Abstract
Recent research indicates that reward-based motivation impacts medial temporal lobe (MTL) encoding processes, leading to enhanced memory for rewarded events. In particular, previous functional magnetic resonance imaging (fMRI) studies of motivated learning have shown that MTL activation is greater for highly rewarded events, with the degree of reward-related activation enhancement tracking the corresponding behavioral memory advantage. These studies, however, do not directly address leading theoretical perspectives that propose such reward-based enhancements in MTL encoding activation reflect enhanced discrimination of the motivational context of specific events. In this study, a high-value or low-value monetary cue preceded a pair of objects, indicating the future reward for successfully remembering the pair. Using representational similarity analysis and high-resolution fMRI, we show that MTL activation patterns are more similar for encoding trials preceded by the same versus different reward cues, indicating a distributed code in this region that distinguishes between motivational contexts. Moreover, we show that activation patterns in hippocampus and PHc that differentiate reward conditions during anticipatory cues and object pairs relate to successful associative memory. Additionally, the degree to which patterns differentiate reward contexts in dentate gyrus/CA2,3 and PHc is related to individual differences in reward modulation of memory. Collectively, these findings suggest that distributed activation patterns in the human hippocampus and PHc reflect the rewards associated with individual events. Furthermore, we show that these activation patterns—which discriminate between reward conditions—may influence memory through the incorporation of information about motivational contexts into stored memory representations.
Keywords: Hippocampus, fMRI, Reward, Memory, MTL
Introduction
Highly motivating events are often well remembered, promoting considerable interest in how motivational factors, including reward, impact memory formation processes supported by the hippocampus and surrounding medial temporal lobe (MTL) structures. Electrophysiological research in rodents has shown that the presentation of reward and differences in motivational state alter the firing properties of hippocampus neurons (Holscher, Jacob, & Mallot, 2003; Kennedy & Shapiro, 2009; Singer & Frank, 2009). In humans, presentation of monetary incentives has been shown to enhance hippocampal activation both prior to (Adcock, Thangavel, Whitfield-Gabrieli, Knutson, & Gabrieli, 2006) and during stimulus encoding (Wolosin, Zeithamova, & Preston, 2012), leading to facilitated memory for rewarding events. It has been speculated that such changes in hippocampal activity during encoding of rewarding events reflect enhanced binding of event elements to the motivational context in which they are experienced (Shohamy & Adcock, 2010; Singer & Frank, 2009). There is much evidence that the hippocampus plays a critical role in representing the both the spatial and temporal context of individual episodic memories (Ekstrom et al., 2003; Hassabis et al., 2009; Jenkins & Ranganath, 2010; Lenck-Santini, Save, & Poucet, 2001; MacDonald, Lepage, Eden, & Eichenbaum, 2011; O’Keefe & Conway, 1978; O’Keefe & Speakman, 1987). Fitting with the theme of this special issue, the goal of the present study was to determine whether or not hippocampal contextual representations extend beyond the domain of episodic memory to include information about the reward values of particular events. As remembering events that lead to reward is critical for survival, it is likely that motivational contexts are also represented within the MTL. In particular, changes in CA3 sharp wave ripple activity have been hypothesized to be a mechanism by which reward contexts are represented and bound to individual events (Singer & Frank, 2009).
Previous functional magnetic resonance imaging (fMRI) studies in humans examining reward-motivated learning have exclusively employed univariate statistical measures to evaluate how mean activation across a particular region-of-interest is modulated by reward (Adcock et al., 2006; Kuhl, Shah, DuBrow, & Wagner, 2010; Wolosin et al., 2012). Specifically, these studies compare mean activation during encoding of events associated with high-value and low-value monetary incentives, finding greater hippocampal and MTL cortical activation during high-value events that relates to superior memory for those events. While these findings indicate that monetary incentives facilitate memory encoding in the MTL, they do not directly address the hypothesis that MTL responses should distinguish between the motivational contexts in and of themselves.
Recent advances in fMRI analysis methods have revealed that multivariate pattern information analysis is a powerful tool for determining how distributed patterns of brain activation differentiate between distinct forms of information content (Kriegeskorte, Mur, & Bandettini, 2008). Such techniques have recently been employed in combination with high-resolution fMRI to examine how spatial context is represented in MTL subregions (Hassabis et al., 2009). The results revealed that distributed patterns of activation within MTL cortex differentiated between distinct spatial environments, and the pattern of activation within the hippocampus differentiated between individual spatial locations within specific environments. Here, we employ similar high-resolution fMRI and pattern information analysis techniques to determine whether or not distributed patterns of MTL subregional activation differentiate between the motivational contexts that distinguish individual associative learning events.
Specifically, we used a monetary incentive encoding task in which a high-value or low-value monetary cue precede presentation of a pair of objects (Wolosin et al., 2012), with the cues indicating how much money a participant would receive if they successfully recalled the object association on a later memory test. First, we predicted that hippocampal activation patterns during both the anticipatory cue and pair encoding trial phases would be more similar for events with the same reward value than events with different reward values, indicating a distributed code within the MTL that differentiates between motivational contexts. Furthermore, we hypothesized that the distinctiveness of hippocampal patterns discriminating the two reward conditions would relate to subsequent associative memory, with remembered associations showing greater pattern consistency within the same reward condition relative to forgotten associations.
We were also interested in how individual differences in hippocampal pattern similarity relate to subsequent memory. If reward facilitates memory by providing additional information about the motivational significance of individual events, then individuals who show more consistent hippocampal patterns within a reward condition should also show superior associative memory in the present task. Given recent evidence that individual differences in reward-related hippocampal activation have been shown to correlate with facilitated memory for high-value events (Adcock et al., 2006; Wolosin et al., 2012), we predicted that individuals who show greater memory facilitation for high-value associations would also show corresponding increases in the pattern similarity for high-value—but not low-value—events in the hippocampus. We further predicted that this brain-behavior relationship would be particularly evident in the CA3 subfield of the hippocampus based on recent rodent (Singer & Frank, 2009) and human (Wolosin et al., 2012) evidence indicating an important role for this region during incentivized learning.
Method
Participants
Thirty-seven healthy, English-speaking individuals (16 females, ages 18–33, mean age = 21) participated in the fMRI study. All participants were right-handed with normal or corrected to normal vision. Prior to the experiment, participants gave informed consent in accordance with a protocol approved by the Institutional Review Boards of Stanford University and The University of Texas at Austin. Participants received $20/hr for their involvement and additional bonus money based on task performance (up to $34). Data from 13 participants were excluded from analysis due to excessive head motion (5 participants), chance memory performance based on a binomial distribution (2 participants, proportion correct less than 0.58), scanner malfunction resulting in loss of data (1 participant), and insufficient number of incorrect trials (10 or fewer incorrect trials) to permit analysis based on memory performance (5 participants). Thus, data from 24 participants (10 female, ages 18–33, mean age = 22) were included in the fMRI analyses.
Procedures
Motivated encoding task
We employed a modified version of the monetary incentive encoding task (Adcock et al., 2006). Stimuli consisted of 320 grayscale photographs of common objects organized into 160 object pairs. Object pairs were assigned to either a high-value or low-value reward condition (80 pairs in each condition).
Across eight event-related functional runs, participants intentionally encoded object pairs, half presented under high reward and half under low reward conditions. Participants were instructed to use the same elaborative encoding strategy for high-value and low-value pairs; specifically, participants were told to learn each pair by forming a story relating the two objects to one another. Each functional run consisted of 10 high-value and 10 low-value trials. On each trial, a monetary cue (either $2.00 or $0.10) was displayed for 2s (Figure 1), indicating how much money a participant could earn for successfully recalling the association at test. The monetary cue was then followed by a 2–7s variable fixation delay, 3s display of the object pair, and 13s of a baseline task. During presentation of the object pairs, participants provided a judgment of learning, indicating how well they learned each association. These judgments were collected to ensure participants’ attention during the encoding phase and were not considered in the analysis of fMRI data. Baseline consisted of six 2s trials of a modified odd/even task (Stark & Squire, 2001), immediately preceded and immediately followed by a 0.5s fixation. During each 2s baseline trial, a pair of digits between one and eight was presented on the screen for 1.75s followed by 0.25s of fixation, and participants indicated whether the sum of the digits was odd or even. Participants were informed that they would be paid 20% of what they earned in the experiment in addition to the base pay of $20/hour.
