Abstract
Purpose
Activation of the hypothalamic–pituitary–adrenal axis, assessed in terms of cortisol levels, may enhance the ability of HIV to infect lymphocytes and downregulate the immune system, accelerating disease progression. This study sought to determine the effects of relaxation techniques on cortisol levels in HIV-sero-positive women.
Methods
Women (n = 150) were randomized to a group cognitive–behavioral stress management (CBSM) condition or an individual information condition and underwent 3 types of relaxation training (progressive muscle relaxation, imagery, and autogenic training). Cortisol levels were obtained pre- and postrelaxation.
Results
Guided imagery was effective in reducing cortisol in the group condition (t = 3.90, P < .001), and muscle relaxation reduced cortisol in the individual condition (t = 3.11, P = .012). Among participants in the group condition attending all sessions, the magnitude of pre- to postsession reduction became greater over time.
Conclusions
Results suggest that specific relaxation techniques may be partially responsible for cortisol decreases associated with relaxation and CBSM.
Keywords: HIV, women, relaxation, cortisol, stress
Introduction
Stress is the result of a state of real or perceived threat to homeostasis. Maintenance of homeostasis in the presence of stressors requires activation of a complex range of responses involving the endocrine, nervous, and immune systems, which are collectively referred to as the stress response.1-3 The principal effector of the stress response lies in the hypothalamic–pituitary–adrenal (HPA) axis. The activity of HPA axis is initiated by pulsatile secretion of corticotrophin-releasing hormone (CRH) from the hypothalamus. The CRH stimulates secretion of adrenocorticotropin hormone (ACTH) from the anterior pituitary. The ACTH acts as a stimulus to the zona fasciculata of the adrenal cortex to produce glucocorticoids (cortisol in humans). Glucocorticoids represent the downstream effectors of the HPA axis and regulate biological changes.4,5 Activation of the HPA axis, commonly assessed in terms of cortisol levels, may contribute to the development of pathologies4,6 and has been recognized as one of the major pathways linking psychological stress to negative systemic outcomes in human beings.7
Many individuals living with HIV infection are confronted with chronic psychosocial stressors8 (eg, stigma, illness) that may lead to chronic cortisol dysregulation and HPA axis activation. Psychosocial factors, for example, anxiety, have been associated with accelerated disease progression among individuals with HIV-1 infection,9 including those on antiretroviral therapy and when controlling for rates of adherence.10 Stress hormones, particularly cortisol, may be a link between stress and HIV-related health outcomes.11,12 Cortisol is capable of enhancing the ability of HIV to infect human lymphocytes and can downregulate the immune system, thereby accelerating the progression of HIV.9,13,14 Several studies have demonstrated abnormalities of the HPA axis in HIV-1 infection15-18 and basal cortisol levels have been inversely related to CD4 counts among HIV-infected men.19
Cognitive–behavioral stress management (CBSM) interventions among HIV-sero-positive men and women have been found to reduce distress,20-25 improve quality of life,26 decrease viral load associated with disease progression,27 improve immune functioning,28 and decrease free testosterone29 and cortisol23 in men. As CBSM interventions typically consist of several components, that is, cognitive–behavioral skill training, coping and problem-solving strategies and relaxation training, the specific mechanism by which these outcomes are achieved is unclear. This substudy of a CBSM intervention found to reduce distress and viral load in women living with HIV20,21,30 examines the specific effects of 3 types of relaxation techniques used in the intervention on cortisol levels in HIV-sero-positive women. It was hypothesized that all 3 relaxation techniques, when administered in a facilitator-led group setting, would significantly reduce stress as measured by a reduction in cortisol.
