Abstract
The NADPH oxidase (NOX) isoform NOX4 has been linked with diabetic kidney disease (DKD). However, a mechanistic understanding of the downstream effects of NOX4 remains to be established. We report that podocyte-specific induction of NOX4 in vivo was sufficient to recapitulate the characteristic glomerular changes noted with DKD, including glomerular hypertrophy, mesangial matrix accumulation, glomerular basement membrane thickening, albuminuria, and podocyte dropout. Intervention with a NOX1/NOX4 inhibitor reduced albuminuria, glomerular hypertrophy, and mesangial matrix accumulation in the F1 Akita model of DKD. Metabolomic analyses from these mouse studies revealed that tricarboxylic acid (TCA) cycle–related urinary metabolites were increased in DKD, but fumarate levels were uniquely reduced by the NOX1/NOX4 inhibitor. Expression of fumarate hydratase (FH), which regulates urine fumarate accumulation, was reduced in the diabetic kidney (in mouse and human tissue), and administration of the NOX1/NOX4 inhibitor increased glomerular FH levels in diabetic mice. Induction of Nox4 in vitro and in the podocyte-specific NOX4 transgenic mouse led to reduced FH levels. In vitro, fumarate stimulated endoplasmic reticulum stress, matrix gene expression, and expression of hypoxia-inducible factor-1α (HIF-1α) and TGF-β. Similar upregulation of renal HIF-1α and TGF-β expression was observed in NOX4 transgenic mice and diabetic mice and was attenuated by NOX1/NOX4 inhibition in diabetic mice. In conclusion, NOX4 is a major mediator of diabetes-associated glomerular dysfunction through targeting of renal FH, which increases fumarate levels. Fumarate is therefore a key link connecting metabolic pathways to DKD pathogenesis, and measuring urinary fumarate levels may have application for monitoring renal NOX4 activity.
Keywords: diabetic nephropathy, NADPH oxidase, fibrosis, reactive oxygen species, podocyte, mitochondria
NADPH oxidase (NOX) is a multicomponent enzyme complex that catalyzes the reduction of molecular oxygen to superoxide and hydrogen peroxide using NADPH as an electron donor. The NOX family consists of seven different members (NOX1–NOX5, DUOX1, and DUOX2). Among the seven members of the NOX family, NOX1, NOX2 (also named gp91phox), and NOX4 are expressed in kidneys of humans and mice, whereas NOX5 is expressed only in humans.1–5 We have been examining the role of NOX isoforms in diabetic kidney disease (DKD) and we recently found that NOX2 did not play a major role in mediating DKD with type 1 diabetes.1,6 However, NOX4 was upregulated in the NOX2 knockout (KO) diabetic kidney, particularly in glomeruli and podocytes, and thus may play a prominent role. Recent studies with total body deletion of NOX4 revealed that NOX4, but not NOX1, is necessary for progression of DKD in ApoE KO mice7; however, in a different mouse model of DKD, the Nox4 KO mouse did not show protection in the B6 background.2 Thus, the major role of NOX4 in the progression of DKD remains unclear. In addition, the key downstream targets of NOX4 that mediate progression of DKD remain to be elucidated.
To clarify the role of increased NOX4, we generated inducible NOX4 transgenic mice with specific expression in podocytes of the glomerulus. We also examined the effectiveness of treatment with GKT137831, a NOX1/NOX4 inhibitor, on the development of CKD and on the urine metabolome in a model of progressive DKD. With the combination of studies, we now identify a new mechanism of action of NOX4 and clarify the role of NOX4 in DKD. Specifically, NOX4 potently inhibits the tricarboxylic acid cycle (TCA) enzyme fumarate hydratase (FH), leading to accumulation of fumarate. Fumarate is a key regulator of hypoxia-inducible factor (HIF)–1α and TGF-β in renal cells and may be the key metabolite linking NOX4 activity to DKD.
Results
Upregulation of NOX4 Induces Apoptosis and Endoplasmic Reticulum Stress
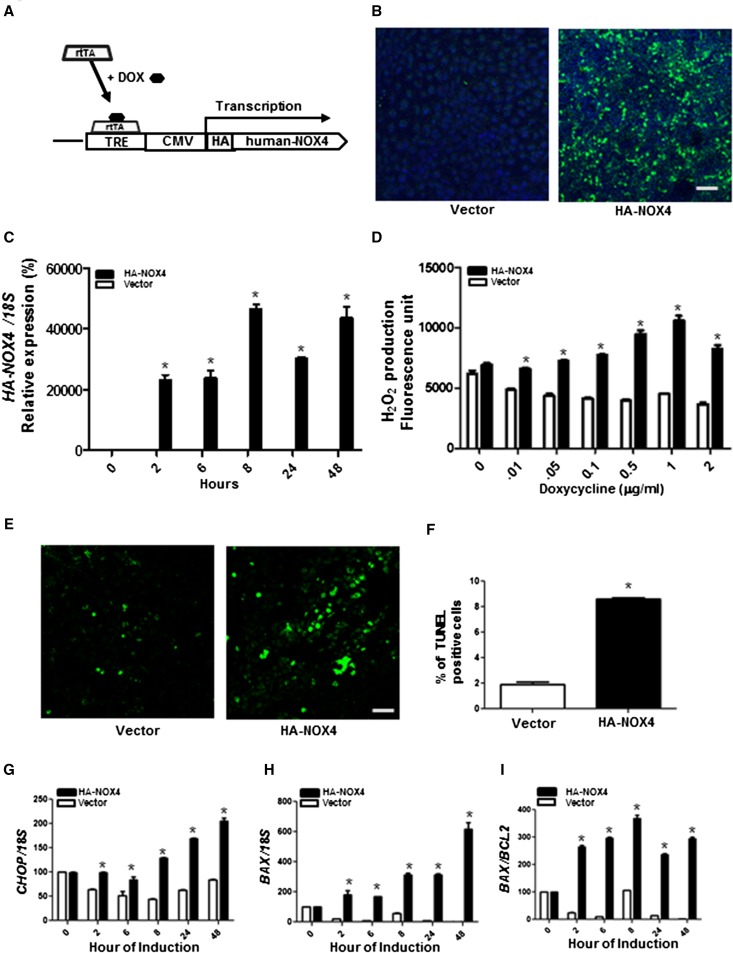
HA-tagged full-length human NOX4 construct (tet-O-NOX4) was stably transfected in Tet-On 293 cells, to induce NOX4 expression under the control of doxycycline (Figure 1, A–C). Cells transfected with the empty vector did not show changes in H2O2 levels, whereas a significant increase in intracellular H2O2 was observed in a doxycycline dose–dependent manner in HA-NOX4–expressing cells (Figure 1D). To determine whether upregulation of NOX4 induces apoptosis, doxycycline was added for 24 hours after HA-NOX4 transfection and the degree of apoptosis was detected with terminal deoxynucleotidyl transferase–mediated digoxigenin-deoxyuridine nick-end labeling (TUNEL) staining. The results indicate that increased expression of NOX4 induces a 4-fold increase in apoptosis (Figure 1, E and F).
Figure 1.
Effects of NOX4 expression in rtTA-293 cells. (A) Schematic representation of the transgene construct of doxycycline-inducible HA-tagged human NOX4. (B) Confocal immunofluorescence images of HA-NOX4 (green) protein expression. (C) HA-Nox4 mRNA expression after normalization against 18S expression. (D) Intracellular H2O2 levels. (E) TUNEL staining of confocal images to detect apoptosis. (F) Quantification of TUNEL-positive cells (n=3) of rtTA-293 cells transfected with pTRE-Tight vector encoding HA-tagged human NOX4 (HA-NOX4) or empty vector with 2 µg/ml doxycycline for 24 hours. (G–J) mRNA expression of CHOP, BAX, and BAX/BCL2 in rtTA-293 cells transfected with HANOX4 or empty vector after 2 µg/ml doxycycline for the indicated time points. Representative results from three independent experiments are shown. *P<0.05 versus vector alone. CMV, cytomegalovirus; DOX, doxycycline. Bar, 100 μm.
The endoplasmic reticulum (ER) stress pathway has been implicated in NOX4-induced apoptosis.8 Gene expression of the ER stress–induced apoptosis mediator CHOP was increased by NOX4 in a time-dependent manner (Figure 1G). The antiapoptotic protein BCL-2 and the proapoptotic protein BAX localize to the mitochondrial outer membrane and their ratio regulates the release of cytochrome c to cytosol.9,10 NOX4 expression resulted in a significant increase in the mRNA levels of proapoptotic BAX and the ratio of BAX/BCL2 (Figure 1, H and I).
