Abstract
Cells expressing both the regulatory T cell (Treg)-inducing transcription factor Foxp3 and the Th17 transcription factor RORγt have been identified (biTregs). It is unclear whether RORγt+Foxp3+ biTregs belong to the Th17-specific Treg17 cells, represent intermediates during Treg/Th17 transdifferentiation, or constitute a distinct cell lineage. Because the role of biTregs in inflammatory renal disease is also unknown, we studied these cells in the nephrotoxic nephritis (NTN) model of acute crescentic GN. Induction of NTN resulted in rapid renal and systemic expansion of biTregs. Notably, analyses of the biTreg expression profile revealed production of both anti-inflammatory (IL-10, IL-35) and proinflammatory (IL-17) cytokines. Additionally, biTregs expressed a signature of surface molecules and transcription factors distinct from those of Th17 cells and conventional Tregs (cTregs), and biTregs were identified in Treg17-deficient mice. Finally, fate reporter and cell transfer studies confirmed that biTregs are not Treg/Th17 transdifferentiating cells. Therapeutic transfer of biTregs suppressed the development of nephritis to an extent similar to that observed with transferred cTregs, but in vitro studies indicated different mechanisms of immunosuppression for biTregs and cTregs. Intriguingely, as predicted from their cytokine profile, endogenous biTregs displayed additional proinflammatory functions in NTN that were abrogated by cell-specific deletion of RORγt. In summary, we provide evidence that RORγt+Foxp3+ biTregs are a novel and independent bifunctional regulatory T cell lineage distinct from cTregs, Treg17 cells, and Th17 cells. Furthermore, biTregs appear to contribute to crescentic GN and hence may be novel therapeutic targets.
Keywords: glomerulonephritis, immunology and pathology, T cells
Even after decades of research, the immune system keeps surprising us with its complexity and ever-expanding number of cellular mediators. Especially in the field of regulatory T cells (Treg) observations of recent years have suggested a previously unrecognized high degree of diversity.1 Importantly, it was suggested that lineage-specific Tregs might exist which correspond to their proinflammatory Th counterpart.2–4 Treg1 cells preferentially downregulate Th1 responses, while Treg17 cells dampen Th17 responses. Interestingly, programming of these lineage-specific Tregs seems to rely on some of the same transcription factors needed for induction of the opposing Th cell population. Recently, using the nephrotoxic nephritis (NTN) model of crescentic GN, we could show that activation of the transcription factor Stat3 is not only crucial for generation of nephritogenic Th17 cells but also programs their protective Treg17 cell counterpart.4 This prompted the question whether other Th17-related transcription factors are active in regulatory T cells as well. Interestingly, pioneering work by the group of Eberl could show that not only Stat3 but also the second Th17 master transcription factor RORγt is expressed by a subpopulation of Foxp3+ Tregs (hereafter referred to as biTregs). These cells were shown to possess potent suppressive capacity and to secrete large amounts of IL-105, identifying them as Tregs. However in a follow-up study the same authors reported proinflammatory IL-17 expression in RORγt+Foxp3+ biTregs under inflammatory conditions mimicking fungal infection.6 This finding indicates both pro- and anti-inflammatory functions of biTregs and suggests a physiologic role during infection.
Importantly, RORγt+Foxp3+ biTregs were described to be regularly present in healthy humans as well, demonstrating trans-species conservation.7,8 Multiple studies by several independent groups could also show the presence of IL-17 secreting biTregs in patients with different inflammatory pathologies including ulcerative colitis,9 colonic cancer,10 psoriasis,11 peridontitis,12 and juvenile arthritis.13 While all these studies reported an association of biTregs with inflammation and tumors, their functional role remains widely unknown. Also, controversy exists over whether biTregs resemble a stable and unique T cell lineage or represent intermediates of Th17/Treg transdifferentiation.14–16 Given these uncertainties, it is not surprising that nothing is known about biTregs with respect to renal disease. Their contribution to renal injury, however, is quite likely because multiple studies by us and others could show crucial dependency of acute glomerulonephritis on Foxp3+ Tregs.17–21 Administration of Foxp3+ Tregs resulted in protection from GN while depletion of Foxp3+ Tregs was shown to greatly aggravate renal injury. Conversely, RORγt is the most potent master transcription factor for induction of pathogenic Th17 responses.22 In line, both RORγt and its target cytokine IL-17 were found to be central proinflammatory mediators of GN.4,20,23,24 It is thus tempting to speculate that cells expressing not only one but both of these potent transcription factors, RORγt and Foxp3, play a significant role in inflammatory renal disease. Importantly, given the dominant role of RORγt in development of pathogenic Th17 responses, multiple research groups and companies are in the process of establishing blocking agents.25–28 Therefore it is of great interest to better characterize all cell populations expressing RORγt, especially in the light of potential clinical applications. We thus decided to study the NTN model of acute GN to address the following aspects: (1) characterize RORγt+Foxp3+ biTreg dynamics during the course of GN, (2) determine whether biTregs represent Treg17 cells, Treg/Th17 intermediates or a unique cell population, (3) investigate the functional role of biTregs in GN.
Results
biTregs Rapidly Expand During the Course of Crescentic GN
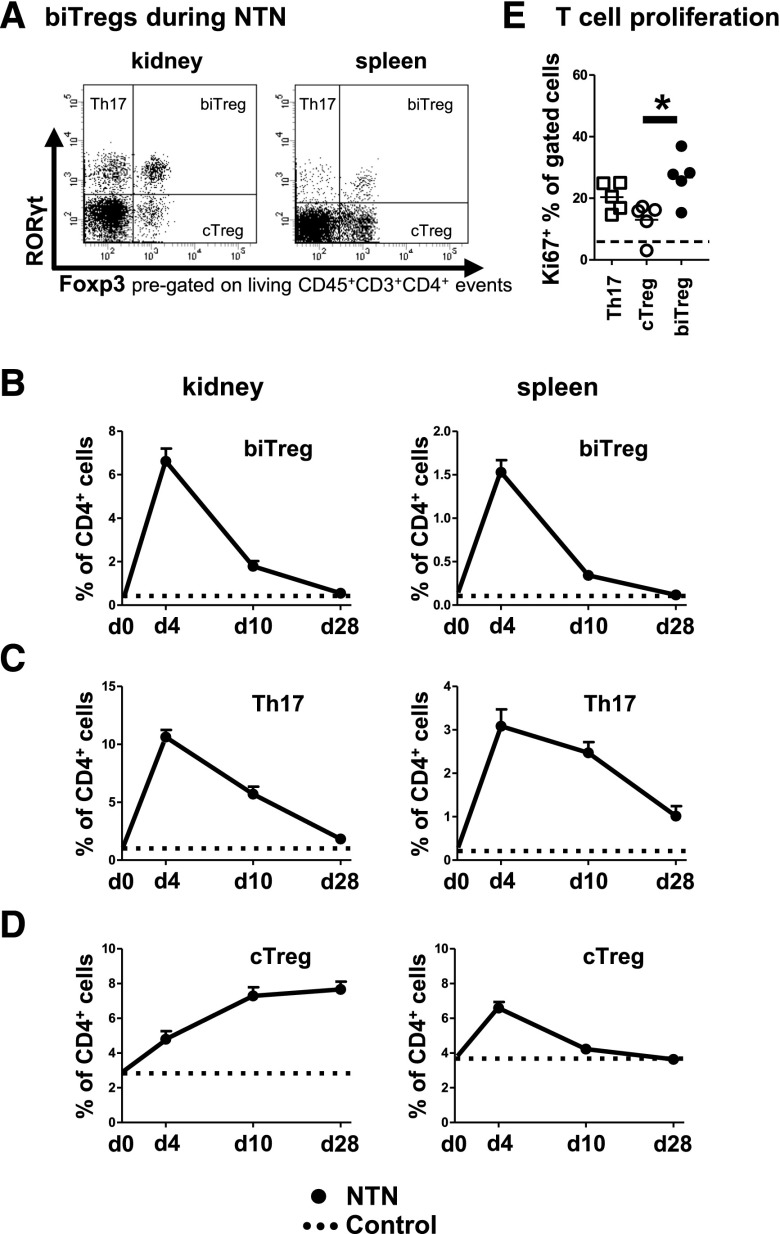
RORγt+Foxp3+ biTregs were found at low frequencies both systemically and locally in the kidneys of healthy mice. Induction of NTN, however, resulted in their rapid expansion in spleens and kidneys as compared with non-immunized control animals (Figure 1A and B). Over time, percentages decreased and were comparable to control levels at day 28 after NTN induction (Figure 1B). Interestingly, the dynamics of biTregs paralleled RORγt+ Th17 responses (Figure 1C). Percentages of conventional Foxp3+ Tregs (cTreg) in contrast, followed a different, more delayed time course and remained increased in the nephritic kidneys (Figure 1D). Expansion of biTregs probably occurred via proliferation, as indicated by very high Ki67 expression, which even exceeded levels observed in Th17 cells and cTregs (Figure 1E).
