi) and contralateral (contra) hemisphere diameters was then calculated and normalized to the diameter of the contralateral hemisphere using the formula: {(diameter (Ipsi) – diameter (Contra))/ diameter (Contra) × 100}.
Evans Blue administration and extravasation analysis
One hour prior to FPI, animals received a 5ml intraperitoneal (IP) injection of EB solution (2% w/v in saline). Six hours post-FPI, animals were deeply anesthetized with sodium pentobarbital (50 mg/kg IP) and transcardially perfused with 200 ml ice-cold heparinized saline, followed by 100 ml freshly prepared 4% paraformaldehyde in PBS. Brains were removed and post-fixed in 4% paraformaldehyde/PBS for four hours at 4°C. Brains were then cryoprotected in 20% sucrose in PBS at 4°C, embedded in O.C.T. (Sakura Finetek USA Inc., Torrance, CA) and stored at -70°C. Whole brains were sectioned coronally at 30μm, and representative slices spanning the entire hippocampus at 270μm increments from each animal were mounted onto slides and cover-slipped with Fluoromount-G containing DAPI (SouthernBiotech, Birmingham, AL). Fluorescent images of whole brain sections were photographed using Surveyor by Objective Imaging software (Cambridge, UK) with a black and white Leica DFC 365FX camera on a Leica DM6000B microscope. A series of 10x images aligned in a grid was obtained using the multiscan setting. Images were stitched together in real time using the extended focus algorithm. Images of EB-positive hippocampal slices were captured using a Zeiss Axioplan2 microscope equipped with a HB0100w/2 lamp, a Photometrics CoolSnapfx camera (Roper Scientific), and IPLab software (BD Biosciences). Images from each slice were stitched together using Fiji/ImageJ (NIH), and EB-positive cells in the hippocampal cell layers were quantified using the cell counter tool.
Electrophysiology
Hippocampal Slice Preparation
Four days after FPI animals were sacrificed and the brains were rapidly removed and immersed in ice-cold, sucrose containing cutting buffer (in mM: 87 NaCl, 2.5, KCl, 7 MgCl2, 0.5 CaCl2, 1.25 NaH2PO4, 25 D-glucose, 35 sucrose, and 25 NaHCO3) for 40-60 s to cool the interior of the brain. Transverse slices (400 μm thickness) were made using a McIlwain Tissue Chopper and the slices were stored individually for recovery (at least 60 min). After recovery, a single slice was transferred to a recording chamber and superfused with artificial cerebrospinal fluid (aCSF) at a bulk flow rate of 2-3 ml/min at 31°C. The aCSF contained the following (in mM): 126 NaCl, 3.0 KCl, 1.0 MgSO4, 2.0 CaCl2, 1.2 NaH2PO4, 11 D-glucose, and 25.9 NaHCO3. A bipolar tungsten stimulating electrode was placed in the Schaffer collateral (SC) pathway to evoke synaptic field excitatory postsynaptic potentials (fEPSPs) recorded in the stratum radiatum using a nearby glass micropipette filled with aCSF.
Baseline Recordings
Before each experimental run on a slice, an input-output curve was generated by increasing the stimulus voltage and recording the synaptic response until either a maximum was reached, or evidence of a population spike was observed on the fEPSP response. Also, a paired-pulse ratio (PPR) was run whereby pairs of stimuli were delivered to CA3 axons at an intrastimulus interval of 50 ms. PPR was quantitated as the amplitude of the second fEPSP / amplitude of the first fEPSP × 100.
Measurement of Long-Term Potentiation
fEPSP responses were evoked with bipolar tungsten electrodes placed in the CA3 to CA1 dendritic field layer. Test stimuli were delivered once every 20 seconds with the stimulus intensity set to 40-50% of the maximum synaptic response. High-frequency stimulation (HFS) consisted of two trains of 100 Hz stimuli lasting 1 second each, with an inter-train interval of 20 seconds, at the control stimulus intensity. fEPSP recordings were made with a glass micropipette filled with aCSF and placed in the stratum radiatum approximately 200-300 μm from the cell body layer. This stimulation produced a potentiated response (LTP) that persisted for more than 60 minutes in control or sham animals. The slopes of fEPSPs were calculated as the slope measured between 10-30% from the origin of the initial negative deflection. Each time point shown is an average of six 20-second interval measurements.
Radial arms water maze testing
The RAWM consists of six 50 cm radial arms emanating from a circular area in a 160 cm diameter tank of 20.5°C water, surrounded by 4 walls, each with a unique pattern. An escape platform was situated at the end of one of the arms and submerged below the surface of black opaque water (non-toxic Dust Free Black Powder Paint, Rich Art). Rats were handled (2 min each) the day before craniotomy and three days after FPI. Training (Day 1; 4 days post-FPI) consisted of placing the animal in one of the arms and giving the animal a maximum of 60 seconds to find the platform in the goal arm. If the animal did not find the escape platform within 60 seconds, it was guided to the goal arm and allowed to stay on the platform for 15 seconds. Fifteen trials were administered with a five-minute inter-trial interval. The start arm for each trial was determined in a pseudorandom fashion with three randomized sequences of the five non-goal arms. The start and goal arms were different for each rat, but equivocal relative to goal arm location, to avoid position and place preferences. Testing (Day 2) the following day consisted of 15 swim trials. The platform remained in the Day 1 goal arm for the first five trials and was then moved to a new arm for trials 6-15. The start arm for each trial was determined in a pseudorandom fashion so the animal did not start in the Day 1 goal arm until after all other arms (trial 10). Videos for each of the 30 trials per animal were analyzed using TopScan (Cleversys Inc.) tracking software for errors and perseverance duration. Errors are defined as entry into a non-goal arm or entry into goal arm without reaching the platform.
Statistical analyses
All data shown are mean +/− standard error of the mean unless otherwise noted. Results were analyzed in SPSS 20 (IBM) or Prism 5.0 (GraphPad). All analysis used two-tailed non-paired student's t-tests for two groups, and one-way ANOVA for two or more groups followed by Tukey's HSD for multiple comparisons unless otherwise noted. LTP I-O curves were analyzed with a two-way repeated measures ANOVA. RAWM day one learning curves were collapsed into groups of three swims and analyzed with a two-way repeated measures ANOVA followed by a one-way ANOVA of each collapsed time point. RAWM perseverance between trials one and three of the reversal task was expressed as a percentage of starting value (swim one=100%; swim three=swim three/swim one × 100) and analyzed with a within subjects two-way repeated measures ANOVA, followed by a two-tailed student's paired t-test between the two trials. Alpha was set as p<0.05 to determine significance in all tests. Region specific outliers in the EB study were identified by SPSS (>2 standard deviations from the mean) and eliminated from all analysis. In total three drug treated and one vehicle treated rat were removed.