Effect of Targeted Podocyte NOX4 Induction on Glomerular Pathology
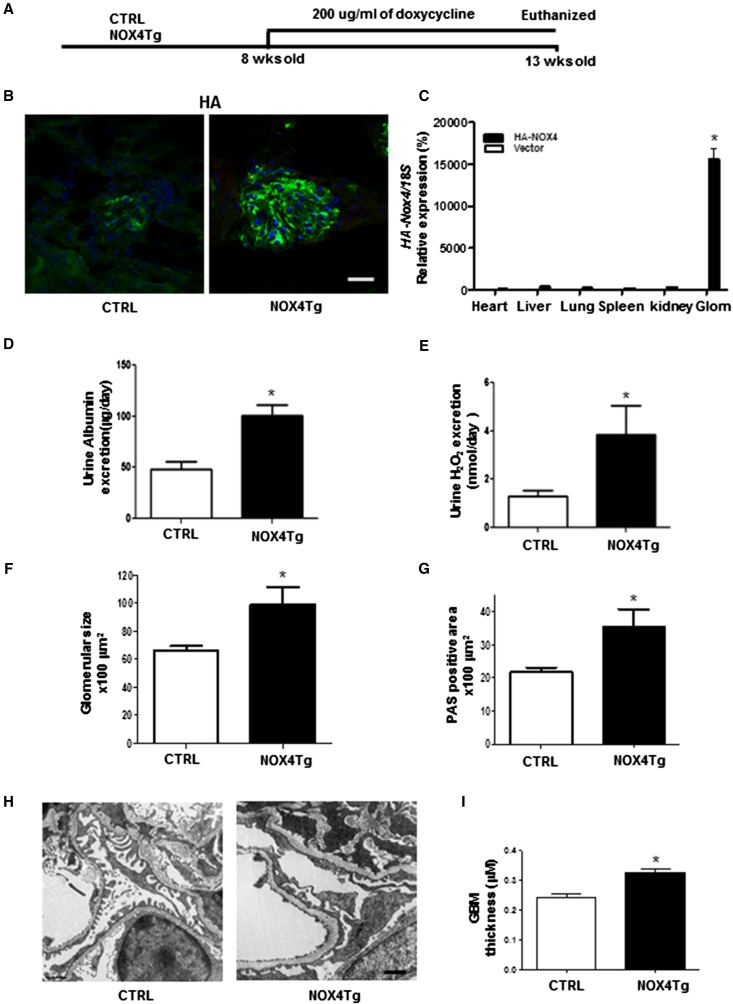
We previously identified that podocytes were a major source of NOX4 production in response to high glucose and with DKD.6 To examine whether podocyte-specific NOX4 upregulation had a primary role in glomerular structure and function, we bred FVB/J mice homozygous for podocin-rtTA with FVB/J heterozygous for tet-O-NOX4 to generate podocin-rtTA:tet-O-NOX4 (NOX4Tg) and podocin-rtTA mice. Male mice were administered doxycycline (200 μg/ml) or vehicle for 5 weeks (Figure 2A), at which point they demonstrated podocyte-specific expression of the HA-NOX4 transgene in NOX4Tg mice (Figure 2, B and C).
Figure 2.
Effects of podocyte-specific NOX4 induction on glomerular pathology. (A) Schematic representation of podocyte-specific NOX4Tg mice and their control littermates (podocin-rtTA) with 200 µg/ml doxycycline for 5 weeks to induce NOX4 transgene. (B) Confocal immunofluorescence images depicting HA-NOX4 expression in podocytes. (C) HA-Nox4 mRNA expression after normalization against 18S expression in the heart, liver, lung, spleen, kidney cortex, and glomeruli of NOX4Tg and control mice. (D and E) Urinary albumin excretions (D) and H2O2 excretion (E) measured with 24-hour urine collections from control and NOX4Tg mice after 5 weeks of doxycycline induction. *P<0.05 versus control (n≥6 per group). (F–I) Glomerular size (F) and mesangial matrix area (G) measured by morphometric analysis, representative TEM images (H), and quantification of glomerular basement membrane thickness from TEM micrographs of NOX4Tg and control mice (I). *P<0.05 versus control (n=3 per group). CTRL, control; Sac, sacrifice; TEM, transmission electron microscopy; GBM, glomerular basement membrane. Bar, 50 μm in B; 1 μm in H and I.
Doxycycline induction of the NOX4 transgene in podocytes did not affect body weight or systemic blood glucose levels (Supplemental Table 1). Albuminuria and urinary H2O2 levels were modestly increased with induction of NOX4 (Figure 2, D and E). Importantly, podocyte-specific Nox4 was sufficient to increase glomerular volume and stimulate mesangial matrix expansion in NOX4Tg doxycycline-treated mice compared with doxycycline-treated control mice (Figure 2, F and G). Electron microscopy was performed to evaluate for ultrastructural changes in glomeruli with NOX4 induction in podocytes. As noted in Figure 2, H and I, podocyte NOX4 induction resulted in a significant increase in glomerular basement membrane thickness.
Profibrotic Markers, ER Stress, and Podocyte Dropout Are Induced by Podocyte NOX4
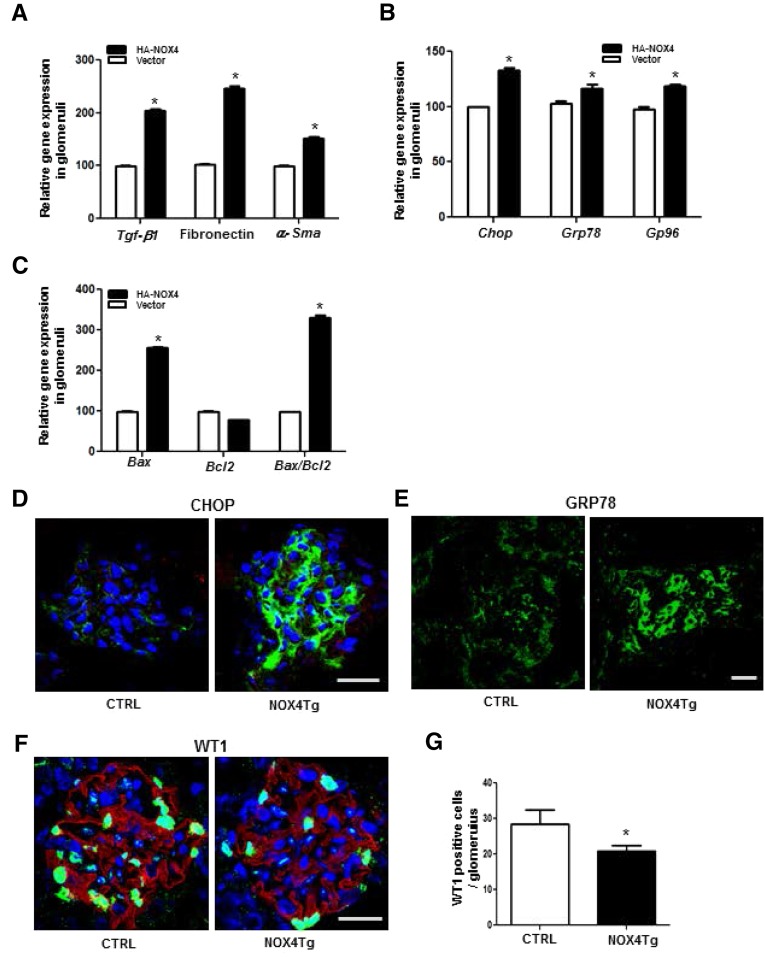
Gene expression analysis was performed for specific pathways based on the histology in the NOX4Tg mice. There was a significant induction of the profibrotic genes TGF-β1, fibronectin, and α-smooth muscle actin (α-SMA) in isolated glomeruli (Figure 3A). Chop, Grp78, and Gp96 were all stimulated in glomeruli (Figure 3B) from NOX4Tg mice. In addition, gene expression levels of Bax and the ratio of Bax/Bcl2 were upregulated in glomeruli from NOX4Tg mice (Figure 3C). Confocal microscopy analysis also revealed that both CHOP and GRP78 were increased in glomeruli of NOX4Tg mice (Figure 3, D and E).
Figure 3.
Effects of podocyte-specific NOX4 on ER stress and podocyte apoptosis. (A and B) mRNA expression analysis of Tgf-b1, fibronectin, and α-Sma (A) and ER stress genes Chop, Grp78, and Gp96 (B) in isolated glomeruli of podocyte-specific NOX4Tg and control mice. (C) mRNA expression analysis of Bax and Bcl2 and Bax/Bcl2 in the NOX4Tg and control mice. *P<0.05 (n=4 per group). (D and E) Confocal immunofluorescence images of CHOP (D; green) and GRP78 (E; green) expression in the glomeruli of the NOX4Tg and control mice after 200 μg/ml doxycycline for 5 weeks. (F and G) DAPI-stained (blue), confocal immunofluorescence image for the expression of WT-1 (F) and quantification of the WT1-positive cell number (G). *P<0.05 (n=3 per group). CTRL, control; DAPI, 4′,6-diamidino-2-phenylindole. Bar, 50 μm.
Podocyte reduction is commonly seen in human and mouse models of DKD. Podocyte numbers was measured using WT1-positive nuclei per glomerulus; a significant decrease in podocyte number was observed in the NOX4Tg mice (Figure 3, F and G). Thus, many of the typical glomerular changes associated with DKD were recapitulated by podocyte-specific NOX4 upregulation.
Inhibition of NOX1/NOX4 by GKT137831 as an Interventional Treatment Improves DKD
To test the functional contribution of NOX4, we evaluated the NOX1/NOX4-specific inhibitor GKT137831 (Supplemental Figure 1A). Treatment with GKT137831 significantly decreased H2O2 production in a concentration-dependent fashion in rtTA-293 cells stably transfected with the HA-tagged human NOX4 transgene (Supplemental Figure 1B).
