Abstract
Hepatocyte nuclear factor 1β (HNF1β)–associated disease is a recently recognized clinical entity with a variable multisystem phenotype. Early reports described an association between HNF1B mutations and maturity-onset diabetes of the young. These patients often presented with renal cysts and renal function decline that preceded the diabetes, hence it was initially referred to as renal cysts and diabetes syndrome. However, it is now evident that many more symptoms occur, and diabetes and renal cysts are not always present. The multisystem phenotype is probably attributable to functional promiscuity of the HNF1β transcription factor, involved in the development of the kidney, urogenital tract, pancreas, liver, brain, and parathyroid gland. Nephrologists might diagnose HNF1β-associated kidney disease in patients referred with a suspected diagnosis of autosomal dominant polycystic kidney disease, medullary cystic kidney disease, diabetic nephropathy, or CKD of unknown cause. Associated renal or extrarenal symptoms should alert the nephrologist to HNF1β-associated kidney disease. A considerable proportion of these patients display hypomagnesemia, which sometimes mimics Gitelman syndrome. Other signs include early onset diabetes, gout and hyperparathyroidism, elevated liver enzymes, and congenital anomalies of the urogenital tract. Because many cases of this disease are probably undiagnosed, this review emphasizes the clinical manifestations of HNF1β-associated disease for the nephrologist.
Keywords: genetic renal disease, clinical nephrology, CKD, cystic kidney, diabetes mellitus, electrolytes
Hepatocyte nuclear factor 1 homeobox β (HNF1β)–associated disease is a recently recognized clinical entity with a variable, multisystemic phenotype. HNF1B mutations were first described in 1997 as a rare genetic cause of maturity-onset diabetes of the young (MODY).1 MODY comprises a group of disorders that are typically characterized by early age of onset of diabetes mellitus, usually before age 25 years, and pancreatic β cell dysfunction. MODY is classified according to the underlying genetic defect and 13 different genes have been identified to date. Most MODY subtypes rarely present with extrapancreatic disease, except for HNF1B mutations, which were associated with renal cystic disease shortly after their recognition as a cause of MODY.2 This disease was therefore renamed renal cysts and diabetes syndrome in 2001.3 However, it now seems to be a clinical entity with a more broad and variable phenotype. Moreover, not all patients with an HNF1B mutation actually have renal cysts and/or diabetes.
Knowledge about the possible clinical manifestations of HNF1β-associated disease is important for the nephrologist in order to recognize these patients in the clinical setting, because many of these clinical manifestations could warrant nephrologic consultation. In this brief review, we emphasize the significance of this disease for the clinician. We illustrate the heterogenic presentation of HNF1β-associated disease on the basis of five selected patients, and we then provide a concise review of the associated signs and symptoms and their molecular pathogenesis.
Heterogenic Presentation of the Multisystem Phenotype of HNF1β-Associated Disease
The first patient, a woman aged 39 years, was referred to the nephrologist with suspected polycystic kidney disease because of reduced eGFR (45 ml/min per 1.73 m2) and renal cysts, without a family history of polycystic kidney disease. Her medical history revealed recurrent urinary tract infections, enuresis nocturna until age 25 years, and type 2 diabetes mellitus and gout at age 32 years. At the time of referral, her serum creatinine was 94 μmol/L, uric acid was 0.46 mmol/L, magnesium was 0.79 mmol/L, hemoglobin A1c was 39 mmol/mol while receiving subcutaneous insulin therapy, and endogenous creatinine clearance was approximately 80 ml/min without proteinuria. A renal ultrasound demonstrated multiple cortical as well as parapelvine cysts in both kidneys. The combination of diabetes at a young age and renal cysts was suggestive of HNF1β-associated disease. Genetic analysis indeed revealed a previously described c.883C>T p.Arg295Cys HNF1B point mutation in an evolutionary conserved amino acid in the homeobox domain of the HNF1β protein.4
The second patient is a man who was diagnosed with renal insufficiency (eGFR 38 ml/min per 1.73 m2) at age 27 years. He was not obese (body mass index 23 kg/m2) and no proteinuria was present. A renal ultrasound revealed a congenital solitary kidney, and multiple small cortical cysts were also reported on repeated ultrasonography. His medical history revealed unexplained elevated liver enzymes and normal serum magnesium levels. His father also had a congenital solitary kidney, chronic renal insufficiency, diabetes at age 37 years, and unexplained elevation of liver enzymes despite multiple liver biopsies. Our patient later developed gout at age 32 years as well as diabetes mellitus, and he now has mildly progressive CKD at age 40 years. Only recently, the combination of symptoms and family history pointed us toward the presence of an HNF1B mutation, which turned out to be a heterozygous missense mutation [c.826C>T; p.(Arg276*)].
Patient 3 was referred to the nephrologist because of chronic electrolyte disturbances, in particular hypomagnesemia (0.43 mmol/L) and a tendency for hypokalemia (3.5 mmol/L), together with a mild metabolic alkalosis. Additional laboratory investigations showed a reduced eGFR of 44 ml/min per 1.73 m2, inappropriately high fractional excretion of magnesium (12%), and a urine calcium concentration <0.5 mmol/L. There was no evidence of diabetes mellitus (hemoglobin A1c of 41 mmol/mol). The patient’s medical history revealed primary amenorrhea with congenital anomalies compatible with the diagnosis of Mayer–Rokitansky–Küster syndrome. She had suffered from recurrent, although self-limiting, arthritis in the past. A renal ultrasound showed two small kidneys with multiple cysts. HNF1β genetic analysis demonstrated a large 1.43-Mb deletion at chromosomal location 17q12 (31,89–33,32 Mb deletion including approximately 17 genes), including the HNF1B gene, which had occurred de novo in this patient but had been previously described in patients with HNF1β-associated phenotypes. The presence of additional mutations in SLC12A3 or CLCNKB, causing Gitelman syndrome, was excluded.
