Abstract
There is a high prevalence of behavioral disorders that feature hyperactivity in individuals with severe inner ear dysfunction. What remains unknown is whether inner ear dysfunction can alter the brain to promote pathological behavior. Using molecular and behavioral assessments of mice that carry null or tissue-specific mutations of Slc12a2, we found that inner ear dysfunction causes motor hyperactivity by increasing in the nucleus accumbens the levels of pCREB and pERK, key mediators of neurotransmitter signaling and plasticity. Hyperactivity was remedied by local administration of the pERK inhibitor SL327. These findings reveal that a sensory impairment, such as inner ear dysfunction, can induce specific molecular changes in the brain that cause maladaptive behaviors, such as hyperactivity, that have been traditionally considered exclusively of cerebral origin.
The inner ear contains the cochlea, devoted to hearing, and the vestibular end organs, devoted to balance. In 20-95% of children with severe hearing loss, auditory and vestibular dysfunction co-occur (1, 2). In such cases, there is a high incidence of behavioral disorders that feature hyperactivity as a core diagnostic symptom (3-5). Although socio-environmental variables have been proposed as risk-factors (6), it is unclear whether sensory impairments such as inner ear defects can directly induce specific changes in the brain that lead to maladaptive behavior. In non-human vertebrates including rodents and frogs, surgical or pharmacological lesions to the vestibulo-auditory system are also linked to long-term changes in locomotor activity, although to date the associations between ear dysfunction and behavior remain unexplained (7-9). Genetic mouse models of inner ear dysfunction can exhibit increased levels of locomotor hyperactivity (10), but because the gene is mutated in the brain as well as the inner ear, the causal neural underpinnings of this behavior remain unknown.
Slc12a2 (also known as Nkcc1) is a gene that encodes a sodium-potassium-chloride co-transporter broadly expressed in tissues including the inner ear and central nervous system (CNS) (11, 12). The Slc12a2 mutant mice used in this study arose spontaneously in our mouse colony and exhibit increased levels of motor hyperactivity, including locomotion, circling, and head tossing (Fig. 1A,B, movie S1, fig. S1A), as with previously characterized Slc12a2 mutants (13-16). The spontaneous mutation was identified as an A to T mutation in exon 17 that changes codon 842 of Slc12a2 from encoding a lysine (K) to a stop codon (*) (hereafter referred to as Slc12a2K842*), resulting in no detectable SLC12A2 protein using antibodies raised to either the N- or C-terminus (fig. S1B-E). The inner ear of Slc12a2K842*/K842* mice revealed a collapse of Reissner's membrane and the membranes of the vestibular compartments (fig. S1F). These morphological defects were associated with profound deafness and balance deficits (fig. S1G, S2; ref 15).
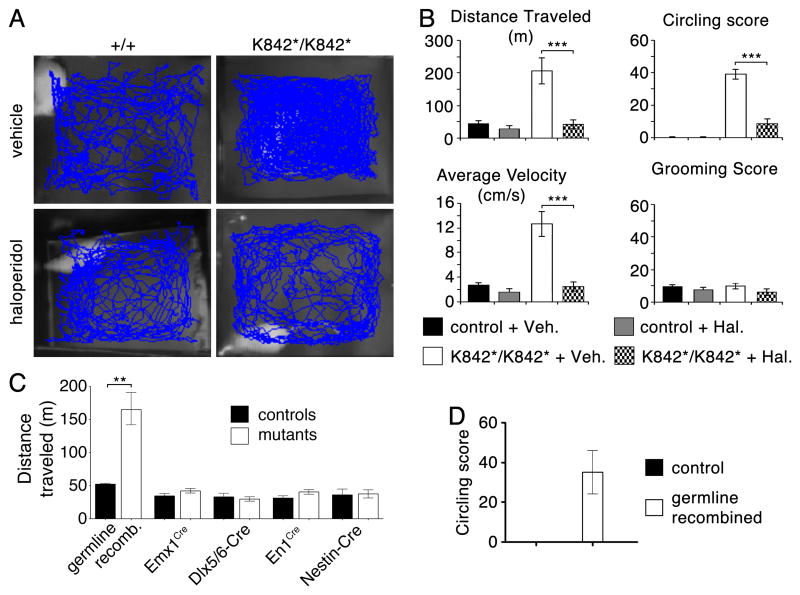
Fig. 1. Slc12a2K842*/K842* mutants display a dopamine receptor-mediated increase in locomotor activity that cannot be explained by disruption of Slc12a2 in the brain.