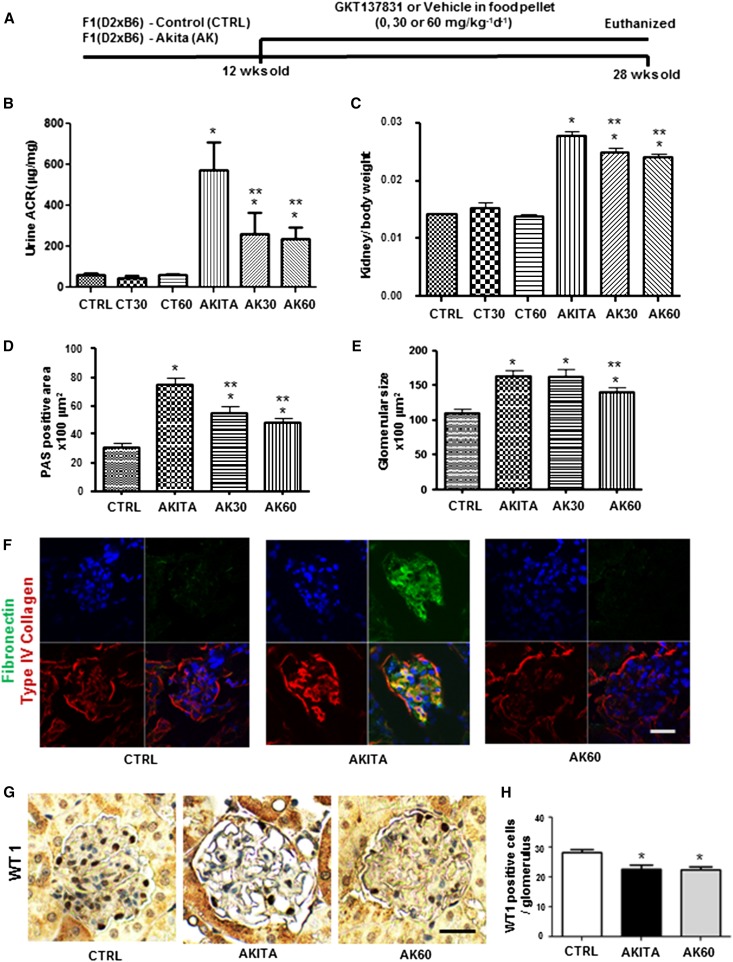
To determine whether the NOX1/NOX4 inhibitor (GKT137831) would have benefit as an interventional agent in DKD, we treated F1 (DBA/2J×C57BL/6J- Ins2Akita [F1 Akita]) mice with food pellets containing the NOX1/NOX4 inhibitor from week 12 to week 28 (Figure 4A). There was no effect of the NOX1/NOX4 inhibitor on body weight, blood glucose levels, or albuminuria (Table 1) in control mice. At 28 weeks of age, there was a significant increase in albuminuria, glomerular volume, and mesangial matrix expansion in F1 Akita in the DBA/C57BL mice compared with F1 control mice. In the F1 Akita mice treated with the NOX1/NOX4 inhibitor for 4 months, there was a significant reduction in albuminuria (Figure 4B), kidney hypertrophy (Figure 4C), mesangial matrix accumulation (Figure 4D, Supplemental Figure 1C), and glomerular volume (Figure 4E). Moreover, the NOX1/NOX4 inhibitor reduced the diabetic stimulation of renal type IV collagen and fibronectin expression to control levels (Figure 4F). However, podocyte depletion measured using WT1-positive nuclei per glomerulus in the F1 Akita mice was not affected with the NOX1/NOX4 inhibitor (Figure 4, G and H).
Figure 4.
Effects of NOX4 inhibitor GKT137831 in F1 Akita mice. (A) Schematic representation of the experimental plan with GKT137831 in the F1 Akita model. (B and C) Twenty-four–hour urinary ACR (B) and kidney/body weight ratio (C) of F1 control mice treated with vehicle, 30 mg/kg per day (CT30), or 60 mg/kg per day GKT137831 (CT60) and F1 Akita mice treated with vehicle (AKITA), 30 mg/kg per day GKT137831 (AK30), or 60 mg/kg per day GKT137831 (AK60) for 16 weeks. *P<0.05 versus control; **P<0.05 versus AKITA (n≥8 per group). (D and E) Mesangial matrix area (D) measured by morphometric analysis of glomeruli and glomerular surface area (E) of F1 control mice treated with vehicle and F1 Akita mice treated with vehicle, 30 mg/kg per day GKT137831, or 60 mg/kg per day GKT137831. *P<0.05 versus control; **P<0.05 versus AKITA (n≥8 per group). (F) Confocal images representative of collagen (red) and fibronectin (green) in glomerular staining (n=3 mice per group). (G) Glomerular WT-1 staining, and (H) quantification of WT-1–positive cells per glomerulus of F1 control mice treated with vehicle and F1 Akita mice treated with vehicle or 60 mg/kg per day GKT137831. *P<0.05 versus control (n=20 glomeruli from each mouse kidney; n=3 per group). CTRL, control; AK, Akita; Sac, sacrifice; ACR, albumin-to-creatinine ratio; PAS, periodic acid–Schiff. Bar, 50 μm.
Table 1.
Physiologic characteristics of NOX1/NOX4 inhibitor GKT137831–treated mice
| Treatment | Number | Body Weight (g) | Blood Glucose (mg/dl) | ACR |
|---|---|---|---|---|
| F1 control 0 mg of GKT137831 (CTRL) | 12 | 34.68±2.16 | 186.91±57.10 | 60.92±7.85 |
| F1 control 30 mg of GKT137831 (CT30) | 14 | 35.71±4.14 | 230.31±60.36 | 43.02±11.90 |
| F1 control 60 mg of GKT137831 (CT60) | 13 | 36.00±3.75 | 237.92±92.76 | 60.85±5.52 |
| F1 Akita 0 mg of GKT137831 (AKITA) | 12 | 26.51±1.83a | >600a | 572.5±137.2a |
| F1 Akita 30 mg of GKT137831 (AK30) | 13 | 27.85±1.84a | >600a | 258.9±105.9a,b |
| F1 Akita 60 mg of GKT137831 (AK60) | 11 | 27.32±2.46a | >600a | 237.2±56.97a,b |
Values are presented as means±SEM. F1 control mice and F1 Akita mice were treated with vehicle, 30 mg/kg per day of GKT137831, or 60 mg/kg per day of GKT137831 for 16 weeks. ACR, albumin-to-creatinine ratio.
P<0.05 versus CTRL group.
P<0.05 versus AKITA group.
Urine Metabolomics Reveals Fumarate as a Target of NOX4 Inhibition
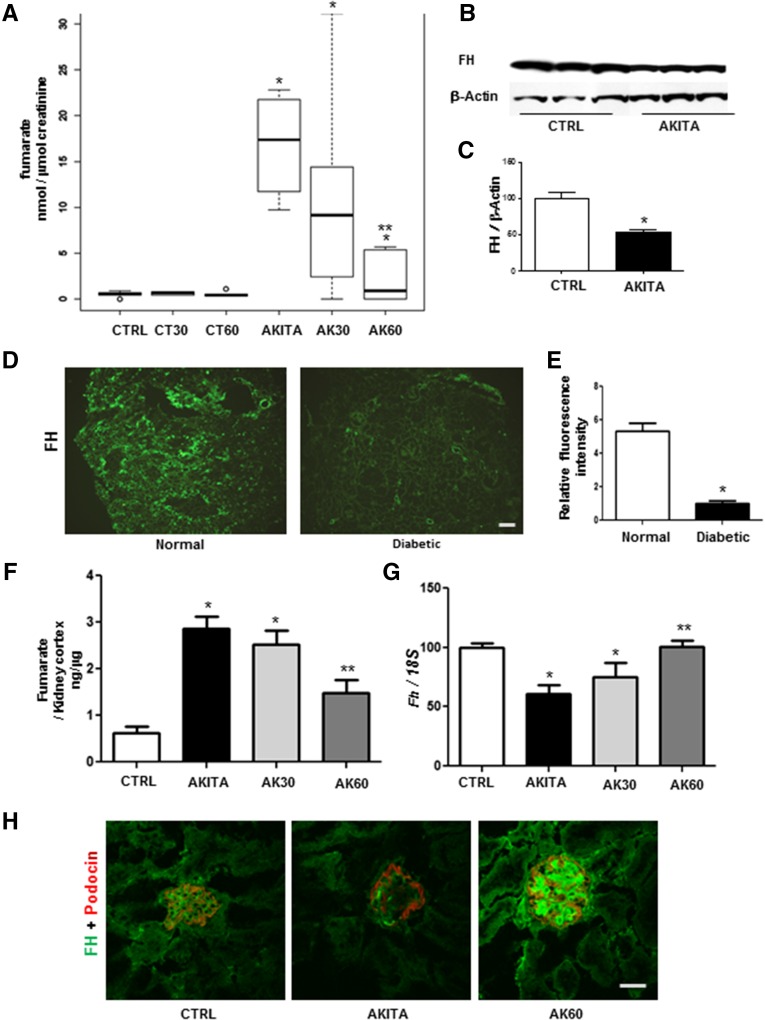
To obtain further insight into how NOX4 excess contributes to DKD, we performed a metabolomic analysis of urine samples obtained from 24-hour urine collections of F1 control mice, F1 Akita mice, and F1 Akita mice treated with the NOX1/NOX4 inhibitor. A prominent pattern was an overall increase in the metabolites of the TCA cycle in the diabetic F1 Akita mice compared with the control group (Supplemental Figure 2). Interestingly, only one of the TCA cycle intermediates, fumarate, was significantly reduced with the NOX1/NOX4 inhibitor in a dose-dependent manner (Figure 5A).
Figure 5.
The GKT137831 NOX4 inhibitor reduces fumarate accumulation in diabetic kidney. (A) Box plots showing reduction in fumarate in F1Akita diabetic urine compared with controls after 16 weeks treatment with GKT137831. F1 control mice treated with vehicle, 30 mg/kg per day GKT137831 (CT30), or 60 mg/kg per day GKT137831 (CT60) and F1 Akita mice treated with vehicle (AKITA), 30 mg/kg per day GKT137831 (AK30), or 60mg/kg per day GKT137831 (AK60). *P<0.05 versus control; **P<0.05 versus AKITA with vehicle (n=5–6; lines indicate the median). (B and C) Immunoblotting of F1 control mice and F1 Akita mice kidney cortex lysates against FH antibody. *P<0.05 versus control. (D and E) Representative immunofluorescence staining of FH (D; green;), and intensity analysis of FH in human renal biopsy specimens (E) from normal patients and patients with diabetes (n=3). (F) Fumarate levels in kidney cortex lysate after treatment of F1 control mice with vehicle and F1 Akita mice with vehicle, 30 mg/kg per day GKT137831, or 60 mg/kg per day GKT137831 using the Fumarate Assay Kit. *P<0.05 versus control; **P<0.05 versus F1 Akita mice with vehicle. (G) mRNA expression levels of Fh in F1 control mice with vehicle and F1 Akita mice with vehicle, 30 mg/kg per day GKT137831, or 60 mg/kg per day GKT137831. *P<0.05 versus control (n=3 per group). (H) Representative confocal microscopy images depicting endogenous expression of FH (green) and podocin (red) in F1 control with vehicle and F1 Akita mice with vehicle or 60 mg/kg per day GKT137831 (n=3 per group). CTRL, control. Bar, 100 μm in D and E; 50 μm in H.
