Abstract
Generalized arterial calcification of infancy (GACI) is an intractable ectopic mineralization disorder caused by mutations in the ENPP1 gene resulting in reduced plasma inorganic pyrophosphate levels. We previously characterized the Enpp1asj mutant mouse as a model of GACI, and we have now explored the potential efficacy of bisphosphonates, non-hydrolyzable PPi analogs, in preventing ectopic mineralization in these mice. These mice were maintained on either basic diet (control) or diets containing etidronate or alendronate in three different concentrations (experimental). Considering low bioavailability of bisphosphonates when administered orally, subsequent studies tested the mice with subcutaneous injections of etidronate. The treatments were initiated at 4 weeks of age, and the degree of mineralization was assessed at 12 weeks of age by quantitation of calcium deposits in the muzzle skin containing dermal sheath of vibrissae and in aorta. We found that bisphosphonate treatments significantly reduced mineralization in skin and aorta. These changes in treated mice were accompanied with restoration of their bone microarchitecture, determined bymicrocomputed tomography. The inhibitory capacity of bisphosphonates, with mechanistic implications, was confirmed in a cell-based mineralization assay in vitro. Collectively, these results suggest that bisphosphonate treatment may be beneficial by a dual effect for preventing ectopic soft tissue mineralization while correcting decreased bone mineralization in GACI caused by ENPP1 mutations.
INTRODUCTION
Generalized arterial calcification of infancy (GACI) (OMIM20800) is an autosomal recessive disorder characterized by ectopic mineralization of the cardiovascular system (Rutsch et al., 2011). Deposition of calcium hydroxyapatite initially on arterial blood vessels, but also on other soft connective tissues, including skin, starts during fetal development, and the disorder is often diagnosed by prenatal ultrasound. The children are born with extensive calcification of arteries, and as a consequence, they in most cases die during the first six months of life from cardiovascular complications. The classic form of this disease, GACI type 1, is caused by mutations in the ENPP1 gene (Ruf et al., 2005; Rutsch et al., 2003), which encodes ectonucleotide pyrophosphatase/phosphodiesterase (ENPP1), an enzyme that hydrolyses ATP to AMP and inorganic pyrophosphate (PPi). Under normal physiologic conditions, PPi is a powerful anti-mineralization factor and the physiological ratio of PPi in relation to inorganic phosphate (Pi) is required to prevent spontaneous precipitation of calcium phosphate complexes on soft connective tissues. Thus, as a result of loss-of-function mutations in the ENPP1 gene, the synthesis of PPi is reduced, resulting in low PPi/Pi ratio which then allows the ectopic mineralization processes to ensue. Loss-of-function ENPP1 mutations can also cause autosomal recessive hypophosphatemic rickets (Lorenz-Depiereux et al., 2010), suggesting an as yet elusive mechanism that balances arterial calcification with bone mineralization. In addition, ENPP1 mutations have been identified in some patients with pseudoxanthoma elasticum (PXE), another ectopic mineralization disorder, but most cases with this disorder harbor mutations in the ABCC6 gene (Li et al., 2012; Nitschke et al., 2012).
There is no effective or specific treatment for GACI. A few studies have suggested that administration of bisphosphonates might be helpful in counteracting the ectopic mineralization in GACI; however, there is no consensus about the efficacy of these compounds. In some studies apparent improvement has been reported, while in other cases there has been very little, if any, effect (Edouard et al., 2011; Galletti et al., 2011; Ramjan et al., 2009; Rutsch et al., 2008). Bisphosphonates have also been reported to be accompanied by severe side effects, particularly on the development of bones (Otero et al., 2013; Silverman et al., 1994; Thomas et al., 1995). These differences can possibly be explained, in part, by the types and doses of bisphosphonates used and the route of administration which vary considerably in different studies.
Bisphosphonates are structural analogs of PPi in which the oxygen linkage to phosphonate (PO3) groups is replaced by a carbon molecule making them stable. The bisphosphonates have two side groups (R1, R2) which determine their potency as well as their pharmacologic characteristics. There are two classes of bisphosphonates, i.e., those containing nitrogen in the side groups and non-nitrogen containing forms. Bisphosphonates have two principal activities relating to mineralization: (a) they can serve as an anti-mineralization factor, and (b) they are inhibitors of osteoclast activity. The latter property is the basis for their use in treatment of bone diseases, particularly osteoporosis, but also for Paget’s disease and bone metastases (Rodan and Fleisch, 1996; Russell, 2006; Uludag, 2002). The first generation bisphosphonates, as exemplified by etidronate (ETD), a non-nitrogen containing bisphosphonate, display considerable anti-mineralization activities but are less potent inhibitors of osteoclasts than currently used third generation nitrogen containing bisphosphonates, such as alendronate (AST). In this study, we have tested the potential use of these prototypic bisphosphonates, ETD and AST, for treatment of GACI. Specifically, these bisphosphonates were administered orally or by subcutaneous injections to Enpp1asj mice, a mouse model of GACI (Li et al., 2013). These mice harbor a homozygous missense mutation in the Enpp1 gene which results in markedly reduced ENPP1 enzymatic activity and lowered plasma PPi concentration which subsequently allows for ectopic mineralization of soft connective tissues in the skin and arterial blood vessels to ensue (Li et al., 2013). In this study we investigated the effects of bisphosphonates in Enpp1asj mice on ectopic mineralization in skin and vascular tissues as well as on bone microarchitecture and mineralization.
RESULTS
GACI is a devastating ectopic mineralization disorder with the demise of affected individuals usually during the first year of life. There is no effective or specific treatment for this disorder. In this study, we tested the hypothesis that bisphosphonates might counteract the ectopic mineralization in skin and vascular tissues, while enhancing bone mineralization, using asj mice as a preclinical platform.
