Abstract
Inflammation is a complex biologic response that is essential for eliminating microbial pathogens and repairing tissue after injury. AKI associates with intrarenal and systemic inflammation; thus, improved understanding of the cellular and molecular mechanisms underlying the inflammatory response has high potential for identifying effective therapies to prevent or ameliorate AKI. In the past decade, much knowledge has been generated about the fundamental mechanisms of inflammation. Experimental work in small animal models has revealed many details of the inflammatory response that occurs within the kidney after typical causes of AKI, including insights into the molecular signals released by dying cells, the role of pattern recognition receptors, the diverse subtypes of resident and recruited immune cells, and the phased transition from destructive to reparative inflammation. Although this expansion of the basic knowledge base has increased the number of mechanistically relevant targets of intervention, progress in developing therapies that improve AKI outcomes by modulation of inflammation remains slow. In this article, we summarize the most important recent developments in understanding the inflammatory mechanisms of AKI, highlight key limitations of the commonly used animal models and clinical trial designs that may prevent successful clinical application, and suggest priority approaches for research toward clinical translation in this area.
Keywords: acute renal failure, clinical nephrology, immunology, pathophysiology of, renal disease and progression
Inflammation is a complex biologic response that is essential for eliminating microbial pathogens and repairing tissue after diverse forms of injury. AKI is known to be associated with intrarenal and systemic inflammation. Improved understanding of the cellular and molecular mechanisms underlying the inflammatory response is important for identifying effective therapies to prevent or ameliorate AKI. The Acute Dialysis Quality Initiative Consensus (ADQI) Conference XIII convened to discuss mechanisms of AKI and identify potential therapeutic targets. This paper summarizes the work of the group focus on inflammation and inflammatory mechanisms of injury.
Methods
The 13th ADQI Consensus Conference on Therapeutic Targets of Human AKI held in Charlottesville, Virginia in April of 2014 (www.adqi.net) was attended by an international group of experts and focused on an objective scientific review of the current literature, developing a consensus of opinion, with evidence where possible, to distill current literature and articulate a research agenda to address important unanswered questions. Similar to other ADQI meetings, a modified Delphi approach was followed. Details of the methods can be found in the summary by Okusa et al.1 in the introduction to the series.
Basic Concepts of Inflammation
Inflammation is a complex response that is needed to eradicate harmful pathogens and mediate tissue repair after injury. However, excess and unresolved inflammation can promote autoimmune disorders, fibrosis, and tissue damage.2 Classically, release of cytokines and recruitment of neutrophils and macrophages to the site of injury are considered the hallmark features of the early inflammatory response followed by cells of adaptive immunity at later stages. More recent data, however, suggest that T cells also participate in early inflammatory responses in AKI.3 The recruitment of immune effector cells is facilitated by the upregulation of adhesion molecules on various cell types within the kidney.4 Subsequently, the balance between pro- and anti-inflammatory mediators significantly affects the extent of tissue injury and repair after an acute event. Inflammation during ischemia-reperfusion (IR) has many similarities in its immune signature to inflammation occurring in response to a microbial pathogen.5
Cellular damage and its associated molecular products are thought to be key triggers for inflammation after acute tissue injury.5 Within the kidney, renal tubular epithelial cells are extremely susceptible to intrinsic oxidative stress, particularly during the reperfusion phase of IR.2,5,6 Necrotic cells release damage–associated molecular patterns, such as high–mobility group box 1, histones, heat shock proteins, fibronectin, and biglycan into the extracellular spaces, which subsequently, activate pattern recognition receptors, such as toll-like receptors (TLRs), and nucleotide–binding oligomerization domain–like receptors, such as the nucleotide–binding oligomerization domain–, LRR–, and pyrin domain–containing 3 inflammasome, expressed in epithelial and endothelial cells, dendritic cells (DCs), monocytes/macrophages, and lymphocytes.6–9 Activated renal parenchyma cells and DCs also secrete chemokines, including CXCL1, CXCL8, CCL2, and CCL5, that promote acute neutrophil– and monocyte/macrophage–dependent inflammatory responses in AKI.6,10 Time-dependent changes in the expression of proinflammatory (e.g., TNF-α, IFN-γ, IL-6, IL-1β, IL-23, IL-17, C3, C5a, and C5b) and anti-inflammatory (e.g., IL-4, TGF-β, IL-10, heme oxygenase 1, resolvins, and protectin D1) mediators by resident and recruited cell populations are important determinants of the injury and repair phases.6 Under ideal conditions, a fine balance between inflammatory and anti-inflammatory factors ensures robust tissue repair and a return of homeostatic conditions. However, AKI often results in an abnormal repair process as a result of prolonged hypoxia and sustained secretion of profibrotic cytokine (e.g., IL-13 and TGF-β1), leading to post-AKI fibrosis and chronic renal dysfunction.6,7
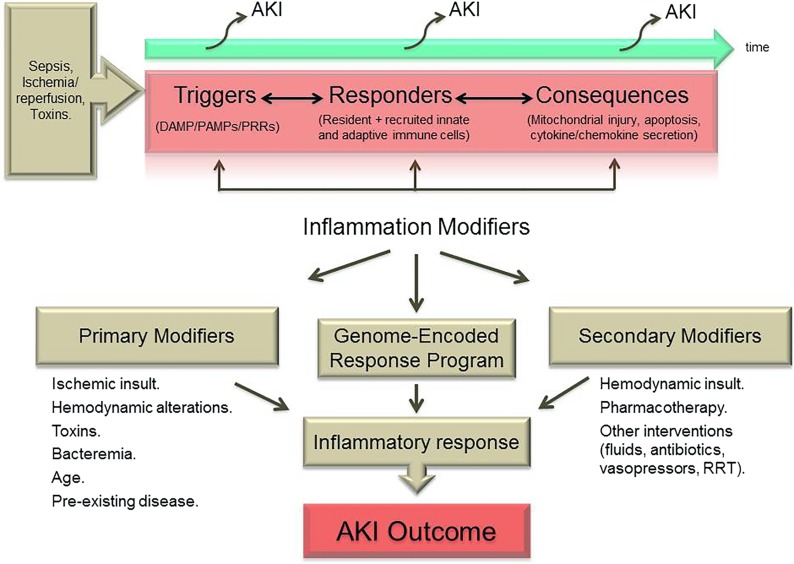
As depicted in Figure 1, recent scientific discoveries regarding the triggers, responders, and consequences of localized inflammatory responses have laid the groundwork for a more rational approach to identifying targets of intervention for AKI. However, in the clinical setting, far more so than in the experimental setting, there is a broad range of primary and secondary modifiers of the archetypal genetically and epigenetically predefined inflammatory responses that remain understudied. In the subsequent sections of this article, we first outline in greater detail some of the important recent insights into the cellular and molecular mechanisms of intrarenal inflammation during AKI. Then, we discuss the current status limitations of preclinical (animal model) investigation and clinical trial design in this area. Finally, we identify key challenges for future successful translation of basic experimental work on inflammation in AKI and suggest how these may be addressed in a more cohesive manner by the research community.