In the TCA cycle, fumarate is generated by oxidation of adenylsuccinate by the enzyme succinate dehydrogenase (SDH) and is converted to malate by the enzyme FH. Inhibition of FH or stimulation of SDH can lead to accumulation of fumarate, as seen in diabetic urine. Previous studies have reported a reduction of SDH in diabetes11–13; however, no studies have examined FH to our knowledge. In addition, because NOX1/NOX4 inhibition led to a reduction in fumarate levels without affecting levels of succinate, FH was considered to be the more likely site of regulation by NOX4. Immunoblotting and immunohistochemistry analysis of mouse kidney samples revealed a substantial reduction in FH protein in diabetic mouse kidney compared with nondiabetic mouse controls (Figure 5, B and C). We also evaluated FH in kidney biopsy samples from patients with normal kidneys and patients with diabetic nephropathy. Indeed, similar to what we observed in the mouse model, there was a reduction of FH in the human diabetic kidney tissue compared with control kidneys (Figure 5, D and E).
To evaluate whether fumarate itself was regulated by NOX4 in the diabetic kidney, fumarate levels were measured and found to be increased in the mouse diabetic renal cortex and reduced by NOX4 inhibition (Figure 5F). Fh gene expression was also significantly reduced in the diabetic kidney cortex and increased with NOX4 inhibition (Figure 5G). Immunostaining showed that the reduced glomerular FH was restored by NOX4 inhibitor treatment in F1 Akita mice (Figure 5H). Together, the metabolomic, gene expression, and protein analyses suggest that FH is a target of NOX4 in the diabetic kidney.
NOX4 Reduces FH Expression and Activity
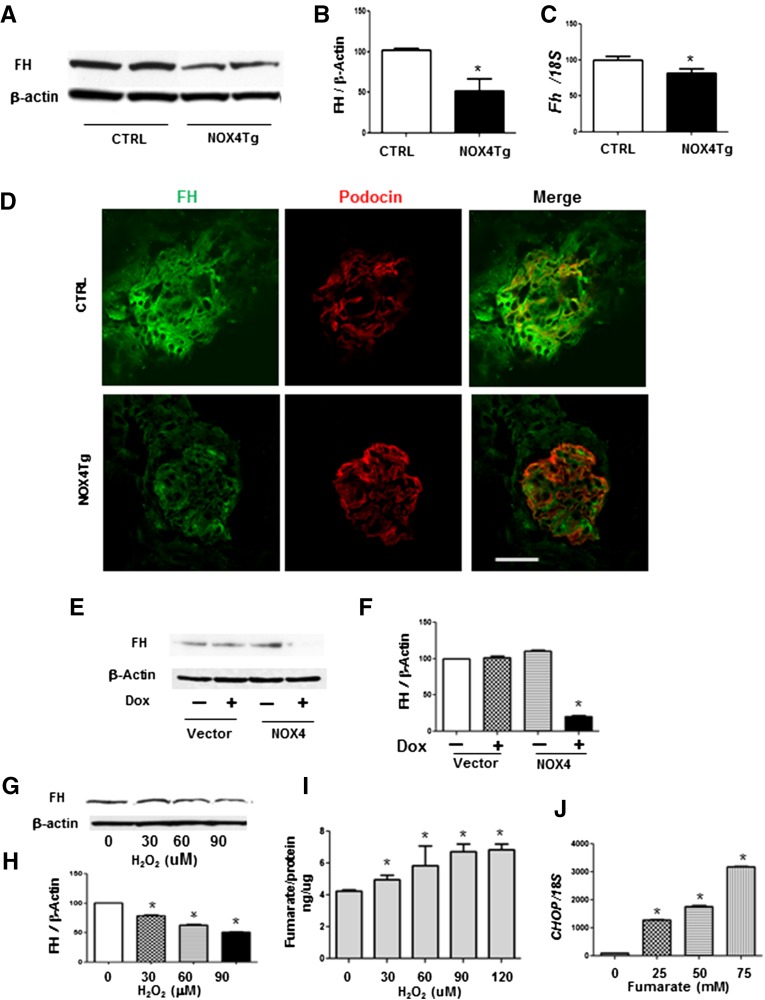
We hypothesized that fumarate levels in diabetes was attributable to regulation of FH activity by upregulation of NOX4. To determine whether podocyte NOX4 upregulation was sufficient to inhibit FH, we evaluated FH levels in the NOX4Tg mice treated with doxycycline. Induction of podocyte NOX4 by doxycycline led to a significant reduction in renal cortical FH protein (Figure 6, A and B) and gene expression (Figure 6C). Immunostaining revealed a clear reduction of glomerular FH with induction of podocyte NOX4 (Figure 6D). Similar to the in vivo studies, induction of NOX4 expression led to a reduction of FH in rtTA-293 cells (Figure 6, E and F). These studies demonstrate that NOX4 is sufficient to inhibit FH in the absence of diabetes.
Figure 6.
NOX4 overexpression reduces FH expression and activity. (A and B) Immunoblotting (A) and quantification (B) of FH protein expression after normalization against β-actin. (C) mRNA expression levels after normalization against 18S expression in the kidney cortex from podocyte-specific (NOX4Tg) and control mice after 200 μg/ml doxycycline for 5 weeks. (n=3–4 mice per group). (D) Confocal microscopic analysis of FH (green) and podocin (red) in the kidney glomerulus from podocyte-specific NOX4Tg and control mice after 200 μg/ml doxycycline for 5 weeks. (E and F) FH protein expression (E) and quantification (F) of immunoblotting after normalization against β-actin from rtTA- 293 cells transfected with HA-NOX4 induced after 2 μg/ml doxycycline for 24 hours. (G and H) Immunoblotting (G) and quantification (H) of FH protein expression after normalization against β-actin. (I) Fumarate production from HEK293 cells treated with H2O2 (0, 30, 60, 90, and 120 μM) for 24 hours. (J) mRNA expression levels of CHOP after normalization against 18S expression in HEK293 cells treated with fumarate (0, 25, 50, and 75 mM) for 24 hours. Representative results from three independent experiments are shown. *P<0.05 versus control mice or no treatment. CTRL, control; Dox, doxycycline. Bar, 50 μm.
Because NOX4 is a potent source of H2O2 production, we questioned whether H2O2 was the signaling molecule mediating effects of NOX4 on FH. Interestingly, H2O2-treated HEK293 cells reduced cellular FH protein levels in a dose-dependent manner (Figure 6, G and H) and increased fumarate levels (Figure 6I). Furthermore, CHOP mRNA expression was dose-dependently upregulated by fumarate (Figure 6J), suggesting that the NOX4-induced fumarate production contributes to ER stress.
NOX4 and Fumarate Are Potent Regulators of HIF-1α and TGF-β
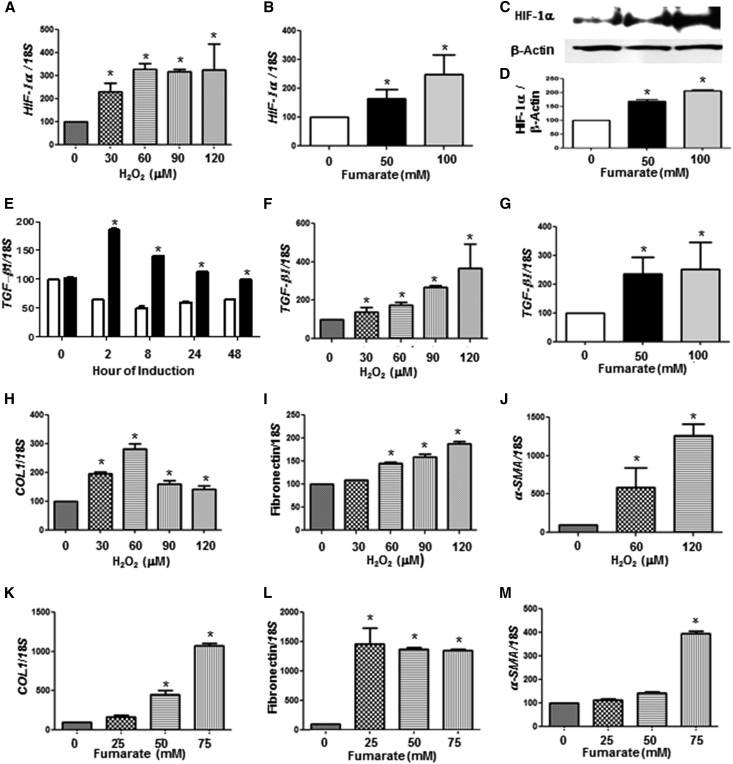
Accumulation of fumarate may contribute to renal pathology, possibly via inhibition of HIF-prolyl hydroxylases and increasing HIF-1α.14 Indeed, both HIF-1α protein and mRNA were elevated with either H2O2 (Figure 7A) or fumarate (Figure 7, B–D) in HEK293 cells in a dose-dependent manner.
Figure 7.
NOX4 and fumarate are potent regulators of HIF-1α and TGF-β in vitro. (A) HIF-1α mRNA expression in HEK293 cells treated with H2O2 (0, 30, 60, 90, and 120 μM) for 24 hours. (B–D) HIF-1α mRNA (B), immunoblotting (C), and quantification (D) of HIF-1α protein in HEK293 cells treated with fumarate (0, 50, and 100 mM) for 24 hours. (E) mRNA expression of TGF-β1 from NOX4 stably transfected into rtTA-293 cells treated with 2 μg doxycycline up to 48 hours. (F and G) mRNA expression of TGF-β1 in HEK293 cells treated with H2O2 (0, 30, 60, 90, and 120 μM) (F) or fumarate (0, 50, and 100 mM) (G) for 24 hours. (H–M) mRNA expression of type 1 collagen (COL1) (H and K), fibronectin (L and J), and α-SMA (J and M) in HEK293 cells treated with H2O2 (0, 30, 60, 90, and 120 mM) (H–J) or fumarate (0, 25, 50, and 75 mM) (K–M) for 24 hours. Data are presented as the ratio of gene to 18S. Representative results from three independent experiments are shown. *P<0.05 versus no treatment.