(A) Traces and (B) quantification of mouse locomotion in an open field showing that haloperidol alleviates locomotor activity and circling in Slc12a2K842*/K842* mice without affecting grooming (*** p < 0.0001; repeated measures ANOVA with Bonferroni post-hoc comparison). (C) Germline recombination of Slc12a2fx/fx mice recapitulates the increased locomotion of the Slc12a2K842*/K842* mutant (p = 0.0032, unpaired two-tailed test). Mice lacking Slc12a2 in the neocortex and hippocampus (Emx1Cre/+;Slc12a2fx/rec), striatum (Dlx5/6-Cre;Slc12a2fx/fx), cerebellum (En1Cre/+;Slc12a2fx/fx), and CNS (Nestin-Cre;Slc12a2fx/fx) display normal levels of motor activity (unpaired two-tailed test). (D) Germline recombination of Slc12a2fx/fx also recapitulates the circling behavior of the Slc12a2K842*/K842* mutants. N = 4-11 mice per genotype. All data are illustrated as mean ± s.e.m.
An increase in locomotor activity is not readily explained by dysfunction of the inner ear but rather points to a disruption of brain functions that regulate movement. Consistent with this notion, the dopaminergic antagonist haloperidol, which acts in the brain to alleviate increased motor behavior in humans (17), normalized the open-field locomotor behavior of Slc12a2K842*/K842* mice (Fig. 1A,B). The same dose of haloperidol did not decrease the locomotor activity in littermate controls, indicating that the dose used was not sedating. Haloperidol did not affect grooming in either mutants or controls, nor did it ameliorate performance of mutants on a rotarod test, which requires an intact inner ear, indicating a specificity in the behaviors modified by haloperidol (Fig. 1B, fig. S2).
To test whether loss of Slc12a2 expression in the brain leads to increased locomotor activity, we deleted a floxed Slc12a2 allele (Slc12a2fx) specifically from individual brain areas that control movement using Emx1Cre for the cortex, Dlx5/6-Cre for the striatum, En1Cre for the cerebellum, and Nestin-Cre for the entire CNS. With these lines, recombination occurs prior to neurogenesis and expression of Slc12a2 (18-21). Western blot analyses confirmed the loss of Slc12a2 specifically in the expected tissues (fig. S3A,C). While mice with a germline deletion of the Slc12a2fx allele, which lack expression in all tissues, are behaviorally indistinguishable from Slc12a2K842*/K842* mice, mice with deletions in the cortex, striatum, cerebellum, or entire CNS are behaviorally normal (Fig. 1C,D, fig. S3B), indicating that loss of Slc12a2 from any single or combination of brain areas does not cause the behavior. Notably, inner ear morphology and Slc12a2 expression were normal in Nestin-Cre;Slc12a2fx/fx mutants (fig. S3C).
To determine whether loss of Slc12a2 expression in the inner ear causes the behavioral phenotype, the Foxg1Cre line was used to delete Slc12a2 from the embryonic precursors of the inner ear (Fig. 2A). However, because Foxg1Cre also targets the telencephalon (neocortex, hippocampus, and striatum; Fig. 2B), Slc12a2 was deleted from the inner ear using a combination of Pax2-Cre and Tbx1Cre, which together sufficiently deleted Slc12a2 to result in the anatomical ear defects indicative of dysfunction (fig. S4). In Pax2-Cre;Tbx1Cre-driven mutants, Slc12a2 deletion also occurred in the mid-hindbrain area, but not the telencephalon. Both the Pax2-Cre;Tbx1Cre and Foxg1Cre-driven mutants recapitulated all the hyperactive features of Slc12a2K842*/K842* mice (Fig. 2C, movie S2, fig. S4). Together with the absence of the behavioral phenotype when Slc12a2 is deleted from the entire CNS, these results demonstrate that inner ear dysfunction caused the abnormal behavior of these mice.
