Abstract
Objective
The aim of this study was to identify the relevant obstetric factors for cerebral palsy (CP) after 33 weeks’ gestation in Japan.
Study design
This retrospective case cohort study (1:100 cases and controls) used a Japanese national CP registry. Obstetric characteristics and clinical course were compared between CP cases in the Japan Obstetric Compensation System for Cerebral Palsy database and controls in the perinatal database of the Japan Society of Obstetrics and Gynecology born as live singleton infants between 2009 and 2011 with a birth weight ≥ 2,000 g and gestation ≥ 33 weeks.
Results
One hundred and seventy-five CP cases and 17,475 controls were assessed. Major relevant single factors for CP were placental abnormalities (31%), umbilical cord abnormalities (15%), maternal complications (10%), and neonatal complications (1%). A multivariate regression model demonstrated that obstetric variables associated with CP were acute delivery due to non-reassuring fetal status (relative risk [RR]: 37.182, 95% confidence interval [CI]: 20.028–69.032), uterine rupture (RR: 24.770, 95% CI: 6.006–102.160), placental abruption (RR: 20.891, 95% CI: 11.817–36.934), and preterm labor (RR: 3.153, 95% CI: 2.024–4.911), whereas protective factors were head presentation (RR: 0.199, 95% CI: 0.088–0.450) and elective cesarean section (RR: 0.236, 95% CI: 0.067–0.828).
Conclusion
CP after 33 weeks’ gestation in the recently reported cases in Japan was strongly associated with acute delivery due to non-reassuring fetal status, uterine rupture, and placental abruption.
Introduction
Cerebral palsy (CP) is a physical disability in children that is strongly associated with public health issues. Despite advances in obstetrics and perinatology, the incidence of CP is reported to be approximately 2 per 1,000 live births [1–3]. Although the risk of CP increases with preterm birth, it is not infrequent even in mature babies [3–5], with an incidence of 1.1 cases per 1,000 live births even at 40 weeks’ gestation [3].
The Japan Obstetric Compensation System for Cerebral Palsy (JOCSC) was launched in January 2009 because of a shortage of young obstetricians and regional gaps in obstetric care provision allegedly due to severe working environments and an increasing number of medical conflicts.
In the present report, obstetric characteristics, clinical course, and the relevant obstetric factors for CP after 33 weeks’ gestation in the JOCSC database (JOCSC-DB) were reviewed and compared with those of controls considered to be representative of the Japanese population in the perinatal database of the Japan Society of Obstetrics and Gynecology (JSOG-DB).
The purpose of the present study was to identify the relevant obstetric factors for CP in Japan. This is the first nationwide study to analyze and identify relevant obstetric factors for CP in Japan.
Patients and Methods
A retrospective case-cohort study was conducted using a nationwide registry in Japan. Obstetric clinical characteristics and disease course were compared between the CP cases and controls.
Cases were infants with CP approved by a review of the Operating Organization of the JOCSC. The objectives of this JOCSC are to provide prompt no-fault compensation for children diagnosed with severe CP caused by trauma during labor and delivery and for their respective families, and to provide information that could help in the prevention, early resolution of disputes, and improvement of quality of obstetric health care. Compensation cases are reviewed by a review committee consisting of obstetricians, pediatricians, midwives, and lawyers according to the rules of the Operating Organization of the JOCSC. After being authorized as eligible to receive compensation by this review committee, the causes for CP are analyzed individually by the Causal Analysis Committee consisting of obstetricians, pediatricians, midwives, and lawyers. Once collected, these individual cases are analyzed from an epidemiological standpoint by the Recurrence Prevention Committee.
Those eligible for inclusion in the present study were infants born between January 2009 and December 2011 and those with a birth weight of ≥2,000 g, gestation of ≥33 weeks, and severe disability due to CP independent of congenital causes or factors during the neonatal period or later, with disability certified to be of first or second degree severity according to the grade of disability definitions in the Act for the Welfare of Persons with Physical Disabilities. The grade of disability range from Level 1 to Level 7, and Levels 1 and 2 correspond to severe locomotor disabilities. Specifically, Levels 1 and 2 indicate the bed-ridden state of a patient, and anticipated inability to walk in the future. According to the rules of the Operating Organization of the JOCSC, CP should be diagnosed by a physician with expertise in CP. Specifically, CP should be diagnosed by a pediatrician qualified for certification of patients with physical disabilities under the Act for the Welfare of Persons with Physical Disabilities or by a physician registered as a specialist in pediatric neurology with the Japanese Society of Child Neurology. Cases were excluded only if congenital anomalies such as bilateral extensive brain malformation, chromosomal aberration, gene anomaly, congenital metabolic abnormality, or other congenital anomaly were present, or neonatal factors such as meningitis and encephalitis were present and plausible causative factors determined by the Review Committee using head imaging data or other test data (medical records, MRI, CT, etc.), and if those factors were apparent principal causes of motor function disturbance.
To restrict cases and controls for demographics, only subjects born between January 2009 and December 2011 as live singleton infants with no major congenital anomalies at a general or university hospital or a perinatal center with a birth weight ≥ 2,000 g and gestation ≥ 33 weeks were enrolled. The analysis included cases wherein the final stages of labor occurred at these hospitals. Particularly, the analysis included cases of women transported from general physicians or private clinics to hospitals, in addition to the cases of women scheduled for delivery at hospitals. Thus, among the controls, all cases of stillbirth and neonatal death were excluded from the present study.