Materials and Methods
Institutional Review Board (IRB) clearance was obtained from the University of Miami Miller School of Medicine prior to study onset. HIV-sero-positive women (n = 328) aged 18 and above were recruited from hospital outpatient clinics, participant referral and community health centers/agencies in Miami-Dade County in South Florida, to participate in the Stress Management And Relaxation Training/Expressive-Supportive Therapy (SMART/EST) Women’s Project. Following provision of informed consent in English, Spanish, or Haitian-Creole, participants were administered baseline assessments. Women were randomized into 1 of the 2 conditions: (1) a CBSM+ group or (2) an individual video presentation. Conditions were time and information equivalent. Data presented in this article represent women (n = 150) drawn from the SMART/EST project who agreed to participate in a substudy examining the effects of the relaxation component of the intervention on cortisol levels.
Treatment Conditions
The CBSM+ group intervention condition consisted of 10 weekly 120-minute sessions (90-minute stress management and 30-minute relaxation components). Intervention sessions included didactic components explaining the physiological effects of stress, cognitive–behavioral interpretation of stress and emotions, identification of cognitive distortions and automatic thoughts, rational thought replacement, coping skills training, cognitive reframing, assertiveness training, anger management, identification of social support, and relaxation.30
The individual condition consisted of videotapes presenting the same relaxation strategies, in combination with videotapes on strategies for stress management and coping with HIV/AIDS.
Relaxation Training
Three widely used relaxation techniques were introduced: sessions 1 to 3, progressive muscle relaxation (PMR)31; sessions 4 to 6, diaphragmatic breathing and guided imagery32; sessions 7 to 9, diaphragmatic breathing and autogenic training33; session 10, participants’ engaged in their own preferred technique. All participants were asked to practice their relaxation skills at home, between sessions.
Assessments
Participants provided demographic information including age, ethnicity, employment status and language. Additionally, participants provided information about their HIV status, including mode of infection, time since HIV diagnosis, and antiretroviral (ARV) medication prescription status.
Salivary Cortisol Assay
Protocol
Saliva samples were collected during sessions 1, 4, 7 and 10 of the group and individual intervention. Sessions were conducted between 10 am and 2 pm; times of collection were equivalent across study conditions. Saliva was collected preand postrelaxation component of the sessions. In order to minimize the impact of the collection procedures on the cortisol end point, saliva samples, rather than blood samples, were collected. In the group condition, saliva samples were collected by the group therapists just before the relaxation exercise and immediately after the relaxation exercise was completed. Saliva samples were collected from participants in the individual condition by research staff just before viewing the relaxation videotape and at the end of the 30-minute videotape.
Assay
Salivary samples were frozen at –20°C until assayed; cortisol was assayed using radioimmunoassay (RIA) method. Frozen samples were thawed and centrifuged to remove the insoluble mucin. The supernatant was used to carry out the assay for free saliva cortisol using an RIA method (kits were obtained from DSL, Webster, Texas). 50 μL of appropriately diluted standards, control, or sample were pipetted at the bottom of glass tubes. Immediately, 500 μL of the cortisol was added to each tube, vortexed, and incubated for 3 hours at room temperature and covered with parafilm. Tubes were centrifuged, decanted, and radioactivity was counted in a gamma counter. Sensitivity of this method is 0.01ug/dL; the intra- and intercoefficient of variance was 6% to 9%. A log transformation was applied to cortisol levels to stabilize the variance, and values are presented as log μg/dL.
Statistical Analyses
This study utilized univariate analyses (frequencies, means, standard deviations) to examine demographic characteristics. Pearson r correlations were used to assess nonindependence in cortisol levels over time. Paired sample t tests were used to compare the effects of each relaxation strategy and condition independently, utilizing false discovery rate (experiment-wide α = .05) to adjust for multiple comparisons. For longitudinal analyses, mixed modeling was used, with repeated pre- and postsession cortisol measurements nested within individuals. Longitudinal mixed models included main effects of condition and time as well as interactions. To assess the impact of ARV medication, main effects of ARV drug status and interactions were included. If any significant main or interaction effects were detected, pairwise comparisons were conducted to examine the differences at each session using Bonferronicorrected significance levels.