Figure 1.
Interactions between injury, inflammatory mediators and outcome during AKI. (Upper panel) Varying types of insult instigate activation of the innate and adaptive immune system within the kidney. Renal dysfunction during AKI may result from the initial receptor–mediated triggers as well the subsequent cellular responses and various secondary sequelae. (Lower panel) In the clinical setting, the nature and outcome of the inflammatory response in AKI are dictated by not only an archetypal genome–encoded response program but also, primary and secondary modifiers (including therapeutic interventions), which have received less attention in experimental studies and must be taken into consideration in the design of clinical trials of inflammation-modifying therapies. DAMP, damage–associated molecular pattern; PAMP, pathogen–associated molecular pattern; PRRs, pattern recognition receptors.
The Renal Immune System and the Phases of the Intrarenal Inflammatory Response to Acute Injury
During the past decade, a remarkable amount of new knowledge has been garnered regarding the intrinsic immunologic cells of the healthy adult kidney as well as the blood-borne counterparts that transit through the renal parenchyma during health and disease.6,11 Not surprisingly, the interactions of these resident and infiltrating leukocytes with the nonimmunologic cells of the kidney after acute injury and the resulting coordinated set of inflammatory responses have been the subject of intensive investigation. As outlined in the opening section, a detailed understanding of the cellular and molecular programs that dictate the course of intrarenal inflammation is considered to be one of the most promising pathways toward developing new therapies for preventing AKI, lessening the severity of initial parenchymal damage, or enhancing subsequent repair and regeneration of the kidney.6,12 Coupled to this is the emerging concept that mechanisms of intrarenal inflammation during AKI also exert potentially harmful effects on distant organs and tissues through release of soluble mediators or re-entry of activated leukocytes into the bloodstream.4,13 The current knowledge base, although exquisite in some of its details, is highly skewed toward experimental work carried out in rodents, and translation to human AKI is in its infancy. Furthermore, it primarily documents events occurring in previously healthy, nonaged kidneys suffering single episodes of injury and typically, addresses the role of one target at a time— limitations that are discussed in greater detail in subsequent sections.
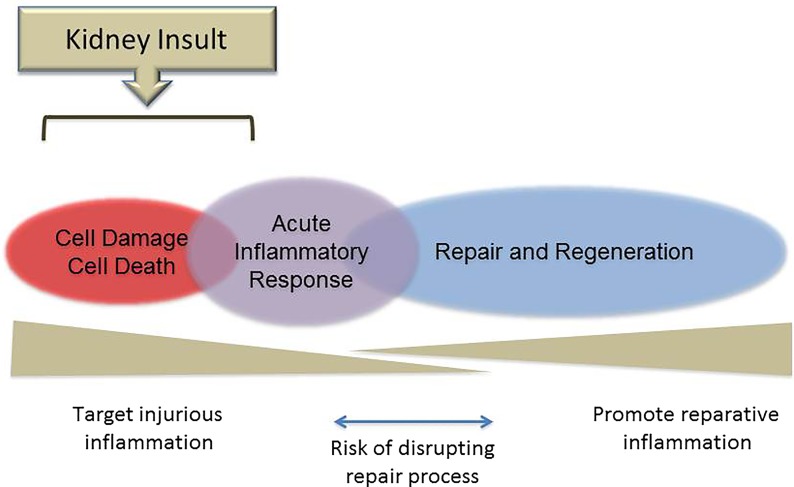
Figure 2 illustrates an idealized model of AKI–associated renal inflammation that has emerged from the recent preclinical literature. It provides a potentially valuable conceptual framework on which to evaluate future approaches for staging and therapeutically targeting episodes of AKI in human subjects.
Figure 2.
Types of inflammatory responses during AKI. An idealized schematic is shown of the phases of inflammation that occur within the healthy kidney after acute insults on the basis of current understanding from preclinical (mostly rodent) models. Initial cell damage and death trigger, over minutes to hours, a primary acute inflammatory response involving resident and infiltrating leukocytes. This phase, if appropriately regulated, evolves over several days into a phase of active repair and regeneration, which is dependent on regulatory and reprogrammed leukocyte subsets. As indicated in the lower panel, molecular and cellular details of the early and later phases of the inflammatory process provide specific therapeutic target opportunities, but strategies that involve blocking/inhibiting elements of the acute inflammatory response may, paradoxically, introduce a risk of disrupting the natural transition to the repair phase if improperly timed.
