Abstract
Idiopathic infantile hypercalcemia (IIH) is characterized by severe hypercalcemia with failure to thrive, vomiting, dehydration, and nephrocalcinosis. Recently, mutations in the vitamin D catabolizing enzyme 25-hydroxyvitamin D3-24-hydroxylase (CYP24A1) were described that lead to increased sensitivity to vitamin D due to accumulation of the active metabolite 1,25-(OH)2D3. In a subgroup of patients who presented in early infancy with renal phosphate wasting and symptomatic hypercalcemia, mutations in CYP24A1 were excluded. Four patients from families with parental consanguinity were subjected to homozygosity mapping that identified a second IIH gene locus on chromosome 5q35 with a maximum logarithm of odds (LOD) score of 6.79. The sequence analysis of the most promising candidate gene, SLC34A1 encoding renal sodium-phosphate cotransporter 2A (NaPi-IIa), revealed autosomal-recessive mutations in the four index cases and in 12 patients with sporadic IIH. Functional studies of mutant NaPi-IIa in Xenopus oocytes and opossum kidney (OK) cells demonstrated disturbed trafficking to the plasma membrane and loss of phosphate transport activity. Analysis of calcium and phosphate metabolism in Slc34a1-knockout mice highlighted the effect of phosphate depletion and fibroblast growth factor-23 suppression on the development of the IIH phenotype. The human and mice data together demonstrate that primary renal phosphate wasting caused by defective NaPi-IIa function induces inappropriate production of 1,25-(OH)2D3 with subsequent symptomatic hypercalcemia. Clinical and laboratory findings persist despite cessation of vitamin D prophylaxis but rapidly respond to phosphate supplementation. Therefore, early differentiation between SLC34A1 (NaPi-IIa) and CYP24A1 (24-hydroxylase) defects appears critical for targeted therapy in patients with IIH.
Keywords: genetic renal disease, molecular genetics, mineral metabolism, activated Vitamin D, hypercalciuria
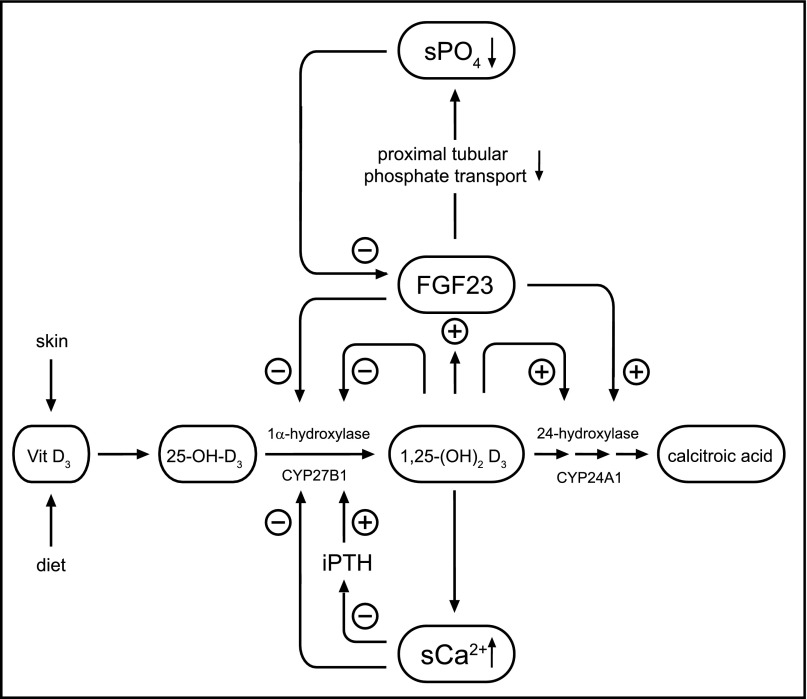
Serum calcium levels are primarily maintained by vitamin D and parathyroid hormone (PTH). The conversion of vitamin D to its biologically active form 1,25-(OH)2D3, as well as its inactivation, involve sequential hydroxylation steps which are tightly regulated (Figure 1). The biologic activity of both key activating and deactivating enzymes 25-OH-D3-1α-hydroxylase (CYP27B1) and 25-OH-D3-24-hydroxylase (CYP24A1) is mainly controlled by 1,25-(OH)2D3 itself, calcium, phosphate, and PTH. In addition, fibroblast growth factor 23 (FGF23) that primarily regulates phosphate metabolism limits the action of vitamin D by inhibiting its activation via 1α-hydroxylase and promoting its degradation via 24-hydroxylase. Defects in vitamin D activation and action cause different forms of vitamin D-dependent rickets whereas impaired degradation of 1,25-(OH)2D3 underlies idiopathic infantile hypercalcemia (IIH).1,2
Figure 1.
Integrated scheme of calcium and phosphate metabolism. The activation of vitamn D to its biologically active form 1,25-(OH)2D3 by 1α-hydroxylase (CYP27B1) as well as its degradation by 24-hydroxylase (CYP24A1) are tightly controlled by 1,25-(OH)2D3 itself, serum calcium, and PTH (lower part). In addition, vitamin D metabolism is critically influenced by phosphate homeostasis via the action of the primary phosphaturic hormone FGF23 that limits the action of active 1,25-(OH)2D3 by inhibiting 1α-hydroxylase (CYP27B1) and activating 24-hydroxylase (CYP24A1) (upper part).
IIH (OMIM #143880) was first described in the 1950s after an epidemic occurrence of unexplained hypercalcemia in infants receiving increased amounts of vitamin D via fortified milk products for the prevention of rickets.3,4 Although a link to exogenous vitamin D was recognized early, the pathophysiology remained unknown until the recent identification of inactivating mutations in CYP24A1.2 Meanwhile, CYP24A1 mutations have also been described in adults who primarily presented with nephrolithiasis while remaining asymptomatic during infancy.5
Here, we demonstrate genetic heterogeneity of IIH by the identification of autosomal-recessive loss-of-function mutations in SLC34A1 encoding the renal sodium-phosphate cotransporter NaPi-IIa. Affected patients, in addition to hypercalcemia and suppressed PTH levels, also exhibit hypophosphatemia due to renal phosphate wasting. The critical role of phosphate deficiency for the development of IIH is replicated in Slc34a1 knockout mice that normalize calcium metabolism following phosphate supplementation but not after omission of vitamin D supplementation alone.