Due to the limited number of participants attending all 4 sessions, Mann-Kendal τ tests were utilized to analyze the dose–response relationship. The Mann-Kendal τ test is a special case of Kendall’s nonparametric rank correlation in which the variable of interest is a time series. Following this analysis, sessions were compared using the Wilcoxin Signed Rank test for related samples to detect significant changes within groups. Statistical analyses were performed using SPSS v20 and SAS v9.3 at a 2-tailed level of significance of P = .05.
Results
Participants had been diagnosed with HIV an average of 8 ± 5 years and were an average of 41 ± 8 years of age. In all 73% reported being infected with HIV through sexual contact, 8% were infected through drug use, and 19% were unsure of the source of their infection. They were primarily African American (83%), 6% were Hispanic, 5% were white non-Hispanic, and the reminder self-classified as Haitian or other. Most participants were unemployed (84%). Nearly half (46%) of participants reported completing less than 12 years of education. No differences between group and individual conditions were observed in demographic variables. There were no associations between cortisol levels and ethnicity, language, age, or time since diagnosis. Presession cortisol levels were correlated across time points; Pearson r correlation statistics and associated significance levels are presented in Table 1.
Table 1.
Pearson Correlations of Presession Cortisol Levels across Time Points.
P < .01.
In order to examine the effects of each relaxation strategy and condition separately, sessions were analyzed independently (session 1, PMR; session 4, diaphragmatic breathing and guided imagery; session 7 diaphragmatic breathing and autogenic training; and session 10, participants utilized their most preferred relaxation technique). For each session, differences in cortisol levels pre- and postsession were assessed within each group. The group condition showed a decrease in cortisol levels in session 4 (t = 3.86, P < .001) and a trend toward decrease in session 10 (t = 2.18, P = .088). Within the individual condition, there was a significant decrease in cortisol levels at session 1 (t = 3.11, P = .012; see Table 2).
Table 2.
Cortisol Level (log μg/dL) Pre- and Postsession by Condition.
| Session | Within-Group Condition
|
Within-Individual Condition
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Mean | Standard Deviation | t | df | Pa | N | Mean | Standard Deviation | t | df | Pa | |
| 1 (Pre) | 45 | −.67 | .36 | 0.33 | 44 | .774 | 42 | −.41 | .44 | 3.11 | 41 | .012 |
| 1 (Post) | −.69 | .40 | −.57 | .51 | ||||||||
| 4 (Pre) | 57 | −.53 | .27 | 3.86 | 56 | < .001 | 26 | −.53 | .47 | 0.29 | 25 | .774 |
| 4 (Post) | −.67 | .32 | −.56 | .43 | ||||||||
| 7 (Pre) | 53 | −.64 | .37 | 1.88 | 52 | .132 | 26 | −.54 | .31 | 0.76 | 25 | .725 |
| 7 (Post) | −.70 | .32 | −.57 | .26 | ||||||||
| 10 (Pre) | 61 | −.52 | .37 | 2.18 | 60 | .088 | 37 | −.51 | .41 | 0.46 | 36 | .774 |
| 10 (Post) | −.58 | .34 | −.52 | .40 | ||||||||
Note: Boldface indicates P < .05.
P values were adjusted for multiple comparisons using false discovery rate, experimentwide α = .05.
To evaluate the longitudinal effects of the relaxation strategies on cortisol levels by condition, mean cortisol over time was examined using mixed modeling. Two analyses were conducted, the first comparing presession cortisol levels between conditions over time and the second comparing postsession levels. For presession levels, there was no significant main effect of condition (F1,140 = .95, P = .33) or time (F3,206 = .92, P = .43), but there was a significant time by condition interaction (F3,206 = 2.65, P = .050). Using a Bonferonni correction to control type 1 error (corrected α = .0125), examination of mean differences between conditions at each time point revealed a difference only at baseline (F1,206 = 6.66, P = .01). Longitudinal examination of postsession levels did not reveal any significant main effects for condition (F1,139 = 2.43, P = .12), time (F3,207 = 46, P = .23) or a significant interaction effect (F3,207= .26, P = .85). Thus, neither the group nor individual condition demonstrated longitudinal changes in pre- or postsession cortisol levels. Figure 1 displays mean cortisol at each time point.