Figure 1.
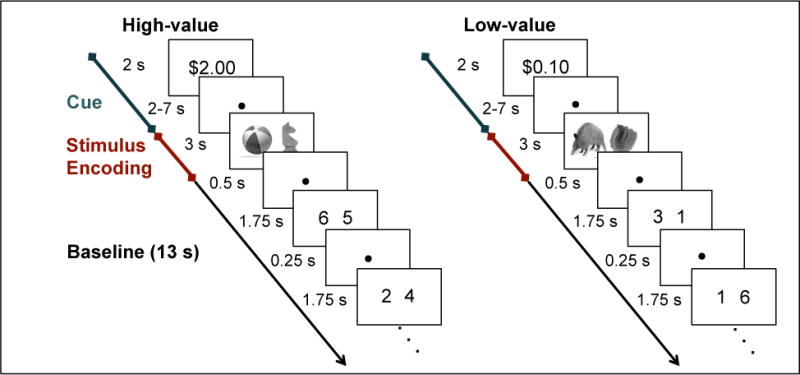
Encoding task. During each encoding trial, participants viewed monetary cues indicating the possible reward for successfully recalling the association at test, and after a variable delay, viewed a pair of objects, followed by 13 s of baseline task.
Within each run, the order of conditions was determined by a sequencing algorithm to optimize the efficiency of the event-related fMRI design (Dale, 1999). The delay durations between the reward cue presentation and object pair presentation were also determined on a per run basis, such that each reward condition contained the same distribution of values (one trial of duration 2 s, two trials of duration 3 s, two trials of duration 4 s, two trials of duration 5 s, two trials of duration 6 s, one trial of duration 7 s). Participants were randomly assigned to one of eight randomization groups. For each of the first four groups, 320 stimuli were randomly organized into 160 pairs and assigned to trials in a unique sequence of 8 runs. To counterbalance the assignment of reward values to object pairings, four additional groups were created by reversing the assignment of high-value and low-value conditions in the original four groups.
Stimuli were generated using Matlab (The MathWorks, Inc., Natick, MA, USA) on a Macbook laptop computer and back-projected via a magnet-compatible projector onto a screen that could be viewed through a mirror mounted above the participant’s head. Participants responded with a button pad held in their right hand.
Associative recognition (not scanned)
Following the motivated encoding task, participants were tested on their memory for all 160 pairs using a 2-alternative forced choice paradigm. This phase of the experiment was performed outside the scanner using a Macbook laptop computer. On each trial, a cue object appeared at the top of the screen, and the participant was instructed to choose the object associated with the cue during the encoding phase from two probe objects presented at the bottom of the screen. Importantly, the incorrect (foil) object was always another previously viewed object from the same reward condition as the correct response, but one that had been paired with a different object than the cue. Prior to scanning, participants practiced the encoding and retrieval tasks using stimuli distinct from those presented during functional scanning.
fMRI Acquisition Procedures
Imaging data were acquired on a 3.0 T GE Signa whole-body MRI system (GE Medical Systems, Milwaukee, WI, USA) with an 8-channel head coil array. Prior to functional scanning, a high-resolution, T2-weighted, flow-compensated spin-echo structural image (TR = 3s, TE = 63 ms, 0.43 × 0.43 inplane resolution) was acquired with 39 1.7-mm thick slices perpendicular to the main axis of hippocampus (oblique coronal) to enable visualization of hippocampal subfields, MTL cortical regions and midbrain structures. A second high-resolution, T2-weighted, flow-compensated spin-echo structural image (TR = 3 s, TE = 65.5 ms, 0.43 × 0.43 inplane resolution) was acquired with 17 2-mm thick slices parallel to the main axis of hippocampus (oblique inplane axial). Functional images were acquired using a high-resolution T2*-sensitive gradient echo spiral in/out pulse sequence (Glover & Law, 2001) with the same slice locations as the second (oblique inplane axial) high-resolution structural image (TR = 2.5s, TE = 31ms, flip angle = 61°, FOV = 22 cm, 1.7 × 1.7 × 2.0 mm resolution). Before functional scanning, a high-order shimming procedure, based on spiral acquisitions, was utilized to reduce B0 heterogeneity (Kim, Adalsteinsson, Glover, & Spielman, 2002).
To obtain a field map for correction of magnetic field heterogeneity, the first time frame of the functional timeseries was collected with an echo time 2 ms longer than all subsequent frames. For each slice, the map was calculated from the phase of the first two time frames and applied as a first order correction during reconstruction of the functional images. In this way, blurring and geometric distortion were minimized on a per-slice basis. In addition, correction for off-resonance due to breathing was applied on a per-time-frame basis using phase navigation (Pfeuffer, Van de Moortele, Ugurbil, Hu, & Glover, 2002). This initial volume was then discarded as well as the following three volumes of each scan (a total of 12 s) to allow for T1 stabilization.
fMRI Analyses
fMRI Preprocessing
fMRI data were analyzed using SPM5 (Wellcome Department of Cognitive Neurology, London, UK) and custom MATLAB routines. Images were realigned to the first volume of the time series to correct for motion. A mean T2*-weighted functional image was computed during realignment, and then to coregistered to the T2-weighted oblique inplane axial image. The T2-weighted oblique inplane axial image was coregistered to the T2-weighted oblique coronal image. The resulting coregistration parameters were then applied to all functional images. A high-pass temporal filter (128s) was applied to all functional images. Functional images were then converted to percent signal change. Finally, a 3 mm smoothing kernel was applied to all functional volumes.
Anatomical region-of-interest definition
We used Advanced Normalization Tools (ANTS: http://www.picsl.upenn.edu/ANTS/) (Avants et al., 2011) to create individual participant regions-of-interest (ROIs) for each MTL subregion. The first fifteen participant’s high-resolution oblique coronal images were used to create a high-resolution group template image. Each participant’s oblique coronal image was then normalized to the group template image. To maximize alignment of MTL regions across participants, a hippocampal mask and an MTL cortex mask, consisting of parahippocampal (PHc), perirhinal (PRc) and entorhinal (ERc) cortices, were drawn on individual participant’s high-resolution oblique coronal images and used as labels to guide normalization to the high-resolution template brain. Regions-of-interest (ROIs) were demarcated on the high-resolution group template image generated in ANTS using techniques adapted for analysis and visualization of MTL subregions (Amaral & Insausti, 1990; Ding & Van Hoesen, 2010; Insausti et al., 1998; Pruessner et al., 2002; Pruessner et al., 2000). Eight MTL subregions were defined in each hemisphere: the hippocampal subfields within the body of the hippocampus (dentate gyrus/CA2,3—hereafter abbreviated DG/CA2,3, CA1, and subiculum) and surrounding MTL cortices (PHc, PRc, and ERc). Because the hippocampal subfields cannot be delineated in the most anterior and posterior extents of the hippocampus at the resolution employed, anterior hippocampal and posterior hippocampal ROIs (inclusive of all subfields) were also demarcated on the most rostral and caudal 1–2 slices of the hippocampus, respectively (Olsen et al., 2009; Preston et al., 2010; Zeineh, Engel, Thompson, & Bookheimer, 2003). Finally, inverse normalizations were performed using ANTS to generate anatomical ROIs for each individual participant in native space. The location of the MTL subfield ROIs was then visually verified on each participant’s oblique coronal image.
Individual participant statistical models
Voxel-based statistical analyses were conducted in SPM5 at the individual participant level according to the general linear model (Worsley & Friston, 1995). To examine how distributed codes within the MTL relate to memory performance, we extracted parameter estimates for the cue and stimulus phase of each trial separately. To optimize estimation, a separate model was created to calculate the parameter estimate for each individual cue and stimulus event using a method described by Mumford and colleagues (Mumford, Turner, Ashby, & Poldrack, 2012). Each model included a regressor for a single phase of an individual trial. Additionally, one regressor was specified for each of the four phases from all of the other trials combined: 1) high-value cue, 2) high-value stimulus, 3) low-value cue, and 4) low-value stimulus. Motion parameters were included in all models as nuisance regressors. As cue and stimulus events are contained in separate regressors, these models account for cue phase activation when modeling stimulus phase activation, and vice-versa. To generate the regressors, events were treated as an impulse convolved with a canonical hemodynamic response function and its temporal derivative.
