Abstract
Childhood chronic kidney disease (CHD) poses multiple threats to bone accrual; however, the associated fracture risk is not well characterized. This prospective cohort study included 537 CKD in Children (CKiD) participants. Fracture histories were obtained at baseline, at years 1, 3, and 5 through November 1, 2009, and annually thereafter. We used Cox regression analysis of first incident fracture to evaluate potential correlates of fracture risk. At enrollment, median age was 11 years, and 16% of patients reported a prior fracture. Over a median of 3.9 years, 43 males and 24 females sustained incident fractures, corresponding to 395 (95% confidence interval [95% CI], 293–533) and 323 (95% CI, 216–481) fractures per 10,000 person-years, respectively. These rates were 2- to 3-fold higher than published general population rates. The only gender difference in fracture risk was a 2.6-fold higher risk in males aged ≥15 years (570/10,000 person-years, adjusted P=0.04). In multivariable analysis, advanced pubertal stage, greater height Z-score, difficulty walking, and higher average log-transformed parathyroid hormone level were independently associated with greater fracture risk (all P≤0.04). Phosphate binder treatment (predominantly calcium-based) was associated with lower fracture risk (hazard ratio, 0.37; 95% CI, 0.15–0.91; P=0.03). Participation in more than one team sport was associated with higher risk (hazard ratio, 4.87; 95% CI, 2.21–10.75; P<0.001). In conclusion, children with CKD have a high burden of fracture. Regarding modifiable factors, higher average parathyroid hormone level was associated with greater risk of fracture, whereas phosphate binder use was protective in this cohort.
Keywords: chronic kidney disease, children, clinical epidemiology
Childhood CKD is associated with nearly universal disturbances in bone and mineral metabolism, collectively termed chronic kidney disease–mineral and bone disorder (CKD-MBD), that present multiple obstacles to bone accrual. In addition to secondary hyperparathyroidism and abnormal mineral metabolism (hyperphosphatemia, hypocalcemia, vitamin D deficiency, and decreased 1-α-hydroxylation of 25-hydroxyvitamin D [25(OH)D]), CKD is associated with delayed pubertal maturation, growth failure, abnormalities in the growth hormone axis, malnutrition, acidosis, and muscle deficits.1–6 Treatment with glucocorticoids may further compromise bone health. The resultant deficits in bone density and micro-architecture likely impact short- and long-term fracture risk.7
It is well established that adults with ESRD have a substantially increased risk of fracture and associated morbidity and mortality.8–17 In a study using the United States Renal Data System, Alem et al. found that compared with healthy controls, young adults on hemodialysis had a 100-fold higher hip fracture rate.9 In a population-based Dutch cohort of 247 adults with a history of ESRD at ≤14 years of age, 89% had osteopenia, 37% had clinical symptoms of bone disease, and 18% were disabled by bone disease.18 Similarly, adults with a history of renal transplantation in childhood reported high rates of fracture.19 Several studies have extended these observations to adults with moderate CKD.20–31
Until recently, studies in children were limited to a report in solid organ transplant recipients, demonstrating a 6-fold higher incidence of all fractures and 160-fold higher incidence of vertebral fractures.32 We subsequently completed a study in 170 children and adolescents with CKD and ESRD. Over an average follow-up of 1 year, 6.5% of participants sustained a fracture (incidence of 556/10,000 person-years), and lower baseline cortical volumetric bone mineral density (BMD) was associated with risk of subsequent fracture.1 The sample size and follow-up duration were insufficient to define gender-specific incidence and identify risk factors for fracture. To our knowledge, no other data are available in children with CKD.
The large prospective Chronic Kidney Disease in Children (CKiD) cohort study represents a unique resource to evaluate fracture burden in pediatric CKD. The study objectives were to determine the gender-specific incidence of fracture in CKiD and to identify risk factors for fracture in this high-risk population.
RESULTS
Fracture Incidence
Participant characteristics at enrollment (Table 1) were representative of the full CKiD cohort.3,33 At enrollment, 16% had a history of prior fracture. Over a median follow-up of 3.9 years (interquartile range 1.8–4.9), 67 participants reported an incident fracture, representing a cumulative incidence of 12.5%. Of these, 43 fractures occurred among males and 24 among females, corresponding to rates of 395 (95% confidence interval [95% CI], 293–533) and 323 (95% CI, 216–481) per 10,000 person-years, respectively. The median age at first incident fracture was 15 years in males and 13 years in females. Table 2 shows fracture incidence stratified by age and gender. Males ≥15 years of age had the highest fracture rate of 570/10,000 person-years.
Table 1.