Results
The effect of MK-886 on brain LTC4 levels after FPI
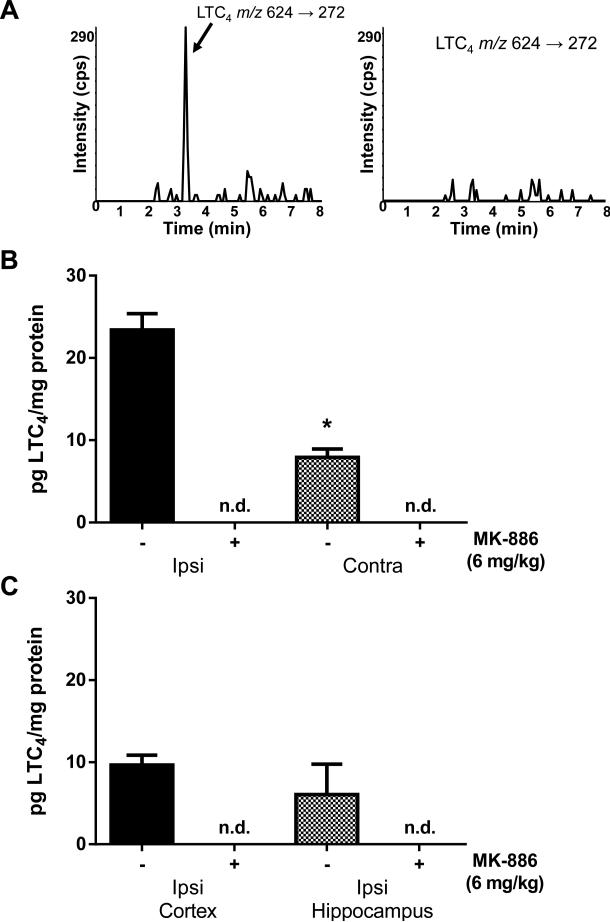
Our previous results indicate that injury-induced leukotriene production is very rapid, peaking at 1-3 hours after FPI and declining to undetectable levels by 24 hours (Farias et al., 2009). To determine the efficacy of post-injury MK-886 administration in blocking leukotriene formation, rats were injected with a single dose of either MK-886 (6mg/kg in 0.9% sterile saline with 10% DMSO) or the same volume of vehicle 15 minutes after FPI. Ninety minutes after injury, the animals were euthanized and the levels of LTC4 were measured in brain regions after extraction of lipids and analysis by RP LC-MS/MS (Fig. 1A). The mean LTC4 level (Fig. 1B) in the ipsilateral hemisphere (23.41 +/− 1.98 pg/mg protein) was significantly higher (p <0.001, student's t-test) than the contralateral hemisphere (7.92 +/− 1.02 pg/mg protein) of vehicle-treated animals. MK-886 reduced the levels of LTC4 to below detectable threshold in both the ipsilateral and contralateral hemispheres (Fig. 1B). To investigate the relative amount of LTC4 production in injured ipsilateral cortex and hippocampus, these brain regions were dissected from injured rats prior to RP LC-MS/MS analysis. LTC4 was detected in both the ipsilateral cortex (9.67 +/− 1.18 pg/mg protein) and hippocampus (6.05 +/− 3.70 pg/mg protein) of vehicle-treated rats. Similar to results in whole brain hemispheres, LTC4 was undetectable in ipsilateral cortex and hippocampus of MK-886-treated rats (Fig. 1C). These results demonstrate that the FLAP inhibitor, MK-886, effectively blocks leukotriene biosynthesis when administered after experimental TBI.
Fig. 1. MK-886 blocks leukotriene synthesis after FPI.
(A) Representative chromatograms from RP LC-MS/MS analysis of LTC4 levels in the ipsilateral hemispheres of FPI-injured rat injected with either vehicle (left) or 6mg/kg MK-886 (right) 15 min after injury. (B) Quantitative analysis of LTC4 levels in the ipsilateral (ipsi) and contralateral (contra) hemispheres in rats injected with either vehicle (-) or MK-886 (+) 15 min after injury. (C) Quantitative analysis of LTC4 levels in ipsilateral cortex and ipsilateral hippocampus of rats treated with either vehicle (-) or MK-886 (+) 15 min after injury. Values are mean -/+ SEM, n=4, n.d.= non-detectable.
*p<0.05, different from ipsilateral hemisphere, student's t-test.
The effect of MK-886 on brain edema after FPI
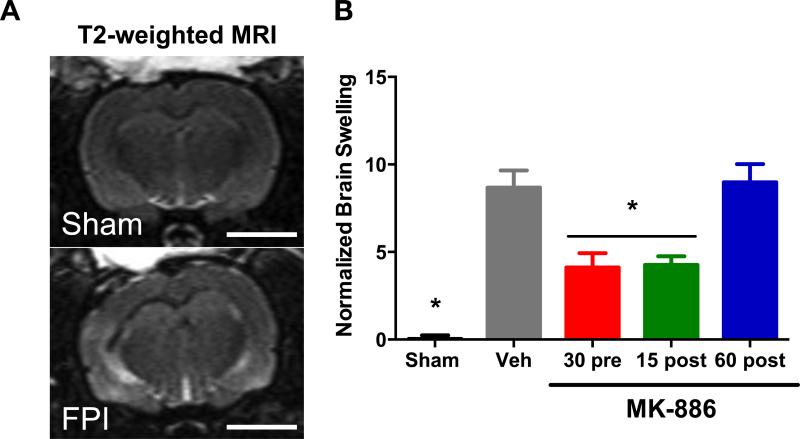
T2-weighted MRI was used to investigate the effect of leukotrienes on TBI-related brain edema 72 hours after FPI. Animals were injected with MK-886 either 30 minutes before, 15 minutes after or 60 minutes after FPI. Additional groups of sham-injured and vehicle-injected rats were used as controls. The ipsilateral (left) and contralateral (right) brain hemispheres of sham rats showed no T2-weighted hyperintensity and were symmetrical in height and width (Fig. 2A top panel). In contrast, the brains from FPI-injured rats consistently demonstrated T2-weighted hyperintensity and unilateral swelling in the ipsilateral hemisphere compared to the contralateral hemisphere (Fig. 2A, bottom panel).
Fig. 2. MK-886 attenuates edema after FPI.
(A) Representative T2-weighted MRI images obtained 72 hours after FPI. The ipsilateral (left) and contralateral (right) hemispheres in the sham brain show no T2 pixel hyperintensity and are symmetric in shape and size. FPI brains demonstrate T2 pixel hyperintensity primarily in the ipsilateral cortex indicative of water content and unilateral swelling. (B) Quantitative MRI analysis of the mean normalized brain swelling was calculated from 5 continuous T2-MRI slices obtained from each animal using Fiji (NIH). Values are mean +/− SEM, Sham (n=4), Vehicle (n=8), MK-886 administered 30 min pre-injury (n=10), 15 min post-injury (n=5), and 60 min post-injury (n=8). Bar = 5mm. *p<0.05, different from vehicle FPI group, not significantly different from sham, one way ANOVA, followed by Tukey's HSD.
Ipsilateral hemispheric edema was quantified relative to the contralateral hemisphere (Fig. 2B). Sham rats exhibited no difference between hemispheres in normalized brain swelling (0.00 +/− 0.03%). In contrast, injured rats given vehicle treatment had significantly more brain swelling than sham rats (vehicle=8.68 +/− 0.09%, p<0.001, one-way ANOVA followed by Tukey's HSD) (Fig. 2B). Rats receiving MK-886 either 30 minutes pre-injury, or 15 min post-injury had significantly lower swelling than vehicle-treated rats (30 pre=4.12 +/− 0.08%, p=0.004; 15 post=4.26 +/− 0.05%, p=0.028; Fig. 2B). Rats injected with MK-886 60 min post-injury did not significantly differ from vehicle-treated rats (60 post=8.98 +/− 1.0%, p=0.999). These results demonstrate that blocking leukotriene production either before or shortly after TBI reduces the amount of injury-related brain swelling at 72 hours.