Representational similarity analysis
To assess how distributed patterns of MTL activation distinguish between high-value and low-value trials, we used representational similarity analysis (RSA) (Kriegeskorte et al., 2008). RSA was conducted at the individual participant level in native space. For each anatomical ROI and each event, a pattern of activation was measured as a vector containing the parameter estimates for all voxels within the ROI for that event. The similarity between a pair of events was measured as the Pearson’s correlation between the two events’ patterns of activations. Correlation coefficients were converted to z-values using Fisher z-transformation to ensure normality of the distribution and permit analysis of variance statistics across participants.
Separate analyses were performed for the cue phase and stimulus phase (Figure 2). For each cue event, similarity was computed between that cue event and all other cue events within the same run. Likewise, for each stimulus event, similarity was computed between that stimulus event and all other stimulus events within the same run. We computed average similarity for all event pairs of the same reward value (within-reward-state similarity) and event pairs of different reward values (between-reward-state similarity). This analysis produced the following measures for the cue and stimulus phases: 1) within-reward-state similarity for high-value events, 2) within-reward-state similarity for low-value events, and 3) between-reward-state similarity. We also obtained an overall measure of within-reward-state similarity by computing the average of (1) and (2). To examine how within-reward-state similarities relate to memory for individual pairs, we further separated events by subsequent memory status (remembered, forgotten). We computed within-reward-state similarities separately for all high-value remembered, high-value forgotten, low-value remembered, and low-value forgotten events.
Figure 2.
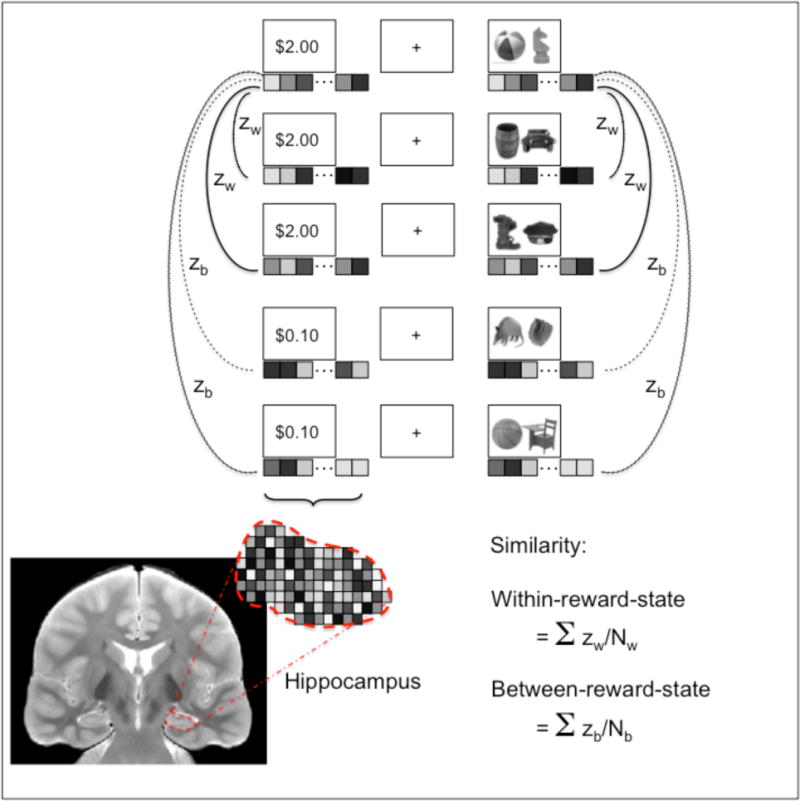
Representational similarity analysis strategy. For each cue and stimulus event within a trial, within-reward-state similarity was computed as the mean of the correlation between the activation pattern for that event and all other events of the same reward value in the functional run. Between-reward-state similarity was computed as the mean correlation between the activation pattern for that event and all events of different reward value in the functional run.
We used paired t-tests to examine the difference between within-reward-state similarities and between-reward-state similarity across the group. To examine the relationship between within-reward-state similarities and subsequent memory status, we conducted 2×2 repeated measures ANOVA for both the cue and stimulus periods, with reward value and subsequent memory status as factors, as well as paired t-tests comparing within-reward-state similarities between remembered and forgotten associations for each reward value. We also examined how individual differences in distributed patterns for high-value and low-value associations are related to behavioral performance by conducting a robust regression analyses with RSA values as the independent variable and behavioral performance as the outcome measure. This robust regression was performed by iteratively reweighting least squares to prevent the influence of outliers.
Analyses were conducted separately for bilateral hippocampus, ERc, PRc, PHc, and the five hippocampal subfields (anterior hippocampus, posterior hippocampus, CA1, DG/CA2,3, subiculum). Left and right hemisphere statistics are reported only for regions showing a hemisphere by condition interaction. To examine whether or not distributed codes in a specific hippocampal subfields are related to behavioral performance, we applied multiple linear robust regression with the RSA values in each hippocampal subregion as regressors and behavioral performance as the outcome measure.
Results
Behavioral Results
At test, memory accuracy (proportion correct) for high-value (mean ± standard error (SEM): 0.80 ± 0.02) and low-value pairs (0.74 ± 0.02) was significantly above chance (both p < 0.001). Participants had better memory for high-value compared to low-value associations (t(23) = 3.31, p = 0.003). The degree to which participants showed better memory for high-value compared to low-value associations was not correlated with overall accuracy across participants (r = 0.03, p = 0.9). Memory performance for high-value pairs was positively correlated with performance for low-value pairs (r = 0.61, p = 0.001), suggesting that there was not a strategic trade-off between learning high-value and low-value pairs. Median reaction times at test did not differ between high-value (2.23 s ± 0.11) and low-value (2.27 s ± 0.2) associations (t(23) = −1.29, p = 0.21).
Distributed MTL Activation Patterns Distinguish Between Reward Conditions
First, we sought to establish whether or not distributed patterns of MTL activation differentiate between high-value and low-value conditions during motivated encoding. We computed the pattern similarity between each pair of events within the same encoding scan and compared mean within-reward-state similarities (high-value events with high-value events and low-value events with low-value events) to mean between-reward-state similarities (high-value events with low-value events) across the group. Within-reward-state similarities were significantly greater than between-reward-state similarities during both the cue period and the stimulus period in all four MTL regions (hippocampus, ERc, PRc, and PHc, all p < 0.01, Table 1). Examination of the distributed pattern of response in hippocampal subfields also revealed significantly greater within-reward-state compared to between-reward-state similarities in all five subfields (all p < 0.01, Table 1). Thus, distributed patterns of activation within the MTL distinguished between events associated with high-value and low-value monetary incentives.
Table 1.
|
A. Cue
| ||||
|---|---|---|---|---|
| Region | Within – Between (mean ± SEM) |
Within – Between t(23) |
High – Low (mean ± SEM) |
High – Low t(23) |
| Hippocampus | 0.015 ± 0.002 | 8.8** | 0.002 ± 0.005 | 0.4 (ns) |
| CA1 | 0.016 ± 0.001 | 10.7** | 0.001 ± 0.005 | 0.3 (ns) |
| DG/CA2,3 | 0.016 ± 0.002 | 7.4** | 0.002 ± 0.005 | 0.3 (ns) |
| Subiculum | 0.013 ± 0.003 | 4.0** | 0.002 ± 0.007 | 0.2 (ns) |
| Anterior hippocampus | 0.012 ± 0.002 | 4.8** | 0.008 ± 0.006 | 1.3 (ns) |
| Posterior hippocampus | 0.019 ± 0.003 | 6.6** | −0.003 ± 0.005 | −0.6 (ns) |
| ERc | 0.014 ± 0.003 | 4.9** | 0.007 ± 0.007 | 1.1 (ns) |
| PRc | 0.016 ± 0.002 | 7.0** | 0.007 ± 0.007 | 1.0 (ns) |
| PHc | 0.014 ± 0.002 | 5.8** | 0.002 ± 0.005 | 0.4 (ns) |
|
B. Stimulus Encoding
| ||||
|---|---|---|---|---|
| Region | Within – Between (mean ± SEM) |
Within – Between t(23) |
High – Low (mean ± SEM) |
High – Low t(23) |
| Hippocampus | 0.011 ± 0.002 | 4.7** | 0.016 ± 0.004 | 3.7** |
| CA1 | 0.015 ± 0.002 | 8.1** | 0.007 ± 0.005 | 1.3 (ns) |
| DG/CA2,3 | 0.010 ± 0.002 | 4.2** | 0.008 ± 0.006 | 1.4 (ns) |
| Subiculum | 0.012 ± 0.003 | 3.9** | 0.018 ± 0.005 | 3.3** |
| Anterior hippocampus | 0.013 ± 0.004 | 3.5** | 0.002 ± 0.007 | 0.3 (ns) |
| Posterior hippocampus | 0.011 ± 0.002 | 6.0** | 0.014 ± 0.005 | 2.7* |
| ERc | 0.009 ± 0.003 | 3.0** | 0.016 ± 0.006 | 2.8* |
| PRc | 0.008 ± 0.002 | 4.1** | 0.024 ± 0.006 | 3.9** |
| PHc | 0.008 ± 0.002 | 3.9** | 0.033 ± 0.008 | 3.9** |
Note.
