Abstract
Cardiovascular mortality is the leading cause of death in ESRD. Whereas innate and adaptive immunity have established roles in cardiovascular disease, the role of humoral immunity is unknown. We conducted a retrospective cohort study in first-time adult kidney transplant candidates (N=161,308) using data from the Scientific Registry of Transplant Recipients and the Centers for Medicare and Medicaid Services to evaluate whether anti–human leukocyte antigen antibodies, measured as panel reactive antibodies (PRAs), are related to mortality in ESRD. Relationships between time-varying PRAs and all-cause or cardiovascular mortality were assessed using Cox proportional hazards models. The analysis was repeated in subcohorts of candidates at lower risk for significant comorbidities, activated on the waiting list after 2007, or unsensitized at activation. Competing risks analyses were also conducted. Fully adjusted models showed increased hazard ratios (HRs [95% confidence intervals]) for all-cause mortality (HR, 1.02 [95% CI, 0.99 to 1.06]; HR, 1.11 [95% CI,1.07 to 1.16]; and HR,1.21 [95% CI,1.15 to 1.27]) and cardiovascular mortality (HR, 1.05 [95% CI,1.00 to 1.10]; HR,1.11 [95% CI,1.05 to 1.18]; and HR,1.21 [95% CI,1.12 to 1.31]) in PRA 1%–19%, PRA 20%–79%, and PRA 80%–100% categories compared with PRA 0%, respectively. Associations between PRA and the study outcomes were accentuated in competing risks models and in lower-risk patients and persisted in other subcohorts. Our findings suggest that PRA is an independent predictor of mortality in wait-listed kidney transplant candidates. The mechanisms by which PRA confers an incremental mortality risk in sensitized patients, and the role of transplantation in modifying this risk, warrant further study.
Keywords: kidney transplantation, immunology, mortality
Cardiovascular mortality is the leading cause of death in patients with ESRD.1,2 Traditional risk factors are highly prevalent in ESRD, yet they tend to underestimate the hazard of mortality in this vulnerable patient population.3 In addition to traditional risk factors, inflammation (as evidenced by proinflammatory cytokines), C-reactive protein,4,5 and T cell–mediated immunity6,7 have been shown to relate to cardiovascular morbidity and mortality in dialysis patients as well as in animal models. How the immune response is triggered,7 and whether humoral immunity8 is associated with cardiovascular disease, remains unknown.
Kidney transplant recipients exhibit a greater risk of cardiovascular mortality than the general population (although to a lesser extent than dialysis and wait-listed patients).1,9 Transplantation, in addition to pregnancies and blood transfusions, is a known cause of immune sensitization against “nonself” human leukocyte antigens (HLAs).10 The breadth of sensitization against HLAs is routinely monitored in wait-listed patients with ESRD using panel reactive antibody (PRA) assays.11–13
This study assessed whether PRA is an independent predictor of all-cause and cardiovascular mortality in first-time wait-listed kidney transplant candidates with ESRD. Sensitivity analyses were carried out to determine whether the results were affected by the patients’ burden of comorbidity, degree of sensitization, or PRA assay. Because sensitization is a known barrier to transplantation and sensitized patients have substantially longer waiting times,14 we also fitted models that accounted for transplantation as a competing event.
Results
Patients
During the study period, a total of 199,484 first-time kidney transplant candidates joined the United Network for Organ Sharing (UNOS) kidney transplant waiting list. A cohort of 161,308 patients was identified after applying the exclusion criteria (Supplemental Figure 1). Table 1 presents the characteristics of the study cohort. There was a preponderance of women in the PRA 80%–100% category (87.5%) versus the PRA 0% category (34.2%). The proportion of African-American transplant candidates was 29.8% in the unsensitized category and 37.1% in the highly sensitized category. A greater proportion of patients fell under PRA categories 20%–79% and PRA 80%–100% from 2007 onward. Traditional cardiovascular risk factors, including age, diabetes, obesity, hypertension, and peripheral vascular disease, were comparable across baseline PRA categories or were somewhat less prevalent among the highly sensitized patients.
Table 1.
Baseline characteristics of the study cohort
