Abstract
Advanced glycation end products (AGEs), a heterogeneous group of compounds formed by nonenzymatic glycation reactions between reducing sugars and amino acids, lipids, or DNA, are formed not only in the presence of hyperglycemia, but also in diseases associated with high levels of oxidative stress, such as CKD. In chronic renal failure, higher circulating AGE levels result from increased formation and decreased renal clearance. Interactions between AGEs and their receptors, including advanced glycation end product–specific receptor (RAGE), trigger various intracellular events, such as oxidative stress and inflammation, leading to cardiovascular complications. Although patients with CKD have a higher burden of cardiovascular disease, the relationship between AGEs and cardiovascular disease in patients with CKD is not fully characterized. In this paper, we review the various deleterious effects of AGEs in CKD that lead to cardiovascular complications and the role of these AGEs in diabetic nephropathy. We also discuss potential pharmacologic approaches to circumvent these deleterious effects by reducing exogenous and endogenous sources of AGEs, increasing the breakdown of existing AGEs, or inhibiting AGE-induced inflammation. Finally, we speculate on preventive and therapeutic strategies that focus on the AGE-RAGE axis to prevent vascular complications in patients with CKD.
Keywords: advanced glycation end product, uremia, CKD
Advanced glycation end products (AGEs) constitute a heterogeneous group of compounds derived from the nonenzymatic glycation of proteins, lipids, and nuclear acids through a complex sequence of reactions referred to as the Maillard reaction.1,2
Generation of AGEs
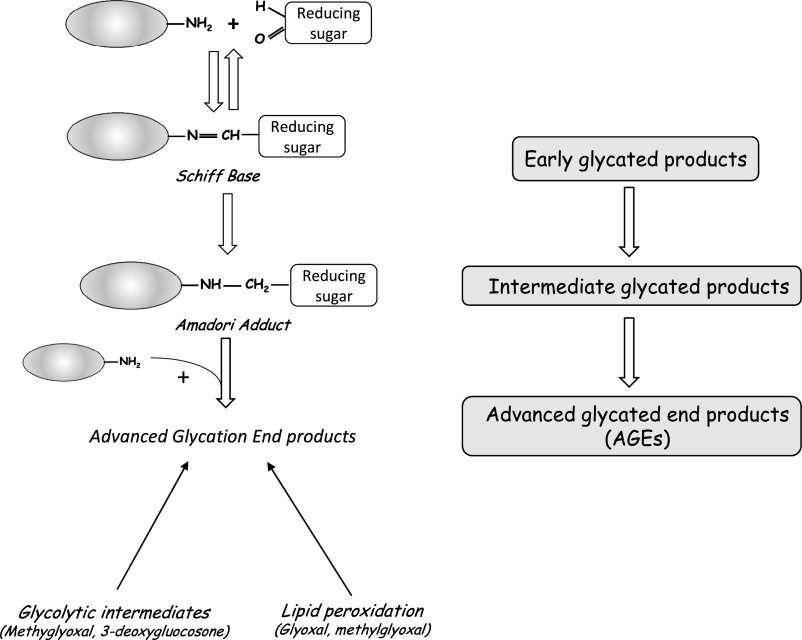
Protein glycation is initiated by a nucleophilic addition reaction between a free amino group from a protein and a carbonyl group from a reducing sugar, with the formation of an unstable, freely reversible Schiff base. This base can be rearranged to form a more stable intermediate called an Amadori product, which in the presence of a transition metal, is oxidized to yield the final AGE (Figure 1) (reviewed in ref. 3). AGEs can also be formed by autoxidation of glucose and oxidative stress. Humans are exposed to exogenous sources of AGE (diet4 and cigarette smoke5) and endogenous sources of AGE when the organism is exposed to high levels of glucose, such as in diabetes.6 AGE precursors include the 1,2-dicarbonyl compounds glyoxal and methylglyoxal (MG), a highly reactive dicarbonyl compound.7 At least 20 different types of AGE have been described: N-carboxymethyllysine (CML), pentosidine, and hydroimidazolone are among the best characterized, are relatively nonreactive, and serve as markers of AGE accumulation in several tissues.8,9 AGEs can be degraded by enzymes, such as glyoxalase I (Glo-1) and II (Glo-2).10 Glo-1 detoxifies reactive α-oxoaldehyde, removing deleterious species, such as MG.11 AGEs can also be modified by innate defense machineries, such as lysozyme, which sequesters AGEs and accelerates their renal excretion in vivo,12 and receptor-dependent uptake and degradation.13
Figure 1.
Main steps in AGE formation.
Receptors for AGEs
Advanced glycation end products receptor 1 (AGER1) binds AGEs14 and leads to their sequestration and detoxification, thus reducing AGE levels in the intracellular and extracellular spaces, resulting in antioxidant properties.15,16 Advanced glycation end product–specific receptor (RAGE), another well characterized receptor for AGEs, is a multiligand transmembrane cell surface receptor that belongs to the Ig protein superfamily17,18 and binds many ligands, including AGEs.19 In the absence of disease, RAGE is usually expressed at very low levels in various cell types (smooth muscle cells, macrophages, and endothelial cells). In several diseases, such as diabetes and autoimmune/inflammatory diseases, RAGE expression is elevated,20 whereas AGER1 levels are decreased, resulting in suppression of the antioxidant defense system and increased levels of pro-oxidant mechanisms. The soluble truncated form of RAGE lacks the full–length transmembrane domain of the receptor. Soluble advanced glycation end product–specific receptor (sRAGE) can be produced by alternative splicing (endogenous secretory advanced glycation end product–specific receptor [esRAGE]) or proteolytic cleavage mediated by metalloproteinase (sRAGE). It is consequently released into the extracellular space, where it can sequester AGEs. High sRAGE levels are associated with an increased incidence of CKD before but not after adjustment for baseline kidney function,21 suggesting that either circulating sRAGE levels are directly affected by an impaired kidney filtration as assessed by GFR or inversely, circulating sRAGE directly affects kidney function. Additional studies are needed to elucidate the mechanisms of the association between sRAGE and kidney disease.
Signaling through RAGE
RAGE was first identified as a signal transduction receptor for AGEs linked to proteins or lipids.22 RAGE can also interact with advanced oxidation protein products, supporting the hypothesis that ligands generated by oxidative stress may signal through RAGE.23 Full-length RAGE contains a single transmembrane region and a short intracellular domain. Upon binding to RAGE, AGEs activate several signaling pathways, including NF-κB, mitogen-activated protein kinases, and Jun N-terminal kinase, which in turn, regulate the transcription of proteins, such as adhesion molecules and proinflammatory chemokines. Signaling through full-length RAGE is known to be essential for both physiologic and pathologic processes.8,24–27 sRAGE acts as a decoy for RAGE ligands and modulates activation or signaling through RAGE.28
Pathophysiologic Effects of AGEs
Mechanisms
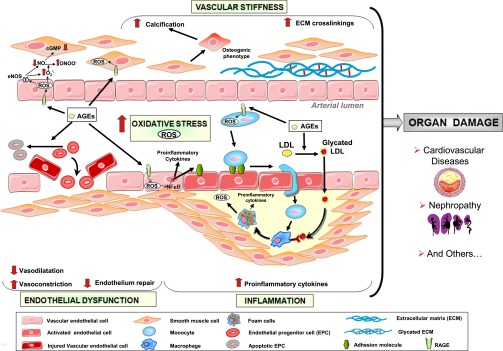
Accumulation of AGEs in patients with CKD has been shown to result from inflammation, oxidative stress, and diet (Figure 2).29,30 AGEs are proinflammatory and pro-oxidative compounds that play a role in the high prevalence of endothelial dysfunction and subsequent cardiovascular disease (CVD) in patients with CKD.31
Figure 2.
Pathophysiologic effects of AGEs. cGMP, cyclic guanosine monophosphate; ECM, extracellular matrix; eNOS, endothelial NO synthase; EPC, endothelial progenitor cell; O2−°, superoxide anion; ONOO−, peroxynitrite.
Oxidative Stress
The oxidative stress induced by reactive oxygen species (ROS) is associated with atherosclerosis and cardiovascular morbidity in patients with CKD.32 AGEs increase the levels of ROS33 through activation of NADPH oxidase34 and mitochondrial pathways in both a receptor-dependent manner (i.e., through RAGE)35 and a receptor-independent manner. In patients with type 2 diabetes mellitus, circulating AGE levels are correlated with RAGE mRNA expression and oxidative markers, such as protein carbonyl formation, advanced oxidation protein product generation, and lipid peroxidation.36 Reciprocally, high levels of ROS lead to increased levels of AGEs,37 because another cause of AGE formation in uremia is the increased oxidative stress generated by an imbalance between oxidized glutathione and GSH levels as well as changes in antioxidant systems, such as superoxide dismutase (SOD)/peroxidase.5 In fact, oxidative stress is closely linked to glycation, because GSH depletion also decreases the in situ activity of Glo-1, thereby increasing glyoxal and MG concentrations.10 Interestingly, AGER1 is downregulated by elevated AGE levels.38 Furthermore, AGEs have been shown to increase the oxidation of LDLs39—a key stage in the development of atherosclerosis.40 Glycated LDLs are, therefore, more susceptible to oxidation,41 are less effectively cleared from the circulation, and also, promote the formation of antibodies that bind AGEs localized in the vessel wall, which amplifies the development of vascular inflammation and atherosclerosis.42 Overexpression of Glo-1 in an animal model has been shown to have beneficial vascular effects, decrease ROS,43 and protect against formation of atherogenic LDL.
Numerous studies have shown that the nuclear factor erythroid 2–related factor (Nrf2) plays an important role in the antioxidant response. Under basal conditions, Nrf2 is associated in the cytoplasm with the repressor kelch like ECH-associated protein1 (Keap1), which promotes its ubiquitination for degradation.44 However, in response to oxidative stress, Nrf2 is released from Keap1 and translocates to the nucleus to induce the expression of genes encoding antioxidant and detoxifying molecules by binding to the antioxidant response element region of their promoter. Moreover, it has been shown that targeting Nrf2 ameliorates oxidative stress.45 Several studies have shown that some Nrf2 target antioxidant genes (SOD and glutathione peroxidase) are decreased in animal models of CKD46 and patients with CKD,47 which may consequently aggravate oxidative stress and inflammation. MG induces oxidative stress partly by negatively affecting Nrf2.48 Interestingly, one of the targets of the Nrf2/Keap system is Glo-1. In Nrf2−/− mice, Glo-1 mRNA is decreased, whereas MG urine excretion is increased.49 Transcriptional control of Glo-1 by Nrf2, therefore, provides protection against dicarbonyl glycation.
Inflammation
AGEs have been shown to amplify inflammatory responses in patients with CKD50,51 through RAGE,52,53 because the RAGE-AGE interaction activates the redox–sensitive transcription factor NF-κB, which leads to gene expression and the release of proinflammatory molecules, such as IL-1α, IL-6, and TNF-α.54,55 The in vitro use of human recombinant lysozyme significantly reduced AGE–induced IL-6 mRNA.56 It has recently been shown that targeting Nrf2 ameliorates inflammation by inhibiting the NF-κB pathway45 and probably, increasing Glo-1, which detoxifies reactive oxoaldehydes, such as MG. RAGE expression by activated endothelium also promotes leukocyte recruitment.57–60 Moreover, AGEs delay spontaneous apoptosis of monocytes and consequently, contribute to the development of inflammatory responses.61 A recent prospective study of patients with AKI and sepsis showed that the association of RAGE and inflammatory mediators contributes to endothelial dysfunction.62 Moreover, exposure of the endothelium to uremic plasma results in a time- and CKD stage–dependent increase in expression of monocyte chemoattractant protein-1 and IL-8, suggesting the presence of a link between systemic inflammation and uremic toxicity.63 In addition to its antioxidant properties, AGER1, by increasing the degradation of AGEs, is a negative regulator of the inflammatory response in inflammatory and parenchymal cells, such as vascular smooth muscle and glomerular mesangial cells.64
Cardiovascular Effects of AGEs
Patients with CKD have a higher CVD burden than the general population, and renal failure is associated with elevated levels of circulating AGEs.65,66 AGEs have an important cardiovascular effect, because AGE accumulation leads to endothelial dysfunction, arterial stiffness, myocardial changes, immune system dysregulation, and atherosclerosis progression. Studies of the relationship between AGEs and CVD in ESRD have yielded conflicting results.67–75 It is noteworthy that many different AGEs have been identified with widely varying tissue localization and composition, which could, therefore, explain these conflicting results. However, recent studies have shown a strong link between serum sRAGE levels and cardiovascular risk factors and disease.29,76 The main deleterious effects of AGEs in the cardiovascular system are described below.