We were asked to evaluate patient 4, a 50-year-old inpatient at the oncology clinic. She had refractory hypomagnesemia during treatment for disseminated ovarian malignancy. The hypomagnesemia had been attributed to cisplatin-based chemotherapy and necessitated weekly intravenous supplementation. Her medical history included congenital anomalies of both ureters, leading to urinary reflux that was surgically corrected at age 10 years but led to chronic renal insufficiency (eGFR approximately 40 ml/min per 1.73 m2) without proteinuria. She was diagnosed with diabetes at age 24 years and had recurrent arthralgias, which were classified as diabetic arthropathy; however, the patient had been using allopurinol because of increased serum uric acid levels for some years. Interestingly, her father suffered from frequent attacks of gout as well as persistent hypomagnesemia after chemotherapeutic treatment for renal cell carcinoma (RCC). No renal anomalies or cysts were apparent on abdominal computed tomography scanning. Genetic analysis showed heterozygous deletion of all exons of the HNF1B gene.
Patient 5 is a man who was diagnosed with renal hypomagnesemia at age 24 years. One year earlier, he underwent parathyroidectomy because of refractory hyperparathyroidism. In addition, there was aplasia of the right kidney with some small cortical cysts in the left solitary kidney. His endogenous creatinine clearance was stable at approximately 74 ml/min but he suffered from gout. Twelve years after presentation, our experience with patients with HNF1β-associated disease eventually led us to confirm the heterozygous full deletion of the HNF1B gene. Because of the concomitant presence of hypomagnesemia and hypocalciuria, the presence of additional mutations in SLC12A3 or CLCNKB was excluded.
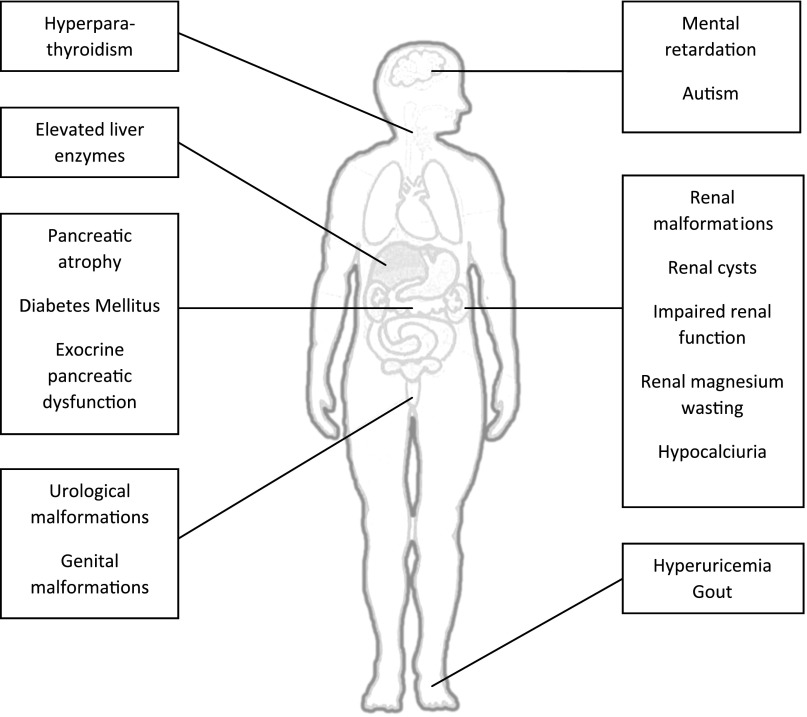
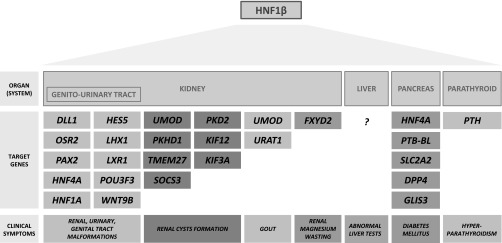
These clinical cases, summarized in Table 1, illustrate the highly variable spectrum of renal and extrarenal phenotypes associated with HNF1β mutations, which is depicted in Figure 1. HNF1β-associated disease occurs as a result of heterozygous mutations or deletions in the HNF1B gene (also known as transcription factor 2 or TCF2) on chromosomal region 17q12.5 It can occur as an autosomal dominant inherited gene defect or as a spontaneous (de novo) mutation, which is seen in 50%–60% of patients.6 HNF1β is a transcription factor that is important in the regulation of the transcription and expression of several different target genes. The multisystem phenotype is probably related to the fact that HNF1β is a rather promiscuous transcription factor, which plays a role in the development of different organs such as the kidney, urinary and genital tract, pancreas, brain, parathyroid gland, and liver. Figure 2 demonstrates that the HNF1β transcription factor regulates many genes, some of them being transcription factors as well, which may be responsible for the diverse clinical phenotype. Why loss of function of only one allele encoding this transcription factor is sufficient to lead to clinical disease is largely unexplained, as is the cause of the clinical variability per se.7 In addition, although the phenotype within the family appears largely conserved in patients 3 and 5, the literature suggests that in general there is only a limited genotype-phenotype correlation.8 Environmental factors or epigenetic regulation of HNF1β expression by microRNA could contribute to the above findings.
Table 1.
Five patients exemplifying the heterogenic presentation of the multisystem phenotype of HNF1β-associated disease
| Characteristic | Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 |
|---|---|---|---|---|---|
| Referral reason | Suspected ADPKD | Renal insufficiency and solitary kidney | Hypomagnesemia | Cisplatin-induced hypomagnesemia | Hypomagnesemia and solitary kidney |
| Signs and symptoms at presentation | |||||
| Renal cysts | Yes | Yes | Yes | No | Yes |
| Diabetes | Yes | No | No | Yes | No |
| Reduced renal function | No | Yes | Yes | Yes | No |
| CAKUT | No | Yes | No | Yes | Yes |
| Hypomagnesemia | No | No | Yes | Yes | Yes |
| Hyperuricemia/gout | Yes/no | No/no | No/no | Yes/no | Yes/yes |
| Elevated liver enzymes | No | Yes | No | No | No |
| Exocrine pancreatic disease | No | No | No | No | No |
| Genital malformations | No | No | Yes | No | No |
| Mental retardation/autism | No | No | No | No | No |
| Hyperparathyroidism | Yes | No | No | Yes | Yes |
| Sex | Woman | Man | Woman | Woman | Man |
| Family history | None | Multipleb | None | Multipled | None |
| HNF1B mutation or deletiona | c.883C>T | c.826C>T | Deletion | Deletion | Deletion |
| Age at presentation (at nephrologist), yr | 39 | 27 | 42 | 50 | 24 |
| Diagnostic delay (from first nephrologic referral) | 3 mo | 13 yr | 1 mo | None | 12 yr |
| HNF1β score at presentation | 12 | 7, 13c | 10 | 8 | 9 |
ADPKD, autosomal dominant polycystic kidney disease.
