Abstract
Cytomegalovirus (CMV) infection in solid-organ transplantation is associated with increased morbidity and mortality, particularly if a CMV mutant strain with antiviral resistance emerges. Monitoring CMV–specific T cell response could provide relevant information for patient care. We and others have shown the involvement of Vδ2neg γδ T cells in controlling CMV infection. Here, we assessed if Vδ2neg γδ T cell kinetics in peripheral blood predict CMV infection resolution and emergence of a mutant strain in high–risk recipients of kidney transplants, including 168 seronegative recipients receiving organs from seropositive donors (D+R−) and 104 seropositive recipients receiving antithymocyte globulins (R+/ATG). Vδ2neg γδ T cell percentages were serially determined in patients grafted between 2003 and 2011. The growing phase of Vδ2neg γδ T cells was monitored in each infected patient, and the expansion rate during this phase was estimated individually by a linear mixed model. A Vδ2neg γδ T cell expansion rate of ˃0.06% per day predicted the growing phase. The time after infection at which an expansion rate of 0.06% per day occurred was correlated with the resolution of CMV DNAemia (r=0.91; P<0.001). At 49 days of antiviral treatment, Vδ2neg γδ T cell expansion onset was associated with recovery, whereas absence of expansion was associated with recurrent disease and DNAemia. The appearance of antiviral–resistant mutant CMV strains was associated with delayed Vδ2neg γδ T cell expansion (P<0.001). In conclusion, longitudinal surveillance of Vδ2neg γδ T cells in recipients of kidney transplants may predict CMV infection resolution and antiviral drug resistance.
Keywords: cytomegalovirus, kidney transplantation, immunology, immunosuppression
Clinical management of cytomegalovirus (CMV) infection remains a challenge in solid-organ transplantation (SOT), because its direct and indirect consequences increase patient morbidity and mortality occurrences. CMV infection resolution involves the synergistic action of an antiviral drug and an efficient CMV immune response. Consequently, a simple measurement of the latter would be of help in the management of infection. In consensus recommendations, the regimen of antiviral drugs includes a minimum course of 2 weeks, with a continuation of treatment until one or two consecutive virologically negative samples are obtained1 (often 49 days on the basis of the randomized Valcyte [valganciclovir po] compared to ganciclovir iv in patients with cytomegalovirus [CMV] disease who are solid organ transplant recipients [VICTOR] Trial).2 Notwithstanding, 30% of patients have virologic and 15% of patients have clinical recurrence of CMV infection,3 meaning that the management of anti–CMV therapy regimens could be improved. Patients with recurrent disease are at risk of developing an antiviral resistance (AVR), which is a serious complication observed in 6%–17% of patients with SOT and CMV disease.4 Risks factors associated with AVR are an extended exposure time to antiviral treatment5 and the absence of preexisting CMV–specific immunity (donor [D]+recipient [R]− patients).6–8 Consequently, the evaluation of cell-mediated immunity (CMI) during the course of infection may help improve the prediction of CMV infection resolution and therefore, the time of therapy discontinuation. Theoretically, CMI could also help predict the risk of emergence of a mutant strain, but it has never been shown until now. During the last decade, a variety of assays has been developed to measure CMV–specific cellular responses,9 but they have failed to identify a threshold of an efficient immune response that can predict CMV infection resolution.10–12 Moreover, there is a lack of data pointing to an immune marker that can help diagnose early the onset of a resistant strain.
Several years ago, we showed how a subset of γδ T cells is involved in the response toward the control of CMV infection. This subset, which is typically located within the epithelia, is characterized by the use of Vδ1, Vδ3, or Vδ5 segments (collectively called Vδ2neg γδ T cells), whereas the most common subset in the peripheral blood uses the association of Vδ2 and Vγ9 variable regions (Vγ9/Vδ2 T cells). After CMV infection and not after other viral infections (such as HSV, VZV, EBV, and influenza), Vδ2neg γδ T cells undergo massive expansion in the blood of recipients of kidney transplants,13 which is associated with the control of CMV infection. This observation has been extended to other SOTs as well as in healthy donors with positive serology, in bone marrow transplants,14 and during fetal life.15
We have shown that these cells displayed a restricted repertoire, which can be regarded as the signature of a CMV-driven selection and amplification of specific T cells in vivo.16 In vitro, data confirm Vδ2neg γδ T cells as an efficient anti–CMV component of the immune response.17,18 Consequently, their monitoring may help guide clinical decision making.19 Because measurement of Vδ2neg γδ T cells in peripheral blood is easy to perform, inexpensive, and highly reproducible, we serially measured the size of this subset by flow cytometry in recipients’ blood during the pre- and post-transplant periods both before and after the onset of CMV infection along with the monitoring of viral load. Our goal was to determine whether longitudinal monitoring of Vδ2neg γδ T cells could predict CMV infection resolution or the occurrence of AVR in high–risk D+R− and R+/antithymocyte globulin (ATG) recipients of kidney transplants.
Results
Baseline Characteristics of Patients
As shown in Table 1, no significant differences were observed in D+R− patients between infected and uninfected patients.
Table 1.
Baseline characteristics of patients
| Criterion | D+R− | R+/ATG | ||||
|---|---|---|---|---|---|---|
| Infected (n=93) | Uninfected (n=75) | P Valuea | Infected (n=74) | Uninfected (n=30) | P Valuea | |
| Recipients | ||||||
| Age, yr (mean±SD) | 50±12.3 | 46.8±14.7 | 0.50 | 56±11.6 | 49±9.6 | <0.01 |
| Sex (men/women) | 65/28 | 55/20 | 0.70 | 48/26 | 17/13 | 0.50 |
| HLA sensitization, N (%) | 26 (28) | 19 (25) | 0.70 | 36 (49) | 25 (83) | 0.001 |
| Nephropathy, N | 0.70 | 0.40 | ||||
| Glomerular | 36 | 28 | 21 | 11 | ||
| Tubulointerstitial | 12 | 8 | 9 | 3 | ||
| Vascular | 7 | 11 | 10 | 1 | ||
| Binephrectomy for cancer | 1 | 0 | 3 | 0 | ||
| Diabetes | 4 | 2 | 4 | 1 | ||
| Congenital | 24 | 18 | 13 | 9 | ||
| Unknown | 9 | 8 | 11 | 5 | ||
| Hemodialysis/peritoneal dialysis, N | 89/4 | 73/2 | 0.60 | 67/5 | 26/2 | >0.99 |
| Donors | ||||||
| Age, yr (mean±SD) | 47.9±13.8 | 44.9±16.6 | 0.20 | 52.6±15.3 | 45.1±15.1 | 0.01 |
| Expanded criteria donors, N (%) | 28 (30) | 17 (23) | 0.30 | 38 (51) | 12 (40) | 0.20 |
| Living donors | 3 | 3 | 0 | 1 | ||
| HLA A/B/DQ/DR mismatches, N | 3.2±1.6 | 3.4±1.3 | 0.60 | 3.4±1.4 | 3.6±1.5 | 0.10 |
| Total ischemia time, h (mean±SD) | 15.6±4.8 | 17.5±6.7 | 0.09 | 18.5±7.3 | 18.4±7.6 | 0.20 |
| Delayed graft function, N (%) | 24 (26) | 20 (27) | >0.99 | 52 (70) | 19 (63) | 0.50 |
| Immunosuppressive treatment, N | ||||||
| Cyclosporin A/tacrolimus/mTORi | 27/62/4 | 17/57/1 | 0.30 | 6/62/5 | 3/26/1 | 0.50 |
| Mycophenolate mofetil | 92 | 74 | 0.60 | 73 | 30 | >0.99 |
| Corticosteroids | 93 | 75 | 0.90 | 73 | 28 | 0.90 |
| Anti–IL-2 receptor antibody/ATG | 61/32 | 53/22 | 0.50 | 0/74 | 30 | — |
| Corticosteroid duration, d (mean±SD) | 656±1110 | 756±1173 | 0.66 | 1322±1247 | 1929±1364 | 0.24 |
| Preemptive therapy/universal prophylaxis 3/6 mo | 48/27/18 | 32/21/22 | 0.30 | 56/18 | 16/14 | 0.03 |
mTORi, mammalian target of rapamycin inhibitor.