A. Similarity statistics during anticipatory cue.
B. Similarity statistics during stimulus encoding.
Within – Between = within-reward-state similarity – between-reward-state similarity
High – Low = high-value within-reward-state similarity – low-value within-reward-state similarity.
p < 0.05,
p < 0.01, ns = non-significant
We also examined whether or not within-reward-state similarities within the MTL were greater for high-value relative to low-value events, reflecting greater consistency in the patterns of activation associated with high-value events. During the cue period, high-value and low-value within-reward-state similarities did not differ in any region (all p > 0.2). During the stimulus period, however, greater within-reward-state similarity was observed for high-value compared to low-value events in all four MTL regions (all p < 0.02, Table 1). Examination of hippocampal subfields responses further revealed greater similarity for high-value compared to low-value events in subiculum and posterior hippocampus (Table 1). These results suggest that within the MTL high-value rewards may facilitate sustained activation patterns reflecting the reward condition during encoding of individual associations.
Reward-Induced MTL Activation Patterns Relate to Subsequent Memory
Given the above evidence for distinct MTL patterns induced by high-value and low-value reward conditions, we next examined how these distributed patterns relate to memory for individual associations. To do so, we further separated events by subsequent memory status (whether the association was later remembered or forgotten) and computed within-reward state similarities separately for high-value remembered, high-value forgotten, low-value remembered, and low-value forgotten pairs.
First, we examined whether or not hemisphere effects were present during the cue period using 2 (memory) × 2 (reward) × 2 (hemisphere) repeated measures ANOVA. A significant hemisphere × reward × memory interaction was observed in hippocampus (F(1,23) = 4.44, p = 0.05). Neither left nor right hippocampus showed a main effect of reward or memory (all p > 0.1). However, left hippocampus showed a memory × reward interaction effect (F(1,23) = 5.10, p = 0.03) that was not observed in right hippocampus (F(1,23) = 0.11, p = 0.8). The interaction effect in left hippocampus was reflected by a significant memory effect (remembered > forgotten) for high-value (t(23) = 2.85, p = 0.009) but not low-value (t(23) = −0.25, p = 0.8) pairs. Results in bilateral hippocampus were similar to results in left hippocampus: no main effects were observed (all p > 0.1), and although there was not a significant memory × reward interaction (F(1,23) = 1.84, p = 0.2), a significant memory effect was observed for high-value (t(23) = 2.06, p = 0.05) but not low-value (t(23) = 0.03, p = 1) pairs (Figure 3A). No other hemisphere effect or hemisphere × condition interaction was observed in hippocampus or any other region (all p > 0.1); thus, all analyses are collapsed across hemisphere for the remaining regions.
Figure 3.
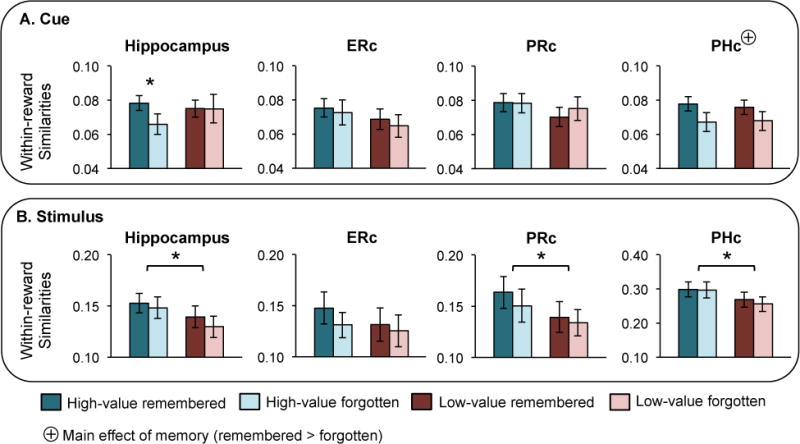
Within-reward-state similarities by memory status and reward condition. (A) Within-reward-state similarities during the cue period for all high-value remembered (blue), high-value forgotten (light blue), low-value remembered (red), low-value forgotten (pink) events. All regions shown are bilateral. Significantly greater within-reward-state similarities were observed for high-value remembered compared to forgotten associations in hippocampus. Additionally, PHc showed a main effect of memory (remembered > forgotten, denoted by a ⊕). (B) Within-reward-state similarities during the stimulus period. A main effect of reward (high-value > low-value) was observed in hippocampus, PRc, and PHc. In all cases, error bars represent standard error of the mean.
Examination of the hippocampal subfields responses during the cue period revealed no memory × reward interaction effects (all p > 0.1) and no main effect of reward (all p > 0.1). A main effect of memory was observed in anterior hippocampus (F(1,23) = 4.87, p = 0.04) and no other subfield (all p > 0.06). Examining memory effects separately for high-value and low-value pairs revealed a significant effect of memory for high-value (t(23) = 2.72, p = 0.01) but not low-value (t(23) = −0.16, p = 0.8) pairs in posterior hippocampus. No other subfield showed a significant memory effect for high-value (all p > 0.3) or low-value pairs (all p > 0.06).
No MTL cortical region showed a memory × reward interaction (all p > 0.5) or a main effect of reward (all p > 0.3) during the cue period. However, PHc showed a main effect of memory across both reward conditions, with greater similarity for remembered, compared to forgotten associations (F(1,23) = 6.87, p = 0.02; Figure 3A). Neither ERc nor PRc showed this effect (both p > 0.4), and no MTL cortical region showed a difference between remembered and forgotten within-reward-state similarities for high-value (all p > 0.08) or low-value (all p > 0.07) pairs.
Next, we examined the effects of reward and memory during stimulus encoding. Hemisphere effects during encoding phase were assessed using a 2 (memory) × 2 (reward) × 2 (hemisphere) repeated measures ANOVA. A significant hemisphere × memory interaction was observed in hippocampus (F(1,23) = 5.38, p = 0.03). Left hippocampus showed a significant reward × memory interaction effect (F(1,23) = 4.14 p = 0.05) that was not observed in right hippocampus (F(1,23) = 0.31 p = 0.6). However, neither the main effect of memory, nor the pairwise differences between remembered and forgotten associations for high-value and low-value pairs were significant in either hemisphere (all p > 0.06). A main effect of reward was observed in both left (F(1,23) = 13.46, p < 0.01) and right (F(1,23) = 4.79, p < 0.04) hippocampus. Bilateral hippocampus also showed a main effect of reward (F(1,23) = 10.41, p < 0.01) but no other significant effects (all p > 0.09) (Figure 3B). No other hemisphere effect or hemisphere × condition interaction was observed in hippocampus or any other region (all p > 0.1).
Examination of the hippocampal subfields responses during stimulus encoding revealed a significant memory × reward interaction in subiculum (F(1,23) = 6.26, p = 0.02), with a significant memory effect for low-value (t(23) = 2.12, p = 0.05) but not high-value (t(23) = −1.49, p = 0.1) pairs. Subiculum also showed a strong main effect of reward (F(1,23) = 16.0, p < 0.01). No other hippocampal subregion showed an interaction effect (all p > 0.3), a main effect of reward (all p > 0.09), a main effect of memory (all p > 0.1), or a memory effect for high-value (all p >0.1) or low-value (all p > 0.2) pairs.
During stimulus encoding, no MTL cortical region showed an interaction effect (all p > 0.2), main effect of memory (all p > 0.01) or a memory effect for high value (all p > 0.06) or low-value (all p > 0.06) pairs. However, PRc, and PHc showed a main effect of reward (t(23) > 8.4, p < 0.01) that was not observed in ERc (t(23) = 2.09, p = 0.2) (Figure 3B).