Endothelial Dysfunction
Endothelial dysfunction, an early marker of atherosclerosis,77 is predictive of the occurrence of cardiovascular events. In CKD, endothelial dysfunction begins early in the course of the disease independently of traditional cardiovascular risk factors.78
Plasma levels of endothelial cell activation markers are elevated in advanced CKD, and exposure of the endothelium to uremic plasma increases soluble vascular cell adhesion molecule-1 (VCAM-1) expression.63 AGE-RAGE–induced oxidative stress is central to VCAM-1 induction, because anti-RAGE antibodies, sRAGE, and N-acetylcysteine inhibit VCAM-1 expression.79 It has recently been shown that chronic CML ingestion induced RAGE–dependent endothelial dysfunction in mice.80 Furthermore, Glo-1 overexpression decreases endothelial dysfunction in a diabetic rat model.81
In patients with CKD, endothelial dysfunction results from increased endothelial injury and decreased endothelial repair82 caused by a decreased endothelial progenitor cell (EPC) count.83–89 Furthermore, circulating EPC levels in patients with ESRD are negatively correlated with skin autofluorescence (a marker of tissue-bound AGEs) but not with serum pentosidine levels.89,90 Recent studies have shown that AGEs impair EPC survival, differentiation, migration, and function.91–93
One of the endothelium’s most important functions is the synthesis and release of endothelium–derived relaxing factors, such as nitric oxide (NO) and prostaglandin I2 (PGI2). Patients with CKD display impaired endothelium–dependent vasodilation.94 AGEs have been shown to impair NO production partly by downregulating NO synthase34,39,95 through increased mRNA degradation.96 In vitro studies show that sera from patients with CKD suppress endothelial NO synthase activity in cultured vascular endothelial cells.97,98 This inhibition of endothelial NO synthase activity is mediated by RAGE activation of peripheral mononuclear cells in patients with CKD.31 AGEs also inhibit NO by increasing NADPH oxidase expression, establishing a link between RAGE and chronic endothelial dysfunction.34,39,95 PGI2 has also been shown to be decreased by AGEs, such as glycated albumin in cultured microvascular endothelial cells.99
Endothelial dysfunction is defined as an imbalance between vasodilating and vasoconstricting substances produced by or acting on the endothelium. The intracellular signaling molecule endothelin-1 (ET-1), best known as a potent endogenous vasoconstrictor peptide produced by endothelial cells, has an important role in the vasculature and is involved in the development of atherosclerosis.100 Serum levels of ET-1 are markedly elevated in patients with chronic renal failure, 101,102 and ET-1 is involved in both the development and progression of CKD.103 Odetti et al.101 observed a significant positive correlation between pentosidine and ET-1 plasma levels in subjects undergoing chronic hemodialysis (HD). Recently, it has been shown that ET-1 transcription in cultured bovine aortic endothelial cells is controlled by the AGE–inducible, redox–sensitive transcription factor NF-κB.104 AGEs, therefore, play an important role in endothelial dysfunction by (1) decreasing levels of two important endothelium–dependent relaxing factors (NO and PGI2) and (2) increasing endothelial production of the potent vasoconstrictor ET-1.104
Another important role of the endothelium is its function as a selective barrier between the blood and the surrounding tissues. AGEs have been shown to be associated with increased permeability of the endothelial layer.105,106
Arterial Stiffness
Arterial stiffening occurs because of loss of compliance of the vessel wall107,108 and is an important independent risk factor for cardiovascular mortality in patients with CKD.
CKD not only accelerates the development of atherosclerosis but also, leads to excessive vascular calcification resulting from the inflammation and oxidative stress present in patients with CKD.109–113 Vascular calcification is an independent predictor of cardiovascular mortality,114,115 and one of its consequences is altered aortic compliance.116 AGE levels117 as well as tissue AGEs118 are associated with vascular calcification in patients with CKD, suggesting that increased AGE may contribute to vascular calcification. It has recently been shown, in an animal model, that AGE-induced calcification is mediated by RAGE and oxidative stress.119 Moreover, lower sRAGE levels were recently associated with carotid plaque calcification.76
Arterial stiffness has a major role not only in the increase in systolic BP and pulse pressure, but also in the decrease in diastolic BP. This BP alteration leads to left ventricular hypertrophy and abnormal pulse wave velocity (PWV), both of which are apparent at the early stages of CKD.120 Measurement of PWV, a noninvasive method for assessing arterial wall stiffness related to vascular calcification,121 is a strong independent predictor of cardiovascular mortality in patients with ESRD.122,123 Several studies suggest that AGEs are involved in arterial stiffness.124 For example, chronic CML ingestion induced RAGE–dependent arterial stiffness in mice.80 Zhou et al.125 showed a positive correlation between arterial stiffness and serum pentosidine levels. Moreover, skin autofluorescence (a marker of AGE accumulation) is associated with arterial PWV (and therefore, arterial stiffness) in patients with ESRD.126 This may be caused by the formation of irreversible cross-links between the end products and long–lived structural proteins, such as collagen and elastin.
Immune System Dysregulation
The immune system plays an important role in the development and progression of CVD. It has been shown that dysregulation of the immune system contributes to atherosclerosis in patients on HD.127,128 Several studies have suggested that AGEs can amplify local immune reactions in the kidney. Other studies in humans have shown that AGE formation and accumulation are partially responsible for the observed immune system dysregulation. Friedlander et al.129 showed a positive association between serum pentosidine and monocyte activation, which may contribute to elevated complication rates. It has also been shown that decreased AGE intake protects against loss of innate immunity.130
Diabetic Nephropathy
Diabetic nephropathy (DN) is the leading cause of ESRD and the most common indication for RRT.131 The kidney has an important role in the metabolism of AGEs, because renal proximal tubule cells absorb AGEs from the glomerular filtrate and catabolize them.132,133 GFR is negatively correlated with not only serum AGE levels but also, RAGE mRNA expression in PBMCs.31 Lysozyme that sequesters AGEs reduces the severity of the early manifestations of DN,56 suggesting that AGEs play an important role in the pathogenesis of DN.134,135 Immunohistochemical studies in patients with DN have shown that AGEs accumulate in the mesangium and glomerular capillary wall.136 Horie et al.137 showed that patients with advanced DN exhibit enhanced CML accumulation in the expanded mesangial matrix and a thicker glomerular capillary wall in early nodular lesions and arterial walls (relative to the healthy kidney). Similar results were found with the AGE imidazole.138
Accumulation of AGEs may be caused by both decreased clearance and increased endogenous AGE formation or higher dietary intake.139 In addition to glomerular accumulation of AGES in DN, the AGE-RAGE interaction can also trigger premature cell senescence, a key process in DN, in response to endoplasmic reticulum stress, partly through p21 signaling activation.140
Therapeutic Interventions
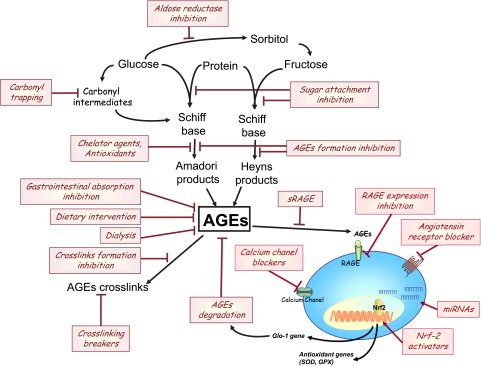
In view of the many harmful effects of AGEs on cell function, it is essential to develop strategies designed to counteract their effects. Several pharmacologic treatment strategies targeting the AGE-RAGE system have been studied in vitro and in vivo for their potential to prevent AGE formation or local AGE accumulation.141 These therapeutic compounds can be divided into several classes as a function of their mechanism of action (Figure 3, Table 1): AGE absorption inhibitors, AGE formation inhibitors, AGE cross–link breakers, RAGE antagonists, and AGE binders, such as sRAGE. Although many compounds are currently being studied, only a few have entered clinical trials, and none have yet been approved for clinical use in patients with CKD.
Figure 3.
Potential strategies for counteracting the effects of AGEs. GPX, glutathione peroxidase; miRNA, microRNA.
Table 1.
Clinical studies to decrease levels of AGEs in patients with CKD
| Therapy and Ref. | Patient Characteristics | Design | Outcome Measures | Results |
|---|---|---|---|---|
| Decreasing exogenous sources of AGEs | ||||
| Dietary restriction | ||||
| 130 | 9 Patients with stage 3 CKD | Interventional study: an isocaloric low–AGE (30%–50% reduction) or regular diet for 4 wk | AGEs: CML, MG, and VCAM-1; PMNC: RAGE and AGER1; OS: 8-isoprostanes | Reduction of AGEs (CML and MG) inflammation and oxidative stress; restore levels of AGER1 and protect against immune dysfunction |
| 143 | 26 Patients without diabetes and with renal failure on maintenance PD (18 completed the study) | Prospective and randomized 4-wk study: high- or low-AGE diet | AGEs: CML and MG | Reduction of AGEs (CML and MG), CML LDL, CML ApoB, dialysate CML, and MG |
| Gastrointestinal absorption inhibition | ||||
| Oral adsorbent AST-120 | ||||
| 144 | 10 Patients with CRF and chronic GN (n=8) or nephrosclerosis (n=2) | 2 g AST-120 three times per day for 3 mo | Serum levels of AGEs (CML) before and after AST-120 treatment | Significant decrease of serum AGEs in patients without diabetes and with CRF |
| SC | ||||
| 153 | Patients with diabetes and stages 2–4 CKD | Single–center, randomized, 2-mo, open–label, intention to treat, cross–over study comparing SC with CC treatment | AGEs: CML and MG; OS: 8-isoprostanes | Decrease CML and MG levels, oxidative stress, and inflammation and increase AGER1 expression |
| 154 | 183 Adult patients on maintenance HD | Randomized trial with parallel-group design: 12 mo of treatment with SC (n=91) or CC (n=92) | CACS; AGEs: pentosidine at study entry, 6 mo, and study completion | Decrease calcification and suppress pentosidine accumulation |
| 159 | 117 Patients with type 2 diabetes mellitus and stages 2–4 DKD | Two–center, randomized, intention to treat, open–label study comparing SC (n=57) with CC treatment (n=60) | Antioxidants: RAGE1, sirtuin1, and Nrf2; pro-oxidants: AGEs (CML and MG), RAGE, and 8-isoprostanes | SC treatment: reduce AGE levels, improve innate defense homeostasis, improve inflammation, and reduce chronic OS |
| Decreasing endogenous sources of AGEs | ||||
| Dialysis | ||||
| 163 | 32 Patients on chronic HD (21 with normoglycemia and 11 with diabetes) and 11 patients without diabetes and with uremia not yet on dialysis | Cross-sectional study: comparison group of ESRD with patients not yet on dialysis; comparison of two different dialysis schedules: 4 h three times per week (SHD) and 2 h six times per week (DHD) | Measurement of glycation indexes on plasma by HPLC: furosine, bound and free pentosidine, and two classes of LMM AGE peptides | DHD: better control of AGE produced in ESRD |
| 164 | 10 Patients with ESRD | Treatment: three times each with DIAPES and HF60, two different synthetic, high–flux HD membranes | Kinetics of AGE removal by fluorescence spectroscopy and ELISA | Both high-flux dialyzers were equally effective to remove low–molecular AGE products |
| 166 | 126 Patients on HD | 29 Patients on HD with low-flux cellulose, 57 patients with high-flux polysulfone, 25 patients with polymenthylmethacrylate, and 15 patients with AN69 | Protein–linked and free plasma pentosidine by HPLC | Similar clearance of free pentosidine with all membranes; reduction levels of protein-linked pentosidine to control levels for three patients who were switched from AN69 to polysulfone |
| 167 | 81 Patients with chronic uremia | Three groups: conventional HD, high-flux HD, and online HDF | Serum AGE levels by ELISA pre- and postdialysis and after 6 mo; AGE clearance | Significant reduction in predialysis serum AGE levels for patients with uremia treated with online HDF for >6 mo |
| Kidney transplantation | ||||
| 183 | HD, CAPD, and renal transplantation | Clearance of Palb and Pfree by different modalities of RRT | Plasma levels of Palb and Pfree | Renal transplantation: best therapeutic modality to normalize both Palb and Pfree levels |
| 184 | 10 Patients on dialysis, 8 patients with renal transplants, 7 controls, 8 patients with mild renal insufficiency and without diabetes | Immunohistochemical study on cardiac tissues from autopsies | Immunohistochemistry with antibodies against CML-modified AGE | Reduction of AGE accumulation in cardiac tissues in patients with renal transplants compared with patients on dialysis |
| 185 | 66 Patients with renal transplants, 1707 patients with CKD (stage 3), and 115 patients on dialysis (53 on HD and 62 on PD) | Comparison between three groups of patients | Tissue AGEs by skin autofluorescence | Reduction of tissue AGE accumulation in patients with renal transplants compared with patients on dialysis and similar to patients with CKD stage 3 |
| Other approaches | ||||
| Calcium channel blockers | ||||
| 221 | 30 Patients with hypertension and nondiabetic stage 1 or 2 CKD | Single–center, randomized, 6-mo, comparison of treatment with azelnidipine (16 mg daily) and amlodipine (5 mg daily) | sRAGE, AGEs OS: urinary 8-OH dG | Azelnidipine treatment but not amlodipine decreases circulating AGEs and sRAGE |
| Antidiabetic drugs | ||||
| 222 | 66 Patients with type 2 diabetes | 6-mo Open–label study: metformin (n=22), pioglitazone (n=22), or control (n=22) | Serum pentosidine at baseline and 6 mo after each treatment | Serum pentosidine levels significantly decreased at 6 mo after treatments in both groups |
| 223 | 64 Patients with type 2 diabetes | Randomized, open–label, parallel group study: comparison of 6-mo treatments with rosiglitazone or sulfonylurea | Serum total sRAGE and esRAGE (before and after 6 mo of treatment) | Increase of circulating sRAGE and esRAGE levels in the rosiglitazone group; significant reduction of AGEs levels in both groups |
| Statins | ||||
| 225 | 70 Patients with type 2 diabetes enlisted to undergo carotid endarterectomy | Simvastatin (40 mg daily; n=35); diet alone (n=35) | Simvastatin inhibits plaque RAGE expression by decreasing MPO–dependent AGE generation |
SC, sevelamer carbonate; CRF, chronic renal failure; DKD, diabetic kidney disease; CAPD, continuous ambulatory peritoneal dialysis; CC, calcium carbonate; SHD, standard hemodialysis; DHD, daily hemodialysis; DIAPES; HF60; AN69; HDF, hemodiafiltration; Palb, albumin-linked pentosidine; Pfree, free form of pentosidine; PMNC, peripheral mononuclear cells; OS, oxidative stress; CACS, coronary artery calcification score; LMM, low molecular mass; 8-OH dG, 8 hydroxy deoxyguanosine; esRAGE, endogenous secretory advanced glycation end product–specific receptor; ApoB, apolipoprotein B; MPO, myeloperoxidase.
Decreasing Exogenous Sources of AGEs
Decreased Dietary AGE Intake
Dietary AGE content is an important contributor to serum AGE accumulation in patients with CKD.142 As mentioned above, AGEs are generated during the thermal processing and storage of foods.4 A recent clinical study in patients with CKD showed that reducing dietary AGEs may lower oxidative stress and inflammation, restore AGER1 levels, and protect against innate immune dysfunction.130 Dietary restriction is, therefore, an effective, feasible, and economic method to reduce the levels of toxic AGEs and possibly, the associated cardiovascular mortality.143
Inhibition of Gastrointestinal Absorption
Another option to reduce the intake of exogenous AGEs is to block or inhibit the gastrointestinal absorption of dietary AGEs. Several compounds have been widely studied, including the spherical carbon adsorbent AST-120 and sevelamer carbonate.
