Abstract
Hypoplastic and/or cystic kidneys have been found in both LDL receptor–related protein 6 (Lrp6)- and β-catenin–mutant mouse embryos, and these proteins are key molecules for Wnt signaling. However, the underlying mechanisms of Lrp6/β-catenin signaling in renal development and cystic formation remain poorly understood. In this study, we found evidence that diminished cell proliferation and increased apoptosis occur before cystic dysplasia in the renal primordia of Lrp6-deficient mouse embryos. The expression of Ret proto-oncogene (Ret), a critical receptor for the growth factor glial cell line–derived neurotrophic factor (GDNF), which is required for early nephrogenesis, was dramatically diminished in the mutant renal primordia. The activities of other representative nephrogenic genes, including Lim1, Pax2, Pax8, GDNF, and Wnt11, were subsequently diminished in the mutant renal primordia. Molecular biology experiments demonstrated that Ret is a novel transcriptional target of Wnt/β-catenin signaling. Wnt agonist lithium promoted Ret expression in vitro and in vivo. Furthermore, Lrp6-knockdown or lithium treatment in vitro led to downregulation or upregulation, respectively, of the phosphorylated mitogen-activated protein kinases 1 and 3, which act downstream of GDNF/Ret signaling. Mice with single and double mutations of Lrp6 and Ret were perinatal lethal and demonstrated gene dosage–dependent effects on the severity of renal hypoplasia during embryogenesis. Taken together, these results suggest that Lrp6-mediated Wnt/β-catenin signaling modulates or interacts with a signaling network consisting of Ret cascades and related nephrogenic factors for renal development, and the disruption of these genes or signaling activities may cause a spectrum of hypoplastic and cystic kidney disorders.
Keywords: cell signaling, cystic kidney, renal agenesis
The mammalian kidney develops through the pronephros, mesonephros, and metanephros stages by reiterated branching, patterning, and segmentation on the base of reciprocal inductions and interactions between the epithelial and mesenchymal tissues originated from the intermediate mesoderm.1,2 Wnt signaling plays crucial roles in organogenesis and disease.3 Among 19 ligands, Wnt4, Wnt9b, and Wnt11 are uniquely expressed in renal tissues and are required for kidney development.4–6 The canonical Wnt/β-catenin signaling is involved in nephron induction and epithelial differentiation.7 Either overactivation or inactivation of Wnt signaling may cause polycystic kidneys in animal models.8–11 The gene-trapped mouse mutants of Lrp6, a key coreceptor in the Wnt/β-catenin signaling pathway, also exhibit hypoplastic and cystic kidneys as embryos,12 yet the detailed phenotypic and mechanistic information remains unavailable.
During early development, the receptor tyrosine kinase Ret and its ligand molecule glial cell line–derived neurotrophic factor (GDNF) play critical roles in the induction and branching of the ureteric bud for kidney morphogenesis.2,13,14 In particular, Ret acts through distinct downstream cascades, including PLCγ and PI3K/MAPK, which have been linked to different types of renal anomalies, including renal agenesis and cystic dysplasia.15–17 This may provide a basis for understanding the related genetic mechanisms. However, the upstream regulators of the Ret signaling remain unclear. Several transcription factors, such as Lim1 and Pax2, which are interactive with Ret signaling, also play critical roles in renal development and related disorders.1,2 This study using Lrp6 knockout mice combined with molecular and cellular approaches demonstrates that Lrp6-mediated Wnt/β-catenin signaling is required for metanephric branching through modulation of or interaction with a signaling network consisting of Ret cascades and related nephrogenic factors. The double knockout analyses demonstrate the genetic interaction between Lrp6 and Ret in urogenital development. These results provide novel insights into the signaling and genetic mechanisms of renal development and cystic dysplasia.
RESULTS
Hypoplastic and Cystic Kidneys with Diminished Proliferation and Increased Apoptosis in the Renal Primordia of the Lrp6-KO Embryos
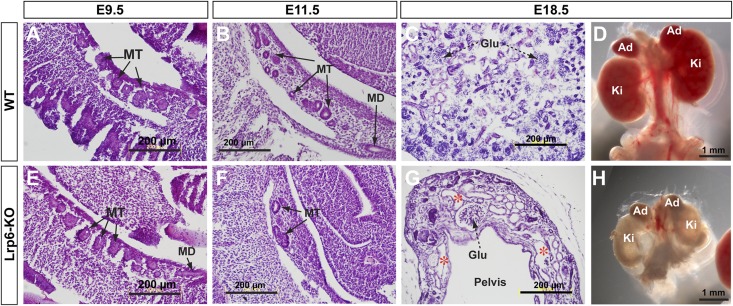
Histologic analyses of the sagittal sections of Lrp6ßgeo/ßgeo (knockout [KO]) mouse embryos demonstrate no obvious morphologic changes of the mutant mesonephros compared with the littermate controls at embryonic day (E) 9.5 (Figure 1, A and E). At E11.5, the mutants exhibit fewer mesonephric tubules compared with the controls (Figure 1, B and F). At E18.5, all examined mutant kidneys (n>40) are hypoplastic and accompanied by multiple cysts (Figure 1, G and H).
Figure 1.
Hypoplastic and cystic kidneys in Lrp6-KO mouse embryos. (A–C, E–G) Hematoxylin and eosin (HE) staining shows the mesonephros at E10.5 and E11.5, and the metanephros at E18.5 of WT and mutant embryos. (D, H) Whole renal structures of the normal and mutant embryos at E18.5. Ad, adrenal gland; Glu, glomerulus; Ki, kidney; MD/MT, mesonephric duct/tubules; asterisk, cysts.
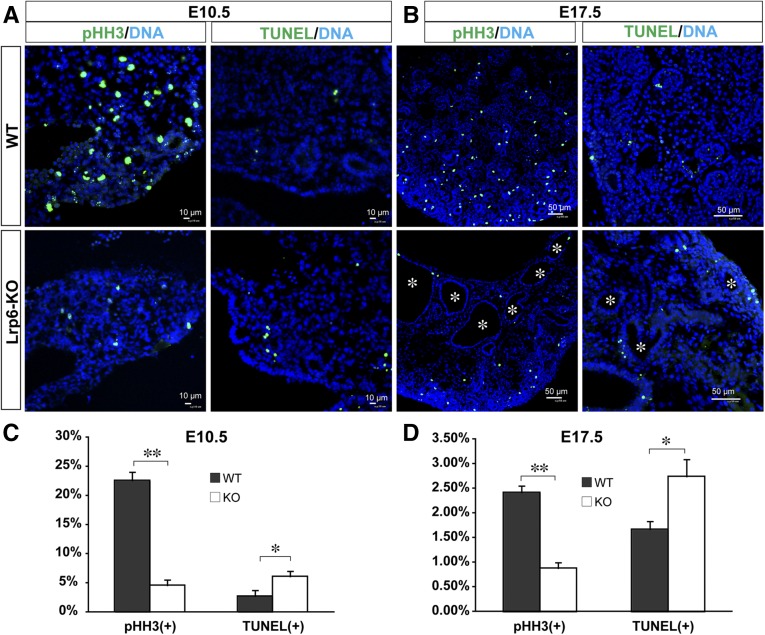
The phospho-histone H3 (pHH3) immunostaining for mitotic cells and the terminal deoxynucleotidyl transferase–mediated digoxigenin-deoxyuridine nick-end labeling (TUNEL) for apoptotic cells were examined in Lrp6-KO mutants and compared with their littermate controls. The percentages of the pHH3(+) cells in the mutant renal primordia dramatically decreased by 5-fold at E10.5 and 2.7-fold at E17.5 (Figure 2, A–D). Conversely, the percentages of the TUNEL(+) cells in the mutant renal primordia increased by 2-fold at E10.5 and 1.6-fold at E17.5, with statistical significance (Figure 2). The diminished proliferation and elevated apoptosis suggest a possible cellular mechanism underlying renal cystic dysplasia in Lrp6-KO embryos.