P values were obtained using Mann–Whitney or χ2 tests for quantitative or discrete variables, respectively.
In infected R+/ATG patients compared with uninfected patients, donors and recipients were significantly older, and recipients were less HLA sensitized. No other differences were noted.
Median CMV DNAemia duration was longer in D+/R− patients: 134 (1st and 3rd quartiles: 14 and 373 days) compared with 24 days (6 and 194 days) in R+/ATG patients (P<0.001). In D+R− infected patients, median CMV DNAemia duration was 21 days (1st and 3rd quartiles: 12 and 30 days) in a 3-month prophylaxis group, 85 days (25 and 167 days) in a 6-month prophylaxis group, and 179 days (123 and 296 days) in the case of preemptive strategy (P<0.001). In R+/ATG infected patients, median CMV duration was not influenced by CMV prevention strategy: 24 (17 and 34) versus 26 (21 and 35) days for preemptive and prophylactic strategies, respectively (P=0.54).
Kinetics of Vδ2neg γδ T Cells in Recipients of Kidney Transplants
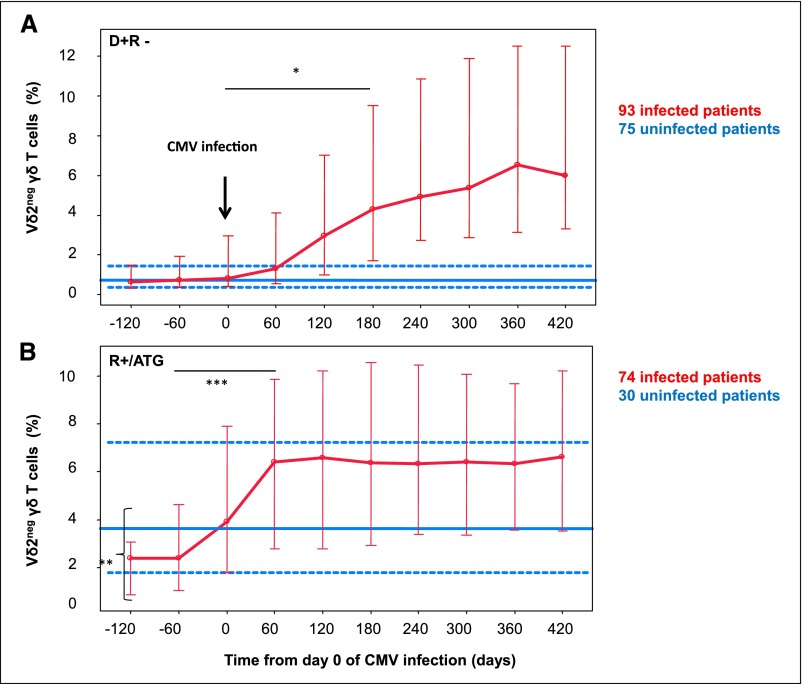
During the 10 years of the study, on average, 10 phenotypic determinations of Vδ2neg γδ T cells were available for each patient. For infected patients, the frequency of sampling averaged 29.2 days, with an SEM of 10.5 days, between day 0 of CMV infection and the end of the growing phase of Vδ2neg γδ T cells. Results of the kinetics of Vδ2neg γδ T cells are expressed in percentages of total lymphocytes (Figure 1), but similar profiles were observed with absolute counts (data not shown). D+R− infected patients (Figure 1A) had a slow and progressive growing phase of Vδ2neg γδ T cells. The median (1st and 3rd quartiles) percentage of Vδ2neg γδ T cells increased from 0.8% (0.4% and 2.9%) before CMV infection to 4.4% (1.4% and 10.7%) thereafter (P=0.001 from day 180). During the same period of time, the median (1st and 3rd quartiles) percentage of Vδ2neg γδ T cells in D+R− uninfected patients (Figure 1A) remained stable at 0.7% (0.5% and 1.06%).
Figure 1.
Kinetics of Vδ2neg γδ T cells in (A) D+R− and (B) R+/ATG patients from day 0 of CMV infection. In infected patients (red lines), Vδ2neg γδ T cells percentage (median; 1st and 3rd quartiles) interpolated data are represented from day 0 of CMV infection. In uninfected patients (blue line), all Vδ2neg γδ T cells percentage values during the follow-up period are pooled and represented (median [solid line]; 1st and 3rd quartiles [dashed lines]). P values were obtained using Mann–Whitney tests. *P<0.05 in D+R− infected patients (comparison of Vδ2neg γδ T cells percentages before and after Vδ2neg γδ T cell expansion); **P<0.05 (comparison of Vδ2neg γδ T cells percentage baselines between R+ infected and uninfected patients); ***P<0.05 in R+/ATG infected patients (comparison of Vδ2neg γδ T cells percentages before and after Vδ2neg γδ T cell expansion).
In infected R+/ATG patients (Figure 1B), the median percentage of Vδ2neg γδ T cells increased from 1.6% (0.8% and 3%) before CMV infection to 6.1% (2.8% and 10.8%) thereafter (P=0.03 from day 60). Interestingly, the growing phase of Vδ2neg γδ T cells occurred before CMV DNAemias became detectable. Moreover, Vδ2neg γδ T cell percentages at the beginning of follow-up were significantly higher in patients who will not develop post–transplant CMV DNAemia (4% [2.1% and 9%]) than in those who will (1.6% [0.8% and 3%]; P=0.01).