Administration of the oral adsorbent AST-120 before initiating dialysis has been shown to not only improve the survival rate in patients on HD144,145 but also, delay the onset of HD in patients with CKD.146 AST-120 is also associated with a reduction in carotid intima media thickness and arterial stiffness in patients with CKD and without diabetes.147 However, recent randomized placebo–controlled Evaluating Prevention of Progression In CKD (EPPIC) Trials of AST-120 in CKD did not support the benefit of adding AST-120 to standard therapy.148 AST-120 has been shown to bind AGE and effectively decrease plasma AGE levels.144 In a recent animal study, AST-120 was shown to reduce the indoxyl sulfate–induced decrease of Nrf2,149 which may, therefore, decrease formation of AGEs, such as MG, by increasing Glo-1.
Sevelamer carbonate is a nonabsorbable, noncalcium–based compound frequently used to lower blood phosphorus levels in patients with advanced CKD or ESRD. It has recently been shown that sevelamer can act as a pleiotropic drug by sequestering cytotoxic AGEs in the gut and thus, preventing their uptake. Moreover, sevelamer decreases CML levels, oxidative stress, and inflammation and increases AGER1 expression.150–153 The results of a randomized trial in patients on HD showed that sevelamer slows calcification and suppresses pentosidine accumulation.154 Although long–term clinical studies in patients with CKD have not yet been performed, a short-term comparison of sevelamer with calcium carbonate showed that it lowered serum AGEs in patients on HD.150 Sirtuin1, an NAD+–dependent histone deacetylase that has anti–inflammatory, antiapoptotic, and antioxidant properties155 and prevents calcification156 and endothelial senescence,157 is decreased by AGEs, such as MG.158 In a recent clinical study in patients with type 2 diabetes mellitus and stages 2–4 diabetic kidney disease, sevelamer significantly increased antioxidant molecules, such as AGER1, Nrf2, and Sirtuin1, while decreasing pro–oxidant molecules, such as RAGE and AGEs.159
Decreasing Endogenous Sources of AGEs
Dialysis
Several dialysis techniques are currently used, such as HD, hemofiltration (HF), hemodiafiltration, and peritoneal dialysis (PD).
A conventional HD session typically reduces blood AGE levels by ≤20%, and only low–molecular mass AGEs (<10-kDa AGE peptide) are efficiently removed from the blood.160 However, this efficiency can be enhanced by increasing the frequency or duration of HD sessions.161–163 In HF, molecules are removed from blood by ultrafiltration of a large blood volume under high hydrostatic pressure through a larger–pore size membrane. Membranes with high permeability can, therefore, decrease blood AGEs by up to 60%164 and up to 80% with high-flux polysulfone.165,166 Lin et al.167 showed that online hemodiafiltration, a combination of both HF and HD, for >6 months in patients with uremia leads to a significant reduction in serum AGE levels. A recent study showed that convective therapies not only effectively cleared uremic toxins but also, decreased levels of inflammatory markers, such as IL-6.168
PD, an important alternative to HD, also reduces AGEs formation.169,170 Advantageously, serum concentrations of AGEs, such as pentosidine, are lower in patients on PD than in patients on HD.171 However, despite higher levels of AGE excretion in patients on PD, free AGE levels in the PD effluent were elevated because of increased synthesis of free AGEs. Conventional PD fluid contains supraphysiologic concentrations of glucose and glucose degradation products (GDPs; generated during sterilization by heating of dialysis fluid) that can increase the formation of AGEs.172–174 In patients on PD, high levels of AGE deposition in tissue correlate with glucose exposure.175 Nonglucose PD solutions or solutions containing low-GDP levels, such as icodextrin, may, therefore, minimize AGE formation.176–179 The acidic pH and the hyperosmolality of conventional PD fluid can also damage the peritoneum and consequently, impair its function as a dialyzing membrane. Other alternatives (such as neutral pH and low–GDP peritoneal solutions180) can also be used to decrease AGE formation during conventional PD.181
Continuous flow PD is another option that also effectively reduces AGE formation.170
Kidney Transplantation
Because AGEs are mainly excreted by the kidneys, serum AGE levels increase with declining kidney function.182 Kidney transplantation is, therefore, an efficient way of decreasing levels of uremic toxins, including AGEs. Thus, pentosidine levels started to fall within 4 weeks of kidney transplantation and reached normal values after 6 months.183 Studies showed that kidney transplantation decreases AGE accumulation in tissues,184,185 which was assessed by skin autofluorescence, thereby significantly reducing cardiovascular events in recipients of transplants compared with patients on dialysis.185
Antioxidants
To decrease endogenous sources of AGEs, several compounds have been developed to attenuate glyoxidation and/or oxidative stress by sequestration of metal ions, reactive dicarbonyl compounds, ROS, and reactive nitrogen species. Thus, the use of antioxidants, such as vitamin E, vitamin A, and lipoic acid, has been studied in vitro to counteract AGE effects.186
Reactive Dicarbonyl Scavengers
Several compounds can trap reactive carbonyl intermediates (AGE precursors) and quench ROS.187,188 For example, the strongly nucleophilic Pimagedine (2-aminoguanidine) can scavenge reactive carbonyl intermediates in the Maillard reaction189,190 and also, decrease VCAM-1 expression in endothelial cells.191 Administration of aminoguanidine to patients with ESRD also led to a reduction of circulating LDL levels.39
The vitamin B6 derivative pyridoxamine (registered as Pyridorin) is a post-Amadori inhibitor192 that traps reactive carbonyl and dicarbonyl compounds derived from Amadori compounds, thereby reducing AGE accumulation.193,194 Pyridoxamine also scavenges ROS and chelates metal ions (oxidation catalysts). In diabetic rats, pyridoxamine has been shown to decrease AGE and calcification.195 Polizzi et al.196 showed that a combination of vitamins B1 and B6 decreases DNA glycation in patients with DN. However, an 8-week randomized, placebo–controlled study in 50 patients on HD found that the combination of pyridoxine and thiamine had no effect on plasma levels of AGEs and pentosidine.197
Renin-Angiotensin System Inhibitors
The renin-angiotensin system plays an important role in the disease processes that leads to DN.198 Inhibitors of the renin-angiotensin axis can improve glomerular hyperfiltration and are known to decrease the severity of DN in animal and clinical studies.199 Several angiotensin receptor blockers (including olmesartan, telmisartan,200,201 and irbesartan202) have been shown to decrease plasma CML and pentosidine levels in patients on HD203 and act as antioxidants against AGE.202 Sebeková et al.204 found that the angiotensin–converting enzyme inhibitor ramipril significantly decreased levels of fluorescent AGEs but not CML.
Aldose Reductase Inhibitors
Aldose reductase is a multifunctional enzyme that reduces aldehydes, and it is involved in the polyol pathway of AGE formation. Inhibiting aldose reductase, therefore, reduces levels of AGE precursors and decreases AGE accumulation.205 Administration of the aldose reductase inhibitor eparlestat decreases serum AGE levels in patients with diabetes.206
Increasing the Breakdown of AGEs
Agents that break AGE cross-links (reviewed in ref. 207) have been shown to improve arterial compliance in humans.208 Animal and human studies have shown that AGE cross–link breakers, such as ALT-711 and alagebrium, decrease AGE levels209,210 and tissue AGEs,211,212 reverse their effects,213–216 such as aortic stiffness, calcification,195 and extracellular matrix accumulation,212 and improve renal function by facilitating urinary excretion of the end products.
RAGE Inhibitors
Many different animal studies have shown that RAGE blockade by decreasing RAGE expression215,217,218 or RAGE competition reduces oxidative stress195 and endothelial dysfunction. Thus, sRAGE prevents the development of structural and functional characteristics of nephropathy in db/db mice.29,219 Recently, a receptor-based bioadsorbent designed to target AGE gave promising results to selectively deplete serum AGEs from human blood.220 This sRAGE–based extracorporeal therapy could be useful to selectively decrease serum AGEs and decrease inflammation in patients with diabetes and/or CKD.
Other Approaches
Other therapeutic approaches, such as calcium channel blockers,221 antidiabetic drugs,93,217,222,223 statins,224,225 and lysozyme,56,226 have also been used. Recent studies have also described the use of alternative medicines with antiglycation activities,227,228 such as extracts from certain plants like Moutan cortex229–231 and Azadirachta indica,232 which exert renoprotective effects by inhibition of the AGE-RAGE axis (Table 1). This section will focus on emerging therapeutic approaches, such as microRNAs (miRs) and Nrf2 and Glo-1 inducers.
MiRs
There is growing evidence that miRs play a key role in kidney physiology and contribute to both the induction and progression of CKD.233,234 miRs are ubiquitously expressed short noncoding RNAs comprising 20–22 nucleotides that regulate key biologic pathways and cellular functions by inhibiting gene expression through post-transcriptional repression of their target mRNAs.235 For example, miR21 has been shown to be a key therapeutic target for renal injury in a mouse model of type 2 diabetes.236 Several other miRs have also been implicated in the process of vascular calcification.111 Interestingly, recent studies have shown the involvement of miRs in the regulation of AGE/RAGE signaling (reviewed in ref. 237). For example, miR200b/miR200c and miR223 levels have been shown to be reduced in AGE–induced endothelial cell apoptosis.238,239 AGEs also strongly increased miR214 in monocytes from patients with chronic renal failure, which in turn, abolished AGE–induced cell survival,61 thereby limiting the inflammatory response. Identifying specific miRs that target the AGE-RAGE axis is, therefore, of interest to correct their expression by in vivo delivery of miR mimics to restore miR levels or inhibitors to block miR function. Chen et al.240 have recently shown that miR29b, which inhibits DN in db/db mice, was downregulated in response to AGEs and that its overexpression by gene therapy attenuated diabetic kidney disease. miRs have consequently emerged as a promising novel therapeutic strategy for the treatment of CKD. However, miR delivery and safety require additional investigation before clinical application.
Nrf2 and Glo-1 Inducers
Nrf2 signaling has been shown to have a protective role in renal injuries. Several Nrf2 target antioxidant genes, such as SOD, are decreased in CKD. Pharmacologic interventions activating Nrf2 could, therefore, be beneficial to protect the kidney in CKD, partly by ameliorating oxidative stress.45 Because another target of Nrf2 is Glo-1,49 targeting Nrf2 can also decrease AGEs, such as MG. Naturally occurring Nrf2 activators have been described in animal studies (reviewed in ref. 241). For example, ankaflavin, a natural peroxisome proliferator-activated receptor γ agonist, has been shown to upregulate Nrf2 and protect against MG-induced diabetes in vivo.242 Monascin, obtained from Monascus-fermented products, has recently been shown to possess anti–inflammatory and antioxidant properties by decreasing RAGE and increasing heme oxigenase 1 by upregulation of Nrf2.48,243 Dimerumic acid, another product extracted from Monascus, has an inhibitory effect on CML–induced RAGE signals by Nrf2-mediated attenuation of oxidative stress in hepatic cells.244 It also decreases hepatic Glo, thereby decreasing serum and hepatic AGEs in MG-treated mice.245 These widely used plant–derived Nrf2 activators have been shown to be safe with shown health benefits in human subjects, suggesting that they could be beneficial for the treatment of CKD. Bardoxolone methyl, a synthetic Nrf2 activator, has been tested as a therapeutic agent in clinical trials.246 Despite promising results in phase II trials, a recent study showed that it contributes to higher rates of cardiovascular events in susceptible patients with an increased risk of heart failure at baseline.247 Additional studies are, therefore, needed to evaluate the safety of these compounds. However, Nrf2 activators seem to be a promising new therapeutic strategy to improve or delay kidney dysfunction.
Conclusion
AGEs have been widely described in hyperglycemic conditions, such as diabetes; however, they can also be generated in conditions associated with elevated levels of oxidative stress, such as CKD. The kidney has an important role in AGE metabolism, because CKD is associated with AGE accumulation with declining kidney function. In this review, we describe the mechanisms of AGE formation and the various pathologic effects of AGEs. The experimental data available to date emphasize the harmful effects of AGEs on vascular function (with endothelial dysfunction, elevated oxidative stress, and arterial stiffness) and the immune system. We also describe the various potential therapeutic strategies designed to decrease exogenous and endogenous sources of AGE precursors or increase the breakdown of AGEs. A large body of evidence has shown that the interaction between AGEs and RAGE plays a key role in vascular damage. Inhibiting the AGE-RAGE axis by means of RAGE inhibitors could become a novel therapeutic strategy. Of interest are the recent new potential therapeutic strategies emerging from in vitro and in vivo studies, such as Nrf2 and Glo-1 inducers as well as miRs regulation.
We provide an overview of the pathobiology of AGEs and information on how AGE accumulation is involved in vascular damage in patients with CKD. Additional preclinical and clinical studies are, nevertheless, needed to (1) determine the relevance of targeting AGEs to reduce the complications of CKD and (2) identify new therapeutic interventions that may reduce or delay morbidity and mortality in this population.