| Characteristic | Overall | PRA 0% | PRA 1%–19% | PRA 20%–79% | PRA 80%–100% | P Value | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of Patients (n=161,308) | Summary Measures | No. of Patients (n=120,888) | Summary Measures | No. of Patients (n=22,112) | Summary Measures | No. of Patients (n=13,000) | Summary Measures | No. of Patients (n=5308) | Summary Measures | ||
| Mean candidate age, yr (SD) | 161,308 | 51.0 (13.0) | 120,888 | 51.0 (13.1) | 22,112 | 51.0 (13.1) | 13,000 | 50.8 (12.4) | 5308 | 50.2 (12.2) | <0.001 |
| Candidate sex, % | |||||||||||
| Men | 96,428 | 59.8 | 79,579 | 65.8 | 12,849 | 58.1 | 3337 | 25.7 | 663 | 12.5 | |
| Women | 64,880 | 40.2 | 41,309 | 34.2 | 9263 | 41.9 | 9663 | 74.3 | 4645 | 87.5 | <0.001 |
| Candidate blood type, % | |||||||||||
| A | 53,312 | 33.0 | 40,468 | 33.5 | 7051 | 31.9 | 4108 | 31.6 | 1685 | 31.7 | |
| B | 23,554 | 14.6 | 17,723 | 14.7 | 3209 | 14.5 | 1878 | 14.5 | 744 | 14.0 | |
| AB | 6249 | 3.9 | 4706 | 3. 9 | 849 | 3.8 | 497 | 3.8 | 197 | 3.7 | |
| O | 78,193 | 48.5 | 57,991 | 47.9 | 11,003 | 49.8 | 6517 | 50.1 | 2682 | 50.5 | <0.001 |
| Candidate race, % | |||||||||||
| White | 75,140 | 46.6 | 58,304 | 48.2 | 9721 | 44.0 | 5108 | 39.3 | 2007 | 37.8 | |
| Black | 48,076 | 29.8 | 34,424 | 28.5 | 6990 | 31.6 | 4693 | 36.1 | 1969 | 37.1 | |
| Hispanic | 25,589 | 15.9 | 18,643 | 15.4 | 3741 | 16.9 | 2258 | 17.4 | 947 | 17.8 | |
| Asian | 9255 | 5.7 | 7054 | 5.9 | 1278 | 5.8 | 673 | 5.2 | 250 | 4.7 | |
| Other | 3248 | 2.0 | 2463 | 2.0 | 382 | 1.7 | 268 | 2.1 | 135 | 2.5 | <0.001 |
| Candidate BMI category | |||||||||||
| <18.5 | 4620 | 2.9 | 3252 | 2.7 | 639 | 2.9 | 490 | 3.8 | 239 | 4.5 | |
| 18.5< BMI <25 | 58,649 | 36.4 | 43,907 | 36.3 | 8073 | 36.5 | 4690 | 36.1 | 1979 | 37.3 | |
| 25< BMI <30 | 51,510 | 31.9 | 39,047 | 32.3 | 6998 | 31.7 | 3939 | 30.3 | 1526 | 28.8 | |
| >30 | 46,529 | 28.8 | 34,682 | 28.7 | 6402 | 29.0 | 3881 | 29.8 | 1564 | 29.5 | <0.001 |
| Cause of ESRD, % | |||||||||||
| GN | 30,624 | 19.0 | 22,802 | 18.9 | 4122 | 18.6 | 2634 | 20.3 | 1066 | 20.1 | |
| Diabetes | 53,133 | 32.9 | 40,226 | 33.3 | 7153 | 32.4 | 4033 | 31.0 | 1721 | 32.4 | |
| PKD | 12,951 | 8.0 | 9848 | 8.2 | 1756 | 7.9 | 1019 | 7.8 | 328 | 6.2 | |
| Hypertension | 38,585 | 23.9 | 28,840 | 23.9 | 5294 | 23.9 | 3159 | 24.3 | 1292 | 24.3 | |
| Other | 26,015 | 16.1 | 19,172 | 15.9 | 3787 | 17.1 | 2155 | 16.6 | 901 | 17.0 | <0.001 |
| Activation era, % | |||||||||||
| 2000–2003 | 54,486 | 33.8 | 42,163 | 34.9 | 7323 | 33.1 | 3578 | 27.5 | 1422 | 26.8 | |
| 2004–2006 | 52,238 | 32.4 | 39,561 | 32.7 | 6912 | 31.3 | 4048 | 31.1 | 1717 | 32.4 | |
| 2007–2009 | 54,584 | 33.8 | 39,164 | 32.4 | 7877 | 35.6 | 5374 | 41.3 | 2169 | 40.9 | <0.001 |
| Median time from dialysis initiation to activation, yr (IQR) | 161,308 | 0.7 (1.6) | 120,888 | 0.7 (1.5) | 22,112 | 0.7 (1.6) | 13,000 | 0.8 (1.7) | 5308 | 0.9 (1.8) | <0.001 |
| Dialysis modality on activation, % | |||||||||||
| HD | 96,907 | 60.1 | 72,188 | 59.7 | 13,346 | 60.4 | 7841 | 60.3 | 3532 | 66.5 | |
| PD | 16,910 | 10.5 | 12,971 | 10.7 | 2082 | 9.4 | 1354 | 10.4 | 503 | 9.5 | |
| No dialysis | 39,104 | 24.2 | 30,106 | 24.9 | 5240 | 23.7 | 2816 | 21.7 | 942 | 17.8 | |
| Modality unknown | 8387 | 5.2 | 5623 | 4.7 | 1444 | 6.5 | 989 | 7.6 | 331 | 6.2 | <0.001 |
| Multilisted, % | |||||||||||
| No | 142,289 | 88.2 | 106,583 | 88.2 | 19,577 | 88.5 | 11,477 | 88.3 | 4652 | 87.6 | |
| Yes | 19,019 | 11.8 | 14,305 | 11.8 | 2535 | 11.5 | 1523 | 11.7 | 656 | 12.4 | 0.24 |
| Diabetes, % | |||||||||||
| No | 95,292 | 59.1 | 71,083 | 58.8 | 13,201 | 59.7 | 7869 | 60.5 | 3139 | 59.1 | |
| Yes | 66,016 | 40.9 | 49,805 | 41.2 | 8911 | 40.3 | 5131 | 39.5 | 2169 | 40.9 | <0.001 |
| Peripheral vascular disease, % | |||||||||||
| No | 152,909 | 94.8 | 114,488 | 94.7 | 21,041 | 95.2 | 12,374 | 95.2 | 5006 | 94.3 | |
| Yes | 8399 | 5.2 | 6400 | 5.3 | 1071 | 4.8 | 626 | 4.8 | 302 | 5.7 | 0.002 |
| Accept HCV-positive kidney, % | |||||||||||
| No | 153,995 | 95.5 | 115,508 | 95.6 | 20,831 | 94.2 | 12,519 | 96.3 | 5137 | 96.8 | |
| Yes | 7313 | 4.5 | 5380 | 4.5 | 1281 | 5.8 | 481 | 3.7 | 171 | 3.2 | <0.001 |
BMI, body mass index; PKD, polycystic kidney disease; HD, hemodialysis; PD, peritoneal dialysis.
The median follow-up period after initial wait-list activation was 2.1 years (interquartile range [IQR], 1.0, 3.6). A total of 28,513 all-cause deaths and 12,792 cardiovascular deaths (44.9%) were reported during 409,202 person-years of follow-up. Frequencies of PRA measurements and changes in PRA scores are presented in Supplemental Table 1.