The effect of MK-886 on BBB disruption after FPI
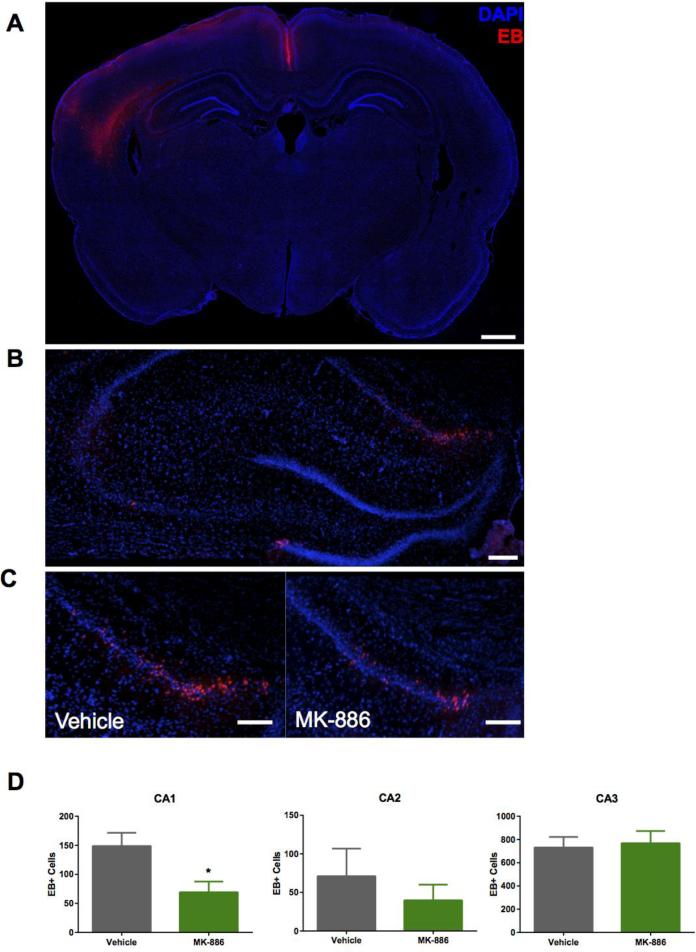
The effect of leukotrienes on TBI-related BBB disruption was assessed using EB fluorescence. EB (<1kDa) is an azo dye that has a strong affinity for serum albumin (67kDa) (Wolman et al., 1981). The resulting EB-albumin complex leaks into the parenchyma upon disruption of the BBB. We used fluorescence microscopy to image brain slices harvested five hours post-injury, taking advantage of EB's intrinsic fluorescent properties (excitation 620nm, emission 680nm). This method is more sensitive than commonly used colorimetric readings of EB in brain homogenates (Uyama et al., 1988) and can be used to localize BBB permeability. As the EB-albumin complex is taken up by cells proximal to sites of permeability, this method allows for microscopic detection of BBB disruption (Loberg and Torvik, 1991). Consistent with this, the brightest EB signal was intracellular. Faint extracellular fluorescence was also observed surrounding EB-positive cell clusters. Not unexpectedly, the greatest density of EB-positive cells was in the ipsilateral cortex adjacent to the injury site and in the ipsilateral hippocampus, with some scattered cells in the thalamus and substantia nigra (Fig. 3A). There was no EB detected in the corresponding contralateral brain regions. Since the hippocampus is relatively distant from the primary injury site and mediates memory and learning processes, we counted EB-positive cells in this area (Fig. 3B). MK-886 administered 15 minutes after FPI significantly reduced the number of EB-positive cells in the CA1 region (FPI vehicle=148.75 +/− 45.65; FPI MK-886=69.25 +/− 36.82, p=0.035, student's t-test) (Fig. 3C,D) but did not statistically reduce EB-positive cells in CA2 (p=0.476) or CA3 (p=0.797). These results indicate that leukotrienes can mediate BBB permeability in selective brain regions vulnerable to injury.
Fig. 3. MK-886 reduces BBB permeability in select regions of hippocampus.
(A) Representative coronal cross-section of Evans Blue (EB, red) uptake in cells (DAPI, blue) in the ipsilateral hemisphere 5 hours after FPI. Bar = 500μm (B) Representative fluorescence images of EB uptake by hippocampal cell layers (DAPI) in the ipsilateral hippocampus 5 hours after FPI. Bar = 200μm (C) Higher magnification images of EB extravasation in the ipsilateral CA1 hippocampal cell layer in animals that received either vehicle or MK-886 15 minutes after FPI. Bar = 200μm (D) Quantitation of EB+ cells (EB-DAPI colocalization) in the hippocampal regions. Values are mean +/− SEM; n=4. *p<0.05, student's t-test.
The effect of MK-886 on hippocampal synaptic plasticity after FPI
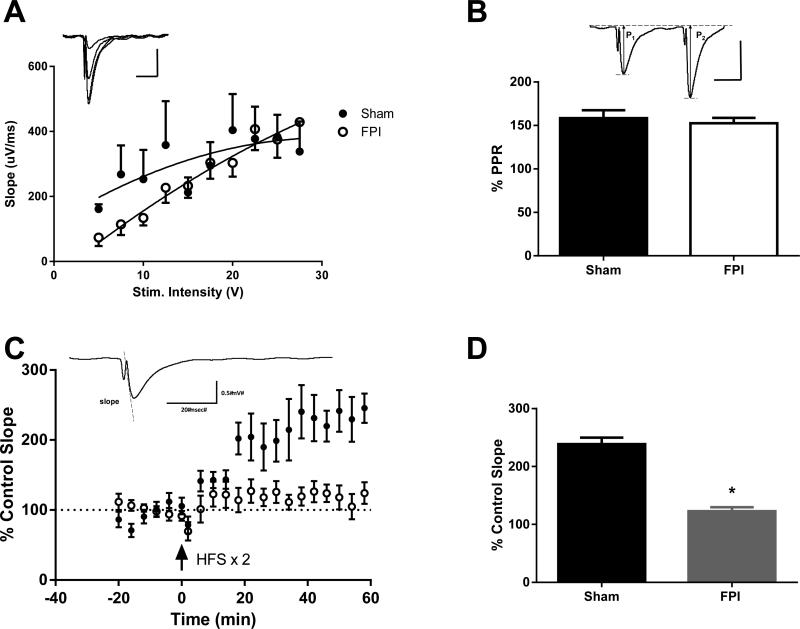
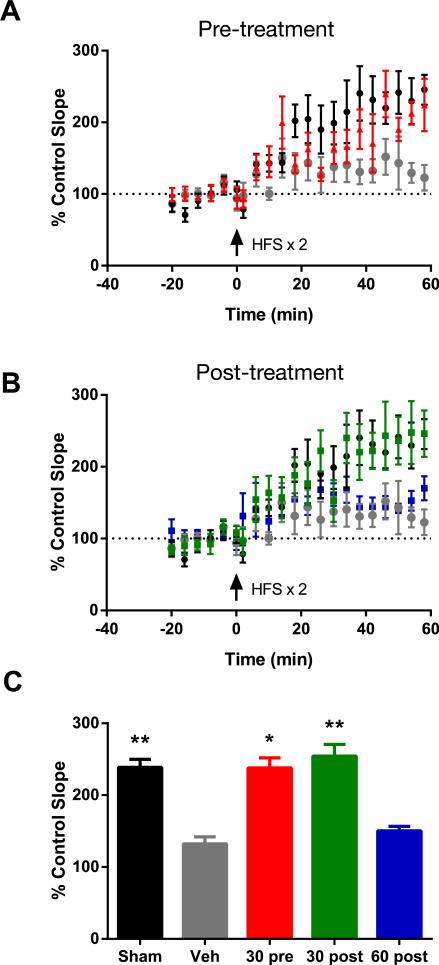
To examine the functional integrity of the hippocampus after FPI, electrophysiological measurements of LTP were recorded in hippocampal slices four days after FPI. LTP is a measure of synaptic plasticity and is thought to represent the molecular mechanisms underlying learning and memory. Synaptic fEPSP responses were evoked by stimulating the CA3 to CA1 Schaffer collateral pathway and recording from the CA1 dendritic field layer. There was no difference in the fEPSP input-output curves (F(4,381)=0.5329, p=0.992, two-way repeated measures ANOVA), nor in the paired pulse ratio measurements between sham and FPI-injured animals, (T(22)=1.883, p=0.073, student's t-test), indicating similar levels of basal synaptic transmission for these groups (Fig. 4B). This is consistent with our findings that there was no substantial hippocampal cell loss by H&E staining and no change in the levels of hippocampal neurofilament protein within one week of FPI (data not shown). Hippocampal slices from sham animals exhibited robust LTP in response to high frequency stimulation (253.51 +/− 69.48%, 58-60 min, last 3 recorded time points). In contrast, hippocampal slices from uninjected FPI-injured animals failed to express LTP (135.06% +/− 42.62%, different from sham rats, p=0.007 one-way ANOVA of all groups included in Fig. 5C followed by Tukey's HSD) (Fig. 4D). Similar to the uninjected FPI-injured animals, hippocampal slices from FPI-injured rats injected with vehicle also failed to exhibit LTP upon high frequency stimulation (Fig. 5A). However, rats that received either an injection of MK-886 30 minutes before or 30 minutes after FPI demonstrated normal LTP. Both were significantly different from vehicle-injected rats (FPI vehicle= 130.86% +/− 55.63%, FPI MK-886 30 min pre-injury= 242.75 +/− 76.94%, p=0.032, FPI MK-886 30 min post-injury= 256.13 +/− 85.27%, p=0.007, one-way ANOVA followed by Tukey's HSD) (Fig. 5C). However, when MK-886 was delivered 60 min post-injury, the drug failed to prevent the LTP deficits observed in vehicle-treated rats (145.46 +/− 18.30, p=0.994). These results indicate that blocking early production of leukotrienes attenuates injury-induced deficits in rat hippocampal synaptic plasticity after injury and suggests a time window of less than one hour after injury for efficacy of MK-886 treatment in rodents.
Fig. 4. FPI leads to marked deficits in hippocampal LTP.