Figure 1.
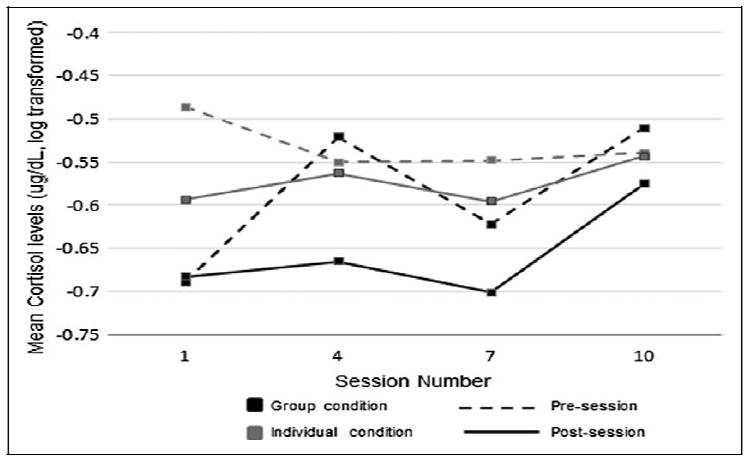
Mean pre- and postsession cortisol levels by condition over time.
To account for the potential differences between participants on ARV drugs and those not on ARV drugs, ARV status was added to models with time and condition. Significant main effects of ARV status were detected in both presession (F1,138 = 2.63, P < .001) and postsession (F1,137 = 17.3, P < .001) cortisol levels, but there was no interaction with condition or time. Using a Bonferonni adjustment (α = .0125), time points were examined individually. Presession cortisol levels were higher in those on ARV medication at sessions 2 and 3 (F1,200 = 07, P = .001 and F = 7.41, P = .01), and postsession levels were higher at sessions 1 and 2 (F1,201 = 18.63, P < .001 and F = 8.31, P = .004).
In order to assess the dose–response of relaxation training sessions on change in cortisol, cortisol change from pre- to postsession was compared over time. Only those participants who attended all sessions (ie, 1, 4, 7, and 10) were examined (n = 26 group condition and n = 9 individual condition). There were no differences in any demographic characteristics or prerelaxation cortisol levels between this participant subset and those not included. Due to the low number of participants attending all sessions, testing of dose–response was conducted using nonparametric tests (ie, Mann-Kendall trend test and Wilcoxon Signed Rank test) on nontransformed cortisol differences.
Evidence of a monotonic trend over time was detected in group condition participants (Mann-Kendall τ = −1, P = .089) but not in individual condition participants (Mann-Kendal τ = −.816, P = .245), although small sample size precluded definitive statistical conclusions. As suggested by the value of the test statistic in the group condition (τ = −1), the reduction in cortisol level from pre- to postsession increases at each time point. Comparisons between time points using the Wilcoxon Signed Rank test revealed a significant difference between the reduction in cortisol from session 1 to session 4 in the group condition (P = .05) but no other significant changes. Figure 2 presents median cortisol changes at each session for each condition.
Figure 2.