Estimation of Vδ2neg γδ T Cell Expansion Rate
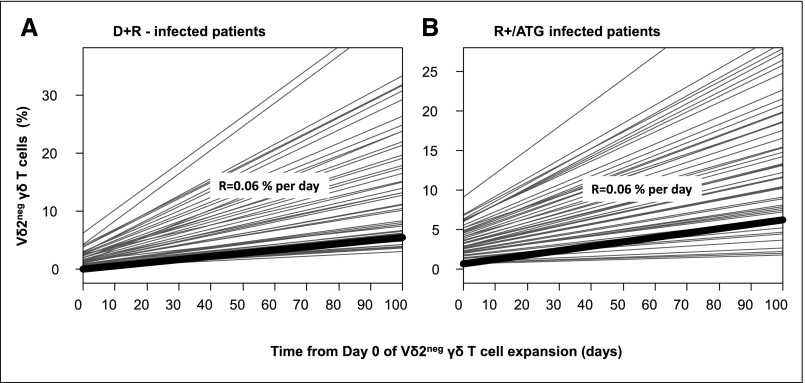
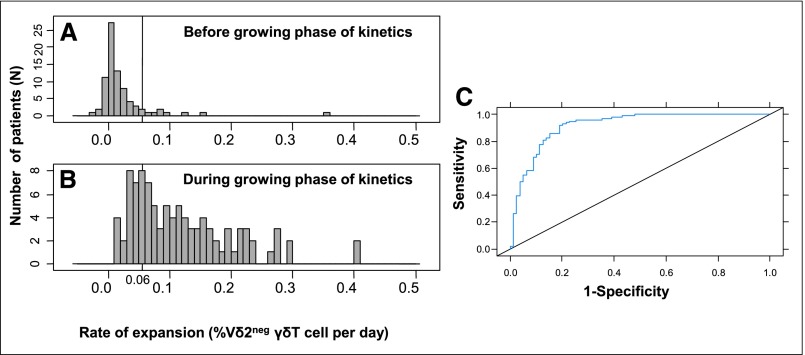
A unique percentage of Vδ2neg γδ T cells could not predict the resolution of DNAemia (Supplemental Figure 1). Instead, we considered, patient by patient, the time at which the growing phase in the whole Vδ2neg γδ T cells kinetics occurred, and then, we estimated the Vδ2neg γδ T cell expansion rate (Figure 2). Estimated expansion rates in infected D+R− (Figure 2A) and R+/ATG patients (Figure 2B) were not significantly different (P=0.35). We were able to determine a threshold of 0.06% Vδ2neg γδ T cells increase per day that maximizes the validity (according to AUC) of the definition of the growing phase of Vδ2neg γδ T cells kinetics (Figure 3, A and B). Thus, for a given patient, an increase of Vδ2neg γδ T cells >0.06% per day between two successive determinations predicted the growing phase, with sensitivity (Se) of 77%, specificity (Spe) of 90%, positive predictive value (PPV) of 88%, and negative predictive value (NPV) of 75% (AUC=0.91) (Figure 3C). Therefore, we were able to define the time of Vδ2neg γδ T cells expansion for each patient (Supplemental Figure 2). It was the time from day 0 of infection to an increase of 0.06% Vδ2neg γδ T cells that was observed between two successive determinations of Vδ2neg γδ T cells.
Figure 2.
Estimation of Vδ2neg γδ T cell expansion rate using a linear mixed model in D+R− infected patients (A) and in R+/ATG infected patients (B). Each infected patient had a growing phase of Vδ2neg γδ T cells identified by careful individual examination of the whole kinetics of Vδ2neg γδ T cells. The rate of expansion (R) was determined, patient by patient, during this growing phase. (A) Represents the estimation of expansion rate in D+R− infected patient. (B) Represents the estimation of expansion rate in R+/ATG infected patients.
Figure 3.
Estimation of the threshold of Vδ2neg γδ T cell expansion rate. A represents a histogram of maximal Vδ2neg γδ T cell increase (in percentage of Vδ2neg γδ T cells per day) between two determinations before the growing phase of Vδ2neg γδ T cells kinetics. B represents a histogram of estimated Vδ2neg γδ T cell expansion rates (in percentages of Vδ2neg γδ T cells per day) with the linear mixed model during the growing phase of Vδ2neg γδ T cells kinetics. A rate of expansion of 0.06% per day was the best threshold to maximize the validity of the definition of the growing phase of Vδ2neg γδ T cells kinetics. (C) Receiver operating characteristics curve of estimated Vδ2neg γδ T cell expansion rates during the growing phase of Vδ2neg γδ T cells kinetics versus maximal Vδ2neg γδ T cell increases between two determinations before this growing phase. Thus, for a given infected patient, an increase of Vδ2neg γδ T cells >0.06% per day between two successive determinations predicted the growing phase, with Se of 77%, Spe of 90%, PPV of 88%, and NPV of 75% (AUC=0.91).
Correlation between the Time of Vδ2neg γδ T Cell Expansion and the CMV DNAemia Resolution
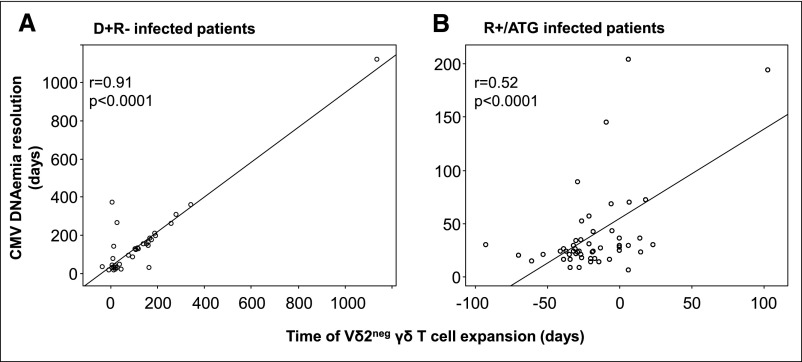
As shown in Figure 4A, the time of Vδ2neg γδ T cell expansion in infected D+R− patients was closely correlated with the CMV DNAemia resolution (r=0.91; P<0.001). Indeed, 88% of infected D+/R− patients had CMV DNAemia resolution within 30 days after they reached 0.06% per day Vδ2neg γδ T cell expansion rate. In infected R+/ATG patients as well, the time of Vδ2neg γδ T cell expansion was correlated with the CMV DNAemia resolution (r=0.52; P<0.001) (Figure 4B). Of note, 92% of infected R+/ATG patients had CMV DNAemia resolution within 30 days after they reached 0.06% per day Vδ2neg γδ T cell expansion rate. Comparing R+/ATG and D+/R− infected patients, the time of Vδ2neg γδ T cell expansion occurred earlier in R+/ATG than D+/R− patients (P<0.001; data not shown). To understand the worst correlation in R+/ATG infected patients compared with D+R− infected patients, we analyzed separately the correlation in D+R− infected patients who received either ATG (n=14) or anti-ILR antibody (n=37) and found that the correlation in D+R− infected patients with ATG was the same as in R+/ATG patients (r=0.55; P<0.001) (Supplemental Figure 3).