(A) Input-output (I-O) curves from sham (n=18) and FPI (n=18) rats prior to induction of LTP. Inset depicts representative signals obtained with successive increases in stimulation voltage (Scale bar x= 20 msec, y= 0.5 mV). There was no significant difference between injured and uninjured animals (two-way repeated measures ANOVA). (B) Paired-pulse responses (PPR) in slices from sham and FPI rats prior to LTP induction. Values are average % PPR +/− SEM (where % PPR = amplitude of peak 2/amplitude of peak 1 x100). Inset depicts representative signals from paired stimulations (50 ms interstimulus interval) including parameters (P1 and P2, peak amplitudes for the 1st and 2nd peaks, respectively) used to calculate % PPR (Scale bar x= 20 msec, y= 0.5 mV). PPR did not differ between injured and sham rats (student's t-test). (C) LTP responses after high frequency stimulation at time 0 of two trains of 100 Hz (1 second each, train interval = 20s). Data are represented as a % of the control fEPSP slope. Each data point shown is an average of six 20-second interval measurements. Sham n=8, FPI n=9. Inset depicts a representative sham fEPSP. (D) Average LTP response in sham and FPI injured brain slices at 58-60 minutes after induction of LTP. Values are mean +/− SEM, Sham n=8, FPI n=8. *p<0.05, one-way ANOVA followed by Tukey's HSD for all groups.
Fig. 5. MK-886 attenuates deficits in hippocampal long-term potentiation after FPI.
(A) LTP measured over time in hippocampal slices from sham rats (black symbols, n=8) and FPI- injured rats injected with vehicle (grey symbols, n=7) or MK-886 (red symbols, n=6) 30 min prior to injury. (B) LTP measured in hippocampal slices from sham rats (black symbols, n=8) and FPI-injured rats injected with vehicle (grey symbols, n=7) or MK-886 30 minutes after FPI (green symbols, n=7) and 60 minutes after FPI (blue symbols, n=6). (C) Average LTP response (mean +/− SEM control slope) in all the groups measured at 58-60 minutes after induction of LTP. *p<0.05, **p<0.01, different from FPI vehicle, not significantly different from sham, one way ANOVA followed by Tukey's HSD.
The effect of MK-886 on memory and learning after FPI
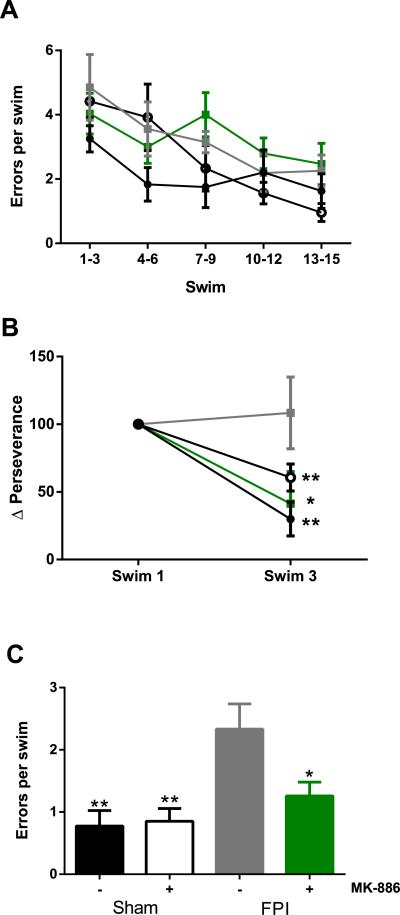
To verify that injury-induced deficits in LTP reflect impairments in hippocampal-dependent spatial learning and memory, sham and FPI-injured animals treated with drug or vehicle were tested in a RAWM four and five days after FPI. The RAWM has an advantage over the Morris water maze in assessing cognitive impairments in rodents, in that the number of entries into an arm lacking the escape platform can be used as an assessment of learning, rather than latency to find the platform, thereby eliminating confounds induced by potential differences in swim speed among the experimental groups. Sham and FPI animals received an injection of either vehicle or MK-886 30 minutes post-injury. On the first day of behavioral testing, the animals completed 15 swim trials in which they used visual cues to navigate the maze to find a hidden escape platform in one of the arms (i.e. goal arm). There were no significant differences in initial task learning between the vehicle and MK-886 treated rats within the sham group or within the FPI group (F(3,155)=2.437, p=0.083, two-way repeated measures ANOVA) and no significant differences between groups for any cluster of 3 swims, (one-way ANOVA for each swim cluster) (Fig. 6A). On the second day of behavioral testing, animals completed five swim trials in the maze with the goal arm in the same position as the previous day. The escape platform was then moved to a new location for ten more trials in order to assess the ability of the animals to learn and remember a new goal arm location (reversal task). In the first three trials of the reversal task, perseverance for the previous goal arm was measured as the percent of total swim duration spent in the arm where the platform used to be. On the first swim, there were no differences in perseverance between any of the groups (F(3,31)=0.713, p=0.522, one-way ANOVA). Both sham groups quickly learned between swims 1 and 3 that the platform was no longer in the previous goal arm. Likewise, FPI rats treated with MK-886 spent less time in the previous goal arm by swim 3 (overall interaction, injury (sham v. FPI) × drug (MK-886 v. vehicle) × trial (swim one to swim three), F(1,62)=5.564, p=0.025, within subjects two-way repeated measures ANOVA followed by student's paired t-test, sham vehicle, p=0.001; sham MK-886, p=0.006; FPI MK-886, p=0.035). However, FPI rats that received a vehicle injection failed to learn that the platform location had changed between swims 1 and 3 (p=0.758) (Fig. 6B). The last five swim trials of the reversal task (swims 11-15) were used for assessment of continued learning. Vehicle-treated FPI rats continued to make significantly more errors per swim than sham rats given either drug or vehicle (FPI vehicle= 2.33 +/− 1.21; sham vehicle= 0.755 +/− 0.705, p=0.003; sham MK-886= 0.850 +/− 0.583, p=0.005; one-way ANOVA followed by Tukey's HSD). FPI rats given MK-886 did not differ from either sham group and were significantly different from FPI-vehicle rats (1.26 +/− 0.700, p=0.044). These data indicate that MK-886 attenuates injury-induced deficits in spatial learning and memory.