Baseline cohort characteristics
| Characteristic | N (%) or median (IQR) | N |
|---|---|---|
| Age (years) | 11.0 (7.4, 14.5) | 537 |
| Male gender | 326 (61) | 537 |
| Black race | 116 (22) | 537 |
| Hispanic | 76 (14) | 531 |
| Tanner stage | 514 | |
| 1 | 307 (60) | |
| 2 | 49 (10) | |
| 3 | 42 (8) | |
| 4 | 72 (14) | |
| 5 | 44 (9) | |
| Height Z-score | −0.7 (−1.4, 0.1) | 523 |
| Body mass index Z-score | 0.4 (−0.4, 1.2) | 521 |
| eGFR (ml/min 1.73 m2) | 46.5 (34.4, 58.5) | 531 |
| CKD stage | 531 | |
| 1/2 | 119 (22) | |
| 3 | 325 (61) | |
| 4/5 | 87 (16) | |
| CKD duration (years) | 8.5 (4.4, 12.9) | 531 |
| Glomerular etiology | 110 (20) | 537 |
| Autoimmune disease | 14 (3) | 530 |
| Prematurity | 65 (13) | 519 |
| Walking difficulty | 64 (12) | 532 |
| Medications | 537 | |
| Vitamin/mineral supplement | 122 (23) | |
| Nutritional vitamin D supplement | 27 (5) | |
| Activated vitamin D | 191 (36) | |
| Phosphate binder | 102 (19) | |
| Alkali therapy | 152 (28) | |
| Growth hormone | 60 (11) | |
| Diuretic | 34 (6) | |
| Corticosteroid | 36 (7) | |
| Albumin (g/dl) | 4.3 (4.1, 4.5) | 533 |
| Corrected calcium (mg/dl) | 9.4 (9.1, 9.6) | 533 |
| Phosphorus (mg/dl) | 4.5 (4.0, 5.0) | 525 |
| Parathyroid hormone (pg/ml) | 48.2 (28.5, 83.1) | 379 |
| 25-hydroxyvitamin D [25(OH)D, ng/ml] | 28.3 (19.1, 35.1) | 388 |
| Vitamin D deficiency [25(OH)D <20 ng/ml] | 105 (27) | |
| FGF23 (RU/ml) | 131.2 (87.9, 197.9) | 388 |
IQR, interquartile range.
Table 2.
Fracture incidence by age and gender
| Age (Years) | Person-Time (Person-Years) | Fractures (n) | Incidence (Per 10,000 Person-Years) | 95% CI | |
|---|---|---|---|---|---|
| Female | <15 | 456 | 17 | 373 | (232, 600) |
| ≥15 | 289 | 7 | 242 | (116, 509) | |
| Male | <15 | 720 | 22 | 306 | (201, 464) |
| ≥15 | 368 | 21 | 570 | (372, 874) |
Fracture Sites
For 17 (25%) of the 67 incident fracture events, a specific fracture site was not recorded. The distribution of fracture sites differed significantly by gender (P=0.03, Table 3). In females, fractures of the ankle/foot were most common, accounting for 26% of fractures. In males, fractures of the wrist/hand and arm/elbow were most common, together accounting for 33% of fractures.
Table 3.
Distribution of fracture site by gender
| Female | Male | |
|---|---|---|
| Site | n (%) | n (%) |
| Shoulder | 0 (0) | 1 (2.2) |
| Arm/elbow | 2 (7.4) | 7 (15.6) |
| Wrist/hand | 0 (0) | 8 (17.8) |
| Finger | 3 (11.1) | 5 (11.1) |
| Hip | 1 (3.7) | 0 (0) |
| Leg/knee | 1 (3.7) | 5 (11.1) |
| Ankle/foot | 7 (25.9) | 6 (13.3) |
| Toe | 3 (11.1) | 0 (0) |
| Collar bone | 1 (3.7) | 3 (6.7) |
| Rib | 0 (0) | 1 (2.2) |
| Back | 1 (3.7) | 0 (0) |
| Unspecified | 8 (29.6) | 9 (20) |
| Totala | 27 (100) | 45 (100) |
The total n exceeds 67 first fracture events as there were fractures involving multiple sites.
Correlates of Incident Fracture
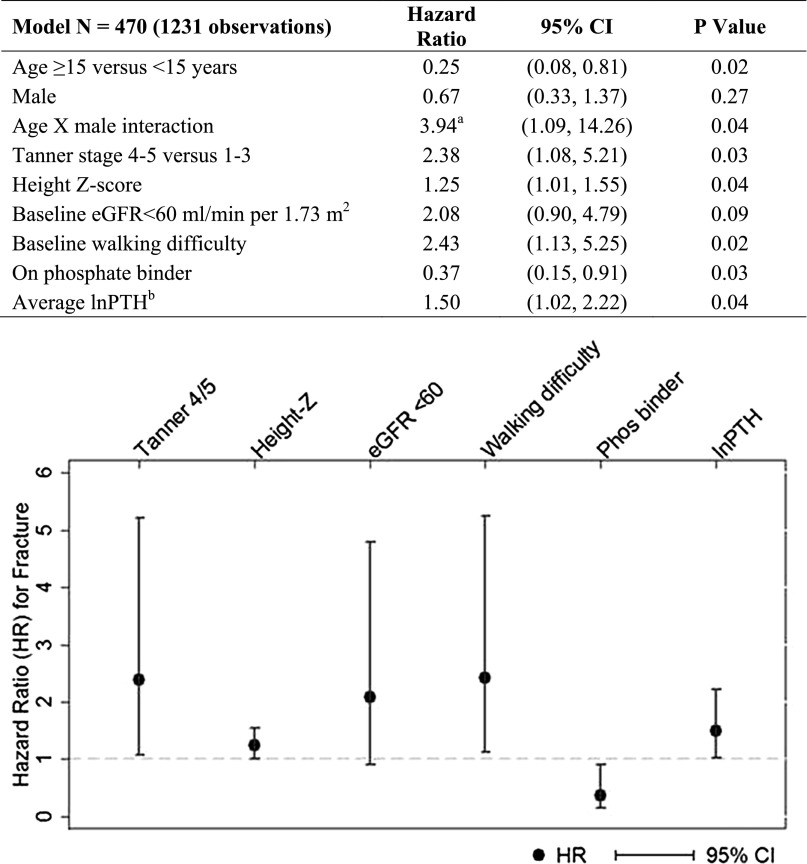
The covariates with P<0.2 when individually assessed in a base Cox regression model including age, gender, and their interaction were: Tanner stage, height Z-score, baseline eGFR<60 ml/min per 1.73 m2, phosphate binder use, calciferol supplementation, history of head injury, and both baseline and average concentrations of log-transformed parathyroid hormone (lnPTH) and 25(OH)D. In addition to these covariates, black race, prematurity, and baseline walking difficulty were also considered in multivariable regression, because of a priori hypotheses regarding their impact on fracture risk. Body mass index Z-score, log-transformed fibroblast growth factor 23 (FGF23) level, active vitamin D therapy, and corticosteroid exposure were not associated with fracture when individually assessed in the base Cox