Sequencing was performed by ion semiconductor and/or Sanger sequencing, and all reported mutations were confirmed by Sanger sequencing.
The patient’s family history included CAKUT, reduced renal function, diabetes, and elevated liver enzymes in the patient’s father.
At the first presentation to the nephrologist, the patient’s HNF1β score would have been 7; on reevaluation 13 years later, the HNF1β score had progressed to 13.
The patient’s family history included gout and hypomagnesemia in the patient’s father.
Figure 1.
Renal and extrarenal features of HNF1β-associated disease. The clinical signs and symptoms that are currently associated with HNF1B mutations and deletions are depicted.
Figure 2.
HNF1β as a promiscuous transcription factor. Target genes known to be regulated by the HNF1β transcription factor in several organ systems, responsible for the diverse multisystem clinical signs and symptoms, are depicted.
HNF1β-Associated Kidney Disease
Cystic and Noncystic Kidney Disease
Nishigori et al. were the first to describe a family with renal cysts, proteinuria, and renal dysfunction that preceded the clinical presentation of diabetes.9 Although the exact role of HNF1β in kidney development awaits further examination, it is clear that HNF1B mutations can lead to abnormal nephron development.10 The latter process involves structures arising from the ureteric bud, giving rise to collecting ducts, the renal pelvis and ureter, and the metanephric mesenchyme responsible for the formation of the biggest part of the nephron. Both embryonic structures express HNF1β, and HNF1β inactivation in mice leads to defective S-shaped body formation resulting in an enlarged Bowman’s capsule and renal tubular dysgenesis.10 In addition to regulating genes involved in nephrogenesis per se, HNF1β affects several genes that are known to be involved in the pathogenesis of renal cystic disease. Examples are the PKD2 and PKHD1 genes that are mutated in polycystic kidney disease. HNF1β mutation in mice inhibited PKHD1 gene expression and induced renal cyst formation.11 Because HNF1β regulates several developmental and cystogenic pathways, there is a wide spectrum of developmental renal abnormalities in patients with HNF1B mutations.
Faguer et al. reported the clinical presentation in 27 adults from 20 families with HNF1B mutations or deletions.12 Renal phenotype appeared to be extremely heterogenic, with 62% of patients having one or more renal cysts. These cysts may be apparent on a renal ultrasound, mostly in the cortex, but they sometimes require magnetic resonance imaging to be detected. Our first patient showed the classical phenotype of renal cysts and diabetes mellitus, but not all patients display typical cortical renal cystic disease. Therefore, the term HNF1β nephropathy has been introduced to encompass all described renal abnormalities, including multicystic renal dysplasia, renal hypoplasia, unilateral renal agenesis, microcystic dysplasia, horseshoe kidney, atypical familial juvenile hyperuricemic nephropathy, and urinary tract malformations. Our second and fifth cases are examples of such renal developmental abnormalities, instead of classical cystic disease.
Renal abnormalities may be detected by ultrasonography when analyzed for renal function decline at a later age or antenatally by prenatal ultrasonography in pregnancy. HNF1β is therefore also recognized as a cause of congenital anomalies of the kidney and urinary tract (CAKUT). Among anomalies found at prenatal screening, CAKUT is present in 20%–30% of the cases.13,14 Renal function ranges from normal to ESRD, but most patients have some degree of impaired kidney function. Urine analysis in general shows no cells and a proteinuria of <1 g/24 h. Occasionally, renal biopsies are performed in patients that do not present with CAKUT, with the histologic diagnosis often being variable and nonspecific. However, glomerulomegaly, glomerulocystic disease, and small tubular cysts may be encountered.15 Faguer et al. reported a renal function decline of −2.45 ml/min per 1.73 m2 per year over a median follow-up of 5.5 years in their cohort of HNF1β patients.12 About 13%–15% of patients eventually develop ESRD.16 In a retrospective study of 377 patients with HNF1β mutations, no correlation was found between the type and location of the genetic mutation and severity of kidney failure.8
Hypomagnesemia and Hypocalciuria
In a 2009 retrospective study in 66 children with CKD who were tested for HNF1B mutations, Adalat et al. found that hypomagnesemia was more prevalent in patients with a HNF1B mutation (48%) versus patients without the mutation (2%).17 In the patients with an HNF1B mutation and hypomagnesemia, there was a renal magnesium leak (median fractional excretion of magnesium of 6.5%) as well as a concomitant hypocalciuria. Because this cohort was highly selected, the true prevalence of hypomagnesemia in HNF1β-associated disease is uncertain, but it appears to be high. Adalat et al. suggested that HNF1β is necessary for the transcription of the FXYD2 gene, encoding the γ-subunit of the sodium-potassium ATPase expressed in the distal convoluted tubule, which is thought to play a role in transcellular magnesium reabsorption.17 Indeed, FXYD2 mutations are associated with hypomagnesemia and hypocalciuria as well.18 Ferrè et al. confirmed that HNF1β specifically acts as an activator of the γ-subunit and that HNF1B mutations identified in patients with hypomagnesemia prevented γ-subunit transcriptional activation, with a dominant negative effect on wild-type HNF1β.19
The combination of hypomagnesemia and hypocalciuria is also encountered in Gitelman syndrome, in which a defect of distal convoluted tubule sodium chloride cotransport is involved. Of note, in two of the above-described patients, the diagnosis of Gitelman syndrome was initially considered. Therefore, HNF1β tubulopathy should also be considered in patients with a phenotype resembling Gitelman syndrome, especially when a genetic confirmation of the latter diagnosis is absent, whether other clinical features of HNF1β-associated disease are present.