Figure 4.
Time of Vδ2neg γδ T cell expansion is correlated with the CMV DNAemia resolution. In (A) D+R− infected and (B) R+/ATG infected patients, linear regressions are represented between the CMV DNAemia resolution (days) and the time of Vδ2neg γδ T cell expansion (days). Scales are different between A and B with regards to the longer CMV DNAemia duration and the Vδ2neg γδ T cell expansion in D+R− compared with R+/ATG patients. P values were obtained using the Fisher test.
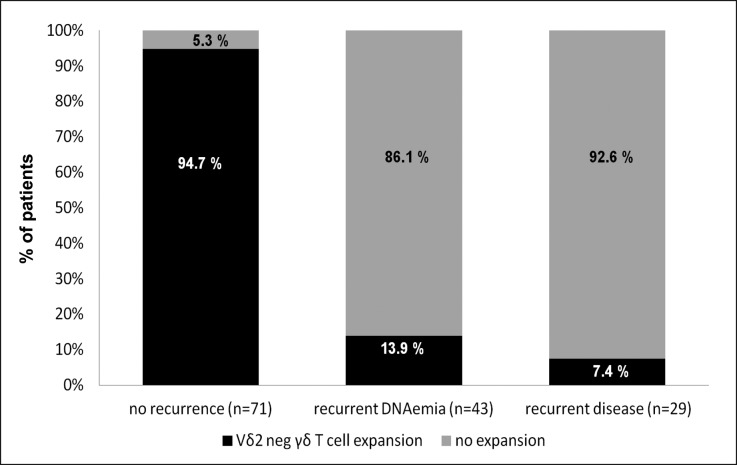
To apply these data in a concrete clinical setting, we analyzed the Vδ2neg γδ T cell expansion onset at the end of the curative antiviral treatment according to the VICTOR Trial (49 days).2 We observed (Figure 5) that Vδ2neg γδ T cell expansion onset was present in 95% of patients without recurrence, whereas absence of Vδ2neg γδ T cell expansion was found in 86% of patients with recurrent DNAemia and 93% of patients with recurrent disease. Consequently, Vδ2neg γδ T cell expansion onset had an Se of 93%, an Spe of 94%, a PPV of 87%, and an NPV of 97% in prediction of CMV resolution at the end of the curative antiviral treatment.
Figure 5.
Absence of Vδ2neg γδ T cell expansion at the end of the antiviral treatment predicts recurrent CMV DNAemia/disease. At the end of antiviral treatment (49 days), data about recovery, recurrent CMV DNAemia, and recurrent disease were collected as well as the occurrence of Vδ2neg γδ T cell expansion; 94.7% (94.67% and 94.73%) of patients with recovery had a Vδ2neg γδ T cell expansion onset, and 86.1% (86% and 86.2%) of patients with recurrent CMV DNAemia and 92.6% (92.51% and 92.69%) of patients with recurrent disease were associated with the absence of Vδ2neg γδ T cell expansion.
The Time of Vδ2neg γδ T Cell Expansion Is Delayed in Infected D+R− Patients with AVR
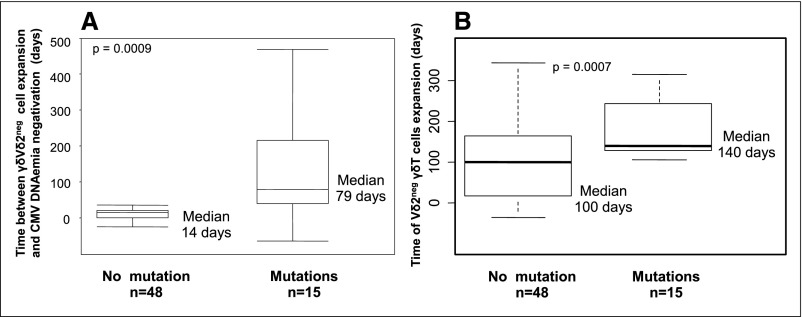
In infected D+R− patients, the median time between Vδ2neg γδ T cell expansion and CMV DNAemia negativation was 14 days in patients without AVR but much longer (79 days) in patients with AVR (P<0.001) (Figure 6A).
Figure 6.
Vδ2neg γδ T cell kinetics in D+R− infected patients with versus without antiviral drug resistance. Prolonged positive CMV DNAemia after Vδ2neg γδ T cell expansion in the case of AVR. A represents box plots comparing time (days) between Vδ2neg γδ T cell expansion (days) and the CMV DNAemia negativation in D+R− infected patients with versus without the emergence of a mutant strain. P value was obtained with a Mann–Whitney test. B) represents box plots comparing the time of Vδ2neg γδ T cell expansion (days) in D+R− infected patients with versus without the emergence of a mutant strain. A time of Vδ2neg γδ T cell expansion above 76 days was predictive of the emergence of a mutant strain (Se of 100%, Spe of 60%, PPV of 47%, and NPV of 100%). P value was obtained using Mann–Whitney test.
In all 15 infected D+R− patients with AVR, the median (1st and 3rd quartiles) time of Vδ2neg γδ T cell expansion was significantly delayed compared with patients without AVR: 140 (128 and 243) versus 100 (18.7 and 163) days, respectively (P<0.001) (Figure 6B). A threshold of 76 days had good predictive values of AVR occurrence (Se=100%; Spe=77%; PPV=60%; NPV=100%; AUC=0.75; data not shown).
Factors Influencing the Delay in Vδ2neg γδ T Cell Expansion in Infected D+R− Patients
To better examine the factors controlling the delay of the time of Vδ2neg γδ T cells expansion in infected D+R− patients (all infected R+/ATG patients had an early expansion), a univariate analysis (Table 2) was undertaken. Interestingly, the type of anti–CMV prevention strategy influenced the time of Vδ2neg γδ T cell expansion, which occurred earlier in patients treated with universal prophylaxis for either 6 or 3 months compared with preemptively treated patients. Moreover, a late-onset infection, occurring in 52.7% of D+R− patients in our study, was also significantly associated with an earlier time of expansion (P<0.001). Universal prophylaxis (3 or 6 months) was highly correlated with late-onset infection, which means that 96% of infected patients treated with universal prophylaxis had a late-onset infection, whereas 87.5% of preemptively treated infected patients had an early infection (relative risk, 7.6; 95% confidence interval, 3.6 to 16.20; P<0.001). A multivariate analysis showed that late-onset infection was the only factor associated with the time of expansion (Supplemental Table 1).
