Abstract
G protein-coupled estrogen receptor (GPER) is a 7-transmembrane receptor implicated in rapid estrogen signaling. Originally cloned from vascular endothelial cells, GPER plays a central role in the regulation of vascular tone and cell growth, as well as lipid and glucose homeostasis. This review highlights our knowledge of the physiological and pathophysiological functions of GPER in the pancreas, peripheral and immune tissues, and the arterial vasculature. Recent findings of its roles in obesity, diabetes, and atherosclerosis, including the GPER-dependent regulation of lipid metabolism and inflammation, are presented. The therapeutic potential of targeting GPER-dependent pathways in chronic diseases such as coronary artery disease and diabetes and in the context of menopause is also discussed.
Keywords: atherosclerosis, diabetes, estrogen receptor, rapid signaling, GPER
Estrogen: modulator of metabolic function
The clinical consequences of obesity, insulin resistance, and diabetes account for the majority of morbidity and mortality in industrialized countries, largely caused by atherosclerosis, leading to coronary artery disease and myocardial infarction, stroke, and peripheral vascular disease [1]. Both atherosclerotic vascular disease and obesity-associated metabolic dysfunction, such as in insulin resistance and diabetes, share a strong inflammatory component, contributing to disease propagation and representing a prognostic marker [1]. The same holds true for obesity, where abnormal increases in fat mass and adipocytes cause metabolic dysfunction, which is partly driven by and perpetuated by inflammation, thereby contributing to organ injury and propelling chronic disease processes such as atherosclerosis and glomerulosclerosis [1-3].
Endogenous estrogens in premenopausal women largely prevent the development of the above pathologies, partly through beneficial effects on blood pressure, lipid metabolism, and glucose homeostasis, and by inhibiting inflammation [2, 4]. However, with the loss of estrogen production after menopause, its beneficial effects prior to menopause are evident as postmenopausal women become prone to the development of obesity, insulin resistance, diabetes, and arterial hypertension, which collectively accelerate the development of atherosclerotic vascular disease [4]. Moreover, the postmenopausal state is characterized by generalized inflammatory activation. Estrogen effects can be divided into chronic (“genomic”) and acute (“non-genomic”) effects that are mediated by specific estrogen receptors [2, 4]. Genomic effects of estrogens such as 17β-estradiol (E2) are largely mediated by the nuclear receptors estrogen receptor alpha and beta (ERα and ERβ) that function as ligand-dependent transcription factors regulating expression of target genes [4]. We [4] and others [5, 6] have recently reviewed the metabolic actions of estrogens in the context of nuclear ER signaling and the related sex differences. Non-genomic effects of estrogens, which include rapid vascular effects as well as signaling in the central nervous system (CNS) [7], are mediated by membrane subpopulations of ERα and ERβ, as well as GPER, a recently identified intracellular transmembrane G protein-coupled receptor [8]. GPER was cloned from multiple sources including lymphocytes, breast cancer cells, and shear stress-exposed endothelial cells [9], which early on suggested possible functions for GPER in cancer, immune and vascular function, which were later confirmed in numerous studies [10].
An intracellular G protein-coupled membrane estrogen receptor
GPER was originally identified through cloning approaches and homology analysis as a 7-transmembrane spanning GPCR in the mid-1990’s [9] [11]. As no ligand could be identified in early studies, it was termed GPR30, an orphan receptor designation. Only in 2007, after several laboratories had independently demonstrated estrogen binding to and function through GPR30 [12-14] did the International Union of Basic and Clinical Pharmacology (IUPHAR) officially designate GPR30 as GPER [9].
GPER cellular localization
GPCRs are almost exclusively depicted as functioning at the plasma membrane, a model consistent with the majority of GPCR ligands that, unlike E2, are not readily membrane permeable [8]. In contrast to early representations [15, 16], initial studies of GPER suggested that the majority of GPER was localized to intracellular membranes such as the endoplasmic reticulum [13]. Although the localization of GPER remained controversial [12, 17, 18], many subsequent studies also observed intracellular localization [19-21], as well as nuclear localization [22, 23]. Studies demonstrating that only cell-permeable E2 derivatives [24] or intracellularly injected ligands [25] can lead to rapid GPER activation, supported the intracellular localization and functionality of GPER. More recent studies reveal that GPER trafficking is complex, with cell surface receptor undergoing constitutive clathrin-mediated internalization to intracellular membranes [26], likely accounting for lack of detectable GPER at the cell surface of many cells. However, regulation of GPER trafficking in varied cell types could lead to complex patterns of differential subcellular expression [26].
GPER ligand selectivity
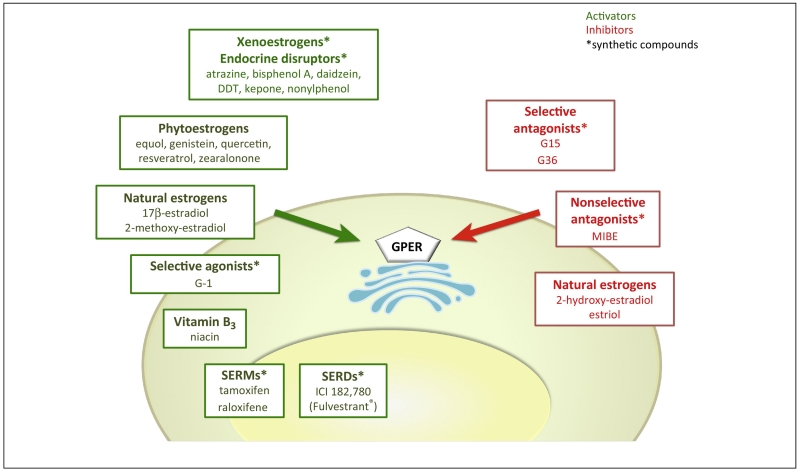
GPER contains specific binding sites for natural ligands such as E2 as well as for synthetic ligands [27]. Binding affinities of E2 for GPER have been reported in the 3-6 nM range [12, 13]; this compares to values for ERα/β in the 0.1-0.5 nM range [14], which suggests GPER is perhaps activated under conditions of higher local or systemic E2 concentrations. E2 binding to GPER exhibits high selectivity over other steroids including testosterone, cortisol, and progesterone [12], and despite studies that suggest a role for GPER expression in specific rapid cellular actions of aldosterone [28], binding of aldosterone could not be detected in GPER-containing membranes that displayed E2 binding activity [26]. Rather, it has been found recently that E2 - via ERα - inhibits mineralocorticoid receptor (MR) function [29]. Natural estrogen metabolites formed by catchol-O-methyltransferase, have recently been shown to bind to and signal through GPER, either as an agonist (2-methoxyestradiol, 2-ME2) [30], or antagonist (2-hydroxy estradiol (2-OH-E2) – a hydroxylated metabolite of E2) [31] (Figure 1). Furthermore, a large number of synthetic and natural estrogenic compounds have been shown to interact with GPER (Figure 1), including the therapeutic anti-estrogens ICI182,780 (a selective ER downregulator, SERD) and 4-hydroxytamoxifen and raloxifene (selective ER modulators, SERMs), which surprisingly all act as GPER agonists [13, 32, 33], and synthetic compounds such as MIBE (ethyl 3-[5-(2-ethoxycarbonyl-1-methylvinyloxy)-1-methyl-1H-indol-3-yl]but-2-enoate)) [34]. Numerous synthetic compounds (e.g. pesticides and plastic precursors) that act as endocrine disruptors with metabolic and reproductive effects [35], including atrazine, bisphenol A, daidzein, zearalonone, nonylphenol, kepone, p,p’-DDT, o,p’-DDE and 2,2’,5′,-PCB-4-OH as well as numerous phytoestrogens, including genistein, quercetin, equol, resveratrol, oleuropein and hydroxytyrosol, have also been reported to bind and/or activate GPER (recently reviewed in [9], Figure 1). With this complex pattern of overlapping ligand specificity between GPER and ERs, the identification of highly GPER-selective ligands, both agonists (G-1 [14]) and antagonists (G15 [36] and G36 [37]) (Figure 1) as well as GPER-selective radioimaging agents [38-41], all of which display no significant binding or function towards either ERα or ERβ has opened the door to pharmacological studies of GPER in cells, tissues, and animals [10]. Molecular modeling and ligand docking simulations have been employed in an attempt to identify possible binding sites on GPER for E2, selective synthetic GPER ligands and therapeutic ligands such as SERMs and SERDs [27, 42].