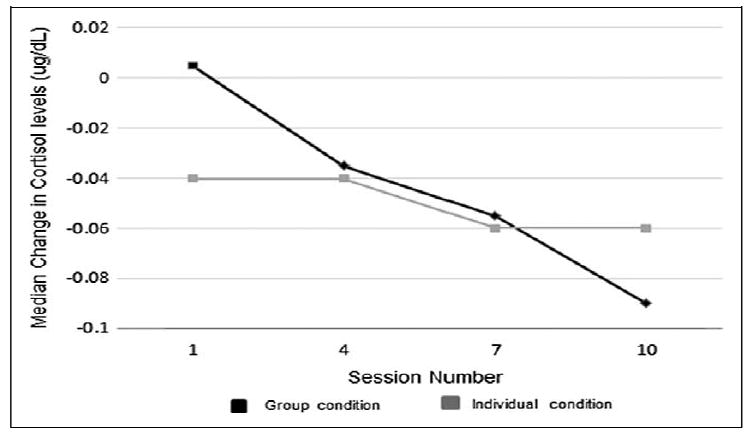
Median change in cortisol levels from pre- to postsession over time.
Discussion
This study sought to determine the specific effects of 3 types of facilitator-administered relaxation techniques contained within a CBSM intervention, that is, PMR, guided imagery with diaphragmatic breathing and autogenic training with diaphragmatic breathing, and participants’ most preferred technique, on cortisol levels in HIV-sero-positive women. While it was hypothesized that all 3 relaxation techniques would significantly reduce cortisol, guided imagery with diaphragmatic breathing was the most effective in cortisol reduction in the group intervention condition. PMR was the most effective strategy in the individual condition, while having no effect on the intervention condition. These findings suggest that specific techniques and delivery methods may be more effective in decreasing cortisol.
Longitudinally, participants’ pre- or postrelaxation cortisol levels did not decrease in either condition. This finding contrasts with an earlier study34 utilizing relaxation in a group intervention with HIV-sero-positive gay men in which presession cortisol levels decreased over a 10-week CBSM intervention. Failure to reduce longitudinal cortisol levels suggests cortisol levels may reflect situational stressors, and it may be difficult to maintain reductions achieved in sessions. This finding implies that there may be a need to maintain a regular pattern of relaxation practice, as session effects may not persist over time in the absence of regular practice. However, no data were collected with regard to relaxation practice outside the formal sessions. In addition, this series of sessions may also have been insufficient to demonstrate a cumulative effect.
Although it is well established that ARV drugs have been instrumental in significantly reducing morbidity and mortality associated with HIV/AIDS, biological side-effects from these powerful agents have been associated with increased risk for other metabolic and cardiovascular risk factors. Consistent with other reasearch,35 our study found that cortisol levels were higher among participants on ARV drugs as compared to those not on ARV drugs, suggesting that one of the mechanisms associated with these risk factors may involve neurohormones such as cortisol. Cortisol is released from the adrenal glands in response to high stress levels and can significantly affect neuroplasticity.36 The hippocampus is particularly sensitive to the acute and chronic effects of circulating cortisol and these effects can be alleviated if the levels of cortisol are reduced by any intervention. It is also well established that cortisol administration can lead to stress induction. However these studies may not be generalized to naturalistic situations with elevated cortisol levels.37 The obtained findings suggest that not all interventions may reduce cortisol levels, as guided imagery and PMR were found to be more efficacious. As noted earlier, the use of relaxation and associated concomitant reductions in cortisol are linked with the central nervous system. The exact mechanism for this effect is unclear. In order to investigate additional mechanisms involved in cortisol reduction, further studies are needed since cortisol can bring about its effects both via genomic and via nongenomic mechanisms.
Dose–response analyses indicated that for those group condition participants attending all of the sessions, the magnitude of cortisol reduction became greater over time, suggesting that a learning effect may have occurred over the course of the intervention. However, the very limited sample of participants attending all sessions, especially in the individual condition, precluded more powerful statistical testing and group comparisons. Extended longitudinal studies would be necessary to confirm whether or not “biobehavioral” learning may occur with repeated practice sessions. Future studies are needed to determine whether extended practice would have more distal, long-lasting effects that could conceivably impact other health indicators for persons living with HIV/AIDS.