Table 2.
Variables associated with the time of Vδ2neg γδ T cell expansion in patients infected with D+R
| Univariate Analysis | Coefficient (SEM) | P Valuec |
|---|---|---|
| Recipient age at the time of the graft,a d | +0.45 (1.1) | 0.70 |
| Recipient’s sex (men versus women) | +8 (32) | 0.80 |
| Induction (anti-ATG versus anti–IL-2 receptor antibody) | +22 (32) | 0.50 |
| Tacrolimus versus cyclosporin A | +14 (4) | 0.70 |
| CMV infection versus CMV disease | +54 (32) | 0.09 |
| Peak viral loadb | −8.6 (4.2) | 0.80 |
| Valganciclovir 3 mo (versus preemptive treatment) | −93 (47) | 0.05 |
| Valganciclovir 6 mo (versus preemptive treatment) | −109 (33) | 0.002 |
| Late-onset infection/disease (yes versus no) | −129 (25) | <0.001 |
Increase in the delay of expansion (days) for each additional 1 year of age.
Decrease in the delay of expansion (days) for each additional 10,000,000 IU CMV copies.
P values were obtained using Fisher test.
Discussion
Our results show that a longitudinal monitoring of Vδ2neg γδ T cells in D+R− and R+/ATG recipients of kidney transplants can predict the fate of CMV infection: CMV DNAemia resolution or CMV DNAemia/disease recurrence and emergence of a mutant strain with AVR; 90% of D+R− and R+/ATG patients had long-term resolution of CMV DNAemia within 30 days after they reached an expansion rate of 0.06% per day. At the end of the antiviral treatment, Vδ2neg γδ T cell expansion onset was closely associated with recovery and absence of expansion with recurrent DNAemia or disease. In addition, we observed that Vδ2neg γδ T cell expansion and CMV resolution were less correlated in D+R− and R+ infected patients with ATG. This rare phenomenon could be explained by two hypotheses. First, after ATG, Vδ2neg γδ T cells expansion could be driven by another homeostatic process than CMV infection. Second, the persistence of CMV DNAemia in these patients with an early Vδ2neg γδ T cells expansion could be explained by a profound and persistent depletion of CMV–specific αβ T cells. Additional studies are needed to analyze more precisely the effect of ATG on both γδ and CMV–specific αβ T cells.
To our knowledge, this study has the highest number of patients (n=272) ever included in a longitudinal immunomonitoring study in organ transplantation. This study also differs from previous CMI studies in several respects. First, we analyzed R+ and R− patients separately, because CMI assays gave conclusive results mainly in R+ patients.20,21 Second, we used longitudinal monitoring, because (1) this approach is more likely to capture the dynamics of the immune response and (2) previous studies aimed at defining a threshold of CMI at a given time point as a predictive marker of CMV events rarely succeeded, especially in D+R− patients.20–22 Indeed, we used this approach to predict CMV recurrence after the first episode of CMV infection, whereas the majority of the studies focused on the prediction of CMV infection on the day of transplantation or at the end of prophylaxis therapy. In keeping with the risk of recurrence, it has been shown in recipients of lung transplants that relapsing viremia was associated with poor induction of T-bet on CMV–specific CD8+ T cells and low frequencies of pp65–specific CD8 effector T cells, although a threshold was not identified.11 Others found a correlation between CMI assay and recurrence but not with a threshold.23–26 Finally, most CMI assays require an in vitro activation step,22,27,28 whereas Vδ2neg γδ T cell monitoring relies on a simple (without stimulation) and standardized whole–blood immunophenotyping.
Uninfected R+/ATG patients had a significantly higher percentage of Vδ2neg γδ T cells at the beginning of follow-up compared with that of infected R+/ATG patients. Moreover, R+/ATG infected patients had an earlier expansion and consequently, a prompt CMV DNAemia resolution compared with D+R− infected patients. This potential protective immune effect is in agreement with the presence of a subset of memory Vδ2neg γδ T cells that we have already described elsewhere.16 In R+/ATG patients, the Vδ2neg γδ T cell response occurs readily, even before CMV DNAemia becomes detectable, which suggests that CMV and memory Vδ2neg γδ T cells do interact early at the sites of entry and replication of the virus in epithelial tissues, where most of the Vδ2neg γδ T cells are located.29
In naïve D+/R− patients, universal prophylaxis is considered to hamper the development of specific immune response, causing more late–onset CMV infections than the preemptive approach,30,31 with increased morbidity and mortality.32 Conversely to this hypothesis, we found that the time of Vδ2neg γδ T cell expansion occurred earlier in patients with a late-onset infection for most under universal prophylaxis, with a faster CMV DNAemia resolution. We hypothesized that the time from transplantation could allow (1) a local priming of Vδ2neg γδ T cells in tissues without systemic dissemination of the virus and (2) a decrease of the immunosuppressive burden. Thus, when a late-onset infection occurs after the end of the universal prophylaxis, Vδ2neg γδ T cells are more prone to undergo an efficient expansion.
AVR is a major concern in recipients of SOTs,19,33 and we have shown a link between a delay in the Vδ2neg γδ T cell expansion and the emergence of mutant strain. Such a relationship between AVR and low CMI had been previously suggested in a single study in a few patients.26 With the caution of a limited size of patient sample, the time of Vδ2neg γδ T cell expansion above 76 days after the beginning of CMV infection could be a useful immune marker to suspect AVR caused by a mutant strain. Indeed, in our cohort, the virologic diagnosis of a mutant strain was performed, on average, 40 days later (namely, 116 days after the beginning of CMV infection) and also, previously reported at about 130 days.4 On one hand, a delayed immune response requiring a longer treatment can favor the emergence of a mutant strain. On the other hand, the immune response can be delayed because of the mutant strain, which has a different fitness as well as a different ability to stimulate immune response compared with a wild-type strain.
Finally, the emergence of a mutant CMV strain was associated with a persistent CMV DNAemia after Vδ2neg γδ T cell expansion. This phenomenon may reflect some degree of clonal exhaustion of Vδ2neg γδ T cells, because it is often observed in chronic viral infections, and it deserves additional investigations.34,35 This observation emphasizes also that infection resolution is the result of a subtle balance between viral replication, specific immune response, antiviral treatment, and immunosuppressive burden, especially in the case of a viral-resistant strain.