Figure 1. Agonists and antagonists with intrinsic activity mediating or inhibiting GPER signaling.
Agonists and antagonists of GPER with intrinsic activity mediating or inhibiting GPER-mediated signaling. Green arrows: activation; red arrows: inhibition; Receptors are activated by natural (endogenous estrogen such as 17β-estradiol, 2-methoxyestradiol) as well as by phytoestrogens (such as equol, quercetin, genistein, zearalonone, resveratrol), highly stable environmental pollutants, also known as xenoestrogens (including atrazine, zearalonone, bisphenol A, nonylphenol, or DDT), SERMS, SERDs, or GPER-selective synthetic drugs. The synthetic GPER-selective compounds G15 and G36, the non-selective MIBE, and the estrogen metabolites 2-hydroxyestradiol (2 OH-E2) and estriol act as GPER antagonists. See text for explanation. * denotes synthetic compounds as opposed to natural substances; DDT, dichlorodiphenyltrichloroethane; SERM, selective estrogen receptor modulator; SERD, selective estrogen receptor downregulator. MIBE, ethyl 3-[5-(2-ethoxycarbonyl-1-methylvinyloxy)-1-methyl-1H-indol-3-yl]but-2-enoate).
GPER signaling
GPER, as other GPCRs, couples to heterotrimeric G proteins that in turn regulate a plethora of downstream cellular effectors [43]. GPER coupling to both Gi/o and Gs proteins has been reported [12, 15, 33, 44]. Downstream pathways include the Src-mediated activation of metalloproteinases (MMP), with release of heparin-binding epidermal growth factor (EGF), followed by transactivation of the EGF receptor (EGFR), which in turn leads to activation of extracellular signal-regulated kinases 1/2 (ERK1/2) and phosphoinositide 3-kinase (PI3K) [13, 33, 45]. GPER also activates endothelial nitric oxide synthase (eNOS) to produce nitric oxide (NO) in endothelial cells [44, 46] and regulates calcium mobilization [13, 47, 48] and potassium channels [49]. Finally, as a result of these and other rapid signaling events, GPER modulates gene expression (distinct from that of ERα and ERβ) [50, 51]. Just how the actions of classical ERs and GPER interact to bring about cellular effects remains to be fully explored, as the multiple receptors may act independently, synergize with each other, or alternatively antagonize the other receptor’s activity [52, 53]. Importantly, functional cross-talk between GPER and nuclear receptors has not only been described for ERα, but also for other nuclear steroid receptors such as the vitamin D receptor (VDR), the glucocorticoid receptor (GR) and the MR [52], whereas no binding of aldosterone [26] or other steroids [12] to GPER has been demonstrated. Under these complex conditions, with the ultimate cellular output being dependent upon the integration of multiple pathways, the specific role(s) of individual receptors is a challenge to ascertain. Not surprisingly, these diverse GPER-regulated pathways impinge upon numerous varied cellular responses ranging from growth to migration to secretion to metabolism, which in turn suggest roles for GPER in multiple aspects of normal physiology and disease. The complexity and open questions regarding GPER function have been discussed in detail elsewhere [52, 54].
Models and phenotypes of GPER deficiency
Given the expansive functions of E2 in both sexes discussed above, ascertaining the distinct contributions of individual ERs requires either their specific genetic or pharmacological manipulation. The use of transgenic mice has been highly informative in the dissection of the roles of ERα and ERβ [55]. As GPER expression has been detected in most organs and tissues throughout the body [52, 56], yet with cell type specificity [26], GPER likely plays important roles in mediating many estrogen-associated aspects of physiology and disease. A phenotype of GPER0 mice was first reported in 2008 [57], with the initial identification of GPER playing a role in E2-induced thymic atrophy; GPER0 mice also develop visceral obesity [47, 58-61]. Genetic ablation of GPER reduces anxiety and alters stress in a sex-dependent manner [62], decreases mammary tumor size and metastasis [63], and modulates immune responses consistent with an anti-inflammatory role for GPER [60, 64]. Unlike mice lacking ERα [55], GPER0 mice are fertile [56], consistent with studies showing GPER exhibits limited activity in uterine and other reproductive functions [36, 65]. This has led to questions regarding the role of GPER as an estrogen receptor [66, 67]. Three additional GPER0 mouse models have also been reported [65, 68, 69] and their genetic and initial basic phenotypic properties have been reviewed [56, 67]. Although discrepancies exist between reports of the phenotypes of these different knockout mice, a number of themes (particularly in the areas of endocrine, metabolic and cardiovascular functions) have emerged in recent years, with apparent discrepancies potentially explained in part by complex age- and sex-dependent effects, or differences in genetic backgrounds in the various studies [52]. Finally, statements questioning the role of GPER as an estrogen receptor based largely on the lack of similarities between GPER and ERα knockout mouse phenotypes would seem imprudent, given the breadth of E2 effects throughout the body.
GPER and obesity
Both male and female GPER0 mice are obese with substantial increases in visceral, subcutaneous, and perivascular fat [47, 58-61]. Following ovariectomy, GPER0 mice also exhibited a reduced response to E2 in terms of reductions in weight and adipocyte size, and no improvement in glucose tolerance with E2 supplementation [58]. While GPER0 mice show a decrease in energy expenditure, suggesting alterations in energy metabolism [58, 60], recent data also indicate that hypothalamic signaling through GPER is involved in the anorectic response mediated by estrogen [70]. There is also evidence supporting a role for GPER in adipogenesis [71]. Consistent with the obesity phenotype observed in mice lacking GPER [47, 60, 61], Zhu et al. recently reported that the known E2-mediated inhibitory effects on adipogenesis in vitro involve GPER-dependent mechanisms, as effects were mimicked by the selective GPER agonist, G-1, and blocked by GPER gene silencing [71]. Furthermore, the GPER-selective agonist G-1 attenuates fatty acid synthesis and triglyceride accumulation in human and rodent pancreatic islets and β-cells [72].