Oral administration of bisphosphonates to asj mice
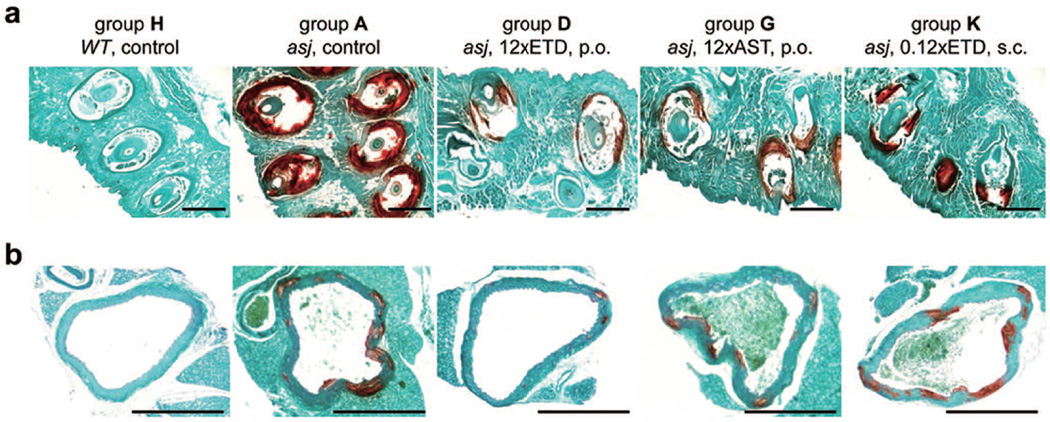
In the first set of experiments (Set 1), two different prototypic bisphosphonates, ETD or AST, in three different concentrations which were calculated to correspond to 1×, 5× and 12× of the corresponding human dose used for treatment of osteoporosis, respectively, were tested by oral administration. Groups of asj mice and wild-type (WT) mice were kept on “acceleration diet”, which facilitates the mineralization process in these mice (Li et al., 2013) without bisphosphonates serving as positive and negative controls of mineralization. For the experimental groups, all mice were kept on the acceleration diet with or without bisphosphonates (see Table 1). The degree of mineralization was first assessed by histopathologic examination of the dermal sheath of vibrissae, a connective tissue capsule surrounding the bulb of vibrissae in the muzzle skin, which serves as an early, progressive biomarker of overall mineralization in these mice (Li et al., 2013). Histopathologic examination of the asj mice on acceleration diet revealed extensive mineralization, while no evidence of mineralization was noted in WT mice on the same diet (Fig. 1). Evidence of mineralization was also noted in the vibrissae of asj mice treated with various doses of ETD or AST, but histopathologic examination suggested a lesser extent of mineral deposits. The presence of tissue mineralization in asj mice was also examined semi-quantitatively by histopathology of kidneys, heart, descending thoracic aorta, and eyes of the asj mice. The majority of asj mice treated with either ETD or AST demonstrated mineralization, and no statistical difference in the proportional mineralization in the kidney, heart, and the eyes was noted (Table S1). It should be noted that the values in Table S1 report the presence of any degree of mineralization. While as shown in Fig. 1, the degree of mineralization was reduced by the bisphosphonate treatment, this treatment did not result in complete absence of mineralization in most cases. Therefore, the values in Table S1, which reflect semi-quantitative assessment of the presence of mineral deposits, do not differ significantly.
Table 1.
Experimental groups of Enpp1asj mice by genotype and treatment*
| Group | Genotype | No. of mice examined (M+F) |
Treatment | |
|---|---|---|---|---|
| Set 1 (p.o.) | ||||
| A | asj | 9 (6+3) | Control diet | |
| B | asj | 9 (6+3) | Diet containing 1×ETD | |
| C | asj | 6 (1+5) | Diet containing 5×ETD | |
| D | asj | 8 (5+3) | Diet containing 12×ETD | |
| E | asj | 6 (4+2) | Diet containing 1×AST | |
| F | asj | 7 (3+4) | Diet containing 5×AST | |
| G | asj | 8 (4+4) | Diet containing 12×AST | |
| H | WT | 9 (6+3) | Control diet | |
| Set 2 (s.c.) | ||||
| I | asj | 7 (3+4) | saline | |
| J | asj | 8 (3+5) | 0.01×ETD | |
| K | asj | 11 (8+3) | 0.12×ETD | |
The mice were placed on either bisphosphonate-containing diets (Set 1) or injected with etidronate (ETD) subcutaneously (Set 2) at 4 weeks of age and followed for another 8 weeks. The mice were sacrificed at the age of 12 weeks for histopathological analysis.
Control diet: acceleration diet TD.00442.
ETD, etidronate; AST, alendronate; M, male; F, female. p.o., perioral; s.c., subcutaneous.
Figure 1. Histopathologic demonstration that bisphosphonate treatment prevents ectopic soft tissue mineralization in asj mice.
The asj mice develop ectopic mineralization of the dermal sheath of vibrissae (a) and aorta (b) when examined at 12 weeks of age by histopathology with Alizarin Red stain (group A). Note that the corresponding wild-type mice have no evidence of mineralization (group H). Feeding the asj mice with diet supplemented with 12×ETD (group D) or 12×AST (group G), and injecting the asj mice with 0.12×ETD (group K) markedly reduced the mineral content of the dermal sheath of vibrissae and aorta. ETD, etidronate disodium; AST, alendronate sodium trihydrate; p.o., perioral; s.c., subcutaneous administration of bisphosphonates. Scale bar = 0.4 mm.
Subcutaneous administration of bisphosphonates to asj mice
In the second set of experiments (Set 2), asj mice, again kept on the acceleration diet, were injected with ETD subcutaneously at 4 weeks of age, followed by twice per week injections up to 12 weeks of age. Two dosages of ETD were delivered, 0.01× and 0.12× (groups J and K) of the doses administered orally, based on the assumption that the absorption of ETD from oral feeding is only 1% or less (Rodan and Fleisch, 1996; Russell, 2006; Uludag, 2002), while subcutaneous injection would result in 100% absorption of the drug. As a control, a group of mice was injected with saline (Group I). Histopathologic examination revealed mineralization of dermal sheath of vibrissae in all mice to varying degrees (Fig. 1), while the presence of mineralization in the kidney, heart, descending thoracic aorta, and eyes in mice injected with ETD was not significantly different from those in mice injected with saline (Table S1).
Quantitation of ectopic mineralization in the skin and aorta by calcium assay
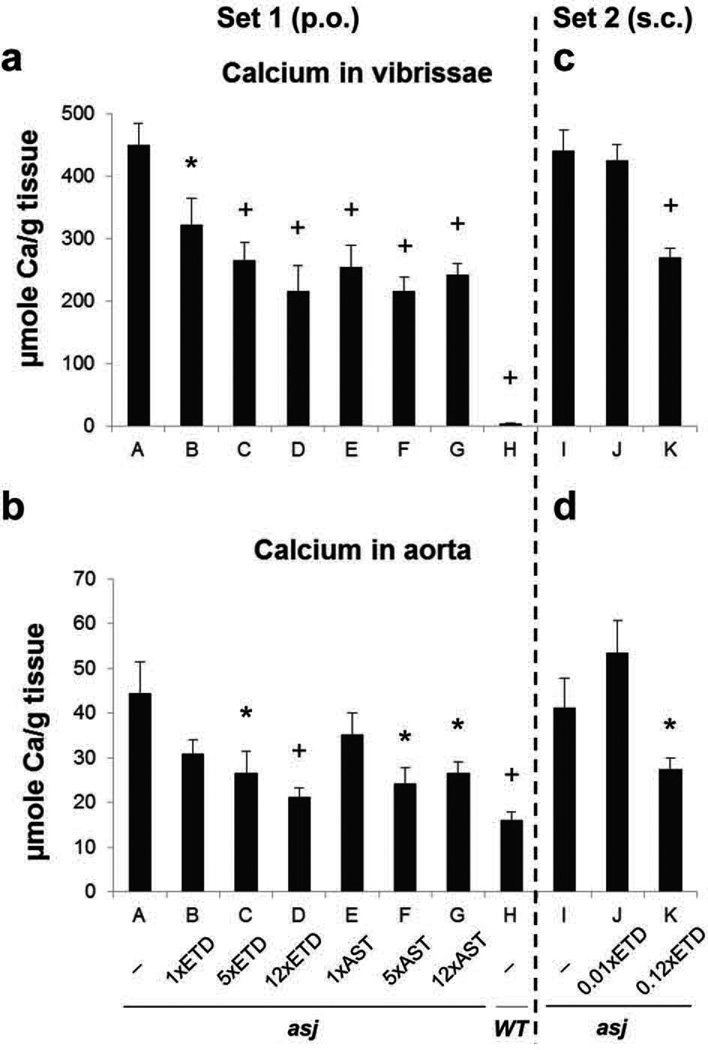
To quantitate the degree of mineralization in the skin and abdominal aorta in different groups of mice, tissue samples were obtained, mineral deposits were solubilized and calcium content was quantitatively measured by a chemical assay. These mineral deposits consist of calcium hydroxyapatite, the Ca/P ratio being ~2:1 (Kavukcuoglu et al., 2012). The amount of calcium in the muzzle skin containing the dermal sheath of vibrissae was significantly reduced in all mice treated with ETD or AST by oral administration (Fig. 2a, Set 1). Similar assay of calcium in vibrissae of mice injected subcutaneously with ETD indicated ~30% reduction with the higher, 0.12× dose (Fig. 2c, Set 2). The extent of mineralization was also determined by calcium assay in abdominal aorta from the same mice (Fig. 2b,d). These analyses revealed a relatively high baseline level of calcium level in the aorta of WT mice (Fig. 2b), but there was no evidence of mineralization by histology (Fig. 1b). Oral feeding of ETD significantly reduced the mineralization of aorta, the reduction with the highest dose (12×) being approximately 50% of the mice on the same diet but without bisphosphonate. Similarly, feeding of AST with 5× or 12× dosages resulted in significant reduction in the calcium content in muzzle skin. Subcutaneous injection of ETD with the higher dosage of ETD (0.12×) resulted in significant reduction of calcium content in aorta (Fig. 2d, Set 2). Thus, administration of ETD or AST orally or ETD by subcutaneous injections reduces the mineralization of muzzle skin as well as in aorta, as suggested by histopathologic observations and quantitated by direct chemical assay of calcium content in these tissues.