Individual Differences in Memory Relate to Reward-Induced Activation Patterns in Hippocampus and PHc
We were also interested in how individual differences in distributed activation patterns within the MTL relate to individual differences in behavioral performance. In particular, we examined whether or not the ability of MTL activation patterns to discriminate reward condition was associated with successful memory. We considered the difference between within-reward-state and between-reward-state similarities as a measure of the strength of the discrimination between reward conditions, hereafter referred to as “reward discrimination”. We hypothesized that reward discrimination within the MTL would be positively associated with memory performance.
To test this hypothesis, we conducted robust regression between reward discrimination in each MTL region and memory performance. During the cue period, no region showed a relationship between associative memory performance and reward discrimination (all p > 0.07). During the stimulus period, however, we observed a positive relationship between associative memory performance and reward discrimination in hippocampus (r = 0.45, p = 0.04) and ERc (r = 0.44, p = 0.05), but not PRc or PHc (p > 0.2) (Figure 4A). To examine whether or not these stimulus period effects could be distinguished from cue related effects, we repeated the correlations between stimulus period activation and performance while controlling for cue period activation with Pearson’s partial correlation analysis. After controlling for cue period activation, only ERc showed a significant positive correlation between associative memory performance and reward discrimination (r = 0.44, p = 0.05, all other p > 0.1).
Figure 4.
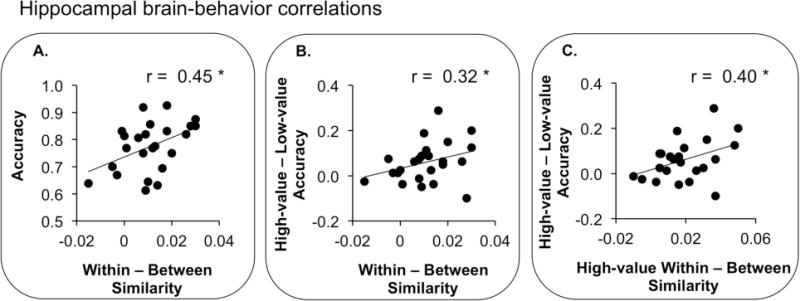
Brain-behavior correlations in bilateral hippocampus. P-values shown are generated using robust regression. (A) Correlation between reward discrimination (within – between reward-state similarities) and overall memory accuracy (B) Correlation between reward discrimination and behavioral reward modulation (high-value – low-value memory accuracy) (C) Correlation between reward discrimination of high-value events (high-value within-reward-state similarities – between reward-state similarities) and behavioral reward modulation.
To examine if the relationship between associative memory performance and reward discrimination observed in hippocampus was driven by the pattern of activation in a specific hippocampal subregion, we conducted a multiple linear robust regression analysis with associative memory performance as the outcome measure and reward discrimination in each hippocampal subregion as regressors. This analysis did not reveal a specific role for any region (all p > 0.5).
Similarly, we were interested in whether or not reward discrimination was associated with behavioral sensitivity to reward, i.e., the degree to which individual participants showed enhanced memory for high-value associations. To examine this possibility, we calculated the relationship between reward discrimination in each MTL region and behavioral reward modulation—the difference between high-value and low-value memory accuracy—across participants. Neither hippocampus nor MTL cortical regions showed this relationship during the cue period (all p > 0.09). During the stimulus period, however, hippocampus showed a positive relationship between behavioral reward modulation and reward discrimination (r = 0.32, p = 0.04) (Figure 4B). No MTL cortical region showed this effect (all p > 0.1). However, the relationship between behavioral reward modulation and reward discrimination in hippocampus during pair encoding was not significant after controlling for cue related activation (all p > 0.2), suggesting this effect may have been driven in part by residual activation from the cue period.
To examine if the relationship between behavioral reward modulation and reward discrimination observed in hippocampus was driven by the pattern of activation in a specific hippocampal subregion, we conducted a multiple linear robust regression analysis with behavioral reward modulation as the outcome measure and reward discrimination in each hippocampal subregion as regressors. This analysis revealed DG/CA2,3 was the only hippocampal subregion to show a positive correlation between behavioral reward modulation and reward discrimination (r = 0.45, p = 0.01, all other p > 0.1). Importantly, this relationship between DG/CA2,3 reward discrimination and behavioral reward modulation was also significant when controlling for possible influences from the cue phase using partial correlation (r = 0.39, p = 0.01).
As within-reward-state similarities were greater for high-value compared to low-value events during stimulus encoding, distributed coding of the high-value, but not low-value condition during stimulus encoding may be associated with greater behavioral sensitivity to the reward manipulation. Specifically, reward discrimination of high value events (within-reward-state similarities for high-value events – between-reward-state similarities) during stimulus encoding may facilitate memory processing for high-value, compared to low-value events. We hypothesized that during stimulus encoding, reward discrimination for high-value associations would be associated with greater behavioral reward modulation across participants. Indeed, we observed a positive correlation between reward discrimination for high-value associations during stimulus encoding and behavioral reward modulation in hippocampus (r = 0.40, p = 0.04) (Figure 4C). This relationship was also observed in PHc (r = 0.30, p = 0.05), but not ERc or PRc (p > 0.6). This positive correlation remained significant in both hippocampus (r = 0.36, p = 0.05) and PHc (r = 0.31, p = 0.05) when controlling for cue period activation.
To examine if this relationship was driven by reward discrimination in a specific hippocampal subregion, we conducted a multiple regression analysis with behavioral reward modulation as the outcome measure and reward discrimination for high-value events in each hippocampal subregion as regressors. This analysis also revealed a specific role for DG/CA2,3, (r = 0.57, p = 0.009) and no other hippocampal subregion (all p > 0.1), which remains significant when controlling for possible influences from the cue phase (r = 0.57, p = 0.006). We also conducted the same analysis using reward discrimination for low-value pairs (within-reward-state similarities for low-value events – between-reward-state similarities), and found no correlation with behavioral reward modulation in any region (all p > 0.1).
Discussion
In the present study, we combined high-resolution fMRI and pattern information analysis techniques to show that patterns of activation within the hippocampus and surrounding MTL cortices distinguish between reward contexts during motivated learning. Distributed patterns of MTL activation were more similar for object associations encoded in the same reward context than associations learned in a different reward context. Furthermore, the degree to which MTL activation patterns discriminated between reward conditions was related to within and across participant measures of subsequent memory. During presentation of anticipatory reward cues, within-reward-state similarities in hippocampus and PHc were greater for remembered relative to forgotten associations, with the hippocampal effect being specific for pairs encoded in the high-value reward condition. During the pair encoding phase, hippocampal discrimination of reward condition was related to across participant measures of overall memory performance as well as the degree of memory enhancement observed for high-value associations. Examination of activation patterns within hippocampal subfields during pair encoding revealed a unique role for DG/CA2,3 in reward modulation of associative memory encoding processes. In fitting with the theme of this special issue, the present findings suggest that hippocampal and PHc representations go beyond spatial and temporal information to include information that differentiates between the motivational contexts of individual events. Moreover, these results indicate that hippocampal discrimination of reward condition in the present study is related to enhanced associative binding processes that promote superior memory.
Hippocampal Discrimination of Motivational Context
In rodents, distributed patterns of neuronal activity within hippocampus have been shown to distinguish between motivational states (water or food deprivation) during contextual retrieval (Kennedy & Shapiro, 2009), providing evidence for a representational code for motivational context in rodent hippocampus. However, in humans, such evidence for discrimination of motivational context within the hippocampus is speculative. Prior human neuroimaging research has shown that encoding-related activation in the MTL is modulated by the presentation of reward (Wittmann et al., 2005) as well as anticipatory cues that predict future rewards (Adcock et al., 2006; Kuhl et al., 2010; Wolosin et al., 2012). Specifically, this work has shown that mean activation is enhanced in hippocampus during presentation of high-value reward cues and the events immediately following those cues, with the degree of enhancement relating to later memory for the events (Adcock et al., 2006; Wolosin et al., 2012).