regression model of age, gender, and their interaction (all P>0.4). Figure 1 shows the final multivariable Cox regression model arrived at using both backward and forward selection. There was a statistically significant interaction between age and gender; there was no gender difference in fracture risk below 15 years of age, but being a male ≥15 years of age was associated with a 2.6-fold higher fracture risk, compared with a female ≥15 years. Tanner stage, height Z-score, walking difficulty, lnPTH, and phosphate binder use remained independent correlates of fracture risk in multivariable analysis. There was a 25% higher risk per standard deviation greater height Z-score. Advanced pubertal stage (Tanner 4–5 versus 1–3) and walking difficulty were both associated with a more than 2-fold greater risk of fracture. There was a 50% higher risk associated with each unit greater average lnPTH. Substituting baseline for average lnPTH yielded similar results with a hazard ratio (HR) of 1.50 (95% CI, 1.04–2.15; P=0.03). Phosphate binder use was protective, with a 63% lower hazard of fracture. Approximately 20% of the cohort was on a binder at each visit, and the majority of this phosphate binder use was calcium-based (median 82%; Supplemental Table 1). Substituting use of a calcium-containing binder and/or vitamin for any phosphate binder was similarly protective in the final multivariable Cox model (HR, 0.37; 95% CI, 0.17–0.82, P=0.02). At each visit, there was no difference in the serum concentrations of corrected calcium among participants who were taking as compared with those who were not taking a phosphate binder, while serum phosphorus at most visits was significantly higher in those on a phosphate binder compared with those not on a phosphate binder (Supplemental Table 2). With the exception of year 4, PTH concentration did not differ by phosphate binder use. Without adjusting for PTH in the model, having a baseline eGFR<60 was associated with a HR of 2.43 (P=0.02); this was attenuated to 2.08 (P=0.09) after adjustment for average lnPTH. Race, history of prevalent fracture, prematurity, underlying glomerular versus non-glomerular disease, CKD duration, and serum concentrations of calcium, phosphorus, and 25(OH)D were not independent risk factors for fracture.
Figure 1.
Final multivariable Cox regression model: correlates of incident fracture. aHR for males ≥15 years versus females ≥15 years=(3.94*0.67)=2.6. bPTH natural log transformed.
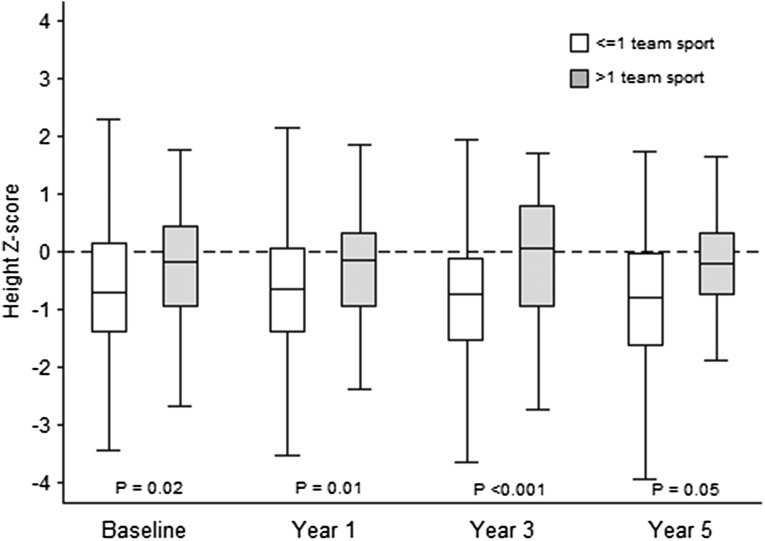
While the availability of physical activity data were limited to participants ≥12 years of age, when these measures were assessed in the final multivariable model derived from analysis of the full cohort, participation in a greater number of team sports was independently associated with higher fracture risk (Table 4). Participation in >1 sport was associated with a nearly 5-fold higher risk of facture (HR, 4.87; 95% CI, 2.21–10.75; P<0.001). Participation in ≥1 team sport was associated with a HR of 2.35 (95% CI, 1.01–5.47; P=0.047). The substantial reduction in sample size attenuated the effect of other covariates; however, the strong protective effect of phosphate binder use persisted, with a HR of 0.12 (95% CI, 0.01–0.91; P=0.04). At each visit when physical activity was assessed, height Z-score was positively associated with the number of team sports in the prior 12 months (spearman’s Rho=0.14–0.23, all P≤0.03). The median height of the participants who played >1 team sport was closer to the 50th percentile as compared with those who participated in ≤1 team sport for whom the median height was consistent with that of the entire cohort around the 25th percentile (Figure 2).
Table 4.