Figure 2. Bisphosphonate treatment reduces ectopic soft tissue mineralization as determined by direct chemical assay of calcium.
Panels a and c, skin biopsies containing the dermal sheath of vibrissae; Panels b and d, aorta. Note the significantly elevated calcium content in asj mice as compared to the WT mice (group A vs. H; + p < 0.01). Treatment of asj mice with diet supplemented with different concentrations of ETD or AST (groups B–D and E–G, respectively; p.o.) resulted in significant reduction in the calcium content in comparison to the asj mice on control diet (group A; * p < 0.05, + p < 0.01). Injecting of mice subcutaneously (s.c.) with 0.12×ETD (group K), but not with 0.01× ETD (group J), significantly reduced the calcium content of the skin and aorta as compared to mice injected with saline (group I). (Mean ± SE; n = 6–11 mice per group). ETD, etidronate disodium; AST, alendronate sodium trihydrate. p.o., perioral; s.c., subcutaneous.
The effects of bisphosphonate administration on serum calcium and phosphate concentrations were also determined. In majority of cases, treatment of the asj mice orally or subcutaneously did not alter the serum calcium or phosphate concentrations or change the Ca/Pi ratio (Table S2).
Effects of bisphosphonates on bone microarchitecture and mineralization
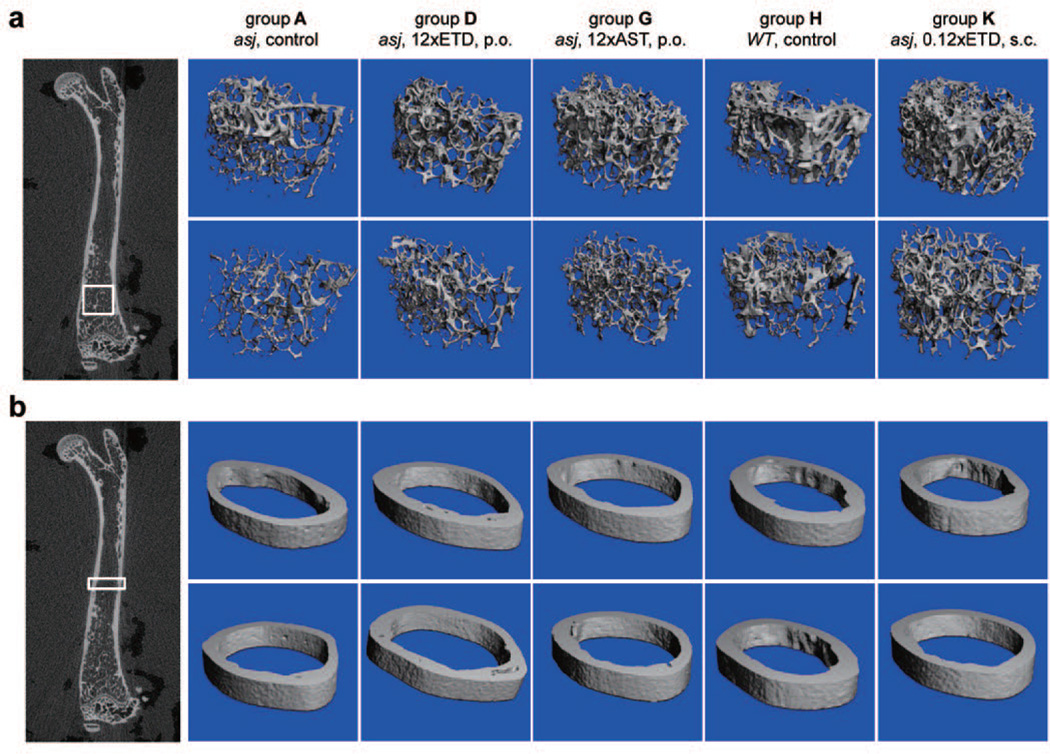
Since bisphosphonates pose both anti-mineralization and anti-osteoclastic activities, and they have been primarily used for treatment of bone disorders, such as osteoporosis, we evaluated the morphometric microarchitecture of the trabecular and cortical bones of femur in treated asj mice by microcomputed tomography (µCT) analysis. The WT mice and asj mice on the acceleration diet, the asj mice on the same diet but supplemented with 12×ETD or 12×AST in their diets, and asj mice, also kept on acceleration diet, receiving subcutaneous injections with 0.12×ETD were analyzed. There were drastic differences between the male and female mice on bone trabecular microarchitecture (Fig. 3), which is consistent with previous publications (Bouleftour et al., 2014; Pennypacker et al., 2009). Therefore, sex-matched comparisons on bone microarchitecture were performed between asj mice and WT mice as well as those treated with bisphosphonates orally or subcutaneously. Drastic differences on bone mineralization were found between WT and asj mice, as well as between asj mice on acceleration diet and those treated with bisphosphonates (Fig. 3, upper panel). These changes of bone morphometric microarchitecture were subsequently quantified using manufacturer-provided software (Table 2). The asj mice of both sexes have reduced bone mineralization, reduced trabecular bone mass and cortical thickness, as compared to WT mice on the same diet (Table 2, groups A vs. H). These observations are in agreement with those seen in Enpp1 KO mice (Enpp1tm1Gdg) (Hajjawi et al., 2014; Mackenzie et al., 2012). Distal femoral bone volume fraction was increased as a result of bisphosphonate treatments, corresponding to the increased trabecular bone mineral density by ~2-fold in treated mice. Trabecular number in asj mice treated with bisphosphonates increased significantly, while trabecular separation was significantly less in bisphosphonate treated mice. The structure model index is significantly less, while connectivity density was significantly higher (~3–4 fold), in sex-matched mice treated with bisphosphonates as compared with untreated asj mice (Table 2). Treated asj mice restored trabecular parameters close to those in the control WT mice. The effects of 12×ETD and 12×AST feeding groups were also compared, the 12×AST treated mice had significantly higher bone mineral density and trabecular number, while trabecular separation is significantly less (Table 2). These results attest to the stronger potency of AST than ETD in anti-osteoclastic activities. The asj mice received 0.01× ETD subcutaneous injections had most of the bone parameters similar to the asj mice treated with 12×ETD orally (Table 2). The cortical parameters were unchanged in treated mice as compared with untreated asj mice (Table 2). Collectively, µCT analysis of the femurs disclosed changes of bone microarchitecture in treated asj mice compared to sex-matched asj control mice as a result of bisphosphonates treatment, observations consistent with previous publications.