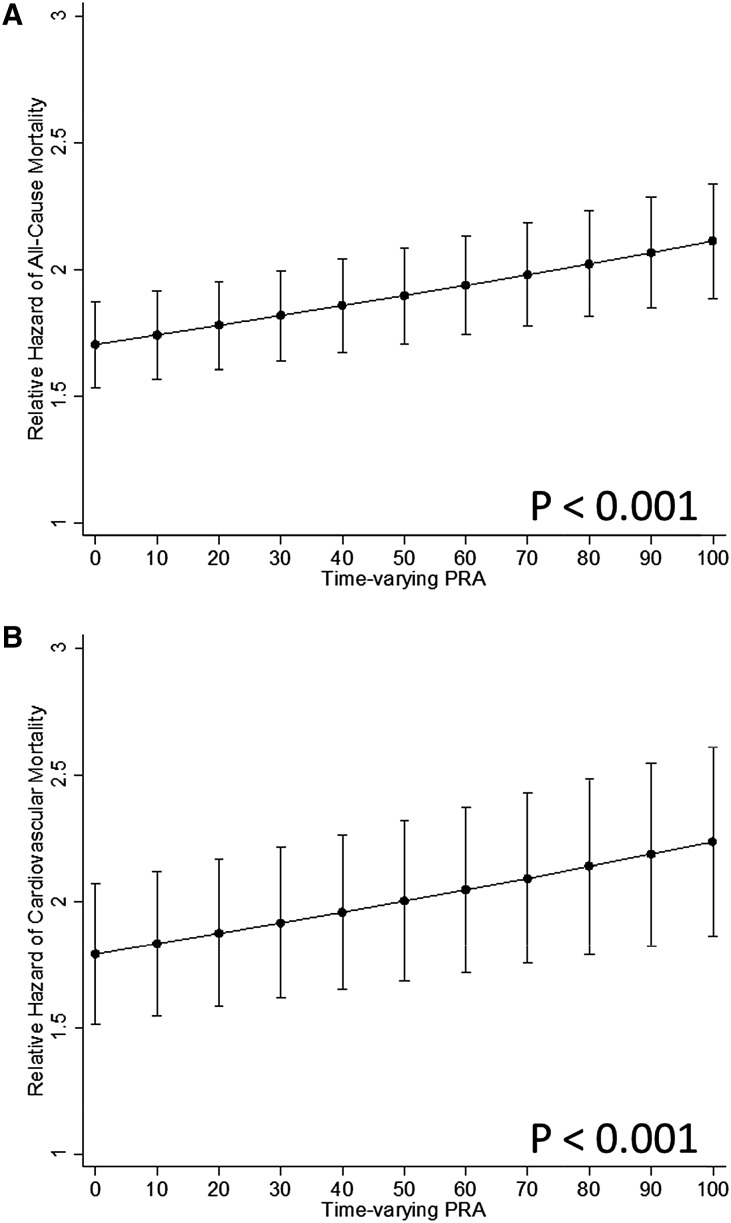
Figure 1 depicts the continuous relationship between PRA and the hazard ratios (HRs) for all-cause and cardiovascular mortality. HRs of 1.02 (95% confidence interval [95% CI], 1.02 to 1.03; P<0.001) and HR, 1.02 (95% CI, 1.01 to 1.03; P<0.001), respectively, were observed per 10% increase in time-varying PRAs by linear models.
Figure 1.
HR of all-cause mortality (A) and cardiovascular mortality (B) on the waiting list for kidney transplantation as a function of time-varying PRAs.
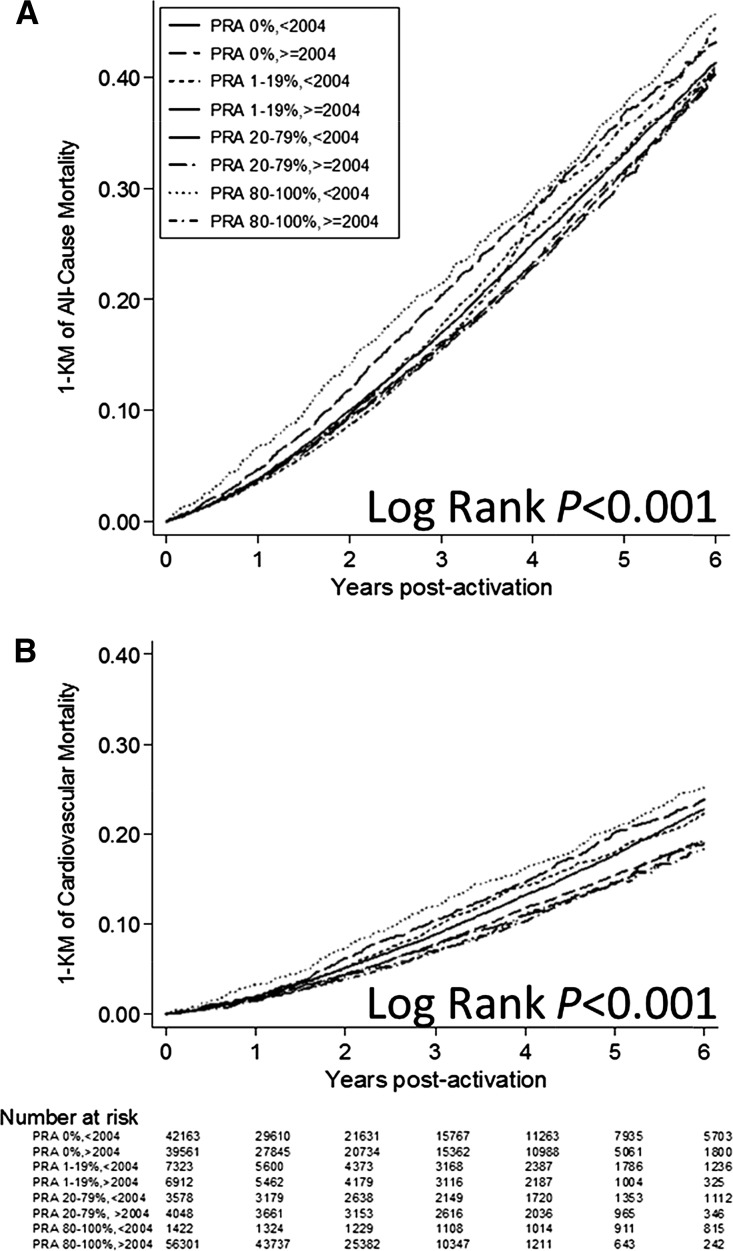
Extended Kaplan–Meier failure curves stratified by PRA category (Supplemental Figure 2) or PRA category and activation era established that the risk for both end points was most pronounced in the highly sensitized category before implementation of UNOS Policy 3.5.11. Cumulative probability of all-cause mortality was 33.1%, 33.4%, 36.8%, and 37.5% before 2004 versus 31.7%, 31.0%, 31.4%, and 35.6% after 2004 (Figure 2A; log-rank P<0.001). Cumulative probability of cardiovascular mortality was 17.6%, 17.9%, 20.2%, and 20.6% before 2004 versus 15.4%, 14.6%, 14.4%, and 14.6% after 2004 (Figure 2B; log-rank P<0.001).
Figure 2.