Hyperuricemia and Early Onset Gout
Most patients with HNF1B mutations display hyperuricemia, and some present with early onset gout like our patients 2, 4, 5, and possibly 3.20 Importantly, for this reason, some patients with HNF1B mutations would meet the diagnostic criteria for familial juvenile hyperuricemic nephropathy. The latter condition is caused by mutations in the UMOD gene, which encodes uromodulin and is suggested to play a role in renal urate transport.21 HNF1B knockout mice also showed reduced UMOD expression, suggesting that HNF1β regulates transcription of UMOD.22 Thus, abnormal urate transport could be the reason why patients with HNF1B mutations present with early onset gout and abnormal elevated urate levels discrepant to their level of renal function decline.
HNF1β-Associated Extrarenal Disease
Diabetes and Exocrine Pancreas Dysfunction
As described above, HNF1B mutations were first described as a cause of MODY. HNF1β has structural similarity to HNF1α, the affected gene in the most common type of MODY (MODY3). Horikawa et al. sequenced the HNF1β transcription factor in 57 patients with MODY and found 1 patient with an HNF1B loss-of-function mutation.1 HNF1β is thought to play an important role in early development and differentiation of the pancreas.23 It regulates the expression of key pancreatic proteins, including the HNF4A and SLC2A2 genes, the latter encoding the glucose transporter GLUT2.24 HNF1B mutations can therefore result not only in pancreatic β cell dysfunction, leading to diabetes mellitus, but also pancreatic atrophy and exocrine pancreatic dysfunction. Pancreatic atrophy affecting either the head or body of the pancreas is frequently observed on computed tomography in patients with HNF1B mutations in combination with exocrine pancreatic dysfunction, which is often subclinical.25 Patients mostly present with diabetes in their early adulthood but with a large variation from the neonatal period to late middle age.7,26 Endogenous insulin production is present and patients with diabetes are not obligatorily insulin dependent.27 However, the majority of patients eventually require insulin therapy for an adequate glycemic control, which is not the case for HNF1α-associated MODY. Overall, HNF1B mutations are an infrequent cause of MODY, occurring in <1% of cases.28
Genital Tract Malformations
Congenital abnormalities of the genital tract are found rather frequently in patients with HNF1B mutations.29 These are often malformations resulting from aplasia and failure of the fusion of the Müllerian ducts, resulting in congenital uterine and upper vaginal abnormalities or aplasia, as was present in our third patient. This indicates HNF1B as a candidate gene for Mayer–Rokitansky–Küster syndrome, as was suggested in publications preceding the description of genital abnormalities in renal cysts and diabetes syndrome.30 Hypospadia and other genital abnormalities are sporadically described in male individuals. These may be coincident findings, or they may be part of the CAKUT spectrum described above, in which HNF1B mutations are the most commonly identified genetic cause.31,32
Elevated Liver Enzymes
In contrast with what its name would suggest, not much is currently known regarding the molecular (patho)physiology of HNF1β in either hepatocytes or the liver and biliary tract in general. Liver enzymes, particularly alanine aminotransferase and γ-glutamyl transpeptidase, are frequently elevated without signs of liver disease or hepatic insufficiency, like in our second patient.15 Liver biopsy in HNF1β patients with abnormal liver enzyme levels generally reveals normal liver tissue.25 Interestingly, hepatic cyst formation does not appear to occur. In contrast with the potential severe neonatal cholestasis that occurs sporadically in neonates and children, adult patients with HNF1B mutations mostly show asymptomatic liver enzyme elevations.33
RCC and Other Malignancies
Case studies suggest that lack of HNF1β expression is related to chromophobe RCC.34,35 Chromophobe RCC is a rare renal cancer that has relatively pale cytoplasm under the microscope, as opposed to the clear cytoplasm in clear cell RCC. This raises the question of whether we should screen patients with HNF1B mutations for renal tumors. Several reports have also suggested a role for HNF1β as a tumor marker in different tumors such as hepatocellular carcinoma,36,37 gynecologic tumors,38,39 and prostate carcinoma.40 The clinical significance of these findings for patients with HNF1B mutations is currently unknown but deserves further attention.
Mental Retardation and Autism
In a cohort of 53 children with HNF1B mutations, 3 children were found to have mental retardation and autism, a higher number than expected based on the prevalence in the general pediatric population.41 Among patients referred for genetic testing for neurodevelopmental or psychiatric disorders, HNF1B mutations were also more frequently present compared with ethnically matched controls.42
Early Onset Hyperparathyroidism
Ferrè et al. observed early hyperparathyroidism and parathyroid hormone (PTH) levels that were judged inappropriately high relative to kidney function in several patients with known HNF1B mutations or deletions.43 HNF1β was demonstrated to be expressed by PTH-producing parathyroid gland cells.43 Wild-type HNF1β inhibited transcription of the PTH gene, thus functioning as a transcriptional repressor of PTH production. HNF1β mutations found in patients lost their repressive effects on PTH transcription, which could explain early onset hyperparathyroidism in patients with HNF1B mutations. However, whether this HNF1β-mediated PTH regulation indeed leads to increased and discrepant hyperparathyroidism needs confirmation in other cohorts of patients with HNF1B mutations.