Fig 6. MK-886 mitigates FPI-induced impairments in memory and learning in radial arms water maze.
(A) Day 1 learning task. The average number of errors (mean +/− SEM) made during the indicated swims in sham rats treated with either vehicle (black circles, n=8) or MK-886 (open circles, n=8) and in FPI-injured rats treated with either vehicle (grey squares, n=9) or MK-886 (green squares, n=8) 30 minutes after injury. There were no significant differences in initial learning curves (one-way repeated measures ANOVA) and no differences between groups at any swim time point for each three-swim cluster (one-way ANOVA). (B) Day 2 reversal task perseverance. The change in perseverance (duration in previous goal arm) at swim 3 expressed as the percentage of perseverance in swim 1. *p<0.05, **p<0.01, within-subjects two-way repeated measures ANOVA followed by paired student's t-test. (C) Day 2 post-perseverance reversal task performance. Errors (mean +/− SEM) made in swims 11-15 of the reversal task. *p<0.05, **p<0.01, different from FPI vehicle, no difference from either sham group, one-way ANOVA followed by Tukey's HSD.
Discussion
FLAP inhibitors target early leukotriene-mediated inflammatory events that are initiated and subsequently amplified by brain trauma. In our study MK-886 effectively reduced brain leukotrienes to levels below the detectable threshold of RP LC-MS/MS. Blocking this inflammatory cascade resulted in an attenuation of brain swelling, reduced BBB damage in area CA1 of the hippocampus, and restored both synaptic (LTP) and behavioral (RAWM) learning and memory to sham levels. Together, these results demonstrate that FLAP inhibitors represent a promising new approach toward mitigating brain damage and neurological deficits resulting from TBI. There is little published data on the role of leukotrienes in the brain due to the difficulty in measuring leukotrienes and a lack of verified antibodies. A few reports demonstrate efficacy of leukotriene receptor antagonists and FLAP inhibitors in reducing detrimental outcomes induced by head injury (Kiwak et al., 1985; Dhillon et al., 1996; Schuhmann et al., 2003; Farias et al., 2009; Voight et al., 2012) or brain ischemia (Minamisawa et al., 1988; Ciceri et al., 2001) when these drugs were administered prior to injury. We demonstrate, for the first time, protective effects of FLAP inhibitors on injury-related outcomes when administered both before and after injury.
FLAP was discovered in the late 1980s and early 1990s in screens for leukotriene inhibitors. Shortly after the discovery of FLAP, inhibitors including the indole MK-886, the quinoline BAY X1005, and the quinoline–indole MK-591 were developed and tested in human trials of asthma (Friedman et al., 1993; Diamant et al., 1995; Dahlen et al., 1997). All demonstrated good safety profiles and efficacy in blocking leukotrienes but were discontinued when the leukotriene receptor antagonists [zafirlukast (Accolate™), montelukast (Singulair™) and pranlukast (Onon™)] and the 5-LO inhibitor zileuton (Zyflo™) were brought to market and approved for treating asthma.
Consistent with our finding that leukotrienes are increased after TBI, leukotriene receptors (cys-LT1 and cys-LT2) are also up-regulated following brain injury (Zhang et al., 2004; Hu et al., 2005; Fang et al., 2006; Ding et al., 2007). The cys-LT1 receptor has been reported to mediate increased permeability of the BBB, vasogenic brain edema, and astrocyte proliferation after brain ischemia, while the cys-LT2 receptor is thought to regulate cytotoxic brain edema after ischemic injury (Wang et al., 2006). In agreement with these findings, the cys-LT1 receptor antagonist, pranlukast, was shown to decrease neutrophil infiltration, IgG extravasation and lesion volumes when administered prior to cold-induced brain injury (Qian et al., 2006) and ischemic brain injury (Yu et al., 2005; Chu et al., 2006). Another cys-LT1 receptor antagonist, montelukast, was reported to decrease BBB permeability and neutrophil infiltration after diffuse brain injury when administered pre-injury (Biber et al., 2009). A caveat of these studies is the lack of specific antibodies to leukotriene receptors and the cross-reactivity of the leukotriene antagonists. Although the receptor antagonists have been used successfully in treatment of asthma for almost 25 years, with the discovery of multiple leukotriene receptors (the cysLT-2 and cys-LT3 receptors were discovered after the cys-LT1 selective antagonists were developed) and increasing evidence that leukotrienes mediate other inflammatory conditions (Evans et al., 2008), there has been renewed interest in FLAP inhibitors. As FLAP inhibitors block the synthesis and action of all three cysteinyl-leukotrienes (LTC4, LTD4, and LTE4) as well as LTB4, they are predicted to have greater efficacy compared to the existing receptor antagonists in blocking inflammatory disorders. Additional studies and more selective antibodies are required to understand the receptor-specific actions of leukotrienes.
In the present study, MK-886 administration attenuated EB extravasation detected by fluorescence microscopy in the brain parenchyma distant from the injury site. Other investigators reported that blocking leukotrienes prior to head injury either had no effect (Schuhmann et al., 2003) or mitigated (Qian et al., 2006; Ding et al., 2007; Biber et al., 2009) BBB permeability. An important factor that could explain these apparent discrepancies is the contribution of mechanical injury to blood vessels following a TBI. Mechanical injury to blood vessels could mask an effect of leukotrienes or other neurochemicals on regulating BBB permeability. Kenne et al. (2012) reported a similar conclusion regarding the potential masking of neurochemical regulation of BBB permeability by mechanical damage to blood vessels. In their study, neutrophil depletion (which reduces leukotriene production (Farias et al., 2009)), reduced vasogenic edema and tissue loss, but had no apparent effect on BBB disruption following CCI in mice (Kenne et al., 2012). Our finding that MK-886 selectively decreased EB accumulation in the CA1 region of the hippocampus, which is furthest away from the injury site, suggests neurochemical rather than mechanical BBB disruption in this region.