Results
Genome-Wide Linkage and Mutational Analysis
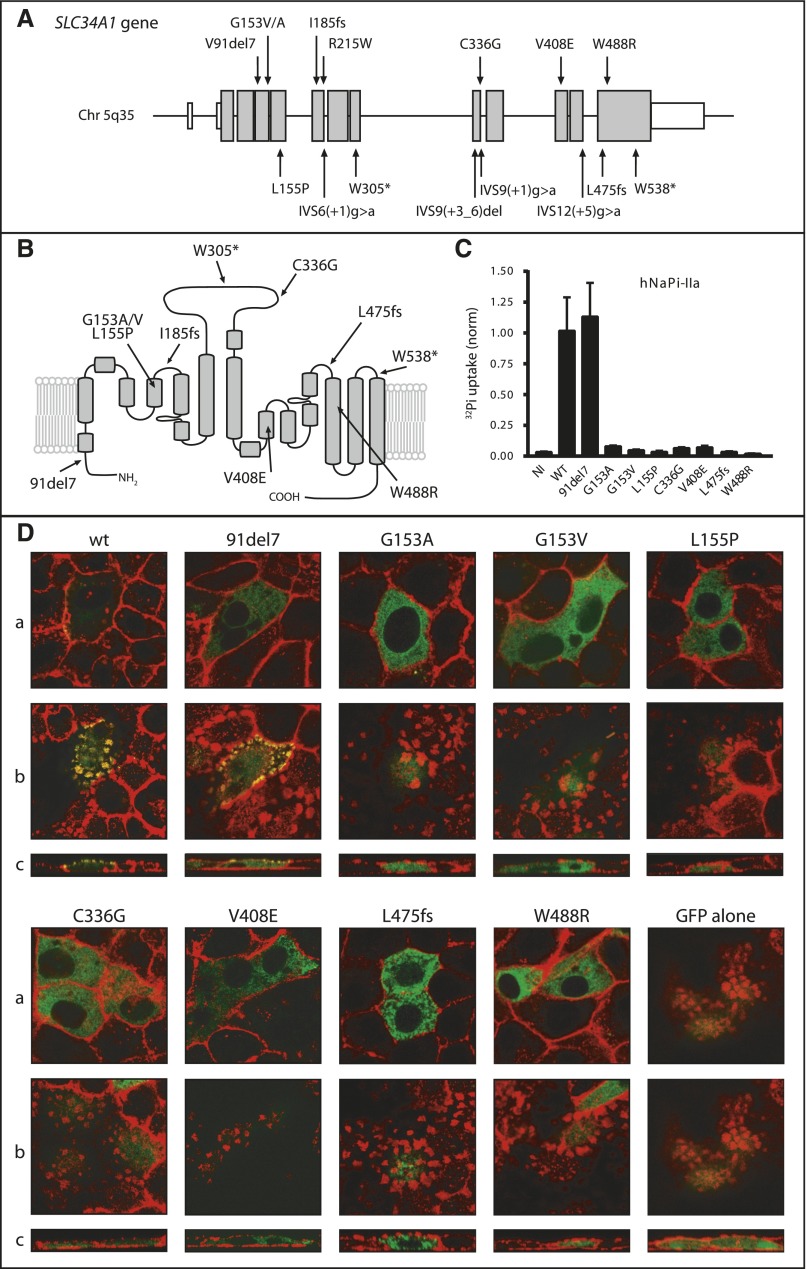
We identified four patients from three consanguineous families (F1–F3) with typical IIH without mutations in CYP24A1 (Supplemental Figure 1). Homozygosity mapping revealed a single region on chromosome 5q35 with a maximum multi-point logarithm of odds score of 6.79 (Supplemental Figure 2). The critical interval of approximately 1.66 Mb contains 30 known RefSeq genes as well as 15 putative transcripts (Supplemental Table 1) including SLC34A1 as the most promising positional candidate. Direct sequencing of SLC34A1 yielded homozygous mutations in all four patients subjected to homozygosity mapping. Next, the SLC34A1 gene was screened in a larger cohort of sporadic IIH patients without CYP24A1 mutations (n=126). Biallelic mutations were identified in 11 patients (Supplemental Figures 1 and 3). Solely in patient F5.1, only one heterozygous mutation could be identified. Cosegregation analysis was compatible with autosomal-recessive inheritance (Supplemental Figure 1). In total, 16 different mutations were identified (Figure 2A/B). All mutations were excluded in at least 204 healthy Caucasian control alleles. Furthermore, we identified an in-frame deletion of seven amino acids (91del7) that has been described previously6 and is listed in the human exome variant server with an allele frequency of approximately 2.6% in the European American population (http://evs.gs.washington.edu). For this variant, a larger sample was tested, yielding an allele frequency of approximately 1.6% (8 out of 512 alleles).
Figure 2.
Genetic and functional analyses of SLC34A1/NaPi-IIa. (A) Identified mutations in the SLC34A1 gene. In total, 16 different mutations were identified including six missense mutations, two frame-shift mutations, one in-frame deletion, two stop mutations, and five splice-site mutations. (B) Secondary topology of the human NaPi-IIa protein (adapted from Fenollar-Ferrer et al.36) with mutations indicated. (C) Phosphate transport activity of wild-type and mutant hNaPi-IIa. Uptakes were performed in Xenopus oocytes 3 days after injection of cRNA encoding hNaPi-IIa. n=2, each 8–10 oocytes; NI, non-injected; wt, wild-type. (D) Expression of human NaPi-IIa cotransporters in OK cells. Cells were transfected with pEGFP plasmids containing either wild-type or mutant hNaPi-IIa, as well as with the empty pEGFP plasmid. Confluent cultures were processed for confocal microscopy. (a) Focal planes of lateral projections. (b) Focal planes of apical projections. (c) Cross-sections. NaPi-IIa signal is shown in green and the actin staining in red.
Clinical Findings
All 15 patients with proven SLC34A1 mutations (F1.1–F14.1) were clinically re-evaluated, as well as one patient with early-onset nephrocalcinosis (F15.1) (Table 1, Supplemental Figure 1). Clinical details of patient F4.1 have been reported previously (patient 3 in Lameris et al.7). All patients received vitamin D supplements from birth according to their home country’s recommendations. The age at onset varied between 20 days and 10 months with failure to thrive and polyuria being the most frequent clinical symptoms. Renal ultrasound demonstrated medullary nephrocalcinosis in all infants. A retrospective analysis of laboratory data at the time of initial manifestation revealed hypercalcemia and suppressed intact parathyroid hormone (iPTH) levels. During hypercalcemia, active 1,25-(OH)2D3 was determined in 11/15 patients and found to be elevated in 6/11 patients (Table 1, Supplemental Table 2, median=82 pg/ml). In patient F15.1, nephrocalcinosis was discovered accidentally at 18 months of age. In this patient, only polyuria had been noticed before. The laboratory evaluation revealed a high–normal serum calcium and an iPTH level at the lower normal limit (Table 1).
Table 1.
Clinical and biochemical characteristics of the patient cohort
| Patient | F1.1 | F1.2 | F2.1 | F3.1 | Sporadic IIH cases F4.1–F14.1 (n=11) | F15.1 nephrocalcinosis |
|---|---|---|---|---|---|---|
| Origin | Turkey | Turkey | Turkey | Turkey | Poland | |
| Age at presentation | 20 days | 1 month | 2 months | 2 months | 1–10 months | 18 months |
| Vitamin D prophylaxis | 400IE | 400IE | 500IE | 400IE | 200–2000IE (n=11) | 800IE |
| Weight loss/failure to thrive | Yes | Yes | No | Yes | 8/11 | No |
| Polyuria/dehydration | Yes | Yes | No | Yes | 8/10 | Yes |
| Muscular hypotonia | Yes | No | No | No | 3/10 | No |
| Nephrocalcinosis | Yes | Yes | Yes | Yes | 11/11 | Yes |
| Hypercalciuria | Yes | Yes | Yes | Yes | 8/11 | No |
| Initial serum calcium (mM) (2.1–2.6) | 3.5 | 2.9 | 3.1 | 3.2 | 3.1 (2.6–3.8) | 2.6 |
| Initial serum phosphate (mmol/l) | 1.0 (−5.8SDS) | 0.5 (−8.6SDS) | 1.5 (−2.8SDS) | 0.7 (−7.2SDS) | 1.2 (0.6–1.9) (−4.1SDS) | 1.7 (+0.4SDS) |
| TmP/GFR (mmol/l GF) | 0.9 (−3.3SDS) | n.d. | 1.4 (−0.3SDS) | n.d. | 0.9 (0.5–1.6) (−2.0SDS) | n.d. |