Final multivariable Cox regression model: correlates of incident fracture, including measures of physical activity
| Model n=290 (483 observations) | HR | 95% CI | P Value |
|---|---|---|---|
| Age ≥15 versus <15 years | 0.39 | (0.10, 1.49) | 0.17 |
| Male | 0.40 | (0.12, 1.36) | 0.14 |
| Age X male interaction | 3.89 | (0.72, 21.04) | 0.12 |
| Tanner stages 4–5 versus 1–3 | 1.42 | (0.58, 3.52) | 0.45 |
| Height Z-score | 1.17 | (0.89, 1.55) | 0.25 |
| Baseline eGFR<60 ml/min per 1.73 m2 | 2.46 | (0.71, 8.50) | 0.15 |
| Baseline walking difficulty | 1.07 | (0.15, 7.69) | 0.95 |
| On phosphate binder | 0.12 | (0.01, 0.91) | 0.04 |
| Average lnPTHa | 1.19 | (0.68, 2.06) | 0.54 |
| >1 sports team in past 12 months | 4.87 | (2.21, 10.75) | <0.001 |
PTH natural log transformed.
Figure 2.
Relationship between height and sports participation.
DISCUSSION
This is the first study to evaluate the burden of fracture risk in a large prospective cohort of children with CKD. Numerous studies have demonstrated increased fracture rates in adults with ESRD8–17 and CKD.20–31 With 90% of peak skeletal mass accrued by age 18, children with CKD are uniquely vulnerable to its multiple threats to bone health, but the study of fractures in this population has been hampered by a dearth of large prospective studies. The strengths of the CKiD cohort allowed us to define gender-specific fracture incidence and identify multiple specific risk factors in pediatric CKD.
The observed fracture rates of 395/10,000 person-years for males and 323/10,000 person-years for females in CKiD were 2.4- and 3-fold higher, respectively than gender-specific rates of 162/10,000 person-years and 103/10,000 person-years reported in a large population-based study of fracture epidemiology in children and adolescents.34 Considering their 95% CIs, the observed fracture rates in Table 2 were 1.8- to 3.3-fold higher in males and 2.1- to 4.7-fold higher in females, suggesting that the relative impact of CKD may be greater among females. Consistent with fracture epidemiology in the general population, incidence varied with age and gender, with males ≥15 years of age having the highest incidence of 570/10,000 person-years, which was again 2-fold higher than previously reported peak incidence in 14–15-year-old males (274–282/10,000 person-years).34 Although as expected teenage males had the highest rate of fracture, the fact that the rate of fracture in females <15 years of age (373/10,000 person-years) far surpassed the peak incidence in the general teenage male population speaks to the possible greater relative risk in females. To underscore the burden of fracture in this population, the observed rates for both genders in the CKiD cohort exceeded that reported in 12,782 adult hemodialysis patients (256/10,000 person-years).14 The higher overall incidence (556/10,000 person-years) in our prior smaller pediatric study1 was likely attributable to the inclusion of participants (30%) with ESRD.
We identified several significant and independent factors associated with higher incident fracture, including baseline walking difficulty, Tanner stage 4–5, greater height Z-score, higher average PTH, and competitive sports participation. Phosphate binder use was the only independent protective factor identified. We previously reported that secondary hyperparathyroidism was associated with declines in peripheral quantitative computed tomography measures of cortical BMD in childhood CKD,1,5 as anticipated given the high bone turnover state of hyperparathyroidism.35 Cross-sectional bone histomorphometry studies in children demonstrated that defective mineralization was present as early as stage 2 CKD, and the prevalence increased with progressive CKD severity, exceeding 90% in CKD 5D.36 In children on peritoneal dialysis, this mineralization defect persisted after therapy with active vitamin D.37 In children with pre-dialysis CKD, lower serum calcium and higher PTH concentrations were associated with defective mineralization.36 Taken together, these findings suggest that hyperparathyroidism contributes to increased fracture risk in children with CKD via impaired mineralization.
The finding of phosphate binder use being protective with a >60% lower fracture risk in multivariable analysis, even more so among the adolescent subset of the cohort with physical activity data, is provocative and warrants further investigation. Given the demonstrated association of lower serum calcium with defective mineralization36 and that the majority of phosphate binder use in CKiD was calcium-based, we hypothesize that the protective effect may be due to calcium supplementation; indeed, substituting use of a calcium-containing binder and/or vitamin was similarly protective in the final multivariable Cox model, with a 63% lower fracture risk. This is supportive of our prior finding that serum calcium was an independent correlate of cortical BMD, particularly in the growing skeleton.1 Furthermore, the protective effect of phosphate binder use was clearly not related to a beneficial effect on serum phosphorus or FGF23 given that neither was associated with fracture and that phosphorus concentrations were higher in those taking versus not taking binders at most visits. At a time of growing concerns about limiting calcium-based binder use due to associations with vascular calcification in adults, these data provide a cautionary note regarding age-appropriate calcium requirements for optimal bone health in children.38
Though not statistically significant after adjustment for PTH, the fully adjusted HR of 2.08 associated with a baseline eGFR<60 ml/min per 1.73 m2 is highly consistent with findings in adults with CKD. In a recent study of >600,000 individuals ≥40 years of age,30 the incidence rate ratio for fracture in 40–65-year-old year old men with an eGFR of 30–44 and 15–29 was 1.7 and 2.2, respectively, compared with the reference group with eGFR≥60. Similarly, the rate ratio for fracture in 40–65-year-old women was 2.4 for both an eGFR of 30–44 and eGFR of 15–29 versus eGFR≥60. The association with walking difficulty may be due to increased risk of falls and osteopenia from immobility. The strong independent relationship with height Z-score was an interesting finding. Childhood CKD is associated with a substantial burden of growth failure and short stature,39 supported by the median height Z-score of -0.7, with 16% of the cohort below the 3rd percentile. A recent CKiD study found a significant association between catch-up growth and parental report of physical functioning.40 Consistent with this, we found that greater height Z-score was related to greater participation in team sports, which was a strong independent correlate of fracture risk. The attenuation of the association of height Z-score with fracture risk in the multivariable analysis including team sports participation may have been due in part to the smaller sample size, but the magnitude and statistical significance of the association with team sports participation in this limited sample indicates that the association with height Z-score was at least in part confounded by team sports participation.