Extended Kaplan–Meier failure curves of all-cause mortality (A) and cardiovascular mortality (B) among wait-listed kidney transplant candidates by PRA category and waiting-list activation era. KM, Kaplan–Meier.
Table 2 shows the results of the Cox proportional hazards models. The fully adjusted model (model 3) showed an increase in the HRs for all-cause mortality (HR, 1.02 [95% CI, 0.99 to 1.06]; HR, 1.11 [95% CI, 1.07 to 1.16]; and HR, 1.21 [95% CI, 1.15 to 1.27]; P<0.001) and cardiovascular mortality (HR, 1.05 [95% CI, 1.00 to 1.10]; HR, 1.11 [95% CI, 1.05 to 1.18]; and HR, 1.21 [95% CI, 1.12 to 1.31]; P<0.001) in PRA 1%–19%, PRA 20%–79%, and PRA 80%–100% categories compared with PRA 0%, respectively.
Table 2.
The relative hazard of all-cause and cardiovascular mortality by time-varying PRA category
| Cox Model | PRA 0% | PRA 1%–19% | PRA 20%–79% | PRA 80%–100% |
|---|---|---|---|---|
| All-cause mortality | ||||
| 1 | Referent | 1.00 (0.96 to 1.03) | 1.05 (1.01 to 1.09) | 1.13 (1.08 to 1.18) |
| 2 | Referent | 1.03 (1.04 to 1.07) | 1.13 (1.09 to 1.18) | 1.26 (1.20 to 1.32) |
| 3 | Referent | 1.02 (0.99 to 1.06) | 1.11 (1.07 to 1.16) | 1.21 (1.15 to 1.27) |
| Cardiovascular mortality | ||||
| 1a | Referent | 1.01 (0.97 to 1.07) | 1.00 (0.97 to 1.07) | 1.09 (1.01 to 1.17) |
| 2 | Referent | 1.06 (1.00 to 1.11) | 1.12 (1.06 to 1.19) | 1.26 (1.17 to 1.36) |
| 3 | Referent | 1.05 (1.00 to 1.10) | 1.11 (1.05 to 1.18) | 1.21 (1.12 to 1.31) |
Data are presented as HRs (95% CIs). Model 1 includes the PRA category. Model 2 includes the PRA category, age, sex, race, body mass index, and cause of ESRD. Model 3 includes the PRA category, age, sex, race, body mass index, cause of ESRD, dialysis modality, time from dialysis initiation to activation, activation era, multilisting, diabetes, peripheral vascular disease, and HCV.
The omnibus test for this model did not reach statistical significance (P=0.15). The P value of the omnibus test in all other models was <0.001.
HRs for both end points increased across time-fixed PRA categories measured at wait-list activation. The risk was accentuated in subdistribution competing risk models, demonstrating HRs of 1.05 (95% CI, 1.01 to 1.08), HR, 1.20 (95% CI, 1.15 to 1.25), and HR, 1.52 (95% CI, 1.44 to 1.62) for all-cause mortality and HR, 1.03 (95% CI, 0.98 to 1.09), HR, 1.14 (95% CI, 1.07 to 1.22), and HR,1.46 (95% CI, 1.34 to 1.60) for cardiovascular mortality across PRA categories.
Subgroup Analyses
HRs for all-cause mortality as a function of time-varying PRAs were numerically higher in men, preemptively activated patients, and patients without diabetes. However, the association between time-varying PRAs and cardiovascular mortality did not exhibit significant effect measure modification across the prespecified subgroups excluding activation era (Supplemental Tables 2 and 3).
Sensitivity Analyses
Table 3 shows the sensitivity analyses results. Adjusted HRs for cardiovascular (HR, 1.29 [95% CI, 0.94 to 1.77]; HR, 1.64 [95% CI, 1.17 to 2.27]; and HR, 2.03 [95% CI, 1.36 to 3.03]) and all-cause mortality (HR, 0.97 [95% CI, 0.78 to 1.20]; HR, 1.25 [95% CI, 1.01 to 1.56]; and HR, 1.53 [95% CI, 1.18 to 2.00]) were accentuated across PRA categories in patients at low risk for significant comorbidity (Supplemental Figure 3). HRs of 1.05 (95% CI, 1.02 to 1.07) and 1.09 (95% CI, 1.05 to 1.13) were observed for all-cause and cardiovascular mortality, respectively, in this subgroup per 10% increase in PRA. The magnitude and trend in HRs for both study end points persisted in subcohorts of kidney transplant candidates who were unsensitized at baseline or activated on the waiting list after 2007. Landmark analyses in the subcohort activated after 2007 with repeated PRA measurements showed an increase in the relative hazard of all-cause mortality as a function of ΔPRA from activation to 3 months and 12 months after activation (Supplemental Table 4).
Table 3.