In addition, there was an early stimulation of TGF-β1 gene expression levels that was noted as early as 2 hours after induction of NOX4 in rt-293 cells (Figure 7E). TGF-β1 gene expression was also stimulated with either H2O2 (Figure 7F) or fumarate (Figure 7G). Increased HIF-1α and TGF-β1 expression with either H2O2 or fumarate in HEK293 cells was also associated with elevated gene expression of classic markers of fibrosis such as type 1 collagen (Figure 7, H and K), fibronectin (Figure 7, I and L), and SMA (Figure 7, J and M) in a dose-dependent manner.
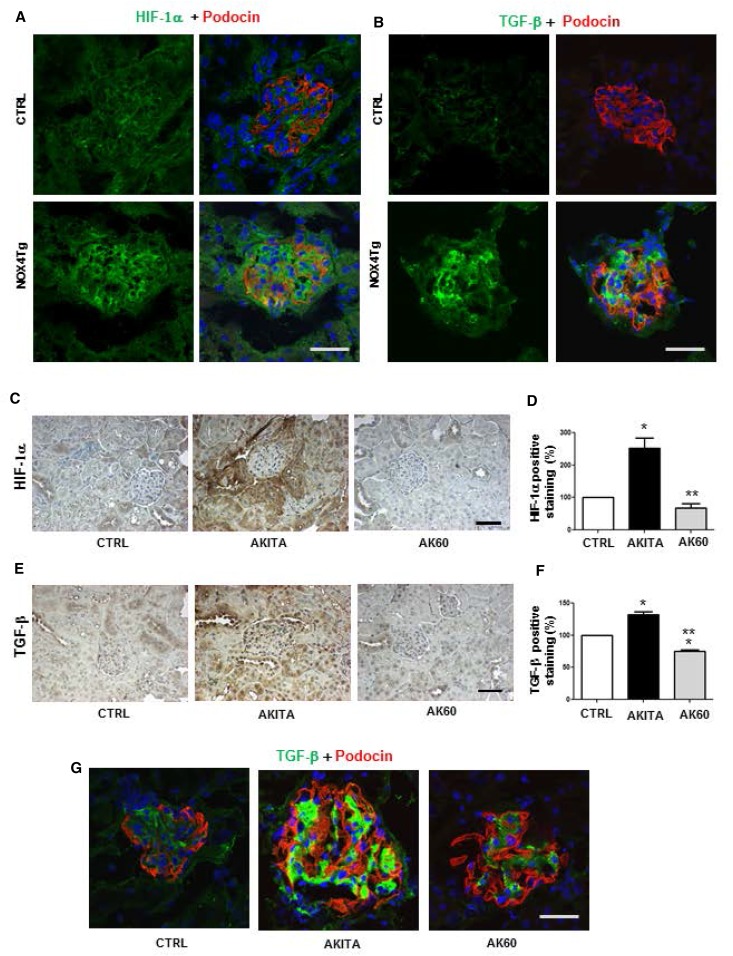
We studied whether a similar effect of FH on HIF-1α and TGF-β was observed in vivo. Interestingly, both HIF-1α (Figure 8A) and TGF-β (Figure 8B) were stimulated in glomeruli of the NOX4 transgenic mice. Moreover, HIF-1α (Figure 8, C and D) and TGF-β (Figure 8, E–G) were also stimulated in the kidney cortex of the diabetic F1 Akita mice compared with controls, whereas Nox4 inhibitor treatment restored FH formation and resulted in significant decrease in HIF-1α and TGF-β expression in kidney.
Figure 8.
NOX4 and fumarate are potent regulators of HIF-1α and TGF-β in mouse kidney. (A and B) Confocal microscopic images of HIF-1α (A; green) and TGF-β1 (B; green) expression in the kidney glomerulus from podocyte-specific NOX4Tg and control mice after 200 μg/ml doxycycline for 5 weeks. (C–F) Immunohistochemistry and image quantitation of HIF-1α (C and D) and TGF-β1 (E and F) of F1 control mice with vehicle and F1 Akita mice with vehicle (AKITA) or 60 mg/kg per day GKT137831 (AK60). *P<0.05 versus control; **P<0.05 versus Akita control group (n=3 mice per group). (G) Confocal expression of TGF-β1 (green) and podocyte-specific marker protein podocin (red) in kidney glomeruli of F1 control mice with vehicle and F1 Akita mice with vehicle or 60 mg/kg per day GKT137831 (n=3 mice per group). CTRL, control. Bar, 50 μm.
Taken together, these data support the hypothesis that loss of FH activity is attributable to increased NOX4 in the diabetic kidney. Increased H2O2 levels through NOX4 in the diabetic kidney can lead to diminished FH and an increase in fumarate. Both H2O2 and fumarate increase HIF-1α and stimulate TGF-β. Pharmacologic inhibition of NOX4 resulted in restored FH activity and reduction of HIF-1α and TGF-β, leading to reduced glomerular matrix and renoprotection (Figure 9).
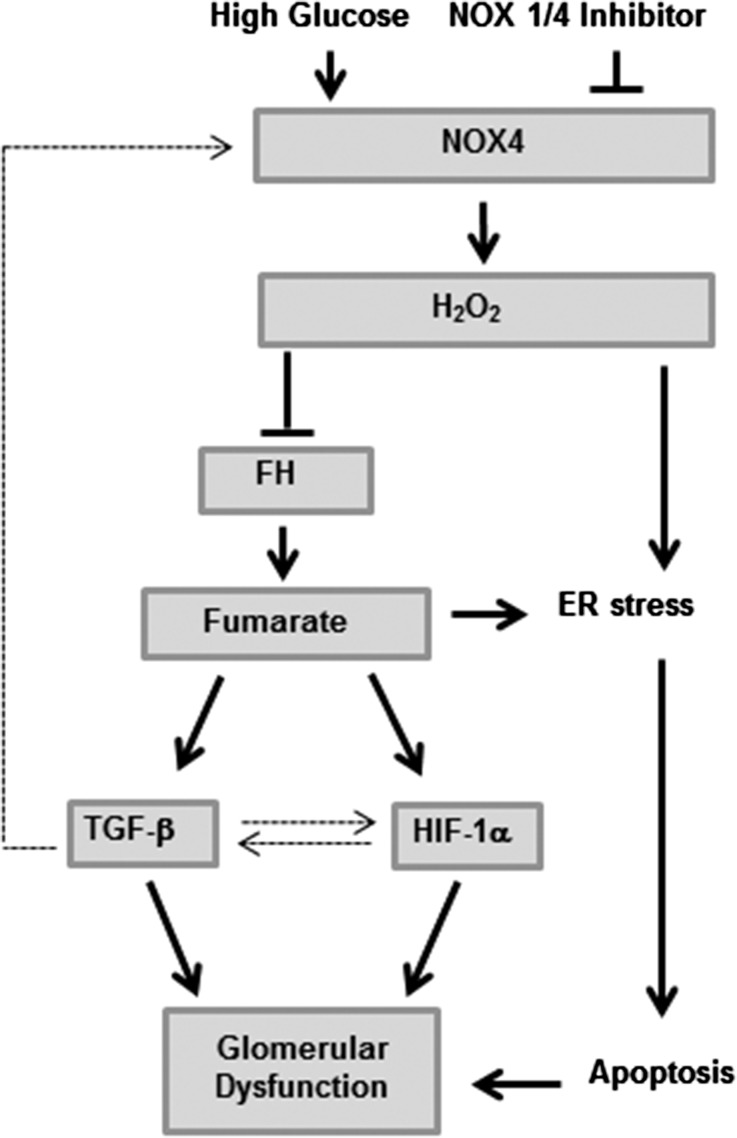
Figure 9.
Model depicting the role of NOX4-induced H2O2 and fumarate in kidney in diabetes or high glucose. High glucose–induced stimulation of NOX4-mediated H2O2 production leads to decreased activity of FH and a consequent increase in fumarate levels. Both H2O2 and fumarate contribute to the elevated HIF-1α level and TGF-β found in kidney diabetic cells and increased glomerular matrix and podocyte dysfunction. In addition, H2O2 and fumarate increase ER stress, which in turn can cause a BCL-2–mediated and mitochondria-dependent apoptosis. Dotted lines represent previous studies, whereas continuous lines represent this study.
Discussion
Diabetic glomerular disease is characterized by glomerular hypertrophy, mesangial matrix expansion, podocyte dropout, and glomerular basement membrane thickening. In this study, upregulation of podocyte Nox4 was sufficient to recapitulate all of these glomerular features of DKD. Pharmacologic intervention with a NOX1/NOX4 inhibitor was able to reduce the major parameters of DKD. A key novel mechanism has been identified via metabolomics, with the finding that Nox4 inhibition regulates fumarate levels, leading to the discovery that the TCA cycle enzyme FH is a downstream target of NOX4. Fumarate has now been identified as a disease-promoting metabolite because it stimulates ER stress, matrix production, and HIF-1α and TGF-β production in the context of DKD.
NOX4 has consistently been implicated as the major Nox isoform that is upregulated in the diabetic kidney15–18; however, recent studies have found conflicting data with NOX4 KO mice.2,7 Our study found that podocyte upregulation of NOX4 was sufficient to develop many of the classic features of diabetic glomerular disease. Coupled with our interventional study demonstrating that the NOX1/NOX4 inhibitor provided protection against diabetic mesangial matrix accumulation and albuminuria in the F1 Akita model and similar beneficial preventive results with the NOX1/NOX4 inhibitor in the ApoE diabetic model,19,20 it is likely that upregulation of NOX4 is a critical contributor to many of the glomerular lesions in DKD. Although complete loss of NOX4 may abrogate its physiologic role and could lead to worsening of renal disease under stressed conditions,21 inhibition with the NOX1/NOX4 inhibitor would not be expected to completely suppress NOX4. Importantly, phase 1 studies in humans have not identified toxicity of a NOX1/NOX4 inhibitor and a phase 2 study in DKD is currently underway.22
The mechanism by which NOX4 contributes to DKD is unknown. In this study, we identified a novel mechanism of action of the Nox1/Nox4 inhibitor using urine metabolomics in the F1 Akita mouse model of DKD. With this model, almost all of the TCA cycle metabolites were found to be increased in the urine of the diabetic mice. A similar pattern has been described in a model of type 2 diabetes early in the course of diabetes23 and in humans with diabetes before a clinical reduction of renal function.24 Of all of the urinary TCA cycle metabolites that were found to be increased by diabetes, only fumarate was significantly reduced in a dose-dependent manner by GKT13781.