Epidemiology
Because of the variable presentation, combined with a likely considerable percentage of patients with HNF1B mutations not being recognized, interpretation of epidemiologic data is difficult. The currently available epidemiologic data mostly concern selected cohorts and are probably not readily applicable to the general population. For instance, a recent Belgian study found a 10% prevalence of HNF1B mutations in 205 patients with CAKUT,44 and similar cohorts yielded prevalence rates of 5%–31%.6,8,45 In a large cohort of 419 children with CKD, 3 patients showed a mutation in the HNF1B gene.46 Other studies noted a prevalence of 9% in kidney transplant recipients with unknown primary disease (mostly CAKUT) and up to 40% in patients with the combination of renal malformations and diabetes.16,47 Clissold et al. summarized the prevalence of HNF1B mutations in nine studies in cohorts with ≥50 patients. These patients were, however, all selected based on renal abnormalities such as cysts and dysplasia, and HNF1B gene anomalies were detected in 19% of these patients.48
Most of the epidemiologic data concerns pediatric (CAKUT) populations, whereas diagnosis can be especially difficult if the patient presents at adult age. We reviewed the existing literature to evaluate differences in the phenotype between pediatric and adult populations. These studies are unable to provide sound quantitative data that can be generalized on a population level. Most studies consisted of selected patient groups, often with specific inclusion criteria. Moreover, there are limited studies in which both children and adults were described. The prevalence of hypomagnesemia ranges from 0 to 100% in the different studies, but the prevalence in adults is consistently higher in those studies that included children and adults.43,44,49 The same appears to be true with regard to the extrarenal phenotypes of elevated liver enzymes, pancreatic hypoplasia, and diabetes mellitus. These differences might be related to a delayed onset of the phenotypical changes. Alternatively, asymptomatic phenotypical changes may be detected during routine investigations during follow-up. With regard to the CAKUT phenotype, there is only one study including both children and adults that clearly reports frequencies of signs and symptoms; unfortunately, CAKUT itself was an inclusion criterion, at least when it came to the pediatric patients.44 With the emergence of whole-exome sequencing in genetic diagnosis, a better estimate of overall prevalence(s) can possibly be attained in the future. However, whole gene deletions could be missed with this technique when no specific post hoc copy number variation analysis or similar is performed.
Which Individuals Should Be Screened for HNF1β Mutations?
The heterogenic presentation of HNF1B mutations in combination with the frequent lack of a positive family history owing to de novo mutations makes recognition of individual cases difficult. Differential diagnosis may vary based on the presenting features in the individual patient. In children, the most prominent presentation of HNF1β-associated disease is with CAKUT. At adult age, however, especially when the presentation deviates from the classical cysts and diabetes phenotype, the disease can be difficult to recognize. The clinical nephrologist can encounter HNF1β-associated disease in the form of suspected polycystic kidney disease, particularly when kidneys are not enlarged, or presumed diabetic nephropathy. CKD in combination with renal cysts is easily incorrectly diagnosed as autosomal dominant polycystic kidney disease or medullary cystic kidney disease. In such cases, the localization of the cysts, the size of the kidneys, the absence of a clear family history, and the presence of extrarenal symptoms could help in being alert for an alternative diagnosis. CKD in combination with diabetes is frequently misdiagnosed as diabetic nephropathy, although the renal impairment mostly precedes the diagnosis of diabetes mellitus in HNF1β-associated disease. Part of the difficulty in recognizing these patients of course lies in the fact that many associated extrarenal signs and symptoms occur commonly in the (nephrologic) population. Gout and/or hyperparathyroidism in the presence of CKD are easily interpreted as complications secondary to the CKD itself but could be a diagnostic clue if they are judged discrepant to the degree of renal insufficiency. HNF1β mutations should also be part of the differential diagnosis of renal hypomagnesemia and can even mimic Gitelman syndrome because of the concomitant hypocalciuria. Other clinical signs or symptoms of HNF1β-associated disease should be actively sought for in these patients. HNF1β-related disease can also be categorized as part of the group of conditions called autosomal dominant tubulointerstitial kidney disease. These are diseases characterized by bland urinary sediment, tubular and interstitial fibrosis, and slowly progressive kidney disease.50 Indeed, the phenotypes associated with HNF1B mutations partly overlap with mutations in REN, UMOD, and MUC1, which are also part of the spectrum of autosomal dominant tubulointerstitial kidney disease.
There are currently no clear directives on which patients should be genetically tested to confirm the diagnosis. In a cohort of 205 CAKUT patients (147 pediatric, 58 adult) with renal anomalies in combination with extrarenal symptoms associated with HNF1β, Raaijmakers et al. recently showed that bilateral renal anomalies, renal cysts from unknown origin, a combination of two major renal anomalies, and hypomagnesemia were all predictive for finding HNF1B mutations, which were detected in 10% of patients.44 Faguer et al. recently developed a 17-item HNF1β risk score to determine which individuals should be genetically screened for HNF1B mutations.49 This score takes into account the presence of renal, pancreatic and genital abnormalities, electrolyte disorders, abnormal liver tests, and family history, all combined into a weighed scoring system. A high risk score with a cutoff of ≥8 points was reported to make the presence of HNF1B mutation more likely (sensitivity 98%, specificity 91%, positive predictive value 19.8%).49 This risk score may facilitate identification of undetected patients with HNF1B mutation-related disease. However, the risk tool has only been validated in the authors’ own cohort, which was a very selected population of mostly pediatric patients with CAKUT who were previously selected for HNF1B mutational screening. The score excludes patients below the cutoff from diagnostic evaluation, although sensitivity might be considerably lower when the score is eventually validated in other cohorts. Although all patients presented above eventually scored above the cutoff on Faguer’s HNF1β score, patient 2 did not at the time of initial presentation, indicating that an unknown percentage of patients with an initial risk score <8 could go undetected. Indeed, even in the rather selected CAKUT cohort of Raaijmakers et al., the HNF1β score would have missed 3 of 20 identified patients.44 Consequent use of this scoring system thus might lead us to falsely exclude HNF1B mutations in a considerable percentage of patients, who would have scored below the cutoff value for the HNF1β scoring system at presentation. Performance of such a score of course also depends on the amount of clinical screening that was done before the moment that the score is applied. Thus, deciding whether a patient should be screened for HNF1B mutations currently still comes down to the presence of clinical suspicion, possibly aided by a clinical risk score, and the attention of the caring physician. In addition, one always needs to keep in mind that proving causality in terms of interpretation of genetic diagnosis is more difficult when the signs and symptoms in the individual patient differ more from the originally associated phenotype. Furthermore, one cannot exclude that mutations or polymorphisms in other genes could partly explain or modulate the phenotype in patients with HNF1B mutations or deletions. The emerging era of whole-exome sequencing might provide opportunities to further examine these issues.
In conclusion, HNF1β-related disease is a variable multisystem disorder with limited genotype-phenotype correlation, with specific significance to the nephrologist. It is likely that an unknown but potentially large percentage of HNF1β-associated kidney disease is currently not being recognized or is misdiagnosed. In addition, there can be a considerable diagnostic delay before the disease is eventually recognized, as exemplified in some of the presented cases. Although no causal therapy is currently available, diagnosing the syndrome is important. Early recognition makes (family) screening for diabetes, renal function decline, hypomagnesemia, and associated hypokalemia possible, and it may prevent unnecessary examinations and biopsies. In addition, genetic counseling should be offered to patients and their family members.