Sensitivity analyses
| Analysis | No. of Patients | All-Cause Mortality | Cardiovascular Mortality | ||||||
|---|---|---|---|---|---|---|---|---|---|
| PRA 0% | PRA 1%–19% | PRA 20%–79% | PRA 80%–100% | PRA 0% | PRA 1%–19% | PRA 20%–79% | PRA 80%–100% | ||
| Primary analysisa | 161,308 | Referent | 1.02 (0.99 to 1.06) | 1.11 (1.07 to 1.16) | 1.21 (1.15 to 1.27) | Referent | 1.05 (1.00 to 1.10) | 1.11 (1.05 to 1.18) | 1.21 (1.12 to 1.31) |
| Adjustment for clustering by centera | 161,308 | Referent | 1.02 (0.98 to 1.06) | 1.11 (1.07 to 1.16) | 1.21 (1.14 to 1.28) | Referent | 1.05 (0.99 to 1.11) | 1.11 (1.04 to 1.18) | 1.21 (1.13 to 1.29) |
| Model with shared frailty by center | 161,308 | Referent | 1.02 (0.99 to 1.07) | 1.11 (1.07 to 1.16) | 1.21 (1.15 to 1.28) | Referent | 1.05 (1.00 to 1.10) | 1.10 (1.04 to 1.17) | 1.22 (1.13 to 1.32) |
| Low risk subcohortb | 18,645 | Referent | 0.97 (0.78 to 1.20) | 1.25 (1.01 to 1.56) | 1.53 (1.18 to 2.00) | Referent | 1.29 (0.94 to 1.77) | 1.64 (1.17 to 2.27) | 2.03 (1.36 to 3.03) |
| Activated after 2007c | 54,584 | Referent | 1.05 (0.97 to 1.13) | 1.06 (0.97 to 1.16) | 1.33 (1.19 to 1.50) | Referent | 1.16 (1.03 to 1.31) | 1.13 (0.99 to 1.30) | 1.26 (1.04 to 1.53) |
| Unsensitized at activationa | 117,804 | Referent | 1.04 (0.98 to 1.09) | 1.15 (1.07 to 1.23) | 1.27 (1.14 to 1.42) | Referent | 1.10 (1.02 to 1.19) | 1.18 (1.07 to 1.31) | 1.33 (1.14 to 1.57) |
| No history of transfusions | 36,323 | Referent | 1.07 (1.01 to 1.14) | 1.20 (1.11 to 1.30) | 1.29 (1.15 to 1.44) | Referent | 1.13 (1.03 to 1.23) | 1.15 (1.02 to 1.30) | 1.34 (1.14 to 1.58) |
| Time-fixed PRA measured at activationa | 161,308 | Referent | 1.02 (1.00 to 1.06) | 1.07 (1.02 to 1.11) | 1.20 (1.14 to 1.28) | Referent | 1.02 (0.97 to 1.07) | 1.04 (0.97 to 1.11) | 1.22 (1.11 to 1.33) |
| Competing risks modela | 161,308 | Referent | 1.05 (1.01 to 1.08) | 1.20 (1.15 to 1.25) | 1.52 (1.44 to 1.62) | Referent | 1.04 (0.98 to 1.09) | 1.14 (1.07 to 1.22) | 1.46 (1.34 to 1.60) |
Data are presented as HRs (95% CIs), unless otherwise indicated.
Model covariates include PRA category, age, sex, race, body mass index, cause of ESRD, dialysis modality, time from dialysis initiation to activation, activation era, multilisting, diabetes, peripheral vascular disease.
Model covariates: PRA category, age, sex, race, body mass index, cause of ESRD, dialysis modality, time from dialysis initiation to activation, activation era, multilisting, and HCV.
Model covariates: PRA category, age, sex, race, body mass index, cause of ESRD, dialysis modality, time from dialysis initiation to activation, multilisting, diabetes, peripheral vascular disease, and HCV.
Analyses Using Centers for Medicare and Medicaid Services or Scientific Registry of Transplant Recipients Data to Ascertain Cause-Specific Mortality
A total of 12,741 deaths (44.7%) reported in the Centers for Medicare and Medicaid Services (CMS) data and 4170 deaths (14.6%) reported in the Scientific Registry of Transplant Recipients (SRTR) were attributed to cardiovascular mortality (Supplemental Table 5). Similar to the main analysis, a trend of increasing HRs of 1.05 (95% CI, 1.00 to 1.10), HR, 1.11 (95% CI, 1.04 to 1.18), and HR, 1.18 (95% CI, 1.10 to 1.28), as well as HR, 1.04 (95% CI, 0.95 to 1.13), HR, 1.23 (95% CI, 1.12 to 1.36), and HR, 1.45 (95% CI, 1.28 to 1.65), was apparent across ascending PRA categories when CMS and SRTR data were used to ascertain cardiovascular mortality, respectively (Supplemental Table 6).
Discussion
We conducted a retrospective cohort study to determine whether immune sensitization is an independent risk factor for mortality in first-time wait-listed kidney transplant candidates. Our analysis established an increased risk for all-cause and cardiovascular mortality as a function of time-varying PRAs. The risk persisted in subcohorts of patients selected to be at low risk for significant comorbidity or activated on the waiting list after 2007. Baseline PRA measurements at activation were also associated with both study end points, and the risk was more pronounced when transplantation was considered a competing event.
In an effort to ensure equitable access to transplantation, highly sensitized (PRA>80%) kidney transplant candidates have received additional points on the UNOS allocation system to compensate for their biologic disadvantage. The 2011 SRTR Annual Data Report stated that, consequent to this allocation scheme,2 30.5% of the highly sensitized candidates underwent transplantation within 5 years of wait-list activation compared with 36.0% of the candidates with PRA <1%. Our analysis showed that immune sensitization is a risk factor for mortality on the waiting list; although immune sensitization is most pronounced in highly sensitized patients (5-year cumulative probability of 31.7% versus 35.6% in unsensitized versus highly sensitized patients, after implementation of Organ Procurement and Transplantation Network [OPTN] Policy 3.5.11), it is not limited to this patient population. Our findings support the “sliding scale of allocation points” based on the calculated PRA, which was recently implemented by UNOS. This system ensures a more continuous prioritization of patients based on their degree of sensitization. Whether earlier kidney transplantation (e.g., from living donors) favorably modifies the risk of mortality conferred by PRA remains to be determined.
Although donor-specific anti-HLA antibodies have been associated with arteriosclerosis of renal transplant arteries,15 our study is the first, to our knowledge, to link PRA and cardiovascular mortality. Atherosclerosis is considered a chronic inflammatory process, involving both the innate and adaptive arms of the immune response.16 Adaptive responses occur when an antigen is recognized as foreign by T cell receptors and Igs on B cell membranes. Antigen recognition drives lymphocyte proliferation and differentiation into effector cells with proinflammatory properties that are meant to be protective in nature. However, this response may lead to tissue damage and disease.16 To date, the bulk of the evidence discusses the interplay between regulatory T cells and the renin-angiotensin-aldosterone system as determinants of vascular oxidative stress and endothelial dysfunction.6,7,17,18 The roles of B cell7 and humoral autoimmunity8 or alloimmunity in the pathogenesis of vascular disease are not as well documented.
Anti-HLA antibodies may be the cause of increased mortality (either directly or through an intermediate process) or there may be a common cause that leads to both increased mortality and sensitization. This causal paradigm implies that anti-HLA antibodies induce a proinflammatory state that contributes to vascular injury. Interestingly, a U-shaped relationship has been described between parity and cardiovascular mortality, with multiparous women demonstrating a decreased risk and grand multiparous women demonstrating an increased risk compared with nonparous women.19–26 Although it is plausible that grand multiparous women are highly sensitized, whether PRA is an independent predictor of cardiovascular mortality in this population is unknown.