GPER and glucose homeostasis
Estrogen and its receptors play critical roles in glucose homeostasis, the result of glucose-induced insulin production by the pancreas and peripheral insulin responsiveness, including metabolism and storage in multiple peripheral tissues (particularly skeletal muscle, adipose and liver) [73-75]. In the pancreas, E2 exhibits several important effects, including promoting insulin secretion [76, 77] and synthesis [74, 78], enhancing β-cell survival [5, 6], increasing islet oxygenation during transplantation and preventing lipotoxicity [72]. The roles of ERα and to a lesser extent ERβ have been extensively investigated and reviewed elsewhere with respect to metabolic and specifically glucose regulation [5, 73, 75], including functions in the CNS [79]. Studies in GPER-deficient mice revealed that ovary-intact female GPER0 mice become glucose intolerant at six months of age, whereas male mice initially appeared unaffected [69]. However, subsequent studies revealed that male GPER0 mice are in fact insulin resistant at six months of age and only become glucose intolerant with increased age [60]. Estrogen receptor-linked, age-dependent phenotypes have also been reported in male ERβ-deficient mice [80]. Pancreatic islets from GPER0 mice are also unresponsive to E2 in terms of insulin secretion [69, 81], and are more sensitive to apoptotic stimuli [82]. Female GPER0 mice also appear to be less sensitive to modulators of food intake such as cholecystokinin (CCK) and leptin, possibly due to impaired hypothalamic E2-dependent ERK phosphorylation [58]. A very recent study has identified GPER as mediating hypothalamic signaling involved in the anorectic eating behavior mediated by estrogen [70].
Under conditions of peripheral insulin resistance (prediabetes), the pancreas is called upon to secrete ever increasing amounts of insulin in an attempt to mitigate the effects of hyperglycemic toxicity. This ultimately leads to β-cell “exhaustion” and apoptosis, leading to a decrease in β-cell mass. To examine whether the observed elevated plasma glucose levels and glucose intolerance in female [69] and older male GPER0 mice [60] could be a result of diminished insulin secretion in GPER0 mice, isolated pancreatic islets were studied in vitro. Whereas high glucose-stimulated insulin secretion was minimally reduced in male GPER0 mice [60, 69], effects in female mice were greater, with moderately reduced insulin mRNA and protein levels in islets also observed in females [69]. Similar results were observed for the decrease in high glucose-stimulated glucagon secretion, although islet glucagon mRNA was unaltered in GPER0 mouse islets [69]. Furthermore, following ovariectomy, E2 treatment failed to increase serum insulin levels in GPER0 mice as it did in wild type mice [69]. To evaluate possible direct effects of GPER on hormone secretion by islets, the effects of E2 stimulation were determined in wild type and GPER0 mouse islets. Whereas E2 stimulated insulin and inhibited glucagon secretion in islets from both male and female wild type mice, these effects were completely absent in islets from GPER0 mice [60, 69, 81]. Furthermore, the GPER-selective agonist G-1 stimulated insulin secretion from islets, similar to that of E2, whereas the GPER-selective antagonist G15 inhibited insulin secretion by both E2 and G-1 [81]. Together, these results demonstrate that GPER plays a critical role in glucose-mediated insulin secretion in female mice and in estrogen-mediated islet secretory function in both female and male mice.
Maintaining functional β-cell mass is a major goal in patients with type 2 diabetes. The lower incidence of diabetes in premenopausal women is consistent with the anti-diabetic functions of estrogen in both humans and rodents [4, 83]. A comparison of mice deficient in ERα, ERβ, or GPER in a streptozotocin-induced type 1 diabetes model revealed that both ERα and GPER mediate protective roles, with that of ERβ being through non-genomic actions [82]. Importantly, in the absence of both ERα and ERβ, E2 partially restored protection in ovariectomized streptozotocin-treated mice, suggesting an alternative mechanism for the actions of E2. Along these lines, female type 1 diabetic mice lacking GPER lost the protective effects of endogenous E2. Furthermore, the GPER-selective agonist G-1 protected from ROS-induced apoptosis in both murine and human islets as effectively as E2 [82]. Although the protective effect of G-1 was absent in islets isolated from GPER0 mice, the effects of E2 remained, suggesting the ERα can compensate for the absence of GPER, unlike the requirement for GPER in insulin secretion [82].
Together, data available to date reveal a complex interplay between estrogen receptors in the E2-mediated protection of β-cells from apoptosis [6]. Consistent with these described survival effects, G-1 as well as ERα- and ERβ-selective estrogenic ligands improved human islet-graft survival in a murine insulin-deficient xenotransplantation model; however, whereas all three ligands improved islet survival, only the ERα- and ERβ-selective ligands improved islet revascularization [84]. Recent results also demonstrate the regulation of GPER expression by insulin [85] and insulin-like growth factor-I [86], as well as the GPER-mediated down regulation of miR-338-3p microRNA during pregnancy, enhancing both proliferation and survival under pro-apoptotic conditions [87], providing additional possible links between GPER, β-cell survival and glucose metabolism.
GPER in lipid metabolism, atherosclerosis, and inflammation
Inflammation has been identified as a unifying theme of obesity, diabetes, dyslipidemia, and atherosclerosis, and inhibition of inflammation is currently pursued as a means to induce plaque stabilization [1, 2]. Dyslipidemia, i.e. high levels of LDL cholesterol and triglycerides, is a major risk factor for the development of coronary atherosclerosis and is typically found in patients with obesity, insulin resistance, or diabetes, translating into an increased cardiovascular risk [1, 2]. Decreased HDL cholesterol and increased triglyceride levels [60] are observed in female GPER0 mice (GPER-lacZ reporter with a partial deletion of the GPER coding sequence) in association with hepatic steatosis [88, 89]. In female mice under atherogenic conditions, deletion of GPER increases total and LDL cholesterol levels [46]. Moreover, aged (but not young) male GPER0 mice exhibit elevated LDL and triglyceride levels [60].
Activation of GPER inhibits proliferation of human vascular smooth muscle cells in vitro [47, 90], one of the key events in early atherogenesis [2]. While part of the estrogen-mediated protection from atherosclerosis in females is mediated by ERα [91], subsequent studies by Villablanca and cowokers demonstrated estrogen-mediated inhibition of athersclerosis in mice deficient in ERα [92]. This indicated the involvement of additional estrogen signaling pathways. Using a model of diet-induced atherosclerosis, it was recently reported that ablation of GPER increases atherosclerotic plaque formation, both in ovary-intact and surgically postmenopausal mice, and that GPER deficiency is associated with increased levels of plasma LDL cholesterol [46]. These data are the first in vivo indication that GPER plays an essential role in vascular disease, the most prevalent health condition in postmenopausal women. Regulation of LDL cholesterol through GPER has also been demonstrated by a recent report where carriers of a GPER polymorphism showed higher circulating levels of plasma LDL cholesterol [93]; furthermore, experiments performed in hepatocytes in vitro demonstrated an inhibitory role for GPER in the regulation of LDL receptor expression [93]. In mice with overt atherosclerosis, it was also observed that the aggravating effect of GPER deficiency on disease progression was associated with pronounced vascular inflammation, involving macrophage and T-cell-dependent mechanisms [46]. Treatment with the highly selective, orally active small molecule agonist of GPER, G-1, was effective in reducing atherosclerosis in postmenopausal mice, and was associated with a pronounced reduction of vascular inflammation [46].