Figure 1.
biTregs rapidly expand during the course of crescentic glomerulonephritis. (A) Representative FACS analysis of renal (left) and systemic (right) RORγt+Foxp3− Th17, RORγt−Foxp3+ conventional Tregs (cTreg) and RORγt+Foxp3+ biTregs 10 days after NTN induction in wild-type (WT) mice. (B–D) For a time course study, frequencies of renal (left panels) and splenic (right panels) biTregs (B), Th17 cells (C) and cTregs (D) were determined at days 4 (n=7), 10 (n=10), and 28 (n=6). Dotted lines in (B–D) indicate untreated WT control mice (n=5). (E) Frequencies of Ki67+ proliferating cells among the indicated T cell subsets. Dashed line represents total CD4+ T cells (n=5). Circles and squares represent individual animals, horizontal lines indicate mean values. Error bars represent standard deviation. *P<0.05.
biTregs Show Co-Expression of Proinflammatory and Immune-Regulatory Molecules
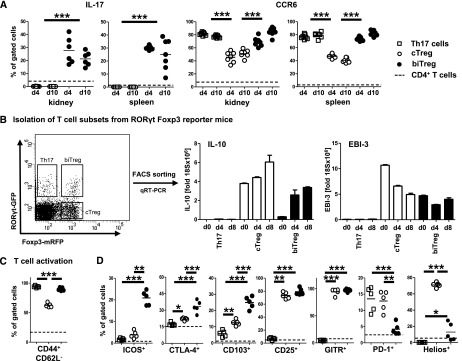
Analysis of splenic and renal biTregs revealed robust expression of IL-17, together with very high levels of the chemokine receptor CCR6 at days 4 and 10 after NTN induction (Figure 2A). In contrast, no IL-17 expression was detected in cTregs. Expression of the Th1-associated molecules IFNγ and chemokine receptor CXCR3 was only found at low levels in biTregs (not shown). Interestingly, analysis of mRNA from FACS sorted spleen cells of RORγt Foxp3 double reporter mice revealed strong expression of anti-inflammatory IL-10 and the IL-35 subunit EBI-3 (Figure 2B) in biTregs at days 4 and 8 after immunization with sheep IgG. In line with their observed rapid expansion, biTregs also showed very high levels of activation (Figure 2C). In addition, biTregs co-expressed a distinctive pattern of regulatory molecules and transcription factors, clearly differentiating them from Th17 cells and cTregs. Surface expression of ICOS, CTLA-4, and CD103 was strikingly enhanced. CD25 and GITR expression were similar to cTregs, while PD-1 and Helios expression in contrast were significantly reduced (Figure 2D). Furthermore, analyses of transcription factor mRNAs revealed lack of PZLF expression in biTregs, distinguishing them from Th17 cells (Supplemental Figure 1A). Finally, as opposed to cTregs, Blimp-1 expression was low in biTregs and similar to levels in Th17 cells (Supplemental Figure 1B).
Figure 2.
biTregs co-express proinflammatory and immune-regulatory molecules. (A) Renal and spleen cell IL-17 secretion (left) and CCR6 expression (right) was determined by flow cytometry for the indicated T cell subsets at 4 and 10 days after NTN induction in wild-type (WT) mice. (B) Th17 cells, biTregs, and cTregs were FACS sorted from MACS-enriched splenic CD4+ T cells from RORγtGFPFoxp3mRFP double reporter mice (FACS plot pre-gated on CD45+CD3+CD4+ events). Quantitation of IL-10 and EBI-3 by qRT-PCR before and at 4 and 8 days after sIgG immunization is shown. (C) T cell activation and (D) regulatory molecules for the indicated T cell subsets from WT spleen cells were FACS analyzed 6 days after sIgG immunization. Circles and squares represent individual animals, horizontal lines indicate mean values. Error bars represent standard deviation. *P<0.05; **P<0.01; ***P<0.001.
biTregs are Different from Treg17 Cells and do not Derive from Th17 Cells or cTregs
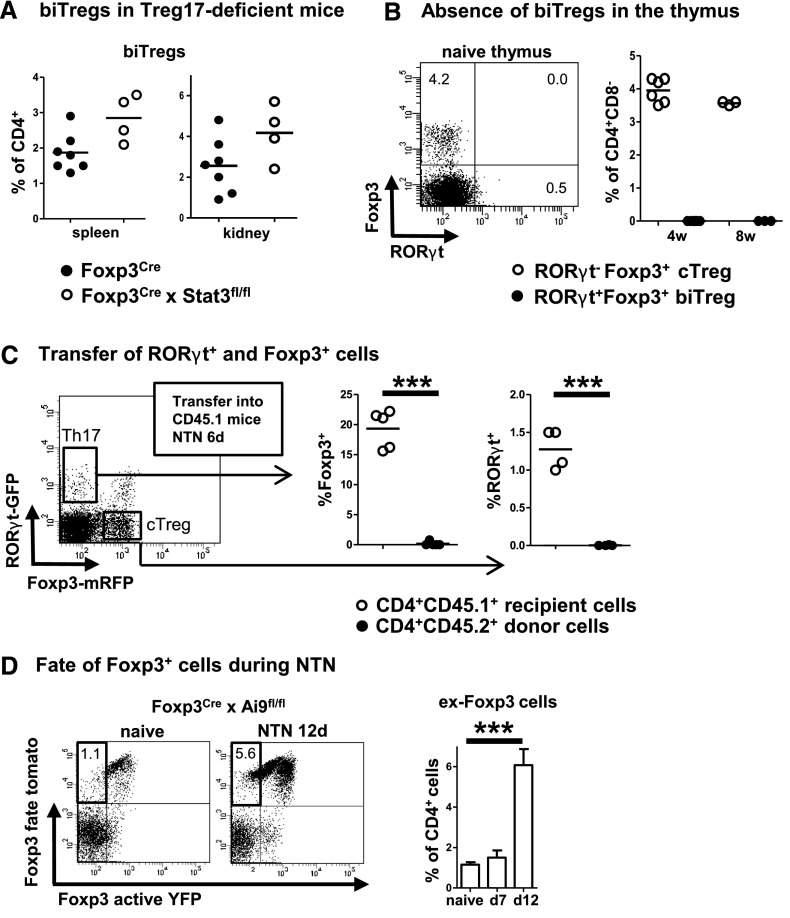
Next we wanted to establish whether biTregs are different from recently described Stat3-dependent Treg17 cells. Indeed, FACS analyses of kidneys and spleens from Treg17-deficient Foxp3Cre × STAT3flox mice revealed similar percentages of biTregs at day 4 after NTN induction, indicating their independent nature (Figure 3A). In order to investigate whether biTregs are a thymic or a peripherally induced Treg population, we analyzed thymi of 4- and 8-week-old naïve wild-type mice. Our analyses revealed complete absence of RORγt expression in CD4+Foxp3+ thymic Tregs in all animals studied (Figure 3B). Because this finding indicated a peripheral origin of biTregs, we next evaluated whether biTregs derive from RORγt+Foxp3− Th17 cells or Foxp3+RORγt− cTregs. Both populations were highly purified by FACS sorting from spleens of RORγt Foxp3 double reporter mice and independently transferred into CD45.1 recipient mice. Analysis of spleens and kidneys at 6 days after NTN induction showed that none of the transferred RORγt+ Th17 cells had upregulated Foxp3 and likewise, none of the Foxp3+ cTregs had upregulated RORγt (Figure 3C). Because transient upregulation of Foxp3 during T helper cell activation has been postulated, we next performed fate reporter studies. Analyses of Foxp3Cre-yfp activity reporter × Ai9 fate reporter mice showed that almost all cells in spleens of naïve mice that had expressed Foxp3 at some stage in life remained Foxp3 positive. NTN induction, however, resulted in growing percentages of fate positive Foxp3 cells that had lost Foxp3 expression (ex-Foxp3 cells). Interestingly, loss of Foxp3 occurred late during NTN between days 7 and 12 (Figure 3D), which coincides with retraction of the pool of biTregs (Figure 1B).