An initial phase of cell damage/cell death occurs that varies in its extent and complexity in accordance with the specific nature of the insult. This phase lasts minutes to hours; may involve epithelial, endothelial, and other renal parenchymal cells in some or all zones of the affected kidneys; and generates a broad range of triggering factors for acute inflammatory response.4,7,14 Recent progress in identifying and elucidating the mechanisms of action of proinflammatory cell death/cell damage–associated factors has been remarkable and is now revealing many novel potential therapeutic targets for prevention or very early treatment of AKI.7,14
Overlapping with this initial phase and lasting hours to days is a phase of acute inflammation that is mediated primarily by immune cells. Among the important immediate responders is the abundant network of resident mononuclear phagocytic cells (often subdivided into renal DCs and macrophages), which is juxtaposed between the tubular epithelium and the peritubular capillaries within the interstitial space.6,11 However, responses of almost equal rapidity occur from the bloodstream involving endothelial adherence and activation of neutrophils and monocytes as well as intraparenchymal infiltration by neutrophils, monocytes, effector memory T cells, and other less frequent myeloid and lymphoid effectors.6,15–19 The initial activation–induced responses of these resident and recruited immune cells are typically dominated by the production of archetypal proinflammatory mediators (cytokines, chemokines, enzymes, free radical species, and lipid mediators) with high cytotoxic potential, which tend to amplify and extend cell damage and cell death.4,6 In this manner, the phase of acute inflammatory response may be seen as consolidating and worsening organ dysfunction after injury. Thus, improved understanding of its cellular and molecular components from preclinical models represents another significant means to identify targets of intervention for limiting organ damage in the early stages of AKI. It has become recognized, however, that the acute inflammatory response incorporates counter-regulatory components, such as recruited regulatory T cells (Tregs) and induced anti–inflammatory cytokines (e.g., IL-10), which are also amenable to manipulation.20–22
Among the most striking advances in knowledge of the past decade is the recognition that many of the same triggers and cellular mediators that are responsible for initial organ damage are also key players in a subsequent phase of repair and regeneration.7,23,24 In the experimental setting, this phase has been shown to be essential for optimal recovery of renal function after AKI and result from programmed transitions in the phenotype of immune effector cells (especially mononuclear phagocytes) involving specific alternative intracellular signaling pathways and soluble mediators.6,11,23–25 The recent progress in understanding these inherent restorative mechanisms has led to a new emphasis on harnessing them to actively promote repair and regeneration after established acute organ injury, including AKI.7,26,27 However, interventions that target earlier phases of inflammation during AKI may bring unintended negative consequences by disrupting the role played by the same mediators in the transition from proinflammatory to prorepair mechanisms (Figure 2).23
Targets for Prevention and Treatment of Renal Inflammation Associated with AKI
As discussed above, experimental models of AKI have shown common inflammatory triggers involving epithelial cells, endothelial cells, resident inflammatory cells, and infiltrating leukocytes. These inflammatory triggers initiate localized and systemic responses through receptors and cells of the innate and adaptive immune systems.6 The innate immune system is a phylogenically conserved early defense system activated by families of membrane-bound and cytoplasmic receptors that are exquisitely sensitive to molecules released from pathogens or dead/dying cells. Several families of innate immune receptors are constitutively expressed in the kidney, and experimental data have clearly shown activation of these receptors to be associated with some of the earliest events of AKI. In preclinical studies, blockade of several of the innate immune receptors (e.g., TLR2,28 nucleotide–binding oligomerization domain–, LRR–, and pyrin domain–containing 3, and nucleotide–binding oligomerization domain 229,30) has been shown to prevent experimental AKI in rodent models. Blockade of TLR2, however, has recently been tested in humans and is in phase 2 trials in the United States and Europe (OPN-305; Opsona Therapeutics).31
Virtually all immune cells have been implicated in AKI, and some are thought to be deleterious (e.g., neutrophils,10,16 monocytes/macrophages,25,32 DCs,33,34 NKT cells,17,35 NK cells,19 and B cells18,36), whereas others are likely protective (e.g., Tregs20,21). Others, such as macrophages, play different roles depending on the type and time at which they arrive in the injured tissue.25 M1 macrophages contribute to inflammation and tissue injury in the injury phase, whereas M2 macrophages exert anti-inflammatory functions in postischemic kidneys and facilitate renal tubular regeneration during the recovery phase.37 Attempts have been made to enhance Tregs using IL-2 complexes in mice,22 and clinical translation of this strategy might be expected in the future. Multiple inflammatory and parenchymal cell types are involved in AKI, and in fact, an important epithelial-endothelial axis exists that promotes the infiltration of inflammatory cells after initial injury.38 Because there are so many different cell types involved in the inflammatory response and because different inflammatory cells contribute to injury and repair, it is very important to be mindful of the phenotype and kinetics of the inflammatory response in designing effective anti–inflammatory approaches to AKI (Figure 2).
Experimental data suggest that it is also very important to consider parenchymal cells in the design of anti-inflammatory approaches, because they can play an essential role in the earliest phases of AKI. Injured parenchymal cells, such as renal tubular epithelial cells and endothelial cells, release damage–associated molecular patterns that trigger innate immune receptors on nearby healthy cells, thereby transducing death signals to the nearby healthy cells and propagating the local tissue injury.38 Experimental data from many laboratories show that parenchymal cell injury can be ameliorated by blockade of membrane–bound or intracytoplasmic innate immune receptors. Rodent models show that these receptors are rapidly activated after renal artery occlusion, leading to a cascade of intracellular signaling events that ultimately cause apoptosis and necrosis of the triggered cells and the production of proinflammatory molecules. Many laboratories have shown in rodent models that interfering with the triggering of pattern recognition receptors prevents the expansion of renal tubular cell injury.6,8,28
Other concepts that require better definition to develop clinically relevant therapeutics involve the soluble mediators of cellular injury, which may be produced locally (within the injured kidney) or systemically. Cytokines and chemokines produced locally recruit a cascade of inflammatory effector cells into injured tissue, and experimental blockade of several cytokines and chemokines can abrogate AKI.39 Cytokines and chemokines lead to upregulation of adhesion molecules on endothelial cells, such as intercellular adhesion molecule-1, P-selectin, and E-selectin, and these adhesion molecules are essential for the recruitment of leukocytes into areas of injury.6 Thus, there has been much interest in adhesion molecule blockade in AKI as a way to interrupt the epithelial-endothelial axis of injury.38,39
Molecules generated within injured cells have also been recently identified as potential targets to abrogate inflammation.40 For example, the cysteine proteases (calpains and caspases) play a role in hypoxia-induced injury in proximal tubular cells, and mitochondrial mediators are gaining interest as potential anti–inflammatory targets in several cell types. Inhibitors of mitochondrial permeability transition pore opening, quinone analogs, superoxide dismutase mimetics, Szeto–Schiller peptides, and mitochondrial division inhibitors have already shown promise in diverse models of AKI, and there have already been limited clinical trials of mitochondrial-targeted therapies.40 Finally, microRNAs generated in injured renal tubular cells are also gaining interest as potential targets.41,42
In summary, a large and rapidly expanding array of inflammatory cell types and molecular mediators has been implicated in the pathogenesis of AKI, and data from rodent models have clearly shown that deletion, blockade, or enhancement of many of these can improve experimental outcomes. Before rational targets are developed, however, a better understanding is needed of the putative cell types involved in injury and repair, the dynamic interactions between parenchymal and immune cells, and the signals that amplify injurious responses and repair. It is imperative that future studies define these important events to allow pharmacologic development of rationale targets to prevent or treat AKI.