Figure 3. Bisphosphonate treatment alters bone microarchitecture in asj mice as determined by µCT analysis.
Panel a: trabecular bone; Panel b: cortical bone. Regions in the box outline the areas of bone where analysis was performed for images and morphometric parameters. Groups D, G and K in which asj mice were treated with bisphosphonates either by perioral (p.o.) or subcutaneous administration (s.c.) were compared with asj mice (group A) and WT mice (group H) on the same diet but without bisphosphonates. Note the distinct difference between male (top row in each panel) and female (bottom row in each panel) asj mice on control diet (group A), assessed by µCT scan. Treatment with bisphosphonates caused changes in femoral microarchitecture, as quantitatively detailed in Table 2 (n = 8–11).
Table 2.
Bone phenotypes by microCT of the right femur of the mice1)
| Trabecular bone: | ||||||||
|---|---|---|---|---|---|---|---|---|
| Group2) | Sex | BMD (mg/cm3) |
BV/TV (%) |
Tb.Th (µm) |
Tb.N (1/mm) |
Tb.Sp (µm) |
SMI | Conn.D (TV/mm3) |
| A | M | 57.5 ± 17.2 | 3.3 ± 0.7 | 27.6 ± 3.1 | 3.9 ± 0.1 | 256.6 ± 7.7 | 3.3 ± 0.1 | 49.8 ± 14.2 |
| A | F | 39.2 ± 11.3 | 1.7 ± 0.1 | 22.3 ± 0.5 | 3.2 ± 0.2 | 310.1 ± 13.9 | 3.2 ± 0.1 | 23.6 ± 6.0 |
| D | M | 96.9 ± 9.5 | 5.0 ± 0.8 | 28.1 ± 2.0 | 4.1 ± 0.1 | 239.5 ± 9.2 | 2.7 ± 0.2* | 129.6 ± 28.7* |
| D | F | 72.4 ± 18.9 | 4.7 ± 0.5+ | 26.7 ± 0.8* | 4.1 ± 0.2* | 241.3 ± 10.6* | 2.7 ± 0.1* | 154.7 ± 18.8+ |
| G | M | 107.7 ± 12.6* | 5.4 ± 1.0 | 25.7 ± 1.8 | 4.9 ± 0.1+ǂ | 200.1 ± 3.3+ǂ | 2.8 ± 0.2* | 164.3 ± 32.9+ |
| G | F | 114.9 ± 7.9+† | 6.9 ± 0.9+ | 28.4 ± 1.5* | 4.6 ± 0.1+ | 213.5 ± 6.2+† | 2.5 ± 0.1+ | 234.9 ± 30.0+ |
| H | M | 158.9 ± 19.8+ | 9.9 ± 1.8+ | 37.7 ± 9.0 | 4.4 ± 0.1+ | 222.7 ± 2.7+ | 2.1 ± 0.4+ | 129.5 ± 31.9* |
| H | F | 90.2 ± 5.4* | 4.1 ± 0.6* | 30.6 ± 2.6* | 3.6 ± 0.1 | 277.5 ± 9.2 | 3.1 ± 0.1 | 90.9 ± 5.9+ |
| K | M | 117.4 ± 10.1+ | 6.2 ± 0.8* | 26.5 ± 0.9 | 4.7 ± 0.1+† | 208.1 ± 6.7+† | 2.5 ± 0.2+ | 209.6 ± 33.2+ |
| K | F | 111.7 ± 18.6* | 6.6 ± 1.4* | 27.1 ± 2.0 | 4.0 ± 0.1* | 245.4 ± 5.0* | 2.1 ± 0.2+† | 252.9 ± 39.9+ |
| Cortical bone: | ||||||||
|---|---|---|---|---|---|---|---|---|
| Group2) | Sex | BMD (mg/cm3) |
Ct.Porosity (%) |
Ct.pMOI (mm4) |
Ct.Th (µm) |
|||
| A | M | 1044.1 ± 7.4 | 11.0 ± 0.5 | 0.28 ± 0.03 | 128.2± 6.9 | |||
| A | F | 1068.2 ± 7.1 | 9.6 ± 0.4 | 0.25 ± 0.02 | 130.3 ± 6.1 | |||
| D | M | 1054.5 ± 1.3 | 10.1 ± 0.6 | 0.37 ± 0.02 | 137.5 ± 5.8 | |||
| D | F | 1073.5 ± 23.4 | 9.6 ± 1.0 | 0.32 ± 0.02 | 138.0 ± 5.5 | |||
| G | M | 1050.3 ± 12.8 | 10.1 ± 0.7 | 0.31 ± 0.02 | 132.0 ± 6.5 | |||
| G | F | 1063.7 ± 8.3 | 8.3 ± 0.8 | 0.31 ± 0.02 | 143.5 ± 5.8 | |||
| H | M | 1072.0 ± 6.7* | 9.4 ± 0.6 | 0.39 ± 0.04* | 161.0 ± 6.1+ | |||
| H | F | 1112.0 ± 1.2* | 7.6 ± 0.2* | 0.31 ± 0.02 | 161.0 ± 4.1* | |||
| K | M | 1041.1 ± 7.3 | 10.2 ± 0.7 | 0.32 ± 0.02 | 129.0 ± 3.2 | |||
| K | F | 1052.7 ± 9.0 | 9.9 ± 0.4 | 0.27 ± 0.02 | 127.7 ± 3.4 | |||
Wild type and asj mice were placed on acceleration diet at 4 weeks of age and maintained another 8 weeks. Groups of asj mice were either placed on different diets supplemented with bisphosphonates, or injected twice a week subcutaneously with saline or etidronate, for an additional 8 weeks. Right femurs of the mice were analyzed by µCT at 12 weeks of age. Values are expressed as mean ± S.E.
Statistical significance in comparison to the asj mice of the same sex on acceleration diet (group A) is indicated: * p < 0.05, + p < 0.01.
Statistical significance in comparison to the asj mice fed on 12×ETD (group D) is indicated: † p < 0.05, ǂ p < 0.01.
BMD, bone mineral density; BV/TV, relative bone volume; Tb.Th, trabecular thickness; Tb.N, trabecular number; Tb.Sp, trabecular separation (marrow thickness, µm); SMI, structure model index; Conn.D, connectivity density; Ct.Porosity, cortical porosity; Ct.pMOI, cortical polar moment of inertia; Ct.Th, cortical thickness; M, male; F, female.
For description of different groups, see Table 1.