This study demonstrates for the first time that blocking the early production of leukotrienes after TBI leads to fewer deficits in hippocampal synaptic plasticity and hippocampal-mediated memory and learning. In our study, the deficits in hippocampal LTP following TBI were observed despite normal basal synaptic responses and no overt hippocampal cell loss, suggesting that leukotrienes likely impair synaptic plasticity by altering synaptic signaling rather than as a by-product of cell loss. Neuronal mechanisms that could result in LTP deficits without loss of neurons involve changes in the morphology or density of hippocampal dendritic spines as well as changes in the levels or activity of postsynaptic density proteins known to regulate LTP. A calcineurin-dependent loss of dendritic spines in rat forebrain has been reported after fluid percussion injury that was followed by an eventual overgrowth of spines (Campbell et al., 2012a; Campbell et al., 2012b). Others have shown decreased hippocampal levels of calcium/calmodulin dependent protein kinase II (CaMKII) and activated CaMKII, a protein kinase that regulates LTP by modulating the phosphorylation state and insertion of AMPA receptors in postsynaptic membranes after TBI (Atkins et al., 2006; Schwarzbach et al., 2006; Folkerts et al., 2007). A recent report by Hartig et al. (2013) shows CD43+ inflammatory cells in the dentate gyrus and CA3 region 24 hours after brain trauma, and MK-886 treatment reduced the number of inflammatory cells at this time point. Future studies involving more detailed investigation of mechanisms that regulate synaptic plasticity after TBI are needed to elucidate the role of leukotriene mediated inflammation in memory deficits. These studies will help provide important insight into the cognitive and executive disabilities that occur after all types of human brain injury including TBI.
Leukotrienes are produced rapidly after brain injury, peaking within 1-3 hours of injury, and returning to basal levels by 24 hour after injury (Farias et al., 2009; Voight et al., 2012). The early production of leukotrienes after head trauma dictates the therapeutic window for FLAP inhibition. In the current studies, MK-886 was efficacious in blocking multiple outcomes of TBI in rats when given 30 minutes after injury. This scenario for intervention is not that different from the therapeutic window of tissue plasminogen activator (t-PA) used in stroke patients (Saver et al., 2013). Because several inflammatory pathways are amplified immediately after injury, early intervention is essential for an optimal therapeutic outcome. On the other hand, Schuhmann et al. (2003) reported a second phase of leukotriene production at 7 days after experimental TBI. It is intriguing to speculate that a second phase of leukotriene production after TBI could mediate reopening of the BBB and a chronic phase of inflammation. If proven true, the second phase of leukotriene production should also be amenable to FLAP inhibition.
In addition to using FLAP inhibitors shortly after brain injury to block or mitigate secondary injury, FLAP inhibitors have a potential preventative role in TBI. Individuals at high risk for head injury, including athletes in high contact sports and military personnel in combat scenarios, could be given FLAP inhibitors chronically or right before an event that predisposes them to risk of head trauma. Second generation FLAP inhibitors have longer half-lives and could theoretically be administered once daily for protection against head injury. FLAP inhibitors have several feasible routes of administration including oral, intravenous, intraperitoneal, and possibly nasal (currently under investigation) and thus far, have no reported toxicity or deleterious side effects in humans. Thus, this class of anti-inflammatory agents are promising new drug candidates for interventional therapy and prevention of functional brain deficits after TBI.
Highlights.
Leukotrienes mediate edema and blood-brain barrier permeability after TBI.
Leukotriene production contributes to lasting deficits in memory and learning.
Blocking leukotriene synthesis attenuates brain injury and cognitive impairments.
FLAP inhibitors are promising therapeutic candidates for traumatic brain injury.
Acknowledgments
The authors would like to thank Dr. Simona Zarini, Dr. Nicolas Busquet, Kendra Huber, and Antoinette Foster for their helpful discussions and technical contributions to this manuscript.
Funding: This study was supported by NIH grants 5T32HD041697 (C.E.C. and D.J.G.), R01NS040710 (M.L.D.), GM069338 (R.C.M.), R21NS079435 (K.A.H.), the Colorado Brain Injury Program (K.A.H.), and the UC Denver Center for Neuroscience Translational Research Award (K.A.H.). Contents are the authors’ sole responsibility and do not necessarily represent official NIH views.
Abbreviations
- 5-LO
5-lipoxygenase
- BBB
blood-brain barrier
- EB
Evan's Blue
- fEPSP
field excitatory post-synaptic potential
- FLAP
5-lipoxygenase activating protein
- FPI
fluid percussion injury
- Gd
gadoinium–diethylenetriamine pentaacetic acid
- LTP
long-term potentiation
- RAWM
radial arms water maze
- RP LC-MS/MS
reverse-phase liquid chromatography coupled to tandem mass spectrometry
- TBI
traumatic brain injury
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Atkins CM, Chen S, Alonso OF, Dietrich WD, Hu B. Activation of calcium/calmodulin-dependent protein kinases after traumatic brain injury. J. Cerebr. Blood. F. Met. 2006;26:1507–1518. doi: 10.1038/sj.jcbfm.9600301. [DOI] [PubMed] [Google Scholar]
- Barkhoudarian G, Hovda DA, Giza CC. The molecular pathophysiology of concussive brain injury. Clin. Sport. Med. 2011;30:33–48. doi: 10.1016/j.csm.2010.09.001. [DOI] [PubMed] [Google Scholar]
- Biber N, Toklu HZ, Solakoglu S, Gultomruk M, Hakan T, Berkman Z, Gul Dulger F. Cysteinyl-leukotriene receptor antagonist montelukast decreases blood-brain barrier permeability but does not prevent oedema formation in traumatic brain injury. Brain Injury. 2009;23:577–584. doi: 10.1080/02699050902926317. [DOI] [PubMed] [Google Scholar]
- Boyce JA. Mast cells and eicosanoid mediators: a system of reciprocal paracrine and autocrine regulation. Immunol. Rev. 2007;217:168–185. doi: 10.1111/j.1600-065X.2007.00512.x. [DOI] [PubMed] [Google Scholar]