In conclusion, Vδ2neg γδ T cell expansion is related to the resolution of CMV infection in high-risk patients, and a delayed expansion is predictive of the occurrence of a mutant strain. A prospective trial could confirm the usefulness of Vδ2neg γδ T cell longitudinal monitoring in patients with SOTs to tailor the optimal duration of treatment.
Concise Methods
Study Design and Patients
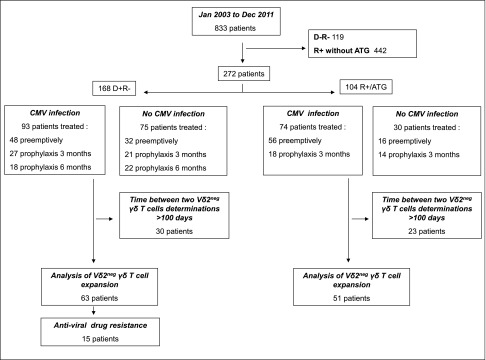
As shown in Figure 7, 168 D+R− patients with either anti–IL-2 receptor antibody (n=114) or ATG (n=54) and 104 R+/ATG patients were included between January 1, 2003 and December 31, 2011, and followed at least 2 years post-transplantation with viral (CMV PCR) and immunologic (peripheral blood immunophenotyping) determinations.
Figure 7.
Distribution of the patients. 168 D+R2 patients with either anti–IL-2 receptor antibody (n=114) or ATG (n=54) and 104 R+/ATG patients were included between January 1, 2003 and December 31, 2011, who experienced or not experienced CMV infection. Peripheral blood immunophenotyping determinations were longitudinally determined for each patient.
From January 1, 2003 to November 30, 2006, patients received universal prophylaxis for 3 months. From December 1, 2006 to June 30, 2010, patients were preemptively followed and treated when the PCR CMV result reached 2000 copies per 1 ml. Finally, from July 1, 2010 to December 31, 2011, patients received universal prophylaxis: 6 months for D+R− patients and 3 months for R+/ATG patients.
CMV manifestations were defined as CMV infection, CMV disease, late-onset infection, CMV tissue–invasive disease, recurrent DNAemia, and recurrent disease on the basis of standardized criteria.1
Intravenous ganciclovir or oral valganciclovir was given for curative treatment and always followed by oral valganciclovir at a prophylactic dose as previously described.2 The anti-CMV treatment was discontinued after two consecutive negative CMV PCR tests. The dose was adjusted according to the manufacturer’s recommendations using the Cockcroft–Gault formula. Expanded criteria for the donor and delayed graft function were defined as previously described.36,37 This study was approved by the Institutional Review Board of the Bordeaux Hospital.
CMV Monitoring
CMV IgG serology was performed following the manufacturer’s recommendations (Enzygnost Anti-CMV/IgM and IgG; Dade Behring, Marburg, Germany and Access CMV IgG and IgM; Beckman Coulter). The CMV PCR assay was used as previously described.38 CMV DNAemia was considered positive when detectable (namely, >500 copies per 1 ml). The assay was performed one time per week for the first 3 months, one time per month between months 3 and 6, and finally, every 2 months up to 1 year. AVR was suspected when persistent viral replication was observed after >2 weeks of appropriate antiviral therapy. AVR was confirmed by full-length sequencing of UL97 and UL54 genes,19 which was performed at the French National Cytomegalovirus Reference Center (Limoges, France). Sequences were compared with the AD169 reference sequence using the Gene Librarian 3.2TM software (Visible Genetics Inc., Siemens, France).6,39
Flow Cytometry Analyses and Monitoring of Vδ2neg γδ T Cells
Vδ2neg γδ T cells count was obtained by immunophenotypic determination after flow cytometry was carried out on 100 µl anticoagulated whole blood taking into account at least 5000 total lymphocytes stained with anti-CD45, antipan-δ (clone IMMU 510; Beckman Coulter, Krefeld, Germany), and anti-TCR Vδ2 (clone 15D; Thermo Fisher Scientific, Rockford, IL). Percentages of cell populations were obtained using CELLQUEST software (BD Bioscience), and absolute counts of lymphocytes were obtained using the Single–Platform Lyse/No–Wash Trucount (BD Bioscience). In our center, the surveillance of Vδ2neg γδ T cells was on the basis of a measurement at day 0 of the graft; months 3, 6, and 12; and then, every year. In case of CMV infection, additional Vδ2neg γδ T cells determinations were performed. A maximal interval of 100 days between two determinations of Vδ2neg γδ T cells phenotype was required; otherwise, these patients were not included in the analysis of the kinetics of Vδ2neg γδ T cells (Figure 7).
Definitions of Time Points of Infection and Vδ2neg γδ T Cells Kinetics
Supplemental Figure 2 illustrates the evolution of CMV DNAemias and Vδ2neg γδ T cells in a representative infected patient. Every time point is considered from the beginning of CMV infection.
Day 0 of infection was the first positive CMV DNAemia.
CMV DNAemia resolution was defined as the first negative CMV DNAemia with successive negative CMV DNAemias (at least two) without subsequent relapse until the end of the follow-up period, which means at least 1 year from day 0 of infection.
The growing phase of Vδ2neg γδ T cells was identified by careful individual examination of the whole kinetics of Vδ2neg γδ T cells.
Day 0 of Vδ2neg γδ T cell expansion was the first Vδ2neg γδ T cells determination from day 0 of infection, after which the Vδ2neg γδ T cell growing phase began.
Statistical Analyses
Analyses were performed with conventional statistical methods using the R statistical software (version 3.10.1) and specifically, the lme4 and ROCR packages.40 Mann–Whitney and Fisher tests were used when appropriate. P<0.05 was considered statistically significant. When required, threshold values and performances were evaluated using receiver operating characteristics. The expansion rate was determined during the growing phase of Vδ2neg γδ T cells kinetics (encompassing from 2 to 12 determinations) and estimated for each patient using a linear mixed model, which is well suited to analyze repeated, correlated, and unbalanced longitudinal data. The individual expansion rate was calculated using empirical Bayes estimates.
Disclosures
None.
Supplementary Material
Acknowledgments
We thank Catherine Rio (nurse coordinator). We also thank the technicians from the Laboratories of Virology and Immunology at Bordeaux University Hospital and the Centre National de Référence des Cytomégalovirus for their significant contribution to this study.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2014100985/-/DCSupplemental.