Demonstration of anti-mineralization effects of bisphosphonates in an in vitro assay
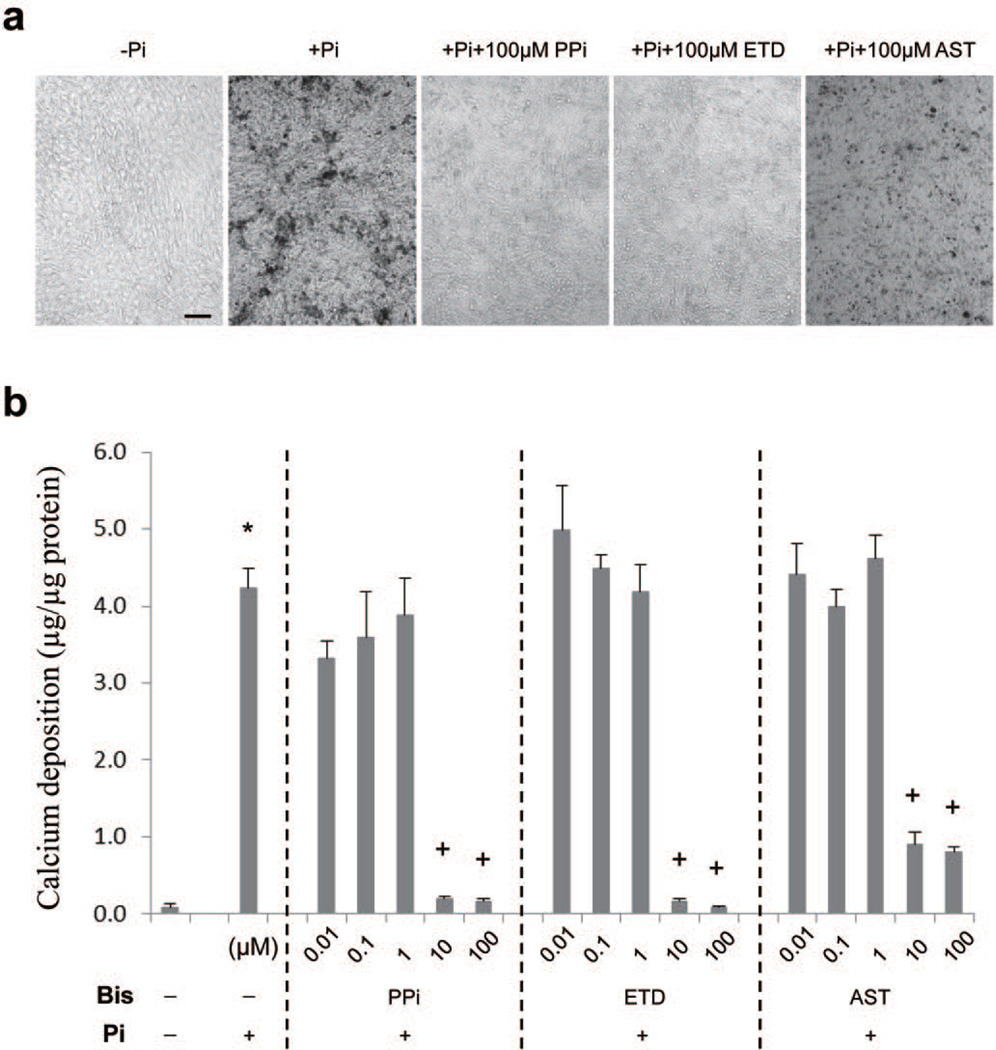
To gain insight into the potential pathomechanisms as to how bisphosphonates might interfere with mineral deposition in vivo, an in vitro mineralization assay was utilized. Specifically, fibroblasts in monolayer culture were incubated at ~80% confluency in DMEM culture medium, and 2 mM Pi is added at day 0. During the subsequent 10 days of incubation, significant cell layer associated mineralization could be observed, as determined by phase contrast microscopy of the cells, and by direct assay of calcium associated with the cell layer and corrected for the protein content (Fig. 4A). A dramatic, about 49-fold increase in the degree of mineralization was noted in cultures upon addition of Pi. Addition of varying concentrations of PPi revealed a dramatic inhibition of the mineralization on cell cultures with 10 or 100 µM, while concentrations 1 µM or lower have no effect. Similarly, addition of ETD resulted in essentially complete inhibition of calcium deposition on the cells in 10 and 100 µM concentrations (Fig. 4B). A similar trend was noted with 10 and 100 µM AST, although the inhibition of mineralization was not as complete as with ETD.
Figure 4. Bisphosphonates prevent mineralization in an in vitro culture system.
NIH3T3 cells were cultured on 96-well plates in DMEM medium containing 10% FBS supplemented with 2 mM Pi. At day 0, PPi, ETD and AST were added to some cultures at concentrations indicated. At day 10, the degree of mineralization was assessed either (a) by phase contrast light microscopy, or (b) by chemical assay of calcium deposition in the cell layer. * p < 0.01, compared with −Pi; + p < 0.01, compared with +Pi (mean ± SE; n = 4 cultures in each group). PPi, sodium pyrophosphate decahydrate; ETD, etidronate disodium; AST, alendronate sodium trihydrate; Pi, inorganic phosphate. Scale bar = 0.1 mm.
DISCUSSION
Generalized arterial calcification of infancy (GACI; OMIM20800) is an autosomal recessive disease characterized by severe vascular calcification in large and medium sized arteries resulting in cardiovascular collapse and death in the early postnatal period. GACI type 1, the classic form, is caused by mutations in the ENPP1 gene which encodes ectonucleotide pyrophosphatase phosphodiesterase (ENPP1), a type II extracellular membrane bound glycoprotein. ENPP1 is the primary source of extracellular inorganic pyrophosphate (PPi) in the body. Under normal physiologic conditions PPi acts as a powerful anti-mineralization factor (Nitschke and Rutsch, 2012b). The patients with GACI type 1 have previously been shown to have a markedly reduced PPi/Pi ratio which would explain the severe mineralization of arterial blood vessels and other soft connective tissue, including the skin. Some patients with GACI type 1 also develop hypophosphatemic rickets characterized by reduced bone mineralization (Lorenz-Depiereux et al., 2010). GACI type 2 is caused by mutations in the ABCC6 gene (Li et al., 2014; Nitschke et al., 2012) which encodes a putative efflux transporter, ABCC6. Mutations in this gene also cause pseudoxanthoma elasticum (PXE; OMIM264800), another heritable ectopic mineralization disorder with late-onset, slowly progressive disease. Recent studies have demonstrated considerable, both genotypic and phenotypic overlap between PXE and GACI (Li et al., 2014; Li et al., 2012; Li and Uitto, 2013; Nitschke and Rutsch, 2012a). There is no effective treatment for these diseases with considerable morbidity and mortality. Recently, patients with PXE with ABCC6 mutations and the Abcc6−/− mouse model of PXE have been reported to have decreased extracellular concentrations of nucleotide triphosphates and PPi through an unknown mechanism (Jansen et al., 2014; Jansen et al., 2013). The identification of mutations both in ENPP1 and ABCC6 as genetic etiologies for GACI therefore links the same biochemical pathway to unregulated tissue mineralization in both types of GACI.