Membership in a specific PRA category is not the result of a random procedure. In this context, the elevated risk for mortality by PRA category membership may be confounded with unmeasured patient characteristics, and the elevated risk for mortality associated with PRA may be an epiphenomenon of a heightened mortality risk related to processes such as severe anemia requiring blood transfusions or an elevated comorbidity burden. To address this possibility, we conducted sensitivity analyses in a subcohort of patients who were not transfused before wait-listing as well as a subcohort with lesser dialysis exposure and a lower comorbidity burden. The effect of PRA on mortality persisted in the former subcohort and was accentuated in the latter. Our study has a number of strengths, including the large sample size and comprehensive coverage of United States kidney transplant candidates in the SRTR. Moreover, the consistency of our findings across subcohorts and various analytical methods increases the robustness of our primary results. Our analysis also demonstrates a temporal and dose-response relationship between PRA and mortality among wait-listed kidney transplant candidates, strengthening the validity of our inferences.
Despite these strengths, some limitations should be noted. First, PRA was only measured in wait-listed patients. Therefore, our findings cannot be generalized to the general population or to nonwaitlisted patients with ESRD. Second, variability in estimates of sensitization may be observed depending on the assay selected to identify anti-HLA antibodies.27 To ensure that our findings were not related to increasing utilization of more sensitive solid-phase assays28 (versus cytotoxicity assays) over time, we repeated the analysis in a subcohort of patients wait-listed after 2007 who were more likely to undergo PRA testing by solid-phase assays, and we found similar results. Third, to address concerns regarding the absence of validation studies to corroborate causes of death obtained from the SRTR, the cause-specific mortality variable in the SRTR was augmented with CMS data. Furthermore, analyses using all-cause mortality, which is obtained by the SRTR from multiple sources, as well as a sensitivity analysis using secondary end points of cardiovascular mortality as reported by the CMS or the SRTR also identified a similar relationship with PRA.
In summary, our study suggests that PRA is a novel predictor of all-cause and cardiovascular mortality in wait-listed patients with ESRD. Multivariable and competing risks models indicate that this relationship is independent of dialysis vintage or comorbid disease burden. Organ allocation schemes may need to consider this additional risk of mortality in sensitized candidates awaiting kidney transplantation. Further research is required to confirm our findings in prospective cohort studies accounting for all relevant covariates (e.g., history of anemia and hospitalizations) and to elucidate the mechanism(s) by which PRA confers an increased risk for mortality. Whether minimizing sensitization and/or receiving a kidney transplant will modify the elevated risk of cardiovascular mortality in sensitized wait-listed patients remains to be determined.
Concise Methods
Study Population
We conducted a retrospective cohort study of all United States adult kidney transplant candidates (aged ≥18 years) placed on the waiting list for their first kidney transplant between January 1, 2000, and October 1, 2009. Pediatric patients (aged ≤8 years), multiorgan transplant recipients, and candidates missing PRA information or with implausible dates (e.g., wait-list activation after death or transplantation) were excluded. Wait-list activity was assessed at a candidate (not registrant) level.29 When candidates were registered at multiple centers, listings were combined such that the first activation date, first event of interest, and patient characteristics were considered for each candidate.
Data Sources
Data from the SRTR and the CMS were used for these analyses. The SRTR includes data on all donors, wait-listed candidates, and transplant recipients in the United States, submitted by the members of the OPTN.30 Data from the CMS are derived from the US ESRD Network program and contain information on baseline demographics, comorbidity, dialysis modality, and outcomes (e.g., cause of death).31
Exposure and Outcome Measurements
The exposure of interest was PRA, measured serially from wait-list activation to transplantation. PRA was modeled as a time-varying continuous and categorical variable (highly sensitized, PRA 80%–100%; moderately sensitized, PRA 20%–79%; mildly sensitized, PRA 1%–19%; and nonsensitized, PRA 0%).2 The nonsensitized PRA category was used as the referent for all analyses. The frequency of PRA testing varied across transplant centers. The most recent PRA value was related to the risk of mortality over the subsequent interval.
The main study end points were all-cause and cardiovascular mortality. All-cause mortality data from the OPTN were supplemented by the SRTR through linkage with the Social Security Death Master File.30 Cardiovascular mortality was defined as death resulting from an acute myocardial infarction, sudden cardiac death, death as a result of heart failure, death due to stroke, and/or death owing to other cardiovascular causes. Cause-specific mortality data from SRTR were supplemented by patient-level linkage with data from the CMS.30 The causes of death among wait-listed patients in the SRTR were obtained from follow-up forms provided by the transplant centers to the OPTN. Cardiovascular mortality, captured in the CMS-2746 Form, has been shown to be highly specific although modestly sensitive, compared with the cause of death determined by an outcomes review committee.32 Patients with relevant death dates, but with missing/unknown causes, were considered as noncardiovascular deaths. Consequently, the incidence of cardiovascular deaths may be underestimated.9 Cardiovascular mortality data from the SRTR or CMS were also considered separately.
Model Covariates
Model covariates included candidate age, sex, race, cause of ESRD, and body mass index. Comorbidity data (including diabetes, peripheral vascular disease, and the candidate’s indication for acceptance of an organ from a donor infected with hepatitis C virus [HCV] used as a surrogate marker for HCV status29) were obtained from the SRTR at the time of wait-listing. The mode of RRT, time from dialysis initiation to activation, activation era, and multilisting for transplantation were also recorded. Dialysis modality was missing in 6% of the final cohort, and a missing indicator was used in multivariable analysis. Other variables were missing in ≤5% of the final cohort, and thus a complete case analysis was conducted.
Statistical Analyses
The distributions of baseline characteristics were evaluated using summary statistics for continuous and categorical variables. Study end points were evaluated across time-varying PRA categories stratified by activation era (before or after the implementation of OPTN Policy 3.5.11) using extended Kaplan–Meier failure curves.33 The time origin for survival analyses was the time of wait-list activation. Time at risk included time spent as status 1, time spent as status 7,29 or time after removal from the waiting list for a reason other than transplantation or death.29 Patients were censored at kidney transplantation or on November 30, 2010, if they neither died nor received a transplant by that time. We used univariable (model 1) and multivariable (models 2 and 3) Cox proportional hazards models to examine the association between PRA categories and study end points. Each multivariable model sequentially incorporated an expanded set of clinically relevant covariates. Model 2 included candidates’ age, sex, race, body mass index, and the cause of ESRD, whereas model 3 also included candidates’ comorbidities (diabetes mellitus, peripheral vascular disease, and HCV), dialysis modality at listing, time from dialysis initiation to activation, activation era, and multilisting. Adjustment for clustering of outcomes by follow-up center was also performed using a robust sandwich estimator34,35 and by fitting Cox proportional hazards models with shared frailty by center.36
To flexibly capture the continuous relationship between time-varying PRAs and study end points, we used restricted cubic splines37 and found better fit using the Bayesian Information Criterion when modeling a linear relationship between exposure and outcomes. To assess whether the effect of PRA on study end points was modified within prespecified subgroups, we introduced interaction terms between PRA categories and the candidates’ baseline characteristics.