Controls in this retrospective case-cohort study were obtained from the perinatal database of the JSOG-DB, which is the nationwide registry in Japan established in 1974. For the JSOG-DB, attending physicians at 192 secondary and tertiary care centers of the Perinatal Research Network in Japan have collected yearly data for each pregnant woman through an off-line clinical database system using a common format. The data are stored by the Perinatal Committee of the JSOG following strict quality control of the information contained in the database [6]. As the JSOB-DB is the largest database, including about 10% of the total number of births and about 40% of cases resulting in perinatal death in Japan, it is available to clinical researchers in Japan and has been used in previous similar studies [7–10].
As a control subcohort, 17,500 newborns were randomly retrieved from the JSOG-DB, and 17,450 samples without data deficiencies were ultimately included as subjects in the present study. The following clinical variables associated with CP were analyzed in these subjects: (i) maternal age, height, weight, parity, and history of hypertension or in vitro fertilization, which were analyzed as maternal characteristics; (ii) pregnancy-induced hypertension (PIH), diabetes mellitus (DM), gestational DM (GDM), preterm labor, premature rupture of membrane (PROM), intra-amniotic infection, placental abruption, polyhydramnios, and oligohydramnios, which were analyzed as variables during pregnancy; and (iii) maternal body weight at delivery, weight gain during pregnancy, oxytocin and prostaglandin augmentation, head presentation of the fetus, uterine rupture, instrumental delivery, acute delivery due to non-reassuring fetal status (NRFS), and both elective and emergency cesarean section, which were analyzed as variables at delivery. Finally, number of gestational weeks, birth weight, male sex, Apgar score, and umbilical artery pH were compared as neonatal variables.
Definitions
Cerebral palsy
CP is defined as the disturbance of motor function or posture of infants that is permanent or variable. The disorder is based on a non-progressive cerebral lesion that develops anytime between conception to the neonatal period (within 4 weeks after birth). However, this definition excludes motor retardation that is either transient or normalizes in the future.
PIH
The definition of PIH included preeclampsia (PE) and gestational hypertension (GH). GH was defined as the onset of hypertension after 20 weeks’ gestation (defined as systolic blood pressure ≥ 140 mmHg or diastolic blood pressure ≥ 90 mmHg) on at least two occasions 4 h apart. PE was defined as GH with proteinuria (0.3 g in a 24-h urine specimen or a protein to creatinine ratio of >0.30).
Intra-amniotic infection
Intra-amniotic infection was defined as an observed infection of the amniotic fluid, membranes, placenta, or decidua, based on overt clinical signs such as PROM, fever, uterine tenderness, maternal and fetal tachycardia, and foul amniotic fluid and/or pathological findings such as chorioamnionitis and/or funisitis or amniotic fluid with a positive bacterial culture.
Acute delivery due to NRFS
Acute delivery included emergency cesarean section, vacuum extraction, and forceps delivery due to a non-reassuring cardiotocogram.
Statistical analysis
A two-side p-value of 0.05 was used to define statistical significance. All analyses were conducted using Stata version 13.0 (STATA Corporation, College Station, TX, USA). Continuous variables are reported as mean ± standard deviation and were compared using the Student t-test. Integer variables are reported as median and range, and were compared using the Mann—Whitney U-test. Categorical variables are reported as frequencies and were compared using the chi-square test. Relationships among clinical variables were evaluated using univariate and multivariate logistic regression with CP as the independent variable. Results are expressed by relative risk (RR) and 95% confidence interval (CI). Considering statistical collinearity among maternal weight, height, and BMI at pregnancy and data deficiency in Apgar score and umbilical artery pH among CP cases; independent variables including maternal age, height, and weight at pregnancy, multiparous birth, IVF, PIH, DM/GDM, preterm labor, PROM, placental abruption, polyhydramnios, oligohydramnios, weight gain during pregnancy, oxytocin and prostaglandin augmentation, uterine rupture, head presentation, acute delivery due to NRFS, instrumental delivery, elective cesarean section, emergency cesarean section, gestational weeks at delivery, neonatal birth weight, and male-sex infant were compulsorily entered into a multivariate model. The goodness of fit of the multivariate model was evaluated using the area under the operating characteristic (AUC) curve and the Hosmer-Lemeshow test. Collinearity among all variables in the final model was evaluated using variance inflation factor (VIF) with a cut-off value of 2.5.
Ethics
The study protocol was approved by the Institutional Review Boards (IRBs) of the JOCSC. Written informed consent was not obtained from patients. However, patients were provided with a supplemental file that contained the announcement of implementation of a “case-control study for cerebral palsy and prevention of its recurrence”. Although the analysis was retrospective, the data for the anonymized JOCSC-DB and the JSOG-DB collected in a normal clinical setting ensured that the confidentiality of the patients involved was protected. All patient records/information was anonymized and de-identified prior to analysis and no personal data were necessary for the present study.