Disclosures
None.
Acknowledgments
Financial support was received from the Innovatiefonds Zorgverzekeraars (Innovation Fund of the Dutch Health Insurace companies).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Horikawa Y, Iwasaki N, Hara M, Furuta H, Hinokio Y, Cockburn BN, Lindner T, Yamagata K, Ogata M, Tomonaga O, Kuroki H, Kasahara T, Iwamoto Y, Bell GI: Mutation in hepatocyte nuclear factor-1 beta gene (TCF2) associated with MODY. Nat Genet 17: 384–385, 1997 [DOI] [PubMed] [Google Scholar]
- 2.Fajans SS, Bell GI: MODY: History, genetics, pathophysiology, and clinical decision making. Diabetes Care 34: 1878–1884, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bingham C, Bulman MP, Ellard S, Allen LI, Lipkin GW, Hoff WG, Woolf AS, Rizzoni G, Novelli G, Nicholls AJ, Hattersley AT: Mutations in the hepatocyte nuclear factor-1beta gene are associated with familial hypoplastic glomerulocystic kidney disease. Am J Hum Genet 68: 219–224, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bellanné-Chantelot C, Clauin S, Chauveau D, Collin P, Daumont M, Douillard C, Dubois-Laforgue D, Dusselier L, Gautier JF, Jadoul M, Laloi-Michelin M, Jacquesson L, Larger E, Louis J, Nicolino M, Subra JF, Wilhem JM, Young J, Velho G, Timsit J: Large genomic rearrangements in the hepatocyte nuclear factor-1beta (TCF2) gene are the most frequent cause of maturity-onset diabetes of the young type 5. Diabetes 54: 3126–3132, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Fischer E, Pontoglio M: HNF1beta and defective nephrogenesis: A role for interacting partners? Kidney Int 74: 145–147, 2008 [DOI] [PubMed] [Google Scholar]
- 6.Ulinski T, Lescure S, Beaufils S, Guigonis V, Decramer S, Morin D, Clauin S, Deschênes G, Bouissou F, Bensman A, Bellanné-Chantelot C: Renal phenotypes related to hepatocyte nuclear factor-1beta (TCF2) mutations in a pediatric cohort. J Am Soc Nephrol 17: 497–503, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Edghill EL, Bingham C, Ellard S, Hattersley AT: Mutations in hepatocyte nuclear factor-1beta and their related phenotypes. J Med Genet 43: 84–90, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heidet L, Decramer S, Pawtowski A, Morinière V, Bandin F, Knebelmann B, Lebre AS, Faguer S, Guigonis V, Antignac C, Salomon R: Spectrum of HNF1B mutations in a large cohort of patients who harbor renal diseases. Clin J Am Soc Nephrol 5: 1079–1090, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nishigori H, Yamada S, Kohama T, Tomura H, Sho K, Horikawa Y, Bell GI, Takeuchi T, Takeda J: Frameshift mutation, A263fsinsGG, in the hepatocyte nuclear factor-1beta gene associated with diabetes and renal dysfunction. Diabetes 47: 1354–1355, 1998 [DOI] [PubMed] [Google Scholar]
- 10.Massa F, Garbay S, Bouvier R, Sugitani Y, Noda T, Gubler MC, Heidet L, Pontoglio M, Fischer E: Hepatocyte nuclear factor 1β controls nephron tubular development. Development 140: 886–896, 2013 [DOI] [PubMed] [Google Scholar]
- 11.Hiesberger T, Shao X, Gourley E, Reimann A, Pontoglio M, Igarashi P: Role of the hepatocyte nuclear factor-1beta (HNF-1beta) C-terminal domain in Pkhd1 (ARPKD) gene transcription and renal cystogenesis. J Biol Chem 280: 10578–10586, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Faguer S, Decramer S, Chassaing N, Bellanné-Chantelot C, Calvas P, Beaufils S, Bessenay L, Lengelé JP, Dahan K, Ronco P, Devuyst O, Chauveau D: Diagnosis, management, and prognosis of HNF1B nephropathy in adulthood. Kidney Int 80: 768–776, 2011 [DOI] [PubMed] [Google Scholar]
- 13.Queisser-Luft A, Stolz G, Wiesel A, Schlaefer K, Spranger J: Malformations in newborn: Results based on 30,940 infants and fetuses from the Mainz congenital birth defect monitoring system (1990-1998). Arch Gynecol Obstet 266: 163–167, 2002 [DOI] [PubMed] [Google Scholar]
- 14.Soliman NA, Ali RI, Ghobrial EE, Habib EI, Ziada AM: Pattern of clinical presentation of congenital anomalies of the kidney and urinary tract among infants and children. Nephrology (Carlton) 20: 413–418, 2015 [DOI] [PubMed] [Google Scholar]
- 15.Bingham C, Hattersley AT: Renal cysts and diabetes syndrome resulting from mutations in hepatocyte nuclear factor-1beta. Nephrol Dial Transplant 19: 2703–2708, 2004 [DOI] [PubMed] [Google Scholar]
- 16.Chen YZ, Gao Q, Zhao XZ, Chen YZ, Bennett CL, Xiong XS, Mei CL, Shi YQ, Chen XM: Systematic review of TCF2 anomalies in renal cysts and diabetes syndrome/maturity onset diabetes of the young type 5. Chin Med J (Engl) 123: 3326–3333, 2010 [PubMed] [Google Scholar]
- 17.Adalat S, Woolf AS, Johnstone KA, Wirsing A, Harries LW, Long DA, Hennekam RC, Ledermann SE, Rees L, van’t Hoff W, Marks SD, Trompeter RS, Tullus K, Winyard PJ, Cansick J, Mushtaq I, Dhillon HK, Bingham C, Edghill EL, Shroff R, Stanescu H, Ryffel GU, Ellard S, Bockenhauer D: HNF1B mutations associate with hypomagnesemia and renal magnesium wasting. J Am Soc Nephrol 20: 1123–1131, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meij IC, Koenderink JB, van Bokhoven H, Assink KF, Groenestege WT, de Pont JJ, Bindels RJ, Monnens LA, van den Heuvel LP, Knoers NV: Dominant isolated renal magnesium loss is caused by misrouting of the Na(+),K(+)-ATPase gamma-subunit. Nat Genet 26: 265–266, 2000 [DOI] [PubMed] [Google Scholar]