The observations of decreased extracellular PPi levels in patients with GACI or PXE caused by ENPP1 mutations have led to the suggestion that administration of PPi or its analogues to the patients could counteract the ectopic mineralization (O'Neill et al., 2011). Bisphosphonates, which are stable, non-hydrolyzable PPi analogues, are typically prescribed for the treatment of osteoporosis to reduce fracture risk, preventing bone loss primarily by the inhibition of osteoclast function. Bisphosphonates also demonstrate anti-mineralization activities, and different chemical structures of bisphosphonates determine their potency, mode of action as well as their pharmacologic characteristics. Etidronate, the first generation of bisphosphonates favoring anti-mineralization property over the anti-osteoclastic activity, was the first molecule used off-label in the treatment of fibrodysplasia ossificans progressiva (Francis and Valent, 2007), a severe soft tissue mineralization disorder caused by mutations in the ACVR1 gene. Recently, bisphosphonates have been used off-label to treat patients with ectopic mineralization, including newborns or infants with GACI type 1 with ENPP1 mutations or GACI type 2 with ABCC6 mutations (Edouard et al., 2011; Ramjan et al., 2009). In 2008, a retrospective observational analysis of 55 patients with GACI revealed survival beyond infancy with etidronate therapy (Rutsch et al., 2008), which was corroborated by another study reporting that 15 out of 22 GACI patients who survived beyond infancy received etidronate treatment (Chong and Hutchins, 2008). However, other studies have found no improvement in vascular mineralization after bisphosphonates treatment (Galletti et al., 2011). A recent case report has highlighted the severe skeletal toxicity with paradoxical joint calcifications following protracted etidronate therapy in a 7-year-old boy with GACI (Otero et al., 2013). Furthermore, a number of studies have suggested detrimental side effects of bisphosphonates treatment, including the development of rickets or osteomalacia during the protracted administration of bisphosphonates (Silverman et al., 1994; Thomas et al., 1995). Taken together, the above observations with limited available clinical data and different patient outcomes make it difficult to determine if bisphosphonates are helpful for treatment of GACI. We, therefore, assessed the effects of two bisphosphonate drugs on ectopic soft tissue calcification and bone microarchitecture in an Enpp1asj mouse model of GACI.
The present study was undertaken in asj mice with features that recapitulate GACI in humans (Li et al., 2013) to test the effects of two bisphosphonates, ETD and AST, in the mineralization processes. All mice were kept on “acceleration diet” that accelerates the mineralization process elicited by the absence of functional ENPP1 (Li et al., 2013). AST was administered orally at lower molar doses than ETD due to its ~1,000 times higher anti-osteoclastic activity (Rodan and Fleisch, 1996; Russell, 2006; Uludag, 2002). In this study, we demonstrated that ETD and AST treatments periorally significantly reduced mineralization in skin and aorta in asj mice. When administered subcutaneously, ETD at much lower dose (0.12×) than when delivered orally, resulted in ~30% reduction in ectopic mineralization of the skin and aorta. Thus, subcutaneous administration could be thought to be better than oral intake for treatment of GACI due to higher bioavailability.
To explore potential mechanisms by which bisphosphonates might exert their anti-mineralization effects, an in vitro mineralization assay was utilized. In this assay, addition of 2 mM Pi into cell culture medium elicits cell-dependent precipitation of calcium hydroxyapatite. Addition of bisphosphonates resulted in abrupt inhibition of the mineralization process, similar to addition of PPi, in the concentration range between 1 and 10 µM. These results suggested that bisphosphonates, stable pyrophosphate analogs, when reaching a certain stoichiometric ratio with Pi, physically prevent the nucleation of calcium phosphate crystals resulting in reduced tissue mineralization.
Since bisphosphonates, besides showing anti-mineralization effects, also have anti-osteoclastic activity, the femurs of asj control mice and those treated with bisphosphonates were analyzed for their bone microarchitecture. Femur was selected as the target bone for analysis, since several previous studies have focused on mineralization of this bone, thus providing appropriate methodologies and relevant reference information for interpretation of our data (Mackenzie et al., 2012; Uveges et al., 2009). µCT analysis of femurs showed drastic sex-dependent differences on bone mineralization between WT and asj mice without any special treatment. As compared with WT mice, the asj mice had reduced bone volume fraction, reduced trabecular and cortical thickness, and reduced trabecular number, as reflected by the significant decrease in bone mineral density in both trabecular bone and cortical bone. The bisphosphonate treatment in asj mice orally or subcutaneously restored these bone microarchitecture phenotypes to nearly similar to those in WT mice. In addition, subcutaneous administration of ETD provided stronger effects on bone microarchitecture than when delivered orally, attesting to its better bioavailability. Furthermore, AST was shown to be more potent than ETD when supplemented in diets to cause changes in bone mineral density and bone microarchitecture. Collectively, the results demonstrated changes in bone morphometry and microarchitecture, attributable to bisphosphonate treatments.
Our results demonstrate the efficacy of bisphosphonates in the treatment of GACI caused by ENPP1 mutations. Except for preventing mineralization in soft connective tissues, an abundance of evidence has been provided to show that bisphosphonates are effective as a treatment for decreased bone density and bone fracture in patients. As compared with PXE, which has no clinical evidence of bone abnormality, patients with GACI have reduced bone mineralization with concomitant ectopic mineralization in vascular tissues, suggesting dysregulated mineralization due to an as yet unknown mechanism that balances arterial calcification with bone mineralization (Rutsch et al., 2011). Some patients with GACI develop hypophosphatemic rickets with a distinct hypomineralizing phenotype. In addition, there are descriptions of patients with a mild GACI phenotype who have both arterial calcification and concomitant hypophosphatemia and hyperphosphaturia (Rutsch et al., 2008). One of the mechanisms of decreased bone mineralization caused by ENPP1 mutations is associated with elevated plasma FGF23 levels, which then mediates hypophosphatemic rickets (Lorenz-Depiereux et al., 2010). Our studies using asj mice as a model of GACI demonstrated dual beneficial effects of bisphosphonates treatment in tissue mineralization and bone mineralization. Bisphosphonate treatment in asj mice not only counteracted soft tissue mineralization in skin and aorta, but also restored bone mineralization parameters similar to those in WT mice which should be considered as a beneficial effect.
Collectively, our results suggest that bisphosphonates may be beneficial for preventing ectopic mineralization while correcting decreased bone mineralization in GACI and PXE caused by mutations in the ENPP1 gene. However, additional long-term studies in mouse models should be conducted to monitor for development of side effects to ensure safety of this approach. It is appropriate to point out that mineral metabolism in the mice may differ from that in humans, and some animals, and perhaps also humans, may be more prone or resistant to these treatments. Consequently, the clinical efficacy and potential side effects in humans remain to be seen in forthcoming clinical trials.
MATERIALS & METHODS
Mice and breeding
C57BL/6J-Enpp1asj/GrsrJ mice on a C57BL/6J background were obtained from The Jackson Laboratory (Bar Harbor, ME). This mouse is referred in this publication as the asj mouse. The wild-type (WT), heterozygous, and homozygous asj mice were generated from heterozygous matings. Mice were maintained on standard rodent laboratory diet (Laboratory Autoclavable Rodent Diet 5010; PMI Nutritional International, Brentwood, MO) under standard conditions. All protocols were approved by the Institutional Animal Care and Use Committee of Thomas Jefferson University.