Limitations and Pitfalls of Animal Models to Study Inflammation in AKI
Rodent models of IR, sepsis, and toxin–induced kidney injury are the commonly used approaches to study the pathophysiology of AKI and examine novel therapeutic approaches. The risk of severe AKI is substantially increased by the presence of preexisting CKD, diabetes mellitus, hypertension, and proteinuria. Developing animal models of renal ischemia superimposed on diabetes, hypertension, and/or CKD would likely advance our understanding of AKI pathogenesis.43,44 Although current models have substantially contributed to our current understanding, there are several important limitations to their use in the study of inflammation and therapeutic targeting of inflammation in human AKI.
Renal IR Injury
There are remarkable sex- and strain-related differences in immune responses that affect susceptibility to AKI. For example, C57BL/6 (B6) mice have a T–helper subset 1–predominant immune system and are highly susceptible to AKI, whereas BALB/C mice have a T–helper subset 2–predominant system and are more resistant to ischemic tissue injury.45 There are fundamental structural and functional differences between the mouse and human kidney that may influence susceptibility to AKI as well as the degree and nature of inflammatory responses. (1) Expression of some molecular markers that determine susceptibility to ischemic injury and inflammation differs substantially between mouse and human kidneys. As an example, tissue factor, expressed by the renal tubules in mice, is not strongly expressed by the human kidney.46,47 (2) Human blood is neutrophil predominant, whereas mouse blood is lymphocyte predominant. (3) Mouse serum has factors that inhibit TNF-α expression and production, and thus, it limits systemic proinflammatory response.47 In this regard, greater use of pig models of AKI might be advantageous, because they may more closely resemble the human anatomy, physiology, immune system, and susceptibility to ischemic AKI.48,49 As an example, renal medullary thickness in pigs is close to that of a human kidney. Thus, pig models may be particularly useful in examining therapeutic targets identified in rodents as a means toward selecting the most promising candidates for translation into human trials. The well recognized limitations to the wider use of pig models of AKI include the cost, specialized expertise, and facilities that are necessary for them to be established.
Animal Models of Sepsis-Induced AKI
An ideal animal model of sepsis would mimic hemodynamic, inflammatory, pathophysiologic, and clinical features as well the course and outcome of human sepsis. Commonly used models include injection of bacterial toxin, such as LPS, injection of bacteria, and perforation of the colon to induce fecal peritonitis. There are several limitations to the use of rodent LPS injection (endotoxemia) as a model of human sepsis. In the first place, mice are less sensitive to LPS than humans. Furthermore, although mice exhibit earlier and higher cytokine response than humans, certain pathogenic features of LPS toxicity, including kidney injury, are ameliorated by simple volume replacement.50 The cecal ligation and puncture (CLP) model also has substantial differences from human sepsis. For example, CLP-induced sepsis lacks the hyperdynamic phase seen in human sepsis and is associated with early lymphocyte apoptosis, which is usually a feature of the recovery phase of human sepsis.50,51 Furthermore, CLP does not reproducibly result in kidney injury. The absence of sepsis-associated comorbidities that are common in human patients and known to affect the immune system (e.g., old age, diabetes mellitus, and CKD) is also a limiting factor that needs to be considered in translating findings derived from the current models.52 It has been reported that the CD-1 outbred strain of mice may be more susceptible to sepsis-induced AKI and as a result of greater genetic heterogeneity, constitute a more clinically relevant model of human disease. The baboon model of sepsis, induced by injecting live gram–negative (Escherichia coli) bacteria, could also be considered as a useful model, because its immune system more closely resembles that of humans, and sepsis in this species has been shown to be associated with a hyperdynamic state.53 An ideal CLP model should include treatment with fluid and antibiotics to more closely mimic the intensive care unit (ICU) scenario.50 However, supportive treatment with fluids and antibiotics is quite variable across laboratories and many times, inadequate.
Toxin-Induced Models of AKI
The advantages of the most commonly used nephrotoxic model of AKI, cisplatin-induced nephrotoxicity, include its technical simplicity, its clinical relevance to one specific form of AKI in human patients, and its amenability to analyzing individual immunologic cell types and mediators. There are also significant disadvantages, however, and these include variable reproducibility of kidney injury and the fact that the inflammatory mechanisms involved differ substantially from those of more common clinical causes of AKI. As an example, CD11c+ DCs have been shown to enhance renal inflammation and exacerbate renal injury in renal IR in the mouse, whereas in cisplatin-induced AKI, depletion of CD11c+ DCs is reported to induce greater neutrophil accumulation and worsen renal injury.54
It is important to be cautious when interpreting pathophysiologic findings and therapeutic targets derived from the existing animal models of AKI. Rodent cisplatin AKI models may not exactly recapitulate human disease; unlike patients treated with cisplatin who undergo repeat dosing with very low short–term mortality, rodent models are usually short term and require high-dose cisplatin, which has a high short–term mortality. Development of animal models that manifest comorbidity and risk factors commonly known to affect the immune response and increase the risk of human AKI is imperative to increase the clinical applicability of such observations. In addition to the more widespread confirmation of key findings in large animal models, the application of other novel approaches, such as the use of humanized mice, in which immunodeficient mice are reconstituted with human immune system, hold promise for experimentally testing the many targeting strategies that have arisen from rodent model experiments. Development of national and international human AKI tissue repositories will be also of great value for more precisely documenting the course and progression of tissue injury and inflammation in human AKI.