Figure 2.
Diminished proliferation and elevated apoptosis in renal primordia or embryonic kidneys of Lrp6-KO mice. Micrographs (A, B) or histograms of the percentages (C, D) of the mitotic cells immunolabeled for pHH3, or apoptotic cells detected by TUNEL in the mesonephric tissues at E10.5 or in the kidney at E17.5. Asterisks in B indicate cysts with varied sizes in the mutant kidney. Asterisks in C, D: *P<0.05; **P<0.01.
Repressed Expression of Ret Followed by other Nephrogenic Factors in the Lrp6-KO Embryos
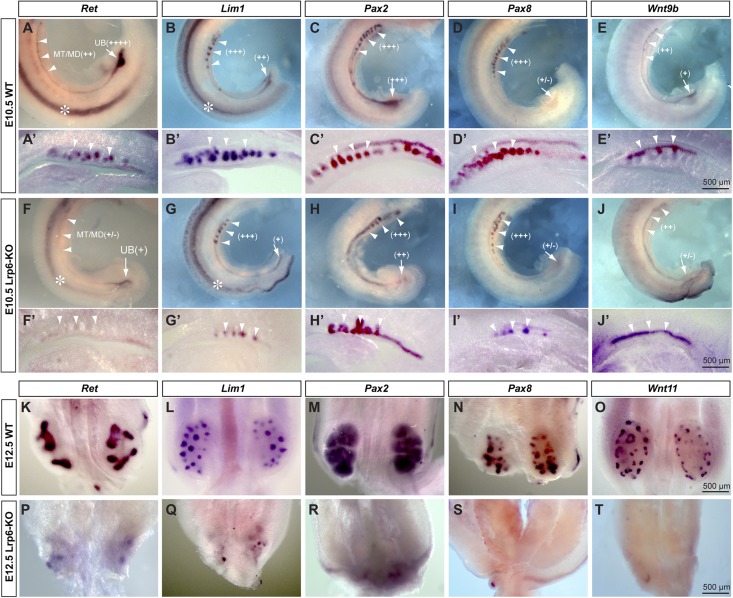
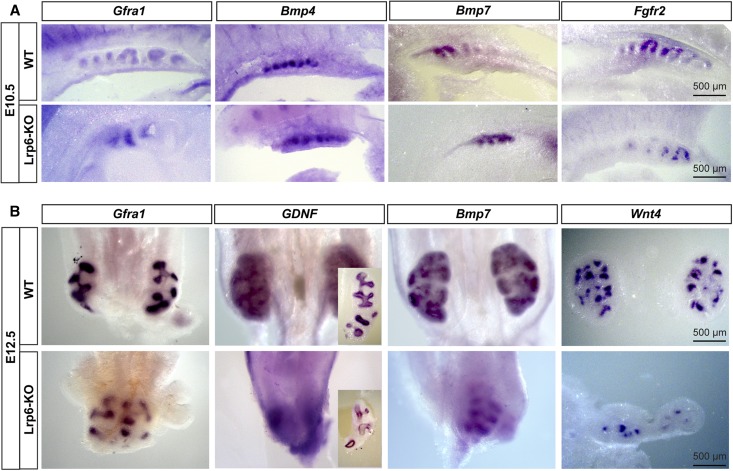
To address the underlying molecular mechanisms of the renal defects in the Lrp6-KO embryos, we performed wholemount in situ hybridization with riboprobes for representative nephrogenic molecules that have been shown to play important roles in early kidney development.1,2 Ret is a GDNF receptor acting as an inductive signaling molecule for renal development. In situ signals for Ret mRNAs are drastically lost in the mutant renal primordia at E10.5 and E12.5 (Figure 3, A, F, K, and P). Among crucial nephrogenic transcription factors, Lim1, Pax2, and Pax8 mRNAs are moderately reduced at E10.5 (Figure 3, B–D, G–I), and are significantly diminished at E12.5 in the mutant renal primordia (Figure 3, L–N, Q–S). Among Wnt ligands expressed in renal primordia, Wnt9b expression is relatively conserved in the mutant mesonephros at E10.5 (Figure 3, E and J). Wnt11 expression is absent in the mutant metanephros at E12.5 (Figure 3, O and T). By contrast, the expression of Gfra1, a GDNF coreceptor, was relatively conserved in the mutant renal primordia at E10.5 and E12.5 (Figure 4, A and B). GDNF expression is also detectable, but diminished in the mutant metanephros at E12.5 (Figure 4B). In addition, Bmp4 and Bmp7 mRNAs are unchanged, and Fgfr2 mRNAs are moderately diminished in the mutant mesonephros at E10.5 (Figure 4A). Wnt4 expression is still detectable in the mutant metanephros at E12.5 (Figure 4B). Because the Ret/GDNF and Wnt11 pathway is critical for ureteric budding and branching,18 our in situ results suggest a possible mechanism of this pathway acting downstream of Lrp6/β-catenin signaling.
Figure 3.
Wholemount in situ hybridization results demonstrate diminished expression of Ret followed by Lim1, Pax2, Pax8, Wnt9b, and Wnt11 in the renal primordia of Lrp6-KO embryos. (A–J) Lateral views of whole renal primordia of the WT controls and mutants at E10.5. (A′–J′) Sagittal vibrotome sections of respective renal primordia at E10.5. (K–T) Front or back views of whole metanephric primordia of E12.5 control and mutant embryos. Arrowheads, mesonephric tubules/ducts (MT/MD); arrows, ureteric buds (UB); asterisks, spinal cords. Relative mRNA signal intensities: +/−, almost none or very weak; +, weak; ++, moderate; +++, strong; ++++, very strong.
Figure 4.
Wholemount in situ hybridization for the expression of the less affected nephrogenic factors in Lrp6-KO mutants. (A) Gfra1, Bmp4, Bmp7, and Fgfr2 mRNA signals are shown in vibrotome sections of E10.5 mesonephric structures. (B) Diminished, but still expressed mRNA signals of Gfra1, GDNF, Bmp7, and Wnt4 in the whole renal organs or vibrotome sections (for GDNF inserts and Wnt4 panels) at E12.5.