| Initial iPTH (pg/ml)(14–72) | 1.0 | 15 | 5.5 | 31 | <3 (<1–4.9) | 13 |
| Initial 25-OH-D3 (ng/ml) (10–65) | 28 | n.d. | 45 | 46 | 33.7 (15.9–56.3) | 21 |
| Initial 1,25-(OH)2D3 (pg/ml) (17–74) | 139 | n.d. | 146 | 26 | 72.3 (50.8–271) | 32 |
| Therapy (acute phase) | Ketoconazole phosphate | Rehydration | No | Rehydration steroids furosemide | Rehydration (10/10) | No |
| Steroids (3/10) | ||||||
| Bisphosphonates (1/10) | ||||||
| Therapy (long term) | Oral phosphate hydrochlorothiazide | Oral phosphate hydrochlorothiazide | No | Low calcium diet | Low calcium diet (3/11) | Potassium citrate |
| Hydrochlorothiazide (1/11) | ||||||
| Potassium citrate (2/11) | ||||||
| Oral phosphate (3/11) | ||||||
| Follow-up | ||||||
| Age at last visit | 1.5 years | 7 years | 6 years | 1.5 years | 5 years (0.5–17) | 3.5 years |
| Last serum calcium (mM) (2.1–2.6) | 2.7 | 2.5 | 2.4 | 2.8 | 2.6 (2.5–2.7) (11/11) | 2.7 |
| Last serum phosphate (mM) | 0.9 (−4.1SDS) | 1.1 (−1.7SDS) | 1.5 (+0.5SDS) | 1.8 (+0.9SDS) | 1.5 (0.9–1.8) (+0.1SDS) | 1.7 (+1.3SDS) |
| TmP/GFR (mmol/l GF) | 0.8 (−3.0SDS) | 1.0 (−2.0SDS) | 1.3 (−0.5SDS) | n.d. | 1.3 (−0.5SDS) | 1.6 (+1.0SDS) |
| Last PTH (pg/ml)(14–72) | 21 | 23 | 20 | 42 | 16 (7.1–33) | 22 |
| FGF23 (kRU/l)(26–110) | 136 | n.d. | n.d. | 77 | 51 (29–114) | n.d. |
| Last 25-OH-D3 (ng/ml) (10–65) | 8 | 12 | 26 | 36 | 26 (15–63) | 38 |
| Last 1,25-(OH)2D3 (pg/ml) (17–74) | 57 | 75 | 58 | n.d. | 59 (35–146) | 54 |
| SLC34A1 mutations nucleotide level | c.644(+1)g>a homoz. | c.644(+1)g>a homoz. | c.458G>T homoz. | c.1006(+1)g>a homoz. | See Supplemental Material | c.271_91del homoz. |
| SLC34A1 mutations protein level | IVS6(+1)g>a homoz. | IVS6(+1)g>a homoz. | p.G153V homoz. | IVS9(+1)g>a homoz. | See Supplemental Material | p.91del7 homoz. |
The individual data are provided for the patients from consanguinous families F1–F3 used for homozygosity mapping as well as for patient F15.1 who presented with polyuria and nephrocalcinosis. The data of sporadic cases of IIH (F4.1–F14.1) are summarized. For clinical symptoms the proportion of affected patients is provided, the values indicated for laboratory parameters correspond to median and range. For serum phosphate and TmP/GFR, age-dependent SDS are provided (for sporadic cases median SDS). GF, glomerular filtrate; n.d., no data available.
A thorough re-evaluation of phosphate metabolism revealed hypophosphatemia (S-PO4 median=1.1 mmol/l (−5.0SDS, adjusted for age)). Impaired renal phosphate conservation could be demonstrated in four out of seven patients with available data (tubular maximum phosphate reabsorption per glomerular filtration rate, TmP/GFR≤−2SDS). During acute treatment of hypercalcemia vitamin D supplements were stopped in all patients, additional therapeutic measures included intravenous rehydration, furosemide, corticosteroids, and ketoconazole (Supplemental Table 2). A low calcium diet was initiated in four patients. Thereafter, serum calcium levels decreased but tended to be continuously elevated during follow-up in six patients. Six out of 16 patients were treated with oral phosphate (sodium phosphate or sodium glycerophosphate). The determination of TmP/GFR (13/16 patients with data) during follow-up revealed low values (TmP/GFR≤−2SDS) in four patients, seven patients exhibited TmP/GFR in the lower part of the normal range. In the majority of patients (11/16), iPTH levels normalized during follow-up (median follow-up of 3.5 years). FGF23 levels were determined in 8/16 patients and found to be within the normal range during follow-up in the presence of normophosphatemia and normalized calcium metabolism.
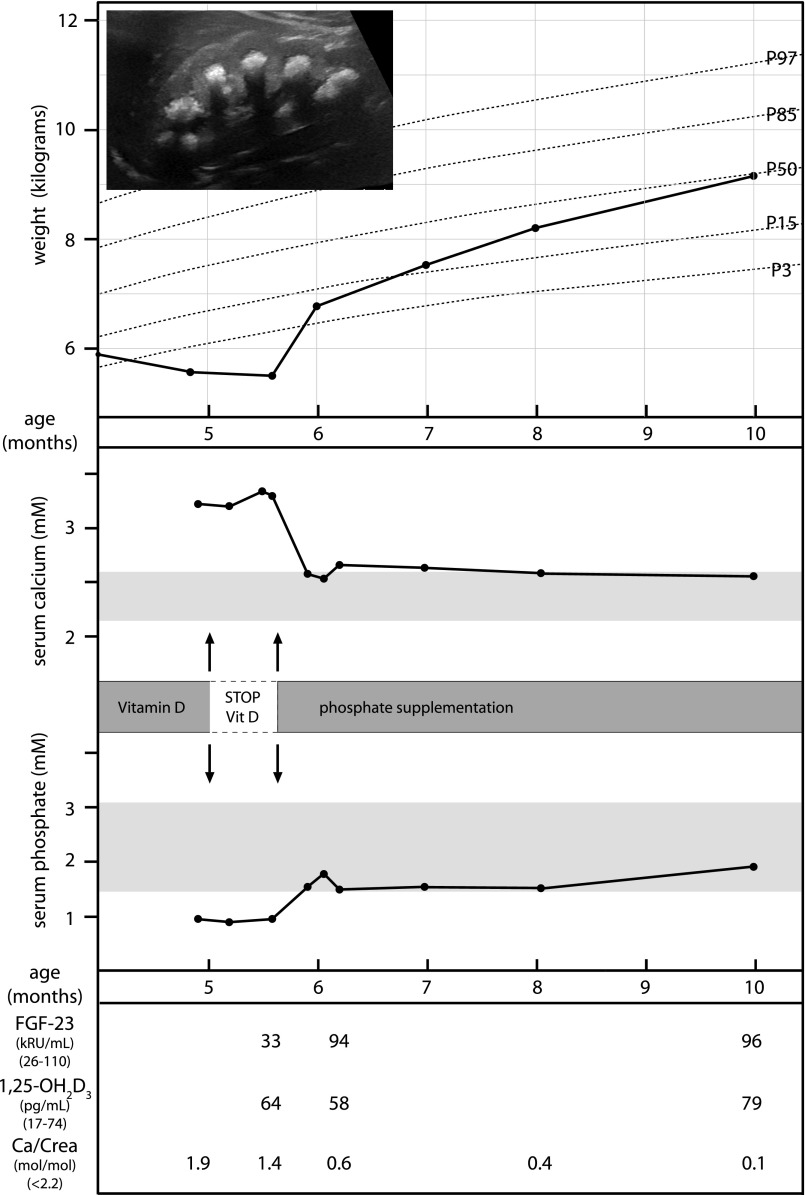
In the context of the efficacy of the mentioned therapeutic measures, patient F9.1 is of special importance because SLC34A1 mutations were detected during acute disease manifestation while still being hypercalcemic and exhibiting phosphate deficiency. Prior to manipulation of dietary phosphate intake, oral rehydration as well as a diet containing low calcium and devoid of vitamin D supplements had been unable to correct the hypercalcemia. Therefore, a supplementation with oral phosphate in a dose of 0.5–1 mmol/kg/day was initiated leading to a rapid correction of hypophosphatemia, a rapid normalization of calcium metabolism, and a significant clinical improvement reflected by a rapid weight gain (Figure 3).
Figure 3.
Clinical course of patient F9.1 during acute disease manifestation. Whereas rehydration and omitting of vitamin D prophylaxis did not lead to correction of hypercalcemia and clinical improvement, phosphate supplementation implemented after genetic diagnosis of SLC34A1 mutations resulted in normophosphatemia, a normalization of calcium metabolism, a reduction in calcium excretion, as well as a rapid clinical recovery and weight gain. The inset shows severe medullary nephrocalcinosis on renal ultrasonography in this infant.