Study Design Considerations and Challenges for Clinical Trials in AKI
Optimally designed clinical trials will be essential for determining the therapeutic value of interventions that target inflammatory response in the setting of AKI. Unfortunately, the vast majority of completed clinical trials related to prevention or treatment of AKI have been negative. This may well be related to the above–described complexity, multifactorial nature, and dynamic progression of AKI as well as our inability to determine the phase that the patient is in and the extent of tissue injury that has already occurred.55,56 However, study design issues may also have played a significant role in the negative outcomes of clinical trials. In the case of both patient inclusion and chosen end points, refinements to current trial approaches may improve the sensitivity to detect beneficial effects of treatment. For example, measurement of known markers of AKI could facilitate early detection and enrollment, thus improving the ability to administer a trial intervention within the window of therapeutic opportunity in patients with subclinical AKI. Regarding chosen end points, it has become clear that, with more sophisticated RRTs, short-term (e.g., 28 days) survival is not an adequate outcome measure to determine whether an investigational compound is effective (i.e., renoprotective) in this specific group of patients. Current Food and Drug Administration (FDA) requirements for phase 3 AKI trials are patient–centered clinical outcomes, such as death, need for acute dialysis, myocardial infarction, stroke, and all–cause nonelective hospitalization, which usually require a larger number of trial participants. Therefore, additional discussion with the FDA is required to clarify the importance of and qualification pathway for short-term changes in renal function and other alternative outcomes. For example, future studies might examine the effect of short-term increases in serum creatinine on long–term clinical outcomes as an alternative to a hard clinical outcome.57 It is now known that AKI acquired in the ICU has important long–term sequelae, including an increased risk for ESRD.58 However, both the number of patients and the duration of follow-up required to address end points, such as ESRD, make it unlikely that pharmacologic interventional trials with sufficient statistical power to show effects on these long-term consequences of ICU-acquired AKI will be conducted in the near future. Therefore, the primary aim of clinical research in AKI should be to determine whether a new intervention actually ameliorates inflammation–associated kidney tissue damage during the patient’s clinical stay. For this reason, measurement of markers of renal injury (e.g., urinary excretion of markers of tubular damage or cell arrest59 and functional assessment, such as endogenous creatinine clearance or iohexol clearance60) may provide more refined evidence of efficacy compared with hard clinical end points, such as patient survival, length of hospital stay, and RRT-free days. Especially for relatively small phase 2 trials with compounds that show promising data in animal studies, a combination of study drug–induced effects on these end points may facilitate the design of larger trials.61
Currently, a cutoff level of 25% or 50% decrease in GFR is the most commonly used outcome measure in clinical trials of intervention for AKI, and this degree of GFR decrease is accepted by the FDA and the EMEA as a clinical end point. An alternative end point with clinical relevance, however, is requirement for initiation of RRT. A therapeutic compound that results in prevention of renal damage, more swift recovery of renal function, or protection of the kidneys from additional damage is likely to result in a lower proportion of treated patients requiring RRT. However, only patients that suffer the most severe forms of AKI are likely to be treated with RRT, implying that many more patients are needed to conduct a trial for which the primary end point is RRT-free days. Importantly, in studies conducted in several thousands of patients, these issues will not influence interpretation. However, in phase 2 trials involving several dozens to a few hundred patients, outcome analyses on the basis of GFR as a continuous variable rather than a 25% or 50% decrease in GFR would increase sensitivity and specificity. Decline in GFR may significantly affect how drugs are metabolized and how patients can manage fluid load. The need for dialysis requires well defined criteria, because in some cases, patients may not be offered dialysis because of the burden of comorbidity and perceived futility, dialysis may be refused by patients or family members on other grounds, or patients may die before dialysis is initiated. Furthermore, because the event rate is critical for power analyses for clinical trials and often on the basis of historical data, in addition to inclusion and exclusion criteria during trial planning, adaptive trial design could offer a useful strategy for phase 2 clinical trials.56,57 Furthermore, in view of the long–term clinical consequences of ICU-acquired AKI,58,62 more attention should be given to relatively small deteriorations in renal function, mainly by clinicians and trialists. It is recognized, of course, that obtaining reliable markers and accurate assessment of renal function in the setting of AKI remains challenging. New techniques, such as contrast-enhanced ultrasonography, which enables the quantification of renal tissue perfusion, could be of distinct value in future trials. Bedside assessment of regional renal blood flow early in the course of AKI could be a valuable diagnostic and prognostic tool,63 although validation studies in patients in the ICU to determine if this technique can facilitate the early diagnosis of AKI and guide the use of therapeutic interventions that target microcirculatory perfusion have yet to be performed.
As described in this article, many potential targets related to inflammation have been identified, and for several of them, drugs have already been developed. In addition, there are older drugs that may have new applications in the prevention or treatment of AKI. In that regard, it is important to realize that, for many clinical trials in sepsis that did not show an effect on overall mortality, a beneficial effect on the kidney may have been missed.64 We would advocate strongly, therefore, for the determination of kidney–specific end points, especially in sepsis trials, because sepsis represents a major cause of AKI.
Key Conclusions
(1) Recent preclinical animal experiments have more accurately documented the phases of inflammation after AKI along with many novel cellular and molecular details. However, the applicability of this knowledge to human AKI remains poorly understood.
(2) Preclinical experiments have typically focused on defining the roles of single mechanistic pathways, cell types, or molecular mediators in young, genetically homogenous animals under idealized circumstances.
(3) The current lack of an effective intervention to treat AKI is partly the result of insensitive trial designs.
Future Guidance
(1) Knowledge acquired in idealized rodent models must be critically evaluated in more clinically relevant animal models and directly investigated in human subjects with AKI using new imaging and biospecimen analysis technologies. Because there is limited knowledge on human inflammatory response during AKI, it is imperative to identifying animal models that best represent human inflammatory response.
(2) Greater emphasis could be placed on using current knowledge of inflammation to develop and test multitargeted approaches aimed at identifying synergistic combinations of interventions in AKI.
(3) Research focused on identifying and measuring inflammatory phases in the setting of human AKI is needed to appropriately target inflammation-modifying therapies and identify optimal times to start and stop such therapies. Additionally, great emphasis should be given to comparative analysis regarding the nature and kinetics of inflammatory response in humans and different experimental models of AKI.
(4) International collaboration to develop a shared resource of tissue samples from human subjects with AKI would be of high value for selecting the most promising inflammation–related therapeutic targets.
(5) Trial designs in AKI should combine renal injury, renal function, and clinical end points. Especially for phase 2 clinical trials, the focus to detect beneficial renal effects should shift from clinical end points, including mortality, to renal damage and renal function end points, such as kidney perfusion and actual GFR.
(6) Data and biologic samples from completed negative sepsis trials could be reanalyzed for evidence of positive effects on renal inflammation and renal injury.
(7) In addition to the design of trials involving novel inflammation–modifying agents, emerging knowledge of the mediators of inflammation in AKI should prompt new clinical trials of older drugs that may be successfully repurposed for AKI prevention or therapy.
(8) Future studies should also try to identify novel inflammation–driven biomarkers to assist in identifying and tracking drug reactions in clinical trials.