The study limitations are largely related to the fact that prospective assessment of fracture outcomes was not an initial aim of the CKiD study. While participants were asked at study visits about interim fractures, the fracture date was not recorded, and information regarding the mechanism of injury was not collected. However, a prior population-based study demonstrated that skeletal fragility contributed to fracture risk in children, regardless of trauma severity.41 Since time between visits was up to 1–2 years, censoring at the midpoint of this interval may have created some misclassification of time and consequent imprecision in the observed incidence rates. This, along with the fact that fracture events were not adjudicated by radiology reports, likely biased the results to the null. Furthermore, the lower bound of the 95% CI for the observed rates far surpassed general population rates. Fracture site was not specified for 25% of the incident fracture events. Physical activity assessments were only available in the adolescent subset of the cohort. However, the association with competitive sports was robust. The etiology of the reported walking difficulty was not ascertained. BMI Z-score did not differ between those with versus without walking difficulty. Finally, since fractures were ascertained through questionnaire, incidence of vertebral fractures, which are often subclinical, was likely underestimated.
In summary, fracture rates in the CKiD cohort were 2- to 3-fold higher than population-based rates, with the greatest burden among males at the time of peak fracture incidence (570/10,000 person-years). Independent modifiable factors included PTH and phosphate binder use. Higher average PTH concentration was associated with greater risk, while phosphate binder use was largely calcium-based and was protective. Team sports participation was a significant risk factor for fracture. Future studies are needed to determine how to optimize CKD-MBD management, including calcium requirements, for fracture prevention.
CONCISE METHODS
Study Design and Participants
We studied 537 children and adolescents with CKD from 48 North American centers who enrolled in CKiD between 2005 and 2011; CKiD methods have been published.42 Eligibility criteria included: age 1–16 years, eGFR 30 to <90 ml/min per 1.73 m2,43 no prior organ transplantation, no diagnosis of malignancy or HIV within 12 months, and no history of structural heart disease, genetic syndromes involving the central nervous system, or severe cognitive disability. Participants were followed annually for up to 6 years. The study adhered to the Declaration of Helsinki, and the Institutional Review Boards of all participating sites approved the CKiD protocol. Informed consent was obtained from a parent/guardian and assent/consent from the participant.
Assessment of Fracture Outcome
Participants/guardians were asked whether the participant ever had a fracture (prevalent fracture) at the baseline visit. They were then interviewed at years 1, 3, and 5 regarding new (incident) fractures in the interim since the prior visit (Supplemental Figure 1). 537 participants provided interval fracture histories and are included in this analysis of incident fractures. Fracture data were available in 98% (n=518), 95% (n=372), and 97% (n=184) of participants completing the year 1, year 3, and year 5 visits, respectively. After November 1, 2009 participants/guardians were interviewed at each annual visit about new fractures in the antecedent year, so data on fracture occurrence were available for 17% (n=78), 87% (n=273), and 97% (n=60) of participants at years 2, 4, and 6, respectively.
Assessment of CKD Characteristics and Risk Factors
Potential correlates of fracture included: age, gender, black versus non-black race, Hispanic ethnicity; time-varying anthropometry (height and body mass index Z-scores, Tanner stage); baseline comorbidities with impact on bone health (prevalent fracture, prematurity, autoimmunity, walking difficulty, history of head injury, coma, or seizure); CKD severity, duration, and glomerular versus non-glomerular etiology; relevant medications (calciferol, active vitamin D, phosphate binder, alkali therapy, corticosteroid); and biochemical markers of mineral metabolism and nutrition (calcium, phosphorus, intact PTH, FGF23, 25(OH)D, albumin).
For CKD severity, eGFR was estimated using the CKiD equation44 and examined both as a continuous and categorical exposure and as a baseline and time-varying covariate. Medication usage was time-varying and categorical (on/off therapy at the time of visit). Calcium, phosphorus, and albumin were time-varying and available for each annual visit.45 Serum PTH and 25(OH)D and plasma C-terminal FGF23 were first measured 3–6 months after baseline in 379, 388, and 388 participants, respectively, and then at alternating visits from the second annual visit onwards45–47; both the baseline and average values were considered for PTH, FGF23, and 25(OH)D. Serum calcium concentrations were corrected for albumin: corrected calcium=measured calcium+0.8×(4.0−albumin).
Questions from the 2005 Youth Risk Behavior Survey were used to assess physical activity in participants ≥12 years of age,48 including measures of exercise-related sweating, non-sweat exercise, hours watching television or playing video games/using computer, physical education classes and duration, and the number of sports teams played on in the past 12 months.
Analysis
Descriptive statistics were reported as median and interquartile range for continuous variables and frequencies for categorical variables. Group differences in continuous variables were assessed using the t-test or Wilcoxon rank-sum test as appropriate and categorical variables using the chi-squared test. PTH and FGF23 levels were skewed and, therefore, natural log transformed.