Figure 3.
biTregs are different from Treg17 cells and do not derive from Th17 or cTregs. (A) Frequencies of splenic and renal biTregs 4 days after NTN induction in Treg17-deficient Foxp3CrexStat3fl/fl mice and Foxp3Cre controls. (B) FACS analysis of thymocytes from naïve 4- and 8-week-old wild-type (WT) mice. A representative FACS plot from a 4-week-old mouse is shown on the left (pre-gated on CD45+CD3+CD4+CD8− cells). Quantitation of the indicated cell populations is shown on the right. (C) RORγt+Foxp3− Th17 cells and Foxp3+RORγt− cTregs were FACS sorted (plot pre-gated on CD45+CD3+CD4+ events) and transferred into CD45.1 recipients. Percentages of spleen cell Foxp3 and RORγt expression among live CD4+CD45.1+ recipient and CD4+CD45.2+ donor cells are shown (data from one representative of two independent experiments for each transfer study are shown). (D) Representative FACS plots of spleen cells from Foxp3Cre-YFP activity × Ai9tomato fate reporter mice from naïve and NTN treated mice at 12 days after induction (pre-gated on CD45+CD3+CD4+ events). ex-Foxp3 cells are found in the upper left quadrant. Quantification of ex-Foxp3 cells is shown as percentage of CD4+ cells (n=4 per group). Numbers in FACS plots indicate percentages of gated cells. Circles represent individual animals, horizontal lines indicate mean values. Error bars represent standard deviation. ***P<0.001.
biTregs do not Trans-Differentiate into cTregs or Th17 Cells
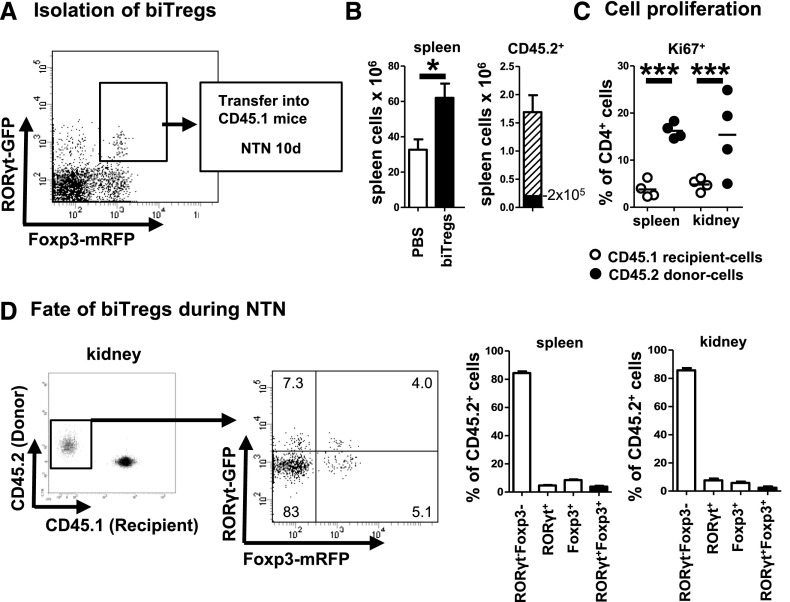
Next, we wanted to clarify the underlying mechanism of the observed contraction of biTregs in the later course of NTN. For this purpose, RORγt+Foxp3+ biTregs were highly purified by FACS sorting from CD45.2 double activity reporter mice and transferred into CD45.1 recipients (Figure 4A). Analyses at day 10 after NTN induction revealed significantly greater enlargement of spleens in transferred versus control animals. Calculated numbers of recovered CD45.2 cells in spleens alone were about 8 times higher than the transferred 2 × 105 cells (Figure 4B). In line, the transferred CD45.2 population in spleens and kidneys showed high proliferative activity much exceeding recipient CD45.1 cells (Figure 4C). Tracking the fate of the transferred biTregs interestingly revealed that the vast majority had downregulated both RORγt and Foxp3 in spleens and kidneys. Minor populations of similar percentages had downregulated either RORγt or Foxp3 while a fourth small population had maintained the RORγt+Foxp3+ biTreg phenotype (Figure 4D).
Figure 4.
biTregs do not trans-differentiate into Th17 or conventional Tregs. (A) biTregs were FACS sorted from spleens of CD45.2 Foxp3mRFP × RORγtGFP double reporter mice (representative FACS plot, pre-gated on CD45+CD3+CD4+ events) and transferred into CD45.1 WT mice 1 day prior to the induction of NTN. (B) Total spleen cells (left panel) were quantified at day 10 of NTN following the transfer of 2 × 105 biTregs (n=4 animals, black bar) or PBS (n=8 controls, white bar). Splenic CD45.2 donor cells had massively expanded (right panel, shaded bar). (C) Proliferation of renal and splenic donor and recipient CD4+ T cells as determined by Ki67 expression. (D) Splenic and renal RORγt and Foxp3 expression of CD45.2 donor cells at day 10 after NTN (FACS plots show a representative kidney, pre-gated on CD3+CD4+ events. Data from one representative of two independent transfer studies are shown). Quantification of the indicated subpopulations in spleens and kidneys is shown. Circles represent individual animals, horizontal lines indicate mean values. Error bars represent standard deviation. *P<0.05; ***P<0.001.
Exogenous biTregs Suppress Crescentic GN by Mechanisms Different from cTregs
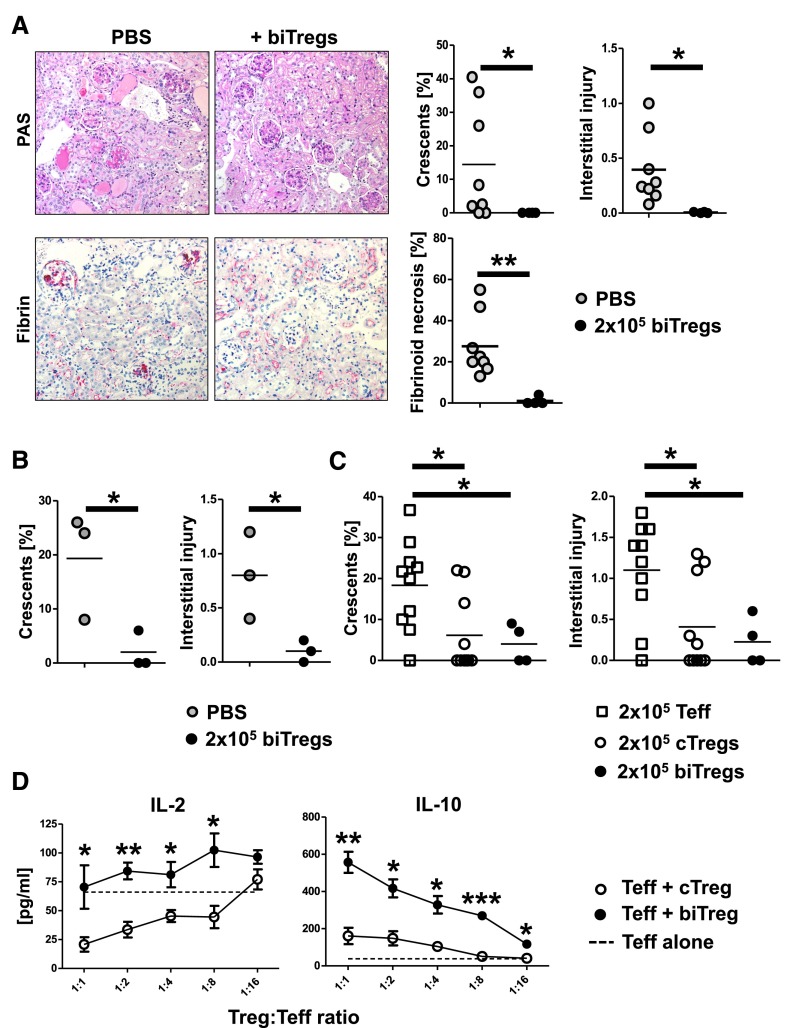
In a next step, we wanted to study the functional role of biTregs in GN. We thus adoptively transferred 2 × 105 biTregs or treated mice with PBS and analyzed kidneys at 10 days after NTN induction. Strikingly, glomerular injury, as measured by crescent formation and fibrinoid necrosis, was almost completely prevented by transfer of biTregs, while controls developed significant disease (Figure 5A). Similarly, tubulo-interstitial injury was greatly ameliorated in the group receiving biTregs (Figure 5A). In line, the renal proinflammatory cell infiltrate was significantly reduced in the biTreg group (Supplemental Figure 2A, C–E), while renal Foxp3+ Tregs were similar between the groups (Supplemental Figure 2B). In order to evaluate whether transfer of biTregs would also result in improved long-term outcome of nephritis, we repeated the experiment and evaluated renal damage at 30 days after NTN induction. Again we found significant protection from disease by transfer of biTregs (Figure 5B). Next, we wanted to compare the effects of exogenous biTregs with cTregs and Foxp3−CD4+ T effector cells (Teff). Analysis of kidneys from mice receiving either biTregs or cTregs showed significant and similar amelioration of renal injury at day 7 after NTN induction, when compared with Teff transferred mice (Figure 5C). Because both Treg populations effectively suppressed NTN, we next wanted to compare their mechanisms of immunosuppression by in vitro suppression assays. Surprisingly, analyses showed that only cTregs but not biTregs sufficiently reduced IL-2 levels in the supernatant of co-cultures with Teff. In contrast, however, biTregs much increased levels of the immunosuppressive cytokine IL-10 (Figure 5D), indicating different modes of action.