Ret is a Transcriptional Target of Wnt/β-Catenin Signaling
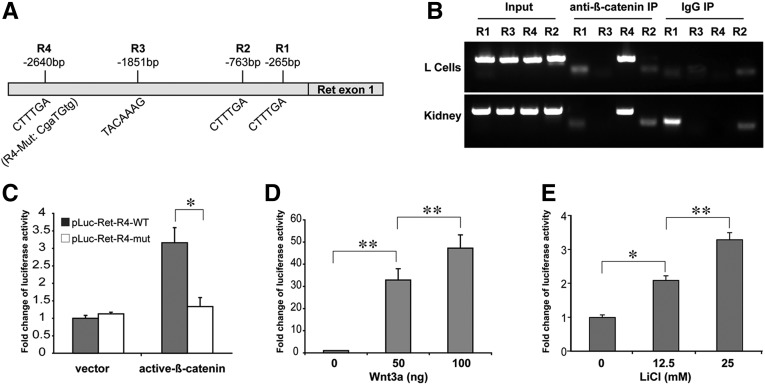
We searched the promoter sequence of the mouse Ret gene19 and identified several conserved Tcf/Lef1-binding sites, or the Wnt-responsive elements, in the upstream of the Ret promoter (Figure 5A). To examine whether the β-catenin/Tcf complex can bind to these sites in vitro and in vivo, we performed chromatin immunoprecipitation assays on both L cells and the freshly isolated kidney cells from the E14.5 wild-type (WT) mouse embryo. Our results demonstrate that the R4 binding site (−2640 bp) in the upstream Ret promoter was specifically amplified by PCR after chromatin immunoprecipitation with anti–β-catenin antibodies (Figure 5B), suggesting that β-catenin/Tcf transcriptional complex was recruited to the R4 binding site of the Ret promoter.
Figure 5.
Transcriptional activation of Ret promoter by Wnt/β-catenin signaling. (A) Schematic structure of the mouse Ret promoter with four putative Tcf/lef1-binding sites or Wnt-responsive elements (R1–R4). (B) Chromatin immunoprecipitation demonstrates the specific amplification of the R4 site immunoprecipitated with β-catenin antibodies, not the control IgG, from either E14.5 WT embryonic kidneys or L cells. (C) Luciferase assay results show specific response of WT Prw-luc, but not the mutant Prm-luc constructs, co-transfected with the persistent active β-catenin and Lef1 constructs. (D) Dose-dependent activation of Prw-luc construct treated with Wnt3a. (E) Dose-dependent activation of Prw-luc construct treated with Wnt agonist lithium chloride. *P<0.05; **P<0.01.
We made a series of deletion constructs with four binding sites in the luciferase reporter pGL2 vectors (including the mutated R4 binding site, shown in Figure 5A). Our results demonstrate that the construct containing the WT binding site R4 in the Ret gene was activated by co-transfection with the persistent active β-catenin and Lef1 cDNA constructs (Figure 5C). Other constructs, including one containing the mutant sequences of the R4 binding site, were not activated by active β-catenin/Lef1. We also found that the luciferase activities of the WT R4 construct is dose-dependently responsive to stimulation by Wnt3a (Figure 5D) or Wnt agonist lithium (Figure 5E). These results suggest that Ret is a transcriptional target of Wnt/β-catenin signaling.
Ret Expression and MAPK1/3 Phosphorylation Can Be Modulated by Wnt Agonists and/or Lrp6-Knockdown
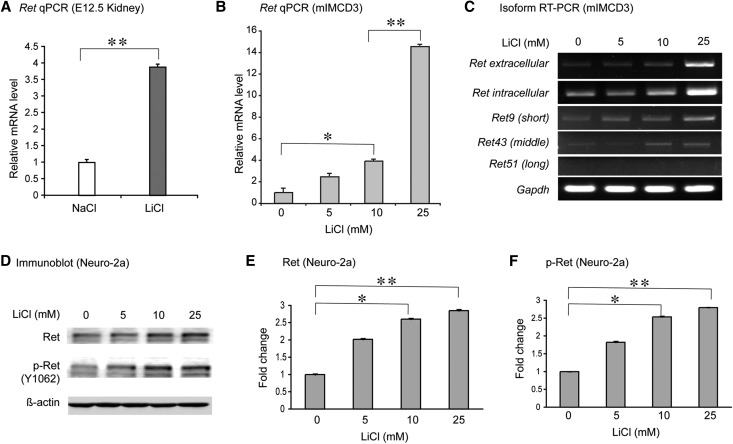
We performed real-time PCR assays to detect whether Wnt-signaling activation can modulate Ret expression in vivo and in vitro. Lithium is a well known small-molecule Wnt-signaling agonist, acting through inhibition of Gsk3β in the β-catenin degradation complex.20 Compared with the control treatments with sodium chloride, intraperitoneal injection of lithium chloride upregulated 4-fold of Ret mRNAs in the E12.5 embryonic kidneys (Figure 6A). The upregulation of Ret mRNA levels stimulated by lithium demonstrated a dose-dependent fashion in the murine inner medullary collecting duct–3 (mIMCD3) cells (Figure 6B). In order to get further details about Ret mRNA upregulation, we designed different primers to amplify all of the Ret splicing isoforms. The Ret intracellular isoform encodes a tyrosine kinase domain, while three variants of Ret9 (short), Ret43 (middle), and Ret51 (long) are encoded in the C-terminal tail. After lithium stimulation, the expression levels of most Ret isoforms (except Ret51) increased in a dose-dependent manner, while the Ret intracellular isoform shows the highest expression level compared with other variants (Figure 6C). Because of the considerably low level of Ret expression in mIMCD3 cells, we performed Western blots on Neuro-2a, another mouse cell line, which has a higher level of Ret expression. We found that both Ret and phosphorylated Ret (Y1062) increased dose-dependently in response to lithium treatment in Neuro-2a cells (Figure 6, D–F).
Figure 6.
Ret expression modulated by small-molecule Wnt agonists in vivo and in vitro. (A) Real-time PCR results show in vivo upregulation of Ret mRNAs in E12.5 kidney primordia after maternal intraperitoneal injection of the Wnt agonist lithium chloride. (B) Real-time PCR results demonstrate a dose-dependent upregulation of Ret mRNAs stimulated by lithium chloride in the mouse kidney-derived mIMCD3 cells. (C) RT-PCR results show the expressions of different Ret isoforms upon different dosages of lithium chloride treatments in mIMCD3 cells. (D–F) Western blot results show the changes of Ret and phosphorylated (p)-Ret (Y1062) expressions upon different dosages of lithium treatments in Neuro-2a cells. *P<0.05; **P<0.01.