The anti-inflammatory effect of GPER activation in the vasculature in vivo is also supported by studies in endothelial cells and macrophages in vitro, demonstrating potent anti-inflammatory effects of drugs acting as GPER agonists [94, 95]. As GPER was also cloned from a human B cell lymphoblast cell line cDNA library [9], and is expressed and functional in white blood cells [96] and macrophages [11, 97], immunomodulating functions of this receptor may be expected. Indeed, studies in models of multiple sclerosis showed that GPER deficiency exacerbated inflammation, and that treatment with the of GPER agonist, G-1, was effective in reducing inflammation [64, 98]. Investigation of the molecular mechanisms underlying the immunomodulating effect of GPER activation identified a prominent role for the forkhead box P3 (FOXOp3) protein pathway in promoting regulatory T cell differentiation and IL-10 secretion [99, 100]. In agreement with these observations indicating inhibitory function of GPER on immune responses, genetic ablation of GPER in aged male mice is associated with a pro-inflammatory state [60].
Enhanced vasoconstriction, just as inflammation, is a hallmark of metabolic diseases such as diabetes or insulin resistance and can also be observed in obese humans [1]. The enhanced vasoconstriction is due to breakdown / inactivation of the vasodilator NO and /or enhanced activity of vasoconstrictor prostanoids and vasoconstrictors such as endothelin-1. [1] The fact that GPER was also cloned from shear-stress exposed endothelial cells [9] suggested a possible vascular role for this receptor. Indeed, its vascular functions are among the best described with numerous mechanisms through which GPER contributes to vascular homeostasis having been identified [101]. The acute estrogen-dependent vasodilator effects mediated by ERα, ERβ and GPER vary markedly between vascular beds [102].
While GPER has been implicated in acute vasodilation and release of nitric oxide [101], its absence or inhibition is associated with enhanced vasoconstriction [61, 103]. Increased vasoconstriction in mice lacking GPER or in arteries treated with a GPER antagonist is brought about in part by enhanced release of endothelium-derived contracting factor (EDCF), mediated by cyclooxygenase-derived prostanoids [103].
Moreover, GPER0 mice show increased activities of the vasoconstrictor endothelin-1 [48] and the recently identified adipose-derived contracting factor (ADCF) [61], and male and female GPER0 mice exhibit excessive formation of perivascular adipose [61]. ADCF released from perivascular adipose contributes to enhanced vasoconstriction in GPER0 mice, an effect that can be prevented by expressing GPER, by ablation of perivascular adipose, or by inhibiting cyclooxygenase enzymes [61]. Thus, GPER is an important endogenous inhibitor of vasoconstriction in the setting of visceral obesity and atherosclerosis, the development of which has been recently linked to the presence of perivascular fat [104].
Given that its activation improves the lipid profile, inhibits inflammation and atherosclerosis, and improves vasodilation, GPER represents a promising therapeutic target for the treatment of coronary artery disease and its risk factors. Furthermore, postmenopausal women may also be eligible for GPER-targeting therapy to provide vascular protection given that selective GPER agonists (unlike natural or equine estrogens [2, 105]) are devoid of uterotrophic effects [10, 36].
Concluding remarks and future perspectives
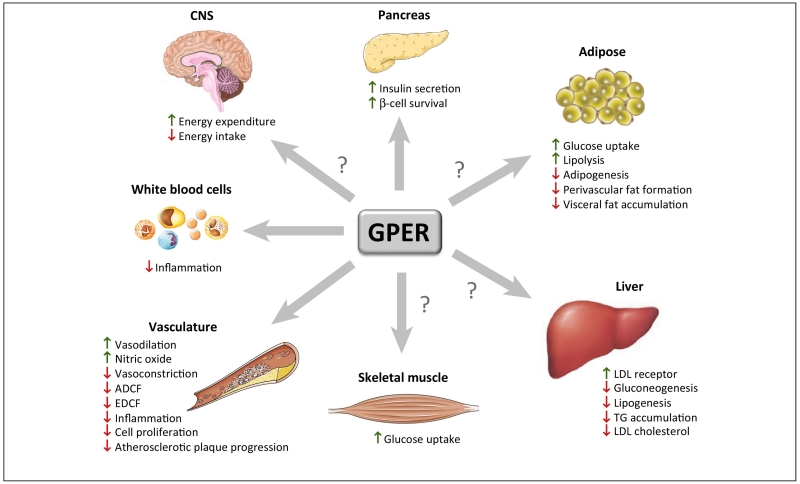
Since the discovery of GPER, research has helped to identify multiple physiological functions and roles in many organs, and we are now beginning to understand its role in disease. There is now good evidence that GPER plays an essential role in metabolic regulation (including lipid and glucose homeostasis, as well as insulin production and action), arterial tone, blood pressure, and immune functions (Figure 2), factors that are all linked to the risk of developing coronary artery disease due to atherosclerosis [1]. Interestingly, widely used drugs such as SERMs and niacin may exert their lipid-lowering effects in part through activation of GPER [8, 52, 106, 107]. There is also data to support a vasoprotective role of GPER in the brain [108-110]. The precise mechanisms of how GPER is involved in this regulation have only been identified in part, and some of the findings are particularly intriguing [52, 54]. For example, although data clearly show that GPER mediates multiple activities of estrogen in vivo (i.e. many physiological effects of estrogen are lost in GPER0 mice), there is accumulating evidence that GPER also has effects in males, in some instances independently of sex steroids (reviewed in [8-10, 52]), and that certain GPER-dependent phenotypes only become apparent with age [60]. Similar observations have been made with regard to physiological functions of ERβ in aged male mice [80]. In addition to its anti-diabetic effects, the vasculotropic expression profile and the numerous vasoprotective functions of GPER (Figure 2) suggest therapeutic potential in the treatment of atherosclerotic vascular disease, the main cause of myocardial infarction and stroke. The currently available data suggest a strong anti-inflammatory component contributes to the activity of GPER in the vascular wall, in endothelial cells, in the pancreas, as well as potentially in adipose tissue, all of which can contribute to the vasoprotective effects of GPER [8, 10]. Continuing research efforts should be directed towards further identifying the precise mechanisms underlying the vasoprotective and metabolic effects of GPER. Development of GPER-selective drugs, such as G-1, which unlike natural estrogen or conjugated equine estrogens, lacks uterotrophic activity [46], may lead to therapeutic applications in other non-communicable chronic diseases driven by chronic inflammation. Finally, targeting GPER may also allow its theranostic use [38-41] and thus offers new approaches for personalized medicine.