Figure 5.
Exogenous biTregs suppress GN by mechanisms different from cTregs. (A) Analyses of renal injury at day 10 of NTN in mice injected with PBS or 2 × 105 biTregs. Representative photographs of PAS or fibrin-stained (red) kidney sections (original magnification, ×200) and quantification of renal histologic damage (crescents, interstitial injury, and fibrinoid necrosis as indicated). (B) Quantification of renal histologic damage at day 30 of NTN in PBS or 2 × 105 biTreg injected mice. (C) Renal histologic damage was quantified at day 7 of NTN after transfer of either 2 × 105 Teff, cTregs, or biTregs. (D) In vitro suppression assays were performed by co-culturing Teff with either cTregs or biTregs at the indicated ratios (n=3 per group). Cytokine levels of IL-2 and IL-10 were analyzed in co-culture supernatants as indicated. Circles and squares represent individual animals, horizontal lines indicate mean values. Error bars represent standard deviation. *P<0.05; **P<0.01; ***P<0.001.
IL-17 Production by biTregs is Dependent on RORγt
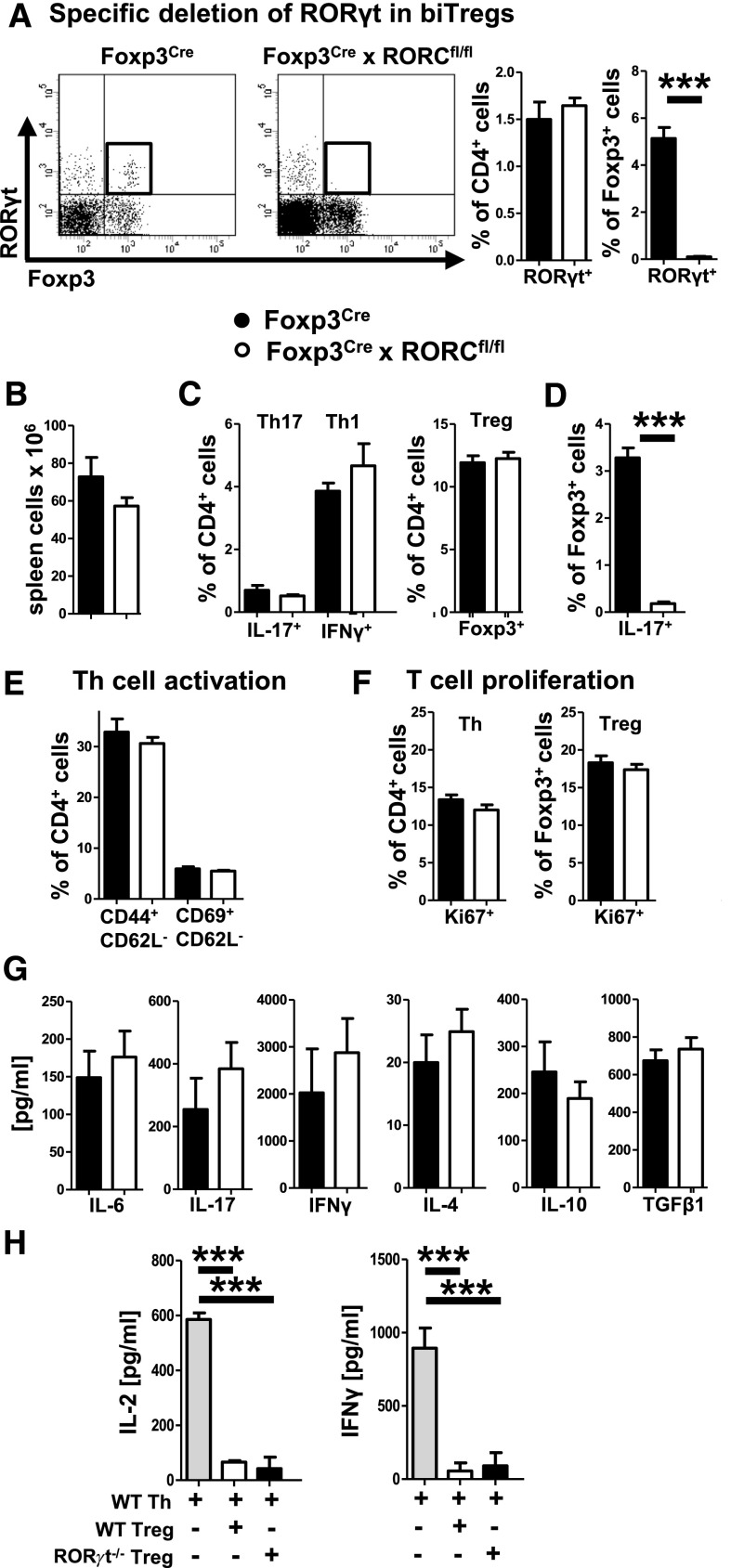
Next, we wanted to study the functional role of RORγt expression in biTregs. For this purpose, we generated Foxp3Cre × RORCflox/flox mice, because our experiments had shown that biTregs are the only Foxp3+ cell population expressing RORγt. In contrast to RORγt pan-knockout mice, Foxp3Cre × RORCflox/flox mice did not lack lymph nodes, indicating preserved RORγt activation in lymphoid tissue inducer cells. In line, spleen cell numbers and subset composition including total Foxp3+ Tregs and CD4+ T helper cells were similar in naïve wild-type and knockout mice (not shown). Importantly, however, analysis of systemic immunity at day 6 after immunization with sIgG showed complete absence of RORγt selectively in Foxp3+ cells of knockout animals (Figure 6A), proving defective RORγt activation in biTregs. Spleen cell numbers (Figure 6B) and IFNγ+ Th1, IL-17+ Th17 as well as total Foxp3+ Treg percentages were not different between the groups (Figure 6C). Most notably, however, IL-17 production by Foxp3+ T cells and thus by biTregs was abrogated in Foxp3Cre × RORCflox/flox mice (Figure 6D). T cell activation and proliferation were comparable (Figure 6, E and F). Similarly, production of various Th1, Th17, and Treg hallmark cytokines by spleen cells was not different (Figure 6G). Finally, we found that in vitro suppressive activity of RORγt-deficient Tregs was unchanged in comparison to wild-type Tregs (Figure 6H).
Figure 6.
IL-17 production by biTregs is dependent on RORγt. (A) Representative FACS plots of spleen cells 6 days after sheep IgG immunization of indicated mice (pre-gated on CD45+CD3+CD4+ events). Quantification of RORγt in CD4+ and Foxp3+ T cells in Foxp3Cre (n=11) and RORCflox/flox × Foxp3Cre mice (n=15). (B) Quantification of spleen cell numbers. (C) Splenic CD4+ subsets determined by FACS (gated as indicated). (D) IL-17 production by splenic Foxp3+ T cells. (E) T cell activation and (F) proliferation of the indicated subsets as determined by flow cytometry from spleen cells. (G) Spleen cell secretion of the indicated cytokines determined by ELISA. (H) In vitro suppression of T helper cell (Th) produced IL-2 and IFNγ by WT and RORγt−/− Tregs as determined by ELISA. Error bars represent standard deviation. ***P<0.001.