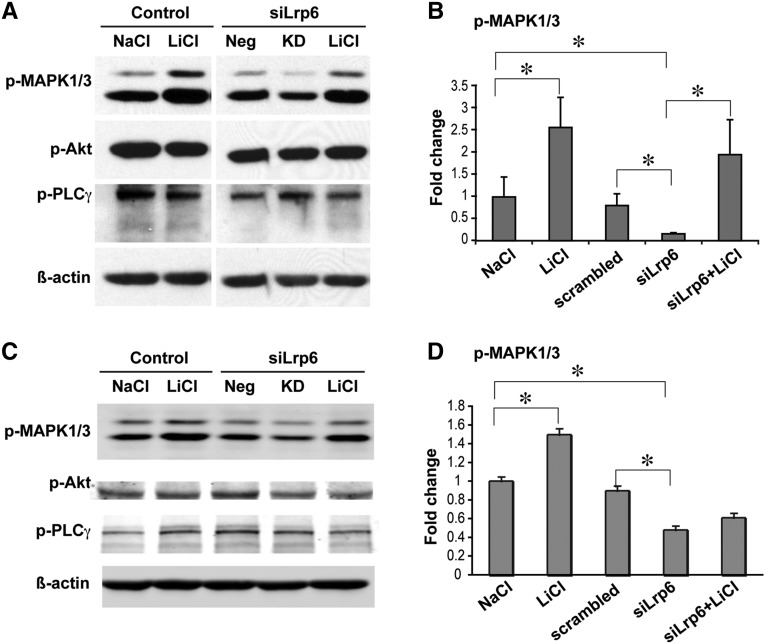
To determine the downstream cascades of Ret signaling in response to Wnt/β-catenin signaling modulation, we carried out further Western blots of PLCγ, PI3K-AKT, and MAPK1/3 (Erk2/1) kinases on both mIMCD3 and Neuro-2a cells after Lrp6-knockdown (KD), with or without stimulation by lithium, which acts downstream of Lrp6 to activate β-catenin signaling. Our results demonstrate that Lrp6-KD significantly diminished, while Wnt/β-catenin signaling activation by lithium significantly restored, the phosphorylation levels of MAPK1/3 in both cell lines compared with their controls after normalization to the levels of β-actin loading controls (Figure 7). However, neither Lrp6-KD nor lithium treatment had effects on Akt and PLCγ phosphorylations (Figure 7 and data not shown). These results suggest that Lrp6-mediated Wnt/β-catenin signaling preferably acts through Ret and MAPK1/3 cascade for signaling transduction in renal and neuronal cells in vitro.
Figure 7.
Alterations of Ret downstream factors modulated by Lrp6 knockdown and restored by Wnt agonists. Western blots were carried out for p-MAPK1/3, p-Akt, and p-PLCγ in mIMCD3 (A, B) and Neuro-2a (C, D) cells after Lrp6-knockdown using siRNAs and treated with or without lithium chloride. Scrambled siRNAs are used as the negative control for the knockdown. Only phosphorylated (p)-MAPK1/3 shows significant changes after different treatments in both cell lines (B, D). *P<0.05.
Genetic Interactions Between Lrp6 and Ret in Renal Disorders
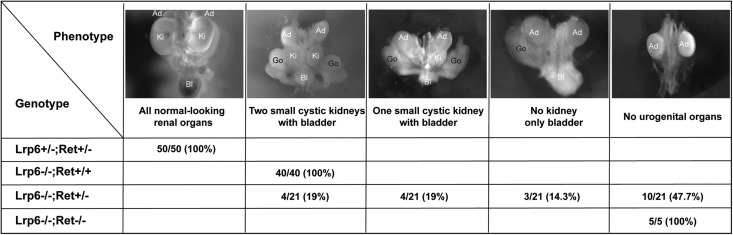
To examine whether Lrp6 and Ret are genetically interactive, we examined the renal phenotypes in the compound mutants of Lrp6 and Ret knockout mice (Figure 8). Single heterozygous Lrp6+/− or Ret+/− and double heterozygous Lrp6+/−;Ret+/− mice are viable and fertile without obvious defects in urogenital organs. All of the examined single homozygous Lrp6−/− embryos exhibit hypoplastic urogenital organs, including two kidneys, a bladder, and two gonads. The compound mutants of Lrp6−/−;Ret+/− mice exhibit more severe urogenital defects than in the single Lrp6−/− embryos, including 19% (4 of 21) compound mutants with only one small cystic kidney and a bladder, 14.3% (3 of 21) without kidneys but with a bladder, and 47.6% (10 of 21) with neither kidneys nor a bladder. The latest phenotype is also observed in 100% (5 of 5) double homozygous Lrp6−/−;Ret−/− embryos (Figure 8). Thus, removal of one or two copies of Ret genes in Lrp6−/− mice synergistically increased the severities of urogenital defects. By contrast, the adrenal primordia remain in all of these single or compound mutant embryos.
Figure 8.
Genetic interactions between Lrp6 and Ret in renal development. From the left to right panels, started with normal renal organs in Lrp6+/−;Ret+/−, increasingly more severe renal defects are seen in the single Lrp6−/−, the compound Lrp6−/−;Ret+/− (with varied severities), and the double KOs of Lrp6−/−;Ret−/− mutant mice around E17.5. Ad, adrenal; Bl, bladder; Go, gonads; Ki, kidney.
Discussion
Lrp6-Mediated Wnt/β-Catenin Signaling in Renal Development and Cystic Dysplasia
This study shows that genetic ablation of Lrp6 resulted in multicystic dysplastic kidneys. Lrp6 is a key coreceptor that mediates Wnt/β-catenin signaling. Lrp6-deficient mice exhibit a spectrum of structural disorders, including limb defects, developmental brain disorders, ocular coloboma, cleft lip/palate, cardiac outflow tract defects, neural tube defects, and urogenital disorders as the consequences of the inactivation of Wnt/β-catenin signaling during organogenesis.12,21–25 β-catenin has been shown to regulate nephron induction, to maintain the precursor state of the Wolffian ducts and the ureteric bud, and is also required for ureteric branching morphogenesis.7,11,26 However, β-catenin has dual roles in Wnt signaling and cell adhesion.27 The consistent renal phenotypes of Lrp6 and β-catenin mutants suggest that the defective transcriptional function of β-catenin is a main cause of renal disorders. Several studies further support the critical role of Wnt/β-catenin signaling in polycystic kidney including cystic kidney ciliopathy.28 Genetic activation of the oncogenic form of β-catenin in the renal epithelial cells causes polycystic lesion in neonatal mice.8 Conditional inactivation of APC, a negative regulator in Wnt/β-catenin signaling, leads to cystic renal neoplasia.10 These observations suggest that overactivation of Wnt/β-catenin signaling is a condition of cystic kidney ciliopathy. Conversely, conditional removal of β-catenin from the mouse Wolffian duct epithelium by Hoxb7-Cre also causes cystic kidneys.11 Downregulation of Wnt/β-catenin activities and cystic kidney ciliopathy are observed in the KO mice of Ahi1 that encodes a cilial protein Jouberin and may be genetically interactive with Lrp6 in polycystic kidney disease (PKD) and related cystogenesis.29 In support of this, a recent study revealed that LRP5 mutations and canonical Wnt signaling are associated with hepatic cystogenesis.30 However, Wnt/β-catenin signaling is not involved in renal cystic formation of the mutant mice of Invs that encodes a cilial protein inversin or nephrocystin-2.31 β-catenin/TCF signaling activity is also not ectopically activated in PKD1 and PKD2 mutants, two different models of polycystic kidney, or renal cystic ciliopathy.32 Together, these contradictory observations suggest that not all cystic kidney ciliopathies have abnormal Wnt/β-catenin signaling, but disruptions of Wnt/β-catenin signaling may trigger cystic dysplasia in kidneys and related organs.