Clinical and/or biochemical data of parents was available for 12 families (Supplemental Tables 3 and 4). Whereas nephrocalcinosis was not identified in any relative, both parents of patient F5.1 had suffered from kidney stone disease, the mother of patient F9.1 underwent nephrectomy in adolescence after recurrent pyelonephritis and a staghorn calculus. The remaining carriers of heterozygous SLC34A1 mutations with available data were free of renal pathology. Biochemical analyses indicated normocalcemia, normophosphatemia and iPTH levels within the normal range; only the father of patient F13.1 with heterozygous SLC34A1 mutation p.V408E displayed hypophosphatemia (0.6 mmol/l) and a suppressed iPTH (<3 pg/ml), while being normocalcemic and free of clinical symptoms. However, in 4/6 carriers of heterozygous SLC34A1 mutations, TmP/GFR was in the lower normal range (0.8 mmol/l).
NaPi-IIa-Mediated Phosphate Uptake in Xenopus Oocytes
Human wild-type and mutant NaPi-IIa constructs were functionally expressed in Xenopus oocytes and transport of labeled phosphate (32Pi) was measured. Overexpression of wild-type NaPi-IIa induced a significant 32Pi uptake as described previously.8 In contrast, injection of mutant NaPi-IIa complementary RNA (cRNA) did not produce 32Pi uptakes significantly different from non-injected controls, compatible with a loss of function and/or defective trafficking to the membrane of identified mutations. Only the 91del7 variant yielded 32Pi uptakes comparable to the wild-type construct (Figure 2C).
Subcellular Localization of Mutant NaPi-IIa in OK Cells
EGFP-tagged human NaPi-IIa constructs were transiently transfected into opossum kidney (OK) cells and the subcellular localization was studied by confocal microscopy (Figure 2D). For wild-type NaPi-IIa, a regular localization at the plasma membrane was observed (visible as patchy apical accumulations on focal as well as cross-sectional planes) in colocalization with actin. Mutant NaPi-IIa constructs displayed a complete intracellular retention and no detectable actin colocalization. The 91del7 variant was found to be expressed both in intracellular compartments as well as at the plasma membrane, indicating a partial retention of this variant inside the cell (Figure 2D).
Animal Data
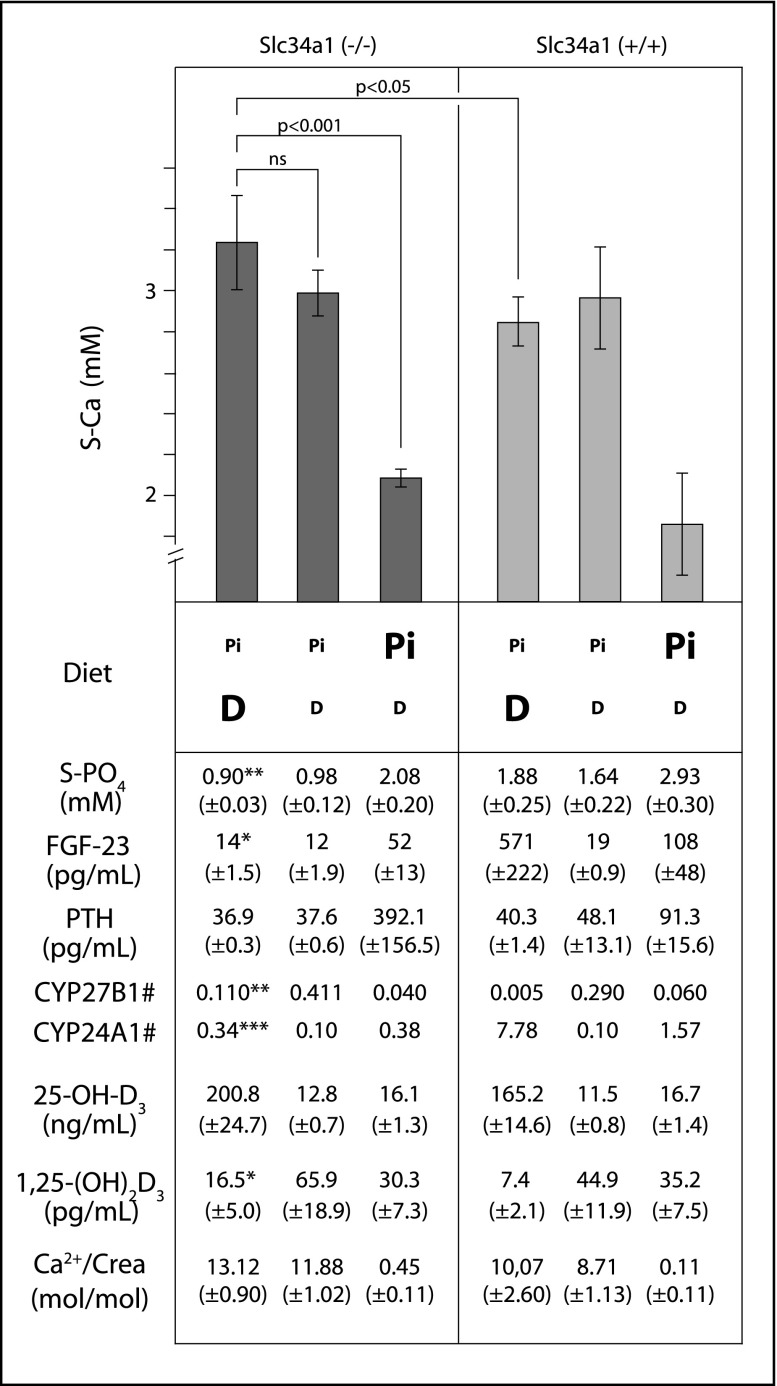
To study the mechanisms behind the immediate clinical improvement upon phosphate supplementation in patient F9.1, the influence of phosphate and vitamin D was studied in Slc34a1 knockout mice. After weaning, mice were fed diets with low or high phosphate (lowP/highP) as well as low or high vitamin D content (lowD/highD). As expected, lowP diets led to severe hypophosphatemia in knockout mice whereas wild-type animals remained normophosphatemic (Figure 4). The highD diets resulted in a vitamin D overload in knockout and wild-type animals reflected by high 25-OH-D3 levels. In response, wild-type mice were able to adequately down-regulate vitamin D activation. They exhibited low CYP27B1 mRNA as well as high CYP24A1 mRNA levels and showed very low serum levels of active 1,25-(OH)2D3. In contrast, knockout animals showed significantly higher levels of CYP27B1 mRNA (0.110 in knockout versus 0.005 in wild-type, P=0.001) while CYP24A1 expression was low (0.34 in knockout versus 7.78 in wild-type, P<0.001). As a consequence, levels of 1,25-(OH)2D3 stayed significantly higher (16.5 ng/ml in knockout versus 7.4 ng/ml in wild-type, P=0.01), contributing to an exacerbation of hypercalcemia and hypercalciuria. The impaired inhibition of vitamin D activation in hypophosphatemic knockout mice likely occurred in consequence of suppressed FGF23 levels (14 pg/ml in knockout versus 571 pg/ml in wild-type, P<0.05). Equally low FGF23 levels were observed in knockout animals on a lowP/lowD diet. Despite low vitamin D supply, these mice showed an augmented vitamin D activation with higher levels of active 1,25-(OH)2D3 resulting in hypercalcemia and hypercalciuria. In contrast, the highP diets normalized serum phosphate levels and FGF23. Consecutively, levels of 1,25-(OH)2D3 as well as serum calcium returned to their physiologic ranges. For a summary of results see Figure 4 (the full data set is provided in Supplemental Table 5).
Figure 4.