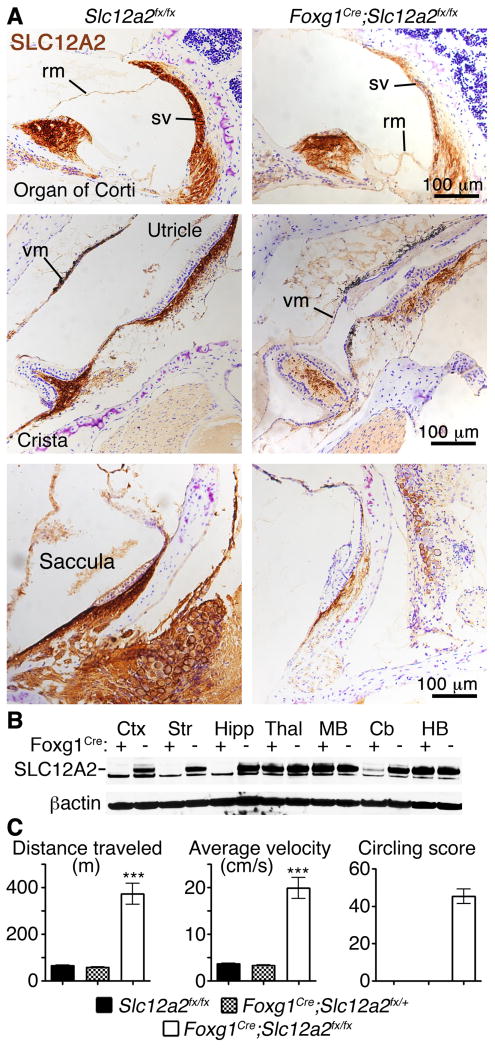
Fig. 2. Increased locomotor activity of Slc12a2 mutants depends on inner ear dysfunction.
(A) The inner ear defects of Foxg1Cre/+;Slc12a2fx/fx mice are identical to those of the Slc12a2K842*/K842* mice: vestibular (vm) and Reissner's (rm) membrane collapse and degeneration of the vestibular end organs (sacculae, utricles, and cristae). In Foxg1Cre/+;Slc12a2fx/fx mice, SLC12A2 immunostaining (brown) is reduced in the endolymph secreting stria vascularis (sv) of the cochlea and in the vestibular end organs. Purple: Nissl counterstain. (B) Western Blot analysis shows loss of SLC12A2 in the neocortex (Ctx), hippocampus (Hipp), and striatum (Str) in Foxg1Cre/+;Slc12a2fx/fx mice, but not in the thalamus (Thal), midbrain (MB), cerebellum (Cb), or hindbrain (HB). (C) Foxg1Cre/+;Slc12a2fx/fx mice recapitulate the abnormal behavior of Slc12a2K842*/K842* mice (n = 7-9 mice per genotype, *** p < 0.0001, unpaired two-tailed test, mean ± s.e.m.).
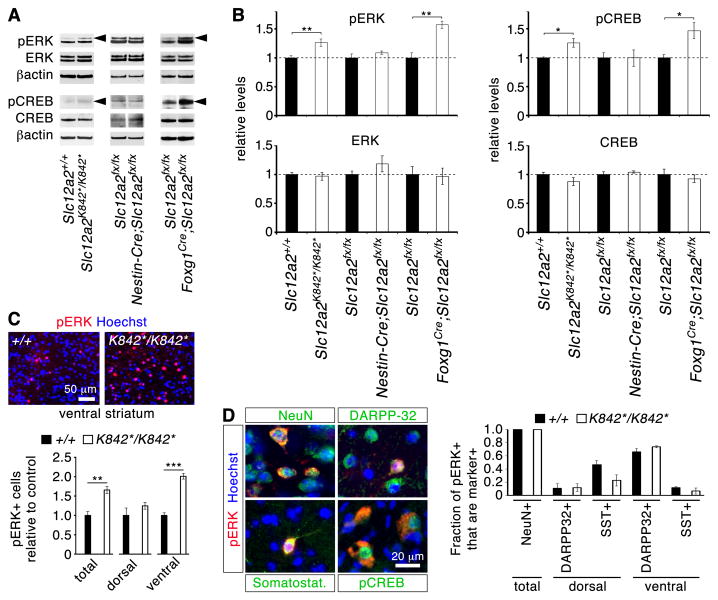
Because increased locomotor activity and a responsiveness to haloperidol are indicative of brain rather than ear dysfunction, we reasoned that inner ear defects may cause abnormal functioning of the striatum, a central brain area regulating motor output levels. Striatal levels of 26 candidate proteins involved in neurotransmitter signaling were examined by Western blot (Fig. 3A,B; fig. S5). Initially, Slc12a2K842*/K842* mutants were examined and proteins showing significant differences in expression levels compared with controls were then also examined in Nestin-Cre;Slc12a2fx/fx and Foxg1Cre/+;Slc12a2fx/fx striatums to identify the relevant changes that correlate with the presence of the ear defects and behavioral phenotype. Amongst the proteins examined, significant increases were observed only in the levels of phosphorylated extracellular signal-regulated kinase (pERK), a key component of dopamine and glutamate neurotransmission in the striatum, and its common downstream target phospho-cAMP response-element binding protein (pCREB-Ser133; refs. 22-24) (Fig. 3A,B). Although phosphorylated forms were elevated, total ERK and CREB were unaffected. Increased pERK and pCREB were specific to the striatum and not observed in other forebrain regions (fig. S5D). Immunohistochemical analysis of Slc12a2K842*/K842* mutants revealed that the number of pERK+ cells was upregulated specifically in the nucleus accumbens, the ventral part of the striatum (Fig. 3C). All pERK+ cells were NeuN+, indicating that they were neurons. Of these, most were DARPP32+ medium-sized spiny neurons (MSNs) and a few were somatostatin+ interneurons (Fig. 3D), but not calretinin+, parvalbumin+, or ChAT+ interneurons. The proportions of pERK+ cell types in the mutants were nevertheless similar to controls. In addition, pCREB-positive cells were pERK-positive (Fig. 3D).