Because FH is a key enzyme in mitochondria that converts fumarate to malate, we evaluated whether reduction of FH may contribute to increased urine fumarate levels. FH was indeed inhibited in the diabetic kidney, both in the F1 Akita mice and in humans with established diabetic nephropathy. FH was previously found to be inhibited by reactive oxygen species25; however, FH has not previously been implicated in DKD nor has the source of reactive oxygen species that regulates FH been previously determined. In this study, we demonstrate that the NOX4/H2O2 pathway is a likely candidate to mediate reduced FH levels in the diabetic kidney. Indeed, inhibition of NOX4 with the NOX1/NOX4 inhibitor was able to restore FH to normal levels.
Homozygous loss-of-function mutations in FH lead to elevated urine fumarate levels and have been associated with encephalopathy in children. Haploinsufficiency of FH caused by a heterozygous loss-of-function mutation predisposes to multiple cutaneous and uterine leiomyomas and hereditary leiomyomatosis with renal cell cancer.26–29 It was recently identified that inhibition of FH leads to increased levels of fumarate, which inhibits the activity of prolyl hydroxylase, resulting in HIF-1α accumulation and activation of HIF-dependent pathways.30–33 To our knowledge, this study is the first to demonstrate that NOX4 mediates upregulation of HIF-1α in DKD. Activation of HIF signaling promotes fibrosis in the context of CKD through increased expression of HIF-1α–regulated factors such as connective tissue growth factor, plasminogen activator inhibitor-1, tissue inhibitor of matrix metalloprotease-1, and/or through induction of TGF-β.34–37 Because NOX4 induction is sufficient to stimulate HIF-1α in vitro and in vivo and inhibition with NOX1/NOX4 reduces HIF-1α in DKD, the major driver of HIF-1α with diabetes may well be NOX4. Diebold et al. found that NOX4 is a novel target gene of HIFs38,39 and several other groups have demonstrated that NOX4 regulates HIF-1 and HIF-2 levels,40,41 suggesting a positive-feedback loop between NOX4 and HIFs. Our study also uniquely demonstrates that fumarate can stimulate matrix production and TGF-β1 in renal cells. We find that TGF-β1 is stimulated by fumarate at both the mRNA and protein levels, suggesting that this metabolite is a key player in mediating matrix accumulation in DKD.
In addition and consistent with previous findings in smooth muscle cells,8 we identified that Nox4 regulates ER stress in podocytes. Both H2O242 and fumarate were found to induce ER stress and may contribute to this effect of Nox4. Further studies are needed to uncover the actual mechanisms governing these observations.
In conclusion, we have identified that NOX4 in podocytes plays a key role in mediating many of the features of DKD at the glomerular level. The mechanisms underlying the damaging effects of NOX4 upregulation involve ER stress, FH reduction, and a H2O2/ fumarate induction of HIF-1α/TGF-β1 upregulation. Inhibition of NOX4 and upregulation of FH may represent novel approaches to treat progressive DKD.
Concise Methods
Generation of Inducible, Podocyte-Specific NOX4 Transgenic Mice
To generate the tet-O-NOX4 construct, HA-tagged NOX4 was placed under the control of a tetracycline-responsive element by subcloning a HA-tagged full-length (1.8 kb) human NOX4 cDNA fragment by PCR into pTRE-Tight vector (Clontech) between the Cla1 and PST1 sites. The tet-O-NOX4 construct was purified by electrophoresis and DNA extraction (QIAEXII gel extraction kit; Qiagen) and injected into pronuclei of FVB/N fertilized zygotes. Transgene-bearing founder mice were identified by PCR using the following primers: 5-TAACCAAGGGCCAGAGTATCACT-3 and 5-AGTGATACTCTGGCCCTTGTTA-3. Transgenic tet-O-NOX4 mice were crossbred with podocin-rtTA mice, which express a reverse transactivator under the control of the podocin-specific promoter (kindly provided by J. Kopp, National Institutes of Health [NIH]). NOX4Tg mice are viable, healthy and fertile. For induction of the NOX4 transgene, 8-week-old NOX4Tg mice were given doxycycline (200 μg/ml) in their drinking water for 5 weeks. All animal studies were approved by the Institutional Animal Care and Use Committee of University of California—San Diego.
Cell Culture and Transfection
All cell culture experiments were performed in HEK293 cells or 293 Tet-On cells (rtTA-293; Clontech) maintained in complete DMEM media (10% FCS, 1% l-glutamine, and 0.1% penicillin–streptomycin), at 37°C and 5% CO2. To overexpress NOX4, the tet-O-NOX4 construct or empty vector was transfected using Lipofectamine 2000 by following the manufacturer’s protocol and was induced with 2 µg/ml of doxycycline for 24 hours. For stable NOX4 generation, the tet-O-NOX4 construct was transfected into rtTA-293 cells and selected with puromycin (2 µg/ml). For treatment with fumarate (Chem Cruz, sodium fumarate dibesic; Santa Cruz Biotechnology), the cells were treated with 0–100 mM for 24 hours. For H2O2 treatment, the cells were treated with 0–120 µM H2O2 for 24 hours. Fumarate was detected in cell and tissue lysates using the Fumarate Assay Kit (Sigma-Aldrich) by following the manufacturer’s protocol.
Interventional Animal Studies with Nox1/Nox4-Specific Inhibitor GKT137831
C57BL/6-Ins2Akita male mice were bred to female DBA/2 mice (The Jackson Laboratory) to produce the F1 (DBA/2 × C57BL/6) control (F1 control) and the F1 (DBA/2 × C57BL/6)-Ins2Akita mice.43 In the interventional study, the Nox1/Nox4-specific inhibitor GKT137831 (Genkyotex, Geneva, Switzerland) was administered by feeding drug-impregnated food pellets (Harlan-Teklad) to the F1 control mice and the F1 Akita mice at dosages of 30 and 60 mg/kg per day or vehicle food pellets at age 12 weeks for 16 weeks. Mice were euthanized after 16 weeks of treatment for further characterization. At the end of the study period, a 24-hour urine collection was obtained in metabolic cages, blood was obtained by venous puncture, and kidneys were harvested as previously described.1 Glomeruli were isolated by magnetic bead–based isolation as described.44 Blood glucose was measured using the Accu-Chek meter system (Roche Diagnostics). Urinary albumin and creatinine were measured by mouse-specific ELISA (Albuwell M ELISA; Exocell) and a Creatinine Companion kit (Exocell). As an index of oxidative stress, urine samples were also analyzed for hydrogen peroxide by the Amplex Red assay (Invitrogen) according to the manufacturer’s protocol.
Histology, Morphometric Analyses, and Transmission Electron Microscopy
Kidneys were fixed in 4% formalin and embedded in paraffin. Periodic acid–Schiff staining was performed to evaluate the extent of the mesangial matrix in the mesangium as previously described.45 The glomerular size and periodic acid–Schiff-positive mesangial area were analyzed with 15 randomly selected glomeruli per animal (n=3 per group) using i-solution software (Advanced Imaging Concepts, Princeton, NJ). Quantitative analysis was performed in a blinded manner. For electron microscopy analysis, the kidney cortex was fixed with 2% paraformaldehyde and 2.5% glutaraldehyde in 0.15 M sodium cacodylate buffer, processed for transmission electron microscopy and viewed on a JEOL 1200EX II transmission electron microscope (JEOL, Peabody, MA), and photographed using a Gatan digital camera (Gatan, Pleasanton, CA). Electron micrographs of four glomeruli per kidney were taken randomly for each mouse to evaluate morphometric analysis of podocyte and glomerular basement membrane thickness according to published methods.6
Human Participants
Slides of normal kidney tissue and kidney tissue from patients with DKD (n=3 per group) were obtained from Agnes Fogo (Vanderbilt University) and Sanjay Jain (Washington University) from archived tissue samples via institutional review board–approved protocols.
Immunofluorescence Staining
Frozen kidney was sectioned into 4- to 6-μm thickness, fixed using 4% paraformaldehyde, and blocked with 5% FBS for 1 hour at room temperature. Sections were incubated overnight at 4°C with primary antibodies, washed with PBS, and incubated for 1 hour at room temperature with Alexa Fluor–conjugated secondary antibodies. The following primary antibodies were used in this study: rabbit anti-HA (Sigma-Aldrich), goat anti–type IV collagen (SouthernBiotech), rabbit anti–TGF-β 1/TGF-β2/TGF-β3 (Santa Cruz Biotechnology), goat anti-fibronectin (Sigma-Aldrich), rabbit anti-WT1 (Santa Cruz Biotechnology), mouse anti-CHOP (Cell Signaling Technology), mouse anti-GRP78 (Cell Signaling Technology), rabbit anti-FH (Sigma-Aldrich), rabbit anti–HIF-1α (Santa Cruz Biotechnology), and goat anti-podocin (Santa Cruz Biotechnology). Sections were visualized by an Olympus FluoView FV1000 device.
Immunohistochemistry
Antigen retrieval from 4- to 5-μm paraffin sections was performed with Vector unmasking reagent (Vector Laboratories, Burlingame, CA). After antigen retrieval, kidney sections were blocked with PBS, 5% (vol/vol) normal goat serum, and 0.01% (vol/vol) Triton. The sections were incubated with primary antibody diluted in the blocking solution overnight at 4°C and were detected with a horseradish peroxidase– conjugated secondary antibody (Jackson ImmunoResearch). The following primary antibodies were used: rabbit anti–TGF-β 1/TGF-β2/TGF-β 3 (Santa Cruz Biotechnology), rabbit anti-WT1 (Santa Cruz Biotechnology), rabbit anti-FH (Sigma-Aldrich), and rabbit anti–HIF-1α (Santa Cruz Biotechnology). Kidney sections were finally incubated with ABC complex (Vector Laboratories) for 30 minutes and bound peroxidase activity was detected with the 3,3′-diaminobenzidine kit. ImageJ software (NIH, Bethesda, MD) was finally used to quantify the percentage of positive area. A total of 10 fields randomly taken at ×400 magnification in the kidney tissue were analyzed from four mice per group. The number of podocytes was analyzed from WT1 staining by estimating the number of total WT1-positive cells (podocytes) in 15 randomly selected glomeruli from three mice per group.