There were several limitations to this substudy, including small sample size, duration of relaxation practice, limited follow-up and time-of-day variations in the sampling protocol, and a lack of data regarding relaxation practice outside of study sessions. In addition, the participant’s menstrual cycle in relation to the saliva sample was not recorded, which may have impacted cortisol levels. Finally, the introduction of relaxation types was not counterbalanced; future studies should apply counterbalancing to reduce the potential for a position effect.
Conclusion
The CBSM interventions typically consist of several components, that is, cognitive–behavioral skill training, coping and problem-solving strategies, and relaxation training, yet the mechanisms underlying the salutary effects of CBSM interventions are unclear. Although not maintained over time, the effects of the cortisol reductions obtained in this study were pronounced and proximal to the relaxation session. Due to its association with disruptions in sleep, overall mood, and quality of life, relaxation practices to reduce cortisol in this population remain especially valuable in this patient population. For women living with HIV/AIDS, practicing relaxation on a daily basis may have salutary health effects through reducing levels of cortisol with its putative negative effects on an already compromised immune system.
Acknowledgments
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by a grant from the National Institutes of Health, R01MH55463.
Footnotes
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Dickerson PA, Lally BE, Gunnel E, Birkle DL, Salm AK. Early emergence of increased fearful behavior in prenatally stressed rats. Physiol Behav. 2005;86(5):586–593. doi: 10.1016/j.physbeh.2005.08.025. [DOI] [PubMed] [Google Scholar]
- 2.Chrousos GP, Gold PW. The concepts of stress and stress system disorders. Overview of physical and behavioral homeostasis. JAMA. 1992;267(9):1244–1252. [PubMed] [Google Scholar]
- 3.Carrasco GA, Van de Kar LD. Neuroendocrine pharmacology of stress. Eur J Pharmacol. 2003;463(1-3):235–272. doi: 10.1016/s0014-2999(03)01285-8. [DOI] [PubMed] [Google Scholar]
- 4.Munck A, Guyre PM, Holbrook NJ. Physiological functions of glucocorticoids in stress and their relation to pharmacological actions. Endocr Rev. 1984;5(1):25–44. doi: 10.1210/edrv-5-1-25. [DOI] [PubMed] [Google Scholar]
- 5.Bamberger CM, Schulte HM, Chrousos GP. Molecular determinants of glucocorticoid receptor function and tissue sensitivity to glucocorticoids. Endocr Rev. 1996;17(3):245–261. doi: 10.1210/edrv-17-3-245. [DOI] [PubMed] [Google Scholar]
- 6.McEwen BS, Stellar E. Stress and the individual. Mechanisms leading to disease. Arch Intern Med. 1993;153(18):2093–2101. [PubMed] [Google Scholar]
- 7.McEwen BS. Protective and damaging effects of stress mediators. N Engl J Med. 1998;338(3):171–179. doi: 10.1056/NEJM199801153380307. [DOI] [PubMed] [Google Scholar]
- 8.McIntosh RC, Rosselli M. Stress and coping in women living with HIV: a meta-analytic review. AIDS Behav. 2012;16(8):2144–2159. doi: 10.1007/s10461-012-0166-5. [DOI] [PubMed] [Google Scholar]
- 9.Chida Y, Vedhara K. Adverse psychosocial factors predict poorer prognosis in HIV disease: a meta-analytic review of prospective investigations. Brain Behav Immun. 2009;23(4):434–445. doi: 10.1016/j.bbi.2009.01.013. [DOI] [PubMed] [Google Scholar]