Cox proportional hazards models were utilized to assess the relationship between time-fixed PRA category at wait-list activation and the main study end points. Subdistribution hazards models handled noncardiovascular mortality as a competing event for cardiovascular mortality and transplantation as a competing event for cardiovascular or all-cause mortality.38,39 Multivariable models were fitted to evaluate the association between time-varying PRAs and the secondary cardiovascular mortality end points reported in the SRTR or CMS. Statistical analyses were performed using Stata/IC 12.0 software. A two-tailed P value of <0.05 was considered statistically significant. The research ethics boards at the University of Toronto and the University Health Network approved the study. The study was reported in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology statement.40
Sensitivity Analyses
To determine the robustness of our main results to various design and analytical assumptions, we conducted sensitivity analyses. These analyses evaluated the relationship between PRA categories and the study end points in subcohorts of kidney transplant candidates who (1) had a lower risk of comorbid disease burden (defined as patients aged <40 years without a history of coronary artery disease, diabetes, or peripheral vascular disease having spent <12 months on dialysis), (2) were unsensitized (i.e., peak PRA 0%) at activation, (3) were not transfused before wait-listing, and (4) were activated after 2007 (during which time PRA was likely measured by the most up-to-date solid-phase assays). Landmark analyses41 evaluating the effect of ΔPRA (difference between PRA at 3, 6, or 12 months versus PRA at activation) on study end points were also conducted in the subcohort activated after 2007.
Disclosures
None.
Supplementary Material
Acknowledgments
We thank Ms. Yanhong Li for assistance with statistical analysis.
Ruth Sapir-Pichhadze was funded by the Eliot Phillipson Clinician Scientist Training Program, Department of Medicine, University of Toronto, Toronto, ON, Canada. Andreas Laupacis is the recipient of a Canada Research Chair in Health Policy and Citizen Engagement.
The data reported here were supplied by the Minneapolis Medical Research Foundation as the contractor for the SRTR. The interpretation and reporting of these data is the responsibility of the authors and in no way should be seen as an official policy of or an interpretation by the SRTR or the US Government. All of the data used for this publication were collected pursuant to Contract No. HHSH250201000018C (Department of Health and Human Services, Health Resources and Services Administration, Rockville, MD).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2014090894/-/DCSupplemental.
References
- 1.US Renal Data System : Mortality. In: 2013 USRDS Annual Data Report, Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2013, pp 263–270 [Google Scholar]
- 2.US Department of Health and Human Services, Health Resources and Services Administration : Kidney. In: OPTN/SRTR 2011 Annual Data Report, Rockville, MD, US Department of Health and Human Services, Health Resources and Services Administration, 2012, pp 11–46 [Google Scholar]
- 3.Longenecker JC, Coresh J, Powe NR, Levey AS, Fink NE, Martin A, Klag MJ: Traditional cardiovascular disease risk factors in dialysis patients compared with the general population: The CHOICE Study. J Am Soc Nephrol 13: 1918–1927, 2002 [DOI] [PubMed] [Google Scholar]
- 4.Yeun JY, Levine RA, Mantadilok V, Kaysen GA: C-reactive protein predicts all-cause and cardiovascular mortality in hemodialysis patients. Am J Kidney Dis 35: 469–476, 2000 [DOI] [PubMed] [Google Scholar]
- 5.Wanner C, Zimmermann J, Schwedler S, Metzger T: Inflammation and cardiovascular risk in dialysis patients. Kidney Int Suppl 80: 99–102, 2002 [DOI] [PubMed] [Google Scholar]
- 6.Kasal DA, Barhoumi T, Li MW, Yamamoto N, Zdanovich E, Rehman A, Neves MF, Laurant P, Paradis P, Schiffrin EL: T regulatory lymphocytes prevent aldosterone-induced vascular injury. Hypertension 59: 324–330, 2012 [DOI] [PubMed] [Google Scholar]
- 7.Schiffrin EL: Immune mechanisms in hypertension and vascular injury. Clin Sci (Lond) 126: 267–274, 2014 [DOI] [PubMed] [Google Scholar]
- 8.Patel RK, Arya R: Does IgA antibody against β2 glycoprotein I increase cardiovascular risk in hemodialysis patients? Kidney Int 81: 1164–1166, 2012 [DOI] [PubMed] [Google Scholar]
- 9.Meier-Kriesche HU, Schold JD, Srinivas TR, Reed A, Kaplan B: Kidney transplantation halts cardiovascular disease progression in patients with end-stage renal disease. Am J Transplant 4: 1662–1668, 2004 [DOI] [PubMed] [Google Scholar]
- 10.Katznelson S, Bhaduri S, Cecka JM: Clinical aspects of sensitization. Clin Transpl 285–296, 1997 [PubMed] [Google Scholar]
- 11.Barama A, Oza U, Panek R, Belitsky P, MacDonald AS, Lawen J, McAlister V, Kiberd B: Effect of recipient sensitization (peak PRA) on graft outcome in haploidentical living related kidney transplants. Clin Transplant 14: 212–217, 2000 [DOI] [PubMed] [Google Scholar]
- 12.Gebel HM, Bray RA, Nickerson P: Pre-transplant assessment of donor-reactive, HLA-specific antibodies in renal transplantation: Contraindication vs. risk. Am J Transplant 3: 1488–1500, 2003 [DOI] [PubMed] [Google Scholar]
- 13.Tambur AR, Leventhal JR, Walsh RC, Zitzner JR, Friedewald JJ: HLA-DQ barrier: Effects on cPRA calculations. Transplantation 96: 1065–1072, 2013 [DOI] [PubMed] [Google Scholar]