Biochemical data of Slc34a1 knockout mice in comparison to wild-type animals. Both mice were fed diets with low or high phosphate content (lowP/highP) and vitamin D (lowD/highD), respectively. HighD diets resulted in a vitamin D overload in both knockout and wild-type mice. Wild-type mice adequately limited vitamin D activation by downregulating Cyp27b1 and activating Cyp24a1 expression. In contrast, phosphate-depleted Slc34a1 knockout mice exhibited low FGF23 levels, provoking a reverse regulation of Cyp27b1 (1α-hydroxylase) and Cyp24a1 (24-hydroxylase) expression. Consequently, these mice were not able to limit vitamin D activation, leading to an aggravation of hypercalcemia. Dysregulated calcium homeostasis in these knockout mice was only slightly improved by limiting vitamin D supply with persistence of hypercalcemia and hypercalciuria. In contrast, high phosphate supplementation restored serum levels of phosphate and FGF23 enabling a normalization of 1,25-(OH)2D3 and serum calcium levels. Significant differences between knockout and wild-type mice under lowP/highD diet are indicated by bold letters, significance levels are: *P<0.05; **P<0.01; ***P<0.001; # = normalized Cyp27b1 and Cyp24a1 expression levels (for details see Supplemental Material).
Discussion
Using a positional candidate gene approach, we identified loss-of-function mutations in SLC34A1 encoding renal proximal tubular sodium-phosphate cotransporter NaPi-IIa in a cohort of infants with IIH without mutations in CYP24A1. Cosegregation analysis indicates autosomal-recessive inheritance.
NaPi-IIa (SLC34A1), together with its close homolog NaPi-IIc (SLC34A3), mediates the conservation of filtered phosphate from primary urine.9 The importance of NaPi-IIa and NaPi-IIc for renal phosphate conservation could be deduced from animal studies as well as hereditary human disease. NaPi-IIa knockout mice exhibit urinary phosphate wasting with consecutive hypophosphatemia.10 They also show high 1,25-(OH)2D3 levels, resulting in hypercalcemia and hypercalciuria. In contrast, NaPi-IIc knockout mice do not exhibit overt renal phosphate wasting, but only display hypercalciuria and elevated 1,25-(OH)2D3 levels.11 These findings for NaPi-IIc in mice differed from initial observations in humans, where inactivating NaPi-IIc mutations were shown to lead to hereditary hypophosphatemic rickets with hypercalciuria (OMIM #241530).12,13 Meanwhile, patients with NaPi-IIc defects presenting with isolated hypercalciuria and nephrolithiasis have been described.14,15
The identification of autosomal-recessive SLC34A1 mutations in infants with IIH now demonstrates a crucial role of NaPi-IIa for calcium metabolism as well as phosphate balance in humans, which are tightly linked because they share major control mechanisms comprising vitamin D, iPTH, and FGF23. FGF23 exerts two major effects in the proximal tubule. In the first place, FGF23 inhibits phosphate reabsorption via NaPi-IIa and NaPi-IIc. Secondly, it inhibits 1α-hydroxylase (CYP27B1) and activates 24-hydroxylase (CYP24A1), decreasing circulating levels of active 1,25-(OH)2D3 (Figure 1). Excess levels of FGF23, as present in different forms of hereditary hypophosphatemic rickets, therefore lead to secondary renal phosphate wasting together with low levels of active vitamin D.
In contrast, IIH patients with NaPi-IIa mutations exhibit a primary defect in proximal tubular phosphate reabsorption. Subsequent hypophosphatemia as present in our patients at initial manifestation (Table 1, Supplemental Table 2) induces a decrease in circulating FGF23 levels.16,17 Both hypophosphatemia and low FGF23 levels are known to increase CYP27B1 expression and 1α-hydroxylase activity as well as inhibiting CYP24A1 expression and 24-hydroxylase activity.16 These effects together promote an increase of 1,25-(OH)2D3 with subsequent hypercalcemia (Figure 5).
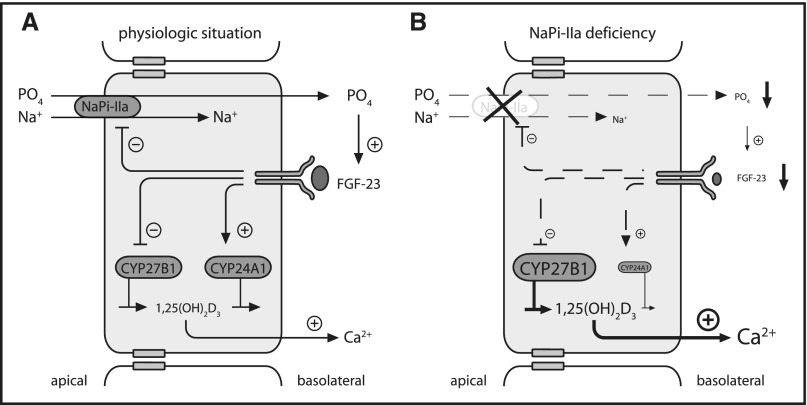
Figure 5.
Pathophysiology of disturbed NaPi-IIa function in the proximal renal tubule. (A) Under physiologic conditions, proximal tubular phosphate reabsorption via NaPi-IIa (and NaPi-IIc, not shown) ensures the maintenance of phosphate homeostasis. Phosphate reabsorption via NaPi-IIa is limited by the concerted action of PTH (not shown) and FGF23. Besides its phosphaturic effect, FGF23 negatively regulates the action of 1,25-(OH)2D3 by inhibiting the expression of 1α-hydroxylase (CYP27B1) and activating 24-hydroxylase (CYP24A1). (B) As a consequence of a NaPi-IIa defect, phosphate depletion leads to a decrease of FGF23 levels. In turn, an unrestricted activation of 1,25-(OH)2D3 results in hypercalcemia, hypercalciuria, and nephrocalcinosis.
As laboratory parameters during acute hypercalcemia were compiled retrospectively, the available data set is incomplete. At initial manifestation, 1,25-(OH)2D3 was elevated in 6/11 IIH patients with available data (Table 1, Supplemental Table 2). Comparable 1,25-(OH)2D3 levels have been identified previously in hypercalcemic patients with CYP24A1 defects.2 In contrast, in non-vitamin D-mediated hypercalcemic conditions, levels of 1,25-(OH)2D3 are expected to be low.
In IIH patients with NaPi-IIa defects, the disturbances in calcium homeostasis clearly outmatch the primary defect in phosphate metabolism. Although hypophosphatemia and renal phosphate wasting are detected at initial presentation and to a lesser extent during follow-up, a clinical correlate, e.g., signs of rickets, is lacking. Unfortunately, an accurate determination of FGF23 levels poses a challenge in the clinical setting due to the hormone’s instability (see Supplemental Material). Therefore data on FGF23 levels in our patients at initial manifestation are mostly lacking. FGF23 was solely measured in patient F9.1 during hypophosphatemia and hypercalcemia (Figure 3) and in the low–normal range (33 kRU/ml) (for details concerning the employed assay please see Supplemental Material). During follow-up, in face of normophosphatemia (median S-PO4=1.5 mmol/l), FGF23 levels were normal (8/16 patients with available data). While this human data incompletely traces all proposed pathophysiologic changes, these have been delineated accurately in Slc34a1 knockout mice.10,16,18
To examine the concomitant disturbances in calcium and phosphate metabolism in more detail and their dependence on phosphate and vitamin D supply, we re-examined Slc34a1 knockout mice fed diets with variable phosphate and vitamin D content. First, the lowP/highD diet was used to simulate the situation of human infants who receive breast milk with low phosphate content (~0.1%–0.15%) together with the recommended vitamin D prophylaxis (500 IU/day orally) for the prevention of rickets. Under this diet, Slc34a1 knockout mice developed hypophosphatemia, hypercalcemia, and hypercalciuria (Figure 4, Supplemental Table 5). Although some changes in calcium metabolism caused by excess vitamin D were also observed in wild-type mice under identical diet, the analysis of hormonal factors and vitamin D metabolizing enzymes revealed the critical changes in the regulation of vitamin D (Figure 4). Wild-type mice are able to limit vitamin D activation by increased action of FGF23, while phosphate-deficient Slc34a1 knockout mice display an unlimited vitamin D activation caused by lack of the regulatory counterpart FGF23. These abnormalities are only slightly mitigated by limiting vitamin D supply with persistence of hypercalcemia and hypercalciuria (Figure 4). In contrast, Slc34a1 knockout mice respond to a high phosphate diet with normalization of serum phosphate and FGF23 levels enabling a return of 1,25-(OH)2D3 into the physiologic range, normocalcemia, and normocalciuria. Similar observations were made by Tenenhouse and colleagues who describe a normalization of calcium metabolism in Slc34a1 knockout mice by either additional ablation of 1α-hydroxylase (CYP27B1) or by high phosphate supplementation.19 Importantly, these data are in line with the clinical observation made in patient P9.1 who remained hypercalcemic after rehydration and cessation of vitamin D supplements (Figure 3), but showed a rapid improvement of his clinical condition and a complete reversal of biochemical abnormalities, including an increase in FGF23, after phosphate supplementation.