Ret as a Downstream Effecter of Lrp6-Mediated Wnt/β-Catenin Signaling in Renal Development
Our results demonstrate that among a panel of critical nephrogenic factors, Ret expression is mostly diminished in the mutant renal primordia at E10.5 onwards. The ureteric buds start to outgrow and invade the metanephric mesenchyme at E10.5 and branch robustly by E12.5, and loss of Ret signaling can prevent these critical processes and cause renal agenesis or severe renal defects.33 Our results showed a dose-dependent upregulation of Ret and its isoforms under the Wnt agonist lithium treatment in vivo or in renal-derived mIMCD3 cells. ChIP and luciferase assays also support that Ret is a downstream target and effector of the Wnt/β-catenin signaling in nephrogenesis. A recent study reports that lithium upregulates Ret expression in mIMCD3 cells through both β-catenin– and mTOR (or mechanistic target of rapamycin)–dependent pathways,34 which is partly consistent with our results. GDNF is a Ret ligand and activates Ret kinase domains in the cytoplasm via phosphorylation.35 We also detected the diminished expression of GDNF in the renal primordia of Lrp6 mutant embryos. A recent study revealed that β-catenin directly regulates GDNF expression in renal mesenchyme, while β-catenin may also reciprocally interact with Ret in renal epithelia,36 which supports a possible role of Ret in mediating Wnt/β-catenin signaling during nephrogenesis. The residual expression of Ret in the renal primordia of Lrp6-null embryos indicates that Ret may also be regulated by other factors, either Wnt-independent factors or the Wnt coreceptor Lrp5; the latter plays a functionally redundant role with Lrp6 during embryonic development.37,38 Our double KO analyses demonstrate the genetic interaction of Lrp6 and Ret on nephrogenesis and related urogenital development. The enhanced urogenital defects in the Lrp6−/−;Ret+/− double mutants also indicate that Lrp6 and Ret may synergistically or in parallel regulate renal development.
An Lrp6-Regulated Signaling Network in Early Nephrogenesis
Our results show that several transcription factors are also severely affected in the mutant renal primordia of Lrp6-null embryos. Pax2, Pax8, and Lim1 play indispensable roles in early mesodermal regionalization, and genetic ablation of these factors causes renal agenesis.39–41 Intriguingly, both Pax2 and Pax8 can activate the Ret promoter.42 It is unclear whether the diminished Pax2 and Pax8 expression in the Lrp6-deficient renal primordia is a direct consequence of the inactivated Wnt/β-catenin signaling or indirectly affected by the loss of Ret signaling. Mice deficient in Lim1 also display renal agenesis associated with diminished expression of Ret in the ureteric bud.43 These results suggest a potential feedback interaction among Ret and Pax2, Pax8, or Lim1 downstream of Lrp6/β-catenin signaling (Figure 9). We also show that Wnt11 was downregulated in the Lrp6-deficient metanephros at E12.5. Wnt11 may regulate GDNF in metanephric development.18 It remains unknown whether Wnt11 acts through Lrp6 to regulate GDNF. We further show that Wnt4 and Wnt9b expression were less affected in the renal primordia of Lrp6-KO mice. Wnt9b acts upstream of Wnt4 to induce the metanephric mesenchyme to undergo a mesenchymal to epithelial transition (MET) for the formation of the epithelial renal vesicles.5 Interestingly, Ret expression does not change, but GDNF, Wnt11, and Wnt4 are downregulated in the Wnt9b-KO mice.5 The Ret expression is also unchanged in the Wnt4-KO mice.4 These results indicate that Wnt4 and Wnt9b may not act though Lrp6 and thus do not regulate Ret signaling in ureteric bud branching, or that these Wnt ligands may have functional redundancy in regulating Lrp6/Ret signaling, which warrants future investigation.
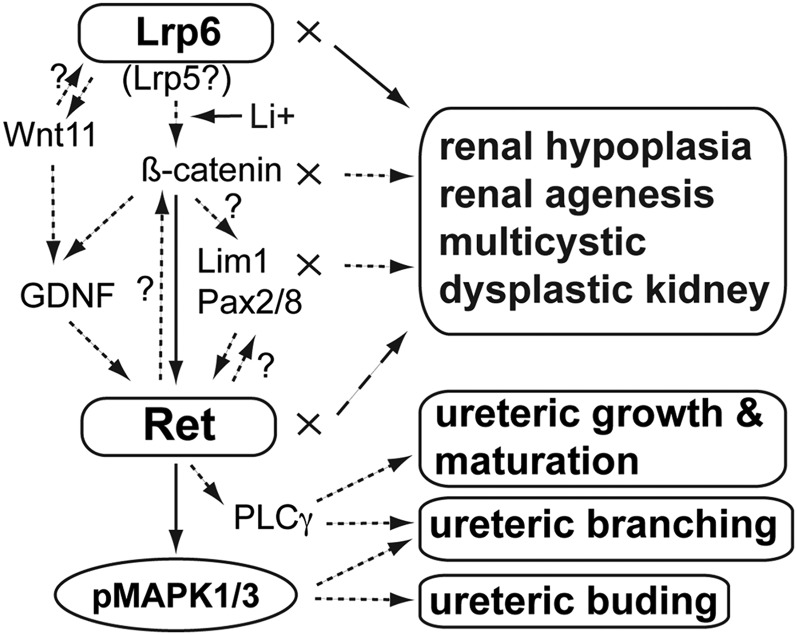
Figure 9.
Schematic summary of Lrp6 and Ret signaling interaction in renal development and cystic dysplasia. We demonstrate that Lrp6-mediated Wnt/β-catenin signaling modulates the expression of Ret that subsequently activates MAPK1/3 (Erk2/1) and PLCγ signaling to promote ureteric budding, branching, growth, and maturation. A recent study suggests a reciprocal interaction between β-catenin and Ret during renal development. Lrp6 may also regulate GDNF (a ligand molecule for Ret) through Wnt11 or through β-catenin. Moreover, Lrp6-mediated Wnt/β-catenin signaling may regulate the expression of nephrogenic transcription factors, such as Lim1, Pax2, and Pax8, which also interact with Ret. Disruption of the signaling network may cause a spectrum of renal defects, such as renal agenesis, renal hypoplasia, and multicystic dysplastic kidney. Another Wnt coreceptor Lrp5 is involved in liver cytogenesis, while it remains unknown whether Lrp5 also plays a role in renal development and cystic dysplasia. The Wnt agonist lithium ion can modulate the Ret signaling at downstream Lrp6 and upstream β-catenin. Solid arrows, demonstrated by the current study; dashed arrows, demonstrated by previous studies; X, disrupted gene activities; ?, potential but as yet unverified.