Experimental design and treatments
For breeding purposes, the homozygous asj male and heterozygous asj female mice were placed on standard rodent diet supplemented with a 5-fold increase in magnesium, to prevent these mice from developing stiffened joints and tissue mineralization, thus capable of breeding. The magnesium-enriched diet has been previously shown to completely prevent connective tissue mineralization in a mouse model of pseudoxanthoma elasticum (Abcc6−/−) (LaRusso et al., 2009; Li et al., 2009). The heterozygous female mice, once pregnant, were switched to standard rodent diet. The offspring were genotyped, and the homozygous asj mice, once weaned, were maintained on “acceleration diet” throughout the experiment (Harlan Teklad, Rodent diet TD.00442, Madison, WI). The “acceleration diet” is enriched in phosphorus (2×) and has reduced magnesium content (20%) in comparison to the standard rodent diet. We have previously shown that the “acceleration diet” is capable of accelerating the onset and severity of tissue mineralization in the asj mice, thus shortening the time required for acquisition of unequivocal results (Li et al., 2013). Four-week old WT and asj mice were either placed on acceleration diet supplemented with ETD or AST (Set 1) or received subcutaneous injections of ETD (Set 2) and maintained on the same treatment regimen for an additional 8 weeks. The groups of mice in the two sets, characterized by genotype and treatment, are described in Table 1.
In Set 1, asj and WT mice at 4 weeks of age were fed either “acceleration diet” (Groups A and H) or the same diet supplemented with either ETD or AST, 6–9 mice per group. ETD treatment consisted of three groups: asj mice were fed with a dose calculated to be equivalent to that used for treatment of humans for osteoporosis (20 mg/kg/day orally), i.e., 8 mg etidronate disodium (Alfa Aesar, Ward Hill, MA) per 100 g of diet (1×ETD), or 5- and 12-fold greater doses (5×ETD and 12×ETD), respectively (Groups B, C and D). AST treatment also consisted of three groups: asj mice were fed with a dose equivalent to human (0.5 mg/kg/day orally), which received 0.2 mg alendronate sodium trihydrate (Alfa Aesar) per 100 g of diet (1×AST), or 5- and 12-fold increase of the human equivalent dose (5×AST and 12×AST) (Groups E, F and G). After 8 weeks of treatment, all mice were euthanized for tissue analysis.
In Set 2, the asj mice were placed on the acceleration diet at 4 weeks of age, and injected with ETD subcutaneously, twice a week, for a total of 8 weeks. Mice were weighed every two weeks to determine the appropriate treatment doses (on a mg/kg basis). Subcutaneous injections consisted of three groups: asj mice were injected with (i) sterile saline, (ii) 0.01×ETD or (iii) 0.12×ETD, 7–11 mice per group (Groups I, J and K). The dose of 0.01×ETD was based on the assumption of 1% of intestinal absorption of ETD while 100% absorption by subcutaneous injection (Rodan and Fleisch, 1996; Russell, 2006; Uludag, 2002). All mice were euthanized at 12 weeks of age for tissue analysis.
Histopathological analysis
Muzzle skin biopsies (left side) and internal organs (liver, kidney, heart, eyes, and descending thoracic aorta) from euthanized mice were collected and processed for histology. Tissue sections were stained with hematoxylin and eosin (H&E) or Alizarin red using standard procedures.
Chemical quantitation of calcium and phosphate
To quantify the mineral deposition in mouse tissues, muzzle skin biopsies (right side) and abdominal aorta were harvested and decalcified with 0.15 mol/L HCl for 48 hours at room temperature. Solubilized calcium was then determined by colorimetric analysis using the ơ-cresolphthalein complexone method (calcium (CPC) LiquiColor; Stanbio Laboratory, Boerne, TX). The values were normalized to tissue weight. Serum calcium concentrations were determined with the same assay. Serum phosphate was measured by the Malachite Green Phosphate Assay Kit (Bioassay Systems, Hayward, CA).
Microcomputed tomography
Microarchitecture of the distal trabecular bone and midshaft region of the right femur was analyzed. A 1.2 mm-thick region located proximal to the distal growth plate of femur and a 0.3 mm-thick midshaft region of the femur were scanned at a 6 µm resolution using the micro-computed tomography (µCT) system (µCT35; Scanco Medical AG, Bassersdorf, Switzerland). The microstructural parameters were obtained through three-dimensional reconstruction and segmentation (using a Gaussian filter and a global threshold of 3685 Hounsfield units) using the manufacturer-provided software.
Cell cultures and induction of mineralization in vitro
NIH3T3 cells were routinely cultured in DMEM growth medium in the presence of 10% fetal bovine serum. At ~80% confluence, the cells were switched to calcification-inducing medium (DMEM supplemented with 2 mM Pi). The first day of culture in calcification-inducing medium was defined as day 0. Sodium pyrophosphate decahydrate (PPi, Thermo Fisher Scientific In., Pittsburgh, PA), etidronate disodium (ETD) or alendronate sodium trihydrate (AST), was added to some cultures at day 0 at the concentration of 0.01, 0.1, 1.0, 10 or 100 µM, for additional 10 days. The medium was changed every other day. All experiments were performed in quadruplicate. At day 10 of cell culture, the media were removed, and the amount of calcium in the cell layer was determined and normalized to the protein content (LaRusso et al., 2009).
Statistical analysis
Comparisons between different groups of mice were performed using the two-sided Kruskal–Wallis nonparametric test. Fisher’s exact test was used to determine proportional differences in different groups. Statistical significance was reached with p < 0.05. All statistical computations were completed using SPSS version 15.0 software (SPSS Inc., Chicago, IL).
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by NIH/NIAMS grants R01AR055225 (JU) and K01AR064766 (QL). The authors acknowledge Penn Center for Musculoskeletal Disorders, supported by NIH/NIAMS P30AR050950, for assistance in analysis of bone morphometry. The authors thank Dian Wang, Jieyu Zhang, Wei-Ju Tseng, and Tingting Zhan for technical assistance. Carol Kelly helped in manuscript preparation.
Abbreviations
- PXE
pseudoxanthoma elasticum
- GACI
generalized arterial calcification of infancy
- ETD
etidronate disodium
- AST
alendronate sodium trihydrate
- KO
knock-out
- WT
wild-type
Footnotes
CONFLICT OF INTEREST
The authors state no conflict of interest.