Results
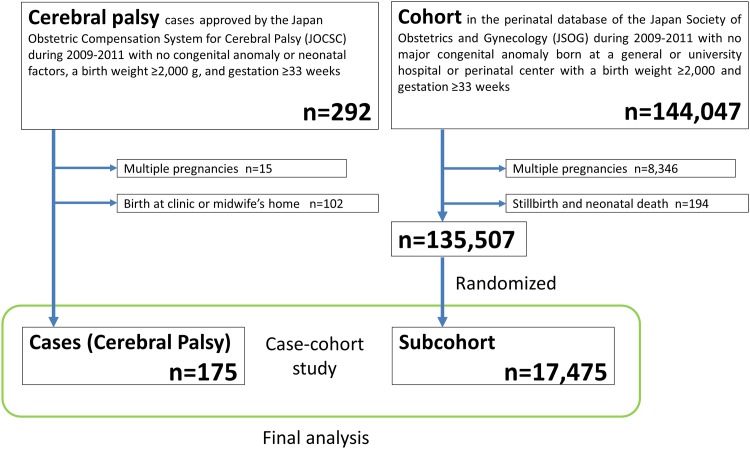
The data of 292 infants with CP obtained from the JOCSC-DB and those of 144,047 infants used to represent the Japanese population from the JSOG-DB restricted for birth weight (≥2,000 g) and pregnancy duration (≥33 weeks) were available for this analysis. The study flow diagram is shown in Fig 1. Fifteen infants with CP and 8,346 infants of multiple births from the general population were excluded from the present study. Moreover, 102 infants with CP born at private clinics or at midwives’ houses were excluded from the CP group because the study populations were limited to patients born at a tertiary hospital. In addition, 194 cases of stillbirths and neonatal deaths were excluded from the general population. After exclusion of the above infants, a total of 135,507 infants were available for analysis as the population control sample pool; of them, 17,475 control infants were randomly selected using computer software. Overall, 175 cases and 17,475 controls were enrolled as the subjects of the present study and analyzed as a 1:100 case-cohort study.
Fig 1. Study flow diagram.
The characteristics of the cases and the major relevant obstetric factors for CP analyzed by the JOCSC Causal Analysis Committee and classified by the Recurrence and Prevention Committee in the 175 CP cases are shown in Table 1. Relevant single factors considered were placental abnormalities (31%), umbilical cord abnormalities (15%), maternal complications (10%), and neonatal complications (1%). About 26% of CP cases were unexplained.
Table 1. Characteristics of cases—major relevant obstetric factors for cerebral palsy reviewed by the Operating Organization (n = 175).
| Single factor | 99 (57%) | |
| Placental abnormalities | 55 (31%) | |
| Placental abruption | 50 | |
| Bleeding of placenta previa | 1 | |
| Feto-maternal blood transfusion | 4 | |
| Umbilical cord abnormalities | 26 (15%) | |
| Umbilical cord prolapse | 9 | |
| Other cord abnormalities | 17 | |
| Maternal complications | 18 (10%) | |
| Uterine rupture | 7 | |
| Intraamniotic infection | 3 | |
| Sepsis | 1 | |
| Herpetic meningoencephalitis | 1 | |
| Maternal cardio-pulmonary arrest | 2 | |
| Amniotic fluid embolism | 1 | |
| PIH | 2 | |
| Uncontrolled DM | 1 | |
| Neonatal complications | 2 (1%) | |
| Blood type incompatibility | 1 | |
| Intraventricular hemorrhage | 1 | |
| Multifactorial | 29 (17%) | |
| Placental abruption | ||
| + cord abnormality | 1 | |
| + FGR | 1 | |
| + uterine rupture | 1 | |
| + intraamniotic infection | 1 | |
| + maternal shock | 1 | |
| Cord abnormality | ||
| + intraamniotic infection | 7 | |
| + intraventricular hemorrhage | 1 | |
| + shoulder dystocia | 1 | |
| FGR | ||
| + intraamniotic infection | 2 | |
| + PIH | 2 | |
| + velamentous insertion | 1 | |
| Uncontrolled DM | ||
| + intraventricular hemorrhage | 1 | |
| Other causes | 9 | |
| Unexplained | 45 (26%) |
PIH; pregnancy induced hypertension, DM; diabetes mellitus, FGR; fetal growth reastriction. Other cord abnormalities included velamentous/marginal cord insertion, vasa previa, hyper coiled cord, cord entanglement, constriction of umbilical cord, and long umbilical cord. Other causes included meconium aspiration syndrome, hyperglycemia, maternal shock, and hypertonic uterine contraction.
Obstetric characteristics, clinical course, and the relevant obstetric factors identified in CP cases and the subcohort are shown in Table 2. Variables up to the time of delivery that had a high crude RR were placental abruption, acute delivery due to NRFS, uterine rupture, and emergency cesarean section. In our series, 8 cases (4.6%) of uterine rupture were observed. Half of these cases occurred suddenly outside the hospital, whereas the other half occurred during labor in a hospital setting. Six cases (75%) had risk factors such as a history of cesarean section and bicornuate uterus. One case was subjected to vacuum extraction with a uterine fundal pressure maneuver. The frequencies of intra-amniotic infection, velamentous cord insertion, marginal cord insertion, and umbilical cord prolapse were not available for controls because JOSG-DB did not collect these data.
Table 2. Obstetric characteristics, clinical course, and relevant obstetric factors in cases with cerebral palsy and subcohort.