Mechanisms of Renal Cystic Dysplasia in Lrp6 and Ret Mutants
Our results show that mitotic cells decreased, while apoptotic cells increased significantly in the renal primordia of Lrp6-deficient embryos. These observations suggest that defective Lrp6 signaling alters cell cycle kinetics and apoptosis, which may subsequently disrupt metanephric branching and morphogenesis, and thus may cause renal hypoplasia. Dysregulated epithelial cell proliferation and apoptosis have also been linked with cystic kidneys in humans and animal models.44–46 At the molecular level, diminished Ret expression may cause renal cystic dysplasia in Lrp6 mutants. Ret has multiple tyrosine residues and mediates cascades for cell proliferation, migration, differentiation and growth by phosphorylation and activation of downstream signaling, such as PLCγ, PI3K and mitogen-activated protein kinases (MAPK).15,17,47 Inactivation of Ret-mediated MAPK signaling caused renal hypoplasia or agenesis, while repression of Ret-mediated PLCγ signaling resulted in cystic kidneys.15 Although we detected only MAPK signaling that is regulated by Lrp6-mediated Wnt signaling in vitro, both Ret-mediated MAPK and PLCγ cascades would be repressed in vivo as the Ret expression is dramatically diminished in the renal primordia of Lrp6 mutants. This data supports a consistent role of Lrp6 and Ret in renal development and also suggests that the repression of Ret signaling cascades is a key molecular mechanism underlying renal cystic dysplasia in Lrp6 mutants.
In conclusion, our results provide strong evidence for the role of Lrp6-mediated Wnt/β-catenin pathway in modulation of a signaling network consisting of Ret cascades and related nephrogenic factors to control cell proliferation, survival, ureteric bud branching, and related nephrogenic processes (Figure 9). These novel results may also provide a molecular and genetic basis for interpreting the mechanisms of congenital renal defects and anomalies.
CONCISE METHODS
Animal Models
The Lrp6ßgeo mice were generated through a gene-trap approach by the Skarnes laboratory12 and conserved by the Mutant Mouse Regional Resource Center at the University of California, Davis (UC Davis). The Ret knockout mice generated by the Costantini laboratory13 were acquired through The Jackson Laboratory (Stock #009085) and were mated with the Lrp6ßgeo mice to generate the double knockout mutants for the study. All mice were housed in the vivarium of the UC Davis. All research procedures using mice were approved by the UC Davis Animal Care and Use Committee and conformed to the National Institutes of Health guidelines.
Hematoxylin and Eosin Staining, Immunofluorescence, and TUNEL Assays
Hematoxylin and eosin staining and immunofluorescence were carried out on 10 μm frozen or paraffin-embedded tissue sections according to standard protocols. Primary antibodies against phospho-histone H3 (pHH3) (1:50; Cell Signaling Technology) and appropriate Alexa fluorescence-conjugated secondary antibodies (1:500; Molecular Probes) were used. DAPI (1:500; Molecular Probes) was used for the nuclear counterstaining. TUNEL assays were performed using the Dead End Fluorometric TUNEL System (Promega) following the manufacturer’s instructions. The micrograph images were acquired with a Nikon C1 confocal microscope. The total numbers of pHH3(+) or TUNEL(+) cells were counted on each photographed renal primordial section and then were divided by the total cell numbers on the same section area as the percentages of the positive cells. In each experiment at least five sections from three mutants or three littermate controls at the same age were counted and averaged for statistical analyses.
Wholemount in situ Hybridization
Embryos or whole renal organs were fixed in 4% paraformaldehyde (PFA) in diethylpyrocarbonate-treated PBS overnight and dehydrated in graded methanol solutions and kept in 100% methanol at −80°C until used. Wholemount in situ hybridization was carried out as previously described,48 using the DIG-labeled cRNA riboprobes (Supplemental Table 1). Samples were photographed using a Carl Zeiss stereological microscope (Lumar V12).
Maternal Administration of Lithium Chloride
In vivo stimulation of Wnt/β-catenin signaling pathway by lithium was carried out as described.23 Pregnant dams were injected intraperitoneally with 200 mg/kg/day LiCl or the control NaCl solution on E8.5 to E11.5. The embryonic kidneys were dissected out at E12.5 for subsequent experiments.
Cell Lines
The mIMCD3, L, and Neuro-2a cells were obtained from ATCC (Manassas, VA) and were grown in the Dulbecco’s modified eagle medium (DMEM, Invitrogen) with 10% fetal bovine serum.
RNA Isolation and Real-Time PCR
Total RNA was isolated from the E12.5 kidneys. Semiquantitative PCR was carried out according to the manual of the Roche 480 Real-Time PCR system using Faststart Universal SYBR GREEN master (ROX) (Roche Diagnostics, Zurich, Switzerland). Levels of mRNAs were normalized to glyceraldehyde-3-phosphate dehydrogenase (Gapdh) to allow comparisons among different experimental groups using the ΔCt method.23
Chromatin Immunoprecipitation and Luciferase Reporter Assay
Chromatin immunoprecipitation (ChIP) assays were performed using isolated E14.5 kidneys from WT mice (in vivo) and the L cell line (in vitro) as described previously.23 Chromatin extraction and immunoprecipitation were performed according to the manufacturer’s protocols using a ChIP assay kit (Upstate Biotechnology, Lake Placid, NY). The equivalent of chromatin was immunoprecipitated with anti-β-catenin (Santa Cruz Biotechnology, Dallas, TX). Normal rabbit IgG was used as a negative control. Four Tcf/Lef1-binding sites (R1–R4; Figure 5A) in the Ret promoter were amplified by PCR with specific primers (Supplemental Table 1) after ChIP. Each assay was repeated three to five times using different chromatin pools.
For luciferase assays, a serial of fragments of 2.4 kb, 1.3 kb, 800 bp, 400 bp, 180 bp flanking the R3, R2, R1, and SP1 binding site and 677 bp region flanking the R4 binding site (or the mutant promoter that was mutated in the binding site) of mouse Ret promoter (Figure 5A) were cloned to a luciferase reporter pGL2-Basic vector. These constructs were transiently transfected into HEK293T cells together with the Renilla construct using the Lipotectamine 2000 reagent (Invitrogen). HEK293T cells were also transiently transfected with the expression vector pGL2-basic or constitutively active β-catenin/Lef1 construct. Twenty-four hours after transfection, luciferase activities were detected using the Dual-Luciferase assay kit (Promega, Madison, WI) according to the manufacturer’s instruction.