- Campbell JN, Register D, Churn SB. Traumatic brain injury causes an FK506-sensitive loss and an overgrowth of dendritic spines in rat forebrain. J. Neurotraum. 2012a;29:201–217. doi: 10.1089/neu.2011.1761. [DOI] [PubMed] [Google Scholar]
- Campbell JN, Low B, Kurz JE, Patel SS, Young MT, Churn SB. Mechanisms of dendritic spine remodeling in a rat model of traumatic brain injury. J. Neurotraum. 2012b;29:218–234. doi: 10.1089/neu.2011.1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu L, Wei E, Yu G, Fang S, Zhou Y, Wang M, Zhang W. Pranlukast reduces neutrophil but not macrophage/microglial accumulation in brain after focal cerebral ischemia in mice. Acta. Pharmacol. Sinic. 2006;27:282–288. doi: 10.1111/j.1745-7254.2006.00290.x. [DOI] [PubMed] [Google Scholar]
- Ciceri P, Rabuffetti M, Monopoli A, Nicosia S. Production of leukotrienes in a model of focal cerebral ischaemia in the rat. Brit. J. Pharmacol. 2001;133:1323–1329. doi: 10.1038/sj.bjp.0704189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlén B, Kumlin M, Ihre E, Zetterström O, Dahlén SE. Inhibition of allergen-induced airway obstruction and leukotriene generation in atopic asthmatic subjects by the leukotriene biosynthesis inhibitor BAYx 1005. Thorax. 1997;52:342–347. doi: 10.1136/thx.52.4.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhillon H, Dose J, Prasad M. Regional generation of leukotriene C4 after experimental brain injury in anesthetized rats. J. Neurotraum. 1996;13:781–789. doi: 10.1089/neu.1996.13.781. [DOI] [PubMed] [Google Scholar]
- Diamant Z, Timmers MC, Van Der Veen H, Friedman BS, De Smet M, Depre M, Hilliard D, Bel EH, Sterk PJ. The effect of MK-0591, a novel 5-1ipoxygenase activating protein inhibitor, on leukotriene biosynthesis and allergen-induced airway responses in asthmatic subjects in vivo. J. Allergy Clin. Immun. 1995;95:42–51. doi: 10.1016/s0091-6749(95)70151-6. [DOI] [PubMed] [Google Scholar]
- Ding Q, Fang S, Zhou Y, Zhang L, Zhang W, Chen Z, Wei E. Cysteinyl leukotriene receptor 1 partially mediates brain cryoinjury in mice. Acta. Pharm. Sinic. 2007;28:945–952. doi: 10.1111/j.1745-7254.2007.00576.x. [DOI] [PubMed] [Google Scholar]
- Evans JF, Ferguson AD, Mosley RT, Hutchinson JH. What's all the FLAP about?: 5-lipoxygenase-activating protein inhibitors for inflammatory diseases. Trends Pharmacol. Sci. 2008;29:72–78. doi: 10.1016/j.tips.2007.11.006. [DOI] [PubMed] [Google Scholar]
- Fang SH, Wei EQ, Zhou Y, Wang ML, Zhang WP, Yu GL, Chu LS, Chen Z. Increased expression of cysteinyl leukotriene receptor-1 in the brain mediates neuronal damage and astrogliosis after focal cerebral ischemia in rats. Neuroscience. 2006;140:969–979. doi: 10.1016/j.neuroscience.2006.02.051. [DOI] [PubMed] [Google Scholar]
- Farias SE, Zarini S, Precht T, Murphy RC, Heidenreich KA. Transcellular biosynthesis of cysteinyl leukotrienes in rat neuronal and glial cells. J. Neurochem. 2007;103:1310–1318. doi: 10.1111/j.1471-4159.2007.04830.x. [DOI] [PubMed] [Google Scholar]
- Farias S, Frey LC, Murphy RC, Heidenreich KA. Injury-related production of cysteinyl leukotrienes contributes to brain damage following experimental traumatic brain injury. J. Neurotraum. 2009;26:1977–1986. doi: 10.1089/neu.2009.0877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faul M, Xu L, Wald MM, Coronado VG. Traumatic brain injury in the United States: emergency department visits, hospitalizations and deaths 2002-2006. Centers for Disease Control and Prevention; Atlanta: 2010. [Google Scholar]
- Folco G, Murphy RC. Eicosanoid transcellular biosynthesis: from cell-cell interactions to in vivo tissue responses. Pharmacol. Rev. 2006;58:375–388. doi: 10.1124/pr.58.3.8. [DOI] [PubMed] [Google Scholar]
- Folkerts MM, Parks EA, Dedman JR, Kaetzel MA, Lyeth BG, Berman RF. Phosphorylation of calcium calmodulin-dependent protein kinase II following lateral fluid percussion brain injury in rats. J. Neurotraum. 2007;24:638–650. doi: 10.1089/neu.2006.0188. [DOI] [PubMed] [Google Scholar]
- Frey LC, Hellier J, Unkart JC, Lepkin A, Howard A, Hasebroock K, Serkova N, Liang L, Patel M, Soltesz I, Staley K. A novel apparatus for lateral fluid percussion injury in the rat. J. Neurosci. Meth. 2009;177:267–272. doi: 10.1016/j.jneumeth.2008.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman BS, Bel EH, Buntinx A, Tanaka W, Han YH, Shingo S, Spector R, Sterk P. Oral leukotriene inhibitor (MK-886) blocks allergen-induced airway responses. Am. Rev. Respir. Dis. 1993;147:839–844. doi: 10.1164/ajrccm/147.4.839. [DOI] [PubMed] [Google Scholar]
- Gennarelli TA. Mechanisms of brain injury. J. Emerg. Med. 1993;11:5–11. [PubMed] [Google Scholar]
- Hartig W, Michalski D, Seeger G, Voigt C, Donat CK, Dulin J, Kacza J, Meixensberger J, Arendt T, Schuhmann MU. Impact of 5-lipoxygenase inhibitors on the spatiotemporal distribution of inflammatory cells and neuronal COX-2 expression following experimental traumatic brain injury in rats. Brain Res. 2013;1498:69–84. doi: 10.1016/j.brainres.2012.12.022. [DOI] [PubMed] [Google Scholar]
- Hu H, Chen G, Zhang J, Zhang W, Zhang L, Ge Q, Yao H, Ding W, Chen Z, Wei E. Distribution of cysteinyl leukotriene receptor 2 in human traumatic brain injury and brain tumors. Acta. Pharm. Sinic. 2005;26:685–690. doi: 10.1111/j.1745-7254.2005.00092.x. [DOI] [PubMed] [Google Scholar]
- Jordan BD. The clinical spectrum of sport-related traumatic brain injury. Nat. Rev. Neurol. 2013;9:222–230. doi: 10.1038/nrneurol.2013.33. [DOI] [PubMed] [Google Scholar]
- Kenne E, Erlandsson A, Lindbom L, Hillered L, Clausen F. Neutrophil depletion reduces edema formation and tissue loss following traumatic brain injury in mice. J. Neuroinflamm. 2012;9:17. doi: 10.1186/1742-2094-9-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiwak KJ, Moskowitz M, Levine L. Leukotriene production in gerbil brain after ischemic insult, subarachnoid hemorrhage, and concussive injury. J. Neurosurg. 1985;62:865–869. doi: 10.3171/jns.1985.62.6.0865. [DOI] [PubMed] [Google Scholar]