Figure 2. Roles of GPER in physiology and disease.
Metabolic, vascular, immunological, physiological, and pathophysiological functions of GPER in the brain, pancreas, adipose tissue, arterial vasculature, skeletal muscle, liver, and white blood cells. ? indicates functions attributed to estrogen activity, where the precise role or contribution of GPER is unknown or requires further study. Abbreviations used: ADCF, adipose-derived contracting factor; CNS, central nervous system; EDCF, endothelium-derived contracting factor; LDL, low-density lipoprotein; TG, triglycerides; VSMC, vascular smooth muscle cells.
HIGHLIGHTS.
The 7-transmembrane G protein-coupled estrogen receptor (GPER) contributes to vascular tone and blood pressure.
GPER deletion augments endothelium-dependent vasoconstriction and the activity of vasoactive peptides.
Mice lacking GPER develop visceral obesity, increased LDL cholesterol levels, a prodiabetic metabolic profile and generalized inflammation.
GPER regulates pancreatic hormone secretion (including insulin) and modulates insulin responsiveness of peripheral tissues.
Loss of GPER accelerates atherogenesis and vascular inflammation in an estrogen-dependent fashion. Both pathologies can be alleviated using GPER-targeting drugs.
Acknowledgment
The authors apologize to those investigators whose work could not be cited due to space limitations.
Funding
Original work by the authors has supported by Swiss National Science Foundation grants 108 258 and 122 504 (M.B.) and NIH R01 grants CA116662, CA118743, CA127731, and CA163890 (E.R.P.)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure
ERP is an inventor on patents for GPER-targeted ligands and imaging agents owned by the University of New Mexico.
References
- 1.Barton M. Obesity and aging: determinants of endothelial cell dysfunction and atherosclerosis. Pflügers Arch. 2010;460:825–837. doi: 10.1007/s00424-010-0860-y. [DOI] [PubMed] [Google Scholar]
- 2.Barton M. Cholesterol and atherosclerosis: modulation by oestrogen. Curr Opin Lipidol. 2013;24:214–220. doi: 10.1097/MOL.0b013e3283613a94. [DOI] [PubMed] [Google Scholar]
- 3.Xu H, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Meyer MR, et al. Obesity, insulin resistance and diabetes: sex differences and role of oestrogen receptors. Acta Physiol (Oxf) 2011;203:259–269. doi: 10.1111/j.1748-1716.2010.02237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mauvais-Jarvis F, et al. The role of estrogens in control of energy balance and glucose homeostasis. Endocr Rev. 2013;34:309–338. doi: 10.1210/er.2012-1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tiano JP, Mauvais-Jarvis F. Importance of oestrogen receptors to preserve functional beta-cell mass in diabetes. Nat Rev Endocrinol. 2012;8:342–351. doi: 10.1038/nrendo.2011.242. [DOI] [PubMed] [Google Scholar]
- 7.Tang H, et al. GPR30 mediates estrogen rapid signaling and neuroprotection. Mol Cell Endocrinol. 2014;387:52–58. doi: 10.1016/j.mce.2014.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Prossnitz ER, Barton M. The G-protein-coupled estrogen receptor GPER in health and disease. Nat Rev Endocrinol. 2011;7:715–726. doi: 10.1038/nrendo.2011.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prossnitz ER, Arterburn JB. International Union of Basic and Clinical Pharmacology. XCVII. G protein-coupled estrogen receptor (GPER) and its pharmacologic modulators. Pharmacol Rev. 2015;67:505–540. doi: 10.1124/pr.114.009712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Prossnitz ER, Barton M. Estrogen biology: new insights into GPER function and clinical opportunities. Mol Cell Endocrinol. 2014;389:71–83. doi: 10.1016/j.mce.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Owman C, et al. Cloning of human cDNA encoding a novel heptahelix receptor expressed in Burkitt’s lymphoma and widely distributed in brain and peripheral tissues. Biochem Biophys Res Commun. 1996;228:285–292. doi: 10.1006/bbrc.1996.1654. [DOI] [PubMed] [Google Scholar]
- 12.Thomas P, et al. Identity of an estrogen membrane receptor coupled to a G protein in human breast cancer cells. Endocrinology. 2005;146:624–632. doi: 10.1210/en.2004-1064. [DOI] [PubMed] [Google Scholar]
- 13.Revankar CM, et al. A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science. 2005;307:1625–1630. doi: 10.1126/science.1106943. [DOI] [PubMed] [Google Scholar]
- 14.Bologa CG, et al. Virtual and biomolecular screening converge on a selective agonist for GPR30. Nat Chem Biol. 2006;2:207–212. doi: 10.1038/nchembio775. [DOI] [PubMed] [Google Scholar]
- 15.Filardo EJ, et al. Estrogen action via the G protein-coupled receptor, GPR30: stimulation of adenylyl cyclase and cAMP-mediated attenuation of the epidermal growth factor receptor-to-MAPK signaling axis. Mol Endocrinol. 2002;16:70–84. doi: 10.1210/mend.16.1.0758. [DOI] [PubMed] [Google Scholar]
- 16.Filardo EJ. Epidermal growth factor receptor (EGFR) transactivation by estrogen via the G-protein-coupled receptor, GPR30: a novel signaling pathway with potential significance for breast cancer. J Steroid Biochem Mol Biol. 2002;80:231–238. doi: 10.1016/s0960-0760(01)00190-x. [DOI] [PubMed] [Google Scholar]
- 17.Funakoshi T, et al. G protein-coupled receptor 30 is an estrogen receptor in the plasma membrane. Biochem Biophys Res Commun. 2006;346:904–910. doi: 10.1016/j.bbrc.2006.05.191. [DOI] [PubMed] [Google Scholar]
- 18.Filardo E, et al. Activation of the novel estrogen receptor, GPR30, at the plasma membrane. Endocrinology. 2007;148:3236–3245. doi: 10.1210/en.2006-1605. [DOI] [PubMed] [Google Scholar]
- 19.Otto C, et al. GPR30 localizes to the endoplasmic reticulum and is not activated by estradiol. Endocrinology. 2008;149:4846–4856. doi: 10.1210/en.2008-0269. [DOI] [PubMed] [Google Scholar]
- 20.Sakamoto H, et al. Expression of G protein-coupled receptor-30, a G protein-coupled membrane estrogen receptor, in oxytocin neurons of the rat paraventricular and supraoptic nuclei. Endocrinology. 2007;148:5842–5850. doi: 10.1210/en.2007-0436. [DOI] [PubMed] [Google Scholar]
- 21.Matsuda K, et al. Expression and intracellular distribution of the G protein-coupled receptor 30 in rat hippocampal formation. Neuroscience Letters. 2008;441:94–99. doi: 10.1016/j.neulet.2008.05.108. [DOI] [PubMed] [Google Scholar]
- 22.Pupo M, et al. The nuclear localization signal is required for nuclear GPER translocation and function in breast Cancer-Associated Fibroblasts (CAFs) Mol Cell Endocrinol. 2013;376:23–32. doi: 10.1016/j.mce.2013.05.023. [DOI] [PubMed] [Google Scholar]