| Variables | Cases | Control subcohort | Crude | 95% Confidence |
|---|---|---|---|---|
| (n = 175) | (n = 17,475) | relative risk | interval | |
| Maternal | ||||
| Age | 31.3 ± 5.7 | 31.9 ± 5.3 | 0.981 | 0.954–1.001 |
| Height (cm) | 157.7 ± 5.6 | 158.3 ± 5.5 | 0.98 | 0.954–1.007 |
| Weight (kg) | 54.8 ± 11.4 | 53.2 ± 9.9 | 1.014 | 1.001–1.027 |
| BMI (kg/m2) | 22.0 ± 4.8 | 21.2 ± 3.7 | 1.047 | 1.015–1.081 |
| Parity (median, range) | 0 (0–5) | 0 (0–9) | 0.955 | 0.797–1.145 |
| Hypertension | 0.0% (0) | 0.8% (143) | n/a | |
| IVF | 5.1% (9) | 4.3% (758) | 1.196 | 0.609–2.348 |
| During pregnancy | ||||
| PIH | 8.0% (14) | 4.0% (704) | 2.072 | 1.194–3.595 |
| DM/GDM | 2.9% (5) | 4.0% (694) | 0.711 | 0.291–1.736 |
| Preterm labor | 32.0% (56) | 12.4% (2,159) | 3.338 | 2.422–4.601 |
| PROM | 15.4% (27) | 11.6% (2,019) | 1.397 | 0.924–2.110 |
| Intraamniotic infection | 18.3% (32) | n/a | n/a | |
| Placental abruption | 32.6% (57) | 0.6% (104) | 80.683 | 55.715–116.841 |
| Velamentous insertion/vasa previa | 4.6% (8) | n/a | n/a | |
| Marginal insertion | 12.6% (22) | n/a | n/a | |
| Polyhydramnios | 1.1% (2) | 0.7% (120) | 1.712 | 0.420–6.980 |
| Oligohydramnios | 2.9% (5) | 1.6% (288) | 1.798 | 0.733–4.409 |
| At delivery | ||||
| Weight (kg) | 64.3 ± 11.1 | 62.9 ± 9.7 | 1.014 | 1.000–1.028 |
| Weight gain (kg) | 9.6 ± 4.2 | 9.7 ± 4.4 | 0.993 | 0.959–1.027 |
| Oxytocin augmentation | 24.6% (43) | 23.7% (4,144) | 1.048 | 0.741–1.481 |
| Prostaglandin augmentation | 6.9% (12) | 6.1% (1,065) | 1.134 | 0.629–2.045 |
| Uterine rupture | 4.6% (8) | 0.1% (17) | 49.195 | 20.942–115.564 |
| Head presentation | 91.4% (160) | 95.4% (16,672) | 0.514 | 0.301–0.876 |
| TV breech presentation | 1.7% (3) | 0.09% (16) | 16.185 | 5.669–46.207 |
| Umbilical cord prolapse | 5.1% (9) | n/a | n/a | |
| Acute delivery due to NRFS | 68.6% (120) | 3.2% (566) | 65.181 | 46.862–90.661 |
| Mode of delivery | ||||
| Normal spontaneous | 21.1% (37) | 65.8% (11,493) | reference | |
| Instrumental | 13.7% (24) | 7.1% (1,238) | 6.021 | 3.591–10.099 |
| Elective CS | 1.7% (3) | 16.6% (2,895) | 0.322 | 0.099–1.045 |
| Emergency CS | 63.4% (111) | 10.6% (1,849) | 18.647 | 12.812–27.140 |
| Neonate | ||||
| Gestational weeks | 38.1 ± 2.0 | 38.6 ± 1.5 | 0.817 | 0.747–0.892 |
| Birth weight (g) | 2,846 ± 457 | 2,969 ± 399 | 0.924 | 0.889–0.960 |
| (SD) | -0.14 ± 1.04 | -0.02 ± 0.98 | 0.879 | 0.752–1.027 |
| Small for gestational age | 11.4% (20) | 5.5% (958) | 2.225 | 1.390–3.560 |
| Male | 51.4% (90) | 50.9% (8,902) | 1.02 | 0.757–1.374 |
| Apgar Score | ||||
| 1 min. | ||||
| > = 7 | 6.3% (11) | 96.7% (16890) | reference | |
| 4–7 | 11.4% (20) | 2.3% (410) | 74.9 | 35.657–157.335 |
| <4 | 56.6% (99) | 1.0% (168) | 904.821 | 476.540–1718.013 |
| Unknown | 25.7% (45) | 0.04% (7) | ||
| 5 min. | ||||
| > = 7 | 10.9% (19) | 99.2% (17338) | reference | |
| 4–7 | 33.1% (58) | 0.6% (101) | 524.024 | 301.168–911.704 |
| <4 | 36.6% (64) | 0.2% (28) | 2085.772 | 1108.473–3924.719 |
| Unknown | 19.4% (34) | 0.05% (8) | ||
| Umbilical artery pH | ||||
| > = 7.2 | 13.7% (24) | 92.0% (16082) | reference | |
| 7.0–7.2 | 16.0% (28) | 7.7% (1338) | 14.023 | 8.106–24.258 |
| 6.8–7.0 | 16.0% (28) | 0.3% (46) | 407.877 | 220.018–756.136 |
| <6.8 | 21.1% (37) | 0.05% (9) | 2754.788 | 1199.741–6325.415 |
| Unknown | 33.1% (58) | 0% (0) |
Data indicate mean ± standard deviation, median (range) or frequency (n). n/a; not available, IVF; in vitro Fertilization, PIH; pregnancy induced hypertension, DM; diabetes mellitus, GDM; gestational diabetes mellitus, PROM; premature rupture of membrane, TV; trans vaginal delivery, NRFS; non-reassuring fetal status, CS; cesarean section, SD; standard deviation.
The multivariate regression model including all the variables is shown in Table 3. Obstetric variables strongly associated with CP were acute delivery due to NRFS (adjusted RR [aRR]: 37.182, 95% CI: 20.028–69.032), uterine rupture (aRR: 24.770, 95% CI: 6.0065.726–102.160), and placental abruption (aRR: 20.891, 95% CI: 11.817–36.934). On the other hand, the obstetric variables associated with reduced CP were head presentation (aRR: 0.199, 95% CI: 0.088–0.450) and elective cesarean section (aRR: 0.236, 95% CI: 0.067–0.828).