Disclosures
The following authors report no conflicts of interest: H.R., M.D.G., D.B.M., S.S., M.H.R., and C.R. P.P. is the principal investigator of the Safety, Tolerability, Efficacy and QoL Study of Human recAP in the Treatment of Patients with SA-AKI Trial (NCT02182440) that investigates the effects of recombinant alkaline phosphatase (AP) in patients with sepsis-associated AKI and has received reimbursement for travel costs and consultancy fees from AM-Pharma (Amsterdam, The Netherlands), the producer of recombinant AP. J.A.K. has received consulting fees from Abbott Laboratories (Chicago, IL), Alere (San Diego, CA), Alung (Pittsburgh, PA), AM-Pharma (Amsterdam, The Netherlands), Astute Medical (San Diego, CA), Atox Bio (Ness Ziona, Israel), Baxter (Chicago, IL), Cytosorbents (Princeton, NJ), Grifols (Barcelona, Spain), Roche (Basel, Switzerland) and Spectral Diagnostics (Toronto, Canada), J.A.K. has also received research grants from Alere (San Diego, CA), Astute Medical (San Diego, CA), Atox Bio (Ness Ziona, Israel), Bard (Covington, GA), Baxter (Chicago, IL), Cytosorbents (Princeton, NJ), Grifols (Barcelona, Spain), and Spectral Diagnostics (Toronto, Canada) and has licensed technologies through the University of Pittsburgh (Pittsburgh, PA) to Astute Medical (San Diego, CA), Cytosorbents (Princeton, NJ), and Spectral Diagnostics (Toronto, Canada).
Supplementary Material
Acknowledgments
A complete list of participants in the Acute Dialysis Quality Initiative Consensus XIII Work Group is provided in the Supplemental Appendix.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2015030261/-/DCSupplemental.
References
- 1.Okusa MD, Rosner MH, Kellum JA, Ronco C: Acute Dialysis Quality Initiative XIII Workgroup: Therapeutic Targets of Human AKI: Harmonizing Human and Animal AKI. J Am Soc Nephrol 27: 371–379, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Medzhitov R: Inflammation 2010: New adventures of an old flame. Cell 140: 771–776, 2010 [DOI] [PubMed] [Google Scholar]
- 3.Linfert D, Chowdhry T, Rabb H: Lymphocytes and ischemia-reperfusion injury. Transplant Rev (Orlando) 23: 1–10, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ratliff BB, Rabadi MM, Vasko R, Yasuda K, Goligorsky MS: Messengers without borders: Mediators of systemic inflammatory response in AKI. J Am Soc Nephrol 24: 529–536, 2013 [DOI] [PubMed] [Google Scholar]
- 5.Chen GY, Nuñez G: Sterile inflammation: Sensing and reacting to damage. Nat Rev Immunol 10: 826–837, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kurts C, Panzer U, Anders HJ, Rees AJ: The immune system and kidney disease: Basic concepts and clinical implications. Nat Rev Immunol 13: 738–753, 2013 [DOI] [PubMed] [Google Scholar]
- 7.Anders HJ, Schaefer L: Beyond tissue injury-damage-associated molecular patterns, toll-like receptors, and inflammasomes also drive regeneration and fibrosis. J Am Soc Nephrol 25: 1387–1400, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim HJ, Lee DW, Ravichandran K, O Keys D, Akcay A, Nguyen Q, He Z, Jani A, Ljubanovic D, Edelstein CL: NLRP3 inflammasome knockout mice are protected against ischemic but not cisplatin-induced acute kidney injury. J Pharmacol Exp Ther 346: 465–472, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vallés PG, Lorenzo AG, Bocanegra V, Vallés R: Acute kidney injury: What part do toll-like receptors play? Int J Nephrol Renovasc Dis 7: 241–251, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bolisetty S, Agarwal A: Neutrophils in acute kidney injury: Not neutral any more. Kidney Int 75: 674–676, 2009 [DOI] [PubMed] [Google Scholar]
- 11.Nelson PJ, Rees AJ, Griffin MD, Hughes J, Kurts C, Duffield J: The renal mononuclear phagocytic system. J Am Soc Nephrol 23: 194–203, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bajwa A, Kinsey GR, Okusa MD: Immune mechanisms and novel pharmacological therapies of acute kidney injury. Curr Drug Targets 10: 1196–1204, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grams ME, Rabb H: The distant organ effects of acute kidney injury. Kidney Int 81: 942–948, 2012 [DOI] [PubMed] [Google Scholar]
- 14.Linkermann A, Chen G, Dong G, Kunzendorf U, Krautwald S, Dong Z: Regulated cell death in AKI. J Am Soc Nephrol 25: 2689–2701, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carlin LM, Stamatiades EG, Auffray C, Hanna RN, Glover L, Vizcay-Barrena G, Hedrick CC, Cook HT, Diebold S, Geissmann F: Nr4a1-dependent Ly6C(low) monocytes monitor endothelial cells and orchestrate their disposal. Cell 153: 362–375, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Awad AS, Rouse M, Huang L, Vergis AL, Reutershan J, Cathro HP, Linden J, Okusa MD: Compartmentalization of neutrophils in the kidney and lung following acute ischemic kidney injury. Kidney Int 75: 689–698, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li L, Huang L, Sung SS, Lobo PI, Brown MG, Gregg RK, Engelhard VH, Okusa MD: NKT cell activation mediates neutrophil IFN-gamma production and renal ischemia-reperfusion injury. J Immunol 178: 5899–5911, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Renner B, Strassheim D, Amura CR, Kulik L, Ljubanovic D, Glogowska MJ, Takahashi K, Carroll MC, Holers VM, Thurman JM: B cell subsets contribute to renal injury and renal protection after ischemia/reperfusion. J Immunol 185: 4393–4400, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang ZX, Wang S, Huang X, Min WP, Sun H, Liu W, Garcia B, Jevnikar AM: NK cells induce apoptosis in tubular epithelial cells and contribute to renal ischemia-reperfusion injury. J Immunol 181: 7489–7498, 2008 [DOI] [PubMed] [Google Scholar]