RNA Knockdown and Western Blot
Lrp6 siRNA oligonucleotides were purchased from Thermo Fisher Scientific (ON-TARGETplus SMARTpool siRNA; Thermo Fisher Scientific. The RNA oligonucleotides were transfected using Lipotectamine 2000 (Invitrogen) according to the manufacturer’s protocols at a 25 nM final concentration. Each target includes four siRNA oligonucleotides for Lrp6, sequence 1: CGACAAAACUCCAUACGAA; sequence 2: GAGAUUAGAUGGACGAUCA; sequence 3: UAUCAAAGUUGGACGGACA; sequence 4: GCUGUUAGCCCGACGGACA. Western blot was carried out on lysates prepared in modified radioimmunoprecipitation assay (RIPA) buffer using the following antibodies: rabbit anti-phospho-p44/42 (MAPK1/3) (1:1000; Cell Signaling Technology), rabbit anti-phospho-Akt (1:1000; Cell Signaling Technology), rabbit anti-phospho-PLCγ (1:500; Cell Signaling Technology), rabbit anti-Ret (1:200; Santa Cruz Biotechnology), rabbit anti-phospho-Ret (Y1062) (1:200; R&D Systems) and mouse anti-β-actin (1:1000; Santa Cruz Biotechnology). All antibodies were diluted in 5% BSA and incubated overnight at 4°C.
Statistical Analyses
Two to five mutant embryos or littermate controls were used for each nonquantitative experiment. At least three mutant embryos or littermate controls were used in each quantitative analysis for statistical significance. Student’s t test was used for statistical comparisons when appropriate, and differences were considered significant at P value <0.05.
Supplementary Material
Acknowledgments
This work was supported by research grants from the National Institutes of Health (1R01-DE021696 to C.J.Z.) and the Shriners Hospitals for Children (86600 and 87500 to C.J.Z.). Y.W. received a postdoctoral training fellowship from the Shriners Hospitals for Children.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2014100998/-/DCSupplemental.
References
- 1.Costantini F, Kopan R: Patterning a complex organ: branching morphogenesis and nephron segmentation in kidney development. Dev Cell 18: 698–712, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dressler GR: The cellular basis of kidney development. Annu Rev Cell Dev Biol 22: 509–529, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Clevers H, Nusse R: Wnt/β-catenin signaling and disease. Cell 149: 1192–1205, 2012 [DOI] [PubMed] [Google Scholar]
- 4.Stark K, Vainio S, Vassileva G, McMahon AP: Epithelial transformation of metanephric mesenchyme in the developing kidney regulated by Wnt-4. Nature 372: 679–683, 1994 [DOI] [PubMed] [Google Scholar]
- 5.Carroll TJ, Park JS, Hayashi S, Majumdar A, McMahon AP: Wnt9b plays a central role in the regulation of mesenchymal to epithelial transitions underlying organogenesis of the mammalian urogenital system. Dev Cell 9: 283–292, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Kispert A, Vainio S, Shen L, Rowitch DH, McMahon AP: Proteoglycans are required for maintenance of Wnt-11 expression in the ureter tips. Development 122: 3627–3637, 1996 [DOI] [PubMed] [Google Scholar]
- 7.Park JS, Valerius MT, McMahon AP: Wnt/beta-catenin signaling regulates nephron induction during mouse kidney development. Development 134: 2533–2539, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Saadi-Kheddouci S, Berrebi D, Romagnolo B, Cluzeaud F, Peuchmaur M, Kahn A, Vandewalle A, Perret C: Early development of polycystic kidney disease in transgenic mice expressing an activated mutant of the beta-catenin gene. Oncogene 20: 5972–5981, 2001 [DOI] [PubMed] [Google Scholar]
- 9.Bridgewater D, Di Giovanni V, Cain JE, Cox B, Jakobson M, Sainio K, Rosenblum ND: β-catenin causes renal dysplasia via upregulation of Tgfβ2 and Dkk1. J Am Soc Nephrol 22: 718–731, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qian CN, Knol J, Igarashi P, Lin F, Zylstra U, Teh BT, Williams BO: Cystic renal neoplasia following conditional inactivation of apc in mouse renal tubular epithelium. J Biol Chem 280: 3938–3945, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Marose TD, Merkel CE, McMahon AP, Carroll TJ: Beta-catenin is necessary to keep cells of ureteric bud/Wolffian duct epithelium in a precursor state. Dev Biol 314: 112–126, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pinson KI, Brennan J, Monkley S, Avery BJ, Skarnes WC: An LDL-receptor-related protein mediates Wnt signalling in mice. Nature 407: 535–538, 2000 [DOI] [PubMed] [Google Scholar]
- 13.Schuchardt A, D’Agati V, Larsson-Blomberg L, Costantini F, Pachnis V: Defects in the kidney and enteric nervous system of mice lacking the tyrosine kinase receptor Ret. Nature 367: 380–383, 1994 [DOI] [PubMed] [Google Scholar]
- 14.Shakya R, Watanabe T, Costantini F: The role of GDNF/Ret signaling in ureteric bud cell fate and branching morphogenesis. Dev Cell 8: 65–74, 2005 [DOI] [PubMed] [Google Scholar]
- 15.Jain S, Encinas M, Johnson EM, Jr, Milbrandt J: Critical and distinct roles for key RET tyrosine docking sites in renal development. Genes Dev 20: 321–333, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Richardson DS, Rodrigues DM, Hyndman BD, Crupi MJ, Nicolescu AC, Mulligan LM: Alternative splicing results in RET isoforms with distinct trafficking properties. Mol Biol Cell 23: 3838–3850, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoshi M, Batourina E, Mendelsohn C, Jain S: Novel mechanisms of early upper and lower urinary tract patterning regulated by RetY1015 docking tyrosine in mice. Development 139: 2405–2415, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Majumdar A, Vainio S, Kispert A, McMahon J, McMahon AP: Wnt11 and Ret/Gdnf pathways cooperate in regulating ureteric branching during metanephric kidney development. Development 130: 3175–3185, 2003 [DOI] [PubMed] [Google Scholar]
- 19.Itoh F, Ishizaka Y, Tahira T, Yamamoto M, Miya A, Imai K, Yachi A, Takai S, Sugimura T, Nagao M: Identification and analysis of the ret proto-oncogene promoter region in neuroblastoma cell lines and medullary thyroid carcinomas from MEN2A patients. Oncogene 7: 1201–1206, 1992 [PubMed] [Google Scholar]
- 20.Hedgepeth CM, Conrad LJ, Zhang J, Huang HC, Lee VM, Klein PS: Activation of the Wnt signaling pathway: a molecular mechanism for lithium action. Dev Biol 185: 82–91, 1997 [DOI] [PubMed] [Google Scholar]