Disclosures
H.V. and G.E.S. received support for an investigator-initiated trial of Sevelamer in the management of diabetic kidney disease from Sanofi.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Maillard L: Action of amino acids on sugars. Formation of melanoidins in a methodical way. Compt Rend 154: 66–68, 1912 [Google Scholar]
- 2.Cho SJ, Roman G, Yeboah F, Konishi Y: The road to advanced glycation end products: A mechanistic perspective. Curr Med Chem 14: 1653–1671, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Vistoli G, De Maddis D, Cipak A, Zarkovic N, Carini M, Aldini G: Advanced glycoxidation and lipoxidation end products (AGEs and ALEs): An overview of their mechanisms of formation. Free Radic Res 47[Suppl 1]: 3–27, 2013 [DOI] [PubMed] [Google Scholar]
- 4.Poulsen MW, Hedegaard RV, Andersen JM, de Courten B, Bügel S, Nielsen J, Skibsted LH, Dragsted LO: Advanced glycation endproducts in food and their effects on health. Food Chem Toxicol 60: 10–37, 2013 [DOI] [PubMed] [Google Scholar]
- 5.Mallipattu SK, He JC, Uribarri J: Role of advanced glycation endproducts and potential therapeutic interventions in dialysis patients. Semin Dial 25: 529–538, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Semba RD, Nicklett EJ, Ferrucci L: Does accumulation of advanced glycation end products contribute to the aging phenotype? J Gerontol A Biol Sci Med Sci 65: 963–975, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thornalley PJ: Pharmacology of methylglyoxal: Formation, modification of proteins and nucleic acids, and enzymatic detoxification--a role in pathogenesis and antiproliferative chemotherapy. Gen Pharmacol 27: 565–573, 1996 [DOI] [PubMed] [Google Scholar]
- 8.Piperi C, Adamopoulos C, Dalagiorgou G, Diamanti-Kandarakis E, Papavassiliou AG: Crosstalk between advanced glycation and endoplasmic reticulum stress: Emerging therapeutic targeting for metabolic diseases. J Clin Endocrinol Metab 97: 2231–2242, 2012 [DOI] [PubMed] [Google Scholar]
- 9.Arsov S, Graaff R, van Oeveren W, Stegmayr B, Sikole A, Rakhorst G, Smit AJ: Advanced glycation end-products and skin autofluorescence in end-stage renal disease: A review. Clin Chem Lab Med 52: 11–20, 2014 [DOI] [PubMed] [Google Scholar]
- 10.Abordo EA, Minhas HS, Thornalley PJ: Accumulation of alpha-oxoaldehydes during oxidative stress: A role in cytotoxicity. Biochem Pharmacol 58: 641–648, 1999 [DOI] [PubMed] [Google Scholar]
- 11.Vander Jagt DL, Hunsaker LA: Methylglyoxal metabolism and diabetic complications: Roles of aldose reductase, glyoxalase-I, betaine aldehyde dehydrogenase and 2-oxoaldehyde dehydrogenase. Chem Biol Interact 143-144: 341–351, 2003 [DOI] [PubMed] [Google Scholar]
- 12.Zheng F, Cai W, Mitsuhashi T, Vlassara H: Lysozyme enhances renal excretion of advanced glycation endproducts in vivo and suppresses adverse age-mediated cellular effects in vitro: A potential AGE sequestration therapy for diabetic nephropathy? Mol Med 7: 737–747, 2001 [PMC free article] [PubMed] [Google Scholar]
- 13.Vlassara H, Uribarri J, Cai W, Striker G: Advanced glycation end product homeostasis: Exogenous oxidants and innate defenses. Ann N Y Acad Sci 1126: 46–52, 2008 [DOI] [PubMed] [Google Scholar]
- 14.Vlassara H, Brownlee M, Cerami A: Novel macrophage receptor for glucose-modified proteins is distinct from previously described scavenger receptors. J Exp Med 164: 1301–1309, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yan SF, Ramasamy R, Schmidt AM: Mechanisms of disease: Advanced glycation end-products and their receptor in inflammation and diabetes complications. Nat Clin Pract Endocrinol Metab 4: 285–293, 2008 [DOI] [PubMed] [Google Scholar]
- 16.Cai W, He JC, Zhu L, Chen X, Striker GE, Vlassara H: AGE-receptor-1 counteracts cellular oxidant stress induced by AGEs via negative regulation of p66shc-dependent FKHRL1 phosphorylation. Am J Physiol Cell Physiol 294: C145–C152, 2008 [DOI] [PubMed] [Google Scholar]
- 17.Stern DM, Yan SD, Yan SF, Schmidt AM: Receptor for advanced glycation endproducts (RAGE) and the complications of diabetes. Ageing Res Rev 1: 1–15, 2002 [DOI] [PubMed] [Google Scholar]
- 18.Fritz G: RAGE: A single receptor fits multiple ligands. Trends Biochem Sci 36: 625–632, 2011 [DOI] [PubMed] [Google Scholar]
- 19.Rojas A, Delgado-López F, González I, Pérez-Castro R, Romero J, Rojas I: The receptor for advanced glycation end-products: A complex signaling scenario for a promiscuous receptor. Cell Signal 25: 609–614, 2013 [DOI] [PubMed] [Google Scholar]
- 20.Ramasamy R, Yan SF, Schmidt AM: Advanced glycation endproducts: From precursors to RAGE: Round and round we go. Amino Acids 42: 1151–1161, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rebholz CM, Astor BC, Grams ME, Halushka MK, Lazo M, Hoogeveen RC, Ballantyne CM, Coresh J, Selvin E: Association of plasma levels of soluble receptor for advanced glycation end products and risk of kidney disease: The Atherosclerosis Risk in Communities study. Nephrol Dial Transplant 30: 77–83, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ramasamy R, Yan SF, Schmidt AM: Receptor for AGE (RAGE): Signaling mechanisms in the pathogenesis of diabetes and its complications. Ann N Y Acad Sci 1243: 88–102, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.D’Agati V, Schmidt AM: RAGE and the pathogenesis of chronic kidney disease. Nat Rev Nephrol 6: 352–360, 2010 [DOI] [PubMed] [Google Scholar]
- 24.Xie J, Méndez JD, Méndez-Valenzuela V, Aguilar-Hernández MM: Cellular signalling of the receptor for advanced glycation end products (RAGE). Cell Signal 25: 2185–2197, 2013 [DOI] [PubMed] [Google Scholar]
- 25.Ott C, Jacobs K, Haucke E, Navarrete Santos A, Grune T, Simm A: Role of advanced glycation end products in cellular signaling. Redox Biol 2: 411–429, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nedić O, Rattan SI, Grune T, Trougakos IP: Molecular effects of advanced glycation end products on cell signalling pathways, ageing and pathophysiology. Free Radic Res 47[Suppl 1]: 28–38, 2013 [DOI] [PubMed] [Google Scholar]
- 27.Franko B, Brault J, Jouve T, Beaumel S, Benhamou PY, Zaoui P, Stasia MJ: Differential impact of glucose levels and advanced glycation end-products on tubular cell viability and pro-inflammatory/profibrotic functions. Biochem Biophys Res Commun 451: 627–631, 2014 [DOI] [PubMed] [Google Scholar]
- 28.Ding Q, Keller JN: Evaluation of rage isoforms, ligands, and signaling in the brain. Biochim Biophys Acta 1746: 18–27, 2005 [DOI] [PubMed] [Google Scholar]
- 29.Park L, Raman KG, Lee KJ, Lu Y, Ferran LJ, Jr., Chow WS, Stern D, Schmidt AM: Suppression of accelerated diabetic atherosclerosis by the soluble receptor for advanced glycation endproducts. Nat Med 4: 1025–1031, 1998 [DOI] [PubMed] [Google Scholar]
- 30.Vlassara H, Fuh H, Makita Z, Krungkrai S, Cerami A, Bucala R: Exogenous advanced glycosylation end products induce complex vascular dysfunction in normal animals: A model for diabetic and aging complications. Proc Natl Acad Sci U S A 89: 12043–12047, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Linden E, Cai W, He JC, Xue C, Li Z, Winston J, Vlassara H, Uribarri J: Endothelial dysfunction in patients with chronic kidney disease results from advanced glycation end products (AGE)-mediated inhibition of endothelial nitric oxide synthase through RAGE activation. Clin J Am Soc Nephrol 3: 691–698, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Massy ZA, Nguyen-Khoa T: Oxidative stress and chronic renal failure: Markers and management. J Nephrol 15: 336–341, 2002 [PubMed] [Google Scholar]
- 33.Yan SD, Schmidt AM, Anderson GM, Zhang J, Brett J, Zou YS, Pinsky D, Stern D: Enhanced cellular oxidant stress by the interaction of advanced glycation end products with their receptors/binding proteins. J Biol Chem 269: 9889–9897, 1994 [PubMed] [Google Scholar]
- 34.Wautier MP, Chappey O, Corda S, Stern DM, Schmidt AM, Wautier JL: Activation of NADPH oxidase by AGE links oxidant stress to altered gene expression via RAGE. Am J Physiol Endocrinol Metab 280: E685–E694, 2001 [DOI] [PubMed] [Google Scholar]
- 35.Ramasamy R, Vannucci SJ, Yan SS, Herold K, Yan SF, Schmidt AM: Advanced glycation end products and RAGE: A common thread in aging, diabetes, neurodegeneration, and inflammation. Glycobiology 15: 16R–28R, 2005 [DOI] [PubMed] [Google Scholar]
- 36.Chawla D, Bansal S, Banerjee BD, Madhu SV, Kalra OP, Tripathi AK: Role of advanced glycation end product (AGE)-induced receptor (RAGE) expression in diabetic vascular complications. Microvasc Res 95: 1–6, 2014 [DOI] [PubMed] [Google Scholar]
- 37.Lee HB, Yu MR, Yang Y, Jiang Z, Ha H: Reactive oxygen species-regulated signaling pathways in diabetic nephropathy. J Am Soc Nephrol 14[Suppl 3]: S241–S245, 2003 [DOI] [PubMed] [Google Scholar]
- 38.He CJ, Koschinsky T, Buenting C, Vlassara H: Presence of diabetic complications in type 1 diabetic patients correlates with low expression of mononuclear cell AGE-receptor-1 and elevated serum AGE. Mol Med 7: 159–168, 2001 [PMC free article] [PubMed] [Google Scholar]
- 39.Bucala R, Makita Z, Vega G, Grundy S, Koschinsky T, Cerami A, Vlassara H: Modification of low density lipoprotein by advanced glycation end products contributes to the dyslipidemia of diabetes and renal insufficiency. Proc Natl Acad Sci U S A 91: 9441–9445, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Witztum JL, Steinberg D: Role of oxidized low density lipoprotein in atherogenesis. J Clin Invest 88: 1785–1792, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Menzel EJ, Sobal G, Staudinger A: The role of oxidative stress in the long-term glycation of LDL. Biofactors 6: 111–124, 1997 [DOI] [PubMed] [Google Scholar]
- 42.Lopes-Virella MF, Baker NL, Hunt KJ, Cleary PA, Klein R, Virella G, DCCT/EDIC Research Group : Baseline markers of inflammation are associated with progression to macroalbuminuria in type 1 diabetic subjects. Diabetes Care 36: 2317–2323, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shinohara M, Thornalley PJ, Giardino I, Beisswenger P, Thorpe SR, Onorato J, Brownlee M: Overexpression of glyoxalase-I in bovine endothelial cells inhibits intracellular advanced glycation endproduct formation and prevents hyperglycemia-induced increases in macromolecular endocytosis. J Clin Invest 101: 1142–1147, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kaspar JW, Niture SK, Jaiswal AK: Nrf2:INrf2 (Keap1) signaling in oxidative stress. Free Radic Biol Med 47: 1304–1309, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ruiz S, Pergola PE, Zager RA, Vaziri ND: Targeting the transcription factor Nrf2 to ameliorate oxidative stress and inflammation in chronic kidney disease. Kidney Int 83: 1029–1041, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim HJ, Vaziri ND: Contribution of impaired Nrf2-Keap1 pathway to oxidative stress and inflammation in chronic renal failure. Am J Physiol Renal Physiol 298: F662–F671, 2010 [DOI] [PubMed] [Google Scholar]
- 47.Puchades MJ, Saez G, Muñoz MC, Gonzalez M, Torregrosa I, Juan I, Miguel A: Study of oxidative stress in patients with advanced renal disease and undergoing either hemodialysis or peritoneal dialysis. Clin Nephrol 80: 177–186, 2013 [DOI] [PubMed] [Google Scholar]
- 48.Hsu WH, Lee BH, Chang YY, Hsu YW, Pan TM: A novel natural Nrf2 activator with PPARγ-agonist (monascin) attenuates the toxicity of methylglyoxal and hyperglycemia. Toxicol Appl Pharmacol 272: 842–851, 2013 [DOI] [PubMed] [Google Scholar]
- 49.Xue M, Rabbani N, Momiji H, Imbasi P, Anwar MM, Kitteringham N, Park BK, Souma T, Moriguchi T, Yamamoto M, Thornalley PJ: Transcriptional control of glyoxalase 1 by Nrf2 provides a stress-responsive defence against dicarbonyl glycation. Biochem J 443: 213–222, 2012 [DOI] [PubMed] [Google Scholar]
- 50.Sebeková K, Podracká L, Heidland A, Schinzel R: Enhanced plasma levels of advanced glycation end products (AGE) and pro-inflammatory cytokines in children/adolescents with chronic renal insufficiency and after renal replacement therapy by dialysis and transplantation--are they inter-related? Clin Nephrol 56: S21–S26, 2001 [PubMed] [Google Scholar]
- 51.Park SH, Stenvinkel P, Lindholm B: Cardiovascular biomarkers in chronic kidney disease. J Ren Nutr 22: 120–127, 2012 [DOI] [PubMed] [Google Scholar]
- 52.Basta G: Receptor for advanced glycation endproducts and atherosclerosis: From basic mechanisms to clinical implications. Atherosclerosis 196: 9–21, 2008 [DOI] [PubMed] [Google Scholar]