RNA Isolation and Real-Time Quantitative PCR
Total RNA was isolated from the kidney cortex using Trizol reagent (Invitrogen) and real-time quantitative PCR was performed as previously described.46 Real-time quantitative was carried out with specific primers (Supplemental Table 2). For quantitative analysis, the samples were normalized to 18S or β-actin gene expression using the Δ cycle threshold value method.
Immunoblotting
Kidney cortex or cell pellets were lysed in lysis buffer (10 mM Tris-HCl [pH 7.5], 100 mM NaCl, 1% NP-40, 50 mM NaF, 20 mM β-glycerophosphate, 2 mM EDTA, 1 mM dithiothreitol, 1 mM PMSF, 10 μg/ml leupeptin, and 10 μg/ml aprotinin) and boiled in SDS sample buffer. Lysates were subjected to SDS-PAGE and Western blotting according to standard techniques. Three separate Western blots were performed for each experiment. The digital quantification of Western blots was performed using ImageJ software. The quantification intensity was normalized against β-actin bands.
TUNEL Analyses
TUNEL analysis was performed to detect apoptotic nuclei using the DeadEnd Fluorometric TUNEL System (Promega) according to the manufacturer’s instructions. TUNEL-positive cells (nuclei) were counted using ImageJ image analysis software.
Metabolomic Analyses
Aliquots of 24-hour urine collection were processed for metabolomic analysis as described previously.47 Briefly, before lyophilization, the ketoacids oximated with pentafluorobenzylhydroxylamine. Organic acids were isolated by liquid partition chromatography on silica (42% 2-methyl-2-butanol in chloroform), the elute was evaporated, and the dry residue was silylated with Tri-Sil N,O-bis (trimethylsilyl) trifluoroacetamide. Urine aliquots corresponding to 1 μmol of creatinine were applied by injection onto a 30 m × 0.32 mm column (Agilent DB-5) in a gas chromatograph (Agilent 5890) and eluted with a 4°C/min gradient of 70°C–300°C, and analytes were detected by electron impact mass spectrometry (Agilent 5973 mass selective detector). Each compound was identified and quantified.
Statistical Analyses
The assessment of differences in metabolite levels among the sample groups was done by the t test after log2 transformation of the metabolite levels. In addition, the metabolite levels were visualized by box plots. These statistical analyses were done using R software. Differences between groups were evaluated for significance using the independent t test or one-way ANOVA with the Student–Newman–Keuls post hoc test. P values <0.05 were considered statistically significant.
Disclosures
K.S. has provided consultancy services to Genkyotex S.A. and is the founder of ClinMet, Inc.
Supplementary Material
Acknowledgments
We thank Manjula Darshi and Maggie Diamond-Stanic (University of California—San Diego, CA) for their critical review of the manuscript.
This work was supported by grants from the NIH (U01-DK076133 and DP3-DK094352 to K.S). Genkyotex S.A. provided the GKT137831 study drug.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “NADPH Oxidase 4 at the Nexus of Diabetes, Reactive Oxygen Species, and Renal Metabolism,” on pages 337–339.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2015030302/-/DCSupplemental.
References
- 1.You YH, Okada S, Ly S, Jandeleit-Dahm K, Barit D, Namikoshi T, Sharma K: Role of Nox2 in diabetic kidney disease. Am J Physiol Renal Physiol 304: F840–F848, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Babelova A, Avaniadi D, Jung O, Fork C, Beckmann J, Kosowski J, Weissmann N, Anilkumar N, Shah AM, Schaefer L, Schröder K, Brandes RP: Role of Nox4 in murine models of kidney disease. Free Radic Biol Med 53: 842–853, 2012 [DOI] [PubMed] [Google Scholar]
- 3.Gorin Y, Block K: Nox as a target for diabetic complications. Clin Sci (Lond) 125: 361–382, 2013 [DOI] [PubMed] [Google Scholar]
- 4.Holterman CE, Thibodeau JF, Towaij C, Gutsol A, Montezano AC, Parks RJ, Cooper ME, Touyz RM, Kennedy CR: Nephropathy and elevated BP in mice with podocyte-specific NADPH oxidase 5 expression. J Am Soc Nephrol 25: 784–797, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Geiszt M, Kopp JB, Várnai P, Leto TL: Identification of renox, an NAD(P)H oxidase in kidney. Proc Natl Acad Sci U S A 97: 8010–8014, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sharma K, Ramachandrarao S, Qiu G, Usui HK, Zhu Y, Dunn SR, Ouedraogo R, Hough K, McCue P, Chan L, Falkner B, Goldstein BJ: Adiponectin regulates albuminuria and podocyte function in mice. J Clin Invest 118: 1645–1656, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Giunti S, Calkin AC, Forbes JM, Allen TJ, Thomas MC, Cooper ME, Jandeleit-Dahm KA: The pleiotropic actions of rosuvastatin confer renal benefits in the diabetic Apo-E knockout mouse. Am J Physiol Renal Physiol 299: F528–F535, 2010 [DOI] [PubMed] [Google Scholar]
- 8.Pedruzzi E, Guichard C, Ollivier V, Driss F, Fay M, Prunet C, Marie JC, Pouzet C, Samadi M, Elbim C, O’dowd Y, Bens M, Vandewalle A, Gougerot-Pocidalo MA, Lizard G, Ogier-Denis E: NAD(P)H oxidase Nox-4 mediates 7-ketocholesterol-induced endoplasmic reticulum stress and apoptosis in human aortic smooth muscle cells. Mol Cell Biol 24: 10703–10717, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McCullough KD, Martindale JL, Klotz LO, Aw TY, Holbrook NJ: Gadd153 sensitizes cells to endoplasmic reticulum stress by down-regulating Bcl2 and perturbing the cellular redox state. Mol Cell Biol 21: 1249–1259, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Minamino T, Komuro I, Kitakaze M: Endoplasmic reticulum stress as a therapeutic target in cardiovascular disease. Circ Res 107: 1071–1082, 2010 [DOI] [PubMed] [Google Scholar]
- 11.Oexle K: Steroids in Duchenne muscular dystrophy. Neurology 44: 1558–1559, 1994 [DOI] [PubMed] [Google Scholar]
- 12.Chen V, Ianuzzo CD: Metabolic alterations in skeletal muscle of chronically streptozotocin-diabetic rats. Arch Biochem Biophys 217: 131–138, 1982 [DOI] [PubMed] [Google Scholar]
- 13.Lashin OM, Szweda PA, Szweda LI, Romani AM: Decreased complex II respiration and HNE-modified SDH subunit in diabetic heart. Free Radic Biol Med 40: 886–896, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Tong WH, Sourbier C, Kovtunovych G, Jeong SY, Vira M, Ghosh M, Romero VV, Sougrat R, Vaulont S, Viollet B, Kim YS, Lee S, Trepel J, Srinivasan R, Bratslavsky G, Yang Y, Linehan WM, Rouault TA: The glycolytic shift in fumarate-hydratase-deficient kidney cancer lowers AMPK levels, increases anabolic propensities and lowers cellular iron levels. Cancer Cell 20: 315–327, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gorin Y, Block K, Hernandez J, Bhandari B, Wagner B, Barnes JL, Abboud HE: Nox4 NAD(P)H oxidase mediates hypertrophy and fibronectin expression in the diabetic kidney. J Biol Chem 280: 39616–39626, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Fujii M, Inoguchi T, Maeda Y, Sasaki S, Sawada F, Saito R, Kobayashi K, Sumimoto H, Takayanagi R: Pitavastatin ameliorates albuminuria and renal mesangial expansion by downregulating NOX4 in db/db mice. Kidney Int 72: 473–480, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Eid AA, Gorin Y, Fagg BM, Maalouf R, Barnes JL, Block K, Abboud HE: Mechanisms of podocyte injury in diabetes: Role of cytochrome P450 and NADPH oxidases. Diabetes 58: 1201–1211, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fujii M, Inoguchi T, Sasaki S, Maeda Y, Zheng J, Kobayashi K, Takayanagi R: Bilirubin and biliverdin protect rodents against diabetic nephropathy by downregulating NAD(P)H oxidase. Kidney Int 78: 905–919, 2010 [DOI] [PubMed] [Google Scholar]
- 19.Jha JC, Gray SP, Barit D, Okabe J, El-Osta A, Namikoshi T, Thallas-Bonke V, Wingler K, Szyndralewiez C, Heitz F, Touyz RM, Cooper ME, Schmidt HH, Jandeleit-Dahm KA: Genetic targeting or pharmacologic inhibition of NADPH oxidase nox4 provides renoprotection in long-term diabetic nephropathy. J Am Soc Nephrol 25: 1237–1254, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gorin Y, Cavaglieri RC, Khazim K, Lee DY, Bruno F, Thakur S, Fanti P, Szyndralewiez C, Barnes JL, Block K, Abboud HE: Targeting NADPH oxidase with a novel dual Nox1/Nox4 inhibitor attenuates renal pathology in type 1 diabetes [published online ahead of print February 4, 2015]. Am J Physiol Renal Physiol 10.1152/ajprenal.00396.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sciarretta S, Zhai P, Shao D, Zablocki D, Nagarajan N, Terada LS, Volpe M, Sadoshima J: Activation of NADPH oxidase 4 in the endoplasmic reticulum promotes cardiomyocyte autophagy and survival during energy stress through the protein kinase RNA-activated-like endoplasmic reticulum kinase/eukaryotic initiation factor 2α/activating transcription factor 4 pathway. Circ Res 113: 1253–1264, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.US National Institutes of Health: Safety and efficacy of oral GKT137831 in patients with type 2 diabetes and albuminuria. Available at https://clinicaltrials.gov/ct2/show/NCT02010242?term=nox4+inhibitor%2C&rank=1. Accessed March 2, 2015