Data were censored at the last visit with available fracture data. For the assessment of fracture incidence, follow-up time was censored at the midpoint of the interval between the visit at which the first incident fracture was reported and the antecedent visit. As time between visits was up to 1–2 years, censoring at the midpoint of this interval ensured that on average, the time of fracture was closer to when the fracture was actually sustained. Multivariable Cox proportional hazards regression was used to evaluate the potential correlates of fracture delineated above. The robust standard error option was used to limit misspecification.49
Prior population-based studies reported greater fracture rates in adolescent males; therefore, we examined multiplicative interactions between age and gender. For regression analyses, all covariates were individually assessed in a base model including age ≥15, gender, and their interaction. Covariates with a P value <0.2 were considered in the multivariable regressions using both forward and backward stepwise selection approaches. Covariates with adjusted P value <0.1 were retained in the final model. Physical activity measures were available in the subset ≥12 years old at baseline and years 1, 3, and 5 and were tested in the final multivariable Cox model including variables derived from analysis of the full cohort. A two-sided P value of <0.05 was considered statistically significant. Analyses were performed using STATA 13.0 software (Stata Corporation, College Station, TX).
DISCLOSURES
M.R.D. has received research funding from Genentech. J.K. has received Honoraria from Alexion Pharmaceuticals. A.A.P. is a speaker for Sanofi. I.B.S. has received research funding from Amgen and AbbVie and is a consultant for Genentech, OPKO Health and Keryx Biopharmaceuticals. B.A.W. is a consultant for Amgen and AbbVie.
Supplementary Material
Acknowledgments
Data in this manuscript were collected by the CKiD prospective cohort study with clinical coordinating centers (principal investigators) at Children’s Mercy Hospital and the University of Missouri-Kansas City (Bradley Warady), Children’s Hospital of Philadelphia (Susan Furth), Central Biochemistry Laboratory (George Schwartz) at the University of Rochester Medical Center, and the data coordinating center (Alvaro Muñoz) at the Johns Hopkins Bloomberg School of Public Health.
The CKiD study is supported by grants from the National Institute of Diabetes and Digestive and Kidney Diseases, with additional funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the National Heart, Lung, and Blood Institute (U01-DK66143, U01-DK66174, U01-DK82194, U01-DK66116).
This project was supported by National Institutes of Heath (NIH) Grants K23-DK093556 (to M.R.D.), K24-DK076808 (to M.B.L.), and K23-DK084339 (to J.K.). Dr. Denburg was also funded by The Nephcure Foundation-American Society of Nephrology Research Grant. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The funders had no role in the design and conduct of the study, collection, management, analysis, and interpretation of the data, or preparation, review, and approval of the manuscript.
Preliminary results of this study were presented at the Pediatric Academic Societies/American Society of Pediatric Nephrology Annual Meeting held in May 2014 in Vancouver, British Columbia.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2015020152/-/DCSupplemental.
REFERENCES
- 1.Denburg MR, Tsampalieros AK, de Boer IH, Shults J, Kalkwarf HJ, Zemel BS, Foerster D, Stokes D, Leonard MB: Mineral metabolism and cortical volumetric bone mineral density in childhood chronic kidney disease. J Clin Endocrinol Metab 98: 1930–1938, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Foster BJ, Kalkwarf HJ, Shults J, Zemel BS, Wetzsteon RJ, Thayu M, Foerster DL, Leonard MB: Association of chronic kidney disease with muscle deficits in children. J Am Soc Nephrol 22: 377–386, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Furth SL, Abraham AG, Jerry-Fluker J, Schwartz GJ, Benfield M, Kaskel F, Wong C, Mak RH, Moxey-Mims M, Warady BA: Metabolic abnormalities, cardiovascular disease risk factors, and GFR decline in children with chronic kidney disease. Clin J Am Soc Nephrol 6: 2132–2140, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kalkwarf HJ, Denburg MR, Strife CF, Zemel BS, Foerster DL, Wetzsteon RJ, Leonard MB: Vitamin D deficiency is common in children and adolescents with chronic kidney disease. Kidney Int 81: 690–697, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tsampalieros A, Kalkwarf HJ, Wetzsteon RJ, Shults J, Zemel BS, Foster BJ, Foerster DL, Leonard MB: Changes in bone structure and the muscle-bone unit in children with chronic kidney disease. Kidney Int 83: 495–502, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wetzsteon RJ, Kalkwarf HJ, Shults J, Zemel BS, Foster BJ, Griffin L, Strife CF, Foerster DL, Jean-Pierre DK, Leonard MB: Volumetric bone mineral density and bone structure in childhood chronic kidney disease. J Bone Miner Res 26: 2235–2244, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leonard MB: A structural approach to the assessment of fracture risk in children and adolescents with chronic kidney disease. Pediatr Nephrol 22: 1815–1824, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beaubrun AC, Kilpatrick RD, Freburger JK, Bradbury BD, Wang L, Brookhart MA: Temporal trends in fracture rates and postdischarge outcomes among hemodialysis patients. J Am Soc Nephrol 24: 1461–1469, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alem AM, Sherrard DJ, Gillen DL, Weiss NS, Beresford SA, Heckbert SR, Wong C, Stehman-Breen C: Increased risk of hip fracture among patients with end-stage renal disease. Kidney Int 58: 396–399, 2000 [DOI] [PubMed] [Google Scholar]