- 53.Kislinger T, Tanji N, Wendt T, Qu W, Lu Y, Ferran LJ, Jr., Taguchi A, Olson K, Bucciarelli L, Goova M, Hofmann MA, Cataldegirmen G, D’Agati V, Pischetsrieder M, Stern DM, Schmidt AM: Receptor for advanced glycation end products mediates inflammation and enhanced expression of tissue factor in vasculature of diabetic apolipoprotein E-null mice. Arterioscler Thromb Vasc Biol 21: 905–910, 2001 [DOI] [PubMed] [Google Scholar]
- 54.Bucciarelli LG, Wendt T, Rong L, Lalla E, Hofmann MA, Goova MT, Taguchi A, Yan SF, Yan SD, Stern DM, Schmidt AM: RAGE is a multiligand receptor of the immunoglobulin superfamily: Implications for homeostasis and chronic disease. Cell Mol Life Sci 59: 1117–1128, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schmidt AM, Yan SD, Yan SF, Stern DM: The biology of the receptor for advanced glycation end products and its ligands. Biochim Biophys Acta 1498: 99–111, 2000 [DOI] [PubMed] [Google Scholar]
- 56.Gallo D, Cocchietto M, Masat E, Agostinis C, Harei E, Veronesi P, Sava G: Human recombinant lysozyme downregulates advanced glycation endproduct-induced interleukin-6 production and release in an in-vitro model of human proximal tubular epithelial cells. Exp Biol Med (Maywood) 239: 337–346, 2014 [DOI] [PubMed] [Google Scholar]
- 57.Basta G, Lazzerini G, Massaro M, Simoncini T, Tanganelli P, Fu C, Kislinger T, Stern DM, Schmidt AM, De Caterina R: Advanced glycation end products activate endothelium through signal-transduction receptor RAGE: A mechanism for amplification of inflammatory responses. Circulation 105: 816–822, 2002 [DOI] [PubMed] [Google Scholar]
- 58.Boulanger E, Wautier MP, Wautier JL, Boval B, Panis Y, Wernert N, Danze PM, Dequiedt P: AGEs bind to mesothelial cells via RAGE and stimulate VCAM-1 expression. Kidney Int 61: 148–156, 2002 [DOI] [PubMed] [Google Scholar]
- 59.Chavakis T, Bierhaus A, Al-Fakhri N, Schneider D, Witte S, Linn T, Nagashima M, Morser J, Arnold B, Preissner KT, Nawroth PP: The pattern recognition receptor (RAGE) is a counterreceptor for leukocyte integrins: A novel pathway for inflammatory cell recruitment. J Exp Med 198: 1507–1515, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kirstein M, Brett J, Radoff S, Ogawa S, Stern D, Vlassara H: Advanced protein glycosylation induces transendothelial human monocyte chemotaxis and secretion of platelet-derived growth factor: Role in vascular disease of diabetes and aging. Proc Natl Acad Sci U S A 87: 9010–9014, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li LM, Hou DX, Guo YL, Yang JW, Liu Y, Zhang CY, Zen K: Role of microRNA-214-targeting phosphatase and tensin homolog in advanced glycation end product-induced apoptosis delay in monocytes. J Immunol 186: 2552–2560, 2011 [DOI] [PubMed] [Google Scholar]
- 62.Sadik NA, Mohamed WA, Ahmed MI: The association of receptor of advanced glycated end products and inflammatory mediators contributes to endothelial dysfunction in a prospective study of acute kidney injury patients with sepsis. Mol Cell Biochem 359: 73–81, 2012 [DOI] [PubMed] [Google Scholar]
- 63.Stinghen AE, Gonçalves SM, Martines EG, Nakao LS, Riella MC, Aita CA, Pecoits-Filho R: Increased plasma and endothelial cell expression of chemokines and adhesion molecules in chronic kidney disease. Nephron Clin Pract 111: c117–c126, 2009 [DOI] [PubMed] [Google Scholar]
- 64.Lu C, He JC, Cai W, Liu H, Zhu L, Vlassara H: Advanced glycation endproduct (AGE) receptor 1 is a negative regulator of the inflammatory response to AGE in mesangial cells. Proc Natl Acad Sci U S A 101: 11767–11772, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Weiss MF, Erhard P, Kader-Attia FA, Wu YC, Deoreo PB, Araki A, Glomb MA, Monnier VM: Mechanisms for the formation of glycoxidation products in end-stage renal disease. Kidney Int 57: 2571–2585, 2000 [DOI] [PubMed] [Google Scholar]
- 66.Del Turco S, Basta G: An update on advanced glycation endproducts and atherosclerosis. Biofactors 38: 266–274, 2012 [DOI] [PubMed] [Google Scholar]
- 67.Wagner Z, Molnár M, Molnár GA, Tamaskó M, Laczy B, Wagner L, Csiky B, Heidland A, Nagy J, Wittmann I: Serum carboxymethyllysine predicts mortality in hemodialysis patients. Am J Kidney Dis 47: 294–300, 2006 [DOI] [PubMed] [Google Scholar]
- 68.Roberts MA, Thomas MC, Fernando D, Macmillan N, Power DA, Ierino FL: Low molecular weight advanced glycation end products predict mortality in asymptomatic patients receiving chronic haemodialysis. Nephrol Dial Transplant 21: 1611–1617, 2006 [DOI] [PubMed] [Google Scholar]
- 69.Meerwaldt R, Hartog JW, Graaff R, Huisman RJ, Links TP, den Hollander NC, Thorpe SR, Baynes JW, Navis G, Gans RO, Smit AJ: Skin autofluorescence, a measure of cumulative metabolic stress and advanced glycation end products, predicts mortality in hemodialysis patients. J Am Soc Nephrol 16: 3687–3693, 2005 [DOI] [PubMed] [Google Scholar]
- 70.Schwedler SB, Metzger T, Schinzel R, Wanner C: Advanced glycation end products and mortality in hemodialysis patients. Kidney Int 62: 301–310, 2002 [DOI] [PubMed] [Google Scholar]
- 71.Suliman ME, Heimbürger O, Bárány P, Anderstam B, Pecoits-Filho R, Rodríguez Ayala E, Qureshi AR, Fehrman-Ekholm I, Lindholm B, Stenvinkel P: Plasma pentosidine is associated with inflammation and malnutrition in end-stage renal disease patients starting on dialysis therapy. J Am Soc Nephrol 14: 1614–1622, 2003 [DOI] [PubMed] [Google Scholar]
- 72.Suliman ME, Stenvinkel P, Jogestrand T, Maruyama Y, Qureshi AR, Bárány P, Heimbürger O, Lindholm B: Plasma pentosidine and total homocysteine levels in relation to change in common carotid intima-media area in the first year of dialysis therapy. Clin Nephrol 66: 418–425, 2006 [DOI] [PubMed] [Google Scholar]
- 73.Kerkeni M, Weiss IS, Jaisson S, Dandana A, Addad F, Gillery P, Hammami M: Increased serum concentrations of pentosidine are related to presence and severity of coronary artery disease. Thromb Res 134: 633–638, 2014 [DOI] [PubMed] [Google Scholar]
- 74.Leurs P, Lindholm B: The AGE-RAGE pathway and its relation to cardiovascular disease in patients with chronic kidney disease. Arch Med Res 44: 601–610, 2013 [DOI] [PubMed] [Google Scholar]
- 75.Fraser SD, Roderick PJ, McIntyre NJ, Harris S, McIntyre CW, Fluck RJ, Taal MW: Skin autofluorescence and all-cause mortality in stage 3 CKD. Clin J Am Soc Nephrol 9: 1361–1368, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Moriya S, Yamazaki M, Murakami H, Maruyama K, Uchiyama S: Two soluble isoforms of receptors for advanced glycation end products (RAGE) in carotid atherosclerosis: The difference of soluble and endogenous secretory RAGE. J Stroke Cerebrovasc Dis 23: 2540–2546, 2014 [DOI] [PubMed] [Google Scholar]
- 77.Davignon J, Ganz P: Role of endothelial dysfunction in atherosclerosis. Circulation 109[Suppl 1]: III27–III32, 2004 [DOI] [PubMed] [Google Scholar]
- 78.Stam F, van Guldener C, Becker A, Dekker JM, Heine RJ, Bouter LM, Stehouwer CD: Endothelial dysfunction contributes to renal function-associated cardiovascular mortality in a population with mild renal insufficiency: The Hoorn study. J Am Soc Nephrol 17: 537–545, 2006 [DOI] [PubMed] [Google Scholar]
- 79.Schmidt AM, Hori O, Chen JX, Li JF, Crandall J, Zhang J, Cao R, Yan SD, Brett J, Stern D: Advanced glycation endproducts interacting with their endothelial receptor induce expression of vascular cell adhesion molecule-1 (VCAM-1) in cultured human endothelial cells and in mice. A potential mechanism for the accelerated vasculopathy of diabetes. J Clin Invest 96: 1395–1403, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Grossin N, Auger F, Niquet-Leridon C, Durieux N, Montaigne D, Schmidt AM, Susen S, Jacolot P, Beuscart JB, Tessier FJ, Boulanger E: Dietary CML-enriched protein induces functional arterial aging in a RAGE-dependent manner in mice. Mol Nutr Food Res 59: 927–938, 2015 [DOI] [PubMed] [Google Scholar]
- 81.Brouwers O, Niessen PM, Miyata T, Østergaard JA, Flyvbjerg A, Peutz-Kootstra CJ, Sieber J, Mundel PH, Brownlee M, Janssen BJ, De Mey JG, Stehouwer CD, Schalkwijk CG: Glyoxalase-1 overexpression reduces endothelial dysfunction and attenuates early renal impairment in a rat model of diabetes. Diabetologia 57: 224–235, 2014 [DOI] [PubMed] [Google Scholar]
- 82.Goligorsky MS, Yasuda K, Ratliff B: Dysfunctional endothelial progenitor cells in chronic kidney disease. J Am Soc Nephrol 21: 911–919, 2010 [DOI] [PubMed] [Google Scholar]
- 83.Eizawa T, Murakami Y, Matsui K, Takahashi M, Muroi K, Amemiya M, Takano R, Kusano E, Shimada K, Ikeda U: Circulating endothelial progenitor cells are reduced in hemodialysis patients. Curr Med Res Opin 19: 627–633, 2003 [DOI] [PubMed] [Google Scholar]
- 84.Choi JH, Kim KL, Huh W, Kim B, Byun J, Suh W, Sung J, Jeon ES, Oh HY, Kim DK: Decreased number and impaired angiogenic function of endothelial progenitor cells in patients with chronic renal failure. Arterioscler Thromb Vasc Biol 24: 1246–1252, 2004 [DOI] [PubMed] [Google Scholar]
- 85.Schlieper G, Hristov M, Brandenburg V, Krüger T, Westenfeld R, Mahnken AH, Yagmur E, Boecker G, Heussen N, Gladziwa U, Ketteler M, Weber C, Floege J: Predictors of low circulating endothelial progenitor cell numbers in haemodialysis patients. Nephrol Dial Transplant 23: 2611–2618, 2008 [DOI] [PubMed] [Google Scholar]
- 86.Jourde-Chiche N, Dou L, Sabatier F, Calaf R, Cerini C, Robert S, Camoin-Jau L, Charpiot P, Argiles A, Dignat-George F, Brunet P: Levels of circulating endothelial progenitor cells are related to uremic toxins and vascular injury in hemodialysis patients. J Thromb Haemost 7: 1576–1584, 2009 [DOI] [PubMed] [Google Scholar]
- 87.Jie KE, Zaikova MA, Bergevoet MW, Westerweel PE, Rastmanesh M, Blankestijn PJ, Boer WH, Braam B, Verhaar MC: Progenitor cells and vascular function are impaired in patients with chronic kidney disease. Nephrol Dial Transplant 25: 1875–1882, 2010 [DOI] [PubMed] [Google Scholar]
- 88.Cheng M, Guan X, Li H, Cui X, Zhang X, Li X, Jing X, Wu H, Avsar E: Shear stress regulates late EPC differentiation via mechanosensitive molecule-mediated cytoskeletal rearrangement. PLoS One 8: e67675, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ueda S, Yamagishi S, Matsui T, Noda Y, Ueda S, Jinnouchi Y, Sasaki K, Takeuchi M, Imaizumi T: Serum levels of advanced glycation end products (AGEs) are inversely associated with the number and migratory activity of circulating endothelial progenitor cells in apparently healthy subjects. Cardiovasc Ther 30: 249–254, 2012 [DOI] [PubMed] [Google Scholar]
- 90.Ueno H, Koyama H, Fukumoto S, Tanaka S, Shoji T, Shoji T, Emoto M, Tahara H, Inaba M, Kakiya R, Tabata T, Miyata T, Nishizawa Y: Advanced glycation end products, carotid atherosclerosis, and circulating endothelial progenitor cells in patients with end-stage renal disease. Metabolism 60: 453–459, 2011 [DOI] [PubMed] [Google Scholar]
- 91.Sun C, Liang C, Ren Y, Zhen Y, He Z, Wang H, Tan H, Pan X, Wu Z: Advanced glycation end products depress function of endothelial progenitor cells via p38 and ERK 1/2 mitogen-activated protein kinase pathways. Basic Res Cardiol 104: 42–49, 2009 [DOI] [PubMed] [Google Scholar]
- 92.Chen Q, Dong L, Wang L, Kang L, Xu B: Advanced glycation end products impair function of late endothelial progenitor cells through effects on protein kinase Akt and cyclooxygenase-2. Biochem Biophys Res Commun 381: 192–197, 2009 [DOI] [PubMed] [Google Scholar]
- 93.Liang C, Ren Y, Tan H, He Z, Jiang Q, Wu J, Zhen Y, Fan M, Wu Z: Rosiglitazone via upregulation of Akt/eNOS pathways attenuates dysfunction of endothelial progenitor cells, induced by advanced glycation end products. Br J Pharmacol 158: 1865–1873, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Jourde-Chiche N, Dou L, Cerini C, Dignat-George F, Brunet P: Vascular incompetence in dialysis patients--protein-bound uremic toxins and endothelial dysfunction. Semin Dial 24: 327–337, 2011 [DOI] [PubMed] [Google Scholar]
- 95.Rashid G, Benchetrit S, Fishman D, Bernheim J: Effect of advanced glycation end-products on gene expression and synthesis of TNF-alpha and endothelial nitric oxide synthase by endothelial cells. Kidney Int 66: 1099–1106, 2004 [DOI] [PubMed] [Google Scholar]
- 96.Rojas A, Romay S, González D, Herrera B, Delgado R, Otero K: Regulation of endothelial nitric oxide synthase expression by albumin-derived advanced glycosylation end products. Circ Res 86: E50–E54, 2000 [DOI] [PubMed] [Google Scholar]