Table 3. Results of the multiple logistic regression analysis.
| Variables | Adjusted relative risk | 95% Confidence interval |
|---|---|---|
| Maternal weight at pregnancy (kg) | 1.035 | 1.017–1.054 |
| Preterm labor | 3.153 | 2.024–4.911 |
| Placental abruption | 20.891 | 11.817–36.934 |
| Oxytocin augmentation | 1.612 | 1.034–2.513 |
| Uterine rupture | 24.77 | 6.006–102.160 |
| Head presentation | 0.199 | 0.088–0.450 |
| Acute delivery due to NRFS | 37.182 | 20.028–69.032 |
| Elective Cesarean section | 0.236 | 0.067–0.828 |
Covariates: maternal age, height, multiparous, IVF, PIH, DM/GDM, PROM, polyhydramnios, oligohydramnios, weight gain during pregnancy, prostaglandin augmentation, instrumental delivery, emergency caesarean section, gestational week at delivery, small for gestational age and male infant; N = 17,633; Hosmer-Lemeshow test (degrees of freedom: 8) P = 0.496; Area under operating characteristic curve: 0.913.
The AUC of the final model of 0.91 indicated a high predictive ability. The Hosmer-Lemeshow test for the final model was non-significant (chi-square: 7.39, degrees of freedom: 8, p = 0.496), indicating that the model performed equally well for all variables. The VIF for all variables in the final model was <2.5, indicating that all variables were orthogonal to each other.
Discussion
In summary, we found a strong association between CP and acute delivery due to NRFS among infants with a birth weight of ≥2,000 g and gestation length of ≥33 weeks. In fact, umbilical artery pH was <7.0 in 37% of CP cases but only in 0.35% of controls. Multiple logistic regression analysis identified eight obstetrical conditions that independently affected the risk of developing of CP; of them, three factors, including placental abruption, uterine rupture, and acute delivery due to NRFS, had extremely large effects.
Intrauterine growth restriction induced by antenatal conditions and resulting in low birth weight for gestational age and preterm delivery are the main risk factors of CP [4, 11–14]. However, in CP infants born almost matured at near term such in the present subjects, adverse conditions involving acute utero-placental under-perfusion including placental abruption and uterine rupture, and required acute delivery due to NRFS were more associated with CP rather than chronically progressive adverse condition such as fetal growth restriction and hypertensive disorder. Previous meta-analysis demonstrated that newborns delivered by emergency Cesarean section at term were more likely to have CP (1.6; 95%CI 1.05–2.44) [15]. It is considered that this result showed risk factors for CP in association with emergency Cesarean section, with the likely confounding that acute or chronic fetal compromise would often precipitate this intervention [15–17].
A high aRR with acute delivery due to NRFS, placental abruption, and umbilical artery pH were also consistent with speculations aboutacidemia, as these factors are known to associate with an increased risk of hypoxemia and academia, resulting in CP. Acute deliveries due to NRFS were thought to involve many umbilical cord complications. Although data relative to frequencies of umbilical cord abnormalities were not available for controls in the present study, umbilical cord prolapse was more frequently observed in our cases with CP (5.1%) than in previous reports (0.12–0.62%)[18–21]. An abnormally positioned fetus is less likely to be engaged in the maternal pelvis, non-vertex presentation is associated with umbilical cord prolapse [22, 23]. Our result that head presentation was protective is consistent with previous studies shown increased risk of CP in term infants born in breech presentation [4, 24, 25]. Velamentous cord insertion was also more frequently observed in our cases with CP (4.6%) than in previous reports ranging from 0.5% to 1.69% of singleton pregnancies [26, 27]. Therefore, strong associations between CP and pathological conditions of the placenta and umbilical cord that require acute delivery are suspected.
A previous study supported the usefulness of fetal heart rate monitoring in the detection of fetal acidemia in low-risk pregnancies and concluded that under continuous monitoring, CP caused by intrapartum asphyxia was restricted to unavoidable hypoxic accidents [28]. The exposure duration to adverse conditions such as hypoxemia and acidemia also increases the risk of adverse outcomes and, hence, could indicate where clinical efforts might result in improvements by either reducing their incidence or shortening the delivery time [29]. Obstetric system improvements consisting of suitable emergency obstetric staffing, education, and training in hospitals might be required in order to reduce CPs caused due to acute intrapartum accident. In particular, iatrogenic accidents during labor, such as umbilical cord prolapse due to amniotomy without confirmation of the descending cord using ultrasound [30], and uterine rupture associated with uterine fundal pressure maneuver [31], should be reduced.
On the other hand, fear of CP litigation is a major influence on the defensive decision-making of the obstetrician to perform a Cesarean section [32, 33]. Nevertheless, the pathogenesis of CP has been attributed to antenatal as well as intrapartum factors [34, 35]. A review concluded that 70–80% of CP cases were due to prenatal factors and that birth asphyxia played a relatively minor role (<10%) [35]. Reportedly >90% of term and near-term singleton infants with CP had experienced no recognized potentially asphyxiating birth event [36, 37]. In the present study, pre-admission central nervous system injury was suspected in some cases because there was a persistent loss of heart rate variability without deceleration of the tracing at the time of admission. In fact, no relevant obstetric factors were identified in 26% of CP cases, and the umbilical artery pH was >7.0 in approximately 30% of CP cases. These results might indicate that CP is not preventable, even if an early emergency or elective cesarean section is performed.