Fig. 3. Inner ear dysfunction contributes to the up-regulation of pERK1 in striatal neurons.
(A) Western blot analyses reveal increased (arrowheads) pERK1 (upper band of doublet) and its common target pCREB in mice with inner ear defects and increased motor activity (Slc12a2K842*/K842* and Foxg1Cre/+;Slc12a2fx/fx mice), but not in phenotypically normal mice. (B) Quantification of Western analyses (pERK: p = 0.009 for Slc12a2K842*/K842*; p = 0.006 for Foxg1Cre/+;Slc12a2fx/fx; pCREB: p = 0.028 for Slc12a2K842*/K842*; p = 0.033 for Foxg1Cre/+;Slc12a2fx/fx; n = 3-6 mice per genotype; unpaired two-tailed t-test). (C) Immunohistochemical analysis showed an increase in the number of pERK+ cells in the ventral striatum of mutants (n = 4 mice per genotype, ** p = 0.0066, *** p = 0.00046, unpaired two-tailed t-test). (D) In both controls and mutants, pERK+ cells in the ventral striatum are primarily DARPP32+, whereas in the dorsal striatum they are somatostatin+ (SST+) interneurons (n = 3 per genotype) (mean ± s.e.m.).
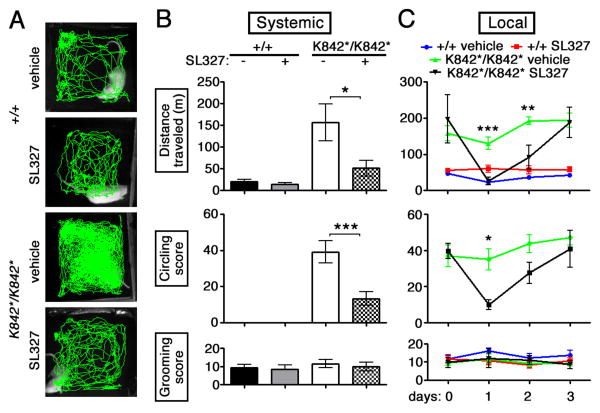
Robust increases in pERK occur in MSNs of the nucleus accumbens in response to psychostimulants and other drugs of abuse and are considered critical for enabling their long-lasting behavioral changes (22 ,25, 26). The induction of such behaviors is inhibited by the local or systemic application of MEK or ERK kinase inhibitors such as SL327 (22, 27-29). To determine whether striatal ERK phosphorylation was necessary for the abnormal increase in locomotor activity, Slc12a2K842*/K842* mice were given an intraperitoneal injection or a local injection of SL327 to the nucleus accumbens. In both sets of experiments, SL327 administration restored locomotor activity to normal levels without affecting the levels of activity in controls (Fig. 4). Mutant mice treated with local SL327 returned to their baseline, pre-surgery locomotor levels of activity by day 3, suggesting that the injection did not cause permanent damage. SL327 administration did not affect grooming, suggesting that increased striatal pERK selectively elevates locomotor activity levels and not general activity.
Fig. 4. The increased striatal pERK levels induced by inner ear defects promote increased locomotor activity.
(A) Traces and (B) quantification of motor activity in an open-field one hour after systemic SL327 administration, which ameliorates both locomotion and circling in the Slc12a2K842*/K842* mutants while having little effect on grooming (n = 6 mice per genotype, * p = 0.015, *** p = 0.0002). (C) Local SL327 administration to the nucleus accumbens of Slc12a2K842*/K842* mice restored open-field activity and circling to control levels, which were unaffected by treatment, for 2-days post-injection, without affecting grooming (n = 5-7 mice per genotype and per treatment condition, *** p = 0.0025, ** p = 0.0082, *** p = 0.018; mean± s.e.m; two-way repeated measures ANOVA with Bonferroni post-hoc comparison).