- 21.Zhou CJ, Pinson KI, Pleasure SJ: Severe defects in dorsal thalamic development in low-density lipoprotein receptor-related protein-6 mutants. J Neurosci 24: 7632–7639, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou CJ, Zhao C, Pleasure SJ: Wnt signaling mutants have decreased dentate granule cell production and radial glial scaffolding abnormalities. J Neurosci 24: 121–126, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Song L, Li Y, Wang K, Wang YZ, Molotkov A, Gao L, Zhao T, Yamagami T, Wang Y, Gan Q, Pleasure DE, Zhou CJ: Lrp6-mediated canonical Wnt signaling is required for lip formation and fusion. Development 136: 3161–3171, 2009 [DOI] [PubMed] [Google Scholar]
- 24.Song L, Li Y, Wang K, Zhou CJ: Cardiac neural crest and outflow tract defects in Lrp6 mutant mice. Dev Dyn 239: 200–210, 2010 [DOI] [PubMed] [Google Scholar]
- 25.Zhou CJ, Wang YZ, Yamagami T, Zhao T, Song L, Wang K: Generation of Lrp6 conditional gene-targeting mouse line for modeling and dissecting multiple birth defects/congenital anomalies. Dev Dyn 239: 318–326, 2010 [DOI] [PubMed] [Google Scholar]
- 26.Bridgewater D, Cox B, Cain J, Lau A, Athaide V, Gill PS, Kuure S, Sainio K, Rosenblum ND: Canonical WNT/beta-catenin signaling is required for ureteric branching. Dev Biol 317: 83–94, 2008 [DOI] [PubMed] [Google Scholar]
- 27.Valenta T, Hausmann G, Basler K: The many faces and functions of β-catenin. EMBO J 31: 2714–2736, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fliegauf M, Benzing T, Omran H: When cilia go bad: cilia defects and ciliopathies. Nat Rev Mol Cell Biol 8: 880–893, 2007 [DOI] [PubMed] [Google Scholar]
- 29.Lancaster MA, Louie CM, Silhavy JL, Sintasath L, Decambre M, Nigam SK, Willert K, Gleeson JG: Impaired Wnt-beta-catenin signaling disrupts adult renal homeostasis and leads to cystic kidney ciliopathy. Nat Med 15: 1046–1054, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cnossen WR, te Morsche RH, Hoischen A, Gilissen C, Chrispijn M, Venselaar H, Mehdi S, Bergmann C, Veltman JA, Drenth JP: Whole-exome sequencing reveals LRP5 mutations and canonical Wnt signaling associated with hepatic cytogenesis. Proc Natl Acad Sci U S A 111: 5343–5348, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sugiyama N, Tsukiyama T, Yamaguchi TP, Yokoyama T: The canonical Wnt signaling pathway is not involved in renal cyst development in the kidneys of inv mutant mice. Kidney Int 79: 957–965, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miller MM, Iglesias DM, Zhang Z, Corsini R, Chu L, Murawski I, Gupta I, Somlo S, Germino GG, Goodyer PR: T-cell factor/β-catenin activity is suppressed in two different models of autosomal dominant polycystic kidney disease. Kidney Int 80: 146–153, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Costantini F, Shakya R: GDNF/Ret signaling and the development of the kidney. BioEssays 28: 117–127, 2006 [DOI] [PubMed] [Google Scholar]
- 34.Kojima N, Saito H, Nishikawa M, Yuri S, Jo OD, Pham PC, Yanagawa N, Yanagawa N: Lithium induces c-Ret expression in mouse inner medullary collecting duct cells. Cell Signal 23: 371–379, 2011 [DOI] [PubMed] [Google Scholar]
- 35.Tang MJ, Worley D, Sanicola M, Dressler GR: The RET-glial cell-derived neurotrophic factor (GDNF) pathway stimulates migration and chemoattraction of epithelial cells. J Cell Biol 142: 1337–1345, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sarin S, Boivin F, Li A, Lim J, Svajger B, Rosenblum ND, Bridgewater D: β-Catenin overexpression in the metanephric mesenchyme leads to renal dysplasia genesis via cell-autonomous and non-cell-autonomous mechanisms. Am J Pathol 184: 1395–1410, 2014 [DOI] [PubMed] [Google Scholar]
- 37.Kelly OG, Pinson KI, Skarnes WC: The Wnt co-receptors Lrp5 and Lrp6 are essential for gastrulation in mice. Development 131: 2803–2815, 2004 [DOI] [PubMed] [Google Scholar]
- 38.Joeng KS, Schumacher CA, Zylstra-Diegel CR, Long F, Williams BO: Lrp5 and Lrp6 redundantly control skeletal development in the mouse embryo. Dev Biol 359: 222–229, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tsang TE, Shawlot W, Kinder SJ, Kobayashi A, Kwan KM, Schughart K, Kania A, Jessell TM, Behringer RR, Tam PP: Lim1 activity is required for intermediate mesoderm differentiation in the mouse embryo. Dev Biol 223: 77–90, 2000 [DOI] [PubMed] [Google Scholar]
- 40.Bouchard M, Souabni A, Mandler M, Neubüser A, Busslinger M: Nephric lineage specification by Pax2 and Pax8. Genes Dev 16: 2958–2970, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pedersen A, Skjong C, Shawlot W: Lim 1 is required for nephric duct extension and ureteric bud morphogenesis. Dev Biol 288: 571–581, 2005 [DOI] [PubMed] [Google Scholar]
- 42.Clarke JC, Patel SR, Raymond RM, Jr, Andrew S, Robinson BG, Dressler GR, Brophy PD: Regulation of c-Ret in the developing kidney is responsive to Pax2 gene dosage. Hum Mol Genet 15: 3420–3428, 2006 [DOI] [PubMed] [Google Scholar]
- 43.Kobayashi A, Kwan KM, Carroll TJ, McMahon AP, Mendelsohn CL, Behringer RR: Distinct and sequential tissue-specific activities of the LIM-class homeobox gene Lim1 for tubular morphogenesis during kidney development. Development 132: 2809–2823, 2005 [DOI] [PubMed] [Google Scholar]
- 44.Ramasubbu K, Gretz N, Bachmann S: Increased epithelial cell proliferation and abnormal extracellular matrix in rat polycystic kidney disease. J Am Soc Nephrol 9: 937–945, 1998 [DOI] [PubMed] [Google Scholar]
- 45.Lanoix J, D’Agati V, Szabolcs M, Trudel M: Dysregulation of cellular proliferation and apoptosis mediates human autosomal dominant polycystic kidney disease (ADPKD). Oncogene 13: 1153–1160, 1996 [PubMed] [Google Scholar]
- 46.Ibrahim S: Increased apoptosis and proliferative capacity are early events in cyst formation in autosomal-dominant, polycystic kidney disease. Sci World J 7: 1757–1767, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jain S: The many faces of RET dysfunction in kidney. Organogenesis 5: 177–190, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang Y, Song L, Zhou CJ: The canonical Wnt/β-catenin signaling pathway regulates Fgf signaling for early facial development. Dev Biol 349: 250–260, 2011 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.