Low blood flow in the placenta, brain, or other organs and inflammation confer a clotting predisposition and increased thrombosis risk [37]. When such events occur relatively early in pregnancy, they can cause brain lesions; later in pregnancy, they can cause cerebral infarction in a vascular territory [37]. In neurologically impaired term infants, umbilical cord abnormalities such as abnormal cord insertion and umbilical cord entanglement are significantly increased in placentas with fetal thrombotic vasculopathy [38]. Even placenta abruption has important antecedents including placental inflammatory and vascular disorders, although the most catastrophic event occurs at birth [39]. Therefore, it is considered that no protective effect of elective Cesarean section in term infant for CP was plausible results [4, 15].
Limitations and Strengths
As this is the first large nationwide study in Japan to analyze obstetrically relevant factors for CP after 33 weeks’ gestation, we believe that the results reported herein will provide useful information and thus reduce the incidence of CP cases associated with acidemia near the time of delivery and with obstetric management.
Limitations of the present study include several biases of the study design. Because there is no registration or record system, archived obstetric and long-term pediatric characteristics, different subjects from two databases, CP cases from the JOCSC-DB and controls from the JSOG-DB were compared. Since characteristics were restricted between the two groups, selection bias was practically undetectable. A subcohort sample to analyze the two groups was collected, but it may have contained a few CP cases. It is likely that the JOCSC-DB may also be subject to information bias, because the JSOG-DB simply contains records from a registration software whereby data are entered by caregivers from obstetric institutions after delivery; whereas, the JOCSC-DB was designed by expert staff according to precise criteria, and data input from medical records required for evaluation of CP cases to qualify for compensation eligibility.
Furthermore, in the present study, the multivariate regression analysis showed that elective Cesarean section was protective for occurrence of CP, in contrast to the result of univariate analysis, while in the previous studies no protective effect for CP was shown for elective Cesarean section in term infant [4, 15]. This discrepancy might be attributable to the lack of available data related to the frequencies of placental and umbilical cord abnormalities, including velamentous/marginal cord insertion, vasa previa, umbilical cord prolapse and intra-amniotic infection, for multivariate regression analysis in JOSG-DB. Therefore, it is difficult to determine whether elective cesarean section has a favorable effect with regard to CP reduction. The collection of further detailed data associated with antenatal causes of CP is needed in JOSG-DB.
Conclusion
In the present study, CP was strongly associated with acute delivery due to NRFS, uterine rupture, and placental abruption. These acute hypoxic academic situations were indicated by a low Apgar score and low umbilical artery blood pH. Although cases of CP with chronic, progressive antenatal causes are unavoidable, we believe that improvements made to obstetric management during labor can at least reduce the incidence of CP cases resulting from delivery-related complications.
Supporting Information
(DOCX)
Acknowledgments
Membership of Prevention Recurrence Committee, Japan Obstetric Compensation System for Cerebral Palsy
Tsuyomu Ikenoue (chair; e-mail: tsuyomu_ikenoue@med.miyazaki-u.ac.jp): JSOG
Junichi Hasegawa: JSOG and JOCSC
Satoshi Toyokawa: JOCSC
Yuri Asano: JOCSC
Shoji Satoh: JSOG and JOCSC
Tomoaki Ikeda: JSOG
Kiyotake Ichizuka: JSOG
Nanako Tamiya: JOCSC
Akihito Nakai: JSOG
Keiya Fujimori: JSOG
Tsugio Maeda: JSOG
Hideaki Masuzaki: JSOG
Hideaki Suzuki: JSOG and JOCSC
Shigeru Ueda: JOCSC
We wish to thank Shinichi Takeda, Natsumi Tsuchiya, Kentaro Kato, and Emi Jojima (Prevention Recurrence Team member, Japan Council for Quality Health Care) for her excellent support in data collection, organization, and statistical analysis.
Data Availability
Ethical and legal restrictions imposed by the Japan Council for Quality Health Care (JCQHC) prevent the sharing of patient data used in this study. Interested researchers may request access to the data in the the Japan Society of Obstetrics and Gynecology database from (nissanfu@jsog.or.jp) or through Dr. Tsuyomu Ikenoue (tsuyomu_ikenoue@med.miyazaki-u.ac.jp).
Funding Statement
The authors received no specific funding for this work.