- 14.Chang P, Gill J, Dong J, Rose C, Yan H, Landsberg D, Cole EH, Gill JS: Living donor age and kidney allograft half-life: Implications for living donor paired exchange programs. Clin J Am Soc Nephrol 7: 835–841, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hill GS, Nochy D, Bruneval P, Duong van Huyen JP, Glotz D, Suberbielle C, Zuber J, Anglicheau D, Empana JP, Legendre C, Loupy A: Donor-specific antibodies accelerate arteriosclerosis after kidney transplantation. J Am Soc Nephrol 22: 975–983, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hansson GK, Hermansson A: The immune system in atherosclerosis. Nat Immunol 12: 204–212, 2011 [DOI] [PubMed] [Google Scholar]
- 17.Barhoumi T, Kasal DA, Li MW, Shbat L, Laurant P, Neves MF, Paradis P, Schiffrin EL: T regulatory lymphocytes prevent angiotensin II-induced hypertension and vascular injury. Hypertension 57: 469–476, 2011 [DOI] [PubMed] [Google Scholar]
- 18.Parikh NI, Cnattingius S, Dickman PW, Mittleman MA, Ludvigsson JF, Ingelsson E: Parity and risk of later-life maternal cardiovascular disease. Am Heart J 159: 215.e216–221.e216, 2010 [DOI] [PubMed] [Google Scholar]
- 19.Steenland K, Lally C, Thun M: Parity and coronary heart disease among women in the American Cancer Society CPS II population. Epidemiology 7: 641–643, 1996 [DOI] [PubMed] [Google Scholar]
- 20.Hinkula M, Kauppila A, Näyhä S, Pukkala E: Cause-specific mortality of grand multiparous women in Finland. Am J Epidemiol 163: 367–373, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Koski-Rahikkala H, Pouta A, Pietiläinen K, Hartikainen AL: Does parity affect mortality among parous women? J Epidemiol Community Health 60: 968–973, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jacobsen BK, Knutsen SF, Oda K, Fraser GE: Parity and total, ischemic heart disease and stroke mortality. The Adventist Health Study, 1976-1988. Eur J Epidemiol 26: 711–718, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jacobs MB, Kritz-Silverstein D, Wingard DL, Barrett-Connor E: The association of reproductive history with all-cause and cardiovascular mortality in older women: The Rancho Bernardo Study. Fertil Steril 97: 118–124, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Simons LA, Simons J, Friedlander Y, McCallum J: Childbearing history and late-life mortality: The Dubbo study of Australian elderly. Age Ageing 41: 523–528, 2012 [DOI] [PubMed] [Google Scholar]
- 25.Dior UP, Hochner H, Friedlander Y, Calderon-Margalit R, Jaffe D, Burger A, Avgil M, Manor O, Elchalal U: Association between number of children and mortality of mothers: Results of a 37-year follow-up study. Ann Epidemiol 23: 13–18, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ryan MJ: An update on immune system activation in the pathogenesis of hypertension. Hypertension 62: 226–230, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cecka JM: Calculated PRA (CPRA): The new measure of sensitization for transplant candidates. Am J Transplant 10: 26–29, 2010 [DOI] [PubMed] [Google Scholar]
- 28.Pei R, Lee JH, Shih NJ, Chen M, Terasaki PI: Single human leukocyte antigen flow cytometry beads for accurate identification of human leukocyte antigen antibody specificities. Transplantation 75: 43–49, 2003 [DOI] [PubMed] [Google Scholar]
- 29.Grams ME, Massie AB, Schold JD, Chen BP, Segev DL: Trends in the inactive kidney transplant waitlist and implications for candidate survival. Am J Transplant 13: 1012–1018, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leppke S, Leighton T, Zaun D, Chen SC, Skeans M, Israni AK, Snyder JJ, Kasiske BL: Scientific Registry of Transplant Recipients: Collecting, analyzing, and reporting data on transplantation in the United States. Transplant Rev (Orlando) 27: 50–56, 2013 [DOI] [PubMed] [Google Scholar]
- 31.US Centers for Disease Control and Prevention: NCHS-CMS Medicare and NCHS-USRDS linked data. Available at: http://www.cdc.gov/nchs/data/datalinkage/description_of_nchs_cms_medicare_linkage_final.pdf. Accessed February 12, 2014
- 32.Rocco MV, Yan G, Gassman J, Lewis JB, Ornt D, Weiss B, Levey AS: Comparison of causes of death using HEMO Study and HCFA end-stage renal disease death notification classification systems. The National Institutes of Health-funded Hemodialysis. Health Care Financing Administration. Am J Kidney Dis 39: 146–153, 2002 [DOI] [PubMed] [Google Scholar]
- 33.Snappin S, Jiang Q, Iglewicz B: Illustrating the impact of a time-varying covariate with an extended Kaplan-Meier estimator. Am Stat 59: 301–307, 2005 [Google Scholar]
- 34.Huber PJ: The behavior of maximum likelihood estimates under nonstandard conditions. In: Proceedings of the Fifth Berkeley Symposium on Mathematical Statistics and Probability, Berkeley, CA, University of California Press, 1967, pp 221–233 [Google Scholar]
- 35.Rogers WH: Regression standard errors in clustered samples. Stata Tech Bull 13: 19–23, 1993 [Google Scholar]
- 36.Gutierrez RG: Parametric frailty and shared frailty survival models. Stata J 2: 22–44, 2002 [Google Scholar]
- 37.Royston P, Sauerbrei W: Multivariable Model-Building: A Pragmatic Approach to Regression Analysis Based on Fractional Polynomials for Modelling Continuous Variables. Chichester, UK, John Wiley & Sons, Ltd, 2008 [Google Scholar]
- 38.Smits JM, van Houwelingen HC, De Meester J, Persijn GG, Claas FH: Analysis of the renal transplant waiting list: Application of a parametric competing risk method. Transplantation 66: 1146–1153, 1998 [DOI] [PubMed] [Google Scholar]
- 39.Fine JP, Gray RJ: A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 94: 496–509, 1999 [Google Scholar]
- 40.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP, STROBE Initiative : The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Lancet 370: 1453–1457, 2007 [DOI] [PubMed] [Google Scholar]
- 41.Dafni U: Landmark analysis at the 25-year landmark point. Circ Cardiovasc Qual Outcomes 4: 363–371, 2011 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.