- 10.Ball AM, Gillen DL, Sherrard D, Weiss NS, Emerson SS, Seliger SL, Kestenbaum BR, Stehman-Breen C: Risk of hip fracture among dialysis and renal transplant recipients. JAMA 288: 3014–3018, 2002 [DOI] [PubMed] [Google Scholar]
- 11.Danese MD, Kim J, Doan QV, Dylan M, Griffiths R, Chertow GM: PTH and the risks for hip, vertebral, and pelvic fractures among patients on dialysis. Am J Kidney Dis 47: 149–156, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Doan QV, Gleeson M, Kim J, Borker R, Griffiths R, Dubois RW: Economic burden of cardiovascular events and fractures among patients with end-stage renal disease. Curr Med Res Opin 23: 1561–1569, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Inaba M, Okuno S, Kumeda Y, Yamakawa T, Ishimura E, Nishizawa Y: Increased incidence of vertebral fracture in older female hemodialyzed patients with type 2 diabetes mellitus. Calcif Tissue Int 76: 256–260, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Jadoul M, Albert JM, Akiba T, Akizawa T, Arab L, Bragg-Gresham JL, Mason N, Prutz KG, Young EW, Pisoni RL: Incidence and risk factors for hip or other bone fractures among hemodialysis patients in the Dialysis Outcomes and Practice Patterns Study. Kidney Int 70: 1358–1366, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Mittalhenkle A, Gillen DL, Stehman-Breen CO: Increased risk of mortality associated with hip fracture in the dialysis population. Am J Kidney Dis 44: 672–679, 2004 [PubMed] [Google Scholar]
- 16.Stehman-Breen CO, Sherrard DJ, Alem AM, Gillen DL, Heckbert SR, Wong CS, Ball A, Weiss NS: Risk factors for hip fracture among patients with end-stage renal disease. Kidney Int 58: 2200–2205, 2000 [DOI] [PubMed] [Google Scholar]
- 17.Tentori F, McCullough K, Kilpatrick RD, Bradbury BD, Robinson BM, Kerr PG, Pisoni RL: High rates of death and hospitalization follow bone fracture among hemodialysis patients. Kidney Int 85: 166–173, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Groothoff JW, Offringa M, Van Eck-Smit BL, Gruppen MP, Van De Kar NJ, Wolff ED, Lilien MR, Davin JC, Heymans HS, Dekker FW: Severe bone disease and low bone mineral density after juvenile renal failure. Kidney Int 63: 266–275, 2003 [DOI] [PubMed] [Google Scholar]
- 19.Bartosh SM, Leverson G, Robillard D, Sollinger HW: Long-term outcomes in pediatric renal transplant recipients who survive into adulthood. Transplantation 76: 1195–1200, 2003 [DOI] [PubMed] [Google Scholar]
- 20.Ensrud KE, Barbour K, Canales MT, Danielson ME, Boudreau RM, Bauer DC, LaCroix AZ, Ishani A, Jackson RD, Robbins JA, Cauley JA: Renal function and nonvertebral fracture risk in multiethnic women: the Women’s Health Initiative (WHI). Osteoporos Int 23: 887–899, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ensrud KE, Parimi N, Fink HA, Ishani A, Taylor BC, Steffes M, Cauley JA, Lewis CE, Orwoll ES, Osteoporotic Fractures in Men Study Group : Estimated GFR and risk of hip fracture in older men: comparison of associations using cystatin C and creatinine. Am J Kidney Dis 63: 31–39, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaji H, Yamauchi M, Yamaguchi T, Shigematsu T, Sugimoto T: Mild renal dysfunction is a risk factor for a decrease in bone mineral density and vertebral fractures in Japanese postmenopausal women. J Clin Endocrinol Metab 95: 4635–4642, 2010 [DOI] [PubMed] [Google Scholar]
- 23.Nitsch D, Mylne A, Roderick PJ, Smeeth L, Hubbard R, Fletcher A: Chronic kidney disease and hip fracture-related mortality in older people in the UK. Nephrol Dial Transplant 24: 1539–1544, 2009 [DOI] [PubMed] [Google Scholar]
- 24.Dooley AC, Weiss NS, Kestenbaum B: Increased risk of hip fracture among men with CKD. Am J Kidney Dis 51: 38–44, 2008 [DOI] [PubMed] [Google Scholar]
- 25.Dukas L, Schacht E, Stähelin HB: In elderly men and women treated for osteoporosis a low creatinine clearance of <65 ml/min is a risk factor for falls and fractures. Osteoporos Int 16: 1683–1690, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Ensrud KE, Lui LY, Taylor BC, Ishani A, Shlipak MG, Stone KL, Cauley JA, Jamal SA, Antoniucci DM, Cummings SR, Osteoporotic Fractures Research Group : Renal function and risk of hip and vertebral fractures in older women. Arch Intern Med 167: 133–139, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Fried LF, Biggs ML, Shlipak MG, Seliger S, Kestenbaum B, Stehman-Breen C, Sarnak M, Siscovick D, Harris T, Cauley J, Newman AB, Robbins J: Association of kidney function with incident hip fracture in older adults. J Am Soc Nephrol 18: 282–286, 2007 [DOI] [PubMed] [Google Scholar]