- 20.Gandolfo MT, Jang HR, Bagnasco SM, Ko GJ, Agreda P, Satpute SR, Crow MT, King LS, Rabb H: Foxp3+ regulatory T cells participate in repair of ischemic acute kidney injury. Kidney Int 76: 717–729, 2009 [DOI] [PubMed] [Google Scholar]
- 21.Kinsey GR, Sharma R, Huang L, Li L, Vergis AL, Ye H, Ju ST, Okusa MD: Regulatory T cells suppress innate immunity in kidney ischemia-reperfusion injury. J Am Soc Nephrol 20: 1744–1753, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim MG, Koo TY, Yan JJ, Lee E, Han KH, Jeong JC, Ro H, Kim BS, Jo SK, Oh KH, Surh CD, Ahn C, Yang J: IL-2/anti-IL-2 complex attenuates renal ischemia-reperfusion injury through expansion of regulatory T cells. J Am Soc Nephrol 24: 1529–1536, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang MZ, Yao B, Yang S, Jiang L, Wang S, Fan X, Yin H, Wong K, Miyazawa T, Chen J, Chang I, Singh A, Harris RC: CSF-1 signaling mediates recovery from acute kidney injury. J Clin Invest 122: 4519–4532, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kulkarni OP, Hartter I, Mulay SR, Hagemann J, Darisipudi MN, Kumar Vr S, Romoli S, Thomasova D, Ryu M, Kobold S, Anders HJ: Toll-like receptor 4-induced IL-22 accelerates kidney regeneration. J Am Soc Nephrol 25: 978–989, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee S, Huen S, Nishio H, Nishio S, Lee HK, Choi BS, Ruhrberg C, Cantley LG: Distinct macrophage phenotypes contribute to kidney injury and repair. J Am Soc Nephrol 22: 317–326, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bonventre JV, Yang L: Cellular pathophysiology of ischemic acute kidney injury. J Clin Invest 121: 4210–4221, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kinsey GR, Li L, Okusa MD: Inflammation in acute kidney injury. Nephron, Exp Nephrol 109: e102–e107, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shigeoka AA, Holscher TD, King AJ, Hall FW, Kiosses WB, Tobias PS, Mackman N, McKay DB: TLR2 is constitutively expressed within the kidney and participates in ischemic renal injury through both MyD88-dependent and -independent pathways. J Immunol 178: 6252–6258, 2007 [DOI] [PubMed] [Google Scholar]
- 29.Shigeoka AA, Mueller JL, Kambo A, Mathison JC, King AJ, Hall WF, Correia JS, Ulevitch RJ, Hoffman HM, McKay DB: An inflammasome-independent role for epithelial-expressed Nlrp3 in renal ischemia-reperfusion injury. J Immunol 185: 6277–6285, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shigeoka AA, Kambo A, Mathison JC, King AJ, Hall WF, da Silva Correia J, Ulevitch RJ, McKay DB: Nod1 and nod2 are expressed in human and murine renal tubular epithelial cells and participate in renal ischemia reperfusion injury. J Immunol 184: 2297–2304, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reilly M, Miller RM, Thomson MH, Patris V, Ryle P, McLoughlin L, Mutch P, Gilboy P, Miller C, Broekema M, Keogh B, McCormack W, van de Wetering de Rooij J: Randomized, double-blind, placebo-controlled, dose-escalating phase I, healthy subjects study of intravenous OPN-305, a humanized anti-TLR2 antibody. Clin Pharmacol Ther 94: 593–600, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Day YJ, Huang L, Ye H, Linden J, Okusa MD: Renal ischemia-reperfusion injury and adenosine 2A receptor-mediated tissue protection: Role of macrophages. Am J Physiol Renal Physiol 288: F722–F731, 2005 [DOI] [PubMed] [Google Scholar]
- 33.Bajwa A, Huang L, Ye H, Dondeti K, Song S, Rosin DL, Lynch KR, Lobo PI, Li L, Okusa MD: Dendritic cell sphingosine 1-phosphate receptor-3 regulates Th1-Th2 polarity in kidney ischemia-reperfusion injury. J Immunol 189: 2584–2596, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li L, Huang L, Ye H, Song SP, Bajwa A, Lee SJ, Moser EK, Jaworska K, Kinsey GR, Day YJ, Linden J, Lobo PI, Rosin DL, Okusa MD: Dendritic cells tolerized with adenosine A₂AR agonist attenuate acute kidney injury. J Clin Invest 122: 3931–3942, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang SH, Lee JP, Jang HR, Cha RH, Han SS, Jeon US, Kim DK, Song J, Lee DS, Kim YS: Sulfatide-reactive natural killer T cells abrogate ischemia-reperfusion injury. J Am Soc Nephrol 22: 1305–1314, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jang HR, Gandolfo MT, Ko GJ, Satpute SR, Racusen L, Rabb H: B cells limit repair after ischemic acute kidney injury. J Am Soc Nephrol 21: 654–665, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jang HR, Rabb H: Immune cells in experimental acute kidney injury. Nat Rev Nephrol 11: 88–101, 2015 [DOI] [PubMed] [Google Scholar]
- 38.Molitoris BA: Therapeutic translation in acute kidney injury: The epithelial/endothelial axis. J Clin Invest 124: 2355–2363, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yalavarthy R, Edelstein CL: Therapeutic and predictive targets of AKI. Clin Nephrol 70: 453–463, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tábara LC, Poveda J, Martin-Cleary C, Selgas R, Ortiz A, Sanchez-Niño MD: Mitochondria-targeted therapies for acute kidney injury. Expert Rev Mol Med 16: e13, 2014 [DOI] [PubMed] [Google Scholar]
- 41.Lorenzen JM, Kaucsar T, Schauerte C, Schmitt R, Rong S, Hübner A, Scherf K, Fiedler J, Martino F, Kumarswamy R, Kölling M, Sörensen I, Hinz H, Heineke J, van Rooij E, Haller H, Thum T: MicroRNA-24 antagonism prevents renal ischemia reperfusion injury. J Am Soc Nephrol 25: 2717–2729, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee CG, Kim JG, Kim HJ, Kwon HK, Cho IJ, Choi DW, Lee WH, Kim WD, Hwang SJ, Choi S, Kim SG: Discovery of an integrative network of microRNAs and transcriptomics changes for acute kidney injury. Kidney Int 86: 943–953, 2014 [DOI] [PubMed] [Google Scholar]
- 43.Anders H, Schlöndorff D: Murine models of renal disease: Possibilities and problems in studies using mutant mice. Exp Nephrol 8: 181–193, 2000 [DOI] [PubMed] [Google Scholar]