It is clear from these prior findings that motivational factors, such as reward, influence memory formation processes in the human hippocampus. However, these findings do not provide definitive evidence for discrimination of motivational context in the human hippocampus, as the observed changes in mean hippocampal encoding signal could reflect facilitated encoding of individual highly rewarding stimuli rather than differences in hippocampal responses related to the motivational context per se. Other recent evidence has shown that the level of hippocampal activation correlates with retrieval of contextual information about reward in value-sensitive prefrontal regions during motivated learning (Kuhl et al., 2010). While this result suggests that the hippocampus may drive reinstatement of information about rewards in neocortical regions, it does not directly address whether or not hippocampal activation patterns distinguish between events that differ by motivational context. The use of representational similarity in the present study thus extends this prior work to directly show that distributed patterns of activation within human hippocampus differentiate between motivational contexts during motivated learning to support encoding of novel information.
Anticipatory MTL Responses Discriminate Between Reward Conditions
The finding that distributed hippocampal and PHc reward representations formed during the anticipatory cue period are related to participants’ later ability to remember individual associations adds to a growing body of literature linking anticipatory brain responses prior to event encoding to successful memory formation. Several studies have shown that brain responses immediately prior to event encoding, including hippocampal activation, are related to subsequent memory (Adcock et al., 2006; Addante, Watrous, Yonelinas, Ekstrom, & Ranganath, 2011; Gruber & Otten, 2010; Gruber, Watrous, Ekstrom, Ranganath, & Otten, 2013; Mackiewicz, Sarinopoulos, Cleven, & Nitschke, 2006; Otten, Quayle, Akram, Ditewig, & Rugg, 2006; Park & Rugg, 2009). One possible interpretation of the present findings is that anticipatory responses within the hippocampus and PHc may reflect the formation of a coherent representation of the motivational context that subsequently impacts encoding of the following events. Within hippocampus, anticipatory patterns of activation showed discriminative coding of cues associated with different reward values, consistent with a representational code of motivational context. As in other studies of motivated learning (Adcock et al., 2006; Gruber & Otten, 2010; Gruber et al., 2013), the relationship between distributed hippocampal anticipatory patterns and subsequent memory was restricted to events that were associated with high-value rewards. This result indicates that the value of monetary incentives modulates anticipatory processes within hippocampus, and one possibility is that this reward-based modulation occurs through enhanced hippocampal representation of reward context.
In contrast to hippocampus, distributed PHc activation patterns during the anticipatory phase were not modulated by reward. Anticipatory patterns in PHc showed a main effect of memory, with greater within-reward-state similarities for remembered compared to forgotten associations for both reward contexts. While several theories emphasize the role of the PHc in the representation of the spatial context surrounding individual events (Epstein, 2008; Knierim, Lee, & Hargreaves, 2006; Manns & Eichenbaum, 2006; Mullally & Maguire, 2011), other perspectives suggest that the PHc represents contextual information beyond the spatial domain (Aminoff, Gronau, & Bar, 2007; Bar & Aminoff, 2003; Davachi, 2006; Diana, Yonelinas, & Ranganath, 2007). For example, two recent human neuroimaging studies have shown PHc responses during event encoding that reflect the temporal context in which those events occur (Jenkins & Ranganath, 2010; Turk-Browne, Simon, & Sederberg, 2012). The present results provide additional evidence for contextual coding in PHc beyond the spatial domain by showing PHc responses that reflect the motivational context of individual experiences.
Moreover, the observed relationship between PHc reward discrimination during the cue and subsequent memory status suggests that motivational modulation of PHc activation patterns may facilitate event encoding regardless of the particular value of the reward. Again, the present PHc finding differs from a prior report of motivated learning that employed univariate measures to isolate functionally defined regions (Adcock et al., 2006). In that study, PHc activation during presentation of monetary incentives was modulated by reward but did not relate to subsequent memory. Here, by examining the entire pattern of activation in PHc, we show that distributed patterns elicited by different reward conditions do impact memory for the events encoded in those contexts.
One limitation of the findings during the anticipatory cue phase is that distributed activation patterns during the cue period may be biased to differentiate between high-value and low-value trials due to differences in the visual properties of the cue. However, a recent study using representational similarity to index content representation within MTL subregions indicates that hippocampus and PHc do not show coherent activation patterns for visually presented text (Liang, Wagner, & Preston, 2013), making it less likely that the perceptual characteristics of the cues alone are the source of the present effects. Furthermore, any potential bias in the representational similarity measures due to the visual properties of the cues would not be expected to produce the relationship between distributed anticipatory patterns and subsequent memory for the associations that are observed here. Future research that manipulates the perceptual characteristics of the incentive cues within each reward value will help further clarify this issue. It is also noteworthy that the nature of the GLM modeling procedure and the partial correlation analyses factoring out cue-period activation from stimulus phases responses suggest that it is unlikely any potential biases from the cue period affect the findings observed during the pair encoding phase.
Discrimination of Reward Condition during Pair Encoding
During the pair encoding phase, distributed activation patterns within the MTL showed greater consistency in the representation of reward context for high-value, compared to low-value pairs. Notably, this observed difference cannot result from bias due to the visible properties of the stimuli, as each trial consists of a different pair of object stimuli. This pattern of results suggests that information reflecting the unique properties of high-value events is maintained during pair encoding and may thus facilitate binding of those events to the motivational context in which they are experienced.
Moreover, we observed positive relationship across participants between hippocampal reward discrimination and degree of behavioral memory facilitation for high-value relative to low-value associations. Importantly, when the distributed activation patterns in hippocampus and PHc were examined separately for high-value and low-value associations, only enhanced pattern similarity for high-value pairs was correlated with reward-related changes in associative memory performance. Importantly, the critical brain-behavior relationships between behavioral reward modulation and hippocampal and PHc pattern similarity for high-value events were significant, even after we explicitly controlled for cue-related activation. Collectively, these findings suggest that the behavioral facilitation in memory for highly motivating events may depend on translating the motivational significance from the time of reward cue presentation to the event itself, thus enabling events to be bound to their motivational context. One caveat to this interpretation is that we did not explicitly measure memory for motivational context in the present study. While our findings indicate that hippocampal and PHc activation patterns differentiate between different reward contexts and further indicate that these MTL activation patterns influence memory for individual events, future research will be necessary to address how distributed MTL representations relate to memory for the motivational context per se.
Finally, the present results suggest that within the hippocampus memory enhancements for highly motivating events may be supported by DG/CA2,3. Multiple regression analysis revealed that DG/CA2,3 was the only hippocampal subregion to show a relationship between reward discrimination and enhanced memory for high-value compared to low-value pairs even when controlling for cue-related activation. These findings thus extend prior work indicating a unique role for DG/CA2,3 in motivated learning (Wolosin et al., 2012). Several neuroanatomical and computational models of hippocampal function propose that CA3 plays a key role in representing events and the context in which they occur through the rich network of intrinsic connections within the region (Levy, 1996; McClelland, McNaughton, & O’Reilly, 1995; O’Reilly & Rudy, 2001; Wallenstein, Eichenbaum, & Hasselmo, 1998). Empirical work in animals further indicates that the CA3 region plays an essential role in binding events to the motivational context in which they are experienced (Luo, Tahsili-Fahadan, Wise, Lupica, & Aston-Jones, 2011; Singer & Frank, 2009). In the present study, the observed relationship between DG/CA2,3 reward discrimination and memory performance may thus be reflective of CA3 encoding processes that represent the motivational context and the event elements experienced within the context.
Alternate Frameworks for Interpretation
It is important to note that there are alternate accounts of the underlying source of the reward discrimination measure used in the present study. The fact that cue- and stimulus-related MTL activation patterns differed as a function of reward condition could reflect the formation of episodic memory representations that include information about reward condition per se, an interpretation that would be consistent with rodent data demonstrating representation of specific motivational states in hippocampus (Kennedy & Shapiro, 2009). Alternatively, differences in MTL pattern similarity across reward conditions could reflect different encoding processes that distinguish the reward conditions but are not directly related to reward value. For example, one possibility is that the reward cues led participants to invoke different strategies when encoding the object pairs. We believe that this strategic account of our findings is less likely in the present study, as participants were instructed to use the same elaborative encoding strategy for all object pairs. In addition, the lack of a correlation between memory performance for high-value and low-value pairs and the lack of an RT difference during recognition suggests there were no strategic tradeoffs in performance between the two reward conditions. Moreover, participants in previous studies using the motivated encoding paradigm failed to report using different encoding strategies for the two reward conditions (Adcock et al., 2006).