The SLC34A1 mutations observed in IIH patients clearly indicate autosomal-recessive inheritance. The spectrum of mutations comprises truncating mutations as well as missense mutations. For the latter, functional analyses demonstrated a loss-of-function character which may be mostly due to impaired trafficking of the mutant transporter to the membrane as indicated by the OK cell experiments (Figure 2D). Mutations in SLC34A1 have been described before, either in homozygous/compound-heterozygous or heterozygous state. A recessive loss-of-function mutation in SLC34A1 was described in two siblings with renal Fanconi’s syndrome, hypophosphatemic rickets, hypercalciuria, and elevated 1,25-(OH)2D3 levels.20,21 Unfortunately, no data regarding the clinical course during infancy were reported. A recent report describes two siblings with a homozygous missense mutation who presented with hypophosphatemia and nephrocalcinosis.22 The laboratory evaluation of the asymptomatic younger sister performed in early infancy revealed hypercalcemia, suppressed iPTH, and elevated levels of 1,25-(OH)2D3 as present in our IIH patients. Thus, the biochemical profile resembles IIH, noteworthy without clinical symptoms related to hypercalcemia. Compound-heterozygous mutations were described in a patient who presented during infancy with early-onset nephrocalcinosis and hyperoxaluria.23
Furthermore, heterozygous missense mutations in SLC34A1 were identified in adult patients with nephrolithiasis, bone demineralization, and renal phosphate leak.6,24 Functional studies demonstrated a loss of phosphate transport activity for the reported mutations, but failed to identify a dominant-negative effect that had initially been postulated.24,25 We observed nephrolithiasis in 3/26 heterozygous first-degree relatives of our patients (Supplemental Table 4). Therefore, a heterozygous carrier status appears to represent a predisposition for the development of kidney stone disease, presumably requiring additional genetic or lifestyle factors as already suggested by Prié and colleagues.24
Previously identified sequence variants included the 91del7 variant,6 for which the authors demonstrated a reduced expression level in HEK293 cells as well as a reduction in phosphate-induced currents in Xenopus oocytes. We here describe three patients (F6.1, F8.1, and F13.1) who carry the 91del7 mutant in compound-heterozygous state together with a second non-functional allele (Supplemental Table 2). These patients display a clinical phenotype that is indistinguishable from that of IIH patients with two non-functional SLC34A1 alleles. Our functional analyses confirmed the impaired trafficking of NaPi-IIa-91del7 in HEK293 cells while phosphate uptake in the Xenopus oocyte system was largely preserved. The pathophysiologic relevance of the 91del7 variant in compound-heterozygous state is also supported by a report on an infant with Sotos syndrome and clinical IIH.26 The patient carried a genomic deletion including SLC34A1 that is typical for Sotos syndrome on one allele and the 91del7 variant on the remaining SLC34A1 allele.26 Finally, we identified this mutant in homozygous state in a girl (F15.1) who presented with incidental nephrocalcinosis and polyuria. Despite no obvious disturbance in phosphate metabolism, she had borderline hypercalcemia and hypercalciuria, a clinical phenotype that could be considered a mild IIH variant.
In summary, the discovery of autosomal-recessive loss-of-function mutations in SLC34A1 (NaPi-IIa) highlights a novel pathophysiologic pathway in IIH. In affected patients, primary renal phosphate wasting and suppression of FGF23 leads to an inappropriate activation of 1,25-(OH)2D3 with subsequent hypercalcemia. As the clinical phenotype strongly resembles that of patients with CYP24A1 defects, all infants clinically presenting with IIH require a careful evaluation of phosphate metabolism. Beyond omitting vitamin D prophylaxis and calcium restriction, infants with defective NaPi-IIa require phosphate supplementation in order to restore serum phosphate levels and normalize vitamin D and calcium metabolism. Future studies will have to address the definite impact of this combined disturbance of phosphate and calcium metabolism for the development of hypercalciuria, nephrocalcinosis, and nephrolithiasis in later life as already described for defects in CYP24A1.5
Concise Methods
Patients
We identified four patients from three consanguineous families (F1–F3) as well as 12 unrelated patients with typical IIH who did not exhibit mutations in CYP24A1. Clinical and laboratory data of affected patients were collected retrospectively from medical charts except for patient F9.1 who was recruited during the acute phase of hypercalcemia. During follow-up, all patients were clinically re-evaluated and current biochemical data obtained. In addition, clinical, radiologic, and laboratory data of available parents were obtained and analyzed using standardized questionnaires (summarized in Supplemental Tables 3 and 4). Detailed descriptions of the analyses of serum iPTH, FGF23, 25-OH-D3, and 1,25-(OH)2D3 are provided in the Supplemental Material. Renal phosphate handling was assessed as TmP/GFR=SPO4–(UPO4×SCr/UCr). For SPO4 and TmP/GFR, age-dependent reference values were used.27,28 All investigations, including genetic studies, were approved by the Ethics Committee of the Westfälische Wilhelms University, Münster. Patients or their parents provided written informed consent.
Genetic Studies
Genomic DNA of affected individuals and available family members was extracted from peripheral blood using standard methods. A genome scan for shared homozygous regions was performed in patients F1.1–F3.1 using Illumina Human660W-Quad and Human OmniExpress BeadChips. Multi-point parametric linkage analysis was performed using Merlin 1.1.2 as described (see Supplemental Material).29 A list of candidate genes within the critical interval was generated on the basis of Ensembl Genome assembly GRCh37 (www.ensembl.org). The coding region and splice sites of SLC34A1 were conventionally Sanger sequenced. Newly identified SLC34A1 sequence variants were tested for their frequency in at least 204 ethnically matched control alleles by Sanger sequencing. The presence of the previously reported sequence variation 91del7 was analyzed in 512 control alleles.
Preparation of Plasmid Constructs
NaPi-IIa mutations 91del7, G153A, G153V, L155P, C336G, V408E, L475fs, and W488R were introduced into human SLC34A1 cDNA subcloned into pEGFP-C1 and KSM vectors as described.25,30 Details concerning mutagenesis are provided in the Supplemental Material. For oocyte expression, capped cRNA was synthesized in vitro using Megascript T3 kit (Ambion) in the presence of cap analog (New England Biolabs).
32Pi Uptake and Two Electrode Voltage Clamp Experiments upon Expression of NaPi-IIa in Xenopus laevis Oocytes
X. laevis oocytes were obtained, selected, and maintained as described previously (for details see Supplemental Material). All animal procedures were approved by the Swiss Cantonal Authority and in accordance with the Swiss Animal Protection Law. Oocytes were injected with 10 ng of wild-type and mutant NaPi-IIa cRNA. Experiments were performed 3 days post-injection. Oocytes were incubated in 100 Na solution containing 1 mM cold Pi and 31Pi (specific activity 10 mCi/mmol Pi; PerkinElmer) for 10 minutes. Scintillation counting (Tri-Carb 29000TR; Packard) was performed after washing and lysis in 2% SDS.