- 19.Ferrè S, Veenstra GJ, Bouwmeester R, Hoenderop JG, Bindels RJ: HNF-1B specifically regulates the transcription of the γa-subunit of the Na+/K+-ATPase. Biochem Biophys Res Commun 404: 284–290, 2011 [DOI] [PubMed] [Google Scholar]
- 20.Bingham C, Ellard S, van’t Hoff WG, Simmonds HA, Marinaki AM, Badman MK, Winocour PH, Stride A, Lockwood CR, Nicholls AJ, Owen KR, Spyer G, Pearson ER, Hattersley AT: Atypical familial juvenile hyperuricemic nephropathy associated with a hepatocyte nuclear factor-1beta gene mutation. Kidney Int 63: 1645–1651, 2003 [DOI] [PubMed] [Google Scholar]
- 21.Han J, Liu Y, Rao F, Nievergelt CM, O’Connor DT, Wang X, Liu L, Bu D, Liang Y, Wang F, Zhang L, Zhang H, Chen Y, Wang H: Common genetic variants of the human uromodulin gene regulate transcription and predict plasma uric acid levels. Kidney Int 83: 733–740, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Igarashi P, Shao X, McNally BT, Hiesberger T: Roles of HNF-1beta in kidney development and congenital cystic diseases. Kidney Int 68: 1944–1947, 2005 [DOI] [PubMed] [Google Scholar]
- 23.Haumaitre C, Barbacci E, Jenny M, Ott MO, Gradwohl G, Cereghini S: Lack of TCF2/vHNF1 in mice leads to pancreas agenesis. Proc Natl Acad Sci U S A 102: 1490–1495, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cha JY, Kim H, Kim KS, Hur MW, Ahn Y: Identification of transacting factors responsible for the tissue-specific expression of human glucose transporter type 2 isoform gene. Cooperative role of hepatocyte nuclear factors 1alpha and 3beta. J Biol Chem 275: 18358–18365, 2000 [DOI] [PubMed] [Google Scholar]
- 25.Bellanné-Chantelot C, Chauveau D, Gautier JF, Dubois-Laforgue D, Clauin S, Beaufils S, Wilhelm JM, Boitard C, Noël LH, Velho G, Timsit J: Clinical spectrum associated with hepatocyte nuclear factor-1beta mutations. Ann Intern Med 140: 510–517, 2004 [DOI] [PubMed] [Google Scholar]
- 26.Yorifuji T, Kurokawa K, Mamada M, Imai T, Kawai M, Nishi Y, Shishido S, Hasegawa Y, Nakahata T: Neonatal diabetes mellitus and neonatal polycystic, dysplastic kidneys: Phenotypically discordant recurrence of a mutation in the hepatocyte nuclear factor-1beta gene due to germline mosaicism. J Clin Endocrinol Metab 89: 2905–2908, 2004 [DOI] [PubMed] [Google Scholar]
- 27.Bingham C, Ellard S, Allen L, Bulman M, Shepherd M, Frayling T, Berry PJ, Clark PM, Lindner T, Bell GI, Ryffel GU, Nicholls AJ, Hattersley AT: Abnormal nephron development associated with a frameshift mutation in the transcription factor hepatocyte nuclear factor-1 beta. Kidney Int 57: 898–907, 2000 [DOI] [PubMed] [Google Scholar]
- 28.Beards F, Frayling T, Bulman M, Horikawa Y, Allen L, Appleton M, Bell GI, Ellard S, Hattersley AT: Mutations in hepatocyte nuclear factor 1beta are not a common cause of maturity-onset diabetes of the young in the U.K. Diabetes 47: 1152–1154, 1998 [DOI] [PubMed] [Google Scholar]
- 29.Thomas CP, Erlandson JC, Edghill EL, Hattersley AT, Stolpen AH: A genetic syndrome of chronic renal failure with multiple renal cysts and early onset diabetes. Kidney Int 74: 1094–1099, 2008 [DOI] [PubMed] [Google Scholar]
- 30.Bernardini L, Gimelli S, Gervasini C, Carella M, Baban A, Frontino G, Barbano G, Divizia MT, Fedele L, Novelli A, Béna F, Lalatta F, Miozzo M, Dallapiccola B: Recurrent microdeletion at 17q12 as a cause of Mayer-Rokitansky-Kuster-Hauser (MRKH) syndrome: Two case reports. Orphanet J Rare Dis 4: 25, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bingham C, Ellard S, Cole TR, Jones KE, Allen LI, Goodship JA, Goodship TH, Bakalinova-Pugh D, Russell GI, Woolf AS, Nicholls AJ, Hattersley AT: Solitary functioning kidney and diverse genital tract malformations associated with hepatocyte nuclear factor-1beta mutations. Kidney Int 61: 1243–1251, 2002 [DOI] [PubMed] [Google Scholar]
- 32.Renkema KY, Verhaar MC, Knoers NV: Diabetes-induced congenital anomalies of the kidney and urinary tract (CAKUT): Nurture and nature at work? Am J Kidney Dis 65: 644–646, 2015 [DOI] [PubMed] [Google Scholar]
- 33.Kotalova R, Dusatkova P, Cinek O, Dusatkova L, Dedic T, Seeman T, Lebl J, Pruhova S: Hepatic phenotypes of HNF1B gene mutations: A case of neonatal cholestasis requiring portoenterostomy and literature review. World J Gastroenterol 21: 2550–2557, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lebrun G, Vasiliu V, Bellanné-Chantelot C, Bensman A, Ulinski T, Chrétien Y, Grünfeld JP: Cystic kidney disease, chromophobe renal cell carcinoma and TCF2 (HNF1 beta) mutations. Nat Clin Pract Nephrol 1: 115–119, 2005 [DOI] [PubMed] [Google Scholar]