- Kochanek PM, Bauman RA, Long JB, Dixon CE, Jenkins LW. A critical problem begging for new insight and new therapies. J. Neurotraum. 2009;814:813–814. doi: 10.1089/neu.2008.0893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langlois JA, Rutland-Brown W, Wald MM. The epidemiology and impact of traumatic brain injury: a brief overview. J. Head Trauma Rehabil. 2006;21:375–378. doi: 10.1097/00001199-200609000-00001. [DOI] [PubMed] [Google Scholar]
- Loberg EM, Torvik A. Uptake of plasma proteins into damaged neurons. Acta. Neuropathol. 1991;81:479–485. doi: 10.1007/BF00310126. [DOI] [PubMed] [Google Scholar]
- McKee AC, Stein TD, Nowinski CJ, Stern RA, Daneshvar DH, Alvarez VE, Lee H, Hall G, Wojtowicz SM, Baugh CM, Riley DO, Kubilus CA, Cormier KA, Jacobs MA, Martin BR, Abraham CA, Ikezu T, Reichard RR, Wolozin BL, Budson AE, Goldstein LE, Kowall NW, Cantu RC. The spectrum of disease in chronic traumatic encephalopathy. Brain. 2013;136:1–22. doi: 10.1093/brain/aws307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minamisawa H, Terashi A, Katayama Y, Kanda Y, Shimizu J, Shiratori T, Inamura K, Kaseki H, Yoshino Y. Brain eicosanoid levels in spontaneously hypertensive rats after ischemia with reperfusion: leukotriene C4 as a possible cause of cerebral edema. Stroke. 1988;19:372–377. doi: 10.1161/01.str.19.3.372. [DOI] [PubMed] [Google Scholar]
- Morganti-Kossmann MC, Satgunaseelan L, Bye N, Kossmann T. Modulation of immune response by head injury. Injury. 2007;38:1392–1400. doi: 10.1016/j.injury.2007.10.005. [DOI] [PubMed] [Google Scholar]
- Murphy RC, Hammarström S, Samuelsson B. Leukotriene C: a slow-reacting substance from murine mastocytoma cells. Proc. Natl. Acad. Sci. 1979;76:4275–4279. doi: 10.1073/pnas.76.9.4275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian X, Wei E, Zhang L, Sheng W, Wang M, Zhang W, Chen Z. Pranlukast, a cysteinyl leukotriene receptor 1 antagonist, protects mice against brain cold injury. Eur. J. Pharmacol. 2006;549:35–40. doi: 10.1016/j.ejphar.2006.07.056. [DOI] [PubMed] [Google Scholar]
- Saver JL, Fonarow GC, Smith EE, Reeves MJ, Grau-Sepulveda MV, Hernandez AF, Peterson ED, Schwamm LH. Time to treatment with intravenous tissue plasminogen activator and outcome from acute ischemic stroke. JAMA. 2013;309:2480–2488. doi: 10.1001/jama.2013.6959. [DOI] [PubMed] [Google Scholar]
- Schmidt OI, Heyde CE, Ertel W, Stahel PF. Closed head injury--an inflammatory disease? Brain Res. Rev. 2005;48:388–399. doi: 10.1016/j.brainresrev.2004.12.028. [DOI] [PubMed] [Google Scholar]
- Schuhmann MU, Mokhtarzadeh M, Stichtenoth DO, Skardelly M, Klinge PM, Gutzki FM, Samii M, Brinker T. Temporal profiles of cerebrospinal fluid leukotrienes, brain edema and inflammatory response following experimental brain injury. Neurol. Res. 2003;25:481–491. doi: 10.1179/016164103101201896. [DOI] [PubMed] [Google Scholar]
- Schwarzbach E, Bonislawski DP, Xiong G, Cohen AS. Mechanisms underlying the inability to induce area CA1 LTP in the mouse after traumatic brain injury. Hippocampus. 2006;550:541–550. doi: 10.1002/hipo.20183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selassie AW, Zaloshnja E, Langlois JA, Miller T, Jones P, Steiner C. Incidence of long-term disability following traumatic brain injury hospitalization, United States, 2003. J. Head Trauma Rehabil. 2008;23:123–131. doi: 10.1097/01.HTR.0000314531.30401.39. [DOI] [PubMed] [Google Scholar]
- Shlosberg D, Benifla M, Kaufer D, Friedman A. Blood-brain barrier breakdown as a therapeutic target in traumatic brain injury. Nat. Rev. Neurol. 2010;6:393–403. doi: 10.1038/nrneurol.2010.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DH, Johnson VE, Stewart W. Chronic neuropathologies of single and repetitive TBI: substrates of dementia? Nat. Rev. Neurol. 2013;9:211–221. doi: 10.1038/nrneurol.2013.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streit WJ, Mrak RE, Griffin WST. Microglia and neuroinflammation: a pathological perspective. J. Neuroinflamm. 2004;1:14. doi: 10.1186/1742-2094-1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taber KH, Warden DL, Hurley RA. Blast-related traumatic brain injury: what is known? J. Neuropsych. Clin. N. 2006;18:141–145. doi: 10.1176/jnp.2006.18.2.141. [DOI] [PubMed] [Google Scholar]
- Uyama O, Okamura N, Yanase M, Narita M, Kawabata K, Sugita M. Quantitative evaluation of vascular permeability in the gerbil brain after transient ischemia using Evans blue fluorescence. J. Cerebr. Blood F. Met. 1988;8:282–284. doi: 10.1038/jcbfm.1988.59. [DOI] [PubMed] [Google Scholar]
- Voigt C, Donat CK, Hartig W, Förschler A, Skardelly M, Stichtenoth D, Arendt T, Meixensberger J, Schuhmann MU. Effect of leukotriene inhibitors on evolution of experimental brain contusions. Neuropath. Appl. Neuro. 2012;38:354–366. doi: 10.1111/j.1365-2990.2011.01211.x. [DOI] [PubMed] [Google Scholar]
- Wang M, Huang X, Fang S, Yuan Y, Zhang W, Lu Y, Ding Q, Wei E. Leukotriene D4 induces brain edema and enhances CysLT2 receptor-mediated aquaporin 4 expression. Biochem. Bioph. Res. Co. 2006;350:399–404. doi: 10.1016/j.bbrc.2006.09.057. [DOI] [PubMed] [Google Scholar]
- Warden D. Military TBI during the Iraq and Afghanistan wars. J. Head. Trauma Rehabil. 2006;21:398–402. doi: 10.1097/00001199-200609000-00004. [DOI] [PubMed] [Google Scholar]
- Wolman M, Klatzo I, Chui E, Wilmes F, Nishimoto K, Fujiwara K, Spatz M. Evaluation of the dye-protein tracers in pathophysiology of the blood-brain barrier. Acta. Neuropathol. 1981;54:55–61. doi: 10.1007/BF00691332. [DOI] [PubMed] [Google Scholar]
- Yu G, Wei E, Wang M, Zhang W, Zhang S, Weng J, Chu L, Fang S, Zhou Y, Chen Z, Zhang Q, Zhang L. Pranlukast, a cysteinyl leukotriene receptor-1 antagonist, protects against chronic ischemic brain injury and inhibits the glial scar formation in mice. Brain Res. 2005;1053:116–125. doi: 10.1016/j.brainres.2005.06.046. [DOI] [PubMed] [Google Scholar]
- Yu F, Zhang Y, Chuang D. Lithium reduces BACE1 overexpression, β amyloid accumulation, and spatial learning deficits in mice with traumatic brain injury. J. Neurotraum. 2012;29:2342–2351. doi: 10.1089/neu.2012.2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Hu H, Zhang L, Ding W, Yao H, Chen K, Sheng W, Chen Z, Wei E. Expression of cysteinyl leukotriene receptor 1 in human traumatic brain injury and brain tumors. Neurosci. Lett. 2004;363:247–251. doi: 10.1016/j.neulet.2004.03.088. [DOI] [PubMed] [Google Scholar]