References
- 1.Kotton CN, Kumar D, Caliendo AM, Asberg A, Chou S, Danziger-Isakov L, Humar A, Transplantation Society International CMV Consensus Group : Updated international consensus guidelines on the management of cytomegalovirus in solid-organ transplantation. Transplantation 96: 333–360, 2013 [DOI] [PubMed] [Google Scholar]
- 2.Asberg A, Humar A, Rollag H, Jardine AG, Mouas H, Pescovitz MD, Sgarabotto D, Tuncer M, Noronha IL, Hartmann A, VICTOR Study Group : Oral valganciclovir is noninferior to intravenous ganciclovir for the treatment of cytomegalovirus disease in solid organ transplant recipients. Am J Transplant 7: 2106–2113, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Asberg A, Humar A, Jardine AG, Rollag H, Pescovitz MD, Mouas H, Bignamini A, Töz H, Dittmer I, Montejo M, Hartmann A, VICTOR Study Group : Long-term outcomes of CMV disease treatment with valganciclovir versus IV ganciclovir in solid organ transplant recipients. Am J Transplant 9: 1205–1213, 2009 [DOI] [PubMed] [Google Scholar]
- 4.Le Page AK, Jager MM, Iwasenko JM, Scott GM, Alain S, Rawlinson WD: Clinical aspects of cytomegalovirus antiviral resistance in solid organ transplant recipients. Clin Infect Dis 56: 1018–1029, 2013 [DOI] [PubMed] [Google Scholar]
- 5.Emery VC, Griffiths PD: Prediction of cytomegalovirus load and resistance patterns after antiviral chemotherapy. Proc Natl Acad Sci U S A 97: 8039–8044, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hantz S, Garnier-Geoffroy F, Mazeron MC, Garrigue I, Merville P, Mengelle C, Rostaing L, Saint Marcoux F, Essig M, Rerolle JP, Cotin S, Germi R, Pillet S, Lebranchu Y, Turlure P, Alain S, French CMV Resistance Survey Study Group : Drug-resistant cytomegalovirus in transplant recipients: A French cohort study. J Antimicrob Chemother 65: 2628–2640, 2010 [DOI] [PubMed] [Google Scholar]
- 7.Boivin G, Goyette N, Rollag H, Jardine AG, Pescovitz MD, Asberg A, Ives J, Hartmann A, Humar A: Cytomegalovirus resistance in solid organ transplant recipients treated with intravenous ganciclovir or oral valganciclovir. Antivir Ther 14: 697–704, 2009 [PubMed] [Google Scholar]
- 8.Myhre HA, Haug Dorenberg D, Kristiansen KI, Rollag H, Leivestad T, Asberg A, Hartmann A: Incidence and outcomes of ganciclovir-resistant cytomegalovirus infections in 1244 kidney transplant recipients. Transplantation 92: 217–223, 2011 [DOI] [PubMed] [Google Scholar]
- 9.Egli A, Humar A, Kumar D: State-of-the-art monitoring of cytomegalovirus-specific cell-mediated immunity after organ transplant: A primer for the clinician. Clin Infect Dis 55: 1678–1689, 2012 [DOI] [PubMed] [Google Scholar]
- 10.Patel M, Stefanidou M, Long CB, Fazzari MJ, Tesfa L, Del Rio M, Lamour J, Ricafort R, Madan RP, Herold BC: Dynamics of cell-mediated immune responses to cytomegalovirus in pediatric transplantation recipients. Pediatr Transplant 16: 18–28, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pipeling MR, John ER, Orens JB, Lechtzin N, McDyer JF: Primary cytomegalovirus phosphoprotein 65-specific CD8+ T-cell responses and T-bet levels predict immune control during early chronic infection in lung transplant recipients. J Infect Dis 204: 1663–1671, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chiereghin A, Gabrielli L, Zanfi C, Petrisli E, Lauro A, Piccirilli G, Baccolini F, Dazzi A, Cescon M, Morelli MC, Pinna AD, Landini MP, Lazzarotto T: Monitoring cytomegalovirus T-cell immunity in small bowel/multivisceral transplant recipients. Transplant Proc 42: 69–73, 2010 [DOI] [PubMed] [Google Scholar]
- 13.Déchanet J, Merville P, Bergé F, Bone-Mane G, Taupin JL, Michel P, Joly P, Bonneville M, Potaux L, Moreau JF: Major expansion of gammadelta T lymphocytes following cytomegalovirus infection in kidney allograft recipients. J Infect Dis 179: 1–8, 1999 [DOI] [PubMed] [Google Scholar]
- 14.Knight A, Madrigal AJ, Grace S, Sivakumaran J, Kottaridis P, Mackinnon S, Travers PJ, Lowdell MW: The role of Vδ2-negative γδ T cells during cytomegalovirus reactivation in recipients of allogeneic stem cell transplantation. Blood 116: 2164–2172, 2010 [DOI] [PubMed] [Google Scholar]
- 15.Vermijlen D, Brouwer M, Donner C, Liesnard C, Tackoen M, Van Rysselberge M, Twité N, Goldman M, Marchant A, Willems F: Human cytomegalovirus elicits fetal gammadelta T cell responses in utero. J Exp Med 207: 807–821, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pitard V, Roumanes D, Lafarge X, Couzi L, Garrigue I, Lafon ME, Merville P, Moreau JF, Déchanet-Merville J: Long-term expansion of effector/memory Vdelta2-gammadelta T cells is a specific blood signature of CMV infection. Blood 112: 1317–1324, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Couzi L, Pitard V, Sicard X, Garrigue I, Hawchar O, Merville P, Moreau JF, Déchanet-Merville J: Antibody-dependent anti-cytomegalovirus activity of human γδ T cells expressing CD16 (FcγRIIIa). Blood 119: 1418–1427, 2012 [DOI] [PubMed] [Google Scholar]
- 18.Halary F, Pitard V, Dlubek D, Krzysiek R, de la Salle H, Merville P, Dromer C, Emilie D, Moreau JF, Déchanet-Merville J: Shared reactivity of Vdelta2(neg) gammadelta T cells against cytomegalovirus-infected cells and tumor intestinal epithelial cells. J Exp Med 201: 1567–1578, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Couzi L, Helou S, Bachelet T, Moreau K, Martin S, Morel D, Lafon ME, Boyer B, Alain S, Garrigue I, Merville P: High incidence of anticytomegalovirus drug resistance among D+R- kidney transplant recipients receiving preemptive therapy. Am J Transplant 12: 202–209, 2012 [DOI] [PubMed] [Google Scholar]