GN is Ameliorated by Deletion of RORγt in biTregs
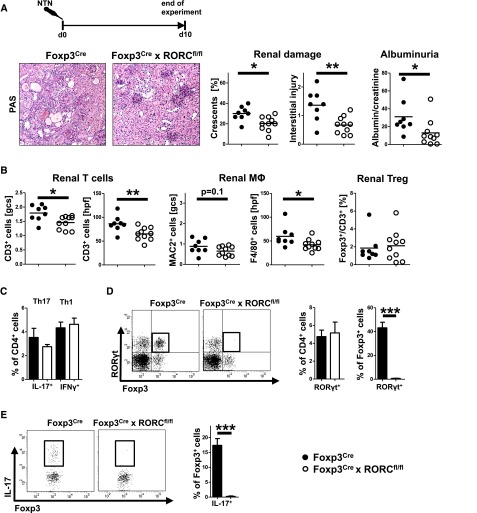
Next, we wanted to know whether RORγt signaling in biTregs confers them with pathogenic properties. Indeed, analyses at day 10 after NTN induction revealed amelioration of nephritis in Foxp3Cre × RORCflox/flox animals in terms of histologic damage and albuminuria (Figure 7A). Furthermore, proinflammatory renal leukocyte infiltration was significantly reduced while renal Foxp3+ total Treg frequencies remained similar (Figure 7B). Likewise, percentages of renal Th1 and Th17 cells were comparable (Figure 7C). Importantly, however, RORγt expression was specifically absent in renal Foxp3+ cells of Foxp3Cre × RORCflox/flox animals, confirming lack of RORγt activation in biTregs (Figure 7D). In line, we found robust expression of IL-17 in renal Tregs of wild-type mice which was completely abrogated in knockouts (Figure 7E). Analysis of systemic immune responses showed slightly reduced spleen cell numbers in knockout mice but otherwise comparable subset composition. In particular, similar percentages of total Tregs and Th17 cells were observed (Supplemental Figure 3A). Also, levels of IL-17 production by spleen cells were not different while IL-6 production was even enhanced in knockouts. Similarly, Treg characteristic cytokines were unaltered (TGF-β) or even elevated (IL-10, Supplemental Figure 3B). Humoral immune responses as measured by antigen-specific antibody production were not altered by RORγt deficiency in biTregs (Supplemental Figure 3C). In order to validate these findings in a second model, we studied accelerated NTN (aNTN).20 Mice were preimmunized with sheep IgG and nephritis was induced 5 days later, at a time point when biTregs have massively expanded. Analysis at day 7 after aNTN induction again showed ameliorated renal damage in Foxp3Cre × RORCflox/flox mice (Supplemental Figure 4A). Renal proinflammatory leukocyte infiltration was reduced while frequencies of renal Tregs were similar (Supplemental Figure 4B). The renal Th1 and Th17 equilibrium again remained unchanged (Supplemental Figure 4C).
Figure 7.
Crescentic GN is ameliorated by deletion of RORγt in biTregs. (A) Experimental set-up and representative photographs of PAS-stained kidney sections (original magnification, ×200) with quantification of renal histologic damage (crescents and interstitial damage) and albuminuria (albumin/creatinine). (B) Quantification of glomerular and interstitial T cells (CD3), macrophages (MAC2, F4/80), and Treg percentages (Foxp3/CD3). (C) FACS analyses of renal Th1 and Th17 cells. (D) Analysis of RORγt expression in the indicated renal leukocyte subsets (a representative FACS plot is shown, pre-gated on CD45+CD3+CD4+ events.). (E) Analysis of IL-17 production by renal Foxp3+ cells (a representative FACS plot is shown, pre-gated on CD45+CD3+CD4+Foxp3+ events). Circles represent individual animals, horizontal lines indicate mean values. Error bars represent standard deviation. *P<0.05; **P<0.01; ***P<0.001.
Discussion
Our study aimed to better characterize the biology of RORγt+Foxp3+ biTregs and define their role in acute crescentic GN. This is of special importance because the Th17 defining transcription factor RORγt has proven to be a potent proinflammatory mediator during glomerulonephritis20 and multiple blocking agents have been developed and await clinical testing.25–28 Our analyses showed regular presence of biTregs in both spleens and kidneys of healthy mice. Interestingly, we observed a rapid and massive biTreg expansion early during the course of NTN which paralleled Th17 cells. Conventional Tregs (cTregs), in contrast, showed different and more delayed dynamics. Proliferation rates of biTregs were very high, indicating that they increase numbers by cell division rather than recruitment or transdifferentiation from other cell types. Further characterization of biTregs showed a bifunctional pro- and anti-inflammatory profile. They express high levels of IL-17 and the Th17-characteristic chemokine receptor CCR6. At the same time, however, biTregs secrete high amounts of anti-inflammatory IL-10 and IL-35. biTregs also showed quite high levels of activation, indicating their functional importance. Detailed analyses of surface expression of immune-modulatory molecules revealed a unique signature differing from both Th17 cells and cTregs. As a main hallmark, we found enhanced levels of ICOS on biTregs, which is in line with two previous reports.5,29 Interestingly, ICOS expression on Tregs has also been reported to be associated with IL-17 production.30 Furthermore, biTregs showed a uniquely strong expression of CTLA-4 and also the Integrin CD103 which was previously described to be a marker for regulatory T cells with the highest suppressive capacity.31 CD25 and GITR expression was high and similar to cTregs, PD-1, and Helios expression in contrast was significantly reduced. Further analyses also revealed a unique transcription factor signature, different from Th17 cells and cTregs. biTregs lacked mRNA expression of the transcription factor PZLF, which was recently shown to be characteristic for naturally occurring nTh17 cells.32 Similarly, we noted that Blimp-1, a transcription factor implicated in Treg effector functions,33 was only weakly expressed in biTregs. Collectively, these findings suggested that biTregs represent a unique and independent cell lineage with both suppressive and proinflammatory properties at the same time.
Next, we wanted to study the developmental origin of biTregs. Recently, a Treg subset specialized at downregulating Th17 responses has been described. These Treg17 cells depend on activation of the transcription factor Stat3.4,34 Because Stat3 is a known inducer of RORγt, we aimed to investigate whether biTregs might belong to this newly identified Treg subset. However, biTregs were present at normal percentages in mice lacking Stat3 activation in Tregs, indicating that they are a population different from Treg17. Next, we addressed the question of whether biTregs are a thymic or a peripherally induced Treg subset. To this end, analyses of thymi showed complete absence of biTregs, which indicates peripheral induction. We thus aimed to investigate whether biTregs might arise in the periphery from RORγt single positive Th17 cells or Foxp3 single positive cTregs and performed fate reporter and cell transfer studies. Highly purified RORγt+Foxp3− Th17 cells and Foxp3+RORγt− cTregs were transferred and their fate was analyzed in spleens and kidneys at day 6 after NTN induction, a time point when biTregs are fully expanded. Results showed that none of the Th17 cells had upregulated Foxp3 and likewise none of the cTregs had started to express RORγt. We could thus conclude that biTregs do not derive from Th17 cells or cTregs. Our findings therefore support the Foxp3 lineage heterogeneity model recently proposed by Hori35 rather than Foxp3/Th17 lineage plasticity. However, it has also been postulated that Foxp3 might be transiently induced in non-Treg CD4+ T cells during their activation.36–39 We therefore tracked the fate of Foxp3+ cells continuously during NTN using fate reporter mice. Analyses showed that almost all Foxp3+ cells in naïve mice had maintained Foxp3 activation during their life. Likewise, during the first 7 days after NTN induction we did not detect loss of Foxp3 expression in fate positive cells, excluding unspecific and temporary Foxp3 upregulation during T effector cell activation. During later stages of NTN, however, a growing fraction of Foxp3 fate positive cells showed loss of Foxp3. These ex-Foxp3 cells were generated at a time point (12 days after NTN) at which numbers of cTregs are stable while numbers of biTregs rapidly decline. We thus wanted to answer the question of whether the observed retraction of biTregs might be due to loss of Foxp3 and/or transdifferentiation into Th17 cells. To answer this question, we adoptively transferred highly purified CD45.2 biTregs cells into CD45.1 animals. At day 10 of NTN we found massive enlargement of spleens in the recipient animals containing numbers of CD45.2 cells that exceeded the transferred numbers by 10-fold. In line, CD45.2 donor-derived cells had a high proliferative activity much exceeding that of CD4+CD45.1 recipient cells. Interestingly, analysis of donor cell fate revealed that the vast majority had downregulated both Foxp3 and RORγt activation. Small and similarly sized fractions had downregulated either Foxp3 or RORγt with no tendency for preferential transdifferentiation into RORγt+Foxp3− Th17 or conventional Foxp3+RORγt− cTregs. These findings support a concept in which RORγt+Foxp3+ biTregs represent a distinct cell lineage which rapidly expands from the pre-existing pool in naïve mice by inflammation-induced proliferation. Subsequently the cells retract and downregulate both transcription factors. After clarifying the dynamics and fate of biTregs, we aimed to study their function. We thus adoptively transferred biTregs into wild-type recipient mice and subsequently induced nephritis. In line with their Foxp3 expression and similar to the observations from Lochner et al.,5 biTregs showed regulatory capacity and potently protected from renal injury. Importantly, protective effects by exogenous biTregs were maintained long term and also ameliorated renal injury at 30 days after disease induction. Furthermore, additional transfer studies comparing biTregs with cTregs showed a similar degree of protection from glomerulonephritis by both populations. However, given the striking differences between cTregs and biTregs on multiple levels as cytokine profile, surface molecule signature and transcription factor expression, we wanted to explore whether they also differ functionally. Indeed, in vitro suppression assays indicated different mechanisms of immunosuppression. While only cTregs sufficiently suppressed IL-2 levels in co-culture with effector T cells, biTregs much enhanced secretion of the anti-inflammatory cytokine IL-10. These data identified biTregs as unique regulatory T cells with a sum effect that is anti-inflammatory in nephritis. However, we suspected that biTregs might have some pathogenic potential as well. In particular, because RORγt expression is strongly associated with proinflammatory properties20 and we observed robust secretion of IL-17 by biTregs. We thus generated mice with selective RORγt deficiency in Foxp3+ Tregs. Because our previous experiments showed that no other Foxp3+ cell population apart from biTregs activates RORγt, this is effectively a knockout of RORγt in biTregs. Development of mice with RORγt-deficient biTregs was normal and they did not show any signs of spontaneous autoimmunity, indicating intact Treg suppressive function. Analysis of immune responses, however, showed complete lack of IL-17 secretion by biTregs in the knockouts. It is of note that Treg and Th17 responses appeared otherwise unaltered, which further underlines the independent character of biTregs. Importantly, NTN severity was much ameliorated in the knockout mice, proving a proinflammatory role of RORγt expression in biTregs. In order to validate these findings in a second model, we also studied the course of accelerated NTN20 and again found protection from renal injury in the absence of RORγt activation in biTregs.