- 23.Smith HO, et al. GPR30 predicts poor survival for ovarian cancer. Gynecol Oncol. 2009;114:465–471. doi: 10.1016/j.ygyno.2009.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Revankar CM, et al. Synthetic estrogen derivatives demonstrate the functionality of intracellular GPR30. ACS Chem Biol. 2007;2:536–544. doi: 10.1021/cb700072n. [DOI] [PubMed] [Google Scholar]
- 25.Deliu E, et al. Mechanisms of G protein-coupled estrogen receptor-mediated spinal nociception. J Pain. 2012;13:742–754. doi: 10.1016/j.jpain.2012.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cheng SB, et al. Anatomical location and redistribution of G protein-coupled estrogen receptor-1 during the estrus cycle in mouse kidney and specific binding to estrogens but not aldosterone. Mol Cell Endocrinol. 2014;382:950–959. doi: 10.1016/j.mce.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 27.Mendez-Luna D, et al. Deciphering the GPER/GPR30-agonist and antagonists interactions using molecular modeling studies, molecular dynamics, and docking simulations. J Biomol Struct Dyn. 2015:1–12. doi: 10.1080/07391102.2014.994102. [DOI] [PubMed] [Google Scholar]
- 28.Gros R, et al. GPR30 expression is required for the mineralocorticoid receptor-independent rapid vascular effects of aldosterone. Hypertension. 2011;57:442–451. doi: 10.1161/HYPERTENSIONAHA.110.161653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barrett Mueller K, et al. Estrogen receptor inhibits mineralocorticoid receptor transcriptional regulatory function. Endocrinology. 2014;155:4461–4472. doi: 10.1210/en.2014-1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koganti S, et al. 2-methoxyestradiol binding of GPR30 down-regulates angiotensin AT(1) receptor. Eur J Pharmacol. 2014;723:131–140. doi: 10.1016/j.ejphar.2013.10.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chourasia TK, et al. The catecholestrogen, 2-hydroxyestradiol-17beta, acts as a G protein-coupled estrogen receptor 1 (GPER/GPR30) antagonist to promote the resumption of meiosis in zebrafish oocytes. Biol Reprod. 2015 doi: 10.1095/biolreprod.114.125674. [DOI] [PubMed] [Google Scholar]
- 32.Petrie WK, et al. G protein-coupled estrogen receptor-selective ligands modulate endometrial tumor growth. Obstet Gynecol Int. 2013;2013:472720. doi: 10.1155/2013/472720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Filardo EJ, et al. Estrogen-induced activation of Erk-1 and Erk-2 requires the G protein-coupled receptor homolog, GPR30, and occurs via trans-activation of the epidermal growth factor receptor through release of HB-EGF. Mol Endocrinol. 2000;14:1649–1660. doi: 10.1210/mend.14.10.0532. [DOI] [PubMed] [Google Scholar]
- 34.Lappano R, et al. MIBE acts as antagonist ligand of both estrogen receptor alpha and GPER in breast cancer cells. Breast Cancer Res. 2012;14:R12. doi: 10.1186/bcr3096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yoon K, et al. Estrogenic endocrine-disrupting chemicals: molecular mechanisms of actions on putative human diseases. J Toxicol Environ Health B Crit Rev. 2014;17:127–174. doi: 10.1080/10937404.2014.882194. [DOI] [PubMed] [Google Scholar]
- 36.Dennis MK, et al. In vivo effects of a GPR30 antagonist. Nat Chem Biol. 2009;5:421–427. doi: 10.1038/nchembio.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dennis MK, et al. Identification of a GPER/GPR30 antagonist with improved estrogen receptor counterselectivity. J Steroid Biochem Mol Biol. 2011;127:358–366. doi: 10.1016/j.jsbmb.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Burai R, et al. Synthesis and characterization of tricarbonyl-Re/Tc(I) chelate probes targeting the G protein-coupled estrogen receptor GPER/GPR30. PLOS One. 2012;7:e46861. doi: 10.1371/journal.pone.0046861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ramesh C, et al. Synthesis and characterization of iodinated tetrahydroquinolines targeting the G protein-coupled estrogen receptor GPR30. J Med Chem. 2010;53:1004–1014. doi: 10.1021/jm9011802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nayak TK, et al. Influence of charge on cell permeability and tumor imaging of GPR30-targeted 111In-labeled nonsteroidal imaging agents. ACS Chem Biol. 2010;5:681–690. doi: 10.1021/cb1000636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nayak TK, et al. GPER-targeted, 99mTc-labeled, nonsteroidal ligands demonstrate selective tumor imaging and in vivo estrogen binding. Mol Cancer Res. 2014;12:1636–1643. doi: 10.1158/1541-7786.MCR-14-0289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rosano C, et al. Recent advances in the rationale design of GPER ligands. Curr Med Chem. 2012;19:6199–6206. [PubMed] [Google Scholar]
- 43.Goupil E, et al. Functional selectivity in GPCR signaling: understanding the full spectrum of receptor conformations. Mini Rev Med Chem. 2012;12:817–830. doi: 10.2174/138955712800959143. [DOI] [PubMed] [Google Scholar]
- 44.Lindsey SH, et al. Vasodilation by GPER in mesenteric arteries involves both endothelial nitric oxide and smooth muscle cAMP signaling. Steroids. 2013;81:99–102. doi: 10.1016/j.steroids.2013.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Scaling AL, et al. GPER mediates estrogen-induced signaling and proliferation in human breast epithelial cells and normal and malignant breast. Horm Cancer. 2014;5:146–160. doi: 10.1007/s12672-014-0174-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Meyer MR, et al. G protein-coupled estrogen receptor protects from atherosclerosis. Sci Rep. 2014;4:7564. doi: 10.1038/srep07564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Haas E, et al. Regulatory role of G protein-coupled estrogen receptor for vascular function and obesity. Circ Res. 2009;104:288–291. doi: 10.1161/CIRCRESAHA.108.190892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Meyer MR, et al. GPER regulates endothelin-dependent vascular tone and intracellular calcium. Life Sci. 2012;91:623–627. doi: 10.1016/j.lfs.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dong WH, et al. Resveratrol inhibits K(v)2.2 currents through the estrogen receptor GPR30-mediated PKC pathway. Am J Physiol Cell Physiol. 2013;305:C547–557. doi: 10.1152/ajpcell.00146.2013. [DOI] [PubMed] [Google Scholar]
- 50.Albanito L, et al. G protein-coupled receptor 30 and estrogen receptor are involved in the proliferative effects induced by atrazine in ovarian cancer cells. Environ Health Perspect. 2015 Jan 16; doi: 10.1289/ehp.11297. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 51.De Francesco EM, et al. GPER Mediates Activation of HIF1alpha/VEGF Signaling by Estrogens. Cancer Res. 2014 doi: 10.1158/0008-5472.CAN-13-3590. [DOI] [PubMed] [Google Scholar]