However, the lack of a strategic difference in the present study would not preclude other theoretically important processing accounts, such as one leading hypothesis that motivation leads to enhanced hippocampal binding (Shohamy & Adcock, 2010). Enhanced binding during high-value trials could also be reflected in distributed patterns and lead to enhanced reward discrimination. While we favor the representational account of our data due to its convergence with electrophysiological research in rodents (Kennedy & Shapiro, 2009; Singer & Frank, 2009), we cannot completely rule out this alternate account of our data. One challenge in adjudicating between these two possibilities is the fact that we used only two types of reward context in the present study, making the context manipulation less discrete than in prior studies examining MTL representations of spatial and temporal context. With only two motivational contexts, the representation of the reward values and the processes elicited by those reward conditions are necessarily confounded. By using a larger range of reward values, future work could help differentiate these two accounts of the present findings.
Finally, our findings could also be interpreted in the framework of the temporal context model (Howard & Kahana, 2002). The motivational state induced by the reward cues, or the reward cues themselves, could be bound to the current temporal context. Object pairs that appear in the same motivational condition could thus be represented more similarly as the overlapping reward cue across those pairs would lead to reinstatement of prior temporal contexts in which that cue was experienced. This account would thus conclude that the patterns of reward discrimination observed in the present study are not about reward values per se, but rather reflect representation of temporal context information that differentiates the two reward conditions. Under this interpretation, the present multivariate findings would provide converging evidence with a recent fMRI study demonstrating univariate hippocampal and PHC responses that track memory for fine and coarse temporal contexts (Jenkins & Ranganath, 2010).
Regardless of which framework—representation of reward value, differences in encoding process, or temporal context coding—is used to interpret the present findings, these data provide novel evidence that individual events evoke different kinds of representational states in the hippocampus and PHc when processed under different motivational conditions. Moreover, our data converge with electrophysiological research in rodents to indicate a particular role for the CA3 region in motivated encoding. Consistent with the theme of this special issue, this work thus contributes to the broader literature, indicating that MTL regions have important functions beyond the domain of episodic memory. The present findings highlight that MTL encoding processes are not only modulated by reward, but may also represent information about motivational states that accompany specific events. Such a representational capacity indicates an critical link between episodic memory and reward-based learning, one that may provide an important memory scaffold for subsequent decision making.
Conclusions
Event memories contain a rich amount of contextual information that goes beyond individual items and the spatial and temporal context in which they occur to include information about the emotional and motivational significance of those events. Prior research utilizing pattern information analyses in humans has provided compelling evidence that the hippocampus and PHc play key roles in representing the spatial context surrounding individual experiences (Hassabis et al., 2009). Using similar methods, the present study demonstrates that distributed codes in hippocampus and PHc also distinguish between reward contexts during motivated learning, providing novel evidence that these regions represent a broader spectrum of contextual information beyond that of space or time. Our findings also suggest that memory enhancements commonly observed for highly motivating events may result, in part, from the formation of coherent representations of motivational context within the MTL.
Acknowledgments
This work was supported by a National Science Foundation CAREER Award (A.R.P.), the National Alliance for Research on Schizophrenia and Depression (A.R.P.), Army Research Office Grant 55830-LS-YIP (A.R.P.), NIMH National Research Service Award F31MH092032 (S.M.W.), F32MH094085 (D.Z.), and an American Psychological Association Diversity Program in Neuroscience Fellowship (S.M.W.).
References
- Adcock RA, Thangavel A, Whitfield-Gabrieli S, Knutson B, Gabrieli JD. Reward-motivated learning: mesolimbic activation precedes memory formation. Neuron. 2006;50(3):507–517. doi: 10.1016/j.neuron.2006.03.036. [DOI] [PubMed] [Google Scholar]
- Addante RJ, Watrous AJ, Yonelinas AP, Ekstrom AD, Ranganath C. Prestimulus theta activity predicts correct source memory retrieval. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(26):10702–10707. doi: 10.1073/pnas.1014528108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaral DG, Insausti R. Hippocampal formation. In: Paxinos G, editor. The Human Nervous System. San Diego: Academic Press; 1990. pp. 711–755. [Google Scholar]
- Aminoff E, Gronau N, Bar M. The parahippocampal cortex mediates spatial and nonspatial associations. Cerebral Cortex. 2007;17(7):1493–1503. doi: 10.1093/cercor/bhl078. [DOI] [PubMed] [Google Scholar]
- Avants BB, Tustison NJ, Song G, Cook PA, Klein A, Gee JC. A reproducible evaluation of ANTs similarity metric performance in brain image registration. Neuroimage. 2011;54(3):2033–2044. doi: 10.1016/j.neuroimage.2010.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar M, Aminoff E. Cortical analysis of visual context. Neuron. 2003;38(2):347–358. doi: 10.1016/S0896-6273(03)00167-3. [DOI] [PubMed] [Google Scholar]
- Dale AM. Optimal experimental design for event-related fMRI. Human Brain Mapping. 1999;8(2–3):109–114. doi: 10.1002/(SICI)1097-0193(1999)8:2/3<109::AID-HBM7>3.0.CO;2-W. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davachi L. Item, context and relational episodic encoding in humans. Current Opinion in Neurobiology. 2006;16(6):693–700. doi: 10.1016/j.conb.2006.10.012. [DOI] [PubMed] [Google Scholar]
- Diana RA, Yonelinas AP, Ranganath C. Imaging recollection and familiarity in the medial temporal lobe: a three-component model. Trends in Cognitive Science. 2007;11(9):379–386. doi: 10.1016/j.tics.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Ding SL, Van Hoesen GW. Borders, extent, and topography of human perirhinal cortex as revealed using multiple modern neuroanatomical and pathological markers. Human Brain Mapping. 2010;31(9):1359–1379. doi: 10.1002/hbm.20940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekstrom AD, Kahana MJ, Caplan JB, Fields TA, Isham EA, Newman EL. Cellular networks underlying human spatial navigation. Nature. 2003;425(6954):184–188. doi: 10.1038/nature01964. [DOI] [PubMed] [Google Scholar]
- Epstein RA. Parahippocampal and retrosplenial contributions to human spatial navigation. Trends in Cognitive Science. 2008;12(10):388–396. doi: 10.1016/j.tics.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover GH, Law CS. Spiral-in/out BOLD fMRI for increased SNR and reduced susceptibility artifacts. Magnetic Resonance in Medicine. 2001;46(3):515–522. doi: 10.1002/mrm.1222. [DOI] [PubMed] [Google Scholar]
- Gruber MJ, Otten LJ. Voluntary control over prestimulus activity related to encoding. Journal of Neuroscience. 2010;30(29):9793–9800. doi: 10.1523/jneurosci.0915-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber MJ, Watrous AJ, Ekstrom AD, Ranganath C, Otten LJ. Expected reward modulates encoding-related theta activity before an event. Neuroimage. 2013;64:68–74. doi: 10.1016/j.neuroimage.2012.07.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassabis D, Chu C, Rees G, Weiskopf N, Molyneux PD, Maguire EA. Decoding neuronal ensembles in the human hippocampus. Current Biology. 2009;19(7):546–554. doi: 10.1016/j.cub.2009.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holscher C, Jacob W, Mallot HA. Reward modulates neuronal activity in the hippocampus of the rat. Behavioral Brain Research. 2003;142(1–2):181–191. doi: 10.1016/S0166-4328(02)00422-9. [DOI] [PubMed] [Google Scholar]
- Howard MW, Kahana MJ. A distributed representation of temporal context. Journal of Mathematical Psychology. 2002;46(3):269–299. doi: 10.1006/Jmps.2001.1388. [DOI] [Google Scholar]