REFERENCES
- Bouleftour W, Boudiffa M, Wade-Gueye NM, et al. Skeletal development of mice lacking bone sialoprotein (BSP)--impairment of long bone growth and progressive establishment of high trabecular bone mass. PLoS One. 2014;9:e95144. doi: 10.1371/journal.pone.0095144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong CR, Hutchins GM. Idiopathic infantile arterial calcification: the spectrum of clinical presentations. Pediatr Dev Pathol. 2008;11:405–415. doi: 10.2350/07-06-0297.1. [DOI] [PubMed] [Google Scholar]
- Edouard T, Chabot G, Miro J, et al. Efficacy and safety of 2-year etidronate treatment in a child with generalized arterial calcification of infancy. Eur J Pediatr. 2011;170:1585–1590. doi: 10.1007/s00431-011-1572-9. [DOI] [PubMed] [Google Scholar]
- Francis MD, Valent DJ. Historical perspectives on the clinical development of bisphosphonates in the treatment of bone diseases. J Musculoskelet Neuronal Interact. 2007;7:2–8. [PubMed] [Google Scholar]
- Galletti S, Nitschke Y, Malavolti AM, et al. Generalized arterial calcification of infancy: Fatal clinical course associated with a novel mutation in ENPP1. JIMD Rep. 2011;1:23–27. doi: 10.1007/8904_2011_11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajjawi MO, MacRae VE, Huesa C, et al. Mineralisation of collagen rich soft tissues and osteocyte lacunae in Enpp1(−/−) mice. Bone. 2014;69:139–147. doi: 10.1016/j.bone.2014.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen RS, Duijst S, Mahakena S, et al. ABCC6-mediated ATP secretion by the liver is the main source of the mineralization inhibitor inorganic pyrophosphate in the systemic circulation-brief report. Arterioscler Thromb Va c Biol. 2014;34:1985–1989. doi: 10.1161/ATVBAHA.114.304017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen RS, Kucukosmanoglu A, de Haas M, et al. ABCC6 prevents ectopic mineralization seen in pseudoxanthoma elasticum by inducing cellular nucleotide release. Proc Nat Acad Sci USA. 2013;110:20206–20211. doi: 10.1073/pnas.1319582110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavukcuoglu NB, Li Q, Pleshko N, et al. Connective tissue mineralization in Abcc6(−/−) mice, a model for pseudoxanthoma elasticum. Matrix Biol. 2012;31:246–252. doi: 10.1016/j.matbio.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaRusso J, Li Q, Jiang Q, et al. Elevated dietary magnesium prevents connective tissue mineralization in a mouse model of pseudoxanthoma elasticum (Abcc6−/−) J Invest Dermatol. 2009;129:1388–1394. doi: 10.1038/jid.2008.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Brodsky JL, Conlin L, et al. Mutations in the ABCC6 gene as a cause of generalized arterial calcification of infancy: Genotypic overlap with pseudoxanthoma elasticum. J Invest Dermatol. 2014;134:658–665. doi: 10.1038/jid.2013.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Guo H, Chou DW, et al. Mutant Enpp1asj mouse as a model for generalized arterial calcification of infancy. Dis Model Mech. 2013;6:1227–1235. doi: 10.1242/dmm.012765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, LaRusso J, Grand-Pierre AE, et al. Magnesium carbonate-containing phosphate binder prevents connective tissue mineralization in Abcc6−/− mice-potential for treatment of pseudoxanthoma elasticum. Clin Transl Sci. 2009;2:398–404. doi: 10.1111/j.1752-8062.2009.00161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Schumacher W, Siegel D, et al. Cutaneous features of pseudoxanthoma elasticum in a patient with generalized arterial calcification of infancy due to a homozygous missense mutation in the ENPP1 gene. Br J Dermatol. 2012;166:1107–1111. doi: 10.1111/j.1365-2133.2012.10811.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Uitto J. Mineralization/anti-mineralization networks in the skin and vascular connective tissues. Am J Pathol. 2013;183:10–18. doi: 10.1016/j.ajpath.2013.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz-Depiereux B, Schnabel D, Tiosano D, et al. Loss-of-function ENPP1 mutations cause both generalized arterial calcification of infancy and autosomal-recessive hypophosphatemic rickets. Am J Hum Genet. 2010;86:267–272. doi: 10.1016/j.ajhg.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie NC, Zhu D, Milne EM, et al. Altered bone development and an increase in FGF-23 expression in Enpp1−/− mice. PLoS One. 2012;7:e32177. doi: 10.1371/journal.pone.0032177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitschke Y, Baujat G, Botschen U, et al. Generalized arterial calcification of infancy and pseudoxanthoma elasticum can be caused by mutations in either ENPP1 or ABCC6. Am J Hum Genet. 2012;90:25–39. doi: 10.1016/j.ajhg.2011.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitschke Y, Rutsch F. Generalized arterial calcification of infancy and pseudoxanthoma elasticum: two sides of the same coin. Front Genet. 2012a;3:302. doi: 10.3389/fgene.2012.00302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitschke Y, Rutsch F. Genetics in arterial calcification: lessons learned from rare diseases. Trends Cardiovasc Med. 2012b;22:145–149. doi: 10.1016/j.tcm.2012.07.011. [DOI] [PubMed] [Google Scholar]
- O'Neill WC, Lomashvili KA, Malluche HH, et al. Treatment with pyrophosphate inhibits uremic vascular calcification. Kidney Int. 2011;79:512–517. doi: 10.1038/ki.2010.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otero JE, Gottesman GS, McAlister WH, et al. Severe skeletal toxicity from protracted etidronate therapy for generalized arterial calcification of infancy. J Bone Miner Res. 2013;28:419–430. doi: 10.1002/jbmr.1752. [DOI] [PubMed] [Google Scholar]
- Pennypacker B, Shea M, Liu Q, et al. Bone density, strength, and formation in adult cathepsin K (−/−) mice. Bone. 2009;44:199–207. doi: 10.1016/j.bone.2008.08.130. [DOI] [PubMed] [Google Scholar]
- Ramjan KA, Roscioli T, Rutsch F, et al. Generalized arterial calcification of infancy: treatment with bisphosphonates. Nat Clin Pract Endocrinol Metab. 2009;5:167–172. doi: 10.1038/ncpendmet1067. [DOI] [PubMed] [Google Scholar]
- Rodan GA, Fleisch HA. Bisphosphonates: mechanisms of action. J Clin Invest. 1996;97:2692–2696. doi: 10.1172/JCI118722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruf N, Uhlenberg B, Terkeltaub R, et al. The mutational spectrum of ENPP1 as arising after the analysis of 23 unrelated patients with generalized arterial calcification of infancy (GACI) Hum Mutat. 2005;25:98. doi: 10.1002/humu.9297. [DOI] [PubMed] [Google Scholar]
- Russell RG. Bisphosphonates: from bench to bedside. Ann N Y Acad Sci. 2006;1068:367–401. doi: 10.1196/annals.1346.041. [DOI] [PubMed] [Google Scholar]
- Rutsch F, Boyer P, Nitschke Y, et al. Hypophosphatemia, hyperphosphaturia, and bisphosphonate treatment are associated with survival beyond infancy in generalized arterial calcification of infancy. Circ Cardiovasc Genet. 2008;1:133–140. doi: 10.1161/CIRCGENETICS.108.797704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutsch F, Nitschke Y, Terkeltaub R. Genetics in arterial calcification: pieces of a puzzle and cogs in a wheel. Circ Res. 2011;109:578–592. doi: 10.1161/CIRCRESAHA.111.247965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutsch F, Ruf N, Vaingankar S, et al. Mutations in ENPP1 are associated with 'idiopathic' infantile arterial calcification. Nature Genet. 2003;34:379–381. doi: 10.1038/ng1221. [DOI] [PubMed] [Google Scholar]
- Silverman SL, Hurvitz EA, Nelson VS, et al. Rachitic syndrome after disodium etidronate therapy in an adolescent. Arch Phys Med Rehabil. 1994;75:118–120. [PubMed] [Google Scholar]
- Thomas T, Lafage MH, Alexandre C. Atypical osteomalacia after 2 year etidronate intermittent cyclic administration in osteoporosis. J Rheumatol. 1995;22:2183–2185. [PubMed] [Google Scholar]
- Uludag H. Bisphosphonates as a foundation of drug delivery to bone. Curr Pharm Des. 2002;8:1929–1944. doi: 10.2174/1381612023393585. [DOI] [PubMed] [Google Scholar]
- Uveges TE, Kozloff KM, Ty JM, et al. Alendronate treatment of the brtl osteogenesis imperfecta mouse improves femoral geometry and load response before fracture but decreases predicted material properties and has detrimental effects on osteoblasts and bone formation. J Bone Miner Res. 2009;24:849–859. doi: 10.1359/JBMR.081238. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.