References
- 1.Reid SM, Carlin JB, Reddihough DS. Rates of cerebral palsy in Victoria, Australia, 1970 to 2004: has there been a change? Developmental medicine and child neurology. 2011;53(10):907–12. Epub 2011/07/15. 10.1111/j.1469-8749.2011.04039.x . [DOI] [PubMed] [Google Scholar]
- 2.Parkes J, Dolk H, Hill N, Pattenden S. Cerebral palsy in Northern Ireland: 1981–93. Paediatric and perinatal epidemiology. 2001;15(3):278–86. Epub 2001/08/08. . [DOI] [PubMed] [Google Scholar]
- 3.Tronnes H, Wilcox AJ, Lie RT, Markestad T, Moster D. Risk of cerebral palsy in relation to pregnancy disorders and preterm birth: a national cohort study. Developmental medicine and child neurology. 2014;56(8):779–85. Epub 2014/03/14. 10.1111/dmcn.12430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thorngren-Jerneck K, Herbst A. Perinatal factors associated with cerebral palsy in children born in Sweden. Obstetrics and gynecology. 2006;108(6):1499–505. Epub 2006/12/02. . [DOI] [PubMed] [Google Scholar]
- 5.Moster D, Lie RT, Markestad T. Long-term medical and social consequences of preterm birth. The New England journal of medicine. 2008;359(3):262–73. Epub 2008/07/19. 10.1056/NEJMoa0706475 . [DOI] [PubMed] [Google Scholar]
- 6.Hayashi M, Nakai A, Satoh S, Matsuda Y. Adverse obstetric and perinatal outcomes of singleton pregnancies may be related to maternal factors associated with infertility rather than the type of assisted reproductive technology procedure used. Fertility and sterility. 2012;98(4):922–8. Epub 2012/07/06. 10.1016/j.fertnstert.2012.05.049 . [DOI] [PubMed] [Google Scholar]
- 7.Yamada T, Yamada T, Morikawa M, Minakami H. Clinical features of abruptio placentae as a prominent cause of cerebral palsy. Early human development. 2012;88(11):861–4. Epub 2012/07/19. 10.1016/j.earlhumdev.2012.06.008 . [DOI] [PubMed] [Google Scholar]
- 8.Yamada T, Cho K, Yamada T, Morikawa M, Minakami H. Labor induction by transcervical balloon catheter and cerebral palsy associated with umbilical cord prolapse. The journal of obstetrics and gynaecology research. 2013;39(6):1159–64. Epub 2013/04/05. 10.1111/jog.12036 . [DOI] [PubMed] [Google Scholar]
- 9.Shiozaki A, Matsuda Y, Hayashi K, Satoh S, Saito S. Comparison of risk factors for major obstetric complications between Western countries and Japan: a case-cohort study. The journal of obstetrics and gynaecology research. 2011;37(10):1447–54. Epub 2011/06/17. 10.1111/j.1447-0756.2011.01565.x . [DOI] [PubMed] [Google Scholar]
- 10.Matsuda Y, Hayashi K, Shiozaki A, Kawamichi Y, Satoh S, Saito S. Comparison of risk factors for placental abruption and placenta previa: case-cohort study. The journal of obstetrics and gynaecology research. 2011;37(6):538–46. Epub 2011/03/08. 10.1111/j.1447-0756.2010.01408.x . [DOI] [PubMed] [Google Scholar]
- 11.Jarvis S, Glinianaia SV, Torrioli MG, Platt MJ, Miceli M, Jouk PS, et al. Cerebral palsy and intrauterine growth in single births: European collaborative study. Lancet. 2003;362(9390):1106–11. Epub 2003/10/11. 10.1016/S0140-6736(03)14466-2 . [DOI] [PubMed] [Google Scholar]
- 12.Jarvis S, Glinianaia SV, Blair E. Cerebral palsy and intrauterine growth. Clinics in perinatology. 2006;33(2):285–300. Epub 2006/06/13. 10.1016/j.clp.2006.03.009 . [DOI] [PubMed] [Google Scholar]
- 13.Ray JG, Redelmeier DA, Urquia ML, Guttmann A, McDonald SD, Vermeulen MJ. Risk of cerebral palsy among the offspring of immigrants. PloS one. 2014;9(7):e102275 Epub 2014/07/16. 10.1371/journal.pone.0102275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O'Callaghan ME, MacLennan AH, Gibson CS, McMichael GL, Haan EA, Broadbent JL, et al. Epidemiologic associations with cerebral palsy. Obstetrics and gynecology. 2011;118(3):576–82. Epub 2011/08/24. . [DOI] [PubMed] [Google Scholar]
- 15.O'Callaghan M, MacLennan A. Cesarean delivery and cerebral palsy: a systematic review and meta-analysis. Obstetrics and gynecology. 2013;122(6):1169–75. Epub 2013/11/10. . [DOI] [PubMed] [Google Scholar]
- 16.Sukhov A, Wu Y, Xing G, Smith LH, Gilbert WM. Risk factors associated with cerebral palsy in preterm infants. J Matern Fetal Neonatal Med. 2012;25(1):53–7. Epub 2011/04/06. 10.3109/14767058.2011.564689 . [DOI] [PubMed] [Google Scholar]
- 17.Mann JR, McDermott S, Griffith MI, Hardin J, Gregg A. Uncovering the complex relationship between pre-eclampsia, preterm birth and cerebral palsy. Paediatric and perinatal epidemiology. 2011;25(2):100–10. Epub 2011/02/02. 10.1111/j.1365-3016.2010.01157.x . [DOI] [PubMed] [Google Scholar]
- 18.Koonings PP, Paul RH, Campbell K. Umbilical cord prolapse. A contemporary look. The Journal of reproductive medicine. 1990;35(7):690–2. Epub 1990/07/01. . [PubMed] [Google Scholar]
- 19.Murphy DJ, MacKenzie IZ. The mortality and morbidity associated with umbilical cord prolapse. British journal of obstetrics and gynaecology. 1995;102(10):826–30. Epub 1995/10/01. . [DOI] [PubMed] [Google Scholar]
- 20.Katz Z, Lancet M, Borenstein R. Management of labor with umbilical cord prolapse. American journal of obstetrics and gynecology. 1982;142(2):239–41. Epub 1982/01/15. . [DOI] [PubMed] [Google Scholar]