- 44.Becker GJ, Hewitson TD: Animal models of chronic kidney disease: Useful but not perfect. Nephrol Dial Transplant 28: 2432–2438, 2013 [DOI] [PubMed] [Google Scholar]
- 45.Kennedy SE, Erlich JH: Murine renal ischaemia-reperfusion injury. Nephrology (Carlton) 13: 390–396, 2008 [DOI] [PubMed] [Google Scholar]
- 46.Luther T, Flössel C, Mackman N, Bierhaus A, Kasper M, Albrecht S, Sage EH, Iruela-Arispe L, Grossmann H, Ströhlein A, Zhang Y, Nawroth PP, Carmeliet P, Loskutoff DJ, Müller M: Tissue factor expression during human and mouse development. Am J Pathol 149: 101–113, 1996 [PMC free article] [PubMed] [Google Scholar]
- 47.Mestas J, Hughes CC: Of mice and not men: Differences between mouse and human immunology. J Immunol 172: 2731–2738, 2004 [DOI] [PubMed] [Google Scholar]
- 48.Giraud S, Favreau F, Chatauret N, Thuillier R, Maiga S, Hauet T: Contribution of large pig for renal ischemia-reperfusion and transplantation studies: The preclinical model. J Biomed Biotechnol 2011: 532127, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lieberthal W, Nigam SK: Acute renal failure. II. Experimental models of acute renal failure: Imperfect but indispensable. Am J Physiol Renal Physiol 278: F1–F12, 2000 [DOI] [PubMed] [Google Scholar]
- 50.Doi K, Leelahavanichkul A, Yuen PS, Star RA: Animal models of sepsis and sepsis-induced kidney injury. J Clin Invest 119: 2868–2878, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ganopolsky JG, Castellino FJ: A protein C deficiency exacerbates inflammatory and hypotensive responses in mice during polymicrobial sepsis in a cecal ligation and puncture model. Am J Pathol 165: 1433–1446, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Leelahavanichkul A, Huang Y, Hu X, Zhou H, Tsuji T, Chen R, Kopp JB, Schnermann J, Yuen PS, Star RA: Chronic kidney disease worsens sepsis and sepsis-induced acute kidney injury by releasing High Mobility Group Box Protein-1. Kidney Int 80: 1198–1211, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kinasewitz GT, Chang AC, Peer GT, Hinshaw LB, Taylor FB, Jr.: Peritonitis in the baboon: A primate model which stimulates human sepsis. Shock 13: 100–109, 2000 [DOI] [PubMed] [Google Scholar]
- 54.Tadagavadi RK, Reeves WB: Renal dendritic cells ameliorate nephrotoxic acute kidney injury. J Am Soc Nephrol 21: 53–63, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jo SK, Rosner MH, Okusa MD: Pharmacologic treatment of acute kidney injury: Why drugs haven’t worked and what is on the horizon. Clin J Am Soc Nephrol 2: 356–365, 2007 [DOI] [PubMed] [Google Scholar]
- 56.Molitoris BA, Okusa MD, Palevsky PM, Chawla LS, Kaufman JS, Devarajan P, Toto RM, Hsu CY, Greene TH, Faubel SG, Kellum JA, Wald R, Chertow GM, Levin A, Waikar SS, Murray PT, Parikh CR, Shaw AD, Go AS, Chinchilli VM, Liu KD, Cheung AK, Weisbord SD, Mehta RL, Stokes JB, Thompson AM, Thompson BT, Westenfelder CS, Tumlin JA, Warnock DG, Shah SV, Xie Y, Duggan EG, Kimmel PL, Star RA: Design of clinical trials in AKI: A report from an NIDDK workshop. Trials of patients with sepsis and in selected hospital settings. Clin J Am Soc Nephrol 7: 856–860, 2012 [DOI] [PubMed] [Google Scholar]
- 57.Okusa MD, Molitoris BA, Palevsky PM, Chinchilli VM, Liu KD, Cheung AK, Weisbord SD, Faubel S, Kellum JA, Wald R, Chertow GM, Levin A, Waikar SS, Murray PT, Parikh CR, Shaw AD, Go AS, Chawla LS, Kaufman JS, Devarajan P, Toto RM, Hsu CY, Greene TH, Mehta RL, Stokes JB, Thompson AM, Thompson BT, Westenfelder CS, Tumlin JA, Warnock DG, Shah SV, Xie Y, Duggan EG, Kimmel PL, Star RA: Design of clinical trials in acute kidney injury: A report from an NIDDK workshop--prevention trials. Clin J Am Soc Nephrol 7: 851–855, 2012 [DOI] [PubMed] [Google Scholar]
- 58.Gammelager H, Christiansen CF, Johansen MB, Tønnesen E, Jespersen B, Sørensen HT: Five-year risk of end-stage renal disease among intensive care patients surviving dialysis-requiring acute kidney injury: A nationwide cohort study. Crit Care 17: R145, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bihorac A, Chawla LS, Shaw AD, Al-Khafaji A, Davison DL, Demuth GE, Fitzgerald R, Gong MN, Graham DD, Gunnerson K, Heung M, Jortani S, Kleerup E, Koyner JL, Krell K, Letourneau J, Lissauer M, Miner J, Nguyen HB, Ortega LM, Self WH, Sellman R, Shi J, Straseski J, Szalados JE, Wilber ST, Walker MG, Wilson J, Wunderink R, Zimmerman J, Kellum JA: Validation of cell-cycle arrest biomarkers for acute kidney injury using clinical adjudication. Am J Respir Crit Care Med 189: 932–939, 2014 [DOI] [PubMed] [Google Scholar]
- 60.Macedo E, Mehta RL: Measuring renal function in critically ill patients: Tools and strategies for assessing glomerular filtration rate. Curr Opin Crit Care 19: 560–566, 2013 [DOI] [PubMed] [Google Scholar]
- 61.Pickkers P, Heemskerk S, Schouten J, Laterre PF, Vincent JL, Beishuizen A, Jorens PG, Spapen H, Bulitta M, Peters WH, van der Hoeven JG: Alkaline phosphatase for treatment of sepsis-induced acute kidney injury: A prospective randomized double-blind placebo-controlled trial. Crit Care 16: R14, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liotta M, Olsson D, Sartipy U, Holzmann MJ: Minimal changes in postoperative creatinine values and early and late mortality and cardiovascular events after coronary artery bypass grafting. Am J Cardiol 113: 70–75, 2014 [DOI] [PubMed] [Google Scholar]
- 63.Kalantarinia K, Okusa MD: Ultrasound contrast agents in the study of kidney function in health and disease. Drug Discov Today Dis Mech 4: 153–158, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Marshall JC: Why have clinical trials in sepsis failed? Trends Mol Med 20: 195–203, 2014 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.