Our data thus lead to the conclusion that biTregs possess both potent anti-inflammatory functions but also some proinflammatory, RORγt-mediated properties. This bifunctional nature might seem illogical at first sight. However, biTregs could have evolutionarily developed to fill an important gap between mediators of host defense and tissue protection. Their unique properties equip them to fight pathogens while they can protect us from collateral tissue injury and development of autoimmunity at the same time. Importantly, their proinflammatory functions can be blocked by abrogation of RORγt signaling, which makes biTregs a promising target for RORγt-directed therapies of inflammatory diseases.
In summary our studies identify biTregs as novel mediators of glomerulonephritis. biTregs represent a previously unrecognized independent and bifunctional regulatory T cell lineage with great potential for future therapeutic strategies.
Concise Methods
Animals
LoxP-site flanked RORCfl/fl and RORC−/− mice were obtained from The Jackson Laboratory. Stat3fl/fl mice were a generous gift from Shizuo Akira, Osaka University, Japan. Foxp3YFP-Cre mice were a kind gift from Alexander Y. Rudensky, Memorial Sloan-Kettering Cancer Center, New York. BAC-transgenic Rorc(γt)-GfpTG x Fir (Foxp3-IRES-mRFP) mice5 were kindly provided by Gerard Eberl, Paris, France and Matthias Lochner, Hannover, Germany. Ai9 mice (B6.Cg-Gt(ROSA)26Sortm9(CAG-tdTomato)Hze/J) were kindly provided by Edgar Kramer, Hamburg, Germany. CD45.1 mice initially derived from The Jackson Laboratory. All animals used in this study were on a C57BL/6 background and were raised under specific pathogen-free conditions at our animal facility.
Animal Experiments and Functional Studies
Nephrotoxic nephritis was induced in 8- to 10-week-old male mice of the indicated genotypes by i.p. injection of 2.5 mg of nephrotoxic sheep serum per gram body weight.4 For accelerated NTN (aNTN), mice were i.p. pre-immunized with 0.5 mg sheep IgG in complete Freund’s adjuvant at day −5. NTN was induced at day 0.20 For immunization studies, mice were i.p. immunized with 0.5 mg sheep IgG in complete Freund’s adjuvant and organs were harvested at day 6. For transdifferentiation studies, FACS sorted CD45.2 splenic Foxp3+ (5 × 105 cells per animal) or RORγt+ single positive CD4+ T cells (1.5 × 105 cells per animal) from naïve Rorc(γt)-GfpTG x Fir (Foxp3-IRES-mRFP) double reporter donor mice were i.v. injected into the CD45.1 recipient tail vein. For nephritis studies, FACS sorted RORγt+Foxp3+ biTregs, RORγt−Foxp3+ cTregs or Foxp3−CD4+ T effector cells (2 × 105 cells per animal) from spleens of double reporter donor mice were i.v. injected into the wild-type recipient tail vein. Organs were harvested between 4 and 30 days after immunization or NTN induction as indicated. Animal experiments were performed according to national and institutional animal care and ethical guidelines and were approved by local committees (approval codes G37/11, G45/12, 73/14, and 07/15). Urine samples were collected after housing the mice in metabolic cages. Albuminuria was determined by standard ELISA (Bethyl Laboratories). Blood urea nitrogen (BUN) and urinary creatinine were quantified using standard laboratory methods.
Morphologic Studies
Crescent formation and glomerular necrosis were determined in a minimum of 50 glomeruli per mouse in 2 µm thick PAS-stained kidney sections in a blinded manner. Semiquantitative analysis of tubulointerstitial damage was performed using ten randomly selected cortical areas (×200) as described previously.20 Paraffin-embedded sections were stained with antibodies directed against murine fibrin alpha chain (UC45; Abcam, Inc., Cambridge, UK), CD3 (A0452; Dako, Hamburg, Germany), F4/80 (BM8; BMA Biomedicals, Hiddenhausen, Germany), MAC2 (M3/38; Cedarlane-Laboratories, Burlington, ON, Canada), GR-1 or Foxp3 and developed with a polymer-based secondary antibody–alkaline phosphatase kit (POLAP; Zytomed, Berlin, Germany), as published previously.40 Fifty glomerular cross-sections (gcs) and 30 tubulointerstitial high power fields (hpf, magnification, ×400) per kidney section were counted in a blinded fashion.
Isolation of Leukocytes from Various Tissues
Spleens or thymi were harvested in HBSS and passed through 70 µm nylon meshes. After lysis of erythrocytes with ammonium chloride, cells were washed and passed over 40 µm meshes. Cells were then washed again, counted and resuspended in PBS for either culture or FACS analysis. Kidneys were minced and incubated in digestion medium (RPMI 1640 medium containing 10% FCS, 1% HEPES, 1% penicillin/streptomycin, 8 µg/ml collagenase D and 0.4 µg/ml DNase) at 37°C for 40 minutes. Tissues were then homogenized, passed over 70 µm nylon meshes and centrifuged at 300 g at 4°C for 8 minutes. After lysis of erythrocytes with ammonium chloride, cells were filtered over 40 µm meshes, washed again and sedimented for 15 minutes at 4°C. The upper leukocyte fraction was aspirated and filtered through another 40 µm nylon mesh. Cells were washed, counted and resuspended in PBS for staining and FACS analysis.
Antigen-Specific Systemic Cellular and Humoral Immune Responses
Splenocytes (4×106 cells/ml) were cultured under standard conditions in the presence of normal sheep IgG (10 µg/ml, Sigma-Aldrich, Taufkirchen, Germany) and supernatants were harvested after 72 hours. Commercially available ELISAs were used for detection of IFNγ, IL-4, IL-6, TNFα, IL-10, IL-17A (Biolegend, San Diego, CA), and TGFβ1, IL-2 (R&D Systems, Minneapolis, MN). Circulating sheep-globulin-specific serum IgG titers were analyzed by ELISA (for total IgG; Biozol, Eching, Germany, for IgG1, IgG2c and IgG3; Invitrogen, Frederick, MD).