- 23.Li M, Wang X, Aa J, Qin W, Zha W, Ge Y, Liu L, Zheng T, Cao B, Shi J, Zhao C, Wang X, Yu X, Wang G, Liu Z: GC/TOFMS analysis of metabolites in serum and urine reveals metabolic perturbation of TCA cycle in db/db mice involved in diabetic nephropathy. Am J Physiol Renal Physiol 304: F1317–F1324, 2013 [DOI] [PubMed] [Google Scholar]
- 24.Salek RM, Maguire ML, Bentley E, Rubtsov DV, Hough T, Cheeseman M, Nunez D, Sweatman BC, Haselden JN, Cox RD, Connor SC, Griffin JL: A metabolomic comparison of urinary changes in type 2 diabetes in mouse, rat, and human. Physiol Genomics 29: 99–108, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Sudarshan S, Sourbier C, Kong HS, Block K, Valera Romero VA, Yang Y, Galindo C, Mollapour M, Scroggins B, Goode N, Lee MJ, Gourlay CW, Trepel J, Linehan WM, Neckers L: Fumarate hydratase deficiency in renal cancer induces glycolytic addiction and hypoxia-inducible transcription factor 1alpha stabilization by glucose-dependent generation of reactive oxygen species. Mol Cell Biol 29: 4080–4090, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tomlinson IP, Alam NA, Rowan AJ, Barclay E, Jaeger EE, Kelsell D, Leigh I, Gorman P, Lamlum H, Rahman S, Roylance RR, Olpin S, Bevan S, Barker K, Hearle N, Houlston RS, Kiuru M, Lehtonen R, Karhu A, Vilkki S, Laiho P, Eklund C, Vierimaa O, Aittomäki K, Hietala M, Sistonen P, Paetau A, Salovaara R, Herva R, Launonen V, Aaltonen LA, Multiple Leiomyoma Consortium : Germline mutations in FH predispose to dominantly inherited uterine fibroids, skin leiomyomata and papillary renal cell cancer. Nat Genet 30: 406–410, 2002 [DOI] [PubMed] [Google Scholar]
- 27.Alam NA, Rowan AJ, Wortham NC, Pollard PJ, Mitchell M, Tyrer JP, Barclay E, Calonje E, Manek S, Adams SJ, Bowers PW, Burrows NP, Charles-Holmes R, Cook LJ, Daly BM, Ford GP, Fuller LC, Hadfield-Jones SE, Hardwick N, Highet AS, Keefe M, MacDonald-Hull SP, Potts ED, Crone M, Wilkinson S, Camacho-Martinez F, Jablonska S, Ratnavel R, MacDonald A, Mann RJ, Grice K, Guillet G, Lewis-Jones MS, McGrath H, Seukeran DC, Morrison PJ, Fleming S, Rahman S, Kelsell D, Leigh I, Olpin S, Tomlinson IP: Genetic and functional analyses of FH mutations in multiple cutaneous and uterine leiomyomatosis, hereditary leiomyomatosis and renal cancer, and fumarate hydratase deficiency. Hum Mol Genet 12: 1241–1252, 2003 [DOI] [PubMed] [Google Scholar]
- 28.Alam NA, Olpin S, Rowan A, Kelsell D, Leigh IM, Tomlinson IP, Weaver T: Missense mutations in fumarate hydratase in multiple cutaneous and uterine leiomyomatosis and renal cell cancer. J Mol Diagn 7: 437–443, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wei MH, Toure O, Glenn GM, Pithukpakorn M, Neckers L, Stolle C, Choyke P, Grubb R, Middelton L, Turner ML, Walther MM, Merino MJ, Zbar B, Linehan WM, Toro JR: Novel mutations in FH and expansion of the spectrum of phenotypes expressed in families with hereditary leiomyomatosis and renal cell cancer. J Med Genet 43: 18–27, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Isaacs JS, Jung YJ, Mole DR, Lee S, Torres-Cabala C, Chung YL, Merino M, Trepel J, Zbar B, Toro J, Ratcliffe PJ, Linehan WM, Neckers L: HIF overexpression correlates with biallelic loss of fumarate hydratase in renal cancer: Novel role of fumarate in regulation of HIF stability. Cancer Cell 8: 143–153, 2005 [DOI] [PubMed] [Google Scholar]
- 31.Pollard PJ, Brière JJ, Alam NA, Barwell J, Barclay E, Wortham NC, Hunt T, Mitchell M, Olpin S, Moat SJ, Hargreaves IP, Heales SJ, Chung YL, Griffiths JR, Dalgleish A, McGrath JA, Gleeson MJ, Hodgson SV, Poulsom R, Rustin P, Tomlinson IP: Accumulation of Krebs cycle intermediates and over-expression of HIF1alpha in tumours which result from germline FH and SDH mutations. Hum Mol Genet 14: 2231–2239, 2005 [DOI] [PubMed] [Google Scholar]
- 32.Selak MA, Armour SM, MacKenzie ED, Boulahbel H, Watson DG, Mansfield KD, Pan Y, Simon MC, Thompson CB, Gottlieb E: Succinate links TCA cycle dysfunction to oncogenesis by inhibiting HIF-alpha prolyl hydroxylase. Cancer Cell 7: 77–85, 2005 [DOI] [PubMed] [Google Scholar]
- 33.O’Flaherty L, Adam J, Heather LC, Zhdanov AV, Chung YL, Miranda MX, Croft J, Olpin S, Clarke K, Pugh CW, Griffiths J, Papkovsky D, Ashrafian H, Ratcliffe PJ, Pollard PJ: Dysregulation of hypoxia pathways in fumarate hydratase-deficient cells is independent of defective mitochondrial metabolism. Hum Mol Genet 19: 3844–3851, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Haase VH: Hypoxia-inducible factors in the kidney. Am J Physiol Renal Physiol 291: F271–F281, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sánchez-Elsner T, Botella LM, Velasco B, Corbí A, Attisano L, Bernabéu C: Synergistic cooperation between hypoxia and transforming growth factor-beta pathways on human vascular endothelial growth factor gene expression. J Biol Chem 276: 38527–38535, 2001 [DOI] [PubMed] [Google Scholar]
- 36.Norman JT, Clark IM, Garcia PL: Hypoxia promotes fibrogenesis in human renal fibroblasts. Kidney Int 58: 2351–2366, 2000 [DOI] [PubMed] [Google Scholar]
- 37.Nangaku M, Eckardt KU: Hypoxia and the HIF system in kidney disease. J Mol Med (Berl) 85: 1325–1330, 2007 [DOI] [PubMed] [Google Scholar]
- 38.Diebold I, Petry A, Hess J, Görlach A: The NADPH oxidase subunit NOX4 is a new target gene of the hypoxia-inducible factor-1. Mol Biol Cell 21: 2087–2096, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Diebold I, Flügel D, Becht S, Belaiba RS, Bonello S, Hess J, Kietzmann T, Görlach A: The hypoxia-inducible factor-2alpha is stabilized by oxidative stress involving NOX4. Antioxid Redox Signal 13: 425–436, 2010 [DOI] [PubMed] [Google Scholar]
- 40.Bonello S, Zähringer C, BelAiba RS, Djordjevic T, Hess J, Michiels C, Kietzmann T, Görlach A: Reactive oxygen species activate the HIF-1alpha promoter via a functional NFkappaB site. Arterioscler Thromb Vasc Biol 27: 755–761, 2007 [DOI] [PubMed] [Google Scholar]
- 41.Block K, Gorin Y, Hoover P, Williams P, Chelmicki T, Clark RA, Yoneda T, Abboud HE: NAD(P)H oxidases regulate HIF-2alpha protein expression. J Biol Chem 282: 8019–8026, 2007 [DOI] [PubMed] [Google Scholar]
- 42.Wu RF, Ma Z, Liu Z, Terada LS: Nox4-derived H2O2 mediates endoplasmic reticulum signaling through local Ras activation. Mol Cell Biol 30: 3553–3568, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gurley SB, Mach CL, Stegbauer J, Yang J, Snow KP, Hu A, Meyer TW, Coffman TM: Influence of genetic background on albuminuria and kidney injury in Ins2(+/C96Y) (Akita) mice. Am J Physiol Renal Physiol 298: F788–F795, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Takemoto M, Asker N, Gerhardt H, Lundkvist A, Johansson BR, Saito Y, Betsholtz C: A new method for large scale isolation of kidney glomeruli from mice. Am J Pathol 161: 799–805, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Miyamoto S, Shikata K, Miyasaka K, Okada S, Sasaki M, Kodera R, Hirota D, Kajitani N, Takatsuka T, Kataoka HU, Nishishita S, Sato C, Funakoshi A, Nishimori H, Uchida HA, Ogawa D, Makino H: Cholecystokinin plays a novel protective role in diabetic kidney through anti-inflammatory actions on macrophage: Anti-inflammatory effect of cholecystokinin. Diabetes 61: 897–907, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim HS, Lee G, John SW, Maeda N, Smithies O: Molecular phenotyping for analyzing subtle genetic effects in mice: Application to an angiotensinogen gene titration. Proc Natl Acad Sci U S A 99: 4602–4607, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sharma K, Karl B, Mathew AV, Gangoiti JA, Wassel CL, Saito R, Pu M, Sharma S, You YH, Wang L, Diamond-Stanic M, Lindenmeyer MT, Forsblom C, Wu W, Ix JH, Ideker T, Kopp JB, Nigam SK, Cohen CD, Groop PH, Barshop BA, Natarajan L, Nyhan WL, Naviaux RK: Metabolomics reveals signature of mitochondrial dysfunction in diabetic kidney disease. J Am Soc Nephrol 24: 1901–1912, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.