- 21.Kahana B, Sheiner E, Levy A, Lazer S, Mazor M. Umbilical cord prolapse and perinatal outcomes. International journal of gynaecology and obstetrics: the official organ of the International Federation of Gynaecology and Obstetrics. 2004;84(2):127–32. Epub 2004/02/12. 10.1016/S0020-7292(03)00333-3 . [DOI] [PubMed] [Google Scholar]
- 22.Holbrook BD, Phelan ST. Umbilical cord prolapse. Obstetrics and gynecology clinics of North America. 2013;40(1):1–14. Epub 2013/03/08. 10.1016/j.ogc.2012.11.002 . [DOI] [PubMed] [Google Scholar]
- 23.Hasegawa J, Ikeda T, Sekizawa A, Ishiwata I, Kinoshita K. Obstetric risk factors for umbilical cord prolapse: A nationwide population-based study in Japan. Archives of gynecology and obstetrics. in press. [DOI] [PubMed] [Google Scholar]
- 24.Krebs L, Topp M, Langhoff-Roos J. The relation of breech presentation at term to cerebral palsy. British journal of obstetrics and gynaecology. 1999;106(9):943–7. Epub 1999/09/24. . [DOI] [PubMed] [Google Scholar]
- 25.Andersen GL, Irgens LM, Skranes J, Salvesen KA, Meberg A, Vik T. Is breech presentation a risk factor for cerebral palsy? A Norwegian birth cohort study. Developmental medicine and child neurology. 2009;51(11):860–5. Epub 2009/05/28. . [DOI] [PubMed] [Google Scholar]
- 26.Eddleman KA, Lockwood CJ, Berkowitz GS, Lapinski RH, Berkowitz RL. Clinical significance and sonographic diagnosis of velamentous umbilical cord insertion. American journal of perinatology. 1992;9(2):123–6. . [DOI] [PubMed] [Google Scholar]
- 27.Heinonen S, Ryynanen M, Kirkinen P, Saarikoski S. Perinatal diagnostic evaluation of velamentous umbilical cord insertion: clinical, Doppler, and ultrasonic findings. Obstetrics and gynecology. 1996;87(1):112–7. . [DOI] [PubMed] [Google Scholar]
- 28.Sameshima H, Ikenoue T, Ikeda T, Kamitomo M, Ibara S. Unselected low-risk pregnancies and the effect of continuous intrapartum fetal heart rate monitoring on umbilical blood gases and cerebral palsy. American journal of obstetrics and gynecology. 2004;190(1):118–23. Epub 2004/01/30. 10.1016/j.ajog.2003.07.014 . [DOI] [PubMed] [Google Scholar]
- 29.Hasegawa J, Sekizawa A, Ikeda T, Koresawa M, Ishiwata I, Kawabata M, et al. Clinical risk factors for poor neonatal outcomes in umbilical cord prolapse. J Matern Fetal Neonatal Med. 2015:1–24. Epub 2015/07/03. 10.3109/14767058.2015.1058772 . [DOI] [PubMed] [Google Scholar]
- 30.Hasegawa J, Sekizawa A, Ikeda T, Koresawa M, Ishiwata I, Kawabata M, et al. Clinical risk factors for poor neonatal outcomes in umbilical cord prolapse. J Matern Fetal Neonatal Med. in press. [DOI] [PubMed] [Google Scholar]
- 31.Hasegawa J, Sekizawa A, Ishiwata I, Ikeda T, Kinoshita K. Uterine rupture after the uterine fundal pressure maneuver. Journal of perinatal medicine. Ahead of print. [DOI] [PubMed] [Google Scholar]
- 32.MacLennan AH, Spencer MK. Projections of Australian obstetricians ceasing practice and the reasons. The Medical journal of Australia. 2002;176(9):425–8. Epub 2002/06/12. . [DOI] [PubMed] [Google Scholar]
- 33.Yang YT, Mello MM, Subramanian SV, Studdert DM. Relationship between malpractice litigation pressure and rates of cesarean section and vaginal birth after cesarean section. Medical care. 2009;47(2):234–42. Epub 2009/01/27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Streja E, Miller JE, Wu C, Bech BH, Pedersen LH, Schendel DE, et al. Disproportionate fetal growth and the risk for congenital cerebral palsy in singleton births. PloS one. 2015;10(5):e0126743 Epub 2015/05/15. 10.1371/journal.pone.0126743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jacobsson B, Hagberg G. Antenatal risk factors for cerebral palsy. Best practice & research. 2004;18(3):425–36. Epub 2004/06/09. 10.1016/j.bpobgyn.2004.02.011 . [DOI] [PubMed] [Google Scholar]
- 36.McIntyre S, Blair E, Badawi N, Keogh J, Nelson KB. Antecedents of cerebral palsy and perinatal death in term and late preterm singletons. Obstetrics and gynecology. 2013;122(4):869–77. Epub 2013/10/03. . [DOI] [PubMed] [Google Scholar]
- 37.Nelson KB, Blair E. Prenatal Factors in Singletons with Cerebral Palsy Born at or near Term. The New England journal of medicine. 2015;373(10):946–53. Epub 2015/09/04. 10.1056/NEJMra1505261 . [DOI] [PubMed] [Google Scholar]
- 38.Redline RW. Clinical and pathological umbilical cord abnormalities in fetal thrombotic vasculopathy. Hum Pathol. 2004;35(12):1494–8. . [DOI] [PubMed] [Google Scholar]
- 39.Oyelese Y, Ananth CV. Placental abruption. Obstetrics and gynecology. 2006;108(4):1005–16. Epub 2006/10/03. . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
Data Availability Statement
Ethical and legal restrictions imposed by the Japan Council for Quality Health Care (JCQHC) prevent the sharing of patient data used in this study. Interested researchers may request access to the data in the the Japan Society of Obstetrics and Gynecology database from (nissanfu@jsog.or.jp) or through Dr. Tsuyomu Ikenoue (tsuyomu_ikenoue@med.miyazaki-u.ac.jp).