Flow Cytometry
Cells were surface-stained for 30 minutes at 4°C with fluorochrome-labeled antibodies against CD45, CD45.1, CD45.2, CD3, CD4, CD8, CD19, CD25, CD44, CD69, CD62L, CCR6, CXCR3, ICOS, CTLA-4, PD-1, GITR, and CD103 (Ebioscience, San Diego, CA) as previously described.4
For intracellular and intranuclear staining, samples were processed using a commercial intranuclear staining kit (Foxp3-Kit; Ebioscience). Fluorochrome-labeled antibodies against IL-17, IFNγ, Foxp3, Ki67, T-Bet (all Ebioscience), RORγt (BD Biosciences, Heidelberg, Germany), and Helios (Biolegend) were employed as recently published.4 For intracellular cytokine staining, cells were activated with PMA (50 ng/ml; Sigma-Aldrich) and ionomycin (1 µg/ml; Calbiochem-Merck) for 4 hours. After 30 minutes of incubation, Brefeldin A (10 µg/ml; Sigma-Aldrich) was added. LIVE/DEAD staining (Invitrogen Molecular Probes, Eugene, OR) was used to exclude dead cells during flow cytometry and to ensure viability of the cells after the stimulation procedure. Experiments were performed on a BD LSRII cytometer (Becton Dickinson, Germany). FACS sorting was performed from single-cell suspensions enriched for CD4+ T cells by MACS sorting (T-cell isolation kit II; Miltenyi Biotec, Germany) from the indicated tissues and animal strains by the institutional HEXT FACS Sorting Core facility using a BD ARIAIII Cytometer (Becton Dickinson, Germany) as previously described.4
Treg Suppression Assay
Total Tregs and effector T cells from wild-type and RORC−/− mice were isolated from splenic single-cell suspensions by MACS according to the manufacturer’s instructions (MACS CD4+ T-Cell-Isolation Kit; Miltenyi Biotec). Briefly, CD4+ T cells were enriched using a biotinylated antibody cocktail, depleting all other blood cell types with anti-biotin microbeads. CD4+CD25+ regulatory T cells were isolated by positive selection using PE-labeled anti-CD25 mAb and anti-PE microbeads. For comparison of cTregs with biTregs, Foxp3+RORγt− cTregs, RORγt+Foxp3+ biTregs and CD4+Foxp3−Teff were isolated by FACS sorting from spleens of naïve Rorc(γt)-GfpTG x Fir (Foxp3-IRES-mRFP) double reporter mice. 1 × 105 effector T cells were cultured for 72 hours in anti-CD3 mAb (5 µg/ml; BD Biosciences) pre-coated 96-well plates either alone or in co-culture with total Tregs, cTregs or biTregs at different ratios as indicated. Suppressive capacity was determined by cytokine ELISAs performed from the supernatants.18
Quantitative Real-Time PCR Analysis
Quantitative RT-PCR from RNA derived from FACS sorted cells was performed in a Stepone Plus detector (Applied Biosystems) as described before.41 For detection of IL-10 and 18S, the sybr green method was used (primer sequences available upon request) while detection of EBI-3, PZLF and Blimp-1 was performed using Taqman probes (Applied Biosystems assay id: Mm00469294_m1, Mm01176868_m1, Mm00476128_m1). Samples were run in duplicates and normalized to 18S rRNA.
Statistical Analyses
Results are expressed as mean±SD. In the case of two groups, comparison was performed by t test and a P value <0.05 was considered statistically significant. For studies with more than two experimental groups, one-way ANOVA was applied using Tukey post hoc testing.
Disclosures
None.
Supplementary Material
Acknowledgments
We are indebted to Professor Gerard Eberl, Paris, France, and Dr. Matthias Lochner, Hannover, Germany for providing Rorc(γt)-GfpTG x Fir (Foxp3-IRES-mRFP) mice and critical evaluation of the manuscript.
We also thank Edgar Kramer, Hamburg, for providing Ai9 mice. We thank M. Schaper, M. Reszka, C. Meyer-Schwesinger and the FACS core unit, UKE Hamburg for their excellent technical help.
This work was supported by grants from the Deutsche Forschungsgemeinschaft (STE 1822/2-1 and KFO 228 STE 1822/3-1) to OMS.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2014090880/-/DCSupplemental.
References
- 1.Feuerer M, Hill JA, Mathis D, Benoist C: Foxp3+ regulatory T cells: differentiation, specification, subphenotypes. Nat Immunol 10: 689–695, 2009 [DOI] [PubMed] [Google Scholar]
- 2.Ohkura N, Kitagawa Y, Sakaguchi S: Development and maintenance of regulatory T cells. Immunity 38: 414–423, 2013 [DOI] [PubMed] [Google Scholar]
- 3.Liston A, Gray DH: Homeostatic control of regulatory T cell diversity. Nat Rev Immunol 14: 154–165, 2014 [DOI] [PubMed] [Google Scholar]
- 4.Kluger MA, Luig M, Wegscheid C, Goerke B, Paust HJ, Brix SR, Yan I, Mittrücker HW, Hagl B, Renner ED, Tiegs G, Wiech T, Stahl RA, Panzer U, Steinmetz OM: Stat3 programs Th17-specific regulatory T cells to control GN. J Am Soc Nephrol 25: 1291–1302, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lochner M, Peduto L, Cherrier M, Sawa S, Langa F, Varona R, Riethmacher D, Si-Tahar M, Di Santo JP, Eberl G: In vivo equilibrium of proinflammatory IL-17+ and regulatory IL-10+ Foxp3+ RORgamma t+ T cells. J Exp Med 205: 1381–1393, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Osorio F, LeibundGut-Landmann S, Lochner M, Lahl K, Sparwasser T, Eberl G, Reis e Sousa C: DC activated via dectin-1 convert Treg into IL-17 producers. Eur J Immunol 38: 3274–3281, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ayyoub M, Deknuydt F, Raimbaud I, Dousset C, Leveque L, Bioley G, Valmori D: Human memory FOXP3+ Tregs secrete IL-17 ex vivo and constitutively express the T(H)17 lineage-specific transcription factor RORgamma t. Proc Natl Acad Sci U S A 106: 8635–8640, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Voo KS, Wang YH, Santori FR, Boggiano C, Wang YH, Arima K, Bover L, Hanabuchi S, Khalili J, Marinova E, Zheng B, Littman DR, Liu YJ: Identification of IL-17-producing FOXP3+ regulatory T cells in humans. Proc Natl Acad Sci U S A 106: 4793–4798, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hovhannisyan Z, Treatman J, Littman DR, Mayer L: Characterization of interleukin-17-producing regulatory T cells in inflamed intestinal mucosa from patients with inflammatory bowel diseases. Gastroenterology 140: 957–965, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blatner, NR, Mulcahy, MF, Dennis, KL, Scholtens, D, Bentrem, DJ, Phillips, JD, Ham, S, Sandall, BP, Khan, MW, Mahvi, DM, Halverson, AL, Stryker, SJ, Boller, AM, Singal, A, Sneed, RK, Sarraj, B, Ansari, MJ, Oft, M, Iwakura, Y, Zhou, L, Bonertz, A, Beckhove, P, Gounari, F, Khazaie, K: Expression of RORγt marks a pathogenic regulatory T cell subset in human colon cancer. Sci Transl Med 4: 164ra159, 2012 [DOI] [PMC free article] [PubMed]
- 11.Bovenschen HJ, van de Kerkhof PC, van Erp PE, Woestenenk R, Joosten I, Koenen HJ: Foxp3+ regulatory T cells of psoriasis patients easily differentiate into IL-17A-producing cells and are found in lesional skin. J Invest Dermatol 131: 1853–1860, 2011 [DOI] [PubMed] [Google Scholar]
- 12.Okui T, Aoki Y, Ito H, Honda T, Yamazaki K: The presence of IL-17+/FOXP3+ double-positive cells in periodontitis. J Dent Res 91: 574–579, 2012 [DOI] [PubMed] [Google Scholar]