- 97.Xu B, Chibber R, Ruggiero D, Kohner E, Ritter J, Ferro A: Impairment of vascular endothelial nitric oxide synthase activity by advanced glycation end products. FASEB J 17: 1289–1291, 2003 [DOI] [PubMed] [Google Scholar]
- 98.Xiao S, Wagner L, Schmidt RJ, Baylis C: Circulating endothelial nitric oxide synthase inhibitory factor in some patients with chronic renal disease. Kidney Int 59: 1466–1472, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yamagishi S, Fujimori H, Yonekura H, Yamamoto Y, Yamamoto H: Advanced glycation endproducts inhibit prostacyclin production and induce plasminogen activator inhibitor-1 in human microvascular endothelial cells. Diabetologia 41: 1435–1441, 1998 [DOI] [PubMed] [Google Scholar]
- 100.Jones GT, van Rij AM, Solomon C, Thomson IA, Packer SG: Endothelin-1 is increased overlying atherosclerotic plaques in human arteries. Atherosclerosis 124: 25–35, 1996 [DOI] [PubMed] [Google Scholar]
- 101.Odetti P, Monacelli F, Storace D, Robaudo C, Rossi S, Deferrari G, Barreca T: Correlation between pentosidine and endothelin-1 in subjects undergoing chronic hemodialysis. Horm Metab Res 38: 817–820, 2006 [DOI] [PubMed] [Google Scholar]
- 102.Kohan DE: Endothelin, hypertension and chronic kidney disease: New insights. Curr Opin Nephrol Hypertens 19: 134–139, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Dhaun N, Goddard J, Webb DJ: The endothelin system and its antagonism in chronic kidney disease. J Am Soc Nephrol 17: 943–955, 2006 [DOI] [PubMed] [Google Scholar]
- 104.Quehenberger P, Bierhaus A, Fasching P, Muellner C, Klevesath M, Hong M, Stier G, Sattler M, Schleicher E, Speiser W, Nawroth PP: Endothelin 1 transcription is controlled by nuclear factor-kappaB in AGE-stimulated cultured endothelial cells. Diabetes 49: 1561–1570, 2000 [DOI] [PubMed] [Google Scholar]
- 105.Wautier JL, Zoukourian C, Chappey O, Wautier MP, Guillausseau PJ, Cao R, Hori O, Stern D, Schmidt AM: Receptor-mediated endothelial cell dysfunction in diabetic vasculopathy. Soluble receptor for advanced glycation end products blocks hyperpermeability in diabetic rats. J Clin Invest 97: 238–243, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Guo XH, Huang QB, Chen B, Wang SY, Li Q, Zhu YJ, Hou FF, Fu N, Brunk UT, Zhao M: Advanced glycation end products induce actin rearrangement and subsequent hyperpermeability of endothelial cells. APMIS 114: 874–883, 2006 [DOI] [PubMed] [Google Scholar]
- 107.McEniery CM, Wallace S, Mackenzie IS, McDonnell B, Yasmin, Newby DE, Cockcroft JR, Wilkinson IB: Endothelial function is associated with pulse pressure, pulse wave velocity, and augmentation index in healthy humans. Hypertension 48: 602–608, 2006 [DOI] [PubMed] [Google Scholar]
- 108.Ketteler M, Biggar PH: Review article: Getting the balance right: Assessing causes and extent of vascular calcification in chronic kidney disease. Nephrology (Carlton) 14: 389–394, 2009 [DOI] [PubMed] [Google Scholar]
- 109.Massy ZA, Mazière C, Kamel S, Brazier M, Choukroun G, Tribouilloy C, Slama M, Andrejak M, Mazière JC: Impact of inflammation and oxidative stress on vascular calcifications in chronic kidney disease. Pediatr Nephrol 20: 380–382, 2005 [DOI] [PubMed] [Google Scholar]
- 110.Massy ZA, Stenvinkel P, Drueke TB: The role of oxidative stress in chronic kidney disease. Semin Dial 22: 405–408, 2009 [DOI] [PubMed] [Google Scholar]
- 111.Massy ZA, Drüeke TB: Vascular calcification. Curr Opin Nephrol Hypertens 22: 405–412, 2013 [DOI] [PubMed] [Google Scholar]
- 112.Shroff R, Long DA, Shanahan C: Mechanistic insights into vascular calcification in CKD. J Am Soc Nephrol 24: 179–189, 2013 [DOI] [PubMed] [Google Scholar]
- 113.Cozzolino MC, Cosa F, Ciceri P, Elli F, Ricca F, Cappelletti L, Bellasi A, Cusi D: Vascular calcification in chronic kidney disease: An update. Eur Med J Nephrol 1: 46–51, 2013 [Google Scholar]
- 114.London GM, Marchais SJ, Guérin AP, Métivier F: Impairment of arterial function in chronic renal disease: Prognostic impact and therapeutic approach. Nephrol Dial Transplant 17[Suppl 11]: 13–15, 2002 [DOI] [PubMed] [Google Scholar]
- 115.Pugliese G, Iacobini C, Blasetti Fantauzzi C, Menini S: The dark and bright side of atherosclerotic calcification. Atherosclerosis 238: 220–230, 2015 [DOI] [PubMed] [Google Scholar]
- 116.Berl T, Henrich W: Kidney-heart interactions: Epidemiology, pathogenesis, and treatment. Clin J Am Soc Nephrol 1: 8–18, 2006 [DOI] [PubMed] [Google Scholar]
- 117.Taki K, Takayama F, Tsuruta Y, Niwa T: Oxidative stress, advanced glycation end product, and coronary artery calcification in hemodialysis patients. Kidney Int 70: 218–224, 2006 [DOI] [PubMed] [Google Scholar]
- 118.Wang AY, Wong CK, Yau YY, Wong S, Chan IH, Lam CW: Skin autofluorescence associates with vascular calcification in chronic kidney disease. Arterioscler Thromb Vasc Biol 34: 1784–1790, 2014 [DOI] [PubMed] [Google Scholar]
- 119.Wei Q, Ren X, Jiang Y, Jin H, Liu N, Li J: Advanced glycation end products accelerate rat vascular calcification through RAGE/oxidative stress. BMC Cardiovasc Disord 13: 13, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Briet M, Bozec E, Laurent S, Fassot C, London GM, Jacquot C, Froissart M, Houillier P, Boutouyrie P: Arterial stiffness and enlargement in mild-to-moderate chronic kidney disease. Kidney Int 69: 350–357, 2006 [DOI] [PubMed] [Google Scholar]
- 121.Kerr PG, Guerin AP: Arterial calcification and stiffness in chronic kidney disease. Clin Exp Pharmacol Physiol 34: 683–687, 2007 [DOI] [PubMed] [Google Scholar]
- 122.Pannier B, Guérin AP, Marchais SJ, Safar ME, London GM: Stiffness of capacitive and conduit arteries: Prognostic significance for end-stage renal disease patients. Hypertension 45: 592–596, 2005 [DOI] [PubMed] [Google Scholar]
- 123.Blacher J, Safar ME, Guerin AP, Pannier B, Marchais SJ, London GM: Aortic pulse wave velocity index and mortality in end-stage renal disease. Kidney Int 63: 1852–1860, 2003 [DOI] [PubMed] [Google Scholar]
- 124.Sell DR, Monnier VM: Molecular basis of arterial stiffening: Role of glycation - a mini-review. Gerontology 58: 227–237, 2012 [DOI] [PubMed] [Google Scholar]
- 125.Zhou Y, Yu Z, Jia H, Sun F, Ma L, Guo R, Peng L, Cui T: Association of serum pentosidine with arterial stiffness in hemodialysis patients. Artif Organs 34: 193–199, 2010 [DOI] [PubMed] [Google Scholar]
- 126.Ueno H, Koyama H, Tanaka S, Fukumoto S, Shinohara K, Shoji T, Emoto M, Tahara H, Kakiya R, Tabata T, Miyata T, Nishizawa Y: Skin autofluorescence, a marker for advanced glycation end product accumulation, is associated with arterial stiffness in patients with end-stage renal disease. Metabolism 57: 1452–1457, 2008 [DOI] [PubMed] [Google Scholar]
- 127.Descamps-Latscha B, Jungers P, Witko-Sarsat V: Immune system dysregulation in uremia: Role of oxidative stress. Blood Purif 20: 481–484, 2002 [DOI] [PubMed] [Google Scholar]
- 128.Hauser AB, Stinghen AE, Kato S, Bucharles S, Aita C, Yuzawa Y, Pecoits-Filho R: Characteristics and causes of immune dysfunction related to uremia and dialysis. Perit Dial Int 28[Suppl 3]: S183–S187, 2008 [PubMed] [Google Scholar]
- 129.Friedlander MA, Witko-Sarsat V, Nguyen AT, Wu YC, Labrunte M, Verger C, Jungers P, Descamps-Latscha B: The advanced glycation endproduct pentosidine and monocyte activation in uremia. Clin Nephrol 45: 379–382, 1996 [PubMed] [Google Scholar]
- 130.Vlassara H, Cai W, Goodman S, Pyzik R, Yong A, Chen X, Zhu L, Neade T, Beeri M, Silverman JM, Ferrucci L, Tansman L, Striker GE, Uribarri J: Protection against loss of innate defenses in adulthood by low advanced glycation end products (AGE) intake: Role of the antiinflammatory AGE receptor-1. J Clin Endocrinol Metab 94: 4483–4491, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Barnett AH, Bain SC, Bouter P, Karlberg B, Madsbad S, Jervell J, Mustonen J, Diabetics Exposed to Telmisartan and Enalapril Study Group : Angiotensin-receptor blockade versus converting-enzyme inhibition in type 2 diabetes and nephropathy. N Engl J Med 351: 1952–1961, 2004 [DOI] [PubMed] [Google Scholar]
- 132.Gugliucci A, Bendayan M: Renal fate of circulating advanced glycated end products (AGE): Evidence for reabsorption and catabolism of AGE-peptides by renal proximal tubular cells. Diabetologia 39: 149–160, 1996 [DOI] [PubMed] [Google Scholar]
- 133.Miyata T, Ueda Y, Horie K, Nangaku M, Tanaka S, van Ypersele de Strihou C, Kurokawa K: Renal catabolism of advanced glycation end products: The fate of pentosidine. Kidney Int 53: 416–422, 1998 [DOI] [PubMed] [Google Scholar]
- 134.Zhou X, Wang B, Zhu L, Hao S: A novel improved therapy strategy for diabetic nephropathy: Targeting AGEs. Organogenesis 8: 18–21, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wada J, Makino H: Inflammation and the pathogenesis of diabetic nephropathy. Clin Sci (Lond) 124: 139–152, 2013 [DOI] [PubMed] [Google Scholar]
- 136.Tanji N, Markowitz GS, Fu C, Kislinger T, Taguchi A, Pischetsrieder M, Stern D, Schmidt AM, D’Agati VD: Expression of advanced glycation end products and their cellular receptor RAGE in diabetic nephropathy and nondiabetic renal disease. J Am Soc Nephrol 11: 1656–1666, 2000 [DOI] [PubMed] [Google Scholar]
- 137.Horie K, Miyata T, Maeda K, Miyata S, Sugiyama S, Sakai H, van Ypersole de Strihou C, Monnier VM, Witztum JL, Kurokawa K: Immunohistochemical colocalization of glycoxidation products and lipid peroxidation products in diabetic renal glomerular lesions. Implication for glycoxidative stress in the pathogenesis of diabetic nephropathy. J Clin Invest 100: 2995–3004, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Niwa T, Katsuzaki T, Miyazaki S, Miyazaki T, Ishizaki Y, Hayase F, Tatemichi N, Takei Y: Immunohistochemical detection of imidazolone, a novel advanced glycation end product, in kidneys and aortas of diabetic patients. J Clin Invest 99: 1272–1280, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Vlassara H, Striker GE: AGE restriction in diabetes mellitus: A paradigm shift. Nat Rev Endocrinol 7: 526–539, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Liu J, Huang K, Cai GY, Chen XM, Yang JR, Lin LR, Yang J, Huo BG, Zhan J, He YN: Receptor for advanced glycation end-products promotes premature senescence of proximal tubular epithelial cells via activation of endoplasmic reticulum stress-dependent p21 signaling. Cell Signal 26: 110–121, 2014 [DOI] [PubMed] [Google Scholar]
- 141.Mallipattu SK, Uribarri J: Advanced glycation end product accumulation: A new enemy to target in chronic kidney disease? Curr Opin Nephrol Hypertens 23: 547–554, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Goldberg T, Cai W, Peppa M, Dardaine V, Baliga BS, Uribarri J, Vlassara H: Advanced glycoxidation end products in commonly consumed foods. J Am Diet Assoc 104: 1287–1291, 2004 [DOI] [PubMed] [Google Scholar]
- 143.Uribarri J, Peppa M, Cai W, Goldberg T, Lu M, He C, Vlassara H: Restriction of dietary glycotoxins reduces excessive advanced glycation end products in renal failure patients. J Am Soc Nephrol 14: 728–731, 2003 [DOI] [PubMed] [Google Scholar]
- 144.Ueda S, Yamagishi S, Takeuchi M, Kohno K, Shibata R, Matsumoto Y, Kaneyuki U, Fujimura T, Hayashida A, Okuda S: Oral adsorbent AST-120 decreases serum levels of AGEs in patients with chronic renal failure. Mol Med 12: 180–184, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Ueda H, Shibahara N, Takagi S, Inoue T, Katsuoka Y: AST-120 treatment in pre-dialysis period affects the prognosis in patients on hemodialysis. Ren Fail 30: 856–860, 2008 [DOI] [PubMed] [Google Scholar]
- 146.Ueda H, Shibahara N, Takagi S, Inoue T, Katsuoka Y: AST-120, an oral adsorbent, delays the initiation of dialysis in patients with chronic kidney diseases. Ther Apher Dial 11: 189–195, 2007 [DOI] [PubMed] [Google Scholar]
- 147.Nakamura T, Kawagoe Y, Matsuda T, Ueda Y, Shimada N, Ebihara I, Koide H: Oral ADSORBENT AST-120 decreases carotid intima-media thickness and arterial stiffness in patients with chronic renal failure. Kidney Blood Press Res 27: 121–126, 2004 [DOI] [PubMed] [Google Scholar]