This study demonstrates that inner ear dysfunction can induce molecular changes in the striatum that promote increased motor hyperactivity. The neural circuits linking inner ear defects to abnormal striatal function are likely transmitted by the normal auditory and vestibular input pathways, primarily via the thalamus and neocortex (30), but this remains to be demonstrated. Our results also suggest that a neurobiological cause, rather than simply socio-environmental factors, contributes to the high incidence of behavioral disorders associated with inner ear dysfunction in children and adolescents. Moreover, disruption of the ERK pathway in the striatum provides a potential target for intervention. Finally, it is intriguing to ponder whether sensory impairments other than those associated with inner ear defects could also cause or contribute to psychiatric or motor disorders that have traditionally been considered exclusively of cerebral origin.
Supplementary Material
Acknowledgments
We thank Kamran Khodakhah and Paola Calderon for support with surgical approaches, rotarod, and early inputs to the project, Gordon Fishell for the Nestin-CreER mice in which Slc12a2K842* arose, Suzanne Zukin for Western blotting, Christoph Heinze for Slc12a2fx genotyping, UC Davis/NIH NeuroMab for antibodies and the Developmental Studies Hybridoma Bank for the T4 antibody developed by Lytle/ Forbush. This work was supported by the Tourette Syndrome Association (JH and Kamran Khodakhah), NIH R21NS073761 and R01MH083804 (JH), and DFG (CAH; HU 800/5-1).
References and Notes
- 1.Selz PA, Girardi M, Konrad HR, Hughes LF. Otolaryngol Head and Neck Surg. 1996;115:70–77. doi: 10.1016/S0194-5998(96)70139-0. [DOI] [PubMed] [Google Scholar]
- 2.Cushing SL, Papsin BC, Rutka JA, James AL, Gordon KA. Laryngoscope. 2008;118:1814–1823. doi: 10.1097/MLG.0b013e31817fadfa. [DOI] [PubMed] [Google Scholar]
- 3.Vostanis P, Hayes M, du Feu M, Warren J. Child Care Health Dev. 1997;23:233–246. doi: 10.1111/j.1365-2214.1997.tb00966.x. [DOI] [PubMed] [Google Scholar]
- 4.Hindley P, Kroll L. J Deaf Stud Deaf Educ. 1998;3:64–72. doi: 10.1093/oxfordjournals.deafed.a014341. [DOI] [PubMed] [Google Scholar]
- 5.van Eldik T, Treffers PD, Veerman JW, Verhulst FC. Am Ann Deaf. 2004;148:390–395. doi: 10.1353/aad.2004.0002. [DOI] [PubMed] [Google Scholar]
- 6.van Gent T, Goedhart AW, Hindley PA, Treffers PD. J Child Psychol Psychiatry. 2007;48:950–958. doi: 10.1111/j.1469-7610.2007.01775.x. [DOI] [PubMed] [Google Scholar]
- 7.Stiles L, Zheng Y, Darlington CL, Smith PF. Behav Brain Res. 2012;227:150–158. doi: 10.1016/j.bbr.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 8.Schirmer M, et al. Brain Res. 2007;1155:179–195. doi: 10.1016/j.brainres.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 9.Lambert FM, Combes D, Simmers J, Straka H. Curr Biol. 2012;22:1649–1658. doi: 10.1016/j.cub.2012.07.019. [DOI] [PubMed] [Google Scholar]
- 10.Deol MS. J Mol Genet. 1968;5:137–158. doi: 10.1136/jmg.5.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crouch JJ, Sakaguchi N, Lytle C, Schulte BA. J Histochem Cytochem. 1997;45:773–778. doi: 10.1177/002215549704500601. [DOI] [PubMed] [Google Scholar]
- 12.Plotkin MD, et al. Am J Physiol. 1997;272:173–183. [Google Scholar]
- 13.Delpire E, Lu J, England R, Dull C, Thorne T. Nat Genet. 1999;22:192–195. doi: 10.1038/9713. [DOI] [PubMed] [Google Scholar]
- 14.Dixon MJ, et al. Hum Mol Genet. 1999;8:1579–1584. doi: 10.1093/hmg/8.8.1579. [DOI] [PubMed] [Google Scholar]