- 13.Pesenacker AM, Bending D, Ursu S, Wu Q, Nistala K, Wedderburn LR: CD161 defines the subset of FoxP3+ T cells capable of producing proinflammatory cytokines. Blood 121: 2647–2658, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou X, Bailey-Bucktrout SL, Jeker LT, Penaranda C, Martínez-Llordella M, Ashby M, Nakayama M, Rosenthal W, Bluestone JA: Instability of the transcription factor Foxp3 leads to the generation of pathogenic memory T cells in vivo. Nat Immunol 10: 1000–1007, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bailey-Bucktrout SL, Martinez-Llordella M, Zhou X, Anthony B, Rosenthal W, Luche H, Fehling HJ, Bluestone JA: Self-antigen-driven activation induces instability of regulatory T cells during an inflammatory autoimmune response. Immunity 39: 949–962, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Komatsu N, Okamoto K, Sawa S, Nakashima T, Oh-hora M, Kodama T, Tanaka S, Bluestone JA, Takayanagi H: Pathogenic conversion of Foxp3+ T cells into TH17 cells in autoimmune arthritis. Nat Med 20: 62–68, 2014 [DOI] [PubMed] [Google Scholar]
- 17.Wolf D, Hochegger K, Wolf AM, Rumpold HF, Gastl G, Tilg H, Mayer G, Gunsilius E, Rosenkranz AR: CD4+CD25+ regulatory T cells inhibit experimental anti-glomerular basement membrane glomerulonephritis in mice. J Am Soc Nephrol 16: 1360–1370, 2005 [DOI] [PubMed] [Google Scholar]
- 18.Paust HJ, Ostmann A, Erhardt A, Turner JE, Velden J, Mittrücker HW, Sparwasser T, Panzer U, Tiegs G: Regulatory T cells control the Th1 immune response in murine crescentic glomerulonephritis. Kidney Int 80: 154–164, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ooi JD, Snelgrove SL, Engel DR, Hochheiser K, Ludwig-Portugall I, Nozaki Y, O’Sullivan KM, Hickey MJ, Holdsworth SR, Kurts C, Kitching AR: Endogenous foxp3(+) T-regulatory cells suppress anti-glomerular basement membrane nephritis. Kidney Int 79: 977–986, 2011 [DOI] [PubMed] [Google Scholar]
- 20.Steinmetz OM, Summers SA, Gan PY, Semple T, Holdsworth SR, Kitching AR: The Th17-defining transcription factor RORγt promotes glomerulonephritis. J Am Soc Nephrol 22: 472–483, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kurts C, Panzer U, Anders HJ, Rees AJ: The immune system and kidney disease: basic concepts and clinical implications. Nat Rev Immunol 13: 738–753, 2013 [DOI] [PubMed] [Google Scholar]
- 22.Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, Cua DJ, Littman DR: The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell 126: 1121–1133, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Paust HJ, Turner JE, Steinmetz OM, Peters A, Heymann F, Hölscher C, Wolf G, Kurts C, Mittrücker HW, Stahl RA, Panzer U: The IL-23/Th17 axis contributes to renal injury in experimental glomerulonephritis. J Am Soc Nephrol 20: 969–979, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Turner JE, Paust HJ, Steinmetz OM, Panzer U: The Th17 immune response in renal inflammation. Kidney Int 77: 1070–1075, 2010 [DOI] [PubMed] [Google Scholar]
- 25.Huh JR, Englund EE, Wang H, Huang R, Huang P, Rastinejad F, Inglese J, Austin CP, Johnson RL, Huang W, Littman DR: Identification of potent and selective diphenylpropanamide RORγ inhibitors. ACS Med Chem Lett 4: 79–84, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Solt LA, Kumar N, Nuhant P, Wang Y, Lauer JL, Liu J, Istrate MA, Kamenecka TM, Roush WR, Vidović D, Schürer SC, Xu J, Wagoner G, Drew PD, Griffin PR, Burris TP: Suppression of TH17 differentiation and autoimmunity by a synthetic ROR ligand. Nature 472: 491–494, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Skepner J, Ramesh R, Trocha M, Schmidt D, Baloglu E, Lobera M, Carlson T, Hill J, Orband-Miller LA, Barnes A, Boudjelal M, Sundrud M, Ghosh S, Yang J: Pharmacologic inhibition of RORγt regulates Th17 signature gene expression and suppresses cutaneous inflammation in vivo. J Immunol 192: 2564–2575, 2014 [DOI] [PubMed] [Google Scholar]
- 28.Kojetin DJ, Burris TP: REV-ERB and ROR nuclear receptors as drug targets. Nat Rev Drug Discov 13: 197–216, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vocanson M, Rozieres A, Hennino A, Poyet G, Gaillard V, Renaudineau S, Achachi A, Benetiere J, Kaiserlian D, Dubois B, Nicolas JF: Inducible costimulator (ICOS) is a marker for highly suppressive antigen-specific T cells sharing features of TH17/TH1 and regulatory T cells. J Allergy Clin Immunol 126: 280–289, 289.e1–7, 2010 [DOI] [PubMed] [Google Scholar]
- 30.Araujo LM, Fert I, Jouhault Q, Labroquere K, Andrieu M, Chiocchia G, Breban M: Increased production of interleukin-17 over interleukin-10 by regulatory T cells implicates ICOS molecule in experimental spondyloarthritis. Arthritis Rheumatol 66: 2412–2422, 2014 [DOI] [PubMed] [Google Scholar]
- 31.Lehmann J, Huehn J, de la Rosa M, Maszyna F, Kretschmer U, Krenn V, Brunner M, Scheffold A, Hamann A: Expression of the integrin alpha Ebeta 7 identifies unique subsets of CD25+ as well as CD25-regulatory T cells. Proc Natl Acad Sci U S A 99: 13031–13036, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Massot B, Michel ML, Diem S, Ohnmacht C, Latour S, Dy M, Eberl G, Leite-de-Moraes MC: TLR-induced cytokines promote effective proinflammatory natural Th17 cell responses. J Immunol 192: 5635–5642, 2014 [DOI] [PubMed] [Google Scholar]
- 33.Cretney E, Xin A, Shi W, Minnich M, Masson F, Miasari M, Belz GT, Smyth GK, Busslinger M, Nutt SL, Kallies A: The transcription factors Blimp-1 and IRF4 jointly control the differentiation and function of effector regulatory T cells. Nat Immunol 12: 304–311, 2011 [DOI] [PubMed] [Google Scholar]
- 34.Chaudhry A, Rudra D, Treuting P, Samstein RM, Liang Y, Kas A, Rudensky AY: CD4+ regulatory T cells control TH17 responses in a Stat3-dependent manner. Science 326: 986–991, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hori S: Lineage stability and phenotypic plasticity of Foxp3⁺ regulatory T cells. Immunol Rev 259: 159–172, 2014 [DOI] [PubMed] [Google Scholar]
- 36.Gavin MA, Torgerson TR, Houston E, DeRoos P, Ho WY, Stray-Pedersen A, Ocheltree EL, Greenberg PD, Ochs HD, Rudensky AY: Single-cell analysis of normal and FOXP3-mutant human T cells: FOXP3 expression without regulatory T cell development. Proc Natl Acad Sci U S A 103: 6659–6664, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang J, Ioan-Facsinay A, van der Voort EI, Huizinga TW, Toes RE: Transient expression of FOXP3 in human activated nonregulatory CD4+ T cells. Eur J Immunol 37: 129–138, 2007 [DOI] [PubMed] [Google Scholar]
- 38.Allan SE, Crome SQ, Crellin NK, Passerini L, Steiner TS, Bacchetta R, Roncarolo MG, Levings MK: Activation-induced FOXP3 in human T effector cells does not suppress proliferation or cytokine production. Int Immunol 19: 345–354, 2007 [DOI] [PubMed] [Google Scholar]
- 39.Miyao T, Floess S, Setoguchi R, Luche H, Fehling HJ, Waldmann H, Huehn J, Hori S: Plasticity of Foxp3(+) T cells reflects promiscuous Foxp3 expression in conventional T cells but not reprogramming of regulatory T cells. Immunity 36: 262–275, 2012 [DOI] [PubMed] [Google Scholar]
- 40.Kluger MA, Ostmann A, Luig M, Meyer MC, Goerke B, Paust HJ, Meyer-Schwesinger C, Stahl RA, Panzer U, Tiegs G, Steinmetz OM: B-cell-derived IL-10 does not vitally contribute to the clinical course of glomerulonephritis. Eur J Immunol 44: 683–693, 2014 [DOI] [PubMed] [Google Scholar]
- 41.Kluger MA, Zahner G, Paust HJ, Schaper M, Magnus T, Panzer U, Stahl RA: Leukocyte-derived MMP9 is crucial for the recruitment of proinflammatory macrophages in experimental glomerulonephritis. Kidney Int 83: 865–877, 2013 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.