- Insausti R, Juottonen K, Soininen H, Insausti AM, Partanen K, Vainio P. MR volumetric analysis of the human entorhinal, perirhinal, and temporopolar cortices. AJNR American Journal of Neuroradiology. 1998;19(4):659–671. [PMC free article] [PubMed] [Google Scholar]
- Jenkins LJ, Ranganath C. Prefrontal and medial temporal lobe activity at encoding predicts temporal context memory. Journal of Neuroscience. 2010;30(46):15558–15565. doi: 10.1523/JNEUROSCI.1337-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy PJ, Shapiro ML. Motivational states activate distinct hippocampal representations to guide goal-directed behaviors. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(26):10805–10810. doi: 10.1073/pnas.0903259106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DH, Adalsteinsson E, Glover GH, Spielman DM. Regularized higher-order in vivo shimming. Magnetic Resonance in Medicine. 2002;48(4):715–722. doi: 10.1002/mrm.10267. [DOI] [PubMed] [Google Scholar]
- Knierim JJ, Lee I, Hargreaves EL. Hippocampal place cells: parallel input streams, subregional processing, and implications for episodic memory. Hippocampus. 2006;16(9):755–764. doi: 10.1002/hipo.20203. [DOI] [PubMed] [Google Scholar]
- Kriegeskorte N, Mur M, Bandettini P. Representational similarity analysis - connecting the branches of systems neuroscience. Frontiers in Systems Neuroscience. 2008;2:4. doi: 10.3389/neuro.06.004.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhl BA, Shah AT, DuBrow S, Wagner AD. Resistance to forgetting associated with hippocampus-mediated reactivation during new learning. Nature Neuroscience. 2010;13(4):501–506. doi: 10.1038/nn.2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenck-Santini PP, Save E, Poucet B. Evidence for a relationship between place-cell spatial firing and spatial memory performance. Hippocampus. 2001;11(4):377–390. doi: 10.1002/hipo.1052. [DOI] [PubMed] [Google Scholar]
- Levy WB. A sequence predicting CA3 is a flexible associator that learns and uses context to solve hippocampal-like tasks. Hippocampus. 1996;6(6):579–590. doi: 10.1002/(SICI)1098-1063(1996)6:6<579::AID-HIPO3>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Liang JC, Wagner AD, Preston AR. Content representation in the human medial temporal lobe. Cerebral Cortex. 2013;23(1):80–96. doi: 10.1093/cercor/bhr379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo AH, Tahsili-Fahadan P, Wise RA, Lupica CR, Aston-Jones G. Linking context with reward: a functional circuit from hippocampal CA3 to ventral tegmental area. Science. 2011;333(6040):353–357. doi: 10.1126/science.1204622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald CJ, Lepage KQ, Eden UT, Eichenbaum H. Hippocampal “time cells” bridge the gap in memory for discontiguous events. Neuron. 2011;71(4):737–749. doi: 10.1016/j.neuron.2011.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackiewicz KL, Sarinopoulos I, Cleven KL, Nitschke JB. The effect of anticipation and the specificity of sex differences for amygdala and hippocampus function in emotional memory. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(38):14200–14205. doi: 10.1073/pnas.0601648103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manns JR, Eichenbaum H. Evolution of declarative memory. Hippocampus. 2006;16(9):795–808. doi: 10.1002/hipo.20205. [DOI] [PubMed] [Google Scholar]
- McClelland JL, McNaughton BL, O’Reilly RC. Why there are complementary learning systems in the hippocampus and neocortex: insights from the successes and failures of connectionist models of learning and memory. Psychological Review. 1995;102(3):419–457. doi: 10.1037/0033-295X.102.3.419. [DOI] [PubMed] [Google Scholar]
- Mullally SL, Maguire EA. A new role for the parahippocampal cortex in representing space. Journal of Neuroscience. 2011;31(20):7441–7449. doi: 10.1523/jneurosci.0267-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumford JA, Turner BO, Ashby FG, Poldrack RA. Deconvolving BOLD activation in event-related designs for multivoxel pattern classification analyses. Neuroimage. 2012;59(3):2636–2643. doi: 10.1016/j.neuroimage.2011.08.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Keefe J, Conway DH. Hippocampal place units in the freely moving rat: why they fire where they fire. Experimental Brain Research. 1978;31(4):573–590. doi: 10.1007/BF00239813. [DOI] [PubMed] [Google Scholar]
- O’Keefe J, Speakman A. Single unit activity in the rat hippocampus during a spatial memory task. Experimental Brain Research. 1987;68(1):1–27. doi: 10.1007/BF00255230. [DOI] [PubMed] [Google Scholar]
- O’Reilly RC, Rudy JW. Conjunctive representations in learning and memory: principles of cortical and hippocampal function. Psychological Review. 2001;108(2):311–345. doi: 10.1037/0033-295X.108.2.311. [DOI] [PubMed] [Google Scholar]
- Olsen RK, Nichols EA, Chen J, Hunt JF, Glover GH, Gabrieli JD. Performance-related sustained and anticipatory activity in human medial temporal lobe during delayed match-to-sample. Journal of Neuroscience. 2009;29(38):11880–11890. doi: 10.1523/jneurosci.2245-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otten LJ, Quayle AH, Akram S, Ditewig TA, Rugg MD. Brain activity before an event predicts later recollection. Nature Neuroscience. 2006;9(4):489–491. doi: 10.1038/nn1663. [DOI] [PubMed] [Google Scholar]
- Park H, Rugg MD. Prestimulus hippocampal activity predicts later recollection. Hippocampus. 2009 doi: 10.1002/hipo.20663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeuffer J, Van de Moortele PF, Ugurbil K, Hu X, Glover GH. Correction of physiologically induced global off-resonance effects in dynamic echo-planar and spiral functional imaging. Magnetic Resonance in Medicine. 2002;47(2):344–353. doi: 10.1002/mrm.10065. [DOI] [PubMed] [Google Scholar]
- Preston AR, Bornstein AM, Hutchinson JB, Gaare ME, Glover GH, Wagner AD. High-resolution fMRI of Content-sensitive Subsequent Memory Responses in Human Medial Temporal Lobe. Journal of Cognitive Neuroscience. 2010;22(1):156–173. doi: 10.1162/jocn.2009.21195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruessner JC, Kohler S, Crane J, Pruessner M, Lord C, Byrne A. Volumetry of temporopolar, perirhinal, entorhinal and parahippocampal cortex from high-resolution MR images: considering the variability of the collateral sulcus. Cerebral Cortex. 2002;12(12):1342–1353. doi: 10.1093/cercor/12.12.1342. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Li LM, Serles W, Pruessner M, Collins DL, Kabani N. Volumetry of hippocampus and amygdala with high-resolution MRI and three-dimensional analysis software: minimizing the discrepancies between laboratories. Cerebral Cortex. 2000;10(4):433–442. doi: 10.1093/cercor/10.4.433. [DOI] [PubMed] [Google Scholar]
- Shohamy D, Adcock RA. Dopamine and adaptive memory. Trends in Cognitive Science. 2010;14(10):464–472. doi: 10.1016/j.tics.2010.08.002. [DOI] [PubMed] [Google Scholar]
- Singer AC, Frank LM. Rewarded outcomes enhance reactivation of experience in the hippocampus. Neuron. 2009;64(6):910–921. doi: 10.1016/j.neuron.2009.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark CE, Squire LR. When zero is not zero: the problem of ambiguous baseline conditions in fMRI. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(22):12760–12766. doi: 10.1073/pnas.221462998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turk-Browne NB, Simon MG, Sederberg PB. Scene representations in parahippocampal cortex depend on temporal context. Journal of Neuroscience. 2012;32(21):7202–7207. doi: 10.1523/JNEUROSCI.0942-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallenstein GV, Eichenbaum H, Hasselmo ME. The hippocampus as an associator of discontiguous events. Trends in Neuroscience. 1998;21(8):317–323. doi: 10.1016/S0166-2236(97)01220-4. [DOI] [PubMed] [Google Scholar]
- Wittmann BC, Schott BH, Guderian S, Frey JU, Heinze HJ, Duzel E. Reward-related FMRI activation of dopaminergic midbrain is associated with enhanced hippocampus-dependent long-term memory formation. Neuron. 2005;45(3):459–467. doi: 10.1016/j.neuron.2005.01.010. [DOI] [PubMed] [Google Scholar]
- Wolosin SM, Zeithamova D, Preston AR. Reward Modulation of Hippocampal Subfield Activation during Successful Associative Encoding and Retrieval. Journal of Cognitive Neuroscience. 2012;24(7):1532–1547. doi: 10.1162/jocn_a_00237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worsley KJ, Friston KJ. Analysis of fMRI time-series revisited–again. Neuroimage. 1995;2(3):173–181. doi: 10.1006/nimg.1995.1023. [DOI] [PubMed] [Google Scholar]
- Zeineh MM, Engel SA, Thompson PM, Bookheimer SY. Dynamics of the hippocampus during encoding and retrieval of face-name pairs. Science. 2003;299(5606):577–580. doi: 10.1126/science.1077775. [DOI] [PubMed] [Google Scholar]