- 15.Flagella M, et al. J Biol Chem. 1999;274:26946–26955. doi: 10.1074/jbc.274.38.26946. [DOI] [PubMed] [Google Scholar]
- 16.Pace AJ, et al. J Clin Invest. 2000;105:441–450. doi: 10.1172/JCI8553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baldessarini RJ. Goodman Gilman's Textbook on Pharmacology. 11. McGraw Hill; USA: 2006. pp. 317–399. [Google Scholar]
- 18.Dubois NC, Hofmann D, Kaloulis K, Bishop JM, Trumpp A. Genesis. 2006;44:355–360. doi: 10.1002/dvg.20226. [DOI] [PubMed] [Google Scholar]
- 19.Gorski JA, et al. J Neurosci. 2002;22:6309–6314. doi: 10.1523/JNEUROSCI.22-15-06309.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kimmel RA, et al. Genes Dev. 2000;14:1377–1389. [PMC free article] [PubMed] [Google Scholar]
- 21.Stenman J, Toresson H, Campbell K. J Neurosci. 2003;23:167–174. doi: 10.1523/JNEUROSCI.23-01-00167.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Valjent E, et al. Proc Natl Acad Sci USA. 2005;102:491–496. doi: 10.1073/pnas.0408305102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Choe ES, Wang JQ. Neuroreport. 2002;13:1013–1016. doi: 10.1097/00001756-200206120-00006. [DOI] [PubMed] [Google Scholar]
- 24.Silva AJ, Kogan JH, Frankland PW, Kida S. Annu Rev Neurosci. 1998;21:27–48. doi: 10.1146/annurev.neuro.21.1.127. [DOI] [PubMed] [Google Scholar]
- 25.Gerdjikov TV, Ross GM, Beninger RJ. Behav Neurosci. 2004;118:740–750. doi: 10.1037/0735-7044.118.4.740. [DOI] [PubMed] [Google Scholar]
- 26.Salzmann J, Marie-Claire C, Le Guen S, Roques BP, Noble F. Br J Pharmacol. 2003;140:831–838. doi: 10.1038/sj.bjp.0705506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Atkins CM, Selcher JC, Petraitis JJ, Trzaskos JM, Sweatt JD. Nat Neurosci. 1998;1:602–609. doi: 10.1038/2836. [DOI] [PubMed] [Google Scholar]
- 28.Favata MF, et al. J Biol Chem. 1998;273:18623–18632. doi: 10.1074/jbc.273.29.18623. [DOI] [PubMed] [Google Scholar]
- 29.Selcher JC, Atkins CM, Trzaskos JM, Paylor R, Sweatt JD. Learn Mem. 1999;6:478–490. doi: 10.1101/lm.6.5.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shiroyama T, Kayahara T, Yasui Y, Nomura J, Nakano K. J Comp Neurol. 1999;407:318–332. [PubMed] [Google Scholar]
- 31.Balordi F, Fishell G. J Neurosci. 2007;27:14248–14259. doi: 10.1523/JNEUROSCI.4531-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schwenk F, Baron U, Rajewsky K. Nucleic Acids Res. 1995;23:5080–5081. doi: 10.1093/nar/23.24.5080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hébert JM, McConnell SK. Dev Biol. 2000;222:296–306. doi: 10.1006/dbio.2000.9732. [DOI] [PubMed] [Google Scholar]
- 34.Huynh T, Chen L, Terrell P, Baldini A. Genesis. 45:470–475. doi: 10.1002/dvg.20317. [DOI] [PubMed] [Google Scholar]
- 35.Ohyama T, Groves AK. Genesis. 38:195–199. doi: 10.1002/gene.20017. [DOI] [PubMed] [Google Scholar]
- 36.Duncan JS, Fritzsch B. PLoS One. 8:e62046. doi: 10.1371/journal.pone.0062046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Franklin KBJ, Paxinos G. The Mouse Brain in Stereotaxic Coordinates. 3rd. Elsevier; Amsterdam: 2008. [Google Scholar]
- 38.Holley FO, Magliozzi JR, Stanski DR, Lombrozo L, Hollister LE. Clin Pharmacol Ther. 1983;33:477–84. doi: 10.1038/clpt.1983.65. [DOI] [PubMed] [Google Scholar]
- 39.Woodard G. Methods of Animal Experimentation. Academic Press; New York: 1965. pp. 343–359. [Google Scholar]
- 40.Zetler G, Baumann GH. Pharmacology. 1985;31:318–327. doi: 10.1159/000138140. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.