- 28.Jassal SK, von Muhlen D, Barrett-Connor E: Measures of renal function, BMD, bone loss, and osteoporotic fracture in older adults: the Rancho Bernardo study. J Bone Miner Res 22: 203–210, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.LaCroix AZ, Lee JS, Wu L, Cauley JA, Shlipak MG, Ott SM, Robbins J, Curb JD, Leboff M, Bauer DC, Jackson RD, Kooperberg CL, Cummings SR, Women’s Health Initiative Observational : Cystatin-C, renal function, and incidence of hip fracture in postmenopausal women. J Am Geriatr Soc 56: 1434–1441, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Naylor KL, McArthur E, Leslie WD, Fraser LA, Jamal SA, Cadarette SM, Pouget JG, Lok CE, Hodsman AB, Adachi JD, Garg AX: The three-year incidence of fracture in chronic kidney disease. Kidney Int 86: 810–818, 2014 [DOI] [PubMed] [Google Scholar]
- 31.Nickolas TL, McMahon DJ, Shane E: Relationship between moderate to severe kidney disease and hip fracture in the United States. J Am Soc Nephrol 17: 3223–3232, 2006 [DOI] [PubMed] [Google Scholar]
- 32.Helenius I, Remes V, Salminen S, Valta H, Mäkitie O, Holmberg C, Palmu P, Tervahartiala P, Sarna S, Helenius M, Peltonen J, Jalanko H: Incidence and predictors of fractures in children after solid organ transplantation: a 5-year prospective, population-based study. J Bone Miner Res 21: 380–387, 2006 [DOI] [PubMed] [Google Scholar]
- 33.Wong CJ, Moxey-Mims M, Jerry-Fluker J, Warady BA, Furth SL: CKiD (CKD in children) prospective cohort study: a review of current findings. Am J Kidney Dis 60: 1002–1011, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cooper C, Dennison EM, Leufkens HG, Bishop N, van Staa TP: Epidemiology of childhood fractures in Britain: a study using the general practice research database. J Bone Miner Res 19: 1976–1981, 2004 [DOI] [PubMed] [Google Scholar]
- 35.Schober HC, Han ZH, Foldes AJ, Shih MS, Rao DS, Balena R, Parfitt AM: Mineralized bone loss at different sites in dialysis patients: implications for prevention. J Am Soc Nephrol 9: 1225–1233, 1998 [DOI] [PubMed] [Google Scholar]
- 36.Wesseling-Perry K, Pereira RC, Tseng CH, Elashoff R, Zaritsky JJ, Yadin O, Sahney S, Gales B, Jüppner H, Salusky IB: Early skeletal and biochemical alterations in pediatric chronic kidney disease. Clin J Am Soc Nephrol 7: 146–152, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wesseling-Perry K, Pereira RC, Sahney S, Gales B, Wang HJ, Elashoff R, Jüppner H, Salusky IB: Calcitriol and doxercalciferol are equivalent in controlling bone turnover, suppressing parathyroid hormone, and increasing fibroblast growth factor-23 in secondary hyperparathyroidism. Kidney Int 79: 112–119, 2011 [DOI] [PubMed] [Google Scholar]
- 38.Rees L, Shroff R: The demise of calcium-based phosphate binders—is this appropriate for children? Pediatr Nephrol 2014, in press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fine RN: Etiology and treatment of growth retardation in children with chronic kidney disease and end-stage renal disease: a historical perspective. Pediatr Nephrol 25: 725–732, 2010 [DOI] [PubMed] [Google Scholar]
- 40.Al-Uzri A, Matheson M, Gipson DS, Mendley SR, Hooper SR, Yadin O, Rozansky DJ, Moxey-Mims M, Furth SL, Warady BA, Gerson AC; Chronic Kidney Disease in Children Study Group: The impact of short stature on health-related quality of life in children with chronic kidney disease. J Pediatr 2013;163: 736-741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Clark EM, Ness AR, Tobias JH: Bone fragility contributes to the risk of fracture in children, even after moderate and severe trauma. J Bone Miner Res 23: 173–179, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Furth SL, Cole SR, Moxey-Mims M, Kaskel F, Mak R, Schwartz G, Wong C, Muñoz A, Warady BA: Design and methods of the Chronic Kidney Disease in Children (CKiD) prospective cohort study. Clin J Am Soc Nephrol 1: 1006–1015, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schwartz GJ, Brion LP, Spitzer A: The use of plasma creatinine concentration for estimating glomerular filtration rate in infants, children, and adolescents. Pediatr Clin North Am 34: 571–590, 1987 [DOI] [PubMed] [Google Scholar]
- 44.Schwartz GJ, Muñoz A, Schneider MF, Mak RH, Kaskel F, Warady BA, Furth SL: New equations to estimate GFR in children with CKD. J Am Soc Nephrol 20: 629–637, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Portale AA, Wolf M, Jüppner H, Messinger S, Kumar J, Wesseling-Perry K, Schwartz GJ, Furth SL, Warady BA, Salusky IB: Disordered FGF23 and mineral metabolism in children with CKD. Clin J Am Soc Nephrol 9: 344–353, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ersfeld DL, Rao DS, Body JJ, Sackrison JL, Jr, Miller AB, Parikh N, Eskridge TL, Polinske A, Olson GT, MacFarlane GD: Analytical and clinical validation of the 25 OH vitamin D assay for the LIAISON® automated analyzer. Clin Biochem 37: 867–874, 2004 [DOI] [PubMed] [Google Scholar]
- 47.Wagner D, Hanwell HE, Vieth R: An evaluation of automated methods for measurement of serum 25-hydroxyvitamin D. Clin Biochem 42: 1549–1556, 2009 [DOI] [PubMed] [Google Scholar]
- 48.CDC : 2005 State and Local Youth Risk Behavior Survey, Atlanta, Centers for Disease Control and Prevention, 2005 [Google Scholar]
- 49.Lin DY, Wei LJ: The robust inference for the cox proportional hazards model. J Am Stat Assoc 84: 1074–1078, 1989 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.