- 35.Wang CC, Mao TL, Yang WC, Jeng YM: Underexpression of hepatocyte nuclear factor-1β in chromophobe renal cell carcinoma. Histopathology 62: 589–594, 2013 [DOI] [PubMed] [Google Scholar]
- 36.Shim JH, Lee HC, Han S, Kang HJ, Yu E, Lee SG: Hepatocyte nuclear factor 1β is a novel prognostic marker independent of the Milan criteria in transplantable hepatocellular carcinoma: A retrospective analysis based on tissue microarrays. Liver Transpl 19: 336–345, 2013 [DOI] [PubMed] [Google Scholar]
- 37.Yuan RH, Lai HS, Hsu HC, Lai PL, Jeng YM: Expression of bile duct transcription factor HNF1β predicts early tumor recurrence and is a stage-independent prognostic factor in hepatocellular carcinoma. J Gastrointest Surg 18: 1784–1794, 2014 [DOI] [PubMed] [Google Scholar]
- 38.Cuff J, Salari K, Clarke N, Esheba GE, Forster AD, Huang S, West RB, Higgins JP, Longacre TA, Pollack JR: Integrative bioinformatics links HNF1B with clear cell carcinoma and tumor-associated thrombosis. PLoS One 8: e74562, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tsuchiya A, Sakamoto M, Yasuda J, Chuma M, Ohta T, Ohki M, Yasugi T, Taketani Y, Hirohashi S: Expression profiling in ovarian clear cell carcinoma: Identification of hepatocyte nuclear factor-1 beta as a molecular marker and a possible molecular target for therapy of ovarian clear cell carcinoma. Am J Pathol 163: 2503–2512, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Debiais-Delpech C, Godet J, Pedretti N, Bernard FX, Irani J, Cathelineau X, Cussenot O, Fromont G: Expression patterns of candidate susceptibility genes HNF1β and CtBP2 in prostate cancer: Association with tumor progression. Urol Oncol 32: 426–432, 2014 [DOI] [PubMed] [Google Scholar]
- 41.Loirat C, Bellanné-Chantelot C, Husson I, Deschênes G, Guigonis V, Chabane N: Autism in three patients with cystic or hyperechogenic kidneys and chromosome 17q12 deletion. Nephrol Dial Transplant 25: 3430–3433, 2010 [DOI] [PubMed] [Google Scholar]
- 42.Moreno-De-Luca D, Mulle JG, Kaminsky EB, Sanders SJ, Myers SM, Adam MP, Pakula AT, Eisenhauer NJ, Uhas K, Weik L, Guy L, Care ME, Morel CF, Boni C, Salbert BA, Chandrareddy A, Demmer LA, Chow EW, Surti U, Aradhya S, Pickering DL, Golden DM, Sanger WG, Aston E, Brothman AR, Gliem TJ, Thorland EC, Ackley T, Iyer R, Huang S, Barber JC, Crolla JA, Warren ST, Martin CL, Ledbetter DH, SGENE Consortium. Simons Simplex Collection Genetics Consortium. GeneSTAR : Deletion 17q12 is a recurrent copy number variant that confers high risk of autism and schizophrenia. Am J Hum Genet 87: 618–630, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ferrè S, Bongers EM, Sonneveld R, Cornelissen EA, van der Vlag J, van Boekel GA, Wetzels JF, Hoenderop JG, Bindels RJ, Nijenhuis T: Early development of hyperparathyroidism due to loss of PTH transcriptional repression in patients with HNF1β mutations? J Clin Endocrinol Metab 98: 4089–4096, 2013 [DOI] [PubMed] [Google Scholar]
- 44.Raaijmakers A, Corveleyn A, Devriendt K, van Tienoven TP, Allegaert K, Van Dyck M, van den Heuvel L, Kuypers D, Claes K, Mekahli D, Levtchenko E: Criteria for HNF1B analysis in patients with congenital abnormalities of kidney and urinary tract. Nephrol Dial Transplant 30: 835–842, 2015 [DOI] [PubMed] [Google Scholar]
- 45.Nakayama M, Nozu K, Goto Y, Kamei K, Ito S, Sato H, Emi M, Nakanishi K, Tsuchiya S, Iijima K: HNF1B alterations associated with congenital anomalies of the kidney and urinary tract. Pediatr Nephrol 25: 1073–1079, 2010 [DOI] [PubMed] [Google Scholar]
- 46.Verbitsky M, Sanna-Cherchi S, Fasel DA, Levy B, Kiryluk K, Wuttke M, Abraham AG, Kaskel F, Köttgen A, Warady BA, Furth SL, Wong CS, Gharavi AG: Genomic imbalances in pediatric patients with chronic kidney disease. J Clin Invest 125: 2171–2178, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Musetti C, Quaglia M, Mellone S, Pagani A, Fusco I, Monzani A, Giordano M, Stratta P: Chronic renal failure of unknown origin is caused by HNF1B mutations in 9% of adult patients: A single centre cohort analysis. Nephrology (Carlton) 19: 202–209, 2014 [DOI] [PubMed] [Google Scholar]
- 48.Clissold RL, Hamilton AJ, Hattersley AT, Ellard S, Bingham C: HNF1B-associated renal and extra-renal disease-an expanding clinical spectrum. Nat Rev Nephrol 11: 102–112, 2015 [DOI] [PubMed] [Google Scholar]
- 49.Faguer S, Chassaing N, Bandin F, Prouheze C, Garnier A, Casemayou A, Huart A, Schanstra JP, Calvas P, Decramer S, Chauveau D: The HNF1B score is a simple tool to select patients for HNF1B gene analysis. Kidney Int 86: 1007–1015, 2014 [DOI] [PubMed] [Google Scholar]
- 50.Eckardt KU, Alper SL, Antignac C, Bleyer AJ, Chauveau D, Dahan K, Deltas C, Hosking A, Kmoch S, Rampoldi L, Wiesener M, Wolf MT, Devuyst O: Autosomal dominant tubulointerstitial kidney disease: Diagnosis, classification, and management-A KDIGO consensus report [published online ahead of print March 4, 2015]. Kidney Int 10.1038/ki.2015.28 [DOI] [PubMed] [Google Scholar]