Voltage clamp experiments were performed as reported25 (for details see Supplemental Material). Steady-state currents were obtained using a protocol in which membrane voltage steps were made from the holding potential (Vh)=−60 mV, to test voltages in the range −180 to +80 mV in 20 mV increments. The steady-state Pi-dependent current (IPi) was obtained by subtracting control traces (in 100Na solution) from the corresponding traces in the presence of Pi.
Cell Culture and Transient Transfections
OK cells were cultured as previously reported31 and transfected with either wild-type or mutant NaPi-IIa fused to EGFP (enhanced green fluorescent protein). Two independent experiments were performed, each in duplicates or triplicates. Upon expression of clear patches of wild-type cotransporter, cells were fixed and permeabilized with saponin as described previously.32 Actin was stained by incubation with Texas Red-X phalloidin (Invitrogen). After washing with saponin/PBS, coverslips were mounted on microscope slides. The subcellular localization of transfected constructs was analyzed by Confocal Laser Scanning Microscopy (Leica SP2).
Animal Studies
Experiments were performed on 4–12 week old C57BL/6 and homozygous Slc34a1 knockout mice obtained from heterozygous crossings (littermates). Generation, breeding, and genotyping of the Slc34a1 knockout mice have been described before.10,33 All experiments were performed according to Swiss animal welfare laws and approved by the local veterinary authority. Animals had free access to water, and received for 8 weeks a standard rodent diet (ssniff, Soest, Germany) supplemented with a high (1.2%) or low (0.1%) Pi content, the latter with high (10 IU/g chow) or low (0.3–0.5 IU/g chow) Vitamin D3. Spontaneous urine samples were collected on the last day and frozen until further analysis. Blood samples were collected before sacrificing the mice by puncture of the vena cava. Serum electrolytes and creatinine were analyzed using commercial kits (Sigma-Aldrich, St. Louis, MO and Wako Chemicals, Neuss, Germany). Plasma concentrations of iPTH and intact FGF23 were determined by ELISA (Immunotopics, San Clemente, CA and Kainos, Tokyo, Japan, respectively).
25-OH-D3 and 1,25-(OH)2D3 were assayed simultaneously by liquid chromatography-tandem mass spectrometry following derivatization as described.34 The detection of 1,25-(OH)2D3 required an immunopurification step involving commercially available antibodies to 1,25-(OH)2D3.
Renal CYP27B1 and CYP24A1 expression was quantified by real-time PCR. After purification of mRNA from kidney (RNeasy Mini Kit, Qiagen), cDNA was synthesized using reverse transcription (TaqMan Reverse Transcription Kit, Applied Biosystems) and used as a template for real-time PCR. The expression of both enzymes was normalized to the expression of hypoxanthine-guanine-phosphoribosyl-transferase (HPRT) (see Supplemental Material).
Disclosures
None.
Supplementary Material
Acknowledgments
We thank the following clinicians and researchers for their contributions to this work: Lea Haisch (Department of General Pediatrics, University Children’s Hospital, Münster, Germany), Carla Bettoni (Institute of Physiology and Zurich Center for Integrative Human Physiology [ZIHP], University of Zurich, Zurich, Switzerland), Tülay Güran (Department of Pediatrics, Division of Pediatric Endocrinology, Marmara University, Istanbul, Turkey), Leo A. Monnens (Pediatric Nephrology, Radboud University Nijmegen Medical Center, Nijmegen, The Netherlands), Anke L. Lameris (Department of Physiology, Radboud University Medical Center, Nijmegen, The Netherlands), Wolfgang Rascher (Department of Pediatrics, Friedrich-Alexander-University, Erlangen, Germany), Einat Azaria (Sheba Medical Center, Tel Aviv, Israel), Yair Anikster (Sheba Medical Center, Tel Aviv, Israel), Joost G. Hoenderop (Department of Physiology, Radboud University Medical Center, Nijmegen, The Netherlands), Francesco Emma (Division of Nephrology and Dialysis, Children’s Hospital Bambino Gesù, IRCCS, Rome, Italy), and Monika Stoll (Department of Human Genetics, University Hospital, Münster, Germany).
This work was supported by the ERA-Net E-Rare Research Programme for Rare Diseases (IIH-ECC) (to K.P.S., G.J., and C.A.W.), the Innovative Medizinische Forschung of the Westfalian Wilhelms-University (to K.P.S. and M.K.), the B’nai B’Brith Leo Baeck (London) Lodge Trust Fund (to A.V., and R.K.) and the European Union, FP7 (grant agreement 2013-305608) (to R.K.), and the Swiss National Science Foundation supported National Center for Excellence in Research NCCR Kidney.CH (to C.A.W.).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2014101025/-/DCSupplemental.
References
- 1.Kato S, Yoshizazawa T, Kitanaka S, Murayama A, Takeyama K: Molecular genetics of vitamin D-dependent hereditary rickets. Horm Res 57: 73–78, 2002 [DOI] [PubMed] [Google Scholar]
- 2.Schlingmann KP, Kaufmann M, Weber S, Irwin A, Goos C, John U, Misselwitz J, Klaus G, Kuwertz-Bröking E, Fehrenbach H, Wingen AM, Güran T, Hoenderop JG, Bindels RJ, Prosser DE, Jones G, Konrad M: Mutations in CYP24A1 and idiopathic infantile hypercalcemia. N Engl J Med 365: 410–421, 2011 [DOI] [PubMed] [Google Scholar]
- 3.Fanconi G: [Chronic disorders of calcium and phosphate metabolism in children]. Schweiz Med Wochenschr 81: 908–913, 1951 [PubMed] [Google Scholar]
- 4.Lightwood R, Stapleton T: Idiopathic hypercalcaemia in infants. Lancet 265: 255–256, 1953 [DOI] [PubMed] [Google Scholar]
- 5.Nesterova G, Malicdan MC, Yasuda K, Sakaki T, Vilboux T, Ciccone C, Horst R, Huang Y, Golas G, Introne W, Huizing M, Adams D, Boerkoel CF, Collins MT, Gahl WA: 1,25-(OH)2D-24 hydroxylase (CYP24A1) deficiency as a cause of nephrolithiasis. Clin J Am Soc Nephrol 8: 649–657, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lapointe JY, Tessier J, Paquette Y, Wallendorff B, Coady MJ, Pichette V, Bonnardeaux A: NPT2a gene variation in calcium nephrolithiasis with renal phosphate leak. Kidney Int 69: 2261–2267, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Lameris AL, Huybers S, Burke JR, Monnens LA, Bindels RJ, Hoenderop JG: Involvement of claudin 3 and claudin 4 in idiopathic infantile hypercalcaemia: a novel hypothesis? Nephrol Dial Transplant 25: 3504–3509, 2010 [DOI] [PubMed] [Google Scholar]
- 8.Virkki LV, Forster IC, Bacconi A, Biber J, Murer H: Functionally important residues in the predicted 3(rd) transmembrane domain of the type IIa sodium-phosphate co-transporter (NaPi-IIa). J Membr Biol 206: 227–238, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Wagner CA, Hernando N, Forster IC, Biber J: The SLC34 family of sodium-dependent phosphate transporters. Pflugers Arch 466: 139–153, 2014 [DOI] [PubMed] [Google Scholar]