- 148.Schulman G, Berl T, Beck GJ, Remuzzi G, Ritz E, Arita K, Kato A, Shimizu M: Randomized placebo-controlled EPPIC trials of AST-120 in CKD. J Am Soc Nephrol 26: 1732–1746, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Bolati D, Shimizu H, Yisireyili M, Nishijima F, Niwa T: Indoxyl sulfate, a uremic toxin, downregulates renal expression of Nrf2 through activation of NF-κB. BMC Nephrol 14: 56, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Vlassara H, Cai W, Chen X, Serrano EJ, Shobha MS, Uribarri J, Woodward M, Striker GE: Managing chronic inflammation in the aging diabetic patient with CKD by diet or sevelamer carbonate: A modern paradigm shift. J Gerontol A Biol Sci Med Sci 67: 1410–1416, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Shantouf R, Budoff MJ, Ahmadi N, Tiano J, Flores F, Kalantar-Zadeh K: Effects of sevelamer and calcium-based phosphate binders on lipid and inflammatory markers in hemodialysis patients. Am J Nephrol 28: 275–279, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Ferramosca E, Burke S, Chasan-Taber S, Ratti C, Chertow GM, Raggi P: Potential antiatherogenic and anti-inflammatory properties of sevelamer in maintenance hemodialysis patients. Am Heart J 149: 820–825, 2005 [DOI] [PubMed] [Google Scholar]
- 153.Vlassara H, Uribarri J, Cai W, Goodman S, Pyzik R, Post J, Grosjean F, Woodward M, Striker GE: Effects of sevelamer on HbA1c, inflammation, and advanced glycation end products in diabetic kidney disease. Clin J Am Soc Nephrol 7: 934–942, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Kakuta T, Tanaka R, Hyodo T, Suzuki H, Kanai G, Nagaoka M, Takahashi H, Hirawa N, Oogushi Y, Miyata T, Kobayashi H, Fukagawa M, Saito A: Effect of sevelamer and calcium-based phosphate binders on coronary artery calcification and accumulation of circulating advanced glycation end products in hemodialysis patients. Am J Kidney Dis 57: 422–431, 2011 [DOI] [PubMed] [Google Scholar]
- 155.Chong ZZ, Wang S, Shang YC, Maiese K: Targeting cardiovascular disease with novel SIRT1 pathways. Future Cardiol 8: 89–100, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Takemura A, Iijima K, Ota H, Son BK, Ito Y, Ogawa S, Eto M, Akishita M, Ouchi Y: Sirtuin 1 retards hyperphosphatemia-induced calcification of vascular smooth muscle cells. Arterioscler Thromb Vasc Biol 31: 2054–2062, 2011 [DOI] [PubMed] [Google Scholar]
- 157.Ota H, Akishita M, Eto M, Iijima K, Kaneki M, Ouchi Y: Sirt1 modulates premature senescence-like phenotype in human endothelial cells. J Mol Cell Cardiol 43: 571–579, 2007 [DOI] [PubMed] [Google Scholar]
- 158.Cai W, Uribarri J, Zhu L, Chen X, Swamy S, Zhao Z, Grosjean F, Simonaro C, Kuchel GA, Schnaider-Beeri M, Woodward M, Striker GE, Vlassara H: Oral glycotoxins are a modifiable cause of dementia and the metabolic syndrome in mice and humans. Proc Natl Acad Sci U S A 111: 4940–4945, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Yubero-Serrano EM, Woodward M, Poretsky L, Vlassara H, Striker GE, AGE-less Study Group : Effects of sevelamer carbonate on advanced glycation end products and antioxidant/pro-oxidant status in patients with diabetic kidney disease. Clin J Am Soc Nephrol 10: 759–766, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Dhondt A, Vanholder R, Van Biesen W, Lameire N: The removal of uremic toxins. Kidney Int Suppl 76: S47–S59, 2000 [DOI] [PubMed] [Google Scholar]
- 161.Vanholder R, Glorieux G, Van Biesen W: Advantages of new hemodialysis membranes and equipment. Nephron Clin Pract 114: c165–c172, 2010 [DOI] [PubMed] [Google Scholar]
- 162.Ledebo I, Blankestijn PJ: Haemodiafiltration-optimal efficiency and safety. NDT Plus 3: 8–16, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Floridi A, Antolini F, Galli F, Fagugli RM, Floridi E, Buoncristiani U: Daily haemodialysis improves indices of protein glycation. Nephrol Dial Transplant 17: 871–878, 2002 [DOI] [PubMed] [Google Scholar]
- 164.Gerdemann A, Lemke HD, Nothdurft A, Heidland A, Münch G, Bahner U, Schinzel R: Low-molecular but not high-molecular advanced glycation end products (AGEs) are removed by high-flux dialysis. Clin Nephrol 54: 276–283, 2000 [PubMed] [Google Scholar]
- 165.Stein G, Franke S, Mahiout A, Schneider S, Sperschneider H, Borst S, Vienken J: Influence of dialysis modalities on serum AGE levels in end-stage renal disease patients. Nephrol Dial Transplant 16: 999–1008, 2001 [DOI] [PubMed] [Google Scholar]
- 166.Jadoul M, Ueda Y, Yasuda Y, Saito A, Robert A, Ishida N, Kurokawa K, Van Ypersele De Strihou C, Miyata T: Influence of hemodialysis membrane type on pentosidine plasma level, a marker of “carbonyl stress”. Kidney Int 55: 2487–2492, 1999 [DOI] [PubMed] [Google Scholar]
- 167.Lin CL, Huang CC, Yu CC, Yang HY, Chuang FR, Yang CW: Reduction of advanced glycation end product levels by on-line hemodiafiltration in long-term hemodialysis patients. Am J Kidney Dis 42: 524–531, 2003 [DOI] [PubMed] [Google Scholar]
- 168.Susantitaphong P, Siribamrungwong M, Jaber BL: Convective therapies versus low-flux hemodialysis for chronic kidney failure: A meta-analysis of randomized controlled trials. Nephrol Dial Transplant 28: 2859–2874, 2013 [DOI] [PubMed] [Google Scholar]
- 169.Agalou S, Ahmed N, Dawnay A, Thornalley PJ: Removal of advanced glycation end products in clinical renal failure by peritoneal dialysis and haemodialysis. Biochem Soc Trans 31: 1394–1396, 2003 [DOI] [PubMed] [Google Scholar]
- 170.Passlick-Deetjen J, Lage C, Jörres A: Continuous flow peritoneal dialysis: Solution formulation and biocompatibility. Semin Dial 14: 384–387, 2001 [DOI] [PubMed] [Google Scholar]
- 171.Oleniuc M, Schiller A, Secara I, Onofriescu M, Hogas S, Apetrii M, Siriopol D, Covic A: Evaluation of advanced glycation end products accumulation, using skin autofluorescence, in CKD and dialysis patients. Int Urol Nephrol 44: 1441–1449, 2012 [DOI] [PubMed] [Google Scholar]
- 172.Friedlander MA, Wu YC, Schulak JA, Monnier VM, Hricik DE: Influence of dialysis modality on plasma and tissue concentrations of pentosidine in patients with end-stage renal disease. Am J Kidney Dis 25: 445–451, 1995 [DOI] [PubMed] [Google Scholar]
- 173.Lee HB, Chung SH, Chu WS, Kim JK, Ha H: Peritoneal dialysis in diabetic patients. Am J Kidney Dis 38[Suppl 1]: S200–S203, 2001 [DOI] [PubMed] [Google Scholar]
- 174.Tauer A, Knerr T, Niwa T, Schaub TP, Lage C, Passlick-Deetjen J, Pischetsrieder M: In vitro formation of N(epsilon)-(carboxymethyl)lysine and imidazolones under conditions similar to continuous ambulatory peritoneal dialysis. Biochem Biophys Res Commun 280: 1408–1414, 2001 [DOI] [PubMed] [Google Scholar]
- 175.McIntyre NJ, Chesterton LJ, John SG, Jefferies HJ, Burton JO, Taal MW, Fluck RJ, McIntyre CW: Tissue-advanced glycation end product concentration in dialysis patients. Clin J Am Soc Nephrol 5: 51–55, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Ueda Y, Miyata T, Goffin E, Yoshino A, Inagi R, Ishibashi Y, Izuhara Y, Saito A, Kurokawa K, Van Ypersele De Strihou C: Effect of dwell time on carbonyl stress using icodextrin and amino acid peritoneal dialysis fluids. Kidney Int 58: 2518–2524, 2000 [DOI] [PubMed] [Google Scholar]
- 177.Dawnay AB, Millar DJ: Glycation and advanced glycation end-product formation with icodextrin and dextrose. Perit Dial Int 17: 52–58, 1997 [PubMed] [Google Scholar]
- 178.Ho-dac-Pannekeet MM, Weiss MF, de Waart DR, Erhard P, Hiralall JK, Krediet RT: Analysis of non enzymatic glycosylation in vivo: Impact of different dialysis solutions. Perit Dial Int 19[Suppl 2]: S68–S74, 1999 [PubMed] [Google Scholar]
- 179.Qi H, Xu C, Yan H, Ma J: Comparison of icodextrin and glucose solutions for long dwell exchange in peritoneal dialysis: A meta-analysis of randomized controlled trials. Perit Dial Int 31: 179–188, 2011 [DOI] [PubMed] [Google Scholar]
- 180.Himmele R, Jensen L, Fenn D, Ho CH, Sawin DA, Diaz-Buxo JA: A new neutral-pH low-GDP peritoneal dialysis fluid. Perit Dial Int 32: 444–452, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Perl J, Nessim SJ, Bargman JM: The biocompatibility of neutral pH, low-GDP peritoneal dialysis solutions: Benefit at bench, bedside, or both? Kidney Int 79: 814–824, 2011 [DOI] [PubMed] [Google Scholar]
- 182.Vlassara H: Serum advanced glycosylation end products: A new class of uremic toxins? Blood Purif 12: 54–59, 1994 [DOI] [PubMed] [Google Scholar]
- 183.Miyata T, Ueda Y, Yoshida A, Sugiyama S, Iida Y, Jadoul M, Maeda K, Kurokawa K, van Ypersele de Strihou C: Clearance of pentosidine, an advanced glycation end product, by different modalities of renal replacement therapy. Kidney Int 51: 880–887, 1997 [DOI] [PubMed] [Google Scholar]
- 184.Yoshida S, Yamada K, Hamaguchi K, Nishimura M, Hatakeyama E, Tsuchida H, Sakamoto K, Kashiwabara H, Yokoyama T, Ikeda K, Horiuchi S: Immunohistochemical study of human advanced glycation end-products (AGE) and growth factors in cardiac tissues of patients on maintenance dialysis and with kidney transplantation. Clin Nephrol 49: 273–280, 1998 [PubMed] [Google Scholar]
- 185.Crowley LE, Johnson CP, McIntyre N, Fluck RJ, McIntyre CW, Taal MW, Leung JC: Tissue advanced glycation end product deposition after kidney transplantation. Nephron Clin Pract 124: 54–59, 2013 [DOI] [PubMed] [Google Scholar]
- 186.Baragetti I, Furiani S, Vettoretti S, Raselli S, Maggi FM, Galli F, Catapano AL, Buccianti G: Role of vitamin E-coated membrane in reducing advanced glycation end products in hemodialysis patients: A pilot study. Blood Purif 24: 369–376, 2006 [DOI] [PubMed] [Google Scholar]
- 187.Nagai R, Murray DB, Metz TO, Baynes JW: Chelation: A fundamental mechanism of action of AGE inhibitors, AGE breakers, and other inhibitors of diabetes complications. Diabetes 61: 549–559, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Singh R, Barden A, Mori T, Beilin L: Advanced glycation end-products: A review. Diabetologia 44: 129–146, 2001 [DOI] [PubMed] [Google Scholar]
- 189.Brownlee M, Vlassara H, Kooney A, Ulrich P, Cerami A: Aminoguanidine prevents diabetes-induced arterial wall protein cross-linking. Science 232: 1629–1632, 1986 [DOI] [PubMed] [Google Scholar]
- 190.Nilsson BO: Biological effects of aminoguanidine: An update. Inflamm Res 48: 509–515, 1999 [DOI] [PubMed] [Google Scholar]
- 191.Menzel EJ, Neumüller J, Sengoelge G, Reihsner R: Effects of aminoguanidine on adhesion molecule expression of human endothelial cells. Pharmacology 55: 126–135, 1997 [DOI] [PubMed] [Google Scholar]
- 192.Khalifah RG, Baynes JW, Hudson BG: Amadorins: Novel post-Amadori inhibitors of advanced glycation reactions. Biochem Biophys Res Commun 257: 251–258, 1999 [DOI] [PubMed] [Google Scholar]
- 193.Voziyan PA, Hudson BG: Pyridoxamine: The many virtues of a maillard reaction inhibitor. Ann N Y Acad Sci 1043: 807–816, 2005 [DOI] [PubMed] [Google Scholar]
- 194.Voziyan PA, Hudson BG: Pyridoxamine as a multifunctional pharmaceutical: Targeting pathogenic glycation and oxidative damage. Cell Mol Life Sci 62: 1671–1681, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Brodeur MR, Bouvet C, Bouchard S, Moreau S, Leblond J, Deblois D, Moreau P: Reduction of advanced-glycation end products levels and inhibition of RAGE signaling decreases rat vascular calcification induced by diabetes. PLoS One 9: e85922, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Polizzi FC, Andican G, Çetin E, Civelek S, Yumuk V, Burçak G: Increased DNA-glycation in type 2 diabetic patients: The effect of thiamine and pyridoxine therapy. Exp Clin Endocrinol Diabetes 120: 329–334, 2012 [DOI] [PubMed] [Google Scholar]
- 197.Nascimento MM, Suliman ME, Murayama Y, Nihi M, Hayashi SY, Stenvinkel P, Riella MC, Lindholm B: Effect of high-dose thiamine and pyridoxine on advanced glycation end products and other oxidative stress markers in hemodialysis patients: A randomized placebo-controlled study. J Ren Nutr 16: 119–124, 2006 [DOI] [PubMed] [Google Scholar]
- 198.Chawla T, Sharma D, Singh A: Role of the renin angiotensin system in diabetic nephropathy. World J Diabetes 1: 141–145, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Thomas MC, Tikellis C, Burns WM, Bialkowski K, Cao Z, Coughlan MT, Jandeleit-Dahm K, Cooper ME, Forbes JM: Interactions between renin angiotensin system and advanced glycation in the kidney. J Am Soc Nephrol 16: 2976–2984, 2005 [DOI] [PubMed] [Google Scholar]
- 200.Miyata T, van Ypersele de Strihou C, Ueda Y, Ichimori K, Inagi R, Onogi H, Ishikawa N, Nangaku M, Kurokawa K: Angiotensin II receptor antagonists and angiotensin-converting enzyme inhibitors lower in vitro the formation of advanced glycation end products: Biochemical mechanisms. J Am Soc Nephrol 13: 2478–2487, 2002 [DOI] [PubMed] [Google Scholar]