- 20.Cantisán S, Lara R, Montejo M, Redel J, Rodríguez-Benot A, Gutiérrez-Aroca J, González-Padilla M, Bueno L, Rivero A, Solana R, Torre-Cisneros J: Pretransplant interferon-γ secretion by CMV-specific CD8+ T cells informs the risk of CMV replication after transplantation. Am J Transplant 13: 738–745, 2013 [DOI] [PubMed] [Google Scholar]
- 21.Kumar D, Chernenko S, Moussa G, Cobos I, Manuel O, Preiksaitis J, Venkataraman S, Humar A: Cell-mediated immunity to predict cytomegalovirus disease in high-risk solid organ transplant recipients. Am J Transplant 9: 1214–1222, 2009 [DOI] [PubMed] [Google Scholar]
- 22.Lisboa LF, Kumar D, Wilson LE, Humar A: Clinical utility of cytomegalovirus cell-mediated immunity in transplant recipients with cytomegalovirus viremia. Transplantation 93: 195–200, 2012 [DOI] [PubMed] [Google Scholar]
- 23.Martín-Gandul C, Pérez-Romero P, Blanco-Lobo P, Benmarzouk-Hidalgo OJ, Sánchez M, Gentil MA, Bernal C, Sobrino JM, Rodríguez-Hernández MJ, Cordero E, Spanish Network for Research in Infectious Diseases (REIPI) : Viral load, CMV-specific T-cell immune response and cytomegalovirus disease in solid organ transplant recipients at higher risk for cytomegalovirus infection during preemptive therapy. Transpl Int 27: 1060–1068, 2014 [DOI] [PubMed] [Google Scholar]
- 24.Sester M, Sester U, Gärtner B, Heine G, Girndt M, Mueller-Lantzsch N, Meyerhans A, Köhler H: Levels of virus-specific CD4 T cells correlate with cytomegalovirus control and predict virus-induced disease after renal transplantation. Transplantation 71: 1287–1294, 2001 [DOI] [PubMed] [Google Scholar]
- 25.Egli A, Silva M, Jr., O’Shea D, Wilson LE, Baluch A, Lisboa LF, Hidalgo LG, Kumar D, Humar A: An analysis of regulatory T-cell and Th-17 cell dynamics during cytomegalovirus replication in solid organ transplant recipients. PLoS ONE 7: e43937, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Egli A, Binet I, Binggeli S, Jäger C, Dumoulin A, Schaub S, Steiger J, Sester U, Sester M, Hirsch HH: Cytomegalovirus-specific T-cell responses and viral replication in kidney transplant recipients. J Transl Med 6: 29, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abate D, Saldan A, Forner G, Tinto D, Bianchin A, Palù G: Optimization of interferon gamma ELISPOT assay to detect human cytomegalovirus specific T-cell responses in solid organ transplants. J Virol Methods 196: 157–162, 2014 [DOI] [PubMed] [Google Scholar]
- 28.Westall GP, Mifsud NA, Kotsimbos T: Linking CMV serostatus to episodes of CMV reactivation following lung transplantation by measuring CMV-specific CD8+ T-cell immunity. Am J Transplant 8: 1749–1754, 2008 [DOI] [PubMed] [Google Scholar]
- 29.Vantourout P, Hayday A: Six-of-the-best: Unique contributions of γδ T cells to immunology. Nat Rev Immunol 13: 88–100, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Khoury JA, Storch GA, Bohl DL, Schuessler RM, Torrence SM, Lockwood M, Gaudreault-Keener M, Koch MJ, Miller BW, Hardinger KL, Schnitzler MA, Brennan DC: Prophylactic versus preemptive oral valganciclovir for the management of cytomegalovirus infection in adult renal transplant recipients. Am J Transplant 6: 2134–2143, 2006 [DOI] [PubMed] [Google Scholar]
- 31.Reischig T, Jindra P, Hes O, Svecová M, Klaboch J, Treska V: Valacyclovir prophylaxis versus preemptive valganciclovir therapy to prevent cytomegalovirus disease after renal transplantation. Am J Transplant 8: 69–77, 2008 [DOI] [PubMed] [Google Scholar]
- 32.Arthurs SK, Eid AJ, Pedersen RA, Kremers WK, Cosio FG, Patel R, Razonable RR: Delayed-onset primary cytomegalovirus disease and the risk of allograft failure and mortality after kidney transplantation. Clin Infect Dis 46: 840–846, 2008 [DOI] [PubMed] [Google Scholar]
- 33.Limaye AP: Ganciclovir-resistant cytomegalovirus in organ transplant recipients. Clin Infect Dis 35: 866–872, 2002 [DOI] [PubMed] [Google Scholar]
- 34.La Rosa C, Krishnan A, Longmate J, Martinez J, Manchanda P, Lacey SF, Limaye AP, Diamond DJ: Programmed death-1 expression in liver transplant recipients as a prognostic indicator of cytomegalovirus disease. J Infect Dis 197: 25–33, 2008 [DOI] [PubMed] [Google Scholar]
- 35.Day CL, Kaufmann DE, Kiepiela P, Brown JA, Moodley ES, Reddy S, Mackey EW, Miller JD, Leslie AJ, DePierres C, Mncube Z, Duraiswamy J, Zhu B, Eichbaum Q, Altfeld M, Wherry EJ, Coovadia HM, Goulder PJ, Klenerman P, Ahmed R, Freeman GJ, Walker BD: PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature 443: 350–354, 2006 [DOI] [PubMed] [Google Scholar]
- 36.Metzger RA, Delmonico FL, Feng S, Port FK, Wynn JJ, Merion RM: Expanded criteria donors for kidney transplantation. Am J Transplant 3[Suppl 4]: 114–125, 2003 [DOI] [PubMed] [Google Scholar]
- 37.Koning OH, Ploeg RJ, van Bockel JH, Groenewegen M, van der Woude FJ, Persijn GG, Hermans J, European Multicenter Study Group : Risk factors for delayed graft function in cadaveric kidney transplantation: A prospective study of renal function and graft survival after preservation with University of Wisconsin solution in multi-organ donors. Transplantation 63: 1620–1628, 1997 [DOI] [PubMed] [Google Scholar]
- 38.Garrigue I, Doussau A, Asselineau J, Bricout H, Couzi L, Rio C, Merville P, Fleury H, Lafon ME, Thiébaut R: Prediction of cytomegalovirus (CMV) plasma load from evaluation of CMV whole-blood load in samples from renal transplant recipients. J Clin Microbiol 46: 493–498, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chou S, Marousek G, Boivin G, Goyette N, Farhan M, Ives JA, Elston R: Recombinant phenotyping of cytomegalovirus sequence variants detected after 200 or 100 days of valganciclovir prophylaxis. Transplantation 90: 1409–1413, 2010 [DOI] [PubMed] [Google Scholar]
- 40.Team RCR: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, 2012. Available at: http://www.R-project.org/. Accessed March 31, 2013 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.