- 10.Beck L, Karaplis AC, Amizuka N, Hewson AS, Ozawa H, Tenenhouse HS: Targeted inactivation of Npt2 in mice leads to severe renal phosphate wasting, hypercalciuria, and skeletal abnormalities. Proc Natl Acad Sci U S A 95: 5372–5377, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Segawa H, Onitsuka A, Kuwahata M, Hanabusa E, Furutani J, Kaneko I, Tomoe Y, Aranami F, Matsumoto N, Ito M, Matsumoto M, Li M, Amizuka N, Miyamoto K: Type IIc sodium-dependent phosphate transporter regulates calcium metabolism. J Am Soc Nephrol 20: 104–113, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bergwitz C, Roslin NM, Tieder M, Loredo-Osti JC, Bastepe M, Abu-Zahra H, Frappier D, Burkett K, Carpenter TO, Anderson D, Garabedian M, Sermet I, Fujiwara TM, Morgan K, Tenenhouse HS, Juppner H: SLC34A3 mutations in patients with hereditary hypophosphatemic rickets with hypercalciuria predict a key role for the sodium-phosphate cotransporter NaPi-IIc in maintaining phosphate homeostasis. Am J Hum Genet 78: 179–192, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lorenz-Depiereux B, Benet-Pages A, Eckstein G, Tenenbaum-Rakover Y, Wagenstaller J, Tiosano D, Gershoni-Baruch R, Albers N, Lichtner P, Schnabel D, Hochberg Z, Strom TM: Hereditary hypophosphatemic rickets with hypercalciuria is caused by mutations in the sodium-phosphate cotransporter gene SLC34A3. Am J Hum Genet 78: 193–201, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yu Y, Sanderson SR, Reyes M, Sharma A, Dunbar N, Srivastava T, Jüppner H, Bergwitz C: Novel NaPi-IIc mutations causing HHRH and idiopathic hypercalciuria in several unrelated families: long-term follow-up in one kindred. Bone 50: 1100–1106, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dasgupta D, Wee MJ, Reyes M, Li Y, Simm PJ, Sharma A, Schlingmann KP, Janner M, Biggin A, Lazier J, Gessner M, Chrysis D, Tuchman S, Baluarte HJ, Levine MA, Tiosano D, Insogna K, Hanley DA, Carpenter TO, Ichikawa S, Hoppe B, Konrad M, Sävendahl L, Munns CF, Lee H, Jüppner H, Bergwitz C: Mutations in SLC34A3/NPT2c are associated with kidney stones and nephrocalcinosis. J Am Soc Nephrol 25: 2366–2375, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Perwad F, Azam N, Zhang MY, Yamashita T, Tenenhouse HS, Portale AA: Dietary and serum phosphorus regulate fibroblast growth factor 23 expression and 1,25-dihydroxyvitamin D metabolism in mice. Endocrinology 146: 5358–5364, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Antoniucci DM, Yamashita T, Portale AA: Dietary phosphorus regulates serum fibroblast growth factor-23 concentrations in healthy men. J Clin Endocrinol Metab 91: 3144–3149, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Tenenhouse HS: Regulation of phosphorus homeostasis by the type iia na/phosphate cotransporter. Annu Rev Nutr 25: 197–214, 2005 [DOI] [PubMed] [Google Scholar]
- 19.Tenenhouse HS, Gauthier C, Chau H, St-Arnaud R: 1alpha-Hydroxylase gene ablation and Pi supplementation inhibit renal calcification in mice homozygous for the disrupted Npt2a gene. Am J Physiol Renal Physiol 286: F675–F681, 2004 [DOI] [PubMed] [Google Scholar]
- 20.Tieder M, Arie R, Modai D, Samuel R, Weissgarten J, Liberman UA: Elevated serum 1,25-dihydroxyvitamin D concentrations in siblings with primary Fanconi’s syndrome. N Engl J Med 319: 845–849, 1988 [DOI] [PubMed] [Google Scholar]
- 21.Magen D, Berger L, Coady MJ, Ilivitzki A, Militianu D, Tieder M, Selig S, Lapointe JY, Zelikovic I, Skorecki K: A loss-of-function mutation in NaPi-IIa and renal Fanconi’s syndrome. N Engl J Med 362: 1102–1109, 2010 [DOI] [PubMed] [Google Scholar]
- 22.Rajagopal A, Braslavsky D, Lu JT, Kleppe S, Clément F, Cassinelli H, Liu DS, Liern JM, Vallejo G, Bergadá I, Gibbs RA, Campeau PM, Lee BH: Exome sequencing identifies a novel homozygous mutation in the phosphate transporter SLC34A1 in hypophosphatemia and nephrocalcinosis. J Clin Endocrinol Metab 99: E2451–E2456, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Halbritter J, Baum M, Hynes AM, Rice SJ, Thwaites DT, Gucev ZS, Fisher B, Spaneas L, Porath JD, Braun DA, Wassner AJ, Nelson CP, Tasic V, Sayer JA, Hildebrandt F: Fourteen monogenic genes account for 15% of nephrolithiasis/nephrocalcinosis. J Am Soc Nephrol 26: 543–551, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Prié D, Huart V, Bakouh N, Planelles G, Dellis O, Gérard B, Hulin P, Benqué-Blanchet F, Silve C, Grandchamp B, Friedlander G: Nephrolithiasis and osteoporosis associated with hypophosphatemia caused by mutations in the type 2a sodium-phosphate cotransporter. N Engl J Med 347: 983–991, 2002 [DOI] [PubMed] [Google Scholar]
- 25.Virkki LV, Forster IC, Hernando N, Biber J, Murer H: Functional characterization of two naturally occurring mutations in the human sodium-phosphate cotransporter type IIa. J Bone Miner Res 18: 2135–2141, 2003 [DOI] [PubMed] [Google Scholar]
- 26.Kenny J, Lees MM, Drury S, Barnicoat A, Van’t Hoff W, Palmer R, Morrogh D, Waters JJ, Lench NJ, Bockenhauer D: Sotos syndrome, infantile hypercalcemia, and nephrocalcinosis: a contiguous gene syndrome. Pediatr Nephrol 26: 1331–1334, 2011 [DOI] [PubMed] [Google Scholar]
- 27.Brodehl J, Gellissen K, Weber HP: Postnatal development of tubular phosphate reabsorption. Clin Nephrol 17: 163–171, 1982 [PubMed] [Google Scholar]
- 28.Stark H, Eisenstein B, Tieder M, Rachmel A, Alpert G: Direct measurement of TP/GFR: a simple and reliable parameter of renal phosphate handling. Nephron 44: 125–128, 1986 [DOI] [PubMed] [Google Scholar]
- 29.Abecasis GR, Cherny SS, Cookson WO, Cardon LR: Merlin—rapid analysis of dense genetic maps using sparse gene flow trees. Nat Genet 30: 97–101, 2002 [DOI] [PubMed] [Google Scholar]
- 30.Patti M, Ghezzi C, Forster IC: Conferring electrogenicity to the electroneutral phosphate cotransporter NaPi-IIc (SLC34A3) reveals an internal cation release step. Pflugers Arch 465: 1261–1279, 2013 [DOI] [PubMed] [Google Scholar]
- 31.Reshkin SJ, Forgo J, Murer H: Functional asymmetry of phosphate transport and its regulation in opossum kidney cells: phosphate transport. Pflugers Arch 416: 554–560, 1990 [DOI] [PubMed] [Google Scholar]
- 32.Pfister MF, Lederer E, Forgo J, Ziegler U, Lötscher M, Quabius ES, Biber J, Murer H: Parathyroid hormone-dependent degradation of type II Na+/Pi cotransporters. J Biol Chem 272: 20125–20130, 1997 [DOI] [PubMed] [Google Scholar]
- 33.Nowik M, Picard N, Stange G, Capuano P, Tenenhouse HS, Biber J, Murer H, Wagner CA: Renal phosphaturia during metabolic acidosis revisited: molecular mechanisms for decreased renal phosphate reabsorption. Pflugers Arch 457: 539–549, 2008 [DOI] [PubMed] [Google Scholar]
- 34.Kaufmann M, Gallagher JC, Peacock M, Schlingmann KP, Konrad M, DeLuca HF, Sigueiro R, Lopez B, Mourino A, Maestro M, St-Arnaud R, Finkelstein JS, Cooper DP, Jones G: Clinical utility of simultaneous quantitation of 25-hydroxyvitamin D and 24,25-dihydroxyvitamin D by LC-MS/MS involving derivatization with DMEQ-TAD. J Clin Endocrinol Metab 99: 2567–2574, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fenollar-Ferrer C, Patti M, Knöpfel T, Werner A, Forster IC, Forrest LR: Structural fold and binding sites of the human Na⁺-phosphate cotransporter NaPi-II. Biophys J 106: 1268–1279, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.