- 52.Barton M. Position paper: the membrane estrogen receptor GPER - clues and questions. Steroids. 2012;77:935–942. doi: 10.1016/j.steroids.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 53.Prossnitz ER, et al. Estrogen action via the transmembrane receptor GPR30. Annu Rev Physiol. 2008;70:165–190. doi: 10.1146/annurev.physiol.70.113006.100518. [DOI] [PubMed] [Google Scholar]
- 54.Lathe R, et al. Steroid promiscuity. J Steroid Biochem Mol Biol. 2015 Jan 15; doi: 10.1016/j.jsbmb.2015.01.008. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 55.Hewitt SC, et al. Lessons in estrogen biology from knockout and transgenic animals. Annual Review of Physiology. 2005;67:285–308. doi: 10.1146/annurev.physiol.67.040403.115914. [DOI] [PubMed] [Google Scholar]
- 56.Olde B, Leeb-Lundberg LM. GPR30/GPER1: searching for a role in estrogen physiology. Trends Endocrinol Metab. 2009;20:409–416. doi: 10.1016/j.tem.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 57.Wang C, et al. GPR30 contributes to estrogen-induced thymic atrophy. Mol Endocrinol. 2008;22:636–648. doi: 10.1210/me.2007-0359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Davis KE, et al. Sexually dimorphic role of G protein-coupled estrogen receptor (GPER) in modulating energy homeostasis. Horm Behav. 2014;66:196–207. doi: 10.1016/j.yhbeh.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ford J, et al. GPR30 deficiency causes increased bone mass, mineralization, and growth plate proliferative activity in male mice. J Bone Miner Res. 2011;26:298–307. doi: 10.1002/jbmr.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sharma G, et al. GPER deficiency in male mice results in insulin resistance, dyslipidemia, and a proinflammatory state. Endocrinology. 2013;154:4136–4145. doi: 10.1210/en.2013-1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Meyer MR, et al. Regulation of vascular smooth muscle tone by adipose-derived contracting factor. PLoS One. 2013;8:e79245. doi: 10.1371/journal.pone.0079245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kastenberger I, Schwarzer C. GPER1 (GPR30) knockout mice display reduced anxiety and altered stress response in a sex and paradigm dependent manner. Horm Behav. 2014;66:628–636. doi: 10.1016/j.yhbeh.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Marjon NA, et al. G protein-coupled estrogen receptor (GPER) regulates mammary tumorigenesis and metastasis. Mol Cancer Res. 2014;12:1644–1154. doi: 10.1158/1541-7786.MCR-14-0128-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yates MA, et al. GPR30, but not estrogen receptor-alpha, is crucial in the treatment of experimental autoimmune encephalomyelitis by oral ethinyl estradiol. BMC Immunol. 2010;11:20. doi: 10.1186/1471-2172-11-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Otto C, et al. GPR30 does not mediate estrogenic responses in reproductive organs in mice. Biol Reprod. 2009;80:34–41. doi: 10.1095/biolreprod.108.071175. [DOI] [PubMed] [Google Scholar]
- 66.Levin ER. Minireview: extranuclear steroid receptors: roles in modulation of cell functions. Molecular Endocrinology. 2011;25:377–384. doi: 10.1210/me.2010-0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Langer G, et al. A critical review of fundamental controversies in the field of GPR30 research. Steroids. 2010;75:603–610. doi: 10.1016/j.steroids.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 68.Isensee J, et al. Expression pattern of G protein-coupled receptor 30 in LacZ reporter mice. Endocrinology. 2009;150:1722–1730. doi: 10.1210/en.2008-1488. [DOI] [PubMed] [Google Scholar]
- 69.Martensson UE, et al. Deletion of the G protein-coupled receptor 30 impairs glucose tolerance, reduces bone growth, increases blood pressure, and eliminates estradiol-stimulated insulin release in female mice. Endocrinology. 2009;150:687–698. doi: 10.1210/en.2008-0623. [DOI] [PubMed] [Google Scholar]
- 70.Kwon O, et al. GPR30 mediates anorectic estrogen-induced STAT3 signaling in the hypothalamus. Metabolism. 2014;63:1455–1461. doi: 10.1016/j.metabol.2014.07.015. [DOI] [PubMed] [Google Scholar]
- 71.Zhu P, et al. GPER mediates the inhibitory actions of estrogen on adipogenesis in 3T3-L1 cells through perturbation of mitotic clonal expansion. Gen Comp Endocrinol. 2013;193:19–26. doi: 10.1016/j.ygcen.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 72.Tiano JP, et al. Estrogen receptor activation reduces lipid synthesis in pancreatic islets and prevents beta cell failure in rodent models of type 2 diabetes. J Clin Invest. 2011;121:3331–3342. doi: 10.1172/JCI44564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Barros RP, Gustafsson JA. Estrogen receptors and the metabolic network. Cell Metab. 2011;14:289–299. doi: 10.1016/j.cmet.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 74.Nadal A, et al. The role of oestrogens in the adaptation of islets to insulin resistance. J Physiol. 2009;587:5031–5037. doi: 10.1113/jphysiol.2009.177188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Faulds MH, et al. The diversity of sex steroid action: regulation of metabolism by estrogen signaling. J Endocrinol. 2012;212:3–12. doi: 10.1530/JOE-11-0044. [DOI] [PubMed] [Google Scholar]
- 76.Nadal A, et al. The pancreatic beta-cell as a target of estrogens and xenoestrogens: Implications for blood glucose homeostasis and diabetes. Mol Cell Endocrinol. 2009;304:63–68. doi: 10.1016/j.mce.2009.02.016. [DOI] [PubMed] [Google Scholar]
- 77.Godsland IF. Oestrogens and insulin secretion. Diabetologia. 2005;48:2213–2220. doi: 10.1007/s00125-005-1930-0. [DOI] [PubMed] [Google Scholar]
- 78.Wong WP, et al. Extranuclear estrogen receptor-alpha stimulates NeuroD1 binding to the insulin promoter and favors insulin synthesis. Proc Natl Acad Sci U S A. 2010;107:13057–13062. doi: 10.1073/pnas.0914501107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Frank A, et al. The role of hypothalamic estrogen receptors in metabolic regulation. Front Neuroendocrinol. 2014;35:550–557. doi: 10.1016/j.yfrne.2014.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhu Y, et al. Abnormal vascular function and hypertension in mice deficient in estrogen receptor beta. Science. 2002;295:505–508. doi: 10.1126/science.1065250. [DOI] [PubMed] [Google Scholar]