- 201.Nangaku M, Miyata T, Sada T, Mizuno M, Inagi R, Ueda Y, Ishikawa N, Yuzawa H, Koike H, van Ypersele de Strihou C, Kurokawa K: Anti-hypertensive agents inhibit in vivo the formation of advanced glycation end products and improve renal damage in a type 2 diabetic nephropathy rat model. J Am Soc Nephrol 14: 1212–1222, 2003 [DOI] [PubMed] [Google Scholar]
- 202.Ishibashi Y, Matsui T, Ueda S, Fukami K, Okuda S, Yamagishi S: Irbesartan inhibits advanced glycation end product-induced increase in asymmetric dimethylarginine level in mesangial cells through its anti-oxidative properties. Int J Cardiol 176: 1120–1122, 2014 [DOI] [PubMed] [Google Scholar]
- 203.Honda H, Hosaka N, Aoshima Y, Hirai Y, Michihata T, Akizawa T: Olmesartan medoxomil is associated with decreased plasma AGEs, pentosidine, and N-(epsilon)-carboxymethyl-lysine levels in hemodialysis patients. Clin Exp Hypertens 34: 17–23, 2012 [DOI] [PubMed] [Google Scholar]
- 204.Sebeková K, Gazdíková K, Syrová D, Blazícek P, Schinzel R, Heidland A, Spustová V, Dzúrik R: Effects of ramipril in nondiabetic nephropathy: Improved parameters of oxidatives stress and potential modulation of advanced glycation end products. J Hum Hypertens 17: 265–270, 2003 [DOI] [PubMed] [Google Scholar]
- 205.Baba SP, Wetzelberger K, Hoetker JD, Bhatnagar A: Posttranslational glutathiolation of aldose reductase (AKR1B1): A possible mechanism of protein recovery from S-nitrosylation. Chem Biol Interact 178: 250–258, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Nakamura N, Yamazaki K, Satoh A, Urakaze M, Kobayashi M, Yamabe H, Osawa H, Shirato K, Sugawara T, Nakamura M, Tamura M, Okumura K: Effects of eparlestat on plasma levels of advanced glycation end products in patients with type 2 diabetes. In Vivo 17: 177–180, 2003 [PubMed] [Google Scholar]
- 207.Susic D, Varagic J, Ahn J, Frohlich ED: Crosslink breakers: A new approach to cardiovascular therapy. Curr Opin Cardiol 19: 336–340, 2004 [DOI] [PubMed] [Google Scholar]
- 208.Kass DA, Shapiro EP, Kawaguchi M, Capriotti AR, Scuteri A, deGroof RC, Lakatta EG: Improved arterial compliance by a novel advanced glycation end-product crosslink breaker. Circulation 104: 1464–1470, 2001 [DOI] [PubMed] [Google Scholar]
- 209.Park J, Kwon MK, Huh JY, Choi WJ, Jeong LS, Nagai R, Kim WY, Kim J, Lee GT, Lee HB, Ha H: Renoprotective antioxidant effect of alagebrium in experimental diabetes. Nephrol Dial Transplant 26: 3474–3484, 2011 [DOI] [PubMed] [Google Scholar]
- 210.Peppa M, Brem H, Cai W, Zhang JG, Basgen J, Li Z, Vlassara H, Uribarri J: Prevention and reversal of diabetic nephropathy in db/db mice treated with alagebrium (ALT-711). Am J Nephrol 26: 430–436, 2006 [DOI] [PubMed] [Google Scholar]
- 211.Forbes JM, Thallas V, Thomas MC, Founds HW, Burns WC, Jerums G, Cooper ME: The breakdown of preexisting advanced glycation end products is associated with reduced renal fibrosis in experimental diabetes. FASEB J 17: 1762–1764, 2003 [DOI] [PubMed] [Google Scholar]
- 212.Thallas-Bonke V, Lindschau C, Rizkalla B, Bach LA, Boner G, Meier M, Haller H, Cooper ME, Forbes JM: Attenuation of extracellular matrix accumulation in diabetic nephropathy by the advanced glycation end product cross-link breaker ALT-711 via a protein kinase C-alpha-dependent pathway. Diabetes 53: 2921–2930, 2004 [DOI] [PubMed] [Google Scholar]
- 213.Wolffenbuttel BH, Boulanger CM, Crijns FR, Huijberts MS, Poitevin P, Swennen GN, Vasan S, Egan JJ, Ulrich P, Cerami A, Lévy BI: Breakers of advanced glycation end products restore large artery properties in experimental diabetes. Proc Natl Acad Sci U S A 95: 4630–4634, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Asif M, Egan J, Vasan S, Jyothirmayi GN, Masurekar MR, Lopez S, Williams C, Torres RL, Wagle D, Ulrich P, Cerami A, Brines M, Regan TJ: An advanced glycation endproduct cross-link breaker can reverse age-related increases in myocardial stiffness. Proc Natl Acad Sci U S A 97: 2809–2813, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Cheng G, Wang LL, Long L, Liu HY, Cui H, Qu WS, Li S: Beneficial effects of C36, a novel breaker of advanced glycation endproducts cross-links, on the cardiovascular system of diabetic rats. Br J Pharmacol 152: 1196–1206, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Cooper ME, Thallas V, Forbes J, Scalbert E, Sastra S, Darby I, Soulis T: The cross-link breaker, N-phenacylthiazolium bromide prevents vascular advanced glycation end-product accumulation. Diabetologia 43: 660–664, 2000 [DOI] [PubMed] [Google Scholar]
- 217.Marx N, Walcher D, Ivanova N, Rautzenberg K, Jung A, Friedl R, Hombach V, de Caterina R, Basta G, Wautier MP, Wautiers JL: Thiazolidinediones reduce endothelial expression of receptors for advanced glycation end products. Diabetes 53: 2662–2668, 2004 [DOI] [PubMed] [Google Scholar]
- 218.Bhatwadekar A, Glenn JV, Figarola JL, Scott S, Gardiner TA, Rahbar S, Stitt AW: A new advanced glycation inhibitor, LR-90, prevents experimental diabetic retinopathy in rats. Br J Ophthalmol 92: 545–547, 2008 [DOI] [PubMed] [Google Scholar]
- 219.Wendt TM, Tanji N, Guo J, Kislinger TR, Qu W, Lu Y, Bucciarelli LG, Rong LL, Moser B, Markowitz GS, Stein G, Bierhaus A, Liliensiek B, Arnold B, Nawroth PP, Stern DM, D’Agati VD, Schmidt AM: RAGE drives the development of glomerulosclerosis and implicates podocyte activation in the pathogenesis of diabetic nephropathy. Am J Pathol 162: 1123–1137, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Zhang Y, Lapidos KA, Gal-Moscovici A, Sprague SM, Ameer GA: A receptor-based bioadsorbent to target advanced glycation end products in chronic kidney disease. Artif Organs 38: 474–483, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Nakamura T, Sato E, Fujiwara N, Kawagoe Y, Koide H, Ueda Y, Takeuchi M, Yamagishi S: Calcium channel blocker inhibition of AGE and RAGE axis limits renal injury in nondiabetic patients with stage I or II chronic kidney disease. Clin Cardiol 34: 372–377, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Kanazawa I, Yamamoto M, Yamaguchi T, Sugimoto T: Effects of metformin and pioglitazone on serum pentosidine levels in type 2 diabetes mellitus. Exp Clin Endocrinol Diabetes 119: 362–365, 2011 [DOI] [PubMed] [Google Scholar]
- 223.Tan KC, Chow WS, Tso AW, Xu A, Tse HF, Hoo RL, Betteridge DJ, Lam KS: Thiazolidinedione increases serum soluble receptor for advanced glycation end-products in type 2 diabetes. Diabetologia 50: 1819–1825, 2007 [DOI] [PubMed] [Google Scholar]
- 224.Okamoto T, Yamagishi S, Inagaki Y, Amano S, Koga K, Abe R, Takeuchi M, Ohno S, Yoshimura A, Makita Z: Angiogenesis induced by advanced glycation end products and its prevention by cerivastatin. FASEB J 16: 1928–1930, 2002 [DOI] [PubMed] [Google Scholar]
- 225.Cuccurullo C, Iezzi A, Fazia ML, De Cesare D, Di Francesco A, Muraro R, Bei R, Ucchino S, Spigonardo F, Chiarelli F, Schmidt AM, Cuccurullo F, Mezzetti A, Cipollone F: Suppression of RAGE as a basis of simvastatin-dependent plaque stabilization in type 2 diabetes. Arterioscler Thromb Vasc Biol 26: 2716–2723, 2006 [DOI] [PubMed] [Google Scholar]
- 226.Cocchietto M, Zorzin L, Toffoli B, Candido R, Fabris B, Stebel M, Sava G: Orally administered microencapsulated lysozyme downregulates serum AGE and reduces the severity of early-stage diabetic nephropathy. Diabetes Metab 34: 587–594, 2008 [DOI] [PubMed] [Google Scholar]
- 227.Elosta A, Ghous T, Ahmed N: Natural products as anti-glycation agents: Possible therapeutic potential for diabetic complications. Curr Diabetes Rev 8: 92–108, 2012 [DOI] [PubMed] [Google Scholar]
- 228.Nagai R, Shirakawa J, Ohno R, Moroishi N, Nagai M: Inhibition of AGEs formation by natural products. Amino Acids 46: 261–266, 2014 [DOI] [PubMed] [Google Scholar]
- 229.Ha T, Ngoc TM, Lee I, Lee YM, Kim JS, Jung H, Lee S, Na M, Bae K, Ha do : Inhibitors of aldose reductase and formation of advanced glycation end-products in moutan cortex (Paeonia suffruticosa). J Nat Prod 72: 1465–1470, 2009 [DOI] [PubMed] [Google Scholar]
- 230.Zhang MH, Feng L, Zhu MM, Gu JF, Jiang J, Cheng XD, Ding SM, Wu C, Jia XB: The anti-inflammation effect of Moutan Cortex on advanced glycation end products-induced rat mesangial cells dysfunction and High-glucose-fat diet and streptozotocin-induced diabetic nephropathy rats. J Ethnopharmacol 151: 591–600, 2014 [DOI] [PubMed] [Google Scholar]
- 231.Zhang M, Feng L, Gu J, Ma L, Qin D, Wu C, Jia X: The attenuation of Moutan Cortex on oxidative stress for renal injury in AGEs-induced mesangial cell dysfunction and streptozotocin-induced diabetic nephropathy rats. Oxid Med Cell Longev 2014: 463815, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Perez Gutierrez RM, de Jesus Martinez Ortiz M: Beneficial effect of Azadirachta indica on advanced glycation end-product in streptozotocin-diabetic rat. Pharm Biol 52: 1435–1444, 2014 [DOI] [PubMed] [Google Scholar]
- 233.Trionfini P, Benigni A, Remuzzi G: MicroRNAs in kidney physiology and disease. Nat Rev Nephrol 11: 23–33, 2015 [DOI] [PubMed] [Google Scholar]
- 234.Chung AC, Yu X, Lan HY: MicroRNA and nephropathy: Emerging concepts. Int J Nephrol Renovasc Dis 6: 169–179, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Hagiwara S, McClelland A, Kantharidis P: MicroRNA in diabetic nephropathy: Renin angiotensin, aGE/RAGE, and oxidative stress pathway. J Diabetes Res 2013: 173783, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Zhong X, Chung AC, Chen HY, Dong Y, Meng XM, Li R, Yang W, Hou FF, Lan HY: miR-21 is a key therapeutic target for renal injury in a mouse model of type 2 diabetes. Diabetologia 56: 663–674, 2013 [DOI] [PubMed] [Google Scholar]
- 237.Piperi C, Goumenos A, Adamopoulos C, Papavassiliou AG: AGE/RAGE signalling regulation by miRNAs: Associations with diabetic complications and therapeutic potential. Int J Biochem Cell Biol 60: 197–201, 2015 [DOI] [PubMed] [Google Scholar]
- 238.Wu XD, Liu WL, Zeng K, Lei HY, Zhang QG, Zhou SQ, Xu SY: Advanced glycation end products activate the miRNA/RhoA/ROCK2 pathway in endothelial cells. Microcirculation 21: 178–186, 2014 [DOI] [PubMed] [Google Scholar]
- 239.Pan Y, Liang H, Liu H, Li D, Chen X, Li L, Zhang CY, Zen K: Platelet-secreted microRNA-223 promotes endothelial cell apoptosis induced by advanced glycation end products via targeting the insulin-like growth factor 1 receptor. J Immunol 192: 437–446, 2014 [DOI] [PubMed] [Google Scholar]
- 240.Chen HY, Zhong X, Huang XR, Meng XM, You Y, Chung AC, Lan HY: MicroRNA-29b inhibits diabetic nephropathy in db/db mice. Mol Ther 22: 842–853, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Choi BH, Kang KS, Kwak MK: Effect of redox modulating NRF2 activators on chronic kidney disease. Molecules 19: 12727–12759, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Lee BH, Hsu WH, Chang YY, Kuo HF, Hsu YW, Pan TM: Ankaflavin: A natural novel PPARγ agonist upregulates Nrf2 to attenuate methylglyoxal-induced diabetes in vivo. Free Radic Biol Med 53: 2008–2016, 2012 [DOI] [PubMed] [Google Scholar]
- 243.Lee BH, Hsu WH, Huang T, Chang YY, Hsu YW, Pan TM: Effects of monascin on anti-inflammation mediated by Nrf2 activation in advanced glycation end product-treated THP-1 monocytes and methylglyoxal-treated wistar rats. J Agric Food Chem 61: 1288–1298, 2013 [DOI] [PubMed] [Google Scholar]
- 244.Lee BH, Hsu WH, Hsu YW, Pan TM: Suppression of dimerumic acid on hepatic fibrosis caused from carboxymethyl-lysine (CML) by attenuating oxidative stress depends on Nrf2 activation in hepatic stellate cells (HSCs). Food Chem Toxicol 62: 413–419, 2013 [DOI] [PubMed] [Google Scholar]
- 245.Lee BH, Hsu WH, Hsu YW, Pan TM: Dimerumic acid attenuates receptor for advanced glycation endproducts signal to inhibit inflammation and diabetes mediated by Nrf2 activation and promotes methylglyoxal metabolism into d-lactic acid. Free Radic Biol Med 60: 7–16, 2013 [DOI] [PubMed] [Google Scholar]
- 246.Wang YY, Yang YX, Zhe H, He ZX, Zhou SF: Bardoxolone methyl (CDDO-Me) as a therapeutic agent: An update on its pharmacokinetic and pharmacodynamic properties. Drug Des Devel Ther 8: 2075–2088, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.de Zeeuw D, Akizawa T, Audhya P, Bakris GL, Chin M, Christ-Schmidt H, Goldsberry A, Houser M, Krauth M, Lambers Heerspink HJ, McMurray JJ, Meyer CJ, Parving HH, Remuzzi G, Toto RD, Vaziri ND, Wanner C, Wittes J, Wrolstad D, Chertow GM, BEACON Trial Investigators : Bardoxolone methyl in type 2 diabetes and stage 4 chronic kidney disease. N Engl J Med 369: 2492–2503, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]