- 10.Remor E, Penedo FJ, Shen BJ, Shneiderman N. Perceived stress is associated with CD4+ cell decline in men and women living with HIV/AIDS in Spain. AIDS Care. 2007;19(2):215–219. doi: 10.1080/09540120600645570. [DOI] [PubMed] [Google Scholar]
- 11.Kopnisky KL, Stoff DM, Rausch DM. Workshop report: the effects of psychological variables on the progression of HIV-1 disease. Brain Behav Immun. 2004;18(3):246–261. doi: 10.1016/j.bbi.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 12.Schneiderman N, Ironson G, Siegel SD. Stress and health: psychological, behavioral, and biological determinants. Annu Rev Clin Psychol. 2005;1:607–628. doi: 10.1146/annurev.clinpsy.1.102803.144141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Antoni MH, August S, LaPerriere A, et al. Psychological and neuroendocrine measures related to functional immune changes in anticipation of HIV-1 serostatus notification. Psychosom Med. 1990;52(5):496–510. doi: 10.1097/00006842-199009000-00002. [DOI] [PubMed] [Google Scholar]
- 14.Leserman J, Petitto JM, Gu H, et al. Progression to AIDS, a clinical AIDS condition and mortality: psychosocial and physiological predictors. Psychol Med. 2002;32(6):1059–1073. doi: 10.1017/s0033291702005949. [DOI] [PubMed] [Google Scholar]
- 15.Azar ST, Melby JC. Hypothalamic-pituitary-adrenal function in non-AIDS patients with advanced HIV infection. Am J Med Sci. 1993;305(5):321–325. doi: 10.1097/00000441-199305000-00012. [DOI] [PubMed] [Google Scholar]
- 16.Laudat A, Blum L, Guechot J, et al. Changes in systemic gonadal and adrenal steroids in asymptomatic human immunodeficiency virus-infected men: relationship with the CD4 cell counts. Eur J Endocrinol. 1995;133(4):418–424. doi: 10.1530/eje.0.1330418. [DOI] [PubMed] [Google Scholar]
- 17.Mayo J, Collazos J, Martinez E, Ibarra S. Adrenal function in the human immunodeficiency virus-infected patient. Arch Intern Med. 2002;162(10):1095–1098. doi: 10.1001/archinte.162.10.1095. [DOI] [PubMed] [Google Scholar]
- 18.Kumar M, Kumar AM, Waldrop D, Antoni MH, Schneiderman N, Eisdorfer C. The HPA axis in HIV-1 infection. J Acquir Immune Defic Syndr. 2002;31(suppl 2):S89–S93. doi: 10.1097/00126334-200210012-00010. [DOI] [PubMed] [Google Scholar]
- 19.Lortholary O, Christeff N, Casassus P, et al. Hypothalamo-pituitary-adrenal function in human immunodeficiency virusinfected men. J Clin Endocrinol Metab. 1996;81(2):791–796. doi: 10.1210/jcem.81.2.8636305. [DOI] [PubMed] [Google Scholar]
- 20.LaPerriere A, Ironson G, Antoni H, et al. CBSM+ group intervention decreases depression in moderately depressed women with AIDS: the SMART/EST women’s project. J Health Psych. 2005;10(2):223–231. doi: 10.1177/1359105305049772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jones D, Owens MI, Lydston D, Tobin JN, Brondolo E, Weiss SM. Self efficacy and distress in women with AIDS: The SMART/EST women’s project. AIDS Care. 2010;22(12):1499–1508. doi: 10.1080/09540121.2010.484454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Antoni MH, Pereira DB, Marion I, et al. Stress management effects on perceived stress and cervical neoplasia in low-income HIV-infected women. J Psychosom Res. 2008;65(4):389–401. doi: 10.1016/j.jpsychores.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Antoni MH, Cruess S, Cruess DG, et al. Cognitive-behavioral stress management reduces distress and 24-hour urinary free cortisol output among symptomatic HIV-infected gay men. Ann Behav Med. 2000;22(1):29–37. doi: 10.1007/BF02895165. [DOI] [PubMed] [Google Scholar]