- 81.Sharma G, Prossnitz ER. Mechanisms of estradiol-induced insulin secretion by the G protein-coupled estrogen receptor GPR30/GPER in pancreatic beta-cells. Endocrinology. 2011;152:3030–3039. doi: 10.1210/en.2011-0091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Liu S, et al. Importance of extranuclear estrogen receptor-alpha and membrane G protein-coupled estrogen receptor in pancreatic islet survival. Diabetes. 2009;58:2292–2302. doi: 10.2337/db09-0257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Louet JF, et al. Antidiabetic actions of estrogen: insight from human and genetic mouse models. Curr Atheroscler Rep. 2004;6:180–185. doi: 10.1007/s11883-004-0030-9. [DOI] [PubMed] [Google Scholar]
- 84.Liu S, et al. Oestrogens improve human pancreatic islet transplantation in a mouse model of insulin deficient diabetes. Diabetologia. 2013;56:370–381. doi: 10.1007/s00125-012-2764-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.De Marco P, et al. GPER1 is regulated by insulin in cancer cells and cancer-associated fibroblasts. Endocr Relat Cancer. 2014;21:739–753. doi: 10.1530/ERC-14-0245. [DOI] [PubMed] [Google Scholar]
- 86.De Marco P, et al. Insulin-like growth factor-I regulates GPER expression and function in cancer cells. Oncogene. 2013;32:678–688. doi: 10.1038/onc.2012.97. [DOI] [PubMed] [Google Scholar]
- 87.Jacovetti C, et al. MicroRNAs contribute to compensatory beta cell expansion during pregnancy and obesity. J Clin Invest. 2012;122:3541–3551. doi: 10.1172/JCI64151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Meoli L, et al. Sex- and age-dependent effects of GPR30 genetic deletion on the metabolic and cardiovascular profiles of diet-induced obese mice. Gene. 2014;540:210–216. doi: 10.1016/j.gene.2014.02.036. [DOI] [PubMed] [Google Scholar]
- 89.Delbeck M, et al. Impaired left-ventricular cardiac function in male GPR30-deficient mice. Mol Med Rep. 2011;4:37–40. doi: 10.3892/mmr.2010.402. [DOI] [PubMed] [Google Scholar]
- 90.Li F, et al. Activation of GPER induces differentiation and inhibition of coronary artery smooth muscle cell proliferation. PLoS One. 2013;8:e64771. doi: 10.1371/journal.pone.0064771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hodgin JB, et al. Estrogen receptor alpha is a major mediator of 17beta-estradiol’s atheroprotective effects on lesion size in Apoe−/− mice. J Clin Invest. 2001;107:333–340. doi: 10.1172/JCI11320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Villablanca AC, et al. 17beta-estradiol prevents early-stage atherosclerosis in estrogen receptor-alpha deficient female mice. J Cardiovasc Transl Res. 2009;2:289–299. doi: 10.1007/s12265-009-9103-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hussain Y, et al. G-protein estrogen receptor as a regulator of low-density lipoprotein cholesterol metabolism: cellular and population genetic studies. Arterioscler Thromb Vasc Biol. 2015;35:213–221. doi: 10.1161/ATVBAHA.114.304326. [DOI] [PubMed] [Google Scholar]
- 94.Chakrabarti S, Davidge ST. G-protein coupled receptor 30 (GPR30): a novel regulator of endothelial inflammation. PLoS One. 2012;7:e52357. doi: 10.1371/journal.pone.0052357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bowling MR, et al. Estrogen effects on vascular inflammation are age dependent: role of estrogen receptors. Arterioscler Thromb Vasc Biol. 2014;34:1477–1485. doi: 10.1161/ATVBAHA.114.303629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tamaki M, et al. Expression and functional roles of G-protein-coupled estrogen receptor (GPER) in human eosinophils. Immunol Lett. 2014;160:72–78. doi: 10.1016/j.imlet.2014.03.012. [DOI] [PubMed] [Google Scholar]
- 97.Rettew JA, et al. GPR30/GPER-1 mediates rapid decreases in TLR4 expression on murine macrophages. Mol Cell Endocrinol. 2010;328:87–92. doi: 10.1016/j.mce.2010.07.017. [DOI] [PubMed] [Google Scholar]
- 98.Blasko E, et al. Beneficial role of the GPR30 agonist G-1 in an animal model of multiple sclerosis. J Neuroimmunol. 2009;214:67–77. doi: 10.1016/j.jneuroim.2009.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Brunsing RL, et al. The G protein-coupled estrogen receptor (GPER) agonist G-1 expands the regulatory T-cell population under TH17-polarizing conditions. J Immunother. 2013;36:190–196. doi: 10.1097/CJI.0b013e31828d8e3b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Brunsing RL, Prossnitz ER. Induction of interleukin-10 in the T helper type 17 effector population by the G protein coupled estrogen receptor (GPER) agonist G-1. Immunology. 2011;134:93–106. doi: 10.1111/j.1365-2567.2011.03471.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Meyer MR, et al. The G protein-coupled estrogen receptor GPER/GPR30 as a regulator of cardiovascular function. Vascul Pharmacol. 2011;55:17–25. doi: 10.1016/j.vph.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Barton M, et al. Alike but not the same: anatomic heterogeneity of estrogen receptor-mediated vasodilation. J Cardiovasc Pharmacol. 2013;62:22–25. doi: 10.1097/FJC.0b013e31829709d4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Meyer MR, et al. Deletion of G protein-coupled estrogen receptor increases endothelial vasoconstriction. Hypertension. 2012;59:507–512. doi: 10.1161/HYPERTENSIONAHA.111.184606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ohman MK, et al. Perivascular visceral adipose tissue induces atherosclerosis in apolipoprotein E deficient mice. Atherosclerosis. 2011;219:33–39. doi: 10.1016/j.atherosclerosis.2011.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Meyer MR, Barton M. ERα, ERβ, and gpER: novel aspects of oestrogen receptor signalling in atherosclerosis. Cardiovasc Res. 2009;83:605–610. doi: 10.1093/cvr/cvp187. [DOI] [PubMed] [Google Scholar]
- 106.Santolla MF, et al. Niacin activates the G protein estrogen receptor (GPER)-mediated signalling. Cell Signal. 2014;26:1466–1475. doi: 10.1016/j.cellsig.2014.03.011. [DOI] [PubMed] [Google Scholar]
- 107.Christodoulakos GE, et al. The cardiovascular effects of selective estrogen receptor modulators. Ann N Y Acad Sci. 2006;1092:374–384. doi: 10.1196/annals.1365.034. [DOI] [PubMed] [Google Scholar]
- 108.Broughton BR, et al. Stroke increases G protein-coupled estrogen receptor expression in the brain of male but not female mice. Neurosignals. 2013;21:229–239. doi: 10.1159/000338019. [DOI] [PubMed] [Google Scholar]
- 109.Broughton BR, et al. Sex-dependent effects of G protein-coupled estrogen receptor activity on outcome after ischemic stroke. Stroke. 2014;45:835–841. doi: 10.1161/STROKEAHA.113.001499. [DOI] [PubMed] [Google Scholar]
- 110.Murata T, et al. G protein-coupled estrogen receptor agonist improves cerebral microvascular function after hypoxia/reoxygenation injury in male and female rats. Stroke. 2013;44:779–785. doi: 10.1161/STROKEAHA.112.678177. [DOI] [PMC free article] [PubMed] [Google Scholar]