- 24.Berger S, Schad T, von Wyl V, et al. Effects of cognitive behavioral stress management on HIV-1 RNA, CD4 cell counts and psychosocial parameters of HIV-infected persons. AIDS. 2008;22(6):767–775. doi: 10.1097/QAD.0b013e3282f511dc. [DOI] [PubMed] [Google Scholar]
- 25.Carrico AW, Antoni MH, Weaver KE, Lechner SC, Schneiderman N. Cognitive-behavioural stress management with HIV-positive homosexual men: mechanisms of sustained reductions in depressive symptoms. Chronic Illn. 2005;1(3):207–215. doi: 10.1177/17423953050010030401. [DOI] [PubMed] [Google Scholar]
- 26.Lechner S, Antoni M, Lydston D, et al. Cognitive-behavioral interventions improve quality of life in women with AIDS. J Psychosomatic Res. 2003;54(3):253–261. doi: 10.1016/s0022-3999(02)00480-4. [DOI] [PubMed] [Google Scholar]
- 27.Ironson G, O’Cleirigh C, Fletcher MA, et al. Psychosocial factors predict CD4 and viral load change in men and women with human immunodeficiency virus in the era of highly active antiretroviral treatment. Psychosom Med. 2005;67(6):1013–1021. doi: 10.1097/01.psy.0000188569.58998.c8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lutgendorf SK, Antoni MH, Ironson G, et al. Cognitive-behavioral stress management decreases dysphoric mood and herpes simplex virus-type 2 antibody titers in symptomatic HIV-seropositive gay men. J Consult Clin Psychol. 1997;65(1):31–43. doi: 10.1037//0022-006x.65.1.31. [DOI] [PubMed] [Google Scholar]
- 29.Cruess DG, Antoni MH, Schneiderman N, et al. Cognitive-behavioral stress management increases free testosterone and decreases psychological distress in HIV-seropositive men. Health Psychol. 2000;19(1):12–20. doi: 10.1037//0278-6133.19.1.12. [DOI] [PubMed] [Google Scholar]
- 30.Weiss SM, Tobin JN, Antoni M, et al. SMART/EST women’s team. Enhancing the health of women living with HIV: the SMART/EST women’s project. Int J Women’s Health. 2011;2:1–15. doi: 10.2147/IJWH.S5947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jacobson E. Progressive Relaxation. Chicago, IL: University of Chicago Press; 1938. [Google Scholar]
- 32.Leuner H. Guided affective imagery (GAI). A method of intensive psychotherapy. Am J Psychother. 1969;23(1):4–22. doi: 10.1176/appi.psychotherapy.1969.23.1.4. [DOI] [PubMed] [Google Scholar]
- 33.Luthe W, Schultz JH. Autogenic Therapy. Vol. 1. New York, NY: Grune and Stratton, Inc; 1969. [Google Scholar]
- 34.Cruess DG, Antoni MH, Kumar M, Schneiderman N. Reductions in salivary cortisol are associated with mood improvement during relaxation training among HIV-seropositive men. J Behav Med. 2000;23(2):107–122. doi: 10.1023/a:1005419917023. [DOI] [PubMed] [Google Scholar]
- 35.Collazos J, Ibarra S, Loureiro M. Cortisol serum levels and their relationship to certain antiretroviral drugs. Scand J Infect Dis. 2004;36(6-7):480–482. doi: 10.1080/00365540410015231. [DOI] [PubMed] [Google Scholar]
- 36.Lupien SG, McEwen BS. The acute effects of cortical steroids on cognition: integration of animal and human studies. Brain Res. 1997;24(1):1–27. doi: 10.1016/s0165-0173(97)00004-0. [DOI] [PubMed] [Google Scholar]
- 37.Van Peer JM, Spinhoven P, Roelofs K. Psychophysiological evidence for cortisol induced reduction in early bias for implicit social threats in social phobia. Psychoneuroendocrinology. 2010;35(1):21–32. doi: 10.1016/j.psyneuen.2009.09.012. [DOI] [PubMed] [Google Scholar]


